Screening assessment - 2-imidazolidinethione (ethylene thiourea)
Official title: Screening assessment - 2-imidazolidinethione (ethylene thiourea)
Chemical Abstracts Service Registry Number
96-45-7
Environment and Climate Change Canada
Health Canada
January 2023
Cat. No.: En84-324/2022E-PDF
ISBN 978-0-660-45974-5
Synopsis
Pursuant to section 74 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of the Environment and the Minister of Health have conducted a screening assessment of 2-imidazolidinethione, hereinafter referred to as ethylene thiourea (ETU). The Chemical Abstracts Service Registry Number (CAS RNFootnote 1 ) for ETU is 96-45-7. ETU was included in the draft screening assessment for the Heterocycles Group published on November 11, 2017. However, ETU was excluded from the final Heterocycles Group assessment to better align with the re-evaluation of certain pesticides.
In 2008, less than 100 kg of ETU was manufactured in Canada and between 10 000 kg and 100 000 kg were imported into Canada according to information submitted pursuant to a CEPA section 71 notice. Non-confidential uses for ETU reported in the survey were as a vulcanization agent, process regulator and plasticizer in plastic and rubber materials, in formed automotive parts, in vehicle imports and as a process regulator in fabric, textile and leather articles. ETU was also reported as an impurity in pest control products. ETU is a degradation product, a metabolite and a residual in ethylene bis-dithiocarbamate (EBDC) fungicides.
The ecological risk associated with ETU was characterized using the ecological risk classification of organic substances (ERC) approach. The ERC is a risk-based approach that employs multiple metrics for both hazard and exposure with weighted consideration of multiple lines of evidence for determining risk classification. Hazard profiles are established primarily on the basis of mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Metrics considered in the exposure profile include potential emission rate, overall persistence, and long-range transport potential. A risk matrix is used to assign a low, moderate or high level of potential concern for substances on the basis of their hazard and exposure profiles. Based on the outcome of the ERC analysis, ETU is considered unlikely to be causing ecological harm.
Considering all available lines of evidence presented in this screening assessment, there is low risk of harm to the environment from ETU. It is concluded that ETU does not meet the criteria under paragraphs 64(a) or (b) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
US EPA classified ETU as a probable human carcinogen (Group B2). Under Europe's harmonised classification and labelling system, ETU is classified as a substance that may damage the unborn child (Repr. 1B: H360D). Laboratory studies showed that ETU had thyroid effects and was carcinogenic. Exposure of the general population to ETU can occur from the diet, including drinking water, as a result of crop treatment with ethylene bis-dithiocarbamate fungicides that break down to ETU. These sources of exposure to ETU have been addressed under the Pest Control Products Act as part of Health Canada’s re-evaluation of ethylene bis-dithiocarbamate fungicides.
The general population may also be exposed by the dermal route to residual ETU through migration from rubber products. Risk to human health from this route was assessed by comparing estimates of exposure to ETU from rubber products with the levels associated with health effects in animal studies, including for carcinogenicity. For both non-cancer and cancer effects, the risk to human health is considered to be low.
Considering all the information presented in this screening assessment, it is concluded that ETU does not meet the criteria under paragraph 64(c) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is concluded that ETU does not meet any of the criteria set out in section 64 of CEPA.
1. Introduction
Pursuant to section 74 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999), the Minister of the Environment and the Minister of Health have conducted a screening assessment of 2-imidazolidinethione, hereinafter referred to as ethylene thiourea (ETU). This substance was identified as a priority for assessment as it met categorization criteria under subsection 73(1) of CEPA (ECCC, HC [modified 2017]). ETU was included in the draft screening assessment for the Heterocycles Group published on November 11, 2017. The screening assessment for Heterocycles Group was finalized and published June 8, 2019 (ECCC, HC 2019). However, ETU was removed to better align with the re-evaluation of certain pesticides (Health Canada 2018a, 2018b, 2020a). As such, the CEPA conclusion for this substance is being provided in this screening assessment.
The ecological risk of ETU was characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a). The ERC describes the hazard of a substance using key metrics including mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity, and considers the possible exposure of organisms in the aquatic and terrestrial environments on the basis of factors including potential emission rates, overall persistence and long-range transport potential in air. The various lines of evidence are combined to identify substances as warranting further evaluation of their potential to cause harm to the environment or as having a low likelihood of causing harm to the environment.
This screening assessment includes consideration of information on chemical properties, environmental fate, hazards, uses and exposure. Relevant data were identified up to July 2021. Empirical data from key studies as well as some results from models were used to reach conclusions. When available and relevant, information presented in assessments from other jurisdictions was considered. The approach includes the use of previously established points of departure for health effects for ETU from Health Canada’s Pest Management Regulatory Agency (PMRA).
This screening assessment was prepared by staff in the CEPA Risk Assessment Program at Health Canada and Environment and Climate Change Canada and incorporates input from other programs within these departments. The ERC document was subject to an external peer-review and a 60-day public comment period. The human health portions of the draft screening assessment for the Heterocycles Group (which included ETU) have undergone external peer review and consultation. Comments on the technical portions relevant to human health were received from scientists (Jeanelle Martinez, Pam Williams, Jennifer Sahmel, Lynne Haber) of the Toxicology Excellence for Risk Assessment Center, Department of Environmental Health, College of Medicine, University of Cincinnati. Additionally, the draft of the Heterocycles Group screening assessment (published November 11, 2017) was subject to a 60-day public comment period. While comments from external peer reviewers and the public were taken into consideration, the final content and outcome of the screening assessment remain the responsibility of Environment and Climate Change Canada and Health Canada.
This screening assessment focuses on information critical to determining whether ETU meets the criteria as set out in section 64 of CEPA, by examining scientific information and incorporating a weight-of-evidence approach and precaution.Footnote 2 The screening assessment presents the critical information and considerations on which the conclusion is based.
2. Identity of substance
The Chemical Abstracts Service Registry Number (CAS RNFootnote 3 ), Domestic Substances List (DSL) name, common name and molecular structure for ETU are presented in Table 2‑1.
| CAS RN (acronym) | DSL name (common name) | Chemical structure and molecular formula | Molecular weight (g/mol) | Reference |
|---|---|---|---|---|
| 96-45-7 (ETU) | 2-Imidazolidinethione (Ethylene thiourea) | 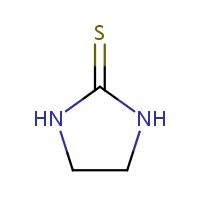 C3H6N2S C3H6N2S |
102.2 | PubChem 2004- |
3. Physical and chemical properties
A summary of physical and chemical property data for ETU is presented in Table 3‑1. Additional physical and chemical properties are reported in ECCC (2016b).
