Guidance for the response and management of a poliovirus event or outbreak in Canada
Download in PDF format
(1,097 KB, 50 pages)
Organization: Public Health Agency of Canada
Date published: September 2024
Cat.: HP40-343/2023E-PDF
ISBN: 978-0-660-49135-6
Pub.: 230128
Update: September 2024
Table of contents
- Acronyms
- Goals and objectives
- 1. Introduction
- 2. Definition of a poliovirus event or outbreak
- 3. Importance of rapid detection, notification, and investigation
- 4. Federal/provincial/territorial roles and responsibilities
- 5. Case classifications
- 6. Detection
- 7. Notification
- 8. Investigation
- 9. Management of cases and contacts
- 10. Risk assessment
- 11. Public health response to a poliovirus event or outbreak in Canada
- Acknowledgements
- Resources
- Annexes
- Selected references
Acronyms
- AFP
- Acute Flaccid Paralysis
- bOPV
- Bivalent Oral Poliovirus Vaccine
- CCDR
- Canada Communicable Disease Report
- CDC
- Centers for Disease Control and Prevention
- CPS
- Canadian Paediatric Society
- CPSP
- Canadian Paediatric Surveillance Program
- CNPHI
- Canadian Network for Public Health Intelligence
- GAP
- Global Action Plan (for poliovirus containment)
- GPEI
- Global Polio Eradication Initiative
- HPTA
- Human Pathogens and Toxins Act
- IHR
- International Health Regulations (2005)
- IPV
- Inactivated Poliovirus Vaccine
- NAC
- National Authority for Containment (of Poliovirus)
- NACI
- National Advisory Committee on Immunization
- nOPV
- Novel Oral Poliovirus Vaccine
- NML
- National Microbiology Laboratory
- OPV
- Oral Poliovirus Vaccine
- PHEIC
- Public Health Emergency of International Concern
- PAHO
- Pan American Health Organization
- PEF
- Poliovirus Essential Facility
- PHAC
- Public Health Agency of Canada
- PIM
- Potentially Infectious Material (poliovirus)
- PT
- Provinces and Territories or Provincial/Territorial
- PID
- Primary Immunodeficiency Disorder
- SOP
- Standard Operating Procedure(s)
- VAPP
- Vaccine-Associated Paralytic Poliomyelitis
- cVDPV
- Circulating Vaccine-Derived Poliovirus
- VDPV
- Vaccine-Derived Poliovirus
- WHO
- World Health Organization
- WPV
- Wild Poliovirus
Goals and objectives
The Guidance for the Response and Management of a Poliovirus Event or Outbreak in Canada provides best practices to help prepare for, detect, and control poliovirus and defines roles and responsibilities for a coordinated response across the country. The objectives of the actions described in the Guidance are:
- Enhance rapid and early detection, notification, and investigation to support timely implementation of public health controls to limit spread and support global polio eradication
- Reduce the health impact of poliovirus on the people of Canada
- Ensure public health responses based on best practices, scientific evidence, and expert input
1. Introduction
1.1 Polio
Polio (poliomyelitis) is a highly infectious viral disease that can infect the central nervous system and damage the nerve cells that activate muscles. While most people infected with poliovirus show no symptoms, others will become symptomatic, including a small proportion who can develop serious symptoms such as paralysis. In countries with low childhood immunization coverage, poliovirus infections are more common in children less than five years of age; however, any person who is not immune to poliovirus, regardless of age, can become infected. Transmission of poliovirus occurs predominantly through the fecal-oral route, particularly in areas with poor sanitation (that is, where excreted virus can contaminate food or water, or spread directly from one person to the other). Spread via the respiratory route is also possible but is less frequent.
The international spread of poliovirus has been identified as a public health emergency of international concern (PHEIC) by the World Health Organization (WHO) Director-General since 2014. The PHEIC introduced temporary recommendations to reduce the international spread to support polio eradication and prevent reintroduction of poliovirus in regions that have achieved elimination.Reference 1
Three serotypes of wild poliovirus (WPV) exist – type 1, type 2, and type 3. Due to the efforts of the Global Polio Eradication Initiative (GPEI), WPV type 2 was declared eradicated in September 2015, with the last case detected in India in 1999.Reference 2 WPV type 3 was declared eradicated in October 2019, with the last case detected in Nigeria in 2012.Reference 3 As of September 2024, WPV type 1 remains endemic in Pakistan and Afghanistan.
There is no specific medication for polio and care is supportive in nature. The disease can be prevented through vaccination. There are two types of polio vaccines in use globally, the inactivated poliovirus vaccine (IPV) and the oral poliovirus vaccine (OPV). IPV, or Salk vaccine, consists of inactivated (killed) poliovirus strains for serotypes 1, 2, and 3 that are not capable of replicating and is administered by injection. IPV does not induce immunity in the gut and, therefore, if someone who has been vaccinated with IPV gets exposed to poliovirus, they can still transmit poliovirus by shedding the virus in their stool. OPV, or Sabin vaccine, contains live weakened virus that is administered orally. It replicates in the intestine to stimulate a protective immune response and is effective at interrupting transmission of the virus. Both IPV and OPV are safe, highly effective in preventing paralytic disease, and have been a critical part of global eradication efforts. In Canada, only IPV has been used since 1995/1996.
Following OPV administration, the vaccine virus is excreted in the stool and can spread from person to person via the fecal-oral route. Therefore, Sabin or Sabin-like vaccine viruses can be found in environmental sewage samples in regions where OPV is used or in an area to which someone who recently received OPV travels.
Along with wild poliovirus, vaccine-derived poliovirus (VDPV) is another form of poliovirus present globally. VDPV can develop in communities with low vaccination coverage when the live OPV virus mutates by spreading from one under-vaccinated individual to another over a long period of time (~12-18 months) or in rare cases, replicating for a prolonged time in a host who is immunocompromised. In this way, OPV strains can lose their attenuated (weakened) properties and revert to a virulent form, resulting in VDPV which behaves similar to the wild-type virus and can be just as pathogenic and transmissible. By definition, a strain will be classified as VDPV if the viral capsid (VP1) genomic region is > 1% divergent (meaning >=10 nucleotide changes) from the serotype 1 and 3 OPV strains or > 0.6% divergent (meaning >=6 nucleotide changes) from the serotype 2 OPV strain. A viral strain that is < 1% (type 1 or 3) or < 0.6% divergent (type 2) to the vaccine strain is classified as Sabin-like or vaccine-like.Reference 4
When there is evidence of person-to-person transmission of VDPV in a community, VDPVs are defined as circulating, or cVDPVs. People who are fully immunized against polio (either with IPV or OPV) will be protected against paralysis caused by both wild-type poliovirus and VDPV.
Vaccine-associated paralytic poliomyelitis (VAPP) is different from vaccine-derived poliovirus (VDPV). VAPP is a rare adverse event from OPV vaccination occurring when one of the attenuated, or weakened, viruses in the OPV causes paralytic polio in someone who has received OPV or their close contacts. In Canada, IPV is used exclusively for vaccination and carries no risk of VAPP. All provinces and territories (PTs) publicly fund IPV as part of routine immunization programs.Reference 5
The WHO recommends that all children are vaccinated against polio. As WPV type 2 was eradicated in 2015 and most cVDPVs detected around the world are type 2, the type 2 component of OPV was removed in favour of a bivalent (types 1 and 3) OPV (bOPV) in 2016.Reference 6 As of April 2019, all countries continuing to use bOPV have incorporated at least one dose of IPV into routine immunization programs.Reference 7 The WHO provides immunization schedule recommendations for countries using a combination of IPV and OPV through position papers on polio vaccines.Reference 8
As of November 2020, the WHO authorized under emergency use a new vaccine, the novel OPV type 2 (nOPV2), in certain situations to respond to outbreaks of cVDPV2.Reference 9 nOPV2 is a modified version of the monovalent OPV2 that provides comparable protection but is more genetically stable and less likely to be associated with the emergence of VDPV.Reference 10 As nOPV2 is being used under the WHO's Emergency Use Listing, countries need to meet specified criteria prior to use and it is being closely monitored for effectiveness and safety, including assessing if it has any ability to revert to a more virulent form that behaves similar to wild-type poliovirus.Reference 11 nOPV2 is being assessed for full vaccine licensure and novel OPVs targeting types 1 and 3 are also in development.Reference 12
1.2 Canadian context
Polio has been nationally notifiable since 1924 and is included in the Canadian Notifiable Disease Surveillance System CNDSS. In addition, to ensure Canada maintains its polio free status in accordance with the WHO, the Public Health Agency of Canada (PHAC), in conjunction with the Canadian Paediatric Society (CPS), conducts surveillance of Acute Flaccid Paralysis (AFP) in children less than 15 years of age. AFP is a possible outcome of poliovirus infection that involves the acute onset of paralysis in one or more limbs but can also be caused by a number of other viruses, as well as other causes. By actively seeking out cases of AFP in Canada and ruling out polio, Canada is able to provide ongoing evidence that the country remains polio free. Canada reports AFP cases to the Pan American Health Organization (PAHO) twice yearly. The performance of AFP surveillance is evaluated based on three key indicators established by the WHO:
- Detect at least one case of non-polio AFP annually per 100 000 population aged less than 15 years.
- All AFP cases should have a full clinical and virological investigation with at least 80% of AFP cases having 'adequate' stool specimens collected.
- At least 80% of AFP cases should have a follow-up examination for residual paralysis at 60 days after the onset of paralysis.
Canada, and many other countries who have achieved polio elimination, do not consistently meet the key AFP surveillance indicators for reasons including the availability of rapid diagnostics that confirm an alternative diagnosis, retrospective reporting of AFP cases, and lack of clinician knowledge about stool testing requirements.Reference 13 Although AFP surveillance in Canada does not conform to the WHO indicators, the Canadian AFP Surveillance System continues to be a sensitive and active surveillance system for the prompt and appropriate investigation of AFP cases to detect polio.
No endemic cases of wild poliovirus have been found in Canada since 1979 and Canada was certified polio free by the WHO in 1994.Reference 14 Cases of wild poliovirus in Canada reported since that time have been associated with importations from countries where the virus was still circulating. Canada has not detected wild poliovirus since 1996. Since 2004, there have been four detections of Sabin-like poliovirus in non-paralytic cases and one likely case of VAPP reported in Canada, all of which were imported from locations outside of Canada where OPV was used.Reference 15 Until polio eradication has been achieved globally and OPV is no longer used, there remains a small risk of importation of poliovirus (wild-type, Sabin or Sabin-like, or vaccine-derived poliovirus) into Canada.
Under the 71st World Health Assembly (2018) resolution, Canada has made international commitments on poliovirus biocontainment. The WHO's Global Action Plan 4th edition (GAPIV) for poliovirus biocontainment identifies facility-related containment breaches (such as a laboratory or vaccine production facility) as a major risk for polio re-emergence in a post-eradication world.Reference 16 In Canada, eradicated polioviruses are stored only in certified Poliovirus Essential Facilities (PEFs) which are audited by the National Authority for Containment (NAC) within the Centre for Biosecurity, PHAC.
The risk of polio in Canada is minimal due to best biosecurity practices, good sanitation, and high polio vaccine coverage. High IPV coverage prevents paralysis should there be any circulation of the virus. Polio vaccination coverage in Canada is high, however, there continue to be communities within Canada with low immunization coverage.
