Risk management scope for triphenyl phosphate (TPHP) and tris(2-butoxethyl) phosphate (TBOEP)
Official title: Risk Management Scope for Triphenyl phosphate (TPHP) and Tris(2-butoxethyl) phosphate (TBOEP) (Updated Human Health Component)
Chemical Abstracts Service Registry Numbers (CAS RNs):
- 115-86-6
- 78-51-3
Environment and Climate Change Canada
Health Canada
November 2025
Summary of proposed risk management
This risk management scope outlines the risk management options under consideration for TPHP and TBOEP. It is proposed to conclude that these substances are harmful to human health under a human health risk characterization. The proposed risk management options under consideration for human health are being considered in addition to the risk management options under consideration to address the risks to the environment of the aryl organophosphates subgroup (which includes TPHP), as outlined in the November 2021 Risk Management Scope for TPHP, BPDP, BDMEPPP, IDDP, IPPP and TEP.
For the purposes of paragraph 77(1)(a) of the Canadian Environmental Protection Act, 1999 (CEPA), the Government of Canada proposes to recommend that TPHP and TBOEP be added to Part 2 of Schedule 1 to CEPAFootnote 1. As a result, the Government of Canada is considering the following new risk management actions:
- Regulatory and/or non-regulatory actions to reduce dermal exposure to TPHP of adults from certain lubricants and greases
- Regulatory and/or non-regulatory actions to reduce prolonged dermal exposure to TPHP of people of all ages from products made with polymeric foams (such as certain mattresses and upholstered furniture)
- Regulatory and/or non-regulatory actions to reduce prolonged dermal exposure to TPHP of infants and children up to 13 years of age from the foam in certain infant and child restraint systems
- Regulatory and/or non-regulatory actions to reduce prolonged dermal exposure of people of all ages to TBOEP in products made with polymeric foams (such as certain mattresses and upholstered furniture)
- Regulatory and/or non-regulatory actions to reduce prolonged dermal exposure of infants and children up to 13 years of age to TBOEP in the foam of certain infant and child restraint systems
The Government of Canada is also considering other risk management actions, as follows:
- Listing TPHP as a prohibited or restricted ingredient on the Cosmetic Ingredient HotlistFootnote 2 to help reduce dermal exposure to TPHP of people 4 years of age and older from certain nail care products
To inform risk management decision-making, information on the following topics should be provided (ideally on or before January 21, 2026) to the contact details identified in section 8 of this document:
- Anticipated economic impacts if the import/export and/or use of TPHP and/or TBOEP are prohibited or restricted in Canada
- Ongoing or anticipated changes in use of the above flame retardants, whether in response to:
- Shifts to alternative substances (please provide commercial name), alternative systems and approaches
- Market forces
- Changes in performance-based flammability requirements and/or standards; and/or
- Other reasons (please provide information on these reasons)
The risk management options outlined in this Updated Risk Management Scope document may evolve through consideration of assessments and risk management options or actions published for other Chemicals Management Plan (CMP) substances as required to ensure effective, coordinated, and consistent risk management decision-making.
Note: The above summary is an abridged list of options under consideration to manage these substances and to seek information on identified gaps. Refer to section 3 of this document for more complete details in this regard. It should be noted that the proposed risk management options may evolve through consideration of additional information obtained from the public comment period, literature and other sources.
1. Context
The Canadian Environmental Protection Act, (CEPA) (Canada 1999) provides the authority for the Minister of the Environment and the Minister of Health (the Ministers) to conduct assessments to determine if substances are toxic to the environment and/or human health as set out in section 64 of CEPAFootnote 3, Footnote 4, and, if so, to manage the associated risks.
The 2 substances, listed in Annex A and referred to throughout this document as TPHP and TBOEP, are included in the Flame Retardants Group of the Substance Groupings Initiative of the Chemicals Management Plan (CMP) (Canada 2025).
2. Issue
Health Canada (HC) and Environment and Climate Change Canada (ECCC) conducted a joint scientific assessment of the Flame Retardants Group in Canada. A notice summarizing the scientific considerations of the draft assessment for these substances was published in the Canada Gazette, Part I on November 6, 2021 (Canada 2021). For further information, please refer to the Draft Assessment for the Flame Retardants Group.
Since the publication of this draft assessment, new information identified through the scientific literature and updated exposure parameters has led to a new proposed conclusion with respect to human health for TPHP and TBOEP. For more information, please refer to the Human health risk characterization document for TPHP and TBOEP.
