Science Integrity Policy – Breach Process Guidelines (Main Document)
Table of content
- Effective date
- Context
- Purpose and objectives
- Application
- Principles
- Breaches of scientific integrity
- Process for addressing breaches of scientific integrity
- Framework and roles
Figure 1 - Breach process framework - References
Figure 2 – Breach process overview
1. Effective date
These guidelines take effect on 1 April 2022.
2. Context
These guidelines are issued pursuant to the DND/CAF Science Integrity Policy and Instructions, subsequently referred to as the “DND/CAF SIP”, which came into effect on 1 April 2019. These guidelines are to be applied congruently with the DND and CAF Code of Values and Ethics and the Treasury Board Secretariat (TBS) Guidelines for Discipline.
Within these guidelines, the overall process described for addressing science integrity breaches will be referred to simply as the “breach process”.
3. Purpose and objectives
These guidelines are intended to assist Department of National Defence (DND) employees and Canadian Armed Forces (CAF) members in understanding the required procedures for the submission, assessment and investigation of breaches of scientific integrity as per provision 7.2.2 of the DND/CAF SIP.
These guidelines will ensure DND employees and CAF members:
- are informed of the procedures available for bringing forward allegations of breaches of scientific integrity;
- fully understand the process by which allegations are evaluated, investigated and adjudicated; and
- have confidence and trust in the procedures for bringing forward, investigating and adjudicating alleged breaches and that they will be conducted fairly, in good faith and without prejudice.
4. Application
These guidelines apply to DND employees and CAF members who design, conduct, communicate, manage, review or make use of defence and security research, science or related activities, and/or DND research facilities (i.e. it also applies to contractors, visiting scientists and students, collaborators and clients).
The guidelines apply to a breach that occurred since the effective date of the original SIP (1 April 2019). Furthermore, the breach must have been reported as required by the SIP within two years of the breach occurrence (or date the alleger ought to have known about it) leading to either an informal resolution with a third party, or the submission of an allegation to the Science Integrity Lead (SIL). The SIL may consider exceptions to this time limit for extraordinary circumstances.
Note that filing an allegation of breach does not prevent a DND employee or CAF member from availing themselves of the grievance procedures provided for in their collective agreement or the military grievance process as applicable. Where the same matter is covered by both the SIP and their collective agreement, employees are encouraged to discuss the appropriate mechanisms to use with their union representative.
In cases where an allegation of a breach is pursuant to a collective agreement or a regulatory or statutory requirement, complaint procedures associated with such agreements, statutes or regulations take precedence.
5. Principles
These guidelines apply to all allegations of breaches of scientific integrity and any assessment, investigation or adjudication of them. They shall be conducted competently and expeditiously with full regard for the following principles:
- Independence and impartiality: all procedures should be conducted by appropriate independent and competent experts in a manner that avoids bias and is impartial and independent of any political, commercial, client or stakeholder interference. In this context, impartiality refers to the absence of both conflicts of interest and bias with respect to a priori beliefs in the truth of the allegation.
- Confidentiality: any confidential (including personal) information brought forward in these procedures shall be maintained in a manner fully consistent with the Privacy ActFootnote 1, the Employment Equity ActFootnote 2 and associated regulations, and in keeping with the federal Code of ConfidentialityFootnote 3.
- Procedural fairness: All parties involved in these procedures shall be fully informed of these principles, all relevant procedures, and their right to be heard in order for them to enhance their awareness and to increase their preparation. Information or evidence gathered or considered in these procedures should be documented. Any disclosure of such information:
- must satisfy the provisions of the Access to Information ActFootnote 4 or one or more of the exemption provisions of the Security of Information ActFootnote 5;
- must not contravene the Privacy Act or the Employment Equity Act and associated regulations; and
- must not contravene the federal Code of Confidentiality.
- Good faith and fair dealing: all parties involved in these procedures, shall conduct themselves in a manner consistent with good faith and fair dealing. DND/CAF should provide safeguards to instil confidence for all involved to bring forward allegations of breaches of scientific integrity or participate in an investigation without fear of reprisal. In the absence of evidence to the contrary it is presumed that all parties are indeed acting in good faith.
- Informal consultation, mediation and dispute resolution: in recommending actions or deciding upon appropriate actions, DND employees and CAF members are encouraged to consider early informal consultation, mediation or dispute resolution as a means of fostering science integrity.
