Archived New Health Labelling for Tobacco Packaging
Notice to the reader:
This consultation is now closed.
Read the New Health-Related Labelling for Tobacco Products: Consultation Summary.
Document for Consultation
October 2018
Table of contents
- Executive summary
- Introduction
- What we are consulting on
- We want your feedback
- Appendix A: Overview of the regulatory process for the Tobacco and Vaping Products Act
- Appendix B: Canada's regulatory framework for health labelling of tobacco products
- Appendix C: Selection of current health labelling on tobacco packages in Canada
Executive summary
Tobacco use is the leading preventable cause of premature death in Canada. It causes diseases and serious health outcomes, leading to immeasurable suffering for thousands of Canadians, their friends and family.
Through Canada's Tobacco Strategy, the Government of Canada has committed $330 million over the next 5 years to help Canadians who smoke to quit or reduce in order to minimize the harmful health effects of tobacco. A key initiative under this strategy is the renewal of health messages for tobacco packaging. Health information on tobacco products is recognized as one of the best approaches to tell people about the health risks of tobacco use. The current labelling requirements for cigarettes and little cigars have been in place since 2011. The current requirements for most other tobacco products have been in place since 2000.
This document describes areas Health Canada is exploring for renewed health labelling for tobacco products. For each consultation section, the current approach is outlined and potential issues with that approach are identified. The approach Health Canada is considering is then presented along with specific questions where your input is requested.
The appendices provide additional information related to the federal regulatory process, the Canadian Regulatory Framework for Health Labelling of Tobacco Products, and additional examples of components of tobacco labels in Canada.
Introduction
The health burden of tobacco use
Tobacco use in Canada is a deadly and costly problem. It causes dozens of diseases in tobacco users as well as those exposed to second-hand smoke. In fact, tobacco use is the leading preventable cause of premature death in Canada.Refernce 1 Every year, more than 45,000 Canadians die from illnesses caused by smoking; Refernce 2 that is about one Canadian every 12 minutes. In a single year, smoking costs in Canada are over $6.5 billion for direct health care and $16.2 billion in combined health and economic costs.Refernce 3 It also impacts families and friends caring for the ill and grieving those who have passed away.
Tobacco control in Canada
In May 2018, the Government of Canada announced Canada's Tobacco Strategy, an enhanced federal strategy to address tobacco use. The Strategy includes $80.5 million in new funding over five years, starting in 2018-19. The Government is committed to reducing tobacco use to less than 5% of Canadians by 2035.
Actions by all levels of government, and many others committed to reducing the burden of smoking, have contributed to reducing tobacco use among Canadians over the last several decades. Despite these efforts, 15% of Canadians still report having used tobacco in the past 30 days. Refernce 4 Smoking rates for youth have not changed since 2013.
Health labelling for tobacco products
Messages on tobacco packages and products have the potential to be seen by millions of people each day and are an important tool in reducing smoking rates to help Canadians live healthy, tobacco-free lives.
Health labels on tobacco products have been put into place all over the world. As of 2016, more than 100 countries, covering 58% of the world's population, require picture-based warnings on cigarette packages. Recent research shows that countries that have implemented the Framework Convention on Tobacco Control labelling requirements have seen smoking rates decrease an average of 1.5% as a result. Refernce 5
We know that health warning messages on tobacco packages are effective at informing and raising awareness of the health hazards and health effects of using tobacco products.Refernce 6,Refernce 7, Refernce 8 We want to build on the achievements of Canada's current labelling system by exploring new ways to maximize the effectiveness of the health-related labels and make them even more impactful. For more information on the regulatory development process under the Tobacco and Vaping Products Act, please see Appendix A. For more information on Canada's Regulatory Framework for Health Labelling of Tobacco Products, please see Appendix B.
Cigarette and little cigar packages in Canada are required to have three health labelling components: Health Warnings (graphic warnings of the health effects of tobacco use, see #1 below), Health Information Messages (messages on the health benefits of quitting smoking, see #2 below), and Toxic Emissions Statements (information on the health effects of toxic substances in cigarettes, see #3 below). Appendix C shows additional examples of current Health Warnings, Health Information Messages, Toxic Emissions Statements, and Toxic Constituents Statements (product-specific toxic constituent information for various products other than cigarettes and little cigars).

Labelling elements for cigarettes and little cigars - Text Equivalent
Image of front of cigarette pack. Front right of pack has number 1 superimposed at far right, while to the left is image of dying woman in bed.
Right justified is text:
WARNING
This is what dying of lung cancer looks like.
Barb Tarbox died at 42 of lung cancer caused by cigarettes.
You can quit. We can help.
1-866-366-3667
gosmokefree.gc.ca/quit
Health Canada
The Edmonton Journal
An insert is partially shown sliding out of top of pack with a number 2 superimposed at the top right corner.
Visible text at left is: Coughing is your lungs warning you.
Visible text at right is: La toux est un signal de vos
Visible in centre is partial illustration of lungs
Bottom of pack has white band with with text: Cigarettes
Number 3 is superimposed at top left side corner.
#1 Health Warnings
#2 Health Information Messages

Health Information Message for cigarettes and little cigars - Morning cough - Text Equivalent
Blue and white box.
Left justified is text: Morning cough? See the back. Health Canada.
Right justified is text: Vous toussez le matin? Voir à l'endos. Santé Canada.
Below box is text: Back view:
Blue and white box with illustrated image of lungs at top centre.
Left justified is text:
Coughing is your lungs warning you it's time to quit.
When you quit smoking:
Within the first few months, you'll cough and wheeze less and you'll be short of breath less often.
In the first 5 years, respiratory problems like bronchitis and pneumonia will decrease significantly.
You can quit and breathe easier!
Health Canada
Right justified is text:
La toux est un signal de vos poumons qu'il est temps d'arrêter.
Quand vous arrêtez de fumer :
Dès les premiers mois, la toux et les râlements diminueront. Vous serez moins essouflé.
Au cours des 5 premières années, les problèmes respiratoires, comme la bronchite et la pneumonie, diminueront de façon marquée.
Vous pouvez arrêter et respirer mieux!
Santé Canada
#3 Toxic Emissions Statements

