Draft assessment - Fourteen Terpene and Terpenoid Substances Group
Official title: Draft assessment - Fourteen Terpene and Terpenoid Substances Group
Environment and Climate Change Canada
Health Canada
January 2025
Synopsis
Pursuant to section 68 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of the Environment and the Minister of Health have conducted an assessment on 14 substances hereinafter referred to as the Fourteen Terpene and Terpenoid Substances Group. The Chemical Abstracts Service Registry Numbers (CAS RNsFootnote 1 ), subgroup, the Domestic Substances List (DSL) names, and common names and/or abbreviations are listed in the table below.
| CAS RN | Subgroup | DSL name | Common name (abbreviation) |
|---|---|---|---|
| 8013-10-3a | Individual | Oils, cade | Cade oil |
| 8023-75-4a | Individual | Oils, jonquil | Jonquil oil |
| 70788-30-6 | Individual | Cyclohexanepropanol, 2,2,6-trimethyl-α-propyl- | Norlimbanol |
| 84961-67-1a | Individual | Verbena officinalis, ext. | Verbena officinalis extract |
| 90045-36-6a | Individual | Ginkgo biloba, ext. | Ginkgo biloba extract |
| 3738-00-9 | Individual | Naphtho[2,1-b]furan, dodecahydro-3a,6,6,9a-tetramethyl- | Amberlyn |
| 8016-37-3a | Individual | Oils, myrrh | Myrrh oil |
| 164288-52-2a | Individual | Cork tree, Phellodendron amurense, ext. | Cork tree extract |
| 8022-56-8a | 1 | Oils, sage | Sage oil |
| 8008-93-3a | 1 | Oils, wormwood | Wormwood oil |
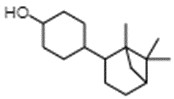
| 3407-42-9 | 2 | Cyclohexanol, 3-(5,5,6-trimethylbicyclo[2.2.1]hept-2-yl)- | Isobornyl cyclohexanol (IBCH) |
| 66068-84-6 | 2 | Cyclohexanol, 4-(5,5,6-trimethylbicyclo[2.2.1]hept-2-yl)- | Sandal cyclohexanol |
| 68877-29-2 | 2 | Cyclohexanol, (1,7,7-trimethylbicyclo[2.2.1]hept-2-yl)- | Bornyl cyclohexanol (BCH) |
| 70955-71-4a | 2 | Phenol, 2-methoxy-, reaction products with 2,2-dimethyl-3-methylenebicyclo[2.2.1]heptane, hydrogenated | Sandela |
a This CAS RN is a UVCB (unknown or variable composition, complex reaction products, or biological materials).
Terpenes are chemicals with repeating isoprene units, and are classified according to the number of isoprene units they contain. Monoterpenes are the smallest unit containing 2 isoprene units and these may be acyclic or cyclic in structure. These substances are components of essential oils and are found in a wide variety of plants.
All of the substances in the Fourteen Terpene and Terpenoid Substances Group have been included in surveys issued pursuant to section 71 of CEPA. Except for amberlyn, none of the substances in this group were reported to be manufactured or imported into Canada in quantities greater than 100 kg during the 2011 reporting year. Amberlyn was reported to be imported in quantities ranging from 100 kg to 1,000 kg. The substances in the Fourteen Terpene and Terpenoid Substances Group are generally used as ingredients in cosmetics, drugs including natural health products (NHPs), cleaning products, air fresheners, as well as essential oils used in do-it-yourself (DIY) applications to create these products, or are added to diffusers, facial steamers, or baths, among others. Some of these substances are also present in pest control products (PCPs) as formulants. In addition, some occur naturally in food and may be used as food flavouring agents.
The ecological risks of the substances in the Fourteen Terpene and Terpenoid Substances Group were characterized using the ecological risk classification of organic substances (ERC), which is a risk-based approach that employs multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. Hazard profiles are based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Metrics considered in the exposure profiles include potential emission rate, overall persistence, and long-range transport potential. A risk matrix is used to assign a low, moderate, or high level of potential concern for substances on the basis of their hazard and exposure profiles. Based on the outcome of the ERC analysis, the substances in the Fourteen Terpene and Terpenoid Substances Group are considered unlikely to be causing ecological harm.
Considering all available lines of evidence presented in this draft assessment, there is low risk of harm to the environment from the substances in the Fourteen Terpene and Terpenoid Substances Group. It is proposed to conclude that the substances in the Fourteen Terpene and Terpenoid Substances Group do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
For the human health risk assessment, 6 of the 14 substances in this group have been addressed under 2 subgroups due to similarities in chemical structure, properties, and/or toxicity, while the remaining substances were addressed individually.
An impact on human health from exposure to the 14 substances through environmental media is not expected due to the low quantities reported in response to a CEPA section 71 survey. Where applicable, exposures were characterized from use of cosmetics, non-prescription drugs (NPDs), NHPs, possible use as food flavouring agents, cleaning products, air fresheners, and DIY applications. Where the health effects datasets were considered to be limited, the toxicological data from its major components were taken into consideration.
Cade oil is the volatile oil extracted from the wood and branches of Juniperus oxycedrus following destructive distillation using high heat. Amongst the major components of cade oil, cresols were associated with the lowest effect levels. Carcinogenicity observed in animal laboratory studies as well as non-cancer central nervous system effects were identified as the critical effects associated with cresols and were used as the basis for characterization of the risk to human health from exposure to cade oil. Comparison of the critical health effect levels to estimates of exposure to cade oil from uses in perfumes (roll-on) and face moisturizers, as well as DIY essential oil uses in aromatic diffusers, facial steamers, bath oils, body moisturizer preparations, and massage oil preparations, and topical preparations applied on abraded/damaged skin, resulted in margins of exposure (MOEs) that are considered potentially inadequate to address uncertainties in the health effects and exposure data used to characterize risk.
Jonquil oil is a substance of unknown or variable composition, complex reaction products, or biological materials (UVCB) defined as the “extractives and their physically modified derivatives obtained from Narcissus jonquilla L., Amaryllidaceae.” Benzyl benzoate and trans-methylisoeugenol were the major components associated with the lowest effect levels in the health effects database. Non-cancer effects (that is, developmental effects, general toxicity) and carcinogenicity observed in animal laboratory studies were identified as the critical effects for risk characterization. Comparison of the critical health effect levels to estimates of exposure to jonquil oil from DIY uses in aromatic diffusers, facial steamers, bath oils, as well as massage oil preparations and body moisturizer preparations resulted in MOEs that are considered potentially inadequate to address uncertainties in the health effects and exposure data used to characterize risk.
Norlimbanol is not present naturally in the environment but is manufactured from the condensation of citral with 2-pentanone. Critical health effects associated with norlimbanol include reproductive and developmental toxicity observed in animal laboratory studies. Comparison of the critical health effects to the estimates of exposure to norlimbanol from the use of spray colognes resulted in MOEs that are considered adequate to address uncertainties in the health effects and exposure data used to characterize risk.
Verbena officinalis extract is an extract of the aerial parts and roots of the Verbena officinalis plant also known as the common vervain. On the basis of the health effects data available on citral (one of its major components), the critical effects identified for Verbena officinalis extract were developmental effects, reduced body weight, and severe respiratory tract irritation observed in laboratory studies. Comparison of the critical health effect levels with estimates of exposure to Verbena officinalis extract from its use in massage oils, body exfoliants, shampoos, hand creams, face moisturizers, oral supplements (NHPs), liquid extracts, and DIY applications of Verbena officinalis essential oil in aromatic diffusers and face steamers resulted in MOEs that are potentially inadequate to address uncertainties in the health effects and exposure data used to characterize risk.
The critical effects associated with Ginkgo biloba extract include developmental effects (reduced fetal body weight and reduced intrauterine growth), as well as increased incidence of liver tumours observed in laboratory studies. Comparison of the critical health effect levels with estimates of exposure to Ginkgo biloba extract from the use of Ginkgo biloba extract in face exfoliants, hair perm/straighteners, hand creams, permanent hair dyes, makeup remover, aftershaves, face masks, body oils, sunless tanning products, massage products, liquid face foundations, genital lubricants, face and body moisturizers, hair mists, spray antiperspirants, face toners, liquid body soaps, face cleansers, shampoos, face sunscreens (NHPs and NPDs), sunscreen lotions (NHPs), oral supplements (including NHPs), and teas (including NHP herbal tea blends) resulted in MOEs that are considered inadequate to address uncertainties in the health effects and exposure data used to characterize risk.
Amberlyn is a naturally occurring terpenoid found in ambergris. The substance can also be obtained from the oxidation of components present in clary sage oil. The critical effects identified for amberlyn include changes in biochemical or blood parameters (for example, platelets, cholesterol levels) and histopathological changes in certain organs (for example, kidneys). Comparison of the critical health effect levels to the estimates of exposure to amberlyn from the use of an air freshener, body lotion, and use as a food flavouring agent resulted in MOEs that are considered adequate to address uncertainties in the health effects and exposure data used to characterize risk.
Myrrh oil is a UVCB defined as the “extractives and their physically modified derivatives from Commiphora, Burseraceae.” The critical effects identified for myrrh oil in laboratory studies included effects on biochemistry (that is, bile acids) and reproductive parameters (that is, sperm levels). Comparison of the critical health effect levels with estimates of exposure to myrrh oil from use in permanent hair dye, hair styling products, massage oils, bath oils, face exfoliants, hair removal aftercare products, sunless tanning products, aftershaves, body moisturizers, face moisturizers, antiperspirants, liquid body soaps, spray perfumes, tooth powders, mouthwashes, teeth whiteners, body lotions (NHPs), pain gels (NHPs), sunscreen lotions (NHPs), hand sanitizers (NHPs), oral capsules (NHPs), resin incense, and use of essential oil in DIY applications such as stomach remedies, aromatic diffusers, and face steamers resulted in MOEs that are potentially inadequate to address uncertainties in the health effects and exposure data used to characterize risk.
Cork tree extract is a UVCB derived from the bark of the Phellodendron amurense tree. On the basis of the health effects data available on the extract, the critical effects identified for cork tree extract were effects on the heart and liver. Comparison of the critical health effects and estimates of exposure to cork tree extract from use in cosmetics and NHPs including face moisturizers, body moisturizers, and analgesic sprays resulted in MOEs that are considered potentially inadequate to address uncertainties in the health effects and exposure databases. Additionally, DIY applications of cork tree extract, including oral ingestion of cork tree extract and use of the substance in aromatic diffusers and massage oil preparations, resulted in MOEs that are also potentially inadequate to address uncertainties in the health effects and exposure data used to characterize risk.
Sage oil is a UVCB that is defined as the extracts derived from the botanical species Salvia officinalis. Wormwood oil is also a UVCB and is defined as the “extractives and their physically modified derivatives from Artemisia absinthium, Compositae”. Thujone, a major component in both substances, was associated with the lowest effect levels in the health effects data sets for sage and wormwood oils. On the basis of the health effects data available on thujone, the critical effects identified for risk characterization were neurological effects (that is, convulsions). As the health effects data sets for both sage oil and wormwood oil were informed by thujone, these substances were assessed together as a subgroup of substances (that is, subgroup 1).
A comparison of the critical health effect levels to estimates of exposure to sage oil from use of massage oils (cosmetics and NHPs), sunless tanning products, douches, face masks, antiperspirants, rinse-off conditioners, spray perfumes, face moisturizers, body moisturizers (cosmetics and NHPs), hair styling products, makeup removers, liquid body soaps, shampoos, hand sanitizers (NHPs), analgesic creams (NHPs), and DIY applications of sage oil in aromatic diffusers and face steamers resulted in MOEs that are potentially inadequate to address uncertainties in the health effects and exposure data used to characterize risk. Oral exposure to sage oil from breath fresheners, mouth washes, toothpastes, oral supplements (NHPs), motion sickness medications (NHPs), and throat sprays (NHPs) also resulted in MOEs that are considered potentially inadequate.
A comparison of the critical health effect levels to estimates of exposure to wormwood oil from use of NHPs and NPDs including hand sanitizers and analgesic creams and when used in DIY applications including aromatic diffusers, facial steamers, bath oils, massage oil preparations, and body moisturizer preparations resulted in MOEs that are potentially inadequate to address uncertainties in the health effects and exposure data used to characterize risk. Oral ingestion of wormwood oil also resulted in MOEs considered potentially inadequate to address uncertainties.
Due to their structural similarities and the use of their common names as synonyms in the literature, isobornyl cyclohexanol (IBCH), sandal cyclohexanol, bornyl cyclohexanol (BCH), and sandela were assessed together as a subgroup of substances (that is, subgroup 2). The toxicological data for sandal cyclohexanol, BCH, and sandela were limited, and the health effects data on IBCH was used to inform the human health risk assessment for all substances in subgroup 2. The critical effects identified included reproductive and developmental effects. Comparison of the critical health effect levels with estimates of exposure to substances in subgroup 2 from use in spray perfumes and body moisturizers resulted in MOEs that are considered potentially inadequate to address uncertainties in the health effects and exposure data used to characterize risk.
The human health assessment for each substance took into consideration those groups of individuals within the Canadian population who, due to greater susceptibility or greater exposure, may be more vulnerable to experiencing adverse health effects. Certain subpopulations are routinely considered throughout the assessment process, such as infants, children, and people of reproductive age. For instance, age-specific exposures are routinely estimated, and developmental and reproductive studies are evaluated for potential adverse health effects. These subpopulations with potential for higher exposure and those who may be more susceptible were taken into account in the risk assessment outcomes.
Considering all the information presented in this draft assessment, it is proposed to conclude that cade oil, jonquil oil, Verbena officinalis extract, Ginkgo biloba extract, myrrh oil, cork tree extract, sage oil, wormwood oil, IBCH, sandal cyclohexanol, BCH, and sandela meet the criteria under paragraph 64(c) of CEPA as they are entering or may enter the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Considering all the information presented in this draft assessment, it is proposed to conclude that norlimbanol and amberlyn do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore proposed to conclude that cade oil, jonquil oil, Verbena officinalis extract, Ginkgo biloba extract, myrrh oil, cork tree extract, sage oil, wormwood oil, IBCH, sandal cyclohexanol, BCH, and sandela meet one or more of the criteria set out in section 64 of CEPA. It is also proposed that norlimbanol and amberlyn do not meet any of the criteria set out in section 64 of CEPA.
1. Introduction
Pursuant to section 68 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999), the Minister of the Environment and the Minister of Health have conducted an assessment on 14 of 76 substances, referred to collectively under the Chemicals Management Plan (CMP) as the Terpenes and Terpenoids Group, to determine whether these 14 substances present or may present a risk to the environment or to human health. These 14 substances, referred to collectively as the Fourteen Terpene and Terpenoid Substances Group, were identified as priorities for assessment as they met categorization criteria or were prioritized through other mechanisms (ECCC, HC [modified 2017]).
Of the other substances in the Terpenes and Terpenoids Group, 46 have been assessed in terms of risk to ecological and human health, and the decisions for these substances are provided in separate reportsFootnote 2 . Decisions on the remaining substances will be communicated in separate assessments.
The ecological risks of the substances in the Fourteen Terpene and Terpenoid Substances Group were characterized using the ecological risk classification (ERC) of organic substances approach (ECCC 2016a). The ERC describes the hazard of a substance using key metrics, including mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity, and considers the possible exposure of organisms in the aquatic and terrestrial environments on the basis of such factors as potential emission rates, overall persistence, and long-range transport potential in air. The various lines of evidence are combined to identify substances as warranting further evaluation of their potential to cause harm to the environment or as having a low likelihood of causing harm to the environment.
Some substances in the Fourteen Terpene and Terpenoid Substances Group or their analogues have been reviewed by the United States Environmental Protection Agency (US EPA), the European Chemicals Agency (ECHA), the European Food Safety Authority (EFSA), the Joint Food and Agriculture (FAO)/World Health Organization (WHO) Expert Committee on Food Additives (JECFA), the Scientific Committee on Consumer Safety (SCCS), or the WHO. Where applicable, reviews conducted by these institutions are used to inform the human health effects characterization in this assessment.
This draft assessment includes consideration of information on chemical properties, environmental fate, hazards, uses, and exposures, including additional information submitted by stakeholders. Targeted literature searches and relevant data were identified up to September 2022. Empirical data from key studies as well as some results from models were used to reach proposed conclusions.
This draft assessment was prepared by staff in the CEPA Risk Assessment Program at Health Canada (HC) and Environment and Climate Change Canada (ECCC) and incorporates input from other programs within these departments. The human health portion of this assessment has undergone external peer review and/or consultation. Comments on the technical portions relevant to human health were received from Jennifer Flippin, Theresa Lopez, and Joan Garey, all affiliates of Tetra Tech. The ecological portion of this assessment is based on the ERC document (published July 30, 2016), which was subject to an external review as well as a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of this draft assessment remain the responsibility of HC and ECCC.
Assessments focus on information critical to determining whether substances meet the criteria as set out in section 64 of CEPA by considering scientific information, including information, if available, on subpopulations who may have greater susceptibility or greater exposure, vulnerable environments and cumulative effectsFootnote 3 , and by incorporating a weight-of-evidence approach and precautionFootnote 4 . This draft assessment presents the critical information and considerations on which the proposed conclusions are based.
2. Identity of terpene and terpenoid substances
The Chemical Abstracts Service Registry Numbers (CAS RNs) and Domestic Substances List (DSL) names for the discrete substances and representative substances for Unknown or Variable composition, Complex reaction products, or Biological materials (UVCBs) in the Fourteen Terpene and Terpenoid Substances Group used to inform the human health assessments are presented in section 2.1 of this assessment. These materials are derived from natural sources or complex reactions. A UVCB is not an intentional mixture of discrete substances and is considered a single substance. The complexity and variability of their compositions can make them difficult to fully and consistently characterize.
Terpenes are simple hydrocarbons consisting of repeating 5-carbon isoprene units (Figure 2-1). Terpenoids are a modified class of terpenes with different functional groups and an oxidized methyl group moved or removed at various positions. Both terpenes and terpenoids are classified according to the number of isoprene units they contain (Caputi and Aprea 2011; Perveen 2018). Monoterpenes contain 2 isoprene units. The prefixes di-, tri-, and tetra- refer to 2, 3, and 4 monoterpene units, respectively. Furthermore, sesquiterpenes and sesterpenes contain 3 and 5 isoprene units, respectively.
Figure 2.1 Isoprene unit

Long description
Figure of structural formula (left) and skeletal formula (right) of an isoprene unit or 2-methyl-1,3-butadiene, with SMILES notation: [C(C=C)(=C)C]
These substances are the components of essential oils found in a wide variety of plants. Essential oils are mixtures of volatile, organic compounds originating from a single botanical source, and contribute to the flavour and fragrance of a plant. These plant-derived essential oils have many components that can be extracted from different parts of the plant (for example, leaves, seeds, stems, flowers, roots, fruits, woods, barks, grasses, gums, tree blossoms, bulbs, flower buds) (Tisserand and Young 2014). In addition, the concentration of these major components can be affected by different factors such as the origin of the plant, species, temperature, soil, and geography, and essential oils extracted from plants of the same genus and species can be chemically different even if their origin is the same.
2.1 Substance identity
Cade oil
The CAS RN, DSL name, and common names for cade oil are presented in Table 2‑1.
| CAS RN | DSL name (common names) |
Chemical structure and molecular formula |
|---|---|---|
| 8013-10-3 | Oils, cade (juniper tar oil, juniper tar, cade oil rectified) | UVCB Unspecified formula |
Abbreviation: UVCB; unknown or variable composition, complex reaction products, or biological materials
Cade oil is a UVCB and is the volatile oil extracted from the wood and branches of Juniperus oxycedrus following destructive distillation with high heat (Wenninger and McEwen 1997; CIR 2001; Salido et al. 2002; Koruk et al. 2005; Loizzo et al. 2007; Barnes and Greive 2017). Juniperus oxycedrus is a shrub or small tree (up to 8 meters in height) that is commonly found in parts of North America, the Mediterranean and Asia (Saab et al. 2013).
Cade oil may be rectified (that is, further distilled under vacuum to remove components or improve colour and solubility) or unrectified (Burdock 2010).
The main components (that is, components of cade oil that have concentrations generally greater than 10%) reported in the literature include: cadinene (14.5% to 19%; including delta, gamma, and alpha isomers), widdrol (11% to 22%), epi-cubenol (8.2% to 13%), τ-muurolol (≤12%), thujopsene (9.2% to 22%), and alpha-cedrene (≤15%) (Barrero et al. 1993; Uçar and Balaban 2002; Loizzo et al. 2007; Saab et al. 2013; Skalli et al. 2014; Tisserand and Young 2014).
Other components of cade oil are formed during the extraction process and the main ones are phenols (Burdock 2010; Barnes and Greive 2017). The concentration of these substances varies considerably; according to Skalli et al. (2014) and Al-Snafi (2018), the phenols content can range between 17% and 26%, composed largely of guaiacol (12%) and cresols. A review of cade oil by the Cosmetic Ingredient Review (CIR) Expert Panel also mentions guaiacol and cresol as chief constituents (CIR 2001). On the other hand, Tisserand and Young (2014) report concentrations of <2.5% for cresols and <1.5% for guaiacol in unrectified cade oil. Based on the above studies, the cresol content of cade oil could range from <2.5% to 14%.
In addition, unrectified cade oil is reported to contain polycyclic aromatic hydrocarbons (PAHs). Compositional data on the type and quantity of PAHs are limited. One of the PAHs reported to be present in unrectified cade oil is benzo[a]pyrene, which was detected in concentrations up to 8 ppm (0.0008%). The content of benzo[a]pyrene in rectified oil has been reported to be much lower (20 ppb) (Tisserand and Young 2014).
Jonquil oil
The CAS RN, DSL name, common names, and major components for jonquil oil are presented in Table 2‑2.
| CAS RN | DSL name (common names)a |
Representative chemical names, structures, and their range of potential concentrations of main componentsb |
|---|---|---|
| 8023-75-4 | Oils, Jonquil (Jonquil oil, Jonquil absolute, Narcissus absolute) | 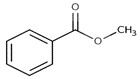 methyl benzoate (23% to 46%) 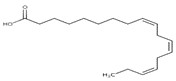 alpha-linolenic acid (19%)  linalool (18%)  alpha-terpineol (0.1% to 23%) 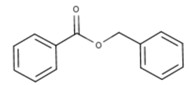 benzyl benzoate (1% to 19%) 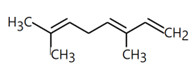 trans-beta-ocimene (26% to 35%) 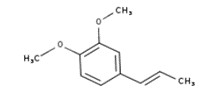 trans-methylisoeugenol (20%) |
a Information extracted from ChemIDplus (1993-).
b Dobson et al. 1997; Hanks 2002; Laboratoire Phytochemia 2017; Tisserand and Young 2014
Jonquil oil is a UVCB with CAS RN 8023-75-4 and is defined as “extractives and their physically modified derivatives derived from Narcissus jonquilla L., Amaryllidaceae” (TSCA definition 2021, as cited in ChemIDplus 1993-). The major components (that is, components with concentrations of generally greater than 10%) of jonquil oil (also known as Jonquil absolute or Narcissus absolute) reported in the literature are presented in Table 2‑2.
Norlimbanol
The CAS RN, DSL name, and common name for Norlimbanol are presented in Table 2‑3.
| CAS RN | DSL name (common name) |
Chemical structure and molecular formula |
|---|---|---|
| 70788-30-6 |
Cyclohexanepropanol, 2,2,6-trimethyl-α-propyl-
(Norlimbanol) |
 C₁₅H₃₀O |
Norlimbanol is a synthetic chemical and is the registered trade name for a diastereomer mixture of racemic alcohols (Margot et al. 2004). At room temperature, the substance is a colourless liquid with a highly diffusive and woody odor. The manufacturing process consists of condensation of citral with 2-pentanone in the presence of bases to produce 8,12-dimethyltrideca-5,7,11-trien-4- one, which is then cyclized and hydrogenated (Chemical Book 2019).
Verbena officinalis extract
The CAS RN, DSL name and common names for Verbena officinalis extract are presented in Table 2‑4.
| CAS RN | DSL name (common names) |
Chemical structure and molecular formula |
|---|---|---|
| 84961-67-1 | Verbena officinalis, ext. (Verbena officinalis extracta, common vervainb,Verbena officinalis flower extractc, Verbena officinalis flower waterc, Verbena officinalis flower/leaf extractc, Verbena officinalis flower/leaf waterc, Verbena officinalis leaf absolutec, Verbena officinalis leaf extractc, Verbena officinalis leaf rectified oilc, and Verbena officinalis leaf/stem waterc ) |
UVCBd Unspecified formula |
a Information extracted from ChemID plus (1993-).
b Kubica et al. 2020
c CosIng c2021
d This substance is a UVCB (unknown or variable composition, complex reaction products, or biological materials).
Verbena officinalis extract is a UVCB, and is extracted from the aerial parts and roots of the Verbena officinalis plant, a perennial plant (up to 1 m tall) that is widespread across the globe (Kubica et al. 2020). A number of extraction techniques have been identified in the scientific literature including: hydrodistillation (Rolim de Almeida et al. 2010; deMartino et al. 2011), steam distillation (Chalchat and Garry 1996), and methanol/ethanol extraction (Rehecho et al. 2011; Zhang et al. 2011; Liu et al. 2012).
Phytochemical analysis of Verbena officinalis extract is difficult since there is a large variability in the type of plant material used (roots or leaves), its origin, environmental conditions, chemotype tested, and the extraction method used (Kubica et al. 2020). For example, Verbena officinalis essential oil extracted via hydrodistillation (a traditional method used in oil extraction) has been found to contain mostly citral (~45%) and isobornyl formate (~45%), while the essential oil obtained from steam distillation has been found to contain spathulenol (>10%), and limonene and eucalyptol (7.5% each); conversely, none of these components have been identified in methanol or ethanol extraction (Kubica et al. 2020).
Despite large variations in phytochemical profiles, the biological activity of Verbena officinalis extract is mainly determined by a group of secondary metabolites, that include iridoid glycosides (for example, verbenalin, hastatoside) and phenylpropanoid glycosides (for example, verbascoside) (Kubica et al. 2020).
Ginkgo biloba extract
The CAS RN, DSL names and common names for Ginkgo biloba extract are presented in Table 2‑5.
| CAS RN | DSL name (common names)a |
Chemical structure and molecular formula |
|---|---|---|
| 90045-36-6 | Ginkgo biloba ext. (Ginkgo biloba extract, Extract of Ginkgo, EGb-761, Ginkgo leaf extract, Ginkgo nut extract, and Ginkgo root extract) |
UVCBb Unspecified formula |
a Information extracted from ChemIDplus (1993-)
b This substance is a UVCB (unknown or variable composition, complex reaction products, or biological materials).
Ginkgo biloba extract is a UVCB extracted from the Ginkgo biloba plant, which is a perennial tree that originated in China and now grows all over the world (IARC 2016). Manufacturing of Ginkgo biloba extracts consists mostly of drying and pressing ginkgo leaves, followed by an extraction with a mixture of acetone and water. Two types of leaf extracts may be produced: whole extracts (containing constituents of variable concentrations) or standardized extracts (for example, CAS RNs 90045-36-6, 122933-57-7, 123009-84-7, and 401901-81-3), which are whole extracts further purified to reach a specific content of flavonoids, lactones, and ginkgolic acids. Extract EGb-761 (CAS RN 90045-36-6) is commonly used as a standardized Ginkgo biloba leaf extract; the composition is reportedly not less than 24% flavonoids (that is, flavonol glycosides), not less than 6% total terpene lactones, and not more than 5 ppm of ginkgolic acids (IARC 2016; Mei et al. 2017; Burnett 2018). Flavonoids include quercetin-3β-D-glucoside, quercitrin, rutin, quercetin, kaempferol, and isorhamnetin, while terpene lactones include ginkgolide A, B, and C as well as bilobalide (IARC 2016). Products with Ginkgo biloba leaf extracts may contain either type of extract. An analysis of Ginkgo biloba leaf extracts in the United States (US) showed variations of 24% to 36% for flavonol glycosides and 4% to 11% for terpene lactones, with a wide range (<500 – 90 000 ppm) of concentrations for ginkgolic acids (Mei et al. 2017). Similarly, large variations were found in more regulated products involving Ginkgo biloba extracts, such as pharmaceutical products or dietary supplements (Mei et al. 2017). In addition, the flavonoid quercetin has been reported to constitute up to 16.7% of Ginkgo biloba extract (Burnett 2018).
While the majority of Ginkgo biloba extracts originate from the leaves, other extracts (that is, Ginkgo biloba nut and root extract) have been associated with CAS RN 90045-36-6 in the literature (Burnett 2018). Compositional data on seed or nut extract are limited. Data suggest that Ginkgo biloba nut (that is, seed) extracts have a similar composition as the leaf extract, with the exception of 4’-O-methylpyridoxine (ginkgotoxin), which may be present in higher concentrations in the seed extract (Arenz et al. 1996; Mei et al. 2017). The limited data available indicate that ginkgotoxin would be present at <1% in the leaf or seed extract (Leistner and Drewke 2010; Lim and Kim 2018).
Amberlyn
Amberlyn is a naturally occurring terpenoid present in ambergris, a waxy like substance produced in the gut of sperm whales (Panten et al. 2014). The substance can also be obtained from the oxidation of sclareolide, which is obtained from sclareol, a natural substance found in clary sage oil (Panten et al. 2014). Amberlyn represents a discrete substance also known as dodecahydro-3a,6,6,9a-tetramethylnaphtho[2,1-b]furan (ChemID 1993-; ECHA 2021a). The CAS RN, DSL name, and common name are presented in Table 2‑6.
| CAS RN | DSL name (common name) |
Chemical structure and molecular formula |
|---|---|---|
| 3738-00-9 | Naphtho[2,1-b]furan, dodecahydro-3a,6,6,9a-tetramethyl- (Amberlyn) |
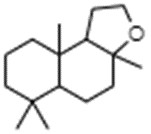 C16H28O |
Myrrh oil
The CAS RN, DSL name, common name, and major components of myrrh oil are presented in Table 2‑7.
| CAS RN | DSL name (common name) |
Representative chemical names, structures, and their range of concentrations for the main componentsa |
|---|---|---|
| 8016-37-3 | Oils, myrrh (Myrrh oil) |
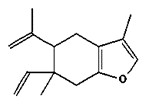 Curzerene (up to 40.1%) 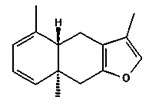 Furanoeudesma-1,3-diene (up to 38.6%) 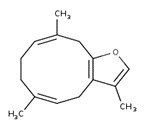 Furanodiene (up to 20%)  Lindestrene (up to 12%) |
a Qureshi et al. 1993; Massoud et al. 2000; Hanuš et al. 2005; EMA 2011; Tisserand and Young 2014; Mahboubi and Kashani 2016
“Myrrh” is a UVCB and is the oleo-gum resin obtained from the stem the Commiphora species, and contains up to 17% volatile oils, 40% resins, and 60% gum (Qureshi et al. 1993; Massoud et al. 2000; El Ashry et al. 2003). “Myrrh oil” (CAS RN 8016-37-3) is a UVCB defined as the “extractives and their physically modified derivatives from Commiphora, Burseraceae” (TSCA definition 2019 as cited in ChemIDplus 1993-). True myrrh oil is obtained from the species Commiphora myrrha, which is synonymous with Commiphora molmol, the main components of which are listed in Table 2‑7. However, there are over 150 species of Commiphora, including Commiphora africana, Commiphora erythraea, Commiphora holtziana, Commiphora kataf, Commiphora mukul, etc., which have chemical compositions that differ from that of Commiphora myrrha (Hanuš et al. 2005). Due to the broad definition of myrrh oil (encompassing the Commiphora genus as a whole), data pertinent to any Commiphora species were taken into consideration in this assessment.
Cork tree extract
The CAS RN, DSL name, common name, and a major component of cork tree extract are presented in Table 2‑8.
| CAS RN | DSL name (common name) |
Representative chemical name, structure, and the range of concentration for the main component |
|---|---|---|
| 164288-52-2 | Cork tree, Phellodendron amurense, ext. (Cork tree extract) |
![Representative chemical structure of
berberine, with SMILES notation:
COc1ccc2cc3c4cc5OCOc5cc4CC[n+]3cc2c1OC](/content/dam/eccc/images/pded/terpenes-group-5/DAR-Table2.8.jpg) Berberine (0.6% to 33%) |
Cork tree extract (CAS RN 164288-52-2) is a UVCB and is defined as “an extract of the powdered bark of the phellodendron, Phellodendron amurense, Rutaceae” (Termium Plus [modified 2022]). With respect to composition, the bark of Phellodendron amurense contains different classes of compounds, including isoquinoline alkaloids, limonoid triterpenes, chlorogenic acids, lignans, flavonoids, glycosides, and monosaccharides (Sun et al. 2019). However, quantitative data regarding the concentration of components in cork tree extract were limited. The only major component (equal to or greater than 10%) identified in the literature was berberine, with a concentration ranging from 0.6% to 33% in cork tree extract (Liu et al. 1993; WHO 2009; James et al. 2011; Xian et al. 2014; Akihisa et al. 2017; Fujii et al. 2017; Kamimura et al. 2019). The abundance of berberine varies depending on the geographical location of harvest, the season of harvest, and the extraction solvents and methods used for its preparation.
Subgroup 1:
Sage oil
The CAS RN, DSL name, common name, and major components of sage oil are presented in Table 2‑9.
| CAS RN | DSL name (common name) |
Representative chemical names, structures, and their range of concentrations for the main componentsa |
|---|---|---|
| 8022-56-8 | Oils, sage (Sage oil) |
![Representative chemical structure of
eucalyptol, with SMILES notation:
C[C@@]12CC[C@@H](CC1)C(C)(C)O2](/content/dam/eccc/images/pded/terpenes-group-5/DAR-Table2.9a.jpg) Eucalyptol (1% to 67.1%) ![Representative chemical structure of
camphor, with SMILES notation:
CC1(C)[C@@H]2CC[C@@]1(C)C(=O)C2](/content/dam/eccc/images/pded/terpenes-group-5/DAR-Table2.9b.jpg) Camphor (5% to 59.03%) ![Representative chemical structure of
thujone, with SMILES notation:
CC(C)[C@@]12C[C@@H]1[C@@H](C)C(=O)C2](/content/dam/eccc/images/pded/terpenes-group-5/DAR-Table2.9c.jpg) Thujone (4% to 56.5%) ![Representative chemical structure of
borneol, with SMILES notation:
CC1(C)[C@@H]2CC[C@@]1(C)[C@@H](O)C2](/content/dam/eccc/images/pded/terpenes-group-5/DAR-Table2.9d.jpg) Borneol (5% to 25%) (CC[C@H]1C(=C)CC[C@H]2C(C)(C)CCC[C@]12C)C=C](/content/dam/eccc/images/pded/terpenes-group-5/DAR-Table2.9e.jpg) Manool (12% to 21%) |
a Raal et al. 2007; Porte et al. 2013; Tisserand and Young 2014; Diab et al. 2018; Jakovljević et al. 2019
Sage oil is a UVCB and is an essential oil obtained from the Salvia officinalis plant through hydrodistillation of the aerial parts (for example, leaves and flowers) (Raj 2021). Both sage oil (derived from Salvia officinalis) and Salvia officinalis (sage) leaf oil correspond to CAS RN 8022-56-8, as well as a generic CAS RN 84082-79-1 (Raj 2021). Sage oil (from Salvia officinalis) is comprised of the major components listed in Table 2‑9.
Various other extracts derived from the Salvia officinalis plant are also associated with CAS RN 84082-79-1. These include Salvia officinalis (sage) extract, Salvia officinalis (sage) flower/leaf/stem extract, Salvia officinalis (sage) flower/leaf/stem juice, Salvia officinalis (sage) flower/leaf/stem water, Salvia officinalis (sage) leaf, Salvia officinalis (sage) leaf extract, Salvia officinalis (sage) leaf powder, Salvia officinalis (sage) leaf water, Salvia officinalis (sage) root extract, and Salvia officinalis (sage) water (Raj 2021). These extracts from Salvia officinalis are therefore considered in the definition of sage oil (Salvia officinalis) for this assessment. In a review examining the composition of different Salvia officinalis preparations, it was found that the major components and their concentrations were similar regardless of extraction method (for example, hydrodistillation, ethanolic extraction, supercritical fluid extraction) and plant part used (for example, leaves, flowers, fresh plant) (Jakovljević et al. 2019). These extracts are therefore expected to exhibit compositions that are comparable to sage oil.
Wormwood oil
The CAS RN, DSL name, common name, and major components of wormwood oil are presented in Table 2‑10.
| CAS RN | DSL name (common name) |
Representative chemical names, structures, and their range of concentrations for the main componentsa |
|---|---|---|
| 8008-93-3 | Oils, wormwood (Wormwood oil) |  Sabinyl acetate (4% to 84%) ![Representative chemical structure of
thujone, with SMILES notation:
CC(C)[C@@]12C[C@@H]1[C@@H](C)C(=O)C2](/content/dam/eccc/images/pded/terpenes-group-5/DAR-Table2.10b.jpg) β-Thujone (10% to 65%) 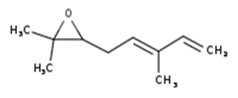 Epoxyocimene (6% to 54%) 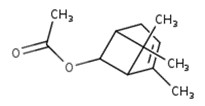 Chrysanthenyl acetate (10% to 42%)  Chamazulene (18% to 39%) ![Representative chemical structure of
b-pinene, with SMILES notation:
CC1(C)[C@H]2CCC(=C)[C@@H]1C2](/content/dam/eccc/images/pded/terpenes-group-5/DAR-Table2.10f.jpg) β-Pinene (5% to 32%) ![Representative chemical structure of
sabinene, with SMILES notation:
CC(C)[C@]12CCC(=C)[C@H]1C2](/content/dam/eccc/images/pded/terpenes-group-5/DAR-Table2.10g.jpg) Sabinene (3% to 24%) 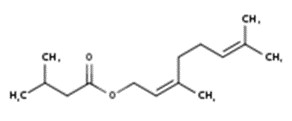 Neryl isovalerate (20%) 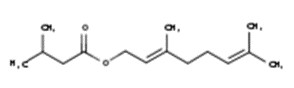 Geranyl isovalerate (16%) ![Representative chemical structure of
eucalyptol, with SMILES notation:
C[C@@]12CC[C@@H](CC1)C(C)(C)O2](/content/dam/eccc/images/pded/terpenes-group-5/DAR-Table2.10j.jpg) Eucalyptol (5% to 16%) 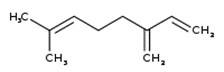 β-Myrcene (5% to 11%) |
a Chialva et al. 1983; Chiasson et al. 2001; Juteau et al. 2003; Kordali et al. 2005; Lopes-Lutz et al. 2008; Judzentiene et al. 2009; Mihajilov-Krstev et al. 2014; Monzote et al. 2014; Tisserand and Young 2014; Stanković et al. 2016; Hodaj-Çeliku et al. 2017; Mathlouthi et al. 2021
Wormwood oil is a UVCB substance derived from the plant species Artemisia absinthium. It can be obtained by the extraction of the whole plant, leaves, or flowering tops of the plant (CosIng c2021; EMA 2021). The Toxic Substances Control Act (TSCA) defines CAS RN 8008-93-3 (associated with wormwood oil) as the “extractives and their physically modified derivatives from Artemisia absinthium, Compositae” (TSCA definition 2021, as cited in ChemID 1993-). Wormwood oil has also been associated with the CAS RN 84929-19-1, which has been used for Artemisia absinthium extract, Artemisia absinthium herb extract, Artemisia absinthium oil, and Artemisia absinthium herb oil (CosIng c2021).
With respect to composition, major components of wormwood oil (that is, components of wormwood oil that have concentrations generally greater than 10%) are presented in Table 2‑10. Wormwood oil can also be classified into different chemotypes depending on the dominant component(s) in the oil (Tisserand and Young 2014). For example, it can be considered to be representative of a β-thujone chemotype, (Z)-epoxy-ocimene chemotype, or sabinene acetate chemotype. There are also “mixed” chemotypes, which describe oils with multiple dominant components (for example, β-thujone + sabinyl acetate chemotype) (Chialva et al. 1983). Although data providing information on the composition of different types of wormwood extracts were limited, the component thujone is present in several types including aqueous and ethanolic extracts (Tegtmeier and Harnischfeger 1994, as cited in EMA 2016). Due to the broad TSCA definition for CAS RN 8008-93-3, which encompasses any extract of Artemisia absinthium, data pertinent to any preparation of Artemisia absinthium were taken into consideration in this assessment.
Sage oil and wormwood oil are assessed together as a subgroup of substances (that is, subgroup 1) given that the health effects datasets for both substances were informed by thujone, a common component for both substances.
Subgroup 2:
IBCH, sandal cyclohexanol, BCH, and sandela
IBCH represents a discrete substance also known as 3-(5,5,6-trimethylbicyclo[2.2.1]hept-2-yl)cyclohexan-1-ol (ChemID 1993-; ECHA 2021b).
Sandal cyclohexanol represents a discrete substance also known as 4-(5,5,6-trimethylbicyclo(2.2.1)hept-2-yl)cyclohexan-1-ol (ECHA 2021c; ChemIDplus 1993-). It represents a structural isomer of IBCH, differing only in the position of the hydroxyl functional group on the cyclohexane ring (that is, meta- position for IBCH and para- position for sandal cyclohexanol).
BCH represents a discrete substance also known as 4-(1,6,6-trimethyl-2-bicyclo[3.1.1]heptanyl)cyclohexan-1-ol (PubChem c2022d). It represents a constitutional isomer of IBCH, differing in the position of the hydroxyl group on the cyclohexane ring (that is, para position for BCH and meta position for IBCH), as well as the position of the bicyclic bridge (for example, [2.2.1] for IBCH and [3.1.1] for BCH]) on the cyclohexane ring. Therefore, BCH is also an isomer of sandal cyclohexanol, differing only in the positioning of the bicyclic bridge.
Sandela represents a UVCB substance (ChemID 1993-; ECHA 2021d). The DSL name for sandela is phenol, 2-methoxy-, reaction products with 2,2-dimethyl-3-methylenebicyclo[2.2.1]heptane, hydrogenated, which suggests that the substance may be a mixture of reaction products, including isomers.
Data available from a product patent application in Europe, as well as data from online retailers, indicate that representative components of sandela can include 3-(5,5,6-trimethylbicyclo[2.2.1]hept-2-yl)cyclohexan-1-ol, which corresponds to IBCH (European Patent Office 2002). Online searches for synonyms and other identifiers of sandela have indicated that the substance may be synonymous with other cyclohexanols in Table 2‑11. These terms include meta-(iso-camphyl-5)-cyclohexanol, sandenol, m-iso camphylcyclohexanol, CAS RN 66068-84-6 (sandal cyclohexanol), 3-Isocamphylcyclohexanol, and isobornyl cyclohexanol (CAS RN 3407-42-9) (PerfumersWorld [accessed 2022]; Scentree [accessed 2022]).
The substance identities for IBCH, sandal cyclohexanol, BCH, and sandela are presented in Table 2‑11.
| - | IBCH | Sandal cyclohexanol | BCH | Sandela (UVCB) |
|---|---|---|---|---|
| CAS RN | 3407-42-9 | 66068-84-6 | 68877-29-2 | 70955-71-4 |
| Chemical formula | C16H28O | C16H28O | C16H28O | N/A |
| Chemical structure | 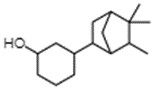 |
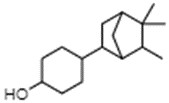 |
 |
Reaction products of the following: phenol, 2-methoxy 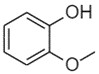 2,2 dimethyl-3-methylenebicyclo[2.2.1]heptane ![Representative chemical structure of
2,2 dimethyl-3-methylenebicyclo[2.2.1]heptane with SMILES notation: CC1(C2CCC(C2)C1=C)C](/content/dam/eccc/images/pded/terpenes-group-5/DAR-Table2.11e.jpg)
|
| Synonyms | Isocamphyl cyclohexanol;a Sandenol;a Sandal hexanol;b Sandal cyclohexanolc |
Sandela (Givaudan);d Sandenol;d Isocamphyl cyclohexanol, mixed isomerse |
Camphylcyclohexanol;f Isobornyl cyclohexanolg,h,i,j |
meta-(iso-Camphyl-5)-cyclohexanol; sandenol;k m-iso camphylcyclohexanol; 3-Isocamphylcyclohexanol;k Isobornyl cyclohexanoll |
Abbreviation: N/A, not applicable.
a Pubchem c2022e
b St-Gelais 2015
c GoodScents 2022a
d GoodScents 2022b
e Consumer Products 2022
f PCPC c2022
g Reincke-Fichtner [accessed 2022]
h Penta Manufacturing [accessed 2022]
i Ventos [accessed 2022]
j Parchem [accessed 2022]
k PerfumersWorld [accessed 2022]
l Scentree [accessed 2022]
Due to their structural similarities and the use of their common names as synonyms in the literature, IBCH, sandal cyclohexanol, BCH, and sandela were assessed together as a subgroup of substances (that is, subgroup 2).
3. Physical and chemical properties
A summary of physical and chemical property data of the substances in the Fourteen Terpene and Terpenoid Substances Group are presented in Table 3-1. When experimental information was limited or not available for a property, data from analogues were used; properties for the analogue substances are presented in Appendix B. Additional physical and chemical properties are reported in ECCC (2016b).
| Substance name | Representative constituent(s) (CAS RN) and percentage composition | MW (g/mol) |
WS (mg/L) |
VP (Pa) |
Log Kow |
|---|---|---|---|---|---|
| Cade oila | Cresols (1319-77-3) (2.5% to 14%) |
108.14 | 25900 | 23 | 1.95 |
| Cade oila | Thujopsene (470-40-6) (9.2% to 22%) |
204.35 | 0.07 | 9.3 | 6.12 |
| Cade oila | Alpha-Cedrene (11028-42-5) (up to 15%) |
204.35 | 0.15 | 0.02 | 5.74 |
| Jonquil oila,b | Methyl benzoate (23% to 46%) |
136.14 | 2100 | 50.66 | 2.12 |
| Jonquil oila,b | Trans-beta-ocimene (26% to 35%) |
136.23 | 2.012 | 358 | 4.80 |
| Jonquil oila,b | Trans-methylisoeugenol (up to 20%) |
178.23 | 169.1 | 1.2 | 2.95 |
| Jonquil oila,b | Alpha-linolenic acid (up to 19%) |
278.43 | 0.1236 | 0.00166 | 6.46 |
| Jonquil oila,b | Linalool (up to 18%) |
154.25 | 1590 | 21.33 | 2.97 |
| Jonquil oila,b | Alpha-terpineol (0.1% to 23%) |
154.25 | 371.7 | 2.62 | 3.28 |
| Jonquil oila,b | Benzyl benzoate (1% to 19%) |
212.24 | 15.39 | 0.0741 | 3.97 |
| Norlimbanolc | N/A | 226.4 | 1.15 | 0.013 | 5.8 |
| Verbena officinalis extractd,e | Citral (5392-40-5) (up to 45%) |
152.23 | 84.71 | 11.9 Pa | 3.45 |
| Ginkgo biloba extractb | Quercetin (117-39-5) (up to 16.7%) |
302.34 | 60 | 3.75 × 10-12 | 1.48 |
| Amberlyn | N/A | 236.4 | 2.44 | 0.4 | 4.76 |
| Myrrh oila,e | Curzerene (17910-09-7) (up to 40.1%) |
216.3 | 0.2 | 0.66 | 6.1 |
| Myrrh oila,e | Furanoeudesma-1,3-diene (87605-93-4) (up to 38.6%) |
214.3 | 2.8 | 0.18 | 4.8 |
| Myrrh oila,e | Furanodiene (19912-61-9) (up to 20%) |
216.3 | 0.1 | 0.08 | 6.5 |
| Myrrh oila,e | Lindestrene (2221-88-7) (up to 12%) |
214.3 | 0.7 | 0.22 | 5.5 |
| Cork tree extracta | N/A | 337.38 | 0.002-9.6 | 6.5 × 10-7 | 2.08 |
| Sage oil,a wormwood oila | α/β – thujone (α: 546-80-5 β: 471-15-8 α/β: 1125-12-8) (up to 57% in sage oil; up to 65% in wormwood oil) |
152 | 408 | 54.9 | 2.65 |
| IBCHa | N/A | 236 | 1.82 | 1.62 × 10-3 | 5.5 |
| Sandal cyclohexanola | N/A | 236 | 1.82 | 1.62 × 10-3 | 5.5 |
| BCHa | N/A | 236a | 1.7-4.7 | 0.002 | 5.5 |
| Sandelaa,f | N/A | N/A (UVCB) | 1.4-3.4 | 0.004 | 5.5 |
Abbreviations: N/A, not applicable; MW, molecular weight; WS, water solubility; VP, vapour pressure
a EPI Suite (c2000-2012)
b ChemIDplus 1993-
c Research Institute for Fragrance Materials [RIFM] (2021a)
d PubChem 2022c
e ChemSpider c2015
f ECHA 2021d
4. Sources and uses
All of the substances in the Fourteen Terpene and Terpenoid Substances Group were included in a survey pursuant to section 71 of CEPA (Canada 2012). With the exception of amberlyn, none of the substances in this group were reported to be manufactured or imported into Canada in quantities greater than the reporting threshold of 100 kg during the 2011 reporting year (Environment Canada 2013). Amberlyn was reported to be imported in quantities ranging from 100 kg to 1,000 kg (Environment Canada 2013).Footnote 5
Table 4‑1 summarizes some uses of substances in the Fourteen Terpene and Terpenoid Substances Group in foods, natural health products (NHPs), non-prescription drugs (NPDs), cosmetics, or PCPs in Canada.
| Substance | Food additive, incidental additive, or FP materialsa,b | MI or NMI in disinfectant, human or veterinary drug productsc | MI or NMI in licensed NHPsd | Notified to be present in cosmetics under the Cosmetic Regulationse | AI or formulant in registered PCPsf |
|---|---|---|---|---|---|
| Cade oil | N | Y (NMI) |
N | Y | Y (formulant) |
| Jonquil oil | N | N | N | N | N |
| Norlimbanol | N | N | N | N | Y (formulant) |
| Verbena officinalis extract | N | N | Y (MI and NMI) |
Y | N |
| Ginkgo biloba extract | N | Y (MI and NMI) |
Y (MI and NMI) |
Y | N |
| Amberlyn | N | N | N | N | Y (formulant) |
| Myrrh oil | N | N | Y (MI and NMI) |
Y | Y (formulant) |
| Cork tree extract | N | N | Y (MI and NMI) |
Y | N |
| Sage oil | N | Y (NMI) |
Y (MI and NMI) |
Y | Y (formulant) |
| Wormwood oil | N | Y (NMI) |
Y (MI and NMI) |
Y | Y (formulant) |
| IBCH | N | N | N | N | Y (formulant) |
| Sandal cyclohexanol | N | N | N | N | Y (formulant) |
| BCH | N | N | N | N | Y (formulant) |
| Sandela | N | N | N | N | Y (formulant) |
Abbreviations: Y, yes, this use was reported for this substance; N, no this use was not reported for this substance; FP, food packaging; MI, medicinal ingredient; NMI, non-medicinal ingredient; AI, active ingredient; PCPs, pest control products
a Personal communication, emails from the Food Directorate (FD), HC, to the Existing Substances Risk Assessment Bureau (ESRAB), HC, dated June 2021; unreferenced.
b While not defined under the Food and Drugs Act (F&DA), incidental additives may be regarded, for administrative purposes, as those substances which are used in food processing plants and which may potentially become adventitious residues in foods (for example, cleaners, sanitizers).
c Personal communication, emails from the Therapeutic Products Directorate (TPD), HC, to the ESRAB, HC, dated June 2021; unreferenced.
d LNHPD 2021; personal communication, emails from the Natural and Non-prescription Health Products Directorate (NNHPD), HC to the ESRAB, HC, dated June 2021; unreferenced
e Personal communication, emails from the Consumer and Hazardous Product Safety Directorate (CHPSD), HC, to the ESRAB, HC, dated June 2021; unreferenced
f Personal communication, emails from the Pest Management Regulatory Agency (PMRA), HC, to the ESRAB, HC, dated June 2021; unreferenced
Do-It-Yourself (DIY) products
Certain substances within the Fourteen Terpene and Terpenoid Substances Group that have aromatic properties are currently available on the Canadian market (as “essential oils”) at a concentration of up to 100%. It is therefore possible that these undiluted substances are purchased and used by consumers to make do-it-yourself (DIY) products. DIY products that may result in high consumer exposures include aromatic diffuser (also known by the popular name of aromatherapy by consumers), massage oil, bath product, body moisturizer, and facial steamer. As a result, uses of undiluted substances in DIY products are evaluated in this assessment. Parameters for estimating dermal and inhalation exposures to DIY products are available in Appendix A.
Additional details and uses of substances in this group are discussed below.
Cade oil
Cade oil has been used to treat chronic eczema and other skin conditions and in traditional medicine as an analgesic and for stomach disorders, because of its alleged anti-microbial and anti-inflammatory properties (Koruk 2005; Achour 2011; Bouhlal et al. 1988, Fernandez et al. 1996 as cited in Salido et al. 2002). The substance has also been reported to be used in aromatherapy (Skalli et al. 2014). The rectified (that is, purified) form of cade oil is reported as a fragrance component in cosmetics and other products available to consumers (Leung and Foster 1996 as cited in Salido et al. 2002). Rectified and unrectified cade oil are listed as fragrance ingredients used in consumer goods by the International Fragrance Association (IFRA 2021). IFRA recommendations indicate that only rectified cade oils should be used in cosmetics. in compliance with the maximum limit for polynuclear aromatic hydrocarbons (PAH) of 1 ppb (IFRA 2013).
Juniperus oxycedrus and its preparations are listed in the Natural Health Products Ingredients Database (NHPID) (NHPID 2022). Juniperus oxycedrus is listed in the NHPID with a medicinal role as classified as a NHP substance falling under Schedule 1, item 1 (a plant or a plant material) of the Natural Health Products Regulations (NHPR). Juniper tar is listed in the NHPID with a medicinal role as classified as an NHP substance falling under Schedule 1, item 2 (an extract) of the NHPR. Juniperus oxycedrus wood essential oil is listed in the NHPID with a non-medicinal role for topical use only as a fragrance ingredient, where only rectified (purified) oils may be used, and the finished product may not contain more than 1 ppb of PAHs. These substances are not found to be listed as being present in NHPs in the Licensed Natural Health Products Database (LNHPD) (LNHPD 2021; personal communication, emails from the Natural and Non-prescription Health Products Directorate [NNHPD], HC, to the Existing Substances Risk Assessment Bureau [ESRAB], HC, dated June 2021; unreferenced).
On the basis of data submitted under the Cosmetic Regulations, cade oil was reported as an ingredient in a limited number of cosmetics including bath products, cleansers, skin care products, hair care products, and fragrance products (personal communication, emails from the Consumer and Hazardous Product Safety Directorate [CHPSD], HC, to the ESRAB, HC, dated June 2021; unreferenced). The substance is also present as a non-medicinal ingredient (NMI) in a NPD formulated as a medicated soap, according to data obtained from HC’s Drug Product Database (DPD) (personal communication, emails from the Therapeutic Products Directorate [TPD], HC, to the ESRAB, HC, dated June 2021; unreferenced).
Cade oil (and other substances in this assessment) is available to consumers as a pure essential oil (that is, present in concentrations of up to 100%). It is possible that these undiluted substances are purchased and used by consumers in Do-It-Yourself (DIY) applications as discussed above. Additional uses of cade oil include treatment of skin conditions such as psoriasis or eczema (Base Formula [accessed 2021]).
No definitive information is available concerning the potential use of cade oil as a food flavouring agent in Canada; however, since the substance is present as a flavouring agent internationally, it is possible that it may be present as a flavouring agent in foods sold in Canada (personal communication, email from the Food Directorate [FD], HC, to the ESRAB, HC, dated June 2021; unreferenced). In the US, annual consumption of cade oil from use in foods was reported to be less than 1 lb (<0.45 kg) (Burdock 2010).
Jonquil oil
Jonquil oil is a naturally occurring substance obtained from various Narcissus spp. of flowers and is primarily used in perfumery as a fragrance ingredient (Poucher 1993). On the basis of information submitted as a follow-up to a survey issued pursuant to section 71 of CEPA (Canada 2012), the substance is reported to be used in a manual dish detergent at a low concentration (<0.00001%) (Environment Canada 2013). In Europe, jonquil oil is listed as a fragrance ingredient used in consumer goods by the IFRA (2021); however, information on types of products that could contain this substance and concentration levels were not identified. Jonquil oil is available to consumers in Canada as a pure essential oil (that is, present at concentrations up to 100%), which could be used in a variety of DIY applications, similarly to other essential oils discussed in this assessment (for example, aromatic diffusers, facial steamers, bath oils, massage oil preparations, body moisturizer preparations).
Norlimbanol
Norlimbanol does not occur naturally in the environment. On the basis of information submitted as a follow-up to a survey issued pursuant to section 71 of CEPA (Canada 2012), norlimbanol is used in a limited number of cosmetics, laundry and dishwashing products, cleaning products, and air care products in Canada (Environment Canada 2013). Available information from the public literature on norlimbanol suggests that the substance is mainly used as a fragrance ingredient for its wood-like scent; it is also listed by the IFRA as a fragrance ingredient used in products available to consumers (RIFM 2021a). In Europe, norlimbanol is reported as being used in cosmetics for its perfuming function (CosIng c2021). As norlimbanol is primarily used as a component in fragrance mixtures, its presence in cosmetic notifications in Canada may not be explicitly stated but may be captured under the general terms for these mixtures used in cosmetics, which are “fragrance” and “parfum” (Canada 2009). This substance was also identified as an ingredient in a spray cologne (SDS 2017).
Verbena officinalis extract
Verbena officinalis has been used in both traditional medicine and modern phytotherapy for the treatment of a number of ailments (Kubica et al. 2020). Verbena officinalis extract is obtained from the aerial parts and roots of the Verbena officinalis plant, which is also known as common vervain (Kubica et al. 2020; CosIng c2021). Other Verbena officinalis-derived extracts are sourced from other parts of the plant, such as flowers, stems, and leaves (CosIng c2021). The following Verbena officinalis-derived extracts are associated with CAS RN 84961-67-1: Verbena officinalis flower extract, Verbena officinalis flower water, Verbena officinalis flower/leaf extract, Verbena officinalis flower/leaf water, Verbena officinalis leaf absolute, Verbena officinalis leaf extract, Verbena officinalis leaf rectified oil, and Verbena officinalis leaf/stem water (CosIng c2021). These extracts are therefore considered as part of the definition of Verbena officinalis extract for this assessment. In addition, for the purpose of this assessment, products deriving from the different types of extraction methods of Verbena officinalis plant will be considered. This includes essential oils which are extracted via hydrodistillation or steam distillation (Kubica et al. 2020).
On the basis of notifications submitted under the Cosmetic Regulations to HC, Verbena officinalis, Verbena officinalis extract, Verbena officinalis leaf extract, and Verbena officinalis flower/leaf extract are used in a wide range of cosmetics within Canada. These include body and face moisturizers, hair care products, makeup products, cleansers, fragrances, and massage products (personal communication, emails from the CHPSD, HC, to the ESRAB, HC, dated June 2021; unreferenced).
Verbena officinalis and its preparations are listed in the NHPID (2022). Verbena officinalis is listed in the NHPID with a medicinal role as classified as an NHP substance falling under Schedule 1, item 1 (a plant or a plant material) of the NHPR. Herba Verbenae is also listed in the NHPID with a medicinal role as classified as an NHP substance falling under Schedule 1, item 1 (a plant or a plant material) of the NHPR. Such substances are listed in the LNHPD as being present as medicinal ingredients (MIs) or NMIs in NHPs, such as oral NHPs (LNHPD 2021; personal communication, emails from the NNHPD, HC, to the ESRAB, HC, dated June 2021; unreferenced).
Verbena officinalis essential oil is also available to Canadian consumers as a pure essential oil (that is, concentration of 100%), which can be used for DIY applications as discussed above. In addition, Verbena officinalis extract has been identified in liquid extracts available for purchase in Canada to be taken orally. It has also been identified as a component present in brewed herbal tea beverages prepared from products containing dried Verbena officinalis leaves.
Ginkgo biloba extract
Within Canada, according to notifications submitted under the Cosmetic Regulations to HC, Ginkgo biloba extract, Ginkgo biloba leaf extract, Ginkgo biloba leaf, Ginkgo biloba leaf powder, Ginkgo biloba nut extract, Ginkgo biloba root extract, and Ginkgo extract are used in a wide range of cosmetics. These include body and face moisturizers, hair care products, makeup products, cleansers, fragrances, deodorants, makeups, and massage products (personal communication, emails from the CHPSD, HC, to the ESRAB, HC, dated 2017 and 2021; unreferenced).
Ginkgo biloba and its preparations are listed in the NHPID (2022). Ginkgo biloba, Folium Ginkgo, and Semen Ginkgo are listed in the NHPID with a medicinal role as classified as NHP substances falling under Schedule 1, item 1 (a plant or a plant material) of the NHPR. Such entries of the NHPID are also associated with the following additional restrictions: in order to reduce toxicity, the following must be processed before use as a Traditional Chinese Medicine ingredient: Ginkgo biloba seeds must be either dry-fried or baked at a medium temperature until they turn deep yellow in order to break down ginkgotoxin. Testing must be performed to ensure that the finished product meets the toxicity restrictions for Ginkgolic acids not more than 5 ppm. Ginkgo biloba leaf extract is listed in the NHPID with a non-medicinal role for topical use as a skin-conditioning agent. Such substances are listed in the LNHPD as being present as MIs or NMIs in NHPs (LNHPD 2021), as well as in NPDs according to data obtained from HC’s DPD, including oral products, herbal tea blends, hand sanitizers, topical creams, and sunscreens (personal communication, emails from the NNHPD and TPD, HC, to the ESRAB, HC, dated 2017 and 2021; unreferenced).
Ginkgo biloba extract sourced from leaves has also been identified in oral supplements (liquid extracts), and leaves from the Ginkgo biloba plant are also used in herbal tea.