2.1 Human health risk characterization conclusion
On the basis of the information available, the human health risk characterization proposes that TPHP and TBOEP are toxic under section 64(c) of CEPA because they constitute or may constitute a danger in Canada to human life or health (Canada 2025). The proposed conclusions with respect to the other substances in the Flame Retardants Group and their corresponding proposed risk management options under consideration remain unchanged from the November 2021 draft assessment and the 2021 risk management scope. It should be noted that TPHP was proposed toxic under section 64(a) of CEPA in the November 2021 draft assessment.
In addition, the November 2021 draft assessment also found that, although risk to the environment has not been identified at current levels of exposure, there may be a concern if exposures to TBOEP were to increase. As a result, this substance may be considered in future initiatives to track its commercial status or identify new uses or exposures.
The exposure sources of concern identified in the human health risk characterization are based on the potential dermal exposure to TPHP and TBOEP from certain products available to consumers. As such, this document will focus on these exposure sources (refer to section 5).
2.2 Proposed recommendation under CEPA
CEPA sets out a 2-track approach for managing risks.
Under sub-section 77(3), the Ministers are required to propose recommending the addition of a substance that meets the criteria set out in paragraph (a), (b) or (c), to Part 1Footnote 5 of Schedule 1 to CEPA and, in developing a proposed regulation or instrument respecting preventive or control actions, to give priority to the total, partial or conditional prohibition of activities in relation to the substance or to the release of the substance into the environment.
For other substances recommended for addition to Part 2 of Schedule 1 to CEPA, the Ministers shall give priority to pollution prevention, and this could include regulatory or non-regulatory measures such as prohibition if warranted.
On the basis of the findings of the human health risk characterization conducted pursuant to CEPA, the Ministers recommend that TPHP and TBOEP be added to Part 2 of Schedule 1 to CEPAFootnote 6 at this time. Addition of a substance to Schedule 1 to CEPA enables the Government to propose certain risk management measures under CEPA to manage potential ecological and human health risks associated with the substance.
The Ministers will take into consideration comments made by stakeholders during the 60-day public comment period on the human health risk characterization and this risk management scope. If the Ministers finalize the recommendation to add TPHP and TBOEP to Part 2 of Schedule 1, risk management instruments must, unless an exception in section 91 of CEPA applies, be proposed within 24 months from the date on which the Ministers recommended that TPHP and TBOEP be added to Schedule 1 to CEPA, and finalized within 18 months from the date on which the risk management instruments are proposed, as outlined in sections 91 and 92 of CEPA (refer to section 8 for publication timelines applicable to this group of substances). Adding a substance to Schedule 1 does not restrict its use, manufacture, or import. Rather, it enables the Government of Canada to take risk management actions under CEPA.
3. Proposed risk management
3.1 Proposed human health objectives
Proposed human health objectives are quantitative or qualitative goals to address human health concerns.
For these substances, the proposed objectives address the exposure sources of concern outlined in section 5 of this document. The proposed human health objectives for TPHP and TBOEP are to reduce dermal exposure of the general population to levels that are protective of human health.
3.2 Proposed risk management objectives
Proposed risk management objectives set quantitative or qualitative targets to be achieved by the implementation of risk management regulations, instruments and/or tools for a given substance or substances. In this case, the proposed risk management objectives for TPHP and TBOEP are to:
- reduce dermal exposure to TPHP of people 4 years of age and older from certain nail care products
- reduce dermal exposure to TPHP of adults from certain lubricants and greases
- reduce prolonged dermal exposure of people of all ages to TPHP in products made with polymeric foams (such as certain mattresses and upholstered furniture)
- reduce prolonged dermal exposure to TPHP of infants and children up to 13 years of age from the foam in certain infant and child restraint systems, including booster seats
- reduce prolonged dermal exposure of people of all ages to TBOEP in products made with polymeric foams (such as certain mattresses and upholstered furniture)
- reduce prolonged dermal exposure of infants and children up to 13 years of age to TBOEP in the foam of certain infant and child restraint systems, including booster seats
These objectives will be refined on the basis of stakeholder consultation and new information, the proposed risk management, the outcome of the assessment, and socio-economic and technical considerations (refer to section 6). Revised human health and risk management objectives will be presented in the Risk Management Approach document that will be published concurrently with the assessment for these substances.