Toxic Emissions Statement - 70 chemicals - Text Equivalent
White rectangular box with text:
Tobacco smoke contains more than 70 chemicals than can cause cancer. Health Canada
La fumée du tabac contient plus de 70 substances chimiques qui peuvent causer le cancer. Santé Canada
Why now?
The current labelling for cigarettes and little cigars has been in place since 2011. The labelling for most other tobacco products has been in place since 2000. Regularly updating the content and styles of tobacco product health labels helps ensure that they are more noticeable, memorable, and engaging.Refernce 9,Refernce 10 In addition, Health Canada has proposed plain and standardized appearance requirements for tobacco packages and certain tobacco products. Some research shows that the noticeability of Health Warnings increases when they are combined with plain packaging. Refernce 11,Refernce 12, Refernce 13 For these reasons, it is an opportune time for Health Canada to be considering changes to its health labelling requirements.
Key considerations for health labelling on tobacco products
Health Canada is committed to developing new health labelling for tobacco products based on the most effective Canadian and international practices. We are guided by peer-reviewed, published studies as well as ongoing consultations with experts in the field, all of which emphasize the importance of the following considerations:
Messages that appeal to all Canadians
Labelling must be understood by people of every age and by a diverse population. New health labelling for tobacco products would continue to feature a range of messages and images to respect different ages, genders, and backgrounds, as well as those who may be at higher risk for developing an addiction to nicotine or other negative health effects.
Personal stories
Personal testimonials or stories are a useful tool in communicating the health hazards of tobacco use and motivating people to quit smoking. Research has shown that Health Warnings are most effective if the viewer can relate to the person or situation described.Refernce 14, Refernce 15 Current health labelling in Canada includes four personal and true stories from former tobacco users whose health has been harmed by tobacco use. Health Canada will make every effort to continue to feature such testimonials on future health labelling.
Messages that cause emotional reactions
Accurate, shocking depictions of the health conditions caused by tobacco use on warning labels are the most likely to be remembered by tobacco users, while messages that are difficult to look at, such as images of rotting teeth, diseased mouths, throat cancer, and disfigurement are generally rated as most effective.Refernce 16,Refernce 17, Refernce 18 Graphic images on warning labels appear to be particularly effective among groups that have been hard to reach, including youth and people with low literacy.Refernce 19, Refernce 20 Other types of messages that cause strong feelings, such as images of children affected by tobacco use, have also been found to be memorable and effective.Refernce 21, Refernce 22 Health Canada will continue to incorporate a mix of images that cause emotion in new Health Warning labels.
What we are consulting on
Today, we are consulting with Canadians on the following issues:
- Labelling on cigarettes
- Labelling content and design
- Health Information Messages
- Toxic Statements (Toxic Emissions Statements and Toxic Constituents Statements)
- Connecting labelling elements
- Quitline information
- Labelling size and placement
- Percentage of coverage of Health Warnings on tobacco products other than cigarettes and little cigars
- Minimum size of Health Warnings on tobacco products other than cigarettes and little cigars
- Labelling for all tobacco products that do not currently require labels
- Labelling rotation
- Other considerations
1) Labelling on cigarettes
Applies to: Cigarettes
Current approach: There is currently no health labelling on cigarettes themselves.
Issue: There is recent but limited research showing that Health Warnings placed directly on a product, such as cigarettes, could be effective in making the product less appealing to users.Refernce 23,Refernce 24, Refernce 25 Please see below for potential examples of how this may look.

Draft potential examples of Health Warnings directly on cigarettes - Text Equivalent
Two cigarettes shown lying horizontally on a grey background, one with a cork-coloured filter and one with a white filter. Printed on the white paper of top cigarette is the text:
SMOKING CAUSES CANCER
Health Canada
Printed on the white paper of bottom cigarette is the text:
FUMER CAUSE LE CANCER
Health Canada

Draft potential examples of Health Warnings directly on cigarette filters - Text Equivalent
Two cigarettes shown lying horizontally on a grey background, one with a cork-coloured filter and one with a white filter. Printed on the filter of the top cigarette is the text:
SMOKING CAUSES CANCER
Health Canada
Printed on the filter of the bottom cigarette is the text:
FUMER CAUSE LE CANCER
Health Canada
Draft potential examples of Health Warnings directly on cigarettes
Approach under consideration: Health Canada is considering introducing requirements for Health Warnings directly on cigarettes. All elements of design, such as size, wording, font and colour are being considered.
- Do you believe that displaying information directly on cigarettes would be effective in informing Canadians of the health hazards and effects of cigarettes?
- Do you have suggestions for types of messages on cigarettes that would be effective in informing Canadians of the health hazards and effects of cigarettes?
- Do you have specific suggestions about the size, font or colour for Health Warnings on cigarettes that would be effective in informing Canadians of the health hazards and effects of cigarettes?
- Are there any studies that would support the measures that you are suggesting? If so, please list the studies.
2) Labelling content and design
2a) Health Information Messages
Applies to: Cigarettes, little cigars, kreteks, cigarette tobacco, leaf tobacco and tobacco sticks
Current approach: Current Health Information Messages focus mainly on the benefits of quitting and provide tips to help people quit. They are either printed on the package or on a leaflet inserted into the package. Examples of current Health Information Messages are shown below.

Example of current Health Information Message for cigarettes and little cigars - It's never too late - Text Equivalent
Box with grey banner at the top with text inside: It's never too late…
Below is light grey background with image at the right of elderly couple walking and holding hands.
Left justified is the text:
Quitting smoking increases life expectancy and improves quality of life.
People who quit smoking increase their chances of living longer. They improve their general health, leading to a better quality of life.
It's never too late to quit. No matter how old you are, you'll start to feel major and immediate health benefits and have more energy to help you live life to the fullest.
Talk to a health care provider.
Health Canada

Example of current Health Information Message for cigarettes and little cigars - Morning cough - Text Equivalent
White and blue box with a cartoon image of a pair of lungs. Inside is the text:
Morning cough?
Coughing is your lungs warning you it's time to quit.
When you quit smoking:
Within the first few months, you'll cough and wheeze less and you'll be short of breath less often.
In the first 5 years, respiratory problems like bronchitis and pneumonia will decrease significantly.
You can quit and breathe easier!
Health Canada

Example of current Health Information Message for cigarettes and little cigars - Quitting... What's in it for me? - Text Equivalent
Black and green box with images of man playing soccer and woman playing the tuba. The text is:
Quitting... What's in it for me?
Quitting…What's in it for me?
WHY SHOULD I QUIT SMOKING?
I want to regain control over myself by getting rid of my tobacco addiction…
I want to be at my best with my activities…
I want to be healthier and have more energy…
I will reward myself with the money saved…
Health Canada
Issue: Health Information Messages should be as noticeable as possible to maximize their effectiveness in communicating with tobacco users.
Approach under consideration: Several options are being explored for maximizing the noticeability of Health Information Messages, including increasing the size of leaflets inserted into packages, requiring them to be stuck onto packages so that they cannot be thrown away, and changing where Health Information Messages are printed on the inside of packages. In recent public opinion research run by Health Canada, participants noted that Health Information Messages on cigarette packs are most likely to be noticed and read when they included new or unusual design features such as vibrant colours, thought bubbles, or cartoons. Refernce 26 An example of a potential new Health Information Message is shown below.