1.3 Guidance development
The Guidance developed for use during a poliovirus event or outbreak in Canada and builds on the following: Protocol for the investigation of AFP and suspected paralytic poliomyelitis (1998 CCDR article) and GPEI SOP: Responding to Poliovirus event or outbreak (Version 4, March 2022)
The Guidance should be used in conjunction with the Federal/Provincial/Territorial Public Health Response Plan for Biological Events.
2. Definition of a poliovirus event or outbreak
The Guidance outlines the response and management to a poliovirus (wild-type poliovirus [(WPV]), vaccine-derived poliovirus [(VDPV]), or Sabin/Sabin-like) event or outbreak. A poliovirus event is intended to capture detections of poliovirus from human, environmental (wastewater), or facility-related sources that do not indicate local circulation. A poliovirus outbreak are detections that suggest community level transmission.
The definitions below are adapted from the GPEI SOP: Responding to Poliovirus event or outbreak (Version 4, March 2022).
The criteria to confirm paralytic poliomyelitis or nonparalytic poliovirus infection in a person is outlined below in Section 5: Case Classifications.
A poliovirus event is defined as:
- Detection in a person:
- Detection of confirmed paralytic poliomyelitis (wild-type or VDPV/cVDPV) in a person with a travel history to an area with poliovirus circulating within 35 days before onset of paralysis, and no evidence of local community transmission in Canada ;or
- Detection of laboratory-confirmed nonparalytic poliovirus infection with a travel history to an area with poliovirus circulating within 6 weeks before poliovirus detectionReference a in the person, and no evidence of local community transmission in Canada; or
- Vaccine-associated paralytic poliomyelitis (VAPP):
- VAPP in a recipient of OPV who received the OPV in the 7 to 35 days before onset of paralysis; or
- VAPP in a close contact of a recipient of OPV, where the exposure in the close contact occurred within the communicable period of the OPV recipient (within up to 6 weeks after receipt of OPV).
- Environmental detection:
- Single environmental (wastewater) laboratory-confirmed (by Canada's National Microbiology Laboratory [NML]) detection of WPV, VDPV, or Sabin/Sabin-like; or
- Multiple environmental (wastewater) NML-confirmed detections of Sabin/Sabin-like type 1 or 3; or
- Multiple environmental (wastewater) NML-confirmed detections if they do not meet the criteria for an outbreak below (for example the detections are from one site within 2 months and no evidence of ongoing viral replication).
- Facility-relatedReference b: Confirmed type-specific poliovirus exposure, spill, or breach in a laboratory or vaccine production facility that handles poliovirus.
A poliovirus outbreak is defined as:
- Detection of confirmed poliovirus infection (paralytic or nonparalytic) in a person (unless defined as a "poliovirus event" above); or
- Two separate NML-confirmed detections from the environment (WPV, VDPV, Sabin/Sabin-like type 2), where "separate" means the samples were collected from two different sites with no overlapping catchment areas or from the same site but at least two months apart; or
- Any newly detected cVDPVReference c, whether in a person or environmental sample; that is, when a VDPV isolated either in human stool or the environment can immediately be genetically linked to another VDPV in the community, thereby confirming circulation in the areas of detection.
3. Importance of rapid detection, notification, and investigation
Rapid identification of poliovirus is critical for identifying possible transmission. Early detection and subsequent investigation support the timely implementation of public health measures to limit the spread of polioviruses and maintain the elimination status achieved by Canada in 1994. Early identification and investigation are necessary for Canada to notify international partners to facilitate knowledge sharing and international public health cooperation. As such, all levels of the public health system play a role in rapid detection, notification, and investigation of a poliovirus event or outbreak.
4. Federal/provincial/territorial roles and responsibilities
Below is an overview of the roles and responsibilities of PHAC, the provincial/territorial and local/regional public health authorities, and the role of laboratories at all levels during a public health response to a poliovirus event or outbreak. Should the severity, scope, or significance require a coordinated federal, provincial, territorial response, please refer to the Federal, Provincial, Territorial Public Health Response Plan for Biological Events - Appendix C for a list of federal health portfolio and PT roles and responsibilities and consider activation of applicable operations centres.
4.1 PHAC
PHAC's roles in a poliovirus event or outbreak, in collaboration with the PHAC's National Microbiology Laboratory (NML) (whose specific roles are detailed below), include providing guidance and expert advice, coordinating inter-provincial/territorial and international collaboration and communication, and developing and coordinating PHAC's risk assessments. PHAC may also play a supporting role in coordinating outbreak investigation committees with PTs in the case of multi-jurisdictional outbreaks, or outbreaks or events requiring national coordination and collaboration. PHAC will provide investigative support if requested by a province or territory. In its role as the National International Health Regulations (IHR) Focal Point for Canada, PHAC is also responsible for notifying the WHO of events that may constitute a public health emergency of international concern (PHEIC). PHAC, in collaboration with the affected PT(s), will share timely event information with other regions in Canada. In addition, Canada's National Authority for Containment (NAC) of poliovirus is responsible for maintaining the national poliovirus inventory, and for auditing and inspecting Poliovirus Essential Facilities (PEFs) under the WHO's Global Action Plan 4th edition (GAPIV) and the Canadian Human Pathogens and Toxins Act (HPTA) legislation. The National Advisory Committee on Immunization (NACI) and NACI Secretariat within PHAC provide guidance on the use of polio vaccines in situations where increased risk of exposure to poliovirus is anticipated.
4.2 Provincial/territorial and local/regional
PTs and local/regional levels are responsible for front-line diagnostics, sample collection of suspected cases, and provision of public health and health care services, including vaccination programs, to individuals within their jurisdictions. Local public health authorities are responsible for initiating investigations following the detection of poliovirus as well as case and contact management, and ensuring appropriate infection prevention and control practices are followed, where applicable. PT and local health authorities should provide information collected through public health investigation and share subject matter expertise with PHAC, to support a collaborative response associated with a poliovirus event or outbreak. PTs are also responsible for collaborating with PHAC to share timely event information with other regions in Canada and international partners. Health and public health authorities should also ensure that there are appropriate procedures in place to collect relevant epidemiologic and clinical information that accompany specimens from suspected polio cases, and that the specimens are transported to the testing laboratory safely and efficiently.
4.3 Laboratory
The NML is the only WHO Regional Reference Laboratory for poliovirus in Canada and is a designated PEF subject to the WHO's GAPIV standard and containment certification scheme. The NML Poliovirus laboratory conducts virus isolation and molecular characterization by genome sequencing and is licensed for controlled activities with polioviruses under the HPTA and Human Pathogens and Toxins Regulations.
As such, all samples that screen positive for poliovirus (if tested) or samples collected from a suspected polio or suspected AFP case should be referred to the NML for poliovirus isolation and molecular characterization. Samples are typically referred to the NML from the provincial/territorial public health laboratory. However, they can also be sent directly to the NML from a hospital or community laboratory. If sending directly, centres are encouraged to report the suspected polio or AFP case to local and/or provincial/territorial public health authority to ensure compliance with PT notifiable disease and conditions reporting guidelines. Due to the possibility of a poliovirus being present, NML will further manipulate samples, as recommended in the WHO Global Action Plan for poliovirus containment.
5. Case classifications
At the time of Guidance development, the national poliovirus case definition was under review. As such, the Guidance refers to the adapted case classification to confirm cases in Table 1. For information about identifying suspected cases, see Section 8.1.1: Index of suspicion for poliomyelitis while awaiting laboratory confirmation.
| Case Classification | Confirmed case |
|---|---|
| Paralytic poliomyelitis |
|
| Nonparalytic poliovirus infection |
|
Table 1 Notes
|
|
6. Detection
All samples suspected of containing poliovirus collected by provincial, territorial, and wastewater laboratories must be sent to the NML for testing to determine the presence of poliovirus. Due to the possibility of a poliovirus being present, respiratory, fecal, sewage concentrated samples and their derivatives that were collected at a time and place where poliovirus was in circulation, including samples that are stored under conditions that maintain virus viabilityReference d are considered poliovirus Potentially Infectious Materials (PIM). When working with and storing PIM, risk mitigation measures must be followed to prevent facility-related events, as outlined in the WHO PIM Guidance Document (2nd edition). See Annex C for details on PIM requirements.
For all cases compatible with the AFP case definition and all cases with suspicion of poliomyelitis, as outlined in Section 8.1.1: Index of suspicion for poliomyelitis while awaiting laboratory confirmation, ensure collection of at least two stool samples (taken at least 24 hours apart and minimum 2 grams per sample) within 14 days after symptom onset for viral studies (although samples can be collected up to 6 weeks after onset if it is not possible to collect earlier). In addition to stool testing, other diagnostic testing and investigations should be conducted, as appropriate, to help detect poliovirus infections or other conditions that may present similarly. These may include:
- Collection of respiratory specimens (collected by two oropharyngeal or nasopharyngeal swabs) or cerebrospinal fluid
- Neurological investigations, including nerve conduction studies and/or electromyography (EMG), magnetic resonance imaging (MRI), or computed tomography (CT) scan
Stool (two samples taken at least 24 hours apart) is the required clinical specimen for the laboratory investigation and diagnosis of poliovirus infections. A stool sample is preferred to a rectal swab because the diagnosis of poliovirus is more reliable. Stool samples are to be placed in a sterile leak proof container, frozen at ≤-20°C until sent for testing and shipped frozen on dry ice. No special medium is required. For information on specimen, collection, and other laboratory details for clinical samples, please refer to the NML's Molecular Detection and Characterization. See more details on laboratory protocols in Annex C.
For environmental wastewater samples suspected of containing poliovirus, samples should be packaged by the collecting lab and sent to the NML, as per the guidance described in the NML's Molecular Detection of SARS-CoV-2 from Wastewater. See more details on submission of wastewater samples for laboratory testing in Annex C.
7. Notification
7.1 Reporting within a province or territory
Any poliovirus event or outbreak (as defined in Section 2: Definition of a poliovirus event or outbreak) should be reported immediately to the local/regional public health authority and the provincial/territorial public health authorities and laboratories. Health authorities should also be notified for cases pending laboratory confirmation with suspicion of poliomyelitis, as outlined in Section 8.1.1: Index of suspicion for poliomyelitis while awaiting laboratory confirmation.
Cases compatible with the AFP case definition must be reported to the local public health authority, if legislatively required within the jurisdiction.
7.2 Reporting between PT authorities and PHAC
Polio is a nationally notifiable disease in Canada. Further, to support compliance with the International Health Regulations (IHR), all PTs are to notify PHAC authorities (email: vpd-mev@phac-aspc.gc.ca and hpoc-cops@phac-aspc.gc.ca) within 24 hours of PT detection of poliovirus for any poliovirus event or outbreak (as defined in Section 2: Definition of a poliovirus event or outbreak).
When reporting a case to PHAC, indicate if the case meets the case classification noted in Section 5: Case classifications. In addition to confirmed cases, consider reporting cases under investigation to PHAC that are compatible with the list of clinical features and have a higher index of suspicion for poliomyelitis, as outlined in Section 8.1.1: Index of suspicion for poliomyelitis while awaiting laboratory confirmation.
For a confirmed environmental detection or a facility-related incident, there are additional notification obligations. The appropriate local and PT public health officials and PHAC must be notified of all environmental samples that are confirmed of containing poliovirus by the NML within 24 hours. In the event of a facility-related event involving poliovirus, the HPTA-regulated PEF must notify the Centre for Biosecurity through the secure Biosecurity Portal (Laboratory Incident Notification Canada) in accordance with the HPTA 12(1) and HPTA 13.
