COVID-19: Provincial and territorial vaccination programs in Canada

 Download this article as a PDF
Download this article as a PDFPublished by: The Public Health Agency of Canada
Issue: Volume 47-5/6: Responding to New Clusters of COVID-19
Date published: May/June 2021
ISSN: 1481-8531
Submit a manuscript
About CCDR
Browse
Volume 47-5/6: Responding to New Clusters of COVID-19
Overview
Environmental scan of provincial and territorial planning for COVID-19 vaccination programs in Canada
Shannon MacDonald1, Hannah Sell1,2, Sarah Wilson3, Samantha Meyer4, Arnaud Gagneur5, Ali Assi1, Manish Sadarangani6, and members of the COVImm Study Team
Affiliations
1 Faculty of Nursing, University of Alberta, Edmonton, AB
2 School of Public Health, University of Alberta, Edmonton, AB
3 Public Health Ontario, Toronto, ON
4 School of Public Health and Health Systems, University of Waterloo, Waterloo, ON
5 Département de pédiatrie, Université de Sherbrooke, Centre de Recherche du CHUS, Sherbrooke, QC
6 University of British Columbia, Vancouver, BC
Correspondence
Suggested citation
MacDonald SE, Sell H, Wilson S, Meyer SB, Gagneur A, Assi A, Sadarangani M and members of the COVImm Study Team. Environmental scan of provincial and territorial planning for COVID-19 vaccination programs in Canada. Can Commun Dis Rep 2021;47(5/6):285-91. https://doi.org/10.14745/ccdr.v47i56a07
Keywords: COVID-19, vaccination, vaccine, pandemic, vaccination program
Abstract
Background: Public health departments in Canada are currently facing the challenging task of planning and implementing coronavirus disease 2019 (COVID-19) vaccination programs.
Objective: To collect and synthesize information regarding COVID-19 vaccination program planning in each province and territory of Canada, including logistic considerations, priority groups, and vaccine safety and effectiveness monitoring.
Methods: Provincial/territorial public health leaders were interviewed via teleconference during the early planning stage of COVID-19 vaccination programs (August–October 2020) to collect information on the following topics: unique factors for COVID-19 vaccination, intention to adopt National Advisory Committee on Immunization (NACI) recommendations, priority groups for early vaccination, and vaccine safety and effectiveness monitoring. Data were grouped according to common responses and descriptive analysis was performed.
Results: Eighteen interviews occurred with 25 participants from 11 of 13 provinces/territories (P/Ts). Factors unique to COVID-19 vaccination included prioritizing groups for early vaccination (n=7), public perception of vaccines (n=6), and differing eligibility criteria (n=5). Almost all P/Ts (n=10) reported reliance on NACI recommendations. Long-term care residents (n=10) and healthcare workers (n=10) were most frequently prioritized for early vaccination, followed by people with chronic medical conditions (n=9) and seniors (n=8). Most P/Ts (n=9) are planning routine adverse event monitoring to assess vaccine safety. Evaluation of effectiveness was anticipated to occur within public health departments (n=3), by researchers (n=3), or based on national guidance (n=4).
Conclusion: Plans for COVID-19 vaccination programs in the P/Ts exhibit some similarities and are largely consistent with NACI guidelines, with some discrepancies. Further research is needed to evaluate COVID-19 vaccination programs once implemented.
Introduction
The race for the development of coronavirus disease 2019 (COVID-19) vaccines is well underway, with the first vaccines now approved for use in CanadaFootnote 1. Canadian public health officials are facing the next major challenge of the pandemic: planning and implementing the COVID-19 vaccination programs. Planning has been particularly challenging in comparison with other vaccines given the speed at which vaccine development has occurred, the need to manage multiple new but differing vaccines, and the fact that a large proportion of the population will need to be vaccinated to significantly interrupt the spread of the virus among the general populationFootnote 2Footnote 3. As initial vaccine supply is limitedFootnote 3Footnote 4, one important consideration is the prioritization of target groups for COVID-19 vaccination. The National Advisory Committee on Immunization (NACI) has released guidance outlining key populations for receiving initial vaccine supplyFootnote 3Footnote 5. However, it is ultimately up to provincial/territorial governments whether to follow these guidelines, and to determine the logistics of COVID-19 vaccination programs, including vaccine dose allocation, delivery, storage, administration, monitoring and reportingFootnote 6. Conversely, the role of the federal government is vaccine approval and procurement, and to provide guidance on vaccine useFootnote 6.
