Selection of Body Weights Values for Use in Human Health Risk Assessment (HHRA) Conducted by Health Canada

Download the alternative format
Organization: Health Canada
Published: 2019-09-03
Cat.: H129-67/2016E-PDF
ISBN: 978-0-660-06290-7
An Exploratory Document on Current Health Canada Practices in the Selection of Body Weights for Use in Risk Assessment and the Potential for Harmonization across the Department in its Use
Prepared by the Task Force on Scientific Risk Assessment's Body Weights Working Group
- Luigi Lorusso, Contaminated Sites Division, Safe Environments Directorate, HECSB
- Christine McEwan, Water Quality Science Division, Safe Environments Directorate, HECSB
- Michele Giddings, Water Air and Climate Change BureauFootnote 1, Safe Environments Directorate, HECSB
- Deborah Watt, Existing Substances, Safe Environments Directorate, HECSB
- Mikin Patel, Existing Substances, Safe Environments Directorate, HECSB
- Kristin Macey, Environmental Health Surveillance Division, Environmental and Radiation Health Sciences Directorate, HECSB
- John Field, Consumer Product Safety Directorate, HECSB
- Gina Coleman, Biologics and Genetic Therapies Directorate, HPFB
- Maya Villeneuve, Food Directorate, HPFB
- Mark Feeley, Food Directorate, HPFB
- Scott Jordan, Marketed Health Products Directorate, HPFB
- Dominique Heon, Marketed Health Products Directorate, HPFB
- Robin Marles, Natural Health Products DirectorateFootnote 2, HPFB
- Yadvinder Bhuller, Therapeutic Products Directorate, HPFB
- Thea Mueller, Therapeutic Products Directorate, HPFB
- Rajinder Sharma, Veterinary Drugs Directorate, HPFB
- Song Gao, Health Evaluation Directorate, PMRA
- Graham White, New Chemical SubstancesFootnote 1
Note: Other groups within the department were consulted with, but were not included in the working group.
Table of Contents
- 1.0 Background
- 2.0 Introduction
- 3.0 Methodology
- 4.0 Results and Discussion
- 5.0 Conclusions and Recommendations
- 6.0 References
- 7.0 Tables
- Appendices
Acronyms
- BFPSI
- Bureau of Food Policy and Science Integration
- BGTD
- Biologics and Genetic Therapies Directorate
- BW
- body weight
- CDC
- Centres for Disease Control
- CCHS
- Canadian Community Health Survey
- CFS
- Canadian Fitness Survey
- CHMS
- Canadian Health Measures Survey
- CIHI
- Canadian Institute for Health Information
- CPSD
- Consumer Product Safety Directorate
- CSD
- Contaminated Sites Division
- CVM
- Centre for Veterinarian Medicine
- DEEM
- Dietary Exposure Evaluation Model
- DRI
- Dietary Reference Intake
- EHB
- Environmental Health Bureau
- ERHSD
- Environmental and Radiation Health Sciences Directorate
- ESRAB
- Existing Substances Risk Assessment Bureau
- F
- Female
- FD
- Food Directorate
- HC
- Health Canada
- HHRA
- Human Health Risk Assessment
- HPFB
- Health Products Food Branch
- Kg
- kilograms
- M
- Male
- MHPD
- Marketed Health Products Directorate
- NA
- not applicable
- NCHS
- National Center for Health Statistics
- NHANES
- National Health and Nutrition Examination Survey
- NHPD
- Natural Health Products Directorate
- NHW
- Department of National Health and Welfare
- NSACB
- New Substances Assessment and Control Bureau
- PHAC
- Public Health Agency of Canada
- PMRA
- Pest Management Regulatory Agency
- RA
- Risk Assessment
- RIVM
- Rijksinstituut voor Volksgezondheid en Milieu (Dutch National Institute for Public Health and the Environment)
- SRA
- Scientific Risk Assessment
- TFSRA
- Task Force on Scientific Risk Assessment
- TPD
- Therapeutic Products Directorate
- UK
- United Kingdom
- US EPA
- United States Environmental Protection Agency
- US FDA
- Unites States Food and Drug Administration
- VDD
- Veterinary Drugs Directorate
- WACCB
- Water, Air and Climate Change Bureau
- WHO
- World Health Organization
- Yrs
- Years
1.0 Background
Health Canada is the federal department responsible for helping the people of Canada maintain and improve their health. Health Canada is committed to improving the lives of all of Canada's people and to making this country's population among the healthiest in the world as measured by longevity, lifestyle and effective use of the public health care system. There are a wide range of activities conducted throughout the department in support of its mandate which require the use of body weights that are representative of the Canadian population in general. For example, Health Canada assesses and regulates the safety of various products (such as pharmaceuticals, foods, consumer goods, pest control products, toxic substances, etc.), establishes guidelines for various media (e.g., air, water, soil), evaluates environmental exposure to chemicals, examines occupational health issues, and gives consideration to the protection of sensitive subpopulations (e.g., children, women of child bearing age, Aboriginals, seniors, etc.).
The TFSRA initiated this project to determine which body weights and associated age groups were currently being used by the various groups within the department, in the course of conducting human health risk assessments (HHRAs) as part of their respective mandated work. The Task Force on Scientific Risk Assessment (TFSRA) is an intradepartmental working group tasked at improving information sharing and enhancing coordination and coherence of scientific risk assessments (SRA) within Health Canada. The main objectives of the TFSRA are: to address broader, cross-cutting issues on SRA; foster a departmental community of risk assessors; and address divergence issues on SRA. This project is one of the current main initiatives of the TFSRA. Body weight is a common factor applied in risk assessments. Body weight appears in risk assessments throughout the department, and has been designated as an important topic for analysis, specifically to examine with regard to how this factor is used, including which values are used in practice.
Given the broad scope with respect to types of health science activities conducted within Health Canada, it is not surprising to find that the body weight values (and associated age groups) used across the department may not necessarily be the same, even for the same chemical being assessed by more than one group within the department (e.g., lead in consumer products, food, and environmental media is assessed by multiple groups within Health Canada, all of which may use different body weights).
This document is also intended as an explanatory document, explaining divergent issues where harmonization is not always possible due to the diverse nature and contexts of various programs. If it is determined that there is a potential to harmonize the body weight values used in risk assessment, such harmonization will be recommended for consideration by the Department. However, the implementation of such harmonization and associated implications will be the responsibility of the various program areas involved.
1.1 Purpose
This project was specifically initiated to address the two tasks listed below:
- Determine current body weight values used as exposure factors in all applicable groups within the department.
- Identify opportunities for harmonization and the process required to implement such harmonization within Health Canada while taking into consideration international approaches.
1.2 Specific Objectives
The expectations of this project include the following:
- To gain a clear understanding of why selected body weight values are used in Human Health Risk Assessment (HHRA).
- To complete a comparison of currently used body weight values and associated age groups to more recent Canadian data (i.e., CCHS 2004 Cycle 2.2), and to determine if there are any differences which could potentially impact the risk assessments being conducted by Health Canada.
- To identify opportunities for harmonization in the use of body weight data in HHRA.
- To generate a better understanding/rationale as to challenges presented by harmonization across the Department.
- To generate recommendations, based on best practices, on agreed updated body weight values that can be used within the Department for HHRA. These recommendations could then be applied by the various program areas within the Department, as practicable.
2.0 Introduction
2.1 General overview on Human Health Risk Assessment
While HHRAs are carried out in many areas of Health Canada, under different circumstances and contexts, they share the same underlying purpose of assessing risks to human health, with the ultimate goal of protecting the health of Canadians. The four general principles of risk assessment are illustrated below in Figure 1.
Body weight is considered a receptor characteristic, which is a critical parameter required in the analysis of determining the risk to humans. During the Problem Formulation step of any HHRA, the risk assessor must evaluate the population demographic (i.e. what age groups, sex, and/or sensitive sub-population such as infants) that may be exposed to the substance (e.g. chemical, drug, etc.) of interest. Additionally, based on the duration of exposure (Ex: acute vs. chronic) an evaluator may choose to be more conservative in their choice of body weight as the assessor may choose to be protective in the event of an acute hazard. The exposure is generally expressed per unit body weight for the age group(s) of interest; therefore, the representative body weight values must be specific to the applicable population demographic(s).
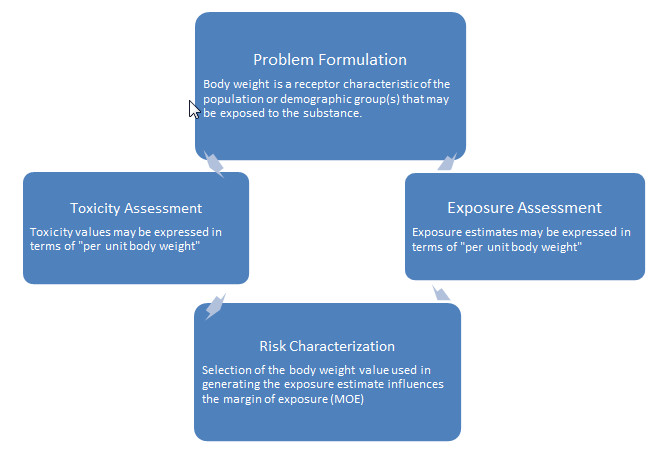
Figure 1 - Text Equivalent
Figure 1 depicts the four general principles of risk assessment: Problem formulation, toxicity assessment, exposure assessment and risk characterization. Further descriptive text relevant to the selection of body weights for use in risk assessment is included under each of the four headings or steps, as follows:
- Problem Formulation - Body weight is considered a receptor characteristic of the population of demographic group(s) that may be exposed to the substance.
- Toxicity Assessment - Toxicity values may be expressed in terms of "per unit body weight."
- Exposure Assessment - Exposure estimates may be expressed in terms of "per unit body weight."
- Risk Characterization - Selection of the body weight value used in generating the exposure estimate influences the margin of exposure (MOE).
The following Health Canada documents relate to HHRA and demonstrate some of the various ways in which HHRA is conducted in HC:
- Health Canada Decision-Making Framework for Identifying, Assessing, and Managing Health Risks. August 1, 2000.
- A Primer on Scientific Risk Assessment at Health Canada. 2010.
- Science Policy Notice: A Decision Framework for Risk Assessment and Risk Management in the Pest Management Regulatory Agency (SPN2000-01).
- Federal Contaminated Sites Risk Assessment in Canada Part I: Guidance on Human Health Risk Assessment (PQRA), 2004.
- Health Products and Food Branch's Guide for Conducting Health Risk Assessments in Humans (HPFB HRA Working Group 2011).
