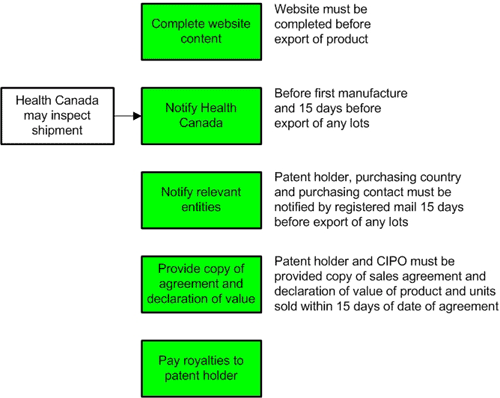
Process map of terms and conditions of a compulsory licence under Canada's Access to Medicines Regime
This process map provides a visual overview of the steps that need to be followed by a pharmaceutical company to meet the terms and conditions of a compulsory licence under Canada's Access to Medicines Regime. For details about these steps, return to Meeting the Terms and Conditions of a Compulsory Licence.
Process Map - Compulsory Licence Terms and Conditions
Complete website content. Website must be completed before export of product. Health Canada may inspect shipment. Notify Health Canada. Before first manufacture and 15 days before export of any lots. Notify relevant entities. Patent holder, purchasing country and purchasing contact must be notified by registered mail 15 days before export of any lots. Provide copy of agreement and declaration of value. Patent holder and Canadian Intellectual Property Office (CIPO) must be provided copy of sales agreement and declaration of value of product and units sold within 15 days of date of agreement. Pay royalties to patent holder.