ARCHIVED - Organized Breast Cancer Screening Programs in Canada - Report on Program Performance in 2003 and 2004
Introduction
An estimated 22,400 women will be diagnosed with breast cancer and 5,300 women will die from the disease in 2008 (1). This makes breast cancer the most common form of cancerFootnote 1 and the second leading cancer cause of death in Canadian women (1). Although breast cancer incidence has risen over the past decades, it has levelled off and is showing statistically non-significant declines since 1999. In addition, deaths attributed to breast cancer continue to decline and are approximately 25% lower than the peak in the mid-1980’s (2) (Figure 1a and 1b).
Figure 1a – Age-standardized incidence rates (ASIR) per 100,000 women for breast cancer in Canada, 1982-2008
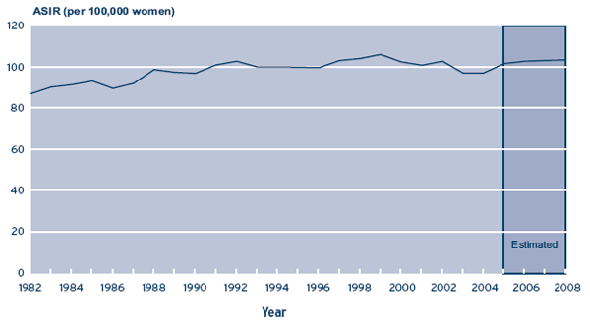
Source: National Cancer Institute of Canada. Canadian Cancer Statistics 2008. Toronto, Canada, 2008.
Notes: Incidence rates are estimated for 2006-2008 and in 2005 for Québec, Manitoba and Alberta. Projected estimates for breast cancer beyond 2004 reflect the long-term increasing trend in breast cancer incidence and are not sensitive to recent decline.
The national rate is an estimate computed from observed case counts for all provinces and territories.
Rates are standardized to the age distribution of the 1991 population.
Figure 1b – Age-standardized mortality rates (ASMR) per 100,000 women for breast cancer in Canada, 1982-2008

Source: National Cancer Institute of Canada. Canadian Cancer Statistics 2008. Toronto, Canada, 2008.
Notes: Mortality rates are estimated for 2005-2008.
The national rate is an estimate computed from the death counts estimated for each province and territory.
Rates are standardized to the age distribution of the 1991 population.
Early detection of breast cancer, through organized mammography screening programs, is an effective method to reduce death and morbidity associated with breast cancer. This is partially because primary prevention of breast cancer has been limited: most known risk factors are not easily modifiable, however, changes in some of the more modifiable risk factors at the population level such as physical activity holds future promise. Of known risk factors, age has the strongest influence on breast cancer incidence; roughly, half of all new cases are among women between 50 and 69 years of age. Modelling exercises have shown that the delivery of high quality breast screening programs to this age group has the potential to reduce breast cancer deaths by as much as one third (4). Among other considerations, this scientific information influences Canadian provinces and territories to provide breast cancer screening services to this age group. Many provinces and territories also provide screening services to other age groups but in a less targeted fashion.
Breast Cancer Screening in Canada
In December 1992, the federal government launched the first phase of the Canadian Breast Cancer Initiative (CBCI) with $25 million over five years including the Canadian Breast Cancer Screening Initiative among its priorities. In June 1998, ongoing funding for the CBCI was put in place at $7 million per year. The Public Health Agency of Canada was created in September 2004 and at that time was given responsibility for overseeing the CBCI.
Organized Breast Cancer Screening Programs
Canada’s first organized breast cancer screening program began in British Columbia in 1988 and was followed quickly by most provinces (Table 1). Organized breast cancer screening programs now exist in all provinces, and the Northwest and Yukon Territories. Nunavut has not developed an organized mammography screening program.
All organized programs provide women between 50 and 69, without a prior diagnosis of breast cancer, with a bilateral, 2-view screening mammogram biennially. Some programs also include women outside of this age group and some provide screening at more frequent intervals (Table 1). In 2003 and 2004, several programs provided clinical breast examination by a nurse or technologist but most programs had phased out this service based on scientific evidence (5). Lastly, some programs include breast cancer survivors; however, survivors within five years of their diagnosis are excluded from this report.