- A Framework for the Application of Precaution in Science-based Decision Making About Risk (Government of Canada 2003).
- Health Products and Food Branch's Guide for Conducting Health Risk Assessments in Humans (HRA Guide).
Note: The above list is not exhaustive; it is based on the information collected in the survey.
2.2 Body weight as an exposure factor
Body weight is a necessary parameter for calculating exposure to substances, as exposure is determined and/or measured on a per unit body weight basis. This is the typical dose expression in human clinical studies and health risk assessments. Body weight values are typically categorized according to age group or life stage as discussed later in Section 2.4 (e.g. infant: 0-6 months, adult: 20-59 years, etc.). The application of body weight as an exposure factor is linked to the age of population of interest; therefore, the use of body weight and age categories in risk assessments must be discussed simultaneously. There are certain cases where body surface area is more applicable than body weight when assessing exposure, both body weight and body surface area are also closely linked. The relationship between body weight and other exposure factors should be considered where applicable (i.e., inhalation rate and dietary intake).
2.3 Body weight in toxicity assessments
While the focus of this document is primarily on the application of body weight as an exposure factor, the information provided within this document could also be applicable to toxicity assessments as the body weight also plays an important role in assessing the toxicity of substances. For example, allometric interspecies scaling of toxicity data for generating a tolerable daily intake (TDI) or toxicological reference value (TRV) may depend on certain receptor characteristics, such as body weight. Another example could be the determination of the maximum safe starting dose in humans for an early phase clinical trial (e.g., Phase 0 or 1). As the underlying objective for these types of trials is to further determine the safety of the product in addition to its pharmacological parameters in humans, the body weight value could have an impact on the identification and selection of this starting dose.
2.4 Age Grouping
Body weight values used in risk assessments are dependent on the age groups of interest. Body weight values change substantially over the course of an individual's lifetime and one must select how to set age groups based on those pertinent lifecycle changes for the purpose of conducting relevant HHRAs. Typically, age groups are established based on biological changes associated with human development or based on behavioural changes.
When conducting HHRAs, Health Canada relies on several Canadian and international guidelines, which provide rationales and other relevant information to aid in the selection of age groupings (as outlined in Tables 3 and 4) for consideration. In certain cases, the age groupings used by Health Canada are based on regulatory requirements (e.g., pharmaceuticals are regulated under the Food and Drug Regulations and as such are assessed based on the age groupings outlined in the regulations). Deviations from such regulatory requirements would present significant challenges for Health Canada. Similarly, changes to the age groupings currently used would also require detailed processes, such as the requirement for regulatory amendments.
From a clinical perspective, attempts are made, where possible, to correlate the age with body weight using validated growth charts, such as those provided by the World Health Organization. However, several challenges exist in correlating age with body weight, such as differences in the grouping of ages across various programs of Health Canada and the selection of which growth charts to use. As a result, different body weight values could be chosen, which could result in different assessment outcomes.
The selected age groups for assessing risks to human health associated with environmental exposures are heavily weighted on behaviour. Exposure to environmental substances is assumed to change substantially over the course of an individual's lifetime, as a result of variations in medium-specific intake rates, activities and physical attributes with age. As an example, six age classes have been developed for the assessment of existing and new substances under the Canadian Environmental Protection Act, 1999 (CEPA 1999), representing the general Canadian population: infants (0-0.5 years), toddlers (0.5-4 years), children (5-11 years), teenagers (12 to 19 years), adults (20-59 years) and seniors (60 years and older). These age classes are intended to reflect more-or-less discrete stages of life in terms of varying potential for exposure to environmental substances. For example, the period of up to 6 months of age is when many infants may be exposed to substances present in breast milk. Toddlers' exposure to contaminants in soil may be significantly higher than that for other age groups, related to increased contact with soil and mouthing behaviours. Children have relatively high intakes of food per unit of body weight. Adulthood is a period of long-term low-level exposure via most environmental media, with relatively high potential exposure to some substances through activities such as the use of consumer products. For many seniors, retirement is associated with substantial changes in activity patterns, with implications for their potential exposure to chemical substances, and seniors are known to be a susceptible age class for some classes of pollutants (HC 1998).
Certain other aspects may need to be considered in the selection of age groups beyond those mentioned above. For example, the use of the chemical being assessed must be considered. Other exposure characteristics, such as body surface area, drinking water intake rates, and dietary intake rates, may need to be consistent with the age groups selected. More complex situations of aggregate exposure (e.g., with different data sources), as opposed to single pathway exposures, may impact the selection of age groups. The sensitivity of certain subpopulations to the chemical being assessed (e.g., children for lead) may influence the selection of a specific age group and associated body weight value. In addition, in the development of guidelines, the adult age group may account for all individuals 20 years of age and older, as the majority of guidelines are intended to protect individuals assuming a lifetime of exposure to the substance of interest.
2.5 Sources of Canadian body weight data currently used
Health Canada relies on several, credible sources for body weight data. However, depending upon the time of the original implementation of these data sources in specific programs within Health Canada, some of the data sets currently used are over 40 years old, which warrants the review of more recent data. Existing sources of body weight data currently used include:
- The 1970/72 Nutrition Canada Survey (NCS):
- This study sampled 12,713 individuals, representative of the general population in Canada (Department of National Health and Welfare 1973; Demirjian 1980). It was selected as the source of body weight data recommended for the infant (0 - 0.5 years of age), toddler (0.5 - 4 years of age) and child (5 - 11 years of age) age groups in Health Canada (1998).
- 1981 Canadian Fitness Survey (CFS) and the 1988 Campbell's Survey:
- The 1981 CFS involved a sample of more than 16,000 individuals selected to be representative of the Canadian population 7 years of age and older. Data were collected as part of an extensive study of leisure physical activity (Government of Canada 1983).
- The 1988 Campbell's Survey used a subset of 3508 individuals from the households studied in 1981 (Stephens and Craig 1990). It was selected as the source of body weight data recommended for the teenage (12 - 19 years of age), adult (20 - 59 years of age) and senior (60 years of age and over) age groups in Health Canada (1998).
- Growth charts are also used for identifying body weights. For example, CDC growth charts published in the year 2000 are recommended by the CDC for children that are 2 years of age and older (CDC 2010). These CDC growth charts are based on data collected by the National Center for Health Statistics (NCHS) in five nationally representative health examination surveys conducted in the U.S. from 1963 to 1994 (Kuczmarski 2002). In addition, the CDC recommends the WHO growth standards published in the year 2006 for children that are less than 2 years of age (CDC 2010). These WHO growth standards are based on data collected as part of the international WHO Multicentre Growth Reference Study (MGRS) from Pelotas (Brazil), Accra (Ghana), Delhi (India), Oslo (Norway), Muscat (Oman) and Davis (California) during the 1997 - 2003 study period (Grummer-Strawn et al. 2010).
Note: The above list is not exhaustive; it is a sample of the information collected in the survey. Please refer to Tables 3 and 4 for a full list of the external and internal reference sources, respectively, used by various programs in Health Canada, based on their survey responses, that are used for body weight data.
2.6 Sources of recent body weight data under consideration for use
The Canadian Community Health Survey (CCHS) was initiated to address a number of issues with the health information system in line with the Health Information Roadmap created by the Canadian Institute for Health Information (CIHI), Statistics Canada and Health Canada. The CCHS is a cross-sectional survey of the Canadian population which collects information related to health status, health care utilization and health determinants. It relies upon a large sample of respondents and is designed to provide reliable estimates at the health region level. Originally, data collection occurred biennially; therefore, data are available for the 2001, 2003 and 2005 periods. In 2007, major changes were made to the survey design and data collection now occurs annually (Statistics Canada 2011).
The objectives of the CCHS include:
- Supporting health surveillance programs by providing health data at the national, provincial and intra-provincial levels;
- Providing a single data source for health research on small populations and rare characteristics;
- The timely release of information easily accessible to a diverse community of users; and,
- Creating a flexible survey instrument that includes a rapid response option to address emerging issues related to the health of the population.
Additional information can be obtained from Statistics Canada, 2011. Canadian Community Health Survey - Annual Component (CCHS).
In 2004, as part of the CCHS, Statistics Canada carried out the Cycle 2.2-Nutrition (CCHS 2.2) survey that served as a separate focus content survey from the then main biennial survey of CCHS. The main objective of the CCHS 2.2 was to provide reliable, timely information about dietary intakes and nutritional well-being, and about the key determinants that may influence both of these variables, to inform and guide programs, policies and activities of federal and provincial governments. Self-reported and measured body weights of participating individuals were also collected as part of this survey. The survey included a sample of 35,107 respondents in the 10 provinces. A complete description of the CCHS 2.2 is available at www.hc-sc.gc.ca/fn-an/alt_formats/hpfb-dgpsa/pdf/surveill/cchs-guide-escc-eng.pdf.
CCHS 2.2 ensured that each age and gender group within each province was representative, with age groups under 1 year old being representative only at the national level (Statistics Canada 2008). The age groups (including gender groups) used in the 2004 CCHS 2.2 were 0-1, 1-3, 4-8, 9-13, 14-18, 19-30, 31-50, 51-70 and 71+ years. However, measured body weight data were obtained only from respondents aged 2 years or older.
The data provided within the 2004 CCHS 2.2-Nutrition were used to calculate averages, percentiles and proportions that are representative of the Canadian population. Sixty-three percent of the 35 107 survey respondents in the CCHS 2.2-Nutrition had their weight and height measured. Self-reported body weight data were obtained from a subset of 10% of participants aged 18 years or above who were asked to self-report their weights and would later have their weights measured. Body weights of children under 2 years of age were not recorded. For accuracy, measured body weight values are preferred over self-reported values. When assessing risks to human health or during the development of human health based guidance and/or guidelines, measured body weight values are thus used. However the actual body weight values used to represent each of the different age groups could be influenced by statistical treatment of the data set and also the choice of statistic chosen (e.g., mean, median, xth percentile).
The CCHS provides a reliable data source for body weights in representing the general Canadian population. The annual component of the CCHS requires 65,000 respondents that are 12 years of age or older, and collects self-reported body weight values of respondents as part of the CCHS questionnaire. Further, given that this study is ongoing (i.e. survey is now conducted annually),trends in body weight can be established and thereby provide valuable insight into the weight of Canadians and help shape policy and decisions being made by Health Canada.
