Biological test method for measuring survival of springtails exposed to contaminants in soil: chapter 1
Section 1
Introduction
1.1 Background
The Method Development and Applications Unit(MDAU) of Environment Canada is responsible for the development, standardization and publication (see Appendix A) of a series of biological test methods for measuring and assessing the toxic effect(s) on single species of terrestrial or aquatic organisms, caused by their exposure to samples of test materials or substances under controlled and defined laboratory conditions. In 1994, MDAU, the Canadian Association of Petroleum Producers (CAPP), and the federal Program for Energy Research and Development (PERD) initiated a multi-year program to research, develop, validate and publish a number of standardized biological test methods for measuring the toxicity of samples of contaminated or potentially-contaminated soil, using appropriate species of terrestrial test organisms. The goal was to develop biological test methods applicable to diverse types of Canadian soils using terrestrial species that were representative of Canadian soil ecosystems. The initial phase of this multi-year program involved a comprehensive review of existing biological test methods used internationally to evaluate the toxicity of contaminated soils to plants and soil invertebrates. The resulting report recommended that Environment Canada support the development, standardization and publication of a number of single-species biological test methods for measuring soil toxicity, including those using springtails (Bonnell Environmental Consulting, 1994). This recommendation was endorsed by both the headquarters and regional offices of Environment Canada (Appendix B) and the Inter-Governmental Ecotoxicological Testing Group (IGETG) (Appendix C).
Numerous soil toxicity tests were coordinated or supported by Environment Canada, using various species of springtails (Orthonychiurus folsomi, Folsomia candida and Folsomia fimetaria) exposed to samples of clean soil and soils contaminated with pesticides, metals, petrochemical wastes or prospective reference toxicants. These studies (AquaTerra Environmental Ltd., 1998; Stephenson et al., 1999a, b, 2000a; AquaTerra Environmental Ltd. and ESG, 2000; ESG, 2000, 2001, 2002; ESG and AquaTerra Environmental Ltd., 2002, 2003; Becker-van Slooten et al., 2003, 2005; Stämpfli et al., 2005; EC, 2007a) focussed on the development and standardization of a biological test method for determining the lethal or sublethal toxicity of samples of contaminated soil to Collembola. Based on the results of these studies, together with the findings of a series on interlaboratory method validation studies (EC, 2007b); Environment Canada proceeded with the preparation and publication of a biological test method for conducting soil toxicity tests that measure the survival and reproduction of three species of springtails (O. folsomi, F. candida and F. fimetaria), as described in the first edition of this report (EC 2007c).
A Scientific Advisory Group (see Appendix D) of international experts experienced with the design and implementation of soil toxicity tests using springtails provided key references that were reviewed and considered as part of this undertaking. These individuals also served actively in providing a critical peer review of two drafts of the first edition of this methodology document. A larger group of knowledgeable persons (see Acknowledgements) provided further review comments in response to the final draft preceding the first edition publication. The experience of the international scientific community when performing similar soil toxicity tests using springtails (see Appendices E and F) was relied on heavily when preparing the first edition of this biological test method.
Two other standardized soil toxicity test methods have been published by Environment Canada including: (1) Biological Test Method: Tests for Toxicity of Contaminated Soil to Earthworms (Eisenia andrei, Eisenia fetida or Lumbricus terrestris), EPS 1/RM/43 (EC, 2004a); and (2) Biological Test Method: Test for Measuring Emergence and Growth of Terrestrial Plants Exposed to Contaminants in Soil, EPS/1/RM/45 (EC 2005a, amended 2007).
In 2003, Environment Canada’s MDAU convened a three-day workshop on the toxicological assessment of Canadian soils and development of standardized testing tools. Based on pre-workshop background materials (a questionnaire), plenary sessions and working group discussions, participants identified areas considered priorities for research and development. It was recommended that priority should be given to dedicating resources for the development of test methods using species that are more reflective of non-agricultural soils and/or habitats. With over 50% of Canada’s total land mass being comprised of the boreal and taiga ecozones, and the contribution of resources within these ecozones to Canada’s economy via oil and gas, mining and forestry industries, priority was given to the development of standardized tests applicable to the assessment of contaminants present in boreal soils. Since then, several years of research have been completed on the selection of suitable and sensitive test organisms for measuring soil toxicity to meet the needs of industry, Canadian regulatory and monitoring requirements, and on the development of appropriate biological test methods. A new Environment Canada test method for measuring growth in contaminated soil using terrestrial plants native to the Boreal Region was prepared and finalized (EC, 2013a). In addition, several collembolan species were investigated for potential use in laboratory toxicity tests using boreal forest soils (EC, 2010, 2013b). Numerous studies were conducted by Environment Canada that focused on developing culturing and testing methods for the sexually reproducing collembolan, Proisotoma minuta, using soils from the boreal and taiga ecozones. Based on the results of these studies, guidance on culturing Proisotoma minuta, as well as procedures and conditions for conducting soil toxicity tests that measure the survival and reproduction of this species in boreal and taiga soils, are described in this second edition report.
Detailed procedures and conditions for preparing and performing this biological test method are defined herein. Universal procedures for preparing and conducting soil toxicity tests using selected species of springtails (i.e., F. candida, O. folsomi, F. fimetaria or P. minuta) are described. Guidance is also provided for specific sets of conditions and procedures that are required or recommended when using this biological test method for evaluating different types of substances or materials (e.g., samples of field-collected soil or similar particulate waste, or samples of one or more chemicals or chemical productsexperimentally mixed into or placed in contact with natural or formulated soil). Special guidance is provided in this updated version of EPS 1/RM/47 for the collection, handling, and testing of boreal forest and taiga soils. The biological endpointsfor this method are: (a) survival (mortality), and (b) reproductive success measured at the end of the test.
