Update on mpox in Canada, March 2023

 Download this article as a PDF
Download this article as a PDFPublished by: The Public Health Agency of Canada
Issue: Volume 49-2/3, February/March 2023: Early Warning in Public Health
Date published: February/March 2023
ISSN: 1481-8531
Submit a manuscript
About CCDR
Browse
Volume 49-2/3, February/March 2023: Early Warning in Public Health
ID News
Update on mpox (monkeypox) in Canada, March 2023
Source: Public Health Agency of Canada. Emerging Science Group: Living evidence profile on the 2022 monkeypox outbreak, Highlights up to December 15, 2022. Full report available from: ocsoevidence-bcscdonneesprobantes@phac-aspc.gc.ca
Introduction
In May 2022, cases of monkeypox—recently renamed mpox by the World Health Organization (WHO)—started to appear in non-endemic countries. As of March 2023, cases have now been reported in over 100 countries Footnote 1. In July 2022, the WHO declared the mpox outbreak a Public Health Emergency of International Concern. This update describes the clinical features of the 2022 mpox outbreak, and provides an overview of the outbreak in Canada, the national public health response, and the implications for emerging pathogen outbreak preparedness in the future.
Current situation in Canada
Cases of mpox have been reported by nine provinces and the Yukon, but were largely centred in Ontario, Québec and British Columbia. The epidemic in Canada peaked in late June/early July when 25–30 new cases were being reported per day. Since the peak, the number of cases has declined rapidly, with only occasional cases reported since mid-November (Figure 1). As of March 3, 2023, Canada reported a cumulative total of 1,460 cases, with 44 reported hospitalizations and no deaths Footnote 2.
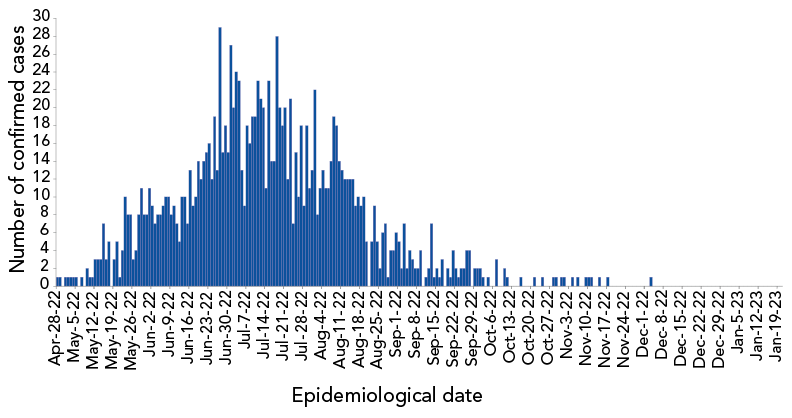
Figure 1 - Text description
| Epidemiological date | Number of confirmed cases |
|---|---|
| 28-Apr-22 | 1 |
| 29-Apr-22 | 1 |
| 1-May-22 | 1 |
| 2-May-22 | 1 |
| 3-May-22 | 1 |
| 4-May-22 | 1 |
| 5-May-22 | 1 |
| 7-May-22 | 1 |
| 9-May-22 | 2 |
| 10-May-22 | 1 |
| 11-May-22 | 1 |
| 12-May-22 | 3 |
| 13-May-22 | 3 |
| 14-May-22 | 3 |
| 15-May-22 | 7 |
| 16-May-22 | 3 |
| 17-May-22 | 5 |
| 19-May-22 | 3 |
| 20-May-22 | 5 |
| 21-May-22 | 1 |
| 22-May-22 | 4 |
| 23-May-22 | 10 |
| 24-May-22 | 8 |
| 25-May-22 | 8 |
| 26-May-22 | 3 |
| 27-May-22 | 4 |
| 28-May-22 | 8 |
| 29-May-22 | 11 |
| 30-May-22 | 8 |
| 31-May-22 | 8 |
| 1-Jun-22 | 11 |
| 2-Jun-22 | 9 |
| 3-Jun-22 | 7 |
| 4-Jun-22 | 8 |
| 5-Jun-22 | 8 |
| 6-Jun-22 | 9 |
| 7-Jun-22 | 10 |
| 8-Jun-22 | 10 |
| 9-Jun-22 | 8 |
| 10-Jun-22 | 9 |
| 11-Jun-22 | 7 |
| 12-Jun-22 | 5 |
| 13-Jun-22 | 10 |
| 14-Jun-22 | 10 |