| Property | Value | Type of data | Reference |
|---|---|---|---|
| Melting point (oC) | 203 | Experimental | EPI Suite 2012 |
| Boiling point (oC) | 347 | Experimental | EPI Suite 2012 |
| Water solubility (g/L) | 20 at 30 °C | Experimental | EPI Suite 2012 |
| Density (g/mL) | 1.417 | Experimental | EPI Suite 2012 |
| Vapour pressure (Pa) | 0.00027 @ 25 °C | Experimental | EPI Suite 2012 |
| Henry’s law constant (Pa m3/mol) | 1.36 | Modelled (bond method) | EPI Suite 2012 |
| Henry’s law constant (Pa m3/mol) | 3.36 × 10-7 | Experimental | EPI Suite 2012 |
| log Kow (dimensionless) | -0.66 | Experimental | EPI Suite 2012 |
| log Koc (dimensionless) | 1.54 - 2.93 | Experimental | Health Canada 2020a |
Abbreviations: Kow, octanol–water partition coefficient; Koc, organic carbon–water partition coefficient
4. Sources and uses
ETU does not occur naturally in the environment. It is used primarily as an accelerator or vulcanizing agent for the curing of polychloroprene (neoprene) and polyacrylate rubbers (IARC 1974; IARC 2001; HSDB 1983-2017; Netherlands 1999). ETU is a degradation product, a metabolite and a residual in ethylene bis-dithiocarbamate (EBDC) fungicides, such as Mancozeb and Metiram. In its proposed re-evaluation decision, Health Canada identified a potential carcinogenic risk from food and drinking water exposures to ETU derived from EBDC fungicides (Health Canada 2014, 2018a). The re-evaluations have been completed, and risk reduction measures have been implemented under the Pest Control Products Act to reduce exposure and risk to ETU (Health Canada 2018b, 2020a).
According to information submitted in response to a CEPA section 71 survey (Canada 2009), less than 100 kg of ETU was manufactured in Canada in 2008 and between 10 000 kg and 100 000 kg was imported into Canada for that same calendar year.Footnote 4 Non-confidential uses for ETU reported in the survey include as a vulcanization agent, process regulator and plasticizer in plastic and rubber materials, in formed automotive parts, in vehicle imports and as a process regulator in fabric, textile and leather articles, and was also reported as an impurity in pest control products (Environment Canada 2009). In the United States, the national production volume for ETU was between 0.45 million kg and 4.5 million kg in 2012 (CDAT 2015).
ETU is reportable under the National Pollutant Release Inventory (NPRI) by facilities manufacturing, processing, or otherwise using ETU in an amount of at least 10 tonnes (NPRI 2021). According to NPRI data from 2010 to 2017, no companies have reported releases of ETU (NPRI 2018).
Given its uses, ETU can be found in low amounts in some manufactured rubber consumer items. Additional information on uses in Canada is presented in Table 4‑1.
| Use | ETU |
|---|---|
| Food packaging materialsa | Yes |
| Formulant in pest control products registered in Canadab | No (contaminant of concern monitored in EBDCs active ingredients or degradate formed in the environment from the use of EBDCs) |
a Personal communication, November 2015 and July 2021 emails from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada; unreferenced.
b Health Canada 2018a.
ETU is on Health Canada’s List of Contaminants and other Adulterating Substances in Foods, which is incorporated by reference into Division 15 of the Food and Drug Regulations. As set out in Part 1 of this List, ETU is prohibited in all food except fruits, vegetables and cereals, and for these foods an amount that exceeds the maximum level of 0.05 mg/kg (ppm) as set out in Part 2, would render those foods adulterated (Health Canada 2020b). ETU may also be present in an antimicrobial agent used in the manufacture of some food packaging materials, such as paper and paperboard (personal communication, November 2015 and July 2021 emails from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada; unreferenced).
5. Characterization of ecological risk
5.1 Potential to cause ecological harm
The ecological risk of ETU was characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a). The ERC is a risk-based approach that considers multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. The various lines of evidence are combined to discriminate between substances of lower or higher potency and lower or higher potential for exposure in various media. This approach reduces the overall uncertainty with risk characterization, in contrast to an approach that relies on a single metric in a single medium (for example, median lethal concentration) for characterization. The following summarizes the approach, which is described in detail in ECCC (2016a).
Data on physical-chemical properties, fate (chemical half-lives in various media and biota, partition coefficients, fish bioconcentration), acute fish ecotoxicity, and chemical import and manufacture volumes in Canada were either collected from scientific literature, from available empirical databases (for example, OECD QSAR Toolbox 2014), from responses to surveys issued pursuant to section 71 of CEPA, or they were generated using selected (quantitative) structure-activity relationship ([Q]SAR) or mass-balance fate and bioaccumulation models. These data were used as inputs to other mass-balance models or to complete the substance hazard and exposure profiles.
Hazard profiles were established primarily on the basis of metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Exposure profiles were based on multiple metrics, including potential emission rate, overall persistence, and long-range transport potential. Hazard and exposure profiles were compared to decision criteria in order to classify the hazard and exposure potentials for each organic substance as low, moderate, or high. Additional rules were applied (for example, classification consistency, margin of exposure) to refine the preliminary classifications of hazard or exposure.
A risk matrix was used to assign a low, moderate or high classification of potential risk for each substance on the basis of its hazard and exposure classifications. ERC classifications of potential risk were verified using a two-step approach. The first step adjusted the risk classification outcomes from moderate or high to low for substances that had a low estimated rate of emission to water after wastewater treatment, representing a low potential for exposure. The second step reviewed low risk potential classification outcomes using relatively conservative, local-scale (i.e., in the area immediately surrounding a point source of discharge) risk scenarios, designed to be protective of the environment, to determine whether the classification of potential risk should be increased.
ERC uses a weighted approach to minimize the potential for both over- and under-classification of hazard and exposure, and of subsequent risk. The balanced approaches for dealing with uncertainties are described in greater detail in ECCC (2016a). The following describes two of the more substantial areas of uncertainty. Error in empirical or modelled acute toxicity values could result in changes in classification of hazard, particularly metrics relying on tissue residue values (i.e., mode of toxic action), many of which are predicted values from QSAR models (OECD QSAR Toolbox 2014). However, the impact of this error is mitigated by the fact that overestimation of median lethality will result in a conservative (protective) tissue residue value used for critical body residue analysis. Error in underestimation of acute toxicity will be mitigated through the use of other hazard metrics, such as structural profiling of mode of action, reactivity and/or estrogen-binding affinity. Changes or errors in chemical quantity could result in differences in classification of exposure as the exposure and risk classifications are highly sensitive to emission rate and use quantity. The ERC classifications thus reflect exposure and risk in Canada on the basis of what is estimated to be the current use quantity, and may not reflect future trends.
Critical data and considerations used to develop the substance-specific profiles for ETU and the hazard, exposure and risk classification results are presented in ECCC (2016b).
On the basis of low hazard and low exposure classifications according to information considered under ERC, ETU was classified as having a low potential for ecological risk. It is unlikely that this substance is resulting in concerns for the environment in Canada.
