Background paper: Evolution of the Existing Substances Risk Assessment Program under the Canadian Environmental Protection Act, 1999
Official title: Chemicals Management Plan Science Committee background paper: Evolution of the Existing Substances Risk Assessment Program under the Canadian Environmental Protection Act, 1999 and considerations looking forward
Chemicals Management Plan (CMP) Science Committee meeting: February 17 to 18, 2021
On this page
- Meeting objectives and scope
- Part I: Evolution of risk assessment of existing substances
- Priority-setting approaches: From categorization to the identification of risk assessment priorities (IRAP)
- Information gathering: From generic mandatory surveys to a tiered approach
- Assessment strategies: Evolution of a fit-for-purpose approach
- Hazard characterization
- Fate and exposure characterization
- Risk characterization
- Governance and engagement
- Part II: Moving forward: Considerations
- Appendix A: Charge questions
Meeting objectives and scope
This meeting of the CMP Science Committee (hereafter referred to as the Committee) will provide members with an opportunity to reflect on how the risk assessment of substances carried out by the Existing Substances Program under authority of the Canadian Environmental Protections Act, 1999 (CEPA 1999) has evolved through the CMP (2006-2020). Importantly, this will also be an opportunity for members to explore potential future directions and provide suggestions to Health Canada (HC) and Environment and Climate Change Canada (ECCC) (hereafter referred to as the Departments).
The CMP was introduced in 2006 to, in part, strengthen the integration of chemicals management programs across the Government of Canada. As such, CMP assessments take into consideration a range of uses and sources, including uses addressed by provisions of various statutes, namely CEPA 1999, the Pest Control Products Act, the Canada Consumer Product Safety Act, and the Food and Drugs Act (that is, one assessment, many uses). In addition, work under the CMP has included extensive research, monitoring and surveillance of chemicals in humans and the environment.
A key element of the CMP has been addressing 4,363 substances that are or may be in commerce in Canada, and that were identified as priorities for assessment in 2006, pursuant to obligations in CEPA 1999. In parallel, pre-market assessments of substances proposed to be introduced into Canadian commerce, as notified through the New Substances Notifications provisions of CEPA 1999, have ensured that potential risks of these new substances are identified and addressed by appropriate risk management measures; as of March 2020, about 6,300 notifications of new substances had been assessed and addressed under the CMP.
It is recognized that there is intersection in risk assessment approaches, tools and methodologies used in New and Existing Substances Programs under CEPA 1999, and under other federal statutes. It is also recognized that a number of CMP elements have had and will continue to have a bearing and relevance to these assessments; for example, research, monitoring and surveillance provide key intelligence important to inform exposure, hazard and risk characterization, and can be critical in setting priorities and work-planning for the assessment and management of substances. Although principles and approaches discussed by the Committee may be in part informed by and have potential relevance on aspects of these various programs and initiatives, this background paper focuses on the assessment of existing substances carried out under the authority of CEPA 1999 since December 2006.
The Committee is requested to reflect upon the evolution of CEPA 1999's Existing Substances Risk Assessment Program (Part I of the background paper) and considerations for moving forward (Part II of the background paper) and, in so doing, consider the charge questions identified in Appendix A. Specifically, the Departments are looking for strategic science input, with a focus on strengthening Canada's risk assessment program for existing substances. The charge questions are open-ended in order to support a broad dialogue.
Part I: Evolution of risk assessment of existing substances
Canada was the first country to systematically prioritize its inventory of existing substances, the Domestic Substances List (DSL), and subsequently assess risk to the environment or human health associated with the identified priorities. Risk assessments were carried out over three phases of the CMP, which launched in 2006. The assessments took into consideration a broad range of uses (including cosmetics, food, consumer products) in addition to levels of the substances in environmental media. While many substances were addressed through streamlined approaches, in-depth risk assessments were required for others. In some cases, Canada was the first country to carry out assessments on these substances and implement risk management. As of December 2020, 24% of those substances not addressed through streamlined approaches have been proposed or concluded to be toxic as defined in section 64 of CEPA 1999. As of December 2020, approximately 180 risk management actions to reduce levels in the environment and human exposure have been put in place.
Key program elements are summarized below, including challenges and lessons learned.
Priority-setting approaches: From categorization to the identification of risk assessment priorities (IRAP)
Under the original CEPA, promulgated in 1988 (CEPA 1988), the DSL was established and included substances reported by industry as being in commerce from 1986 to 1988. By exclusion, substances not on the DSL were deemed to be "new," for the purposes of CEPA 1988, and were henceforth required to be notified prior to manufacture or import, as provided for in the Act and New Substances Notification Regulations. "Existing" substances were not defined in the Act. Under the Act, the Ministers of the Environment and of Health may assess any substance (including complex effluents, emissions, mixtures and classes of substances, and any matter that can be dispersed in the environment) for the purpose of determining whether it meets the definition of toxic in the Act, namely whether it has or may have harmful effects on human health or the environment.
Under CEPA 1988, assessment of chemicals and other substances focused on substances notified as new substances, and on existing substances included on the Priority Substances List (PSL); substances were added to the PSL based on recommendations from 2 multi-stakeholder ministerial expert advisory panels. Sixty-nine substances, including individual chemicals, classes of chemicals, and complex effluents or emissions were assessed under the PSL provisions.