3.3 Proposed risk management actions under consideration
To achieve the proposed risk management objectives and to work towards achieving the proposed human health objectives, for the purposes of paragraph 77(1)(a) of CEPA, the Government of Canada proposes to recommend that TPHP and TBOEP be added to Part 2 of Schedule 1 to CEPA. As a result, the Government of Canada is considering the following new risk management actions:
- Regulatory and/or non-regulatory actions to reduce dermal exposure to TPHP of adults from certain lubricants and greases. This could include regulatory measures under the Canada Consumer Product Safety Act (CCPSA) or under CEPA. Non-regulatory measures under CEPA, such as performance agreements or codes of practice, could also be considered and developed in partnership with industry stakeholders
- Regulatory and/or non-regulatory actions to reduce prolonged dermal exposure to TPHP of infants and children up to 13 years of age from the foam in certain infant and child restraint systems, including booster seats. This could include regulatory measures under the CCPSA or under CEPA. Non-regulatory measures under CEPA, such as performance agreements or codes of practice, could also be considered and developed in partnership with industry stakeholders
- Regulatory and/or non-regulatory actions to reduce prolonged dermal exposure of people of all ages to TPHP in products made with polymeric foams (such as certain mattresses and upholstered furniture). This could include regulatory measures under the CCPSA or under CEPA. Non-regulatory measures under CEPA, such as performance agreements or codes of practice, could also be considered and developed in partnership with industry stakeholders
- Regulatory and/or non-regulatory actions to reduce prolonged dermal exposure of people of all ages to TBOEP in products made with polymeric foams (such as certain mattresses and upholstered furniture). This could include regulatory measures under the CCPSA or under CEPA. Non-regulatory measures under CEPA, such as performance agreements or codes of practice, could also be considered and developed in partnership with industry stakeholders
- Regulatory and/or non-regulatory actions to reduce prolonged dermal exposure of infants and children up to 13 years of age to TBOEP in the foam of certain infant and child restraint systems, including booster seats. This could include regulatory measures under CCPSA or under CEPA. Non-regulatory measures under CEPA, such as performance agreements or codes of practice, could also be considered and developed in partnership with industry stakeholders
The Government of Canada is also considering other risk management options, as follows:
- Listing TPHP as a prohibited or restricted ingredient on the Cosmetic Ingredient HotlistFootnote 7 to help reduce dermal exposure to TPHP of people 4 years of age and older from certain nail care products
Cosmetics are regulated under the Food and Drugs Act and its regulations. For the purposes of subparagraph 77(1)(b) of CEPA, human health risks associated with substances are regulated in cosmetics by section 16 of the Food and Drugs Act.
Note that these proposed risk management options are preliminary and subject to change. Following the publication of this document, additional information obtained from the public comment period and from other sources will also be considered in the instrument selection and development processFootnote 8. The risk management options may also evolve through consideration of assessments and risk management options or actions published for other CMP substances (such as other chemical flame retardant substances) to ensure effective, coordinated, and consistent risk management decision-making.
3.4 Performance measurement evaluation
Performance measurement evaluates the ongoing effectiveness and relevance of the actions taken to manage risks from toxic substancesFootnote 9. ECCC and HC have developed a Performance Measurement Evaluation Strategy that sets out the approach to evaluate the effectiveness of actions taken on substances found toxic under CEPA. The aim is to determine whether human health and/or environmental objectives have been met and whether there is a need to revisit the risk management approach for these substances. Selection of a substance for performance measurement evaluation is conducted through readiness, prioritization and work planning as outlined in the Performance Measurement Evaluation Strategy. In evaluating progress and revisiting risk management, as warranted, these activities together will aim to manage risks effectively over time.
The Government of Canada may measure the effectiveness of the risk management actions and the progress towards meeting the risk management human health objectives for TPHP and TBOEP.
To do so, the Government of Canada may collect and analyze data, such as industry reporting on the presence of TPHP and TBOEP in the products of concern.
When undertaken, the results of performance measurement and evaluation are used to inform whether further risk management action is warranted and are made available to people in Canada along with recommendations for further action, if applicable.
3.5 Risk management information gaps
Interested stakeholders can provide further information to inform risk management decision-making regarding TPHP and TBOEP, including:
- Quantity and current use of TPHP by Canadian importers/exporters in different products (for example, adhesives and sealants, paints and coatings, lubricants and greases, and plastic and rubber formulation)
- The use of TPHP and TBOEP in textiles, including textile backings in furniture
- The use of TPHP and TBOEP in polymeric foams other than polyurethane foams (PUFs), which may be used in products such as furniture, mattresses, and other foam-based products to which prolonged skin contact may be expected
- Changes to TPHP and TBOEP use patterns and economic impacts, including:
- Anticipated economic impacts if the import/export and/or use of the flame retardants in question are prohibited or restricted in Canada
- Ongoing or anticipated changes in use of the above flame retardants, whether in response to:
- Market forces
- Shifts to alternative substances (please provide commercial name), alternative systems and approaches
- Changes in performance-based flammability requirements and/or standards; and/or
- Other reasons (please provide information on these reasons)
- Chemical and non-chemical alternatives to TPHP and TBOEP for the uses of concern
Stakeholders that have information to help address these gaps should provide it on or before January 21, 2026 to the address identified in section 8.