| 15-Jun-22 | 7 |
| 16-Jun-22 | 13 |
| 17-Jun-22 | 9 |
| 18-Jun-22 | 10 |
| 19-Jun-22 | 14 |
| 20-Jun-22 | 12 |
| 21-Jun-22 | 14 |
| 22-Jun-22 | 15 |
| 23-Jun-22 | 16 |
| 24-Jun-22 | 12 |
| 25-Jun-22 | 19 |
| 26-Jun-22 | 13 |
| 27-Jun-22 | 29 |
| 28-Jun-22 | 15 |
| 29-Jun-22 | 18 |
| 30-Jun-22 | 15 |
| 1-Jul-22 | 27 |
| 2-Jul-22 | 20 |
| 3-Jul-22 | 24 |
| 4-Jul-22 | 23 |
| 5-Jul-22 | 13 |
| 6-Jul-22 | 9 |
| 7-Jul-22 | 18 |
| 8-Jul-22 | 16 |
| 9-Jul-22 | 19 |
| 10-Jul-22 | 19 |
| 11-Jul-22 | 23 |
| 12-Jul-22 | 21 |
| 13-Jul-22 | 20 |
| 14-Jul-22 | 11 |
| 15-Jul-22 | 23 |
| 16-Jul-22 | 14 |
| 17-Jul-22 | 14 |
| 18-Jul-22 | 28 |
| 19-Jul-22 | 20 |
| 20-Jul-22 | 18 |
| 21-Jul-22 | 20 |
| 22-Jul-22 | 12 |
| 23-Jul-22 | 21 |
| 24-Jul-22 | 7 |
| 25-Jul-22 | 15 |
| 26-Jul-22 | 10 |
| 27-Jul-22 | 18 |
| 28-Jul-22 | 9 |
| 29-Jul-22 | 18 |
| 30-Jul-22 | 11 |
| 31-Jul-22 | 13 |
| 1-Aug-22 | 22 |
| 2-Aug-22 | 8 |
| 3-Aug-22 | 11 |
| 4-Aug-22 | 13 |
| 5-Aug-22 | 11 |
| 6-Aug-22 | 11 |
| 7-Aug-22 | 14 |
| 8-Aug-22 | 19 |
| 9-Aug-22 | 18 |
| 10-Aug-22 | 14 |
| 11-Aug-22 | 13 |
| 12-Aug-22 | 12 |
| 13-Aug-22 | 12 |
| 14-Aug-22 | 12 |
| 15-Aug-22 | 12 |
| 16-Aug-22 | 9 |
| 17-Aug-22 | 10 |
| 18-Aug-22 | 9 |
| 19-Aug-22 | 10 |
| 20-Aug-22 | 5 |
| 22-Aug-22 | 5 |
| 23-Aug-22 | 9 |
| 24-Aug-22 | 5 |
| 25-Aug-22 | 2 |
| 26-Aug-22 | 6 |
| 27-Aug-22 | 7 |
| 28-Aug-22 | 1 |
| 29-Aug-22 | 4 |
| 30-Aug-22 | 4 |
| 31-Aug-22 | 6 |
| 1-Sep-22 | 5 |
| 2-Sep-22 | 2 |
| 3-Sep-22 | 7 |
| 4-Sep-22 | 2 |
| 5-Sep-22 | 4 |
| 6-Sep-22 | 3 |
| 7-Sep-22 | 2 |
| 8-Sep-22 | 2 |
| 9-Sep-22 | 4 |
| 11-Sep-22 | 1 |
| 12-Sep-22 | 2 |
| 13-Sep-22 | 7 |
| 14-Sep-22 | 1 |
| 15-Sep-22 | 2 |
| 16-Sep-22 | 1 |
| 17-Sep-22 | 3 |
| 19-Sep-22 | 2 |
| 20-Sep-22 | 1 |
| 21-Sep-22 | 4 |
| 22-Sep-22 | 2 |
| 23-Sep-22 | 1 |
| 24-Sep-22 | 2 |
| 25-Sep-22 | 2 |
| 26-Sep-22 | 4 |
| 27-Sep-22 | 4 |
| 29-Sep-22 | 2 |
| 30-Sep-22 | 2 |
| 1-Oct-22 | 2 |
| 2-Oct-22 | 1 |
| 4-Oct-22 | 1 |
| 7-Oct-22 | 3 |
| 10-Oct-22 | 2 |
| 11-Oct-22 | 1 |
| 16-Oct-22 | 1 |
| 21-Oct-22 | 1 |
| 24-Oct-22 | 1 |
| 28-Oct-22 | 1 |
| 29-Oct-22 | 1 |
| 31-Oct-22 | 1 |
| 1-Nov-22 | 1 |
| 4-Nov-22 | 1 |
| 6-Nov-22 | 1 |
| 9-Nov-22 | 1 |
| 10-Nov-22 | 1 |
| 11-Nov-22 | 1 |
| 14-Nov-22 | 1 |
| 17-Nov-22 | 1 |
| 3-Dec-22 | 1 |
Epidemiologic and clinical features of 2022 mpox outbreak
The outbreak began in May 2022, and some 680 studies had been published by mid-December 2022. Based on the global scientific literature, the median incubation period spans 7–9.6 days from exposure until the appearance of first symptoms, with a range of 2–21 days. This current estimate is similar to historical data for this disease.