A Canadian Network for Public Health Intelligence (CNPHI) Public Health Alert should be considered to allow for the timely notification and/or dissemination of information between local/regional, PT and national public health stakeholders. PHAC/NML can collaborate with local/regional and PT authorities to issue an alert, where necessary.
7.3 Reporting by PHAC to WHO
Day "0" for international reporting (to the WHO) is defined as the day the NML confirms a positive laboratory result for poliovirus by genetic sequencing (VDPV, WPV, Sabin/Sabin-like) from either human or environmental wastewater samples.
Under the IHR, Canada has an obligation to report, through an Article 6 Notification, all events which may constitute a public health emergency of international concern within its territoryReference 17. The relevant technical teams at PHAC have 48 hours to conduct an assessment (from Day 0) using the IHR Annex 2 "decision instrument for the assessment and notification of events that may constitute a public health emergency of international concern" (Annex D), by working in collaboration with the reporting province/territory and the National IHR Focal Point Office.
If, by using the decision instrument, it is determined that the event meets the criteria for Article 6 (Notification) or Article 7 (Information sharing during unexpected or unusual public health events), Canada must alert WHO within 24 hours of assessment. Reporting an Article 6 or Article 7 requires a description of the event with the available details and the written assessment using the IHR Annex 2. The WHO headquarters will inform relevant Global Polio Eradication Initiative (GPEI) partners.
As indicated in the IHR case definitions for diseases requiring notification, all detections of paralytic poliomyelitis due to wild-type poliovirus requires immediate Article 6 Notification in all circumstances. PHAC must send the notification to the respective WHO regional office within 24 hours of NML sequencing a notifiable poliovirus (Day 0) through the National IHR Focal point (hpoc-cops@phac-aspc.gc.ca with a Cc: to ihr-rsi@phac-aspc.gc.ca). Note that in these instances there is no 48-hour period to complete the IHR Annex 2 assessment.
In addition to laboratory confirmed cases of paralytic poliomyelitis due to wild-type poliovirus, the isolation of the following must generally also be reported to the WHOReference 18 as an Article 6 or Article 7, as determined by the public health authorities completing the assessment using the IHR Annex 2:
- Wild poliovirus isolation from a case of nonparalytic poliovirus infection
- Wild poliovirus isolation from a non-human source (environmental detection)
- Vaccine-derived poliovirus (types 1, 2, 3) isolation from human or non-human sources
- Sabin/Sabin-like type 2 isolation from human or non-human sources if they were detected from an area where Sabin OPV2 has not been used in the previous four months
For poliovirus events that do not meet the Article 6 Notification or Article 7 criteria, other reporting and information-sharing requirements to the WHO and other National IHR Focal Points may still apply under the Regulations to support transparency and public health response.
Note: Notification to PAHO/WHO may lead to the publication of a Disease Outbreak News or Epidemiological Alert report on the PAHO/WHO public websites, as appropriate, based on virus type, risk assessment and outbreak status as well as on the WHO Event Information Site, which is only accessible to National IHR Focal Points. Further, based on data sharing requirements from the GPEI and the Global Polio Laboratory Network, Canada has the responsibility to share specific geolocation of a poliovirus sample or case testing positive for poliovirus. The location data will be published on a secure site available to the National IHR Focal Points and members of the GPEI.
If an outbreak is declared, consult the Temporary Recommendations issued by the WHO Director-General upon the advice of the IHR Emergency Committee on the international spread of poliovirus. Visit World Health Organization's website for past statements from the IHR.
Annex A contains figures to illustrate the key initial steps and notification requirements for detecting polio.
8. Investigation
8.1 Investigation of a case
8.1.1 Index of suspicion for poliomyelitis while awaiting laboratory confirmation
If a case with AFP is awaiting laboratory confirmation and is epidemiologically linked to a laboratory confirmed polio case, proceed to Section 8.1.2: Epidemiologic characteristics of poliovirus relevant to investigation and case/contact management, as this meets the case classification for confirmed paralytic poliomyelitis.
For other cases awaiting laboratory results of cases presenting with AFP, the following are high risk factors that raise the index of suspicion regarding poliovirus and would warrant managing the AFP case as a poliomyelitis case:
- If the person with AFP received OPV within the previous 35 days
- If the person with AFP had exposure to someone who received OPV within the 6 weeks prior to the exposure
- If the person with AFP was a contact of someone being investigated for paralysis or poliomyelitis
The management for these higher risk cases awaiting laboratory confirmation include:
- Isolating and managing case, as per the considerations in Table 3
- Considering initiating contact identification and management, as per the considerations in Table 2, Table 4, and Table 5
Other factors may also increase suspicion that a person with AFP may have poliomyelitis and may warrant beginning early public health management:
- Recent travel to area where there is poliovirus
- Contact with a recent traveller from an area where there is poliovirus
- Comes from or visited a community with a large unimmunized population or where poliovirus has been circulating
- Unvaccinated or under-vaccinated against poliovirus
The management for these cases awaiting laboratory confirmation could include the considerations for case and contact management in Tables 2 to 5.
If poliovirus is confirmed, management guidance is provided in Section 8.1.3: Investigation of poliovirus infection (with or without paralysis).
8.1.2 Epidemiologic characteristics of poliovirus relevant to investigation and case/contact management
Period of communicability
Poliovirus can be identified from throat secretions as early as 36 hours after exposure and from stool as early as 72 hours after exposure. The virus persists in the throat for approximately 1 week and in the stool for 3 to 6 weeks. Cases are most infectious in the days before and after onset of first symptoms (which could be non-specific symptoms).
Incubation period
The incubation period is 3 to 6 days for onset of non-specific symptoms. In those that progress to paralysis, this occurs in 7 to 21 days from exposure (can range up to 35 days).
Assumptions for this document
Communicability starts very soon after acquisition of the poliovirus, so in some circumstances for this document, communicability will be considered to start at the time of acquisition of the virus. Therefore, for those with paralytic polio, 35 days (5 weeks) before onset of paralysis is the outer limit of the time of acquisition and approximately the start of the period of communicability. For those with nonparalytic poliovirus infection with poliovirus detected in their stool, 6 weeks before time of detection in stool is the outer limit of the time of acquisition and approximately the start of the period of communicability. For simplicity, 6 weeks will be used in some circumstances as the longest incubation period and start of the period of communicability for those with confirmed poliovirus infection (with or without paralysis) in some places in this document. Circumstances of a particular case or contact could further limit these periods, such as the specific date of return from travel.
Note that in some instances the incubation period and/or period of communicability can be longer. As an example, individuals with primary immunodeficiency disorder can excrete poliovirus for prolonged periods, thereby extending the period of communicability, and meaning the time of acquisition could be considerably earlier than 6 weeks.
In the sections of this document that follows, "detection" refers to either the date of onset of paralysis or date of stool sample collection that was positive for poliovirus (for those with nonparalytic poliovirus infection).
8.1.3 Investigation of poliovirus infection (with or without paralysis)
If poliovirus is detected, public health authorities should initiate investigation within 24 hours after PT or NML detection (do not wait for sequencing or final classification of the virus by the NML). The information collected from investigations will be important to inform risk assessments and international notification obligations to the WHO. PTs and local/regional leads will collaborate to conduct investigations, with support from PHAC where applicable.
In absence of a case investigation form in the corresponding jurisdiction, PHAC recommends using the PAHO polio case investigation form (all sections except Section VII) as a guide to inform public health investigations. The following minimum data requirements should be collected and included in the investigation for each case:
- Date of birth and sex
- Polio immunization status including total number of doses of OPV and/or IPV received, as well as the date(s) of administration
- Exposure to someone who received the OPV, where the exposure occurred within the communicable period (up to 6 weeks after receipt of OPV)
- History of travel to/from or residing in another country where there is a risk of poliovirus or OPV exposure within 6 weeks prior to onset of paralysis or collection of positive stool sample (for those without paralytic polio); Details of travel history, including travel dates and locations
- Exposure to someone with a travel history to/residing in another country where there is a risk of poliovirus or OPV exposure, where the exposure occurred within the potential communicable period (up to 6 weeks after the traveller returned home) and onset of paralysis or collection of positive stool sample (for those without paralytic polio) occurred within approximately 6 weeks afterward; Details of travel history, including travel dates and locations
- Summary of the clinical presentation, course of illness, and final diagnosis (if known)
- Results of stool testing (if not available for testing, this should also be indicated in the report)
- Results of other appropriate testing/investigations (including oropharyngeal and/or nasopharyngeal swabs, cerebrospinal fluid, nerve conduction studies and/or EMG, MRI, or CT scan), if available (also indicate if tests were not done)
- Relevant medical history including immunocompromised status or abnormal neurological history
- Whether a member of a group/community that is known to be unvaccinated or under-vaccinated
- Final outcome obtained after 60 days after onset of illness
The following data elements should be collected to inform case and contact management:
- Information on how to reach the case (phone, email, text, address)
- Occupation/school or child care centre attendance
- List and information on how to reach household members and close contacts, including if the case lives in a group living arrangement (such as dormitories, shelters, detention centres, group homes, settlement houses) and other contacts as outlined in Table 2, as appropriate
- Preparation of meals for others during the period of communicability, with names, dates and information on how to reach them
- Possible sources of exposure in the incubation period, if the acquisition source is not clear (such as food shopping stores, imported food consumed, restaurants, meals prepared by others, bathrooms used)
8.1.4 Contact tracing
Follow-up of close contacts is important to identify a potential source of infection if it is not readily apparent (acquisition source) and also to determine those who may have been infected by the case (transmission risk) to prevent further onward spread. Certain contacts may require exclusions (see Section 9.2: Management of contacts) and should thereby be identified without delay. Contact tracing for acquisition is only required if the source is not apparent and possible sources of acquisition are still being investigated (meaning it would not be required if the case had an obvious source of acquisition such as recent travel to a country where poliovirus transmission is occurring or a facility-related exposure). Potential contacts are outlined in Table 2.