4. Background
4.1 General information on TPHP and TBOEP
TPHP is one of the aryl organophosphate (OP) subgroup substances addressed in the Flame Retardants Group draft screening assessment. It is an organophosphate ester containing 3 aryl groups (that is, triaryl).
TBOEP is part of the alkyl OP subgroup addressed in the Flame Retardants Group draft screening assessment. Substances in the alkyl OP subgroup contain 3 alkyl substituents, with alkyl chain length and branching varying throughout the subgroup. TBOEP is a phosphate ester that contains 3 2-butoxyethyl groups.
Neither TPHP nor TBOEP occur naturally in the environment.
4.2 Current uses and identified sectors
According to information submitted in response to surveys under section 71 of CEPA (Canada 2012, 2016), TPHP was not manufactured in Canada in 2011 and/or 2015; however, a total of 100 000 kg to 10 000 000 kg of TPHP was imported into Canada in each of the 2011 and 2015 reporting years (Environment Canada 2013; ECCC 2016).
In Canada, according to information submitted in response to CEPA section 71 surveys (Environment Canada 2013; ECCC 2016b), TPHP is primarily used as either an additive flame retardant and/or as a plasticizer in products available to consumers and commercial products, such as adhesives and sealants, paints and coatings, lubricants and greases, and in plastic and rubber formulation. TPHP has been detected in foam-containing products available to consumers in Canada such as mattresses, upholstered furniture and children’s products including infant and child restraint seats (CEC 2015; HC 2019).
TPHP is used in cosmetics in Canada, primarily nail care products. It may also be used in food packaging materials and as a formulant in pest control products. It is currently present in 2 registered domestic class pest control products, both of which are anti-fouling paints with marine applications.
While there is no confirmed textile use in Canada, TPHP has been identified in infant clothing and textiles/fabrics in the United States (US) (Zhu et al. 2020).
In 2011, a total of 1 000 kg to 10 000 kg of TBOEP was manufactured in and between 10 000 kg and 100 000 kg was imported into Canada (Canada 2012).
In general, TBOEP is used as an additive flame retardant and plasticizer (Ash and Ash 2009). In Canada, according to CEPA section 71 surveys (Environment Canada 2013), TBOEP is used in paints and coatings, and floor coverings. It may also be used as a component in the manufacture of a limited number of adhesives used in the middle layers of food packaging materials and as a formulant in pest control products.
TBOEP can also be found in rust paint, leather repair solution, shoe waterproofing spray, marine mildew block spray, and glass marker products in Canada.
5. Exposure sources of concern and identified risks
As outlined in the Human Health Risk Characterization Document, dermal exposure to TPHP from prolonged contact with certain foam-containing mattresses or upholstered furniture (all age groups), prolonged contact with certain foam-containing infant and child restraints systems (including booster seats), and dermal contact to certain nail care products (4 years of age and older) and lubricants and greases (for example, power steering fluids, engine oils, synthetic greases) (adults) were identified as exposures of concern.
Dermal exposure to TBOEP from prolonged contact with foam-containing mattresses or upholstered furniture (all age groups) and prolonged contact with infant or child restraint systems (0 to 13 years of age) were also identified as scenarios of concern.
6. Risk management considerations
6.1 Alternatives and alternate technologies
Flame retardant substances are generally used to meet performance-based flammability requirements and/or standards. These requirements do not specify that chemical flame retardants need to be used; rather, they may require a product or components thereof to pass a laboratory test such as a cigarette smolder or open flame ignition test (for restraint systems and booster seats only). Performance-based flammability requirements exist internationally for various types of products, including electronics, building materials, mattresses and upholstered furniture, among others (US CPSC 2006; California 2013; UL 2014; ASTM 2014; Canada 2016a). They can be regulatory, and may exist at different levels of government (California 2013; Canada 2016b). Voluntary standards for flammability are also developed by independent standard development organizations (ASTM 2014; ISO 2014; UL 2014). Using chemical flame retardants in their products is one means through which companies can help ensure that their products meet performance-based flammability requirements. However, technologies other than chemical flame retardants that allow products to meet performance-based flammability requirements also exist. Products may be made of materials with a low flammability or materials that require lower quantities of flame retardants to meet standards. For example, concentrations of flame retardants tend to be higher in lower density foams, which tend to ignite more easily than higher density foams (CEC 2015). Materials such as foams may also be covered with a protective barrier made of a material that does not burn easily (US EPA 2005).