It is important to understand and document the processes and strategies that have been employed by each of the provinces/territories (P/Ts) in their COVID-19 vaccination planning. Identifying the range of strategies, highlighting new and innovative approaches, and learning from successful and unsuccessful approaches will enhance our capacity to respond to similar challenges we will undoubtedly face in the future. As such, the objective of this study was to use key informant interviews to collect and synthesize information regarding planned COVID-19 vaccination programs in each of the P/Ts, including logistic considerations, priority groups, and vaccine safety and effectiveness monitoring.
Methods
This pan-Canadian environmental scan involved structured key informant interviews of public health leaders from P/Ts across Canada. The research team included researchers from six P/Ts, as well as knowledge users from the NACI Secretariat and three P/T health departments. Knowledge users assisted in recruitment, identified topics of value to include in the interview guide, and were provided the study findings for their reference in decision-making. The goal of the scan was to capture and synthesize the perspectives of public health leaders actively involved in P/T immunization program planning. We recruited P/T members of the Canadian Immunization Committee (CIC), and when they were unavailable to participate, we asked them to designate a replacement. Additional participants were identified through our research team members, P/T health departments and the NACI Secretariat who are knowledgeable of P/T vaccine program leadership. Key informants were contacted via an initial email sent by the NACI Secretariat, inviting them to participate in the study. Interested individuals were emailed an information sheet and consent form. To optimize response rate, up to two email reminders were sent. Some participants were identified through snowball sampling, with study participants suggesting additional key informants. Interviews took place from August to October 2020, prior to release of NACI’s preliminary guidanceFootnote 3 and the approval of any COVID-19 vaccines in Canada. Interviews (35–60 minutes long) were conducted by members of the research team (HS, AA, MK) via teleconference.
Interview questions included key topics related to COVID-19 vaccination, as identified in scientific literature and news articles, and augmented with input from the immunization experts on the research team and knowledge users, including the NACI Secretariat (see Supplemental material). The structured interview guide consisted of mainly open-ended questions about the following topics: unique factors to be considered in COVID-19 vaccination program planning, the extent of reliance on NACI recommendations, the use of a geographical prioritization framework for vaccine allocation, target groups for prioritization for early vaccination, and plans for monitoring vaccine safety and effectiveness. The interview guide was reviewed and edited by immunization experts and pilot tested with an individual who worked in provincial immunization program planning, but was not involved in the study, to check face and content validity, flow, and comprehension. The inclusion of multiple perspectives within and between P/Ts enhanced the credibility of findings. The guide was shared with participants prior to the interview. Ethical approval for this study was obtained from the Health Research Ethics Board at the University of Alberta.
Interviews were audio-recorded and transcribed verbatim by one member of the research team and any personally identifying information was removed. The same team member then coded and categorized participant responses. Given the very structured nature of the interviews, analysis involved little subjective interpretation. However, to ensure rigor, coding and categorization were validated by three other team members to ensure they accurately reflected and were fully representative of participants’ responses. Descriptive analysis of response counts was performed using Microsoft Excel. Participant responses were synthesized and presented by P/T.
Results
Invitation emails from NACI were sent to 35 potential participants: 13 agreed to participate; one declined; and 21 did not respond. Twelve participants were recruited from other participants, five through referrals and seven joined the interviews of their colleagues. Therefore, some interviews contained more than one participant. In total, there were 18 interviews with 25 participants from 11 of the 13 P/Ts. Table 1 shows the demographics of the study sample.