Draft Health Information Message teaser for cigarettes and little cigars tested in public opinion research - Tips for quitting - Text Equivalent
Rectangular blue box with red band at the bottom.
Left justified is the following text:
Tips for quitting
Gosmokefree.gc.ca/quit
See back (a white arrow points to the left)
Health Canada
Right justified is the following text:
Des trucs pour arrêter
Vivezsansfumee.gc.ca/abandon
Voir à l'endos (a white arrow points to the right)
Santé Canada

Draft Health Information Message for cigarettes and little cigars tested in public opinion research - Tips for quitting - Text Equivalent
A white box with a blue border. At the centre are three fingers with smiling faces drawn on.
Justified to the left is the following text:
Most people try many times before they quit for good because tobacco products are highly addictive. To stay on track, try to:
Talk about it with someone who's already quit
Exercise daily to help deal with stress
Drink water and eat healthy
Challenge a buddy to a quit smoking competition
You've got what it takes to be smoke-free.
1-866-366-3667
Health Canada
Justified to the right is the following text:
La plupart des personnes essaient plusieurs fois avant d'arrêter de fumer pour de bon, car les produits du tabac créent une forte dépendance.
Afin de rester sur la bonne voie, essayez de :
parler à quelqu'un qui a déjà arrêté de fumer
faire de l'exercice tous les jours pour vous aider à gérer votre stress
boire de l'eau et manger mieux
défier un ami dans une compétition pour arrêter de fumer
Vous avez ce qu'il faut pour vous libérer du tabac
1 866 JARRETE (1 866-527-7383)
Santé Canada
- Do you have specific suggestions related to the size, colour and design of new Health Information Messages that would maximize the noticeability of the information?
- Do you have specific suggestions related to the placement of new Health Information Messages that would maximize the noticeability of the information?
- Do you have suggestions for topics for future Health Information Messages?
- Are there any studies that would support the measures that you are suggesting? If so, please list the studies.
2b) Toxic Statements (includes Toxic Emissions Statements and Toxic Constituents Statements)
Applies to: All tobacco products
Current approach: Toxic Emissions Statements are required on packages of cigarettes and little cigars. Certain other tobacco products display a list of some of the toxic emissions or the amount of toxic constituents found in the product. Examples of Toxic Emissions Statements for packages of cigarettes and little cigars are shown below.

Example of current Toxic Emissions Statement for cigarettes and little cigars - 70 Chemicals - Text Equivalent
White rectangular box with text:
Tobacco smoke contains more than 70 chemicals than can cause cancer. Health Canada
La fumée du tabac contient plus de 70 substances chimiques qui peuvent causer le cancer. Santé Canada

Example of current Toxic Emissions Statement for cigarettes and little cigars - Hydrogen cyanide - Text Equivalent
White box with black text:
Tobacco smoke contains hydrogen cyanide, a poisonous gas. Health Canada
La fumée du tabac contient du cyanure d'hydrogène, un gaz toxique. Santé Canada
Issue: Toxic Statements on products that currently have them should be updated to maximize their effectiveness.
Approach under consideration: Health Canada is considering updating requirements related to current Toxic Statements. This includes considering changes to the messages, design and location of the Toxic Statements.
In recent public opinion research run by Health Canada, participants found Toxic Emissions Statements on cigarette packages most eye-catching and easiest to read when: English and French texts were presented side-by-side; black text was on a yellow background and white text was on a red background; and a red "WARNING" banner was placed above the text. Refernce 27 Please refer to the potential example below.

Example of Potential Toxic Statement - Hydrogen cyanide - Text Equivalent
Rectangular box. Left 2/3 of box has red banner at the top with white text: WARNING AVERTISSEMENT.
Below is yellow with a black bar separating the left and right sides of the box.
Left justified is black text: Hydrogen cyanide in tobacco smoke damages the cleaning system of your lungs, allowing toxins to build up. Health Canada.
Right justified is black text: L'acide cyanhydrique dans la fume de tabac endommage le système de nettoyage de vos poumons, ce qui permet d'accumuler des toxines. Santé Canada.
Right 1/3 of the box is brown with white text: Name and address of manufacturer. Nom et adresse du fabricant
- Do you have specific suggestions related to the size, colour and design of new Toxic Statements that would serve to make them even easier to notice and read?
- Do you have specific suggestions related to the placement of new Toxic Statements that would serve to make them even easier to notice and read?
- Do you have specific suggestions regarding the content of future Toxic Statements?
- Are there any studies that would support the measures that you are suggesting? If so, please list the studies.
2c) Connecting labelling elements
Applies to: All tobacco products
Current approach: There is currently no requirement for the various health messages on tobacco products and packages to relate to one another.
Issue: There may be benefits to thematically linking the labelling elements on tobacco packages in order to provide more information about a particular health condition, increasing understanding of the health effect and its causes.
Approach under consideration: Since 2012, Australia has connected labelling elements on tobacco packages so that they have messages that relate to one another.Refernce 28 An example of the Australian theme "Smoking Causes Blindness" is shown here.Refernce 29

Example of the "Smoking Causes Blindness" theme from Australian tobacco packaging - Front of pack - Text Equivalent
Box with black band at the top with white text: SMOKING CAUSES BLINDNESS
Image below is of eye held open with metal prongs.
At bottom is brown band with white text: Brand variant
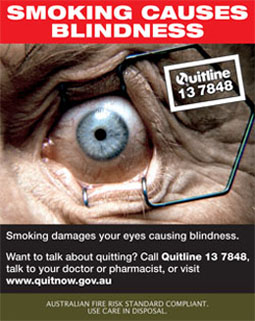
Example of the "Smoking Causes Blindness" theme from Australian tobacco packaging - Back of pack - Text Equivalent
Box with red band at the top with white text: SMOKING CAUSES BLINDNESS
Image below is of eye held open with metal prongs. Superimposed on top is white outlined box with text: Quitline 13 7848
Below image is dark brown band with white text: Smoking damages your eyes causing blindness. Want to talk about quitting? Call Quitline 13 7848, talk to your doctor or pharmacist, or visit www.quitnow.gov.au.
At bottom is brown band with white text: Brand variant
AUSTRALIAN FIRE RISK STANDARD COMPLIANT. USE CARE ON DISPOSAL.
Similar measures to those in Australia could be implemented for future labelling in Canada. One example could be a Health Warning showing lung cancer, a Toxic Statement about chemicals in tobacco smoke that damage lungs, and a Health Information Message that explains how quitting smoking reduces the risk of developing lung cancer.
- Do you feel that linking the information on tobacco packages by theme would improve understanding of the information presented?
- Do you have any suggestions related to linking this information by theme on tobacco packages that would improve understanding of the information presented?
- Are there any studies that would support the measures that you are suggesting? If so, please list the studies.
2d) Quitline information
Applies to: All tobacco products
Current approach: Toll-free quitline and web site information is currently on Health Warnings for cigarettes and little cigars, as well as on some Health Information Messages. Other products do not require quitline information. Please refer to the example below.