6. Potential to cause harm to human health
6.1 Exposure assessment
Environmental media and food
The primary source of exposure to ETU through diet and drinking water intake is expected to be from the use of ethylene bis-dithiocarbamate fungicides, which has been evaluated, and risk to human health characterized, by Health Canada’s PMRA (Health Canada 2018a, 2018b, 2020a). There is the potential for soil and dust to contain ETU in the vicinity of farms or agricultural sites, which have used EBDC fungicides as noted and considered by Health Canada (2018b, 2020a).
ETU may be present in an antimicrobial agent used in the manufacture of some food packaging materials, such as paper and paperboard. Dietary exposure, if any, from these sources is expected to be negligible (personal communication, November 2015 and July 2021 emails from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada; unreferenced).
No other data on levels of ETU in environmental media or food have been identified. According to NPRI data from 2010 to 2017, no companies have reported releases of ETU (NPRI 2018). In addition, given ETU’s total usage quantity, applications, and physical-chemical properties, potential human exposure resulting from industrial releases to the environment is expected to be limited.
As part of the fifth (2016 to 2017) and sixth (2018 to 2019) cycles of the Canadian Health Measures Survey, ETU (total ETU) was measured in the urine samples of 97% of 2704 individuals (fifth cycle) and 99% of 2508 individuals (sixth cycle) representing the Canadian population aged 3 to 79 years (Health Canada 2019, 2021). The CHMS is an ongoing nationally representative survey that collects important health and wellness data as well as biological samples from individuals across the country. The geometric mean and 95th percentiles of total ETU (with 95% confidence intervals (CIs)) were 0.42 (0.35–0.51) µg/L and 3.5 (2.0–4.9) µg/L, respectively, in the fifth cycle, and 0.40 (0.36–0.45) µg/L and 2.7 (2.1–3.2) µg/L, respectively, in the sixth cycle. The limit of detection was 0.033 µg/L. While these data may not capture acute or unique intermittent exposure, they support that there are potential chronic exposures to ETU in the general Canadian population, likely from use of multiple EBDC pesticides (Health Canada 2021).
Products available to consumers
As ETU is used in the curing of rubbers such as polychloroprene (neoprene), potential dermal exposure to ETU from use of rubber-based products available to consumers was examined. Although the curing of rubbers converts ETU to other compounds, residual amounts of ETU may be present (IARC 1974). Therefore, there is the potential for ETU to migrate from rubber surfaces.
Products available to consumers that contain neoprene include shoes and certain soft rubber containers (lunch bags) and diving gear. To determine concentrations of ETU in neoprene and associated rubber products, Health Canada undertook a marketplace analysis of 33 different rubber-based products available to consumers, including footwear, children’s products, neoprene-based clothing (for example, swimwear, wetsuits), and rubber grips for steering wheels, bicycles, and tools (Health Canada 2016). Products were cut into 1 cm3 pieces of approximately 1 gram and incubated overnight at 40ºC in an equal parts mixture of methanol and water. The released concentration of ETU for each product was then determined, with the majority of samples containing less than 0.16 mg/kg. Only one product, a steering wheel cover, contained 11.0 mg of ETU per kg of material. The neoprene-based products were shown to release from 0.0022 to 0.0838 mg of ETU per kilogram of material (Health Canada 2016).
Given there were limited data available on the migration of ETU, and in order to calculate systemic exposure to ETU through the dermal route from a solid rubberized material matrix, the migration of diethyl thiourea, was considered. Diethyl thiourea is a substance that can be found in chloroprene rubber products, where its function is the same as ETU, and its presence was more easily measured than ETU’s in samples in a study by the Danish EPA (2012). The migration rate of the substance from the material is dependent on its concentration in the rubber product, and the ratio of concentration versus migration was considered across several samples (Danish EPA 2012). An analysis of data for diethyl thiourea from five samples showed a range of ratios spanning from 339 000:1 to 609 000:1, relating concentration of diethyl thiourea in the material to migration rate. The upper and lower 95% confidence intervals for the ratio between concentration and migration rate were 342 000:1 and 635 000:1 respectively. A lower ratio of 300 000:1 was selected to conservatively estimate the migration rate of ETU. The lower ratio presumes that a larger amount of ETU will migrate from the material (Danish EPA 2012). Using this ratio, a dermal absorption value of 45% established by Health Canada (Health Canada 2020a), as well as other assumptions as described in Appendix A, potential dermal exposures to ETU across several durations were estimated for an adult and a child wearing a wetsuit as well as an adult using a steering wheel cover (Table 6‑1). Although adolescents aged 16 and older may also use steering wheel covers while driving, their exposure is likely lower than that of an adult as it is assumed they would be driving less frequently and for shorter durations.
| Exposure scenario | Adult (19+ years) (mg/kg-bw/day) | Child (5 to 11 years) (mg/kg-bw/day) |
|---|---|---|
| Wetsuit (single day) | 2.4 × 10-4 | 3.15 × 10 -4 |
| Wetsuit (10-day exposure) | 6.0 × 10-5 | 8.0 × 10-5 |
| Wetsuit (30-day exposure) | 2.0 × 10-5 | 3.0 × 10-5 |
| Wetsuit (yearly average exposure)a | 1.65 × 10-6 | 2.17 × 10-6 |
| Steering wheel cover (single day) | 4.24 x 10-4 | N/A |
| Steering wheel cover (30-day) | 3.74 x 10-4 | N/A |
| Steering wheel cover (daily exposure over ~10 years)b | 1.34 x 10-5 | N/A |
Abbreviations: N/A, not applicable
a Assume an adult or child would use a wetsuit 30 days of the year.
b Assume life of a steering wheel cover is about 10 years (Autos.com Editor, 2013).
It should be recognized that the yearly average value for children (5- to 11-year olds) is an overestimate of true daily lifetime exposures, as the majority of the lifetime exposures would occur as an adult, where calculated daily exposures are lower due to differences in skin surface area and body weight between children (5- to 11-year olds) and adults.
6.2 Health effects assessment
Health Canada (2018a, 2018b, 2020a), the United States Environmental Protection Agency (US EPA 2020), the National Toxicology Program (NTP 1992), and the International Agency for Research on Cancer (IARC 1974, 2001) summarized the health effects literature and/or characterized the hazard of ETU. The Health Canada report (2020a) was used to inform the health effects characterization in this screening assessment. US EPA (2020) classified ETU as a probable human carcinogen (Group B2) whereas IARC (2001) considered ETU as not classifiable as to its carcinogenicity to humans (Group 3). Under Europe's harmonized classification and labelling system, ETU is classified as a substance that may damage the unborn child (Repr. 1B: H360D) (ECHA 2021). Targeted literature searches were conducted until May 2021. No additional health effect studies that would impact the risk characterization (i.e., result in different critical endpoints or lower points of departure than those stated herein) were identified.
The following paragraphs provide critical endpoints and corresponding effect levels for ETU that are used for risk characterization, as cited from Health Canada (2020a). Short-term dermal and inhalation toxicity studies were not available (Health Canada 2018a).
