Discussion paper on the third legislative review of the Tobacco and Vaping Products Act
Table of Contents
- Disclaimer
- Introduction
- Background
- Areas to explore
- Conclusion
- Annex 1: Other laws regulating tobacco and vaping products
- Annex 2: Regulations under the TVPA
- Annex 3: What we heard and areas for potential action identified through the first and second legislative reviews of the TVPA
- Annex 4: Overview of Canada's Tobacco Strategy partners
- Endnotes
Disclaimer
This document does not constitute part of the Tobacco and Vaping Products Act (TVPA) or its associated Regulations. In the event of any inconsistency or conflict between the TVPA or its Regulations and this document, the TVPA or its Regulations take precedence. This document is intended to seek the perspectives of Canadians, experts and other stakeholders as part of the legislative review of the TVPA. This document is not intended to provide legal advice regarding the interpretation or application of the TVPA or its Regulations. If a regulated party has questions about legal obligations or responsibilities under the TVPA or its Regulations, they should seek their own legal advice.
Introduction
Tobacco use remains a significant preventable cause of disease and premature death in Canada, with approximately 46,000 people dying from tobacco-related illnesses every year.Footnote 1 In Canada, responsibility over health-related matters such as tobacco control is shared between the federal, provincial and territorial governments. This allows for both a comprehensive and tailored approach to health-related matters in Canada. Federal legislation is one of several tools used to advance Canada's Tobacco Strategy (CTS) and helps protect Canadians from tobacco-related death and disease.
The Tobacco Act, originally enacted in 1997, was amended on May 23, 2018, and renamed the Tobacco and Vaping Products Act (TVPA). The TVPA is the federal legislation that regulates the manufacture, sale, labelling and promotion of tobacco and vaping products. The TVPA, along with other pieces of legislation that were also amended in 2018, created a new federal legislative framework for vaping products. This new legislative framework made it possible for adults to legally access vaping products, for which no therapeutic claim is made and to protect young persons and non-users of tobacco products from inducements to use vaping products.
The TVPA's overall purpose is to provide a legislative response to a national public health problem of substantial and pressing concern. It also aims to protect the health of Canadians given the conclusive evidence implicating tobacco use in the incidence of numerous debilitating and fatal diseases. Tobacco and vaping products are also subject to other federal Acts (Annex 1) and Regulations (Annex 2).Footnote 2
Legislative review
The TVPA requires a review of its provisions and operation three years after coming into force, and every two years thereafter. This third Legislative Review of the Tobacco and Vaping Products Act (TVPA) will focus on compliance and enforcement issues identified in the final report for the first and the second review. This includes a potential need for additional or enhanced tools for a progressive enforcement approach to help address non-compliance. See Annex 3 for compliance and enforcement comments received, and areas for potential action identified, in the previous reviews.
The review will be informed by relevant data and studies, and consultations to gather the perspectives of Canadians, including subject matter experts. Some stakeholders may wish to provide feedback related to tobacco or vaping products that apply to other pieces of federal legislation or other key tobacco and vaping-related priorities. Relevant feedback will be shared with the appropriate departments as necessary.
We want to hear from you
A key part of this legislative review is seeking the perspectives of Canadians, experts and other stakeholders as they relate to compliance and enforcement of the TVPA. Through this consultation, we want to learn more about your ideas, your experiences and your perspectives on compliance and enforcement challenges related to tobacco and vaping products both online and in physical points of sale. You are encouraged to submit any data (e.g., statistics, studies, reports, research findings, etc.) that you may have to support your input. To assist in providing a submission, a list of key questions has also been provided in the discussion paper. This list is not exhaustive and any input relating to compliance and enforcement of tobacco and vaping-related provisions of the TVPA is welcome.
You may participate by sending your written submission by September 12, 2025 to: legislativereviewtvpa.revisionlegislativeltpv@hc-sc.gc.ca
Please do not include any personal information when providing your input. The Government of Canada will not be retaining your e-mail address or contact information when receiving your submission and will only retain the comments you provide.
Submissions will be summarized in the legislative review's final report. Comments will not be attributed to any specific individual or organization. The final report will be tabled in Parliament and made public on Canada.ca at that time.
Please note: You must declare any perceived or actual conflicts of interest with the tobacco industry when providing a submission to this consultation. If you are part of the tobacco industry, an affiliated organization, an individual acting on its behalf, or otherwise support the interests of the tobacco industry, you must clearly state this in your submission.
Health Canada is also interested in being made aware of perceived or actual conflicts of interest with the vaping industry or pharmaceutical industry. Therefore, please declare any perceived or actual conflicts of interest, if applicable, when providing input. If you are a member of the vaping industry, pharmaceutical industry, an affiliated organization or an individual acting on their behalf, you are asked to clearly state this in your submission.
Inviting the perspectives of First Nations, Inuit, and Métis Peoples
The Government of Canada recognizes the sacred and ceremonial role that traditional tobacco has for many First Nations and Métis Peoples, which differs from the use of commercial tobacco. We also understand the economic role that the manufacture and sale of regulated commercial tobacco plays in certain First Nations communities. The focus of this paper is compliance and enforcement of commercial products.
We also recognize that the TVPA was developed before the coming into force of the United Nations Declaration on the Rights of Indigenous Peoples Act and the process of developing and amending the legislation in 2018 lacked extensive consultation and engagement with First Nations, Inuit and Métis Peoples.
We acknowledge that the diversity among and within First Nations, Inuit and Métis Peoples necessitates addressing distinct needs and priorities concerning tobacco and vaping. Health Canada is committed to fostering ongoing dialogue and building strong relationships with First Nations, Inuit and Métis partners. This will include ensuring that there are meaningful opportunities to contribute to the current, and future, legislative reviews of the TVPA. We welcome all comments and feedback from First Nations, Inuit and Métis Peoples on how to enhance opportunities to contribute to current and future legislative reviews of the TVPA. We want to ensure the rights and voices of these distinct communities are carefully considered in reviewing and updating federal laws.
Background
Canada's Tobacco Strategy was launched in 2018 as the federal strategy to address tobacco use in Canada, setting a goal to reduce tobacco use to less than 5 percent by 2035.Footnote 3 The strategy aims to help Canadians who smoke to quit or reduce the harms of nicotine addiction. The strategy also seeks to protect youth and people who do not use tobacco from the dangers of tobacco use and nicotine addiction.Footnote 4 The strategy features population-based approaches, as well as focused approaches on specific populations, in order to reach the Strategy's ambitious goal. In addition, Health Canada has set interim targets of reducing tobacco use among those 18 years and older to 11 percent by 2025 and 9 percent by 2030. It also has set a target of reducing vaping use among those aged 12 to 17 to below 10 percent by 2025.Footnote 5
Smoking and vaping prevalence in Canada
The smoking rate has seen a steady and continuing decline over the years. In 2018, 16 percent (4.9 million people) of Canadians aged 15 years and older reported cigarette useFootnote 6 compared to 12 percent (3.8 million people) in 2022.Footnote 7 Vaping products emerged on the Canadian market in 2007Footnote 8 and by 2017, 2.9 percent (863,000 people) of Canadians over the age of 15 had reported past 30-day use of vaping productsFootnote 9. This number had increased to 5.8 percent (1.8 million people) in 2022.Footnote 10 Youth vaping rates in particular were rising at a rapid pace, doubling between 2017 and 2019.Footnote 11Footnote 12 However, the latest results report vaping rates for youth are declining. According to the Canadian Health Survey for Children and Youth, in 2019, 13.2 percent (299,000 people) of youth aged 12 to 17 years reported vaping in the last 30 days. This number had declined to 7.2 percent (174,000 people) in 2023.Footnote 13
First Nations, Inuit and Métis Peoples have among the highest smoking and vaping rates of any population group in Canada. The Indigenous Peoples Survey is a national survey on the social and economic conditions of First Nations people living off-reserve, Métis and Inuit living in the 10 provinces and 3 territories.Footnote 14 This survey collects information on smoking and collected information on vaping for the first time in 2022. The First Nations Information Governance Centre leads the Regional Health Survey (RHS), which collects wide-ranging information, including smoking rates, about First Nations living on-reserve and in northern communities.Footnote 15 These surveys found the following smoking and vaping rates:
- 40.3% of First Nations people living on-reserve aged 18 years and older smoke cigarettes daily, and 13.1% smoke occasionally (RHS 2015-16)
- 20.5% of First Nations people living off-reserve aged 15 years and older smoke daily, 8.4% smoke occasionally, 7.2% vape daily and 16.1% vaped within the past 30 days (IPS, 2022)
- 46.2% of Inuit living in Canada aged 15 years and older reported smoking daily, 9.4% smoked occasionally, 3.8% vaped daily and 10.7% vaped within the past 30 days (IPS, 2022)
- 17.2% of Métis aged 15 years and older smoke daily, 6.1% smoke occasionally, 6.5% vape daily and 14.3% vaped within the past 30 days (IPS, 2022)
Compliance and enforcement of the TVPA
As a regulator, Health Canada's role includes regulating various products and activities in order to protect and maintain Canadians' health. Compliance and enforcement activities are key in protecting Canadians against risks to their health and safety. These activities help ensure that the relevant laws and regulations are being followed and that their objectives are being met.Footnote 16
The development and enforcement of federal acts and regulations is one of several tools used to advance Canada's Tobacco Strategy and protect Canadians from tobacco-related death and disease. Compliance and enforcement measures are in place to ensure these protections are upheld and to support the objectives of the TVPA and the Strategy.