Example of a current Health Warning on cigarettes and little cigars - Tobacco smoke hurts everyone - Text Equivalent
Image of cigarette package with image at left of empty crib
To the right is yellow box with the text:
WARNING
Tobacco smoke hurts everyone.
Infants who are exposed to tobacco smoke are at greater risk of dying from Sudden Infant Death Syndrome (SIDS).
Circled in red is the text:
Need help to quit?
1-866-366-3667
gosmokefree.gc.ca/quit
Health Canada
Issue: Quitline information should appear on all tobacco products and be as noticeable as possible to maximize their effectiveness in communicating with tobacco users.
Approach under consideration: Health Canada is considering changes to the size, colour, and placement of quitline and website information on labelling to maximize its noticeability. It is also considering adding quitline and website information to other tobacco products, such as smokeless, heated tobacco, and fine cut tobacco products.
- Do you have specific suggestions related to the size, colour, and placement of quitline and website information on new labelling that would maximize the noticeability of the information on various types of tobacco product packaging?
- Do you have suggestions for improving how tobacco packages show information about available quitline and web-based cessation services to tobacco users beyond simply displaying the telephone number and website (i.e. smartphone application, #tag, QR code, @symbol, or other methods)?
- Are there any studies that would support the measures that you are suggesting? If so, please list the studies.
3) Labelling size and placement
3a) Percentage of coverage of Health Warnings on tobacco products other than cigarettes and little cigars
Applies to: Tobacco products other than cigarettes and little cigars
Current approach: Health Warnings on cigarette and little cigar packages must cover at least 75% of the two largest sides of the package or primary display area. For certain other tobacco products, Health Warnings must either cover up to 50% of the main surface or meet size requirements (that are less than 50%), depending on the type of product. Only toxic constituent amounts are required on packages of smokeless tobacco. Examples of these requirements are shown below using proposed Plain and Standardized Appearance* requirements.
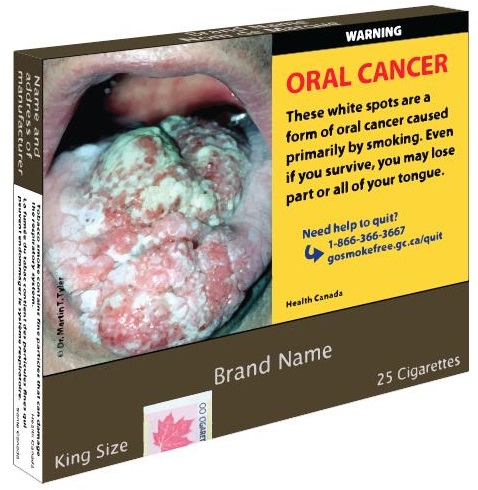
Example of the 75% requirement for cigarette and little cigar packages - Text Equivalent
Image of the front of a cigarette pack. To the left is a photograph of an open moth with oral cancer.
Justified to the right is a yellow background with the text:
WARNING
ORAL CANCER
These white spots are a form of oral cancer caused primarily by smoking. Even if you survive, you may lose part or all of your tongue.
Need help to quit?
1-866-366-3667
Gosmokefree.gc.ca/quit
At the bottom is a brown band with the text:
Brand Name
King Size
25 Cigarettes

Example of the Health Warning size requirement (that is less than 50%) for a tobacco pouch - Text Equivalent
Image of the back of a brown rectangular pouch of tobacco.
On the top flap in the centre is a black box with image of young boy using an inhaler.
Left justified is text:
WARNING
Tobacco smoke hurts children
Tobacco smoke can trigger asthma attacks in children.
Health Canada
Right justified is text:
AVERTISSEMENT
La fumée du tabac nuit aux enfants
La fumée du tabac peut provoquer des crises d'asthme chez les enfants.
Santé Canada
At the bottom of the package is the text:
Name and address of manufacturer
Nome et adresse du fabricant