| Characteristic | Number of participants, n |
|---|---|
| Province/territory | |
| British Columbia | 1 |
| Alberta | 4 |
| Saskatchewan | 3 |
| Manitoba | 4 |
| Ontario | 3 |
| Québec | 3 |
| Newfoundland and Labrador | 1 |
| Nova Scotia | 3 |
| New Brunswick | 0 |
| Prince Edward Island | 1 |
| Nunavut | 1 |
| Northwest Territories | 1 |
| Yukon | 0 |
| Perspective | |
| Provincial/territorial | 12 |
| Regional/municipal | 9 |
| Both | 4 |
| Job title | |
| Director of Immunization or Communicable Disease Control | 2 |
| Immunization Program or Policy Manager | 7 |
| Medical Officer of Health | 5 |
| Public Health or Medical Consultant | 3 |
| Policy Analyst | 2 |
| Public Health or Communicable Disease Specialist | 2 |
| Other | 4 |
Unique factors for COVID-19 vaccination programs
A wide array of factors that are unique to planning for COVID-19 vaccination programs were identified (see Table 2). Participants from slightly over half of P/Ts (n=7) indicated the need to prioritize target groups for early vaccination. Many P/Ts (n=5) also highlighted the possibility of having different eligibility criteria for each vaccine (i.e. if one vaccine is more effective in older adults), which may impact the order of priority groups.
| Unique factor | Number of P/TsTable 2 footnote a, n |
|---|---|
| Priority groups | |
| Prioritization of target groups | 7 |
| Differing eligibility criteria | 5 |
| Equity in delivery | 1 |
| Public engagement | |
| Public perception of the vaccine, including vaccine hesitancy | 6 |
| Clear communication with public | 3 |
| Logistics and supply | |
| Logistics, storage, cold-chain management | 4 |
| Limited vaccine supply, availability of vaccine | 3 |
| Availability of PPE and other vaccination supplies (other than the vaccine itself) | 3 |
| Vaccine distribution | 2 |
| Resource issues (in general) | 2 |
| Vaccine procurement | 1 |
| Delivery | |
| COVID-19 related restrictions, public health measures, PPE | 4 |
| Vaccine provider (e.g. physicians, pharmacists, public health) | 4 |
| Appointment-based delivery versus mass clinics | 3 |
| Need to vaccinate everyone, large volume of people | 3 |
| Less human resources due to COVID-19 redeployment | 3 |
| Training for providers | 2 |
| Uncertainty, not having enough information to plan | 2 |
| Vaccine characteristics | |
| Possibility of needing more than one dose | 4 |
| Vaccine safety | 3 |
| Dealing with a new vaccine | 3 |
| Considerations for the route of administration | 3 |
| Possibility of having more than one vaccine | 2 |
| Speed with which vaccine development is occurring | 2 |
Some participants from P/Ts also discussed factors related to public engagement, including having clear communication with the public regarding safety implications, eligibility criteria and priority groups (n=3 P/Ts). Likewise, six P/Ts highlighted the need to manage public perception of COVID-19 vaccines. Specifically, one P/T felt that vaccine hesitancy for COVID-19 vaccines would be greater than for previous vaccines.
The P/Ts also discussed unique factors related to logistics and supply of COVID-19 vaccines. Four P/Ts highlighted the unique storage requirements of some of the vaccines, with some P/Ts stating that it was unlikely that all providers currently had the capacity to store vaccines at the appropriate temperature. Others noted that supply of the vaccine (n=3) and other vaccination supplies (n=3) will likely be limited.
Planning for the delivery of the COVID-19 vaccines was anticipated to be challenging, with some P/Ts (n=4) reporting that they were unsure about which providers would deliver the vaccines (e.g. public health, physicians, pharmacists), or whether they would have appointment-based clinics or mass clinics (n=3). Similarly, four P/Ts mentioned the need for adapting vaccination clinics to follow COVID-19 recommendations, including physical distancing, personal protective equipment, layout, one-way flow of traffic and ventilation. One P/T mentioned including industrial engineers on their planning team to consider these factors. A full list of P/T responses is provided in Table 2.
Reliance on NACI recommendations
Almost all P/Ts (n=10) indicated that they would likely rely on the NACI recommendations for target groups in planning their COVID-19 vaccination strategies. One P/T indicated that they would more likely rely on their provincial/territorial immunization committee recommendations.