Tobacco and vaping products on the Canadian market must comply with the requirements set out in the TVPA, its regulations and any other laws that apply to them. A number of regulations under the TVPA regulate tobacco and vaping products and set out the specific rules that industry must follow. A list of these regulations is provided in Annex 2.
There are a number of policy instruments that support compliance and enforcement of the TVPA. Health Canada has a Compliance and Enforcement Policy Framework in place that aims to promote fairness, consistency, transparency and predictability in its compliance and enforcement actions across product lines. It overlays and is supported by other established departmental policies related to specific products, such as the Compliance and Enforcement Policy for the Tobacco and Vaping Products Act. To help ensure regulated parties meet their legal obligations, Health Canada enforces the TVPA through a variety of compliance and enforcement activities. These include compliance promotion, compliance monitoring and enforcement actions.
The TVPA sets out the powers of designated inspectors. For example, an inspector can enter any place if there are reasonable grounds to believe that:
- Tobacco or vaping products are being manufactured, tested, stored, promoted, transported or furnished at the location;
- There is anything used in the manufacture, testing, promotion or furnishing of a tobacco or vaping product at the location; or
- There is any information related to the above activities at the location.
In verifying compliance with the Act, the designated inspector has various powers including:
- Visually examining tobacco or vaping products, ordering their production for examination and opening containers
- Taking samples for laboratory analysis or for compliance assessment
- Collecting, copying and reviewing documents and records
- Seizing any tobacco or vaping product if they believe it was involved in a violation of the Act
Health Canada proactively engages in compliance promotion activities to educate industry, the public and other stakeholders, of the requirements under the TVPA. Health Canada inspectors provide compliance promotional material such as copies of applicable regulations, information letters and fact sheets.
Health Canada monitors the activities of regulated parties to verify compliance with the TVPA and its Regulations and to prevent non-compliance. Compliance monitoring includes gathering and analyzing information, carrying out compliance verification activities and collaborating with other regulatory agencies as appropriate.Footnote 17
The TVPA does not require an authorization be issued for tobacco and vaping products under the TVPA before they can be sold on the market. Once products reach the market, monitoring occurs throughout the supply chain to help ensure products are compliant with the TVPA and its regulations. A robust market oversight is essential to detect and address non-compliances effectively.
When non-compliance is identified by Health Canada, several actions may be taken to inform Canadians about risk and support a regulated party with coming into compliance: publishing advisories on non-compliant products, warning letters, seizures, prosecutionsFootnote 18 and ticketing through the Contraventions Act.Footnote 19 Health Canada takes the most appropriate and effective compliance and enforcement action required specific to each situation. This will depend on the level of risk associated with the non-compliance.Footnote 20
Snapshot of TVPA compliance
Health Canada uses information from internal and external sources to identify possible non-compliance.Footnote 21 External sources of information include the public, a company within a supply chain and/or federal, provincial, territorial and international partners. Sources are encouraged to report any suspected violations to Health Canada by e-mail at tcp.questions-plt@hc-sc.gc.ca.Footnote 22
Approximately 2900 enquiries and complaints have been received since 2020. For the 2024-2025 fiscal year, 28 percent of all complaints were related to restrictions under the Nicotine Concentration in Vaping Products Regulations, followed by online ads at 16 percent. As shown in Figure 1, most complaints for the 2024-2025 fiscal year concerned vaping products. Enquiries and complaints received are a potential data source to understand what Canadians are observing and identify areas of concern that should be prioritized. Those relating to potential non-compliance can trigger an inspection.
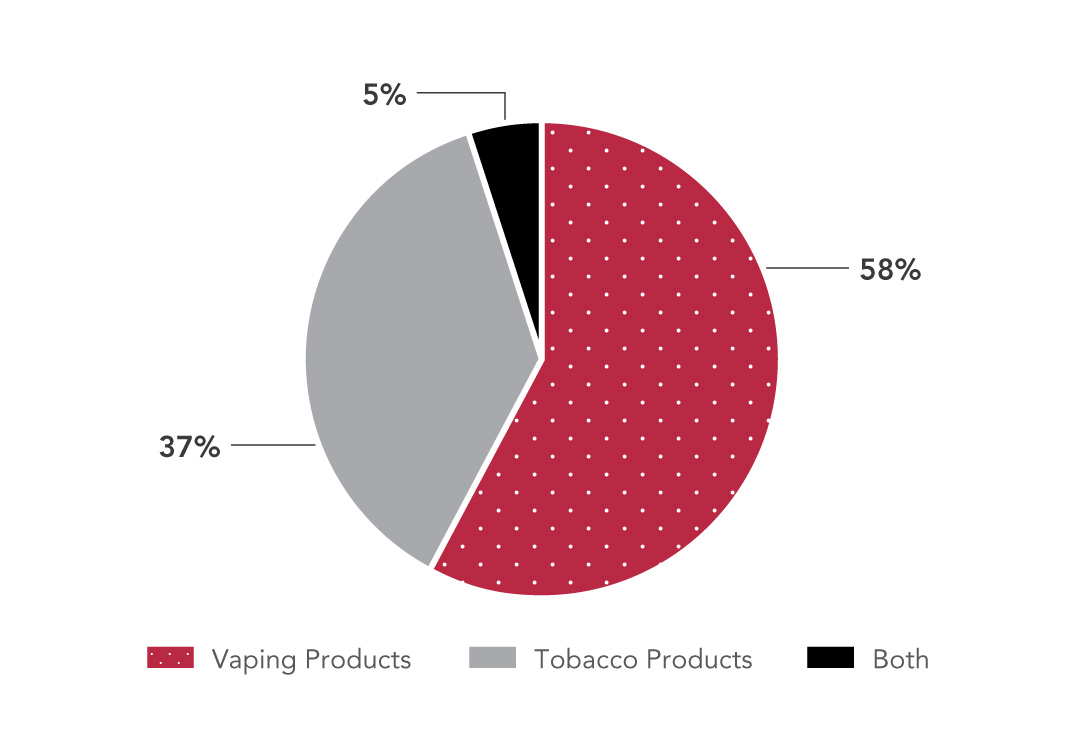
Figure 1: Text description
| Product type | Complaints (%) |
|---|---|
| Vaping products | 58 |
| Tobacco products | 37 |
| Both | 5 |
Health Canada conducts inspections of regulated parties at brick-and-mortar establishmentsFootnote 23, festivals and events, and online. The number and scope of inspections conducted by Health Canada are based on operational priorities and may change from one year to the next.