Table 2: Potential contacts of a case infected with poliovirus and the possible acquisition and/or transmission risk
Higher risk contacts include: household contacts and those who stayed overnight in the same household as the case; sexual contacts; contacts in group living settings who shared a bathroom with the case or had close interactions with the case (for example, dormitories, shelters, detention centres, group homes, settlement houses); children who attended child care with the case and the child care workers; and those who had contact with the feces of the case (excluding health care providers who used appropriate infection prevention and control practices). Other contacts are generally considered lower risk. The transmission risk for a contact is dependent on the timing and nature of the exposure with the case and is not influenced by the contact's vaccination status, since those vaccinated with IPV can still become infected and spread infection to others.
| Potential contact or exposure source (generally listed from highest to lowest transmission risk) |
To determine acquisition source (only relevant if a source of acquisition is not apparent) |
To determine transmission risk | Transmission risk level |
|---|---|---|---|
Household and other close contacts: including those who stayed overnight in the same household as the case, sexual contacts, and contacts in group living settings who shared a bathroom with the case or had close interactions with the case (such as dormitories, shelters, detention centres, group homes, settlement houses), if applicable |
If exposure occurred in the case's incubation period (could include up to 6 weeks before detection) |
If exposure occurred while the case was communicable (could include up to 6 weeks before detection until the case was recognized and proper infection prevention and control measures were initiated) |
Higher risk contact – See Table 4 for management considerations |
Child care contacts: including staff and children in the group/class with the case and groups/classes who had regular interactions with or routinely shared a bathroom with the case's group/class at the child care centre |
If exposure occurred in the case's incubation period (could include up to 6 weeks before detection) |
If exposure occurred while the case was communicable (could include up to 6 weeks before detection until the case was recognized and excluded from the child care setting) |
Higher risk contact – See Table 4 for management considerations |
Known contact with feces (including changing diapers and assisting with toileting) See last row for health care and laboratory workers handling specimens from the case |
If exposure occurred in the case's incubation period (could include up to 6 weeks before detection) |
If exposure occurred while the case was communicable (could include up to 6 weeks before detection until the case was recognized and proper infection prevention and control measures were initiated) |
Higher risk contact – See Table 4 for management considerations |
Toilet exposure (such as using a common bathroom in a workplace) |
If exposure occurred in the case's incubation period (could include up to 6 weeks before detection) |
Those who frequently shared toilets with the case if exposure occurred while the case was communicable (could include up to 6 weeks before detection up until the toilet was appropriately cleaned, disinfected, and not used again by the case) |
Generally lower risk contact– See Table 5 for management considerations |
Food exposure / handling |
Food shopping stores, imported food consumed, restaurants, meals prepared by others in the case's incubation period (could include up to 6 weeks before detection) |
If the case prepared food for others while the case was communicable (could include up to 6 weeks before detection until proper infection prevention and control instituted, particularly if the food was not cooked after handling by the case) |
Generally lower risk contact– See Table 5 for management considerations |
Health care workers who provided care for the case and laboratory workers who handled specimens from the case |
NA |
If exposure occurred while the case was communicable (could include up to 6 weeks before detection) |
Generally lower risk contact– See Table 5 for management considerations. If the health care worker is determined to not have used adequate personal protective equipmenttable 2 note * and infection prevention and control practices in addition to having substantial close contact or contact with the case's feces, they could be considered higher risk - See Table 4 for management considerations If the health care worker / laboratory worker is determined to have used adequate personal protective equipmenttable 2 note * and infection prevention and control practices, they could be considered not a contact. |
Table 2 Notes
|
|||
The following minimum data requirements should be collected from household members or other higher risk contacts. Collection of some or all of this information on lower risk contacts may also be relevant depending on the circumstances:
- Information on how to reach the contact (phone, email, text, address)
- Date of birth and sex
- Polio immunization status including total number of doses of OPV and/or IPV received, as well as the date(s) of administration
- Travel history within 12 weeks before the onset of illness in the case and details of travel history, including dates and locations if the acquisition sources is unclear and the contact could be a possible acquisition source
- Whether the contact is experiencing any symptoms, including details of the symptoms and their onset
- Whether the contact is a member of a group/community that is known to be unvaccinated or under-vaccinated
- Relevant medical history including immunocompromised status or abnormal neurological history
- Occupation/school or child care centre attendance
- Group living arrangements (for example, dormitories, shelters, detention centres, group homes, settlement houses)
- If not a household contact, determine dates, times, and locations of exposure to the case, in the 6 weeks before the case was detected and up until proper infection prevention and control was used. Include if and when the contact prepared food for the case (if the acquisition source of the case is not apparent) or the case prepared food for the contact
- For health care workers who provided care for the case and laboratory workers who handled specimens from the case while the case was communicable, determine the dates and types of exposure, and if appropriate, personal protective equipment (see table 2 note * note above in Table 2 for further information) and infection prevention and control practices were used
In addition to the case and contact investigation, collecting information about the local population, such as vaccination coverage and socio-demographic characteristics of unvaccinated and under-vaccinated individuals in the area, may be warranted to inform risk assessments to assess the potential for transmission.
8.2 Investigation of a detection from environmental wastewater sampling
If a poliovirus of concern (WPV, VDPV, or Sabin/Sabin-like 2) is confirmed in an environmental wastewater sample by NML, local/regional health authorities should initiate investigation within 24 hours of confirmation. Best efforts should be made to gather the following information specific to the sampling site catchment area and neighbouring sites:
- Population characteristics (for example, population size included in catchment area, demographics of affected population, polio vaccination coverage, presence of unvaccinated and under-vaccinated groups, vaccine acceptance)
- Population movement (for example, migration and travel patterns, cultural/travel links with other affected areas)
- Description of the catchment area (for example, location, links with other sites, closed or converging sewer network, density of dwellings)
- Relevant institutions (for example, health facilities, schools, poliovirus essential facility (PEF) like a poliovirus vaccine manufacturer or laboratory, transportation centres such as airports, settlement houses for newcomers)
- Collection schedule, number and frequency of samples collected, timeliness and completeness of collection
More data elements for consideration are outlined in the GPEI SOP: Responding to Poliovirus event or outbreak (Version 4, March 2022) - Chapter 3
8.3 Investigation of a facility-related event involving poliovirus
The PEF performs investigations of facility-related events with poliovirus in the facility and the NAC would provide containment guidance and oversee the investigation of root causes leading to an event. The contact tracing recommendations included in Section 8.1.4: Contact tracing and other public health follow-up may still be required, and as such, local/regional and PT health authorities should be notified as per Section 7.1: Reporting within a province or territory.
9. Management of cases and contacts
Detecting a poliomyelitis case or non-paralytic poliovirus infection in Canada is likely to be very rare. However, in the event of a case detection, immediate public health precautions should be initiated to prevent transmission. Transmission of poliovirus can put people in Canada who are unvaccinated or under-vaccinated at risk and could endanger Canada's polio free status. As such, the recommendations regarding case and contact management provided below are restrictive in nature to minimize the risk of transmission. The management recommendations are outlined to support PT decision making and are not intended to supersede existing guidelines or protocols within a jurisdiction.
9.1 Management of cases
General recommendations:
- Notify local public health for all cases with suspicion of poliomyelitis, as outlined in the Section 8.1.1: Index of suspicion for poliomyelitis while awaiting laboratory confirmation.
- Investigate all cases to help maintain Canada's polio elimination status, to determine the source of infection, and prevent further cases. Information that should be obtained from the case is outlined above in Section 8.1.3: Investigation of poliovirus infection (with or without paralysis).
- Follow all appropriate infection prevention and control measures. Hospitalization is recommended for cases where clinically indicated. For hospitalized patients, admit the patient to a private room with routine practices and contact precautions. Cases that do not require hospitalization are permitted to isolate at home with appropriate infection prevention and control measures. An overview of possible case management recommendations and considerations are outlined in Table 3.
- Handle secretions, feces, and contaminated articles using routine practices and contact precautions. In areas where there is modern sewage disposal, feces and urine can be discharged directly into the sewers. Collect and incinerate waste if sanitary sewage is not connected to a municipal wastewater management system.
- Report cases compatible with the AFP case definition to the Canadian Paediatric Surveillance Program (CPSP) using the CPSP case questionnaire, in addition to local public health if legislatively required within the jurisdiction.
- For cases meeting the AFP case definition, conduct a follow-up assessment of the outcome of paralysis 60 days after onset of paralysis. A follow-up report should be submitted when the information is available.
- Note: the initial report should not be delayed because of incomplete information; however, all relevant case information should be sent in a follow-up report as soon as it is available.
Table 3: Overview of possible polio (with or without paralysis) case management recommendations and considerations
Notes:
The following applies to confirmed cases. Some aspects may also apply to suspect cases while awaiting laboratory results, depending on the level of suspicion that the case has poliomyelitis (such as if the case has paralysis and received OPV within the previous 35 days or is a member of a community with an ongoing outbreak).
Some of the following possible recommendations/considerations are relevant until the case is deemed to be no longer infectious (marked with a *). A case can be considered no longer infectious based on three consecutive negative stool samples each collected at least 24 hours apart. All stool sample testing must be conducted by the NML. The turnaround time for poliovirus isolation in cell culture at the NML is 14 days (See Annex C for details).
Poliovirus in the stool is usually cleared within 3 to 6 weeks in an immunocompetent person. For cases that do not clear the infection in this time frame, such as individuals with primary immunodeficiency disorder, public health management and stool testing will be determined on a case-by-case basis in consultation with public health and infectious disease specialists. Further recommendations can be found in the GPEI's Guidelines for Implementing Poliovirus Surveillance among Patients with Primary Immunodeficiency Disorders (PIDs). The recommendations/considerations below also reflect that there may be unrecognized cases in the household, as the contact investigations may still be ongoing.
| Factor | Recommendations and considerations |
|---|---|
| Isolation and infection prevention and control in health care facilitiestable 3 note * |
|
Isolation and infection prevention and control at hometable 3 note * |
|
Stool testingtable 3 note * |
|
Food preparationtable 3 note * |
|
Exclusionstable 3 note * |
|
Visitorstable 3 note * |
|
Clinical management |
|
Monitoring |
|
Vaccination |
|
Table 3 Notes
|
|
9.2 Management of contacts
General recommendations:
- A guide to identification of contacts can be found in Table 2 and information that should be obtained from contacts is outlined above in Section 8.1.4: Contact tracing.
- A summary of contact management recommendations/considerations are outlined in Table 4 for household and other close and/or higher risk contacts and Table 5 for lower risk contacts.
Table 4: Higher risk contact management recommendations and considerations for poliovirus
Applies to: Household and other close and/or higher risk contacts, including those who stayed overnight in the same household as the case; sexual contacts; contacts in group living settings who shared a bathroom with the case or had close interactions with the case (such as dormitories, shelters, detention centres, group homes, settlement houses); children who attended child care with the case and the child care workers; and those who had contact with the feces of the case (excluding health care providers who used appropriate infection prevention and control practices).
Notes:
Some of the following recommendations/considerations are relevant until the contact is determined not to be infected (marked with a *), based on two consecutive negative stool samples taken at least 48 hours apart, with the first collected at least 4 days after the contact's last exposure to the case before infection prevention and control measures were initiated. Note that this is a different procedure for stool sample testing compared to the process outlined in Table 3 for a polio case. All stool sample testing must be conducted by the NML. The turnaround time for poliovirus isolation in cell culture at the NML is 14 days (See Annex C for details).
| Factor | Recommendations and considerations |
|---|---|
Infection prevention at hometable 4 note * |
|
Stool testingtable 4 note * |
|
Food preparationtable 4 note * |
|
Exclusionstable 4 note * |
|
Visitorstable 4 note * |
|
Monitoring |
|
Vaccination |
Note: Vaccination is recommended to protect an individual against ongoing exposure and not intended as post-exposure immunoprophylaxis for contacts. |
Table 4 Notes
|
|
Table 5: Lower risk contact management recommendations and considerations for poliovirus
Applies to: Those who shared toilets with the case; ate food prepared by the case; health care workers who provided care for the case or laboratory workers who handle specimens from the case (unless they are classified as higher risk or no risk, see Table 2).
Notes:
If contacts shared toilets with the case, ate food prepared by the case, or health care workers who provided care for the case or laboratory workers who handle specimens from the case are deemed, based on a risk assessment, to be at higher risk of contracting poliovirus from their exposure to the case, they should follow the recommendations in Table 4, otherwise they should follow the recommendations in Table 5. Health care workers / laboratory workers determined to have used adequate personal protective equipment and infection prevention and control practices could be considered not a contact.
For information on adequate personal protective equipment for poliovirus, please refer to PHAC's Routine Practices and Additional Precautions for Preventing the Transmission of Infection in Healthcare Settings (2016) - page 83
| Factor | Recommendations and considerations |
|---|---|
Infection prevention and control |
|
Stool testing |
|
Monitoring |
|
Vaccination |
|
Further possible control measures for management of a person exposed to poliovirus material from a facility-related event are contained in Annex B.