Where chemical flame retardants are concerned, a number of factors come into play in determining whether one flame retardant is a good alternative to another. Different flame retardants are appropriate for application to different materials and for different end uses. Their physical and chemical properties affect their ability to meet performance-based flammability requirements as well as the uses in which they can be effective. Flame retardant properties such as pH, viscosity, the ability to mix evenly and stability in exothermic reactions can impact the quality of end products such as foams (CEC 2015b; Danish EPA 2016a). The potential for effects on health and the environment are key considerations in the selection of alternatives. The US Environmental Protection Agency (US EPA) updated its ‘Design for the Environment’ report comparing the health and environmental properties of a variety of flame retardants for use in flexible PUF in 2015 (US EPA 2015). The Danish Environmental Protection Agency (Danish EPA 2016b) also published a report on some flame retardants which may be used in flexible PUF. Finally, cost is also a factor companies consider in decisions regarding alternative flame retardants.
6.2 Socio-economic and technical considerations
Changes to performance-based flammability requirements are affecting the need for chemical flame retardants, particularly flame retardants used in flexible PUF. Companies producing foam may manufacture their foam so that it can pass the most stringent flammability standards for any product in which it is used, even if many of the end products containing the foam are not subject to those stringent flammability standards.
Within North America, California standard Technical Bulletin (TB) 117 previously required that upholstered furniture in the California marketplace pass an open flame test, creating a market for certain flame retardants in flexible PUF (Stapleton et al. 2011, 2012). However, the revised standard, California TB 117-2013 (California 2013), which came into effect on January 1, 2015, requires a cigarette smoulder test and no longer requires an open flame test. It also allows for the use of barrier materials in upholstered furniture to allow the furniture to pass the flammability tests without the use of added chemical flame retardants.
Two federal flammability standards exist for mattresses in the US: the Standard for the Flammability of Mattresses and Mattress Pads, 16 CFR 1632 (US CPSC 1984); and the Standard for the Flammability (Open Flame) of Mattress Sets; 16 CFR 1633 (US CPSC 2006). Comments on the proposed rule raised concerns about the possible need for flame retardants to meet the open flame test. The standard 16 CFR 1633 applies to mattresses and mattress foundation sets. A mattress is defined as a resilient material, used alone or in combination with other materials, enclosed in a ticking and intended or promoted for sleeping upon. It does not include uncovered foam mattress toppers or pads (US CPSC 2006); however, the Standard 16 CFR 1632 does apply to mattress pads or toppers (excludes convoluted foam pads which are not totally encased in ticking).
The United Kingdom has consulted on proposed changes to its Furniture and Furnishings (Fire Safety) Regulations 1988, which could reduce the use of flame retardants and better reflect modern furniture manufacturing processes, while maintaining safety (UK Department for Business, Energy and Industrial Strategy, 2016). To date, though, no new regulations have been published.
Canada has regulations under the CCPSA that set out performance-based flammability requirements for mattresses and textile products that are manufactured, imported, sold or advertised in Canada (Canada 2016a, 2016b). As with other performance-based standards, the applicable regulations under the CCPSA do not prescribe how the requirements are to be met. Various options are available to manufacturers, including the use of materials that are less flammable, or that are inherently flame resistant (such as wool), fire barrier systems, or flame retardant chemicals. It is not expected that Canada’s federal flammability requirements for mattresses drive the flame retardant market. Canada does not have a federal flammability standard for upholstered furniture other than mattresses. Uncovered foam mattress pads are not considered mattresses, but may be subject to the Textile Flammability Regulations (Canada 2016b), as they may be considered ‘bedding’ under those regulations.
Socio-economic factors will be considered in the selection process for a regulation or instrument respecting preventive or control actions, and in the development of the risk management objective(s) as per the guidance provided in the Treasury Board document Assessing, Selecting, and Implementing Instruments for Government Action [PDF] (TBS 2007). In addition, socio-economic factors will be considered in the development of regulations, instrument(s) or tool(s) to address risk management objective(s), as identified in the Cabinet Directive on Regulation (TBS 2018), the Red Tape Reduction Action Plan (TBS 2012) and the Red Tape Reduction Act (Canada 2015).
7. Overview of existing risk management
7.1 Related Canadian risk management context
Existing risk management for TPHP and TBOEP in Canada is summarized below. In general, cosmetics are regulated under the Food and Drugs Act and its regulations as follows.
Cosmetics
The human health risks of substances in cosmetics are primarily managed under the Food and Drugs Act and the Cosmetic Regulations. The addition or modification of the entries in the Cosmetic Ingredient Hotlist (Hotlist) inform stakeholders and the public about substances that, according to HC, may contravene section 16 of the Food and Drugs Act or may contravene one or more provisions of the Cosmetic Regulations when they are present in a cosmetic. Section 16 of the Food and Drugs Act states, among other things, that “No person shall sell any cosmetic that has in or on it any substance that may cause injury to the health of the user.”