Priority group ranking
Participants were asked to rank their top five priority groups, with rank 1 representing the group that should receive COVID-19 vaccination first. For reporting purposes, we used the ranking of the respondent from each P/T that had the most cross-provincial perspective based on their job position and whether they stated they had a provincial perspective as opposed to regional/municipal. One P/T did not answer, for a total of 10 P/Ts. All of the P/Ts ranked long-term care residents (n=10) and healthcare workers (n=10) in the top five priority groups for receiving COVID-19 vaccination. Specifically, six P/Ts indicated long-term care residents as top priority, three indicated healthcare workers and one indicated seniors. Three P/Ts ranked healthcare workers second, followed by long-term care residents (n=2), people with chronic medical conditions (n=2), seniors (n=2), and essential workers (n=1). Groups ranked third included seniors (n=3), long-term care residents (n=2), healthcare workers (n=1), people with chronic medical conditions (n=1), people of Indigenous ancestry (n=1), those with socio-economic disadvantage (n=1) and people living in remote communities (n=1). Figure 1 provides a full summary of P/T rankings.
Figure 1: Provinces/territories’ priority group choices to include in their top five groups to be considered for early vaccination in the presence of limited vaccine supply(N=10)Figure 1 footnote aFigure 1 footnote b
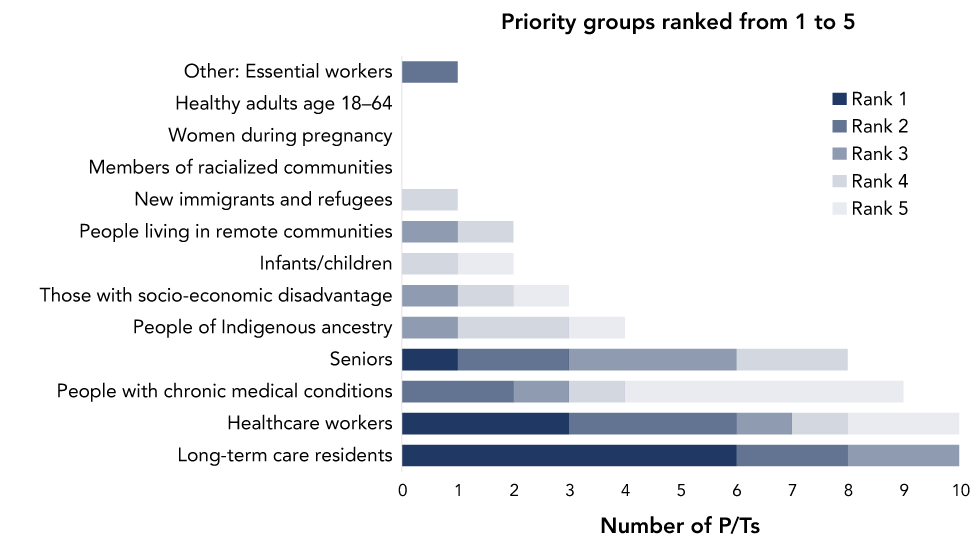
Text description: Figure 1
| Priority group | Number of P/TsFigure 1 footnote a, n | ||||
|---|---|---|---|---|---|
| Rank 1 | Rank 2 | Rank 3 | Rank 4 | Rank 5 | |
| Long-term care residents | 6 | 2 | 2 | 0 | 0 |
| Healthcare workers | 3 | 3 | 1 | 1 | 2 |
| People with chronic medical conditions | 0 | 2 | 1 | 1 | 5 |
| Seniors | 1 | 2 | 3 | 2 | 0 |
| People of Indigenous ancestry | 0 | 0 | 1 | 2 | 1 |
| Those with socio-economic disadvantage | 0 | 0 | 1 | 1 | 1 |
| Infants/children | 0 | 0 | 0 | 1 | 1 |
| People living in remote communities | 0 | 0 | 1 | 1 | 0 |
| New immigrants and refugees | 0 | 0 | 0 | 1 | 0 |
| Members of racialized communities | 0 | 0 | 0 | 0 | 0 |
| Women during pregnancy | 0 | 0 | 0 | 0 | 0 |
| Healthy adults age 18-64 | 0 | 0 | 0 | 0 | 0 |
| Other: Essential workers | 0 | 1 | 0 | 0 | 0 |
Use of a geographical prioritization framework
None of the P/Ts had firm plans for a geographical prioritization framework based on disease incidence (i.e. target groups in high COVID-19 incidence areas are prioritized over target groups in low incidence areas). The majority of P/Ts (n=7) were open to this approach if advised by NACI (n=1), or if the vaccine characteristics (n=1) or number of doses available (n=3) warrants it. Three P/Ts were against using a geographical prioritization framework due to concerns with the equity of this approach (n=1) or due to their jurisdiction’s small geography or dense population (n=2). One P/T did not know if they were planning on using a geographical prioritization framework.