Example of a toxic constituent label for a smokeless tobacco tin - Text Equivalent
Image of the top of a round metal tin
There is a white band around the centre of the tin with the following text:
Toxic constituents/gram:.016 mg Nitrosamines,.0004 mg Lead, 26.4 mg Nicotine
Health Canada
Constituants toxiques/gramme:.016 mg Nitrosamines,.0004 mg Plomb, 26.4 mg Nicotine
Santé Canada
In the centre of the tin is a brown circle with the following text:
Brand Name
25 g smokeless tobacco/tabac sans fume
*At the time of publication, the Tobacco Products Regulations (Plain and Standardized Appearance) have not yet been published in Canada Gazette Part II. Please refer to Appendix A for information on the regulatory process. For more information on Plain and Standardized Appearance please visit www.canada.ca/en/health-canada/news/2018/06/plain-and-standardized-appearance-for-tobacco-packaging.html
Issue: There is a great deal of research that larger Health Warnings are more effective and generally seen as more believable for both youth and adult cigarette smokers.Refernce 30,Refernce 31, Refernce 32
Approach under consideration: Health Canada is considering expanding the 75% Health Warning requirements for cigarettes and little cigars to other tobacco products.
- Should the amount of space required for Health Warnings be the same for all tobacco products?
- If not, what should the exceptions be? What is the rationale for these exceptions?
- Are there any studies that would support the measures that you are suggesting? If so, please list the studies.
3b) Minimum size of health warnings on tobacco products other than cigarettes and little cigars
Applies to: Tobacco products other than cigarettes and little cigars
Current approach: Labelling requirements for tobacco products other than cigarettes and little cigars are varied, with minimum size requirements (in cm2) applying only to a small number of package types.
Issue: In Canada, tobacco products are sold in a variety of package sizes and formats, including pouches, cans, tins, and kits, among others. New tobacco products with novel package designs continue to emerge on a regular basis. Health Warnings should be large enough to be clear, visible, easy to read, and effective at informing users of the health hazards and effects of tobacco product use.
Approach under consideration: Health Canada is considering establishing a minimum size for Health Warnings on tobacco products other than cigarettes and little cigars. Minimum sizes for Health Warnings for products other than cigarettes and little cigars have also been adopted in AustraliaRefernce 33 and the European Union.Refernce 34
- Should we require tobacco products other than cigarettes and little cigars to have minimum sizes for Health Warnings?
- Do you have specific suggestions regarding minimum size requirements for Health Warnings on tobacco products other than cigarettes and little cigars in Canada in the context of plain and standardized appearance measures?
- Are there any studies that would support the measures that you are suggesting? If so, please list the studies.
4) Labelling for all tobacco products that do not currently require labels
Applies to: Tobacco products not currently required to have health labels
Current approach: Some tobacco products, such as heated tobacco products, water pipe tobacco and blunt wraps, are not currently covered by health labelling requirements.
Issue: Tobacco products packaged without health labelling could be seen as safe.
Approach under consideration: Health Canada is considering extending health labelling requirements to all packages of products containing tobacco, including products not currently subject to existing labelling requirements.
- Should health labelling be required on the packaging of all tobacco products?
- Which types of labelling (Health Warnings, Health Information Messages, Toxic Statements) should be required?
- Should different messages be considered for different types of tobacco products?
- Are there additional requirements related to extending health labelling to all tobacco products you would suggest?
- Are there any studies that would support the measures that you are suggesting? If so, please list the studies.
5) Labelling rotation
Applies to: All tobacco products
Current approach: Health messages on tobacco products are not currently required to rotate after a specified period of time.
Issue: Smokers and non-smokers get used to the health information they see on tobacco product packages even when many different labels are displayed equally on tobacco packages sold across Canada.Refernce 35, Refernce 36 As a result, many countries have rotation periods for their messages on tobacco product packages.
Approach under consideration: Heath Canada is considering implementing several rotating suites of health labelling for tobacco products. Some research suggests that the ideal rotation time between sets of labels is 12-18 months, Refernce 37 and the most common rotation period for countries appears to be 12 months per set.
- What would be the ideal period of time for the rotation of health labelling on tobacco products?
- Are there any different considerations for rotation of different labelling elements or for different products?
- How many labels should be included in each set for rotation?
- Are there any other measures you would suggest related to the rotation of health labelling for tobacco products?
- Are there any studies that would support the measures that you are suggesting? If so, please list the studies.
6) Other considerations
In addition to the feedback on the specific elements above, we encourage you to provide any other comments and suggestions. We are open to all ideas that would improve labelling for tobacco products.
More generally,
- Do you feel that there are other significant measures to regulate the health labelling for tobacco products in Canada that we have not addressed?
- Do you generally support Health Canada's considerations and approach to changing health labelling for tobacco products?
- Are there any studies that would support the measures that you are suggesting? If so, please list the studies.
We want your feedback
We are seeking input from interested Canadians on the considerations put forward in this document. All feedback received on or before January 4, 2019 will be considered.
The Department will not retain your email address or contact information when receiving your feedback, and will only retain the comments you provide.
You can provide your input using the contact information below.
Health Canada will summarize the results of this consultation which will be published online. No identifying information will be used in the summary without your explicit permission.
Thank you for taking the time to consider this document and for contributing to helping Canadians to lead healthier, tobacco-free lives.
Mailing Address:
Labelling and Plain Packaging Office
Tobacco Control Directorate
Address Locator 0301A, 150 Tunney's Pasture Driveway
Ottawa, ON K1A 0K9
Email: hc.pregs.sc@canada.ca
Note:
Canada is a Party to the World Health Organization Framework Convention on Tobacco Control (FCTC). Article 5.3 of the FCTC obliges Parties, in setting and implementing their public health policies with respect to tobacco control, to protect these policies from commercial and other vested interests of the tobacco industry in accordance with national law. You must declare any perceived or actual conflicts of interest with the tobacco industry when providing input to this consultation.
The personal information you provide is protected in accordance with the Privacy Act and collected under the authority of the Department of Health Act, section 4. The information is being collected to allow you to provide your opinions regarding new health labelling for tobacco products in Canada. Your comments, without identifying information such as your name or email address, may be used to brief senior management and inform policy decisions. A summary of comments will also be posted online. To further safeguard privacy, you should ensure that any written comments you may provide are sufficiently general that you cannot be identified as the author and that individual identities are not disclosed. For more information about the use of your personal information please contact the Labelling and Plain Packaging Office at hc.pregs.sc@canada.ca or the Privacy Management Division at hc.privacy-vie.privee.sc@canada.ca. You also have the right to file a complaint with the Privacy Commissioner of Canada if you think your personal information has been handled improperly.
Appendix A: Overview of the regulatory process for the Tobacco and Vaping Products Act
Establishing regulations under the Tobacco and Vaping Products Act typically follows these steps:
- Public consultation, where the proposal is made public and comments are invited from interested parties
- Pre-publication of the proposed regulations, and their accompanying Regulatory Impact Analysis Statement, in the Canada Gazette, Part I, followed by a comment period of 30 or 75 days (the latter where the proposed regulations may affect international trade)
- Consideration of comments received from the public and adjustments of the proposed regulations where appropriate
- Final approval by the Governor in Council
- Registration of the regulations and final publication in the Canada Gazette, Part II
- Coming Into Force of the regulations on the day of registration, unless otherwise specified. For instance, the Coming Into Force may happen six months after their registration, where a delay is necessary to meet Canada's trade obligations
Appendix B: Canada's regulatory framework for health labelling of tobacco products
In 2000, Canada became the first country in the world to have graphic Health Warnings on tobacco packages. The Tobacco Products Information Regulations (TPIR) currently apply to cigars, pipe tobacco, fine cut tobacco, bidis, kreteks and smokeless tobacco. These regulations require picture-based Health Warnings to be displayed on at least 50% of most tobacco packages, including cigarette tobacco, pipe tobacco, and chewing tobacco. The TPIR also requires text-based Health Information Messages on packages, which tell tobacco users about the health effects and health hazards of tobacco use and the benefits of quitting. It also sets out requirements for toxic emissions information and toxic constituents information.
In 2011, the Tobacco Products Labelling Regulations (Cigarettes and Little Cigars) (TPLR) replaced the requirements of the TPIR for cigarettes and little cigars. The TPLR required Health Warnings to cover at least 75% of the main panels. New images were also introduced. These larger Health Warnings include testimonials and information about available smoking cessation resources (a toll-free, pan-Canadian quitline number and web address). Under the TPLR, numerical values of toxic emissions information, which were generally not well understood, were replaced with text-based Toxic Emissions Statements on the sides of most packages. These new Toxic Emissions Statements were designed to provide clear, concise and easy-to-understand information in a plain-language format. Coloured, pictorial Health Information Messages emphasizing the benefits of quitting replaced text-based Health Information Messages, either as leaflets or printed on the inside of packages. These new Health Information Messages were re-designed to be more engaging and encourage users to read the information, including "teasers" displayed on the upper slide flap to draw attention to the inside of the package or the leaflets through the use of colour and images.
The Tobacco Act was recently amended to create, among other things, new rules for vaping products in Canada and to change its title to the Tobacco and Vaping Products Act. The Act will also allow Health Canada to enhance the public awareness of the health hazards of using tobacco products by requiring that the tobacco product and package display information about the product and its emissions, and about the health hazards and health effects arising from the use of the product and from its emissions. The Act also allows Health Canada to put in place regulations to make sure all tobacco packaging looks the same, reducing their appeal to youth and others. These measures would apply to all tobacco products. The Tobacco and Vaping Products Act also provides the authority to make regulations for information to appear directly on tobacco products such as cigarettes.
Appendix C: Selection of current health labelling on tobacco packages in Canada
Health Warnings - cigarettes:

Warning for Cigarettes - Cigarettes are a major cause of heart disease - Text Equivalent
Box with image at left of gloved hands holding a human heart.
The right of box is yellow with the text:
Smoking is a major cause of heart disease.
Smokers are up to 4 times more likely to develop heart disease than non-smokers.
You can quit. We can help.
1-866-366-3667
gosmokefree.gc.ca/quit
Health Canada
Above the yellow is a black band with white text:
WARNING

Warning for Cigarettes - Your kids are sick of your smoking - Text Equivalent
Black box with image at left of child wearing oxygen mask.
The right of the box has the text:
Your kids are sick of your smoking.
Second-hand smoke causes more frequent and severe asthmatic attacks in children.
You can quit. We can help.
1-866-366-3667
gosmokefree.gc.ca/quit
Health Canada
At the top is a red band with white text:
WARNING

Warning for Cigarettes - Dying of lung cancer - Text Equivalent
Box with image at left of dying woman in bed.
Right justified is text:
This is what dying of lung cancer looks like.
Barb Tarbox died at 42 of lung cancer caused by cigarettes.
You can quit. We can help.
1-866-366-3667
gosmokefree.gc.ca/quit
Health Canada
The Edmonton Journal
At the top is a red band with white text:
WARNING
Health Warnings - little cigars:

Warning for Little Cigars - Throat cancer - Text Equivalent
Box with image at left of man with tracheal tube.
The right of box is yellow with the text:
Throat cancer. It's tough to swallow.
Smoking causes throat cancer. This cancer often requires extensive surgery and may require a hole cut in your throat to breathe.
Need help to quit?
1-866-366-3667
gosmokefree.gc.ca/quit
Health Canada
Above the yellow is a black band with white text:
WARNING

Warning for Little Cigars - Tobacco is addictive - Text Equivalent
Box with image at left of girl being offered a little cigar.
The right of box is yellow with the text:
Tobacco is addictive, even for occasional smokers.
A single puff is the first step towards addiction. Nicotine is a highly addictive drug.
Need help to quit?
1-866-366-3667
gosmokefree.gc.ca/quit
Health Canada
Above the yellow is a black band with white text:
WARNING

Warning for Little Cigars - Tobacco addiction affects generations - Text Equivalent
Box with image to the left of two women. The older woman is ill and wears an oxygen tube while the younger woman beside her is holding a lit cigarette.
Justified to the right is the text:
Cigarette addiction affects generations.
Mother and daughter are both addicted to tobacco. Nicotine is the drug in tobacco that causes addiction.
You can quit. We can help.
1-866-366-3667
gosmokefree.gc.ca/quit
Health Canada
©Brenda Ann Kenneally
Above text is a black band with white text:
Health Information Messages - cigarettes and little cigars:

Information Message for Cigarettes and Little Cigars - Never Quit Trying to Quit - Text Equivalent
Red and white box with image of calendar and the text:
Never Quit Trying to Quit
Never quit trying to quit.
Most smokers try to quit several times before they succeed.
Think of every attempt as a learning experience, not a failure. Never quit trying to quit.
Pick a quit date, write it down or tell someone about it.
Health Canada

Health Information Message for Cigarettes and Little Cigars - Quitting ... What's in it for me? - Text Equivalent
Black and green box with images of man playing soccer and woman playing the tuba. The text is:
Quitting... What's in it for me?
Quitting…What's in it for me?
WHY SHOULD I QUIT SMOKING?
I want to regain control over myself by getting rid of my tobacco addiction…
I want to be at my best with my activities…
I want to be healthier and have more energy…
I will reward myself with the money saved…
Health Canada

Information Message for Cigarettes and Little Cigars - I had enough of feeling guilty - Text Equivalent
Purple and white box with image of smiling woman. The text is:
I had enough of feeling guilty "I had enough of feeling guilty…"
1-866-366-3667
"Quitting is hard and it takes a lot of will power.
"I had enough of feeling guilty. I was ashamed of being a smoker.
"When I was ready to quit, I called a quitline and, with their support, I made it through the first few days of cravings.
"As the days went by, I was more and more proud of myself and my will to keep going got stronger."
- Susan
Tobacco products are highly addictive.
Health Canada
Toxic Emissions Statements - cigarettes and little cigars:

Toxic Emissions Statement for Cigarettes and Little Cigars - Benzene - Text Equivalent
White box with black text:
Tobacco smoke contains benzene, a chemical that causes cancer. Health Canada
La fumée du tabac contient du benzène, un produit chimique qui donne le cancer. Santé Canada

Toxic Emissions Statement for Cigarettes and Little Cigars - Hydrogen cyanide - Text Equivalent
White box with black text:
Tobacco smoke contains hydrogen cyanide, a poisonous gas. Health Canada
La fumée du tabac contient du cyanure d'hydrogène, un gaz toxique. Santé Canada