The flowchart in Figure 1illustrates the universal topics covered herein, and lists topics specific to testing samples of field-collected soil, similar particulate waste (e.g., sludge, drilling mud or dredged material), or soil spiked experimentally with chemical(s) or chemical product(s).
Figure 1 Considerations for Preparing and Performing Soil Toxicity Tests Using Springtails and Various Types of Test Materials or Substances
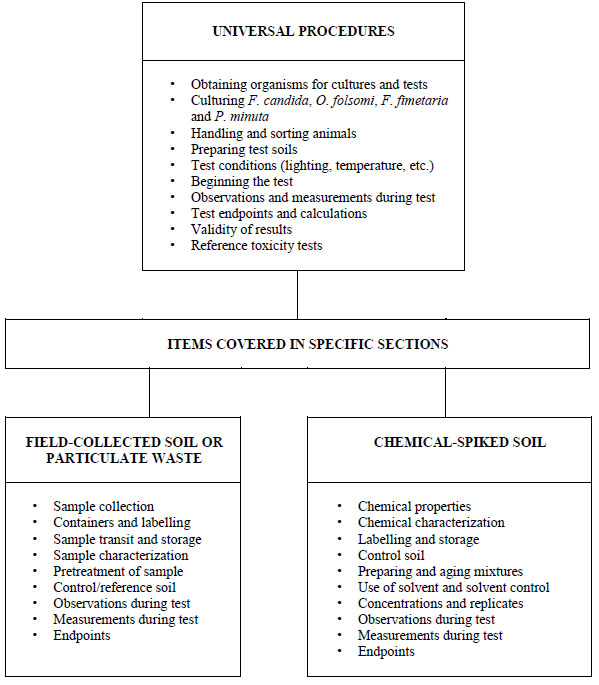
Diagram delineating test conditions and procedures appropriate for the testing of various types of materials
This figure divides the methods contained in this document into two specialized testing categories in addition to listing universal procedures which are common to the testing of any substance. The two specialized categories are specific to the testing of chemical-spiked soil and field-collected soil or particulate waste.
This biological test method is intended for use in evaluating the lethal and sublethal toxicity of samples of material such as the following:
- field-collected soil that is contaminated or potentially contaminated;
- soils under consideration for removal and disposal or remediation treatment;
- soils that have undergone remediation treatment;
- dredged material destined or under consideration for land disposal after dewatering;
- industrial or municipal sludge and similar particulate wastes that might be deposited on land; and
- clean or contaminated soil (natural or artificial), spiked with one or more chemicals or chemical products (e.g., for risk assessment of new or current-use chemicals).
In formulating this biological test method, an attempt has been made to balance scientific, practical and cost considerations, and to ensure that the results will be sufficiently precise for the majority of situations in which they will be applied. It is assumed that the user has a certain degree of familiarity with soil toxicity tests. Explicit instructions that might be required in a regulatory protocol are not provided in this report, although it is intended as a guidance document useful for that and other applications. The current report represents a revised and updated version of EPS 1/RM/47 and is intended to supersede and replace the guidance for measuring survival and reproduction of springtails exposed to contaminants in soil provided in Environment Canada’s earlier version of Report EPS 1/RM/47 (EC, 2007c).
For guidance on the implementation of this and other biological test methods, and on the interpretation and application of endpoint data
for soil toxicity, the reader should consult Sections 4.12, 5.5 and 5.6.4 in Environment Canada’s Guidance Document on Application and Interpretation of Single-Species Tests in Environmental Toxicology (EC, 1999).
1.2 Identification, Distribution, and Life History of Folsomia candida, Orthonychiurus folsomi, Folsomia fimetaria, and Proisotoma minuta
The test species to be used for the biological test method described herein (i.e., Folsomia candida, Orthonychiurus folsomi, Folsomia fimetaria and Proisotoma minuta) belong to the class Collembola (phylum, Arthropoda; subphylum, Pancrustacea; superclass, Hexapoda). The Collembola, commonly known as springtails, are currently considered to be a monophyletic (i.e., evolved from a single common ancestor) class of the phylum Arthropoda (Hopkin, 2002; Bellinger et al., 2013). They are historically considered to be an order within the class Insecta; however, their position relative to other arthropods is subject to much debate and, based on modern theories of evolution and advancement in molecular phylogeny, their placement is yet to be settled (Hopkin, 1997, 2002).
Collembola are the most abundant and widely occurring arthropods in terrestrial ecosystems and are ubiquitous to the wide variety of soil types occurring in Canada. Definitive information regarding the identification, systematics, distribution, biology, physiology and life history of springtails, including Folsomia candida, Orthonychiurus folsomi, Folsomia fimetaria and Proisotoma minuta, can be found in several publications and websites, including Hopkin, 1997, 2006 (www.stevehopkin.co.uk); Fountain and Hopkin, 2005; and Bellinger et al., 2013 (www.collembola.org).