10. Risk assessment
GPEI Initial risk assessment: Consider using the GPEI risk assessment tool Annex E in this document and Annex 1 of the GPEI SOP) in the early stages of the investigation for WPV, VDPV, and Sabin/Sabin-like type 2 detections. The initial risk assessment can be used as a guide to inform the type and scale of response, including a review of virologic risk and epidemiologic characteristics, and to determine the risk of further transmission in the area of the event or outbreak and internationally. The assessment can be completed by subject matter experts at PHAC and the affected PT.
An overview of the GPEI risk instrument is outlined in GPEI SOP: Responding to Poliovirus event or outbreak (Version 4, March 2022) - Chapter 4
Rapid risk assessment: A comprehensive Rapid Risk Assessment is an important tool to describe the risk to people in Canada and assess gaps in knowledge using a standard framework and method. Where applicable, based on established information, the RRA would be conducted by PHAC, with input from the investigating PT. This process is consistent with the Initial Assessment phase (3.2) of the Federal/Provincial/Territorial Public Health Response Plan for Biological Events.
11. Public health response to a poliovirus event or outbreak in Canada
Public health authorities in Canada manage responses to public health events in various ways such as surveillance and monitoring activities, public health measures and leveraging laboratory networks. Emergency management authorities facilitate and support coordination of responses to public health events, for example by providing logistical guidance and support, and using platforms and tools such as emergency operations centres for planning and coordination of integrated response activities. The Federal/Provincial/Territorial Public Health Response Plan for Biological Events describes how the two programs will work together using the governance structure and concept of operations of the plan, and that response activities and coordination required will vary depending on response objectives of the event.
The following activities should be considered in response to a poliovirus event or outbreak.
11.1 Enhanced case surveillance
The objective of enhanced case surveillance activities is to increase the probability of detecting cases and enrich quality of reporting. In the event of a WPV/VDPV case or environmental detection in Canada, enhancements to existing active surveillance networks are warranted to ensure sensitivity.
Further considerations include:
- Enhanced communications to health professionals at all levels regarding the current situation and the importance of:
- Detecting cases presenting with symptoms compatible with AFP (at all ages)
- Monitoring for non-specific symptoms in those with poliovirus exposure
- Collecting stool samples within 14 days of symptom onset and sending to the laboratory for all AFP cases, and also when there is suspicion of poliovirus
- Reporting AFP with suspicion of poliovirus and confirmed cases to local public health
- Expanding AFP surveillance to be PT notifiable in all jurisdictions
- Inclusion of adolescents and adults in AFP reporting (the geographic area will be informed by the initial case investigation)
11.2 Expanded environmental wastewater sampling
In the event of a WPV/VDPV case or environmental detection in Canada or international detections presenting a risk of importation to Canada, expanded strategic environmental wastewater sampling (such as increased number of sites sampled or increased frequency of sampling) may be warranted to complement AFP surveillance. It is important to note that wastewater testing for poliovirus is different from that for other pathogens such as COVID-19. Poliovirus wastewater testing is not routinely or broadly recommended, and there are strict laboratory safety requirements. To ensure public health plans are in place to confirm, interpret and follow up on environmental detections, decisions to implement/expand environmental wastewater sampling in a region is the joint responsibility between local, PT and federal stakeholders. If wastewater sampling in a region is agreed upon, all samples at this time should be sent to the NML for testing.
If a case is identified, the case's area of residence and other catchment areas (depending on identified epidemiological links) could be considered for environmental sampling to assess the geographic scope of detections, community transmission, and duration of poliovirus circulation. The identification of highest risk areas should be based on the location of populations considered at risk and behavioural characteristics that represent potential transmission risk including:
- Known or suspected population immunity gaps, such as specific age cohorts that missed vaccination, and groups who are unvaccinated or under-vaccinated on religious, philosophical or other grounds
- Known or suspected links to areas with circulating polioviruses
- Areas with a large mobile population (for example, daily movement of population for work, migrants, nomads, refugees, informal settlements, undocumented guest workers)
- Low coverage in routine poliovirus vaccination (<90%)
The sampling frequency is recommended to be every 2 weeks, but the minimum frequency is monthly. Sampling will cease after there have been no further WPV/VDPVs isolated in a six-month period.
For more information on enhancing environmental surveillance refer to GPEI SOP: Polio Environmental Surveillance Enhancement Following Investigation of a Poliovirus Event or Outbreak (Version 15, December 2020)
11.3 Community stool sampling
In some instances, consensual community stool sampling may be considered when trying to determine a possible source or the extent of transmission. This may be appropriate in closed groups such as child care centres, congregate living settings, or religious communities that are known to be unvaccinated or under-vaccinated. Sampling of routinely collected stool samples or samples from diapers in health care offices, health care facilities or child care centres could be considered, with parental/guardian consent.
11.4 Vaccination
Assessing immunization coverage and targeted IPV immunization campaigns are warranted in populations at high risk, such as communities near the detected event and/or communities with populations who are known or suspected to be unvaccinated or under-vaccinated. Given that delivery of immunization programs is a PT responsibility, vaccination activities will be led by the respective PT health authority and will be supported by PHAC where necessary. The National Advisory Committee on Immunization (NACI) may also be involved in providing specific vaccination recommendations and the Canadian Immunization Committee (CIC) may be involved in programmatic and operational discussions. Where applicable, engagement from trusted community members, such as faith leaders, may be included as part of vaccination campaign efforts.
The extent of further IPV immunization activities is dependent on the event or outbreak and determined based on factors related to the case/contacts, rapid risk assessment, and in consultation with public health and other partners, as appropriate. Further immunization activities could include:
- Catch-up vaccines for unvaccinated or under-vaccinated people
- Immunization with a single adult lifetime booster with IPV-containing vaccine for high risk adults if they have not previously had one at or after 18 years of age (such as close contacts, health care workers, adults at risk of exposure)
- Outbreak vaccination among certain groups (for example, all children under 5 years of age regardless of vaccination status).
If PTs require additional vaccination supplies, PHAC may be involved to assist coordinating procurement. Federal and PT partners will need to be vigilant in disseminating public communication to ensure vaccinations are up to date, as per the Provincial and territorial routine and catch-up vaccination schedule for infants and children in Canada. Recommendations on routine immunization schedules, booster doses and immunization of travelers are available in the Canadian Immunization Guide: Poliomyelitis vaccine.
11.5 Communication
Consistent, clear, and coordinated communication is important following a poliovirus event or outbreak to support response activities, inform people in Canada about any risks and actions to protect their health, increase vaccination uptake and support robust surveillance with early notification of AFP and poliovirus.
During an event/outbreak, it is important to develop communication strategies and resources to:
- Advise health professionals to consider polio in patients with polio-like symptoms (for clinical symptoms of polio, refer to Canadian Immunization Guide: Poliomyelitis vaccine), especially if the patient is unvaccinated or under-vaccinated and/or recently traveled or resided in a country where polio still occurs or was exposed to a person who traveled to these areas or was potentially exposed as part of a local event or outbreak.
- Advise health professionals to ensure their patients are up to date on polio vaccinations as per recommendations in the Canadian Immunization Guide.
- Raise awareness of the risks of polio to children, families, and communities.
- Strengthen community confidence in vaccination and encourage vaccination uptake.
Communication considerations:
- Coordinate and align communication approaches and messaging between FPT authorities, where appropriate.
- Engage/leverage stakeholders to assist in reaching target audiences, where appropriate.
- Employ targeted approaches and translation to communities that have been marginalized and remote populations, as needed.
- Display openness, transparency, and timeliness with information and messaging that provides advice, acknowledges uncertainties, and explains next steps.
- In addition to traditional media and social media, leverage existing platforms for communication such as professional networks, CNPHI public health alerts, and yellow fever and travel health clinics.
11.6 Surveillance evaluation and assessment following investigation
Following the initial investigation of any poliovirus event or outbreak, it is critical to assess and enhance AFP and poliovirus surveillance.
The current Canadian Acute Flaccid Paralysis Surveillance System should be evaluated and enhanced, where needed, to increase data management capacity to ensure all databases and analyses are up to date, ensure data harmonisation with the laboratory, and work with health professionals to increase case compliance of full clinical and virological investigation and follow-up of cases.
As per the recommendations of the GPEI, complete the following outbreak response assessments:
- First assessment within 3 to 4 months of lab notification.
- Follow-up quarterly assessments during the course of the outbreak.
- Final assessment after at least 6 months without poliovirus detection.
- Further desk reviews in prolonged outbreaks, as appropriate.
For more information on Surveillance following investigation refer to GPEI SOP: Responding to a poliovirus event or outbreak (Version 4, March 2022) - Chapter 7.
Acknowledgements
The Public Health Agency of Canada would like to express sincere gratitude to all individuals and organizations who contributed to the development of the Guidance for the Response and Management of a Poliovirus Event or Outbreak. The collaborative efforts from centres across the Agency as well as external partners were invaluable in shaping the robust and comprehensive framework.
We extend our appreciation to the following groups for their valuable contributions and expertise:
- Canadian Immunization Committee
- Provincial and Territorial partners
- Special Advisory Committee / Pan-Canadian Public Health Network
- Centre for Biosecurity, Public Health Agency of Canada
- Centre for Border and Travel Health, Public Health Agency of Canada
- Communications and Public Affairs Branch, Health Canada/Public Health Agency of Canada
- Centre for Emergency Preparedness, Public Health Agency of Canada
- Centre for Immunization Readiness, Public Health Agency of Canada
- Centre for Immunization and Respiratory Infectious Diseases, Public Health Agency of Canada
- Centre for Immunization Surveillance, Public Health Agency of Canada
- National Microbiology Laboratory, Public Health Agency of Canada
Resources
Biosecurity
- Canada Human Pathogens and Toxins Act
- GPEI Strategy for Global Poliovirus Containment
- World Health Assembly. WHA 71.16 Poliomyelitis – containment of polioviruses
- WHO Containment Certification Scheme
- WHO Global Action Plan for Poliovirus Containment (GAPIV)
- WHO Global Poliovirus Containment Action Plan 2022–2024
- WHO Guidance to minimize risks for facilities collecting, handling, or storing materials potentially infectious for polioviruses (2nd edition, 2021)
- WHO Public Health management of Facility Related Exposure to Live Polioviruses
Case definitions
- Case Definitions for the four diseases requiring notification to WHO in all circumstances under the IHR (2005)
- CDC Nonparalytic Poliovirus Infection Case Definition
- CPSP AFP Case Definition
- Government of Canada Poliomyelitis Case Definition
Case questionnaires
Lab processes
- NML's Molecular Detection and Characterization (for submitting clinical samples to lab)
- NML's Molecular Detection of SARS-CoV-2 from wastewater (process for submitting environmental samples to lab)
- NML Requisition for Detection
Reporting
- CNPHI Alert
- CPSP Acute flaccid paralysis protocol
- WHO Disease Outbreak News
- WHO Guidance for the Use of Annex 2
- International Health Regulations (2005) (IHR)
- Poliovirus IHR Emergency Committee Statements
SOPs and reference
- Canadian SOP (1998 CCDR)
- Canadian Immunization Guide: Poliomyelitis vaccine
- Canadian provincial and territorial routine and catch-up vaccination schedule
- GPEI AFP Surveillance Indicators
- GPEI Classification and reporting of vaccine-derived polioviruses (VDPV)
- GPEI SOP for Responding to a Poliovirus Event or Outbreak (March 2022)
- GPEI SOP for Polio Environmental Surveillance Following Investigation of a Poliovirus Event of Outbreak
- GPEI SOP Guidelines for Implementing Poliovirus Surveillance among Patients with Primary Immunodeficiency Disorders (PIDs)
- Federal, Provincial, Territorial Public Health Response Plan for Biological Events
- PAHO Epidemiological Update. Detection of a circulating vaccine-derived poliovirus type 2 (VDPV2) in the United States: Considerations for the Region of the Americas
- Routine Practices and Additional Precautions for Preventing the Transmission of Infection in Healthcare Settings
- Surface disinfectants for emerging viral pathogens
Annexes
Annex A: Key initial steps and notification requirements
Key initial steps and notification requirements to be taken are illustrated in the case of a poliomyelitis case or nonparalytic poliovirus infection (Figure 1), positive environmental wastewater sample (Figure 2), or facility-related event involving poliovirus (Figure 3).