When preparing for an on-site inspection, the inspector will review all available information about the establishment, including past compliance history. On site, the inspector may sample products for laboratory analysis or label assessment, in addition to any on-site label and promotional assessment.
Online inspections monitor websites and social media accounts of manufacturers and retailers. These inspections are conducted to verify compliance with legislative and regulatory requirements online, such as the prohibition on promotion of certain flavours appealing to youth (e.g., confectionary, dessert, cannabis, soft drink and energy drink).
Health Canada also conducts inspections at festivals and events to ensure advertising of tobacco or vaping products and other promotional activities at the event, such as promotion associated with sponsors, are compliant with the TVPA and its regulations. Over the past three years, 65 festivals and events were inspected for both tobacco and vaping products. Health Canada also sends compliance promotion material to festival and event organizers each year to help ensure they are aware of their responsibilities under the TVPA.
In addition to inspections, Health Canada audits reports submitted by manufacturers, including importers, as per the Tobacco Reporting Regulations and Vaping Product Reporting Regulations to ensure industry compliance. These regulations set out the requirements for the reporting of information on, among other things, sales data and ingredients used in tobacco and vaping products.
When a non-compliance is identified, the inspector will take enforcement action, taking into account several factors, such as the severity or impact of the non-compliance, the level of due diligence or the degree of cooperation of the establishment. When appropriate, the inspector may provide direction to support the correction of non-compliances. In these cases, additional activities are conducted to verify non-compliances have been addressed, including possible follow-up inspections.
Health Canada is committed to transparency and openness, providing information to consumers and industry about Health Canada's compliance and enforcement activities concerning the sale, labelling and promotion of vaping products. Results of the compliance and enforcement activities for retail establishments are posted in the Vaping Compliance and Enforcement Reports section of Health Canada's website.
Tobacco compliance
The number of inspections conducted at tobacco retailers and manufacturers, along with the compliance rates over a six-year period, are listed in Table 1.
| Fiscal Year | Retailers | Manufacturers | ||
|---|---|---|---|---|
| # of inspections conducted | Compliance rate % | # of inspections conducted | Compliance rate % | |
| 2018-2019 | 6,716 | 91 | 73 | 98 |
| 2019-2020 | 691 | 96 | 2 | 100 |
| 2020-2021 | 0Footnote * | N/AFootnote * | 10Footnote ** | 90 |
| 2021-2022 | 1,320 | 95 | 4 | 34 |
| 2022-2023 | 2,038 | 82 | 21 | 77 |
| 2023-2024 | 2,015 | 95 | 17 | 49 |
|
||||
It is important to note that an inspection is rated non-compliant if just one of the products inspected at a manufacturing site does not meet the regulatory requirements.
The decline in compliance rates for 2021-2022 in Table 1 was due in part to the coming-into-force of the Tobacco Products Regulations (Plain and Standardized Appearance) TPR(PSA).Footnote 24 It is not uncommon to observe lower rates of compliance following the introduction of new regulations.
Detailed assessments of products are conducted at the manufacturer level. This allows Health Canada to identify systemic minor non-compliances across several products that cannot be detected at retail.
Vaping compliance
The number of inspections conducted at vaping retailers (brick-and-mortar and online), which include gas and convenience stores, specialty vaping establishments, and manufacturers, along with the corresponding compliance rates, conducted over a five-year period are listed in Table 2.
| Fiscal Year | Retailers (Gas and Convenience Stores) | Retailers (Specialty Vaping Establishments) | Manufacturers | |||
|---|---|---|---|---|---|---|
| # of inspections conducted | Compliance rate % | # of inspections conducted | Compliance rate % | # of inspections conducted | Compliance rate % | |
| 2019-2020 | 2,038 | 88 | 1,080 | 17 | 3 | 0 |
| 2020-2021 | 0Footnote * | N/A | 304 (online) | 47 | 16Footnote *** | 0 |
| 2021-2022 | 1,320 | 89 | 191 76 (online)Footnote ** |
40 20 |
35Footnote *** | 64 |
| 2022-2023 | 835 | 93 | 345 255 (online)Footnote ** |
65 10 |
80 | 72 |
| 2023-2024 | 1,609 | 97 | 288 21 (online)Footnote ** |
62 0 |
80 | 51 |
|
||||||
Despite initially low rates of vaping industry compliance since the coming into force of the TVPA, compliance rates have improved at brick-and-mortar locations. The online space continues to have high non-compliance.
Enforcement activities
Health Canada addresses industry non-compliance through various enforcement measures under the TVPA, particularly the use of warning letters and conducting product seizures. A warning letter is a formal document issued by Health Canada to inform the recipient of an instance of non-compliance, indicate their requirement to come into compliance immediately or within a certain timeframe and specify the consequences of failing to comply. A seizure is an immediate and effective enforcement action for controlling non-compliant products or removal of promotional material. Table 3 shows the enforcement activities resulting from inspections of retailers and manufacturers of tobacco and vaping products, for the years 2019-2020 to 2023-2024. Following an issuance of a warning letter or a seizure, Health Canada can negotiate with the regulated party to establish an appropriate timeframe to come into compliance.
| Fiscal Year | Tobacco Enforcement Activities | Vaping Enforcement Activities | ||
|---|---|---|---|---|
| Warning letters | Seizures | Warning letters | Seizures | |
| 2019-2020 | 22 | 60 | 2 | 1036 |
| 2020-2021 | 1 | 0Footnote * | 161 | 0Footnote * |
| 2021-2022 | 3 | 66 | 40 | 265 |
| 2022-2023 | 13 | 348 | 250 | 199 |
| 2023-2024 | 2 | 80 | 32 | 197 |
|
||||
Interpreting the data
The volume of inspections, products sampled and subset of requirements inspected can vary from one year to the next. For example, inspectors may assess compliance with a smaller subset of requirements across a larger number of products one year, and a more comprehensive verification of compliance for a limited type and number of products another year.
The prioritization of inspections of regulated parties with a history of repeat non-compliance can also impact compliance rates, as it may be more likely to find non-compliance. Priority inspections also change based on market and user trends, as well as the introduction of new regulations. Therefore, the same variables are not being compared from year to year.
The compliance rates reported for tobacco and vaping products are based on observed compliance with both the TVPA and the Canada Consumer Products Safety Act (see Annex 1).
Areas to explore
This legislative review will examine compliance and enforcement of the TVPA. Some compliance and enforcement challenges were identified in the final report for the first review and the second review. See Annex 3 for compliance and enforcement comments received and areas for potential action identified in the previous reviews. We have identified three themes that highlight challenges related to enforcing the TVPA. Each theme includes a summary of the current situation and questions designed to gather feedback on potential improvements.
Changing market
The Government of Canada began regulating tobacco products in 1989 with the coming into force of the Tobacco Products Control Act. Canada has a mature tobacco market where the industry is well-acquainted with the regulations and laws in place. The 2018 amendments to the Tobacco Act, renamed the TVPA, and other legislations introduced a new legislative framework for vaping products that brought several new regulated parties into the fold.Footnote 25 In turn, compliance and enforcement responsibilities of the TVPA were expanded to include vaping products.Footnote 26 Tobacco and vaping products have unique markets, each with their own challenges for compliance and enforcement activities.
Tobacco market
The Canadian tobacco market is concentrated, with four key manufacturers accounting for over 95 percent of sales. Several small importers of tobacco products make up the remainder of the market. Most manufacturers are solely producing for the domestic market. In 2023, 16.2 billion domestic cigarettes were sold.Footnote 27 Cigarettes represented 94 percent of the total tobacco market share in 2023.Footnote 28 There are over 100 brands of cigarettes offered in Canada.