Collembola are apterygote (wingless) soil invertebrates. The basic body parts of the Collembola species to be used in this test method are illustrated in Figure 2. The Collembolan body can be divided into three main parts: (i) the head, which bears a pair of antennae, a pair of eyes (if present), and mouthparts, which are held inside the head capsule; (ii) the thorax, which consists of three segments, each bearing a pair of legs; and (iii) the abdomen, which is comprised of six segments. In several species, some of the abdominal segments are fused, making it difficult to distinguish them (Hopkin, 1997; Bellinger et al., 2013). The furca or the springing organ is what gives the Collembola their common name of springtails. If present (i.e., the furca is absent or has become a vestigial structure in some species confined to the soil), it is located on the ventral side of the fourth abdominal segment and is usually folded under the body. The furca originated from a pair of appendages, which fused basally to form the manubrium. The two distal parts remained separate and developed into a pair of structures called dentes (singular, dens). On the end of each of these is a modified claw called a mucro. The springtails use their mucros to push or hook against the ground, providing the leverage to enable them to jump (Hopkin, 1997). All Collembola have a ventral tube (a pair of thin- walled, closely apposed, eversible vessicles on the ventral side of the first abdominal segment) that plays an important role in fluid exchange with the external environment (i.e., the regulation of water and salt content) (Rundgren and van Gestel, 1998; Hopkin, 2000; Fountain and Hopkin, 2005), and which also plays an important role in the uptake of toxicants dissolved in porewater (Lock and Janssen, 2003). The ventral tube can also function as a sticky appendage to enable springtails to adhere to slippery surfaces (Hopkin, 2002).
Springtails occupy a key position in the soil food web, being consumers of fungi, detritus, nematodes and bacteria (Lee and Widden, 1996; Laskowski et al., 1998). They are also one of the important prey groups for generalist invertebrate predators in agro-ecosystems such as mites, centipedes, spiders, carabidae and rove beetles (Bilde et al., 2000; OECD, 2009). Collembola contribute to decomposition and respiration processes in soil, mainly through feeding on fungal hyphae (Hopkin, 2000), although their role in humus formation is not well known. In soil, they have been shown to influence the growth of mycorrhizae and the control of fungal diseases of some plants (Laskowski et al., 1998; Hopkin, 2000; Fountain and Hopkin, 2005). In acidic forest soils, they may be the most important invertebrates, as earthworms and diplopods are absent (OECD, 2009). Collembola population densities of 105/m2 are commonly observed in soil and leaf litter layers under favourable conditions (OECD, 2009). Springtails are important members of the soil fauna and are appropriate organisms for use in the assessment of potentially toxic soils, and compared to soft- bodied invertebrates (e.g., earthworms), the Collembola might represent organisms with a different route (or at least rate) of exposure (OECD, 2009).
Figure 2 Adult Female Folsomia candida
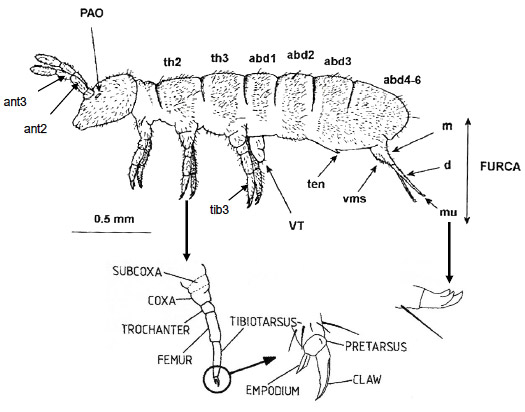
Diagram of an adult Folsomia candida
This figure illustrates the basic body parts of all four species described in this test method. The furca is normally held underneath the abdomen by the tenaculum. The first thoracic segment is reduced dorsally compared with the second and third. The last three abdominal segments are fused together. The antennae are divided into four segments. Other key body parts include: dens, manubrium, mucro, post-antennal organ, ventral manubrial satae, ventral tube, and tibiotarsus.
1.2.1 Folsomia candida
Folsomia candida Willem 1902, also known as the “compost” springtail (Römbke et al., 2006) is among the most intensively studied of all species of Collembola (Hopkin, 1997). It belongs to the family Isotomidae:
- class, Collembola;
- order, Entomobryomorpha;
- superfamily, Isotomoidea;
- family, Isotomidae;
- subfamily, Proisotominae
(Bellinger et al., 2013). F. candida resembles O. folsomi in that it is unpigmented, eyeless and has no anal spines. Unlike O. folsomi, F. candida is parthenogenetic (i.e., asexual). Females lay unfertilized eggs that develop into viable offspring, and males are completely absent from the population. F. candida is hemiedaphic in nature (Schrader et al., 1997) and possesses a well-developed furca (Hopkin, 1997). Adults are 1.5−3.0 mm in length at maturity (Fountain and Hopkin, 2005).
Diagnostic features of F. candida include:
- the absence of ocelli;
- the ratio of the length of the longest setae and the tip of the abdomen/length of mucro is between about two and four;
- the manubrium has numerous stout (16−32) ventral (anterior) setae;
- the dens has 20−40 ventral (anterior) setae and 7−10 dorsal (posterior) setae; and
- the POA is quite broad and is shorter than the width of the first antennal segment (Figure 2) (Fountain and Hopkin, 2005; Hopkin, 2006).
F. candida can be found in most regions of the world except for Africa and India (Hopkin, 1997). Its original biogeographical locations are difficult to ascertain, since it has been carried all over the world in small portions of soil (Fountain and Hopkin, 2005). In Canada, its distribution is limited mainly to southern areas (Christiansen and Bellinger, 1980). F. candida is an indigenous species to forest soils in Ontario and Quebec (Addison, 1996); however, it has low ecological relevance (i.e., it is not abundant) in soils of the Canadian boreal forests and northern lands (Römbke et al., 2006). This species has also been recorded in British Columbia (Skidmore, 1995). F. candida is found in a variety of habitats including caves, mines, agricultural systems, soils high in organic matter, forests, stream banks, and greenhouses (Fountain and Hopkin, 2005; Hopkin, 2006). F. candida is well adapted to dry soil conditions. It has physiological adaptations to avoid desiccation and the ability to absorb water vapor (Fountain and Hopkin, 2005). Oxygen uptake is via the cuticle (no tracheae), and they can survive for up to 18 h in completely anaerobic conditions, or under conditions of elevated carbon dioxide (Fountain and Hopkin, 2005).