The Canadian Health Measures Survey (CHMS) is another survey that is collecting useful health-related data. This biennial survey, conducted by Statistics Canada in partnership with Health Canada and the Public Health Agency of Canada, was initiated in 2007 in order to obtain measured health related parameters from Canadians, including body weight data, during collection cycles of two-year periods. The body weight data are both self-reported, based on responses to a personal interview at the household, followed by measured body weight data obtained at the mobile examination centres (MECs). Cycle 1 of this survey collected measured data from approximately 5,600 people aged 6 to 79 across the country. Cycle 2 collected data over the period of August 2009 to November 2011 from 6,400 respondents aged 3 to 79 years.
The CCHS Cycle 2.2-Nutrition (2004) survey is considered to be the largest recent Canadian data set of measured body weight values representative at the national level. While more recent body weight data are available from the annual component of CCHS, this data set is comprised of self-reported values and is therefore not considered as reliable. This lack of reliability was confirmed in a comparison of self-reported and measured body weight values obtained from 4,567 respondents aged 12 years or older in a subsample of respondents in the 2005 collection year of the then biennial CCHS. This comparison indicated an "underreporting bias," where female respondents underreported their weight by an average of 2.5 kg while the male respondents by 1.8 kg (Shields et al. 2008). In addition, while more recent measured body weight data are available from the CHMS, the biennial sample size of CHMS (i.e., 5,600 for Cycle 1; 6,400 for Cycle 2) is much smaller than the measured body weight sample size in CCHS Cycle 2.2 (2004) (i.e., 63% of 35,107 respondents). As such, the measured body weight data obtained as part of the CCHS Cycle 2.2-Nutrition (2004) are considered to be the most reliable source of recent Canadian body weight data at this time. However, it should be noted that another CCHS "Nutrition" focus content component is scheduled for the 2015 calendar year, which, if similar in design to CCHS Cycle 2.2, may serve as a more updated source of Canadian measured body weight data in the future (see Trainor 2012).
It is important to note that neither of these surveys (CCHS and CHMS) captures body weight information for infants and toddlers less than two years old. This leaves a data gap in terms of gathering recent body weight data for infants and toddlers less than two years old. This is potentially a significant limitation of both surveys (CCHS and CHMS). However, it is important to note that both the NHANES and CSFII (US EPA 2005, US EPA 2011) surveys have body weight data for this age group (< 2 years) that may be relevant for risk assessment activities conducted within Health Canada.
3.0 Methodology
A working group (led by SED's Contaminated Sites Division) was established to facilitate the collection of data and gather input from all applicable areas within Health Canada. Working group members included representatives from Bureaus and Directorates across Health Canada identified as using body weight as an exposure factor in risk assessments. Appendix A summarizes the working group members. The relevant directorates and mandates are also summarized and provided in Appendix B.
Data were gathered by distributing a questionnaire to the working group members to collect data regarding: currently used body weight values, references for values in use, international affiliations, ability to harmonize and the consideration of more recent body weight data. The questions from the questionnaire are provided below. The questions were designed to create a central database which would capture the vast use of body weight values used across Health Canada programs.
- Question #1: Indicate the body weight values and associated age groups that your group uses for the purposes of conducting Human Health Risk Assessments.
- Question #2: What is the reference for the body weight values and age groups provided above? If possible, please attach a copy when returning this questionnaire.
- Question #3: Outline the rationale for the use of the body weight values and associated age groups from the above noted reference. Be sure to indicate if affiliation with international groups (i.e., WHO or US EPA) plays a role in the rationale.
- Question #4: Identify any issues your group may have in harmonizing to body weight values different from those currently in use.
- Question #5: Please review the Drinking Water Consumption and Body Weight Report - Draft for Discussion dated August 11, 2010 produced by the Water, Air and Climate Change Bureau. This document addressed the data collected in the 2004 Canadian Community Health Survey Cycle 2.2. Would your group be able to adopt the body weight values from this document? Please provide comments regarding the applicability of this document to body weight values used by your group.
- Question #6: Please review the External Review Draft US EPA Exposure Factors Handbook, July 2009 (Chapter 8 - Body Weight). Has there been any consideration of the body weight values in this document by your group? Please provide comments regarding the applicability of this document to body weight values used by your group.
The 2004 CCHS Cycle 2.2 was considered the most applicable and current Canadian data source and was therefore, the main item of discussion in terms of using more current Canadian data recognizing the changes in demographics since the 1970's and 1980's (Canadian data currently used within department were collected during this time period).
It is recognized that some areas of the department have working relationships with regulators from other countries and international organizations. Thus, it is important to consider how other countries with similar demographics are addressing the trend of increasing body weight with respect to assessing exposure. The External Review Draft US EPA Exposure Factors Handbook, July 2009 (Chapter 8 - Body Weight) was considered by the working group, as one example of an international perspective.
It is noted that the final versions of the documents listed above in Questions #5 and #6 have been made available since the issuing of the questions. The final versions are titled:
- Drinking Water Consumption and Body Weight Estimates for Developing the Guidelines for Canadian Drinking Water Quality - Final Report dated November 2011; and
- US EPA Exposure Factors Handbook: 2011 Edition. Dated September 2011.
4.0 Results and Discussion
Upon analysing the data collected, it became evident that the different groups within HC generally fell into one of two "streams" based on the type of assessments conducted within their respective groups. These two broad streams are summarized as follows:
- Body weight values, used in assessments submitted to HC (e.g., drug submissions to the Department), based on reference sources that are a result of acceptance of such values between different nations (i.e., internationally adopted guidelines such as the International Conference on Harmonisation) or are bound by Canadian Law: minimal flexibility in terms of altering what body weight value to use.
- Body weight values developed or selected by HC (e.g., pesticides, drinking water or soil quality guideline development by the Department) and are not bound by Canadian Law or international guidelines adopted by HC: more flexibility in terms of body weight value selection for use in a risk assessment.
In both streams, the exposures being assessed might be a result of either deliberate or unintentional exposure to the substance. It is important to highlight that the application of body weight in a clinical/therapeutic setting primarily addresses risk as a result of deliberate exposure (i.e., dose) and reflects the health risk at the sub-population level (or individual level). By contrast, the application within environmental settings typically addresses incidental chemical/contaminant exposure to the general population (thus at a population level). It should be noted that some groups within HC (e.g., Existing Substances Risk Assessment Bureau, SED, HECSB) consider some environmental exposure as direct (i.e., intended product or manufactured item use) and that in some cases the exposure assessment may need to take into account both deliberate and unintentional exposure.
Given that body weights are dependent on (and thus linked to) the age of individuals, the age groups are also varied amongst streams. This can be seen in the following sections.
4.1 Body Weight Values Used by HC
As mentioned above, for body weight values that are either bound by Canadian Law or that are based on international guidelines adopted by HC, there is limited flexibility with respect to the selection of body weight values to use in risk assessments. As outlined in Table 2 (refer to Section 7, Tables), there are several international affiliations, agreements and/or collaborations within the department that control how body weight is used in risk assessments.
Alternatively, some groups within the Department select the body weight values that are used or that are prescribed for use in risk assessments. The rationale for the body weight value(s) selected is generally outlined in various guidance documents prepared by HC that are not bound by Canadian Law or that are not constrained by international guidelines adopted by HC. The current body weight value(s) and associated age groups selected by the different programs within HC are displayed in Table 4 (refer to Section 7, Tables).
As seen in Table 3 and Table 4 there is much diversity in the age groups, associated body weights and even the body weight for a given age group that are currently being used within Health Canada. The rationales for selecting such body weights by the various groups within HC are documented in the survey results. While the age groupings and associated body weight values are from various sources (both Canadian and international sources), only a few groups within HC (i.e., WACCB drinking water group and most of the HPFB groups) currently use data from Statistics Canada's CCHS Cycle 2.2 - Nutrition (2004) survey, which is viewed as being the largest data set of recent measured Canadian body weight data for all age groups excluding under the age of 2 years.
4.2 Differences between body weight values in use and more recent values under consideration
The Biostatistics Section of Health Canada (ERHSD) completed a comparison of the body weight values currently in use in Tables 3 and 4 with the CCHS Cycle 2.2 - Nutrition (2004) survey data for the age groups currently used across Health Canada programs. This comparison is presented in Tables 5 and 6 (refer to Section 7, Tables). One limitation of this data set is that it does not include data for infants less than two years of age.
In almost all cases the comparison indicates that the currently used average body weights (for each of the associated age groups being used by the different programmes within HC), that are based on measured body weight data, have increased between the time period in which most of these data were collected (i.e., 1970s and 1980s) and 2004 when the CCHS Cycle 2.2 - Nutrition survey body weight data were collected. For much of the data collected in questionnaire responses from the various programs within HC, the statistical parameter (e.g., mean, median, etc...) provided was assumed to be the "average" for purposes of this comparison. The reasons for this increase in body weight of Canadians may be numerous and not part of the scope of this project. However, based on the self-reported body weight data collected through the ongoing annual component of Statistics Canada's Canadian Community Health Survey (CCHS) since 2004, the body weights of Canadians have remained relatively stable.
4.3 International Perspective
As discussed in preceding sections, there are some programs within HC that are closely associated with international organizations and accordingly make use of international references for body weight values in their risk assessments. For other groups within the Department that do not rely on the use of international references, it may be important to remain consistent in approach, or at least be aware of changes to international approaches (i.e., WHO or the US EPA), while not necessarily adopting their values.
Note: The list provided below is not exhaustive; it is based on the information collected in the survey.