The number of cigarettes sold in Canada has declined by almost 42 percent from 2013 to 2022. Two-thirds of that decline occurred since 2018, the year the new TVPA was implemented in Canada.Footnote 29
Over 95 percent of tobacco products in Canada are sold in person through gas and convenience stores.Footnote 30 The remainder are sold in grocery stores, drug stores and mass merchandise stores. The number of gas and convenience stores in the country are decreasing, with a 17.5 percent decrease between 2017 and 2023.Footnote 31 Products such as cigars and pipe tobacco are also found in speciality smoke stores. In 2023, Canada had over 22,000 retailers where tobacco products could be sold.Footnote 32
Vaping market
An estimated 99 percent of vaping devices and accessories and 95 percent of pods pre-filled with vaping liquid available in Canada are imported from China.Footnote 33 About 80 percent of these imported products are from five importers. However, during the early 2020's, numerous smaller entities started buying products directly from China.Footnote 34
E-liquids, the liquid used for heating and inhalation in open vaping systemsFootnote 35, can be sold separate from open system devices. In 2021 there were approximately 100 Canadian-based e-liquid manufacturers. This number had decreased to approximately 75 in 2023.Footnote 36 The majority of stand-alone e-liquids in Canada are manufactured by four major companies.Footnote 37
Canadians can purchase vaping products from a variety of sources, such as convenience stores, specialty vaping establishments and online. In response to the COVID-19 pandemic, many vaping retailers developed an online presence and now over 90 percent have their own online store. Consumer preferences are shifting toward online channels for greater variety and convenience. Online sales currently make up around 23 percent of the total vaping market in Canada but are projected to grow to 33 percent by 2028.Footnote 38
Since 2018, new regulations have come into force that strengthen the TVPA and address emerging issues. Annex 2 lists the regulations currently under the TVPA. The introduction of new regulations in a relatively short period of time may present challenges for regulated parties, especially while the industry is relatively young. The implementation of several regulations has also increased administration of regulatory oversight for Health Canada. The rate of non-compliance observed in the vaping industry has been higher than anticipated, making it necessary to reallocate some resources to support the enforcement of vaping-related regulations implemented since 2018. Currently, Health Canada has 25 inspectors for both tobacco and vaping products.
The large number of manufacturers, importers and thousands of points of sale presents challenges for the oversight of the market and the enforcement of regulations. Health Canada currently uses several enforcement tools to address non-compliance, particularly warning letters and product seizures.
Discussion questions:
- How could the Government of Canada improve monitoring/oversight of the tobacco and vaping product markets considering these products are widely available?
- Should the Government of Canada consider additional enforcement tools to effectively address non-compliance? If so, which ones?
- Are there other measures the Government of Canada could take to increase compliance with the TVPA and its regulations?
Regulating in a digital era
The TVPA generally prohibits the promotion of a tobacco product or a tobacco product-related brand element, including by means of packaging, except as authorized by the Act or its regulations. This general prohibition permits the TVPA to keep pace with changing marketing practices in tobacco product promotion (e.g., online promotion). Some adult-targeted advertising is authorized under the TVPA. However, it is limited to information and brand-preference advertising in signs in a place where young persons are not permitted by law, or in a publication that is addressed and sent to an adult identified by name. There is no general promotion prohibition for vaping products in the TVPA. However, vaping products are subject to several specific promotion prohibitions and restrictions, while leaving room for vaping manufacturers and retailers to communicate product and brand information to their adult customers. For example, promoting a vaping product by means of advertising that is appealing to young persons, or lifestyle is prohibited. The Vaping Products Promotion Regulations, implemented in 2020, prohibit advertising that can be seen or heard by young persons. This includes the displaying vaping products and vaping product-related brand elements at points of sale. Retailers' websites and social media are inspected to verify compliance with the promotion prohibitions under the TVPA. Non-compliance results in warning letters sent to regulated parties.
The TVPA also prohibits furnishingFootnote 39 tobacco and vaping products to persons under 18 years of age, including online. This establishes a uniform minimum protection for youth across Canada. In previous consultations, stakeholders suggested more needs to be done about online sales of vaping products and raised concerns regarding the ease with which youth can purchase tobacco and vaping products (see Annex 3). While studies indicate most youth access tobacco and vaping products through social (e.g., a family member or a friend) and retail sources, young persons who vaped daily or occasionally reported that they can obtain vaping products easily, including online.Footnote 40
With the evolution of online marketing practices and the growing use of social media, ensuring adherence to these regulations requires robust oversight and innovative compliance promotion and enforcement strategies. The dynamic nature of online vaping product promotions presents significant challenges for compliance and enforcement. Advertisers continually adapt their strategies to leverage emerging digital platforms and social media, creating a rapidly changing promotional landscape. This constant evolution requires regulatory bodies to remain vigilant and flexible in their enforcement approaches. Assessing compliance is further complicated by the global reach of the internet, where content can originate from multiple jurisdictions with varying regulations. Additionally, the advanced targeting capabilities of online marketing allow advertisers to reach specific audiences. This makes it challenging to monitor and control the exposure of tobacco and vaping promotions to certain populations, particularly youth.
The online marketplace brings other unique and complex challenges with compliance and enforcement. This includes challenges in identifying website operators, determining IP addresses, demonstrating whether site operators have a brick-and-mortar site in Canada and obtaining addresses and contact information of regulated parties. These challenges limit the types of enforcement actions taken for online retailers, such as warning letters or product seizures if a regulated party cannot be identified, their address or contact information cannot be obtained, or they are located outside of Canada.
Discussion questions:
- Which measures, technologies or tools would be useful to better monitor/have better oversight of internet and social media?
- How can the Government of Canada improve the monitoring and enforcement of the TVPA and its regulations in digital and social media spaces where traditional enforcement tools such as seizure of products may not apply?
- Are there enforcement tools to effectively address non-compliance with the TVPA and its regulations in the online context? Should additional tools be considered, and why? Please provide examples and supporting data.
Facilitating collaboration through the legislative framework of the TVPA
To support the compliance and enforcement objectives of the TVPA, Health Canada collaborates with other federal partners and works closely with provincial and territorial governments.Footnote 41 Collaboration is important to help build better communication, increased productivity, efficiency gains and improved outcomes. However, coordinating with multiple stakeholders can present operational challenges. Health Canada is interested in exploring opportunities to improve collaboration through the legislative framework of the TVPA.
Canada's Tobacco Strategy partners
Canada's Tobacco Strategy is the federal strategy to address tobacco use in Canada. Under the authority of the Tobacco and Vaping Products Act, the Canada Consumer Product Safety Act and the Food and Drugs Act, Health Canada leads the legislative and regulatory program for tobacco and vaping products. This includes the development and implementation of policies and regulations, research, public outreach and compliance. Several other federal departments also play important roles in implementing this Strategy (see Annex 4).
The Department of Finance Canada develops and evaluates federal tax policies and draft legislation for excise duty. The implementation and interpretation of the excise duty legislation and framework are the responsibility of the Canada Revenue Agency (CRA). Pursuant to the Customs Act, the Canada Border Services Agency has the authority to administer and enforce the collection of duties and taxes, including excise duties on tobacco and vaping products. The CRA has estimated that for the 2018 tax year there was $400 million in revenue loss in federal excise duties from the illegal production and smuggling of cigarettes.Footnote 42
Only holders of tobacco or vaping products licences under the Excise Act, 2001, can legally manufacture tobacco products and vaping products in Canada and import these products into Canada. Importers of tobacco products or vaping products who do not manufacture in Canada require "prescribed person" status under the Excise Act, 2001, in order to legally import these products.