In carcinogenicity studies, mice and rats orally administered ETU exhibit thyroid tumours with a clear mode of action, i.e., neoplasia of thyroid follicular cells due to increased secretion of thyroid stimulating hormone (TSH) from the pituitary. TSH production occurs in response to chronic inhibition of thyroid peroxidase by ETU, resulting in decreased thyroid hormone production. In mice, chronic exposure to ETU has also resulted in pituitary gland neoplasia and liver adenomas and carcinomas. Using the most sensitive tumour (i.e., liver tumour induction in female mice), Health Canada previously derived a cancer slope factor of 0.0601 (mg/kg-bw per/day)-1 and indicated a lack of evidence to support a threshold mode of action for this effect (Health Canada 2020a). Using the same data from mice, the US EPA also calculated a cancer slope factor of 0.0601 (mg/kg per day)-1 (US EPA 2020). On the basis of thyroid gland tumours in CD rats, other groups have derived cancer slope factors for ETU ranging from 0.006 (mg/kg-bw per day)-1 (Frakes 1988) to 0.045 (mg/kg-bw per day)-1 (OEHHA 2009).
In an extended one-generation reproductive toxicity (EOGRT) study submitted to PMRA, Crl:CD(SD) rats were administered 0.2, 2, 10 mg/kg bw/day of ETU in diet (Health Canada 2020a). The EOGRT study also included a developmental neurotoxicity component and characterization of thyroid effects at multiple life stages. In both male and female rats at multiple life stages, there were significant changes in the thyroid hormone profile, thyroid weights, and thyroid histopathology (follicular cell hypertrophy/hyperplasia) at the mid and high dose levels. At 0.2 mg ETU/kg bw/day, there was a change in thyroid histopathology (follicular cell hypertrophy) in P1 males (20 of 27 animals affected) and F1 Cohort 1A males (15 of 26 animals affected), which was accompanied by pituitary gland hypertrophy (very slight 9/26), demonstrating a perturbation of the hypothalamus-pituitary-thyroid (HPT) axis at this dose level. A lowest observed adverse effect level (LOAEL) of 0.2 mg/kg bw/day was selected by PMRA based on HPT axis perturbation (hypertrophy of thyroid and pituitary in parental animals) (Health Canada 2020a).
While reproductive parameters were not affected in the P generation, a no observed adverse effect level (NOAEL) of 2 mg/kg bw/day for reproductive effects was identified based on the increased proportion of abnormal sperm and increased ovarian follicle count for the F1 generation in high dose males and females, respectively (Health Canada 2020a).
The neurotoxicity cohort of EOGRT study was considered a screening level test and a NOAEL for developmental neurotoxicity was identified at a dose level of 2 mg/kg bw/day (Health Canada 2020a).
A developmental study conducted in 2015 in Sprague Dawley rats via the oral (gavage) route was submitted to the PMRA (Health Canada 2020a). There were no maternal treatment-related effects at any of the dose levels tested (5, 15 or 30 mg/kg bw/day) on gestation days 6 to 19. However, there was an increase in incidence of hydrocephaly at the 15 mg/kg bw/day dose and numerous fetal head and skeletal developmental malformations were observed at the 30 mg/kg bw/day dose level. These fetal effects were observed in the absence of maternal toxicity. A developmental NOAEL of 5 mg/kg bw/day was selected based on the increased incidence of hydrocephalus (Health Canada 2020a). These effects were consistent with those seen in rats after dermal exposure to 50 mg/kg-bw/day ETU on gestation days 12 to 13 (Health Canada 2018a). All fetal rats had marked skeletal malformations, at non-maternally toxic doses (Health Canada 2018a).
6.3 Characterization of risk to human health
The primary source of exposure to ETU for the general population of Canada is expected to be from the use of EBDC fungicides, which have been evaluated, and risk to human health characterized, by Health Canada’s PMRA (Health Canada 2018a, 2018b, 2020a).
The general population of Canada may also be exposed to ETU when using rubber products.
Potential dermal exposures to ETU from wearing a neoprene-based wetsuit or using a rubber-based steering wheel cover were considered relative to health effects of ETU identified in laboratory animals. Adults and children (5- to 11-year olds) were assessed for single and multiple (10 and 30 days) exposures from wearing a neoprene-based wetsuit for 8 hours. Daily exposure was considered to be a function of the ETU concentration remaining in the wetsuit on a given day. Adult exposures to ETU from use of a steering wheel cover were assessed for single (for 4 hours) and yearly exposure (for 1.27 hours/day).
Regarding the potential risk of carcinogenicity, the highest derived yearly average (i.e., corrected for leaching/loss) daily exposure to ETU associated with wearing a neoprene wetsuit for 30 days (2.16 × 10-6 mg/kg-bw per day for children (5- to 11-year olds)) was multiplied by the cancer slope factor of 0.0601 (mg/kg-bw per day)-1 derived by Health Canada (2020a) on the basis of liver tumours in female mice, resulting in a risk level of 1.3 × 10-7 (less than 1 in 1 millionFootnote 5 ). It should be recognized that this value is an overestimate of true daily lifetime exposure and risk, as the majority of the lifetime exposures would occur as an adult, where calculated daily exposures are lower due to differences in skin surface area and body weight between children (5- to 11-year olds) and adults. The potential risk of carcinogenicity associated with use of rubber-based steering wheel covers was derived using the average daily exposure to ETU over ten years of use (1.34 x 10-5 mg/kg-bw per day for adults). This was multiplied by the cancer slope factor of 0.0601 (mg/kg-bw per day)-1 resulting in a risk level of 8.17 x 10-7 (less than 1 in 1 million).
Regarding the potential risk from non-cancer effects, no short-term dermal toxicity studies were available (Health Canada 2018a). There are dermal developmental toxicity studies available for ETU. However, these studies were limited to 1-2 doses and did not assess thyroid toxicity directly compared to the oral toxicity studies, such as the EOGRT, that assessed thyroid toxicity adequately.
Table 6‑2 provides all relevant exposure estimates, critical effect levels, and resulting margins of exposure (MOEs) for characterization of non-cancer risk to human health for ETU from use of products available to consumers.
| Exposure scenario | Exposure estimate (mg/kg-bw/day) | Critical effect level ( mg/kg-bw/day) | Margins of exposure (MOEs) |
|---|---|---|---|
| Wetsuit (single day) | 2.4 × 10-4 (adult) – 3.15 × 10-4 (5 to 11 year olds) | 5 (malformations in the absence of maternal toxicity) | 15 873 – 20 833 |
| Steering wheel cover (single day) | 4.24 x 10-4 (adult) | 5 (malformations in the absence of maternal toxicity) | 11 792 |
| Wetsuit (10 days) | 6.0 × 10-5 (adult) – 8.0 × 10-5 (5 to 11 years) | 0.2 (HPT axis perturbation (hypertrophy of thyroid and pituitary in parental animals)) | 2500 – 3333 |
| Wetsuit (30 days) | 2.0 × 10-5 (adult) – 3.0 × 10-5 (5 to 11 years) | 0.2 (HPT axis perturbation (hypertrophy of thyroid and pituitary in parental animals)) | 6667 – 10 000 |
| Steering wheel cover (30 days) | 3.74 x 10-4 (adult) | 0.2 (HPT axis perturbation (hypertrophy of thyroid and pituitary in parental animals)) | 534 |
The calculated MOEs associated with wearing a neoprene-based wetsuit or using a rubber-based steering wheel cover are considered adequate to address uncertainties in the health effects and exposure datasets. Risk to human health is therefore considered to be low for dermal exposure to ETU from rubber- and neoprene-based products.