Data obtained globally, including from a study in Montréal, Québec, show the mpox outbreak largely affected the gay, bisexual and men who have sex with men (gbMSM) community, and transmission was predominantly associated with intimate sexual contact Footnote 3. Other exposures not associated with intimate contact occurred among household members who had close (non-sexual) contact. Sporadic cases of mpox among healthcare workers were reported, but only a small number of cases, all outside Canada, were associated with workplace exposure. Fomite transmission was rare, based on a small number of transmission events in healthcare workers, and contaminated piercing and tattoo establishments, despite the fact the mpox virus can survive on hard surfaces for days.
The clinical presentation of cases in the 2022 outbreak had notable new characteristics when compared with historical descriptions. Most cases had at least one skin lesion and, in about half of the cases, lesions appeared before other symptoms—including fever, headache and malaise. Often the first lesion appeared at the place of inoculation and then spread to other areas of the body; for many cases, the first lesion was in the mouth or anogenital region. Mpox lesions can be hard to differentiate from lesions of other sexually transmissible infections, such as chancre or syphilis. Furthermore, some cases presented with few or no lesions, had lesions that did not spread or had asynchronous lesions (appearing at different stages of development). Proctitis and pharyngitis were common and were sometimes accompanied by significant pain. Some cases required health care, largely for pain control or treatment of secondary infections. Symptoms usually lasted 2–4 weeks. Vision impairment due to ocular mpox is rare but has been reported. Other complications, such as myocarditis or encephalitis, have also been described.
There are eight recent studies reporting on the clinical use and safety of tecovirimat (TPOXX) or cidofovir for treatment of mpox, but there is limited evidence on treatment effectiveness. Emerging data on the Bavarian Nordic vaccine, Imvamune®, has shown variable effectiveness across studies. The early COSMOS study, with data up to October 31, 2022, found vaccine efficacy was 37% after one dose and 69% after two doses, with no difference by sex, immune status or type of injection Footnote 4. However, a more recent publication estimated 86% effectiveness from a single subcutaneous dose Footnote 5
Canada's response
Health is a shared responsibility in Canada; so the mpox outbreak required a federal/provincial/territorial (FPT) public health response plan to contain it Footnote 6. The objectives of this plan were to reduce the impact of mpox, stop the chains of transmission, minimize the risk that mpox becomes established in Canada, and ensure the clinical and public health response were based on the best available evidence.
Canada's National Microbiology Laboratory was the first to provide diagnostic testing for mpox and worked closely with provincial and territorial (PT) public health laboratories to increase their testing capacity. Information on testing procedures was developed and distributed locally. The Public Health Agency of Canada (PHAC) established a national surveillance system, in collaboration with FPT partners. The PT health authorities compiled and forwarded de-identified data to PHAC, which collated the data to produce national statistics, and shared regular reports with the WHO.
Federal/provincial/territorial collaboration led to clinical and public health guidance documents that were distributed nationally, such as the case and contact management guidance Footnote 7 and guidance on reducing the risk of spread in community settings Footnote 8. PHAC and PTs facilitated the deployment of nearly 100K doses of Imvamune vaccine across the country, and implemented vaccine safety surveillance through systems already in place. The National Advisory Committee on Immunization issued vaccine guidelines for at-risk populations Footnote 9. PHAC's Chief Public Health Officer and Chief Science Officer established an expert panel to engage researchers and clinical experts from across Canada to share knowledge and shape the national public health response. PHAC participated in the World Health Organization Research and Development Blueprint meetings held to discuss mpox science and research gaps, and how best to assess vaccine effectiveness. These science leadership activities helped to inform domestic science and research priorities, which in turn were leveraged for funding of international mpox research by the Canadian Institutes of Health Research and the International Development Research Centre.