Like other Collembola, F. candida feeds on fungal hyphae. In lab microcosm studies, F. candida showed a preference for fungi growing on the surfaces of leaf litter rather than on soil particles, and there is good evidence that they are an important stimulant of decomposition (Fountain and Hopkin, 2005). The type of fungus on which F. candida feeds has been shown to influence their growth and fecundity (i.e., some taxa of fungi are more nutritious than others) (Fountain and Hopkin, 2005).
F. candida can reproduce 12−16 days after hatching (Spahr, 1981). Typically, however, the first egg laying occurs between 17 and 26 days, most often after 21−22 days (K. Becker-van Slooten, personal communication, Laboratory of Environmental Chemistry and Ecotoxicology, ENAC-ISTE, Ecolé polytechnique fédérale de Lausanne, Lausanne, Switzerland, 2006). It has a high reproductive rate, and populations consist exclusively of parthenogenetic females. Eggs are laid in small batches or on top of those already deposited by other females forming aggregates that can be easily seen with the naked eye in laboratory cultures. Crowding or high population densities reduce the number of eggs laid (Hopkin 1997; Fountain and Hopkin, 2005). During early instars, about 20 eggs are laid in each batch, but this increases to 100 around the 20th instar before declining back to 60 at the 30th instar (Snider, 1973; Hopkin, 1997). F. candida moults every 3 to 8 days, with a short reproductive instar (~1.5 days) alternating with longer nonreproductive instars (~8.5 days) (Fountain and Hopkin, 2005). Oviposition occurs every 5 to 10 days, depending on the age of the organism (Snider, 1973). Eggs, which are white, spherical and 80 to 110 μm in diameter, take 7 to 10 days to hatch. The optimal temperature for hatching success is 21°C, and eggs maintained above 28°C will fail to hatch (Fountain and Hopkin, 2005). F. candida lives for about 140 days (maximum 190 days) and goes through up to 45 moults under laboratory conditions at 21°C. Longevity is almost doubled and egg production is ~30% greater at 15°C compared with 21°C (Hopkin, 1997). F. candida is used widely by ecotoxicologists in standard toxicity tests (Hopkin, 1997). These organisms are easily cultured in the laboratory, and their biology and ecology is very well known. Figure 3 presents an example of age-synchronized 10-12 day-old F. candidathat are ready for addition to a toxicity test.
1.2.2 Orthonychiurus folsomi
Orthonychiurus folsomi Schäffer 1900 (formerly identified as Onychiurus folsomi) belongs to the family Onychiuridae:
- class, Collembola;
- order, Poduromorpha;
- superfamily, Onychiuroidea;
- family, Onychiuridae;
- subfamily, Onychiurinae
(Bellinger et al., 2013). O. folsomiis a small, blind, poorly pigmented, euedaphic springtail that occupies the interstitial spaces between soil particles, or under stones and rotting wood on the soil surface. O. folsomi have several characteristics that are typical of those species that live permanently in the interstitial spaces in soil. These characteristics allow greater access to habitat space and enhance movement within soils (Kamplichler and Hauser, 1993) and include: lack of a furca, lack of eyes, pale white integument, elongate body (up to 1.9 mm in length) with rounded abdomen, downward-pointing mouthparts and the absence of anal spines.
Diagnostic features of O. folsomi include:
- absence of ocelli(eye lenses);
- a complex elliptical post antennal organ (PAO) with 10−12 complex vesicles;
- the absence of anal spines;
- a dorsal sensory organ on the third antennal segment with four papillae;
- an inner unguis with a small tooth;
- an unguiculus slightly shorter than its unguis and without a lamella; and
- a ventral tube in the male consisting of four modified setae on the second abdominal segment.
The tibiotarsi of the legs bear nine distal setae, and the empodium is long and filamentous, reaching the same length as the claw (Figure 2). Pseudocelli are absent from the first thoracic segment, and form a dorsal pattern of 32/022/33342 (or 3) and a ventral pattern of 2/010/0101 (Hopkin, 2006).Footnote1 Pseudocelli are defensive pores (i.e., small areas of thin cuticle) from which a fluid is extruded as a defense mechanism in response to perceived threats (e.g., predation).
Orthonychiurus folsomi is a species common in soil environments of North America. O. folsomi is a detritivore, playing an important functional role in nutrient cycling in soils. It is a sexually reproducing species with indirect sperm transfer. The sperm are produced from paired testes and ejaculated from a simple genital opening in a spermatophore, which is deposited on the substrate, or placed directly onto the female. Females have paired ovaries, and the eggs are laid singly, but often in clumps or clutches. Conspicuous sexual dimorphism is rare, and it is difficult to distinguish males from females. The females are generally slightly larger, especially if they are fecund. The organisms can be sexed by examining the genital plate, but this requires high magnification. Subtle secondary sex characteristics can sometimes be used to distinguish males from females. For example, the setae on the males might be marginally shorter in comparison to the female, and males occasionally have extra spines on their legs. The sexual dimorphism of test-aged O. folsomi is illustrated in Figure 4.
Snider (1983) conducted a study on the oviposition, egg development and fecundity of O. folsomi. The author found that temperature affected the development time of eggs, in that the time to eclosiondecreased with increased temperature. At 15 and 21°C, eggs hatched in 21 and 14 days, respectively, and at 27°C this time was reduced to 11 days. At 15 and 21°C, time to eclosion was least variable and egg viability was highest. There was a shorter time to the onset of egg laying at 21°C (four weeks), relative to 15°C (five weeks). Egg mass size varied between 15 and 45 eggs at 15°C, and between 12 and 36 eggs at 21°C.
Snider (1983) also found that crowding negatively affected fecundity (i.e., there were four times more eggs in small cultures than in large ones) and that paired breeding was the most efficient technique for breeding. AquaTerra Environmental Ltd.’s results (1998) differed from those of Snider in that cultures at greater population densities were more productive than cultures containing fewer individuals.