U.S.: Environmental Protection Agency
The US EPA Exposure Factors Handbook: 2011 Edition (Chapter 8 - Body Weight) recommends various age groups and body weights based on their analysis of NHANES 1999-2006 data (see Table 5). The body weight of an adult American has increased from the 70 kg standard commonly used in HHRA to 80 kg (for combined male and female). This value exceeds the 76 kg (arithmetic mean value for females and males aged 20 years of age or older) obtained in the CCHS 2.2 - Nutrition (2004) survey data. The Child-Specific Exposure Factors Handbook (2008) is also available from the US EPA.
| Age | Mean Body Weight (kg) |
|---|---|
| Birth to <1 month | 4.8 |
| 1 to <3 months | 5.9 |
| 3 to <6 months | 7.4 |
| 6 to <11 months | 9.2 |
| 1 to <2 years | 11.4 |
| 2 to <3 years | 13.8 |
| 3 to <6 years | 18.6 |
| 6 to <11 years | 31.8 |
| 11 to <16 years | 56.8 |
| 16 to <21 years | 71.6 |
| Adults | 80 |
The PMRA has generated a comparison table between NHANES and CCHS for internal discussion regarding BW data (see Table 2 below). Note the CCHS mean body weight values were generated from PMRA's analysis for the internal BW SOP to be completed in 2012.
| Population Group | NHANES (1999-2006) | CCHS Cycle 2.2 (2004) | ||
|---|---|---|---|---|
| Mean BW (kg) | Sample Size | Mean BW (kg) | Sample Size | |
| 16 to < 80 yrs | 80 | 21582 | 76 | 12407 |
| 11 to < 16 yrs | 57 | 5297 | 55 | 3204 |
| 6 to <11 yrs | 32 | 3593 | 31 | 2339 |
| 3 to <6 yrs | 19 | 2318 | 18 | 1209 |
| 2 to <3 yrs | 14 | 1144 | 14 | 332 |
| 1 to <2 yrs | 11 | 1176 | NA | 0 |
| 6 to <12 months | 9 | 927 | NA | 0 |
| 3 to < 6 months | 7 | 489 | NA | 0 |
| 1 to < 3 months | 6 | 284 | NA | 0 |
| Birth to < 1 month | 5 | 158 | NA | 0 |
| NA = not available | ||||
Australia: National Health and Medical Research Council
Based on the Australian Drinking Water Guidelines, the average adult in Australia is assumed to weigh 70 kg. This value was selected because it is used in Canada and other developed countries. They also assume that the average body weight of a two-year old child is 13 kg, in order to be line with other developed countries such as Canada (NHMRC 2004).
The default human body weight values used in the risk assessment of environmental hazards in Australia include: 70 kg (adult male), 58 kg (adult female), 13.2 kg (2 year old child), 64 kg (average male and female combined) (Department of Health and Ageing and enHealth Council 2004).
European Union: European Commission, Rijksinstituut voor volksgezondheid en milieu (National Institute of Public Health and the Environment), and European Food Safety Authority
Default body weight values outlined by the European Commission include 70 kg (adult male) and 60 kg (adult female) (EC 2003).
Based on data collected during the period of 1995-1997 by the Dutch National Institute of Public Health and the Environment (RIVM), default body weight values were selected as the 25th percentile values. The reason for selecting a lower percentile was to generate more conservative exposure estimates since body weight tends to be in the denominator of most exposure algorithms. These values include 74 kg (adult male), 61 kg (adult female) and 65 kg (combined adult male and female). Additionally, default body weight values for children are provided based on different data sources. The 25th percentile is selected in generating the default values for children as well. The values for children (sexes combined) include the following: 6.21 kg (3-6 months); 7.62 kg (6-12 months); 9.47 kg (12-18 months); 9.85 kg (1.5-3 years); 16.3 kg (3-9 years based on 4.5 year old); 20.6 kg (3-9 years based on 6.5 year old); and 39.3 kg (9-14 years) (Bremmer et al. 2006).
The European Food Safety Authority (EFSA) has also published default body weight values for use in dietary risk assessments (EFSA 2012). A default body weight of 70 kg for adults (> 18 years of age) is recommended while for toddlers (1 - 3 years) and infants (0 - 12 months) body weights of 5 kg and 12 kg, respectively, are recommended.
World Health Organization
The "WHO Human Health Risk Assessment Toolkit: Chemical Hazards" highlights the use of default body weight values of 60 kg by the WHO and 64 kg by the IPCS (International Programme on Chemical Safety) (WHO 2010). The WHO Guidelines for Drinking Water Quality indicate the use of default body weight values of 60 kg (adult), 10 kg (child) and 5 kg (infant) (WHO 2011). Additionally, the WHO growth charts are referenced as a source of body weight values with respect to children (WHO 2006).
4.4 Implications of harmonizing to CCHS Cycle 2.2 body weight data
When considering adopting the increased body weight values as exposure factors, there are a number of implications to evaluate. These implications are primarily policy based and will vary depending on the application (e.g., developing risk-based environmental guidelines or tolerable or allowable intakes, versus establishing safe clinical trial doses).
As seen in the previous section, the CCHS Cycle 2.2 - Nutrition (2004) survey data indicate that body weight values have increased, compared to measured body weight values currently used across most programs in HC, for all age groups (for which data have been collected). Regarding the significance of this change in impacting risk assessments, this is dependent on many factors and cannot be easily quantified. General risk assessment principles dictate that as body weight increases, the tolerable or allowable exposure must increase to maintain the same risk level. Accordingly, given that body weights have increased, current guidelines established by HC would still be protective (from the perspective of the body weight variable). Furthermore, if current body weight values were to be maintained, guidelines, for example, would be more protective then they would if the new CCHS body weight data were used.
Some programmes within Health Canada do not have the flexibility to adopt updated body weight values in their assessments for various reasons. For example, if a programme's assessment is linked to an international agreement or bound by Canadian law, that group may have limited flexibility in terms of adopting the recent CCHS Cycle 2.2 - Nutrition (2004) survey body weight data without renegotiation of said agreements or legislative changes. While both options may be theoretically possible, their implementation would require significant effort, time and resources and may not be warranted given that currently used body weight values may provide a higher level of protection. However, there may be some value in at least comparing the hypothetical outcome of a risk assessment through the use of the updated CCHS Cycle 2.2 - Nutrition (2004) survey body weight data to determine if the outcome would be considered overly conservative given the context of the risk assessment.
In a clinical setting, patients for whom the dosing of some drug is being evaluated may weigh less than the "average" person due in part to their illness. Thus, if dosing is based solely on the average body weight of a person, the potential for overestimating the required dose may exist. In such cases, where the body weights of a subpopulation may be significantly different from the general population, the use of representative or clinically appropriate BW data (not necessarily the average) for that subpopulation would be more appropriate than use of the data that are representative of the general population at large.
In the development of environmental media quality guidelines (e.g., soil, water and air), body weight is incorporated into the risk assessment. The proportionality between body weights and guideline values is not clear since other factors such as drinking water consumption and source apportionment complicates this relationship. As such, the body weight value selected for the development of a guideline value needs to be reflective of the target population to ensure the desired level of protection is achieved. For example, as indicated in the drinking water report, drinking water guidelines based on the currently used BW values result in more protective guidelines than guidelines established using the new CCHS Cycle 2.2 - Nutrition (2004) survey BW data (Health Canada 2011). However, this does not suggest that use of the new CCHS Cycle 2.2 - Nutrition (2004) survey body weight data would not be sufficiently protective as such a statement is dictated HC Branch/Programme Policy on the level of protection desired. For example, at a given level of protection (e.g., 50th percentile), the use of the new CCHS Cycle 2.2 - Nutrition (2004) survey BW data would result in higher guideline values compared to guideline values developed using the current BW values. In reality, the level of protection (or acceptable level of risk) is subjective and is typically set to a level with which the individual Branches/Programmes would be comfortable. However, such decisions require the support of sound science and reliable data.
In terms of environmental exposure, retaining the body weight values currently used may result in risk assessments and guidelines that are "more protective" of human health than would occur through use of the updated CCHS Cycle 2.2 - Nutrition (2004) survey body weight data.
This example highlights the effect of not using the updated CCHS Cycle 2.2 - Nutrition (2004) survey body weight data, but instead retaining currently used body weight data. By continuing to use the older data, HC's approach in some program areas continues to be more protective (i.e., mean or 50th percentiles are typically selected for the various exposure parameters used when characterizing the receptor characteristics of Canadian populations, whereas retaining body weight data currently used in most Health Canada programs compared to recently measured body weight data from the CCHS Cycle 2.2 - Nutrition [2004] survey dataset would result in using body weight values greater than the mean or 50th percentile values which would effectively increase the protectiveness of any guideline developed). However, it is important to note that the use of the CCHS Cycle 2.2 - Nutrition (2004) survey body weight data would not result in guidelines being insufficiently protective. Furthermore, becoming more protective can run the risk of being overly protective or conservative. In such cases, the use of recently measured body weight data could help further refine the risk assessment.
There is no overarching policy at HC for the level of protection that is deemed acceptable.
The level of protection would depend on the program area within Health Canada and what their mandate and/or internal policies are. The level of protectiveness for one program within Health Canada may not be sufficiently protective for another or it may be overly protective for another based on the purpose and context of the assessment. Typically the average values for the different exposure parameters (e.g., body weight) are used in human health risk assessments; however, this may not be always the case. For example, there may be a need to select exposure parameters that are representative of some sensitive sub-population to ensure that the decisions being made are sufficiently protective of these sub-populations.
There may also be a need to consider the association of the body weight variable to other exposure factors that may be related to body weight. For example, if body weight values have increased, receptor characteristics such as body surface area and dietary intake rates may also have changed from values currently used across Health Canada programs. In order to determine the impact a change of body weight value may have on a risk assessment, the combined impact of updating the values of body weight and these other associated receptor characteristics may warrant consideration.
Body weight should be considered a dynamic variable and thus regular monitoring of population data (such as those data being generated by the CCHS and CHMS) will be required so that information within the Department remains relevant to the current Canadian context.
5.0 Conclusions and Recommendations
Recent Canadian data have demonstrated that the body weights of Canadians have increased over the past several decades and preceding sections describe how these data could be used in HHRAs conducted across Health Canada programs.
It is clear that there is a continuum of flexibility with respect to adopting new values for use in HHRAs ranging from programs that are mandated to follow specific values prescribed by Canadian law, or are constrained to follow internationally agreed-upon guidelines, to those that can set their own exposure factors internally. In turn, while Department-wide harmonization may not be achievable, the recommendations outlined below are intended to highlight areas where the Department could strive to align internally with respect to the underlying use of body weight as an exposure factor in HHRA.
It is recommended that, where possible, all programs within the Department consider using a common data set for the purposes of HHRA. More specifically, it is recommended that the CCHS Cycle 2.2 - Nutrition (2004) survey data be considered the primary source for recent Canadian measured body weight data for use in human health risk assessments, recognizing that there may be other Canadian data sets which may be more appropriate in specific situations (i.e., sensitive subpopulations). The CCHS Cycle 2.2 - Nutrition (2004) survey provides measured body weight data from the largest number of participants across Canada. It is noted that other Canadian studies (e.g., CHMS) also provide recent measured body weight data and thus may represent other potential sources of body weight data that could be considered when conducting HHRAs.
The CCHS and CHMS data are collected from Canadians on a regular basis (i.e., self-reported data on an annual basis for CCHS and measured data on a biennial basis for CHMS), which could enable programs using these data in their HHRAs to compare values in use to the most up to date data and make any necessary changes to the body weight values applied in risk assessments. However, it is equally important to note that not using the CCHS Cycle 2.2 - Nutrition (2004) survey body weight data does not result in current approaches being insufficiently protective. On the contrary, maintaining currently used body weight values could result in assessments being even more protective or conservative, which, in addition to reasons of being bound by Canadian law or constrained by international guidelines, could be a reason as to why certain program areas choose not to use the body weight values from the CCHS Cycle 2.2 - Nutrition (2004) dataset.