- Neither TPHP nor TBOEP are on the Hotlist, and TPHP is used in nail care products in Canada
- TPHP and TBOEP may be used in food packaging materials in Canada
- TPHP is permitted for use as a formulant in pest control products in Canada
7.2 Pertinent international risk management context
In the US, a number of risk management actions have been taken for TPHP and TBOEP, or have implications for these substances:
- California has passed legislation prohibiting the sale of new covered upholstered furniture, children's products, and mattresses that contain halogenated, phosphorus-based, nitrogen-based or nanoscale flame retardants above 1 000 ppm (parts per million). The repair of upholstered furniture with foam containing flame retardants above 1 000 ppm is also prohibited. The prohibition took effect on January 1, 2020 (California 2018)
- Maine has passed legislation to prohibit the sale of new residential upholstered furniture containing halogenated, phosphorus-based, nitrogen-based and nanoscale flame retardants above 1 000 ppm. The prohibition took effect on January 1, 2019 (Maine 2017)
- New York has passed legislation that prohibits the use of halogenated, organophosphorus, and organonitrogen flame retardant chemicals in upholstered furniture, mattresses and electronic enclosures. This law came into effect in January 2024 (New York 2021)
- Maryland has passed legislation prohibiting the sale of any juvenile product, mattress, upholstered furniture or reupholstered furniture that contains more than 0.1 percent of flame retardant chemicals by mass (Maryland 2020)
- New Hampshire has passed legislation that prohibits the manufacture and sale of upholstered furniture containing in its fabric or other covering or in its cushioning materials more than 0.1 percent of a halogenated, phosphorus-based, nitrogen-based or nanoscale flame-retardant chemical or more than 0.1 percent of a mixture that includes flame-retardant chemicals. The prohibition fully came into effect in January 2021 (New Hampshire 2019)
In addition, TPHP is used as a plasticizer in cosmetics in the European Union (EU) and TPHP and TBOEP are both permitted for use in certain food packaging materials in the US and the EU and for non-food use in pesticides in the US.
8. Next steps
8.1 Public comment period
Industry and other interested stakeholders are invited to submit comments on the content of this document or other information that would help to inform decision-making (such as the information gaps outlined in section 3.5). Please submit additional information and comments prior to January 21, 2026.
If the assessment confirms that TPHP and TBOEP are toxic, a risk management approach document outlining and seeking input on the proposed risk management instruments would be published concurrently with the assessment. At that time, there would be further opportunity for consultation.
Comments and information submissions on the risk management scope should be submitted to the address provided below:
Environment and Climate Change Canada
Gatineau, Quebec K1A 0H3
Telephone: 1-800-567-1999 (in Canada) or 819-938-3232
Fax: 819-938-5212
Email: substances@ec.gc.ca
Companies who have a business interest in TPHP and/or TBOEP are encouraged to identify themselves as stakeholders. The stakeholders will be informed of future decisions regarding TPHP and TBOEP and may be contacted for further information.
Stakeholders and members of the public who are interested in being notified of CMP publications are invited to subscribe for the latest news on the CMP. Stakeholders and members of the public who would like to receive CMP Publication Plans on a quarterly basis by email can contact: substances@ec.gc.ca.
When a statement identifying the first regulation or instrument is published in relation to TPHP and TBOEP, a statement outlining the estimated timeframe for the development of subsequent proposed regulations or instruments will be made available.
8.2 Timing of actions
Electronic consultation on the human health risk characterization and Risk Management Scope: November 22, 2025 to January 21, 2026. This should include the submission of public comments, additional studies and/or information on TPHP and TBOEP.
Publication of responses to public comments on the human health risk characterization and Risk Management Scope: concurrent to the publication of the assessment and, if required, the Risk Management Approach document.
Publication of responses to public comments on the Risk Management Approach, if applicable and if required, on the proposed instruments: At the latest, 24 months from the date on which the Ministers recommended that TPHP and TBOEP be added to Part 2 of Schedule 1 to CEPA.
Consultation on the proposed instruments: 60-day public comment period starting upon publication of each proposed instrument.
Publication of the final instruments: At the latest, 18 months from the publication of each proposed instrument.
These are planned timelines, and are subject to change. Please consult the schedule of risk management activities and consultations for updated information on timelines.
9. References
Ash M, Ash I. 2009. Specialty Chemicals - Source Book. 4th ed. Endicott (NY). Synapse Information Resources, Inc. [accessed 2018 May 4 from Knovel].