Amberlyn
Amberlyn is mainly used as a fragrance ingredient and as a food flavouring agent. When used in perfumery applications, it creates sweet earthy notes (woody, pine, cedar-like) (Panten et al. 2014). On the basis of information submitted as a follow-up to a survey issued pursuant to section 71 of CEPA (Canada 2012), there were reported uses of amberlyn in a limited number of cosmetics, laundry and dishwashing products, cleaning products, and air care products (Environment Canada 2013).
The substance is listed in the NHPID (2022) with a non-medicinal role for oral use only as a flavour enhancer, but it is not found to be listed in the LNHPD (2021) as being present in NHPs in Canada (personal communication, emails from the NNHPD, HC, to the ESRAB, HC, dated June 2021; unreferenced).
No definitive information is available concerning the use of amberlyn in foods sold in Canada; however, amberlyn is identified as a food flavouring agent internationally and it is possible that it may be present as a flavouring agent in foods sold in Canada (JECFA 2004a). In the US, annual consumption of amberlyn from use in food is reported to be less than 1 lb (<0.45 kg) (Burdock 2010). It is reported to be used in baked goods, non-alcoholic beverages, confection/frosting, frozen dairy, fruit ices, gelatins/puddings, jams/jellies and candy (Burdock 2010). The substance is listed in the US Food and Drug Administration (FDA) Substances Added to Food Database, where it can be used as a flavouring agent or adjuvant (US FDA 2022). Amberlyn has been granted a Generally Recognized As Safe (GRAS) status by the Flavor and Extract Manufacturers Association (FEMA) for its use as a food flavouring agent (Oser and Ford 1975) and has been deemed safe for use as a food flavouring agent by JECFA (JECFA 2004a).
Amberlyn is not reported to be an ingredient in cosmetics in Canada, on the basis of notifications submitted to HC under the Cosmetic Regulations (personal communication, emails from the CHPSD, HC, to the ESRAB, HC, dated June 2021; unreferenced). As amberlyn may be used as a component in fragrance mixtures, its presence in cosmetic notifications in Canada may not be explicitly stated but may be captured under the general terms for these mixtures used in cosmetics, which are “fragrance” and “parfum” (Canada 2009). In Europe, amberlyn is reported as being used in cosmetics for its perfuming function (CosIng c2021). It is also listed by the IFRA (2021) as a fragrance ingredient used in consumer goods and was assessed by the Research Institute for Fragrance Materials (RIFM) in 2021 for its use as a fragrance ingredient in consumer products (RIFM 2021b). In searches of public databases, amberlyn is listed as an ingredient in products available to consumers, most notably in cleaning and laundry products, air care products, and personal care products, according to information from the Consumer Product Information Database (CPID) (CPID c2021).
Myrrh oil
According to notifications submitted under the Cosmetic Regulations to HC, cosmetics are available in Canada containing the ingredients Commiphora myrrha, Commiphora myrrha extract, Commiphora myrrha leaf cell extract, Commiphora myrrha oil, Commiphora myrrha essential oil, Commiphora myrrha gum oil, Commiphora myrrha gum extract and gum powder, Commiphora myrrha resin oil, Commiphora myrrha resin and resin extract, Commiphora mukul resin extract, myrrh essential oil, and myrrh oil (personal communication, emails from the CHPSD, HC, to the ESRAB, HC, dated January 2022; unreferenced). These products include fragrances, body and face moisturizers, cleanser, exfoliants, antiperspirants, tooth powders, mouthwashes, and hair products.
Commiphora species and preparations are listed in the NHPID (2022). Commiphora myrrha is listed in the NHPID with a medicinal role as classified as NHP substances falling under Schedule 1, item 1 (a plant or a plant material) of the NHPR. Myrrh absolute and myrrh essential oil are listed in the NHPID with a medicinal role as classified as NHP substances falling under Schedule 1, item 2 (an extract) of the NHPR. Commiphora myrrha gum oil, Commiphora myrrha leaf cell extract, Commiphora myrrha resin, Commiphora myrrha resin extract, and myrrh oil are listed in the NHPID with a non-medicinal role for topical or oral use as a fragrance ingredient, masking agent, skin protectant, and/or flavour enhancer. Such substances are listed in the LNHPD (2021) as being present as MIs or NMIs in NHPs in Canada, including topical pain relief products, toothpastes, sunscreens, hand sanitizers, oral capsules, and throat lozenges (personal communication, emails from the NNHPD, HC, to the ESRAB, HC, dated June 2021; unreferenced).
Myrrh oil is available as an essential oil on the Canadian market at concentrations of up to 100% for use in DIY applications as described above. Additional DIY uses of myrrh oil include as a stomach remedy (doTERRA 2022b). In addition, myrrh oil has been identified as an ingredient in a liquid laundry detergent available in Canada (CPID 2021). Myrrh resin, from which myrrh oil is derived, is also burned as incense. Internationally, the IFRA has reported myrrh oil as a fragrance ingredient used in products available to consumers (IFRA 2021).
In Canada, myrrh oil is reported to be used as a formulant in PCPs, such as insect repellents and insecticides (personal communication, emails from the PMRA, HC, to the ESRAB, HC, dated June 2021; unreferenced).
No definitive information is available concerning the potential use of myrrh oil as a food flavouring agent in Canada; however, since the substance is identified as a food flavouring agent internationally, it is possible that the substance is present as a flavouring agent in foods sold in Canada (personal communication, emails from the FD, HC, to the ESRAB, HC, dated June 2021; unreferenced). Myrrh oil is listed as a natural flavouring permitted in foods in the US under Title 21 Part 172 of the US 21CFR172.510 (personal communication, emails from the FD, HC, to the ESRAB, HC, dated June 2021; unreferenced). Myrrh oil is listed as number 2766 in the FEMA’s Flavor Ingredient Library (FEMA c2022). The Council of Europe (1981 as cited in Pelkonen et al. 2013) identified myrrh as a natural source of flavourings (that is, materials of vegetable or animal origin, whether or not they are normally consumed as food, from which flavourings may be obtained) acceptable for use in food, and myrrh oil has reported uses as a flavouring agent in a variety of foods (Burdock 2010).
Cork tree extract
Cork tree extract is a naturally occurring substance obtained from the powdered bark of the Phellodendron amurense tree, which is native to Asia (Kumar et al. 2007). Cork tree extract has a long history of use in traditional herbal medicine for treating a variety of ailments, particularly in Asia (Swanson et al. 2015).
Cork tree extract and its preparations are listed in the NHPID (2022). Phellodendron amurense and Cortex Phellodendri Amurensis are listed in the NHPID with a medicinal role as classified as NHP substances falling under Schedule 1, item 1 (a plant or a plant material) of the NHPR. Phellodendron amurense bark extract is listed in the NHPID with a non-medicinal role for topical use as a skin-conditioning agent. Such substances are listed in the LNHPD (2021) as being present as MIs or NMIs in NHPs in Canada. Types of products containing such substances as NMIs include acne treatment products, face creams with sun protection factors (SPF), and analgesic sprays (personal communication, emails from the NNHPD, HC, to the ESRAB, HC, dated June 2021; unreferenced).
On the basis of data submitted under the Cosmetic Regulations, cork tree extract was reported as an ingredient in a variety of cosmetics including antiperspirants/deodorants, cleansers, skin care products, hair care products, make-up, and perfumes (personal communication, emails from the CHPSD, HC, to the ESRAB, HC, dated June 2021; unreferenced). Cork tree extract is available as a pure extract to consumers in Canada (that is, present at concentrations of up to 100%), where it could be used in a variety of DIY applications, similar to those of other essential oils discussed in this assessment.
Subgroup 1 (sage oil and wormwood oil)
Sage oil
Sage oil is obtained from the perennial plant Salvia officinalis, which is grown in many Mediterranean countries (Altindal and Altindal 2016). Sage has been traditionally used for its antimicrobial, antiviral and immunosuppressive properties, and for food flavouring purposes (Abu-Darwish et al. 2013; Altindal and Altindal 2016).
According to notifications submitted under the Cosmetic Regulations to HC, cosmetics are available in Canada containing the ingredients sage oil, Salvia officinalis (sage) oil, Salvia officinalis (sage) leaf oil, and Salvia officinalis oil (personal communication, emails from the CHPSD, HC to the ESRAB, HC, dated June 2021; unreferenced). These include body and face moisturizers, massage products, hair care products, mouthwashes, and fragrances.
Salvia officinalis and its preparations are listed in the NHPID (2022). Salvia officinalis and Dalmatian sage essential oil are listed in the NHPID with a medicinal role as classified as NHP substances falling under Schedule 1, items 1 and 2 (a plant or a plant material and extract, respectively) of the NHPR. Sage leaf dry, sage leaf powder, Salvia officinalis (sage) leaf extract, Salvia officinalis (sage) leaf oil, and Salvia officinalis (sage) oil are listed in the NHPID with a non-medicinal role for oral or topical use as a flavour enhancer, fragrance ingredient, masking agent, skin-conditioning agent, skin protectant, and/or tonic. The non-medicinal role of Salvia officinalis (sage) leaf oil is further associated with the restrictions detail “for topical use only, up to 0.02%, when formulated to be non-sensitizing”. Such substances are listed in the LNHPD (2021) as being present as MIs or NMIs in NHPs in Canada, such as toothpastes, oral supplements, teas, nasal inhaler sticks, room sprays, essential oil blends, hand sanitizers, and sunscreens (personal communication, emails from the NNHPD, HC, to the ESRAB, HC, dated June 2021; unreferenced). Sage oil is also present as an NMI in NPD sunscreen products in Canada according to data obtained from HC’s DPD (personal communication, emails from the TPD, HC, to the ESRAB, HC, dated June 2021; unreferenced).
Sage oil is also available as a pure essential oil in the Canadian market at concentrations of up to 100% for use in DIY applications as described above; it is also a component present in herbal teas prepared from products containing dried sage leaves. Sage oil is reported by the IFRA (2021) as a fragrance ingredient used in products available to consumers.
In Canada, sage oil is also reported to be used as a formulant in PCPs, such as animal repellents, insect repellents, and sanitizers (personal communication, emails from the PMRA, HC, to the ESRAB, HC, dated June 2021; unreferenced).
No definitive information is available concerning the potential use of sage oil as a food flavouring agent in Canada; however, since the substance is identified as a food flavouring agent internationally, it is possible that the substance is present as a flavouring agent in foods sold in Canada (personal communication, emails from the FD, HC, to the ESRAB, HC, dated June 2021; unreferenced). Sage oil is listed as being GRAS under Title 21 Part 182 of the US 21CFR182.20 (personal communication, emails from the FD, HC, to the ESRAB, HC, dated June 2021; unreferenced). Sage oil (Salvia officinalis) is listed as number 3001 in the FEMA’s Flavor Ingredient Library (FEMA c2022). Internationally, sage oil has reported uses as a flavouring agent in a variety of foods (Burdock 2010).
Wormwood oil
Wormwood oil is a dark green to brown, bitter, naturally occurring substance obtained from the leaves and tops of the wormwood (Artemisia absinthium) plant (TermiumPlus 2022). Wormwood oil is primarily used as a fragrance ingredient in products available to consumers. Wormwood oil also has a long history of use in traditional herbal medicine (Lachenmeier 2010).
Artemisia absinthium and its preparations are listed in the NHPID (2022). Artemisia absinthium is listed in the NHPID with a medicinal role as classified as an NHP substance falling under Schedule 1, item 1 (a plant or a plant material) of the NHPR, and is further associated with the following restriction details: “Artemisia absinthium contains thujone. For adults, the upper limit for total daily intake of thujone from health products is 6 mg. Product licence applications for oral products should include a copy of a certificate of analysis or any other equivalent document demonstrating that the thujone content of a daily dose of the product is acceptable. Because thujone content of the herbal materials can vary, the thujone content should be determined for each batch during production of the product”. Artemisia absinthium extract is listed in the NHPID with a non-medicinal role for topical use only as a skin-conditioning agent. Such substances are listed in the LNHPD (2021) as being present as MIs or NMIs in NHPs in Canada. NMI uses include a limited number of products such as hand sanitizers (personal communication, emails from the NNHPD, HC, to the ESRAB, HC, dated June 2021; unreferenced). There were no reported uses of wormwood oil as an MI in NPDs in Canada, but the substance was reported to be used as an NMI in analgesic creams, according to data obtained from HC’s DPD (personal communication, emails from the TPD, HC, to the ESRAB, HC, dated June 2021; unreferenced).
No definitive information is available concerning the potential use of wormwood oil as a food flavouring agent in Canada; however, since the substance is identified as a food flavouring agent internationally, it is possible that the substance is present as a flavouring agent in foods sold in Canada. In the US, annual consumption of wormwood oil from use in food is reported to be 316.67 lbs (143.6 kg) (Burdock 2010). It is reported to be used in alcoholic and non-alcoholic beverages, baked goods, frozen dairy, gelatins/puddings and candy (Burdock 2010). Wormwood is also known for being used to flavour liqueurs such as vermouth and absinthe (Burdock 2010).
On the basis of data submitted under the Cosmetic Regulations, wormwood oil was reported as an ingredient in a limited number of cosmetics, including face serums, masks, and hair care products (personal communication, emails from the CHPSD, HC, to ESRAB, HC, dated June 2021; unreferenced). Wormwood oil is also available to consumers in Canada as a pure essential oil (that is, present at 100%).
Subgroup 2 (IBCH, sandal cyclohexanol, BCH, and sandela)
IBCH, sandal cyclohexanol, BCH, and sandela do not occur naturally in the environment. Available information for IBCH, sandal cyclohexanol, BCH, and sandela suggests that these substances are primarily used as fragrance ingredients. These substances have a characteristic woody odour and are synthetic alternatives to sandalwood fragrance (Scentree [accessed 2022]; de Groot 2020). IBCH, sandal cyclohexanol, BCH, and sandela are listed by the IFRA (2021) as fragrance ingredients used in products available to consumers. In Europe, IBCH, sandal cyclohexanol, BCH and sandela are reported as being used in cosmetics for their perfuming function (CosIng c2021).
According to notifications submitted under the Cosmetic Regulations to HC, IBCH, sandal cyclohexanol, BCH, or sandela were not reported in cosmetics (personal communication, emails from the CHPSD, HC, to the ESRAB, HC, dated June 2021; unreferenced). As these substances are primarily used as components in fragrance mixtures, their presence in cosmetic notifications in Canada may not be explicitly stated, but may be captured under the general terms for these mixtures used in cosmetics, which are “fragrance” and “parfum” (Canada 2009).
On the basis of information obtained through follow-up to the section 71 survey, sandela, IBCH, or sandal cyclohexanol were also identified as ingredients in a limited number of products including candles, air fresheners, liquid cleaners, laundry detergents, softeners, body washes, conditioners, shampoos, shave gels, perfumes, and body lotions (Environment Canada 2013). The CPID website reports that IBCH, sandal cyclohexanol, and BCH (under the synonym camphylcyclohexanol) are ingredients in products available to consumers such as candles, fabric softeners, air fresheners (home and auto), and laundry detergents; however, concentration data were not available for these substances in the identified products (CPID 2022). Through searches of publicly available data, IBCH, sandal cyclohexanol, and sandela were identified as ingredients in a limited number of cosmetics and air care products available to consumers, including fragrances, body moisturizers, body cleansers, and air freshener products (SDS 2015a, 2015b).
5. Environmental fate and behaviour
5.1 Environmental persistence
According to models used in ERC (ECCC 2016b), norlimbanol, Verbena officinalis extract, Ginkgo biloba extract, amberlyn, cork tree extract, sandal cyclohexanol, and BCH are expected to persist in water, sediment, and soil but are not expected to persist in air.
According to models used in ERC (ECCC 2016b), wormwood oil is expected to persist in sediment and air but is not expected to persist in water and soil.
According to models used in ERC (ECCC 2016b), cade oil, jonquil oil, myrrh oil, sage oil, IBCH, and sandela are not expected to persist in air, water, sediment, or soil.
5.2 Potential for bioaccumulation
Given its high log Kow and high bioconcentration factor (ECCC 2016b), cade oil is expected to significantly bioaccumulate in organisms.
Although the log Kow values for norlimbanol, amberlyn, myrrh oil, IBCH, sandal cyclohexanol, BCH, and sandela are high, the bioconcentration factors for these substances are low to moderate (1157.4, 2115.9, 1149.5, 577.2, 2046.4, 1688.5, and 416.9 L/kg, respectively). As a result, these substances are not expected to significantly bioaccumulate in organisms (ECCC 2016b).
Given their low log Kow and low bioconcentration factors (ECCC 2016b), jonquil oil, cork tree extract, wormwood oil, and sage oil are not expected to significantly bioaccumulate in organisms. Based on a manual judgement-based approach, Verbena officinalis extract and Ginkgo biloba extract are also not expected to significantly bioaccumulate in organisms.
6. Potential to cause ecological harm
6.1 Characterization of ecological risk
The ecological risks of the substances in the Fourteen Terpene and Terpenoid Substances Group were characterized using the ERC approach (ECCC 2016a). The ERC is a risk-based approach that considers multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. The various lines of evidence are combined to discriminate between substances of lower or higher potency and lower or higher potential for exposure in various media. This approach reduces the overall uncertainty associated with risk characterization compared with an approach that relies on a single metric in a single medium (for example, median lethal concentration) for characterization. Since Verbena officinalis extract and Ginkgo biloba extract are UVCBs and could not be suitably represented by a single chemical structure, a manual judgment-based approach to classification was used. The following summarizes the approach, which is described in detail in ECCC (2016a).
Data on physical-chemical properties, fate (chemical half-lives in various media and biota, partition coefficients, and fish bioconcentration), acute fish ecotoxicity, and chemical import or manufacture volume in Canada were collected from the scientific literature, available empirical databases (for example, OECD QSAR Toolbox 2014), or responses to surveys issued pursuant to section 71 of CEPA, or they were generated using selected (quantitative) structure-activity relationship ([Q]SAR) or mass-balance fate and bioaccumulation models. These data were used as inputs to other mass-balance models or to complete the substance hazard and exposure profiles.
Hazard profiles were based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Exposure profiles were also based on multiple metrics, including potential emission rate, overall persistence, and long-range transport potential. Hazard and exposure profiles were compared to decision criteria in order to classify the hazard and exposure potentials for each organic substance as low, moderate, or high. Additional rules were applied (for example, classification consistency, margin of exposure) to refine the preliminary classifications of hazard or exposure. However, in the case of Verbena officinalis extract and Ginkgo biloba extract, hazard and exposure could not be fully profiled because of the lack of a representative structure to estimate needed properties and the lack of empirical data for these properties. Therefore, manual classification of hazard and exposure was performed by examining the UVCB constituents, analyzing information submitted in response to a CEPA section 71 survey, making decisions by taking into consideration similar substances, and/or application of expert judgment.
A risk matrix was used to assign a low, moderate, or high classification of potential risk for each substance on the basis of its hazard and exposure classifications. ERC classifications of potential risk were verified using a 2-step approach. The first step adjusted the risk classification outcomes from moderate or high to low for substances that had a low estimated rate of emission to water after wastewater treatment, representing a low potential for exposure. The second step reviewed low risk potential classification outcomes using relatively conservative, local-scale (that is, in the area immediately surrounding a point source of discharge) risk scenarios, designed to be protective of the environment, to determine whether the classification of potential risk should be increased.
ERC uses a weighted approach to minimize the potential for both over- and under classification of hazard and exposure, and of subsequent risk. The balanced approaches for dealing with uncertainties are described in greater detail in ECCC (2016a). The following describes 2 of the more substantial areas of uncertainty. Error with empirical or modelled acute toxicity values could result in changes to classification of hazard, particularly metrics relying on tissue residue values (that is, mode of toxic action), many of which are predicted values from (Q)SAR models (OECD QSAR Toolbox 2014). However, the impact of this error is mitigated by the fact that overestimation of median lethality will result in a conservative (protective) tissue residue value used for critical body residue analysis. Error with underestimation of acute toxicity will be mitigated through the use of other hazard metrics such as structural profiling of mode of action, reactivity, and/or estrogen binding affinity. Changes or errors in chemical quantity could result in differences in classification of exposure as the exposure and risk classifications are highly sensitive to emission rate and use quantity. The ERC classifications thus reflect exposure and risk in Canada on the basis of what is estimated to be the current use quantity and may not reflect future trends.
In addition, it should be noted that in this assessment, evaluation of the potential to cause ecological harm considered each substance individually. If exposure to multiple substances occurs simultaneously, this could result in cumulative effects on organisms and potentially present a higher risk. The potential for cumulative effects and how they may manifest in the environment were not further investigated due to the low ecological risk classification of these substances considering both ecological exposure and hazard under the ERC approach.
Critical data and considerations used to develop the substance-specific profiles for the substances in the Fourteen Terpene and Terpenoid Substances Group and the hazard, exposure, and risk classification results are presented in ECCC (2016b).
The hazard and exposure classifications for the substances in the Fourteen Terpene and Terpenoid Substances Group are summarized in Table 6‑1.
| Substance | ERC hazard classification | ERC exposure classification | ERC risk classification |
|---|---|---|---|
| Cade oil | high | low | low |
| Jonquil oil | low | low | low |
| Norlimbanol | low | low | low |
| Verbena officinalis extract | high | low | low |
| Ginkgo biloba extract | high | low | low |
| Amberlyn | low | low | low |
| Myrrh oil | low | low | low |
| Cork tree extract | low | low | low |
| Sage oil | low | low | low |
| Wormwood oil | low | low | low |
| Isobornyl cyclohexanol (IBCH) | low | low | low |
| Sandal cyclohexanol | low | low | low |
| Bornyl cyclohexanol (BCH) | low | low | low |
| Sandela | low | low | low |
On the basis of low hazard and low exposure classifications according to information considered under ERC, amberlyn, myrrh oil, jonquil oil, norlimbanol, cork tree extract, wormwood oil, sage oil, IBCH, sandal cyclohexanol, BCH, and sandela were classified as having a low potential for ecological risk. It is unlikely that these substances are resulting in concerns for the environment in Canada.
According to information considered under ERC, cade oil, Verbena officinalis extract, and Ginkgo biloba extract were classified as having a low exposure potential. Cade oil was classified as having a high hazard potential on the basis of a high potential to cause adverse effects on aquatic food webs given its high bioaccumulation potential. Verbena officinalis extract and Ginkgo biloba extract were classified as having a high ecological hazard potential using a conservative manual classification that was applied due to uncertainties in the model outcomes for these substances. These 3 substances were classified as having a moderate potential for ecological risk; however, the risk classification was decreased to low following the adjustment of risk classification on the basis of current use quantities (see section 7.1.1 of the ERC approach document [ECCC 2016a]). The potential effects and how they may manifest in the environment were not further investigated given the low exposure of these substances. On the basis of current use patterns, these substances are unlikely to be resulting in concerns for the environment in Canada.
7. Potential to cause harm to human health
7.1 Cade oil
7.1.1 Exposure assessment
Environmental media and food
No measured concentrations of cade oil in environmental media were identified in Canada or elsewhere. The substance was not reported to be manufactured or imported in quantities greater than the reporting threshold of 100 kg according to information submitted in response to a CEPA section 71 survey (Environment Canada 2013). In consideration of low reported quantities of the substance in Canada, exposure to cade oil from its presence in environmental media is expected to be minimal.
Fenaroli’s Handbook of Flavor Ingredients reports the per capita ('individual') estimated intake of cade oil from its use as a food flavouring agent to be 0.00014 mg/kg bw/day for the US population on the basis of annual production volumes reported by the food industry (NAS 1989 as cited in Burdock 2010). In the absence of data on the actual use, if any, of cade oil as a flavouring agent in foods sold in Canada, the per capita intake estimate for the US population (Burdock 2010) is considered to be an acceptable estimate of possible Canadian dietary intake of cade oil for the general population, that is, 1 year of age and older (personal communication, email from FD, HC, to the ESRAB, HC, dated May 2022; unreferenced).
Products available to consumers
Cade oil is reported as an ingredient in a limited number of cosmetics and NPDs in Canada. In order to evaluate the potential exposure of the general population to cade oil from use of these products, representative sentinel exposure scenarios were selected. These scenarios describe the highest level of exposure to the substance from use of products, taking into consideration the frequency of use and concentration of cade oil. Other products reported to contain this substance are expected to be associated with a lower potential for exposure than these sentinel scenarios. For cade oil, the use of perfumes (roll-on) at 30% and use of face moisturizers at 1% were selected as the sentinel cosmetic scenarios.
Cade oil is also available to consumers in Canada as a pure essential oil (that is, present at 100%). The oil can be used in DIY applications such as massage oil preparations or body moisturizer preparations, or it can be added to a bath, facial steamer, or aromatic diffuser. Table 7‑1 outlines the estimated exposure to cade oil when used as an essential oil and in preparations for topical use on abraded/damaged skin (Tisserand Institute [accessed 2021]; doTERRA 2022a.; Base Formula [accessed 2021]).
Exposure estimates were adjusted to account for the percent composition of the major components of cade oil (that is, cresols) used to characterize the risk of the substance. For oral, dermal, and inhalation scenarios an adjustment factor of 0.14 is used, which is based on the upper-bound percent composition of cresols in cade oil identified in the literature. Furthermore, data from a similar substance, chlorocresol—which was evaluated by ECCC, HC (2021a)—was used to inform the potential dermal absorption of cresols. The chlorocresol assessment noted that in studies with guinea pigs, 0.2% to 1.6% of applied doses remained as free chlorocresol at the exposed (patch) site and 75% of chlorocresol in aqueous suspension permeated the skin (Andersen et al. 1985). A dermal absorption value of 75% was also applied in a risk assessment for chlorocresol completed under Regulation (EU) No 528/2012, which was adopted from the EFSA guidance (2012). Chlorocresol and cresols have very similar structure, molecular weights, water solubilities, log Kow values, and vapour pressures. In consideration of this information and given the uncertainties associated with using data for chlorocresol to inform cresols, a dermal absorption value of 80% is used to estimate dermal exposures. However, it is considered that up to 100% dermal absorption would be possible when the products are applied to abraded/damaged skin.
Estimates of exposure to cade oil from its use in cosmetics and as an essential oil in DIY applications are presented in Table 7‑1 and Table 7‑2; for each scenario, exposure estimates were derived for a range of relevant age groups and only the highest one is presented. Parameters used to estimate the exposure scenarios presented are outlined in Appendix A. Given the number of products containing cade oil available on the Canadian market, exposure may result from the use of several types of products (for example, cosmetics and NHPs) containing the substance on the same day (that is, aggregate exposure).
| Scenario | Route of exposure | Concentration (%) | Exposurea,b |
|---|---|---|---|
| Perfume (roll-on) | Dermal and inhalation | 30 | Dermal (mg/kg bw/day): 0.63 (2 to 3 years) Inhalation (24-hr TWAc, mg/m3): 0.03 (19+ years) |
| Face moisturizer | Dermal and inhalation | 1 | Dermala (mg/kg bw/day): 0.05 (19+ years) Inhalation (24-hr TWAc, mg/m3): 0.003 (19+ years) |
a Exposure estimates are adjusted to account for the percent composition of the major components of cade oil used to characterize risk to the substance (that is, cresols). For dermal, oral, and inhalation scenarios, an adjustment factor of 0.14 is used for cresols.
b For dermal exposures an absorption value of 80%, based on dermal absorption data for chlorocresol (ECCC, HC 2021a), is used.
c 24-hr TWA = 24-hour time-weighted-average (TWA). This value describes the 24-hour average air concentration on the day of product use.
| Scenario | Route of exposure | Concentration (%) | Exposurea,b (age range) |
|---|---|---|---|
| Essential oil use in aromatic diffuser | Dermal and inhalation | 100c | Dermal, usersd (mg/kg bw/day): 0.27 (9 to 13 years) Inhalation, users and bystanders (24-hr TWAe, mg/m3): 0.38 (0 to 19+ years) |
| Essential oil use in facial steamer, users | Dermal and inhalation | 100f | Dermal, users (mg/kg bw/day) 0.58 (4 to 8 years) Inhalation, users (24-hr TWAe, mg/m3): 0.23 (4 to 19+ years) |
| Essential oil use in facial steamer, bystanders | Inhalation | 100f | Inhalation, bystanders (24-hr TWAc, mg/m3): 0.05 (0 to 19+ years) |
| Essential oil use in bath | Dermal and inhalation | 100f | Dermal (mg/kg bw/day): 0.001 (9 to 13 years) Inhalation (24-hr TWAe, mg/m3): 0.07 (4 to 19+ years) |
| Essential oil use in massage oil preparations | Dermal and inhalation | 3 (after dilution)g,h | Dermal (mg/kg bw/day): 0.96 (0 to 5 months) Inhalation (24-hr TWAe, mg/m3): 0.009 (19+ years) |
| Essential oil use in body moisturizers | Dermal, inhalation | 3 (after dilution)h | Dermal (mg/kg bw/day): 1.07 (0 to 5 months) Inhalation (24-hr TWAe, mg/m3): 0.03 (19+ years) |
| Essential oil use in topical preparations for abraded/damaged skin | Dermali | 1 (after dilution)j | Dermal (mg/kw bw/day) 0.51 (0 to 5 months) |
a Exposure estimates are adjusted to account for the percent composition of the major components of cade oil used to characterize risk to the substance (that is, cresols). For dermal, oral, and inhalation scenarios, an adjustment factor of 0.14 is used for cresols.
b For dermal exposures, an absorption value of 80%, based on dermal absorption data for chlorocresol (ECCC, HC 2021a), is used.
c Assumes that 920 mg of the pure essential oil is added to the device (~ 18 drops of cade oil).
d Dermal exposure from this use is expected to be incidental during refilling of an aromatic diffuser and is considered to be applicable to users only (for example, those 9 to 13, 14 to 18, and 19+ years).
e 24-hr TWA = 24-hr time-weighted-average. This value describes the 24-hr average air concentration on the day of product use.
f Assumes that approximately 10 drops of pure essential oil is used. Details on the corresponding product amounts are presented in Appendix A.
g Essential oils used in massage oil preparations are typically diluted prior to use (Bremmer et al. 2006)
h Tisserand Institute [accessed 2021]
i This scenario assumes that the amount applied is completely absorbed via the dermal route as it is applied to abraded/damaged skin.
j Base Formula [accessed 2021]
Lifetime-average-daily doses (LADDs) were also derived for the scenarios described above and were adjusted to account for the maximum cresol content in cade oil identified in the literature (up to 14%). Table 7‑3 presents the LADDs for cade oil. The parameters used to estimate these LADDs are presented in Appendix A.
| Scenario | Route of exposure | Concentration(%) | LADDa,b (mg/kg bw/day) |
|---|---|---|---|
| Perfume (roll-on) | Dermal and inhalation | 30 | 0.24 |
| Face moisturizer | Dermal and inhalation | 1 | 0.04 |
| Essential oil use in aromatic diffusera, users | Dermal and inhalation | 100 | 0.24 |
| Essential oil use in facial steamerc, users | Dermal and inhalation | 100 | 0.26 |
| Essential oil use in bath | Dermal and inhalation | 100 | 0.004 |
| Essential oil use in massage oil preparations | Dermal and inhalation | 3 (after dilution) | 0.02 |
| Essential oil use in body moisturizers | Dermal and inhalation | 3 (after dilution) | 0.48 |
| Essential oil use in topical preparation on abraded/damaged skin | Dermald | 1 (after dilution) | 0.08 |
a Exposure estimates are adjusted to account for the percent composition of the major components of cade oil used to characterize risk to the substance (that is, cresols). For dermal, oral, and inhalation scenarios, an adjustment factor of 0.14 is used for cresols.
b For dermal exposures an absorption value of 80%, based on dermal absorption data for chlorocresol (ECCC, HC 2021a), is used.
c LADD estimates for bystander exposures were not derived.
d This scenario assumes that the amount applied is completely absorbed via the dermal route as it is applied to abraded/damaged skin.
7.1.2 Selection of analogues
As cade oil and its major components had limited health effects data, a read-across approach using data from analogues was used to inform the human health assessment of cade oil. As cade oil (CAS RN 8013-10-3) is a UVCB, an analogue was selected that is structurally similar and/or functionally similar to cade oil major components cadinene, τ-muurolol, widdrol and epi-cubenol, and that is associated with relevant empirical data.
The chemical structure and molecular weight of cade oil major components and their analogue are presented in Table 7‑4. For information on the physical-chemical properties of cade oil major components and their analogue, refer to Appendix B, Table B-1.
| CAS RN | DSL or other name (common name) |
Chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
| 29350-73-0 (target) |
Naphthalene, decahydro-1,6-dimethyl-4-(1-methylethyl)-, [1S-(1α,4α,4aα,6α,8aβ)]-, didehydro deriv. (Cadinene) |
 C12H24 |
204.35 |
| 19912-62-0 (target) |
τ-Muurolol |  C15H26O |
222.37 |
| 6892-80-4 (target) |
Widdrol |  C15H26O |
222.37 |
| 19912-67-5 (target) |
epi-Cubenol | 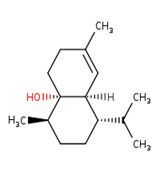 C15H26O |
222.37 |
| 98-55-5 | 3-Cyclohexene-1-methanol, α,α,4-trimethyl- (alpha-Terpineol) |
 C10H18O |
154.25 |
7.1.3 Health effects assessment
Cade oil (CAS RN 8013-10-3)
A Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) dossier is available with minimal information on cade oil toxicity (ECHA 2021e). A safety review was conducted by the CIR Expert Panel on a range of Juniper oxycedrus extracts, including juniper tar (that is, cade oil) which concluded that the available data were insufficient to support the safety of cade oil for use in cosmetics (CIR 2001). A similar conclusion was adopted by the European Commission (SCCNFP 2003).
Since no quality toxicity studies on cade oil were identified in the available published literature, data on the main components were used to inform the health effects characterization of cade oil.
Genotoxicity and carcinogenicity
The genotoxicity of cade oil was examined in a reverse gene mutation assay comprised of 3 experiments, in which Salmonella typhimurium strains TA 1535, TA 1537, TA 98, TA 100 and TA 102 were exposed to cade oil (composition and rectification state not specified) in the presence and absence of metabolic activation (10% liver S9 fraction). In these experiments, the test material was identified as non-mutagenic (with or without metabolic activation) (ECHA 2021e).
In a review by the CIR (2001), cade oil (rectification state not specified) was reported to be genotoxic in a number of in vitro studies (for example, rec-assay and reverse mutation assays) using Bacillus subtilis, Salmonella typhimurium, and Escherichia coli strains. Furthermore, in vivo and in vitro experiments using cade oil on human and mouse skin showed the formation of DNA adducts at a level greater than that induced by coal tar, which contains known carcinogens such as benzo[a]pyrene (CIR 2001).
Coal-tar ointment produced very low levels of adducts (< 0.03 fmol/µg DNA) in mice lungs while juniper tar produced higher levels of adducts (0.7 fmol/µg DNA) that were persistent in the tissue (CIR 2001; SCCNFP 2003).
A search of the scientific literature has not identified any carcinogenicity studies conducted with cade oil (juniper tar).
Cadinene (CAS RN 29350-73-0), τ-Muurolol (CAS RN 19912-62-0), Widdrol (CAS RN 6892-80-4) and Epi-cubenol (CAS RN 19912-67-5)
No suitable toxicological studies were found for cadinene, τ-muurolol, widdrol and epi-cubenol. Considering their similar chemical structures and physical-chemical properties, they were treated as one group for the purpose of read-across. Alpha-terpineol was found to be the closest analogue with sufficient quality toxicological studies to fill the data gaps.
The health effects of alpha-terpineol have been characterized in the draft assessment of the Terpenes and Terpenoids - Monocyclic and Bicyclic Sesquiterpenes Group conducted under the CMP (ECCC, HC 2021b). The lowest critical effect level identified for alpha-terpineol was a no-observed-adverse-effects level (NOAEL) of 250 mg/kg bw/day based on impairment of male fertility due to a reduced number or complete absence of spermatozoa in the presence of degenerate spermatogenic cells in the epididymides and seminiferous tubular atrophy/degeneration in the testes at 750 mg/kg bw/day from a combined repeated-dose and reproduction/developmental toxicity study (OECD TG 422) in Sprague-Dawley rats (ECCC, HC 2021b). Alpha-terpineol was not found to be mutagenic in bacterial assays, did not induce gene mutations in mammalian cells, and was not found to be carcinogenic in A/He mice (ECCC, HC 2021b).
Thujopsene (CAS RN 470-40-6) and α-Cedrene (CAS RN 469-61-4)
No suitable toxicological data were found on thujopsene or α-cedrene; however, Virginia cedarwood oil is a mixture that contains mainly thujopsene and alpha-cedrene (up to 35% and 45%, respectively) and data on the toxicity of Virginia cedarwood oil was used to inform the assessment of health effects of thujopsene and alpha-cedrene.
A REACH dossier for Virginia cedarwood oil is available (ECHA 2021f) and a summary of key studies is presented in this section.
Repeated-dose/reproductive and developmental toxicity
In a combined repeated dose toxicity study with a reproduction/developmental toxicity screening test, groups of Wistar Crl:WL(Han) rats were exposed in diet to Virginia cedarwood oil (10/sex/dose) at concentrations of 750, 1500, and 5000 ppm (actual dose of 62, 120, and 381 mg/kg bw/day in males, and 104, 207 and 560 mg/kg bw/day in females). Main study males were exposed for 28 days, while females were exposed for between 49 and 62 days. Recovery animals (5/sex/dose) were exposed for 28 days (males) and 49 days (females). No mortalities occurred, and no treatment-related or toxicologically relevant changes were noted in clinical appearance, functional observations, or haematology parameters. A dose-dependent reduction in body weight gain was observed in males treated with middle and highest doses and in females treated with the highest dose. A reduction in food consumption of 15% (pre-mating) and 25% to 30% (gestation and lactation) was observed in females of the highest dose group. Treatment-related increases in liver weights (absolute and relative to body weight) and decreases in thymus weights (absolute and relative to body weight) were noted in females treated with the highest dose, though they returned to normal ranges by the end of the recovery period. Serum T4 levels in male and female rats of the F0 generation showed a dose dependent decrease at all dose levels. However, no morphological effects were observed in the thyroid gland and no statistically significant changes were observed in thyroid-stimulating hormone (TSH) levels. Hepatocellular hypertrophy was observed in female rats at the middle and highest doses, as well as increased levels of alkaline phosphatase ALP (both sexes), alanine aminotransferase (ALT), cholesterol, and bilirubin (females). Hyaline droplet accumulation in the kidney was observed in males treated at all doses; however, the authors did not consider this effect to be adverse as it was male specific and did not lead to degeneration or inflammation. No reproductive toxicity was observed up to the highest tested dose. With respect to pup development, body weight gains of male and female pups in the high dose group were decreased by 16% and 18% respectively, on post-natal day (PND) 13. A parental lowest-observed-adverse-effects level (LOAEL) of 750 ppm (62 mg/kg bw/day for females) was identified on the basis of reduced T4 serum levels in male and female rats at all dose levels. The reproductive NOAEL was > 5,000 ppm (381 mg/kg bw/day in males and 560 mg/kg bw/day in females). The developmental NOAEL was 1500 ppm (207 mg/kg bw/day in females) on the basis of reduced body weight gain of pups at the next dose level of 5000 ppm (560 mg/kg bw/day in females) (ECHA 2021f).
In a repeated dose dermal toxicity study by the US National Toxicology Program (NTP)(NTP 2016), groups of F344 rats and B6C3F1/N mice (10/sex/dose) were treated topically with Virginia cedarwood oil in 95% aqueous ethanol at 6.25%, 12.5%, 25%, 50% or 100% for 14 weeks (5 days/week). This dosage range corresponds to 31, 62, 125, 250 or 500 mg/kg bw/day for rats and to 125, 250, 500, 1000 or 2000 mg/kg bw/day for mice.
In the rat experiment, all rats survived to the end of the study, with the exception of 2 males in the 500 mg/kg bw/day group. There was a decrease in mean body weights and body weight gains in males and females treated with 250 and 500 mg/kg bw/day Virginia cedarwood oil (8% to 13% relative to control); irritation, thickening, non-neoplastic lesions and ulceration of the skin at the site of application in males and females at all dose groups; significant increases in relative liver weight (males at 250 and 500 mg/kg bw/day, females at 500 mg/kg bw/day [relative and absolute]), relative kidney weight (males at 250 and 500 mg/kg bw/day, females at 125 mg/kg bw/day and greater) and absolute kidney weight (males at 500 mg/kg bw/day); significant decreases in relative thymus weight (females at 500 mg/kg bw/day) and absolute thymus weight (males at 125 and 500 mg/kg bw/day, females at 500 mg/kg bw/day) weights; and significantly increased incidence of renal tubule degeneration and hyaline droplet accumulation in males at 125 mg/kg bw/day and higher dose groups (NTP 2016).
In the mice experiment, due to severity of skin lesions (for example, mild to moderate epidermis hyperplasia, hyperkeratosis, ulcers, fibrosis) at the site of application, all male and female mice in the 2000 mg/kg bw/day group, one male and one female each in the 1000 mg/kg bw/day group, and one male in the 250 mg/kg bw/day group were euthanized during weeks 10, 11, and 12, respectively. A significant decrease in final mean body weights occurred in males at the 500 and 1000 mg/kg bw/day dose groups, and in females exposed to 250 mg/kg bw/day or higher doses (>10% relative to control); further, mean body weight gains were significantly decreased in males and females exposed to 250 mg/kg bw/day or higher doses. Most dosed mice manifested irritation, thickening, fibrosis, chronic active inflammation and ulceration of the skin at the site of application. Absolute liver weights were significantly increased in males and females at 1000 mg/kg bw/day, while relative liver weights significantly increased in males and females at 125 mg/kg bw/day or higher. Absolute kidney weights increased in females at 1000 mg/kg bw/day, while relative kidney weights increased in males at 1000 mg/kg bw/day and in females at 250 mg/kg bw/day or higher. Absolute thymus weight significantly decreased in treated males (at 500 mg/kg bw/day or higher) and females (at 250 mg/kg bw/day or higher). In the thymus, incidences of atrophy were significantly increased at 500 mg/kg bw/day or greater in males. In the kidney, the incidence of nephropathy was significantly increased in males exposed to 2000 mg/kg bw/day compared with those in the untreated controls (NTP 2016).
For local dermal effects, a LOAEL of 6.25%, equivalent to 31 mg/kg bw/day in female rats and 125 mg/kg bw/day in female and male mice, was determined on the basis of increased incidences of skin lesions at all doses. As increased incidences of skin lesions were only seen in animals exposed dermally, these are considered to be local effects. The authors concluded that the organ weight changes in the liver, kidneys, and thymus were secondary effects to the skin lesions at the site of application in both rats and mice.
For systemic effects, a NOAEL of 12.5%, equivalent to 62 mg/kg bw/day in rats, and a NOAEL of 6.25%, equivalent to 125 mg/kg bw/day in mice, were interpreted on the basis of decreased thymus and body weights in rats and mice at higher doses.
Genotoxicity and carcinogenicity
Virginia cedarwood oil was not mutagenic in bacterial reverse mutation assay using S. typhimurium strains (TA98, TA 100, TA102) with or without metabolic activation up to 333 µg/plate. Results from in vivo micronucleus studies in mice were equivocal; no significant increase in micronucleated erythrocytes occurred in males after 3 months of dermal exposure (up to 50% concentration); however, in females small increases in the frequency of micronucleated erythrocytes were observed at the middle and highest doses (25% and 50%). The authors suggested that bone marrow toxicity is not induced by dermal application of cedarwood oil (NTP 2016).
Cresols (o-, m-, p-cresol)
Cresols (including o-, p-, and m-cresols as well as their mixture) have been previously assessed by ECCC and HC (2016a). A literature search was conducted for the period of 2015 to 2021, but no studies that could result in a health effects characterization differing from that in the ECCC, HC (2016a) assessment were identified.
In ECCC, HC (2016a), a lowest-observed-adverse-effects concentration (LOAEC) of 9 mg/m3 was identified as the critical effect level for general population exposure via inhalation, on the basis of a sub-chronic inhalation toxicity study in rats using o-cresol which resulted in irritation and morphological changes of the respiratory tract, signs of liver toxicity, leukocytosis and a decreased myeloid-erythroid ratio in the bone marrow.
For oral exposures, the most conservative point of departure (POD) was a NOAEL of 30 mg/kg bw/day, which was based on the occurrence of tremors under contact stimulus in rat neonates exposed to 100 mg/kg bw/day of m-cresol by gavage from PND 4 to 21 (ECCC, HC 2016a). Other central nervous system (CNS) effects (for example, convulsions, rapid laboured breathing, hypoactivity tremors, excessive salivation, coma) were observed in adult rodents exposed for longer durations (that is, 13 weeks) at 50 mg/kg bw/day or higher of o-, m- or p-cresols (ECCC, HC 2016a).
Carcinogenicity studies in mice and rats are available. In these studies, animals were exposed to a mixture of cresol isomers in the diet at maximum doses of 720 mg/kg bw/day (rats) or 1040 mg/kg bw/day (mice) for 105 weeks, and an increase in the incidence of squamous cell papillomas in the forestomach of female mice was observed. Using these studies, the HC assessment conducted a benchmark dose analysis and derived a Benchmark Dose Level (BMDL10) of 376 mg/kg bw/day (ECCC, HC 2016a).
When tested separately, each of the 3 isomers (o-, p- and m-) was negative in mutagenicity studies using S. typhimurium and mouse lymphoma cells. Results in other in vitro and in vivo genotoxicity assays were mixed. Given the equivocal results of the in vitro tests and limited in vivo genotoxicity studies, HC could not clearly define the genotoxic potential of cresol isomers (ECCC, HC 2016a).
Guaiacol (CAS RN 90-05-1)
In a sub-acute toxicity study, male Fischer 344 rats (5/dose) were administered guaiacol in their diet at a dose of 2% (1500 mg/kg bw/day) for 28 days. A LOAEL of 1500 mg/kg bw/day was identified by the study authors on the basis of an increase in relative liver and kidney weights and an increase in the thickness of glandular stomach and esophagus (ECHA 2021g).
In a limited carcinogenicity study, 16 male Fischer 344 rats were exposed to 1.5% guaiacol (1000 mg/kg bw/day) orally via diet for 51 weeks. Forestomach hyperplasia was observed in 94% of the rats receiving the test substance; however, no papillomas or carcinomas were detected. Forestomach lesions were detected and were characterized by downward basal cell growth. The authors identified a LOAEL for non-carcinogenic effects of 1000 mg/kg bw/day on the basis of the forestomach lesions and hyperplasia (Hirose et al.1989).
In an in vivo mammalian erythrocyte micronucleus study, guaiacol mixed in corn oil was administered orally at 125, 250 and 500 mg/kg to NMRI mice. The number of polychromatic erythrocytes (PCEs) did not substantially decrease when compared to control in the highest tested dose. The authors concluded that there was no biologically relevant enhancement in the frequency of the detected micronuclei at any dose used. Therefore, guaiacol was considered to be non-mutagenic (ECHA 2021g).
In a bacterial reverse mutation assay, strains of Salmonella typhimurium were exposed to guaiacol at 0 µg/plate to 5000 µg/plate in the presence and absence of metabolic activation. The test substance was not found to be genotoxic up to cytotoxic concentrations of 5000 µg/plate (ECHA 2021g).
Polycyclic aromatic hydrocarbons (PAHs)
Cade oil is reported to contain PAHs.
The IFRA generates standards which provides guidance to formulators in terms of recommendation of use of ingredients (IFRA Standards 2013). As part of their Standards, the IFRA recommend that crude cade oil be prohibited and that only the purified cade oil be used with a maximum limit of 1 ppb of PAHs. The levels reported by Tisserand and Young (2014) are above the recommended threshold identified by IFRA.
7.1.4 Characterization of risk to human health
A very limited number of toxicity studies conducted with cade oil are available from the published literature; therefore, the cade oil health effects characterization was based on information available on its main components or their relevant analogues.
A NOAEL of 30 mg/kg bw/day, identified in an 18-day toxicity study conducted with m-cresol, was considered to be the most appropriate health effects endpoint for the risk characterization of cade oil from oral and dermal exposures. This NOAEL was based on clinical signs of CNS-stimulation post-dosing, such as salivation, rapid respiration, tremors and hypoactivity observed in neonatal and adult rodents at the next dose of 50 mg/kg bw/day (or higher). While dermal toxicity studies were available, they did not assess neurological function and as a result were not considered for risk characterization.
For the risk characterization of cade oil from inhalation exposure, a LOAEC of 9 mg/m3, determined on the basis of irritation and morphological changes in the respiratory tract, signs of liver toxicity, leukocytosis, and a decreased myeloid-erythroid ratio in the bone marrow, was considered to be the most appropriate endpoint. These effects were observed in rats in a sub-chronic inhalation toxicity study conducted with o-cresol.
Table 7‑5 and Table 7‑6 provide relevant exposure estimates, critical effect levels, and resulting margins of exposure (MOEs) for the characterization of risk to human health from exposure to cade oil from food, cosmetics, and other products available to consumers.
| Scenarioa | Exposureb,c (mg/kg bw/day) |
MOEd |
|---|---|---|
| Perfume (roll-on) (2 to 3 years) |
0.63 | 48 |
| Face moisturizer (19+ years) |
0.05 | 600 |
| Essential oil use in aromatic diffuser, users (9 to 13 years) | 0.27 | 111 |
| Essential oil use in facial steamer, users (4 to 8 years) |
0.58 | 52 |
| Essential oil use in bath, (9 to 13 years) |
0.001 | 30 000 |
| Essential oil use in massage oil preparations (0 to 5 months) |
0.96 | 31 |
| Essential oil use in body moisturizer preparations (0 to 5 months) |
1.07 | 28 |
| Essential oil use in topical preparations for abraded/damaged skine (0 to 5 months) |
0.51 | 59 |
| Food flavoring agent (1+ years) |
0.00002 | > 100 000 |
Abbreviations: MOE, margin of exposure; NOAEL, no observed adverse effects level
a Exposure estimates are presented for the highest exposed age group relevant for each scenario.
b Exposure estimates are adjusted to account for the percent composition of the major components of cade oil used to characterize risk to the substance (that is, cresols). For dermal, oral, and inhalation scenarios, an adjustment factor of 0.14 is used for cresols.
c For dermal exposures an absorption value of 80%, based on dermal absorption data for chlorocresol (ECCC, HC 2021a), is used.
d MOEs are presented using the selected NOAEL of 30 mg/kg bw/day, based on clinical signs of CNS stimulation in juvenile and adult rodents exposed orally to m-cresol from 18 to 90 days.
e This scenario assumes that the amount applied is completely absorbed via the dermal route as it is applied to abraded/damaged skin.
For dermal exposures to cade oil, comparisons between the critical effect level and estimates of exposure from uses in perfumes (roll-on) as well as from DIY consumer product uses in facial steamers, massage oil preparations, body moisturizer preparations, and topical preparations on abraded/damaged skin resulted in MOEs ranging from 28 to 59, which are below 100 and are considered potentially inadequate to address uncertainties in the health effects and exposure data used to characterize risk. Factors including interspecies and intraspecies differences were considered during the determination of the adequacy of the MOEs. Given the number of products containing cade oil available on the Canadian market, it is possible that exposure from several different types of products may occur on the same day that is, aggregate exposure), in which case the MOE would be even lower.
Resulting MOEs from uses in face moisturizers and essential oil uses in aromatic diffusers and baths range from 111 to 30,000, and are considered adequate to address uncertainties in the health effects and exposure data used to characterize risk.
Comparisons between the critical effect level and the estimates of exposure from use as a food flavouring agent resulted in an MOE that is considered adequate to address uncertainties in the health effects and exposure data used to characterize risk.
| Scenario | Adjusted exposure estimate (mg/m3)a,b | MOEc |
|---|---|---|
| Perfume (roll-on) (19+ years) | 0.03 | 300 |
| Face moisturizer (19+ years) |
0.003 | 3000 |
| Essential oil use in aromatic diffuser, users and bystanders (0 to 19+ years) |
0.38 | 24 |
| Essential oil use in facial steamer, users (4 to 19+ years) | 0.23 | 39 |
| Essential oil use in facial steamer, bystanders (0 to 19+ years) |
0.05 | 180 |
| Essential oil use in baths, (4 to 19+ years) |
0.07 | 129 |
| Essential oil use in massage oil preparations (19+ years) |
0.009 | 1000 |
| Essential oil use in body moisturizers (14+ years) |
0.03 | 300 |
Abbreviations: MOE, margin of exposure; LOAEC, lowest observed adverse effect concentration
a Exposure estimates are presented for the highest exposed age group relevant for each scenario.
b Exposure estimates are adjusted to account for the percent composition of the major components of cade oil used to characterize risk to the substance (that is, cresols). For dermal, oral, and inhalation scenarios, an adjustment factor of 0.14 is used for cresols.
c MOEs are presented using the selected LOAEC of 9 mg/m3, based on irritation and morphological changes in the respiratory tract of rats exposed to o-cresol by whole body inhalation for 90 days.
Comparison between the critical effect level and the estimates of inhalation exposure from uses of cade essential oil in aromatic diffusers, facial steamers, and baths resulted in MOEs ranging from 24 to 180, which are below 300 and are considered potentially inadequate to address uncertainties in the health effects and exposure data used to characterize risk. Factors including interspecies and intraspecies variability as well as the use of a LOAEC for determination of risk were considered in the determination of adequacy of the MOEs. Inhalation exposure from the use of cade oil in body moisturizer preparations and massage oil preparations as well as in perfumes (roll-on) and face moisturizers resulted in MOEs of 300 to 3000, which are considered adequate.
A BMDL10 of 376 mg/kg bw/day, identified in the screening assessment report for cresols (ECCC, HC 2016a) on the basis of neoplastic effects in the forestomach of female mice exposed orally to a cresol mixture over 2 years, was identified as the most appropriate endpoint to characterize the risk from lifetime exposure to cade oil. LADDs and resulting MOEs are presented in Table 7‑7.
| Scenario | LADD (mg/kg bw/day)a |
MOEb |
|---|---|---|
| Perfume (roll-on) | 0.24 | 1 567 |
| Face moisturizer | 0.04 | 9 400 |
| Essential oil use in aromatic diffuser | 0.24 | 1 566 |
| Essential oil use in facial steamer | 0.26 | 1 446 |
| Essential oil use in bath | 0.004 | 94 000 |
| Essential oil use in massage oil preparations | 0.02 | 18 800 |
| Essential oil use in body moisturizers | 0.48 | 783 |
| Essential oil use in topical preparations to treat abraded skin | 0.08 | 4 700 |
| Food intake from use as a flavouring agent | 0.00002 | > 100 000 |
Abbreviations: MOE, margin of exposure; BMDL, benchmark dose level
a Exposure estimates are adjusted to account for the percent composition of the major components of cade oil used to characterize risk to the substance (that is, cresols). For dermal, oral, and inhalation scenarios, an adjustment factor of 0.14 is used for cresols.
b MOEs are presented using the selected BMDL10 of 376 mg/kg bw/day, identified in the screening assessment for cresols (ECCC, HC 2016a), on the basis of neoplastic effects in the forestomach of female mice exposed orally to a cresol mixture over 2 years
With respect to neoplastic effects, the MOEs between the critical effect level and the estimates of lifetime exposure to cade oil from perfumes (roll-on) and face moisturizers, as well as from essential oil uses in aromatic diffusers, facial steamers, body moisturizers, and topical preparations for abraded/damaged skin, range from 783 to 9400 which are below 10000, (to account for uncertainties with respect to interspecies extrapolation, intraspecies extrapolation, the POD, and the adequacy of the database) and are considered potentially inadequate to address uncertainties in the health effects and exposure data used to characterize risk. Given the number of products containing cade oil available on the Canadian market, it is possible that exposure may result from the use of several types of products containing this substance on the same day (that is, aggregate exposure), in which case the MOE would be even lower. MOEs for cade oil used in baths, massage oils, and as a food flavouring agent are considered adequate.
7.1.5 Uncertainties in evaluation of risk to human health
The key sources of uncertainty are presented in Table 7‑8.
| Key source of uncertainty | Impact |
|---|---|
| The concentration of the main components of cade oil depends on the conditions of oil extraction and the origin, species, and growing conditions (temperature, soil, and geography) of the plant. Therefore, the precise composition of cade oil present in products available to consumers is unknown, which represents an uncertainty in the assessment. | +/- |
| Data from a structurally similar chemical, chlorocresol, was used to inform the potential dermal absorption of cresols, the major components of cade oil used to characterize risk. | +/- |
| There are no chronic studies, reproductive/developmental toxicity studies, or carcinogenicity studies identified for cade oil. | +/- |
| There are no adequate animal studies examining the repeated-dose toxicity of cade oil for any of the relevant routes of exposure ( that is, dermal, oral, inhalation). Hazard data from the main components (cresols, cadinene, thujopsene, τ-muurolol, alpha-cedrene, widdrol and epi-cubenol) were used to inform the health effects assessment, where applicable. | +/- |
| Most of the health effects endpoints are derived from analogue data due to a lack of adequate data to inform the hazard database. | +/- |
| Cresols were used in support of oral, dermal, and inhalation PODs with a concentration range of 2% to 14% in cade oil. The maximum inclusion value of 14% (upper-bound concentration identified in the literature) was considered in deriving the appropriate exposure estimates. | + |
| Virginia cedarwood oil, an analogue of thujopsene and cedrene, was used in support of the dermal POD. | +/- |
| The presence and concentration of PAHs in cade oil extracts cannot be ascertained. | +/- |
| The risk characterization did not take into consideration the potential for additive, synergistic, or antagonistic effects of components within UVCBs. | +/- |
| The potential use of more than one product by a single person in a day (that is, aggregate exposure) was not considered. This may potentially underestimate exposure to some individuals. | - |
+ = uncertainty with potential to cause over-estimation of exposure/risk; - = uncertainty with potential to cause under-estimation of exposure risk; +/- = unknown potential to cause over- or under-estimation of risk.
7.2 Jonquil oil
7.2.1 Exposure assessment
Environmental media
No measured data on concentrations of jonquil oil in environmental media were identified in Canada or elsewhere. The substance was not reported to be manufactured or imported in quantities greater than the reporting threshold of 100 kg, on the basis of information submitted in response to a CEPA section 71 survey (Environment Canada 2013). In consideration of low reported quantities of the substance in Canada, exposure to jonquil oil from environmental media is expected to be minimal.
Products available to consumers
Jonquil oil is not permitted to be used as a food additive, and was not identified as an ingredient in food-packaging materials or incidental additives, or as an ingredient in NHPs, NPDs, cosmetics, or pesticides in Canada. The substance was identified as potentially present at a low concentration (that is, less than 0.00001%) in a manual dish detergent. Jonquil oil is available to consumers in Canada as a pure essential oil (that is, present at 100%). The oil can be used in DIY applications such as formulating into massage oils or body moisturizers, or it can be added to baths, facial steamers, or aromatic diffusers (Tisserand Institute [accessed 2021]; doTERRA 2022a). Table 7‑9 presents estimates of exposure to jonquil oil when it is used as an essential oil in these applications.
Exposure estimates were adjusted by 19% (Hanks 2002) to account for the upper-bound percent composition of the major component associated with non-neoplastic effects, benzyl benzoate, present in the substance (see section 7.2.3 for additional information on the health effects of the major components of jonquil oil). The extent of dermal absorption of benzyl benzoate applied to the skin was determined in an in vivo study on rhesus monkeys. The mean absorption of benzyl benzoate under non-occluded conditions was reported to be 57% in an acetone vehicle. The authors noted that absorption of benzyl benzoate increased to approximately 70% when it was used in a moisturizing lotion vehicle (Bronaugh et al. 1990). Absorption occurred primarily within the first 24 -hours after topical application and may be predictive of the ability of benzyl benzoate to be absorbed when applied to human skin (Bronaugh et al. 1990). On the basis of this study data, an absorption value of 70% is used to estimate the potential dermal exposure to benzyl benzoate present in jonquil oil, to characterize risk of non-neoplastic effects.
| Scenario | Route | Concentration (%) |
Exposurea,b, mg/kg bw/day (age range) |
|---|---|---|---|
| Essential oil use in aromatic diffuser | Dermal and inhalation | 100c | Dermal, usersd: 0.32 (9 to 13 years) Inhalatione, users and bystanders: 0.37 (1 year) |
| Essential oil use in facial steamer, user | Dermal and inhalationc | 100f | 0.85 (4 to 8 years) |
| Essential oil use in facial steamer, bystander | Inhalationc | 100f | 0.04 (1 year) |
| Essential oil use in baths | Dermal and inhalationc | 100f | 0.05 (9 to 13 years) |
| Essential oil formulated in body moisturizers | Dermal and inhalationc | 3g | 1.27 (0 to 5 months) |
| Essential oil formulated in massage oils | Dermal and inhalationc | 3g | 1.15 (0 to 5 months) |
| Manual dish detergent | Dermal | <0.00001 | 7.40E-06 |
a Exposure estimates are adjusted to account for the percent composition of the major component of jonquil oil use to characterize risk of non-neoplastic effects (that is, benzyl benzoate). For dermal, oral, and inhalation scenarios, an adjustment factor of 0.19 is used for benzyl benzoate (Hanks 2002).
b For dermal exposures an absorption value of 57%, based on dermal absorption data for benzyl benzoate (Bronaugh et al. 1990), is used.
c Assumes that 920 mg of pure essential oil is added to the device (~18 drops of jonquil oil).
d Dermal exposure from this use is expected to be incidental during refilling of an aromatic diffuser and considered to be applicable to users only (for example, those 9 – 13, 14 – 18, and 19+ years).
e Inhalation exposures were converted to internal doses using default inhalation rates and body weights from Health Canada (2021). Additional details are presented in Appendix A.
f Assumes approximately 10 drops of pure essential oil is used. Details on the corresponding product amount are presented in Appendix A.
g Essential oils used in massage oil and body moisturizer preparations are typically diluted prior to use (Bremmer et al. 2006, Tisserand Institute [accessed 2021]).