The extent to which each group can incorporate these data into their HHRAs will differ based on their ability to introduce recently measured body weight data into their practices. Those groups that cannot introduce such values may use, where appropriate, this dataset as a check to put their HHRAs into a current Canadian perspective or could consider using them to refine risk assessments, where possible. Those groups with the flexibility to incorporate such values may go further with respect to harmonization by ensuring the similar use of body weight as an exposure factor across those programs within Health Canada. It is worth noting that this agreed upon dataset could be incorporated into Standard Operating Procedures or guiding principles within program areas of Health Canada. It is also important to consider the consistent use of certain metrics (i.e., arithmetic mean, geometric mean, xth percentile, etc.) and the selection of age group categories in the application of body weight as an exposure factor.
Finally, consideration should also be given to the following comments:
Given that there is a current trend towards increasing body weights in the Canadian general population, the CCHS Cycle 2.2 - Nutrition (2004) survey body weight data may only remain reflective of the general Canadian population for a limited period of time. This generates the need to continue regular demographic monitoring to ensure risk assessments completed within the Department are as representative as possible of the current Canadian population. As mentioned previously in this document, the CCHS and CHMS are both ongoing surveys and will act as useful sources of regular contemporary data for tracking changes in body weight trends of Canadians.
Maintaining the currently used values is thought to reach a balance between protecting sensitive-subpopulations and the general population as well as maintaining an acceptable level of public health protection given the current shifts in data (i.e., increased body weights). Maintaining current values also keeps methods internationally consistent for some applications just as the development of drinking water guidelines (Health Canada 2011).
It has been noted that there is a data gap for recent body weight values of Canadians less than 2 years old. It is recommended that Health Canada and other relevant departments (i.e., Statistics Canada) initiate discussions regarding how to best collect the data from this group of the population so that the values being used remain relevant or representative of that subpopulation in the current Canadian context.
6.0 References
- Bremmer, H.J., L.C.H. Prud'homme de Lodder, and J.G.M. Engelen. 2006. General Fact Sheet. Limiting conditions and reliability, ventilation, room size, body surface area. Updated version for ConsExpo 4. Rijksinstituut voor volksgezondheid en milieu/National Institute of Public Health and the Environment (RIVM). RIVM report 320104002/2006. http://www.rivm.nl/bibliotheek/rapporten/320104002.pdf
- Canadian Paediatric Society. http://www.cps.ca/english/index.htm
- [CDC] Centers for Disease Control and Prevention. 2010. Growth Charts. Atlanta (GA): Centers for Disease Control and Prevention. [cited 2012 Oct 15]. Available from: http://www.cdc.gov/growthcharts/
- ConsExpo (RIVM) software available for download. http://www.rivm.nl/en/healthanddisease/productsafety/Main.jsp
- Department of National Health and Welfare. 1973. Nutrition: A National Priority. Nutrition Canada, Department of National Health and Welfare, Ottawa.
- Demirjian, A. 1980. Anthropometry Report: Height, Weight and Body Dimensions. A report from Nutrition Canada. Health Promotion Directorate, Bureau of Nutritional Sciences, Health and Welfare Canada, Ottawa. 133 pages + unpublished data of the Nutrition Canada Survey.
- Department of Health and Ageing and enHealth Council. 2004. Environmental Health Risk Assessment Guidelines for assessing human health risks from environmental hazards. www.health.gov.au/internet/main/publishing.nsf/Content/A12B57E41EC9F326CA257BF0001F9E7D/$File/w.Aust-Exposure-Factor-Guide.docx
- European Commission (EC). 2003. Technical Guidance Document on Risk Assessment Part 1. Institute for Health and Consumer Protection. European Chemicals Bureau. https://echa.europa.eu/documents/10162/16960216/tgdpart1_2ed_en.pdf
- Government of Canada. 1983. Canada Fitness Survey. Fitness and Lifestyle in Canada. Fitness and Amature Sport, Government of Canada, Ottawa.
- Grummer-Strawn LM, Reinold C, Krebs NF. 2010. Use of World Health Organization and CDC Growth Charts for Children Aged 0 - 59 Months in the United States: Recommendations and Reports. Morbidity and Mortality Weekly Report (MMWR). Atlanta (GA): Centers for Disease Control and Prevention. [cited 2012 Oct 15]. Available from: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5909a1.htm
- Hayward, S. 2009. Consumption data based on CCHS Cycle 2.2 2004. Food Directorate, Bureau of Food Policy and Science Integration.
- Health Canada. 2011. Drinking Water Consumption and Body Weight Estimates for Developing the Guidelines for Canadian Drinking Water Quality. Water, Air and Climate Change Bureau. November 2011.
- Health Canada. 2010. Draft for Discussion: The Drinking Water Consumption and Body Weight and Body Weight Report. Water, Air and Climate Change Bureau.
- Health Canada. 2010. A Primer on Scientific Risk Assessment at Health Canada.
- Health Canada.2010. Draft Abbreviated Labelling Standard (AbLS) for Caffeinated Energy Drinks. Natural Health Products Directorate.
- Health Canada. 2007. Natural Health Product Directorate (NHPD) Guidance Document: Evidence for Quality of Finished Natural Health Products.
- Health Canada. 2000. Health Canada Decision-Making Framework for Identifying, Assessing, and Managing Health Risks.
- Health Canada. 1998. Exposure Factors for Assessing Total Daily Intake of Priority Substances by the General Population of Canada. Unpublished report. Environmental Health Directorate, Ottawa, ON.
- Health Canada. 1995. A Handbook for Exposure Calculations.
- Health Canada. 1994. Canadian Environmental Protection Act. Human Health Risk Assessment for Priority Substances.
- Institute of Medicine of the National Academies. 2006. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements. The National Academies Press, Washington, D.C.
- Kalant, H. 1988. Sources of Individual Variation in Drug Response (Chapter 62). Principles of Medical Pharmacology (6th edition).
- Kuczmarski RJ, Ogden CL, Guo SS, et al. 2002. 2000 CDC growth charts for the United States: Methods and development. National Center for Health Statistics. Vital Health Stat 11(246). [cited 2012 Oct 15]. Available from: http://www.cdc.gov/growthcharts/2000growthchart-us.pdf
- National Health and Medical Research Council. 2004. National Water Quality Management Strategy. 2006 Australian Drinking Water Guidelines 6. Australian Government. http://www.nhmrc.gov.au/_files_nhmrc/publications/attachments/eh34_adwg_11_06.pdf
- Nutrition Canada. 1970s. Consumption Data.
- Public Health Agency of Canada and Canadian Institute for Health Information. 2011. Obesity in Canada.
- Richardson, G.M. 1997. Compendium of Canadian Human Exposure Factors for Risk Assessment. O'Connor Associates Environmental Inc.
- Ritter, L., Totman, C. and T.Watson. 2005. Evaluation of Assumptions for Chemical Drinking Water Guidelines: Evaluation of the scientific rationale for the assumptions used for developing drinking water guidelines for chemical contaminants.
- Shields M, Connor Gorber S, Tremblay MS. 2008. Estimates of obesity based on self-report versus direct measures. Ottawa (ON): Statistics Canada. [cited 2012 Oct 15]. Available from: http://www.statcan.gc.ca/pub/82-003-x/2008002/article/10569-eng.pdf
- Statistics Canada. 2011. Canadian Community Health Survey - Annual Component (CCHS). Available at: http://www.statcan.gc.ca/cgi-bin/imdb/p2SV.pl?Function=getSurvey&SDDS=3226&lang=en&db=imdb&adm=8&dis=2Statistics Canada. 2010. Canadian Health Measures Survey: Cycle 1 Data Tables 2007 to 2009 Published.
- Statistics Canada. 2008.User Guide: Canadian Community Health Survey (CCHS), Cycle 2.2 (2004), Nutrition - General Health (including Vitamin & Mineral Supplements) & 24-hour Dietary recall components. Ottawa.
- Statistics Canada. 2005-2008 (data release period).Canadian Community Health Survey - Nutrition (CCHS). Detailed information for 2004 (Cycle 2.2). http://www.statcan.gc.ca/cgi-bin/imdb/p2SV.pl?Function=getSurvey&SDDS=5049&lang=en&db=imdb&adm=8&dis=2
- Stephens, T. and C.L. Craig. 1990. The Well-Being of Canadians: Highlights of the 1988 Campbell's Survey. Canadian Fitness and Lifestyle Research Institute, Ottawa. 95p. + appendices + data.
- Trainor C. 2012. Introducing the 2015 Canadian Community Health Survey (CCHS) - Nutrition: Setting the Stage, Starting the Process. CNS-SCN Annual Meeting. Statistics Canada. [cited 2012 Oct 15]. Available from: http://www.cns-scn.ca/CONFERENCE2012/PDF-
Presentations/Thursday/Cathy-Trainor-
Introducing%20the%202015%20Canadian%20Community%20Health%20Survey%20on%20Nutrition.pdf - United States Environmental Protection Agency (US EPA). 2011. Exposure Factor Handbook: 2011 Edition. National Center for Environmental Assessment. Office of Research and Development. U.S. Environmental Protection Agency. Washington, DC. September 2011.
- United States Environmental Protection Agency (US EPA). 2009. External Review Draft Exposure Factors Handbook. July 2009.
- United States Environmental Protection Agency (US EPA). 2008. Child-Specific Exposure FactorsHandbook. National Center for Environmental Assessment. Office of Research and Development. U.S. Environmental Protection Agency. Washington, DC. September 2008.
- United States Environmental Protection Agency (US EPA). 1996. Exposure Handbook.
- US EPA. 2005. Analysis of Total Food Intake and Composition of Individual's Diet Based on the U.S. Department of Agriculture's 1994-96, 1998 Continuing Survey of Food Intakes By Individuals (CSFII) (Final). U.S. Environmental Protection Agency, Washington, DC, EPA/600/R-05/062F.
- US FDA. 2005. Guidance for Industry: Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers. U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER).