[ASTM] ASTM International. 2014. Fire Standards and Flammability Standards.
Biomonitoring California. 2024. Triphenyl phosphate (TPP) | Biomonitoring California. [accessed 2024 Aug 12].
[California] California Department of Consumer Affairs. 2013. Technical Bulletin 117-2013: Requirements, Test Procedure and Apparatus for Testing the Smolder Resistance of Materials Used in Upholstered Furniture [PDF].
[California] California Legislative Information. 2018. AB-2998 Consumer products: Flame retardant materials.
Canada. 1978. Food and Drug Regulations. C.R.C., c.870, s.C.01.040.2.
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C. 1999, c.33. Canada Gazette, Part III, vol. 22, no. 3.
Canada. 2000. Canadian Environmental Protection Act, 1999: Persistence and Bioaccumulation Regulations [PDF]. P.C. 2000-348, 23 March 2000, SOR/2000-107.
Canada. 2015. Red Tape Reduction Act. S.C. 2015, c.12.
Canada. 2016a. Mattresses Regulations (Canada Consumer Product Safety Act). SOR/2016-183.
Canada. 2016b. Textile Flammability Regulations (Canada Consumer Product Safety Act). SOR/2016-194.
Canada, Department of the Environment. 1995. Toxic Substances Management Policy. [accessed2023 Nov 15].
Canada, Department of the Environment. 2006. Canadian Environmental Protection Act, 1999: Notice with respect to selected substances identified as priority for action [PDF]. Canada Gazette, Part I, vol. 140, no. 9, p. 435-459.
Canada, Department of the Environment. 2012. Canadian Environmental Protection Act, 1999: Notice with respect to certain substances on the Domestic Substances List [PDF]. Canada Gazette, Part I, vol. 146, no. 48, Supplement.
Canada, Department of the Environment, Department of Health. 2011. Canadian Environmental Protection Act, 1999: Announcement of planned actions to assess and manage, where appropriate, the risks posed by certain substances to the health of Canadians and the environment [PDF]. Canada Gazette, Part I, vol. 145, no. 41, p. 3125-3129.
Canada, Department of the Environment, Department of Health. 2016. Canadian Environmental Protection Act, 1999: Early stakeholder engagement to help inform the plan to address the remaining 1 550 substances under the Chemicals Management Plan [PDF]. Canada Gazette, Part I, vol. 150, no. 6, p. 152-154.
Canada, Department of the Environment, Department of Health. 2016.Canadian Environmental Protection Act, 1999: Announcement of planned actions to assess and manage, where warranted, the risks posed by certain substances to the health of Canadians and the environment.Canada Gazette, Part I, vol. 150, no. 25, p. 1989-1994.
[CEC] Commission for Environmental Cooperation. 2015. Enhancing Trilateral Understanding of Flame Retardants and Their Use in Manufactured Items: Supply Chain Analysis of Select Flame Retardants Contained in Manufactured Items Used in Indoor Environments [PDF].
Danish EPA. 2016a. Chlorinated phosphorous-based flame retardants in children’s articles containing foam [PDF]. Background for content and possibilities for prevention in the EU. Environmental project No. 1855.
Danish EPA. 2016b. Environmental and health screening profiles of phosphorous flame retardants: A LOUS follow-up project [PDF].
[ECCC] Environment and Climate Change Canada. 2013. DSL Inventory Update data collected under theCanadian Environmental Protection Act, 1999, section71: Notice with respect to certain substances on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
[ECCC] Environment and Climate Change Canada. 2016a. Supporting documentation: data used to create substance-specific hazard and exposure profiles and assign risk classifications. Gatineau (QC): ECCC. Information in support of the science approach document: ecological risk classification of organic substances. Available from: substances@ec.gc.ca.
[ECCC] Environment and Climate Change Canada. 2016b. Supporting documentation: data used to create substance-specific hazard and exposure profiles and assign risk classifications. Gatineau (QC): ECCC. Information in support of the science approach document: Ecological risk classification of organic substances. Available from: substances@ec.gc.ca.
[ECCC, HC]. Environment and Climate Change Canada, Health Canada. 2021a. Draft Screening Assessment for the Flame Retardants Group.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. [2021b]. Risk Management Scope for TPHP, BPDP, BDMEPPP, IDDP, IPPP and TEP.
Environment Canada. 1995. Toxic Substances Management Policy [PDF]. Ottawa (ON): Government of Canada. [accessed 2018 Aug 29].