Table 7‑10 outlines the estimated LADDs from exposure to jonquil oil. These values were adjusted by 20% (Hanks 2002) to account for the upper-bound percent composition of the major component associated with neoplastic effects, trans-methylisoeugenol, present in the substance (see section 5.2.6 for additional information on the health effects of the major components of jonquil oil). In the absence of chemical-specific dermal absorption data on trans-methylisoeugenol, information on the isomer methyl eugenol is used. The substances have the same molecular weight, and very similar vapour pressure, water solubility, and log Kow values (EC, HC 2010). In HC and Environment Canada’s assessment of methyl eugenol, a dermal absorption value of 40% was used for products applied to skin (European Commission 2000, as cited in EC, HC 2010). On the basis of data for methyl eugenol, a dermal absorption value of 50% for trans-methylisoeugenol is used to estimate the potential dermal exposure of this component present in jonquil oil, to characterize risk from neoplastic effects. Further details on the parameters used to estimate exposures are presented in Appendix A.
| Scenario | Route of exposure | Concentration (%) | LADDa,b (mg/kg bw/day) |
|---|---|---|---|
| Essential oil use in aromatic diffuserc | Dermal and inhalation | 100 | 0.27 |
| Essential oil use in facial steamerc | Dermal and inhalation | 100 | 0.26 |
| Essential oil use in baths | Dermal and inhalation | 100 | 0.01 |
| Essential oil formulated in body moisturizer | Dermal and inhalation | 3 | 0.44 |
| Essential oil formulated in massage oil | Dermal and inhalation | 3 | 0.02 |
| Cleaning product use | Dermal | <0.00001 | 4.21E-06 |
Abbreviation: LADD, lifetime average daily dose
a Exposure estimates are adjusted to account for the percent composition of the major component of jonquil oil use to characterize risk of neoplastic effects (that is, trans-methylisoeugenol). For dermal, oral, and inhalation scenarios, an adjustment factor of 0.20 is used for benzyl benzoate (Hanks 2002).
b For dermal exposures an absorption value of 50%, based on dermal absorption data for methyl eugenol (EC, HC 2010), is used.
c LADDs for bystander exposure were not derived
7.2.2 Selection of analogues
A read-across approach using data from analogues, where appropriate, has been used to inform the health effects assessment of jonquil oil. As jonquil oil is a UVCB, 2 analogues were selected that were structurally similar and/or functionally similar to 2 major components (that is, trans-beta-ocimene and trans-methylisoeugenol) and had relevant empirical data that could be used to read across to toxicological endpoints with limited empirical data.
| CAS RN | DSL name (common name) |
Chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
| 123-35-3 | 1,6-Octadiene, 7-methyl-3-methylene- (Beta-myrcene) |
 C10H16 |
136.24 |
| CAS RN | DSL name (common name) |
Chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
| 93-16-3 | Benzene, 1,2-dimethoxy-4-(1-propenyl)-(Methylisoeugenol) |  C11H14O2 |
178.23 |
| 93-15-2 | Benzene, 1,2-dimethoxy-4-(2-propenyl)- (Methyl eugenol) |
 C11H14O2 |
178.23 |
7.2.3 Health effects assessment
No international assessments and no relevant toxicological studies were identified for jonquil oil and information on major components was used to inform the jonquil oil health effects assessment. The main components (that is, components of jonquil oil that have concentrations of generally greater than 10%) reported in the literature include methyl benzoate (23% to 46%), alpha-linolenic acid (19%), linalool (18%), alpha-terpineol (0.1% to 23%), benzyl benzoate (1% to 19%), trans-beta-ocimene (26% to 35%), and trans-methylisoeugenol (20%).
Methyl benzoate
Methyl benzoate was evaluated in the screening assessment on the Benzoates Group conducted under the CMP (ECCC, HC 2019). It was concluded that methyl benzoate exhibited low hazard properties, and the potential risk to human health was considered to be low. A literature search was conducted from one year prior to the Benzoates Group screening assessment (that is, 2018). No health effects studies that could impact the health effects assessment were identified.
Alpha-linolenic acid
Alpha-linolenic acid was evaluated in the screening assessment on the Fatty Acids and Derivatives Group conducted under the CMP (ECCC, HC 2020b). Alpha-linolenic acid was not identified as having systemic health effects of concern, and the risk to human health is considered to be low. A literature search was conducted from one year prior to the publication of the Fatty Acids and Derivatives Group screening assessment (that is, 2019). No health effects studies that could impact the health effects assessment, were identified.
Linalool
The health effects of linalool were characterized in the draft screening assessment of the Terpenes and Terpenoids - Acyclic, Monocyclic, and Bicyclic Monoterpenes Group conducted under the CMP. No health effects of concern were identified, and its overall toxicity was deemed to be low (ECCC, HC 2020b).
Alpha-terpineol
The health effects of alpha-terpineol acid have been characterized in the draft screening assessment of the Terpenes and Terpenoids - Monocyclic and Bicyclic Sesquiterpenes Group conducted under the CMP (ECCC, HC 2021b). The lowest critical effect level identified for alpha-terpineol was a NOAEL of 250 mg/kg bw/day based on impairment of male fertility due to a reduced number or complete absence of spermatozoa in the presence of degenerate spermatogenic cells in the epididymides and seminiferous tubular atrophy/degeneration in the testes at 750 mg/kg bw/day from a combined repeated-dose and reproduction/developmental toxicity study (OECD TG 422) in rats (ECCC, HC 2021b). Alpha-terpineol was not found to be mutagenic in bacterial assays, did not induce gene mutations in mammalian cells, and was not found to be carcinogenic in A/He mice (ECCC, HC 2021b).
Benzyl benzoate
The health effects of benzyl benzoate have also been characterized in the draft assessment of the Terpenes and Terpenoids - Phenylpropanoids and Aldehydes Group conducted under CMP (ECCC, HC 2024). In a developmental toxicity study, pregnant Wistar rats (5/dose) were administered 0, 25, or 100 mg/kg bw/day benzyl benzoate by gavage from gestational day (GD) 0 to 20 (Koçkaya and Kιlιç 2014). A LOAEL of 25 mg/kg bw/day was identified from the study on the basis of the dose-dependent increase in resorptions, edema in the maternal brains and fetal skeletal variations.
In another developmental study, pregnant Wistar rat (21/dose) were administered 0, 0.4, or 1% benzyl benzoate (equivalent to 0, 26, or 646 mg/kg bw/day, as determined by the authors) via their diet from GD 1 to PND 20 (Morita et al. 1981, as cited in ECHA 2022a). A LOAEL of 26 mg/kg bw/day was selected on the basis of the decrease in body weight in pups (no further details were provided) at both doses in the absence of maternal toxicity, and the increase in fetal malformations in the absence of maternal toxicity at 646 mg/kg bw/day.
In an oral subchronic study, male Sprague-Dawley rats (n=8/group) were administered benzyl benzoate (99% purity) at doses of 0, 25, or 100 mg/kg bw/day by gavage for 90 days (Süloğlu et al. 2022). Clinical signs, body weights, food/water consumption were recorded throughout the study. Hematological, clinical chemistry, morphological, and histopathological examinations (liver, kidney, thymus, cauda epididymis, prostate, testes) were also conducted. No treatment-related changes in body weights, clinical signs, food/water consumption, hematological, or clinical chemistry changes were identified. However, significant histopathological changes were observed in the liver, kidneys (that is, congestion, tubular degeneration, mononuclear cell infiltration, tissue lysis), thymus (that is, increase in Hassall’s bodies, lipid, degeneration, congestion, fibrosis, and decreased lymphocytes), and reproductive system tissues (that is, vacuolization and irregular secretion in the prostate, increased connective tissue in the epididymis, and presence of cells in the lumen) in the treated animals. No changes in sperm count, sperm production, or sperm morphologies were noted. A LOAEL of 25 mg/kg bw/day was considered for this study on the basis of histopathological changes observed.
Benzyl benzoate was not mutagenic in bacterial mutagenicity assays (Florin 1980, as cited in Api et al. 2020). Studies examining the potential carcinogenic effects following exposure to benzyl benzoate have not been identified.
Trans-beta-ocimene
The health effects dataset for trans-beta-ocimene was considered to be limited and a read-across approach was used. Beta-myrcene was identified to be a suitable analogue for trans-beta-ocimene since the substances are structurally and functionally similar and beta-myrcene had relevant empirical data for use in hazard characterization.
The health effects of beta-myrcene have been characterized in the draft screening assessment of the Terpenes and Terpenoids - Acyclic, Monocyclic, and Bicyclic Monoterpenes Group conducted under the CMP (ECCC, HC 2020b). The lowest effect level identified in the health effects database for non-neoplastic effects was 115 mg/kg bw/day based on effects on the liver and developmental effects (for example, reduced pup weights) at the next dose level of 250 mg/kg bw/day (ECCC, HC 2020b). The International Agency for Research on Cancer (IARC) has classified beta-myrcene as possibly carcinogenic to humans in Group 2B (IARC 2014). The Office of Environmental Health Hazard Assessment (OEHHA) has added beta-myrcene to the list of chemicals established by California State to cause cancer for purposes of Proposition 65 (OEHHA 2018). EFSA deemed beta-myrcene not to pose a safety concern when used as flavouring substances at estimated levels of intake.
In a 2-year study conducted by the NTP, F344/N rats (50/sex/dose) were administered 0, 250, 500 or 1,000 mg/kg bw/day of beta-myrcene by gavage (NTP 2010). A statistically significant increase in the incidence of renal tubule adenomas and carcinomas was observed in the male rats. The authors concluded that under the conditions of this study, there was clear evidence of carcinogenic activity of beta-myrcene in male rats and equivocal evidence in females (NTP 2010). The same authors conducted another 2-year study in which B6C3F1 mice received 0, 250, 500 or 1000 mg/kg bw/day beta-myrcene by gavage, 5 days per week (NTP 2010). Incidences of hepatocellular adenoma, carcinoma and hepatoblastoma were significantly greater than controls. The authors concluded that there was clear evidence of carcinogenic activity of beta-myrcene in male mice and equivocal evidence in females (NTP 2010).
The US FDA analyzed the data from the NTP (2010) studies and concluded that under the test conditions of the studies, myrcene induced renal tubular tumours in F344 rats and hepatocellular tumours in B6C3F1 mice (US FDA 2018a). The US FDA derived a BMDL10 of 90 mg/kg bw/day based on the most sensitive endpoint, the combined renal tubular adenomas and carcinomas, using Benchmark Dose Software (BMDS) (unknown version). Because this value was based on exposures that occurred 5 days per week, the value was adjusted to calculate a 7-day exposure. The resultant BMDL10 value was 64 mg/kg bw/day (US FDA 2018b).
All in vitro genotoxicity studies, including bacterial mutagenicity assays, a chromosomal aberration study and a mouse lymphoma assay, did not show any evidence of genotoxicity. Beta-myrcene is not considered to be genotoxic (ECCC, HC 2020b).
Trans-methylisoeugenol
Trans-methylisoeugenol is one of 2 isomers of methylisoeugenol, with cis-methylisoeugenol being the other one. No international assessments and no relevant health effects studies were identified for trans-methylisoeugenol alone and the health effects data associated with methylisoeugenol (naturally occurring as a mixture of cis- and trans- isomers) were taken into consideration.
In a study by Purchase et al. (1992), Sprague-Dawley rats (n=16/sex/group) were fed 0, 30, 100, or 300 mg/kg bw/day of methyl isoeugenol (containing 11.7% cis-isomer and 86.8% trans-isomer) in the diet for 28 to 31 days. At the highest dose level of 300 mg/kg bw/day, there were significant increases in the levels of lymphocytes, white blood cells, reticulocytes, and mean cell volume. Effects were also observed in the liver (that is, significant increase in liver weights, levels of ALT, and multifocal necrosis), the kidneys (that is, interstitial nephritis), and the Harderian gland (that is, inflammation with acinar degeneration). A NOAEL of 100 mg/kg bw/day was selected from the study on the basis of hematological/clinical chemistry changes, as well as histopathological changes in the liver/kidneys/Harderian gland at the next dose level of 300 mg/kg bw/day.
In a subchronic toxicity study, F344/DuCrj rats (n=10/sex/group) were administrated methylisoeugenol at doses of 8, 40, and 200 mg/kg bw/day by gavage for 13 weeks (Akagi et al, 2019). In males given 200 mg/kg bw/day, there was a decrease in body weight gain and triglyceride levels. At the same dose level, hepatocyte hypertrophy was observed in the animals, as well as an increase in absolute/relative liver weights. On the basis of these results, the authors identified a NOAEL of 40 mg/kg bw/day based on histological and functional changes in the liver of animals at the next dose tested of 200 mg/kg bw/day.
In a reproduction/developmental toxicity study conducted according to Organisation for Economic Co-operation and Development (OECD) Test Guideline (TG) 421, Crl:CD (SD) rats were fed diet with methylisoeugenol at concentrations of 1500, 4500, or 15000 ppm, equivalent to 94, 272 or 769 mg/kg bw/day for males and 103, 289 or 826 mg/kg bw/day for females, respectively (Anonymous 2015, as cited in ECHA, 2022b). During lactation, the mean achieved doses were higher at 207, 601 or 1793 mg/kg bw/day, respectively. Males were treated for a minimum of 4 consecutive weeks, including 2 weeks prior to pairing. Females were treated for 2 weeks prior to pairing, throughout mating and gestation and until day 7 of lactation. At the highest dose tested, food intake was significantly lower than controls and body weight loss was recorded during the first week of treatment and stayed low for females throughout gestation and lactation. At the same dose level, a significantly lower mean number of implantations and live litter sizes was observed. In addition, body weights of one-day-old offspring were significantly lower than controls, and their growth between PNDs 1 and 7 was significantly decreased at the highest dose. Pups were cold to touch and/or had little milk in their stomach. The authors identified a NOAEL of 4500 ppm (equivalent to 272 mg/kg bw/day) based on decreased food consumption and weight gain at the next dose tested of 15000 ppm (769 mg/kg bw/day) for parental toxicity (Anonymous 2015, as cited in ECHA, 2022b). With respect to reproductive and developmental effects, the same NOAEL of 272 mg/kg bw/day was considered on the basis of reduced implantations, litter sizes, and pup weights at the next dose level of 769 mg/kg bw/day.
No chronic toxicity or carcinogenicity studies were identified for methyl isoeugenol. Therefore, data on methyl eugenol, which is structurally very similar to trans-methylisoeugenol, was used to address these data limitations. Methyl eugenol was evaluated under the CMP in 2010 (EC, HC 2010) and also as a major component of certain substances in the draft assessment of the Terpenes and Terpenoids Phenylpropanoids and Aldehydes Group (ECCC, HC 2024). Based principally on the weight of evidence–based assessments of international or other national agencies, the critical health effect for methyl eugenol was identified to be carcinogenicity. A literature search was conducted from one year prior to the methyl eugenol screening assessment (that is, 2009). No health effects studies which could impact the health effects assessment were identified. In 2013 (that is, after the publication of the screening assessment on methyl eugenol), the IARC published an assessment on methyl eugenol and concluded it to be a Group 2B carcinogen (possibly carcinogenic to humans) (IARC 2013).
In experimental animal studies, methyl eugenol induced tumours in 2 species, in both genders, and at multiple sites. In the standard 2-year NTP carcinogenicity studies with rats and mice, methyl eugenol induced multiple types of tumours in liver and neuroendocrine tumours in the glandular stomach in both males and females in a dose-related manner. In addition, methyl eugenol significantly increased the incidences of kidney neoplasms, mammary gland fibroadenoma, malignant mesothelioma, subcutaneous fibroma or fibrosarcoma in male rats; and the liver tumours, hepatocholangioma or hepatocholangiocarcinoma, in both male and female rats (NTP 2000).
The US FDA analyzed the data from NTP (2000) and concluded that under the test conditions of the studies, methyl eugenol induced cancer in rodents (US FDA 2018a). The US FDA derived BMDL10 values of 7.9 and 7.7 mg/kg bw/day (using BMDS and PROAST software, respectively) based on the combined hepatic carcinomas and adenomas (US FDA 2018c). However, the details regarding their derivation were limited. There are also several BMDL10 values available in the scientific literature based on the same 2-year NTP carcinogenic study (2000). For example, BMDL10 values were determined to be 15.3 to 34 mg/kg bw/day for male rats and 48.8 to 73.6 mg/kg bw/day for female rats for methyl eugenol (Van den Berg et al. 2011). In another study by Suparmi et al. (2019), the BMDL10 value for male rats was determined to be 22.2 mg/kg bw/day and 66.5 mg/kg bw/day for female rats (Suparmi et al. 2019). EFSA and the French Agency for Food, Environmental and Occupational Health & Safety (ANSES) selected the BMDL10 of 22.2 mg/kg bw/day to assess the natural occurring levels of methyl eugenol in essential oils in food. Other studies were also considered but did not follow US EPA or EFSA guidelines for cancer analysis (Rietjens et al. 2008; Smith et al. 2010). It has been noted that there were other tumours (for example, malignant mesothelioma, considered to be rare) observed in the carcinogenicity study on rats (NTP 2000). These tumours were not amenable to benchmark dose modelling due to data limitations.
With respect to non-cancer critical effects, a significantly increased dose-related incidence of non-neoplastic lesions in the liver and glandular stomach was observed in male and female rats and mice dosed with methyl eugenol in the 2-year chronic studies. Based on the effects of the non-neoplastic lesions (hypertrophy, hyperplasia, etc.), the LOAEL was identified to be 37 mg/kg bw/day in male and female rats and mice (NTP 2000).
With respect to genotoxicity, methyl eugenol was genotoxic in a number of in vivo assays in experimental animals, in vitro assays in mammalian cells and its metabolites were mutagenic in some Salmonella test strains. A summary of genotoxicity assays conducted on methyl eugenol can be found in ECCC, HC (2010). Genotoxicity studies conducted after the publication of the screening assessment on methyl eugenol (ECCC, HC 2010) revealed that, overall, the genotoxicity data support a conclusion that methyl eugenol is genotoxic to mammalian somatic cells in vitro and in vivo. This conclusion is consistent with the opinion of the Scientific Committee on Food on the safety of the presence of methyl eugenol in food that methyl eugenol is genotoxic and carcinogenic and therefore “the existence of a threshold can not be assumed” (European Commission 2001, as cited in EC HC 2010).
7.2.4 Characterization of risk to human health
The health effects datasets for jonquil oil are considered to be limited. In order to inform the risk assessment and address data limitations, the health effects data available on the major components and their analogues was used. The components associated with the lowest effect levels in the hazard databases were used to inform the characterization of risk following exposure to jonquil oil.
For non-neoplastic effects, a developmental toxicity study conducted using benzyl benzoate in rats (Koçkaya and Kιlιç 2011) was considered to be the most relevant study for the characterization of risk following exposure to jonquil oil. A LOAEL of 25 mg/kg bw/day was identified based on developmental effects (for example, resorptions, fetal skeletal variations). This critical effect level is also consistent with that identified in a 90-day oral subchronic study (with the same substance) in rats based on general toxicity (Suloglu et al. 2022) and was considered applicable to both short-term intermittent and daily exposure scenarios.
For carcinogenic effects, a BMDL10 of 22.2 mg/kg bw/day derived on the basis of hepatocellular carcinomas in male rats administered methyl eugenol in a 2-year study (NTP 2000) was considered to be the most relevant endpoint for the characterization of risk.
Table 7‑13 and Table 7‑14 provides all the relevant exposure estimates, critical effect levels, and resulting MOEs for characterization of risk to human health from exposure to jonquil oil in DIY consumer applications and from use in a cleaning product.
| Scenario | Exposurea,b,c (mg/kg bw/day) | MOEd |
|---|---|---|
| Essential oil use in aromatic diffuser, users (9–13 years) |
0.49 | 51 |
| Essential oil use in aromatic diffuser, bystander (1 year) |
0.37 | 68 |
| Essential oil use in facial steamer, user (4–8 years) |
0.85 | 29 |
| Essential oil use in facial steamer, bystander (1 year) |
0.04 | 624 |
| Essential oil use in baths (9–13 years) |
0.05 | 500 |
| Essential oil formulated in body moisturizers (0–5 months) |
1.27 | 20 |
| Essential oil formulated in massage oils (0–5 months) |
1.15 | 22 |
| Cleaning product use (19+ years) |
7.40E-05 | > 100 000 |
Abbreviations: MOE, margin of exposure; LOAEL, lowest observed adverse effects level
a Exposure estimates are presented for the highest exposed age group relevant for each scenario.
b Exposure estimates are adjusted to account for the percent composition of the major component of jonquil oil used to characterize risk of non-neoplastic effects of the substance (that is, benzyl benzoate). For these scenarios, an adjustment factor of 0.19 is used (Hanks 2002).
c For dermal exposures, an absorption value of 70%, based on dermal absorption data for benzyl benzoate (Bronaugh et al. 1990), is used.
d MOEs are presented using the selected LOAEL of 25 mg/kg bw/day, based on developmental effects and general toxicity from oral, short-term and sub-chronic studies in rats.
Comparisons between the critical effect level and estimates of exposure to jonquil essential oil from uses in DIY consumer applications such as aromatic diffusers (for users and bystanders) and facial steamers (for users and bystanders), as well as from use in bath oils, massage oil preparations and body moisturizer preparations resulted in MOEs ranging from 20 to 500, which are less than 1000 and considered potentially inadequate to address uncertainties in the health effects and exposure data used to characterize risk. Factors including interspecies and intraspecies differences and the inability to identify a NOAEL from the database were considered when determining the adequacy of the MOEs. The resulting MOE from the use of jonquil oil in a manual dish detergent is considered adequate.
| Scenario | LADD (mg/kg bw/day)a,b |
MOEc |
|---|---|---|
| Essential oil use in aromatic diffuser | 0.27 | 82 |
| Essential oil use in facial steamer | 0.26 | 85 |
| Essential oil use in baths | 0.01 | 2 220 |
| Essential oil formulated in body moisturizer | 0.44 | 101 |
| Essential oil formulated in massage oil | 0.02 | 1 110 |
| Cleaning product use | 4.21E-06 | > 100 000 |
Abbreviations: MOE, margin of exposure; BMDL, benchmark dose level
a Exposure estimates are adjusted to account for the percent composition of the major component of jonquil oil used to characterize risk of neoplastic effects to the substance (that is, trans-methylisoeugenol). For these scenarios, an adjustment factor of 0.20 is used (Hanks 2002).
b For dermal exposures, an absorption value of 50%, based on dermal absorption data for methyl eugenol (HC 2021), is used.
c MOEs are presented based on the selected BMDL10 of 22.2 mg/kg bw/day, based on hepatocellular carcinomas in male rats from an oral, 2-year study.
With respect to neoplastic effects, MOEs between the critical effect level and the estimates of lifetime exposure to jonquil oil from DIY consumer uses (use of jonquil essential oil in aromatic diffusers, facial steamers, and bath oils, as well as in massage oil preparations and body moisturizer preparations) range from 82 to 2 220, which are below 10 000 (to account for uncertainties with respect to interspecies extrapolation, intraspecies extrapolation, the POD, and the adequacy of the database) and are considered potentially inadequate to address uncertainties in the health effects and exposure data used to characterize risk. The use of jonquil oil in product manual dish detergent is considered adequate.
7.2.5 Uncertainties in evaluation of risk to human health
The key sources of uncertainty are presented in Table 7‑15.
| Key source of uncertainty | Impact |
|---|---|
| The concentration of the main components of jonquil oil depends on the conditions of oil extraction and the origin, species, and growing conditions (temperature, soil, and geography) of the plant. Therefore, the precise composition of jonquil oil present in products available to consumers is unknown, which represents an uncertainty in the assessment. | +/- |
| Data from a structurally similar chemical, methyl eugenol, was used to inform the potential dermal absorption of trans-methyl isoeugenol, a major component of jonquil oil used to characterize risk from neoplastic effects. | +/- |
| The health effects data for jonquil oil were considered to be limited. Health effects data from the major components and their analogues were used for risk characterization purposes. | +/- |
| The risk characterization did not take into consideration the potential for additive, synergistic, or antagonistic effects of components within UVCBs. | +/- |
+ = uncertainty with potential to cause over-estimation of exposure/risk; - = uncertainty with potential to cause under-estimation of exposure risk; +/- = unknown potential to cause over- or under-estimation of risk.
7.3 Norlimbanol
7.3.1 Exposure assessment
No measured data regarding concentrations of norlimbanol in environmental media were identified in Canada or elsewhere. The substance was not reported to be manufactured or imported in Canada above the reporting threshold of 100 kg, according to information submitted in response to a CEPA section 71 survey (Environment Canada 2013). In consideration of low reported quantities of the substance in Canada, exposure of the general population to norlimbanol from its presence in environmental media is expected to be minimal.
Norlimbanol is primarily used as a fragrance ingredient at low concentrations in products available to consumers. Data from a 2021 RIFM assessment on norlimbanol reported a 95th percentile concentration of 0.62% from its use in fine fragrances (RIFM 2021a). Other types of products reported to contain norlimbanol include body moisturizers and lotions, deodorants, shaving products, hair care products, perfumes and colognes, dishwashing liquids, laundry detergents, and air fresheners (Environment Canada 2013). Although it is used as a fragrance ingredient in products, norlimbanol has a low vapour pressure (0.013 Pa), and therefore, the main route of exposure is from dermally applied products available to consumers. Inhalation exposure may still occur from the use of air care or other spray-based products.
From the available data on products containing norlimbanol, a spray cologne containing the substance at a maximum concentration of 3% was identified as the sentinel dermal and inhalation exposure scenario (SDS 2017). Norlimbanol was reported at lower concentrations in the other products available to consumers described above, and exposure from these products is addressed in the exposure from spray cologne scenario. The total systemic exposure (that is, combined dermal and inhalation) from the use of a spray cologne containing 3% norlimbanol was estimated for a range of relevant age groups and only the highest one is presented in Table 7‑16, assuming dermal and inhalation absorption is equivalent to oral absorption. Details on the parameters used to derive these estimates are presented in Appendix A.
| Scenario | Route of exposure | Concentration | Systemic exposure (mg/kg bw/day) |
|---|---|---|---|
| Spray cologne | Dermal and inhalationa,b | 3% | 0.29 (4 to 8 years) |
a Data on the partitioning of norlimbanol between the dermal and inhalation routes were unavailable. Therefore, dermal and inhalation exposures were evaluated separately and combined to estimate systemic exposure. This is considered a conservative approach to estimate exposure to norlimbanol. Dermal absorption was assumed to be equivalent to oral absorption (that is, 100%).
b Inhalation exposures were converted to internal doses using default inhalation rates and body weights from Health Canada (2021).
7.3.2 Health effects assessment
Norlimbanol has been previously assessed by the RIFM (2021a). Two separate REACH dossiers were identified for norlimbanol for the racemic mixture (ECHA 2021h) and relative configuration of the molecule (ECHA 2021i).
Repeated-dose toxicity
In a combined repeated-dose toxicity study with a reproduction/developmental screening test, groups of 12 Sprague-Dawley Crl:CD rats (male and female) were given 0, 60, 200, and 600 mg/kg bw/day of norlimbanol by gavage. Males were treated once daily for 49 days while females were treated once daily for 2 weeks prior to mating, throughout gestation and 13 days after delivery. An additional 6 animals/sex/dose treated with 0 and 600 mg/kg bw/day were included as recovery groups for an additional 14 days. No treatment-related adverse effects were reported for body weight, food consumption, clinical chemistry, urinalysis, organ weights, or histopathology at any dose level. Mucous stools were reported at 600 mg/kg bw/day (males and females) in the main study and recovery groups. RIFM identified a NOAEL of 200 mg/kg bw/day, based on adverse effects observed (mucous stools) in the highest dose groups (RIFM 2021a). Reproductive and developmental effects are described in the next section.
In another combined repeated dose toxicity study with a reproduction/developmental screening test, groups of 10 Wistar Crl:Wi(Han) rats/sex/dose were given 0, 1000, 3000 or 10 000 ppm (approximately 72/96, 215/291 or 697/923 mg/kw bw/day for males/ females, respectively) of norlimbanol orally via feed for a minimum of 28 days followed by a 28-day recovery period. Only 5 animals/sex/dose were considered in the recovery group.
A reduction in body weight gain (up to -37% when compared to controls) was seen in males treated with the highest dose; after the second week of the recovery period, normal body weight gain was observed which did not result in significantly lower terminal body weights. A reduction in food consumption (up to 0.83x of controls) was observed in the pregnant and lactating females treated with the highest dose, which resulted in a 10% decrease in body weight at the end of the treatment. An increase in the incidence and severity of hyaline droplet accumulation was identified in the kidneys of males at the middle and highest doses which was accompanied by granular casts (degenerative alteration of kidneys). Effects observed at the highest dose were considered adverse as they were associated with tubular damage. However, hyaline droplet accumulation is considered to occur via an alpha2- globulin mechanism, which is specific to male rats and is irrelevant to humans. Hepatocellular hypertrophy was also observed in the liver of high-dose females which led to slightly higher liver weights. This effect was considered adaptive by the study authors in the absence of any degenerative or inflammatory changes. Increased incidence and severity of decreased lymphoid cellularity in the thymus in high-dose females was observed. No other toxicologically significant changes were reported in the remaining study parameters (that is, functional observations, mortality, clinical appearance, haematology and clinical biochemistry). In conclusion, a parental toxicity NOAEL of 3000 ppm, equivalent to 215 and 291 mg/kg bw/day for males and females respectively, was considered on the basis of reduced body weight gains in rats at the higher dose level (ECHA 2021h).
Norlimbanol is considered a skin sensitizer on the basis of positive results obtained from OECD guideline-compliant in vivo local lymph node assays (LLNAs) in mice (ECHA 2021h, 2021i). However, it is not considered a skin or eye irritant according to results from in vitro and in vivo assays (ECHA 2021h, 2021i).
No repeated-dose inhalation studies were identified in the scientific literature.
Reproductive and Developmental Toxicity
In the combined repeated-dose toxicity study with a reproduction/developmental toxicity screening test mentioned above (RIFM 2021a), no adverse effects related to mating, estrous cycle, or sperm parameters were observed in any dose groups. For developmental outcomes, no adverse effects were reported in the gestation period, gestation index, post-implantation loss rate, live birth index, clinical observations of pups, sex ratio of pups, viability index, T4, TSH, anogenital distance, nipple retention, or endocrine-disruption potential at any dose level. A significant reduction in the total litter size was reported in animals treated with 600 mg/kg bw/day. No additional details on these results were available (RIFM 2021). These effects were observed in conjunction with clinical signs in parental animals as described above.
In the second combined repeated dose toxicity study with a reproduction/developmental screening test mentioned above (ECHA 2021h), no treatment-related adverse effects were observed in any of the dose groups. Reproductive parameters that were examined included mating and fertility indices, precoital time, number of implantations, estrous cycle, and histopathological examination of reproductive organs. A lower body weight gain was observed in the pups of the highest dose group on PNDs 7 and 13. No other toxicologically relevant changes were noted in any other developmental parameters (that is, gestation, viability, lactation indices, duration of gestation, parturition, sex ratio, maternal care, litter size, postnatal pup development, clinical signs, and T4 thyroid hormone levels) (ECHA 2021h).
Genotoxicity and carcinogenicity
The genotoxicity of norlimbanol was assessed in multiple in vitro assays (for example, bacterial reverse mutation assay, mammalian cell gene mutation test, micronucleus test) with and without metabolic activation. Norlimbanol was found to be negative for cytotoxicity and genotoxicity (ECHA 2021hi; RIFM 2021a).
No carcinogenicity studies have been identified in the scientific literature for norlimbanol or its synonyms. Due to lack of neoplastic or pre-neoplastic effects observed in short-term repeated dose toxicity studies as well as its negative genotoxicity, norlimbanol is not expected to be carcinogenic.
7.3.3 Characterization of risk to human health
A NOAEL of 200 mg/kg bw/day from a combined repeated dose toxicity study with a reproduction/developmental toxicity screening test was identified as the most appropriate end point to characterize the risk to human health from short- and long-term dermal and inhalation exposure to norlimbanol. This was based on significant reductions in the total litter size and production of mucous stools observed in rats at the next dose tested (600 mg/kg bw/day).
Table 7‑17 describes the exposure to norlimbanol from a spray cologne (sentinel scenario), the critical effect level, and the resultant MOE for the characterization of risk to human health.
| Scenario | Exposure estimate (mg/kg bw/day)a,b |
MOEc |
|---|---|---|
| Spray cologne (4 to 8 years) (daily dermal and inhalation exposure) |
0.29 | 690 |
Abbreviations: MOE, margin of exposure; NOAEL, no observed adverse effects level
a For risk characterization, exposure estimates are presented for the highest exposed age group.
b Dermal absorption was assumed to be equivalent to oral absorption (that is, 100%).
c MOEs are presented using the selected NOAEL of 200 mg/kg bw/day, based on reduced litter size and increased mucous stools in rats exposed to 600 mg/kg bw/day norlimbanol by oral gavage for 49 days.
This MOE is considered adequate to address uncertainties in the health effects and exposure data used to characterize risk. Factors including interspecies and intraspecies differences were considered during the determination of the adequacy of the MOEs.
7.3.4 Uncertainties in evaluation of risk to human health
The key sources of uncertainty are presented in Table 7‑18.
| Key source of uncertainty | Impact |
|---|---|
| Dermal absorption was considered to be equivalent to oral absorption (that is, 100%). | + |
| No short-term repeated-dose toxicity studies were identified for norlimbanol via the dermal or inhalation route of exposure. | +/- |
| There are no chronic repeated-dose toxicity studies identified for norlimbanol. | +/- |
| For dermal and inhalation exposures, absorption was assumed to be equivalent to oral absorption. | + |
+ = uncertainty with potential to cause over-estimation of exposure/risk; - = uncertainty with potential to cause under-estimation of exposure risk; +/- = unknown potential to cause over- or under-estimation of risk.
7.4 Verbena officinalis extract
7.4.1 Exposure assessment
Environmental media and food
No reports of monitoring were identified for Verbena officinalis extract in environmental media in Canada or elsewhere. The substance was not reported to be manufactured or imported in quantities greater than the reporting threshold of 100 kg according to information submitted in response to a CEPA section 71 survey (Environment Canada 2013). In consideration of low reported quantities of the substance in Canada, exposure to Verbena officinalis extract from environmental media is expected to be minimal.
No information indicating the potential use of Verbena officinalis extract in food packaging or as a flavouring agent in foods sold in Canada was identified. Oral exposure is possible through the consumption of herbal tea products brewed from dried Verbena officinalis leaves, which may contain Verbena officinalis extract as a component. The resulting daily oral exposure estimates for adults aged 19+ years is 4.86 × 10-2 mg/kg bw/day (personal communication, emails from the FD, HC, to the ESRAB, HC, dated December 2022; unreferenced), where the estimate was adjusted by a factor of 0.45 to account for the upper-bound percent composition of citral (Kubica et al. 2020), which is used for the risk characterization of Verbena officinalis extract.
Products available to consumers
Exposure to Verbena officinalis extract is possible through the use of cosmetics, NHPs, and other products available to consumers. Representative “sentinel” scenarios were selected to evaluate the potential for exposure to Verbena officinalis extract.
Exposure is also possible through the use of pure (that is, present at 100%) Verbena officinalis essential oil which can be used in DIY applications as described in section 4. Exposure to Verbena officinalis essential oil formulated in massage oils and body moisturizers is considered to be captured by the assessment of cosmetic scenarios.
Data on the dermal absorption of Verbena officinalis extract were not identified. However, data on the dermal absorption of citral, one of the main components of Verbena officinalis extract, were available. A 30% dermal absorption value for citral was determined from an in vitro dermal absorption study using human skin after variability in the reported absorption values was accounted for (Charles River Laboratories 2019). This value was used to derive estimates systemic exposure from the use of dermally applied products.
The estimated 11.9 Pa vapour pressure of citral (predicted using a model) is considered high and would indicate that citral is volatile. Therefore, for dermally applied products, the product amount available for inhalation was adjusted by 70% to account for the product amount absorbed by the dermal route.
Exposure estimates were adjusted to account for the percent composition of the major component of Verbena officinalis extract, citral, which serves as the basis for the risk characterization. For oral, dermal, and inhalation scenarios, an adjustment factor of 0.45 is used, on the basis of the upper-bound percent composition of citral in Verbena officinalis extract identified in the literature (Kubica et al. 2020). Details on the parameters used to estimate exposure to Verbena officinalis extract are presented in Appendix A.
Estimates of exposure from use of products available to consumers and DIY applications are summarized in Table 7‑19, and estimated air concentrations from use of cosmetics and DIY applications are presented in Table 7‑20. For each scenario, exposure estimates and air concentrations were derived for a range of relevant age groups, and only the highest one is presented. Given the number of products containing Verbena officinalis extract available on the Canadian market, exposure may result from the use of several types of products (for example, cosmetics and NHPs) containing the substance on the same day (that is, aggregate exposure).
| Exposure scenario | Route of exposure | Concentration | Exposure (mg/kg bw/day)i (age) |
|---|---|---|---|
| Massage oil | Dermala | 3%d | 1.2 (0 to 5 months) |
| Body exfoliant | Dermala | 3%d | 0.18 (14+ years) |
| Face moisturizer | Dermala | 10%d | 0.55 (19+ years) |
| Hand cream | Dermala | 3%d | 0.23 (2 to 3 years) |
| Shampoo | Dermala | 30%d | 0.25 (0 to 11 months) |
| Essential oil use in aromatic diffuser (user) | Dermala,b | 100%e,f | 0.30 (9 to 13 years) |
| Essential oil use in face steamer (user) | Dermala,c | 100%e,g | 0.28 (4 to 8 years) |
| Oral supplement (NHP) | Oral | 5 mg/tableth | 9.1 × 10-2 (19+ years) |
| Liquid extract | Oral | 12.5% | 3.1 (19+ years) |
a From a dermal absorption value of 30% based on an in vitro dermal absorption study of citral (Charles River Laboratories 2019).
b Dermal exposure from this use is expected to be incidental during refilling of an aromatic diffuser and considered to be applicable to users only (for example, those 9+ years).
c Assuming dermal exposure for product deposited onto the face of a user. Additional details are provided in Appendix A.
d Personal communication, emails from the CHPSD HC, to the ESRAB, HC, dated June 2021; unreferenced.
e Tin and Feather [accessed 2022]
f Assumes that 920 mg of pure essential oil (~ 21 drops of Verbena officinalis essential oil) is added to the device (RIVM 2021b).
g Assumes that approximately 10 drops of pure essential oil are added to the device. Details on the corresponding product amounts are provided in Appendix A.
h Personal communication, emails from NNHPD, HC, to the ESRAB, HC, 2021; unreferenced).
i Exposure estimates were adjusted by a factor of 0.45 to account for the upper-bound percent composition of citral, used as the basis for the risk characterization of Verbena officinalis extract (Kubica et al. 2020).
| Exposure scenario (inhalation) |
Concentration (%) |
Inhalation exposure (24-hr TWAd, mg/m3)e,f (age) |
|---|---|---|
| Massage oil | 3a | 4.1 × 10-2 (19+ years) |
| Body exfoliant | 3a | 6.8 × 10-3 (19+ years) |
| Face moisturizer | 10a | 0.32 (19+ years) |
| Hand cream | 3a | 2.1 x 10-2 (14+ years) |
| Shampoo | 30a | 5.7 × 10-3 (19+ years) |
| Essential oil use in aromatic diffuser | 100b | 1.2 (0 to 19+ years) |
| Essential oil use in face steamer (user) | 100c | 0.30 (4+ years) |
| Essential oil use in face steamer (bystander) | 100c | 0.15 (0 to 19+ years) |
a Personal communication, emails from the CHPSD, HC, to the ESRAB, HC, dated June 2021; unreferenced.
b Assumes that 920 mg of pure essential oil ) (~ 21 drops of Verbena officinalis essential oil) is added to the device (RIVM 2021b).
c Assumes that approximately 10 drops of pure essential oil are added to the device. Details on the corresponding product amounts are provided in Appendix A.
d 24-hr TWA = 24-hour time-weighted-average. This value describes the 24-hour average air concentration on the day of product use.
e For inhalation exposures, the product amount available for inhalation was adjusted by 70% to account for product absorbed by the dermal route (that is, 30% dermal absorption).
f Exposure estimates were adjusted by a factor of 0.45 to account for the upper-bound percent composition of citral, used for the risk characterization of Verbena officinalis extract (Kubica et al. 2020).
7.4.2 Selection of analogues
A read-across approach using data from analogues and the results of (Q)SAR models, where appropriate, has been used to inform the human health assessment. As Verbena officinalis extract is an UVCB, analogues were selected that were structurally and/or functionally similar to the major components and bioactive components of Verbena officinalis extract and that had relevant empirical data that could be used to read across to substances with limited empirical data.
Suitable analogues with relevant toxicological data were identified for 2 major components of Verbena officinalis extract, verbascoside and isobornyl formate, and are presented in Table 7‑21 and Table 7‑22, respectively. For further information on the physical-chemical properties of analogues, please refer to Appendix B, Tables B-5 and B-6.
| CAS RN | DSL or other name (common name) |
Chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
| 61276-17-3 (target) |
Glucopyranoside, 2-(3,4-dihydroxyphenyl)ethyl 3-O-(6-deoxy-alpha-L-mannopyranosyl)-, 4-(3-(3,4-dihydroxyphenyl)-2-propenoate), (E)-beta-D-, Actoside (Verbascoside) |
 C29-H36-O15 |
624.59 |
| 20702-77-6 | 1-(4-((2-O-(6-Deoxy-alpha-L-mannopyranosyl)-beta-D-glucopyranosyl)oxy)-2,6-dihydroxyphenyl)-3-(3-hydroxy-4-methoxyphenyl)- (Neohesperidin dihydrochalcone) |
 C28-H36-O15 |
612.57 |
| CAS RN | DSL or other name (common name) |
Chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
| 1200-67-5 (target) |
Bicyclo(2.2.1)heptan-2-ol, 1,7,7-trimethyl-, 2-formate, (1R,2R,4R)-rel- (Isobornyl formate) |
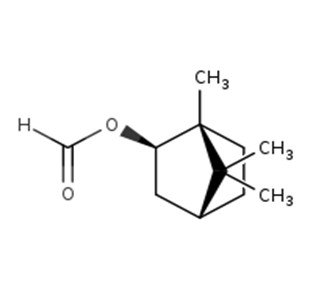 C11-H18-O2 |
182.26 |
| 125-12-2 | Bicyclo(2.2.1)heptan-2-ol, 1,7,7-trimethyl-, 2-acetate, (1R,2R,4R)-rel- (Pichtosin, isobornyl acetate) |
 C12-H20-O2 |
196.29 |
| 5888-33-5 | 2-Propenoic acid, (1R,2R,4R)-1,7,7-trimethylbicyclo(2.2.1)hept-2-yl ester, rel- (Isobornyl acrylate) |
 C13-H20-O2 |
208.30 |
7.4.3 Health effects assessment
The database for health effects of Verbena officinalis whole extract was limited. As a result, the health effects of the major and bioactive components of Verbena officinalis extract (that is, verbenalin, verbascoside, hastatoside, citral and isobornyl formate) were considered in this assessment. Only citral had a sufficient health effects data set available. The data on analogues of verbascoside and isobornyl formate identified in section 7.4.2 were used to inform the health effects assessment of Verbena officinalis extract; no suitable analogues were identified for verbenalin or hastatoside.
Verbena officinalis extract (CAS RN 84961-67-1)
Developmental toxicity
A prenatal developmental toxicity study conducted with Verbena officinalis leaf extract in pregnant female Sprague-Dawley rats (10/dose) was identified (Fateh et al. 2019a). However, the test substance may not represent the Verbena officinalis extract contained in products available to consumers; the extraction method does not involve hydrodistillation, steam distillation, or alcohol extraction, which are the common extraction methods for Verbena officinalis extract. Moreover, the compositional analysis conducted by the study authors did not identify typical components of Verbena officinalis extract (for example, verbascoside, verbenalin, hastatoside, citral). Hence, the study was not considered further.
Genotoxicity
The in vitro mutagenicity of Verbena officinalis aqueous leaf extract was assessed in an Ames assay in different strains of Salmonella typhimurium (TA97a, TA98, TA100, and TA1535) and Escherichia coli WP2 uvrA (pKM101) in the presence or absence of metabolic activation. Study results showed that 0.625, 1.25, 2.5 and 5 mg/ml of the extract induced a significant mutagenic effect against TA100 and TA98 strains (with and without metabolic activation) (Fateh et al. 2019b).
The in vivo mutagenicity of Verbena officinalis aqueous leaf extract was assessed in a micronucleus experiment where groups of 6 male and 6 female rats were administered 500, 1000 and 2000 mg/kg bw/day of Verbena officinalis extract daily for 3 days. Study results showed no significant increase in the micronucleated polychromatic erythrocytes (MNPE) and no significant alterations in the polychromatic erythrocytes (PCE) to normochromic erythrocytes (NCE) ratio of treated rats compared with the negative control (Fateh et al. 2019b). The uncertainty associated with the test substance used in this study is similar to that of the substance used in the prenatal developmental toxicity study described in the previous section.
The anti-carcinogenic properties of Verbena officinalis extract were explored in a therapeutic context, where the extract demonstrated anti-tumour activity against H22 tumour-bearing mice (Kou et al. 2013).
Verbascoside (CAS RN 61276-17-3)
Verbascoside is reported to be one of the bioactive components of Verbena officinalis extract, displaying anti-oxidant and anti-inflammatory effects (Kubica et al. 2020). No suitable toxicity studies were identified for verbascoside, and a read-across approach was used. Neohesperidin dihydrochalcone (CAS RN 20702-77-6) was selected as an appropriate analogue, and data on its toxicity were used to inform the health effects assessment of verbascoside.
Neohesperidin dihydrochalcone has chemical moieties in common with verbascoside, as well as similar physical-chemical properties (Table C-5). It has been reviewed by EFSA (2010) for its use as a sweetener, with the EFSA panel reviewing available genotoxicity, repeated-dose toxicity and reproductive, and developmental toxicity studies.
Repeated-dose toxicity
Neohesperidin dihydrochalcone has been studied in subchronic and chronic repeated-dose toxicity studies in rats and dogs (EFSA 2010). No adverse effects were observed when rats and dogs were fed neohesperidin dihydrochalcone in the diet at a range of 0.64 mg/kg bw/day to 10 000 mg/kg bw/day over the course of 90 to 730 days. The EFSA panel assigned a no-observed-effects level (NOEL) of 500 mg/kg bw/day on the basis of the highest dose tested in a 90-day repeated-dose diet study in rats in order to determine an acceptable daily intake (ADI) level of 5 mg/kg bw/day.
Reproductive and developmental toxicity
Neohesperidin dihydrochalcone has been studied in 4 developmental and reproductive studies in rats (EFSA 2010). No developmental or reproductive toxicity was reported when rats were fed the substance in diet at 64 to 3300 mg/kg bw/day. The EFSA panel determined that no adverse effects were observed, except for cecal enlargement in dams exposed to 3300 mg/kg bw/day from GD 0 to 21; however, they determined that this is a well-known physiological response to the ingestion of high doses of a low-digestible substance and thus lacks toxicological relevance (EFSA 2010).
Genotoxicity and carcinogenicity
Neohesperidin dihydrochalcone has been studied for genotoxicity in several in vitro and in vivo assays (EFSA 2010). Results from bacterial reverse mutagenicity assays were negative, with and without metabolic activation, using several strains of Salmonella typhimurium (TA97, TA98, TA100, TA1537, TA1535, TA1538) at doses up to 10 000 µg/plate. A micronucleus test in male mice treated with 200, 400, or 800 mg/kg bw/day yielded a positive result; however, the EFSA panel concluded that the study was inadequate and suffered from methodological flaws. Therefore, the EFSA panel concluded that there was no concern in regard to the genotoxicity of the substance (EFSA 2010).
Furthermore, in 2-year dog and rat diet studies, no carcinogenic effects were reported when neohesperidin dihydrochalcone was administered at up to 2500 mg/kg bw/day (EFSA 2010).
Isobornyl formate (CAS RN 1200-67-5)
Isobornyl formate is reported to be one of the major components of Verbena officinalis extract obtained using the hydrodistillation method (Kubica et al. 2020). Although it was evaluated by EFSA (2014) as a flavouring substance and was deemed to have no safety concerns at the relevant consumption levels, no toxicological studies were identified for isobornyl formate. As a result, a read-across approach was used, isobornyl acetate (CAS RN 125-12-2) and isobornyl acrylate (CAS RN 5888-33-5) were considered suitable analogues, and the information on their toxicity was used to inform the health effects assessment of isobornyl formate.
Both isobornyl acetate and isobornyl acrylate share a bicyclic ring structure with isobornyl formate, which has the same methyl substituents but differs in one substituent (formate vs. acetate or acrylate). The analogues have some similar physical-chemical properties as isobornyl formate (Table C-6). REACH dossiers are available for both analogues (ECHA 2021j, 2021k).
Repeated-dose toxicity
In a repeated-dose toxicity test, groups of 15 CFE rats/sex/dose were administered isobornyl acetate at 15, 90, or 270 mg/kg bw/day by gavage for 13 weeks. Study results showed no differences in body weight gain, food intake, or hematological parameters between dose levels. Water intake increased in males of the highest dose group. At the middle dose (90 mg/kg bw/day), increased cell excretion in the urine was observed in male rats at week 13, indicative of proximal tubular damage, which was also observed at the highest dose at week 6. At 270 mg/kg bw/day, there was a decrease in urine concentrating ability, exfoliation of tubular cells, increased kidney and liver weights, and vacuolation of the renal tubular cells and epithelium of the intrahepatic bile-duct in male rats. The authors identified a NOEL of 15 mg/kg bw/day on the basis of nephrotoxicity effects at the middle and highest doses in male rats. However, as these effects did not occur in females, the US EPA suggested that the nephrotoxicity was driven by an alpha-2u-globulin mechanism, which is specific to the male rat and is irrelevant to other mammals, including humans (Gaunt et al. 1971; US EPA 2006; ECHA 2021j).
Developmental and reproductive toxicity
In a one-generation reproductive toxicity study (Politano et al. 2017), adult Sprague-Dawley rats (25/sex/dose) were given 30, 100, or 300 mg/kg bw/day isobornyl acetate in corn oil by oral gavage for 14 days premating in females, and for 84 days premating in males, as well as during the mating, gestation, parturition and lactation periods. The authors examined the parental animals, as well as F1 generation pups (25/sex/dose) for general and reproductive toxicity parameters. The authors concluded that isobornyl acetate did not elicit any adverse effects at any of the tested doses, and thus determined a NOAEL of 300 mg/kg bw/day for both general and reproductive toxicity in both the parental and F1 generations.
In a combined repeated-dose and reproduction developmental toxicity screening test, groups of 10 Sprague-Dawley rats/sex/dose were administered isobornyl acrylate at 25, 100, and 500 mg/kg bw/day for 5 or 6 weeks (for males and females, respectively) from 2 weeks before pairing, during pairing, and up to day 3 of post-partum. Clinical signs of salivation were noted in the mid- and high-dose groups as well as increased values of blood urea in the high-dose groups of both sexes. Proteinuria was also recorded in the mid- and high-dose males. Increased kidney weight was identified in the mid- and high-dose males, and a decrease in thymus weight was seen in the high-dose females. No differences were found in mating performance including pre-coital interval, copulatory index, and fertility index in all groups. Significant differences were found in total litter size and litter weight in the high-dose group compared to the control. The percentage of cumulative pup loss post-partum starting from birth was increased in the high-dose group. The authors identified a NOAEL for general toxicity and reproduction and developmental toxicity of 100 mg/kg bw/day in both sexes on the basis of increased blood urea, decreased litter size and litter weight, and cumulative pup loss post-partum at the highest dose (ECHA 2021k).
Genotoxicity
Isobornyl acetate and isobornyl acrylate were both negative for genotoxicity in several in vitro and in vivo assays, including bacterial reverse mutation assays conducted with Salmonella typhimurium strains TA 1535, TA 1537, TA 98, and TA 100 and Escherichia coli WP2 up to 5000 µg/plate; forward mammalian gene mutation assays with Chinese hamster lung fiborblasts up to 5000 µg/plate; and micronucleus assays conducted with mice up to 2000 mg/kg bw/day (ECHA 2021j, 2021k).
Citral (CAS RN 5392-40-5)
Citral is reported to be one of the main components of Verbena officinalis extract using the hydrodistillation method (Kubica et al. 2020). The health effects of citral have been previously described in the draft screening assessment of the Terpenes and Terpenoids – Acyclic, Monocyclic, and Bicyclic Monoterpenes Group (ECCC, HC 2020). Since the publication of that draft screening assessment, the health effects characterization of citral was updated based on new information and is described below.
Developmental and Reproductive Toxicity
Doses of 40, 200, or 1000 mg/kg bw/day of citral were administered to male and female rats by gavage (unknown solvent) for approximately 46 days prior to and through mating, and gestation. At the highest dose (1000 mg/kg bw/day), decreases in body weight and food consumption were observed in animals, as well as histopathological effects in the forestomach in dams, while pups experienced decreases in body weight. On the basis of these results, a NOAEL of 200 mg/kg bw/day was determined for maternal toxicity, and citral was not reported as a reproductive or a developmental toxicant (MHW, Japan 2002 as cited in OECD 2001).
In a prenatal developmental toxicity study, New Zealand White rabbits (25/dose) were administered 20, 60, or 200 mg/kg bw/day of citral suspended in water and 0.5 carboxymethylcellulose by gavage on GDs 6 to 28. Dams in the 200 mg/kg bw/day dose group showed decreases in body weight and weight gain, reduced food consumption, mortality (2 rabbits) and abortion (1 rabbit). In addition, a litter of pups from a dam administered 200 mg/kg bw/day developed external malformations due to severe maternal toxicity (ECHA 2023). However, evidence of stomach irritation (severe reddening of the stomach mucosa) was observed in rabbits that died in the high dose group. On the basis of these results, a NOAEL of 60 mg/kg bw/day was noted for maternal toxicity.
In an inhalation study, pregnant rats (25/dose) were exposed (whole body) to 10, 34, 68 ppm of citral for 6 hours/day from GD 6 to 15 (Gaworski et al. 1992). The concentrations of 10 and 34 ppm were generated as vapours, while the 68 ppm concentration was a combination of aerosol and vapour. Maternal effects observed at the 68 ppm concentration included mortality (1 female on day 10 of gestation), abortion (1 female on day 17 of gestation), reduced body weights, ocular opacity, breathing difficulty, nasal discharge, and salivation, and were associated with severe respiratory tract irritation. Changes in body weight and signs of toxicity were reversed upon cessation of exposure. At the 68 ppm concentration, the fetus showed a non-significant decrease in body weight and hypoplastic (small size) bones in the presence of maternal toxicity. No maternal or offspring effects were noted at the 2 lower concentrations. A no-adverse-effect concentration (NOAEC) was identified at a concentration of 34 ppm, which is equivalent to 215 mg/m3, on the basis of maternal toxicity observed at the 68 ppm concentration (or 423 mg/m3). The NOAEC was further adjusted to a value of 54 mg/m3 to account for the short exposure duration (6 hours per day) used in the study. The developmental toxicity effects were observed in the presence of maternal toxicity (Gaworski et al. 1992).
In an extended one-generation reproductive toxicity study, Wistar rats (20-25/sex/dose) were given 25, 80, or 250 mg/kg bw/day of citral suspension in water by oral gavage. F0 and F1 parental animals were dosed prior to, during, and after mating (F0 animals for a maximum 10 weeks, F1 animals [that is, cohort 1B] for a maximum of 18 weeks). F1 non-mated animals were treated post-weaning until the age of 13 weeks (that is, cohort 1A); F2 animals were not exposed after birth. No treatment related mortalities were observed in any group. Observed effects included transient salivation shortly after gavage, and increased food and water consumption. Mild intermittent body weight increases were also observed in all animals and some animals in the high-dose group, and in mid-dose groups in the F0 and F1 cohorts, likely due to the taste of the substance. The most notable adverse effect occurred in the forestomach of animals in the F0 and F1 cohorts, which showed thickening of the stomach wall, hyperplasia, hyperkeratosis, ulcers, and lesions at the middle- and high-doses, with increasing severity. Significant increases in cauda epididymis (F1 high-dose males), liver (F0 middle- and high-dose males/females, and F1 high-dose males), and kidney (F0 and F1 high-dose males) weights occurred, but were not considered adverse due to absence of histopathological effects. No reproductive or developmental parameters were affected by citral treatment (that is, sex organ weights and histopathology, fertility, estrous cycle, sperm quality, conception, gestation, lactation, weaning, pup weight, postnatal survival and post-weaning development were comparable to controls). Based on these results, the authors identified a parental NOAEL of 25 mg/kg bw/day, on the basis of effects on the forestomach and effects observed at the 80 and 250 mg/kg bw/day doses; a reproductive and developmental NOAEL of 250 mg/kg bw/day was identified for all generations, which is the highest tested dose (ECHA 2023).
In a developmental toxicity study, pregnant Wistar rats were exposed to 0, 60, 125, 250, 500, and 1000 mg/kg bw/day of citral dissolved in corn oil via intragastric cannula from GD 6 to 15; caesarean sections were carried out on GD 21. In rats treated with 60 mg/kg bw/day, citral induced a slight reduction in body weight gain from day 6, and a dose-dependent reduction in body weight gains were seen across all treatment groups. In rats treated with 125 and 250 mg/kg bw/day of citral, a decrease in gravid uterus weight resulted in the reduction in body weight. However, statistically significant reduced body weight gains were observed in the dams in the 500 and 1000 mg/kg bw/day groups that were independent of the gravid uterus weight. A slight but statistically significant increase in post-implantation losses was observed in the 60 and 125 mg/kg bw/day groups. Signs of fetal growth retardation and a higher incidence of minor skeletal abnormalities were found in doses higher than 60 mg/kg bw/day. Doses higher than 125 mg/kg bw/day dose-dependently reduced the number of pregnant females. No increase in the frequency of visceral anomalies was found at any dose level, but a significant increase in fetal spleen weight (both absolute and relative weights) was observed in doses higher than 125 mg/kg bw/day. The only death among citral-treated dams occurred at the 250 mg/kg bw/day dose, 5 days after the end of the treatment period (Nogueira et al, 1995). On the basis of increased post-implantation losses and reduced body weight gains in the dams, a LOAEL of 60 mg/kg bw/day was identified.
Genotoxicity
Citral was reviewed previously in an OECD screening information dataset (SIDS) initial assessment report (OECD/SIDS 2001). This report concluded that on the basis of the weight of evidence, citral was not considered to be a genotoxic hazard.
The NTP conducted several in vitro and in vivo genotoxicity assays with citral, where citral was negative for genotoxicity in a bacterial reverse mutation assay using Salmonella typhimurium bacterial strains TA98, TA100, TA1535, and TA1537 at 1 to 220 µg/plate. Citral was also negative for genotoxicity in a chromosome aberration test in Chinese Hamster Ovary cells (CHO), with and without metabolic activation. Furthermore, negative results were obtained in in vivo micronucleus tests with male B6C3F1 mice bone marrow treated intraperitoneally with 250 to 750 mg/kg bw/day of citral daily for 3 days; the next highest dose of 1000 mg/kg bw/day was lethal. In addition, no micronuclei were detected in the peripheral blood of male and female mice exposed to up to 8110 mg/kg bw/day of microencapsulated citral for 14 weeks (NTP 2003). In vitro micronucleus tests using human leukocytes and HepG2 tumour cells exposed to 0.625 to 1000 µg/mL of citral also generated negative results (NTP 2003; Souza et al. 2020).
Several in vitro genotoxicity assays produced positive results with citral exposure, however limitations were noted. A sister chromatid exchange (SCE) assay tested positive with citral exposure, but the authors noted that the substance was toxic to the cells (NTP 2003). DNA strand breaks induced by citral have been detected by different in vitro comet assays. Citral significantly increased DNA migration in mice macrophages at 0 hours, but effects were reversed at 4,18, and 24 hours, suggesting DNA repair (De Paula Porto et al., 2014). The mice macrophages, as well as HepG2 cells, were positive, without examination of the cytotoxicity at the tested concentrations (De Paula Porto et al. 2014; Souza et al. 2020). In B16F10 cells, citral was positive in the comet assay in the presence of cytotoxicity, and in human lymphocytes and leukocytes in the absence of cytotoxicity (Sinha et al. 2014; Sanches et al. 2017; Souza et al. 2020). A known limitation of the comet assay is that it can produce false positives when the test substance is cytotoxic (Brendler-Schwaab et al., 2005). Positive results reported in the assay indicate that citral induced DNA damage, but this may reflect cytotoxicity rather than genotoxicity. In the in vitro p53 transcription factor- and TUNEL assays, citral tested positive; however, effects can also be attributed to cytotoxicity (Duerksen-Hughes et al. 1999; Sanches et al. 2017). On the basis of the weight of evidence, citral is considered likely non-genotoxic.
Carcinogenicity
In 2 carcinogenicity studies conducted by the NTP (2003), rats and mice (50/sex/dose) were fed diets containing microcapsules of 1000, 2000, or 4000 ppm, and 500, 1000, or 2000 ppm citral, respectively (that is, 50, 100 or 210 mg/kg bw/day for rats; 60, 120 or 260 mg/kg bw/day for mice), for 2 years. Male and female rats exposed to the highest dose had reduced body weight gains, with no change in food consumption levels, when compared to controls. Mean body weights of mice exposed to the mid- and high-doses were generally less than those of the vehicle controls throughout the study, and the mean body weights of female mice in the 60 mg/kg bw/day group decreased from week 30 until the end of the study; feed consumption was not affected. On the basis of the results of histopathology, the authors concluded that there was no carcinogenic activity of citral in rats or male mice, while there was equivocal evidence of carcinogenic activity in female mice based on a positive trend of increasing incidences of malignant lymphoma, which reached statistical significance at 260 mg/kg bw/day compared to the vehicle control. The authors also stated that the incidence could not clearly be related to citral administration (Ress et al., 2003). Therefore, a LOAEL of 60 mg/kg bw/day was identified on the basis of reduced body weight in mice.