The Screening Process
Organized breast cancer screening programs offer screening to women who are asymptomatic for breast cancer. Organized programs in Canada typically involved three steps:
- Identification and invitation of the target population
- Provision of a screening examination
- Follow-up of any abnormalities detected at screening
A number of methods are used to invite women to a screening examination and include media campaigns targeting women, population-based invitations, physician education to increase referrals, and personal invitations. Women may enter into organized programs through their personal letter of invitation, physician referral or self referral.
Table 1 – Breast cancer screening programs in CanadaTable 1 - Footnote 1 a – usual practices, 2003 and 2004 screen years
| Province/territory | Program start date | Clinical breast examination on site | Program practices for women outside the 50-69 year age group | ||
|---|---|---|---|---|---|
| Age group | Accept | Recall | |||
|
|||||
| Northwest Territories | 2003 | No | 40-49 | Yes | Annual |
| 70+ | Yes | Biennial | |||
| Yukon Territory | 1990 | No | 40-49 | Yes | None |
| 70+ | Yes | None | |||
| British Columbia | 1988 | No | <40 | YesTable 1 - Footnote 2b | None |
| 40-49 | Yes | Annual | |||
| 70-79 | Yes | Biennial | |||
| 80+ | YesTable 1 - Footnote 2b | None | |||
| Alberta | 1990 | No | 40-49 | Yes | Annual |
| 70-74 | Yes | Biennial | |||
| 75+ | Yes | None | |||
| Saskatchewan | 1990 | No | 40-49 | No | N/A |
| 70-74 | Yes | Biennial | |||
| 75+ | Yes | None | |||
| Manitoba | 1995 | YesTable 1 - Footnote 6 f | 40-49 | YesTable 1 - Footnote 3 c | Biennial |
| 70+ | YesTable 1 - Footnote 3 c | None | |||
| Ontario | 1990 | YesTable 1 - Footnote 7 g | 40-49 | No | N/A |
| 70-74 | Yes | Biennial | |||
| 75+ | Yes | None | |||
| Québec | 1998 | No | 35-49 | YesTable 1 - Footnote 4 d | Biennial |
| 70+ | YesTable 1 - Footnote 4 d | None | |||
| New Brunswick | 1995 | No | 40-49 | YesTable 1 - Footnote 2 b | None |
| 70+ | YesTable 1 - Footnote 2 b | None | |||
| Nova Scotia | 1991 | YesTable 1 - Footnote 8 h | 40-49 | Yes | Annual |
| 70+ | Yes | None | |||
| Prince Edward Island | 1998 | YesTable 1 - Footnote 8 h | 40-49 | Yes | Annual |
| 70-74 | Yes | Biennial | |||
| Newfoundland and Labrador | 1996 | YesTable 1 - Footnote 9 i | 40-49 | No | N/A |
| 70+ | YesTable 1 - Footnote 5 e | None | |||
Screening mammograms are provided at both fixed and mobile sites. Fixed sites are located in larger urban areas while mobile sites are used to provide service to rural and distant communities.
Results of a screening mammogram are provided to both the woman and her physician. In general, women who have normal screening results are invited back for subsequent screening through a letter of invitation. The interval is generally 24 months; however, some women are invited back after 12 months based on their age, breast density, family history, and results of their screening. After receipt of normal results, women are encouraged to follow-up with their family physicians if they become symptomatic prior to their next scheduled screening visit.
In the case of abnormal results, both the woman and her family physician are informed. The family physician or the screening program then provides coordination of follow-up. This process varies by region. The follow-up process is resolved when a final diagnosis of cancer or normal / benign is concluded (Figure 2).
Figure 2 – Pathway of a breast cancer screening program

Canadian Breast Cancer Screening Database
Monitoring and evaluation of organized breast cancer screening programs through the systematic collection, analysis, and interpretation of health data, allows for the enhancement of programming across Canada. The Canadian Breast Cancer Screening Database (CBCSD) provides a method to examine and assess Canadian organized breast cancer screening programs. The CBCSD was established in 1993 and is operated and maintained by the Public Health Agency of Canada on behalf of the Canadian Breast Cancer Screening Initiative. Participating provincial and territorial screening programs contribute to the national database while retaining ownership over their data.
The CBCSD contains screening information from the inception of each organized screening program up to December 2004. At the present time Yukon does not keep electronic records and Nunavut does not have an organized program so they are excluded from the database. At every screening event, data including demographic characteristics, risk factors, the screen event, screening results and subsequent referral, diagnostic tests, outcomes, and cancer information is collected.