- Right of union, legal or other representation: any party involved in these procedures shall have the right to union, legal or other representation at any stage of the process. Further, these guidelines in no way replace or abridge any employee rights pursuant to applicable collective agreements or member rights under the National Defence Act.
- Positive working relationships: everyone is encouraged to work together to establish and restore positive working relationships, particularly when allegations and investigations are resolved.
6. Breaches of scientific integrity
A breach of scientific integrity is behaviour or actions by an individual that could reasonably be construed as inconsistent with or violating one or more of the principles of scientific integrity as described in the SIP.
Breaches may be of two types:
- Scientific misconduct: actions or behaviours, normally in the direct conduct or preparation of scientific activity by individuals, that could reasonably be construed as inconsistent with or violating ss. 7.3.3, 7.4.2-7.4.4, 7.4.9, 7.5.1 – 7.5.3, 7.5.6 or 7.8 of the SIP as indicated in the table below:
| 7.3.3 | Transparency and timeliness - suppression, alteration, or impeding timely release |
| 7.4.2 | Public communications - respecting legal restrictions on information disclosure |
| 7.4.3 | Public communications – planned events - formal notifications required and compliance with applicable orders and directives |
| 7.4.4 | Public communications - unplanned events (no lead time) |
| 7.4.9 | Public communications – requirements as an official spokesperson and speaking on the record |
| 7.5.1 | Dissemination – review of drafts by approval and/or release authorities |
| 7.5.2 | Dissemination – approval required if explicit discussions on statutory, regulatory or policy matters are involved |
| 7.5.3 | Dissemination – release authority required for DND and CAF research or scientific communications |
| 7.5.6 | Dissemination – requirements to be ensured by author of any research or scientific communication including permissions, accreditation, affiliation, peer review, intellectual property (IP), copyright, Official Languages managed and Open Science principles considered |
| 7.8 (entire article) |
Responsible conduct of research (RCR) – application of RCR practices including RCR concepts and standards (7.8.1) and avoiding RCR breaches (including plagiarism, mismanaged conflict of interest (COI), inaccurate attribution, applications, information, etc.) |
- Unethical or unprofessional behaviour: actions or behaviour of managers, supervisors or other relevant personnel, normally in the support of scientific activity, that could reasonably be construed as inconsistent with or violating ss. 7.3.3, 7.3.4, 7.4.6, 7.4.7, 7.5.4, or 7.5.7 of the DND/CAF SIP as indicated in the table below:
| 7.3.3 | Transparency and timeliness – suppression, alteration, or impeding timely release |
| 7.3.4 | Transparency and timeliness – scientific information produced and disseminated in a timely and transparent manner |
| 7.4.6 | Public communications – no obligation to act as DND/CAF subject matter expert (SME) or appear in public unless explicitly directed |
| 7.4.7 | Public communications – use of work by researchers/scientists must be approved and accredited, or the interpretation of the work supported |
| 7.5.4 | Dissemination – actions in the event of disagreement on editorial changes required for approval |
| 7.5.7 | Dissemination – review of drafts by approval and/or release authorities |
Severity of a breach: with recommendations obtained in the course of the assessment and/or investigation of an allegation, the SIL will make a determination of the severity of a breach of scientific integrity based on an evaluation of:
- the potential impact on public health, public security, the defence of Canada or the environment;
- the extent to which it would undermine the perception of a professional non-partisan DND employee or CAF member or the federal public service;
- its deviation from relevant professional codes of conduct or standards of practice;
- its likely effect on the research or scientific credibility of, or public trust in, research or scientific information produced or employed by DND or the CAF;
- its potential impact on the research or scientific community – whether broadly or amongst our allies – and the accumulation of knowledge;
- whether the breach concerns discretionary actions versus dutiesFootnote 6;
- the level of associated intent (e.g. accidental, unknowing or unwitting, wilful, reckless, etc.);
- whether the associated actions appear to represent isolated incidents or chronic or persistent conduct; and
- the extent to which the breach has a direct or urgent impact on others outside of the immediate parties.