Monitoring vaccine safety and effectiveness
With regards to post-market vaccine safety monitoring, most P/Ts (n=9) planned to conduct their routine adverse event monitoring, while some (n=3) anticipated enhanced surveillance of adverse events (see Table 3). Some P/Ts (n=4) anticipated that this will be done by federal/provincial/territorial committees and groups. For post-market vaccine effectiveness monitoring, some P/Ts (n=3) anticipated that their P/T public health departments would do this, with a similar number (n=3) stating that this will be routine information collected. Others (n=3) expected that this will be done by researchers or research organizations.
| Planned approach | Number of P/TsTable 3 footnote a, n |
|---|---|
| Safety | |
| Regular adverse event reporting | 9 |
| Enhanced surveillance of adverse events | 3 |
| Reliance on federal/provincial/territorial committees and groups (e.g. CIC, CIRC) | 4 |
| Reliance on what NACI recommends | 1 |
| Undecided | 1 |
| Do not know | 2 |
| Effectiveness | |
| Reliance on provincial public health (e.g. surveillance teams) | 3 |
| Reliance on researchers/research organizations | 3 |
| Reliance on NACI or other national guidance | 4 |
| Collect routine monitoring information (e.g. number of clients who tested positive after vaccination, vaccine coverage) | 3 |
| Undecided | 2 |
| Do not know | 1 |
| No answer | 2 |
Discussion
Although P/T rankings of potential priority groups were collected prior to the publication of NACI’s guidance documents, the overall P/T rankings aligned somewhat with NACI recommendations. Specifically, the groups ranked highest in this study were healthcare workers and long-term care residents, followed by people with chronic medical conditions and seniors. The most recent NACI recommendations prioritize healthcare workers, long-term care residents and staff, seniors aged 70 years and older (with those 80+ years having highest priority) and adults in Indigenous communitiesFootnote 5. The notable difference between the P/T rankings in our study and NACI recommendations is that less than half of P/Ts ranked Indigenous communities in the top five prioritized groups, and that people with chronic medical conditions (ranked third by most P/Ts) were not included in NACI’s most recent guidance on early vaccinationFootnote 5.
A common consideration among P/Ts was the potential negative public perception of COVID-19 vaccines. Many P/Ts recognized the important role public health will have in the development of communication strategies to counter these concerns. A Statistics Canada survey in June 2020 reported that 76.5% of Canadians would be very likely or somewhat likely to get a COVID-19 vaccine when availableFootnote 7, but data from a national Leger survey in November 2020 estimate this number to be 65%Footnote 8.
The P/Ts also highlighted the challenging logistics of vaccine delivery and the need to ensure that vaccination clinics follow public health recommendations on distancing, using personal protective equipment, disinfection and ventilation, etc. Multiple P/Ts viewed the 2020–2021 seasonal influenza program as a trial for how COVID-19 vaccine delivery may occur. Following the H1N1 pandemic, it was noted that well-functioning influenza vaccination programs are essential for ensuring that adequate infrastructure is available for pandemic vaccination responseFootnote 9. Guidance on strategies for influenza vaccine delivery during the pandemic were provided by NACI early in the pandemicFootnote 10.