This conclusion is supported by a comparison between the general population biomonitoring data from CHMS and available biomonitoring equivalents (BEs). A BE is the concentration of a substance in a biological medium that corresponds to an exposure at an existing health-based guidance value. BEs are screening tools used to interpret population-level biomonitoring data in a health risk context. BE values are available for total ETU (Tetra Tech 2021). These BEs were developed based on the Health Canada’s toxicological reference values (TRVs). For the non-cancer TRV (ADI), the associated BE was 27 ug/L or µg/g creatinine ETU in urine. The BE associated with the cancer risk assessments and an excess cancer risk of 1 x 10-6 was 0.7 ug/L or µg/g creatinine ETU in urine. In CHMS, the geometric mean levels of total ETU (with 95% CIs) measured in the Canadian general population aged 3 to 79 years were 0.42 (0.35–0.51) µg/L in the fifth cycle (2016-2017), and 0.40 (0.36–0.45) µg/L in the sixth cycle (2018-2019). These concentrations are lower than both the cancer and non-cancer BEs (Health Canada 2019, 2021). This indicates that exposures for the Canadian general population are below the current level of concern. It was not considered appropriate to compare the ETU BEs with upper percentile values from CHMS, as the BEs are associated with chronic reference doses.
While exposure of the general population to ETU is not of concern at current levels, this substance is considered to have a health effect of concern related to its potential carcinogenicity and reproductive/developmental toxicity as a result of US EPA (2020) classification of ETU as a probable human carcinogen (Group B2) and under Europe's harmonized classification and labelling system classification as a substance that may damage the unborn child (Repr. 1B: H360D) (ECHA 2021). Therefore, there may be a concern for human health if exposures were to increase.
7. Uncertainties in evaluation of risk to human health
Overall confidence in the exposure and hazard datasets for ETU is moderate.
There is uncertainty for the durations and frequencies of exposure to residual ETU from rubber- and neoprene-based products. However, given the conservative nature of the exposure scenarios, the risk characterization is not expected to underestimate risk.
There is some uncertainty in using oral route endpoints to characterize risk of dermal exposure to ETU for the general population. This endpoint is considered to be conservative and supports the conclusion.
8. Conclusion
Considering all available lines of evidence presented in this screening assessment, there is low risk of harm to the environment from ETU. It is concluded that ETU does not meet the criteria under paragraphs 64(a) or (b) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
Considering all the information presented in this screening assessment, it is concluded that ETU does not meet the criteria under paragraph 64(c) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore concluded that ETU does not meet any of the criteria set out in section 64 of CEPA.
References
Autos.com Editor. 2013. How Long Does a Leather Steering Wheel Wrap Last Usually? [Internet]. El Segundo (CA): MH Sub I, LLC [accessed 2012 Oct].
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C. 1999, c. 33, Canada Gazette. Part III. Vol. 22, no. 3.
Canada, Dept. of the Environment. 2009. Canadian Environmental Protection Act, 1999: Notice with respect to certain inanimate substances (chemicals) on the Domestic Substances List. Canada Gazette, Part I, vol. 143, no. 40, p. 2945-2956.
[CDAT] Chemical Data Access Tool. 2015. Non-confidential 2012 Chemical Data Reporting Information: search results for CAS RN 108-24-7. Washington (DC): US Environmental Protection Agency. [updated 2014 Jun; accessed 2015 Sep 3].
[Danish EPA] Danish Environmental Protection Agency. 2012. Survey and health assessment of thiourea compounds in chloroprene rubber. Copenhagen (DE): Danish Ministry of the Environment.
[ECCC] Environment and Climate Change Canada. 2016a. Science approach document: ecological risk classification of organic substances. Ottawa (ON): Government of Canada.
[ECCC] Environment and Climate Change Canada. 2016b. Supporting documentation: data used to create substance-specific hazard and exposure profiles and assign risk classifications. Gatineau (QC): ECCC. Information in support of the science approach document: ecological risk classification of organic substances. Available from: substances@ec.gc.ca.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2017. Draft Screening Assessment: Substances Identified as Being of Low Concern based on the Ecological Risk Classification of Organic Substances and the Threshold of Toxicological Concern (TTC)-based Approach for Certain Substances. Ottawa (ON): Government of Canada.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2019. Screening assessment - Heterocycles group. Ottawa (ON): Government of Canada. [accessed 2021 Nov 29].
[ECHA] European Chemicals Agency. 2021. Brief profile: Imidazolidine-2-thione; CAS RN 96-45-7. Helsinki (FI): ECHA. [updated 2021 May 19].
Elcombe CR, Odum J, Foster JR, Stone S, Hasmall S, Soames AR, Kimber I, Ashby J. 2002. Prediction of rodent nongenotoxic carcinogenesis: evaluation of biochemical and tissue changes in rodents following exposure to nine nongenotoxic NTP carcinogens. Environ Health Perspect. 110(4):363-375.
Environment Canada. 2009. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain inanimate substances (chemicals) on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
[EPI Suite] Estimation Program Interface Suite for Microsoft Windows [estimation model]. 2012. Version 4.1. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse Research Corporation.
Frakes RA. 1988. Drinking water guideline for ethylene thiourea, a metabolite of ethylene bisdithiocarbamate fungicides. Reg Toxicol Pharmacol. 8:207-218.
Health Canada. 1998. Exposure Factors for Assessing Total Daily Intake of Priority Substances by the General Population of Canada. Ottawa (ON): Health Canada, Environmental Health Directorate, Bureau of Chemical Hazards.
Health Canada. 2014. Metiram: Proposed Re-evaluation Decision. PRVD2014-03. Catalogue #113-27/2014-3E. Ottawa (ON): Health Canada.
Health Canada. 2016. Ethylenethiourea (ETU) Content in Consumer Products: Testing Samples with Various Matrix/Polymer Types. Ottawa (ON): Health Canada, Product Safety Laboratory. Project Number 2016-2205.
Health Canada. 2018a. Mancozeb: Proposed Re-evaluation Decision. PRVD2018-17 Ottawa (ON): Health Canada.
Health Canada. 2018b. Metiram and Its Associated End-use Products: Final Decision. RVD2018-20. Ottawa (ON): Health Canada.
Health Canada. 2019. Fifth report on human biomonitoring of environmental chemicals in Canada: Results of the Canadian Heath Measures Survey Cycle 5 (2016-2017) [PDF]. Ottawa (ON): Health Canada. [accessed 2021 Jul 13].