1.2.3 Folsomia fimetaria
Like Folsomia candida, Folsomia fimetaria Linnaeus 1758 belongs to the family Isotomidae:
- class, Collembola;
- order, Entomobryomorpha;
- superfamily, Isotomoidea;
- family, Isotomidae;
- subfamily, Proisotominae
(Bellinger et al., 2013). Also like F. candida, F. fimetaria is a hemiedaphic (Folker-Hansen et al., 1996; Bilde et al., 2000; Kanal, 2004), non- pigmented, eyeless species possessing a well- developed furca (Jensen et al., 2003). F. fimetaria, however, is a sexually reproducing species, unlike the parthenogenetic F. candida (see Section 1.2.1), and adults are smaller (0.8−1.4 mm long).
Diagnostic features of F. fimetaria include:
- the absence of ocelli;
- the ratio of the length of the longest setae and the tip of the abdomen/length of mucro is between 3.2 and 4.0;
- the manubrium has 4 + 4 apical ventral (anterior) setae with 3 + 3 in a transverse row and 1 + 1 above them; and
- the dens has 18−24 ventral (anterior) setae and 5 dorsal (posterior) setae.
The PAO is narrow and is about the same length as the width of the first antennal segment (Hopkin, 2006). Discrimination from species of the same genus is not problematic today, with the unique position of manubrial seta and other characteristics (Fjellberg, 1980); however, care should be taken to avoid confusion with other white and eyeless members of the same genus like F. candida, F. lawrencei and F. litsteri (Krogh, 2004). F. candida (see Section 1.2.1) can be misidentified for F. fimetaria and vice versa; however, a good characteristic for separating the species is that F. candida has 2 + 2 or 3 + 3 setae on the ventral side of the third thoracic segment; these are absent in F. fimetaria (Hopkin, 2006).
Folsomia fimetariais widely distributed and common in several soil types ranging from sandy to loamy soils and from mull to mor soils (OECD, 2009). It has been recorded in agricultural soils all over Europe (Römbke et al., 2006); however, there is little evidence of this species inhabiting boreal forests or northern lands. In Canada, F. fimetaria has been found in the Northwest Territories, British Columbia, Alberta, Manitoba, Ontario, New Brunswick and Newfoundland (Skidmore, 1995).
F. fimetaria has an omnivorous feeding habit, with a diet that includes fungal hyphae, bacteria, protozoa and detritus (OECD, 2009). In farmland soils, it is considered to be an important prey for the beneficial arthropod predators, which are recognized for their role of suppressing insect pests. Thus, the presence of F. fimetaria may stabilize these populations of beneficial insects at a level that is desirable in integrated and organic farming systems (Laskowski et al., 1998). F. fimetaria has shown a high degree of food selectivity, preferring fungi that optimize their growth, survival and fecundity. This species could even select the optimal food when a fungal species was grown in different soil substrates. The high degree of selectivity corresponding to food quality that was seen in this species might be due to a production of fungal odour that can be detected by the collembolans (Jørgensen et al., 2003).
F. fimetaria is sexually mature after 18 days, when the sixth instar has been reached. Sexual differences between males and females are difficult to discern before 20 days after hatching. The males have a more slender body, and they are only half the size of the females (Krogh, 2004). F. fimetaria has many characteristics desirable for a toxicity test species, including their ease of culturing in sufficient numbers, and they reproduce readily, continuously and year-round, ensuring the routine availability of test organisms (Riepert and Kula, 1996). The sexual dimorphism of age- synchronized F. fimetaria before addition to a toxicity test is illustrated in Figure 5.
1.2.4 Proisotoma minuta
Like F. candidaand F. fimetaria, P. minuta Tullberg 1871 belongs to the family Isotomidae:
- class, Collembola;
- order, Entomobryomorpha;
- superfamily, Isotomoidea;
- family, Isotomidae;
- subfamily, Proisotominae
(Bellinger et al., 2013). P. minuta is a common, widespread, grayish-brown species with adults reaching a maximum length of ~1.1 mm long (Hopkin, 2006). Like F. fimetaria and 0.folsomi, P. minuta is sexually reproducing; however, what distinguishes them from these species is their visible ocelli and gray/brown pigmentation.
Diagnostic features of P. minuta include:
- the presence of ocelli (8 + 8);
- the ratio of the length of the longest setae and the tip of the abdomen/length of mucro is between 3.2 and 4.0;
- the mucro has three teeth;
- the manubrium has 1 + 1 ventral manubrial setae; and
- the dens has six ventral (anterior) setae, and the dorsal side is crenulated.
P. minuta has often been confused with similar species, Proisotoma tenella and Proisotoma subminuta. Like P. minuta, P. tenella has 8 + 8 ocelli but differs in that it has 3 + 3 ventral manubrial setae, numerous setae on the ventral side of the dens, and the mucro has two teeth. P. subminuta differs from P. minuta in that they lack ventral setae on the thorax and on the second section of the abdomen (abd2; see Figure 2) (Fjellberg, 2007).
Distribution of P. minuta is worldwide, having been recorded in soils from Australia (Park, 2007), Asia (Stach, 1964), Europe (Dromph, 2003), and South (Heckman, 2001) and North America (Lartey et al., 1989). In Canada, the species is considered to be widely distributed (Dodd and Addison, 2010) and has been identified in Alberta, Manitoba, Ontario and New Brunswick soils (Skidmore, 1995). Laboratory cultures have been initiated from specimens isolated from samples of central Saskatchewan soil (EC, 2010).