This result is aligned with findings in the evaluation of citral conducted by the JECFA in 2004. The FAO/WHO concluded that citral does not present a safety concern as a food flavouring agent based on estimated levels of intake (WHO 2004c).
7.4.4 Characterization of risk to human health
For evaluating the risk of Verbena officinalis extract in oral and dermal exposure scenarios, a LOAEL of 60 mg/kg bw/day is considered the most appropriate POD on the basis of increased post-implantation losses, and reduced body weight gain in dams observed in an oral developmental study on rats treated with citral (Nogueira et al, 1995). This POD is similar to the LOAEL of 60 mg/kg bw/day or higher, which is based on reduced body weight gains in mice observed in the 2-year carcinogenicity dietary study (NTP 2003).
A NOAEC of 54 mg/m3 was identified as the most appropriate POD for characterization of the human health risk from inhalation exposure to Verbena officinalis extract. This NOAEC was identified on the basis of maternal toxicity (that is, mortality, abortion, reduced body weights, ocular opacity, breathing difficulty, nasal discharge, salivation, and severe respiratory tract irritation) observed at the next dose in a rat developmental toxicity study using citral administered as an aerosol or vapour via whole-body inhalation (Gaworski et al. 1992). Since it is unknown whether the toxic effect observed by the inhalation route was the result of a single exposure or repeated exposure events, in addition to severe respiratory tract irritation observed in the study, it was considered appropriate to compare air concentrations associated with the inhalation exposure scenario directly with the adjusted NOAEC of 54 mg/m3 from the inhalation toxicity study.
Exposure estimates and resulting MOEs for the highest exposed age groups are presented in Table 7‑23. In addition, estimated air concentrations and resulting MOEs from the use of products available to consumers and DIY applications are presented in Table 7‑24.
| Exposure scenario | Exposureb (mg/kg bw/day) |
MOEc |
|---|---|---|
| Massage oila (0 to 5 months) |
1.2 | 50 |
| Body exfolianta (14+ years) |
0.18 | 333 |
| Face moisturizera (19+ years) |
0.55 | 109 |
| Hand creama (2 to 3 years) |
0.23 | 261 |
| Shampooa (0 to 5 months) |
0.25 | 240 |
| Essential oil use in aromatic diffuser (user)a (9 to 13 years) |
0.30 | 200 |
| Essential oil use in face steamer (user)a (4 to 8 years) |
0.28 | 214 |
| Oral supplement (NHP) (19+ years) |
9.1 × 10-2 | 659 |
| Liquid extract (19+ years) |
3.1 | 19 |
| Herbal tea (19+ years) |
4.86 × 10-2 | 1 235 |
Abbreviations: MOE, margin of exposure; LOAEL, lowest observed adverse effect level
a Dermal exposure estimates were adjusted by a dermal absorption factor of 30% (Charles River Laboratories 2019).
b Exposure estimates were adjusted by a factor of 0.45 to account for the upper-bound percent composition of citral used for the risk characterization of Verbena officinalis extract (Kubica et al. 2020).
c MOEs are presented using the selected LOAEL of 60 mg/kg bw/day, which is based on reduced body weight gains in dams and significant increases in the ratio of resorptions per implantation exposed to citral at 60, 125, 250, 500, or 1000 mg/kg bw/day from GD 6 to 15.
Comparison of the critical effect level with the estimated level of dermal exposure to Verbena officinalis extract from the use of massage oils, body exfoliants, shampoos, hand creams, and face moisturizers, and from use in aromatic diffusers and face steamers resulted in MOEs ranging from 50 to 333, which are below 1000 and are considered potentially inadequate to account for uncertainties in the health effects and exposure databases. Factors including interspecies and intraspecies differences and the inability to identify a NOAEL from the database were considered during the determination of adequacy of the MOEs. Given the number of products containing Verbena officinalis extract available on the Canadian market, it is possible that exposure may result from the use of several types of products containing this substance on the same day (that is, aggregate exposure), in which case the MOE would be even lower.
Comparison of the critical effect level with the estimated level of oral exposure to liquid extracts and oral supplements (NHPs) resulted in MOEs ranging from 19 to 659, which are below 1000 and are considered potentially inadequate to address uncertainties in the health effects and exposure data used to characterize risk. Comparison of the critical effect level with the estimated level of oral exposure from herbal teas resulted in an MOE of 1235, which is above 1000 and is considered adequate.
| Exposure scenarioa | Air concentration (mg/m3)b | MOEc |
|---|---|---|
| Massage oil (19+ years) |
4.1 × 10-2 | 1317 |
| Body exfoliant (19+ years) |
6.8 × 10-3 | 7941 |
| Face moisturizer (19+ years) |
0.32 | 169 |
| Hand cream (14+ years) |
2.1 × 10-2 | 2571 |
| Shampoo (19+ years) |
5.7 × 10-3 | 9474 |
| Essential oil use in face steamer (bystander) (0 to 19+ years) |
0.15 | 360 |
| Essential oil use in face steamer (user) (4+ years) |
0.30 | 180 |
| Essential oil use in aromatic diffuser (0 to 19+ years) |
1.2 | 45 |
Abbreviations: MOE, margin of exposure; NOAEC, no observed adverse effect concentration
a Estimated air concentrations are presented for the highest exposed age group relevant for each scenario.
b Estimated air concentrations were adjusted by a factor of 0.45 to account for the upper-bound percent composition of citral used for the risk characterization of Verbena officinalis extract (Kubica et al., 2020).
c MOEs are presented using the selected NOAECadj of 54 mg/m3, which is based on maternal toxicity (that is, mortality, abortion, reduced body weights, ocular opacity, breathing difficulty, nasal discharge, salivation, and severe respiratory tract irritation) observed at 106 mg/m3 (LOAECadj) in a rat developmental toxicity study using citral via whole body inhalation.
With respect to the massage oils, body exfoliants, hand creams, and shampoos, comparison of the critical effect level with exposure concentrations resulted in MOEs of above 300, which are considered adequate to address uncertainties in the health effects and exposure data used to characterize risk. With respect to the face moisturizer, and use of essential oil in aromatic diffusers and face steamers, the resulting MOEs were below 300 and considered potentially inadequate to address uncertainties in the health effects and exposure data used to characterize risk. Factors including interspecies and intraspecies differences and the duration of the pivotal study were considered during the determination of the adequacy of the MOEs. Given the number of products containing Verbena officinalis extract available on the Canadian market, it is possible that exposure may result from the use of several types of products containing this substance on the same day (that is, aggregate exposure), in which case the MOE would be even lower.
7.4.5 Uncertainties in evaluation of risk to human health
The key sources of uncertainty are presented in Table 7‑25.
| Key source of uncertainty | Impact |
|---|---|
| The concentration of the main components in Verbena officinalis extract differs depending on the type of plant material used (for example, roots or leaves), its origin, environmental conditions, chemotype tested, and the extraction method used. Therefore, the composition of Verbena officinalis extract present in products available to consumers is unknown, which represents an uncertainty in the assessment. | +/- |
| The upper-bound concentration of 45% identified in the literature for the citral content in Verbena Officinalis extract was considered in deriving the appropriate exposure estimates. Although citral is only identified in extracts obtained via hydrodistillation, its presence in products available to consumers containing Verbena officinalis extract cannot be ruled out. | +/- |
| There are no subchronic or chronic repeated-dose toxicity, or carcinogenicity studies identified for Verbena officinalis extract. | +/- |
| There are no adequate studies examining the repeated-dose toxicity of Verbena officinalis extract. Hazard data from the main or bioactive components (that is, verbascoside, citral, and isobornyl formate) were used to inform the health effects assessment, where applicable. | +/- |
| The risk characterization did not take into consideration the potential for additive, synergistic, or antagonistic effects of components within UVCBs. | +/- |
| The potential use of more than one product by a single person in a day (that is, aggregate exposure) was not considered. This may potentially underestimate exposure to some individuals. | - |
+ = uncertainty with potential to cause over-estimation of exposure/risk; - = uncertainty with potential to cause under-estimation of exposure risk; +/- = unknown potential to cause over- or under-estimation of risk.
7.5 Ginkgo biloba extract
7.5.1 Exposure assessment
Environmental media and food
No reports of monitoring for Ginkgo biloba extract in environmental media in Canada or elsewhere were identified. The substance was not reported to be manufactured or imported in quantities greater than the reporting threshold of 100 kg on the basis of information submitted in response to a CEPA section 71 survey (Environment Canada 2013). In consideration of low reported quantities of the substance in Canada, exposure to Ginkgo biloba extract from environmental media is expected to be minimal.
No information indicating the potential use of Ginkgo biloba extract in food packaging or as a flavouring agent in foods sold in Canada was identified. Oral exposure is possible through consumption of herbal tea products brewed from dried Ginkgo biloba leaves, which may contain Ginkgo biloba extract as a component. The resulting daily oral exposure estimates for adults aged 19+ years is 1.01 mg/kg bw/day, and the LADD is estimated to be 0.77 mg/kg bw/day (personal communication, emails from the FD, HC, to the ESRAB, HC, dated June 2022; unreferenced).
Products available to consumers
Based on reports of uses of Ginkgo biloba extract in cosmetics, NHPs, NPDs, and oral supplements (liquid extracts), the main routes of exposure are expected to be dermal and oral. The potential dermal exposure of the general population to Ginkgo biloba extract from the use of products applied to the skin was estimated using representative sentinel scenarios. Inhalation exposures from the use of dermally applied products are not expected due to the negligible vapour pressure of main components in the substance, such as quercetin (3.75 × 10-12 Pa).
No dermal absorption data were identified for Ginkgo biloba extract. A major component of Ginkgo biloba extract is the flavonoid quercetin (IARC 2016), which may constitute up to 16.7% of Ginkgo biloba extract (Burnett 2018). As quercetin is a major component of Ginkgo biloba extract and may contribute to its toxicological profile (as indicated in more detail in section 7.5.2), dermal penetration data on quercetin have been considered. An in vitro study by dal Belo et al. (2009) examined the dermal penetration of quercetin; following application of a cosmetic formulation supplemented with 6.0% Ginkgo biloba glycolic leaf extract, the final concentration of quercetin in the extract was 0.12% (dal Belo et al. 2009). 10 mg/cm2 of the formulation was applied to human skin samples (n=6) mounted onto Franz diffusion cells for 24 hours, and samples of receptor fluid were taken after 6 hours and 24 hours. Skin cells were washed after the exposure time, and the stratum corneum (SC) was removed by tape stripping. Following analysis, quercetin was identified in the SC at 24% of the applied dose, while 33% of the applied dose was in the epidermis; quercetin was not detected in the dermis or receptor fluid (dal Belo et al. 2009). The amount of the substance that penetrated the epidermis and the SC is considered to be available for dermal absorption. Accounting for the standard deviation of the doses applied to the epidermis and the SC, the total amount that may be potentially absorbed is 65% of the applied dose. Dermal absorption was conservatively assumed to be equivalent to oral absorption (that is, 100%) for products applied to mucosal membranes (for example, genital lubricants).
Details on the method and parameters used to derive exposure estimates are provided in Appendix A. Exposure estimates and LADDs from these products are summarized in Table 7‑26; for each scenario, exposure estimates were derived for a range of relevant age groups, and only the highest one is presented. Given the number of products containing Ginkgo biloba extract available on the Canadian market, exposure may result from the use of several types of products (e.g., cosmetics, NHPs, and NPDs) containing the substance on the same day (that is, aggregate exposure).
| Exposure scenario | Route of exposure | Concentration (%) | Exposure (mg/kg bw/day) (age) |
LADD (mg/kg bw/day) |
|---|---|---|---|---|
| Hair perm/ straightener | Dermala | 3c | 2.1 (19+ years) |
2.6 × 10-2 |
| Permanent hair dye | Dermala | 1c | 1.4 (14 to 18 years) |
2.1 × 10-2 |
| Face exfoliant | Dermala | 1c | 3.3 × 10-2 (14 to 18 years) |
6.6 × 10-3 |
| Aftershave | Dermala | 5c | 1.8 (9 to 13 years) |
0.67 |
| Sunless tanning product | Dermala | 0.3c | 0.36 (9 to 13 years) |
4.9 × 10-2 |
| Massage product | Dermala | 1c | 1.9 (0 to 5 months) |
4.0 × 10-2 |
| Face mask | Dermala | 10c | 1.0 (14 to 18 years) |
7.1 × 10-2 |
| Body oil | Dermala | 10c | 3.0 (14 to 18 years) |
0.26 |
| Hair mist | Dermala | 0.3c | 8.6 × 10-3 (19+ years) |
6.8 × 10-3 |
| Liquid face foundation | Dermala | 30c | 2.9 (4 to 8 years) |
1.7 |
| Genital lubricant | Dermalb | 1c | 0.88 (19+ years) |
0.66 |
| Face moisturizer | Dermala | 45c | 11.9 (19+ years) |
9.9 |
| Face toner | Dermala | 94c | 32.0 (9 to 13 years) |
22.7 |
| Body moisturizer | Dermala | 3c | 6.2 (0 to 5 months) |
2.8 |
| Hand cream | Dermala | 0.1c | 3.8 × 10-2 (2 to 3 years) |
2.6 × 10-2 |
| Body soap (liquid) | Dermala | 3c | 0.17 (0 to 5 months) |
4.8 × 10-2 |
| Spray antiperspirant | Dermala | 0.1c | 3.7 × 10-2 (14 to 18 years) |
2.9 × 10-2 |
| Shampoo | Dermala | 10c | 0.40 (0 to 11 months) |
0.12 |
| Face cleanser | Dermala | 3c | 1.7 × 10-2 (9 to 13 years) |
1.2 × 10-2 |
| Makeup remover | Dermala | 1c | 4.6 × 10-2 (14 to 18 years) |
3.4 × 10-2 |
| Face sunscreen (NHP) | Dermala | 3d | 0.79 (19+ years) |
0.60 |
| Sunscreen lotion (NHP) | Dermala | 3.1 mg/gd | 1.9 (6 to 11 months) |
0.72 |
| Face sunscreen (NPD) | Dermala | 0.1e | 2.6 × 10-2 (19+ years) |
2.2 × 10-2 |
| Oral supplement (capsule) (NHP) | Oral | 187 mg/capsuled | 7.6 (19+ years) |
5.7 |
| Oral supplement (liquid extract) | Oral | 1000 mg/mL | 81.1 (19+ years) |
61.3 |
| Herbal tea blend (NHP) | Oral | 3d | 7.3 × 10-2 (19+ years) |
5.5 × 10-2 |
a Systemic exposure was estimated using a dermal absorption value of 65% (dal Belo et al. 2009).
b Dermal absorption was assumed to be equivalent to oral absorption.
c Personal communication, emails from the CHPSD, HC, to the ESRAB, HC, dated June 2021; unreferenced.
d Personal communication, emails from the NNHPD, HC, to the ESRAB, HC, dated June 2021; unreferenced.
e Personal communication, emails from the TPD, HC to the ESRAB, HC, dated June 2021; unreferenced.
7.5.2 Health effects assessment
For the health effects characterization of Ginkgo biloba extract, toxicity studies conducted on the whole extract, rather than individual components, were considered. A number of international organizations have evaluated the health effects of Ginkgo biloba extract including the IARC (2016), the European Medicines Agency (EMA) (EMA 2014), and the US NTP (NTP 2013). Ginkgo biloba extract was also evaluated by the CIR Expert Panel (Burnett 2018).
Toxicokinetics
Few toxicokinetics studies using the whole Ginkgo biloba leaf extract were identified.
In male and female Sprague-Dawley rats exposed to single oral doses of radiolabelled Ginkgo biloba extract, analysis showed that at least 60% of the radiolabelled extract was absorbed, likely from the upper gastrointestinal tract, and that it followed a 2-compartment model with an apparent first order phase and a half-life of around 4.5 hours (Burnett 2018).
In a human plasma study using 3 different Ginkgo biloba extract preparations, including EGb-761, the levels of bilobalide, ginkgolide A, and ginkgolide B were measured in 24 healthy volunteers. Results showed that all 3 components are bioavailable after oral exposure, with bilobalide having the greatest plasma concentration and EGb-761 yielding the greatest concentration of all 3 components compared to the other formulations of Ginkgo biloba extract (Burnett 2018).
In a human study, 12 healthy volunteers were given 80 mg once daily or 40 mg twice daily of Ginkgo biloba extract in the form of standardized EGb 761, containing a standard active ingredient composition of 1.25% ginkgolide A, 0.9% ginkgolide B, and 3% bilobalide via oral administration for 7 days. The level of ginkgolide B was measured in plasma over time. The results showed that a dosage of 40 mg twice daily resulted in a significantly longer half-life (11.6 hours vs. 4.3 hours) and mean residence time (13.1 hours vs. 7.25 hours) than did a single 80 mg dose. The maximum concentration (Tmax) was reached in 2.3 hours for both dosages (IARC 2016).
In a separate clinical study, the presence of metabolites was examined in humans following oral administration of a Ginkgo biloba leaf extract. Six healthy volunteers were given a single dose of 4 g of Ginkgo biloba leaf extract per day. Urine samples were then collected for 2 days, and blood samples were taken every 30 minutes for 5 hours. Study results showed that only urine samples contained detectable amounts of metabolites such as substituted benzoic acids (for example, 4-hydroxybenzoic acid conjugate and 4-hydroxyhippuric acid). None of the measured metabolites were found at detectable levels in the blood (IARC 2016).
The excretion of quercetin and its conjugates (one of the main components of Ginkgo biloba extract) was examined. Results showed that quercetin and its conjugates were excreted in human urine at a range of 0.07% to 17.4% of food intake and that only quercetin glucuronides (and not free quercetin) were detected (IARC 2016).
Repeated-dose toxicity
In one repeated-dose toxicity study conducted by the NTP (2013), groups of 10 male and 10 female F344/N rats were given 62.5, 125, 250, 500, or 1000 mg/kg bw/day of Ginkgo biloba leaf extract in corn oil by gavage, 5 days per week for 14 weeks. In tandem, a clinical pathology study was conducted where additional groups of 10 male and 10 female rats were given the same dose 5 days per week for 23 days. The study results showed a significant increase in liver weights for all dose groups in both male and female rats when compared to the controls. Hepatocyte hypertrophy incidence was also significantly higher in all dosed male groups (considered mild to moderate in severity) and in the 500 and 1000 mg/kg bw/day female groups (considered mild in severity). Thyroid gland follicular cell hypertrophy was also observed in the 500 and 1000 mg/kg dose male groups and the 1000 mg/kg bw/day female group. The incidence of pigmentation in the olfactory epithelium of the nose was also significantly increased in the 500 and 1000 mg/kg bw/day male groups and the 125 mg/kg bw/day female group (NTP 2013). A LOAEL of 62.5 mg/kg bw/day can be inferred based on signs of liver toxicity in male rats.
In a second repeated-dose toxicity study conducted by the NTP (2013), groups of 10 male and 10 female B6C3F1/N mice were given 125, 250, 500, 1000, or 2000 mg/kg bw/day of Ginkgo biloba leaf extract in corn oil by gavage, 5 days per week for 14 weeks. A decrease in mean body weights was observed in the highest test dose female group when compared to controls. The study results showed adverse effects in the liver and olfactory epithelium of male and female mice. Liver weights of males in the 250 mg/kg bw/day or higher dose groups and liver weights of all female dose groups were significantly higher than control groups. Hepatocyte hypertrophy incidence was significantly higher in male and female mice in the groups given doses of 250 mg/kg bw/day or greater. This was accompanied by focal hepatocyte necrosis in the 1000 and 2000 mg/kg bw/day male groups. Hyaline droplet accumulation in the respiratory epithelium was significantly higher in 500 mg/kg bw/day males, as well as in the 1000 and 2000 mg/kg bw/day females. Similar results were seen in the olfactory epithelium, where hyaline droplet accumulation was significantly higher in the 125 (females only), 500, and 1000 mg/kg bw/day treatment groups (males and females). The incidence of pigment accumulation in macrophages of the olfactory epithelium was also significantly increased in the 500 mg/kg bw/day or greater male groups and in the 1000 and 2000 mg/kg bw/day female groups (NTP 2013).
No repeated-dose inhalation or dermal toxicity studies were identified in the scientific literature.
Carcinogenicity
Ginkgo biloba leaf extract has been classified by IARC as possibly carcinogenic to humans (Group 2B) on the basis of inadequate evidence in humans and sufficient evidence in experimental animals.
In a 2-year carcinogenicity study (NTP 2013), groups of F344/N rats (50/sex/dose) were given 100, 300, or 1000 mg/kg bw/day of Ginkgo biloba leaf extract in corn oil by gavage, 5 days per week for 104 weeks (males) or 105 weeks (females). The Ginkgo biloba extract contained 31.2% flavonol, 15.4% terpene lactones (bilobalide, 6.94%; ginkgolide A, 3.74%; ginkgolide B, 1.62%; ginkgolide C, 3.06%), and ginkgolic acid at 10.45 ppm. In tandem, as part of a “special” study, another group of rats (10/sex/dose) was administered the same doses for 14 weeks. Mortality was observed in the males treated at the highest dose (14/50 animals). In the main study, a decrease in mean body weights was observed after week 93 in the 300 mg/kg bw/day males and females and after week 89 in the 1000 mg/kg bw/day group (males and females).
Rats showed a significant increase in liver weights at all doses when compared with the control as well as an increase in levels of TSH in all dosed males and in females treated with 1000 mg/kg bw/day. In the main study, the incidence of liver effects in the form of hepatocellular adenomas was higher in males treated with 100 and 300 mg/kg bw/day. Non-neoplastic effects in the form of hepatocyte hypertrophy and bile duct hyperplasia were observed in animals at all doses. Focal fatty change was observed in all females at all doses, cystic degeneration was observed in males treated with 100 and 1000 mg/kg bw/day, and oval cell hyperplasia and necrosis were observed in males treated with 1000 mg/kg bw/day.
A significant increase in the incidence of follicular cell hypertrophy of the thyroid gland was observed in all test groups (males and females), and follicle hyperplasia was observed in the thyroid of all dosed males. An increased incidence of follicular cell adenomas was observed in animals treated with 300 (male and female) and 1000 mg/kg bw/day (male). Single incidences of follicular cell carcinomas were observed in females treated with 300 and 1000 mg/kg bw/day.
A dose-related increase in the severity of kidney nephropathy was noted in all male groups.
The incidence of transitional epithelium and respiratory epithelium hyperplasia was significantly increased in all dosed male and female groups. Significant increases were also seen in the incidence of atrophy, pigmentation, and respiratory metaplasia for all treatment groups (males and females). Goblet cell hyperplasia of the respiratory epithelium also showed significant increases in the 300 and 1000 mg/kg bw/day male and female groups. Chronic active inflammation and submucosa fibrosis (male) also showed significant increases at the highest dose tested (male and female).
Mononuclear cell leukemia showed a significant increase in the 300 and 1000 mg/kg bw/day male group when compared with controls (NTP 2013).
In another 2-year carcinogenicity study, groups of B6C3F1/N mice (50/sex/dose) were given 200, 600, or 2000 mg/kg bw/day of Ginkgo biloba leaf extract in corn oil by gavage, 5 days per week for 104 weeks (NTP 2013). The Ginkgo biloba extract contained 31.2% flavonol, 15.4% terpene lactones (bilobalide, 6.94%; ginkgolide A, 3.74%; ginkgolide B, 1.62%; ginkgolide C, 3.06%), and ginkgolic acid at 10.45 ppm Mixed results were seen with respect to the mean body weights and survival of animals, with males having a lower survival rate than controls in the high-dose groups (600 and 2000 mg/kg bw/day) and females having a significantly higher survival rate than controls in the 600 mg/kg bw/day group. Mean body weight of males in the middle and high-dose groups were less than the controls after 85 weeks, while the mean body weight of females was lower only in the highest-dose group.
With respect to liver effects, a statistically significant increase in the incidences of hepatocellular adenomas was observed in all treated females. Incidences of hepatocellular carcinomas were significantly increased in all treated males and in females treated with 2000 mg/kg bw/day of Ginkgo biloba leaf extract. Incidences of hepatoblastomas were significantly increased in all treated males, and in females treated with 600 and 2000 mg/kg bw/day of Ginkgo biloba extract.
Overall, a significant increase in the incidence of hepatocellular adenoma/carcinoma (combined) and hepatocellular carcinoma/hepatoblastoma (combined) was observed in all dosed groups of males and females. Non-neoplastic liver lesions included hypertrophy in all male and female groups, erythrophagocytosis in all males and 600 mg/kg bw/day or higher in females, hematopoietic cell proliferation, inflammation, cytoplasmic vacuolization and necrosis (only in ≥600 mg/kg bw/day males).
Thyroid follicular cell adenomas were observed in 2 cases of the 600 and 2000 mg/kg bw/day male groups. This was also accompanied by significant increases in follicle hyperplasia in males treated with 2000 mg/kg bw/day and significant increases in follicular cell hypertrophy in females treated with 600 mg/kg bw/day and males and females treated with 2000 mg/kg bw/day.
Hyaline droplet accumulation in the olfactory epithelium was significantly increased in the males and females treated with the highest dose. In addition, a significant increase in the incidence of pigmentation was observed in males treated at the highest dose, and in females treated with doses of 600 mg/kg bw/day and higher (NTP 2013).
The NTP (2013) concluded that there was some evidence of carcinogenic activity of Ginkgo biloba extract in male and female F344/N rats on the basis of increased incidences of thyroid gland follicular cell adenoma and follicular cell neoplasms, respectively.
There was clear evidence of carcinogenic activity of Ginkgo biloba extract in male and female B6C3F1/N mice on the basis of increased incidences of hepatocellular adenomas, carcinomas, and hepatoblastomas. Considering the results of the above 2 carcinogenicity studies, tumour response data based on the combined incidence of hepatocellular carcinoma and adenoma in mice were selected for benchmark dose modelling to calculate the 10% excess risk of the benchmark response (BMDL10) associated with the lower 95% confidence limit. Incidence data from male mice were used as they displayed a higher tumour incidence; these are presented in Table 7‑27.
| Dose (mg/kg bw/day) | Number of animals (N) | Overall tumour incidence |
|---|---|---|
| 0 | 50 | 39 |
| 200 | 50 | 46 |
| 600 | 50 | 46 |
| 2000 | 50 | 49 |
The US EPA’s BMDS v3.2 was used to evaluate multiple dichotomous models and identify the most sensitive output. The calculated BMDL10 value is 45 mg/kg bw/day, based on the best fitting multistage model. Details on the BMDL analysis are presented in Appendix D.
Genotoxicity
According to the NTP (2013), Ginkgo biloba leaf extract was found to be mutagenic in Salmonella typhimurium strains TA98 and TA100, and in Escherichia coli strain WP2 uvrA/pKM101, with and without exogenous metabolic activation (NTP 2013).
In a separate in vitro genotoxicity study, Ginkgo biloba extract and 8 of its constituents (quercetin; quercetin-3-β-D-glucoside; kaempferol; isorhamnetin; ginkgolide A; ginkgolide B; ginkgolide C; and bilobalide) were evaluated in mouse L5178Y cells using a lymphoma assay and a comet assay. Results showed a dose-dependent increase in mutant frequency and DNA double strand breaks with Ginkgo biloba leaf extract, quercetin, quercetin-3-β-D-glucoside, and kaempferol. The authors concluded that Ginkgo biloba extract, quercetin, and kaempferol are mutagenic in mouse L5178Y cells (Burnett 2018).
In vivo test data from a peripheral blood micronucleus test in male and female B6C3F1/N mice administered Ginkgo biloba leaf extract for 3 months by gavage was negative for males and equivocal for females (NTP 2013).
According to a reporter gene mutation assay, B6C3F1 gpt delta mice (males) were administered Ginkgo biloba leaf extract orally in corn oil in doses up to 2000 mg/kg bw/day for 90 days. No significant increases in in gpt or Spi- mutation frequencies in liver DNA were noted. No treatment-related clinical signs were observed. At 2000 mg/kg bw/day, an increase in relative liver weights was observed as well as hepatocellular hypertrophy in the centrilobular area, along with slight focal necrosis (Burnett 2018).
Overall, IARC postulated that the genotoxicity of Ginkgo biloba leaf extract could be one of the mechanisms responsible for its possible carcinogenicity. Two main components found at high levels, quercetin and kaempferol, have been found to be mutagenic in several assays and may be responsible for the genotoxicity of Ginkgo biloba extract. These substances are believed to have an inhibitory effect on DNA topoisomerase, leading to chromosomal damage (IARC 2016).
Epidemiological studies examining carcinogenicity
IARC (2016) concluded that there is inadequate evidence in support of the carcinogenicity of Ginkgo biloba extract in humans.
Reproductive and developmental toxicity
In a developmental toxicity study, Ginkgo biloba extract (with a composition of 28% flavonoids, 8% terpene lactones, and less than 5 ppm ginkgolic acids, reportedly similar to standardized Ginkgo biloba leaf extract) was administered to pregnant female Wistar rats (15 in each group) at 3.5, 7, or 14 mg/kg bw/day in distilled water by gavage daily from pregnancy day 8 to 20. On pregnancy day 20, the animals were sacrificed. No maternal toxicity was observed. However, fetal mean body weights were significantly decreased at 7 and 14 mg/kg bw/day compared with controls. Absolute, but not relative, fetal liver weights were also significantly decreased compared with the control. The authors concluded that, at 7 and 14 mg/kg bw/day, Ginkgo biloba extract induced intra-uterine growth retardation in the fetuses when exposed during the organogenesis and post-implantation phase, in the absence of maternal toxicity. As a result, the NOAEL is determined to be 3.5 mg/kg bw/day (Pinto et al. 2007).
In a reproductive toxicity study, a total of 240 Swiss albino mice (60/sex/dose) were administered an aqueous suspension of Ginkgo biloba leaf extract, containing 24% flavonoids (12 mg of a 50 mg Ginkgo biloba leaf extract), by gastric intubation at 25, 50, and 100 mg/kg bw/day for 90 days. Study results showed a significant increase in the mean weight of caudae epididymis in animals treated with 50 and 100 mg/kg bw/day as well as a significant increase in the mean weight of the prostate in animals treated with 100 mg/kg bw/day. No significant changes in spermatozoa morphology were observed, and no adverse effects were seen in either sperm motility or count at any tested dose. Significant changes were seen in the frequency of aneuploidy and the total percentage of chromosomal aberrations in the testes at 50 and 100 mg/kg bw/day. A decrease in the rate of pregnancy and embryonic losses before implantation were observed in females treated with 100 mg/kg bw/day. Based on these results, a NOAEL of 25 mg/kg bw/day is considered given the effects on the caudae epididymis and an increase in the frequency of aneuploidy and chromosomal aberrations in the testes of male mice exposed to 50 or 100 mg/kg bw/day (Al-Yahya et al. 2006).
In a developmental toxicity study, female pregnant Swiss albino mice (6/dose) were exposed for 18 days to 78 mg/kg bw/day or 100 mg/kg bw/day Ginkgo biloba extract dissolved in distilled water. Results showed a significant decrease in body weight and crown-rump length, and a significant increase in the incidence of malformations (for example, round-shaped eye and orbits, syndactyly, malformed pinnae, nostrils, lips and jaws) of fetuses in the high dose group compared with controls (Zehra et al. 2010). Certain key information was missing from the report, including data on the composition of the Ginkgo biloba extract, on maternal effects, and on the derivation of the tested doses.
In a reproductive and developmental toxicity study, groups of 10 to 20 female Swiss albino mice were administered 3.7, 7.4, or 14.8 mg/kg bw/day of Ginkgo biloba leaf extract (reported as standardized purified extracts of Ginkgo biloba EGb-761) by gavage in saline for 28 days prior to mating and during pregnancy, from day 1 to 7 of gestation or from day 10 to 18 of gestation. Study results showed no clinical signs of toxicity or unscheduled deaths in the dams. A significant decrease in body weight gain and relative weight of the gravid uterus was observed in the mice of the highest dose group when compared with the control after exposure for 28 days. Hormone levels showed a significant increase for prolactin and estradiol and a significant decrease in progesterone, luteinizing hormone (LH) and follicle-stimulating hormone (FSH) at 14.8 mg/kg bw/day. A significant reduction in ovarian follicle counts, implantation index, and fetal viability was also observed at the highest tested dose, along with vaginal bleeding and a significant increase in resorption index, disruption of estrous cycle, and maternal toxicity. The authors identified a NOAEL of 7.4 mg/kg bw/day on the basis of adverse effects on the estrous cycle, fertility, abortifacient parameters, reproductive performance, and hormone levels of female mice at the highest dose level (El Mazoudy and Attia 2012).
In a developmental toxicity study, female CD-1 mice (20/dose) were administered 100, 350, or 1225 mg/kg bw/day of Ginkgo biloba leaf extract (reported as standardized extracts of EGb-761, containing 24% flavonoids, 6% terpene lactones, and 0.4 ppm ginkgolic acids) in tap water by gavage on days 6 to 15 of gestation. Study results showed no clinical signs of toxicity or treatment-related effects on body weight gain or feed/water consumption of the dams. No embryotoxic effects were observed during internal and external fetal examinations. There were no increases in the incidences of malformations, variations, or retardations. The authors identified a NOAEL of 1225 mg/kg bw/day, which is the highest tested dose (Koch et al. 2013).
Ginkgotoxin
While the majority of Ginkgo biloba extracts originate from the leaves, a number of dermally applied cosmetics in Canada are reported to contain root or seed extract. The limited compositional data identified for these extracts suggest that ginkgo nuts (that is, seeds) extracts have a similar composition to the leaf extract, with the exception of 4’-O-methylpyridoxine (ginkgotoxin), which is present in a higher concentration in the seed (Arenz et al. 1996; Mei et al. 2017). While it is not a major component of Ginkgo biloba extract (<1%), ginkgotoxin has been reported to be associated with health effects observed in humans ingesting seeds.
Neurotoxic effects were observed with the ingestion of 50 to 80 of Ginkgo biloba whole seeds or nuts (Mei et al. 2017). Several human case reports, mainly originating from Japan and China, are available; the ingestion of Ginkgo biloba seeds resulted in vomiting, convulsions, and loss of consciousness (Mei et al. 2017). These effects have been linked to ginkgotoxin (that is, 4’-O-methylpyridoxine), which has been shown to inhibit the biosynthesis of vitamin B6, leading to disruption of the synthesis of the gamma-aminobutyric acid (GABA) neurotransmitter (Mei et al. 2017).
Limited animal toxicity data are available on the effects of ginkgo seeds and ginkgotoxin. In acute toxicity studies, an LD50 of 30 mg/kg bw was determined when rabbits were administered ginkgotoxin by intraperitoneal injection, while guinea pigs experienced seizures at 11 mg/kg bw and ventricular fibrillation and death at 30 to 50 mg/kg bw using the same route of administration (Leistner and Drewke 2010). In a single oral dose study, mice exposed to 0.1 mmol/kg bw (18 mg/kg bw) of ginkgotoxin experienced convulsions within 30 minutes, while exposure to 0.2 mmol/kg bw (37 mg/kg bw) of ginkgotoxin resulted in deaths (Kobayashi et al. 2010).
7.5.3 Characterization of risk to human health
On the basis of the overall available information, the critical effects associated with exposures to Ginkgo biloba extract are reproductive and developmental toxicity and carcinogenicity.
A NOAEL of 3.5 mg/kg bw/day was identified as the most appropriate non-neoplastic endpoint to characterize the risk from dermal and oral exposures to Ginkgo biloba extract. This is based on reduced body weight and reduced intrauterine growth in rat fetuses in the absence of maternal toxicity observed when parental animals were exposed orally to 7 or 14 mg/kg bw/day Ginkgo biloba leaf extract for 14 days.
Exposure estimates for age groups with the highest exposure and resulting MOEs are presented in Table 7‑28.
| Exposure scenario | Exposure (mg/kg bw/day) |
MOEc |
|---|---|---|
| Hair perm/straightenera (19+ years) |
2.1 | 2 |
| Permanent hair dyea (14 to 18 years) |
1.4 | 3 |
| Face exfolianta (14 to 18 years) |
3.3 × 10-2 | 106 |
| Aftershavea (9 to 13 years) |
1.8 | 2 |
| Sunless tanning producta (9 to 13 years) | 0.36 | 10 |
| Massage producta (0 to 5 months) |
1.9 | 2 |
| Face maska (14 to 18 years) |
1.0 | 4 |
| Body oila (14 to 18 years) |
3.0 | 1 |
| Hair mista (19+ years) | 8.6 × 10-3 | 407 |
| Liquid face foundationa (4 to 8 years) | 2.9 | 1 |
| Genital lubricantb (19+ years) | 0.88 | 4 |
| Face moisturizera (19+ years) |
11.9 | < 1 |
| Face tonera (19+ years) |
32.0 | < 1 |
| Body moisturizera (0 to 5 months) |
6.2 | < 1 |
| Hand creama (2 to 3 years) |
3.8 × 10-2 | 92 |
| Body soap (liquid)a (0 to 5 months) |
0.17 | 21 |
| Spray antiperspiranta (9 to 13 years) |
3.7 × 10-2 | 95 |
| Shampooa (0 to 11 months) |
0.40 | 9 |
| Face cleansera (9 to 13 years) | 1.7 × 10-2 | 206 |
| Makeup removera (14 to 18 years) |
4.6 × 10-2 | 76 |
| Face sunscreen (NHP)a (19+ years) |
0.79 | 4 |
| Sunscreen lotion (NHP)a (6 to 11 months) |
1.9 | 2 |
| Face sunscreen (NPD)a (19+ years) |
2.6 × 10-2 | 135 |
| Oral supplement (capsule) (NHP) (19+ years) | 7.6 | < 1 |
| Oral supplement (liquid extract) (19+ years) |
81.1 | < 1 |
| Herbal tea blend (NHP) (19+ years) |
7.3 × 10-2 | 48 |
| Herbal tea (19+ years) |
1.01 | 3 |
Abbreviations: MOE, margin of exposure; NOAEL, no observed adverse effect level
a Dermal exposure estimates were adjusted by a dermal absorption value of 65% (dal Belo et al. 2009).
b Dermal absorption was assumed to be equivalent to oral absorption.
c MOEs are presented using the selected NOAEL of 3.5 mg/kg bw/day, based on reduced fetal body weight and intrauterine growth in rat fetuses observed when parental animals were exposed orally to 7 or 14 mg/kg bw/day Ginkgo biloba leaf extract for 14 days.
For exposure to Ginkgo biloba extract in hair mists, the resulting MOE between the critical effect level and the estimate of exposure was 407, which is above 300 and is considered adequate to address uncertainties in the health effects and exposure data used to characterize risk. For all other products for consumers listed in Table 7‑28, the resulting MOEs between the critical effect level and the estimates of exposure were between <1 and 206, which are below 300 and are considered potentially inadequate to address uncertainties in the health effects and exposure data used to characterize risk. Factors including interspecies and intraspecies differences and database deficiencies were considered during the determination of the adequacy of the MOEs. Given the number of products containing Ginkgo biloba extract available on the Canadian market, it is possible that exposure may result from the use of several types of products containing this substance on the same day (that is, aggregate exposure), in which case the MOE would be even lower.
A BMDL10 of 45 mg/kg bw/day, based on an increased incidence of hepatocellular adenomas and/or carcinomas in male mice exposed orally to Ginkgo biloba leaf extract for 2 years, was identified as the most appropriate endpoint to characterize the risk from lifetime exposure to Ginkgo biloba extract. LADDs and resulting MOEs are presented in Table 7‑29.
| Exposure scenario | LADD (mg/kg bw/day) |
MOEc |
|---|---|---|
| Hair perm/straightenera | 2.6 × 10-2 | 1731 |
| Permanent hair dyea | 2.1 × 10-2 | 2143 |
| Face exfolianta | 6.6 × 10-3 | 6818 |
| Aftershavea | 0.67 | 67 |
| Sunless tanning producta | 4.9 × 10-2 | 918 |
| Massage producta | 4.0 × 10-2 | 1125 |
| Face maska | 7.1 × 10-2 | 634 |
| Body oila | 0.26 | 173 |
| Hair mista | 6.8 × 10-3 | 6618 |
| Liquid face foundationa | 1.7 | 26 |
| Genital lubricantb | 0.66 | 68 |
| Face moisturizera | 9.9 | 5 |
| Face tonera | 22.7 | 2 |
| Body moisturizera | 2.8 | 16 |
| Hand creama | 2.6 × 10-2 | 1731 |
| Body soap (liquid)a | 4.8 × 10-2 | 938 |
| Spray antiperspiranta | 2.9 × 10-2 | 1552 |
| Shampooa | 0.12 | 375 |
| Face cleansera | 1.2 × 10-2 | 3750 |
| Makeup removera | 3.4 × 10-2 | 1324 |
| Face sunscreen (NHP)a | 0.60 | 75 |
| Sunscreen lotion (NHP)a | 0.72 | 63 |
| Face sunscreen (NPD)a | 2.2 × 10-2 | 2045 |
| Oral supplement (capsule) (NHP) | 5.7 | 8 |
| Oral supplement (liquid extract) | 61.3 | < 1 |
| Herbal tea (NHP) | 5.5 × 10-2 | 818 |
| Herbal tea | 0.77 | 58 |
Abbreviations: MOE, margin of exposure; BMDL, benchmark dose level
a Dermal exposure estimates were adjusted by a dermal absorption value of 65% (dal Belo et al. 2009).
b Dermal absorption was assumed to be equivalent to oral absorption .
c MOEs are presented using the selected BMDL of 45 mg/kg bw/day, based on an increased incidence of hepatocellular adenomas and/or carcinomas in male mice exposed orally to Ginkgo biloba leaf extract for 2 years.
For all scenarios listed in Table 7‑29, the resulting MOEs between the critical effect level and the LADDs were between <1 and 6 818, which are below 10 000 and are considered potentially inadequate to address uncertainties in the health effects and exposure data used to characterize risk. Given the number of products containing Ginkgo biloba extract available on the Canadian market, it is possible that exposure may result from the use of several types of products containing this substance on the same day (that is, aggregate exposure), in which case the MOE would be even lower.
7.5.4 Uncertainties in evaluation of risk to human health
The key sources of uncertainty are presented in Table 7‑30.
| Key source of uncertainty | Impact |
|---|---|
| Although Ginkgo biloba leaf extracts have a number of standardized formulations such as EGb-761, variations in the composition of these products have been identified on the US market and may also be present on the Canadian market. | +/- |
| Although quercetin is a major component of Ginkgo biloba extract, there is a degree of uncertainty in extrapolating the dermal absorption of quercetin to Ginkgo biloba extract. | +/- |
| There are no repeated-dose toxicity studies identified for Ginkgo biloba extract via the dermal route of exposure. | +/- |
| There is limited information on ginkgotoxin. Although it is not a major component of Ginkgo biloba leaf extract (<1%), ginkgotoxin has been reported to be associated with health effects observed in humans ingesting seeds. | - |
| The potential use of more than one product by a single person in a day (that is, aggregate exposure) was not considered. This may potentially underestimate exposure to some individuals. | - |
+ = uncertainty with potential to cause over-estimation of exposure/risk; - = uncertainty with potential to cause under-estimation of exposure risk; +/- = unknown potential to cause over- or under-estimation of risk.
7.6 Amberlyn
7.6.1 Exposure assessment
Environmental media and food
No measured data regarding concentrations of amberlyn in environmental media were identified in Canada or elsewhere. The substance was reported to be imported into Canada at a quantity ranging from 100 kg to 1,000 kg, according to information submitted in response to a CEPA section 71 survey (Environment Canada 2013). On the basis of low reported quantities of the substance in Canada, exposure of the general population to amberlyn from environmental media is expected to be minimal.
Amberlyn is not reported to occur naturally in foods (JECFA 2004a; Burdock 2010). The JECFA evaluated amberlyn as a food flavouring agent and estimated the corresponding per capita intake to be 0.1 μg/day (approximately 0.002 μg/kg bw/day) for the US population (National Academy of Science 1987; International Organization of the Flavour Industry 1995, both cited in JECFA 2004a and JECFA 2004b). The JECFA concluded that the substance presents “no safety concern[s] at current levels of intake when used as a flavouring agent.” In the absence of data on the actual use, if any, of amberlyn as a flavouring agent in foods sold in Canada, the per capita intake estimate for the US population derived by JECFA is considered to be an acceptable estimate of possible Canadian dietary exposure to this substance for the general population 1 year of age and older (personal communications, emails from the FD, HC to the ESRAB, HC, dated June 2021; unreferenced).
Products available to consumers
From the available public data, types of products reported to contain amberlyn include cosmetics, laundry detergents, cleaning sprays and liquids, and air fresheners for home and auto (CPID 2021). Concentrations of amberlyn in these products were not available; however, on the basis of follow-up information submitted in response to a CEPA section 71 survey, amberlyn was identified as an ingredient in similar cosmetics, laundry and dishwashing products, cleaning products, and air care products. Types of products reported to contain the substance included body lotions and creams, bath soaps, gels and scrubs, dish and laundry detergents, laundry beads, liquid cleaners and sprays, and air fresheners (Environment Canada 2013).
The main route of exposure to amberlyn is expected to be the dermal route as the vapour pressure of the substance is low to moderate (0.4 Pa); however, inhalation exposure to amberlyn may occur from the use of air care products (for example, air fresheners) or spray products. For dermal exposure, a body lotion containing 0.15% and 0.04% amberlyn was selected as the sentinel scenario to characterize exposure of older age groups (that is, 9+ years) and younger age groups (that is, 0 to 9 years), respectively. An air freshener with a concentration of 1% was selected as the sentinel scenario to characterize inhalation exposure to amberlyn. Amberlyn was identified in lower concentrations in the other products reported to contain it and the body lotion and air freshener scenario are expected to capture these uses. For the dermal and inhalation exposure scenarios presented, absorption was assumed to be equivalent to oral absorption (that is, 100%). Estimates of exposure to amberlyn from the use of a body lotion and air freshener were derived for a range of relevant age groups, and only the highest one is presented in Table 7‑31. Details on the parameters used to estimate these scenarios are presented in Appendix A.
| Scenario | Route of exposure | Concentration | Systemic exposurea (mg/kg bw/day) |
|---|---|---|---|
| Body lotion | Dermal | 0.15 (9+ years) 0.04 (0 to 9 years) |
0.28 (9 to 13 years) |
| Air freshener | Inhalation | 1 | 0.18 (1 year) |
a For dermal exposures, absorption was assumed to be equivalent to oral absorption (that is, 100%).
b Inhalation exposure estimates were converted to internal doses on the basis of default inhalation rates and body weights from HC (2021).
7.6.2 Health effects assessment
No international assessments were identified for amberlyn. The health effects assessment of amberlyn was informed by the studies available in a REACH registration dossier available from ECHA (2021a).
Repeated-dose toxicity
In a short-term dose range-finding study, Wistar rats (n=3/sex/group) were administered amberlyn (in corn oil) by gavage at doses of 0, 100, 300, or 1000 mg/kg bw/day during a pre-pairing period of 14 days until GD 14 (Anonymous 2009, as cited in ECHA 2021l). On GD 14, the female animals were sacrificed and subjected to gross pathological examination to determine fertility, the number and distribution of implantation sites, the number of corpora lutea, the number of live/dead embryos, and the number of early/late embryonic deaths. The male animals were sacrificed and subjected to gross pathological examination following treatment for 28 days. At 300 mg/kg bw/day, there were clinical signs such as ruffled fur, discomfort, and salivation. At the highest dose level of 1000 mg/kg bw/day, all of the animals displayed clinical signs (for example, discomfort, ruffled fur, diarrhea, salivation) and one female animal was found dead on day 4. At the same dose level, there were transient, yet significant reductions in body weight gains and food consumption. A NOAEL of 100 mg/kg bw/day is determined based on clinical signs observed at higher doses.
In a combined repeated-dose and reproductive/developmental toxicity screening study, Wistar rats (n=10/sex/dose) were administered amberlyn (in corn oil) by gavage at doses of 0, 100, 400, or 800 mg/kg bw/day during a pre-pairing phase, a cohabitation phase (14 days), and during gestation up to PND 4 (Anonymous 2009, as cited in ECHA 2021l; unnamed study report 2021). Male animals were treated for 4 weeks (28 days) while female animals were treated for approximately 7 weeks. At 100 mg/kg bw/day, liver weights were significantly increased (+11% compared to control animals). However, there were no accompanying histopathological changes or changes in clinical chemistry parameters that would be indicative of liver damage. Therefore, the increase in liver weights was considered to be adaptive and non-adverse. At the mid- and high doses, there were signs of discomfort (defined by the authors as animals pushing their heads through the bedding and salivation) following treatment. In addition, there were increased levels of platelets, cholesterol, globulin, increased liver weights, centrilobular hepatocellular hypertrophy, and hyaline inclusions, and tubular basophilia in the kidneys. Furthermore, diffuse follicular hypertrophy was noted in the thyroid of high dose animals, and neurobehavioural examinations also noted a reduction in the number of rearings in male animals. A NOAEL of 100 mg/kg bw/day was identified on the basis of clinical signs, changes in hematological and clinical chemistry parameters, and histopathological changes at the next dose level of 400 mg/kg bw/day.
Genotoxicity and carcinogenicity
Amberlyn has been examined in bacterial mutagenicity assays and tested against Salmonella typhimurium strains TA1535, TA1537, TA98, TA100, and Escherichia coli WP2 in the presence and absence of metabolic activation (Anonymous 2008 as cited in ECHA 2021l). The test substance was not found to be mutagenic up to the highest tested concentration. Amberlyn has also been tested in a mammalian cell gene mutation assay on Chinese hamster lung fibroblasts (CHL V79) and did not induce any statistically significant increases in mutation frequency at any dose level with or without metabolic activation (Anonymous 2009 as cited in ECHA 2021l). In terms of chromosome aberrations, amberlyn was tested on CHL V79 fibroblast cells with and without metabolic activation (Anonymous 2009 as cited in ECHA 2021l). Exposure to amberlyn did not induce any increases in the frequency of cells with aberrations or polyploidy cells. Therefore, it was not considered to be clastogenic. Although no in vivo genotoxicity studies were identified, the available data suggest that exposure to amberlyn is not likely to result in genotoxicity.
Studies examining the potential carcinogenic effects following exposure to amberlyn have not been identified.
Reproductive and developmental toxicity
In the combined repeated-dose and reproductive/developmental screening study noted previously, Wistar rats (n=10/sex/dose) were administered amberlyn (in corn oil) by gavage at doses of 0, 100, 400, or 800 mg/kg bw/day during a pre-pairing phase, a cohabitation phase (14 days), and during gestation up to PND 4 (Anonymous 2009, as cited in ECHA 2021l). There were no effects on fertility, duration of gestation, corpora lutea count, implantation rate, post-implantation loss, or sperm effects up to the highest dose tested of 800 mg/kg bw/day reported in this limited study.
7.6.3 Characterization of risk to human health
On the basis of the health effects data available, amberlyn is not expected to be carcinogenic or genotoxic.
A NOAEL of 100 mg/kg bw/day was identified in a combined repeated-dose, reproductive/developmental toxicity study conducted on rats, which was considered to be the most appropriate endpoint to use for characterization of the human health risk from exposure to amberlyn (Anonymous 2009 as cited in ECHA 2021l; unnamed study report 2021). This NOAEL was identified on the basis of clinical signs, changes in hematological/clinical chemistry parameters, and histopathological changes at the next dose level of 400 mg/kg bw/day.
Table 7‑32 describes the exposure to amberlyn from a body lotion, air freshener, and potential use as a food flavouring agent as well as the critical effect level and resultant MOEs for the characterization of risk to human health.
| Scenario | Exposure estimatea,b (mg/kg bw/day) |
MOEc |
|---|---|---|
| Body lotion (9 to 13 years) |
0.28 | 357 |
| Air freshener (1 year) |
0.18 | 556 |
| Food flavouring agent (individuals 1 year and older) |
0.000002 | > 100 000 |
Abbreviations: MOE, margin of exposure; NOAEL, no observed adverse effect level
a For dermal exposures, absorption was assumed to be equivalent to oral absorption (that is, 100%).
a Exposure estimates are presented for the highest exposed age group.
c MOEs are presented using the selected NOAEL of 100 mg/kg bw/day, based on clinical signs, hematological/clinical chemistry changes, and histopathological changes at the next dose level of 400 mg/kg bw/day in a combined repeated-dose, reproductive/developmental toxicity study in rats.
These MOEs are above 300 and considered adequate to address uncertainties in the health effects and exposure data used to characterize risk. Factors including interspecies and intraspecies differences and database deficiencies were considered during the determination of the adequacy of the MOEs.
7.6.4 Uncertainties in evaluation of risk to human health
The key sources of uncertainty are presented in Table 7‑33.
| Key source of uncertainty | Impact |
|---|---|
| Dermal absorption was considered to be equivalent to oral absorption (that is, 100%). | + |
| Studies examining chronic administration of amberlyn were not identified. The health effects data from a combined repeated-dose and reproductive/developmental toxicity study were used to inform long-term exposure scenarios. | +/- |
| Studies conducted through the dermal and inhalation routes were not identified. Health effects data from oral studies were used to inform the characterization of risk for these routes (that is, route-to-route extrapolation). | +/- |
+ = uncertainty with potential to cause over-estimation of exposure/risk; - = uncertainty with potential to cause under-estimation of exposure risk; +/- = unknown potential to cause over- or under-estimation of risk.
7.7 Myrrh oil
7.7.1 Exposure assessment
Environmental media and food
Data on myrrh oil in environmental media such as air, water, dust, soil, or sediment in Canada or elsewhere were not identified. The substance was not reported to be manufactured or imported in quantities greater than the reporting threshold of 100 kg on the basis of information submitted in response to a CEPA section 71 survey (Environment Canada 2013). In consideration of low reported quantities of the substance in Canada, exposure to myrrh oil from its presence in environmental media is expected to be minimal.
No definitive information is available concerning the potential use of myrrh oil in food packaging or as a food flavouring agent in foods sold in Canada. However, since myrrh oil is identified as a food flavouring agent internationally, it is possible that this substance is present as a flavouring agent in foods sold in Canada.
Fenaroli’s Handbook of Flavour Ingredients reports the per capita (“individual”) estimated intake of myrrh oil from its use as a food flavouring agent to be 1.553 × 10-4 mg/kg bw/day for the US population on the basis of production volumes reported by the food industry (Burdock 2010). In the absence of data on the use of myrrh oil as a food flavouring agent in foods sold in Canada, the per capita intake estimate for the US population (Burdock 2010) is an acceptable estimate of possible Canadian dietary exposure to this substance for the general population aged 1 year and older (personal communication, emails from the FD, HC, to the ESRAB, HC, dated June 2021; unreferenced).
Products available to consumers
Myrrh oil, including ingredients derived from Commiphora myrrha and Commiphora mukul, are present in products available to consumers, including cosmetics, NHPs, and resin incense. Myrrh oil is also available as a pure essential oil (that is, present at 100%) available to consumers in Canada. Representative sentinel exposure scenarios were selected to evaluate potential exposures to myrrh oil from use of these products. Parameters used to estimate the exposure scenarios are presented in Appendix A. For dermal exposures, absorption was conservatively assumed to be equivalent to oral absorption. Inhalation exposure is not expected for cosmetics and NHPs due to the low vapour pressure of the main components of myrrh oil (see section 3); however, inhalation exposure from spray-based products (for example, spray perfumes) is possible.
Short-term intermittent and daily exposure estimates are summarized in Table 7‑34 and Table 7‑35, respectively. For each scenario, exposure estimates were derived for a range of relevant age groups, and only the highest one is presented. Given the number of products containing myrrh oil available on the Canadian market, exposure may result from the use of several types of products (e.g., cosmetics and NHPs) containing the substance on the same day (that is, aggregate exposure).
| Exposure scenario | Route of exposure | Concentration (%) | Exposure (mg/kg bw/day)c (age range) |
|---|---|---|---|
| Permanent hair dye | Dermal | 3a | 6.4 (14 to 18 years) |
| Hair styling product | Dermal | 30a | 5.6 (2 to 3 years) |
| Massage oil | Dermal | 3a | 8.6 (0 to 5 months) |
| Bath oil | Dermal | 100a | 0.38 (9 to 13 years) |
| Face exfoliant | Dermal | 10a | 0.50 (14 to 18 years) |
| Hair removal aftercare | Dermal | 3a | 3.9 (9 to 13 years) |
| Sunless tanning product | Dermal | 0.3a | 0.55 (9 to 13 years) |
| Aftershave | Dermal | 3a | 1.6 (9 to 13 years) |
| Pain gel (NHP) | Dermal | 9.1b | 7.3 (19+ years) |
a Personal communication, emails from the CHPSD, HC, to the ESRAB, HC, dated January 2022; unreferenced.
b Personal communication, emails from the NNHPD, HC, to the ESRAB, HC, dated June 2021; unreferenced.
c Exposure estimates are based on product use on day of exposure.
| Exposure scenario | Route of exposure | Concentration | Exposure (mg/kg bw/day) (age range) |
|---|---|---|---|
| Body moisturizer | Dermal | 10%d | 31.7 (0 to 5 months) |
| Face moisturizer | Dermal | 30%d | 12.2 (19+ years) |
| Antiperspirant | Dermal | 3%d | 0.53 (14+ years) |
| Liquid body soap | Dermal | 10%d | 0.86 (0 to 5 months) |
| Spray perfume | Dermal and inhalationb,c | 10%d | Dermal: 1.9 (2 to 3 years) Inhalation: 2.9 × 10-4 (2 to 3 years) |
| Tooth powder | Oral | 30%d | 12.2 (2 to 3 years) |
| Mouthwash | Oral | 1%d | 0.43 (4 to 8 years) |
| Teeth whitener | Oral | 50%d | 2.4 (14 to 18 years) |
| Body lotion (NHP) | Dermal | 0.5%e | 5.0 (2 to 3 years) |
| Sunscreen lotion (NHP) | Dermal | 0.049%e | 0.47 (6 to 11 months) |
| Hand sanitizer (NHP)a | Dermal | 0.04%e | 4.0 × 10-2 to 1.0 (2 to 3 years) |
| Oral capsule (NHP) | Oral | 25 mg/capsulee | 2.0 (19+ years) |
| Resin incense | Inhalationb | 100% | 2.3 (1 year old) |
a The range of exposure estimates represent a range of use frequencies for hand sanitizers. The upper end of the range describes situations of public health concern, where the use of hand sanitizers among the general population may increase up to 25 uses per day (personal use by individuals 20+ years, increased use by children in schools and childcare facilities) (RIVM 2021a; Lopez et al. 2022).
b Inhalation exposures were converted to internal doses using default inhalation rates and body weights (HC 2021).
c Data on the partitioning of myrrh oil between the dermal and inhalation routes for this scenario were unavailable. Therefore, dermal and inhalation exposures were evaluated separately.
d Personal communication, emails from the CHPSD, HC, to the ESRAB, HC, 2022; unreferenced.
e Personal communication, emails from the NNHPD, HC, to the ESRAB, HC, 2021; unreferenced.
Myrrh oil is available to consumers in Canada as a pure essential oil (that is, present at 100%). Information from the available literature on uses of essential oils note that they can be used by consumers in DIY applications (see section 4). Exposure to myrrh essential oil formulated in massage oils and body moisturizers is captured in the assessment of cosmetic products in Table 7‑34 and Table 7‑35. Additional indications of use for myrrh oil in DIY applications include use as a stomach remedy (doTERRA 2022b). Parameters used to estimate the exposure scenarios are presented in Appendix A. For each scenario, exposure estimates were derived for a range of relevant age groups, and only the highest one is presented in Table 7‑36.
| Exposure scenario | Route of exposure | Concentration | Exposure range (mg/kg bw/day) (age range) |
|---|---|---|---|
| Essential oil use as a stomach remedy (short-term intermittent exposure) |
Oral | 100% | 6.7 (2 to 3 years) |
| Essential oil use in aromatic diffuser (daily exposure) |
Dermala and inhalationb | 100%d | 3.3 (9 to 13 years) |
| Essential oil use in face steamer (user) (daily exposure) |
Dermal and inhalationb,c | 100%e | 0.61 (4 to 8 years) |
a Dermal exposure from this use is expected to be incidental during refilling of an aromatic diffuser and considered to be applicable to users only (for example, those 9+ years).
b Based on a 20-minute mean event concentration of 23 mg/m3, assuming that 50% of this air concentration value (that is, 23 mg in a 1 m3 volume) is available for inhalation (11.5 mg/m3) and that 50% (11.5 mg) is deposited onto the face of a user. Additional details provided in Appendix A.
c Inhalation exposures were converted to internal doses using default inhalation rates and body weights (HC 2021).
d Assumes that 920 mg of pure essential oil is added to the device (~18 drops of myrrh oil).
e Assumes that approximately 10 drops of pure essential oil are added to the device. Details on the corresponding product amounts are provided in Appendix A.
7.7.2 Health effects assessment
No international assessments with relevant health effects data were identified for myrrh oil. The health effects assessment of myrrh oil was informed by taking into consideration toxicological data from any Commiphora species and their extracts.
Repeated-dose toxicity
The health effects data set for myrrh oil (derived from Commiphora molmol) was limited to 2 studies. The first study examined the effects of myrrh oil (prepared by fractional distillation of the resins from Commiphora molmol) through the subcutaneous route of administration (Lamichhane et al. 2019). ICR mice (n=6/group) were administered approximately 0, 33, 167, 334, 669, 1337, or 2675 mg/kg bw/day of myrrh oil by subcutaneous injection for 3 days. No treatment-related effects were observed up to 334 mg/kg bw/day. All treated animals from the 669 mg/kg bw/day group and above developed palpable subcutaneous nodules, accompanied by pruritis, had significantly reduced body weights and food consumption, had significant increases in the levels of white blood cells (WBC), aspartate aminotransferase (AST), and creatinine, and had significant decreases in the levels of ALT and blood urea nitrogen (BUN). Animals at those dose levels also showed effects on the liver (that is, presence of dilated sinusoids/central vein) and the spleen (that is, increased weights, loss of distinction between red and white pulps, and extensive accumulation of megakaryocytes), while effects on the kidneys were only observed at the highest dose.