Health Canada. 2020a. Mancozeb and Its Associated End-use Products: Final Decision. RVD2020-12. Catalogue # H113-28/2020-12-PDF. Ottawa (ON): Health Canada.
Health Canada. 2020b. List of contaminants and other adulterating substances in foods [modified 2020 July 03]. Ottawa (ON): Health Canada. [Accessed 2021 July 20].
Health Canada. 2021. Sixth report on human biomonitoring of environmental chemicals in Canada: Results of the Canadian Heath Measures Survey Cycle 6 (2018-2019) [PDF]. Ottawa (ON): Health Canada. [accessed 2022 May 11].
[HSDB] Hazardous Substances Data Bank [database]. 1983- . Bethesda (MD): US National Library of Medicine (US). [updated 2016 Oct 28; accessed 2017 Jan 9]. Data Bank.
[IARC] International Agency for Research on Cancer. 1974. Ethylene thiourea. IARC Monogr Eval Carcinog Risks Hum. 7:45-52.
[IARC] International Agency for Research on Cancer. 2001. Ethylene thiourea. IARC Monogr Eval Carcinog Risks Hum. 79:659-702.
Maranghi F, De Angelis S, Tassinari R, Chiarotti F, Lorenzetti S, Moracci G, Marcoccia D Gilardi E, Di Virgilio A, Eusepi A, et al. 2013. Reproductive toxicity and thyroid effects in Sprague Dawley rats exposed to low doses of ethylenethiourea. Food Chem Toxicol. 59:261-271.
Matz Carlyn J, Stieb David M, Davis Karelyn, Egyed Marika, Rose Andreas, Chou Benedito, Brion Orly. Effects of age, season, gender and urban-rural status on time-activity: Canadian Human Activity Pattern Survey 2 (CHAPS 2). Int J Env Res Publ Health. 11: 2108-2124.
[Netherlands] Health Council of the Netherlands: Dutch Expert Committee on Occupational Standards (DECOS). 1999. Ethylene thiourea. The Hague: Health Council of the Netherlands, Publication No. 1999/03OSH.
[NPRI] National Pollutant Release Inventory. 2018. NPRI Data Search [database on the Internet]. Gatineau (QC): Environment and Climate Change Canada. [last updated 2018 Sep 13].
[NPRI] National Pollutant Release Inventory. 2021. Substance list by threshold [database on the Internet]. Gatineau (QC): Environment and Climate Change Canada. [last updated 2021 Nov 15].
[NTP] National Toxicology Program (US). 1992. Technical report on the perinatal toxicology and carcinogenesis studies of ethylene thiourea (CAS No. 96-45-7) in F344/N rats and B6C3F1 mice (feed studies). National Toxicology Program, U.S. Dept. of Health and Human Services, Public Health Service. Report No. TR 388. pp. 245.
OECD QSAR Toolbox [Read-across tool]. 2014. Version 3.3. Paris (FR): Organisation for Economic Co-operation and Development, Laboratory of Mathematical Chemistry.
[OEHHA] Office of Environmental Health Hazard Assessment. 2009. Air Toxics Hot Spots Risk Assessment Guidelines Part II: Technical Support Document for Cancer Potency Factors. Appendix B. Chemical-specific summaries of the information used to derive unit risk and cancer potency values. (OEHHA) of California. Report. pp. 626.
[PubChem [database]. 2004- . Bethesda (MD): US National Library of Medicine, National Center for Biotechnology Information.
Tetra Tech. 2021. Biomonitoring Equivalents for Ethylene Thiourea. Final report March 19, 2021. Prepared for Health Evaluation Directorate Pest Management Regulatory Agency Health Canada. Prepared by Tetra Tech and Sean Hays Summit Toxicology Bozeman, MT. Unpublished.
[US EPA] United States Environmental Protection Agency. 2020. Mancozeb and Ethylene Thiourea (ETU): Draft Human Health Risk Assessment (DRA) for Registration Review. Washington (DC): US Environmental Protection Agency, Office of Chemical Safety and Pollution Prevention. Document ID: EPA-HQ-OPP-2015-0291-0022
Appendix A. Exposure to ETU from products available to consumers
Exposures to ETU from neoprene-based wetsuit
Adult exposure to ETU from wearing a full body wetsuit covering 16 925 cm2 (body surface area minus surface area of the head) (Health Canada 1998) for up to 30 days per year was considered. A stepwise daily loss of ETU was determined for each period of use on the basis of the migration rate (Table A-1). ETU migration was considered to occur from both the inside and outside surface of the wetsuit (i.e., into the skin and out into the surrounding environment). Assuming a daily exposure of 8 hours/day, the concentration of ETU in the product was recalculated for the following day to give a new daily migration rate based on the remaining concentration (Table A-2 and Table A-3). Similarly, exposure of a child (5- to 11-year olds) was also considered, where body surface area was considered to be 8450 cm2 and body weight to be 27 kg, with a neoprene wetsuit weight of 1.34 kg containing 0.095 mg of ETU.
| Parameter | Adult (19+ years) | Child (5 to 11 years) |
|---|---|---|
| Skin surface area minus head (cm2)a | 16 925 | 8450 |
| Body weight (kg)a | 70.9 | 27 |
| Initial ETU concentration (mg/kg)b | 0.0838 | 0.0838 |
| Mass of suit (kg)c | 2.268 | 1.134 |
| Initial ratiod | 300 000-1 | 300 000-1 |
| Initial migration rate (mg/cm2/hr)e | 2.793E-07 | 2.793E-07 |
| Initial mass of ETU in suit (mg)f | 0.1901 | 0.0950 |
| Total mass extracted (mg)g | 0.095050 | 0.04749 |
| 45% dermally absorbed (mg)h | 0.042772 | 0.02137 |
| Amortized over a year (mg/day)i | 0.000117 | 5.855E-05 |
| Dose for adult (mg/kg-bw-day) j | 1.65E-06 | 2.17E-06 |
| Unit risk (unitless)k | 9.92E-08 | 1.30E-07 |
a Health Canada 1988.
b Health Canada 2016.
c Professional judgement based on retailer websites and potential wet weight.
d Danish EPA 2012.
e Initial concentration multiplied by the initial ratio.
f ETU concentration (for a neoprene water sock) of 0.0838 mg/kg (Health Canada 2016) and initial mass of suit were used to calculate the initial mass of ETU in suit.
g Total mass transferred in over 30 days (Table A-2 for adult, Table A-3 for child).
h Amount of ETU absorbed through the skin assuming 45% is dermally absorbed, calculated using total mass extracted and a dermal absorption of 45%.