Illegal tobacco and vaping products can pose a serious threat to the health and safety of Canadians.Footnote 43Footnote 44 Illegal tobacco products undermine government tobacco control efforts and fuel organized crime groups, who use this lucrative trade to finance other criminal activities, such as illegal drugs, firearms and human smuggling. It impacts the regulated market and is harmful to legitimate businesses. Public Safety Canada, Canada Border Services Agency, the Royal Canadian Mounted Police and police of jurisdiction across the country work together to understand the illegal market and support efforts to disrupt and dismantle related criminal activity.Footnote 45
For the fiscal year 2018-2019, the Canada Border Services Agency reported that just under 14,000 cartons of tobacco products, approximately 161,000 kilograms of tobacco and 252,800 inadmissible tobacco products were seized at ports of entry across Canada. Reports for the first 3 quarters of 2024-2025 fiscal year show just under 80,000 cartons, approximately 560,000 kilograms of tobacco and over 1.2 million inadmissible tobacco products have been seized.Footnote 46
Provinces, territories and municipalities
In Canada, provincial, territorial and local governments may enact their own tobacco and vaping legislation, and corresponding compliance and enforcement programs. All provinces and territories have legislation and/or strategies in place to address tobacco use. Their legislation may duplicate, enhance or be more restrictive than the requirements of the TVPA.Footnote 47 Many provinces and territories are also either extending tobacco laws or developing new strategies to address vaping. Certain provinces have increased the minimum age for the sale and provision of tobacco and vaping products and implemented additional restrictions on these products or their promotion based on their jurisdiction's needs. Many municipalities also have measures in place to regulate tobacco and vaping products and their use.Footnote 48
Provincial, territorial and local governments are responsible for enforcing their own legislation. For example, inspectors from most provinces and territories conduct compliance verification and enforcement activities mainly at retail locations, which include youth access restrictions and flavour bans. Although not all provinces and territories conduct inspections of manufacturers and online sites, there is some overlap between federal and provincial/territorial inspections in the retail space. Shared responsibility for regulating tobacco and vaping products, including compliance and enforcement efforts, means industry could be inspected by both federal and provincial inspectors in the same time period. Provinces and territories also collaborate with federal partners on illegal tobacco enforcement activities.
Regulated parties
Regulated parties are responsible for understanding and complying with relevant legislation and regulations as it applies to them and their products.Footnote 49 Health Canada proactively engages in compliance and promotion activities with industry to inform them of requirements under the TVPA. For example, backgrounders to provide information are posted on Health Canada's website. Inspectors can also share information with industry during inspections.
Canada is a Party to the WHO Framework Convention on Tobacco Control (FCTC). Article 5.3 of the Convention obliges Parties, in setting and implementing their public health policies with respect to tobacco control, to protect these policies from commercial and other vested interests of the tobacco industry in accordance with national law. The FCTC guidelines recommend Parties should only interact with the tobacco industry when required and only to the extent necessary to enable them to effectively regulate the tobacco industry and tobacco products. Health Canada has published a guidance document, Interacting with the tobacco industry - Guidance for Federal Public Service Representatives and Employees for Canada's policy and decision-makers on identifying ties to the tobacco industry and how to interact with the tobacco industry pursuant to Canada's obligations under Article 5.3 of the WHO FCTC. In line with the guidance document, Health Canada can meet with industry stakeholders, for instance, during public consultations on new regulations and provide webinars on new regulatory requirements. In support of transparency, information on industry meetings that take place with the Tobacco Control Directorate are regularly posted online.
Discussion questions:
- How can federal, provincial, territorial and local governments collaborate further to strengthen compliance and enforcement of the TVPA?
- Would it be useful to explore transparency initiatives of compliance & enforcement findings? If yes, how would this be useful?
- Is there additional compliance promotion measures the Government of Canada could take to increase compliance with the TVPA and its regulations? Please provide examples and supporting data.
Conclusion
Since the Tobacco Act was amended and renamed the TVPA in 2018, the introduction of numerous vaping product regulations in a relatively short period of time has increased Health Canada's regulatory oversight responsibilities. At the same time, the vaping market in Canada has evolved rapidly and many industry members have had difficulty adjusting to the regulatory environment.
The presence of online sales and dynamic nature of online marketing and promotional practices has also led to unique compliance and enforcement challenges. As a result, regulatory bodies need to develop robust, innovative and flexible strategies to ensure regulated parties adhere to regulations when selling and promoting products online.
Protecting the health of Canadians by supporting compliance and enforcement activities for tobacco and vaping products is a shared responsibility between Health Canada, other federal partners, provincial and territorial governments, and regulated parties. Everyone has a role to play, and there may be opportunities to improve collaboration between parties to increase effectiveness and reduce duplication of efforts.
Health Canada is interested in hearing the views of Canadians, experts and other stakeholders on these challenges and their suggestions to strengthen compliance and enforcement of the TVPA. In addition to the questions posed throughout the paper, interested parties are also invited to provide responses to the questions below.
Discussion questions:
- Is there anything else that you would like to add as it relates to any of the topics covered in this discussion paper? If so, please include your comments, with supporting data/evidence where possible.
- What key issues remain that, if successfully addressed, would result in further strengthening compliance and enforcement of the TVPA and its regulations?
- How can the federal government support the needs of First Nations, Inuit and Métis communities with regards to tobacco and vaping compliance and enforcement?
Annex 1: Other laws regulating tobacco and vaping products
Canada Consumer Product Safety Act
The Canada Consumer Product Safety Act (CCPSA) establishes legislative and regulatory requirements to help protect the public by addressing or preventing dangers to human health or safety posed by consumer products in Canada.
The manufacturing, importation, advertisement and sale of vaping products that do not make therapeutic claims (e.g., helping to quit smoking) are subject to the CCPSA, while also subject to the TVPA.
The CCPSA does not apply to tobacco products except in respect to cigarette ignition propensity and to any devices, other than a water pipe, that are necessary for the use of a tobacco product and parts used with a device.
In addition to other authorities, the CCPSA allows the federal government to carry out inspections and order recalls or other measures. The CCPSA requires industry to prepare and maintain certain documents (Section 13) and report health or safety incidents involving a consumer product (Section 14).
Food and Drugs Act
The Food and Drugs Act (FDA) applies to vaping products that make a therapeutic claim (e.g., helping to quit smoking). This includes products that contain nicotine, or any other drugs as defined by the FDA. These products must receive an authorization from Health Canada before they can be commercially imported, advertised and sold in Canada. Before issuing a market authorization, Health Canada carefully reviews the evidence provided by the product sponsor to confirm the product meets safety, efficacy and quality requirements of the FDA and its regulations. A valid establishment or site licence from Health Canada is also required before a vaping product under the FDA can be labelled, imported, packaged, or manufactured.
Non-smokers' Health Act
The Non-smokers' Health Act addresses issues related to the use of tobacco, vaping and cannabis products, including second-hand smoke and aerosol, in federally-regulated workplaces such as banks, commercial aircrafts and federal government offices.
Legislation related to the use of tobacco, vaping and cannabis products in most other public places and private places, including workplaces, restaurants and multi-unit dwellings, is the responsibility of provincial, territorial and municipal governments.
Cannabis Act
The Cannabis Act creates a legal framework for controlling the production, distribution, sale and possession of cannabis in Canada. Vaping products containing cannabis are regulated under the Cannabis Act and its regulations.
Excise Act, 2001
Excise duties have been applied to tobacco products manufactured in Canada or imported into Canada for decades. An excise duty on vaping products was introduced in 2022. The excise duty applies to vaping substances that are manufactured in Canada or imported and that are intended for use in a vaping device in Canada.
Provincial, territorial and municipal laws
Provincial, territorial and municipal laws also regulate tobacco and vaping products and their use.
Annex 2: Regulations under the TVPA
The Tobacco and Vaping Products Act (TVPA) provides, among other things, the authority for Health Canada to regulate the manufacture, sale, labelling and promotion of tobacco and vaping products. Those activities respecting tobacco and vaping products are allowed in Canada if they meet the requirements of the TVPA and its regulations, and any other piece of legislation to which tobacco and vaping products are subject. Below is a brief description of each of the Regulations made under the TVPA.
Tobacco product regulations under the TVPA
In 1999 the Tobacco (Access) Regulations and the Tobacco (Seizure and Restoration) Regulations were in place to set out the types of documentation that may be used to verify the age of a person wanting to purchase tobacco products and set out the information that an owner of a seized product must provide to the Minister to apply for restoration of a seized product, and the time and manner to do so.