The second study examined the effects of a hydrodistillate of myrrh oil on cardiac function of Wistar rats (n=6/group), which were administered 0 or 50 mg/kg bw/day of myrrh oil orally for 28 days, and injected subcutaneously with the same substance on days 29 and 30. There were no treatment related effects on cardiac function (Younis and Mohamed 2021).
Although the health effects dataset for myrrh oil was considered to be limited, there were several studies conducted on different myrrh extracts (derived from Commiphora myrrha or Commiphora molmol). According to data available, ethanolic, petroleum ether, and hexane extracts from the aforementioned botanical species contain similar components as the essential oil (Hanuš et al. 2005; Mahboubi and Kashani 2016). Therefore, these studies were also taken into consideration in this assessment.
The short-term effects of an ethanolic extract of myrrh (from Commiphora myrrha oleo-gum resin) were examined in a study where Wistar rats (n=6 males/group) were given 0, 500, or 1000 mg/kg bw/day of extract (in drinking water) for 14 days (Omer et al. 1999). Clinical signs, mortality, and changes in hematological and clinical chemistry parameters were examined. Gross pathology and histopathological examinations were also conducted on the intestines, liver, spleen, kidneys, and the heart. At 500 mg/kg bw/day, there was a statistically significant increase in serum ALT levels. However, in the absence of histopathological changes in the liver or other relevant clinical chemistry parameters, this effect was not considered to be adverse at this dose level. At 1000 mg/kg bw/day, clinical signs (for example, depression, soft feces, and ruffled fur) were observed, with 2 animals dying from unknown causes. There were also significant decreases in red blood cells (RBC), hemoglobin, packed cell volume, WBC, neutrophils, and increases in mean corpuscular volume (MCV) and lymphocytes. With respect to clinical chemistry parameters, there were significant differences in ALP, ALT, cholesterol, bilirubin, total protein, albumin, and creatinine. In addition, animals exhibited histopathological changes in the heart, intestines (for example, accumulation of lymphocytes), liver (for example, fatty vacuolation, necrosis of hepatocytes), kidneys (for example, degeneration of renal tubular cells), and spleen (for example, hemosiderin in the splenic red pulp). A NOAEL of 500 mg/kg bw/day was interpreted from the study on the basis of the systemic effects observed at the next dose level of 1000 mg/kg bw/day.
In another study, the effects of a water extract of myrrh (from Commiphora molmol oleo-gum resin) following subchronic exposure was examined. In this limited study, Swiss albino mice (n=10/sex/group) were given the extract (in drinking water) at a dose level of 100 mg/kg bw/day for 90 days (Rao et al. 2001). At the end of the study, animals in the treated group exhibited significantly higher weights of the testes, caudae epididymides and seminal vesicles compared with animals in the control group, although no effects on sperm were observed. There were also significant increases in certain hematological parameters such as RBC and hemoglobin levels (+11% and 14%, respectively) in the treated animals compared with the control animals.
Mitsumoto et al. (2021) examined the effects of pulverized grains of myrrh (unknown botanical species) when mixed in dietary feed. F344 rats (n=10/sex/group) were fed 0, 290, 850, or 3000 mg/kg bw/day of myrrh in the diet for 90 days. At the highest dose level of 3000 mg/kg bw/day, the animals had significantly lower body weights, spleen weights, adrenal weights, and lower levels of AST, ALT, BUN, and triglycerides. There was also a significant increase in liver and kidney weights, which was accompanied by the presence of hyaline droplets. The authors identified a NOAEL of 850 mg/kg bw/day on the basis of the systemic effects observed at the next dose level of 3000 mg/kg bw/day.
Studies have also been conducted on other species of Commiphora, predominantly on Commiphora mukul. In 2020, the NTP published a report on a series of studies conducted on rats and mice using an extract of Commiphora mukul. In the first study, Sprague-Dawley rats (n=10/sex/group) were administered 0, 62.5, 125, 250, 500, or 1000 mg/kg bw/day of extract (in corn oil) by gavage for 28 days. At doses equal to or greater than 125 mg/kg bw/day, significant increases in liver weights were observed. However, since no histopathological changes in the liver or relevant clinical chemistry parameters were observed, this effect was considered to be adaptive and not adverse. This was supported by the observations of enzyme induction (for example, CYP2B, CYP3A) at all dose levels (2- to 6-fold increases). At 500 mg/kg bw/day, there were also significant increases in globulin levels and decreased levels of phospholipids, but no histopathological or functional changes were observed. At 1000 mg/kg bw/day, in addition to the aforementioned effects, there were also significantly increased kidney weights, increased protein levels, and decreased levels of cholesterol, bile acids, and albumin/globulin (A/G) ratios. A NOAEL of 500 mg/kg bw/day was identified on the basis of effects on the kidneys and changes in clinical chemistry parameters observed at the next dose level of 1000 mg/kg bw/day.
The same study was also conducted over a longer duration of 3 months (NTP 2020). The study also included examinations of sperm motility and vaginal cytology. At all dose levels, a significant reduction in bile acids was observed (up to 53% lower than controls). At doses equal to or greater than 250 mg/kg bw/day, there was also a significant increase in liver weights. In addition to these effects, treatment with 500 mg/kg bw/day also resulted in significant increases in globulin levels and decreased A/G ratios. At the highest dose level of 1000 mg/kg bw/day, significant increases in kidney weights and significant decreases in thymus weights, hematocrit, and hemoglobin levels were noted. A LOAEL of 62.5 mg/kg bw/day was identified by the authors on the basis of reduced levels of bile acids.
In similar studies conducted on mice (B6C3F1, n=15/sex/group), the animals were administered 0, 15.5, 31, 62.5, 125, or 250 mg/kg bw/day of Commiphora mukul extract (in corn oil) by gavage for 28 days (NTP 2020). At 125 mg/kg bw/day, a significant decrease in relative thymus weights was observed. However, this observation was not considered to be treatment-related by the authors due to the lack of dose-dependence. No other treatment-related, adverse effects were observed up to 250 mg/kg bw/day.
Another group of B6C3F1 mice (n=15/sex/group) was administered 0, 62.5, 125, or 250 mg/kg bw/day of Commiphora mukul extract (in corn oil) by gavage for 3 months (NTP 2020). All dose levels were associated with significantly increased cholesterol levels and effects on the sperm (that is, lower mean total number of sperm heads, cauda epididymal sperm). At the highest dose level of 250 mg/kg bw/day, there were also increased levels of phospholipids and decreased testes weights. The NTP (2020) indicated that the testes may be a target organ of Commiphora mukul extract and identified a LOAEL of 62.5 mg/kg bw/day.
Carcinogenicity and genotoxicity
Studies examining the potential carcinogenic effects of myrrh oil were not identified. With respect to genotoxicity, no studies examining the effects of myrrh oil were available. An aqueous extract of myrrh (from Commiphora molmol oleo-gum resin) was not found to result in chromosome aberrations when administered to Swiss albino mice (n=5 females/group) at doses of 125, 250, or 500 mg/kg bw/day by gavage for 7 days in a micronucleus test (Qureshi et al. 1993). Similar results were found when an aqueous extract of myrrh (from Commiphora molmol oleo-gum resin) was administered to SWR mice (n=5 males/group) by gavage for 7 days (Al-Harbi et al. 1994). No chromosome aberrations were observed up to the highest tested dose of 250 mg/kg bw/day. Chromosome aberration studies were also negative (Massoud et al. 2000 ;Omar et al. 2005). With respect to genotoxicity data on Commiphora mukul, no increases in the frequency of micronuclei were observed when an extract was administered by gavage to rats (at doses up to 1000 mg/kg bw/day) and mice (at doses up to 250 mg/kg bw/day) for 3 months (NTP 2020). Overall, these studies suggest that exposure to myrrh oil is not expected to result in genotoxicity.
Reproductive and developmental toxicity
Studies examining the potential effects of myrrh oil on reproduction and development were not identified. However, in a limited study conducted using a commercial extract of Commiphora molmol, the fertility of male rats was assessed before and during treatment (Massoud et al. 2002; Massoud et al. 2012 as cited in Al-Kazzaz 2018). In the first experiment of this study, male albino rats were divided into 3 groups (n=10/group) given 0, 100, or 200 mg/kg bw/day of the extract by gavage for 8 weeks. Four weeks after commencement, the males from each group were mated with females. No differences in mating or fertility rates were observed up to the highest level tested of 200 mg/kg bw/day.
In another study male rats (n=20/group) were given 0, 100, or 200 mg/kg bw/day of a commercial extract (derived from Commiphora molmol) for 8 weeks (Massoud et al. 2002). At the end of the 1st, 2nd, 4th, and 8th week of treatment, animals from each group (n=5/group) were sacrificed. Testosterone levels were measured and the testes, epididymides, seminal vesicles, and prostate glands were extracted for examination. Sperm parameters (for example, motility, concentration, abnormalities) were also evaluated. No treatment-related effects were observed up to the highest level tested of 200 mg/kg bw/day.
In a limited study where female pregnant rats (n=5/group) were administered a commercial extract derived from Commiphora molmol (extracted with light petrol and methanol), dose levels of 0, 50, 100, or 200 mg/kg bw/day by gavage from GD 6 to 15 did not reveal any skeletal abnormalities (Massoud et al. 2000; Al-Kazzaz 2018). The results of other examinations of the morphology of the fetuses or organ development were not reported. Another group of female animals was treated with 0 or 200 mg/kg bw/day of the extract from GD 16 and throughout the 3-week lactation period (Massoud et al. 2000, as cited in Al-Kazzaz 2018). Observations on the offspring were made at PND 0, 4, 14, and 21. No treatment-related effects were observed on the offspring, litter size, growth rate, or body weights.
In another limited study examining the potential effects of a commercial extract (derived from Commiphora molmol) on reproduction and development, pregnant albino rats (n=5/group) were given 0 or 200 mg/kg bw/day of extract by gavage from GD 6 to 11 (Shaaban 2005). The number of abortions and newly born fetuses were counted and examined for any congenital abnormalities. No abortions or congenital abnormalities were observed. Furthermore, the number of pups and pup weights in the treatment group was comparable to those in the control group. However, the studies were conducted on a limited number of animals and examined a limited number of toxicological parameters.
There were also limited studies that examined the effects of Commiphora mukul on reproduction. In a study examining the effects of Commiphora mukul oleoresin emulsion, female albino rats (n=12/group) were orally given 0 or 200 mg/kg bw/day of extract for 7 days (Amma et al. 1978). Following treatment, the animals were sacrificed, and reproductive organs (ovaries, uterus, cervix, and vagina) were examined. The treatment caused a significant decrease in the absolute and relative weights of all the reproductive organs studied, except for the vagina, where the weight was significantly increased. The treated animals also exhibited significantly increased glycogen and sialic acid levels in the ovaries, uterus, and cervix, while protein levels were decreased in the cervix. The authors indicated that the increase in glycogen and sialic acid levels in the uterus/ovaries with a concomitant reduction in their weight is suggestive of a potential anti-fertility effect.
In another study, adult male Wistar rats (unknown sample size) were administered 0 or 300 mg/kg bw/day of an ethanolic extract of Commiphora mukul gum by gavage for 60 days (Rezaei et al. 2020). The epididymal sperm count, weight, motility, morphology, viability, and serum testosterone and glucose levels were determined. No treatment-related adverse effects were observed in any of the parameters examined (for example, glucose levels, final body weight, absolute/relative testis weight, sperm parameters, testosterone levels). However, in a subchronic study previously mentioned in the Repeated-dose toxicity subsection (NTP 2020), when a group of B6C3F1 mice (n=15/sex/group) were administered 0, 62.5, 125, or 250 mg/kg bw/day of Commiphora mukul extract (in corn oil) by gavage for 3 months, all dose levels were associated with effects on the sperm (that is, lower mean total number of sperm heads, cauda epididymal sperm). At the highest dose level of 250 mg/kg bw/day, decreased testicular weights were also observed. The NTP (2020) indicated that the testes may be a target organ of Commiphora mukul extract and identified a LOAEL of 62.5 mg/kg bw/day.
7.7.3 Characterization of risk to human health
On the basis of the health effects data available, exposure to myrrh oil is not expected to result in genotoxicity. Studies examining the potential carcinogenic effects of myrrh oil were not identified.
For both short and long-term exposure scenarios, the subchronic (3 months) oral studies conducted on rats and mice using an extract of Commiphora mukul were considered to be relevant for the characterization of risk from daily exposure to myrrh oil (NTP 2020). A LOAEL of 62.5 mg/kg bw/day was identified on the basis of reduced levels of bile acids in rats and reduced levels of sperm in mice.
Estimated short-term intermittent and daily exposures for the most highly exposed age groups and resulting MOEs are presented in Table 7‑37 and Table 7‑38, respectively.
| Exposure scenario | Exposure (mg/kg bw/day) |
MOEa |
|---|---|---|
| Permanent hair dye (14 to 18 years) |
6.4 | 10 |
| Hair styling product (2 to 3 years) |
5.6 | 11 |
| Massage oil (0 to 5 months) |
8.6 | 7 |
| Bath oil (9 to 13 years) |
0.38 | 164 |
| Face exfoliant (14 to 18 years) |
0.50 | 125 |
| Hair removal aftercare (9 to 13 years) |
3.9 | 16 |
| Sunless tanning product (9 to 13 years) |
0.55 | 114 |
| Aftershave (9 to 13 years) |
1.6 | 39 |
| Pain gel (NHP) (19+ years) |
7.3 | 9 |
| Essential oil use as a stomach remedy (2 to 3 years) | 6.7 | 9 |
Abbreviations: MOE, margin of exposure; LOAEL, lowest observed adverse effect level
a MOEs are presented using the selected LOAEL of 62.5 mg/kg bw/day, based on reduced levels of bile acids and reduced levels of sperm in oral subchronic studies (3 months) conducted on rats and mice, respectively.
With respect to exposure to myrrh oil from the use of permanent hair dye, hair styling products, massage oils, bath oils, face exfoliants, hair removal aftercare products, sunless tanning products, aftershaves, pain gels (NHPs), and use of myrrh oil essential oil in a stomach remedies, a comparison of the critical effect level to the estimated levels of exposure resulted in MOEs ranging from 7 to 164, which are below 300 and considered potentially inadequate to address uncertainties in the health effects and exposure data used to characterize risk. Factors including interspecies and intraspecies variability and the inability to identify a NOAEL were taken into consideration during the determination of the adequacy of MOEs. Given the number of products containing myrrh oil available on the Canadian market, it is possible that exposure may result from the use of several types of products containing this substance on the same day (that is, aggregate exposure), in which case the MOE would be even lower.
| Exposure scenario | Exposure (mg/kg bw/day) |
MOEb |
|---|---|---|
| Body moisturizer (0 to 5 months) |
31.7 | 2 |
| Face moisturizer (19+ years) |
12.2 | 5 |
| Antiperspirant (14+ years) |
0.53 | 120 |
| Liquid body soap (0 to 5 months) |
0.86 | 73 |
| Spray perfume (2 to 3 years) |
1.9 | 33 |
| Tooth powder (2 to 3 years) |
12.2 | 5 |
| Mouthwash (4 to 8 years) |
0.43 | 145 |
| Teeth whitener (14 to 18 years) |
2.4 | 26 |
| Body lotion (NHP) (2 to 3 years) |
5.0 | 13 |
| Sunscreen lotion (NHP) (6 to 11 months) | 0.47 | 133 |
| Hand sanitizer (NHP)a (2 to 3 years) | 1.0 | 63 |
| Oral capsule (NHP) (19+ years) |
2.0 | 31 |
| Resin incense (1 year) |
2.3 | 27 |
| Food flavouring intake (1 year and older) |
1.55 × 10-4 | >100 000 |
| Essential oil use in aromatic diffuser (9 to 13 years) |
3.3 | 20 |
| Essential oil use in face steamer (user) (4 to 8 years) |
0.61 | 102 |
Abbreviations: MOE, margin of exposure; LOAEL, lowest observed adverse effect level
a Estimates for situations of public health concern.
b MOEs are presented using the selected LOAEL of 62.5 mg/kg bw/day, based on reduced levels of bile acids and reduced levels of sperm in oral subchronic studies (3 months) conducted on rats and mice, respectively.
With respect to exposure to myrrh oil from the use of body moisturizers (cosmetics), face moisturizers, antiperspirants, liquid body soaps, spray perfumes, shampoos, tooth powders, mouthwashes, teeth whiteners, body lotions (NHPs), sunscreen lotions (NHPs), hand sanitizers (NHPs), oral capsules (NHPs), resin incense, and use of myrrh essential oil in aromatic diffusers and face steamers, a comparison of the critical effect levels to the estimated levels of exposure resulted in MOEs ranging from 2 to 145, which are below 300 and considered potentially inadequate to address uncertainties in the health effects and exposure data used to characterize risk. Factors including interspecies and intraspecies variability and the inability to identify a NOAEL were taken into consideration during the determination of the adequacy of MOEs. Given the number of products containing myrrh oil available on the Canadian market, it is possible that exposure may result from the use of several types of products containing this substance on the same day (that is, aggregate exposure), in which case the MOE would be even lower. The MOE for exposure to myrrh oil from food flavouring intake is greater than 300 and is considered to be adequate.
7.7.4 Uncertainties in evaluation of risk to human health
The key sources of uncertainty are presented in Table 7‑39.
| Key source of uncertainty | Impact |
|---|---|
| For dermal exposures, absorption was assumed to be equivalent to oral absorption (that is, 100%). | + |
| Myrrh oil may be used in hand sanitizers. There is uncertainty regarding the duration of increased hand sanitizer use that may occur in a situation of public health concern. | +/- |
| The hazard dataset for myrrh oil was considered to be limited. Health effects data from different extracts and from different Commiphora species were taken into consideration, where applicable. | +/- |
| Given the potential differences in composition between different Commiphora species, there is uncertainty regarding whether the substances tested in the critical studies are representative of the myrrh oil present in products that Canadians are exposed to. | +/- |
| The potential use of more than one product by a single person in a day (that is, aggregate exposure) was not considered. This may potentially underestimate exposure to some individuals. | - |
+ = uncertainty with potential to cause over-estimation of exposure/risk; - = uncertainty with potential to cause under-estimation of exposure risk; +/- = unknown potential to cause over- or under-estimation of risk.
7.8 Cork tree extract
7.8.1 Exposure assessment
Environmental media
No measured data regarding concentrations of cork tree extract in environmental media were identified in Canada or elsewhere. The substance was not reported to be manufactured or imported in quantities greater than the reporting threshold of 100 kg, according to information submitted in response to a CEPA section 71 survey (Environment Canada 2013). In consideration of low reported quantities of the substance in Canada, exposure to cork tree extract from its presence in environmental media is expected to be minimal.
Products available to consumers
Cork tree extract was reported as an ingredient in cosmetics and NHPs, and is also available as a pure extract. In order to evaluate the potential exposure of the general population to cork tree extract from use of these products, representative sentinel exposure scenarios were selected. For dermal exposures, absorption was assumed to be equivalent to oral absorption (that is, 100%). Estimates of exposure to cork tree extract from these sentinel scenarios were derived for a range of relevant age groups and only the highest one is presented in Table 7‑40 and Table 7‑41. Parameters used to estimate the exposure scenarios are presented in Appendix A.
| Scenario | Route of exposure | Concentration (%) | Systemic exposurea (mg/kg bw/day) |
|---|---|---|---|
| Face moisturizer | Dermal | 3 | 1.22 (19+ years) |
| Body moisturizer | Dermal | 3 | 9.52 (0 to 5 months) |
| Analgesic spray (NHP) | Dermal | 25 | 8.11 (19+ years) |
a For dermal exposures, absorption was assumed to be equivalent to oral absorption (that is, 100%).
Cork tree extract is available to consumers in Canada as a pure extract (that is, present at 100%) and may be associated with consumer DIY applications (Tisserand Institute [accessed 2022]; DoTERRA 2022a). These uses can include formulating into other products such as massage oil preparations or body moisturizer preparations, and other uses can include adding to aromatic diffusers (Tisserand Institute [accessed 2021]; DoTERRA 2022a). Exposure to cork tree extract from body moisturizers is captured in the assessment of cosmetic products. Table 7‑41 presents estimates of exposure to cork tree extract from uses in an aromatic diffuser, when formulated in a massage oil, and when ingested orally.
| Scenario | Route of exposure | Concentration (%) | Exposurea (age range) |
|---|---|---|---|
| Essential oil use in aromatic diffuser | Dermal and inhalation | 100b | Dermal, usersc (mg/kg bw/day): 2.38 (9 to 13 years) Inhalation, users and bystanders (mg/kg bw/day)d: 2.7 E-05 (1 year) |
| Essential oil use in massage oil preparations | Dermal | 3 | 8.57 (0 to 5 months) |
| Oral ingestion of cork tree extract | Oral | 100 | 64.5 (14 to 18 years) |
a For dermal exposures, absorption was assumed to be equivalent to oral absorption (that is, 100%).
b Assumes that 920 mg of pure essential oil is added to the device (~ 18 drops of cork tree extract).
c Dermal exposure from this use is expected to be incidental during refilling of an aromatic diffuser and considered to be applicable to users only (for example, those 14 to 18, and 19+ years).
d Inhalation exposure converted to internal doses using default body weight and inhalation rates from HC (2021).
7.8.2 Health effects assessment
Repeated-dose toxicity
The effects of cork tree extract following short-term exposure have been examined in a study where male Wistar rats (n=5/group) were administered 0, 250, 500, and 750 mg/kg bw/day of a commercial extract of the bark of Phellodendron amurense (trade name: Nexrutine®) by gavage for 28 days (Alam et al. 2021). At 250 and 500 mg/kg bw/day, significant decreases in RBC, hemoglobin, and hematocrit were observed, with the decreases being less than 10% compared to controls. These changes did not appear to be dose-dependent and did not occur at the highest dose level of 750 mg/kg bw/day. The lowest dose level was also associated with significantly decreased levels of triglycerides (-10%), urea (-17%), and glucose (-8%) compared to controls. At 500 mg/kg bw/day, there were histopathological changes in the heart (that is, presence of lymphocytes, congested blood vessels) and effects on the liver (for example, lymphatic infiltration, increased levels of AST and ALP). In addition to these effects, the highest dose level of 750 mg/kg bw/day was associated with a significant decrease in relative kidney weights, a significant decrease in the levels of triglycerides (-18%), urea (-25%), and glucose (-11%), and histopathological changes in the lungs (that is, lymphocytic infiltration, congested blood vessels without edema) and kidneys (that is, tubular degeneration). On the basis of the presence of histopathological changes in the heart and effects on the liver at 500 mg/kg bw/day, a NOAEL of 250 mg/kg bw/day was considered from this study.
Carcinogenicity and genotoxicity
Studies examining the potential carcinogenic effects of cork tree extract following chronic exposure have not been identified. With respect to genotoxicity, the health effects dataset was considered to be limited. However, different cork tree extracts (aqueous, alcoholic) were not found to be mutagenic in bacterial mutagenicity assays using Salmonella typhimurium TA98 and TA100, or Bacillus subtilis H17 (Morimoto et al. 1982).
Reproductive and developmental toxicity
Studies examining the potential effects of cork tree extract on reproduction and development were considered to be too limited for regulatory use (Lee 1982; WHO 2009; Lee et al. 2018).
Summary
Overall, with the exception of one 28-day, repeated-dose toxicity study, the health effects dataset for cork tree extract was considered to be too limited to characterize the potential effects of cork tree extract. Therefore, health effects data on the main component berberine (0.6% to 33%) were also taken into consideration.
Berberine
Berberine is a quaternary ammonium compound from the protoberberine group of isoquinoline alkaloids (Cicero and Baggioni 2016). It is the major alkaloid component found in cork tree extract and can be found at concentrations ranging from 0.6% to 33% (Liu et al. 1993; James et al. 2011). Since berberine can be manufactured as chloride or sulfate salts, which are expected to dissociate under physiological conditions, data available on these salts were also taken into consideration.
Repeated-dose toxicity
Sprague-Dawley rats (n=5/sex/group) were fed 0 or 156 mg/kg bw/day of berberine in the diet for 90 days (Yi et al. 2013). Female animals in the berberine group had lower body weight (-4%) compared to controls. Relative weights of the heart, lungs, and stomach were lower while the relative weights of the liver, spleen, kidneys, and brain were higher in the treatment group compared to controls. There were also decreases in ALT, AST, gamma-glutamyl transferase (GGT), and A/G. The study was deemed too limited for POD determination.
Carcinogenicity and genotoxicity
Studies examining the potential carcinogenic effects of berberine following chronic exposure have not been identified. With respect to genotoxicity, berberine was not mutagenic when tested in bacterial strains such as Salmonella typhimurium TA97, TA100 and TA1535 (NTP 2018a). When Nozaka et al. (1990) examined mutagenicity in Salmonella typhimurium TA98 and TA100, berberine hydrochloride was found to be weakly mutagenic in TA98 in the absence of metabolic activation, but not in the presence of metabolic activation. In vivo, intraperitoneal administration of berberine chloride was not associated with an increase of chromosome aberrations (NTP 2018b). Studies conducted using emerging test protocols (for example, single-cell gel electrophoresis [COMET]) or micronucleus assays in vitro have found that berberine can result in DNA damage in cancer cells or rapidly proliferating cells (Inbaraj et al. 2006; Liu et al. 2009; Chen et al. 2013; Gu et al. 2015). Exposure to berberine has also been found to inhibit mutagen-induced DNA damage in different cell systems (for example, Euglena garcilis) (Čerňáková et al. 2002).
Reproductive and developmental toxicity
Berberine has the ability to displace bilirubin from serum albumin and potentially result in the accumulation of bilirubin in the brain (Bateman et al. 1998; Lactmed® 2006-). Although the extent of transfer from the mother to infant is unknown, this has resulted in concerns regarding the potential manifestation of jaundice and kernicterus (a type of brain damage) in neonates (Chan 1993; Chan 1994; Linn et al. 2012). Therefore, exposure of pregnant women to berberine has not been recommended (Chan 1993; Kumar et al. 2015).
In a developmental toxicity study pregnant Sprague-Dawley rats (n=25/group) were fed 0, 3625, 7250, or 14500 ppm of berberine chloride dehydrate (87.7% purity) in the diet from GD 6 to 20 (Jahnke et al. 2006). This was equivalent to approximately 0, 282, 531, or 1313 mg/kg bw/day (Health Canada 1994). At 531 and 1313 mg/kg bw/day, maternal body weight gain was significantly less than controls (9% to 26%) during the gestation period, with a significant trend of reduced weight gain with and without correction for gravid uterine weight. In addition, animals at the highest dose showed significantly reduced absolute/relative liver weights (5% to 14%) and, at the same dose level, the fetuses exhibited a significantly lower body weight than controls (6%). A maternal NOAEL of 282 mg/kg bw/day (202 mg/kg bw/day berberine) was identified by the authors on the basis of reduced body weight gains) and a developmental toxicity NOAEL of 531 mg/kg bw/day (380 mg/kg bw/day berberine) was identified on the basis of reduced fetal body weights at higher doses.
Jahnke et al. (2006) repeated the same study in pregnant Sprague-Dawley rats (n=25/group), except the animals were administered 0 or 1000 mg/kg bw/day of berberine chloride dehydrate (approximately 779 to 800 mg/kg bw/day berberine, in 0.5% methylcellulose) by gavage. Similar effects on maternal body weight, and liver weights were observed at 1000 mg/kg bw/day, but no effects on fetuses were observed.
Jahnke et al. (2006) also conducted similar studies in mice where pregnant Swiss albino (CD-1) mice (n=25/group) were fed 0, 3500, 5250, or 7000 ppm berberine chloride dehydrate (87.7% purity) in the diet from GD 6 to 17. This was equivalent to approximately 0, 569, 841, or 1155 mg/kg bw/day (0, 408, 602, 827 mg/kg bw/day berberine), respectively. No maternal adverse effects were observed. The percent incidence of malformations in fetuses increased in a dose-dependent manner (that is, 1.21%, 1.23%, 3.29%, 5.13% for the 0, 569, 841, and 1155 mg/kg bw/day dose groups, respectively), without reaching statistical significance. The incidence of cleft palate was 0%, 0.3%, 0.7%, and 2.3%, respectively. Discontinuous rib was also mentioned to be a malformation that occurred with a higher incidence (no further details). At the highest dose level, fetal body weights were 4% lower than controls, although not statistically significant. The authors identified a NOAEL of 569 mg/kg bw/day (408 mg/kg bw/day berberine) for developmental toxicity on the basis of malformations at the next dose level.
When the same study was conducted by gavage in mice (n=25/group) administered 0 or 1000 mg/kg bw/day berberine chloride dehydrate (in 0.5% methylcellulose, equivalent to approximately 740 to 742 mg/kg bw/day berberine), 7 animals were found moribund or dead. However, the authors indicated that 6 of the 7 animals effluxed dosing solution before death/moribundity, suggestive of issues related to gavage administration. With respect to effects on the offspring, fetal body weights were 5% to 6% less than the control group, which were statistically significant in males. The authors identified a LOAEL of 1000 mg/kg bw/day for maternal toxicity and developmental toxicity on the basis of mortality and decreased fetal body weights, respectively.
7.8.3 Characterization of risk to human health
On the basis of the health effects data available, cork tree extract is not expected to be genotoxic.
The 28-day, oral study conducted on rats was identified to be the most appropriate study for the risk characterization of exposure to cork tree extract (Alam et al. 2021). A NOAEL of 250 mg/kg bw/day was identified on the basis of effects on the heart and liver at the next dose level of 500 mg/kg bw/day. This effect level was selected from extract data, which were considered to be more representative of cork tree extract than the data available on berberine.
Table 7‑42 describes the exposure estimates to cork tree extract, the critical effect level, and resultant MOEs for the characterization of risk to human health.
| Scenario | Exposurea,b estimate (mg/kg bw/day) | MOEc |
|---|---|---|
| Face moisturizer (19+ years) (daily dermal exposure) |
1.22 | 205 |
| Body moisturizer (0 to 5 months) (daily dermal exposure) |
9.52 | 26 |
| Analgesic spray (NHP) (19+ years) (short-term intermittent dermal exposure) |
8.11 | 31 |
| Essential oil use in aromatic diffuser, users (9 to 13 years) (daily dermal and inhalation exposure) |
2.38 | 105 |
| Essential oil use in massage oil preparations (0 to 5 months) (short-term intermittent dermal exposure) |
8.57 | 29 |
| Oral ingestion of cork tree extract (14 to 18 years) (daily oral exposure) | 64.5 | 4 |
Abbreviations: MOE, margin of exposure; NOAEL, no observed adverse effect level
a Exposure estimates are presented for the highest exposed age group relevant for each scenario.
b For dermal exposures, absorption was assumed to be equivalent to oral absorption (that is, 100%).
c MOEs are presented using the selected NOAEL of 250 mg/kg bw/day, on the basis of effects on the heart and liver in a 28-day study in rats.
For short-term intermittent scenarios described above, comparisons between the critical effect level and the estimates of exposure to cork tree extract from the use of analgesic sprays (NHPs) and the use of cork tree extract essential oil in massage oil preparations resulted in MOEs of 31 and 29, respectively, which are less than 100 and considered potentially inadequate to address uncertainties in the health effects and exposure data used to characterize risk. Factors including interspecies and intraspecies differences were taken into consideration during the determination of the adequacy of the MOEs.
For daily scenarios, comparisons between the critical effect level and the estimates of exposure to cork tree extract from oral ingestion or the use of face moisturizers, body moisturizers, and aromatic diffusers resulted in MOEs ranging from 4 to 205, which are below 300 and considered potentially inadequate to address uncertainties in the health effects and exposure data used to characterize risk. Factors, including interspecies and intraspecies differences and the duration of the critical study relative to the potential duration of human exposure, were taken into consideration during the determination of the adequacy of the MOEs.
7.8.4 Uncertainties in evaluation of risk to human health
The key sources of uncertainty are presented in Table 7‑43.
| Key source of uncertainty - Exposure | Impact |
|---|---|
| The concentration of the main components of cork tree extract depends on the conditions of extraction of the substance and the origin, species, and growing conditions (temperature, soil, and geography) of the plant. Therefore, the precise composition of cork tree extract present in products available to consumers is unknown, which represents an uncertainty in the assessment. | +/- |
| For dermal scenarios, absorption was assumed to be equivalent to oral absorption. | + |
| Robust compositional data on cork tree extract were considered to be limited. Therefore, it is not known with certainty whether the composition of the cork tree extract tested in the critical study is representative of the cork tree extract to which Canadians are exposed. | +/- |
| The health effects database for cork tree extract was considered to be limited (for example, lack of robust studies on carcinogenicity, reproduction, and development). Relevant data for the major components (for example, berberine) were also taken into consideration. Overall, substance-specific data on cork tree extract were selected for risk characterization purposes. | +/- |
+ = uncertainty with potential to cause over-estimation of exposure/risk; - = uncertainty with potential to cause under-estimation of exposure risk; +/- = unknown potential to cause over- or under-estimation of risk.
7.9 Subgroup 1: Sage oil and wormwood oil
7.9.1 Exposure assessment
Sage oil
Environmental media and food
No reports of monitoring for sage oil in environmental media in Canada or elsewhere were identified. The substance was not reported to be manufactured or imported in quantities above the reporting threshold of 100 kg on the basis of information submitted in response to a CEPA section 71 survey (Environment Canada 2013). In consideration of low reported quantities of the substance in Canada, exposure to sage oil from environmental media is expected to be minimal.
No definitive information is available concerning the potential use of sage oil in food packaging or as a flavouring agent in foods sold in Canada. However, since sage oil is identified as a food flavouring agent internationally, it is possible that this substance is present as a flavouring agent in foods sold in Canada (personal communication, emails from the FD, HC, to the ESRAB, HC, dated June 2021; unreferenced).
Fenaroli’s Handbook of Flavour Ingredients reports the per capita ('individual') estimated intake of sage oil from its use as a food flavouring agent to be 0.0155 mg/kg bw/day for the US population on the basis of annual production volumes reported by the food industry (NAS 1989 as cited in Burdock 2010). In the absence of data on the use of sage oil as a flavouring agent in foods sold in Canada, the per capita intake estimate for the US population (Burdock 2010) is considered to be an acceptable estimate of possible Canadian dietary exposure for the general population aged 1 year and older to this substance (personal communication, emails from the FD, HC, to the ESRAB, HC, dated June 2021; unreferenced). Oral exposure is possible through consumption of herbal teas brewed from dried Salvia officinalis leaves, which may contain sage oil as a component. The resulting daily oral exposure estimates for adults aged 19+ years is 0.0089 mg/kg bw/day; this is based on the amount of thujone, one of the main components of sage oil, extracted from sage leaf tea (personal communication, emails from the FD, HC, to the ESRAB, HC, dated June 2022; unreferenced).
Products available to consumers
Sage oil (Salvia officinalis) and extracts from Salvia officinalis are present in products available to consumers, including cosmetics and NHPs, where dermal, oral and inhalation exposures are possible. Representative sentinel scenarios were selected to evaluate the potential exposure to sage oil.
Sage oil is available to consumers in Canada as a pure essential oil (that is, present at 100%). Information from the available literature on uses of essential oils notes that they can be used by consumers in DIY applications (see section 4). Exposures to sage essential oil formulated in massage oils and body moisturizers are considered as being captured in the assessment of products available for consumers outlined in Table 7‑44.
Wormwood oil
Environmental media and food
Data on wormwood oil in environmental media such as air, water, dust, soil, or sediment in Canada or elsewhere were not identified. The substance was not reported to be manufactured or imported in quantities greater than the reporting threshold of 100 kg, according to information submitted in response to a CEPA section 71 survey (Environment Canada 2013). In consideration of low reported quantities of the substance in Canada, exposure to wormwood oil from its presence in environmental media is expected to be minimal.
No definitive information is available concerning the use of wormwood oil in foods sold in Canada; however, since the substance is identified as a food flavouring agent internationally, it is possible that the substance is present as a flavouring agent in foods sold in Canada (personal communication, email from the FD, HC, to the ESRAB, HC, June 2021; unreferenced).
Fenaroli’s Handbook of Flavor Ingredients reports the per capita ('individual') estimated intake of wormwood oil to be 0.00027 mg/kg bw/day for the US population on the basis of annual production volumes reported by the food industry (NAS 1989 as cited in Burdock 2010). In the absence of data on the actual use, if any, of wormwood oil as a flavouring ingredient in foods sold in Canada, the per capita intake estimate for the US population (Burdock 2010) is an acceptable estimate of possible Canadian dietary exposure for the general population 1 year of age and older to this substance (personal communication, email from FD, HC, to ESRAB, HC, 2021; unreferenced).
Products available to consumers
Wormwood oil was reported as an ingredient in a limited number of products available to consumers in Canada such as hand sanitizers, and analgesic creams.
Wormwood oil is also available to consumers in Canada as a pure essential oil (that is, present at 100%). Information from the available literature on uses of essential oils note that they can be used by consumers in DIY applications (Tisserand Institute [accessed 2021]; DoTERRA 2022a). Product label information for wormwood oil also note that the substance can be consumed orally. Estimates of exposure to wormwood oil were derived for these uses.
To evaluate potential exposures to wormwood oil from these products, “sentinel” scenarios were selected. Other products reported to contain this substance are expected to be associated with a lower potential for exposure than these “sentinel” scenarios.
Dermal absorption
With respect to dermal absorption, studies on sage oil or wormwood oil were not identified in the literature. Information on dermal absorption of thujone, one of the major components of sage oil and wormwood oil which constitutes up to 57% of sage oil and up to 65% of wormwood oil (Raal et al. 2007; Blagojevic et al. 2006), was used.
A dermal absorption value of 20% for occluded skin conditions was used for the Terpenes and Terpenoids—Acyclic, Monocyclic, and Bicyclic Monoterpenes Group (ECCC, HC 2020b) on the basis of in vitro human dermal absorption studies conducted with geraniol, citronellol, and linalool (Gilpin et al. 2010; ECHA 2022d). For these substances, dermal absorption ranged from 4.3% to 19.5% depending on whether the site is occluded, including skin-bound residues. Thujone (α/β) has a similar molecular weight, log Kow, and water solubility (moderately soluble) to geraniol, citronellol, and linalool. Geraniol, citronellol, and linalool have vapour pressures ranging from 4 to 21 Pa, whereas thujone has a higher vapour pressure (~55 Pa). In an in vitro dermal absorption study of another similar substance, citral (the major component of Verbena officinalis extract), percutaneous absorption through human skin was determined to be 30% after accounting for variability in the reported absorption values (Charles River 2019) (see section 7.4.1). Thujone has the same molecular weight as citral, and similar log Kow and water solubility (moderately soluble). Thujone has a higher vapour pressure than citral. In consideration of the absorption data from similar terpene and terpenoid components, and to account for uncertainties in the use of these data to inform the dermal absorption of thujone, a dermal absorption of 50% for thujone is used. This value is used to estimate systemic exposure in the dermal scenarios.
As thujone has a vapour pressure of 55 Pa, it is considered to be volatile. For dermally applied products, the amount available for inhalation was adjusted by 50% to account for the portion of product absorbed through the skin. Dermal absorption was conservatively assumed to be equivalent to oral absorption for products applied to mucosal membranes (for example, douches).
Exposure estimates for sage oil from the use of products available to consumers and DIY applications are summarized in Table 7‑44. For each scenario, exposure estimates were derived for a range of relevant age groups, and only the highest one is presented. Details on the parameters used to estimate exposure are presented in Appendix A. Given the number of products containing sage oil available on the Canadian market, exposure may result from the use of several types of products (for example, cosmetics, and NHPs) containing the substance on the same day (that is, aggregate exposure).
| Exposure scenario | Route of exposure | Concentration (%) | Exposure (mg/kg bw/day) |
|---|---|---|---|
| Massage oil | Dermal and inhalationb,c | 3g | 4.3 (0 to 5 months) |
| Hair styling product | Dermal and inhalationb,c | 1h | 0.11 (2 to 3 years) |
| Sunless tanning product | Dermal and inhalationb,c | 0.1h | 0.11 (9 to 13 years) |
| Douche | Dermald | 0.3h | 0.64 (14 to 18 years) |
| Face mask | Dermal and inhalationb,c | 3h | 0.24 (14 to 18 years) |
| Massage oil (NHP) | Dermal and inhalationb,c | 3i | 3.3 (19+ years) |
| Analgesic cream (NHP) | Dermal and inhalationb,c | 0.098j | 7.7 × 10-2 (9 to 13 years) |
| Throat spray (NHP) | Oral | 0.5j | 4.8 × 10-2 (19+ years) |
| Motion sickness medication (NHP) | Oral | 5j | 1.0 (14 to 18 years) |
| Antiperspirant | Dermal and inhalationb,c | 10%h | 0.91 (14 to 18 years) |
| Conditioner (rinse-off) | Dermal and inhalationb,c | 10%h | 0.18 (2 to 3 years) |
| Spray perfume | Dermal and inhalationb,c | 10%h | 0.94 (2 to 3 years) |
| Makeup remover | Dermal and inhalationb,c | 3%h | 9.0 × 10-2 (9 to 13 years) |
| Body moisturizer | Dermal and inhalationb,c | 3%h | 4.8 (0 to 5 months) |
| Face moisturizer | Dermal and inhalationb,c | 3%h | 0.64 (19+ years) |
| Liquid body soap | Dermal and inhalationb,c | 10h | 0.43 (0 to 5 months) |
| Shampoo | Dermal and inhalationb,c | 3h | 9.3 × 10-2 (0 to 5 months) |
| Breath freshener | Oral | 0.1h | 4.3 × 10-2 (4 to 8 years) |
| Mouthwash | Oral | 0.1h | 4.3 × 10-2 (4 to 8 years) |
| Toothpaste | Oral | 0.3h | 0.12 (2 to 3 years) |
| Hand sanitizer (NHP)a | Dermal and inhalationb,c | 0.25%j | 0.12 to 3.1 (2 to 3 years) |
| Body moisturizer (NHP) | Dermal and inhalationb,c | 1%j | 1.5 (9 to 13 years) |
| Oral supplement (capsule) (NHP) | Oral | 200 mg/capsulej | 40.5 (19+ years) |
| Oral supplement (loose herbs) (NHP) | Oral | 180 mg/gj | 21.9 (19+ years) |
| Herbal tea blend (NHP) | Oral | 6.7%j | 1.8 × 10-3 (19+ years) |
| Essential oil use in aromatic diffuser (user) | Dermale and inhalationb,c | 100%k | 2.0 (9 to 13 years) |
| Essential oil use in aromatic diffuser (bystander) | Inhalationc | 100%k | 2.0 (1 year old) |
| Essential oil use in face steamer (user) | Dermal and inhalationb,c,f | 100%l | 3.8 (4 to 8 years) |
| Essential oil use in face steamer (bystander) | Inhalationc,f | 100%l | 0.57 (1 year old) |
a The range of exposure estimates represent a range of use frequencies for hand sanitizers. The upper end of the range describes situations of public health concern, where the use of hand sanitizers among the general population may increase up to 25 uses per day (personal use by individuals 20+ years, increased use by children in schools and childcare facilities) (RIVM 2021a; Lopez et al. 2022).
b On the basis of a dermal absorption value of 50% assumed for thujone, used for the risk characterization of sage oil. For inhalation exposures, to account for product absorbed by the dermal route (that is, 50% dermal absorption), the product amount available for inhalation was adjusted by 50%.
c Inhalation exposure estimates (expressed as 24-hour TWA, mg/m3) were converted to internal doses using default inhalation rates and body weights (HC 2021).
d Dermal absorption was assumed to be equivalent to oral absorption (that is, 100%).
e Dermal exposure from this use is expected to be incidental during refilling of an aromatic diffuser and considered to be applicable to users only (for example, those 9+ years).
f On the basis of a 20-minute mean event concentration of 124 mg/m3, assuming 50% of this air concentration value (that is, 124 mg in a 1 m3 volume) is available for inhalation (62 mg/m3) and that 50% (62 mg) is deposited onto the face of a user. Additional details provided in Appendix A.
g Although the upper concentration reported for massage oil containing sage oil was 10% (personal communication, emails from the CHPSD, HC, to the ESRAB, HC, dated 2021; unreferenced), massage oils are typically diluted prior to use. Thus, the maximum concentration of sage oil in massage oil was assumed to be 3% (Bremmer et al. 2006).
h Personal communication, emails from the CHPSD, HC, to the ESRAB, HC, dated June 2021; unreferenced
i Although the upper concentration reported for massage oil containing sage oil was 13.8% (personal communication, emails from the NNHPD, HC to the ESRAB, HC, dated June 2021; unreferenced), massage oils are typically diluted prior to use. Thus, the maximum concentration of sage oil in massage oil was assumed to be 3% (Bremmer et al. 2006).
j Personal communication, emails from the NNHPD, HC, to the ESRAB, HC, dated June 2021; unreferenced.
k Assumes that 920 mg of pure essential oil is added to the device (~20 drops of sage oil).
l Assumes that approximately 10 drops of pure essential oil are added to device. Details on the corresponding product amounts are provided in Appendix A.
Estimates of exposure to wormwood oil from use of NHPs, NPDs, or as an essential oil in DIY applications are summarized in Table 7‑45 and Table 7‑46. Details on parameters used to estimate exposures are presented in Appendix A.
| Scenario | Route of exposure | Concentration (%) | Exposurea (mg/kg bw/day) |
|---|---|---|---|
| Hand sanitizerb (NHP) | Dermal | 1.3 | 0.65 to 16.3 (2 to 3 years) |
| Analgesic cream (NPD) |
Dermal and inhalation | 0.58 | 0.45 (19+ years) |
a Based on a dermal absorption value of 50%. For inhalation exposures, to account for product absorbed by the dermal route (that is, 50% dermal absorption) the product amount available for inhalation (Appendix A) was adjusted by 50%. Inhalation exposure estimates (expressed as 24-hr TWA, mg/m3) were converted to internal doses using default inhalation rates and body weights from HC (2021).
b The range of exposure estimates represents a range of use frequencies for hand sanitizers. The upper end of the range describes situations of public health concern, where the use of hand sanitizers among the general population may increase up to 25 uses per day (personal use by individuals 20+ years, increased use by children in schools and childcare facilities) (RIVM 2021a; Lopez et al. 2022).
| Scenario | Route | Concentration (%) | Exposurea, mg/kg bw/day (age range) |
|---|---|---|---|
| Essential oil use in aromatic diffuserb | Dermalc and inhalationd | 100 | Dermal, usersd: 1.07 (9 to 13 years) Inhalation, users and bystanders: 1.97 (1 year) |
| Essential oil use in facial steamer, usere | Dermal and inhalationd | 100 | 2.99 (4 to 8 years) |
| Essential oil use in facial steamer, bystandere | Inhalationd | 100 | 0.21 (1 year) |
| Essential oil use in bathsf | Dermal and inhalationd | 100 | 0.24 (9 to 13 years) |
| Essential oil formulated in body moisturizer | Dermal and inhalationd | 3 | 4.79 (0 to 5 months) |
| Essential oil formulated in massage oilf | Dermal and inhalationd | 3 | 4.34 (0 to 5 months) |
| Oral ingestion of wormwood essential oil | Oral | 100 | 19.1 (14 to 18 years) |
a For dermal exposures, an absorption value of 50% is used. For inhalation exposures, to account for product absorbed by the dermal route (that is, 50% dermal absorption) the product amount available for inhalation (Appendix A) was adjusted by 50%. Inhalation exposure estimates (expressed as 24-hr TWA, mg/m3) were converted to internal doses using default inhalation rates and body weights from HC (2021).
b Assumes that 920 mg of pure essential oil is added to the device (~ 20 drops of wormwood oil).
c Dermal exposure from this use is expected to be incidental during refilling of an aromatic diffuser and considered to be applicable to users only (for example, those 9 to 13,14 to 18, and 19+ years of age).
d Inhalation exposures were converted to internal doses using default inhalation rates and body weights from HC (2021). Additional details are presented in Appendix A.
e Assumes that approximately 10 drops of pure essential oil are added. Details on the corresponding product amounts are provided in Appendix A.
f Exposure estimated on a per-event basis (that is, on the day of product use).
7.9.2 Health effects assessment
Sage oil
Repeated-dose toxicity
Information on the effects of sage oil (from Salvia officinalis) was limited to a report of an 8-week study where rats (n=5/group) were orally administered sage oil at doses of 0, 250, 500, 1000, and 1250 mg/kg bw/day (Skramlik 1959 as cited in EMA 2009). The authors reported a daily dose of 250 mg/kg bw/day to be “well tolerated.” When the dose was increased to 500 mg/kg bw/day, convulsions were observed in some of the animals. Higher dose levels were associated with mortality in the majority of the animals. Due to the lack of details available on the original study, this study was considered of limited use.
Carcinogenicity and genotoxicity
With respect to carcinogenicity, no good laboratory practice (GLP)-compliant guideline study was identified for sage oil derived from the leaves of Salvia officinalis. With respect to genotoxicity, sage oil (obtained from hydrodistillation or steam distillation of Salvia officinalis) was not found to be mutagenic when tested in bacterial strains Salmonella typhimurium TA98, TA100, and TA102 and Escherichia coli WP2 and K12 (Vuković-Gačić et al. 2006). α-Thujone (38%), eucalyptol (14%), and camphor (14%) were identified to be the major components. Furthermore, sage oil from Salvia officinalis (containing 17% camphor, 7% α-thujone, and 5% β-thujone) was not mutagenic in Salmonella typhimurium TA1535 and TA1536 and in a rec-assay with Bacillus subtilis PB1652 and P1791 (Zani et al. 1991). In in vivo studies, when male Swiss mice (n=10/group) were orally administered 0, 0.1, 0.2, or 0.4 mL/kg bw/day sage oil for 2 weeks (equivalent to approximately 0, 91, 182, or 365 mg/kg bw/day, respectively), no micronuclei, DNA fragmentation, or chromosome aberrations were observed in a micronucleus test, a COMET assay, and cytogenetic evaluation of bone marrow cells, respectively (Diab et al. 2018). In these studies, only eucalyptol (29%) and caryophyllene (17%) were identified as major components. β-thujone was detected at a level of 1%. Data available on other extracts of Salvia officinalis (for example, infusions, ethanolic extracts) demonstrated a lack of treatment-related genotoxic responses (Vujosević and Blagojević 2004; Patenković et al. 2009; Horváthová et al. 2016).
Reproductive and developmental toxicity
Studies examining the effects of sage oil (from Salvia officinalis) on reproduction and development were considered to be limited. Aqueous and ethanolic extracts derived from Salvia fruticosa were examined for their potential effects on reproduction and development, as reported in 4 experiments conducted on Sprague-Dawley rats (Elbetieha et al. 1998). In these experiments, the animals were administered by gavage up to 800 mg/kg bw/day of an aqueous extract (range: 200 mg/kg bw/day to 800 mg/kg bw/day) or 400 mg/kg bw/day of an ethanolic extract prior to mating, during the implantation period, or during organogenesis. Both extracts were associated with treatment-related effects such as significant decreases in the number of implantation sites and viable fetuses and increases in the number of resorptions at 400 mg/kg bw/day and higher. However, the surviving offspring were considered to be “normal” by the authors in terms of their physical appearance and no external abnormalities were observed. In addition, there were no treatment-related effects on the timing of testicular descent, day of vaginal opening, and pup weights.
Neurological effects
Several case studies have been published that report on the appearance of CNS effects following ingestion of sage oil (from Salvia officinalis) in humans. Adverse effects such as fainting, sweating, dizziness, tachypnea, generalized tonic-clonic convulsions, seizures, and coma are commonly described in the scientific literature (Burkhard et al. 1999; Halicioglu et al. 2011). Although exposure estimates were not well-characterized in these case studies, the EMA (2009) noted that ingestion of a few drops of sage oil (from Salvia officinalis) can result in the appearance of neurological effects. The onset of symptoms is also rapid, often occurring within minutes of exposure.
Although no neurotoxicity studies on sage oil have been conducted through conventional routes of exposure (for example, oral, dermal, and inhalation), relevant data from studies conducted through the intraperitoneal route were identified. When Wistar rats (n=15) were administered single intraperitoneal injections of a commercial sage oil (from Salvia officinalis) at increasing doses (300, 500, 3200 mg/kg bw), doses equal to or greater than 500 mg/kg bw/day were associated with tonic-clonic seizures and mortality (Millet et al. 1981). The authors noted that the dose limit below which the neurological effects were subclinical was 300 mg/kg bw/day.
Wormwood oil
Repeated-dose toxicity
Studies examining the potential effects following repeated exposure to wormwood oil were not identified. However, health effects data on an unspecified extract of Artemisia absinthium were available (Muto et al. 2003). The extract was supplied by a manufacturer and contained a mixture of components, including α- and β-thujone (at unknown concentrations). In this study, Wistar Hannover (GALAS) rats (n=10/sex/group) were given water containing 0%, 0.125%, 0.5%, or 2% of the extract for 13 weeks, equivalent to approximately 0, 64, 257, or 1027 mg/kg bw/day, respectively. At levels equal to or greater than 0.5% (257 mg/kg bw/day), the A/G ratio was significantly reduced compared to controls. At 2% (1027 mg/kg bw/day), there were significant increases in certain clinical chemistry parameters (that is, protein, albumin, BUN, sodium, and chloride levels) and significant increases in liver weights. The same dose level was also associated with histopathological changes in the liver (microgranulomas), kidneys (basophilic tubules, calcification), heart (focal necrosis), submandibular gland, pituitary gland (hypertrophy), and thyroid (hypertrophy). The authors noted that these changes were not considered to be toxicologically significant and identified a NOAEL of 2% (1027 mg/kg bw/day), representing the highest dose tested. However, given the effects observed on different organs at the highest dose level (for example, liver, kidneys, etc.), a NOAEL of 0.5% (257 mg/kg bw/day) was considered.
Carcinogenicity and genotoxicity
Health effects data related to carcinogenicity were not identified for either wormwood oil or wormwood extracts. However, an ethanolic extract of Artemisia absinthium leaves (unknown composition) was found to be genotoxic when male Swiss albino mice (n=12/group) were administered 900 or 1200 mg/kg bw/day by intraperitoneal injection for up to 3 days (Yousef Alshibly 2014). At these dose levels, there was a significant increase in the incidence of chromosome aberrations.
Reproductive and developmental toxicity
Studies examining the potential effects of wormwood oil on reproduction and development were not identified. However, limited health effects data on a decoction (prepared by boiling water) of Artemisia absinthium were available (Desaulniers et al. 2016). In this study, pregnant ICR mice (n=10/group) were administered 0% or 1% (w/v) of the extract from GD 8 to 18. Water consumption and tail temperature were recorded on a daily basis. After parturition, the pups were weighed and observed with respect to development. At maturity (PND 50), the male offspring were mated with non-littermate females. These females were then sacrificed on GD 16, and reproductive performance was examined (for example, conception rate, pregnancy rate, percentage of females mated). Furthermore, gravid body weights, gravid uterine weights, litter size, number of implantation sites/corpora lutea, and resorptions were recorded. The males were sacrificed and their testes weights were documented. Other than a significant increase in water consumption, no treatment-related adverse effects were observed on any of the examined outcomes in the study.
Neurological effects
It has been reported that the long-term use of wormwood oil may cause psychological disorders in humans, with clinical manifestations including convulsions, sleeplessness, and hallucinations (McGuffin et al. 1995, as cited in Batiha et al. 2020). Other reported side effects include stomach cramps, vertigo, vomiting, nausea, insomnia, and restlessness. There have also been case reports of acute poisoning following accidental ingestion of large quantities of wormwood oil (Smith 1862; Weisbord et al. 1997; Aloui et al. 2010; Brown 2017). In these cases, the subjects experienced seizures, convulsions, and acute renal failure.
Summary
Overall, the health effects datasets for sage oil (from Salvia officinalis) and wormwood oil were considered to be limited due to the lack of GLP-compliant studies conducted in accordance with internationally recognized test guidelines. The limited data available for certain toxicological endpoints (for example, repeated-dose toxicity, reproductive and developmental toxicity) suggest that neurological effects (for example, convulsions) may be a critical effect following exposure to sage oil (from Salvia officinalis) or wormwood oil. In order to further characterize the potential health effects associated with these substances, information available for their major components (as outlined in section 2.1) were also taken into consideration.
Thujone (α-isomers, β- isomers, or both)
Thujone is a monoterpene ketone that can be found in sage oil at concentrations ranging between 4% and 57% (Jakovljević et al. 2019). In wormwood oil, it can be found in concentrations ranging between 10% and 65% (Blagojević et al. 2006). Thujone can be comprised of α- and β-isomers, but the α-isomer is the predominant isomer found in sage oil, while the β-isomer is the predominant isomer in wormwood oil. Thujone has been assessed by different international agencies including the US FDA, EFSA, and JECFA. As a result of these assessments, different jurisdictions have imposed different measures to limit exposure to thujone.
Repeated-dose toxicity
The effects of thujone following short-term exposure have been examined in rats and mice by the NTP (2011). In a study conducted on F344 rats (n=5/sex/group), α-thujone (in 0.5% methylcellulose) was administered by gavage at doses of 0, 1, 3, 10, 30, or 100 mg/kg bw/day for 16 days. At the highest dose level of 100 mg/kg bw/day, effects such as convulsions/seizures, reduced thymus weights, and mortality were observed on day 15. No other treatment-related adverse effects were reported. When F344 rats (n=5/sex/group) were administered a thujone mixture containing approximately 71% α-thujone and 12% β-thujone at 0, 1, 3, 10, 30, or 100 mg/kg bw/day for 16 days, no convulsions/seizures, morphological findings, or histopathological changes were observed, although mortality was observed in one animal at the highest dose level of 100 mg/kg bw/day. These findings suggest that the α-thujone isomer may be more potent than a mixture of thujone isomers with respect to the manifestation of neurological effects. For α-thujone, a NOAEL of 30 mg/kg bw/day was identified on the basis of neurological effects (for example, convulsions/seizures), histopathological changes in the thymus, and mortality observed at the next dose level of 100 mg/kg bw/day. The same critical effect level was also identified for the thujone mixture (that is, a NOAEL of 30 mg/kg bw/day), on the basis of the one animal that died at the next level of 100 mg/kg bw/day.
The health effects observed in mice were similar to those reported in the previous studies conducted on rats. For example, when B6C3F1 mice (n=5/sex/dose) were administered α-thujone (in 0.5% methylcelluose) by gavage at doses of 0, 1, 3, 10, 30, or 100 mg/kg for 17 days, seizures, tremors, hyperactivity, and mortality were observed at the highest dose level of 100 mg/kg bw/day. However, when B6C3F1 mice (n=5/sex/dose) were administered the thujone mixture of isomers at the same doses for the same duration, no neurological effects were observed, although the majority of the animals died at the highest dose level of 100 mg/kg bw/day. Similar to the rat studies, the studies in mice suggest that α-thujone may be more potent than a mixture of thujone isomers with respect to neurological effects. A NOAEL of 30 mg/kg bw/day was identified for α-thujone on the basis of neurological effects (for example, convulsions/seizures) and mortality observed at the next dose level of 100 mg/kg bw/day. The same critical effect level was also identified for the thujone mixture (that is, a NOAEL of 30 mg/kg bw/day) on the basis of mortality occurring at the next dose level of 100 mg/kg bw/day.
Studies of longer duration have also been identified in the literature. In a report of an oral, subchronic study, weanling rats (n=20/sex/group) were administered a commercial mixture of α- and β-thujone by gavage at doses of 0, 12.5, 25, and 50 mg/kg bw/day for 13 weeks (Surber 1962, as cited in Lachenmeier and Uebebacker 2010). Throughout the study, convulsions were frequently observed amongst the treated animals. By the end of the study period, convulsions were observed in 5%, 28%, and 85% of the animals in the low-, medium-, and high-dose groups, respectively. No other treatment-related effects on body weight, hematology, or histopathology were reported. The authors established a lowest-observed-effects level (LOEL) of 12.5 mg/kg bw/day on the basis of the observation of convulsions. In another report of a subchronic study, rats (n=10/sex/group) were administered thujone (unknown composition of isomers) by gavage at doses of 0, 5, 10, or 20 mg/kg bw/day, 6 days/week for 14 weeks (Margaria 1963 as cited in Lachenmeier and Uebebacker 2010). At 10 mg/kg bw/day, one animal experienced one convulsion on the thirty-eighth day of treatment. At the highest dose level of 20 mg/kg bw/day, 75% of the animals experienced convulsions and 20% of the animals died. There were no other treatment-related effects noted with respect to body weight, hematology, organ weights, gross pathology or histopathology. For this study, the authors identified a NOAEL of 5 mg/kg bw/day on the basis of convulsions observed at the next dose level of 10 mg/kg bw/day. The Council of Europe derived a tolerable daily intake (TDI) value on the basis of the findings from the study by Margaria (1963 as cited in Lachenmeier and Uebebacker 2010) (Council of Europe as cited in Pelkonen et al. 2013). Specifically, a TDI of 0.01 mg/kg bw/day was established on the basis of a NOAEL of 5 mg/kg bw/day for convulsions, to which a safety factor of 500 was applied.
In a subchronic study conducted by the NTP (2011), F344 rats (n=10/sex/group) were administered 0, 12.5, 25, 50, 75, or 100 mg/kg bw/day of thujone (α- and β-isomers in 0.5% methylcellulose) by gavage for 14 weeks. In all dose groups, the incidence of renal tubular mineralization was significantly increased in female rats. At doses equal to or greater than 25 mg/kg bw/day, seizures were observed beginning from week 4, which were accompanied by lesions in the brain (for example, pigmentation, congestion and/or hemorrhage), organ weight changes (for example, in the liver and thymus), and histopathological changes in other organs (for example, pituitary, spleen), as well as mortality at higher dose levels. At the highest dose level, the majority of the animals died beginning from week 3. A LOAEL of 12.5 mg/kg bw/day was identified on the basis of kidney effects observed (that is, findings of renal tubular mineralization at all dose levels). Similar findings were observed when B6C3F1 mice (n=10/sex/group) were administered 0, 6.25, 12.5, 25, 50, or 75 mg/kg bw/day thujone (α- and β-isomers in 0.5% methylcellulose) by gavage using the same 14-week protocol as the previous study in rats (NTP 2011). At doses equal to or greater than 25 mg/kg bw/day, seizures were observed (starting from week 8 in mice receiving 25 mg/kg bw/day, and from week 2 at 50 and 75 mg/kg bw/day), which were accompanied by congestion, hemorrhage, and mortality at higher dose levels. A NOAEL of 12.5 mg/kg bw/day was identified on the basis of convulsions observed at the next dose level of 25 mg/kg bw/day.