Toxic Emissions Statement for Cigarettes and Little Cigars - Fine particles - Text Equivalent
White box with black text:
Tobacco smoke contains fine particles that can damage the respiratory system. Health Canada
La fumée du tabac contient des particules fines qui peuvent endommager le système respiratoire. Santé Canada
Toxic Constituents Statements* - Kreteks, Cigarette Tobacco, Leaf Tobacco, Tobacco Sticks, Chewing Tobacco and Snuff:
Toxic constituents/gram:.005 mg Nitrosamines,.0004 mg Lead, 27.8 mg Nicotine
Health Canada
Toxic constituents/gram:.016 mg Nitrosamines,.0004 mg Lead, 26.4 mg Nicotine
Health Canada
*Toxic constituents information is provided by the manufacturer and is product -specific.
Health Warnings - cigars and pipe tobacco:

WARNING - Where there's smoke, there's poison - Text Equivalent
Black box with smoke in background.
Left justified is text:
WARNING - Where there's smoke, there's poison
Tobacco smoke contains more than 50 cancer-causing agents.
Health Canada
Right justified is text:
AVERTISSEMENT - Qui dit fumée dit poison
La fumée contient plus de 50 agents cancérigènes.
Santé Canada
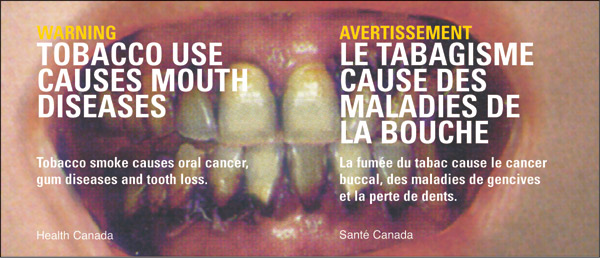
WARNING - Tobacco use causes mouth diseases - Text Equivalent
Box with image of open mouth with inflamed gums and rotting teeth.
Left justified is text:
WARNING
Tobacco use causes mouth diseases
Tobacco smoke causes oral cancer, gum diseases and tooth loss.
Health Canada
Right justified is text:
AVERTISSEMENT
Le tabagisme cause des maladies de la bouche
La fumée du tabac cause le cancer buccal, des maladies de gencives et la perte de dents.
Santé Canada

WARNING - Tobacco smoke hurts children - Text Equivalent
Black box with image of young boy using an inhaler.
Left justified is text:
WARNING
Tobacco smoke hurts children
Tobacco smoke can trigger asthma attacks in children.
Health Canada
Right justified is text:
AVERTISSEMENT
La fumée du tabac nuit aux enfants
La fumée du tabac peut provoquer des crises d'asthme chez les enfants.
Santé Canada
Health Warnings - kreteks, cigarette tobacco, leaf tobacco and tobacco sticks:

WARNING - Cigarettes are highly addictive - Text Equivalent
Dark grey box with image to the left of ashtray full of cigarette butts and a burning cigarette.
Justified to the right is the text:
WARNING
Cigarettes are highly addictive
Studies have shown that tobacco can be harder to quit than heroin or cocaine.
Health Canada

WARNING - Don't poison us - Text Equivalent
White box with image to the left of two young boys looking serious.
Justified to the right is the text:
Don't poison us
WARNING: Second-hand smoke contains carbon monoxide, ammonia, formaldehyde, benzo[a]pyrene and nitrosamines. These chemicals can harm your children.
Tobacco smoke hurts children.
Health Canada

WARNING - Each year, the equivalent of a small city dies from tobacco use - Text Equivalent
White box with horizontal bar graph justified to the left with the information:
Estimated deaths in Canada, 1996
Murders -- 510;
Alcohol -- 1,900;
Car accidents -- 2,900;
Suicides -- 3,900;
Tobacco -- 45,000
Health Canada
Justified to the right is the text:
WARNING
Each year, the equivalent of a small city dies from tobacco use
Health Information Messages - kreteks, cigarette tobacco, leaf tobacco and tobacco sticks:

You CAN quit smoking! - Text Equivalent
White box with black text:
You CAN quit smoking!
Tobacco products are highly addictive
If you're thinking of quitting, having a plan and sticking to it really helps overcome your addiction.
Psych yourself up. If you want others to help you, tell them your quit date.
Take a moment and write down your reasons for quitting. If you are tempted to light up, look at this list to remind yourself why you stopped.
For more information on tobacco, its health effects and ways to overcome a tobacco addiction, talk to a doctor, nurse or pharmacist or visit www.infotobacco.com
Health Canada 1

If I have lung cancer, what are my chances of surviving? - Text Equivalent
White box with black text:
If I have lung cancer, what are my chances of surviving?
Your chances of surviving this disease are low.
60% of lung cancer victims die within one year.
Less than 15% of lung cancer victims will be alive 5 years after diagnosis.
Smoking causes 85% of all lung cancers.
Quitting smoking reduces your chance of getting lung cancer.
For more information on tobacco, its health effects and ways to overcome a tobacco addiction, talk to a doctor, nurse or pharmacist or visit www.infotobacco.com
Health Canada 9