In the field, this species is hemiedaphic and resides within leaf litter and upper horizons of soils (Bahrndorff et al., 2009); however, experimentation in a laboratory setting has shown P. minuta to be capable of survival and reproduction in subsurface soil horizons, and across a wide variety of soil pH and organic matter compositions (EC, 2013b). Generally, this species is considered to have a cosmopolitan distribution and can be found within disturbed agronomic soils (Laterley et al., 1989) as well as undisturbed, stratified, forest soils (EC, 2010). As with other springtail species, P. minuta feeds on fungal hyphae found within the soil.
Sexual maturity is reached (under laboratory conditions) approximately 14 days after organisms hatch. Oviposition occurs in clusters ranging from 30-50 eggs. These eggs require a minimum of 6 days of incubation before hatching (EC, 2013b). In culture, male and female organisms can be distinguished visually by their relative size and shape, with females being larger and possessing more rounded abdomens while males possess an erect, thin, ventral sensillum on the second segment of their antennae (ant2) and 3 short, erect sensilla on the ventral position of ant3 (see Figure 2). Additionally, males lack modified seta on tibiotarsi 3 (tib3; see Figure 2) (Fjellburg, 2007). The sexual dimorphism of age- synchronized P. minuta before addition to a toxicity test is illustrated in Figure 6.
Figure 3 Age-synchronized 10- to 12-day-old Folsomia candida

Age-synchronized 10-to 12-day-old Folsomia candida
This is a photographic image, taken through a microscope, of a 10 to 12 day-old F. candida. The body is approximately 600 µm long with antennae extending another ~150 µm beyond.
Figure 4 Sexual Dimorphism of Age-synchronized 28- to 31-day-old Orthonychiurus folsomi

Sexual dimorphism of age-synchronized 28-to 30-day-old Orthonychiurus folsomi
This is a photographic image, taken through a microscope, of two 28 to 31 day-old O. folsomi; one male and one female. The female is approximately 800 µm long with antennae extending another ~150 µm beyond. The pictured male is about 15% smaller than the female.
Figure 5 Sexual Dimorphism of Age-synchronized 23- to 26-day-old Folsomia fimetaria

Sexual dimorphism of age-synchronized 23-to 26-day-old Folsomia fimetaria
This is a photographic image, taken through a microscope, of two 23 to 26 day-old F. fimetaria; one male and one female. The female is approximately 1 mm long with antennae extending another ~220 µm beyond. The pictured male is about 20% smaller than the female.
Figure 6 Sexual Dimorphism of Age-synchronized 14-day-old Proisotoma minuta

Sexual dimorphism of age-synchronized 14-day-old Proisotoma minuta
This is a photographic image, taken through a microscope, of two 14 day-old P. minuta; one male and one female. The female is approximately 750 µm long with antennae extending another 160 µm beyond. The pictured male is about 25% smaller than the female and has slightly narrower lower abdominal segments.
1.3 Historical Use of Springtails in Toxicity Tests
The development of biological test methods for soil toxicity testing lags behind that for other media (e.g., water and sediment) (Bonnell Environmental Consulting, 1994). This delay is partially due to the fact that research and regulators have focused on the aquatic environment. Soil systems are more complex than aquatic systems, with many problems inherent in their lack of homogeneity. The variety of exposure routes available to investigators (e.g., via pore water, soil vapours or direct contact with soil particles), coupled with the high cost of running soil toxicity tests, in the past have led investigators to rely on extrapolations from aquatic test methods to soil- based exposures (Bonnell Environmental Consulting, 1994).
Assessment of soil quality before the 1980s primarily involved the evaluation of the physicochemical properties of soil, and not until the 1980s did the initial use of standardized biological test methods for measuring soil toxicity emerge from agencies responsible for pesticide registration and application (e.g., the United States Environmental Protection Agency [USEPA], and the Office of Pesticides Programs [Holst and Ellanger, 1982]). Historically, Collembola have been incorporated into a wide range of ecotoxicological assessments. One of the earliest laboratory studies involving Collembola was undertaken by Sheals (1956), who studied the effects of organochlorine compounds on microarthropod communities and screened various species for differences in susceptibility to DDT, using filter paper for the exposure (Wiles and Krogh, 1998). In a later study, Scopes and Lichtenstein (1967) used F. fimetaria in an acute test, also using the filter paper method of exposure. Thompson and Gore (1972) were among the first to promote the use of F. candida as a laboratory test species in their bioassay assessments of 29 insecticides. Many laboratory studies followed in the 1970s to 1990s using various species of Collembola, of which four species were used most commonly: Folsomia candida, Folsomia fimetaria, Onychiurus armatus (Protaphorura armata) and Orchesella cincta (Scott-Fordsmand and Krogh, 2005).
The toxicity of site soils became a “new” concern in the mid-1980s, and regulatory programs such as SUPERFUND in the United States, and the National Contaminated Sites Remediation Program (NCSRP) in Canada, were established to address the urgent need for guidance on the assessment and remediation of high-priority contaminated sites. Under the NCSRP, a review of existing whole-organism bioassays for soil, freshwater sediment, and fresh water (Keddy et al., 1995) was conducted to lead to the establishment of a suite of tests that could be used immediately for contaminated-site assessment in Canada (Bonnell Environmental Consulting, 1994). Keddy et al. (1995) concluded that most of the existing methods or procedures for measuring the toxicity of samples of soil from contaminated sites were inadequate for proper ecotoxicological assessment, and recommended that attempts be made to develop a suite of standardized biological test methods for soil that used test species and conditions applicable to Canadian soil ecosystems. The Canadian Council of Ministers of the Environment (CCME) published a framework for ecological risk assessment (ERA) in 1994 (CCME, 1994), which had a subsequent impact on the management of contaminated sites (CCME, 1996, 1997). The ERA approach, which relied on the results of single-species toxicity tests, led to the need to develop reliable, reproducible and realistic soil toxicity tests with ecologically relevant terrestrial test species for the assessment of contaminated site soils (Bonnell Environmental Consulting, 1994). In the late 1990s, biological assessments in the form of toxicity testing were becoming a useful complement to chemical analyses, especially when applied to site-specific risk assessments.