Having a unified approach to COVID-19 vaccination in Canada may be beneficial for providing consistent public messaging and clarifying why certain priority groups have been selected for early vaccination. Public communication strategies are important to prevent vaccine hesitancy and mistrust Footnote 9. Furthermore, a unified approach to vaccination may improve equity and produce cost-savingsFootnote 11. Critics of Canada’s long-standing provincial and territorial variability in immunization programs and schedules have argued that lack of consistency in eligibility and modes of delivery results in inconsistencies in public messaging which can undermine public confidence when the rationale for differences is unclearFootnote 11Footnote 12. Conversely, diversity across P/Ts enables flexibility to adapt to the unique circumstances of each jurisdiction, given the variation in geography, population, and COVID-19 cases across P/TsFootnote 13. Although P/Ts will inevitably develop their own plans for COVID-19 vaccination, results from this study suggest that there will likely be many similarities.
Strengths and limitations
The strength of this study is the wide variety of perspectives that were obtained on COVID-19 vaccination program planning from most P/Ts. As well, the use of key informant interviews allowed us to gather in-depth perspectives on COVID-19 vaccination program planning in each P/T. However, as only a few select individuals were interviewed from each P/T, the perspectives gathered are not representative of the entire P/Ts. Furthermore, there may be variation in individual perspectives across a single P/T, although the perspectives shared were very consistent within a given P/T. Generalizability may be limited due to the small sample size and non-random sampling. Interviews were conducted during a period when COVID-19 vaccination planning was in its early stages. It will be interesting to follow whether early plans have changed since the release of NACI guidance documents Footnote 3Footnote 5.
Implications
The implementation of COVID-19 vaccination programs in Canada is in the early stages. There is an opportunity to expand on this study’s findings through a variety of research avenues, including the assessment of each P/T’s finalized COVID-19 vaccination plan, and how variation in vaccination programs ultimately affects vaccine uptake and effectiveness in each P/T.
This study adds to existing literature by synthesizing P/T public health perspectives on COVID-19 vaccination programs at a planning stage. Results can inform policymakers and program planners and can assist NACI in future development of national guidelines. We anticipate that the information in this study will enable P/Ts to learn from one another by comparing their approach to COVID-19 vaccination with others across Canada.
Conclusion
The key informant interview findings show that Canadian P/Ts are facing similar challenges in planning for COVID-19 vaccination. The majority will be relying on NACI recommendations regarding how to allocate limited vaccine supply. Further research is needed to evaluate provincial/territorial COVID-19 vaccination programs once they are implemented.
Authors’ statement
- SMacDonald — Conceptualization, methodology, funding acquisition, supervision, formal analysis, writing (original draft, and review and editing)
- HS — Conceptualization, investigation, data curation, formal analysis, writing (original draft, and review and editing)
- SW — Conceptualization, methodology, writing (review and editing)
- SMeyer — Conceptualization, methodology, writing (review and editing)
- AG — Conceptualization, methodology, funding acquisition, writing (review and editing)
- AA — Conceptualization, investigation, data curation, supervision, project administration, formal analysis, writing (review and editing)
- MS — Conceptualization, methodology, writing (review and editing)
The content and view expressed in this article are those of the authors and do not necessarily reflect those of the Government of Canada.
Competing interest
S MacDonald is supported by a salary award from the Canadian Child Health Clinician Scientist Program. MS is supported by salary awards from the BC Children’s Hospital Foundation, the Canadian Child Health Clinician Scientist Program and the Michael Smith Foundation for Health Research. MS has been an investigator on projects funded by GlaxoSmithKline, Merck, Pfizer, Sanofi-Pasteur, Seqirus, Symvivo and VBI Vaccines. All funds have been paid to his institute, and he has not received any personal payments.
Acknowledgements
This was part of a larger project conducted by the COVImm study team, which included the named authors, as well as: M Tunis, K Benzies, J Bettinger, M Driedger, E Dubé, R Humble, M Kiely, N MacDonald, E Rafferty, and J Robinson. We express particular thanks to M Kiely for her assistance with the interviews.
Funding
This work was funded by the Canadian Institutes of Health Research.