Policy non-compliance: by contrast, policy non-compliance are actions or behaviour by DND employees or CAF members that could reasonably be construed as inconsistent with or violating ss. 7.1, 7.2.1, 7.2.2, 7.3.1, 7.3.2, 7.6, 7.7, or 7.9 of the DND/CAF SIP. Policy non-compliance would likely be subject to organizational corrective measures. Instances of policy non-compliance should not be brought under these procedures. Rather, they should be documented and brought to the attention of the Chief of Staff (Defence Research and Development Canada) [COS(DRDC)] who will determine the appropriate course of action. The general subject behind each of these articles is indicated in the table below:
| 7.1 | Implementation |
| 7.2.1 | Science virtues – learning policies support training, education, and professional development (PD) about science virtues, RCR, research ethics |
| 7.2.2 | Science virtues – appoint a SIL, review and properly investigate breaches |
| 7.3.1, 7.3.2 | Transparency and timeliness – posting of policy and amendments |
| 7.6 | Encourage and facilitate contribution to the scientific community (domestic, international, allied, academic, government, industry, professional, civil society, etc.) |
| 7.7 | Consultations and mechanism in support of science advice to evidence informed decision making (EIDM) |
| 7.9 | Monitoring implementation and performance of SIP |
Suspicion of malfeasance or malicious intent: the SIP fosters an environment that encourages and assumes that disclosures of potential breaches are made in good faith. Allegations raised otherwise detract from confidence and trust in these procedures. An allegation of breach for which there are indications of malfeasance or malicious intent may itself be considered a breach of scientific integrity, as a case of employee or member misconduct, or as case for disciplinary action.
7. Process for addressing breaches of scientific integrity
The process for addressing a potential breach of scientific integrity involves the following sequence of Phases:
- Phase 1. Detection and consultation
- Phase 2. Notification and assessment
- Phase 3. Structured alternative dispute resolution (ADR)
- Phase 4. Breach investigation
- Phase 5. Resolution and conclusion
The framework for the key positions reviewing a breach is shown graphically at figure 1. An overview of the full process is presented at figure 2. Details of the steps in each Phase are elaborated upon in Appendix A.
8. Framework and roles
Science Integrity Lead (SIL): In accordance with the SIP, the Deputy Minister / Chief of Defence Staff (DM/CDS) via Assistant Deputy Minister (Defence Research and Development Canada [ADM (DRDC)] has appointed the COS(DRDC) as the SIL for the department. The SIL:
- is responsible for ensuring that all allegations, investigations and adjudications of a breach, and any actions taken as a result of an allegation, are conducted in manner consistent with the principles and procedures of these guidelines.
- is informed of every step in the process, and any decisions resulting therefrom.
The Office of Science Integrity (OSI), part of the COS(DRDC) organization dedicated to the departmental administration of the SIP, provides responses to inquiries, and guidance on the breach process. For the breach process, the OSI will:
- serve as a case manager to support the particular case through the Phases, unless otherwise directed by the SIL;
- support the SIL and breach head of response in carrying out their responsibilities; and,
- For Phases 1 and 2:
- provide guidance on these procedures to anyone considering or involved in a potential breach allegation;
- provide information as requested, including recommending avenues for alternative and informal resolution mechanisms; and,
- arrange for a proper initial assessment and validation to the SIL of an allegation once formally submitted.
Case Manager (CM): is responsible to the SIL and ensures that the case is conducted properly. As such, the CM is a point of contact for all parties involved in the case. In addition to the initial validation and assessment of the allegation(s) in Phase 2, the CM will support the designated head of response and selected teams in Phase 4 as well as coordinate the tracking and sharing of reports as directed at Appendix A.
Alleger: is the originator or source of the allegation of a breach of scientific integrity. The alleger is a person, persons or the representative of a group of persons to whom the breach process applies as per Section 4 of these guidelines and shall:
- consider attempting to resolve alleged breaches through informal means, where possible and appropriate;
- submit allegation(s) of a breach to the SIP in writing;
- ensure that allegations are made without prejudice or malicious intent; and,
- provide sufficient evidence to support the allegation and participate in these breach procedures fully and in a manner that correctly informs potential assessment/investigation activity.
Respondent: is a person, persons or the representative of a group of persons to whom the breach process applies as per section 4 of these guidelines and have allegedly breached scientific integrity. They shall:
- consider attempting to resolve alleged breaches through informal means, where possible and appropriate;
- participate in these breach procedures fully and in a manner that correctly informs potential assessment/investigation activity.
SIP Breach Head of Response (BHR):an investigation is initiated, the BHR is the individual designated to oversee and manage a breach allegation investigation. The Director Research and Development Strategic Resource Planning and Management (DRDSRPM) is the default DND SIP BHR, unless designated otherwise by the SIL.