The Tobacco Reporting Regulations were created in 2000 to set out the requirements for the reporting to Health Canada of sales data, manufacturing information, tobacco product ingredients, constituents, emissions, research and development activities and promotional activities by tobacco manufacturers and importers. These regulations were amended in 2005 and in 2019. As part of the latest changes, the official methods for the sampling and testing of tobacco products were updated to reflect technological advances.
The Promotion of Tobacco Products and Accessories Regulations (Prohibited Terms) were created in 2011 to protect the public from misleading and deceptive information by prohibiting the use of terms "light" and "mild," and variations thereof, from various tobacco products, their packaging, promotions, retail displays, as well as from tobacco accessories.
Tobacco Products Appearance, Packaging and Labelling Regulations (TPAPLR) are the result of the 2023 amendments made to the Tobacco Products Regulations (Plain and Standardized Appearance) (2019) and repeal of the previous Tobacco Products Information Regulations (2000) and Tobacco Products Labelling Regulations (Cigarettes and Little Cigars) (2011).The TPAPLR consolidate all the appearance, packaging and labelling requirements for tobacco products under a single set of regulations.
The Tobacco Charges Regulations (TCR) was created in 2025, and set out the requirements for designated tobacco manufacturers, including importers, to pay an annual charge to recover the costs of tobacco-related activities undertaken by the Government of Canada in relation to the carrying out of the purpose of the TVPA. These regulations specify the formula to calculate an annual charge for each designated manufacturer that is proportionate to their domestic market share of total tobacco product net sales revenue in the previous fiscal year (from April 1 to March 31).
Vaping product regulations under the TVPA
In 2018, Regulations Excluding Certain Vaping Products Regulated Under the Food and Drugs Act from the Application of the Tobacco and Vaping Products Act were created. These regulations exclude certain categories of vaping products authorized under the Food and Drugs Act from the application of the TVPA.
Regulatory requirements for vaping products under the TVPA and Canada Consumer Product Safety Act began in 2019 with the Vaping Products Labelling and Packaging Regulations which set out labelling requirements pursuant to the TVPA (Part 1) and labelling requirements, child-resistant container requirements and maximum nicotine concentration pursuant to the CCPSA (Part 2).
The Vaping Products Promotion Regulations were introduced in 2020, which set out the requirements related to advertising and point of sale promotions of vaping products as well as the required information in advertising of vaping products.
The Nicotine Concentration in Vaping Products Regulations came into force in 2021, which establish a maximum nicotine concentration of 20 mg/mL for vaping products manufactured or imported for sale in Canada and also prohibit the packaging and sale of vaping products if the nicotine concentration displayed on the package exceed 20 mg/mL.
The Vaping Products Reporting Regulations were made in 2023 and set out the requirements for the reporting of sales and ingredients information to Health Canada by manufacturers and importers of vaping products. This information is submitted in an electronic format using forms established by the Minister that are incorporated by reference in the Regulations and may be amended from time to time.
Annex 3: What we heard and areas for potential action identified through the first and second legislative reviews of the TVPA
Changing market
- Almost all those who responded to the consultation identified the need for increased compliance monitoring and enforcement to address current and future issues in tobacco and vaping control. Many stakeholders suggested increasing the number of inspectors and frequency of inspections.
- Regional health authorities, non-governmental organizations and provincial/territorial governments noted the need for new enforcement tools to support the efficiency and effectiveness of compliance and enforcement activities, such as new or increased penalties, recalls and orders to comply (e.g., cease and desist orders).
- The vaping industry felt the TVPA and regulations are adequate to protect both youth and non-users of tobacco products. In their view, the problem lies with insufficient enforcement of existing prohibitions and more needs to be done on this front before implementing new restrictions.
- Some industry respondents, non-governmental organizations and provinces suggested that vaping products be submitted to Health Canada for a pre-market review in order to reduce the number of non-compliant products on the market, or that a protocol be put in place to verify compliance of product labels and promotional material with Health Canada.
- Some non-governmental organizations and members of the general public suggested actively monitoring compliance through the use of methods such as randomized product testing for prohibited ingredients and nicotine dosage, using mystery shoppers to audit retailers and developing a whistleblower hotline allowing Canadians to notify Health Canada of non-compliance.
- A Métis organization recommended greater initiatives to promote the consequences of furnishing tobacco or vaping products to youth.
- A member of the general public recommended the implementation of a product registration system.
Regulating in a digital era
- There was consensus amongst all stakeholder groups that more needs to be done about online sales of vaping products, and many highlighted the need for increased compliance monitoring and enforcement related to online activities (e.g., online sales, marketing, and promotion), including stronger penalties for industry, notably for non-compliant promotion on social media platforms that are popular among youth.
- Many consultation respondents indicated that current measures to prevent youth from accessing tobacco products could be strengthened to better address online sales. Some recommended online sales and marketing be banned.
- Industry respondents requested guidance from Health Canada on how to verify the age of those who visit websites and social media pages.
Facilitating collaboration through the legislative framework of the TVPA
- Some regional health authorities, non-governmental organizations, provincial/territorial governments, industry stakeholders and members of the general public recommended improving the coordination of enforcement efforts with provinces and territories and publishing more comprehensive and frequent compliance monitoring and enforcement reports.
- Vaping product manufacturers and their associations highlighted a need for Health Canada to provide more detailed guidance documents to assist in better understanding the promotion prohibitions under the TVPA and its associated regulations.
- Provincial and territorial governments suggested the federal government increase collaboration with provincial and territorial governments, enforcement bodies and neighbouring jurisdictions to reduce duplication of enforcement efforts and prevent cross-border sales. They also recommended the government work with health organizations, schools, community groups and law enforcement agencies to coordinate efforts and share information.
- A member of the general public recommended the federal government work with provincial and territorial partners and local law enforcement to empower them to adequately enforce new regulations.
- A First Nations organization recommended enhancing and co-developing the process for reviewing the TVPA as tobacco pertains to the rights of Nations to practice their cultures and any changes made may impact these rights.
- A Métis organization suggested the federal government collaborate with various agencies to ensure culturally distinct Métis representation and sufficient sample sizes to research and evaluate tobacco regulations within individual Métis communities.
- A vaping industry member suggested strengthening enforcement at the border to stop shipments of non-compliant products or shipments without recipient age-verification mechanisms. Another industry member suggested prohibiting international direct-to-consumer shipments.
- A public health unit recommended banning the importation of vape products that are over the 20mg/mL nicotine limit and providing relevant training to CBSA staff to reduce the availability of high nicotine vape products in Canada.
- Industry members suggested that there should be more of a focus on the illegal tobacco market.
Areas for Potential Action Identified in the First and Second Legislative Reviews
Changing market
- Explore the development of additional tools that could respond to repeated non-compliance with a progressive enforcement approach.
- Enhance Health Canada's understanding of how the market is changing by collecting new information about technical innovations.
Regulating in a digital era
- Conduct a comprehensive assessment of current industry practices and government guidance as a first step in addressing age verification requirements as they relate to online sales.
- Consider developing guidance or regulatory requirements to set out in greater detail the actions that regulated parties involved in online and distance sales may take to verify age and identity.
- Establish a Federal/Provincial/Territorial working group to examine online access to vaping products with a view to assessing the current provisions and practices to ensure that youth are adequately protected.
- Enhance compliance and enforcement actions under existing authorities to prevent youth from accessing tobacco products online.
Facilitating collaboration through the legislative framework of the TVPA
- Continue to examine the retail environment with provinces and territories with a view to assessing the current provisions and practices, identifying collaborative opportunities and considering all possible regulatory, policy, program and research activities to ensure that youth remain adequately protected.
- Explore the development and publication of guidance material for the vaping industry to provide additional direction on how to comply with the provisions of the TVPA to promote compliance with current obligations.
- Expand efforts to make compliance and enforcement more transparent by regularly publishing information about actions resulting from compliance and enforcement activities.