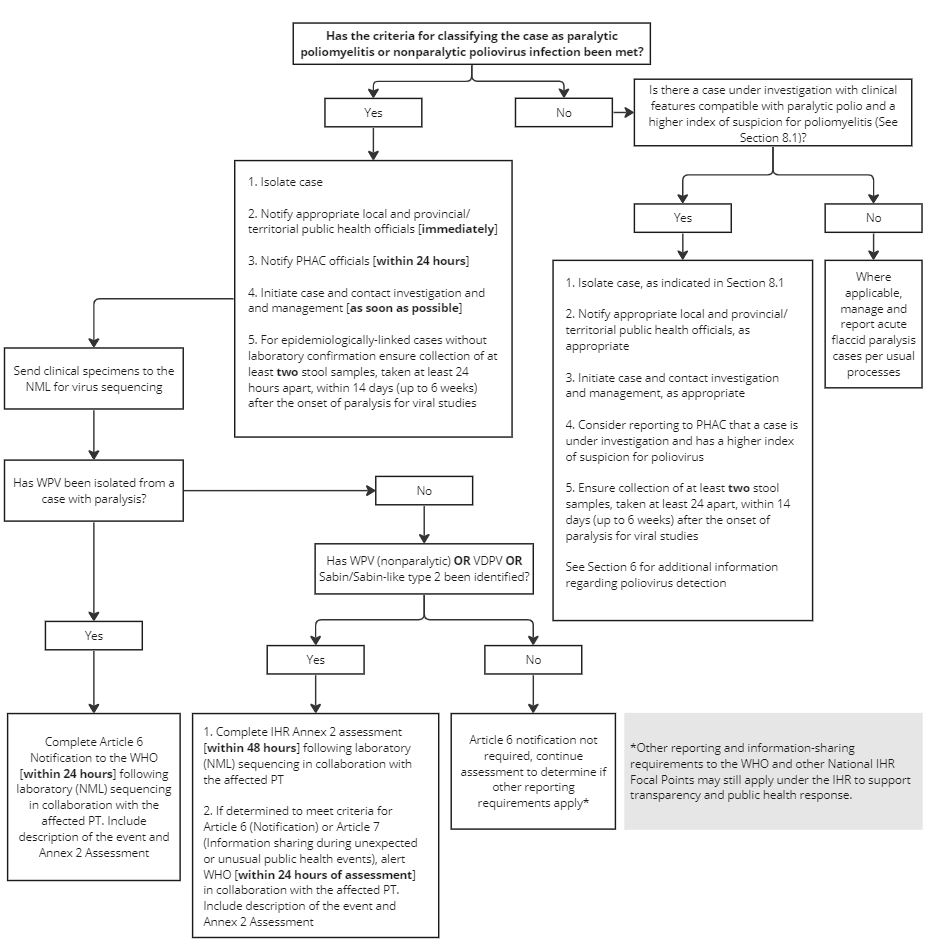
Figure 1 - Text description
The graphic is a flow chart illustrating the key initial steps and notification requirements for paralytic poliomyelitis or nonparalytic poliovirus infection in a person.
The first step is determining if the criteria for classifying the case as paralytic poliomyelitis or nonparalytic poliovirus infection has been met.
If the answer is 'yes', then the next steps would be to:
- Isolate the case
- Notify appropriate local and provincial/territorial public health officials immediately
- Notify PHAC officials within 24 hours
- Initiate case and contact investigation and management as soon as possible
- For epidemiologically-linked cases without laboratory confirmation ensure collection of at least 2 stool samples, taken at least 24 hours apart, within 14 days (up to 6 weeks) after the onset of paralysis for viral studies
- The next step is to send clinical specimens to the NML for virus sequencing to detect if WPV has been isolated from a case with paralysis.
- If yes, then complete Article 6 Notification to the WHO (within 24 hours) following laboratory (NML) sequencing in collaboration with the affected PT and include a description of the event and Annex 2 Assessment.
- If no, has WPV (nonparalytic) or VDPV or Sabin/Sabin-like type 2 been identified?
- If yes, complete IHR Annex 2 assessment (within 48 hours) following laboratory (NML) sequencing in collaboration with the affected PT. Then, if determined to meet criteria for Article 6 (Notification) or Article 7 (Information sharing during unexpected or unusual public health events), alert WHO (within 24 hours of assessment) in collaboration with the affected PT and include a description of the event and Annex 2 Assessment
- If no, then Article 6 notification is not required, continue assessment to determine if other reporting requirements apply under the IHR to support transparency and public health response.
If the answer is 'no' and the criteria for classifying the case as paralytic poliomyelitis or nonparalytic poliovirus infection has not been met, then it must be determined if there is a case under investigation with clinical features compatible with paralytic polio and a higher index of suspicion for poliomyelitis (more details in Section 8.1).
If the answer is 'yes', then complete the following steps:
- Isolate the case as indicated in Section 8.1
- Notify appropriate local and provincial/territorial public health officials, as appropriate
- Initiate case and contact investigation and management, as appropriate
- Consider reporting to PHAC that a case is under investigation and has a higher index of suspicion for poliovirus, and ensure collection of at least 2 stool samples, taken at least 24 hours apart, within 14 days (up to 6 weeks) after the onset of paralysis for viral studies
- Section 6 contains additional information regarding poliovirus detection
If there is no case under investigation with clinical features compatible with paralytic polio and a higher index of suspicion for poliomyelitis then where applicable, where applicable, manage and report acute flaccid paralysis cases per usual processes.
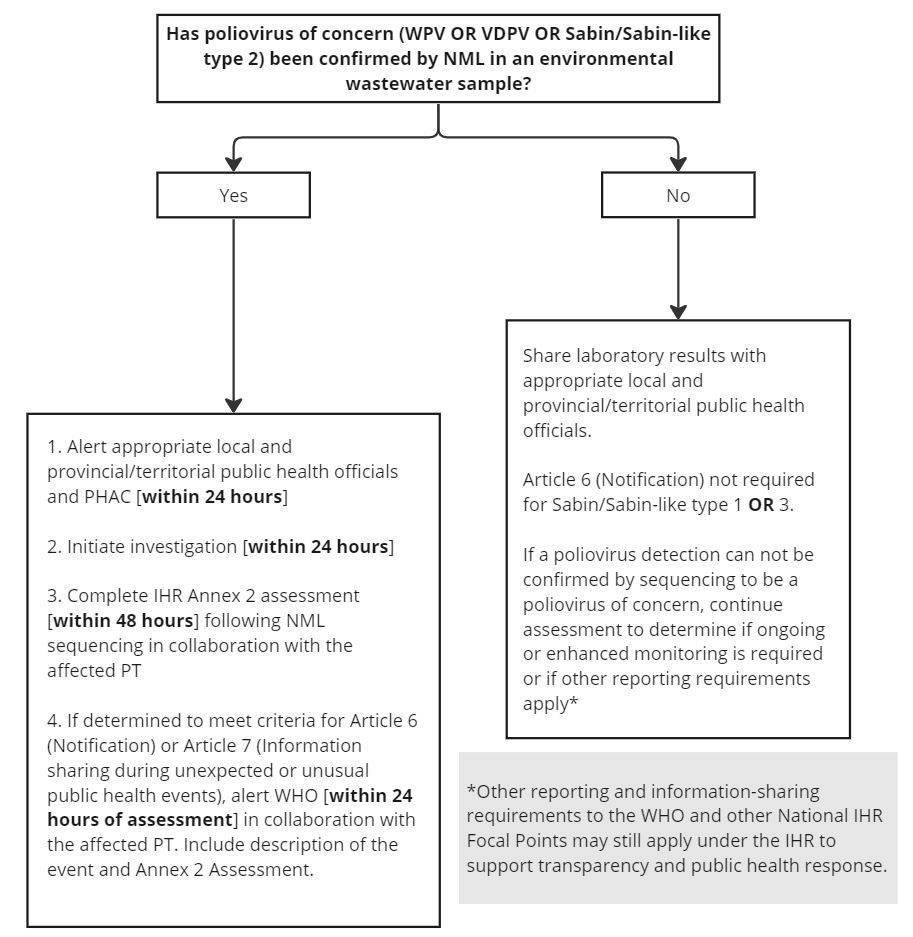
Figure 2 - Text description
The graphic is a flow chart illustrating the key initial steps and notification requirements for a detection of poliovirus from environmental wastewater sampling.
The first step is determining if a poliovirus of concern (WPV OR VDPV OR Sabin/Sabin-like type 2) has been confirmed by the NML in an environmental wastewater sample.
If the answer is yes, then the next steps would be:
- Alert appropriate local and provincial/territorial public health officials and PHAC within 24 hours
- Initiate investigation within 24 hours
- Complete the IHR Annex 2 assessment within 48 hours following NML sequencing in collaboration with the affected PT
- If determined to meet criteria for Article 6 (Notification) or Article 7 (Information sharing during unexpected or unusual public health events), alert WHO within 24 hours of assessment in collaboration with the affected PT. Include description of the event and Annex 2 Assessment.
If the answer is no, and a poliovirus of concern has not been confirmed, then the laboratory results will be shared with appropriate local and provincial/territorial public health officials.
An article 6 (Notification) is not required for Sabin/Sabin-like type 1 or 3.
If a poliovirus detection can not be confirmed by sequencing to be a poliovirus of concern, continue assessment to determine if ongoing or enhanced monitoring is required or if other reporting requirements apply under the IHR to support transparency and public health response.