Chronic toxicity, carcinogenicity and genotoxicity
The potential carcinogenic effects of thujone were examined in a 2-year oral study where F344 rats (n=50/sex/group) were administered 0, 12.5, 25, or 50 mg/kg bw/day thujone (α- and β-isomers in 0.5% methylcellulose) by gavage for 105 weeks (NTP 2011). In all treatment groups, a significantly increased incidence of kidney mineralization was observed when compared to controls. At the lowest dose level of 12.5 mg/kg bw/day, there were observations of seizures, which occurred in 8% of the animals starting from week 58. At 25 mg/kg bw/day, there were also significant reductions in survival (34% vs. 54% in controls), and most rats (90%) experienced seizures from week 6 onwards. This dose level was also associated with a significantly increased incidence of preputial gland adenomas or carcinomas in male animals (18% vs. 6% in control animals). In addition, there was one case of malignant pheochromocytoma of the adrenal medulla and a significant increase in the incidence of benign pheochromocytoma (24% vs. 12% in control animals). Other histopathological changes included pigmentation of the spleen, epithelial hyperplasia in the lungs, and dilatation of Rathke's cleft in the pituitary gland. At the highest dose level of 50 mg/kg bw/day, all rats experienced seizures from week 3 onwards and died throughout the study. This dose level was also associated with an increased incidence of lesions in the brain (necrosis, pigmentation), pituitary gland (atrophy and dilatation of Rathke's cleft), spleen (pigmentation), and testes (interstitial cell adenomas). The NTP (2011) concluded that there was some evidence of carcinogenic activity in male rats on the basis of the neoplasms identified in the preputial gland and adrenal gland pheochromocytomas. However, these tumours were observed at doses that resulted in overt toxicity (for example, convulsions and mortality), which compromised the interpretations of the findings of the study. For these reasons, the tumours in the preputial and adrenal glands were not considered for risk characterization purposes as they were likely to represent effects secondary to overt toxicity. For non-neoplastic effects, a LOAEL of 12.5 mg/kg bw/day was identified on the basis of the observations of kidney mineralization and seizures.
No carcinogenic effects were observed in B6C3F1 mice (n=50/sex/group) administered 0, 3, 6, 12, or 25 mg/kg bw/day thujone (α- and β-isomers in 0.5% methylcellulose) by gavage for 105 weeks using the same study protocol (NTP 2011). However, observations such as significantly reduced body weights, seizures (affecting approximately 94% of the animals, starting from day 1), and mortality were observed at the highest dose level of 25 mg/kg bw/day. A NOAEL of 12 mg/kg bw/day was identified on the basis of neurological effects (that is, seizures) and mortality observed at the next dose level of 25 mg/kg bw/day.
Lachenmeier and Uebelacker (2010) and Gwinn et al. (2020) evaluated the data available on thujone (including the studies included in this health effects assessment) using a benchmark dose modelling approach. Benchmark dose modelling was performed to derive PODs for different apical outcomes. For each dataset, the dose-response curve was used to derive a lower one-sided 95% confidence limit for the benchmark dose (BMDL) predicted to result in a 10% response rate (BMDL10). Only the results of the best-fitting model (selected according to p-value and Akaike’s information criterion) were considered. A BMDL10 of 11 mg/kg bw/day was derived for the clonic seizures (that is, seizures causing twitching) observed in the long-term studies conducted by the NTP on rats (Lachenmeier and Uebelacker 2010).
With respect to genotoxicity, α-thujone was not genotoxic when tested in bacterial mutagenicity assays in Salmonella typhimurium TA98 and TA100 and Escherichia coli SY252 and WP2 uvrA with or without metabolic activation up to cytotoxic concentrations (Vuković-Gačić et al. 2006; NTP 2011). A mixture of thujone isomers (α- and β-thujone) was also not mutagenic in Salmonella typhimurium TA97, TA98, TA100, and TA1535, or Escherichia coli WP2 uvrA (NTP 2011). However, when tested in a mammalian cell gene mutation assay, α-thujone was mutagenic in the presence of, but not without, metabolic activation (Seifried et al. 2006 as cited in ACToR 2008-). This secondary reference, however, was considered to be limited as it was associated with uncertainties and deviated from current internationally-accepted test guidelines, compromising the interpretation of the results. In vivo exposure to thujone (α- and β-isomers) for up to 3 months was also associated with a significant increase in micronucleated erythrocytes in female mice (NTP 2011). However, it was noted that this response was only detected at the highest dose (that is, 50 mg/kg bw/day), which was associated with overt toxicity (that is, convulsions and mortality). In assays examining the levels of DNA damage (that is, COMET), thujone was associated with spontaneous mutagenesis and DNA damage in bacterial and mammalian cells deficient in DNA repair capacity, but not in proficient cells (Nikolić et al. 2011, 2015). Overall, the findings from these studies suggest that the potential for thujone to result in genotoxicity is limited (weak).
Reproductive and developmental toxicity
Studies examining the potential reproductive and developmental effects following exposure to thujone have not been identified in the literature.
Human data
There are numerous case reports in the scientific literature reporting the effects of thujone poisoning. The majority of these reports describe effects on the CNS such as mania and impairment (Di Lorenzo et al. 2018). The potential neurological effects of thujone in humans have also been examined in a study whereby healthy volunteers (n=25) were administered an alcoholic beverage containing 0, 10, or 100 mg/L α-thujone (Dettling et al. 2004). The total amount of thujone consumed by each subject was estimated to be between 0.024 and 0.028 mg/kg bw for the low concentration alcoholic beverage and between 0.24 and 0.28 mg/kg bw for the high concentration alcoholic beverage. In this study, effects on attention performance (as measured by the number of correct reactions to visual stimulus and reaction time) and mood (as assessed by subjective questionnaires) were recorded. Consumption of the beverage with the high concentration of thujone resulted in a significant reduction in attention, which suggests an impairment on performance. These effects were not observed when the subjects consumed the beverages with low or no thujone content. In 2011, the EMA conducted an assessment on the human data available on thujone and indicated that 3 mg (approximately 0.04 mg/kg bw) was the maximum safe daily dose for humans. Since then, the EMA has published a final public statement on the use of herbal medicinal products containing thujone, which identified 6 mg (approximately 0.08 mg/kg bw in a 74 kg adult) as a limit of daily exposure to thujone (EMA 2012). In order to derive this limit, the EMA selected the BMDL10 of 11 mg/kg bw/day derived by Lachenmeier and Uebelacker (2010) for convulsions and applied an uncertainty factor of 100, resulting in an ADI of 0.11 mg/kg bw/day. For a “standard human being” (according to the EMA), this would result in a limit dose of approximately 7 mg per day. However, the EMA noted that the average amount of dietary intake of thujone was 1 mg per day. Therefore, the highest safe amount (that is, 7 mg per day) was reduced by the possible intake by food (that is, 1 mg per day) to give 6 mg as a limit of daily exposure. This limit has been adopted by HC. Currently, product licence applications for oral NHPs of sage oil and wormwood oil should include a copy of a certificate of analysis or any other equivalent document demonstrating that the thujone content of a daily dose of the product is acceptable (NHPID [modified 2022]).
7.9.3 Characterization of risk to human health
The health effects data sets for sage oil (from Salvia officinalis) and wormwood oil are considered to be limited. In order to inform the risk assessment and address data limitations, the health effects data available on the major components were taken into consideration. Thujone represents the component associated with the lowest effect levels in the hazard databases and is present in high concentrations in sage oil and wormwood oil (that is, up to approximately 57% and 65%, respectively). For this reason, the health effects data from thujone were used to inform the characterization of risk following exposure to sage oil (from Salvia officinalis) and wormwood oil.
A chronic oral 2-year study conducted using α- and β-thujone in rats was considered to be the most relevant study for the characterization of risk following exposure to sage oil and wormwood oil (NTP 2011). A BMDL10 of 11 mg/kg bw/day derived by Lachenmeier and Uebelacker (2010) on the basis of clonic seizures observed in male rats in the NTP study was found to be the most relevant endpoint. This endpoint is also consistent with that used by the EMA (2012) to derive daily limits of thujone in herbal medicinal preparations.
Sage oil
Estimates of exposure to sage oil for the highest exposed age group, the critical effect level, and resulting MOEs are presented in Table 7‑47.
| Exposure scenario | Exposure (mg/kg bw/day) |
MOEd |
|---|---|---|
| Massage oila (0 to 5 months) |
4.3 | 3 |
| Hair styling producta (2 to 3 years) |
0.11 | 100 |
| Sunless tanning producta (9 to 13 years) |
0.11 | 100 |
| Doucheb (14 to 18 years) |
0.64 | 17 |
| Face maska (14 to 18 years) |
0.23 | 48 |
| Massage oil (NHP)a (19+ years) |
3.3 | 3 |
| Analgesic cream (NHP)a (9 to 13 years) |
7.7 × 10-2 | 143 |
| Throat spray (NHP) (19+ years) |
4.8 × 10-2 | 229 |
| Motion sickness medication (NHP) (14 to 18 years) |
1.0 | 11 |
| Antiperspiranta (19+ years) |
0.91 | 12 |
| Conditioner (rinse-off)a (2 to 3 years) |
0.18 | 61 |
| Spray perfumea (2 to 3 years) |
0.94 | 12 |
| Makeup removera (9 to 13 years) |
9.0 × 10-2 | 122 |
| Body moisturizera (0 to 5 months) |
4.8 | 2 |
| Face moisturizera (19+ years) |
0.64 | 17 |
| Liquid body soapa (0 to 5 months) |
0.43 | 26 |
| Shampooa (0 to 5 months) |
9.3 × 10-2 | 118 |
| Breath freshener (4 to 8 years) |
4.3 × 10-2 | 256 |
| Mouthwash (4 to 8 years) |
4.3 × 10-2 | 256 |
| Toothpaste (2 to 3 years) |
0.12 | 92 |
| Hand sanitizer (NHP)a.c (2 to 3 years) |
3.1 | 4 |
| Body moisturizer (NHP)a (9 to 13 years) |
1.5 | 7 |
| Oral supplement (capsule) (NHP) (19+ years) |
40.5 | <1 |
| Oral supplement (loose herbs) (NHP) (19+ years) |
21.9 | <1 |
| Herbal tea blend (NHP) (19+ years) |
1.8 × 10-3 | 6111 |
| Herbal tea (19+ years) |
8.9 × 10-3 | 1236 |
| Food flavouring intake (general population 1 year and older) |
1.55 × 10-2 | 710 |
| Essential oil use in aromatic diffuser (user)a (9 to 13 years) |
2.0 | 6 |
| Essential oil use in aromatic diffuser (bystander) (1 year) |
2.0 | 6 |
| Essential oil use in face steamer (user)a (4 to 8 years) |
3.8 | 3 |
| Essential oil use in face steamer (bystander) (1 year) |
0.57 | 19 |
Abbreviations: MOE, margin of exposure; BMDL, benchmark dose level
a Dermal exposure estimates were adjusted by a dermal absorption factor of 50%.
b Dermal absorption was assumed to be equivalent to oral absorption (that is, 100%).
c Estimate for situations of public health concern.
d MOEs are presented using the selected BMDL of 11 mg/kg bw/day, based on convulsions observed in a 2-year study in rats.
With respect to exposures to sage oil from the use of massage oils (cosmetics and NHPs), hair styling products, sunless tanning products, douches, face masks, analgesic creams (NHPs), throat sprays (NHPs), motion sickness medications (NHPs), antiperspirants, conditioners, spray perfumes, face moisturizers, body moisturizers (cosmetics and NHPs), makeup removers, liquid body soaps, shampoos, breath fresheners, mouth washes, toothpastes, oral supplements (capsules and loose herbs) (NHPs), hand sanitizers (NHPs), and essential oil use in aromatic diffusers and face steamers, the comparison of the critical effect level to the estimated level of exposure resulted in MOEs ranging from <1 to 256, which are below 300 and considered potentially inadequate to address uncertainties in the health effects and exposure data used to characterize risk. Factors including interspecies and intraspecies differences and database deficiencies (for example, lack of reproductive and developmental toxicity studies) were considered during the determination of adequacy of the MOEs. Given the number of products containing sage oil available on the Canadian market, it is possible that exposure may result from the use of several types of products containing this substance on the same day (that is, aggregate exposure), in which case the MOE would be even lower.
The resulting MOEs derived for use in herbal tea blends (NHPs) and herbal teas and as a food flavouring are 710 and 6111, respectively, which are greater than 300 and considered adequate.
Wormwood oil
Table 7‑48 describes the exposure scenarios associated with wormwood oil, the critical effect level, and resultant MOEs for the characterization of human health risk.
| Scenario | Exposure (mg/kg bw/day)a.b |
MOEb |
|---|---|---|
| Hand sanitizer (NHP) (2 to 3 years) |
0.65 to 16.3 | < 1 to 17 |
| Analgesic cream (NPD) (19+ years) |
0.45 | 24 |
| Essential oil use in aromatic diffuser, user (9 to 13 years) |
3.04 | 4 |
| Essential oil use in aromatic diffuser, bystander (1 year) |
1.97 | 6 |
| Essential oil use in facial steamer, users (4 to 8 years) |
2.99 | 4 |
| Essential oil use in facial steamer, bystanders (4 to 8 years) |
0.21 | 52 |
| Essential oil use in baths (9 to 13 years) |
0.24 | 46 |
| Essential oil formulated in body moisturizer (0 to 5 months) |
4.79 | 2 |
| Essential oil formulated in massage oil (0 to 5 months) |
4.34 | 3 |
| Oral ingestion of wormwood essential oil (14 to 18 years) |
19.1 | < 1 |
| Food flavouring agent (general population 1 year and older) |
0.00027 | > 40 000 |
Abbreviations: MOE, margin of exposure; BMDL, benchmark dose level
a Exposure estimates are presented for the highest exposed age group. Individual exposure estimates for each age group are presented in the exposure assessment section 5.9.4.
b MOEs are presented using the selected BMDL of 11 mg/kg bw/day, based on convulsions observed in a 2-year study in rats.
The MOEs between the critical effect level and the dermal and inhalation estimates of exposure to wormwood oil in hand sanitizers (NHPs), analgesic creams (NPDs), aromatic diffusers, facial steamers, and baths and when formulated in massage oils and body lotions, ranged from less than 1 to 52, which are below 300 and considered potentially inadequate to address uncertainties in the health effects and exposure data used to characterize risk. The MOE from oral ingestion of wormwood essential oil is also below 300 and considered potentially inadequate. Factors including interspecies and intraspecies differences and database deficiencies were considered during the determination of adequacy of the MOEs.
The potential for oral exposure to wormwood oil from its use as a food flavouring agent resulted in an MOE of > 40 000, which is greater than 300 and considered adequate to address uncertainties in the health effects and exposure data used to characterize risk.
7.9.4 Uncertainties in evaluation of risk to human health
The key sources of uncertainty for sage oil and wormwood oil are presented in Table 7‑49.
| Key source of uncertainty | Impact |
|---|---|
| The composition of the main components in sage oil and wormwood oil differs depending on the origin of the plant, its species, temperature, soil, and geography. Therefore, the composition of sage oil and wormwood oil present in products available to consumers is unknown, which represents an uncertainty in the assessment. | +/- |
| Data from similar terpene and terpenoid compounds, including geraniol, citronellol, linalool (ECCC, HC 2020a), and citral was used to inform the potential dermal absorption of thujone, the major component of sage oil and wormwood oil, used to characterize risk. | +/- |
| Sage oil and wormwood oil may be used in hand sanitizers. There is uncertainty regarding the duration of increased hand sanitizer use that may occur in a situation of public health concern. | +/- |
| The health effects data for different sage and wormwood oils were considered to be limited. Health effects data from the major component with the lowest effect level in the hazard database (that is, thujone) was selected for risk characterization purposes for both substances. | + |
| The health effects database for thujone indicates that the α-isomer may be more potent than the β-isomer. The health effects studies available is based on thujone containing up to 71% α-thujone. However, the predominant isomer found in wormwood oil is β-thujone. | + |
| The risk characterization did not take into consideration the potential for additive, synergistic, or antagonistic effects of components within mixtures. It is possible that the health effects of thujone may be modified in the presence of other major components in sage oil and wormwood oil. | +/- |
| The potential use of more than one product by a single person in a day (that is, aggregate exposure) was not considered. This may potentially underestimate exposure to some individuals. | - |
+ = uncertainty with potential to cause over-estimation of exposure/risk; - = uncertainty with potential to cause under-estimation of exposure risk; +/- = unknown potential to cause over- or under-estimation of risk.
7.10 Subgroup 2: Isobornyl cyclohexanol (IBCH), sandal cyclohexanol, bornyl cyclohexanol (BCH), and sandela
7.10.1 Exposure assessment
Environmental media
These substances do not occur naturally in the environment. No reports of monitoring in environmental media in Canada or elsewhere were identified for these substances. These substances were not reported to be manufactured or imported in quantities greater than the reporting threshold of 100 kg on the basis of information submitted in response to a CEPA section 71 survey (Environment Canada 2013). In consideration of low reported quantities of these substances in Canada, exposure to IBCH, sandal cyclohexanol, BCH, and sandela from their presence in environmental media is expected to be minimal.
Products available to consumers
Although IBCH, sandal cyclohexanol, BCH, and sandela are distinct substances, their names are used as synonyms and used interchangeably in the literature, including the use information. Therefore, these 4 substances were assessed together and, for the scenarios presented, it is assumed that the products may contain any one of IBCH, sandal cyclohexanol, BCH, or sandela at the reported concentration.
In order to evaluate the potential exposure of the general population to substances in subgroup 2 from the use of products available to consumers, representative sentinel exposure scenarios were selected. Sentinel scenarios include exposure from the use of a spray perfume (5%) and body moisturizer (0.4%). Details on the method and parameters used to derive exposure estimates are provided in Appendix A. For the scenarios described in Table 7‑50, estimates were derived for a range of relevant age groups and only the highest one is presented. Dermal absorption was assumed to be equivalent to oral absorption (that is, 100%).
| Scenario | Route of exposure | Reported concentration (%) | Exposure (mg/kg bw/day) |
|---|---|---|---|
| Spray perfume | Dermala | 5b | 0.94 (2 to 3 years) |
| Body moisturizer | Dermal | 0.4c | 1.27 (0 to 5 months) |
a Though inhalation exposure is possible from the use of the spray perfume, exposure via this route was not quantified as the resulting exposure estimates are lower than those derived for the dermal route. Therefore, absorption via the dermal route was assumed to be equivalent to oral absorption (that is, 100%).
b SDS 2015b
c SDS 2015a
7.10.2 Selection of analogues
A read-across approach was used to inform the human health assessment of sandal cyclohexanol, BCH, and sandela and address toxicological endpoints with limited data. IBCH was selected as an analogue on the basis of its structural and/or functional similarity (for example, isomerism) to these substances and the availability of relevant empirical data for use in hazard characterization. For further information on the physical-chemical properties of IBCH, sandal cyclohexanol, BCH, and sandela, refer to Table 3‑1.
7.10.3 Health effects assessment
Health effects data were available for IBCH, and the toxicological studies on IBCH were identified in the REACH registration dossiers for IBCH and sandela. The health effects data sets for sandal cyclohexanol, BCH, and sandela were considered to be limited. The toxicological data available for IBCH were used to inform the health effects assessment of all substances within subgroup 2.
Repeated-dose toxicity
In a short-term study, Sprague-Dawley rats (n=7/sex/group) were administered 0, 125, 375, or 1125 mg/kg bw/day of IBCH (in corn oil) by gavage for 28 days (Anonymous 2015 as cited in ECHA 2021b). Locomotor activity was also assessed. At the lowest dose level of 125 mg/kg bw/day, there were significant increases in the levels of creatinine, monocytes, and basophils in the blood and significant decreases in heart relative weights of males, but were not dose-dependent. At 375 mg/kg bw/day, there were significantly increased absolute/relative liver weights, increased levels of WBC and mean corpuscular hemoglobin (MCH), and decreased levels of RBC in females when compared with controls. At the highest dose level of 1125 mg/kg bw/day, there were significant increases in absolute/relative liver weights, increases in the levels of monocytes, basophils, WBC, MCV, and MCH, and significant decreases in the levels of RBC and hematocrit in females. Changes in clinical chemistry were also observed at this dose, including significant increases in the levels of protein, cholesterol, sodium, potassium, and BUN, and significant decreases in ALT and glucose levels. In addition, female mean body weights were reduced compared to control. One female animal exhibited an enlarged stomach and an enlarged uterus filled with uterine fluid. Histopathological changes at the highest dose level included a reactive spleen and excessive number of lymphocytes in the lungs. Based on these results, a NOAEL of 125 mg/kg bw/day is determined based on effects on female liver weight, clinical biochemistry and hematology at higher doses.
The effects of IBCH were also examined in a report of a combined repeated-dose and reproduction/developmental toxicity screening study conducted in accordance with OECD TG 422 (Anonymous 2018 as cited in ECHA 2021d). Sprague-Dawley rats (n=10/sex/group) were administered 0, 50, 150, or 500 mg/kg bw/day IBCH (in corn oil) by gavage for 2 weeks before mating, during mating, and throughout gestation until PND 14. Male animals were treated for a total of 6 weeks, while female animals were treated for approximately 9 weeks. Clinical signs, body weight, food consumption, and mortality were recorded throughout the study. A functional observation battery (FOB) was also conducted for the assessment of motor activity and reactivity to different stimuli (n=5/sex/group). At the end of the study, the animals were sacrificed, and gross necropsy was performed for all parental (F0) animals, with special attention given to reproductive organs. Histopathological examinations were also performed on all organs. The thyroid and parathyroid of F0 animals and pups were also examined. At doses equal to and greater than 50 mg/kg bw/day, salivation was noted in the animals. However, no treatment-related neurological effects were noted in the FOB analysis. At 150 mg/kg bw/day, additional clinical signs such as piloerection, round back, and closed eyes were observed. Two female animals in this dose group were also sacrificed during lactation due to poor condition. There were statistically significant reductions in body weight, food consumption, levels of urea, and thyroid hormones (T4). Significant changes in organ weights (for example, increased kidney, brain, and testes/epididymides weights, and decreased heart weight) were also observed at this dose level. In addition to these effects, animals treated with the highest dose level of 500 mg/kg bw/day also exhibited significantly higher levels of creatinine, protein, calcium, and bilirubin. Significant changes in organ weights (for example, increased liver, adrenal, thyroid weights, decreased weight of levator ani plus bulbocavernous muscle) were also observed. A NOAEL of 50 mg/kg bw/day was considered on the basis of poor condition, changes in body and organ weights (for example, kidney, brain, heart, testes/epididymides), and changes in clinical chemistry parameters (that is, urea), food consumption, and thyroid hormones at the next dose level of 150 mg/kg bw/day.
Genotoxicity and carcinogenicity
The hazard data available suggest that IBCH is not expected to result in mutagenicity or clastogenicity. In bacterial mutagenicity assays conducted using Salmonella typhimurium strains TA98, TA100, TA102, TA1535, and TA1537, no genotoxicity was observed up to cytotoxic concentrations with or without metabolic activation (Anonymous 2018 as cited in ECHA 2021b). Furthermore, no genotoxicity was observed in a mammalian cell gene mutation assay when tests were conducted in Chinese hamster ovary cells up to limit concentrations of 10 µM with or without metabolic activation (Anonymous 2015 as cited in ECHA 2021b). A similar mammalian cell gene mutation assay using mouse lymphoma L5178Y cells did not result in genotoxicity up to cytotoxic concentrations with or without metabolic activation (Anonymous 2016 as cited in ECHA 2021d). When IBCH was tested in chromosome aberration tests using human blood lymphocytes, no clastogenicity was observed with or without metabolic activation up to cytotoxic concentrations (Anonymous 2016 as cited in ECHA 2021d; Anonymous 2018 as cited in ECHA 2021b). Although no in vivo genotoxicity studies were identified, the available data suggests that IBCH is not likely genotoxic.
Studies examining the potential carcinogenic effects following chronic exposure to IBCH were not identified.
Reproductive and developmental toxicity
No guideline studies were identified for reproductive toxicity. However, the short-term repeated-dose toxicity study mentioned previously provided relevant health effects data on certain reproductive parameters (Anonymous 2015 as cited in ECHA 2021a). In this study, Sprague-Dawley rats (n=7/sex/group) were administered 0, 125, 375, or 1125 mg/kg bw/day of IBCH (in corn oil) by gavage for 28 days. At the end of the study, examinations of hormone levels (that is, testosterone, estrogen), gross pathology, and histopathology (for example, testes, ovaries, uterus) were conducted. At the highest dose level of 1125 mg/kg bw/day, one female animal exhibited an enlarged uterus filled with uterine fluid. No treatment-related adverse effects were identified with respect to hormone levels and the absolute or relative weights of reproductive organs. A NOAEL of 375 mg/kg bw/day was identified on the basis of effects on the uterus at the next dose level of 1125 mg/kg bw/day.
In the combined repeated-dose and reproduction/developmental toxicity screening study mentioned above, IBCH (in corn oil) was administered by gavage at doses of 0, 50, 150, or 500 mg/kg bw/day to rats prior to mating, during mating, and throughout gestation until PND 14 (Anonymous 2018 as cited in ECHA 2021d). At 150 mg/kg bw/day, the dams had a significantly lower mean number of implantation sites, accompanied by a lower mean number of pups delivered. Histopathological examinations revealed increased mucification of the vaginal epithelium in 50% of the animals. The pups at this dose level exhibited an increase in the incidence of clinical signs due to the lack of maternal care. There were significant reductions in viability, pup weights, and the number of live pups. These effects were also observed at the dose level of 500 mg/kg bw/day, but with greater incidence and severity. At 500 mg/kg bw/day, there were also dams that did not get pregnant (50%) and one failed to deliver. Two dams were sacrificed because of poor condition, while ovarian atrophy was observed in 30% of the dams, 2 of which also had atrophy of the thymus. Histopathological examinations also revealed increased mucification of the vaginal epithelium in 30% of the animals. The authors of the report identified a NOAEL of 50 mg/kg bw/day for parental and reproductive toxicity on the basis of effects on the reproductive organs and performance at the next dose level of 150 mg/kg bw/day. A NOAEL of 50 mg/kg bw/day was also identified by the authors for developmental toxicity on the basis of effects on the pups (for example, viability, pup weight) at the next dose level of 150 mg/kg bw/day. The effects on pups were observed in the presence of maternal toxicity.
7.10.4 Characterization of risk to human health
On the basis of the health effects data available, IBCH, sandal cyclohexanol, BCH, and sandela are not expected to be carcinogenic or genotoxic.
An oral combined repeated-dose and reproduction/developmental screening study (OECD TG 422) using IBCH was identified as the most appropriate study for the risk characterization of exposure to substances in this subgroup (Anonymous 2018 as cited in ECHA 2021d). A NOAEL of 50 mg/kg bw/day was identified on the basis of effects observed at the next dose level of 150 mg/kg bw/day (that is, parental toxicity, effects on reproductive organs, and effects on pups).
Table 7‑51 provides all relevant exposure estimates for the highest exposed age group, critical effect levels, and resulting MOEs for characterization of risk to human health.
| Scenario | Exposure (mg/kg bw/day)a | MOEb |
|---|---|---|
| Spray perfume (2 to 3 years) | 0.94 | 53 |
| Body moisturizer (0 to 5 months) | 1.27 | 39 |
Abbreviations: MOE, margin of exposure; NOAEL, no observed adverse effect level
a Exposure estimates are presented for the highest exposed age group for comparison with the critical effect level.
b MOEs are presented using the selected NOAEL of 50 mg/kw bw/day on the basis of effects observed at the next dose level of 150 mg/kg bw/day, including parental toxicity (poor condition, changes in body and organ weights such as kidney, brain, and heart, changes in clinical chemistry parameters, food consumption, thyroid hormones), effects on reproductive organs, and effects on pups in an oral combined repeated-dose and reproduction/developmental toxicity study in rats.
With respect to daily exposures to members of this subgroup from the use of spray perfumes and body moisturizers, comparison of the critical effect level with the estimated levels of exposure resulted in MOEs of 39 and 53, respectively, which are below 300 and considered potentially inadequate to address uncertainties in the health effects and exposure data used to characterize risk. Factors including interspecies and intraspecies differences, and the duration of the critical study relative to the duration of human exposure were taken into consideration during the determination of the adequacy of the MOEs.
7.10.5 Uncertainties in evaluation of risk to human health
The key sources of uncertainty are presented in Table 7‑52.
| Key source of uncertainty | Impact |
|---|---|
| For concentrations of sandela and IBCH in safety data sheets (used to characterize exposure to all substances in this subgroup), the reported upper-bound concentration was used to estimate dermal and inhalation exposures. | + |
| For dermal exposures, absorption was assumed to be equivalent to oral absorption (that is, 100%). | + |
| No chronic studies have been conducted using IBCH for any relevant routes of exposure. Health effects data from a combined repeated-dose and reproductive/developmental toxicity study were used to inform the assessment. | +/- |
| The health effects assessment was based on summaries of unpublished studies that were retrieved from REACH registration dossiers. Study quality could not be completely assessed because of the limited details for certain methodologies and results. | +/- |
| The health effects data sets for sandal cyclohexanol, BCH, and sandela were considered to be limited. Toxicological data from IBCH were used to inform the assessment. | +/- |
| The chemical composition of sandela is currently unknown. Given that there is some evidence that sandela may be associated with IBCH, health effects data from IBCH were used to inform the health effects assessment of sandela. | +/- |
+ = uncertainty with potential to cause over-estimation of exposure/risk; - = uncertainty with potential to cause under-estimation of exposure risk; +/- = unknown potential to cause over- or under-estimation of risk.
8. Consideration of subpopulations who may have greater susceptibility or exposure
There are groups of individuals within the Canadian population who, due to greater susceptibility or greater exposure, may be more vulnerable to experiencing adverse health effects from exposure to substances. Certain populations are routinely considered throughout the assessment process, such as infants, children, and people of reproductive age. For instance, age-specific exposures are routinely estimated, and developmental and reproductive toxicity studies are evaluated for potential adverse health effects. These subpopulations with potential for higher exposure and those who may be more susceptible were taken into account in the risk assessment outcomes.
9. Conclusion
Considering all available lines of evidence presented in this draft assessment, there is low risk of harm to the environment from the substances in the Fourteen Terpene and Terpenoid Substances Group. It is proposed to conclude that the substances in the Fourteen Terpene and Terpenoid Substances Group do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
Considering all the information presented in this draft assessment, it is proposed to conclude that cade oil, jonquil oil, Verbena officinalis extract, Ginkgo biloba extract, myrrh oil, cork tree extract, sage oil, wormwood oil, isobornyl cyclohexanol, sandal cyclohexanol, bornyl cyclohexanol, and sandela meet the criteria under paragraph 64(c) of CEPA as they are entering or may enter the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Considering all the information presented in this draft assessment, it is proposed to conclude that norlimbanol and amberlyn do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore proposed to conclude that cade oil, jonquil oil, Verbena officinalis extract, Ginkgo biloba extract, myrrh oil, cork tree extract, sage oil, wormwood oil, isobornyl cyclohexanol, sandal cyclohexanol, bornyl cyclohexanol, and sandela meet one or more of the criteria set out in section 64 of CEPA and that norlimbanol, and amberlyn do not meet one or more of the criteria set out in section 64 of CEPA.
References
Abu-Darwish MS, Cabral C, Ferreira IV, Gonçalves MJ, Cavaleiro C, Cruz MT, Al-bdour TH, Salgueiro L. 2013. Essential oil of common sage (Salvia officinalis L.) from Jordan: assessment of safety in mammalian cells and its antifungal and anti-inflammatory potential. BioMed Res Int. 2013;538940.538940.
Achour S, Abourazzak S, Mokhtari A, Soulaymani A, Soulaymani R, Hida M. 2011. Juniper tar (cade oil) poisoning in new born after a cutaneous application. BMJ Case Rep.: bcr0720114427.
[ACToR] Aggregated Computational Toxicology Resource [database]. 2008. Washington (DC): United States Environmental Protection Agency. [updated 2009 May 21].
Akagi J, Cho YM, Mizuta Y, Tatebe C, Sato K, Ogawa K. 2019. Subchronic toxicity evaluation of isoeugenyl methyl ether in F344/DuCrj rats by 13-week oral administration. Regul Toxicol Pharmacol. 102:34-39.
Akihisa T, Yokokawa S, Ogihara E, Matsumoto M, Zhang J, Kikuchi T, Koike K, Abe M. 2017. Melanogenesis-inhibitory and cytotoxic activities of limonoids, alkaloids, and phenolic compounds from Phellodendron amurense bark. Chem Biodivers. 14(7):e1700105.
Alam S, Payal M, Jagdale PR, Ayanur A, Ansari KM. 2021. Safety studies of Nexrutine, bark extract of Phellodendron amurense through repeated oral exposure to rats for 28 days. Heliyon. 7(7): e07654.
Al-Harbi MM, Qureshi S, Ahmed MM, Rafatullah S, Shah AH. 1994. Effect of Commiphora Molmol (oleo-gum-resin) on the cytological and biochemical changes induced by cyclophosphamide in mice. Am J Chin Med. 22(1):77-82.
Al-Kazzaz MA. 2018. Toxicological profile of Mirazid: a review. Biol Res Rev. 1:9-16.
Aloui S, Skhiri H, Ltaief A, Elmay M. 2010. An exceptional case of acute renal failure: is there a renal toxicity of Artemisia herba-alba? Ren Fail. 32(8): 1009-1011.
Al-Snafi AE. 2018. Pharmacological and therapeutic effects of Juniperus oxycedrus- a review. Indo Am J Pharm Sci. 5(4):2198-2205.
Altindal D, Altindal N. 2016. Sage (Salvia officinalis) oils. In: Preedy VR, editor. Essential oils in food preservation, flavor and safety. Boston (MA): Academic Press. Chapter 81. p. 715-721.
Al-Yahya AA, Al-Majed AA, Al-Bekairi AM, Al-Shabanah OA, Qureshi S. 2006. Studies on the reproductive, cytological and biochemical toxicity of Ginkgo Biloba in Swiss albino mice. J Ethnopharmacol. 107:222-228.
Amma MKP, Malhotra N, Suri RK, Arya OP, Dani HM, Sareen K. 1978. Effect of oleoresin of gum guggul (Commiphora mukul) on the reproductive organs of female rat. Indian J Exp Biol. 16(9):1021-1023.
Api AM, Belmonte F, Belsito D, Biserta S, Botelho D, Bruze M, Burton Jr GA, Buschmann J, Cancellieri MA, Dagli ML, et al. 2020. RIFM fragrance ingredient safety assessment, benzyl benzoate, CAS Registry Number 120-51-4. Food Chem Toxicol. 144 Suppl 1: 111500.
Arenz A, Klein M, Fiehe K, Groß J, Drewke C, Hemscheidt T, Leistner E. 1996. Occurrence of neurotoxic 4′-O-methylpyridoxine in Ginkgo biloba leaves, Ginkgo medications and Japanese Ginkgo food. Planta Med. 62(6):548-551.
Azizan A, Blevins RD. 1995. Mutagenicity and antimutagenicity testing of 6 chemicals associated with the pungent properties of specific spices as revealed by the Ames Salmonella/ microsomal assay. Arch Environ Contam Toxicol. 28(2):248-258.
Banerjee S, Welsch CW, Rao AR. 1995. Modulatory influence of camphor on the activities of hepatic carcinogen metabolizing enzymes and the levels of hepatic and extrahepatic reduced glutathione in mice. Cancer Lett. 88(2):163-169.
Bánsághi S, Soule H, Guitart C, Pittet D, Haidegger T. 2020. Critical reliability issues of common type alcohol-based handrub dispensers. Antimicrob Resist Infect Control. 9:90.
Barnes TM, Greive KA. 2017. Topical pine tar: history, properties and use as a treatment for common skin conditions. Australas J Dermatol. 58(2):80-85.
Barrero AF, Oltra JE, Altarejos J, Barragán A, Lara A, Laurent R. 1993. Minor components in the essential oil of Juniperus oxycedrus L. wood. Flavour Fragr J. 8(4):185-189.
Base Formula. [accessed 2021 Dec 2]. Cade (Rectified) Essential Oil: Recipes.
Bastaki M, Aubanel M, Bauter M, Cachet T, Demyttenaere J, Diop MM, Harman CL, Hayashi S, Krammer G, Li X, et al. 2018. Absence of renal adverse effects from β-myrcene dietary administration in OECD guideline-compliant subchronic toxicity study. Food Chem Toxicol. 120:222-229.
Bateman J, Chapman RD, Simpson D. 1998. Possible toxicity of herbal remedies. Scott Med J. 43(1):7-15.
Batiha GES. Olatunde A, El-Mleeh A, Hetta HF, Al-Rejaie S, Alghamdi S, Zahoor M, Beshbishy AM, Murata T, Zaragoza-Bastida A, et al. 2020. Bioactive compounds, pharmacological actions, and pharmacokinetics of wormwood (Artemisia absinthium). Antibiotics (Basel). 9(6):353.
Bellakhdar, J. 1997. La pharmacopée marocaine traditionnelle: médecine arabe ancienne et savoirs populaires. Paris (FR): Ibis Press.
Bernard A, Houssin A, Ficheux AS, Wesolek N, Nedelec AS, Bourgeois P, Hornez N, Batardière A, Misery L, Roudot AC. 2016. Consumption of hair dye products by the French women population: usage pattern and exposure assessment. Food Chem Toxicol. 88: 123-132.
Bernotienė G, Nivinskienė O, Butkienė R, Mockutė D. 2007. Essential oil composition variability in sage (Salvia officinalis L.). Chemija. 18(4):38-43.
Blagojević P, Radulović N, Palić R, Stojanović G. 2006. Chemical composition of the essential oils of Serbian wild-growing Artemisia absinthium and Artemisia vulgaris. J Agric Food Chem. 54(13):4780-4789.
Boguski TK. 2006. Understanding units of measurement. Manhattan (KS): Kansas State University, Center for Hazardous Substance Research. [accessed 2021 Jul 14].
Bonassi S, Prinzi G, Lamonaca P, Russo P, Paximadas I, Rasoni G, Rossi R, Ruggi M, Malandrino S, Sanchez-Flores M, et al. 2018. Clinical and genomic safety of treatment with Ginkgo biloba L. leaf extract (IDN 5933/ Ginkgoselect®Plus) in elderly: a randomised placebo-controlled clinical trial [GiBiEx]. BMC Complement Altern Med. 18(1):22.
Bone K, Mills S. 2013. Principles and practice of phytotherapy: modern herbal medicine. 2nd ed. Toronto (ON): Churchill Livingstone/Elsevier.
Bremmer HF, Prud’homme de Lodder LCH, van Engelen JGM. 2006. Cosmetics Fact Sheet [PDF]. RIVM report 320104001/2006.
Bronaugh RL, Wester RC, Bucks D, Maibach HI, Sarason R. 1990. In vivo percutaneous absorption of fragrance ingredients in rhesus monkeys and humans. Food Chem Toxicol. 28(5):369-373.
Brown AC. 2017. Kidney toxicity related to herbs and dietary supplements: online table of case reports. Part 3 of 5 series. Food Chem Toxicol. 107(Pt A):502-519.
Burdock GA. 2010. Fenaroli’s handbook of flavor ingredients. 6th ed. Boca Raton (FL): CRC Press.
Burkhard PR, Burkhardt K, Haenggeli CA, Landis T. 1999. Plant-induced seizures: reappearance of an old problem. J Neurol. 246(8):667-670.
Burnett, CL. 2018. Safety assessment of Ginkgo biloba-derived ingredients as used in cosmetics [PDF]. Washington (DC): Cosmetic Ingredient Review. [accessed 2022 Feb 25].
Caldas GFR, Limeira MMF, Araújo AV, Albuquerque GS, Silva-Neto JC, da Silva TG, Costa-Silva JH, de Menezes IRA, da Costa JGM, Wanderley AG. 2016. Repeated-doses and reproductive toxicity studies of the monoterpene 1,8-cineole (eucalyptol) in Wistar rats. Food Chem Toxicol. 97:297-306.
Canada. 1978. Food and Drug Regulations. C.R.C., c.870.
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C. 1999, c.33.
Canada. 2009. Guide to cosmetic ingredient labelling. Ottawa (ON): Government of Canada. [accessed 2022 Apr 11].
Canada, Dept. of the Environment. 2012. Canadian Environmental Protection Act, 1999: Notice with respect to certain substances on the Domestic Substances List [PDF]. Canada Gazette, Part I, vol. 146, no. 48, Supplement.
Caputi L, Aprea E. 2011. Use of terpenoids as natural flavouring compounds in food industry. Recent Pat Food Nutr Agric. 3(1): 9-16.
Čerňáková M, Košt'álová D, Kettmann V, Plodová M, Tóth J, Dřímal J. 2002. Potential antimutagenic activity of berberine, a constituent of Mahonia aquifolium. BMC Complement Altern Med. 2:2.
Chalchat JC, Garry RP. 1996. Chemical composition of the leaf oil of Verbena officinalis L. J Essent Oil Res. 8(4):419-420.
Chan E. 1993. Displacement of bilirubin from albumin by berberine. Biol Neonate. 63(4):201-208.
Chan TY. 1994. The prevalence use and harmful potential of some Chinese herbal medicines in babies and children. Vet Hum Toxicol. 36(3):238-240.
Charles AK, Darbre PD. 2009. Oestrogenic activity of benzyl salicylate, benzyl benzoate and butylphenylmethylpropional (Lilial) in MCF7 human breast cancer cells in vitro. J Appl Toxicol. 29(5):422-34.
Charles River. 2019. The in vitro percutaneous absorption of radiolabelled alpha-pinene and radiolabelled citral in 2 test preparations through human skin (OECD 428 and SCCS). Tranent (GB). 109 p. Report No. 40807. [restricted access].
Chemical Book. 2019. 2,2,6-Trimethyl-α-propylcyclohexanpropanol Produkt Beschreibung. [accessed 2021 Sep 13].
ChemIDplus [database]. 1993-. Bethesda (MD): US National Library of Medicine. [accessed 2022 Feb 14]
ChemSpider [database]. c2015. London (UK): Royal Society of Chemistry. [accessed 2019 May 3].
ChemSrc. 2018. Ginkgo biloba extract. [accessed 2021 Aug 10].
Chen S, Wan L, Couch L, Lin H, Li Y, Dobrovolsky VN, Mei N, Guo L. 2013. Mechanism study of goldenseal-associated DNA damage. Toxicol Lett. 221(1):64-72.
Chen YX, Gao QY, Zou TH, Wang BM, Liu SD, Sheng JQ, Ren JL, Zou XP, Liu ZJ, Song YY, et al. 2020. Berberine versus placebo for the prevention of recurrence of colorectal adenoma: a multicentre, double-blinded, randomised controlled study. Lancet Gastroenterol Hepatol. 5(3):267-275.
Chialva F, Liddle PAP, Doglia G. 1983. Chemotaxonomy of wormwood (Artemisia absinthium L.). Z Lebensm Unters Forsch. 176(5):363-366.
Chiasson H, Bélanger A, Bostanian N, Vincent C, Poliquin A. 2001. Acaricidal properties of Artemisia absinthium and Tanacetum vulgare (Asteraceae) essential oils obtained by 3 methods of extraction. J Econ Entomol. 94(1):167-171.
Cicero AFG, Baggioni A. 2016. Berberine and its role in chronic disease. In: Gupta SC, Prasad S, Aggarwal BB, editors. Anti-inflammatory nutraceuticals and chronic diseases. Switzerland: Springer International Publishing. p. 27-45. (Advances in experimental medicine and biology 928).
[CIR] Cosmetic Ingredients Review expert panel. 2001. Final Report on the Safety Assessment of Juniperus Communis Extract, Juniperus Oxycedrus Extract, Juniperus Oxycedrus Tar, Juniperus Phoenicea Extract, and Juniperus Virginiana Extract [PDF]. [accessed 2022 Feb 25]
[CosIng] Cosmetic Ingredient Database. [database]. c2021. Brussels (BE): European Commission. [accessed 2021 Dec 8]
[CPID] Consumer Product Information Database [database]. c2021. McLean(VA): DeLima Associates. [accessed 2021 Dec 9].
[CPID] Consumer Product Ingredients Database [database]. c2022. Search results for: 66068-84-6. [accessed 2022 Feb 14].
[CTFA] Cosmetic, Toiletry and Fragrance Association. 1983. Summary for the Results of Surveys of the amount and Frequency of use of cosmetic products by Women. Report Prepared by Pitkin B, Rodericks JV, Turnbull D. Environ Corporation 1850 K Street, N.W, Washington, DC.
dal Belo SE, Gaspar LR, Maia Campos PMBG, Marty JP. 2009. Skin penetration of epigallocatechin-3-gallate and quercetin from green tea and Ginkgo biloba extracts vehiculated in cosmetic formulations. Skin Pharmacol Physiol. 22(6):299-304.
de Groot AC. 2020. Fragrances and essential oils. In: John SM, Johansen JD, Rustemeyer T, Elsner P, Maibach HI, editors. Kanerva’s occupational dermatology. p. 579-605.
de Martino L, Arena GD, Minervini MM, Deaglio S, Sinisi NP, Cascavilla N, de Feo V. 2011. Active caspase-3 detection to evaluate apoptosis induced by Verbena officinalis essential oil and citral in chronic lymphocytic leukaemia cells. Brazilian J Pharmacogn. 21:869-873
de Paula Porto M, da Silva GN, Luperini BCO, Bachiega TF, de Castro Marcondes JP, Sforcin JM, Salvadori DMF. 2014. Citral and eugenol modulate DNA damage and pro-inflammatory mediator genes in murine peritoneal macrophages. Mol Biol Rep. 41:7043-7051
Desaulniers AT, Lamberson WR, Safranski TJ. 2016. Prenatal heat stress reduces male anogenital distance at birth and adult testis size, which are rescued by concurrent maternal Artemisia absinthium consumption. J Therm Biol. 57:84-91.
Dettling A, Grass H, Schuff A, Skopp G, Strohbeck-Kuehner P, Haffner HT. 2004. Absinthe: attention performance and mood under the influence of thujone. J Stud Alcohol. 65(5):573-581.
De Vincenzi M, Silano M, De Vincenzi A, Maialetti F, Scazzocchio B. 2002. Constituents of aromatic plants: eucalyptol. Fitoterapia. 73(3):269-275.
Diab KA, Fahmy MA, Hassan ZM, Hassan EM, Salama AB, Omara EA. 2018. Genotoxicity of carbon tetrachloride and the protective role of essential oil of Salvia officinalis L. in mice using chromosomal aberration, micronuclei formation, and comet assay. Environ Sci Pollut Res Int. 25(2):1621-1636.
Di Lorenzo C, Ferretti F, Moro E, Ceschi A, Colombo F, Frigerio G, Lüde S, Restani P. 2018. Identification and quantification of thujone in a case of poisoning due to repeated ingestion of an infusion of Artemisia Vulgaris L. J Food Sci. 83(8):2257-2264.
Di Sotto A, Durazzi F, Sarpietro MG, Mazzanti G. 2013. Antimutagenic and antioxidant activities of some bioflavours from wine. Food Chem Toxicol. 60:141-146.
Dobson HEM, Arroyo J, Bergström G, Groth, I. 1997. Interspecific variation in floral fragrances within the genus Narcissus (Amaryllidaceae). Biochem. Syst. Ecol. 25(8): 685-706.
Dörsam B, Wu CF, Efferth T, Kaina B, Fahrer J. 2015. The eucalyptus oil ingredient 1,8-cineole induces oxidative DNA damage. Arch Toxciol. 89(5):797-805.
doTERRA. c2022a. 100 uses for essential oils [PDF]. [accessed 2021 Dec 2].
doTERRA. c2022b. doTERRA eBOOK: Internal Use of Essential Oils [PDF]. [accessed 2021 Dec 2].
Duerksen-Hughes PJ, Yang J, Ozcan O. 1999. P53 induction as a genotoxic test for 25 chemicals undergoing in vivo carcinogenicity testing. Environ Health Perspect. 107(10):805-812.
[ECCC] Environment and Climate Change Canada. 2016a. Science approach document: ecological risk classification of organic substances. Ottawa (ON): Government of Canada.
[ECCC] Environment and Climate Change Canada. 2016b. Supporting documentation: data used to create substance-specific hazard and exposure profiles and assign risk classifications. Gatineau (QC): ECCC. Information in support of the science approach document: ecological risk classification of organic substances. Available from: substances@ec.gc.ca.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2015. Identification of risk assessment priorities: results of the 2015 review. Ottawa (ON): Government of Canada.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2016a. Screening Assessment Internationally Classified Substance Grouping: Cresol (phenol, methyl-) Substances. Ottawa (ON): Government of Canada. [accessed 2021 Jul 15].
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2016b. Draft screening assessment - boric acid, its salts and its precursors. Ottawa (ON): Government of Canada.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2017. Rapid screening of substances with limited general population exposure. Ottawa (ON): Government of Canada.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. [modified 2017 Mar 12]. Categorization of chemical substances. Ottawa (ON): Government of Canada. [accessed 2022 Mar 31].
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2019. Screening assessment benzoates. Ottawa (ON): Government of Canada.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2020a. Screening assessment fatty acids and derivatives group. Ottawa (ON), Canada: Government of Canada.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2020b. Draft screening assessment: terpenes and terpenoids: Acyclic, Monocyclic, and Bicyclic Monoterpenes Group. Ottawa (ON): Government of Canada.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2020c. Draft screening assessment: Parabens Group. Ottawa (ON): Government of Canada. [accessed 2022 Feb 15].
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2021a. Screening assessment phenol, 4-chloro-3-methyl- (Chlorocresol). Ottawa (ON): Government of Canada.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2021b. Draft screening assessment terpenes and terpenoids monocyclic and bicyclic sesquiterpenes group. Ottawa (ON): Government of Canada.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2021c. Screening assessment coal tars and their distillates. Ottawa (ON): Government of Canada. [accessed 2022 Feb 15].
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2024. Draft assessment terpenes and terpenoids phenylpropanoid and aldehyldes group. Ottawa (ON): Government of Canada.
[ECHA] European Chemicals Agency. 2009. Registration dossier for beta-myrcene. [accessed 2021 Jun 1].
[ECHA] European Chemicals Agency. 2016. Registration dossier for citrate. [accessed 2021 Jun 1].
[ECHA] European Chemicals Agency. 2021a. Brief profile: Dodecahydro-3a,6,6,9a-tetramethylnaphtho[2,1-b]furan; CAS RN 3738-00-9. Helsinki (FI): ECHA. [accessed 2021 Aug 17].
[ECHA] European Chemicals Agency. 2021b. Brief profile, toxicological information: 3-(5,5,6-trimethylbicyclo[2.2.1]hept-2-yl)cyclohexan-1-ol; CAS RN 3407-42-9. Helsinki (FI): ECHA. [updated 2021 Aug 2; accessed 2021 Jun 1].
[ECHA] European Chemicals Agency. 2021c. Brief profile: 4-(5,5,6-trimethylbicyclo[2.2.1]hept-2-yl)cyclohexan-1-ol; CAS RN 66068-84-6. Helsinki (FI): ECHA. [updated 2019 Jan 5; accessed 2021 Jun 1].
[ECHA] European Chemicals Agency. 2021d. Brief profile: Phenol, 2-methoxy-, reaction products with 2,2-dimethyl-3-methylenebicyclo[2.2.1]heptane, hydrogenated; CAS RN 70955-71-4. Helsinki (FI): ECHA. [updated 2020 Nov 5; accessed 2021 Sept 15].
[ECHA] European Chemicals Agency. 2021e. Brief profile: Juniper, Juniperus oxycedrus, ext; CAS RN 90046-02-9. Helsinki (FI): ECHA. [updated 2021 Jul 06; accessed 2021 Jul 06].
[ECHA] European Chemicals Agency. 2021f. Brief profile: Juniper, Juniperus virginiana, ext.; CAS RN 85085-41-2. Helsinki (FI): ECHA. [updated 2021 Dec 21; accessed 2022 Jan 31].
[ECHA] European Chemicals Agency. 2021g. Brief profile: Guaiacol; CAS RN: 90-05-1. Helsinki (FI): ECHA. [updated 2021 Jul 14; accessed 2021 Jul 14].
[ECHA] European Chemicals Agency. 2021h. Brief profile: Nimberol EC number: 942-425-2. Helsinki (FI): ECHA. [updated 2020 Dec 08; accessed 2021 Sep 13].
[ECHA] European Chemicals Agency. 2021i. Brief profile: rel-1-[(1R,6S)-2,2,6-trimethylcyclohexyl]hexan-3-ol. EC number: 814-113-5. Helsinki (FI): ECHA. [updated 2021 Dec 21; accessed 2022 Feb 03].
[ECHA] European Chemicals Agency. 2021j. Brief profile: (isobornyl acetate) Exo-1,7,7-trimethylbicyclo[2.2.1]hept-2-yl acetate; CAS RN 125-12-2. Helsinki (FI): ECHA. [updated 2021 Dec 23; accessed 2022 Feb 07].
[ECHA] European Chemicals Agency. 2021k. Brief profile: (isobornyl acrylate) Exo-1,7,7-trimethylbicyclo[2.2.1]hept-2-yl acrylate; CAS RN 5888-33-5. Helsinki (FI): ECHA. [updated 2021 Aug 09; accessed 2021 Oct 28].
[ECHA] European Chemicals Agency. 2021l. Brief profile, toxicological information: [3aR-(3aα,5aβ,9aα,9bβ)]-dodecahydro-3a,6,6,9a-tetramethylnaphtho[2,1-b]furan; CAS RN 6790-58-5. Helsinki (FI): ECHA. [updated 2020 Dec 21; accessed 2021 Aug 17].
[ECHA] European Chemicals Agency. 2022a. Brief profile: benzyl benzoate. CAS RN:120-51-4. Helsinki (FI): ECHA. [updated 2022 Jan 07; accessed 2022 Feb 14].
[ECHA] European Chemicals Agency. 2022b. Brief profile: (trans-methylisoeugenol) 4-trans-propenylveratrole. CAS RN: 6379-72-2. Helsinki (FI): ECHA. [updated 2022 Mar 01; accessed 2022 Mar 15].
[ECHA] European Chemicals Agency. 2022d. Brief profile: Linalool. CAS RN: 78-70-6. Helsinki (FI): ECHA. [accessed 2022 March 18].
[ECHA] European Chemicals Agency. 2022e. Brief profile: o-cresol; CAS RN:95-48-7. Helsinki (FI): ECHA. [updated 2022 Jan 07; accessed 2022 Feb 14].
[ECHA] European Chemicals Agency. 2022f. Brief profile: m-cresol; CAS RN: 108-39-4. Helsinki (FI): ECHA. [updated 2022 Jan 07; accessed 2022 Feb 14].
[ECHA] European Chemicals Agency. 2022g. Brief profile: p-cresol; CAS RN:106-44-5. Helsinki (FI): ECHA. [updated 2022 Jan 07; accessed 2022 Feb 14].
[ECHA] European Chemicals Agency. 2022h. Brief profile: 1-[4-[[2-O-(6-deoxy-α-L-mannopyranosyl)-β-Dglucopyranosyl]oxy]-2,6-dihydroxyphenyl]-3-(3-hydroxy-4-methoxyphenyl)propan-1-one; CAS RN 20702-77-6. Helsinki (FI): ECHA. [updated 2022 Jan 07; accessed 2022 Feb 14].
[ECHA] European Chemicals Agency. 2023. REACH registration dossier; CAS RN 5392-40-5. Helsinki (FI): ECHA. [updated 2023 May 19; accessed 2023 Jul 01]
[EC, HC] Environment Canada, Health Canada. 2010. Screening assessment for the Challenge: Benzene, 1,2-dimethoxy-4-(2-propenyl)-(Methyl eugenol): Chemical Abstracts Service Registry Number 93-15-2. Ottawa (ON): Government of Canada.
[EFSA] European Food Safety Authority. 2009. Flavouring group evaluation 78 (FGE.78). Consideration of aliphatic and alicyclic and aromatic hydrocarbons evaluated by JECFA (63rd meeting) structurally related to aliphatic and aromatic hydrocarbons evaluated by EFSA in FGE.25. Scientific opinion of the panel on food additives, flavourings, processing aids and materials in contact with food (AFC). EFSA J. 931:1-59.
[EFSA] European Food Safety Authority. 2010. Flavouring Group Evaluation 32 (FGE.32): Flavonoids (Flavanones and dihydrochalcones) from chemical groups 25 and 30. EFSA J. 8(9):1065.
[EFSA] European Food Safety Authority. 2012. Conclusion on pesticide peer review: conclusion on the peer review of the pesticide risk assessment of the active substance extract from tea tree. EFSA J. 10(2): 2542.
[EFSA] European Food Safety Authority. 2014. Scientific Opinion on Flavouring Group Evaluation 87, Revision 2 (FGE.87Rev2): Consideration of bicyclic secondary alcohols, ketones and related esters evaluated by JECFA (63rd meeting) structurally related to bicyclic secondary alcohols, ketones and related esters evaluated by EFSA in FGE.47Rev1 (2008). EFSA J. 12(10):3864
[EFSA] European Food Safety Authority. 2015. Scientific opinion on flavouring group evaluation 78, revision 2 (FGE.78Rev2): consideration of aliphatic and alicyclic and aromatic hydrocarbons evaluated by JECFA (63rd meeting) structurally related to aliphatic hydrocarbons evaluated by EFSA in FGE.25Rev3. EFSA J. 13(4):4067.
[EFSA] European Food Safety Authority. 2016. Safety and efficacy of α,β-unsaturated straight-chain and branched-chain aliphatic primary alcohols, aldehydes, acids and esters belonging to chemical group 3 when used as flavourings for all animal species. EFSA J. 8(9):1065
[EFSA] European Food Safety Authority. 2017. Update: use of the benchmark dose approach in risk assessment. EFSA J. 15(1):4658.
El Ashry ESH, Rashed N, Salama OM, Saleh A. 2003. Components, therapeutic value and uses of myrrh. Pharmazie. 58(3):163-168.
Elbetieha A, Al-Hamood MH, Alkofahi A, Bataineh H. 1998. Reproductive toxicity potentials of Salvia fruticosa (Labiatae) in rats. J Ethnopharmacol. 61(1):67-74.
El Mazoudy RH, Attia AA. 2012. Efficacy of Ginkgo biloba on vaginal estrous and ovarian histological alterations for evaluating anti-implantation and abortifacient potentials in albino female mice. Birth Defects Res B Dev Reprod Toxicol. 95(6):444-459
El-Shahat M, El-Abd S, Alkafafy M, El-Khatib G. 2012. Potential chemoprevention of diethylnitrosamine-induced hepatocarcinogenesis in rats: myrrh (Commiphora molmol) vs. turmeric (Curcuma longa). Acta Histochem. 114(5):421-428.
[EMA] European Medicines Agency. 2009. Assessment report on Salvia officinalis L., folium and Salvia officinalis L., aetheroleum. London (GB): EMA. Doc. Ref.: EMA/HMPC/330383/2008.
[EMA] European Medicines Agency. 2011. Assessment report on Commiphora molmol Engler, gummi-resina. London (GB): EMA. EMA/HMPC/96910/2010.
[EMA] European Medicines Agency. 2012. Public statement on the use of herbal medicinal products containing thujone [PDF]. London (GB): EMA. EMA/HMPC/732886/2010 Rev.1.
[EMA] European Medicines Agency. 2014. Assessment report on Ginkgo biloba L., folium. London (GB): EMA. EMA/HMPC/321095/2012.
[EMA] European Medicines Agency. 2017. Assessment report on Artemisia absinthium L., herba [PDF]. London (UK): European Medicines Agency. Doc. Ref: EMA/HMPC/751484/2016.
[EMA] European Medicines Agency. 2021. European Union herbal monograph on Artemisia absinthium L., herba [PDF]. London (UK): European Medicines Agency Report No. EMA/HMPC/751490/2016 Corr.
Environment Canada. 2013. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain substances on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
Environment Canada, Health Canada. 2014. Approach for identification of chemicals and polymers as risk assessment priorities under Part 5 of the Canadian Environmental Protection Act, 1999 (CEPA 1999). Ottawa (ON): Government of Canada.
[EPI Suite] Estimation Program Interface Suite for Microsoft Windows [estimation model]. c2000-2012. Ver. 4.11. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Fateh AH, Mohamed Z, Chik Z, Alsalahi A, Zin SRM, Alshawsh MA. 2019a. Prenatal developmental toxicity evaluation of Verbena officinalis during gestation period in female Sprague-Dawley rats. Chem Biol Interact. 304:28-42.
Fateh AH, Mohamed Z, Chik Z, Alsalahi A, Zin SRM, Alshawsh MA. 2019b. Mutagenicity and genotoxicity effects of Verbena officinalis leaves extract in Sprague-Dawley rats. J. Ethnopharmacol. 235:88-99.
Faust HR, Casserly EW. 2003. Petrolatum and regulatory requirements. In Penreco. NPRA International Lubricants & Waxes Meeting, November (pp. 13-14). Citeseer.
[FEMA] Flavor and Extract Manufacturers Association. c2022. Flavor Ingredient Library [database]. [accessed 2022 Feb 25].
Fernandes ES, Pinto RM, Reis JE, Guerra MdO, Peters VM. 2010. Effects of Ginkgo biloba extract on the embryo-fetal development in Wistar rats. Birth Defects Res B Dev Reprod Toxicol. 89(2):133-138
Ferreira-da-Silva FW, Barbosa R, Moreira-Júnior L, Dos Santos-Nascimento T, De Oliveira-Martins MD, Coelho-de-Souza AN, Cavalcante FSA, Ceccatto VM, De Lemos TLG, Magalhães PJC, et al. 2009. Effects of 1,8-cineole on electrophysiological parameters of neurons of the rat superior cervical ganglion. Clin Exp Pharmacol Physiol. 36(11):1068-1073.