- Continue to work closely with provinces and territories to identify, monitor and address issues of non-compliance. Explore legislative and regulatory options when considering additional tools to address compliance and enforcement issues.
Annex 4: Overview of Canada's Tobacco Strategy partners
The Canada Border Services Agency, through the Customs Act, is responsible for disrupting the supply of illegal tobacco, illegal vaping and related products being illegally imported into Canada. It does this through monitoring, compliance and the interception and seizure of illegal tobacco and tobacco-related products at Canadian ports of entry. The CBSA also collects excise duties and taxes on tobacco products and vaping products imported into Canada.
The Canada Revenue Agency, through the Excise Act, 2001, is responsible for the Excise Duty Program and oversees the collection and payment of federal taxes on legal tobacco products and vaping products manufactured in Canada.Footnote 50 Canadian companies manufacturing tobacco and vaping products must obtain a licence and only licensees can legally manufacture these products in Canada. The Canada Revenue Agency conducts periodic audits and regulatory reviews of licensees such as confirming volumes of tobacco or vaping products produced and verifying whether excise stamps are applied correctly to tobacco or vaping products to support excise duties paid.
The Department of Finance Canada develops and evaluates federal tax policies and legislation in the areas of personal income tax, corporate income tax and sales and excise tax, including the excise tax on legal tobacco products and vaping products sold in Canada. The actual collection of taxes and interpretation of tax law are the responsibility of the Canada Revenue Agency. The Canada Border Services Agency also has authority to administer and enforce the collection of duties and taxes for imported products.
Employment and Social Development Canada's Labour Program is responsible for administering and enforcing the Non-Smokers' Health Act, which restricts smoking and vaping in federally regulated workplaces such as government offices and commercial airplanes.
Health Canada leads the implementation of Canada's Tobacco Strategy through its Tobacco Control Directorate. Health Canada also administers and enforces the TVPA and other legislation that regulate tobacco and vaping products, including the Canada Consumer Product Safety Act and the Food and Drugs Act. In addition, Health Canada's Substance Use and Addictions Program supports innovative initiatives that respond to drug and substance use issues, including nicotine and tobacco. The program's projects aim to protect people from the harms of smoking and nicotine addiction by means of prevention, health promotion, harm reduction and cessation initiatives.
Indigenous Services Canada transfers funding to Indigenous communities and organizations to develop and implement self-led distinct approaches to reducing commercial tobacco use. The Department also works collaboratively with partners to improve access to high quality services for First Nations, Inuit and Métis,Footnote 51 in support of Indigenous self-determination and control over culturally appropriate service design and delivery.
The Public Health Agency of Canada's Healthy Canadians and Communities Fund invests in tobacco cessation and prevention projects that support populations who face health inequalities and higher rates of tobacco use such as Indigenous Peoples, 2SLGBTQIA+ communities and people living on low incomes.
As the federal department responsible for leading efforts to counter crime, Public Safety Canada is responsible for providing leadership and coordinating with federal, provincial and territorial law enforcement partners, including police of jurisdiction, to support collective efforts to disrupt and dismantle illegal activity related to tobacco and vaping products. Public Safety Canada supports law enforcement efforts to monitor, enforce and intercept illegal activity in Canada. Additionally, it also provides funding to support law enforcement efforts across jurisdictions to disrupt illegal activities related to organized crime through the First Nations Organized Crime Initiative.
The Royal Canadian Mounted Police, through the Criminal Code, is responsible for preventing, detecting and deterring crime in partnership with police of jurisdiction. Under the Excise Act, 2001, the RCMP's Federal Policing has a wide-ranging mandate to prevent, detect and investigate serious and organized crime, financial crime and cybercrime, as well as crimes related to national security. It also enforces federal statutes such as the TVPA.
Endnotes
- Footnote 1
-
Canadian Substance Use Costs and Harms. 2023. Retrieved from: https://csuch.ca/assets/documents/reports/english/Canadian-Substance-Use-Costs-and-Harms-Report-2007-2020-en.pdf
- Footnote 2
-
Health Canada. Regulating tobacco and vaping products. 2024. Retrieved from: https://www.canada.ca/en/health-canada/services/smoking-tobacco/regulating-tobacco-vaping.html
- Footnote 3
-
Health Canada. Canada's Tobacco Strategy. 2025. Retrieved from: https://www.canada.ca/en/health-canada/services/publications/healthy-living/canada-tobacco-strategy.html
- Footnote 4
-
Health Canada. Addressing Tobacco and Vaping Product Use in Canada. 2025. Retrieved from: https://www.canada.ca/en/health-canada/services/health-concerns/tobacco/about-tobacco-control/tobacco-control-canada.html
- Footnote 5
-
Addressing Tobacco and Vaping Product Use in Canada. 2025. Retrieved from: https://www.canada.ca/en/health-canada/services/health-concerns/tobacco/about-tobacco-control/tobacco-control-canada.html
- Footnote 6
-
Canadian Community Health Survey (CCHS) 2018. Statistics Canada.
- Footnote 7
-
Canadian Community Health Survey (CCHS) 2022. Statistics Canada.
- Footnote 8
-
House of Commons Standing Committee on Health. Vaping: Towards a Regulatory Framework for E-Cigarettes. Report of the Standing Committee on Health. 2015. Retrieved from: https://www.ourcommons.ca/documentviewer/en/41-2/HESA/report-9/
- Footnote 9
-
Canadian Tobacco, Alcohol and Drugs Survey (CTADS) 2017. Health Canada. Retrieved from: https://www.canada.ca/en/health-canada/services/canadian-tobacco-alcohol-drugs-survey/2017-summary.html
- Footnote 10
-
Canadian Tobacco and Nicotine Survey (CTSN) 2022. Health Canada. Retrieved from: https://www.canada.ca/en/health-canada/services/canadian-tobacco-nicotine-survey/2022-summary.html
* CTNS was previously known as the Canadian Tobacco, Alcohol, and Drugs Survey (CTADS). In 2019, CTADS split into CTNS and the Canadian Alcohol and Drugs Survey (CADS).
- Footnote 11
-
Canadian Tobacco, Alcohol and Drugs Survey (CTADS) 2017. The survey can be accessed here: https://www.canada.ca/en/health-canada/services/canadian-tobacco-alcohol-drugs-survey/2017-summary.html
- Footnote 12
-
Canadian Tobacco and Nicotine Survey (CTNS) 2019. The survey can be accessed here: https://www.canada.ca/en/health-canada/services/canadian-tobacco-nicotine-survey/2019-summary.html
- Footnote 13
-
Canadian Health Survey for Children and Youth (CHSCY) Master File 2019, 2023 Note: CHSCY 2019 and 2023.
- Footnote 14
-
Indigenous Peoples Survey (IPS) 2022. Statistics Canada. Retrieved from: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=4110007101
- Footnote 15
-
Regional Health Survey (RHS) Phase 3 2015-16. First Nations Information Governance Centre. Retrieved from:
https://fnigc.ca/wp-content/uploads/2020/09/713c8fd606a8eeb021debc927332938d_FNIGC-RHS-Phase-III-Report1-FINAL-VERSION-Dec.2018.pdf
- Footnote 16
-
Health Canada. Compliance and enforcement policy framework. 2018. Retrieved from: https://www.canada.ca/en/health-canada/corporate/mandate/regulatory-role/what-health-canada-does-as-regulator/compliance-enforcement-framework.html
- Footnote 17
-
Health Canada. Compliance and Enforcement Policy for the Tobacco and Vaping Products Act. 2024. Retrieved from: https://www.canada.ca/en/health-canada/services/smoking-tobacco/regulating-tobacco-vaping/compliance-enforcement-policy-tobacco-vaping-products-act.html
- Footnote 18
-
Health Canada. Compliance and Enforcement Policy for the Tobacco and Vaping Products Act. 2024. Retrieved from: https://www.canada.ca/en/health-canada/services/smoking-tobacco/regulating-tobacco-vaping/compliance-enforcement-policy-tobacco-vaping-products-act.html
- Footnote 19
-
Contraventions Act (SC 1992, c. 47), (last amended 2019). Retrieved from: https://laws-lois.justice.gc.ca/eng/acts/c-38.7/
- Footnote 20
-
Health Canada. Compliance and Enforcement Policy for Health products (POL-0001). 2019. Retrieved from: https://www.canada.ca/en/health-canada/services/drugs-health-products/compliance-enforcement/good-manufacturing-practices/policies-standards/compliance-enforcement-policy-0001.html#s8
- Footnote 21
-
Health Canada. Compliance and Enforcement Policy for the Tobacco and Vaping Products Act. 2024. Retrieved from: https://www.canada.ca/en/health-canada/services/smoking-tobacco/regulating-tobacco-vaping/compliance-enforcement-policy-tobacco-vaping-products-act.html
- Footnote 22
-
Health Canada. Vaping compliance and enforcement. 2024. https://www.canada.ca/en/health-canada/services/smoking-tobacco/vaping/compliance-enforcement.html
- Footnote 23
-
Brick-and-mortar establishments include gas and convenience stores, specialty vaping establishments, as well as manufacturers, distributors, importers or any other physical place tobacco and vaping products are sold, promoted, manufactured or labelled.