Environment Canada. 2009. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain inanimate substances (chemicals) on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
Environment Canada. 2012. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain substances on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
[HC] Health Canada. 2019. Determination of Flame Retardants in a Survey of Consumer Products: Isopropylphenyl phosphate (IPPP). Project Report number P2019-00015A5; 27 June, 2019. Ottawa (ON): Product Safety Laboratory. 35 pp.
[ISO] International Organization for Standardization. 2014. Standards Catalogue.
Maine. 2017. An Act To Protect Firefighters by Establishing a Prohibition on the Sale and Distribution of New Upholstered Furniture Containing Certain Flame-retardant Chemicals [accessed 2023 Feb 15].
Maryland. 2020. Public Health – Products Containing a Flame Retardant Chemical. [accessed 2023 Feb 15].
New Hampshire. 2019. Prohibiting the sale of certain furniture and carpeting with flame retardant chemicals. [accessed 2013 Feb 16].
New York. 2021. An act to amend the environmental conservation law, in relation to the regulation of chemicals in upholstered furniture, mattresses and electronic enclosures. [accessed 2023 Feb 20].
Stapleton, H.M., Klosterhaus, S., Keller, A. et al. 2011. Identification of flame retardants in polyurethane foam collected from baby products. Environmental Science and Technology 45: 5323-5331.
Stapleton, H.M., Sharma, S., Getzinger, G., et al. 2012. Novel and high volume use flame retardants in US couches reflective of the 2005 PentaBDE phase out. Environmental Science and Technology 24(24): 13432-13439.
[TBS] Treasury Board of Canada Secretariat. 2007. Assessing, Selecting, and Implementing Instruments for Government Action [PDF]. Ottawa (ON): Government of Canada. [accessed 2018 Aug 29].
[TBS] Treasury Board of Canada Secretariat. 2012. Red Tape Reduction Action Plan. Ottawa (ON): Government of Canada. [accessed 2018 Aug 29].
[TBS] Treasury Board of Canada Secretariat. 2018. Cabinet Directive on Regulation. Ottawa (ON): Government of Canada. [accessed 2018 Aug 29].
UK Department for Business, Energy and Industrial Strategy. 2016. Consultation on updating the Furniture and Furnishings (Fire) (Safety) Regulations.
[UL] Underwriters Laboratories. 2014. UL 94, the Standards for Safety of Plastic Materials for Parts in Devices and Appliances.
[US CPSC] United States Consumer Product Safety Commission. 1984. Standard for the Flammability of Mattresses and Mattress Pads. eCFR. [accessed 2024 Jan 5].
[US CPSC] United States Consumer Product Safety Commission. 2006. Standard for the Flammability (Open Flame) of Mattress Sets: 16 CFR Part 1633 [PDF].
[US CPSC] United States Consumer Product Safety Commission. 2016. CPSC Staff Preliminary risk assessment of flame retardant chemicals in upholstered furniture foam [PDF]. Bethesda, MD: United States Consumer Product Safety Commission. [accessed 2018 Mar 29].
[US EPA] United States Environmental Protection Agency. 2005. Furniture Flame Retardancy Partnership: Environmental Profiles of Chemical Flame-Retardant Alternatives for Low-Density Polyurethane Foam [PDF].
[US EPA] United States Environmental Protection Agency. 2015. Flame retardants used in flexible polyurethane foam: an alternatives assessment update [PDF].
Zhu H, Al-Bazi MM, Kumosani TA, Kannan K. 2020. Occurrence and Profiles of Organophosphate Esters in Infant Clothing and Raw Textiles Collected from the United States. Environ. Sci. Technol. Lett. 7:415-420.
Annex A. Substance identities
| CAS RN | Domestic Substances List Name (English) | Common Name/ Simplified Name | Acronym | Chemical Structure | Molecular Weight (g/mol) | Chemical Formula |
|---|---|---|---|---|---|---|
| 115-86-6 | Phosphoric acid, triphenyl ester | Triphenyl phosphate | TPHP | 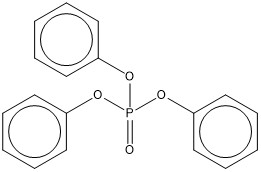 |
326.29 | C18H15O4P |
| 78-51-3 | ethanol, 2-butoxy-, phosphate (3:1) | Tris(2-butoxethyl) phosphate) | TBOEP | 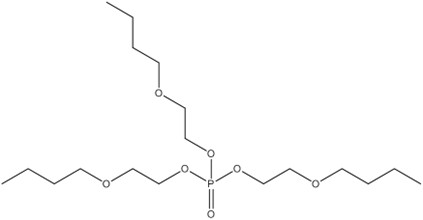 |
398.47 | C18H39O7P |