I The amount that was dermally absorbed divided by 365 days/year.
j Calculated by dividing amount amortized over a year by body weight.
k The dose multiplied by the cancer slope factor of 0.061 (mg/kg-bw per day)-1 derived by Health Canada on the basis of liver tumours in female mice.
| Day | Migration ratea (mg/cm2/hr) | Mass transferred inb (mg) | Mass transferred outc (mg) | Final massd (mg) | New concentratione (mg/kg) |
|---|---|---|---|---|---|
| 1 | 2.79E-07 | 0.03782 | 0.03782 | 0.11446 | 0.05047 |
| 2 | 1.68E-07 | 0.02278 | 0.02278 | 0.06890 | 0.03038 |
| 3 | 1.01E-07 | 0.01371 | 0.01371 | 0.04148 | 0.01829 |
| 4 | 6.10E-08 | 0.00825 | 0.00825 | 0.02497 | 0.01101 |
| 5 | 3.67E-08 | 0.00497 | 0.00497 | 0.01503 | 0.00663 |
| 6 | 2.21E-08 | 0.00299 | 0.00299 | 0.00905 | 0.00399 |
| 7 | 1.33E-08 | 0.00180 | 0.00180 | 0.00545 | 0.00240 |
| 8 | 8.01E-09 | 0.00108 | 0.00108 | 0.00328 | 0.00145 |
| 9 | 4.82E-09 | 0.00065 | 0.00065 | 0.00197 | 0.00087 |
| 10 | 2.90E-09 | 0.00039 | 0.00039 | 0.00119 | 0.00052 |
| 11 | 1.75E-09 | 0.00024 | 0.00024 | 0.00072 | 0.00032 |
| 12 | 1.05E-09 | 0.00014 | 0.00014 | 0.00043 | 0.00019 |
| 13 | 6.33E-10 | 0.00009 | 0.00009 | 0.00026 | 0.00011 |
| 14 | 3.81E-10 | 0.00005 | 0.00005 | 0.00016 | 0.00007 |
| 15 | 2.29E-10 | 0.00003 | 0.00003 | 0.00009 | 0.00004 |
| 16 | 1.38E-10 | 0.00002 | 0.00002 | 0.00006 | 0.00002 |
| 17 | 8.31E-11 | 0.00001 | 0.00001 | 0.00003 | 0.00002 |
| 18 | 5.01E-11 | 0.00001 | 0.00001 | 0.00002 | 0.00001 |
| 19 | 3.01E-11 | 0.00000 | 0.00000 | 0.00001 | 0.00001 |
| 20 | 1.81E-11 | 0.00000 | 0.00000 | 0.00001 | 0.00000 |
| 21 | 1.09E-11 | 0.00000 | 0.00000 | 0.00000 | 0.00000 |
| 22 | 6.57E-12 | 0.00000 | 0.00000 | 0.00000 | 0.00000 |
| 23 | 3.96E-12 | 0.00000 | 0.00000 | 0.00000 | 0.00000 |
| 24 | 2.38E-12 | 0.00000 | 0.00000 | 0.00000 | 0.00000 |
| 25 | 1.43E-12 | 0.00000 | 0.00000 | 0.00000 | 0.00000 |
| 26 | 8.63E-13 | 0.00000 | 0.00000 | 0.00000 | 0.00000 |
| 27 | 5.20E-13 | 0.00000 | 0.00000 | 0.00000 | 0.00000 |
| 28 | 3.13E-13 | 0.00000 | 0.00000 | 0.00000 | 0.00000 |
| 29 | 1.88E-13 | 0.00000 | 0.00000 | 0.00000 | 0.00000 |
| 30 | 1.13E-13 | 0.00000 | 0.00000 | 0.00000 | 0.00000 |
a Migration rate for day 1 is the initial migration rate (Table A-1). Subsequent migration rates were calculated by taking the previous day’s new concentration value multiplied by the initial ratio value (Table A-1).
b Mass Transferred In is the amount available on skin. It was calculated as migration rate multiplied by the surface area minus head value (Table A-1) multiplied by 8 hours.
c Mass Transferred Out is the amount transferred to external environment and is equivalent to mass transferred in.
d Final Mass for day 1 was calculated as initial mass of ETU in suit (Table A-1) minus mass transfer in and mass transfer out. Subsequent days’ final mass calculation was calculated by using the previous day’s final mass and subtracting the mass transfer in and mass transfer out values.
e New concentration was calculated as final mass divided by initial mass of suit (Table A-1).
| Day | Migration ratea (mg/cm2/hr) | Mass transferred inb (mg) | Mass transferred outc (mg) | Final massd (mg) | New concentratione (mg/kg) |
|---|---|---|---|---|---|
| 1 | 2.79E-07 | 0.01888 | 0.01888 | 0.05723 | 0.05047 |
| 2 | 1.68E-07 | 0.01137 | 0.01137 | 0.03449 | 0.03041 |
| 3 | 1.01E-07 | 0.00685 | 0.00685 | 0.02078 | 0.01833 |
| 4 | 6.11E-08 | 0.00413 | 0.00413 | 0.01252 | 0.01104 |
| 5 | 3.68E-08 | 0.00249 | 0.00249 | 0.00755 | 0.00665 |
| 6 | 2.22E-08 | 0.00150 | 0.00150 | 0.00455 | 0.00401 |
| 7 | 1.34E-08 | 0.00090 | 0.00090 | 0.00274 | 0.00242 |
| 8 | 8.05E-09 | 0.00054 | 0.00054 | 0.00165 | 0.00146 |
| 9 | 4.85E-09 | 0.00033 | 0.00033 | 0.00099 | 0.00088 |
| 10 | 2.92E-09 | 0.00020 | 0.00020 | 0.00060 | 0.00053 |
| 11 | 1.76E-09 | 0.00012 | 0.00012 | 0.00036 | 0.00032 |
| 12 | 1.06E-09 | 0.00007 | 0.00007 | 0.00022 | 0.00019 |
| 13 | 6.40E-10 | 0.00004 | 0.00004 | 0.00013 | 0.00012 |
| 14 | 3.86E-10 | 0.00003 | 0.00003 | 0.00008 | 0.00007 |
| 15 | 2.32E-10 | 0.00002 | 0.00002 | 0.00005 | 0.00004 |
| 16 | 1.40E-10 | 0.00001 | 0.00001 | 0.00003 | 0.00003 |
| 17 | 8.44E-11 | 0.00001 | 0.00001 | 0.00002 | 0.00002 |
| 18 | 5.08E-11 | 0.00000 | 0.00000 | 0.00001 | 0.00001 |
| 19 | 3.06E-11 | 0.00000 | 0.00000 | 0.00001 | 0.00001 |
| 20 | 1.85E-11 | 0.00000 | 0.00000 | 0.00000 | 0.00000 |
| 21 | 1.11E-11 | 0.00000 | 0.00000 | 0.00000 | 0.00000 |
| 22 | 6.70E-12 | 0.00000 | 0.00000 | 0.00000 | 0.00000 |
| 23 | 4.04E-12 | 0.00000 | 0.00000 | 0.00000 | 0.00000 |
| 24 | 2.43E-12 | 0.00000 | 0.00000 | 0.00000 | 0.00000 |
| 25 | 1.47E-12 | 0.00000 | 0.00000 | 0.00000 | 0.00000 |
| 26 | 8.84E-13 | 0.00000 | 0.00000 | 0.00000 | 0.00000 |
| 27 | 5.33E-13 | 0.00000 | 0.00000 | 0.00000 | 0.00000 |
| 28 | 3.21E-13 | 0.00000 | 0.00000 | 0.00000 | 0.00000 |
| 29 | 1.93E-13 | 0.00000 | 0.00000 | 0.00000 | 0.00000 |
| 30 | 1.17E-13 | 0.00000 | 0.00000 | 0.00000 | 0.00000 |
a Migration rate for day 1 is the initial migration rate (Table A-1). Subsequent migration rates were calculated by taking the previous day’s new concentration value multiplied by the initial ratio value (Table A-1).
