Overview of the licence application process for a cannabis drug licence
Important: Effective March 12, 2025, Health Canada announced the coming into force of the Regulations Amending Certain Regulations Concerning Cannabis (Streamlining of Requirements). This web page will be updated in the coming months to reflect the amended regulations. You can refer to Summary of changes following the streamlining of regulations for how the changes may impact you.
On this page
- Who’s this for
- What’s a drug containing cannabis
- Foreword and disclaimer
- Licence application process
- Application checklists
- Contact us
Who’s this for
Use the content in these pages to apply for a cannabis drug licence. For other licences, refer to:
- Application requirements for micro-cultivation, nursery and standard cultivation, micro-processing and standard processing and sale for medical purposes
- Application requirements for analytical testing licences
- Application requirements for cannabis research licences
- Industrial hemp licensing application guide
What’s a drug containing cannabis
A drug containing cannabis is any cannabis that is produced, packaged, labelled, and sold or distributed as a drug. This includes synthesized cannabis. The Cannabis Regulations define “drug” as any substance or mixture of substances manufactured, sold or represented for use in:
- the diagnosis, treatment, mitigation or prevention of a disease, disorder or abnormal physical state, or its symptoms, in human beings or animals
- restoring, correcting or modifying organic functions in human beings or animals
A drug containing cannabis can only have health claims if it has received a Drug Identification Number (DIN) and a Notice of Compliance (NOC). If you want more information about this, refer to Health products containing cannabis or for use with cannabis.
In most cases, a drug establishment licence will also be required to conduct activities involving drugs containing cannabis. Refer to the Guidance on drug establishment licences to help understand and comply with Part C, Division 1A of the Food and Drug Regulations with regards to drug establishment licence requirements.
A drug containing cannabis is different from cannabis for medical purposes. If you want to sell cannabis products (e.g., dried cannabis, extracts, edibles or topicals) to registered clients for medical purposes, you need a sale for medical purposes licence, not a cannabis drug licence. Refer to the Application requirements for cannabis cultivation, processing and medical sales licences.
Foreword and disclaimer
Foreword
The Cannabis Act sets out that an applicant must file a licence application in the form and manner set by the Minister of Health, and include all the required information. These pages set out:
- the application process, including the requirements of a licence application
- the information that the applicant must submit
Under the Cannabis Act, Health Canada may require more information to process your licence application. If you don’t submit the information requested, the Minister may refuse to consider your application.
Disclaimer
You need to read these pages along with the Cannabis Act and the Cannabis Regulations. If there are differences between these pages and the Cannabis Act and the Cannabis Regulations, the Act and the Regulations are correct. If there are differences between the Cannabis Tracking and Licensing System (CTLS) and these pages, these pages are correct.
Health Canada’s CTLS is a Secure Web Portal and a single point of access for licence application submissions. You can access the CTLS directly at Health Canada’s Secure Web Portal. You should familiarize yourself with the use of this system and should refer to the CTLS Getting Started Guide for more information.
Licence application process
Important: You can only start your authorized cannabis activities after you’ve received your initial cannabis drug licence from Health Canada.
When starting the application process, Before you start applying for a licence has more information about what you should consider before applying. It may also be helpful to use the application checklist that can guide you through this process.
Figure 1 provides an overview of the licence application process and the things you need to do as an applicant.
Figure 1: Overview of licence application process
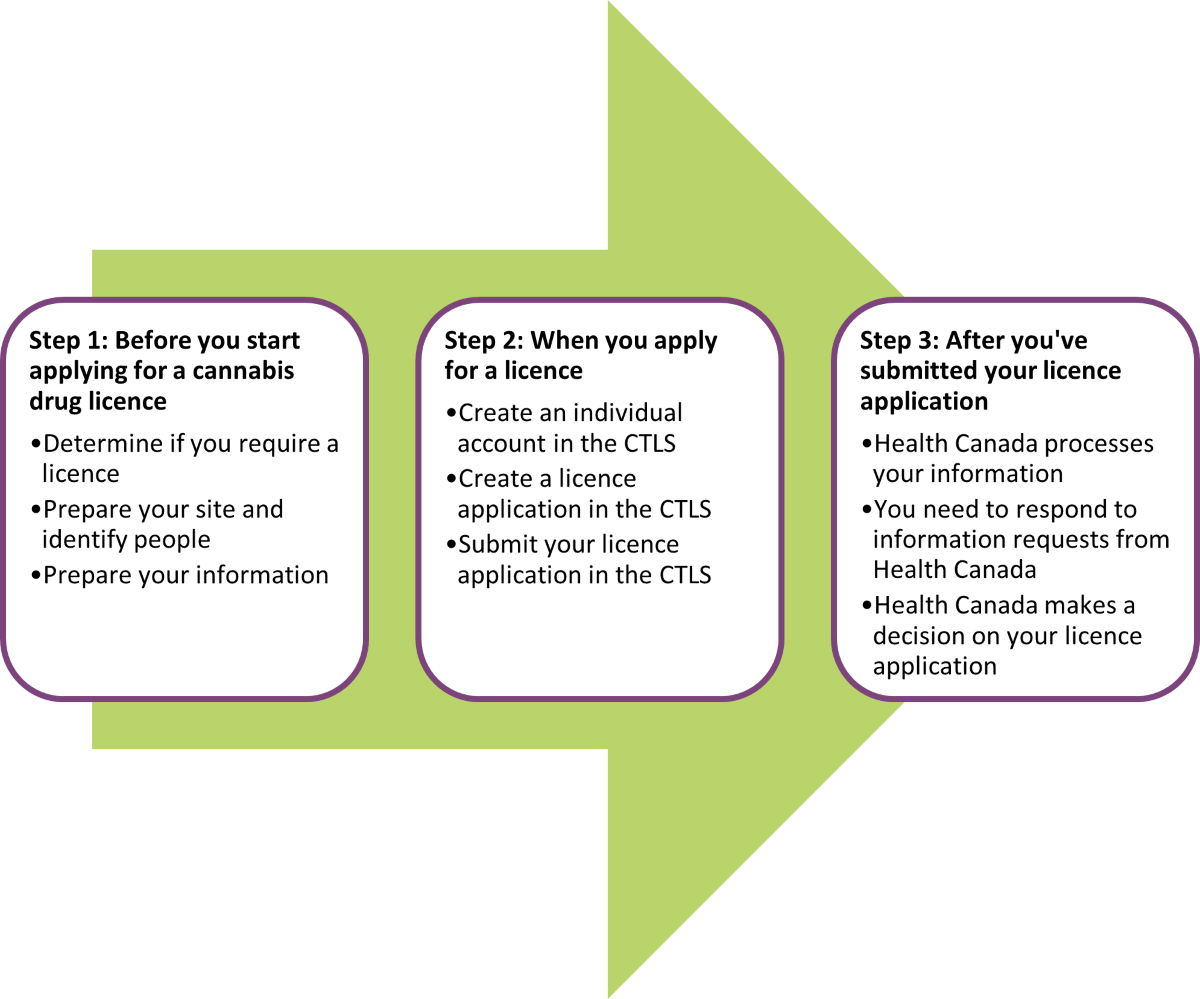
Licence application process: Key steps