Ficheux AS, Wesolek N, Chevillotte G, Roudot AC. 2015. Consumption of cosmetic products by the French population. First part: Frequency data. Food Chem Toxicol. 78:159-169.
Ficheux AS, Chevillotte G, Wesolek N, Morisset T, Dornic N, Bernard A, Bertho A, Romanet A, Leroy L, Mercat AC, et al. 2016. Consumption of cosmetic products by the French population second part: Amount data. Food Chem Toxicol. 90:130-141.
Fujii A, Okuyama T, Wakame K, Okumura T, Ikeya Y, Nishizawa M. 2017. Identification of anti-inflammatory constituents in Phellodendri Cortex and Coptidis Rhizoma by monitoring the suppression of nitric oxide production. J Nat Med. 71(4):745-756.
Garcia-Hidalgo E, von Goetz N, Siegrist M, Hungerbühler K. 2017. Use-patterns of personal care and household cleaning products in Switzerland. Food Chem Toxicol. 99:24-39.
Gaunt JF, Agrelo E, Colley J, Lansdown ABG, Grasso P. 1971. Short-term Toxicity of Isobornyl Acetate in Rats. Food Cosmet Toxicol. 9(3):355-366.
Gaworski CL, Vollmuth TA, York RG, Heck JD, Aranyj C. 1992. Developmental toxicity evaluation of inhaled citral in Sprague-Dawley rats. Food Chem Toxicol. 30:269-275.
Geller RJ, Spyker DA, Garrettson LK, Rogol AD. 1984. Camphor toxicity: development of a triage strategy. Vet Hum Toxicol. 26(Suppl 2):8-10.
Gibson DE, Moore GP, Pfaff JA. 1989. Camphor ingestion. Am J Emerg Med. 7(1):41-43.
Gilman EF, Watson DG. 1993. Ginkgo biloba: Maidenhair Tree. [place unknown]: US Forest Service; Southern Group of State Foresters. 3 p. Fact Sheet ST-273.
Gilpin S, Hui X, Maibach H. 2010. In vitro human skin penetration of geraniol and citronellol. Dermatitis 21(1):41-48.
Givaudan SA. 2002. European patent application: antibacterial composition comprising Sandela [PDF]. [place unknown]: European Patent Office. EP 1 181 866 A1. [accessed 2021 Sep 15].
Goel HC, Singh S, Adhikari JS, Rao AR. 1985. Radiomodifying effect of camphor on the spermatogonia of mice. Jpn J Exp Med. 55(6):219-223.
Goel HC, Roa AR. 1988. Radiosensitizing effect of camphor on transplantable mammary adenocarcinoma in mice. Cancer Lett. 43(1-2):21-27.
Goel HC, Singh S, Singh SP. 1989. Radiomodifying influence of camphor on sister-chromatid exchange induction in mouse bone marrow. Mutat Res. 224(2):157-160.
Gomes-Carneiro MR, Felzenszwalb I, Paumgartten FJR. 1998. Mutagenicity testing of (±)-camphor, 1,8-cineole, citral, citronellal, (-)-menthol and terpineol with the Salmonella / microsome assay. Mutat Res. 416(1-2):129-136.
Gomez-Berrada MP, Gautier F, Parent-Massin D, Ferret PJ. 2013. Retrospective exposure data for baby and children care products: an analysis of 48 clinical studies. Food Chem Toxicol. 57:185-194.
Gomez-Berrada MP, Ficheux AS, Dahmoul Z, Roudot AC, Ferret PJ. 2017. Exposure assessment of family cosmetic products dedicated to babies, children and adults. Food Chem Toxicol. 103:56-65.
[GoodScents] The Good Scents Company [database]. c2022a. Search results for 3407-42-9. [accessed 2022 Feb 14].
[GoodScents]. The Good Scents Company [database]. c2022b. Search results for 66068-84-6. [accessed 2022 Feb 14].
Gu M, Xu J, Han C, Kang Y, Liu T, He Y, Huang Y, Liu C. 2015. Effects of berberine on cell cycle, DNA, reactive oxygen species, and apoptosis in L929 murine fibroblast cells. Evid Based Complement Alternat Med. 2015:796306.
Gwinn WM, Auerbach SS, Parham F, Stout MD, Waidyanatha S, Mutlu E, Collins B, Paules RS, Merrick BA, Ferguson S, et al. 2020. Evaluation of 5-day in vivo rat liver and kidney with high-throughput transcriptomics for estimating benchmark doses of apical outcomes. Toxicol Sci. 176(2):343-354. Supplementary data; p. 353.
Halicioglu O, Astarcioglu G, Yaprak I, Aydinlioglu H. 2011. Toxicity of Salvia officinalis in a newborn and a child: an alarming report. Pediatr Neurol. 45(4):259-260.
Hall B, Tozer S, Safford B, Coroama M, Steiling W, Leneveu-Duchemin MC, McNamara C, Gibney M. 2007. European consumer exposure to cosmetic products, a framework for conducting population exposure assessments. Food Chem Toxicol 45(11):2097-2108.
Hanks GR, editor. 2002. Narcissus and daffodil: the genus narcissus. 1st ed. London (GB): CRC Press.
Hanuš LO, Řezanka T, Dembitsky VM, Moussaieff A. 2005. Myrrh – commiphora chemistry. Biomed
Pap Med Fac Univ Palacky Olomouc Czech Repub. 149(1):3-28.
[HC] Health Canada. 1994. Human health risk assessment for priority substances [PDF]. Ottawa (ON): Government of Canada. Cat. No.: En40-215/41E.
[HC] Health Canada. 2016. Science approach document: threshold of toxicological concern (TTC)-based approach for certain substances. Ottawa (ON): Government of Canada.
[HC] Health Canada. [modified 2019 Dec 03]. Cosmetic Ingredient Hotlist: list of ingredients that are prohibited for use in cosmetic products. Ottawa (ON): Government of Canada.
[HC] Health Canada. 2021. Canadian exposure factors used in human health risk assessments. [modified 2021-06-25]. Ottawa (ON): Government of Canada. [accessed 2022 Feb 8].
[HERA] Human and Environmental Risk Assessment. 2005. Guidance document methodology [PDF]. Brussels (BE): HERA Secretariat.
Hirono I, Ueno I, Hosaka S, Takanashi H, Matsushima T, Sugimura T, Natori S. 1981. Carcinogenicity examination of quercetin and rutin in ACI rats. Cancer Lett. 13(1):15-21.
Hirose M, Yamaguchi S, Fukushima S, Hasegawa R, Takahashi S. Ito N. 1989. Promotion by dihydroxybenzene derivatives of N-methyl-N′-nitro-N-nitrosoguanidine-induced F344 rat forestomach and glandular stomach carcinogenesis. Cancer Res. 49(18):5143-5147.
Hodaj-Celiku E, Tsiftsoglou O, Shuka L, Abazi S, Hadjipavlou-Litina D, Lazari D. 2017. Antioxidant activity and chemical composition of essential oils of some aromatic and medicinal plants from Albania. Nat Prod Commun. 12(5):785-790.
Horváthová E, Turcaniova V, Slameňová D. 2007. Comparative study of DNA-damaging and DNA-protective effects of selected components of essential plant oils in human leukemic cells K562. Neoplasma. 54(6):478-483.
Horváthová E, Slameňová D, Maršálková L, Šramková M, Wsólová L. 2009. Effects of borneol on the level of DNA damage induced in primary rat hepatocytes and testicular cells by hydrogen peroxide. Food Chem Toxicol. 47(6):1318-1323.
Horváthová E, Kozics K, Srančíková A, Hunáková L, Gálová E, Ševčovičová A, Slameňová D. 2012. Borneol administration protects primary rat hepatocytes against exogenous oxidative DNA damage. Mutagenesis. 27(5):581-588.
Horváthová E, Navarova J, Galova E, Ševčovičová A, Chodakova L, Snahnicanova Z, Melusova M, Kozics K, Slamenova D. 2014. Assessment of antioxidative, chelating, and DNA-protective effects of selected essential oil components (eugenol, carvacrol, thymol, borneol, eucalyptol) of plants and intact Rosmarinus officinalis oil. J Agric Food Chem. 62(28):6632-6639.
Horváthová E, Srančíková A, Regendová-Sedláčková E, Melušová M, Meluš V, Netriová J, Krajčovičová Z, Slameňová D, Pastorek M, Kozics K. 2016. Enriching the drinking water of rats with extracts of Salvia officinalis and Thymus vulgaris increases their resistance to oxidative stress. Mutagenesis. 31(1):51-59.
Hu LM. 2005. Experimental compare of synthetic borneol and D-borneal on PCE micronucleus in mice. Chin Trad Herb Drugs. 36(4):571-572.
[IARC] International Agency for Research on Cancer. 2013. Some chemicals present in industrial and consumer products, food and drinking-water. Lyon (FR): IARC. (IARC monographs on the evaluation of carcinogenic risks to humans; vol. 101).
[IARC] International Agency for Research on Cancer. 2014. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans [PDF]. Report of the Advisory Group to Recommend Priorities for IARC Monographs during 2015-2019. Lyon (FR).
[IARC] International Agency for Research on Cancer. 2016. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Ginkgo biloba. IARC Monogr Eval Carcinog Risks Hum. 108: 91-116.
[IFRA] International Fragrance Association. 2013. IFRA standard: Cade oil. Geneva (SZ): The international fragrance association. [accessed 2021 Dec 2]
[IFRA] International Fragrance Association. 2021. International Fragrance Association Ingredient List [database]. [accessed 2017 Jun].
Inbaraj JJ, Kukielczak BM, Bilski P, He YY, Sik RH, Chignell CF. 2006. Photochemistry and photocytotoxicity of alkaloids from Goldenseal (Hydrastis canadensis L.). 2. Palmatine, hydrastine, canadine, and hydrastinine. Chem Res Toxicol. 19(6):739-744.
InformedHealth.org. 2017. Cologne, Germany: Institute for Quality and Efficiency in Health Care (IQWiG); 2006-. Eczema: Overview [Internet]. 2013 Sep 26 [updated 2017 Feb 23].
[IQWiG] Institute for Quality and Efficiency in Health Care. 2017. Eczema: Steroids and other topical medications. Berlin (GE): Institute for Quality and Efficiency in Health Care
Jagetia GC, Rao. 2015. Isoquinoline alkaloid berberine exerts its antineoplastic activity by inducing molecular DNA damage in HeLa cells: a COMET assay study. Biol Med (Aligarh). 7(1):1000223.
Jahnke GD, Price CJ, Marr MC, Myers CB, George JD. 2006. Developmental toxicity evaluation of berberine in rats and mice. Birth Defects Res B Dev Reprod Toxicol. 77(3):195-206.
Jakovljević M, Jokić S, Molnar M, Jašić M, Babić J, Jukić H, Banjari I. 2019. Bioactive profile of various Salvia officinalis L. preparations. Plants (Basel). 8(3):55.
James MA, Fu H, Liu Y, Chen DR, You M. 2011. Dietary administration of berberine or Phellodendron amurense extract inhibits cell cycle progression and lung tumorigenesis. Mol Carcinog. 50(1):1-7.
[JECFA] Joint FAO/WHO Expert Committee on Food Additives. 2004a. Safety evaluation of certain food additives and contaminants. Geneva (CH): World Health Organization, International Programme on Chemical Safety. (WHO Food Additive Series 52). Prepared by the sixty-first meeting of the JECFA.
[JECFA] Joint FAO/WHO Expert Committee on Food Additives. 2004b. Evaluation of certain food additives and contaminants [PDF]. Geneva (CH): World Health Organization. (WHO Technical Report Series 922). Sixty-first report of the JECFA.
[JECFA] Joint FAO/WHO Expert Committee on Food Additives. 2004c. Safety evaluation of certain food additives and contaminants. [PDF] Who Food Additives Series: 61. Geneva: World Health Organization.
Judzentiene A, Tomi F, Casanova J. 2009. Analysis of essential oils of Artemisia absinthium L from Lithuania by CC, GC(RI), GC-MS and 13C NMR. Nat Prod Commun. 4(8):1113-1118.
Juteau F, Jerkovic I, Masotti V, Milos M, Mastelic J, Bessière JM, Viano J. 2003. Composition and antimicrobial activity of the essential oil of Artemisia absinthium from Croatia and France. Planta Med. 69(2):158-161.
Kamimura S, Nishihara M, Osumi Y, Shiota H. 2019. Simultaneous quantitative analysis of berberine and other alkaloids in powdered Phellodendron bark. Yakugaku Zasshi. 139(11):1471-1478.
Kampf G, Ruselack S, Eggerstedt S, Nowak N, Bashir M. 2013. Less and less-influence of volume on hand coverage and bactericidal efficacy in hand disinfection. BMC Infect Dis. 13:472.
Kauffman RE, Banner W Jr, Berlin CM Jr, Blumer JL, Gorman RL, Lambert GH, Wilson GS. 1994. Camphor revisited: focus on toxicity (RE9422). Pediatrics. 94(1):127-128.
Kobayashi D, Yoshimura T, Johno A, Sasaki K, Wada K. 2011. Toxicity of 4′-O-methylpyridoxine-5′-glucoside in Ginkgo biloba seeds. Food Chem. 126(3):1198-1202.
Koch E, Nöldner M, Leuschner J. 2013. Reproductive and developmental toxicity of the Ginkgo biloba special extract EGb 761® in mice. Phytomedicine. 21(1):90-97.
Koçkaya EA, Kιlιç A. 2014. Developmental toxicity of benzyl benzoate in rats after maternal exposure throughout pregnancy. Environ Toxicol. 29(1):40-53.
Kolya Naturals. [accessed 2022 Feb 25]. Earth's Aromatique | Myrrh Gum Resin.
Kordali S, Cakir A, Mavi A, Kilic H, Yildirim A. 2005. Screening of chemical composition and antifungal and antioxidant activities of the essential oils from 3 Turkish Artemisia species. J Agric Food Chem. 53(5):1408-1416.
Kou WZ, Yang J, Yang QH, Wang Y, Wang ZF, Xu SL, Liu J. 2013. Study on in-vivo anti-tumor activity of Verbena officinalis extract. Afr J Tradit Complement Altern Med. 10(3):512-517.
Koruk ST, Ozyilkan E, Kaya P, Colak D, Donderici O, Cesaretli Y. 2005. Juniper tar poisoning. Clin Toxicol (Phila). 43(1):47-49.
Kowalski Z, Dutkiewicz T, Szymczykiewicz K. 1962. Investigations on value of the maximum allowable concentration of natural essential oils in the air. Med Pr. 13(2):69-84.
Kubica P, Szopa A, Dominiak J, Luczkiewicz M, Ekiert H. 2020. Verbena officinalis (common vervain) - A review on the investigations of this medicinally important plant species. Planta Med. 86(17):1241-1257.
Kumar AP, Bhaskaran S, Ganapathy M, Crosby K, Davis MD, Kochunov P, Schoolfield J, Yeh IT, Troyer DA, Ghosh R. 2007. Akt/cAMP-responsive element binding protein/cyclin D1 network: a novel target for prostate cancer inhibition in transgenic adenocarcinoma of mouse prostate (TRAMP) model mediated by Nexrutine, a Phellodendron amurense bark extract. Clin Cancer Res. 13(9):2784-2794.
Kumar A, Ekavali, Chopra K, Mukherjee M, Pottabathini R, Dhull DK. 2015. Current knowledge and pharmacological profile of berberine: an update. Eur J Pharmacol. 761:288-297
Kurata Y, Fukushima S, Hagiwara A, Ito H, Ogawa K, Ito N. 1990. Carcinogenicity study of methyl hesperidin in B6C3F1 mice. Food Chem. Toxicol. 28(9):613-618.
[Laboratoire Phytochemia] 2017. Report on Narcissus jonquilla essential oil analysis. Chicoutimi (QC)
Lachenmeier DW. 2010. Wormwood (Artemisia absinthium L.)—A curious plant with both neurotoxic and neuroprotective properties? (letter to the editor). J Ethnopharmacol. 131: 224–227.
Lachenmeier DW, Uebelacker M. 2010. Risk assessment of thujone in foods and medicines containing sage and wormwood – evidence for a need of regulatory changes? Regul Toxicol Pharmacol. 58(3):437-443.
[LactMed®] Drugs and Lactation Database [database]. 2006- Bethesda (MD): US National Library of Medicine.
Lamichhane R, Lee KH, Pandeya PR, Sung KK, Lee S, Kim YK, Jung HJ. 2019. Subcutaneous injection of myrrh essential oil in mice: acute and subacute toxicity study. Evid Based Complement Alternat Med. doi: 10.1155/2019/8497980.
Lee EB. 1982. Teratogenicity of the extracts of crude drugs. Korean J Pharmacogn. 13(3):116-121.
Lee SH, Lee HJ, Lee SH, Kim YS, Lee D, Chun J, Lee JY, Kim H, Chang GT. 2018. Effects of Huang Bai (Phellodendri Cortex) on bone growth and pubertal development in adolescent female rats. Chin Med. 13:3.
Leistner E, Drewke C. 2010. Ginkgo biloba and ginkgotoxin. J Nat Prod. 73(1):86-92.
Leuschner J. 1997. Reproductive toxicity studies of D-camphor in rats and rabbits. Arzneimittelforschung. 47(2):124-128.
Li WR, Chen RY, Yang L, Huang TL, Xu QW, Mi SQ, Wang NS. 2012. Pharmacokinetics of natural borneol after oral administration in mice brain and its effect on excitation ratio. Eur J Drug Metab Pharmacokinet. 37(1):39-44.
Lim HB, Kim DH. 2018. Effect of heat treatment on 4′‐O‐methylpyridoxine (MPN) content in Ginkgo biloba seed extract solution. J Sci Food Agric. 98(13):5153-5156.
Linn YC, Lu J, Lim LC, Sun H, Sun J, Zhou Y, Ng HS. 2012. Berberine-induced haemolysis revisited: safety of Rhizoma coptidis and Cortex phellodendri in chronic haematological diseases. Phytother Res. 26(5):682-686.
Liu YM, Sheu SJ, Chiou SH, Chang HC, Chen YP. 1993. A comparative study on commercial samples of Phellodendri cortex. Planta Med. 59(6):557-561.
Liu Y, Hu L, Yi Y, Fan X, Du R, Zhang B. 2004. A comparative study on the mutagenicity of natural borneol and synthetic borneol. Trad Chin Drug Res Clin Pharmacol. 6:233-235.
Liu Z, Liu Q, Xu B, Wu J, Guo C, Zhu F, Yang Q, Gao G, Gong Y, Shao C. 2009. Berberine induces p53-dependent cell cycle arrest and apoptosis of human osteosarcoma cells by inflicting DNA damage. Mutat Res. 662(1-2):75-83.
Liu Z, Xu Z, Zhou H, Cao G, Cong XD, Zhang Y, Cai BC. 2012. Simultaneous determination of 4 bioactive compounds in Verbena officinalis L. by using high-performance liquid chromatography. Pharmacogn Mag. 8(30):162-165.
[LNHPD] Licensed Natural Health Products Database [database]. [modified 2021 Aug 10]. Ottawa (ON): Government of Canada. [accessed 2022 Oct 19].
Loizzo MR, Tundis R, Conforti F, Saab, AM, Statti GA, Menichini F. 2007. Comparative chemical composition, antioxidant and hypoglycaemic activities of Juniperus oxycedrus ssp. oxycedrus L. berry and wood oils from Lebanon. Food Chemistry. 105(2): 572-578
Lopes-Lutz D, Alviano DS, Alviano CS, Kolodziejczyk PP. 2008. Screening of chemical composition, antimicrobial and antioxidant activities of Artemisia essential oils. Phytochemistry. 69(8):1732-1738.
Lopez TK, Jones K, Roseberry-Lincoln A, Zidek A, MacKinnon M, Marro L. 2022. Adult and children's use of hand sanitizer during a pandemic - an observational study. J Expo Sci Environ Epidemiol. 1-9.
Loretz LG, Api AM, Barraj LM, Burdick J, Dressler WE, Gettings SD, Han Hsu H, Pan YHL, Re TA, Renskers KJ, et al. 2005. Exposure data for cosmetic products: lipstick, body lotion, and face cream. Food Chem Toxicol. 43(2):279-291.
Loretz L, Api AM, Barraj L, Burdick J, Davis DA, Dressler W, Gilberti E, Jarrett G, Mann S, Pan YHL, Re T, et al. 2006. Exposure data for personal care products: hairspray, spray perfume, liquid foundation, shampoo, body wash, and solid antiperspirant. Food Chem Toxicol. 44(12):2008-2018.
Loretz LG, Api AM, Babcock L, Barraj LM, Burdick J, Cater KC, Jarrett G, Mann S, Pan YHL, Re TA, et al. 2008. Exposure data for cosmetic products: facial cleanser, hair conditioner, and eye shadow. Food Chem Toxicol. 46(5):1516-1524.
Love JN, Sammon M, Smereck J. 2004. Are 1 or 2 dangerous? camphor exposure in toddlers. J Emerg Med. 27(1):49-54.
Macinga DR, Edmonds SL, Campbell E, Shumaker DJ, Arbogast JW. 2013. Efficacy of novel alcohol-based hand rub products at typical in-use volumes. Infect Control Hosp Epidemiol. 34(3):299-301.
Mahboubi M, Kashani LMT. 2016. The anti-dermatophyte activity of Commiphora molmol. Pharm Biol. 54(4):720-725.
Mahmoud AM, Zaki AR, Hassan ME, Mostafa-Hedeab G. 2017. Commiphora molmol resin attenuates diethylnitrosamine/phenobarbital-induced hepatocarcinogenesis by modulating oxidative stress, inflammation, angiogenesis and Nrf2/ARE/HO-1 signaling. Chem Biol Interact. 270:41-50.
Margot C, Simmons DP, Reichlin D, Skuy D. 2004. Amber-woody scent: alcohols with divergent structure present common olfactory characteristics and sharp enantiomer differentiation. Helv Chim Acta. 87(10):2662-2684.
Massoud AM, El-Ashmawy IM, Hemeda SA, Salama OM. 2000. Hematological, chromosomal and teratogenic studies of a new schistosomicidal agent derived from myrrh. Alex J Pharm Sci. 14(1):61-68.
Massoud AM, El-Ashmawy IM, Nasr MA, Salama OM. 2002. Mirazid; a new schistosomicidal agent derived from Myrrh: studies on its influence on male reproductive organs and bile flow. Alex J Pharm Sci. 16(2):82-87.
Mathlouthi A, Saadaoui N, Ben-Attia M. 2021. Essential oils from Artemisia species inhibit biofilm formation and the virulence of Escherichia coli EPEC 2348/69. Biofouling. 37(2):174-183.
Mei N, Guo X, Ren Z, Kobayashi D, Wada K, Guo L. 2017. Review of Ginkgo biloba-induced toxicity, from experimental studies to human case reports. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 35(1):1-28.
[MHW, Japan] Ministry of Health, Labour and Welfare: Japan. 2002. Toxicity Testing Reports of Environmental Chemicals, 9 [Cited in: [OECD/SIDS] The Organisation for Economic Co-operation and Development/Screening Information Dataset. 2001. Screening Information Dataset Initial Assessment Profile: Citral [PDF]. Switzerland, 2001].
Mihajilov-Krstev T, Jovanović B, Jović J, Ilić B, Miladinović D, Matejić J, Rajković J, Đorđević L, Cvetković V, Zlatković B. 2014. Antimicrobial, antioxidative, and insect repellent effects of Artemisia absinthium essential oil. Planta Med. 80(18):1698-1705.
Millet Y, Jouglard J, Steinmetz MD, Tognetti P, Joanny P, Arditti J. 1981. Toxicity of some essential plant oils. clinical and experimental study. Clin Toxicol (Phila). 18(12):1485-1498.
Mitsumoto T, Ishii Y, Namiki M, Nakamura K, Takasu S, Ogawa K. 2021. A 90-day subchronic toxicity study of Myrrh in F344 rats. Reg Toxicol Pharmacol. 127:105076.
Monzote L, Piñón A, Scull R, Setzer WN. 2014. Chemistry and leishmanicidal activity of the essential oil from Artemisia absinthium from Cuba. Nat Prod Commun. 9(12):1799-1804.
Morimoto I, Watanabe F, Osawa T, Okitsu T, Kada T. 1982. Mutagenicity screening of crude drugs with Bacillus subtilis rec-assay and Salmonella/microsome reversion assay. Mutat Res. 97(2):81-102.
Morino K, Matsukura N, Kawachi T, Ohgaki H, Sugimura T, Hirono I. 1982. Carcinogenicity test of quercetin and rutin in golden hamsters by oral administration. Carcinogenesis. 3(1):93-97.
[MSDS] Material Safety Data Sheet [accessed 2022 Aug 15]. Jonquil absolute oil Product code: PAO2052. [PDF].
Mühlbauer RC, Lozano A, Palacio S, Reinli A, Felix R. 2003. Common herbs, essential oils, and monoterpenes potently modulate bone metabolism. Bone. 32(4):372-380.
Muto T, Watanabe T, Okamura M, Moto M, Kashida Y, Mitsumori K. 2003. Thirteen-week repeated dose toxicity study of wormwood (Artemisia absinthium) extract in rats. J Toxicol Sci. 28(5):471-478.
Našel C, Našel B, Samec P, Schindler E, Buchbauer G. 1994. Functional imaging of effects of fragrances on the human brain after prolonged inhalation. Chem Senses. 19(4):359-364.
[NHPID] Natural Health Products Ingredients Database [database]. [modified 2022 Mar 07]. Ottawa (ON): Government of Canada.
Nicolella HD, De Oliveira PF, Munari CC, Costa GFD, Moreira MR, Veneziani RCS, Tavares DC. 2014. Differential effect of manool – a diterpene from Salvia officinalis, on genotoxicity induced by methyl methanesulfonate in V79 and HepG2 cells. Food Chem Toxicol. 72:8-12.
Nicolella HD, Fernandes G, Ozelin SD, Rinaldi-Neto F, Ribeiro AB, Furtado RA, Senedese JM, Esperandim TR, Veneziani RCS, Tavares DC. 2021. Manool, a diterpene from Salvia officinalis, exerts preventive effects on chromosomal damage and preneoplastic lesions. Mutagenesis. 36(2):177-185.
Nikolić B, Mitić-Ćulafić D, Vuković-Gačić B, Knežević-Vukčević J. 2011. Modulation of genotoxicity and DNA repair by plant monoterpenes camphor, eucalyptol and thujone in Escherichia coli and mammalian cells. Food Chem Toxicol. 49(9):2035-2045.
Nikolić B, Vasilijević B, Mitić-Ćulafić D, Vuković-Gačić B, Knežević-Vukćević J. 2015. Comparative study of genotoxic, antigenotoxic and cytotoxic activities of monoterpenes camphor, eucalyptol and thujone in bacteria and mammalian cells. Chem Biol Interact. 242:263-271
Nogueira ACMA, Carvalho RR, Souza CAM, Chahoud I, Paumgartten FJR. 1995. Study on the embryofeto-toxicity of citral in the rat. Toxicology. 96(2):105-113.
Nozaka T, Watanabe F, Tadaki SI, Ishino M, Morimoto I, Kunitomo JI, Ishii H, Natori S. 1990. Mutagenicity of isoquinoline alkaloids, especially of the aporphine type. Mutat Res. 240(4):267-279.
[NTP] National Toxicology Program (US). 1992a. Cytogenetic study of d-camphor in Chinese hamster ovary cell chromosome aberrations test. Research Triangle Park (NC): US Department of Health and Human Services, National Toxicology Program.
[NTP] National Toxicology Program (US). 1992b. Prenatal developmental toxicity study (TER91018) of d-camphor (464-49-3) in Sprague-Dawley rats exposed via gavage. Research Triangle Park (NC): US Department of Health and Human Services, National Toxicology Program.
[NTP] National Toxicology Program (US). 1992c. Prenatal developmental toxicity study (TER91019) of d-camphor (464-49-3) in New Zealand White rabbits exposed via gavage. Research Triangle Park (NC): US Department of Health and Human Services, National Toxicology Program.
[NTP] National Toxicology Program (US). 2000. NTP Technical Report on the toxicology and carcinogenesis studies of methyl eugenol (CAS No. 93-15-2) in F344/N rats and B6C3F1 mice (gavage studies). Research Triangle Park (NC): US Department of Health and Human Services, National Toxicology Program. NTP Technical Report 491. NIH Publication No. 00-3950.
[NTP] National Toxicology Program (US). 2003. NTP Technical Report on the toxicology and carcinogenesis studies of citral (microencapsulated) in F344/N rats and B6C3F1 mice. [PDF] Research Triangle Park (NC): US Department of Health and Human Services, National Toxicology Program. NTP Toxicity Report 505.
[NTP] National Toxicology Program (US). 2010. NTP Technical Report on the toxicology and carcinogenesis studies of β-myrcene (CAS No. 123-35-3) in F344/N rats and B6C3F1 mice (gavage studies). NTP TR557. Research Triangle Park (NC): US Department of Health and Human Services, National Toxicology Program.
[NTP] National Toxicology Program (US). 2011. NTP Technical Report on the toxicology and carcinogenesis studies of α,β-thujone (CAS No. 76231-76-0) in F344/N rats and B6C3F1 mice (gavage studies). NTP TR570. Research Triangle Park (NC): US Department of Health and Human Services, National Toxicology Program.
[NTP] National Toxicology Program (US). 2013. NTP Technical Report on the toxicology and carcinogenesis studies of Ginkgo biloba extract (CAS NO. 90045-36-6) in F344/N rats and B6C3F1/N mice. Research Triangle Park (NC): US Department of Health and Human Services, National Toxicology Program.
[NTP] National Toxicology Program (US). 2014a. 3-month evaluation of the toxicity (C87003) of DL-camphor (76-22-2) in F344 rats exposed via topical application. NTP special study. Research Triangle Park (NC): US Department of Health and Human Services, National Toxicology Program.
[NTP] National Toxicology Program (US). 2014b. 3-month evaluation of the toxicity (C87003) of DL-camphor (76-22-2) in B6C3F1 mice rats exposed via topical application. NTP special study. Research Triangle Park (NC): US Department of Health and Human Services, National Toxicology Program.
[NTP] National Toxicology Program (US). 2016. Technical Report on the toxicity studies of cedarwood oil (Virginia) (CASRN 8000-27-9) administered dermally to F344/N rats and B6C3F1/N mice. Research Triangle Park (NC): US Department of Health and Human Services, National Toxicology Program.
[NTP] National Toxicology Program (US). 2018a. Genetic toxicity evaluation of berberine chloride in salmonella/E.coli mutagenicity test or Ames test. Study A24049 [PDF]. Research Triangle Park (NC): US Department of Health and Human Services, National Toxicology Program.
[NTP] National Toxicology Program (US). 2018b. Genetic toxicity evaluation of berberine chloride (633-65-8) in micronucleus study A64425 in B6C3F1 mice [PDF]. Research Triangle Park (NC): US Department of Health and Human Services, National Toxicology Program.
[NTP] National Toxicology Program (US). 2018c. Genetic toxicity evaluation of d-camphor in salmonella/E.coli mutagenicity test or AMES test. Study 374268 [PDF]. Research Triangle Park (NC): US Department of Health and Human Services, National Toxicology Program.
[NTP] National Toxicology Program (US). 2018d. Genetic toxicity evaluation of DL-camphor (76-22-2) in micronucleus study A69097 in B6C3F1 mice [PDF]. Research Triangle Park (NC): US Department of Health and Human Services, National Toxicology Program.
[NTP] National Toxicology Program (US). 2020. NTP Technical Report on the toxicity studies of a gum guggul extract formulation administered by gavage to Sprague Dawley (HSD: Sprague Dawley® SD®) rats and B6C3F1/N mice. NTP Tox 99. Research Triangle Park (NC): US Department of Health and Human Services, National Toxicology Program.
[OECD] Organisation for Economic Co-operation and Development. 2001. Screening Information Dataset Initial Assessment Profile: Citral [PDF]. Paris (FR): OECD
[OECD] Organisation for Economic Co-operation and Development. 2009. Emission scenario document on coating industry (paints, lacquers and varnishes) [PDF]. Paris (FR): OECD, Environment Directorate. (Series on Emission Scenario Documents No. 22; Report No.: ENV/JM/MONO(2009)24, JT03267833).
OECD QSAR Toolbox [read-across tool]. 2014. Ver. 3.3. Paris (FR): Organisation for Economic Co-operation and Development, Laboratory of Mathematical Chemistry.
OECD QSAR Toolbox [read across tool]. 2017. Ver. 4.2. Paris (FR): Organisation for Economic Co-operation and Development, Laboratory of Mathematical Chemistry.
[OEHHA] Office of Environmental Health Hazard Assessment. 2018. Chemical Listed Effective March 27, 2015 as Known to the State of California to Cause Cancer: Beta-Myrcene.
Omar A, Elmesallamy GES, Eassa S. 2005. Comparative study of the hepatotoxic, genotoxic and carcinogenic effects of praziquantel distocide & the natural myrrh extract Mirazid® on adult male albino rats. J Egypt Soc Parasitol. 35(1):313-329.
Omer SA, Adam SE, Khalid HE. 1999. Effects on rats of Commiphora myrrha extract given by different routes of administration. Vet Hum Toxicol. 41(4):193-196.
Oser BL, Ford RA. 1975. Recent progress in the consideration of flavouring ingredients under the food additives amendment, 9. GRAS Substances. Food Technol. 70-72.
Oyajobi BO, Gupta A, Mann M, Kumar AP. 2011. Abstract 4216: antitumor effect of Nexrutine, a phellodendron amurense bark extract, in multiple myeloma. Cancer Res. 71(Suppl 8): Abstract nr 4216.
Pages N, Fournier G, Baduel C, Tur N, Rusnac M. 1996. Sabinyl acetate, the main component of Juniperus Sabina L’Herit. essential oil, is responsible for antiimplantation effect. Phytother Res. 10(5): 438-440.
Panten J, Surburg H, Hölscher B. 2014. Recent results in the search for new molecules with ambergris odor. Chem Biodivers. 11(10):1639-1650.
Parchem. [accessed 2022 Feb 14]. Isobornyl cyclohexanol. New Rochelle (NY): Parchem fine & speciality chemicals.
Patenković A, Stamenkovic-Radak M, Banjanac T, Andjelkovic M. 2009. Antimutagenic effect of sage tea in the wing spot test of Drosophila melanogaster. Food Chem Toxicol. 47(1):180-183.
Paumgartten FJR, De-Carvalho RR, Souza CAM, Madi K, Chahoud I. 1998. Study of the effects of ß-myrcene on rat fertility and general reproductive performance. Braz J Med Biol Res. 31(7):955-965.
[PCPC] Personal Care Products Council [database]. c2022. Washington (DC): Personal Care Products Council. [accessed 2022 Feb 14].
Pelkonen O, Abass K, Wiesner J. 2013. Thujone and thujone-containing herbal medicinal and botanical products: toxicological assessment. Regul Toxicol Pharmacol. 65(1):100-107.
Penta Manufacturing. [accessed 2022 Feb 14]. Product search: 68877-29-2. Livingston(NJ): Penta Manufacturing Company
PerfumersWorld. [accessed 2022 Feb 14]. Sandela 50. PerfumersWorld.
Perveen S. 2018. Introductory Chapter: Terpenes and Terpenoids. In: IntechOpen. [accessed 2022 Feb 14].
Pinto RM, Fernandes ES, Reis JE, Peters VM, Guerra MDO. 2007. Intra-uterine growth retardation after prenatal administration of Ginkgo biloba to rats. Reprod Toxicol. 23(4):480-485.
Politano VT, Lewis EM, Hoberman AM, Diener RM, Api AM, Patel A. 2017. Oral 1-Generation Rat Reproduction Study of Isobornyl Acetate: An Evaluation Through Sexual Maturity in the F1 Generation. Int J Toxicol. 36(3):252-259.
Porte A, Godoy RLO, Maia-Porte LH. 2013. Chemical composition of sage (Salvia officinalis L.) essential oil from the Rio de Janeiro State (Brazil). Rev Bras Pl Med. 15(3):438-441.
Poucher WA. 1993. Perfumes, cosmetics and soaps, volume II: the production, manufacture and application of perfumes. 9th ed. London (GB): Chapman & Hall.
PubChem. c2022a. Compound summary for CID 637531, Isobornyl acetate. National centre for biotechnology information. Bethesda (MD). [accessed 2022 Feb 9].
PubChem. c2022b. Compound summary for CID 639970, Isobornyl acrylate. National centre for biotechnology information. Bethesda (MD). [accessed 2022 Feb 9].
PubChem. c2022c. Compound summary for CID 638011, Citral. National centre for biotechnology information. Bethesda (MD). [accessed 2022 Feb 25].
PubChem. c2022d. Compound Summary for CID 111394, Bornylcyclohexanol. National centre for biotechnology information. Bethesda (MD). [accessed 2022 Feb 14].
PubChem. c2022e. Compound Summary for CID 103005. Isocamphylcyclohexanol. National centre for biotechnology information. Bethesda (MD). [accessed 2022 Feb 14].
PubChem. c2022f. Compound summary for CID 261491. Thujone. National centre for biotechnology information. Bethesda (MD). [accessed 2022 Feb 25].
Purchase R, Ford GP, Creasy DM, Brantom PG, Gangolli SD.1992. A 28-day feeding study with methyl isoeugenol in rats. Food Chem Toxicol. 30(6):475-481.
Qian Y, Peng Y, Shang E, Zhao M, Yan L, Zhu Z, Tao J, Su S, Guo S, Duan JA. 2017. Metabolic profiling of the hepatotoxicity and nephrotoxicity of Ginkgolic acids in rats using ultra-performance liquid chromatography-high-definition mass spectrometry. Chem Biol Interact. 273(1):11-17.
Qureshi S, Al-Harbi MM, Ahmed MM, Raza M, Giangreco AB, Shah AH. 1993. Evaluation of the genotoxic, cytotoxic, and antitumor properties of Commiphora molmol using normal and Ehrlich ascites carcinoma cell-bearing Swiss albino mice. Cancer Chemother Pharmacol. 33(2):130-138.
Raal A, Orav A, Arak E. 2007. Composition of the essential oil of Salvia officinalis L. from various European countries. Nat Prod Res. 21(5):406-411.
Raj PS. 2021. Safety assessment of Salvia officinalis (sage)-derived ingredients as used in cosmetics [PDF]. Washington (DC): Cosmetic Ingredient Review. [accessed 2022 Feb 25].
Ramirez-Martinez A, Granda-Torres P, Wesolek N, Ficheux AS, Roudot AC. 2015. Exposure of hairdressers to the main cosmetics used in hairdressing salons in France: a preliminary study. Arch Environ Occup Health. 71(5):247-258.
Rao VSN, Menezes AMS, Gadelha MGT. 1988. Antifertility screening of some indigenous plants of Brasil. Fitoterapia. 59(1):17-20.
Rao RM, Khan ZA, Shah AH. 2001. Toxicity studies in mice of Commiphora molmol oleo-gum-resin. J Ethnopharmacol. 76(2):151-154.
Rehecho S, Hidalgo O, García-Iñiguez de Cirano M, Navarro I, Astiasarán I, Ansorena D, Cavero RY, Calvo MI. 2011. Chemical composition, mineral content and antioxidant activity of Verbena officinalis L. LWT – Food Sci Technol. 44(4):875-882
Reincke-Fichtner. [accessed 2022 Feb 14]. Products: essential oils aromatic-chemicals extracts. Reincke und Fichtner GmbH.
Ress NB, Hailey JR, Maronpot RR, Bucher JR, Travlos GS, Haseman JK, Hejtmancik MR. 2003. Toxicology and carcinogenesis studies of microencapsulated citral in rats and mice. Toxicol Sci. 71(2):198-206.
Rezaei AA, Salehi I, Karimi SA, Rahnama M. 2020. The effects of Commiphora mukul extract on spermatogenesis and testosterone levels in male diabetic rats. Clin Exp Reprod Med. 47(1):34-41.
Ribeiro DA, Marques MEA, Salvadori DMF. 2006. In vitro cytotoxic and non-genotoxic effects of gutta-percha solvents on mouse lymphoma cells by single cell gel (comet) assay. Braz Dent J. 17(3):228-232.
Ribeiro DA, Matsumoto MA, Marques MEA, Salvadori DMF. 2007. Biocompatibility of gutta-percha solvents using in vitro mammalian test-system. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 103(5):e106-e109.
Rietjens IMCM, Slob W, Galli C, Silano V. 2008. Risk assessment of botanicals and botanical preparations intended for use in food and food supplements: emerging issues. Toxicol Lett. 180(2):131-136.
[RIFM] Research Institute for Fragrance Materials. 2020. RIFM fragrance ingredient safety assessment, citral, CAS registry number 5392-40-5 [PDF]. Food Chem Toxicol. 141:111339.
[RIFM] Research Institute for Fragrance Materials. 2021a. RIFM fragrance ingredient safety assessment,1-(2,2,6-trimethylcyclohexyl)-3-hexanol, CAS registry number 70788-30-6 [PDF]. Food Chem Toxicol. 153:112358.
[RIFM] Research Institute for Fragrance Materials. 2021b. RIFM fragrance ingredient safety assessment, naphtho[2,1-b]furan, dodecahydro-3a,6,6,9a-tetramethyl-, (3aR,5aS,9aS,9bR)-, CAS registry number 6790-58-5. Food Chem Toxicol. 156:112454.
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment (NL)]. 2010. New default values for the spray model [PDF]. Bilthoven (NL): RIVM, March 2010.
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment (NL)]. 2018. Cleaning products fact sheet: default parameters for estimating consumer exposure - Updated version 2018 [PDF]. Report No. 320104003/2006.
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment (NL)]. 2021a. Health risk assessment of ethanol-containing hand sanitizer [PDF]. Bilthoven (NL): RIVM. Report No.: 2021-0026.
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment (NL)]. 2021b. Air fresheners fact sheet [PDF]. Bilthoven (NL): RIVM. Report No.: 2021-0233.
Roe FJC, Palmer AK, Worden AN, Van Abbé NJ. 1979. Safety evaluation of toothpaste containing chloroform: I. long-term studies in mice. J Environ Pathol Toxicol. 2(3):799-819.
Rolim de Almeida LF, Frei F, Mancini E, De Martino L, De Feo V. 2010. Phytotoxic activities of Mediterranean essential oils. Molecules. 15(6):4309-4323
Russin WA, Hoesly JD, Elson CE, Tanner MA, Gould MN. 1989. Inhibition of rat mammary carcinogenesis by monoterpenoids. Carcinogenesis. 10(11):2161-2164.
Saab AM, Guerrini A, Sacchetti G, Maietti S, Zeino M, Arend J, Gambari R, Bernardi F, Efferth T. 2013. Phytochemical analysis and cytotoxicity towards multidrug-resistant leukemia cells of essential oils derived from Lebanese medicinal plants. Planta Med. 78(18):1927-1931
Salido S, Altarejos J, Nogueras M, Sánchez A, Pannecouque C, Witvrouw M, De Clercq E. 2002. Chemical studies of essential oils of Juniperus oxycedrus ssp. badia. J Ethnopharmacol. 81(1):129-134.
Sanches LJ, Marinello PC, Panis C, Fagundes TR, Morgado-Díaz JA, de-Freitas-Junior JCM, Cecchini R, Cecchini AL, Luiz RC. 2017. Cytotoxicity of citral against melanoma cells: The involvement of oxidative stress generation and cell growth protein reduction. Tumour Biol. 39(3):1010428317695914.
Santos FA, Rao VSN. 1997. Mast cell involvement in the rat paw oedema response to 1,8-cineole, the main constituent of eucalyptus and rosemary oils. Eur J Pharmacol. 331(2-3):253-258.
Santos FA, Rao VSN. 2000. Antiinflammatory and antinociceptive effects of 1,8-cineole a terpenoid oxide present in many plant essential oils. Phytother Res. 14(4):240-244.
Sasaki YF, Imanishi H, Ohta T, Yasuhiko S. 1989. Modifying effects of components of plant essence on the induction of sister-chromatid exchanges in cultured Chinese hamster ovary cells. Mutat Res. 226(2):103-110.
[SCCNFP] Scientific Committee on Cosmetic Products and Non-Food Products. 2003. Opinion of the scientific committee on cosmetic products and non-food products intended for consumers concerning wood tars and wood tar preparations [PDF] [accessed 2022 Feb 25].
[SCCS] Scientific Committee on Consumer Safety. 2010. Basic criteria for the in vitro assessment of dermal absorption of cosmetic ingredients [PDF]. Brussels (BE): European Commission, Directorate-General for Health & Consumers.
[SCCS] Scientific Committee on Consumer Safety. 2015. The SCCS notes of guidance for the testing of cosmetic ingredients and their safety evaluation. 9th revision [PDF]. European Commission. [updated 2016 Apr 25].
Scentree. [accessed 2022 Feb 14]. Sandela®. c2019 – 2021. Paris (FR): ScenTree SAS.
Schoket B, Horkay I, Kósa A, Páldeák L, Hewer A, Grover PL, Phillips DH. 1990. Formation of DNA adducts in the skin of psoriasis patients, in human skin in organ culture, and in mouse skin and lung following topical application of coal-tar and juniper tar. J Invest Dermatol. 94(2):241-246.
[SDS] Safety Data Sheet. 2015a. WONDERSTRUCK ENCHANTED TAYLOR SWIFT SCENTED BODY LOTION. Stamford (CT): Elizabeth Arden Industries. [accessed 2021 December 8] [restricted access]
[SDS] Safety Data Sheet. 2015b. Laura Perfume by Laura Biagiotti. Jamesburg (NJ): P&G Prestige Products. [accessed 2021 Dec 21] [restricted access]
[SDS] Safety Data Sheet. 2017. The Art of Shaving Cologne Intense Bourbon Amber. Proctor and Gamble. [accessed 2021 Dec 8]
[SDS] Safety Data Sheet. 2020a. Cade Essential Oil (Rectified). Mississauga (ON): New Directions Aromatics. [accessed 2021 Dec 1] [restricted access]
[SDS] Safety Data Sheet. 2020b. Wormwood oil. Hilden (GER): Caesar & Loretz GmbH. [accessed 2021 Dec 1] [restricted access]
Segal S, Cohen SN, Freeman J, Hill RM, Kagan BM, Kauffman R, Pruitt AW, Soyka LF, Vickers SM. 1978. Committee on drugs. Camphor: who needs it? Pediatrics. 62(3):404-406.
Shaaban IA. 2005. A study on the effect of Mirazid on some of the internal organs and its possible prenatal effect in albino rat. Mansoura Med J. 36(3,4):323-344.
Simple Loose Leaf Tea Company.2019. How to Mmeasure loose leaf tea? Easy steps for the best brew. [accessed 2022 Feb 25].
Sinha S, Jothiramajayam M, Ghosh M, Mukherjee A. 2014. Evaluation of toxicity of essential oils palmarosa, citronella, lemongrass and vetiver in human lymphocytes. Food Chem Toxicol. 68:71-77.
Skalli S, Chebat A, Badrane N, Bencheikh RS. 2014. Side effects of cade oil in Morocco: An analysis of reports in the Moroccan herbal products database from 2004 to 2012. Food Chem Toxicol. 64:81–85
Slameňová D, Horváthová E, Wsólová L, Šramková M, Navarová J. 2009. Investigation of anti-oxidative, cytotoxic, DNA-damaging and DNA-protective effects of plant volatiles eugenol and borneol in human-derived HepG2, Caco-2 and VH10 cell lines. Mutat Res. 677(1-2):46-52.
Smith W. 1862. Case of poisoning by oil of wormwood (artemisia absinthium). Med Chir Trans. 46:23-24.
Smith B, Cadby P, Leblanc JC, Setzer RW. 2010. Application of the margin of exposure (MoE) approach to substances in food that are genotoxic and carcinogenic example: methyleugenol, CASRN: 93-15-2. Food Chem Toxicol. 48(Supp 1):S89-S97.
Sobreira Dantas Nóbrega de Figuêiredo FR, Monteiro AB, de Menezes IRA, Sales VS, Nascimento EP, Rodrigues CKS, Primo AJB, Cruz LP, Amaro EN, Delmondes GA, et al. 2019. Effects of the Hyptis martiusii Benth. leaf essential oil and 1,8-cineole (eucalyptol) on the central nervous system of mice. Food Chem Toxicol. 133:110802.
Souza ACS, Silva LK, Queiroz TB, Marques ES, Hiruma-Lima CA, Gaivão IOM, Maistro EL. 2020. Citral presents cytotoxic and genotoxic effects in human cultured cells. Drug Chem Toxicol. 43(4):435-440.
Stanković N, Mihajilov-Krstev T, Zlatković B, Matejić J, Stankov Jovanović V, Kocić B, Čomić L. 2016. Comparative study of composition, antioxidant, and antimicrobial activities of essential oils of selected aromatic plants from Balkan Peninsula. Planta Med. 82(7):650-661.
Statistics Canada. 2017. Custom tabulation of grooming products data from the Canadian Health Measures Survey Cycle 2 (2010-2011). Prepared for Existing Substances Risk Assessment Bureau, Health Canada by Statistics Canada. [restricted access].
St-Gelais A. 2015. Isobornyl cyclohexanol and you. Saguenay (QC): Phytochemica. [accessed 2022 Feb 14].
Stoner GD, Shimkin MB, Kniazeff AJ, Weisburger JH, Weisburger EK, Gori GB. 1973. Test for carcinogenicity of food additives and chemotherapeutic agents by the pulmonary tumor response in strain A mice. Cancer Res. 33(12):3069-3085.
Strittholt CA, McMillan DA, He T, Baker RA, Barker ML 2016. A randomized clinical study to assess ingestion of dentrifrice by children. Regul Toxicol Pharmacol. 75:66-71.
Süloğlu AK, Koçkaya EA, Selmanoğlu G. 2022. Toxicity of benzyl benzoate as a food additive and pharmaceutical agent. Toxicol Ind Health. 38(4):221-233.
Sun Y, Lenon GB, Yang AWH. 2019. Phellodendri cortex: a phytochemical, pharmacological, and pharmacokinetic review. Evi Based Complement Alternat Med. 2019(7621929):1-45.
Suparmi S, Ginting AJ, Mariyam S, Wesseling S, Rietjens IMCM. 2019. Levels of methyleugenol and eugenol in instant herbal beverages available on the Indonesian market and related risk assessment. Food Chem Toxicol. 125:467-478.
Swanson GP, Jones WE 3rd, Ha CS, Jenkins CA, Kumar AP, Basler J. 2015. Tolerance of Phellodendron amurense bark extract (Nexrutine®) in patients with human prostate cancer. Phytother Res. 29(1):40-42.
TERMIUMPlus® [database]. Ottawa (ON): Government of Canada [accessed 2022 Feb 8].
Tisserand R, Young R. 2014. Essential oil safety: a guide for health care professionals. 2nd ed. Toronto (ON): Churchill Livingstone/Elsevier.
Tisserand Institute. [accessed 2021 December 2]. How to use essential oils safely.
Uçar G, Balaban M. 2002. The composition of volatile extractives from the wood of Juniperus excelsa, Juniperus foetidissima and Juniperus oxycedrus. Holz Roh Werkst. 60(5):356-362.
Unnamed study report. 2021. Unpublished confidential study submitted to Health Canada. Ottawa (ON): Existing Substances Risk Assessment Bureau. Submission received on 2021 Dec 2. [restricted access]
[US EPA] United States Environmental Protection Agency. 2006. lnert reassessment-isobornyl acetate (CAS Reg. No. 125-1 2-2) [PDF]. Washington (DC): Office of Prevention, Pesticides, and Toxic Substances (OPPT).
[US EPA] United States Environmental Protection Agency. 2010. Screening-Level Hazard Characterization: Benzyl Derivatives Category. Hazard Characterization Document. US Environmental Protection Agency.
[US EPA] United States Environmental Protection Agency. 2011. Exposure factors handbook. Washington (DC): US EPA, National Center for Environmental Assessment, Office of Research and Development.
[US FDA] United States Food and Drug Administration. Substances added to food (formerly EAFUS) [database]. [updated 2022 Feb 3; accessed 2022 Feb 9]. Silver Spring (MD): US Food and Drug Administration
[US FDA] United States Food and Drug Administration. 2018a. Food additive regulations; synthetic flavoring agents and adjuvants. Fed Regist. 83(195). Docket No. FDA-2015-F-4317. Silver Spring (MD): US FDA.
[US FDA] United States Food and Drug Administration. 2018b. Memorandum. FAP A4810: review of the carcinogenicity potential of myrcene as a flavoring substance in food (FAP 5A4810). Silver Spring (MD): US FDA.
[US FDA] United States Food and Drug Administration. 2018c. Memorandum. FAP 5A4810: review of the carcinogenicity potential of methyl eugenol as a flavoring substance in food. Silver Spring (MD): US FDA.
van den Berg SJPL, Restani P, Boersma MG, Delmulle L, Rietjens IMCM. 2011. Levels of genotoxic and carcinogenic compounds in plant food supplements and associated risk assessment. Food Nutr Sci. 2(9):989-1010.
Ventos. [accessed 2022 Feb 14]. Isobornyl cyclohexanol (IBCH). Barcelona(ESP): ERNESTO VENTÓS S.A.
Vujošević M, Blagojević J. 2004. Antimutagenic effects of extracts from sage (Salvia officinalis) in mammalian system in vivo. Acta Vet Hung. 52(4):439-443.
Vuković-Gačić B, Nikčević S, Berić-Bjedov T, Knežević-Vukčević J, Simić D. 2006. Antimutagenic effect of essential oil of sage (Salvia officinalis L.) and its monoterpenes against UV-induced mutations in Escherichia coli and Saccharomyces cerevisiae. Food Chem Toxicol. 44(10):1730-1738.
Weisbord SD, Soule JB, Kimmel PL. 1997. Poison on line – acute renal failure caused by oil of wormwood purchased through the Internet. N Eng J Med. 337(12):825-827.
Wenninger JA, McEwen GN Jr. 1997. International cosmetic ingredient dictionary and handbook, 7th ed., 701-702. Washington (DC): Cosmetic, Toiletry, and Fragrance Association
[WHO] World Health Organization. 2009. WHO monographs on selected medicinal plants. Volume 4 [PDF]. [PDF]. Geneva (CH): World Health Organization.
Wu X, Bennett DH, Ritz B, Cassady DL, Lee K, Hertz-Picciotto I. 2010. Usage pattern of personal care products in California households. Food Chem Toxicol. 48(11):3109-3119.
Xian X, Sun B, Ye X, Zhang G, Hou P, Gao H. 2014. Identification and analysis of alkaloids in cortex Phellodendron amurense by high-performance liquid chromatography with electrospray ionization mass spectrometry coupled with photodiode array detection. J Sep Sci. 37(13):1533-1545.
Xu J, Hu ZQ, Wang C, Yin ZQ, Wei Q, Zhou LJ, Li L, Du YH, Jia RY, Li M, et al. 2014. Acute and subacute toxicity study of 1,8-cineole in mice. Int J Clin Exp Pathol. 7(4):1495-1501.
Xue L, Chen XW, Fan HX, Fan SY, Liang Y, Xia JX, Zhou H, Hu Z. 2006. Effect of borneol on levels of monoamine neurotransmitters in prefrontal cortex of rat after long term continuous operations. Acta Academiae Medicinae Militaris Tertiae. 28(18):1867-1869.
Yi J, Ye X, Wang D, He K, Yang Y, Liu X, Li X. 2013. Safety evaluation of main alkaloids from Rhizome coptidis. J Ethnopharmacol. 145(1):303-310.
Younis NS, Mohamed ME. 2021. Protective effects of myrrh essential oil on isoproterenol-induced myocardial infarction in rats through antioxidant, anti-inflammatory, Nrf2/HO-1 and apoptotic pathways. J. Ethnopharmacol. 270: 113793
Yousef Alshibly NM. 2014. Effect of Artemisia absinthium L. on genotoxicity on mice bone marrow cells. World Appl Sci J. 30(6):770-777.
Zani F, Massimo G, Benvenuti S, Bianchi A, Albasini A, Melegari M, Vampa G, Bellotti A, Mazza P. 1991. Studies on the genotoxic properties of essential oils with Bacillus subtilis rec-assay and Salmonella/microsome reversion assay. Planta Med. 57(3):237-241.
Zehra U, Tahir M, Lone KP. 2010. Ginkgo biloba induced malformations in mice. J Coll Physicians Surg Pak. 20(2):117-121.
Zhang Y, Jin H, Qin J, Fu J, Cheng X, Zhang W. 2011. Chemical constituents from Verbena officinalis. Chem Nat Compd. 47(2):319–320
Appendix A. Exposure parameters used to estimate exposure to substances in the Fourteen Terpene and Terpenoid Substances Group
Anthropometric data used to characterize exposure to relevant sub-populations were derived from the Canadian exposure factors used in human health risk assessments factsheet (Canada 2021).
For products that are not used everyday, exposure on the day of use was estimated (that is, frequency of at least once per day).