Can my baby be harmed if I smoke while I'm pregnant? - Text Equivalent
White box with black text:
Can my baby be harmed if I smoke while I'm pregnant?
Yes.
Smoking during pregnancy creates serious risks for the baby. Infants born to smoking mothers are more likely to need hospital care. Their risk of death is also higher. "Crib death" (sudden infant death syndrome) has been linked to smoking during pregnancy.
Don't run the risk of harming your baby.
The best solution is to stop smoking.
For more information on tobacco, its health effects and ways to overcome a tobacco addiction, talk to a doctor, nurse or pharmacist or visit www.infotobacco.com
Health Canada 15
To view all labelling visit:
References
- Footnote 1
-
Public Health Agency of Canada. (2008). Risk Factor Atlas. Retrieved from the Government of Canada website: https://www.canada.ca/en/public-health/services/chronic-diseases/risk-factor-atlas.html
- Footnote 2
-
Dobrescu, A., Bhandari A., Sutherland G. and Dinh, T. (2017). The Costs of Tobacco Use in Canada, 2012. The Conference Board of Canada. Retrieved from the Government of Canada website: https://www.canada.ca/en/health-canada/services/publications/healthy-living/costs-tobacco-use-canada-2012.html
- Footnote 3
-
Dobrescu et al. (2017)
- Footnote 4
-
Health Canada. (2015). The Canadian Tobacco, Alcohol and Drugs Survey, 2015 summary. Retrieved from the Government of Canada website: https://www.canada.ca/en/health-canada/services/canadian-tobacco-alcohol-drugs-survey/2015-summary.html
- Footnote 5
-
Gravely, S., Giovino, G.A., Craig, L., Commar, A., D'Espaignet, E.T., Schotte, K., and Fong, G.T. (2017). Implementation of key demand-reduction measures of the WHO Framework Convention on Tobacco Control and change in smoking prevalence in 126 countries: an association study. The Lancet Public Health, Volume 2, Issue 4, e166-e174. https://doi.org/10.1016/S2468-2667(17)30045-2
- Footnote 6
-
Hammond, D. (2011). Health warning messages on tobacco products: a review. Tobacco Control, 2011;20:327-337. http://dx.doi.org/10.1136/tc.2010.037630
- Footnote 7
-
Retrieved from the National Cancer Institute, Cancer Intervention and Surveillance Modeling Network (CISNET) website: https://resources.cisnet.cancer.gov/registry/packages/simsmoke-georgetown/#basics
- Footnote 8
-
Levy, D. (2017). Canada SimSmoke: The effect of past policies and the potential effect of future demand reduction policies - Final Report. Prepared for Health Canada.
- Footnote 9
-
World Health Organization Framework Convention on Tobacco Control. (2008)
- Footnote 10
-
Hammond, D. & Reid, J.L. (2012). Health warnings on tobacco products: International practices. Salud Publica Mex 2012;54:270-280.
- Footnote 11
-
Wakefield, M., Coomber, K., Zacher, M., Durkin, S., Brennan, E., & Scollo, M. (2015). Australian adult smokers' responses to plain packaging with larger graphic health warnings 1 year after implementation: results from a national cross-sectional tracking survey. Tobacco control, 24, ii17--ii25. http://dx.doi.org/10.1136/tobaccocontrol-2014-052050
- Footnote 12
-
Maynard, O. M., Munafò, M. R., & Leonards, U. (2012, Oct). Visual attention to health warnings on plain tobacco packaging in adolescent smokers and non-smokers. Addiction, 108, 413-419.
https://doi.org/10.1111/j.1360-0443.2012.04028.x - Footnote 13
-
Al-Hamdani, M. (2013). The Effect of Cigarette Plain Packaging on Individuals' Health Warning Recall. Healthcare Policy, 8(3), 68-77. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3999559/
- Footnote 14
-
Hammond, D. (2009). Tobacco labelling and packaging toolkit: A guide to FCTC article 11. Waterloo, Ontario: Department of Health Studies, University of Waterloo. Retrieved from the Tobacco Labelling Resource Centre website: http://www.tobaccolabels.ca/toolkit/
- Footnote 15
-
Decima Research (2009)
- Footnote 16
-
Hammond (2011)
- Footnote 17
-
Environics Research Group. (2007b). The health effects of tobacco and health warning messages on cigarette packages- survey of youth: Wave 12 surveys. Prepared for Health Canada.
- Footnote 18
-
Phoenix Strategic Perspectives Inc. (2018a). Qualitative Testing of New Health Warnings and Contact Information Taglines for Cigarette Packages - 2017. Prepared for Health Canada.
- Footnote 19
-
Hammond (2009)
- Footnote 20
-
Hammond (2011)
- Footnote 21
-
Sambrook Research International (2009)
- Footnote 22
-
Phoenix Strategic Perspectives Inc. (2018a)
- Footnote 23
-
Crawford Moodie, C., MacKintosh, A.M., Gallopel-Morvan, K., Hastings, G. and Ford, A. (2017). Adolescents' Perceptions of an On-cigarette Health Warning. Nicotine & Tobacco Research, Volume 19, Issue 10, 1 October 2017, 1232-1237, https://doi.org/10.1093/ntr/ntw165
- Footnote 24
-
Hoek J., Gendall P., Eckert C., and Louviere, J. (2016). Dissuasive cigarette sticks: the next step in standardised ('plain') packaging? Tobacco Control 2016;25:699-705. https://doi.org/10.1136/TOBACCOCONTROL-2015-052533
- Footnote 25
-
Hoek, J. and Robertson, C. (2015). How do young adult female smokers interpret dissuasive cigarette sticks?: A qualitative analysis. Journal of Social Marketing, Vol. 5 Issue: 1, pp.21-39, https://doi.org/10.1108/JSOCM-01-2014-0003
- Footnote 26
-
Phoenix Strategic Perspectives Inc. (2018b)
- Footnote 27
-
Phoenix Strategic Perspectives Inc. (2018b)
- Footnote 28
-
Government of Australia. (2013). Competition and Consumer (Tobacco) Information Standard 2011 (as amended and in force on 26 July 2013). Retrieved from the Government of Australia Federal Register of Legislation website: https://www.legislation.gov.au/Details/F2013C00598
- Footnote 29
-
Retrieved from the Australian Government Department of Health website: http://www.health.gov.au/internet/main/publishing.nsf/Content/tobacco-label-images
- Footnote 30
-
Les Études de Marché Créatec (2008). Quantitative study of Canadian youth smokers and vulnerable non-smokers: Effects of modified packaging through increasing the size of warnings on cigarette packages. Prepared for Health Canada.
- Footnote 31
-
Les Études de Marché Créatec (2008). Quantitative study of Canadian adult smokers: Effects of modified packaging through increasing the size of warnings on cigarette packages. Prepared for Health Canada.
- Footnote 32
-
Shanahan & Elliott (2009)
- Footnote 33
-
Government of Australia (2013)
- Footnote 34
-
The European Parliament and the Council of the European Union. (2014). Tobacco Products Directive (2014/40EU). Retrieved from the European Commission - Public Health website: https://ec.europa.eu/health//sites/health/files/tobacco/docs/dir_201440_en.pdf
- Footnote 35
-
Borland, R., Wilson, N., Fong, G.T., Hammond, D., Cummings, K.M., Yong, H.H., Hosking, W., Hastings, G., Thrasher, J. and McNeill, A. (2009). Impact of graphic and text warnings on cigarette packs: findings from four countries over five years. Tobacco Control. 2009;18(5):358-364. http://dx.doi.org/10.1136/tc.2008.028043
- Footnote 36
-
Swayampakala, K., Thrasher, J.F., Yong, H., Nagelhout, G.E., Li, L., Borland, R., Hammond, D., O'Connor, R.J. & Hardin, J.W. (2017). Over-Time Impacts of Pictorial Health Warning Labels and their Differences across Smoker Subgroups: Results from Adult Smokers in Canada and Australia. Nicotine & Tobacco Research, 2017, 1-9. https://doi.org/10.1093/ntr/ntx134
- Footnote 37
-
Sambrook Research International (2009)