- Footnote 24
-
The TPR(PSA) were amended in 2023 and renamed as Tobacco Products Appearance, Packaging and Labelling Regulations.
- Footnote 25
-
Health Canada. Regulating tobacco and vaping products. 2024. Retrieved from: https://www.canada.ca/en/health-canada/services/smoking-tobacco/regulating-tobacco-vaping.html
- Footnote 26
-
Health Canada. Evaluation of the Health Portfolio Tobacco and Vaping Activities 2016-17 to 2020-21. 2021. Retrieved from: https://www.canada.ca/en/health-canada/corporate/transparency/corporate-management-reporting/evaluation/tobacco-vaping-activities-2016-2017-2020-2021.html
- Footnote 27
-
Data derived from industry reports submitted under the Tobacco Reporting Regulations (SOR/2000-273), Section 13.
- Footnote 28
-
Data derived from industry reports submitted under the Tobacco Reporting Regulations (SOR/2000-273), Section 13.
- Footnote 29
-
Data derived from industry reports submitted under the Tobacco Reporting Regulations (SOR/2000-273), Section 13.
- Footnote 30
-
Cigarette retail sales data derived from industry reports submitted under the Tobacco Reporting Regulations (SOR/2000-273), Section 13 and obtained from NielsenIQ Market Track Report, Tobacco Cigarettes, 2022. Data purchased from NielsenIQ under contact to Health Canada.
- Footnote 31
-
Convenience Industry Council of Canada. Pre-budget submission to the House of Commons Standing Committee on Finance. 2023. Retrieved from: https://convenienceindustry.ca/wp-content/uploads/2023/08/CICCPreBudget2023.pdf
- Footnote 32
-
Convenience Industry Council of Canada. Pre-budget submission to the House of Commons Standing Committee on Finance. 2023. Retrieved from: https://convenienceindustry.ca/wp-content/uploads/2023/08/CICCPreBudget2023.pdf
- Footnote 33
-
Euromonitor International. Study of the market size, characteristics, and growth trends of the vaping products market in Canada. A customer report compiled by Euromonitor International for Health Canada. 2024.
- Footnote 34
-
Euromonitor International. Study of the market size, characteristics, and growth trends of the vaping products market in Canada. A customer report compiled by Euromonitor International for Health Canada. 2024.
- Footnote 35
-
An open vaping system is a vaping device there the user manually refills the e-liquid tank.
- Footnote 36
-
Euromonitor International. Study of the market size, characteristics, and growth trends of the vaping products market in Canada. A customer report compiled by Euromonitor International for Health Canada. 2024.
- Footnote 37
-
Euromonitor International. Study of the market size, characteristics, and growth trends of the vaping products market in Canada. A customer report compiled by Euromonitor International for Health Canada. 2024.
- Footnote 38
-
Euromonitor International. Study of the market size, characteristics, and growth trends of the vaping products market in Canada. A customer report compiled by Euromonitor International for Health Canada. 2024.
- Footnote 39
-
Furnish means to sell, lend, assign, give or send, with or without consideration, or to barter or deposit with another person for the performance of a service. Tobacco and Vaping Products Act (SC 1997, c. 13), (last amended 2018). Section 2. Retrieved from: https://laws-lois.justice.gc.ca/eng/acts/t-11.5/
- Footnote 40
-
Environics Research. Vapers Panel Survey to Measure Attitudes and Behaviours Regarding Vaping Products Final Report. 2019. Retrieved from: https://publications.gc.ca/collections/collection_2020/sc-hc/H14-316-2019-1-eng.pdf
- Footnote 41
-
Health Canada. Compliance and Enforcement Policy for the Tobacco and Vaping Products Act. 2024. Retrieved from: https://www.canada.ca/en/health-canada/services/smoking-tobacco/regulating-tobacco-vaping/compliance-enforcement-policy-tobacco-vaping-products-act.html
- Footnote 42
-
Overall federal tax gap report: Estimates and key findings for non-compliance, tax years 2014-2018. Canada Revenue Agency. Retrieved from: https://www.canada.ca/content/dam/cra-arc/corp-info/aboutcra/tax-canada-conceptual-study/rv4-149-1-2022-ovrll-tx-gp-rpt-en.pdf
- Footnote 43
-
Stephens WE, Calder A, & Newton J. Source and health implications of high toxic metal concentrations in illicit tobacco products. Environmental Science and Technology 2005; 39(2): 479-488. Retrieved from: https://pubmed.ncbi.nlm.nih.gov/15707047/
- Footnote 44
-
Baker M, et al. Vaping-associated lung illness (VALI) in Canada: a descriptive analysis of VALI cases reported from September 2019 to December 2020. Health Promotion and Chronic Disease Prevention in Canada: Research, Policy, and Practice 2022; 42(1): 37. Retrieved from: https://www.canada.ca/content/dam/phac-aspc/documents/services/reports-publications/health-promotion-chronic-disease-prevention-canada-research-policy-practice/vol-42-no-1-2022/hpcdp-vol-42-no-1-2022.pdf
- Footnote 45
-
Health Canada. Addressing Tobacco and Vaping Product Use in Canada. 2025. Retrieved from: https://www.canada.ca/en/health-canada/services/health-concerns/tobacco/about-tobacco-control/tobacco-control-canada.html
- Footnote 46
-
Canada Border Services Agency. Canada Border Services Agency enforcement action statistics. 2025. Retrieved from: https://www.cbsa-asfc.gc.ca/security-securite/seizure-saisie-eng.html
- Footnote 47
-
Health Canada. Compliance and Enforcement Policy for the Tobacco and Vaping Products Act. 2024. Retrieved from: https://www.canada.ca/en/health-canada/services/smoking-tobacco/regulating-tobacco-vaping/compliance-enforcement-policy-tobacco-vaping-products-act.html
- Footnote 48
-
Health Canada. Addressing Tobacco and Vaping Product Use in Canada. 2025. Retrieved from: https://www.canada.ca/en/health-canada/services/health-concerns/tobacco/about-tobacco-control/tobacco-control-canada.html
- Footnote 49
-
Health Canada. Compliance and enforcement policy framework. 2018. Retrieved from: https://www.canada.ca/en/health-canada/corporate/mandate/regulatory-role/what-health-canada-does-as-regulator/compliance-enforcement-framework.html
- Footnote 50
-
Health Canada. Addressing Tobacco and Vaping Product Use in Canada. 2025. Retrieved from: https://www.canada.ca/en/health-canada/services/health-concerns/tobacco/about-tobacco-control/tobacco-control-canada.html
- Footnote 51
-
Government of Canada. Indigenous Services Canada. 2025. Retrieved from: https://www.canada.ca/en/indigenous-services-canada.html