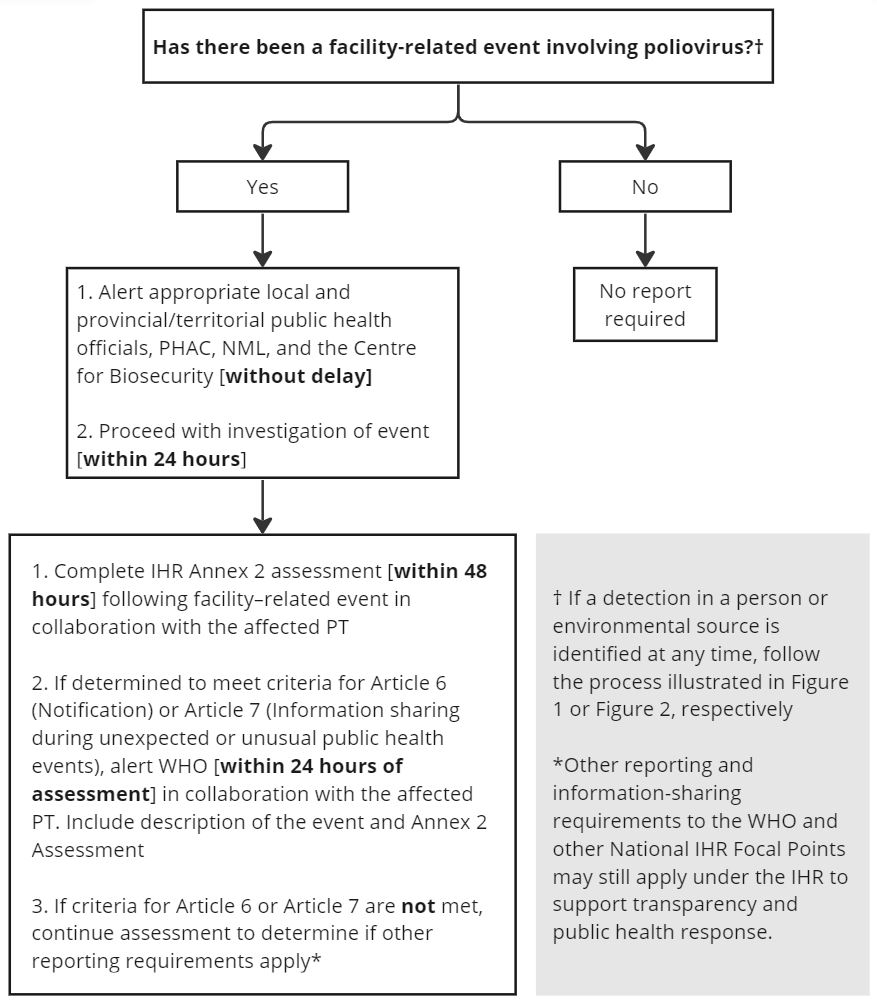
Figure 3 - Text description
The graphic is a flow chart illustrating the key initial steps and notification requirements for a facility-related event involving poliovirus.
It is noted that if a detection in a person or environmental source is identified at any time to follow the process illustrated in Figure 1 or Figure 2, respectively.
The first step is to determine if there has been a facility-related event involving poliovirus.
If the answer is no, then no report is required.
If the answer is yes, the next steps would be:
- Alert appropriate provincial/territorial public health officials, PHAC, NML, and the Centre for Biosecurity without delay
- Proceed with investigation of event within 24 hours
Once the steps above have been completed, then follow the next steps:
- Complete IHR Annex 2 Assessment within 48 hours following facility-related event in collaboration with the affected PT
- If determined to meet criteria for Article 6 (Notification) or Article 7 (Information sharing during unexpected or unusual public health events), alert WHO within 24 hours of assessment in collaboration with the affected PT and include a description of the event and Annex 2 Assessment
If the criteria for Article 6 or Article 7 are not met, continue assessment to determine if other reporting requirements apply under the IHR to support transparency and public health response.
Annex B: Public health management of a facility-related event involving poliovirus
Public health risk assessment of a facility-related exposure event for poliovirus
| Public Health Risk | Poliovirus Exposure Event |
|---|---|
Very High Risk |
|
High Risk |
|
Low Risk |
|
Minimal Risk |
|
Note 1: Where a breach involves multiple polioviruses, assess the risk to the virus that poses the highest public health risk.
Note 2: When assessing public health risk, consider the immunization history/profile of the exposed persons close contacts and/or local community.
Viral shedding monitoring post exposure in a facility-related event: specimen collection and sampling schedule
Collect both throat swab in Universal Transport medium (UTM) and stool (2-8 g) samples on days 3/4 and days 7/8 post exposure. If sample(s) test negative for poliovirus by RT-PCR, the probable exposure has not led to measurable virus excretion. Discontinuing isolation of individual is possible.
If any sample(s) test positive for poliovirus, continue to collect stool samples daily until 3 consecutive samples collected 24 hours apart test negative for poliovirus.
Possible control measures for management of a potentially exposed person in a facility-related event by the type of poliovirus material
| Activity | Sabin/Sabin-like type 1 or 3 | Sabin/Sabin-like type 2 (with > 90% IPV coverage and access to safely managed sewage) |
WPV/VDPV (any type) Sabin/Sabin-like type 2 - (< 90% IPV coverage and lower access to safely managed sewage) |
|---|---|---|---|
| Isolation | None | At home | At home, provided good compliance |
| Isolation Duration | N/A | Min. 7 days at home if all testing negative | Min. 7 days at home if all testing negative |
| Management of Stool | General Sewage; Disinfection of toilet following use | General Sewage. Dedicated (own use) toilet if possible. | General Sewage. Dedicated (own use) toilet or Collect and incinerate if toilet is not connected to municipal wastewater management system |
| Cleaning/ Disinfection |
Household bleach/0.5% Accelerated Hydrogen Peroxide (AHP) containing disinfectant (i.e., PREempt RTU) | Household bleach/0.5% AHP containing disinfectant (i.e., PREempt RTU) | Household bleach/0.5% AHP containing disinfectant (i.e., PREempt RTU) |
| Food Handling (for other people) | Not Permitted | Not Permitted | Not Permitted |
| Close Contacts/Visitors | Should be limited to those with proven vaccination history and in good general health (i.e., not immunocompromised); should be educated on proper handwashing. | Should be limited to those with proven vaccination history and in good general health (i.e., not immunocompromised); should be educated proper handwashing. | Should be limited to those with proven vaccination history and in good general health (i.e., not immunocompromised); should be educated on proper handwashing. |
For additional recommendations of control measures based on the public health risk posed by the exposure, refer Guidance in managing exposed persons for countries hosting facilities that maintain live polioviruses.
Relevant Canadian legislation: Human Pathogens and Toxins Act
- Under the HPTA (12(1)): "Obligation to Inform Minister: Inadvertent release: 12(1) if a licence holder has reason to believe that a human pathogen or toxin has been released inadvertently from the facility in the course of an activity that is otherwise authorized by the licence, the licence holder shall, without delay, inform the Minister of the release and provide the Minister with the information referred to in subsection (3) that is under the licence holder's control."
- In the case of a poliovirus biocontainment incident that involves human disease: "Obligation to Inform Minister: Disease (13) if a licence holder has reason to believe that an incident involving a human pathogen or toxin that is in their possession has, or may have, caused disease in an individual, the licence holder shall, without delay, inform the Minister of the incident and provide the Minister with the following information that is under the licence holder's control: (a) a description of the incident; (b) the name of the human pathogen or toxin; and (c) any other information relating to the incident that the Minister may require."
Annex C: Laboratory protocols
Clinical specimen collection protocol
For diagnostics, ensure collection of at least two stool samples (taken at least 24 hours apart and minimum 2 grams per sample) within 14 days after symptom onset for viral studies.
Stool (two samples taken at least 24 hours apart) is the required clinical specimen for the laboratory investigation and diagnosis of poliovirus infections. Stool collected from diapers are acceptable samples. A stool sample is preferred to a rectal swab because the diagnosis of poliovirus is more reliable.
A case can be considered no longer infectious based on three consecutive negative stool samples each collected at least 24 hours apart.
A high risk contact subject to stool sampling can be determined not to be infected, based on two consecutive negative stool samples taken at least 48 hours apart, with the first collected at least 4 days after the contact's last exposure to the case before infection prevention and control measures were initiated.
Minimum Volume Required |
2-8 g |
|---|---|
Turnaround Time |
Total turnaround time for the complete polio testing algorithm is 28 calendar days:
|
Collection, Storage, and Preservation of Specimen Prior to Shipping |
At least two stool specimens collected at least 24 hours apart, ideally within 14 days after onset Samples can be collected up to 6 weeks after onset if it is not possible to collect earlier Place in sterile leak proof container Keep specimens frozen (≤ -20C) Do not add transport medium |
Shipping Instructions |
NML does not accept routine shipments on weekends or holidays. Please make sure packages arrive Monday – Friday. Ship specimens frozen on dry ice according to TDG regulations. |
- NML Requisition for Poliovirus Detection
- NML's Molecular Detection and Characterization (for submitting clinical samples to lab)
| Collection, Storage, and Preservation of Specimen Prior to Shipping | 500 mL of composite or grab samples should be sent to PHAC NML Wastewater Surveillance Unit. Ideally the samples should be maintained at 4οC during collection and storage. The impact of common water/wastewater preservatives has not been assessed and submitters are discouraged from sending preserved samples. Please contact the NML (NMLWastewater.NMLWastewater@phac-aspc.gc.ca) before planning to ship samples to the NML for testing wastewater for poliovirus |
|---|---|
| Shipping Instructions | NML does not accept routine shipments on weekends or holidays. Please make sure packages arrive Monday – Friday. Please refer to Packaging wastewater samples for shipment protocol. Accompany each shipment with a sample requisition sheet available online.
Essential details:
|
Working and storing potentially infectious materials (poliovirus)
When working with and storing PIM, risk mitigation measures must be followed to prevent facility-related events, as outlined in the WHO PIM Guidance Document (2nd edition). These requirements include:
- Verification of polio vaccination of personnel handling the virus. If vaccination cannot be verified, they must receive a single lifetime adult booster of IPV-containing vaccine. Enhanced Biosecurity protocols - this includes storing the PIM in locked freezers, segregated from the general inventory, with access limited to trained personnel. Also, detailed inventories must be maintained and reported to PHAC's Centre of Biosecurity as Canada's National Authority of Containment (NAC) for Poliovirus. For additional information, containment questions can be directed to Canada's NAC at polio@phac-aspc.gc.ca; use the subject line – "Poliovirus Containment Wastewater"; use the subject line – "Poliovirus Containment Wastewater"
- Note: Although sewage/concentrates may not pose an immediate risk to vaccinated personnel (virus concentration is below the infectious dose), the intent of the guidelines is to ensure the facility identifies these materials so that no high risk activities (such as inoculation into polio permissive cells) are inadvertently performed.
- Detailed biological risk assessments, which will inform best biosafety practices (such as measures to reduce aerosol exposure, what disinfectants and additional PPE are to be used) need to be on file.
- If the presence of an eradicated poliovirus (type 2), or of any type or a VDPV/WPV, is confirmed, this material is subject to full containment requirements. For laboratories who are not officially designated by the NAC as PEFs, the PIM must be destroyed or inactivated using validated procedures. The Canadian NAC can be contacted for guidance on containment and disposition of PIM at polio@phac-aspc.gc.ca.
- If the facility wishes to retain this material, the options are to:
- Transfer the PIM to a certified PEF (the NML), or,
- Apply to the NAC to become a PEF that is certified to meet the biosafety and biosecurity requirements outlined in the Global Action Plan for Poliovirus Containment (GAPIV) and Strategy for Poliovirus Containment.