In 1998, Wiles and Krogh published a test procedure using three species of Collembola (Isotoma viridis, Folsomia candida and Folsomia fimetaria). The procedures were formatted like an International Organization for Standardization (ISO) standard since it was the European Union’s intention to standardize the method according to the ISO system of test guidelines (Scott-Fordsmand and Krogh, 2005). The first standardized whole-soil toxicity test using springtails, applicable to both pesticide and non-pesticide exposures in artificial soil, was a reproduction test-method published by the ISO in 1999. This method describes the use of Folsomia candida as the test species, and was developed to assess chemical-spiked soils only. In 2005, the National Environmental Research Institute in Denmark released a proposal to the Organisation for Economic Co-operation and Development (OECD) for a new test guideline that assesses the effects of chemical-spiked soils on the reproduction of two species of Collembola (Folsomia fimetaria and Folsomia candida) (OECD, 2005), and in 2009, the guideline was adopted (OECD, 2009).
Today, Collembola are widely used as test organisms in single-species toxicity tests intended to measure the toxicity of pure chemicals, chemical products, or samples of soil contaminated or potentially contaminated with chemicals in the field or (for experimental purposes) in the laboratory. Collembola play a key role in soil functioning and are vital indicators for soil ecotoxicology (Cortet et al., 1999). They are frequently exposed to numerous toxic chemicals in soil such as fertilizers, insecticides, herbicides and fungicides from agricultural and domestic applications, as well as heavy metals, petroleum hydrocarbons, or other chemicals such as wood preservatives (e.g., pentachlorophenol) or nitroaromatic explosive compounds in contaminated soils. Springtails possess many attributes that make them appropriate organisms for use in the assessment of potentially toxic soils. Their life history characteristics, distribution and ecological function make them ecologically important (Riepert and Kula, 1996). They are ubiquitous in nature, widely distributed in diverse soil environments, often highly abundant, easily sampled in the field, can be cultured or maintained in the laboratory and have a relatively rapid life cycle with a high reproductive rate (Scott-Fordsmand and Krogh, 2005). Besides the standard test using earthworms, tests involving Collembola are becoming more routine for testing the effects of chemicals on non-target organisms.
In Canada, the use of Collembola toxicity tests as “ecotoxicological assessment tools” for assessing the toxicity of contaminated or potentially contaminated site soil is also increasing (AquaTerra Environmental Ltd., 1998; Stephenson et al., 1999a, b, 2000a; AquaTerra Environmental Ltd. and ESG, 2000; ESG 2000, 2001, 2002; ESG and AquaTerra Environmental Ltd., 2002, 2003), and results of soil toxicity tests are used to:
- derive national soil quality criteria;
- establish site-specific, risk-based, cleanup objectives (e.g., remediation targets); and
- assess the efficacy of remediation technologies (Stephenson et al., 2002).
Extensive reviews on the use of springtail toxicity tests as “ecological assessment tools” for appraising the toxicity of contaminated or potentially contaminated soils in tiered testing or risk assessments have been carried out by various authors (NERI, 1993; Leon and van Gestel, 1994; Keddy et al., 1995; Römbke et al., 1996; van Gestel et al., 2001; Achazi, 2002; Lanno, 2003; Princz et al., 2012). Other ecotoxicological assessments involving the use of springtails include field monitoring of population trends (e.g., Neuhauser et al., 1989), field bioassays (e.g., Wiles and Frampton, 1996), meso- and microcosm studies (e.g., Addison and Holmes 1995; Addison 1996; Cortet et al., 2003) and a wide variety of laboratory tests (e.g., Crommentuijn et al., 1993; Addison and Holmes, 1995; Martikainen and Krogh, 1999; Fountain and Hopkin, 2001).
A number of diverse laboratory methods have been investigated to measure the effects of specific chemicals or chemical products on springtails. Some of the less “standard” endpoints reported include: growth (e.g., Folker-Hansen et al., 1996), population growth (e.g., Crommentuijn et al., 1993), bioaccumulation through ingestion of contaminated food, toxicant uptake and body burden (e.g., Janssen et al., 1991; Pedersen et al., 2000; Fountain and Hopkin, 2001; Markweise et al., 2001), and biomarkers (Stämpfli et al., 2002). Test methodology improvements, as well as the effects of variations on soil characteristics and/or laboratory test conditions, have also been investigated and/or reviewed (Sandifer and Hopkin, 1996, 1997; Riepert and Kula, 1996; Smit and van Gestel, 1996, 1997, 1998; and van Diepen, 1997; Crouau et al., 1999; Martikainen and Krogh, 1999; Martikainen and Rantalainen, 1999; Lock and Janssen, 2001; Crouau and Cazes, 2003).