1. The key steps you need to do before you start applying for a licence are to:
2. The key steps you need to do when you apply for a licence are to:
- Create an individual account in the CTLS
- Create a licence application in the CTLS
- Submit your licence application in the CTLS
3. The key steps after you’ve submitted your licence application are:
- Health Canada processes your information
- You need to respond to information requests from Health Canada
- Health Canada makes a decision on your licence application
Application checklists
The checklist is a tool that can guide you through the licence application process. It summarizes the key tasks:
- before you start applying for a licence
- when you’re applying for a licence
- after you’ve submitted your licence application
The checklist contains links to the sections where you can find more information.
Health Canada recommends using a document naming convention for all the information you have to submit. We can process your application more efficiently when you follow this document naming convention. It generally follows this layout:
“MainCategory”_APP-#_”SubCategory”_”YYYY-MM-DD”.”PDF”
- MainCategory: Main category of the information type
- APP-#: Your Licence application ID, which is available after you’ve created a licence application in the CTLS
- SubCategory: Sub-category of the information type
- YYYY-MM-DD: The date (year-month-date) of when your document was created
- PDF: Document file type
Section 1: Before you apply
- Determine if you require a licence
- Familiarize yourself with the legislation
- If you intend to self-identify as an Indigenous or Indigenous-affiliated applicant, you may want to contact the Indigenous Navigator Service at navigator-navigateur@hc-sc.gc.ca for additional guidance on the application process
- Prepare your site
- Identify the following people:
- senior person in charge
- qualified person in charge and their alternates
- persons with consent to communicate with Health Canada
- All identified people need to create their own CTLS account and give you their CTLS account ID
Section 2: Information to prepare
- For an individual applicant
- Copy of a government-issued photo ID
- For corporation, cooperatives or partnerships
- Corporate organizational chart
- If you’re a corporation: copy of your certificate of incorporation, amalgamation or amendment
- If you’re a cooperative or a partnership: Copy of your business name registration or partnership agreement
- Site details
- Site plan
- Description of physical security
- Appendix for the directives (only if the security measures are based on the Directive on Physical Security Requirements for Controlled Substances)
- Loss and theft prevention plan
- Site additional information form
- Drug establishment licence
- Document detailing written order form
- Identified people
- Senior person in charge
- Criminal record check or security clearance
- Copy of a government-issued photo ID
- Declaration for designated individuals
- Travel summary, if applicable
- Qualified person in charge and their alternates
- Curriculum vitae
- Criminal record check or security clearance
- Copy of a government-issued photo ID
- Declaration for designated individuals
- Certification (diploma, certificate, credential, equivalency assessment)
- Travel summary, if applicable
- Senior person in charge
- Record keeping
Important: If you’ve reached the upload limit of 5 documents in the CTLS, combine similar documents together. If you have any questions or need further instructions, email sp-licensing-cannabis-licences-sp@hc-sc.gc.ca. The subject line should be “Document upload limit for APP #”.
Section 3: Create a licence application
- Create an account in the CTLS, if you don’t have one already
- If you’re applying as a part of a corporation, a cooperative, or a partnership, create a corporate profile, if you don’t already have one
- Create a new licence application in the CTLS
Section 4: Submit your information in the CTLS
Contact us
For questions related to a specific licence application, contact Health Canada by email at sp-licensing-cannabis-licences-sp@hc-sc.gc.ca. Include your Application ID found in the CTLS. The subject line should be, "Questions about APP #".
For questions about the CTLS, contact Health Canada by email at ctls-sscdl@hc-sc.gc.ca or by phone at 1-866-337-7705 (toll free).
For general questions, contact Health Canada by email at cannabis@hc-sc.gc.ca or by phone at 1-866-337-7705 (toll free).