Annex D: IHR reporting
Event description prompts
Insert event description, where possible including:
- number of cases and deaths
- case definitions
- laboratory results
- source and type of the risk
- conditions affecting the spread of the disease and
- public health measures employed
Note: if this information is not yet available at the time of reporting, then provide it to PAHO/WHO as soon as possible in a follow-up / update report
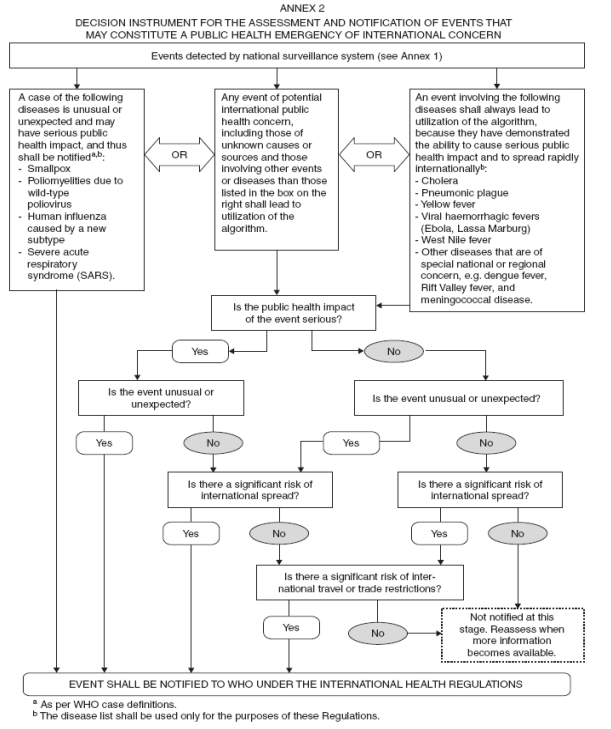
Figure 4 - Text description
The graphic is a flow chart illustrating the points of decision for the assessment and notification of events that may constitute a public health emergency of international concern.
Events detected by national surveillance systems fall into one of three categories.
The first category is cases of certain diseases that are unusual or unexpected and may have serious public health impact, and thus shall be notified as per WHO case definitions. The diseases are smallpox, poliomyelitis due to wild-type, human influenzas caused by a new subtype, or severe acute respiratory syndrome (SARS). The illustration notes that the disease list shall be used only for the purposes of these Regulations.
- If the event is a case of one of the identified diseases, the event shall be notified to WHO under the International Health Regulations
The second category of event, are any events of potential international public health concern, including those of unknown causes or sources and those involving other events or diseases than those listed in the first or third category shall lead to utilization of the algorithm.
The third category are events involving certain diseases shall always lead to utilization of the algorithm because they have demonstrated the ability to cause serious public health impact and to spread internationally. The diseases listed are cholera, pneumonic plague, yellow fever, viral haemorrhagic fevers (Ebola, Lassa Marburg), West Nile fever, and other diseases that are of special national or regional concern, e.g. dengue fever, rift valley fever and meningococcal disease.
For the second and third category of event, the user is prompted to assess the event using four questions:
- Is the public health impact of the event serious?
- Is the event unusual or unexpected?
- Is there a significant risk of international spread?
- Is there a significant risk of international travel or trade restrictions.
If two or more of the questions are answered in the affirmative, the flow chart indicates that the event shall be notified to WHO under the International Health Regulations. If fewer than two of the questions are answered in the affirmative the flow chart indicates the event does not require notification at this stage and to reassess when more information becomes available.
Source: International Health Regulations (2005) (IHR) Annex 2
Note: The decision for each prompt, as well as a rationale, will need to be documented and submitted as part of the assessment.
Annex E: GPEI initial risk assessment
| Risk category | High Risk | Low Risk | Remarks |
|---|---|---|---|
| Virology | |||
cVDPV |
Automatically defined as high risk situation |
- |
- |
| Virologic factors | |||
Genetic deviation from parent vaccine virus (nucleotide changes) |
Substantial |
Not substantial |
Seek expert virologist assessment |
Relatedness, if any, to past isolations |
Related |
Not related |
|
Virologist characterization / interpretation |
Yes |
No |
|
Co-circulation with WPV |
Yes |
No |
|
Detection of other (un-related) VDPVs in region |
Yes |
No |
|
| Human Source | |||
Co-isolation with other Sabin or enterovirus |
Yes |
No |
|
Evidence of primary immunodeficiency |
No |
Yes |
|
| Environmental Science | |||
Number of virus isolates |
High |
Medium/low |
|
Genetic diversity (number of genetic clusters) |
|||
| Context | |||
| Case Characteristics | |||
Member of known "high risk"/underserved population (slum, minority, refugee, mobile, internally displaced, etc.) |
Yes |
No |
Review and discussion by technical experts, between country, region and global levels |
0 dose or "under"-vaccinated |
Yes |
No |
|
Aged above 5 years |
Yes |
No |
|
| Coverage data | |||
RI coverage (IPV if available-otherwise diphtheria-tetanus-pertussis (DPT3) in infected Admin 1 level |
Poor |
Good/high |
Population Immunity for type 2 polioviruses, should factor time since switch and use of IPV to estimate type 2 naïve population |
Quality of prior (>5% missed children by IM data, >80% LQAS lots passed) |
Poor |
Fair/good |
|
| Surveillance quality | |||
Surveillance quality (factors such as sub-standard AFP indicators, infrequent or absent environmental surveillance, orphan virus) in infected Admin 1 level |
Evident |
Fair/good |
- |
Other recent poliovirus detection |
Yes |
No |
- |
| Admin level 1 context | |||
Large, densely populated are |
Yes |
No |
- |
Known high risk populations (such as mobile, refugee, trade, pilgrimage, displacement) |
Yes |
No |
- |
Insecure and/or inaccessible area affecting surveillance and/or immunization |
Yes |
No |
- |
Any type of sentinel events suggesting higher risk of rapid spread |
Yes |
No |
- |
Evidence of containment breach |
Yes |
No |
- |
Finding tOPV/mOPV2/nOPV2 in a sweep of the vaccine distribution chain |
Yes |
No |
- |
Environmental conditions associated with high levels of fecal-oral transmission |
Poor water and sanitation |
Fair/good water and sanitation |
- |
| International spread | |||
| Linkages with International Border | |||
Contiguous or direct transport link to int'l border (especially if other area is known high risk) |
Yes |
No |
Local case investigation / based on available data Review and discussion by GPEI technical experts, in consultation with country, regional levels |
Links between site or person with poliovirus to other countries (such as markets, transport routes) |
Yes |
No |
|
Travel history of poliovirus case or household (such as refugee, nomadic, pilgrimage, stateless persons) to the neighbouring (or any other) country |
Yes |
No |
|
Prior history of polio transmission patterns and outbreaks between countries |
Yes |
No |
|
| Population mobility/migration | |||
Common service points between infected area and neighbouring areas like markets, pilgrim sites, common trading sites etc. |
Yes |
No |
- |
Evidence of high levels of migration (from sequencing data, available cell phone data, prior migration patterns, etc.) |
Yes |
No |
- |
| Context of neighbouring areas | |||
Evidence of surveillance gaps or other high risk factors in neighbouring areas susceptible to importation from affected areas |
Yes |
No |
- |
Population immunity in neighbouring countries/areas |
Low |
Good/high |
- |
Conflict |
Present |
None |
- |
Source: GPEI SOP: Responding to a poliovirus event or outbreak
Selected references
- Reference 1
-
World Health Organization. Poliovirus IHR Emergency Committee Statements. 2023; Available at: https://www.who.int/groups/poliovirus-ihr-emergency-committee
- Reference 2
-
Global Polio Eradication Initiative. Global eradication of wild poliovirus type 2 declared. 2015; Available at: https://polioeradication.org/news-post/global-eradication-of-wild-poliovirus-type-2-declared
- Reference 3
-
Global Polio Eradication Initiative. Two out of three wild poliovirus strains eradicated. 2019; Available at: https://polioeradication.org/news-post/global-eradication-of-wild-poliovirus-type-2-declared
- Reference 4
-
Global Polio Eradication Initiative. Classification and reporting of vaccine-derived polioviruses (VDPV). 2016; Available at: https://polioeradication.org/wp-content/uploads/2016/09/Reporting-and-Classification-of-VDPVs_Aug2016_EN.pdf
- Reference 5
-
Public Health Agency of Canada. Provincial and territorial routine and catch-up vaccination schedule for infants and children in Canada. 2023; Available at: https://www.canada.ca/en/public-health/services/provincial-territorial-immunization-information/provincial-territorial-routine-vaccination-programs-infants-children.html
- Reference 6
-
Global Polio Eradication Initiative. OPV Cessation. 2023; Available at: https://polioeradication.org/polio-today/preparing-for-a-polio-free-world/opv-cessation/#:~:text=The%20switch,with%20significant%20public%20health%20benefitsalertalert
- Reference 7
-
Global Eradication Initiative. Inactivated polio vaccine now introduced worldwide. 2019; Available at: https://polioeradication.org/news-post/inactivated-polio-vaccine-now-introduced-worldwide/
- Reference 8
-
World Health Organization. Polio vaccines: WHO position paper - June 2022. Wkly Epidemiol Rec 2022 June 24;25(97):277-300
- Reference 9
-
World Health Organization. First ever vaccine listed under WHO emergency use. 2020; Available at: https://www.who.int/news/item/13-11-2020-first-ever-vaccine-listed-under-who-emergency-use
- Reference 10
-
Global Polio Eradication Initiative. nOPV2. 2023; Available at: https://polioeradication.org/nopv2
- Reference 11
-
Global Polio Eradication Initiative. Preparing for nOPV2 Use: An overview on requirements for countries. 2022; Available at: https://polioeradication.org/wp-content/uploads/2022/06/nOPV2-requirements-overview-for-countries-.pdf
- Reference 12
-
Macklin GR, Peak C, Eisenhawer M, Kurji F, Mach O, Konz J, et al. Enabling accelerated vaccine roll-out for Public Health Emergencies of International Concern (PHEICs): Novel Oral Polio Vaccine type 2 (nOPV2) experience. Vaccine 2023 April 06;41:A122-A127
- Reference 13
-
Desai S, Smith T, Thorley BR, Grenier D, Dickson N, Altpeter E, et al. Performance of acute flaccid paralysis surveillance compared with World Health Organization standards. J Paediatr Child Health 2014 July 14;51(2):209-214
- Reference 14
-
Public Health Agency of Canada. Polio: For health professionals. 2023; Available at: https://www.canada.ca/en/public-health/services/diseases/poliomyelitis-polio/health-professionals.html
- Reference 15
-
Booth TF, Grudeski E, McDermid A, Rotondo J. The polio eradication endgame: Why immunization and continued surveillance is critical. Can commun dis rep 2015 October 01;41:233
- Reference 16
-
World Health Organization. WHO Global Action Plan for Poliovirus Containment (GAPIV). 2022; https://polioeradication.org/wp-content/uploads/2022/07/WHO-Global-Action-Plan-for-Poliovirus-Containment-GAPIV.pdf
- Reference 17
-
World Health Organization. International Health Regulations (2005), 3rd ed. 2016; Available at: https://apps.who.int/iris/handle/10665/246107
- Reference 18
-
World Health Organization. Case definitions for the four diseases requiring notification in all circumstances under the International Health Regulations. 2005; Available at: https://cdn.who.int/media/docs/default-source/documents/emergencies/case-definitions-ihr-four-diseases7f1ee707-3d13-4581-a1af-d5f44f86423a.pdf?sfvrsn=9c68df20_1&download=true
References
- Reference a
-
Detection meaning the date of stool sample collection that was positive for poliovirus for those with nonparalytic poliovirus infection.
- Reference b
-
See Annex B for details on facility-related events. For more details on laboratory and vaccine manufacturing related poliovirus events, refer to Public health management of facility-related exposure to live polioviruses: Guidance in managing exposed persons for countries hosting facilities that maintain live polioviruses.
- Reference c
-
The criteria for cVDPV classification can be found in the GPEI's Classification and reporting vaccine-derived polioviruses (VDPV) - page 3.
- Reference d
-
Poliovirus in clinical and environmental samples survive indefinitely in the laboratory freezer (≤ -20° C), and for many months in the refrigerator.