Federal/provincial/territorial collaboration also included partnerships with community organizations, particularly those serving the gbMSM population and focusing on sexual health. This led to raised awareness about the mpox outbreak at PRIDE events, including information on ways to limit its spread, including vaccination. Surveys of high-risk populations during the outbreak indicated that, 40%–69% of people reduced their number of sexual partners to reduce their risk of mpox infection and transmission.
Conclusion
Work continues across Canada and abroad to increase our understanding of the virus and our ability to prevent, detect and treat new cases. The response to mpox occurred while the Canadian health system and public health authorities were dealing with the continuing COVID-19 pandemic, resurgent respiratory infections, and the need to ensure domestic preparedness due to the Sudan virus outbreak in Uganda. Addressing these epidemics illustrates the urgent and ongoing need for Canada's clinical and public health systems further develop and sustain their capacity to detect and manage multiple national infectious disease emergencies simultaneously.
References
- Footnote 1
-
World Health Organization. Multi-country outbreak of mpox, External situation report #13 - 5. Geneva (CH): WHO; January 5, 2023. [Accessed 2023 Jan 26]. https://www.who.int/publications/m/item/multi-country-outbreak-of-mpox--external-situation-report--13---5-january-2023
- Footnote 2
-
Public Health Agency of Canada. Monkeypox epidemiology update. Ottawa, ON: PHAC. [Accessed 2023 Mar 6]. https://health-infobase.canada.ca/monkeypox/
- Footnote 3
-
Harrison LB, Bergeron G, Cadieux G, Charest H, Fafard J, Levade I, Blais AC, Huchet E, Trottier B, Vlad D, Szabo J, Thomas R, Poulin S, Greenaway C, Zaharatos GJ, Oughton M, Chakravarti A, Pilarski R, Bui-Nguyen A, Benomar K, Libman MD, Vinh DC, Duggan AT, Graham M, Klein MB, Barkati S. Monkeypox in Montréal: Epidemiology, phylogenomics, and public health response to a large North American outbreak. Ann Intern Med 2023;176(1):67–76. https://doi.org/10.7326/M22-2699
- Footnote 4
-
Center for Disease Control and Prevention. Preliminary JYNNEOS Vaccine Effectiveness Estimates Against Medically Attended Mpox Disease in the U.S., August 15, 2022 – October 29, 2022. [Accessed 2023 Jan 26]. https:///www.cdc.gov/poxvirus/monkeypox/cases-data/mpx-JYENNOS-vaccine-effectiveness.html
- Footnote 5
-
Sagy YW, Zucker R, Hammerman A, Markovits H, Arieh NG, Ahmad WA, Battat E, Ramot N, Carmeli G, Mark-Amir A, Wagner-Kolasko G, Duskin-Bitan H, Yaron S, Peretz A, Arbel R, Lavie G, Netzer D. Real-world effectiveness of a single dose of mpox vaccine in males. Nat Med 2023. https://doi.org/10.1038/s41591-023-02229-3
- Footnote 6
-
Public Health Agency of Canada. Federal, Provincial and Territorial Public Health Response Plan for the Management of the Monkeypox Outbreak. Ottawa, ON: PHAC; 2022. [Accessed 2023 Jan 26]. https://www.canada.ca/en/public-health/services/diseases/monkeypox/technical-documents/federal-provincial-territorial-public-health-response-plan-management.html
- Footnote 7
-
Public Health Agency of Canada. Monkeypox: Public health management of cases and contacts in Canada. Ottawa, ON: PHAC; October 2022. [Accessed 2023 Jan 26]. https://www.canada.ca/en/public-health/services/diseases/monkeypox/health-professionals/management-cases-contacts.html
- Footnote 8
-
Public Health Agency of Canada. Monkeypox: How operators can reduce the risk of spread in community settings. Ottawa, ON: PHAC; December 2022. [Accessed 2023 Jan 26]. https://www.canada.ca/en/public-health/services/diseases/monkeypox/risks/how-operators-can-reduce-spread-community-settings.html
- Footnote 9
-
Public Health Agency of Canada. An Advisory Committee Statement (ACS) National Advisory Committee on Immunization (NACI). NACI Rapid Response: Updated interim guidance on the use of Imvamune in the context of monkeypox outbreaks in Canada. Ottawa, ON: PHAC; November 2022. [Accessed 2023 Jan 26]. https://www.canada.ca/en/public-health/services/publications/vaccines-immunization/rapid-response-updated-interim-guidance-imvamune-monkeypox-outbreaks.html