Where applicable and on the basis of the defaults above, inhalation exposures were converted to internal doses using the following equation:
Inhalation exposure (mg/kg bw/day) = [Air concentration (24-hr TWA, mg/m3) * Inhalation rate (m3/day)] ÷ Body weight (kg)
Combined exposure (mg/kg bw/day) = Dermal exposure (mg/kg bw/day) + Inhalation exposure (mg/kg bw/day)
Lifetime average daily dose (LADD)
LADDs were calculated to estimate the potential cancer risk from exposure to certain substances in this assessment group. Where applicable, inhalation exposures were converted to internal doses using default inhalation rates from HC (2021) and combined with the corresponding dermal exposure for each scenario. The general equation for estimating LADDs is as follows:
LADD = [(DSE0 to 5 months × AD0 to 5 months) + (DSE6 to 11 months × AD6 to 11 months) + (DSE1 to 2 years × AD1 to 2 years) + (DSE2 to 3 years × AD2 to 3 years) + (DSE4 to 8 years × AD4 to 8 years) + (DSE9 to 13 years × AD9 to 13 years) + (DSE14 to 18 years × AD14 to 18 years) + (DSE19 to 78 years × AD19 to 78 years) ] / [AL]
Where:
DSE = daily systemic exposure (individual DSEs may be 0 for situations where exposure to a specific age group is not expected [for example, products not intended for use by children])
AL = Average lifetime (78 years [US EPA 2011])
AD = Age group durations (0 to 5 months [0.5 years], 6 to 11 months [0.5 years], 1 to ≤ 2 years [1 year], 2 to 3 years [2 years], 4 to 8 years [5 years], 9 to 13 years [5 years], 14 to 18 years [5 years], 19 to 78 years [59 years])
Parameters for estimating dermal, inhalation, and oral exposures to products available to consumers are presented in Table A-1, and parameters for estimating exposures to DIY products are presented in Table A-2.
| Exposure scenario | Assumptions |
|---|---|
| Aftershave (Ginkgo biloba extract; myrrh oil) |
Concentration: Ginkgo biloba extract: 5% Myrrh oil: 3% (personal communication, emails from the CHPSD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Frequency (/day): 1 (assumed, to estimate exposure on the day of product use) Frequency (for LADD estimates, /day): 19+ years: 0.67 (Wu et al. 2010) 9 to 18 years: 0.73 (Wu et al. 2010) Dermal exposure: Product amount (g): 14 to 19+ years: 2.4 (Ficheux et al. 2016) 9 to 13 years: 2.3 (Ficheux et al. 2016) (surface area [SA] adjustment) Retention factor: 1 |
| Air freshener (Amberlyn) |
Concentration: 1% (Environment Canada 2013) Inhalation exposures were estimated using ConsExpo Web v.1.1.0 – Instantaneous release and defaults from RIVM (2021b) for an aerosol air freshener. Exposure duration: 4 hours Product amount (g): 8 Room volume (m3): 20 Ventilation rate (/hour): 0.6 |
| Analgesic cream (NHP) (Sage oil; wormwood oil) |
Concentration: Sage oil: 0.098% (as sage essential oil) Wormwood oil: 0.58% (personal communication, emails from the NNHPD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Age groups: Sage oil: 12+ years Wormwood oil: 9+ years (personal communication, emails from the NNHPD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Frequency (/day): Sage oil: 4 Wormwood oil: 4 (assumed short-term, intermittent use based on product instructions indicated for temporary pain relief) (personal communication, emails from the NNHPD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Dermal exposure: Sage oil, wormwood oil Product amount (g): corresponds to amounts for a body moisturizer (Ficheux et al. 2016), adjusted for exposed area, assuming applied to half trunk (Canada 2021) Example: 19+ years: 10 g × () = 1.97 g 14 to 18 years: 2.12 9 to 13 years: 1.61 Inhalation exposure: Sage oil, wormwood oil: Model: ConsExpo web v.1.1.0 exposure to vapour–evaporation–constant release area model Product amount: As above adjusted to account for the amount remaining on the skin surface following dermal absorption of the substance. Exposure and emission duration (hours): 6 (assumed based on application frequency of 4x/day) Molecular weight matrix (g/mol): 1000 Temperature (°C): 32 Room volume (m3): 58 Ventilation rate (per hour): 0.5 SA (cm2) (based on defaults from HC 2021): 19+ years: 3445 (half trunk) 14 to 18 years: 3490 (half trunk) 9 to 13 years: 2655 (half trunk) |
| Analgesic spray (NHP) (Cork tree extract) |
Concentration (%): 25 (as Phellodendron amurense bark extract) (personal communication, emails from the NNHPD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Frequency (/day): 3 (personal communication, emails from the NNHPD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Product amount (g): derived based on an assumed mass generation rate of 0.1g/sec (RIVM 2010) and a spray duration of 8 seconds (assumed) 19+ years: 0.8 |
| Antiperspirant (solid/roll-on) (Myrrh oil; sage oil) |
Concentration: Myrrh oil: 3% Sage oil: 10% (personal communication, emails from the CHPSD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Frequency (/day): 19+ years: 1.3 (Loretz et al. 2006) 14 to 18 years: 1.2 (Ficheux et al. 2015) 9 to 13 years: 1.1 (Wu et al. 2010) Dermal exposure: Myrrh oil, sage oil: Product amount (g): 14 to 19+ years: 1 (Ficheux et al. 2016) 9 to 13 years: 0.4 (Ficheux et al. 2016) Inhalation exposure: Sage oil: Model: ConsExpo web v.1.1.0 exposure to vapour–evaporation–constant release area model Product amount: As above adjusted to account for the amount remaining on the skin surface following dermal absorption of the substance (that is, 50%). Exposure and emission duration (hours) 19+ years: 18 9 to 18 years: 22 Molecular weight matrix (g/mol): 1000 Temperature (°C): 32 Room volume (m3): 58 Ventilation rate (per hour): 0.5 SA (cm2): 19+ years: 240 (underarms) 14 to 18 years: 234 (underarms) 9 to 13 years: 179 (underarms) |
| Antiperspirant (spray) (Ginkgo biloba extract) |
Concentration: Ginkgo biloba extract: 0.1% (personal communication, emails from the CHPSD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Frequency (/day): 19+ years: 1.3 (Loretz et al. 2006) 14 to 18 years: 1.2 (Ficheux et al. 2015) 9 to 13 years: 1.1 (Wu et al. 2010) Dermal exposure: Product amount (g): 14 to 19+ years: 3.48 (Hall et al. 2007) 9 to 13 years: 0.98 (Ficheux et al. 2016) |
| Bath oil (Myrrh oil) |
Concentration: Myrrh oil: 100% (personal communication, emails from the CHPSD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Frequency (/day): 1 (assumed, to estimate exposure on the day of product use) Dermal exposure: Product amount (g): assumes 15 g of product (CTFA 1983) is diluted in a bathtub (assuming 120 L of water in the tub (Bremmer et al. 2006)). Product amount on skin (g): Calculated using a film-thickness approach where the concentration of the substance in the tub was multiplied by the amount of water in contact with skin. Amount of water in contact with skin (19+ years): 0.01 cm (ECHA 2015, for film thickness) * 17530 cm2 (total body SA minus head) = 175.3 cm3 = 0.1753 L Example for product amount on skin: individual 19+ years = (15 g / 120 L) x 0.1753 L = 0.0219 g 14 to 18 years: 0.0206 9 to 13 years: 0.0159 Retention factor: 1 |
| Body exfoliant (Verbena officinalis extract) |
Concentration: Verbena officinalis extract: 3% (personal communication, emails from the CHPSD, HC, to ESRAB, HC, dated June 2021; unreferenced) Frequency (/day): 1 (assumed, to estimate exposure on the day of product use) Dermal exposure: Product amount (g): 19+ years: 32 (ECCC, HC 2020c) 14 to 18 years: 28 (ECCC, HC 2020c) Retention factor: 0.1 Inhalation exposure: Model: ConsExpo web v.1.1.0 exposure to vapour–evaporation–constant release area Product amount (g): As above adjusted to account for the amount remaining on the skin surface following dermal absorption of the substance. Exposure and emission duration (minutes): 5 Molecular weight matrix (g/mol): 1000 Temperature (°C): 32 Room volume (m3): 10 Ventilation rate (per hour): 2 SA (cm2) (adapted from body moisturizer product and equivalent to unclothed skin SA, which was assumed to be half of the total body SA (based on defaults from HC 2021): 19+ years: 9058 14 to 18 years: 8415 |
| Body lotion (NHP) (Myrrh oil; sage oil) |
Concentration: Myrrh oil: 0.5% (as myrrh essential oil) Sage oil: 1% (as Salvia officinalis [sage] leaf extract) (personal communication, emails from the NNHPD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Age groups: Myrrh oil: children 2 to 19+ years Sage oil: 12+ years (personal communication, emails from the NNHPD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Frequency (/day): Myrrh oil: 3 Sage oil: 4 (personal communication, emails from the NNHPD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Dermal exposure: Myrrh oil, sage oil: Product amount (g): Myrrh oil: 2 to 19+ years: 5 (based on product instructions to apply 5 mL of product, assuming density of myrrh oil 1 is g/mL) Sage oil: 12+ years; 3 (based on product instructions to apply up to 3 mL of product, assuming density of product is 1 g/mL) (personal communication, emails from the NNHPD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Inhalation exposure: Sage oil: Model: ConsExpo web v.1.1.0 exposure to vapour–evaporation–constant release area Product amount (g): As above, adjusted to account for the amount remaining on the skin surface following dermal absorption of the substance. Exposure and emission duration (hours): 24 Molecular weight matrix (g/mol): 1000 Temperature (°C): 32 Room volume (m3): 58 Ventilation rate (per hour): 0.5 SA (cm2) (equivalent to unclothed skin SA, which was assumed to be half of the total body SA based on defaults from HC 2021): 19+ years: 9058 14 to 18 years: 8415 9 to 13 years: 6525 |
| Body moisturizer (Ginkgo biloba extract; amberlyn; myrrh oil; cork tree extract; sage oil; sandela) |
Concentration: Ginkgo biloba extract: 3% (personal communication, emails from the CHPSD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Amberlyn: 0.15% (9+ years), 0.04% (0 to 9 years) (Environment Canada 2013) Myrrh oil: 10% (personal communication, emails from the CHPSD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Cork tree extract: 3% (personal communication, emails from the CHPSD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Sage oil: 3% (personal communication, emails from the CHPSD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Sandela: 0.4% (SDS 2015a) Frequency (/day): 19+ years: 1 (Wu et al. 2010; Ficheux et al. 2015) 0 months to 18 years: 1 (assumed, to estimate exposure on the day of product use) Frequency (for LADD estimates, /day): 19+ years: 1 (Wu et al. 2010; Ficheux et al. 2015) 0 months to 18 years: 0.8 (Wu et al. 2010; Ficheux et al. 2015) Dermal exposure: Ginkgo biloba extract, amberlyn, myrrh oil, cork tree extract, sage oil, sandela: Product amount (g): 19+ years: 10 (Ficheux et al. 2016) 14 to 18 years: 10 (Ficheux et al. 2016) 9 to 13 years: 7.7 (Ficheux et al. 2016, SA adjustment) 4 to 8 years: 5 (Ficheux et al. 2016, SA adjustment) 2 to 3 years: 4.1 (Ficheux et al. 2016) 1 year: 3.1 (Ficheux et al. 2016, SA adjustment) 6 to 11 months: 2.5 (Ficheux et al. 2016, SA adjustment) 0 to 5 months: 2 (Ficheux et al. 2016, SA adjustment) Inhalation exposure: Sage oil: Model: ConsExpo Web v.1.1.0 exposure to vapour–evaporation–constant release area Product amount (g): The product amount available for inhalation was adjusted to account for the presence of clothing and was adjusted by half of the values used for dermal exposure (see above). Since there is already a reduction in the product amount for this, no further refinement was applied to the product amount based on dermal absorption factors. 19+ years: 5 14 to 18 years: 5 9 to 13 years: 3.85 4 to 8 years: 2.5 2 to 3 years: 2.05 1 year: 1.55 6 to 11 months: 1.25 0 to 5 months: 1 Exposure and emission duration (hours): 24 Molecular weight matrix (g/mol): 1000 Temperature (°C): 32 Room volume (m3): 58 Ventilation rate (per hour): 0.5 SA (cm2) (equivalent to unclothed skin SA, which was assumed to be half of the total body SA (HC 2021): 19+ years: 9058 14 to 18 years: 8415 9 to 13 years: 6525 4 to 8 years: 4298 2 to 3 years: 3113 1 year: 2433 6 to 11 months: 2045 0 to 5 months: 3180 |
| Body oil (Ginkgo biloba extract) |
Concentration: Ginkgo biloba extract: 10% (personal communication, emails from the CHPSD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Frequency (/day): 1 (assumed, to estimate exposure on the day of product use; adapted from massage oil scenario) Frequency (for LADD estimates, /day; adapted from massage oil scenario): 14 to 19+ years: 0.11 (Ficheux et al. 2015) Dermal exposure: Product amount (g) (adapted from massage oil scenario): 19+ years: 3.2 (Ficheux et al. 2016) 14 to 18 years: 2.9 (Ficheux et al. 2016, SA adjustment) |
| Body soap (liquid) (Ginkgo biloba extract; myrrh oil; sage oil) |
Concentration: Ginkgo biloba extract: 3% Myrrh oil: 10% Sage oil: 10% (personal communication, emails from the CHPSD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Frequency (/day): 19+ years: 1.4 (Loretz et al. 2006) 14 to 18 years: 1.2 (Ficheux et al. 2015) 4 to 13 years: 1 (assumed, to estimate exposure on the day of product use) 0 months to 3 years: 1.2 (Ficheux et al. 2015) Frequency (for LADD estimates, /day): 19+ years: 1.4 (Loretz et al. 2006) 14 to 18 years: 1.2 (Ficheux et al. 2015) 4 to 13 years: 0.93 (Ficheux et al. 2015) 0 months to 3 years: 1.2 (Ficheux et al. 2015) Dermal exposure: Ginkgo biloba extract, myrrh oil: Product amount (g): 14 to 19+ years: 11 (Loretz et al. 2006; Ficheux et al. 2016) 4 to 13 years: 10.9 (Garcia-Hidalgo et al. 2017) 2 to 3 years: 6.7 (Garcia-Hidalgo et al. 2017) 1 year: 5.4 (Ficheux et al. 2016) 6 to 11 months: 4.9 (Gomez-Berrada et al. 2017) 0 to 5 months: 4.5 (Gomez-Berrada et al. 2017) Retention factor: 0.01 Inhalation exposure: Sage oil: Model: ConsExpo web v.1.1.0 exposure to vapour–evaporation–constant release area Product amount (g): As above, adjusted to account for the amount remaining on the skin surface following dermal absorption of the substance. Exposure and emission duration (minutes): 5 Molecular weight matrix (g/mol): 1000 Temperature (°C): 32 Room volume (m3): 10 Ventilation rate (per hour): 2 SA (cm2) (equivalent to total body SA minus the head based on defaults from HC 2021): 19+ years: 17530 14 to 18 years: 16460 9 to 13 years: 12700 4 to 8 years: 8290 2 to 3 years: 5950 1 year: 4430 6 to 11 months: 3690 0 to 5 months: 2860 |
| Breath freshener (Sage oil) |
Concentration: Sage oil: 1% (personal communication, emails from the CHPSD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Frequency (/day) (adapted from mouthwash scenario): 14 to 19+ years: 1 (Ficheux et al. 2015) 4 to 13 years: 1 (assumed, to estimate exposure on the day of product use) Oral exposure: Product amount (g) (adapted from mouthwash scenario): 14 to 19+ years: 1.7 (Ficheux et al. 2016; SCCS 2015) 4 to 13 years: 1 (SCCS 2015) |
| Cleaning product use (Jonquil oil) |
Concentration: < 0.00001% Frequency (/day): 2 Product amount (g): 20.58, on the basis of film thickness, where 2058cm2 is the surface area of skin contacted (HERA 2005 values for hands and forearms *0.01cm layer of product on skin* 1g/cm3 density, RIVM 2018) |
| Conditioner (rinse-off) (Sage oil) |
Concentration: Sage oil: 10% (personal communication, emails from the CHPSD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Frequency (/day): 19+ years: 1.1 (Loretz et al. 2008) 2 to 18 years: 1 (assumed, to estimate exposure on the day of product use) Dermal exposure: Product amount (g): 19+ years: 13.1 (Loretz et al. 2008) 14 to 18 years: 10 (Ficheux et al. 2016) 4 to 13 years: 7.8 (Ficheux et al. 2016) 2 to 3 years: 5.2 (Garcia-Hidalgo et al. 2017) Retention factor: 0.01 Inhalation exposure: Model: ConsExpo web v.1.1.0 exposure to vapour–evaporation–constant release area model Product amount (g): As above, adjusted to account for the amount remaining on the skin surface following dermal absorption of the substance. Exposure and emission duration (minutes): 5 Molecular weight matrix (g/mol): 1000 Temperature (°C): 32 Room volume (m3): 10 Ventilation rate (per hour): 2 SA (cm2) (HC 2021): 19+ years: 1040 (half hands and half head) 14 to 18 years: 755 (half hands and half head) 9 to 13 years: 655 (half hands and half head) 4 to 8 years: 530 (half hands and half head) 2 to 3 years: 275 (half head) |
| Douche (Sage Oil) |
Concentration: Sage oil: 10% (personal communication, emails from the CHPSD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Frequency (/day): 14 to 19+ years: 1 (assumed, to estimate exposure on the day of product use) Dermal exposure: Product amount (g): 1 to 19+ years: 133 (professional judgement) Retention factor: 0.1 |
| Face cleanser (Ginkgo biloba extract) |
Concentration: Ginkgo biloba extract: 3% (personal communication, emails from the CHPSD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Frequency (/day): 19+ years: 1.6 (Loretz et al. 2006) 9 to 18 years: 1.2 (Ficheux et al. 2015) Dermal exposure: Product amount (g): 14 to 19+ years: 3.3 (Ficheux et al. 2016) 9 to 13 years: 3.1 (Ficheux et al. 2016) Retention factor: 0.01 |
| Face exfoliant (Ginkgo biloba extract; myrrh oil) |
Concentration: Ginkgo biloba extract: 1% Myrrh oil: 10% (personal communication, emails from the CHPSD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Frequency (/day): 1 (assumed, to estimate exposure on the day of product use) Frequency (for LADD estimates, /day): 14 to 19+ years: 0.29 (Ficheux et al. 2015) Dermal exposure: Product amount (g): 14 to 19+ years: 3.1 (Ficheux et al. 2016) Retention factor: 0.1 |
| Face mask (Ginkgo biloba extract; sage oil) |
Concentration: Ginkgo biloba extract: 10% Sage oil: 3% (personal communication, emails from the CHPSD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Frequency (/day): 1 (assumed, to estimate exposure on the day of product use) Frequency (for LADD estimates, /day): 14 to 19+ years: 0.1 (Ficheux et al. 2015) Dermal exposure: Product amount (g): 14 to 19+ years: 9.7 (Ficheux et al. 2016) Retention factor: 0.1 Inhalation exposure: Sage oil: Model: ConsExpo web v.1.1.0 exposure to vapour-evaporation–constant release area model Product amount (g): As above, adjusted to account for the amount remaining on the skin surface following dermal absorption of the substance. Exposure and emission duration (minutes): 5 Molecular weight matrix (g/mol): 1000 Temperature (°C): 32 Room volume (m3): 10 Ventilation rate (per hour): 2 SA (cm2) (HC 2021): 19+ years: 585 (half head) 14 to 18 years: 370 (half head) |
| Face moisturizer (Cade oil; Verbena officinalis extract; myrrh oil; cork tree extract; sage oil) |
Concentration: Cade oil:1% Verbena officinalis extract: 10% Myrrh oil: 30% myrrh oil Cork tree extract: 3% Sage oil: 3% (personal communication, emails from the CHPSD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Frequency, including values used for LADD estimates (/day): 19+ years: 2.0 (Loretz et al. 2005) 14 to 18 years: 1.0 (Ficheux et al. 2015) 9 to 13 years: 1.0 (Ficheux et al. 2015) Dermal exposure: Cade oil, Verbena officinalis extract, myrrh oil, cork tree extract, sage oil Product amount (g): 19+ years: 1.5 (Ficheux et al. 2016) 14 to 18 years: 1.5 (Ficheux et al. 2016) 9 to 13 years: 1.1 (Ficheux et al. 2016) Inhalation exposure: Cade oil, Verbena officinalis extract, sage oil Model: ConsExpo web v1.1.0 exposure to vapour– evaporation–constant release area Product amount (g): As above, adjusted to account for the amount remaining on the skin surface following dermal absorption of the substance. Exposure and emission duration (hour): 19+ years: 12 14 to 18 years: 24 9 to 13 years: 24 Molecular weight matrix (g/mol): 1000 (assumed) Room volume (m3): 20 Ventilation rate (/hour): 0.6 Application temperature: 32°C Release area (cm2): equivalent to SA of face, calculated using half SA of head (HC 2021) 19+ years: 585 14 to 18 years: 370 9 to 13 yeas: 350 |
| Face sunscreen (NHP) (Ginkgo biloba extract) |
Concentration: Ginkgo biloba extract: 3% (personal communication, emails from the NNHPD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Age groups: Adults (19+ years) (personal communication, emails from the NNHPD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Frequency (/day): 19+ years: 1.2 (Loretz et al. 2006) Dermal exposure:< Product amount (g): 19+ years: 0.54 (Loretz et al. 2006) |
| Face sunscreen (NPD) (Ginkgo biloba extract) |
Concentration: Ginkgo biloba extract: 0.1% (personal communication, emails from the TPD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Frequency (/day) (adapted from face moisturizer scenario): 19+ years: 2 (Loretz et al. 2008) 9 to 18 years: 1 (Ficheux et al. 2015) Dermal Exposure : Product amount (g): 14 to 19+ years: 1.5 (Ficheux et al. 2016) 9 to 13 years: 1.1 (Ficheux et al. 2016) |
| Face toner (Ginkgo biloba extract) |
Concentration: Ginkgo biloba extract: 94% (personal communication, emails from the CHPSD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Frequency (/day) (adapted from face moisturizer scenario): 19+ years: 2 (Loretz et al. 2005) 9 to 18 years: 1 (Ficheux et al. 2015) Dermal exposure: Product amount (g) (adapted from face moisturizer scenario): 14 to 19+ years: 1.5 (Ficheux et al. 2016) 9 to 13 years: 1.1 (Ficheux et al. 2016) |
| Genital lubricant (Ginkgo biloba extract) |
Concentration: Ginkgo biloba extract: 1% (personal communication, emails from the CHPSD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Frequency (/day): 19+ years: 1 (ECCC, HC 2016b) Dermal exposure: Product amount (g): 19+ years: 10 (ECCC, HC 2016b) Dermal absorption is assumed to be equivalent to oral absorption because of application to a mucosal membrane. |
| Hair mist (Ginkgo biloba extract) |
Concentration: Ginkgo biloba extract: 0.3% (personal communication, emails from the CHPSD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Frequency (/day) (adapted from hair spray pump scenario): 19+ years: 1.49 (Loretz et al. 2008) 14 to 18 years: 1 (assumed, to estimate exposure on the day of product use) Frequency (for LADD estimates, /day) (adapted from massage oil scenario): 19+ years: 1.49 (Loretz et al. 2008) 14 to 18 years: 0.63 (Wu et al. 2010) Dermal exposure: Product amount (g): 19+ years: 2.6 (Loretz et al. 2008) 14 to 18 years: 2.3 (Ficheux et al. 2016) Retention factor: 0.085 |
| Hair perm/straightener (Ginkgo biloba extract) |
Concentration: Ginkgo biloba extract: 3% (personal communication, emails from the CHPSD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Relevant age group: 19+ years (personal communication, emails from the CHPSD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Frequency (/day): 1 (assumed, to estimate exposure on the day of product use) Frequency (for LADD estimates, /day): 0.016 (Wu et al. 2010; Ficheux et al. 2015) Dermal exposure: Product amount (g): 19+ years: 80 (Bremmer et al. 2006) Retention factor: 0.1 |
| Hair removal aftercare (Myrrh oil) |
Concentration: Myrrh oil: 3% (personal communication, emails from the CHPSD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Frequency (/day): 1 (assumed, to estimate exposure on the day of product use) Dermal exposure: Product amount (g): 14 to 19+ years: 7.1 (Ficheux et al. 2016) 9 to 13 years: 5.5 (SA adjustment) Retention factor: 1 |
| Hair styling product (wax/gel/putty) (Myrrh oil; sage oil) |
Concentration: Myrrh oil: 30% Sage oil: 1% (personal communication, emails from the CHPSD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Frequency (/day): 1 (assumed, to estimate exposure on the day of product use) Dermal exposure: Myrrh oil, sage oil: Product amount (g): 14 to 19+ years: 3.7 (Ficheux et al. 2016) 9 to 13 years: 3.5 (Ficheux et al. 2016, SA adjustment) 4 to 8 years: 3.1 (Ficheux et al. 2016, SA adjustment) 2 to 3 years: 2.8 (Ficheux et al. 2016, SA adjustment) Retention factor: 0.1 Inhalation exposure: Sage oil: Model: ConsExpo web v.1.1.0 exposure to vapour–evaporation–constant release area model: Product amount (g): As above, adjusted to account for the amount remaining on the skin surface following dermal absorption of the substance. Exposure and emission duration (hour): 24 Molecular weight matrix (g/mol): 1000 Temperature (°C): 32 Room volume (m3): 58 Ventilation rate (per hour): 0.5 SA (cm2) (HC 2021): 19+ years: 1040 (half hands and half head) 14 to 18 years: 755 (half hands and half head) 9 to 13 years: 655 (half hands and half head) 4 to 8 years: 530 (half hands and half head) 2 to 3 years: 275 (half head) |
| Hand cream (Verbena officinalis extract; Ginkgo biloba extract) |
Concentration: Verbena officinalis extract: 3% Ginkgo biloba extract: 0.1% (personal communication, emails from the CHPSD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Frequency (/day): 19+ years: 2 (Loretz et al. 2005) 4 to 18 years: 1 (Wu et al. 2010; Ficheux et al. 2015) 2 to 3 years: 1 (assumed, to estimate exposure on the day of product use) Frequency (for LADD estimates, /day): 19+ years: 2 (Loretz et al. 2005) 4 to 18 years: 1 (Wu et al. 2010; Ficheux et al. 2015) 2 to 3 years: 0.63 (Wu et al. 2010; Ficheux et al. 2015) Dermal exposure: Verbena officinalis extract, Ginkgo biloba extract: Product amount (g): 14 to 19+ years: 1.6 (Ficheux et al. 2016) 4 to 13 years: 1.2 (Ficheux et al. 2016) 2 to 3 years: 0.87 (Ficheux et al. 2016, SA adjustment) Inhalation exposure: Verbena officinalis extract: Model: ConsExpo web v.1.1.0 exposure to vapour–evaporation–constant release area model Product amount (g): As above, adjusted to account for the amount remaining on the skin surface following dermal absorption of the substance. Exposure and emission duration (hours): 24 Molecular weight matrix (g/mol): 1000 Temperature (°C): 32 Room volume (m3): 58 Ventilation rate (per hour): 0.5 SA (cm2) (equivalent to SA of both hands, based on defaults from HC 2021): 19+ years: 910 14 to 18 years: 770 9 to 13 years: 610 4 to 8 years: 430 2 to 3 years: 310 |
| Hand sanitizer (NHP) (Myrrh oil; sage oil; wormwood oil) |
Concentration: Myrrh oil: 0.04% (as myrrh essential oil) Sage oil: 0.25% (as sage leaf extract) Wormwood oil: 1.3% (as Artemisia absinthium extract) (personal communication, emails from the NNHPD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Frequency (/day): 19+ years: 2.9 (Wu et al. 2010) 4 to 18 years: 1.4 (Wu et al. 2010) 2 to 3 years: 1 (assumed, to estimate exposure on the day of product use) Frequency (for situations of public health concern, /day): 2 to 19+ years: 25 (RIVM 2021a; Lopez et al. 2022) Dermal exposure: Myrrh oil, sage oil, wormwood oil: Product amount (g): 2 to 19+ years: 1.5 (Kampf et al. 2013; Macinga et al. 2013; Bánsághi et al. 2020) Inhalation exposure: Wormwood oil, sage oil: Model: ConsExpo web v.1.1.0 exposure to vapour–evaporation–constant release area model Product amount (g): As above, adjusted to account for the amount remaining on the skin surface following dermal absorption of the substance. Exposure and emission duration (minutes): 20 Molecular weight matrix (g/mol): 1000 Temperature (°C): 32 Room volume (m3): 20 Ventilation rate (per hour): 0.6 SA (cm2) (HC 2021): 19+ years: 910 (hands) 14 to 18 years: 770 (hands) 9 to 13 years: 610 (hands) 4 to 8 years: 420 (hands) 2 to 3 years: 310 (hands) |
| Herbal tea blend (NHP) (Ginkgo biloba extract; sage oil) |
Concentration: Ginkgo biloba extract: 3% (as Ginkgo biloba) Sage oil: 6.7% (as sage leaf dry) (personal communication, emails from the NNHPD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Age groups: Ginkgo biloba extract, sage oil: 19+ years (personal communication, emails from the NNHPD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Frequency (/day): Ginkgo biloba extract, sage oil: 3 (personal communication, emails from the NNHPD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Oral exposure: Ginkgo biloba extract: Product amount (g): 2 (assuming amount used is equivalent to one tea bag; Simple Loose Leaf Tea Company 2019) Extraction fraction of Ginkgo biloba extract from one cup of Ginkgo biloba leaf tea: 0.03 (personal communication, emails from the FD, HC, to the ESRAB, HC, dated October 2022; unreferenced) Oral daily exposure (mg/kg bw/day) = [product amount (g) * extraction fraction of Ginkgo biloba extract * concentration of Ginkgo biloba in tea blend * frequency (/day) * conversion factor (1000 mg/g)] ÷ body weight (kg) Sage oil: Mean amount of thujone extracted per cup of sage leaf tea (mg): 0.66 (personal communication, emails from the FD, HC, to the ESRAB, HC, dated October 2022; unreferenced) Oral daily exposure (mg/kg bw/day) = [amount of thujone (mg) * concentration of sage leaf in tea blend * frequency (/day)] ÷ body weight (kg) |
| Liquid face foundation (Ginkgo biloba extract) |
Concentration: Ginkgo biloba extract: 30% (personal communication, emails from the CHPSD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Frequency (/ day): 19+ years: 1.2 (Loretz et al. 2006) 14 to 18 years: 1 (Ficheux et al. 2015) 4 to 13 years: 1 (assumed, to estimate exposure on the day of product use) (Garcia-Hidalgo et al. 2017; professional judgment) Dermal exposure: Product amount (g): 19+ years: 0.54 (Loretz et al. 2006) 14 to 18 years: 0.41 (Ficheux et al. 2016) 9 to 13 years: 0.39 (Ficheux et al. 2016; SA adjustment) 4 to 8 years: 0.24 (Ficheux et al. 2016; SA adjustment) |
| Makeup remover (liquid non-oily) (Ginkgo biloba extract; sage oil) |
Concentration: Ginkgo biloba extract: 1% (personal communication, emails from the CHPSD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Frequency (/day): 9 to 19+ years: 1 (Ficheux et al. 2015) Dermal exposure: Ginkgo biloba extract: Product amount (g): 14 to 19+ years: 4.4 (Ficheux et al. 2016) 9 to 13 years: 2.2 (Ficheux et al. 2016) Retention factor: 0.1 |
| Makeup remover (liquid oil/milk) (Sage oil) |
Concentration: Sage oil: 3% (personal communication, emails from the CHPSD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Frequency (/day): 9 to 19+ years: 1 (Ficheux et al. 2015) Dermal exposure : Ginkgo biloba extract, myrrh oil : Product amount (g): 14 to 19+ years: 2.6 (Ficheux et al. 2015) 9 to 13 years: 2.5 (Ficheux et al. 2015; SA adjustment) Retention factor: 0.1 Inhalation exposure: Sage oil: Model: ConsExpo web v.1.1.0 exposure to vapour–evaporation–constant release area model Product amount (g): As above, adjusted to account for the amount remaining on the skin surface following dermal absorption of the substance. Exposure and emission duration (minutes): 5 Molecular weight matrix (g/mol): 1000 Temperature (°C): 32 Room volume (m3): 10 Ventilation rate (per hour): 2 SA (cm2) (Health Canada 2021): 19+ years: 585 (half head) 14 to 18 years: 370 (half head) |
| Massage oil (Verbena officinalis extract; Ginkgo biloba extract; myrrh oil; sage oil) |
Concentration: Verbena officinalis extract: 3% (diluted from 30%) Ginkgo biloba extract: 1% Myrrh oil: 3% (diluted from 30%) Sage oil: 3% (diluted from 10%) (personal communication, emails from the CHPSD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Frequency (/day): 1 (assumed, to estimate exposure on the day of product use) Frequency (for LADD estimates, /day): 0.016 (Wu et al. 2010; Ficheux et al. 2015) Dermal exposure: Verbena officinalis extract, Ginkgo biloba extract, myrrh oil, sage oil: Product amount (g): 19+ years: 3.2 (Ficheux et al. 2016) 14 to 18 years: 2.9 (Ficheux et al. 2016, SA adjustment) 9 to 13 years: 2.3 (Ficheux et al. 2016, SA adjustment) 4 to 8 years: 1.9 (Ficheux et al. 2016, SA adjustment) 1 to 3 years: 1.8 (Ficheux et al. 2016) 0 to 11 months: 1.8 (Ficheux et al. 2016) Inhalation exposure: Verbena officinalis extract, sage oil: Model: ConsExpo web v.1.1.0 exposure to vapour–evaporation–constant release area Product amount: As above, adjusted to account for the amount remaining on the skin surface following dermal absorption of the substance. Exposure and emission duration (hours): 24 Molecular weight matrix (g/mol): 1000 Temperature (°C): 32 Room volume (m3): 58 Ventilation rate (per hour): 0.5 SA (cm2) (based on total body SA, minus half-head and half-torso; HC 2021): 19+ years: 14 670 14 to 18 years: 13 340 9 to 13 years: 13 050 4 to 8 years: 8 595 2 to 3 years: 6 225 1 year: 4 865 6 to 11 months: 4 090 0 to 5 months: 3 180 |
| Massage oil (NHP) (Sage oil) |
Concentration: Sage oil: 3% (diluted from 13.8%) (as sage essential oil) (personal communication, emails from the NNHPD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Age groups: 19+ years (personal communication, emails from the NNHPD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Frequency (/day): 3 (assumed short-term, intermittent use based on product instructions indicated for temporary relief of aches and pains) (personal communication, emails from the NNHPD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Dermal exposure: Product amount (g): 19+ years: 3.2 (Ficheux et al. 2016) Inhalation exposure: Model: ConsExpo web v.1.1.0 exposure to vapour–evaporation–constant release area model Product amount: As above, adjusted to account for the amount remaining on the skin surface following dermal absorption of the substance. Exposure and emission duration (hours): 24 Molecular weight matrix (g/mol): 1000 Temperature (°C): 32 Room volume (m3): 58 Ventilation rate (per hour): 0.5 SA (cm2) (based on total body SA, minus half-head and half-torso; HC 2021): 19+ years: 14 670 |
| Mouthwash (Myrrh oil; sage oil) |
Concentration: Myrrh oil: 1% Sage oil: 0.1% (personal communication, emails from the CHPSD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Frequency (/day): 14 to 19+ years: 1 (Ficheux et al. 2015) 4 to 13 years: 1 (assumed, to estimate exposure on the day of product use) Oral exposure: Product amount (g): 14 to 19+ years: 1.7 (SCCS 2015; Ficheux et al. 2016) 4 to 13 years: 1 (SCCS 2015) |
| Motion sickness medication (NHP) (Sage oil) |
Concentration: Sage oil: 5% (as sage essential oil) (personal communication, emails from the NNHPD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Age groups: 15+ years and older (personal communication, emails from the NNHPD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Frequency (/day): 1 (assumed short-term, intermittent use based on intended use of product to relieve motion sickness) (personal communication, emails from the NNHPD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Product amount (mg): 1300 (based on product instructions to consume up to 26 drops at one time, assuming 1 drop ~0.05 mL and density of 1 g/mL) (personal communication, emails from the NNHPD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Daily oral exposure (mg/kg bw/day) = [product amount (mg) * frequency (/day)] ÷ body weight (kg) |
| Liquid extract (Verbena officinalis extract) |
Concentration: Frequency (/day): |
| Oral supplement (liquid extract) (Ginkgo biloba extract) |
Dose per serving: Relevant age groups: 19+ years (as per product instructions) |
| Oral ingestion of essential oil (Cork tree extract; wormwood oil) |
Concentration (%): 100 Frequency (/day): based on product label instructions Cork tree extract: 4 Wormwood oil: 3 Product amount (g): Cork tree extract: directions of use noting ingestion of 0.7–1.0 mL of liquid, and an assumed density of 1.0g/mL 14 to 19+ years: 1 Wormwood oil: based on label instructions noting consumption of 0.45 mL of wormwood oil and an assumed density of 0.9 g/mL 1 to 19+ years: 0.395 |
| Oral supplement (NHP) (loose herbs) (Sage oil) |
Dose: Sage oil: 180 mg/g (as sage) (personal communication, emails from the NNHPD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Age groups: adults (19+ years) (personal communication, emails from the NNHPD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Frequency (/day): 1 Product amount (mg): 1620 mg Based on product instructions to take up to 9 g at one time (personal communication, emails from the NNHPD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Daily oral exposure (mg/kg bw/day) = [product amount (mg) * frequency (/day)] ÷ body weight (kg) |
| Oral supplement (NHP) (tablet/capsule) (Verbena officinalis extract; Ginkgo biloba extract; myrrh oil; sage oil) |
Dose: Verbena officinalis extract: 5 mg per tablet (as Verbena officinalis flower) Ginkgo biloba extract: 187 mg per capsule (as Ginkgo) Myrrh oil: 25 mg per capsule (as Commiphora myrrha resin extract) Sage oil: 200 mg per capsule (as Salvia officinalis/Dalmatian sage) (personal communication, emails from the NNHPD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Age group: Verbena officinalis extract, Ginkgo biloba extract, myrrh oil, sage oil: 19+ years (personal communication, emails from the NNHPD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Frequency (tablets per day): Verbena officinalis extract: 1 tablet 3 times per day Ginkgo biloba extract: 1 capsule 3 times per day Myrrh oil: 2 capsules 3 times per day Sage oil: 3 capsules 5 times per day (personal communication, emails from the NNHPD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Daily oral exposure (mg/kg bw/day) = [dose * frequency (/day)] ÷ body weight (kg) |
| Pain gel (NHP) (Myrrh oil) |
Concentration: Myrrh oil: 9.1% (as Commiphora myrrha resin extract) (personal communication, emails from the NNHPD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Age groups: adults (19+ years) (personal communication, emails from the NNHPD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Frequency (/day): 3 (assumed short-term, intermittent use based on product instructions indicated for temporary pain relief) (personal communication, emails from the NNHPD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Dermal exposure: Product amount (g): Corresponds to amounts for a body moisturizer (Ficheux et al. 2016), adjusted for exposed area, assuming application to ½ trunk (Health Canada 2021). 19+ years: 10 g × () = 1.97 g |
| Perfume (roll-on) (Cade oil) |
Concentration: Cade oil: 30% (personal communication, emails from the CHPSD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Frequency: 19+ years: 1.7 (Loretz et al. 2006) 9 to 18 years: 1.4 (Statistics Canada 2017) 2 to 8 years: 1 (assumed, to estimate exposure on the day of product use) Frequency (for LADD estimates, /day): 19+ years: 1.7 (Loretz et al. 2006) 9 to 18 years: 1.4 (Statistics Canada 2017) 4 to 8 years: 0.8 (Statistics Canada 2017) 2 to 3 years: 0.4 (Statistics Canada 2017) Dermal exposure: Product amount (g): 2 to 19+ years: 0.33 (Loretz et al. 2006) Retention factor: 0.85 Inhalation exposure: Model: ConsExpo web v.1.1.0 exposure to vapour– evaporation Product amount (g): 0.106 (Loretz et al. 2006, based on amount for dermal exposure adjusted to account for retention factor and amount dermally absorbed) Room volume: 20m3 Air exchange rate: 0.6/hour Exposure and emission durations: adjusted to account for frequency of use cited above 19+ years: 14.1 hours 14 to 18 years: 17.1 hours 2 to 8 years: 24 hours Molecular weight matrix: 1000 g/mol (assumed) Vapour Pressure: 24 Pa (from o-cresol) Application Temperature: 32°C Molecular Weight: 108.2 g/mol (from o-cresol) Mass Transfer coefficient: 10 m/hr Release area: 200 cm2 |
| Perfume (spray) (Norlimbanol; myrrh oil; sage oil; IBCH) |
Concentration: Norlimbanol: 3% (SDS 2017) Myrrh oil: 10% (personal communication, emails from the CHPSD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Sage oil: 10% (personal communication, emails from the CHPSD, HC, to the ESRAB, HC, dated June 2021; unreferenced) IBCH: 5% (SDS 2015b) Frequency: 19+ years: 1.7 (Loretz et al. 2006) 9 to 18 years: 1.4 (Statistics Canada 2017) 2 to 8 years: 1 (assumed, to estimate exposure on the day of product use) Dermal exposure: Norlimbanol, myrrh oil, sage oil, IBCH: Product amount (g): 2 to 19+ years: 0.33 (Loretz et al. 2006) Retention factor: 0.85 Inhalation exposure: Norlimbanol, sage oil: Exposure model: ConsExpo web v.1.1.0 Exposure to Spray–Spraying Spray duration: 3.3 seconds Exposure duration: 5 minutes Room volume: 10 m3 Room height: 2.5m Ventilation rate: 2/hour Cloud volume: 0.0625 m3 Mass generation rate: 0.1 g/sec Airborne fraction: 0.02 Density of non-volatile: 1.5 g/cm3 Inhalation cut off diameter: 15 µm Aerosol diameter: lognormal, median = 2.7 µm, CV = 0.73 µm Maximum diameter: 50 µm |
| Permanent hair dye (Ginkgo biloba extract; myrrh oil) |
Concentration: Ginkgo biloba extract: 1% Myrrh oil: 3% (personal communication, emails from the CHPSD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Frequency (/day): 1 (assumed, to estimate exposure on the day of product use) Frequency (for LADD estimates, /day): 19+ years: 0.022 (Bernard et al. 2016) 14 to 18 years: 0.011 (Bernard et al. 2016) Dermal exposure: Ginkgo biloba extract, myrrh oil: Product amount (g): 14 to 19+ years: 132.6 (Ramirez-Martinez et al. 2015) Retention factor: 0.1 |
| Resin incense (Myrrh oil) |
Concentration: Myrrh oil: 100% Frequency (/day): 1 (assumed, to estimate exposure on the day of product use) Inhalation exposure: Model: ConsExpo exposure to vapour–evaporation–constant release area model (RIVM 2021b) Product amount: 1.4 g Exposure duration: 4 hours Temperature: 20°C Room volume: 20 m3 Ventilation rate: 0.6/hour Emission duration: 65 minutes |
| Shampoo (liquid) (Verbena officinalis extract; Ginkgo biloba extract; sage oil) |
Concentration: Verbena officinalis extract: 30% Ginkgo biloba extract: 10% Sage oil: 3% (personal communication, emails from the CHPSD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Frequency (/day): 19+ years: 1.1 (Loretz et al. 2008) 0 months to 18 years: 1 (assumed, to estimate exposure on the day of product use) Frequency (for LADD estimates, /day): 19+ years: 1.1 (Loretz et al. 2008) 9 to 18 years: 0.7 (Wu et al. 2010; Ficheux et al. 2015) 4 to 8 years: 0.64 (Gomez-Berrada et al. 2013) 2 to 3 years: 0.65 (Gomez-Berrada et al. 2013) 0 months to 1 year: 0.64 (Ficheux et al. 2015) Dermal exposure: Verbena officinalis extract, Ginkgo biloba extract: Product amount (g): 19+ years: 11.8 (Loretz et al. 2008) 14 to 18 years: 10.4 (Ficheux et al. 2016) 9 to 13 years: 7.5 (Ficheux et al. 2016) 4 to 8 years: 9.7 (Gomez-Berrada et al. 2013) 2 to 3 years: 7.9 (Gomez-Berrada et al. 2013) 1 year: 6.1 g (Gomez-Berrada et al. 2013) 6 to 11 months: 5.6 (Gomez-Berrada et al. 2013) 0 to 5 months: 3.9 (Gomez-Berrada et al. 2013) Retention factor: 0.01 Inhalation exposure: Verbena officinalis extract, sage oil: Model: ConsExpo web v.1.1.0 exposure to vapour–evaporation–constant release area model Product amount (g): As above, adjusted to account for the amount remaining on the skin surface following dermal absorption of the substance. Exposure and emission duration (minutes): 5 Molecular weight matrix (g/mol): 1000 Temperature (°C): 32 Room volume (m3): 10 Ventilation rate (per hour): 2 SA (cm2) (based on defaults from HC 2021): 19+ years: 1040 (half hands and half head) 14 to 18 years: 755 (half hands and half head) 9 to 13 years: 655 (half hands and half head) 4 to 8 years: 520 (half hands and half head) 2 to 3 years: 275 (half head) 1 year: 435 (half head) 6 to 11 months: 410 (half head) 0 to 5 months: 320 (half head) |
| Sunless tanning product (Ginkgo biloba extract; myrrh oil; sage oil) |
Concentration: Room volume (m3): 58 SA (cm2) (adapted from body moisturizer product) (equivalent to unclothed skin SA, which was assumed to be half of the total body SA, based on defaults from HC 2021): |
| Sunscreen lotion (NHP) (Ginkgo biloba extract; myrrh oil) |
Concentration: Ginkgo biloba extract: 3.1 mg/g (as Ginkgo biloba leaf extract; personal communication, emails from the NNHPD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Myrrh oil: 0.049% (personal communication, emails from the NNHPD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Frequency (/day): 4 to 19+ years: 1.4 (Ficheux et al. 2015) 6 months to 3 years: 1.6 (Ficheux et al. 2015) Dermal exposure: Ginkgo biloba extract, myrrh oil: Product amount (g): 14 to 19+ years: 18.2 (Ficheux et al. 2016) 4 to 13 years: 6.3 (Ficheux et al. 2016) 6 months to 3 years: 5.4 (Ficheux et al. 2016) Dermal exposure for Ginkgo biloba extract (mg/kg bw/day): [concentration (mg/g) * product amount (g) * frequency (/day) * dermal absorption] ÷ Body weight (kg) |
| Teeth whitener (Myrrh oil) |
Concentration: Myrrh oil: 50% (personal communication, emails from the CHPSD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Frequency of use (/day): 2 (product website) Assuming use by 14 to 19+ years Oral exposure: Product amount: 0.15 g Product instructions indicate addition of up to 3 drops to toothpaste (product website). Assuming 1 drop ~0.05 mL, and density of myrrh oil is 1 g/mL |
| Toothpaste (Sage oil) |
Concentration: Sage oil: 0.3% (personal communication, emails from the CHPSD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Frequency (/day): 19+ years: 2.5 (Garcia-Hidalgo et al. 2017) 9 to 18 years: 2.6 (Garcia-Hidalgo et al. 2017) 2 to 8 years: 2.9 (Ficheux et al. 2015) Oral exposure: Product amount (g): 14 to 19+ years: 0.08 (SCCS 2015; Ficheux et al. 2016) 9 to 13 years: 0.14 (Strittholt et al. 2016) 2 to 8 years: 0.21 (Strittholt et al. 2016) |
| Tooth powder (Myrrh oil) |
Concentration: Myrrh oil: 30% (personal communication, emails from the CHPSD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Frequency (/day) (adapted from toothpaste scenario): 19+ years: 2.5 (Garcia-Hidalgo et al. 2017) 9 to 18 years: 2.6 (Garcia-Hidalgo et al. 2017) 2 to 8 years: 2.9 (Ficheux et al. 2015) Oral exposure: Product amount (g) (adapted from toothpaste scenario): 14 to 19+ years: 0.08 (SCCS 2015; Ficheux et al. 2016) 9 to 13 years: 0.14 (Strittholt et al. 2016) 2 to 8 years: 0.21 (Strittholt et al. 2016) |
| Throat spray (NHP) (Sage oil) |
Concentration: Sage oil: 0.5% (as Salvia officinalis [sage] leaf extract) (personal communication, emails from the NNHPD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Age groups: 19+ years (personal communication, emails from the NNHPD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Frequency (/day): 3 (assumed short-term, intermittent use based on product’s intended use for soothing the throat) (personal communication, emails from the NNHPD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Product amount (mg): 473.5 Based on product instructions to spray up to 6 times per use, assuming each spray contains 0.1 mL and a density of 0.7892 g/mL (for alcohol, the main solvent in the product) (personal communication, emails from the NNHPD, HC, to the ESRAB, HC, dated June 2021; unreferenced) Daily oral exposure (mg/kg bw/day) = [product amount (mg) * frequency (/day)] ÷ body weight (kg) |
| Exposure scenario | Assumptions |
|---|---|
| Essential oil use in aromatic diffuser (Cade oil; jonquil oil; Verbena officinalis extract; myrrh oil; cork tree extract; sage oil; wormwood oil) |
Concentration: 100% for all substances. Frequency, including values used for LADD estimates (/day): 1 (assumed) Age group: 9 to 19+ years (users) 0 to 9 years (bystanders) Dermal exposure: Product amount (g): assumes 2 drops of the oils are spilled onto hands, where 1 drop ~0.05mL; assumed for users only Cade oil: 0.103 (based on density of 1.03 g/mL, SDS 2020a) Jonquil oil: 0.101 (based on density of 1.01 g/mL, MSDS [accessed 2022]) Verbena officinalis extract: 0.09 (based on density of 0.897 g/mL for citral, PubChem c2022c) Myrrh oil: 0.1 (based on an assumed density of 1 g/mL) Cork tree extract: 0.1 (based on an assumed density of 1 g/mL) Sage oil: 0.092 (based on density of 0.92 g/mL for thujone, PubChem c2022f) Wormwood oil: 0.09 (based on density of 0.9g/mL, SDS 2020b) Inhalation exposure: Estimated using ConsExpo web v.1.1.0 and defaults for a nebula diffuser (RIVM 2021b) Product amount: 0.920 g Exposure duration: 10 hours Room volume: 20 m3 Ventilation rate: 0.6/hr Emission duration: 10 hours Application temperature: 25°C |
| Essential oil use in baths (Cade oil; jonquil oil; wormwood oil) |
Concentration: 100% for all substances. Dermal exposure: Frequency (/day): 1 (assumed, to estimate exposure on the day of product use) Frequency (for LADD estimates, /day): 9 to 19+ years: 0.29 (Bremmer et al. 2006) Product amount (g): assumes approximately 10 drops of pure essential oil is added to the bath, where 1 drop ~ 0.05mL Cade oil: 0.513 (based on density of 1.03 g/mL, SDS 2020a) Jonquil oil: 0.505 (based on density of 1.01 g/mL, MSDS [accessed 2022]) Wormwood oil: 0.450 (based on density of 0.9g/mL, SDS 2020b) Volume of water in tub: 120 L Product amount on skin (mg): Calculated using a film-thickness approach where the concentration of the substance in the tub was multiplied by the amount of water in contact with skin. Amount of water in contact with skin (19+ years): 0.01 cm (ECHA 2015, for film thickness) * 17 530 cm2 (total body SA minus head) = 175.3 cm3 = 0.1753 L Example for product amount on skin: individual 19+ years = 0.513 g/120L × 1000 mg/g × 0.1753 L = 0.750 mg Inhalation exposure: Air concentrations were modeled using ConsExpo web v.1.1.0 exposure to vapour – evaporation – constant release area model: Product amount (g): ~0.450 to ~0.513, amount added to bath minus amount in contact with skin Exposure and emission duration: 45 min Molecular weight matrix: 18 g/mol (water) Temperature: 32°C Room volume: 10 m3 Ventilation rate: 2/hr SA: 11552 cm2 (equivalent to the surface area of a bathtub, standard tub dimensions of 76 cm × 152 cm) |
| Essential oil use in body moisturizer preparations (Cade oil; jonquil oil; wormwood oil) |
Concentration: diluted to 3% (Tisserand Institute [accessed 2021]) Frequency: 1 (assumed, to estimate exposure on the day of product use) Frequency (for LADD estimates, /day): 19+ years: 1 (Ficheux et al. 2015) 0 to 18 years: 0.8 (Wu et al. 2010; Ficheux et al. 2015) Dermal exposure: cade oil, jonquil oil, wormwood oil Product amount (g): 19+ years: 10 (Ficheux et al., 2016) 14 to 18 years: 10 (Ficheux et al. 2016, SA adjustment) 9 to 13 years: 7.7 (Ficheux et al. 2016, SA adjustment) 4 to 8 years: 5.0 (Ficheux et al. 2016, SA adjustment) 2 to 3 years: 4.1 (Ficheux et al. 2016) 1 year: 3.1 (Ficheux et al. 2016, SA adjustment) 6 to 11 months: 2.5 (Ficheux et al. 2016, SA adjustment) 0 to 5 months: 2.0 (Ficheux et al. 2016, SA adjustment) Inhalation exposure: Cade oil, jonquil oil, wormwood oil: Model: ConsExpo Web v.1.1.0 exposure to vapour–evaporation, constant release area mode (personal communication, RIVM to the ESRAB, HC, datedDecember 2020; unreferenced) Product amount (g): As above, adjusted to account for the amount remaining on the skin surface following dermal absorption of the substance. Exposure and emission duration (hr): 24 Molecular weight matrix (g/mol): 1000 Room volume (m3): 58 Ventilation rate (/hr): 0.5 Mass transfer coefficient (m/hr): 10 Release area (cm2): The release area is considered equivalent to unclothed skin SA, which was assumed to be half of the total body SA. 19+ years: 9058 14 to 18 years: 8415 9 to 13 years: 6525 4 to 8 years: 4298 2 to 3 years: 3113 1 year: 2433 6 to 11 months: 2045 0 to 5 months: 3180 |
| Essential oil use in facial steamer (Cade oil; jonquil oil; Verbena officinalis extract; myrrh oil; sage oil; wormwood oil) |
Concentration: 100% for all substances. Frequency, including values used for LADD estimates (/day): 1 (assumed) Age group: 4 to 19+ years (users) 0 to 3 years (bystanders) Inhalation exposure: Model: ConsExpo Web v.1.1.0-exposure to vapour-constant rate Product amount (g): assumes that approximately 10 drops of pure essential oil are added to the device, where 1 drop ~0.05mL Cade oil: 0.513 (based on density of 1.03 g/mL, SDS 2020a) Jonquil oil: 0.505 (based on density of 1.01 g/mL, MSDS [accessed 2022]) Verbena officinalis extract: 0.45 (based on density of 0.897 g/mL for citral, PubChem c2022c) Myrrh oil: 0.5 (based on an assumed density of 1 g/mL) Cork tree extract: 0.1 (based on an assumed density of 1 g/mL) Sage oil: 0.5 (based on density of 0.92 g/mL for thujone, PubChem c2022f) Wormwood oil: 0.450 (based on density of 0.9 g/mL, SDS 2020b) Exposure and emission duration: 20 min (assumed) Molecular weight matrix: Pure substance Temperature: 32°C Room volume: 1 m3 (personal cloud volume, assumed) Ventilation rate: 0.5/hr (assumed) Result: 20-min TWA: Cade oil: 240 mg/m3 Jonquil oil: 240 mg/m3 Verbena officinalis extract: 96 mg/m3 Myrrh oil: 23 mg/m3 Sage oil: 240 mg/m3 Wormwood oil: 210 mg/m3 20-min TWA, adj. (It was assumed that 50% of the 20-min TWA is available for inhalation and that the remainder is available for dermal exposure.): Cade oil: 120 mg/m3 Jonquil oil: 120 mg/m3 Verbena officinalis extract: 48 mg/m3 Myrrh oil: 11.5 mg/m3 Sage oil: 120 mg/m3 Wormwood oil: 105 mg/m3 Inhalation exposure, 24hr-TWA (mg/m3) = [20-min TWA, adj. (mg/m3) × (20 min/1440 mins)] Dermal exposure: It is assumed that 50% of the 20-min TWA will be inhaled and 50% will be exposed dermally (that is, 105 mg/m3 × 1 m3 = 105 mg). Dermal exposure (mg/kg bw/day) was calculated using the following formula: [20-min TWA, adj (mg/m3) * room volume (1 m3)] ÷ body weight (kg) |
| Essential oil use in massage oil preparations (Cade oil; jonquil oil; cork tree extract; wormwood oil) |
Concentration: diluted to 3% (Tisserand Institute [accessed 2021]) Frequency: 1 (assumed, to estimate exposure on the day of product use) Frequency (for LADD estimates, /day): 19+ years: 0.11 (Ficheux et al. 2015) 14 to 18 years: 0.11 (Ficheux et al. 2015) –13 years: 0.13 (Ficheux et al. 2015) Dermal exposure: Cade oil, jonquil oil, cork tree extract, wormwood oil: Product amount (g): 19+ years: 3.2 (Ficheux et al., 2016) 14 to 18 years: 2.9 (Ficheux et al., 2016, SA adjustment) 9 to 13 years: 2.3 (Ficheux et al., 2016, SA adjustment) 4 to 8 years: 1.9 (Ficheux et al., 2016, SA adjustment) 0 to 3 years: 1.8 (Ficheux et al., 2016) Inhalation exposure: Cade oil, jonquil oil, wormwood oil: Model: Consexpo Web v.1.1.0 exposure to vapour–evaporation, constant release area (personal communication, RIVM to the ESRAB, HC, dated December 2020; unreferenced) Product amount (g): As above, adjusted to account for the amount remaining on the skin surface following dermal absorption of the substance. Exposure and emission duration (hr): 8.5 Molecular weight matrix: 452 (based on mineral oil as carrier oil) Room volume (m3 ): 20 Ventilation rate (/hr): 0.6 Mass transfer coefficient (m/hr): 10 Release area (cm2): total body SA, minus half-head and half-torso (HC 2021) 19+ years: 14670 14 to 18 years: 13340 9 to 13 years: 13050 4 to 8 years: 8595 2 to 3 years: 6225 1 year: 4865 6 to 11 months: 4090 0 to 5 months: 3180 |
| Essential oil use in stomach remedy (Myrrh oil) |
Concentration: Myrrh oil: 100% Frequency (/day): 2 to 19+ years: 1 (assumed) (assumed short-term, intermittent use based on intended use to relieve the stomach) Oral exposure: Product amount (g): 0.1 Assumes approximately 2 drops of pure essential oil are added to water (doTERRA 2022b), where 1 drop ~0.05 mL, and density of myrrh oil is 1 g/mL |
| Essential oil use in topical preparations to treat damaged/abraded skin (Cade oil) |
Concentration: dilution to 1% (Base Formula [accessed 2021]) Frequency (/day): 3 (assumed, to estimate exposure on the day of product use) Frequency (for LADD estimates, /day): 1 (professional judgment) Product amount (g): Product amount data were estimated from IQWiG (2017), on typical amounts applied for topical ointments. Values assumed use on back and buttocks. 19+ years: 3.5 (IQWiG 2017) 14 to 18 years: 3.5 (IQWiG 2017) 9 to 13 years: 3.5 (IQWiG 2017) 4 to 8 years: 2.5 (IQWiG 2017) 2 to 3 years: 1.63 (age adjustment, IQWiG 2017) 1 year: 1.5 (IQWiG 2017) 6 to 11 months: 0.75 (IQWiG 2017) 0 to 5 months: 0.75 (IQWiG 2017) |
Appendix B. Additional information on main components and analogues
| Physical property | Cadinene (CAS RN 29350-73-0)a |
τ-Muurolol (CAS RN 19912-62-0) |
Widdrol (CAS RN 6892-80-4) |
Epi-cubenol (CAS RN 19912-67-5) |
Alpha-terpineol (CAS RN 98-55-5) |
|---|---|---|---|---|---|
| Melting point (°C) | 35.55 | 80.5 to 81.5b | 78.80c | N/Ac | 31 to 40.5b |
| Boiling point (°C) | 262.43 | 292.61c | 291.53c | 303.4c | 210 to 219b |
| log P (octanol-water) | 6.32 | 4.77c | 4.84c | 4.97c | 2.37b |
| Water solubility (mg/L) @ 25°C | 0.04864 | 9.13c | 7.93c | N/A | 371.7c |
| Vapour pressure (mmHg) @ 25°C | 0.0188 | 8.24 ×10-5c | 7.13 × 10-5c | 0.0±1.4c | 1.96 × 10-2c |
| Average mass (Da) | 204.351 | 222.37 | 222.37 | 222.37 | 154.25 |
a Predicted value using EpiSuite 4.11 from ChemSpider c2015
b Experimental values using EpiSuite 4.11 from ChemSpider c2015
c Predicted values using EpiSuite 4.11 from ChemSpider c2015
| Physical property | Thujopsene (CAS RN 470-40-6) |
α-Cedrene (CAS RN 469-61-4) |
Virginia cedarwood oil (CAS RN 85085-41-2) |
|---|---|---|---|
| Melting point (°C) | 48.59a | 262.5a | -80c |
| Boiling point (°C) | 258 to 260b | 245.05a | 252c |
| log P (octanol-water) | 6.12 a | 5.74a | 4.33 to 7.02c |
| Water solubility (mg/L) @ 25°C | 0.071a | 0.15a | 0.03 to 29.25c |
| Vapour pressure (mmHg) @ 25°C | 0.07a | 1.38 × 10-4a | 1.9 × 10-2 c |
| Average mass (Da) | 204.19 | 204.35 | NA |
a Predicted values using EpiSuite 4.11 from ChemSpider c2015
b Experimental values using EpiSuite 4.11 from ChemSpider c2015
c Experimental values from ECHA 2021b
| Physical property | o-Cresol (CAS RN 470-40-6)a |
m-Cresol (CAS RN 469-61-4)a |
p-Cresol (CAS RN 85085-41-2) |
|---|---|---|---|
| Melting point (°C) | 31 | 11.8 | 35.5 |
| Boiling point (°C) | 191 | 202 | 202 |
| log P (octanol-water) | 1.95 | 1.96 | 1.94 |
| Water solubility (mg/L) @ 25°C | 26 × 103 | 23 × 103 | 22 × 103 |
| Vapour pressure (mmHg) @25°C | 0.28 | 0.11 | 0.11 |
| Average mass (Da) | 108.14 | 108.14 | 108.14 |
| Density (mg/l) | 1.05 | 1.03 | 1.03 |
a Experimental values from ECHA 2022e, 2022f, 2022g
| Physical property | Guaiacol (CAS RN 90-05-1)a |
|---|---|
| Melting point (°C) | 32 |
| Boiling point (°C) | 205 |
| log P (octanol-water) | 1.32 |
| Water solubility (mg/L) @ 25°C | 1.84 × 104 |
| Vapour pressure (mmHg) @ 25°C | 0.10 |
| Average mass (Da) | 124 |
a Experimental values from ECHA 2021c
| Physical property | Verbascoside (CAS RN 61276-17-3) | Neohesperidin dihydrochalcone (CAS RN 20702-77-6) |
|---|---|---|
| Melting point (°C) | N/A | 157b |
| Boiling point (°C) | 908.8b | 927±65.0a |
| log P (octanol-water) | -0.71b | -0.31b |
| Water solubility (mg/L) @ 25°C | N/A | N/A |
| Vapour pressure (mmHg) @ 25°C | N/A | N/A |
| Average mass (Da) | 624.59 | 612.20 |
| Density (g/mL) | 1.6a | 1.38c |
a Predicted values using ACD/LABs from ChemSpider c2015
b Experimental values from ChemSpider 2021
c Experimental values from ECHA 2022h
| Physical property | Isobornyl formate (CAS RN 1200-67-5) |
Isobornyl acetate (CAS RN 125-12-2) |
Isobornyl acrylate (CAS RN 5888-33-5) |
|---|---|---|---|
| Melting point (°C) | 29.06a | 29e | -35e |
| Boiling point (°C) | 208.55a | 106 to 233e | 119 to 121e |
| log P (octanol-water) | 3.3a | .47b | 4.21a |
| Water solubility (mg/L) @ 25°C | 80.06a | 9.72a | 10.04a |
| Vapour pressure (mmHg) @ 25°C | 0.199a | 0.107a | 0.0077a |
| Average mass (Da) | 182.26 | 196.29 | 208.30 |
| Density (g/mL) | 1.1c | 0.98c | 0.99d |
a Predicted values using EPISuite from ChemSpider c2015
b Experimental values from ChemSpider c2015
c PubChem c2022a, c2022b
d Experimental values from ECHA 2021g
| Physical property | Citral (CAS RN 5392-40-5) |
|---|---|
| Melting point (°C) | -26.7a |
| Boiling point (°C) | 217a |
| log P (octanol-water) | 3.45a |
| Water solubility (mg/L) @ 25°C | 84.71a |
| Vapour pressure (mmHg) @ 25°C | 0.089a |
| Average mass (Da) | 152.24 |
| Density (g/mL) | 0.897b |
a Predicted values using EPISuite from ChemSpider c2015
b PubChem c2022c
Appendix C. Derivation of benchmark dose level (BMDL) for ginkgo biloba leaf extract
| Dose (mg/kg bw/day) | Number of animals (N) | Tumor incidence |
|---|---|---|
| 0 | 50 | 39 |
| 200 | 50 | 46 |
| 600 | 50 | 46 |
| 2000 | 50 | 49 |
| Model | Analysis type | Restriction | Risk type | BMRF | BMD | BMDL | BMDU | P value | AIC | BMDS recommendation |
|---|---|---|---|---|---|---|---|---|---|---|
| Multistage Degree 3 | frequentist | Restricted | Extra risk | 0.1 | 82.87592 | 44.6936 | 641.52976 | 0.3350422 | 124.5513528 | Viable - Alternate |
| Multistage Degree 2 | frequentist | Restricted | Extra risk | 0.1 | 82.87591 | 44.69359 | 530.20522 | 0.3350422 | 124.5513528 | Viable – Recommended (Lowest AIC) |
| Multistage Degree 1 | frequentist | Restricted | Extra risk | 0.1 | 82.87616 | 44.69373 | 209.45036 | 0.3350422 | 124.5513528 | Viable - Alternate |
Abbreviations: AIC, Akaike’s Information Criterion; BMRF, factor defining the benchmark response level; BMD, benchmark dose; BMDL, lower 95% confidence limit of the BMD; BMDU, upper 95% confidence limit of the BMD.
Figure C-1. BMD modelling for combined incidence of hepatocellular adenomas and/or carcinomas in male mice exposed to Ginkgo biloba extract

Long description
This is a dose-response graph, with dose on the X-axis and response on the Y-axis. Tumour response data based on the combined incidence of hepatocellular carcinoma and adenoma in mice (open circles) were selected for benchmark dose modelling to calculate the 10% excess risk of the benchmark response (BMDL10) associated with the lower 95% confidence limit. The calculated BMDL10 value is 45 mg/kg bw/day, based on the best fitting multistage model.