- US FDA. Centre for Veterinary Medicine (CVM) http://www.fda.gov/AnimalVeterinary/default.htm
- World Health Organization (WHO). 2011. Guidelines for Drinking-water Quality. Fourth edition. http://whqlibdoc.who.int/publications/2011/9789241548151_eng.pdf
- WHO. 2010. WHO Human Health Risk Assessment Toolkit: Chemical Hazards. http://www.who.int/ipcs/publications/methods/harmonization/toolkit.pdf
- WHO. 2006. Environmental Health Criteria 237. Principles for Evaluation Health Risks in Children Associated with Exposure to Chemicals. http://www.who.int/ipcs/publications/ehc/ehc237.pdf
- WHO. 2006. Multicentre Growth Reference Study Group. WHO Child Growth Standards: Length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: Methods and development. Geneva: World Health Organization.
7.0 Tables
- Table 1 - (in text) - Recommended Body Weights for different age groups (US EPA Exposure Factors Handbook 2011)
- Table 2 - (in text) - Comparison of NHANES (1996-2006) and CCHS Cycle 2.2 - Nutrition (2004) survey mean body weight values for various age groups
- Table 3 - Variability in Body Weight Values based on Credible, External Reference Sources
- Table 4 - Variability in Body Weight Values based on Internal Reference Sources
- Table 5 - Currently Used Body Weight Values: External Reference Sources vs. CCHS Cycle 2.2 2004 data
- Table 6 - Currently Used Body Weight Values: Internal Reference Sources vs. CCHS Cycle 2.2 2004 data
| External Reference | Area of Health Canada Used | Age Groups (yrs) | Body Weight (kg) |
|---|---|---|---|
| The Institute of Medicine of the National Academies. 2006. Dietary Reference Intakes: The essential Guide to Nutrient Requirements. The National Academies Press, Washington, D.C. | HPFB | 2-6 months | 6 |
| 7-12 months | 9 | ||
| 1-3 | 12 | ||
| 4-8 | 20 | ||
| 9-13 | 36 (M) | ||
| 37(F) | |||
| 14-18 | 61 (M) | ||
| 54 (F) | |||
| 19+ | 70 (M) | ||
| 57 (F) | |||
| WHO and/or CDC growth charts by age. | HPFB | Values provided in the growth chart | Values provided in the growth chart |
| CDC/NCHS growth charts used to determine weights to be used to develop dietary reference intakes (DRI) used in HHRA | HPFB's FD (Bureau of Nutritional Science) | 2-6 months | 6 |
| 7-12 months | 9 | ||
| 1-3 | 12 | ||
| 4-8 | 20 (based on median height and median BMI) | ||
| 9-13 | 36 (M) (based on median height and median BMI) | ||
| 37 (F) (based on median height and median BMI) | |||
| 14-18 | 61 (M) (based on median height and median BMI) | ||
| 54 (F) (based on median height and median BMI) | |||
| 19+ | 70 (M) | ||
| 57 (F) | |||
| US FDA's Guidance for Industry: Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers | HPFB, TPD and BGTD (risk benefit analysis) | 70kg is often used for Phase 1 of clinical trials, but the reference BW values in guidance are 20kg for children and 60kg for adults. 50-80 kg is provided to determine SA dose conversion factors. | |
| Canadian Paediatric Society and CDC growth charts | HPFB (health risk perspective) | NA | NA |
| US FDA Centre for Veterinary Medicine (CVM). | HPFB's VDD | NA | 60kg |
| USDA's Continuing Survey of Food Intakes by Individuals for 1994-1996, and 1998. | PMRA | infants (<1) | This information is imbedded in the dietary risk assessment model software we use called Dietary Exposure Evaluation Model (DEEM). |
| children (1-2) | |||
| children (3-5) | |||
| children (6-12) | |||
| youth (13-19) | |||
| adults (20-49) | |||
| females (13-49) | |||
| adults (50+) | |||
| US EPA's Exposure Factor Handbook (1996) Data sources: NHANES II |
PMRA | Toddler (3yrs) | 15 |
| Youth (12 yrs) | 39 | ||
| Adult (18+) | 70 | ||
| ConsExpo (RIVM) software (default values) | PSP, CPS | Many age categories | Built into software http://www.rivm.nl/bibliotheek/rapporten/320104002.pdf |
| Reference | Area of Health Canada Used | Age Groups (yrs) | Body Weight (kg) |
|---|---|---|---|
| Health Canada Body Reference Weights (http://www.hc-sc.gc.ca/fn-an/nutrition/reference/table/index-eng.php#rhw) and the CDC growth charts (http://www.cdc.gov/growthcharts). Note: The smallest body weight of the two subpopulations (male and female) is used for ages 9-18. | HPFB's NHPD | Infant (0 months) | 3.4 |
| Infant (1 months) | 4.2 | ||
| Infant (2-6 months) | 6 | ||
| Infant (7-12 months) | 9 | ||
| Toddler (1-3) | 12 | ||
| Early Child (4-8) | 20 | ||
| Puberty (9-13) | 36 | ||
| Adolescents (14-18) | 54 | ||
| Adults (19+) | 70 | ||
| Draft Abbreviated Labelling Standard (AbLS) for energy drinks using ICH as a reference in establishing an age reference for adults as 18 years and older. | HPFB's NHPD | Not specified | Not specified |
| Nutrition Canada (1970's) consumption data. | HPFB's FD (Bureau of Chemical Safety) | 1-4 | 14.4 |
| 5-11 | 26.4 | ||
| 12-19 | 53.8 | ||
| 20-39 | 60 | ||
| CCHS cycle 2.2 data (2004) with Nutrition Canada (1970's age groups). | HPFB's FD (Bureau of Chemical Safety) | 1-4 | 15 (M&F) (based on weighted mean values based on female body weights only) |
| 15.8 (F) | |||
| 5-11 | 30 (M&F) (based on weighted mean values based on female body weights only) | ||
| 31.5 (F) | |||
| 12-19 | 60 (M&F) (based on weighted mean values based on female body weights only) | ||
| 59.4 (F) | |||
| 20+ | 70 (M&F) (based on weighted mean values based on female body weights only) | ||
| 69.6 (F) | |||
| Stephen Hayward (BFPSI, FD). He provides consumption data from CCHS Cycle 2.2. These age/sex groups were proposed in 2009 based on Dietary Reference Intakes (DRI's). | HPFB's FD (Bureau of Chemical Safety) | 0-6months | No data |
| 6months - <1yr | No data | ||
| 1-3 | 15.3 | ||
| 4-8 | 24.07 | ||
| 9-13 | 46.2 (M) | ||
| 44.27 (F) | |||
| 14-18 | 69.22 (M) | ||
| 61.06 (F) | |||
| 19-50 | 81.91 (M) | ||
| 68.69 (F) | |||
| 51-70 | 84.86 (M) | ||
| 71.07 (F) | |||
| 71+ | 78.65 (M) | ||
| 67.14 (F) | |||
| Canadian Environmental Protection Act. Human Health Risk Assessment for Priority Substances. Health Canada. 1994. (Note: Refer to section 2.5 for raw data source information). | - WACCB (Indoor Air and Drinking Water) - CPSD |
0 - 6 months | 7 Footnote 1 |
| 7 months - 4yrs | 13 Footnote 1 | ||
| 5-11 | 27 Footnote 1 | ||
| 12-19 | 57 Footnote 1 | ||
| 20+ | 70 Footnote 1 | ||
| Drinking Water Consumption and Body Weight Estimates for Developing the Guidelines for Canadian Drinking Water Quality. Health Canada, Water, Air and Climate Change Bureau. 2011. |
WACCB (Drinking Water) | 0 - 6 months | 7 Footnote 1 |
| 7 months - 4yrs | 13 Footnote 1 | ||
| 5-11 | 27 Footnote 1 | ||
| 12-19 | 57 Footnote 1 | ||
| 20+ | 70 Footnote 1 | ||
| Exposure Factors for Assessing Total Daily Intake of Priority Substances by the General Population of Canada. 1998. Unpublished report. Ottawa, ON: Health Canada, Environmental Health Directorate. A Handbook for Exposure Calculations. Health Canada. 1995. | NSACB | 0 - 6 months | 7.5 Footnote 1 |
| 7 months - 4yrs | 15.5 Footnote 1 | ||
| 5-11 | 31.0 Footnote 1 | ||
| 12-19 | 59.4 Footnote 1 | ||
| 20-59 | 70.9 Footnote 1 | ||
| 60+ | 72.0 Footnote 1 | ||
| Exposure Factors for Assessing Total Daily Intake of Priority Substances by the General Population of Canada. 1998. Unpublished report. Ottawa, ON: Health Canada, Environmental Health Directorate | WACCB (Fuels Assessment Section) | 0 - 6 months | 7.7 Footnote 2 |
| 7 months - 4yrs | 15.4 Footnote 2 | ||
| 5-11 | 30.6 Footnote 2 | ||
| 12-19 | 59.1 Footnote 2 | ||
| 20-59 | 69.5 Footnote 2 | ||
| 60+ | 70.3 Footnote 2 | ||
| Exposure Factors for Assessing Total Daily Intake of Priority Substances by the General Population of Canada. 1998. Unpublished report. Ottawa, ON: Health Canada, Environmental Health Directorate. | ESRAB Footnote * | 0 - 6 months | 7.5 Footnote 1 |
| 7 months - 4yrs | 15.5 Footnote 1 | ||
| 5-11 | 31 Footnote 1 | ||
| 12-19 | 59.4 Footnote 1 | ||
| 20-59 | 70.9 Footnote 1 | ||
| 60+ | 72.0 Footnote 1 | ||
| Compendium of Canadian Human Exposure Factors for Risk Assessment. Richardson, 1997. | EHB, CSD | 0 - 6 months | 8.2 Footnote 1 |
| 7 months - 4yrs | 16.5 Footnote 1 | ||
| 5-11 | 32.9 Footnote 1 | ||
| 12-19 | 59.7 Footnote 1 | ||
| 20+ | 70.7 Footnote 1 | ||
|
|||
| Reference | Area of Health Canada Used | Age Groups (yrs) | Body Weight (kg) | Body Weight (kg) arithmetic weighted average from CCHS data |
|---|---|---|---|---|
| The Institute of Medicine of the National Academies. 2006. Dietary Reference Intakes: The essential Guide to Nutrient Requirements. The National Academies Press, Washington, D.C. |
HPFB | 2-6months | 6 | No data |
| 7-12months | 9 | No data | ||
| 1-3 | 12 | 15 | ||
| 4-8 | 20 | 24 | ||
| 9-13 | 36 (M) | 46 (M) | ||
| 37(F) | 44 (F) | |||
| 14-18 | 61 (M) | 69 (M) | ||
| 54 (F) | 61 (F) | |||
| 19+ | 70 (M) | 83 (M) | ||
| 57 (F) | 70 (F) | |||
| WHO and/or CDC growth charts by age. | HPFB | Values provided in the growth chart | Values provided in the growth chart | NA |
| CDC/NCHS growth charts used to determine weights to be used to develop dietary reference intakes (DRI) used in HHRA | HPFB's FD (Bureau of Nutritional Science) | 2-6 months | 6 | No data |
| 7-12 months | 9 | No data | ||
| 1-3 | 12 | 15 | ||
| 4-8 | 20 (based on median height and BMI) | 24 | ||
| 9-13 | 36 (M) (based on median height and BMI) | 46 (M) | ||