Table 2 – Annual screening volume by program, all ages, 1988 to 2004 screen years
| Year | Program | |||||
|---|---|---|---|---|---|---|
| NT | BC | AB | SK | MB | ON | |
| 1988 | --- | 4,395 | --- | --- | --- | --- |
| 1989 | --- | 9,188 | --- | --- | --- | --- |
| 1990 | --- | 22,482 | 616 | 6,355 | --- | 590 |
| 1991 | --- | 54,564 | 5,873 | 14,305 | --- | 15,380 |
| 1992 | --- | 80,893 | 15,442 | 15,778 | --- | 40,295 |
| 1993 | --- | 100,276 | 16,146 | 26,057 | --- | 45,541 |
| 1994 | --- | 118,878 | 15,372 | 25,540 | --- | 55,480 |
| 1995 | --- | 143,412 | 14,170 | 29,603 | 2,671 | 58,287 |
| 1996 | --- | 166,738 | 14,679 | 28,901 | 13,594 | 67,729 |
| 1997 | --- | 173,908 | 23,336 | 33,915 | 19,163 | 80,132 |
| 1998 | --- | 189,963 | 18,887 | 34,094 | 23,457 | 98,597 |
| 1999 | --- | 217,551 | 22,408 | 35,050 | 28,204 | 114,059 |
| 2000 | --- | 223,610 | 21,714 | 35,265 | 28,566 | 138,308 |
| 2001 | --- | 224,566 | 23,745 | 36,133 | 28,728 | 163,862 |
| 2002 | --- | 234,873 | 23,338 | 34,344 | 29,263 | 192,237 |
| 2003 | --- | 220,933 | 21,806 | 35,477 | 31,637 | 211,926 |
| 2004 | 1,103 | 230,830 | 23,098 | 35,950 | 32,301 | 248,551 |
| Total | 1,103 | 2,417,060 | 260,630 | 426,767 | 237,584 | 1,530,974 |
Table 2 – Annual screening volume by program, all ages, 1988 to 2004 screen years (con’t)
| Year | Program | |||||
|---|---|---|---|---|---|---|
| QCTable 2b - Footnote 1 a | NB | NS | PE | NL | Canada | |
| Table 2b - Footnote 1 a Although Québec accepts women aged 35-49 and 70+ with physician referral if done at a program screening centre, they are not officially considered within the program. Notes: Yukon Territory and Nunavut programs are still in development. Data include all screens; figures have been updated and may vary slightly from previous reports. | ||||||
| 1988 | --- | --- | --- | --- | --- | 4,395 |
| 1989 | --- | --- | --- | --- | --- | 9,188 |
| 1990 | --- | --- | --- | --- | --- | 30,043 |
| 1991 | --- | --- | 1,877 | --- | --- | 91,999 |
| 1992 | --- | --- | 4,354 | --- | --- | 156,762 |
| 1993 | --- | --- | 4,891 | --- | --- | 192,911 |
| 1994 | --- | --- | 8,461 | --- | --- | 223,731 |
| 1995 | --- | 5,853 | 12,491 | --- | --- | 266,487 |
| 1996 | --- | 18,441 | 15,547 | --- | 3,120 | 328,749 |
| 1997 | --- | 18,247 | 19,477 | --- | 4,694 | 372,872 |
| 1998 | 43,987 | 26,044 | 25,459 | --- | 5,521 | 466,009 |
| 1999 | 145,107 | 30,623 | 29,285 | 5,578 | 6,087 | 633,952 |
| 2000 | 152,989 | 32,620 | 35,260 | 6,268 | 6,790 | 681,390 |
| 2001 | 172,062 | 33,681 | 35,260 | 6,700 | 8,054 | 732,791 |
| 2002 | 194,368 | 37,196 | 38,612 | 6,267 | 8,859 | 799,357 |
| 2003 | 207,816 | 37,433 | 44,998 | 6,094 | 11,038 | 829,158 |
| 2004 | 220,821 | 37,344 | 48,655 | 6,060 | 9,819 | 894,532 |
| Total | 1,137,150 | 277,482 | 324,627 | 36,967 | 63,982 | 6,714,326 |
The database is currently used for monitoring, evaluation, and applied screening research. Research priorities are identified on an ongoing basis and the CBCSD is made available to researchers external to the Canadian Breast Cancer Screening Initiative.