The BHR will:
- reach out to expert/technical advisors to review evidence as necessary, and make recommendations to the SIL regarding approach (e.g. whether a formal investigation is required);
- coordinate the preparation of fact-finding and investigation reports, with the assistance of the expert advisor as needed; and,
- participate in the Science Integrity Committee.
Expert advisor to the head of response: is an impartial DND employee or CAF member (appropriate to but not involved in the case at hand) with expert knowledge of the scientific/research field central to the complaint. The expert advisor reports directly to the BHR to provide insight and/or advice if required on the findings of the breach case assessors and breach case committee.
Breach case assessor(s) (BCA): is/are appointed by the designated head of response to conduct the “fact-finding” stage of Phase 4. Depending on the nature and complexity of the case, the head of response may consider selecting a panel of two assessors, preferably from within DND/CAF.
As per the DND and CAF Code of Values and Ethics, there must be no actual or perceived personal or organizational conflict of interest associated to the selection of the BCA(s). BCA(s) will be selected with consideration of appropriate knowledge and competencies including their organizational awareness, demonstrated science integrity, communications skills, and ability to conduct a thorough evaluation of the case leading to well-founded conclusions.
Breach case committee (BCC): is selected by the designated head of response should the fact-finding report establish reasonable grounds for further or more formal investigation of the evidence available, or the severity of the case warrants a more in-depth examination. The BCC is a panel that shall consist of three to five members, at least two of whom should be from outside the federal government. It should include:
- an independent subject matter expert from the department or agency by whom the alleger or the respondent are employed;
- one independent expert who has an ethics background such as a Defence Ethics Program employee or member and;
- one independent expert who has scientific or research background relevant to the subject of the dispute.
- Current or prior participation in research ethics committees would be considered an asset.