| 37 (F) (based on median height and BMI) | 44 (F) | |||
| 14-18 | 61 (M) (based on median height and BMI) | 69 (M) | ||
| 54 (F) (based on median height and BMI) | 61 (F) | |||
| 19+ | 70 (M) | 83 (M) | ||
| 57 (F) | 70 (F) | |||
| US FDA's Guidance for Industry: Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers | HPFB, TPD and BGTD (risk benefit analysis) | 70kg is often used for Phase 1 of clinical trials, but the reference BW values in guidance are 20kg for children and 60kg for adults. 50-80 kg is provided to determine SA dose conversion factors. | Overall average BW is 69kg across all age groups | |
| Canadian Paediatric Society and CDC growth charts | HPFB (health risk perspective) | NA | NA | NA |
| US FDA Centre for Veterinary Medicine (CVM). | HPFB's VDD | NA | 60kg | Overall average BW is 69kg across all age groups |
| USDA's Continuing Survey of Food Intakes by Individuals for 1994-1996, and 1998. | PMRA | Infants (<1) | This information is imbedded in the dietary risk assessment model software we use called Dietary Exposure Evaluation Model (DEEM). | No data |
| children (1-2) | 14 | |||
| children (3-5) | 18 | |||
| children (6-12) | 36 | |||
| youth (13-19) | 65 | |||
| adults (20-49) | 76 | |||
| females (13-49) | 68 (F) | |||
| adults (50+) | 76 | |||
| US EPA's Exposure Factor Handbook (1996) Data sources: NHANES II | PMRA | Toddler (3yrs) | 15 | 15 (1-3 yrs) |
| Youth (12 yrs) | 39 | 33 (4-12 yrs) | ||
| 63 (13-17 yrs) | ||||
| Adult (18+) | 70 | 76 (18+ yrs) | ||
| ConsExpo (RIVM) software (default values) | PSP, CPS | Many age categories | Built into software http://www.rivm.nl/ bibliotheek/rapporten/ 320104002.pdf |
NA |
| Reference | Area of Health Canada Used | Age Groups (yrs) | Body Weight (kg) | Body Weight (kg) arithmetic weighted average from CCHS data |
|---|---|---|---|---|
| Health Canada Body Reference Weights (http://www.hc-sc.gc.ca/fn-an/nutrition/reference/table/index-eng.php#rhw) and the CDC growth charts (http://www.cdc.gov/growthcharts). Note: The smallest body weight of the two subpopulations (male and female) is used for ages 9-18. | HPFB's NHPD | Infant (0 months) | 3.4 | No data |
| Infant (1 months) | 4.2 | No data | ||
| Infant (2-6 months) | 6 | No data | ||
| Infant (7-12 months) | 9 | No data | ||
| Toddler (1-3) | 12 | 15 | ||
| Early Child (4-8) | 20 | 24 | ||
| Puberty (9-13) | 36 | 45(M=46, F=44) | ||
| Adolescents (14-18) | 54 | 65 (M=69, F=61) | ||
| Adults (19+) | 70 | 76 (M=83, F=70) | ||
| Draft Abbreviated Labelling Standard (AbLS) for energy drinks using ICH as a reference in establishing an age reference for adults as 18 years and older. | HPFB's NHPD | Not specified | Not specified | NA |
| Nutrition Canada (1970's) consumption data. | HPFB's FD (Bureau of Chemical Safety) | 1-4 | 14.4 | 16 |
| 5-11 | 26.4 | 32 | ||
| 12-19 | 53.8 | 63 | ||
| 20-39 | 60 | 75 | ||
| CCHS cycle 2.2 data (2004) with Nutrition Canada (1970's age groups). | HPFB's FD (Bureau of Chemical Safety) | 1-4 | 15 (M&F) (based on weighted mean values based on female body weights only) |
16 (M&F) |
| 15.8 (F) | 16 (F) | |||
| 5-11 | 30 (M&F) (based on weighted mean values based on female body weights only) | 32 (M&F) | ||
| 31.5 (F) | 31 (F) | |||
| 12-19 | 60 (M&F) (based on weighted mean values based on female body weights only) | 63 (M&F) | ||
| 59.4 (F) | 59 (F) | |||
| 20+ | 70 (M&F) (based on weighted mean values based on female body weights only) | 76 (M&F) | ||
| 69.6 (F) | 70 (F) | |||
| Stephen Hayward (BFPSI, FD). He provides consumption data from CCHS Cycle 2.2. These age/sex groups were proposed in 2009 based on Dietary Reference Intakes (DRI's). | HPFB's FD (Bureau of Chemical Safety) | 0-6months | No data | No data |
| 6months - <1yr | No data | No data | ||
| 1-3 | 15.3 | 15 | ||
| 4-8 | 24.07 | 24 | ||
| 9-13 | 46.2 (M) | 46 (M) | ||
| 44.27 (F) | 44 (F) | |||
| 14-18 | 69.22 (M) | 69 (M) | ||
| 61.06 (F) | 61 (F) | |||
| 19-50 | 81.91 (M) | 83 (M) | ||
| 68.69 (F) | 69 (F) | |||
| 51-70 | 84.86 (M) | 85 (M)) | ||
| 71.07 (F) | 71 (F) | |||
| 71+ | 78.65 (M) | 79 (M) | ||
| 67.14 (F) | 67 (F) | |||
| Canadian Environmental Protection Act. Human Health Risk Assessment for Priority Substances. Health Canada. 1994. |
|
0 - 6 months | 71 | No data |
| 7 months - 4 (1-4) | 131 | 16 | ||
| 5-11 | 271 | 32 | ||
| 12-19 | 571 | 63 | ||
| 20+ | 701 | 77 | ||
| Drinking Water Consumption and Body Weight Estimates for Developing the Guidelines for Canadian Drinking Water Quality. Health Canada, Water, Air and Climate Change Bureau. 2011. |
|
0 - 6 months | 7 Footnote 1 | No data |
| 7 months - 4yrs | 13 Footnote 1 | 16 | ||
| 5-11 | 27 Footnote 1 | 32 | ||
| 12-19 | 57 Footnote 1 | 63 | ||
| 20+ | 70 Footnote 1 | 77 | ||
| Exposure Factors for Assessing Total Daily Intake of Priority Substances by the General Population of Canada. 1998. Unpublished report. Ottawa, ON: Health Canada, Environmental Health Directorate. A Handbook for Exposure Calculations. Health Canada. 1995. |
|
0 - 6 months | 7.5 Footnote 1 | No data |
| 7 months - 4yrs | 15.5 Footnote 1 | 16 | ||
| 5-11 | 31.0 Footnote 1 | 32 | ||
| 12-19 | 59.4 Footnote 1 | 63 | ||
| 20-59 | 70.9 Footnote 1 | 77 | ||
| 60+ | 72.0 Footnote 1 | 75 | ||
| Exposure Factors for Assessing Total Daily Intake of Priority Substances by the General Population of Canada. 1998. Unpublished report. Ottawa, ON: Health Canada, Environmental Health Directorate | WACCB (Fuels Assessment Section) | 0 - 6 months | 7.7 Footnote 2 | No data |
| 7 months - 4yrs | 15.4 Footnote 2 | 16 | ||
| 5-11 | 30.6 Footnote 2 | 32 | ||
| 12-19 | 59.1 Footnote 2 | 63 | ||
| 20-59 | 69.5 Footnote 2 | 77 | ||
| 60+ | 70.3 Footnote 2 | 75 | ||
| Exposure Factors for Assessing Total Daily Intake of Priority Substances by the General Population of Canada. 1998. Unpublished report. Ottawa, ON: Health Canada, Environmental Health Directorate. | ESRAB | 0 - 6 mo | 7.5 | No data |
| 7 mo - 4 (1-4) | 15.5 | 16 | ||
| 5-11 | 31 | 32 | ||
| 12-19 | 59.4 | 63 | ||
| 20-59 | 70.9 | 77 | ||
| 60+ | 72 | 75 | ||
| Compendium of Canadian Human Exposure Factors for Risk Assessment. Richardson, 1997. | EHB, CSD | 0 - 6 mo | 8.2 | No data |
| 7 mo - 4 (1-4) | 16.5 | 16 | ||
| 5-11 | 32.9 | 32 | ||
| 12-19 | 59.7 | 63 | ||
| 20+ | 70.9 | 76 | ||
|
||||
Appendices
- Appendix A - Body Weight Working Group
- Appendix B - Branch and Directorate Information
Appendix A - Body Weight Working Group
| Health Canada Area | Name | Title |
|---|---|---|
| HECSB/SED/CSD | Luigi Lorusso | Senior Advisor - CEAA and Contaminated Sites Remediation |
| HECSB/SED/CSD | Christine McEwan | Soil Quality Guidelines Specialist |
| HECSB/SED/WACCB/Water Quality Science Division | Michele Giddings | Manager |
| HECSB/SED/Risk Assess Bureau/ExSD | Deborah Watt | Evaluator |
| HECSB/SED/Risk Assess Bureau/ExSD | Mikin Patel | Evaluator |
| HECSB/ERHSD/Environmental Health Surveillance Division | Kristin Macey | Scientific Evaluator, Environmental Health Surveillance Division |
| HECSB/CPSD | John Field | Toxicologist |
| HPFB/ Biologics & Genetic Therapy Directorate | Dr. Gina Coleman | Chief, Clinical Trials Evaluation Division |
| HPFB/Food Directorate/Bureau of Nutritional Science | Maya Villeneuve | Associate Director, Bureau of Nutritional Sciences |
| HPFB/Food Directorate/Bureau of Chemical Safety | Mark Feeley | Associate Director, Bureau of Chemical Safety |
| HPFB/Marketed Health Products Directorate | Dr. Scott Jordan | Evaluator/Toxicologist, Marketed Biologicals, Biotechnology and Natural Health Products Bureau |
| HPFB/Marketed Health Products Directorate | Dr. Dominique Heon | Medical Evaluator, Marketed Pharmaceuticals and Medical Devices Bureau |
| HPFB/Natural Health Products Directorate | Robin Marles | Director, Bureau of Clinical Trials and Health Sciences |
| HPFB/Therapeutic Products Directorate | Yadvinder Bhuller (alt. Dr. Thea Mueller) | Manager, Clinical Trials Group 2 Note: Yadvinder is now with PMRA (Director, Health Evaluation Directorate) |
| HPFB/Veterinary Drugs Directorate | Dr. Rajinder Sharma | Team Leader, Human Safety Division |
| PMRA/Health Evaluation Directorate | Song Gao | Evaluation Officer, Exposure Re-evaluation |
| NSARC/New Chemical Substances 1 | Graham White | Chemist/Evaluator |
| Note: Other groups within the department were consulted with but it was determined that the project was not of direct relevance to them; therefore, they were not included in the working group. Some contacts did request to be notified of progress of this project (Details are available from project lead). | ||
Appendix B -Branch and Directorate Information
Health Products Food Branch (HPFB)
Mandate:
- Minimizing health risk factors to Canadians while maximizing the safety provided by the regulatory system for health products and food; and
- Promoting conditions that enable Canadians to make healthy choices and providing information so that they can make informed decisions about their health
Marketed Health Products (MHPD)
MHPD takes a leadership role in coordinating and implementing a consistent approach to the conduct of post-market surveillance monitoring, assessing and intervening on all regulated marketed health product types.