b Mass Transferred In is the amount available to skin. It was calculated as migration rate multiplied by the surface area minus head value (Table A-1) multiplied by 8 hours.
c Mass Transferred Out is the amount transferred to external environment and is equivalent to mass transferred in.
d Final Mass for day 1 was calculated as initial mass of ETU in suit (Table A-1) minus mass transfer in and mass transfer out. Subsequent days’ final mass calculation was done by using the previous day’s final mass and subtracting the mass transfer in and mass transfer out values.
e New concentration was calculated as final mass divided by Initial mass of suit (Table A-1).
Exposures to ETU from steering wheel cover
Adult exposure to ETU from using a steering wheel cover in a vehicle in contact with both hand palms (455 cm2) (Health Canada 1998) for up to 365 days per year was considered. A stepwise daily loss of ETU was determined for each period of use on the basis of the migration rate (Table A-4). ETU migration was considered to occur from both the outside surface of the cover directly to the palms of both hands. No information was available on possible loss of ETU into the surrounding environment. Following each daily exposure (i.e., 4 hr/day for single day, 1.27 hr/day for other durations (Matz et al. 2014)) the concentration of ETU in the product was recalculated to give a new daily migration rate based on the remaining concentration (Table A-5). To derive the estimates, the average daily time spent in-vehicle for all participants was chosen from the study (Matz et al. 2014), even though this average would include those to young to drive (i.e., individuals under 15 to 16 years of age). Additionally, while the “in vehicle” time in the study (Matz et al. 2014) included time spent as a passenger in a private vehicle, on a bus, in a taxi, on a plane train, subway in addition to time spent driving, it was conservatively assumed for the calculation that all this time was spent driving. Similarly, exposure of a teen (14 to 18 years old) was also considered but were lower than that of an adult and are not presented.
| Parameter | Adult (19+ years) |
|---|---|
| Skin surface area of two palms (cm2)a | 455 |
| Body weight (kg)a | 70.9 |
| Initial ETU concentration (mg/kg)b | 11.0 |
| Mass of steering wheel cover (kg)c | 0.7 |
| Initial ratiod | 300,000-1 |
| Initial migration rate (mg/cm2/hr)e | 3.67 x 10-5 |
| Initial mass of ETU in steering wheel cover (mg)f | 7.7 |
| Mass transferred in on first day (mg)g | 6.67 x 10-2 |
| Dermal exposure on first day (mg/kg-bw/day)h | 4.24 x 10-4 |
| Mass transferred in over 30 days (mg)i | 1.77 |
| Dermal exposure over 30 days (mg/kg-bw/day)j | 3.74 x 10-4 |
| Mass transferred in over ~10 years or 3650 days (mg)k | 7.7 |
| Daily dermal exposure over 10 years of use (mg/kg-bw/day)l | 1.34 x 10-5 |
| Unit risk (unitless)m | 5.18 x 10-6 |
a Health Canada 1998.
b Health Canada 2016.
c Professional judgement based on retailer websites.
d Danish EPA 2012.
e Initial concentration multiplied by the initial ratio.
f ETU concentration (for a steering wheel cover) of 11.0 mg/kg (Health Canada 2016) and initial mass of the cover were used to calculate the initial mass of ETU in steering wheel cover.
g Total mass transferred in on the first day, assuming 4 hours spent in a vehicle (P95 value from Matz et al. 2014).
h Calculated by multiplying “mass transferred in on first day” by dermal absorption of 45% and dividing by body weight [6.67E-02 x 0.45/70.9 = 4.24E-04 mg/kg-bw/day].
i Total mass transferred in over 30 days (Table A-5), assuming 4 hours spent in a vehicle (P95 value from Matz et al. 2014).
j Calculated by dividing “mass transferred in over 30 days” by 30 days and by multiplying dermal absorption of 45% and dividing by body weight [1.77/30 x 0.45/70.9 = 3.74E-04 mg/kg-bw/day].
k Given this value is meant to compare to possible long-term effects, mean value for time spent in a vehicle of 1 hr 16 min (or 1.27 hours) (Matz et al. 2014) was used in the derivation of loss of ETU over time. Mass Transferred In over ~10 years or 3650 days (approximate life of cover) (Autos.com Editor 2013) was calculated by summing the Mass Transferred In over all 3650 days (7.70 mg).
l Calculated by 7.7/3650 x 0.45/70.9 = 1.34E-05 mg/kg-bw/day.
m The dose multiplied by the cancer slope factor of 0.0601 (mg/kg-bw per day)-1 derived by Health Canada on the basis of liver tumours in female mice.
| Day | Migration ratea (mg/cm2/hr) | Mass transferred inb (mg) | Final massc (mg) | New concentrationd (mg/kg) |
|---|---|---|---|---|
| 1 | 3.67E-05 | 2.12E-02 | 7.68 | 10.97 |
| 2 | 3.66E-05 | 2.11E-02 | 7.66 | 10.94 |
| 3 | 3.67E-05 | 2.11E-02 | 7.64 | 10.91 |
| 4 | 3.67E-05 | 2.10E-02 | 7.62 | 10.88 |
| 5 | 3.67E-05 | 2.10E-02 | 7.59 | 10.85 |
| 10 | 3.58E-05 | 2.07E-02 | 7.49 | 10.70 |
| 30 | 3.39E-05 | 1.96E-02 | 7.09 | 10.13 |
| 365 | 1.34E-05 | 7.77E-03 | 2.82 | 4.03 |
| 3650 | 1.58E-09 | 9.11E-07 | 0.00033 | 0.00047 |
a Migration rate for day 1 is the initial migration rate (Table A-4). Subsequent migration rates were calculated by taking the previous day’s new concentration value multiplied by the initial ratio value (Table A-4).
b Mass Transferred In is the amount available on skin. It was calculated as migration rate multiplied by the surface area of the palms of the hand (Table A-4) multiplied by 1.27 hours (mean value of time spent in vehicle Matz et al. 2014).
c Final Mass for day 1 was calculated as initial mass of ETU in cover (Table A-4) minus mass transfer in. Subsequent days’ final mass calculation was calculated by using the previous day’s final mass and subtracting the mass transfer in value.
d New concentration was calculated as final mass divided by Initial mass of cover (Table A-4).