Supplemental material
Study interview guide (PDF)
References
- Footnote 1
-
Government of Canada. Vaccines for COVID-19: Authorized vaccines. Ottawa (ON): Government of Canada; (updated 2021; accessed 2021-03-05). https://www.canada.ca/en/health-canada/services/drugs-health-products/covid19-industry/drugs-vaccines-treatments/vaccines.html
- Footnote 2
-
MacDonald NE, Comeau JL, Dubé È, Bucci LM. COVID-19 and missed routine immunizations: designing for effective catch-up in Canada. Can J Public Health 2020;111(4):469-72. https://doi.org/10.17269/s41997-020-00385-4
- Footnote 3
-
Ismail SJ, Zhao L, Tunis MC, Deeks SL, Quach C; National Advisory Committee on Immunization. Key populations for early COVID-19 immunization: preliminary guidance for policy. CMAJ 2020;192(48):E1620-32. https://doi.org/10.1503/cmaj.202353
- Footnote 4
-
World Health Organization. WHO SAGE roadmap for prioritizing uses of COVID-19 vaccines in the context of limited supply. Geneva, Switzerland: WHO; 2020 (accessed 2021-03-05). https://www.who.int/publications/m/item/who-sage-roadmap-for-prioritizing-uses-of-covid-19-vaccines-in-the-context-of-limited-supply
- Footnote 5
-
National Advisory Committee on Immunization. Guidance on the prioritization of initial doses of COVID-19 vaccine(s). Ottawa (ON): Government of Canada; 2020 (accessed 2020-12-14). https://www.canada.ca/en/public-health/services/immunization/national-advisory-committee-on-immunization-naci/guidance-prioritization-initial-doses-covid-19-vaccines.html
- Footnote 6
-
Government of Canada. COVID-19 immunization: Federal, provincial and territorial statement of common principles. Ottawa (ON): Government of Canada; 2020 (accessed 2021-03-05). https://www.canada.ca/en/public-health/services/diseases/2019-novel-coronavirus-infection/canadas-reponse/covid-19-immunization-federal-provincial-territorial-statement-common-principles.html
- Footnote 7
-
Frank K, Arim R. Canadians' willingness to get a COVID-19 vaccine: Group differences and reasons for vaccine hesitancy. Ottawa (ON): StatCan; 2020 (accessed 2020-12-11). https://www150.statcan.gc.ca/n1/pub/45-28-0001/2020001/article/00073-eng.htm
- Footnote 8
-
Leger. North American tracker. Montreal (QC): Leger; 2020 (accessed 2020-12-11). https://leger360.com/wp-content/uploads/2020/11/Legers-North-American-Tracker-November-30th-2020-min.pdf?x43558
- Footnote 9
-
Sultana J, Mazzaglia G, Luxi N, Cancellieri A, Capuano A, Ferrajolo C, de Waure C, Ferlazzo G, Trifirò G. Potential effects of vaccinations on the prevention of COVID-19: rationale, clinical evidence, risks, and public health considerations. Expert Rev Vaccines 2020;19(10):919-36. https://doi.org/10.1080/14760584.2020.1825951
- Footnote 10
-
National Advisory Committee on Immunization (Advisory Committee Statement). Canadian Immunization Guide Chapter on Influenza and Statement on Seasonal Influenza Vaccine for 2020-2021. Ottawa (ON): PHAC; 2020 (accessed 2020-12-15). https://www.canada.ca/content/dam/phac-aspc/documents/services/publications/healthy-living/canadian-immunization-guide-statement-seasonal-influenza-vaccine-2020-2021/naci-2020-2021-seasonal-influenza-stmt-eng.pdf
- Footnote 11
-
Macdonald N, Bortolussi R. A harmonized immunization schedule for Canada: A call to action. Paediatr Child Health 2011;16(1):29-31. https://doi.org/10.1093/pch/16.1.29
- Footnote 12
-
A patchwork policy: vaccination in Canada. CMAJ 2003;168(5):533. https://www.cmaj.ca/content/168/5/533
- Footnote 13
-
Government of Canada. COVID-19 daily epidemiology update. Ottawa (ON): Government of Canada; 2021 (accessed 2021-03-09). https://health-infobase.canada.ca/covid-19/epidemiological-summary-covid-19-cases.html