Note: While MHPD is not a regulatory authority, it does collaborate regularly with HPFB directorates who are. These include the Health Products and Food Branch Inspectorate, the Biologics and Genetic Therapies Directorate and the Natural Health Products Directorate and TPD.
Biologics and Genetic Therapies (BGTD)
The Biologics and Genetic Therapies Directorate (BGTD) is the regulatory authority in Canada that is responsible for ensuring the safety, efficacy and quality of all biologics and radiopharmaceuticals for human use, which are marketed in Canada. These include blood and blood products, viral and bacterial vaccines, genetic therapeutic products, tissues, organs and xenografts, which are manufactured in Canada or elsewhere.
Health Canada's Biologics and Genetic Therapies Directorate (BGTD) is the Canadian federal authority that regulates biological drugs (products derived from living sources) and radiopharmaceuticals for human use. Prior to being issued a Notice of Compliance (NOC), a manufacturer must present substantive scientific evidence of a product's safety, efficacy and quality as required under the Food and Drugs Act and Regulations. Some of the products regulated by BGTD include, blood and blood products, viral and bacterial vaccines, gene therapy products, tissues, organs and xenografts, which are manufactured in Canada or elsewhere.
Before a manufacturer/sponsor is eligible to market the product in Canada they must obtain a Drug Identification Number and / or a Notice of Compliance, as applicable. Manufacturers must provide sound evidence of the safety and effectiveness of their products such that upon conclusion of the review, BGTD can determine that the benefits of the product outweigh its risks and the risks can be mitigated. Once a product is approved for marketing in Canada, BGTD, in concert with other Directorates of the Health Products and Food Branch (HPFB), namely the Inspectorate (HPFBI), the Marketed Health Products Directorate (MHPD) and the Public Health Agency of Canada (PHAC) all play a role in monitoring the product's safety and effectiveness for the lifecycle of the product in Canada.
BGTD's work provides health care professionals (including physicians and other key partners) with the information that they need to make informed recommendations to Canadians about biologics including: vaccines, blood products, radiopharmaceuticals and other treatments.
Food Directorate
The Food Directorate is responsible for the development of policies, standards, procedures, regulations and guidelines directed towards the achievement of a high standard of safety and nutritional quality of food. These responsibilities are carried out through coordinated programs of scientific research, surveillance, pre-market evaluation, and regulatory activities under the authority of the Food and Drugs Act and Regulations, the Canadian Food Inspection Agency Act, and the Health Canada Act. In addition, the Directorate is responsible for assessing the effectiveness of the activities of the Canadian Food Inspection Agency related to food safety.
Note: The body weights used in HRAs within the FD may vary depending on the source of food consumption figures that are used for a given HRA. The food consumption figures employed are based on the availability of consumption figures for a certain type of food, turnaround time for the HRA, etc. CHHAD, BCS, FD would like to use CCHS cycle 2.2 food consumption figures for all HRAs; these data are available upon request from the Bureau of Food Policy and Science Integration (BFPSI), FD, although consumption data from the CHHS cycle 2.2 for some foods have been provided to BCS, FD in summary form. Thus, CHHS 2.2 data are not available for all HRAs that are conducted by BCS, particularly those with a short deadline (eg. HRAs conducted upon request of the CFIA).
Natural Health Products (NHPD)
The Natural Health Products Directorate (NHPD) ensures that Canadians have ready access to natural health products that are safe, effective, and of high quality by maintaining proper labelling and implementing a regulatory framework that supports freedom of informed choice and cultural diversity.
NHPD participates in the establishment of IOM values and has set a precedence by using the IOM life stages as they are supported by detailed rationale and published by an authoritative body.
Therapeutic Products (TPD)
Health Products and Food Branch's (HPFB) Therapeutic Products Directorate (TPD) is the federal authority that regulates pharmaceutical drugs and medical devices for human use. Prior to being given market authorization, a manufacturer must present substantive scientific evidence of a product's safety, efficacy and quality as required by the Food and Drugs Act and Regulations. Drugs, regulated by TPD, include prescription and non prescription pharmaceuticals, disinfectants and sanitizers with disinfectant claims. Medical devices cover a wide range of health or medical instruments used in the treatment, mitigation, diagnosis or prevention of a disease or abnormal physical condition.
Veterinary Drugs (VDD)
The Veterinary Drugs Directorate (VDD) is responsible for ensuring the safety of foods such as milk, meat, eggs, fish and honey from animals treated with veterinary drugs and that
veterinary drugs sold in Canada are safe and effective for animals. The Veterinary Drugs Directorate also leads the Government of Canada's work in science, policy and regulation to combat antimicrobial resistance.
Healthy Environments and Consumer Safety Branch (HECSB)
Mandate:
HECS' mandate is keyed to health protection and health promotion; a dual role which ensures that Canadians receive high calibre national services delivered to meet local needs.
Water, Air and Climate Change Bureau (WACCB)
The Water, Air and Climate Change Bureau leads the development of health risk assessments to protect the health of Canadians from contaminants in drinking and recreational waters and outdoor and indoor air, and participates in developing regulations and standards to address risks from air and water contaminants. The Bureau advances the understanding of health impacts of climate change, and provides advice on adaptation strategies. The Bureau works in close collaboration with partners and stakeholders, and participates actively in developing broad federal and national water, air and climate change policies.
Existing Substances Risk Assessment Bureau (ESRAB)
To protect the health of Canadians by assessing, and providing expert advice on, the health risks and impacts posed by chemical substances in commerce.
Assessment completed under the Canadian Environmental Protection Act, 1999.
Contaminated Sites Division (CSD)
Develops soil quality guidelines, and other tools to guide the development of risk mitigation strategies for the protection of human health. Provides custodial departments with expert advice, guidance, training and tools on best practices and innovative methods for human health risk assessment and incorporating stakeholders into contaminated site management
Product Safety Program, Consumer Product Safety (PSP, CPS)
The PSP assists in the protection of Canadians by researching, assessing and collaborating in the management of the health and safety hazards associated with:
- consumer products;
- cosmetics;
- workplace chemicals;
- new chemical substances;
- products of biotechnology;
- radiation-emitting devices;
- environmental noise;
- solar UV radiation.
CPS works closely with partners and stakeholders to protect consumers from product-related hazards and to promote the safe use of products. Research and tests performed by the Product Safety Laboratory support the development of standards and product safety regulations that are enforced by Product Safety Officers in the Regions who oversee product investigations, inspections, seizures, recalls and prosecutions. CPS is actively involved in injury prevention through promoting consumer awareness of actual and potential product-related hazards. CPS also encourages the design of safer products for the Canadian market by providing importers and manufacturers with hazard and product technical information.
New Substances Assessment and Control Bureau (NSACB)
The New Chemical Substances Sections of NSACB are responsible for the pre-manufacture or pre-import assessment of the potential health risks to the general population associated with approximately 1,000 new chemicals and polymers such as fabric dyes and fuel additive components, that manufacturers and importers wish to introduce into the marketplace each year. This work is carried out according to the Guidelines for the Notification and Testing of New Substances so as to ensure that no new chemical is introduced into Canada on a commercial scale before it has been reviewed to determine the potential risks to human health and the environment. Some substances are reviewed at the research and development stage. The legal responsibility of importers and manufacturers to notify new substances is set out in the New Substances Notification Regulations (NSNR) of the Canadian Environmental Protection Act, 1999 (CEPA 1999). This proactive approach to managing the potential risks of new substances is one of the key objectives of CEPA. If a risk is identified, measures are taken to reduce the risk by controlling or even banning the substance or product.
Pest Management Regulatory Agency (PMRA)
The PMRA is responsible for pesticide regulation in Canada. Created in 1995, this branch of Health Canada consolidates the resources and responsibilities for pest management regulation.
Pesticides are stringently regulated in Canada to ensure they pose minimal risk to human health and the environment. Under authority of the Pest Control Products Act, Health Canada:
- registers pesticides after a stringent, science-based evaluation that ensures any risks are acceptable;
- re-evaluates the pesticides currently on the market on a 15-year cycle to ensure the products meet current scientific standards; and
- promotes sustainable pest management.
Health Canada also promotes and verifies compliance with the Act and enforces situations of non compliance warranting action. Our programs and initiatives look to improve the regulatory process and provide Canadians pest control products and strategies with acceptable risk and value. Health Canada is committed to providing an open, transparent and participatory process for pesticide regulation.
Health Canada works with provincial, territorial and federal departments in Canada to help refine and strengthen pesticide regulation across the country. These partnerships ensure that the diverse needs of the Canadian public are addressed at all levels of government, and that the policies developed by Health Canada meet these needs.
Beyond Canada, Health Canada also works closely with a number of international organizations including: United States Environmental Protection Agency, the North American Free Trade Agreement Technical Working Group, and the Organization for Economic Co-operation and Development. These close ties contribute to the development of policies and regulations.
Footnotes
- Footnote 1
-
Now the Water and Air Quality Bureau, Safe Environments Directorate, HECSB
- Footnote 2
-
Now the Natural and Non-Prescription Health Products Directorate, HPFB