These independent experts are to be outside of the chain of command and, as per the DND and CAF Code of Values and Ethics, there must be no actual or perceived personal or organizational conflict of interest associated to their appointment. When applicable and possible, a representative of the professional code by which the respondent is bound can be appointed as a member. No member shall have been a BCA in the fact-finding stage of this case.
Figure 1 - Breach process framework
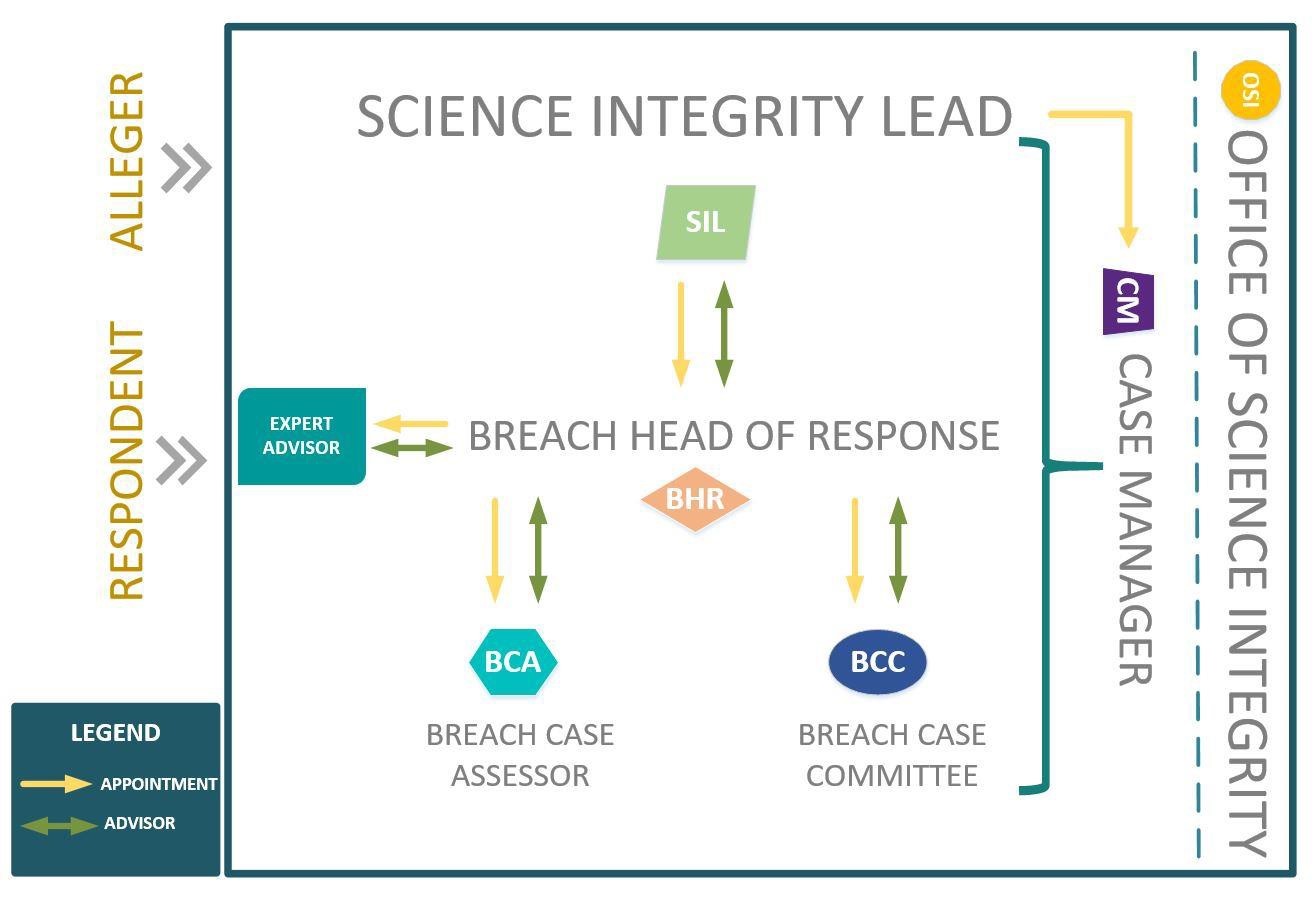
Long description of Figure 1
Figure 1 provides an overview of the different roles involved within a Science Integrity Policy (SIP) breach investigation.
Following an assessment of the validity and complexity of an alleged SIP breach, the science integrity lead (SIL) will determine how to best resolve the issue. A SIP breach investigation involves the following key roles:
- The alleger who makes the allegation
- The respondent who is named in the allegation
- The office of science integrity (OSI) assists in the coordination and management of the allegation.
- The SIL oversees the response to the allegation by:
- appointing a case manager (CM)
- appointing a breach head of response (BHR) if an investigation is required, who is an advisor to the SIL
- The BHR first appoints a breach case assessor (BCA). The BCA advises the BHR.
- If needed, the BHR will appoint a breach case committee (BCC). The BCC advises the BHR.
- The BHR may appoint an independent expert advisor who advises on the case.
Science Integrity Committee: will be established to act as an advisory body to the SIL to consider the findings and recommendations of an assessment or investigation prior to finalizing an official decision. This may include reviewing recommended corrective measures or the need for disciplinary action. It may also be used to review ethics and integrity issues related to the SIP that are not part of an active breach case.
- Membership will include: SIL, DRDC ethics champion, the breach head of response, expert advisor and representatives from Director General Military Personnel Research and Analysis (DGMPRA), the Defence Ethics Program (DEP), and the Professional Institute of the Public Service of Canada (PIPSC). Membership may also include legal services or other representation as required.
For questions about the SIP and/or this breach process, please contact us.