Toxic effects resulting from exposure of Collembola to a wide range of environmental contaminants have been documented in laboratory studies involving samples of soil spiked or contaminated with:
- pesticides (Thompson and Gore, 1972; Tomlin, 1975; Mola et al., 1987; Addison and Holmes, 1995; Addison, 1996; Folker- Hanset et al., 1996; Peterson and Gjelstrup, 1998; Martikainen and Krogh, 1999; ESG and AquaTerra Environmental Ltd., 2002; Indiger, 2002; Campiche et al., 2006);
- metals (Crommentuijn et al., 1993, 1997; Posthuma and van Straalen, 1993; Pedersen et al., 1997, 1999; Sandifer and Hopkin, 1996, 1997; Smit and van Gestel, 1996, 1997, 1998; van Gestel and Van Diepen, 1997; Scott-Fordsmand et al., 1999; AquaTerra Environmental Ltd. and ESG, 2000; Pedersen and van Gestel 2001; Fountain and Hopkin, 2001);
- petroleum hydrocarbons (Neuhauser et al., 1989; ESG 2000, 2001; ESG and AquaTerra, 2003; van Gestel et al., 2001; Jensen and Sverdrup, 2002; Sverdrup et al., 2002; Princz et al., 2012); and
- other chemicals including reference toxicants(AquaTerra Environmental Ltd., 1998; Addison and Bright, 2002; Jensen et al., 2003; Becker-van Slooten et al., 2003, 2005; Stämpfli et al., 2005; EC, 2007a).
In addition, database reviews have been summarized in reports discussing trends of Collembola toxicity to various contaminants (Leon and van Gestel, 1994).
Historically, Folsomia candida has been the preferred species for studying the effects of prolonged exposure to contaminants on the survival and reproduction of springtails, due to widespread knowledge and experience in culturing this species, its rapid life cycle, its international distribution, and its frequent use in toxicity tests. The development, growth and reproductive biology of F. candida under laboratory conditions have been extensively studied and are well documented (Hopkin, 1997; Fountain and Hopkin, 2005). Following a review of the use of this species as a “standard” test organism, Fountain and Hopkin concluded that, although there has been some criticism toward the field relevance of the ISO test with F. candida, this species plays an important role in risk assessment and will continue to be included in the development of new environmental standards (Fountain and Hopkin, 2005).
Results from experiments on F. candida cannot, for the most part, be extrapolated to other species of Collembola because of the differences in sensitivity among species (Krogh, 1995; Hopkin 1997). For example, the NOEC for atrazine is 600 μg/g for F. candida but only 40 μg/g for Orchesella cincta(Badejo and van Straalen, 1992). For dimethoate and copper, however, no differences in sensitivity between F. candida and F. fimetaria were detected (Scott-Fordsmand and Krogh, 2005), and for boric acid, the differences in sensitivity between these two species is small (K. Becker-van Slooten, personal communication, Laboratory of Environmental Chemistry and Ecotoxicology, ENAC-ISTE, Ecolé polytechnique fédérale de Lausanne, Lausanne, Switzerland, 2006).
Folsomia fimetaria is a relatively new test species for use in sublethal soil ecotoxicity tests. The development of a test using F. fimetaria was initiated in Denmark in 1990 while investigating the effects of pesticides (Wiles and Krogh, 1998). Since then, this species has been used for assessing the toxic effects of many different compounds such as copper, nickel, phthalates, linear alkyl benzene sulphonates (LAS), pyrene, dimethoate, polycyclic aromatic hydrocarbons (PAHs), polycyclic aromatic compounds (PACs), veterinary pharmaceutical products and sewage sludge (Fabian and Petersen, 1994; Folker-Hansen et al., 1996; Scott-Fordsmand et al., 1997, 1999, 2000; Jensen et al., 2001; Holmstrup and Krogh, 2001; Jensen and Sverdrup, 2002; Scott- Fordsmand and Krogh, 2004; Becker-van Slooten et al., 2005; EC, 2007a).
Although the biology and ecological relevance of Orthonychiurus folsomi is well known (Snider, 1983 and Section 1.2.2), use of this species in laboratory toxicity testing is relatively unknown and limited to several Canadian studies (AquaTerra Environmental Ltd., 1998; Stephenson et al., 1999a, b, 2000a; AquaTerra Environmental Ltd. and ESG, 2000; ESG 2000, 2001, 2002; Addison and Bright, 2002; ESG and AquaTerra Environmental Ltd., 2002, 2003; EC, 2007a).
The use of Proisotoma minuta as a test species has been recommended independently by researchers in Australia (Greenslade and Vaughan, 2003), as well as in Canada (Dodd and Addison, 2010). The species has been praised for its rapid reproduction potential in laboratory conditions. Conversely, the species has also proven difficult to manipulate due to its small size and pigmentation (Greenslade and Vaughan, 2003), and difficulty distinguishing adult and juvenile organisms (Dodd and Addison, 2010).
P. minuta have comparable survival and reproduction responses to inorganic toxicants such as zinc, arsenic and cadmium, as F. candida, while being relatively more sensitive to copper (Greenslade and Vaughan, 2003; Nursita et al., 2005). As has been noted with F. candida(Sandifer and Hopkin, 1996), P. minuta can tolerate high concentrations of lead in soil with no toxic responses (>3000 mg/kg) (Nursita et al., 2005).
Relative to F. candida, P. minuta displayed less toxic responses to organic compounds such as phenol and methyl tert butyl ether (Greenslade and Vaughan, 2003; Dodd and Addison, 2010). Experimental exposure to acutely toxic levels of α-endosulfan and β-endosulfan pesticides indicated that the α- endosulfan was significantly more toxic than the β- form. In sublethal doses, P. minuta reproduction was inhibited, but the organisms were able to metabolize the pesticide from highly toxic α- endosulfan and β-endosulfan to endosulfan sulphate, and eventually resume egg production (Park and Lees, 2004). P. minuta was also more sensitive than F. candida to mixtures of contaminants in soils from both brine- and petroleum-hydrocarbon-contaminated boreal forest sites (Princz et al., 2012).
The methodology documents summarized in Appendices E and F were used as guidance in developing the first edition of this test method document. The updated version of Environment Canada’s standardized biological test method for performing a test that measures the toxic effects of prolonged exposure to chemical- spiked soil or site soil on the survival and reproduction of Collembola is described herein.