Monitoring and Evaluation Using the CBCSD
Monitoring and evaluation of organized screening programs is essential to ensure Canadian women are receiving high quality services that result in the reduction of morbidity and mortality from breast cancer while minimizing the unwanted effects of screening. The results of monitoring and evaluation stemming from the CBCSD are used to enhance the performance of organized screening programs in Canada.
In order to provide fair evaluation for Canadian organized breast screening programs, standardized methods of evaluation have been developed. For detailed information please refer to the most recent Evaluation Indicators Working Group ReportFootnote 2 . In general, agreed upon performance indicators for women aged 50 to 69 include those related to recruitment and retention (participation rate, retention rate), timeliness (diagnostic interval), mammography interpretation (abnormal call rate, positive predictive value), diagnosis (invasive and in situ cancer detection rate, benign:malignant open surgical biopsy ratio, benign:malignant core biopsy ratio, benign open surgical biopsy rate, benign core biopsy rate), and cancers (tumour size, node negative rate in invasive cancers, post-screen invasive cancer rate) (Table 3).
Table 3 – Performance measures for organized breast cancer screening programs in Canada, women aged 50-69
| Indicator | Definition | Target |
|---|---|---|
Source: Public Health Agency of Canada. Report from the Evaluation Indicators Working Group: Guidelines for Monitoring Breast Cancer Screening Program Performance: Second edition. Ottawa: Minister of Health, 2007. Note: Table adapted from the Program Performance Measures. |
||
| 1. Participation rate | Percentage of women who have a screening mammogram (calculated biennially) as a proportion of the eligible population. | ≥70% of the eligible population. |
| 2. Retention rate | The estimated percentage of women who are re-screened within 30 months of their previous screen. | ≥75% initial re-screen within 30 months; ≥90% subsequent re-screens within 30 months. |
| 3. Abnormal call | rate Percentage of women screened who are referred for further testing because of abnormalities found with a program screen. | <10% (initial screen); <5% (subsequent screens). |
| 4. Invasive cancer detection rate | Number of invasive cancers detected per 1,000 screens. | >5 per 1,000 (initial screen) >3 per 1,000 (subsequent screens). |
| 5. In situ cancer detection rate | Number of ductal carcinoma in situ cancers (rather than invasive cancer) during a screening episode per 1,000 screens. | Surveillance and monitoring purposes only. |
| 6. Diagnostic interval | Total duration from abnormal screen to resolution of abnormal screen. | ≥90% within 5 weeks if no tissue biopsyTable 3 - Footnote 1 a performed; ≥90% within 7 weeks if tissue biopsyTable 3 - Footnote 1 a performed. |
| 7. Positive predictive value | Proportion of abnormal cases with completed follow-up found to have breast cancer (invasive or in situ) after diagnostic work-up. | ≥5% (initial screen); ≥6% (subsequent screens). |
| 8. Benign open surgical biopsyTable 3 - Footnote 2 b rate | The number of benign open surgical biopsies per 1,000 screens. | Surveillance and monitoring purposes only. |
| 9. Benign to malignant open surgical biopsyTable 3 - Footnote 2 b ratio | Among open surgical biopsies, the ratio of the number of benign cases to the number of malignant cancer cases. | ≤1:1 (initial screen); ≤1:1 (subsequent screens). |
| 10. Benign core biopsy rate | The number of benign core biopsies per 1,000 screens. | Surveillance and monitoring purposes only. |
| 11. Benign to malignant core biopsy ratio | Among core biopsies, the ratio of number of benign cases to the number of malignant cancer cases. | Surveillance and monitoring purposes only. |
| 12. Invasive cancer tumour size | Percentage of invasive cancers with tumour size of .10mm and .15mm in greatest diameter as determined by the best available evidence: 1) pathological, 2) radiological, and 3) clinical. | >25% ≤10mm; >50% ≤15mm. |
| 13. Node negative rate in cases of invasive cancer | Proportion of invasive cancers in which the cancer has not invaded the lymph nodes. | >70% (initial and subsequent screens). |
| 14. Post-screen invasive cancer rateTable 3 - Footnote 3 c | Number of women with a diagnosis of invasive breast cancer after a normal screening within 12 AND 24 months of the screen date. | <6 per 10,000 person-years (within 12 months); <12 per 10,000 person-years (within 24 months). |