9. References
- DND/CAF Science Integrity Policy and Instructions
- DND and CAF Code of Values and Ethics
- Framework for the Management of Compliance, Treasury Board
- Treasury Board Secretariat (TBS) Guidelines for Discipline
Figure 2 - Breach process overview
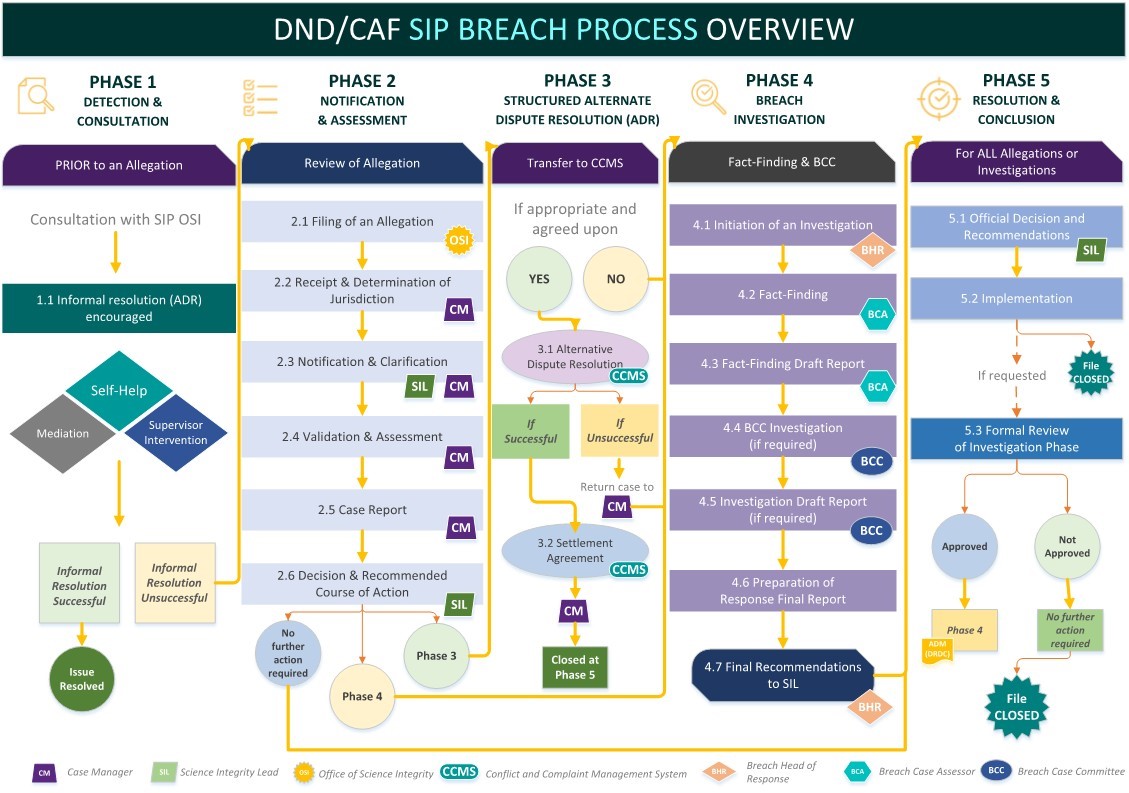
Long description of Figure 2
The review and resolution of a Science Integrity Policy (SIP) breach allegation will generally follow a sequence through up to five phases.
Phase 1 is a preliminary phase prior to an allegation being submitted, that focuses on detection and consultation regarding the issue at hand. The objective is to provide guidance and interpretations to anyone looking for information on the SIP or the breach process. It also ensures that when possible, options for informal resolution are explored prior to an allegation being submitted. These options include mediation, self-help and supervisor intervention.
If the informal resolution is successful, the issue is resolved.
If the informal resolution is unsuccessful, the breach process proceeds to Phase 2.
Phase 2 is the phase of the breach process that facilitates the preparation and initial evaluation of an allegation, and ensures that notifications are submitted as required. An initial validation and assessment of the incident is then conducted for the science integrity lead (SIL). With this information, the SIL determines how to best proceed with resolution.
Steps within Phase 2 include:
- filing of an allegation
- receipt and determination of jurisdiction
- notification and clarification
- validation and assessment
- case report
- decision and recommended course of action
It may be necessary to proceed to phase 3 for alternative dispute resolution (ADR), if suitable to the allegation and both parties agree. In phase 3, the allegation is turned over to the conflict and complaint management service (CCMS) for a structured ADR process, leading to a settlement agreement.
If, however, the parties disagree or the issue is too complex or urgent for ADR, the allegation may be investigated within the SIP breach process, by the breach head of response (BHR) at Phase 4.
Steps within Phase 4 include:
- Initiation of an investigation, led by the BHR
- Fact-finding, led by the breach case assessor (BCA)
- Fact-finding draft report, led by the BCA
- Breach case committee (BCC) investigation
- Investigation draft report (if required) from the BCC
- Preparation of response final report
The BHR will then prepare a final recommendation to the SIL.
Phase 5 is the resolution and conclusion phase of the process for all allegations and investigations.
Steps within Phase 5 include:
- Official decision and recommendations by the SIL
- Implementation
- Formal review of investigation phase, if requested
Ultimately, resolutions of allegations determined at phases 2, 3, or 4 are reviewed and finalized by a final decision by the SIL at phase 5.