With the assent in 1999 of the revised CEPA, the Departments were required to examine the 23,000 substances on the DSL to "categorize" them according to specific criteria (see Figure 1). The exercise was based on criteria for persistence and bioaccumulation (as prescribed in the Persistence and Bioaccumulation Regulations) and inherent toxicity to humans and non-human organisms, or "greatest potential for exposure" of people in Canada. Substances meeting the criteria were required to be subjected to a "screening assessment". Screening assessments are not defined in the Act but have ranged from succinct reviews of commercial status in Canada to in-depth risk assessments. Based on a screening assessment, Ministers may take no further action, add a substance to the PSL for further assessment, or recommend adding the substance to the List of Toxic Substances and undertake actions to manage risks (see Figure 1).
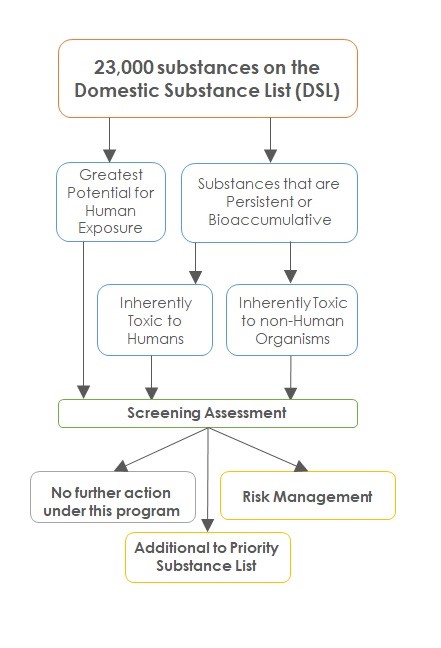
Figure 1: Text equivalent
Figure 1 is a flow diagram that outlines the decision criteria that was applied to the 23, 000 substances on the DSL during categorization progressing to assessment outcomes. HC and ECCC were required to examine the 23,000 substances on the DSL to categorize them according to specific criteria. They were categorized to identify those that were inherently toxic to humans or to the environment and that might be:
persistent (take a very long time to break down), and/or
bioaccumulative (collect in living organisms and end up in the food chain) and those that might be substances to which people might have greatest potential for exposure.
Substances meeting the criteria were required to be subjected to a "screening assessment". Based on a screening assessment, 3 outcomes were possible. Ministers may take no further action, addition of a substance to the Priority Substance List for further assessment, or recommend adding the substance to the List of Toxic Substances and undertake actions to manage risks.
Approaches used by HC and ECCC to carry out categorization were outlined in various documents, including in the Final Integrated Framework for the Health-related Components of Categorization of the DSL under CEPA (Health Canada 2009) and a series of guidance manuals and approach documents used by ECCC (Environment Canada 2003, 2005a, 2005b, 2005c).
The categorization exercise identified 3965 substances that legally required screening assessments and 398 other substances that were deemed important to assess because of potential concerns for human health. These 4,363 substances have been the focus of risk assessment activities of existing substances under the CMP.
Based on new scientific information, other substances, including those not meeting the categorization criteria, may be of potential concern (for example, certain bisphenols) and mechanisms were required to identify priorities for assessment post-categorization. Reflecting upon the categorization exercise, and informed by subsequent risk assessment activities under the CMP, the Departments and the stakeholder community have identified a number of lessons learned pertaining to priority-setting, including:
- The determination of which substances met the categorization criterion for "greatest potential for human exposure" used information available at the time, specifically, information for 1986-1988 submitted by industry at the time of development of the DSL. Mandatory surveys subsequently conducted under CEPA 1999 to inform the risk assessments consistently showed that at least 30% of substances surveyed were no longer imported or manufactured within the reporting period.Footnote 1 Yet, the Departments were obligated to conduct assessments on them.
- Streamlined approaches were required to address categorized substances of lower concern given the large number of substances with limited commercial activity. These approaches collectively addressed 2,599 of the 4,363 substances (60%).
- Due to limitations related to domain of applicability of fate and effect modelling, there was considerable uncertainty in attempting to address categorization criteria for chemicals with certain chemistries (for example, organometallics). Though some of these substances may not have been found to meet the criteria, they may nonetheless pose a risk.
- When substances identified as bioaccumulative through categorization were assessed during the first phase of the CMP, it became evident that bioaccumulation potential was frequently overestimated and required correction for metabolism rate.
- Data-poor substances presented a challenge. Human health categorization was biased towards data-rich substances. For example, for human health, identification of high hazard to humans was largely based on classifications of various international agencies. For ecological categorization, empirical data (often only a single value) for persistence or bioaccumulation or inherent toxicity were only available for approximately 5-10% of all organic substances and there was a heavy reliance on in silico approaches for determining persistence, bioaccumulation and inherent toxicity. Moving forward, new and emerging technologies, including new approach methodologies (NAMs),Footnote 2 will help identify data-poor substances of potential concern (see Hazard characterization section).
- The categorization exercise was prescriptive and was generally not driven by weight of evidence (that is, pass/fail decision making). The hard-wired nature of categorization restricted program flexibility and required the Departments to conduct screening assessments for some substances that were of lower concern and that constrained, in the short-term, their ability to address other priorities, such as substances of high inherent ecotoxicity that did not meet categorization criteria for persistence and bioaccumulation.
The need for CMP to continue to keep pace with emerging science was noted in internal and external audits of the first phase of the CMP. Taking the above learnings into consideration, in 2014, the Departments developed a formal ongoing priority-setting framework that enhances the way new information from multiple sources is acquired and evaluated to determine if further action may be necessary. These enhancements are outlined in the , which provides for IRAP (see Figure 2).
The need for CMP to continue to keep pace with emerging science was noted in internal and external audits of the first phase of the CMP. Taking the above learnings into consideration, in 2014, the Departments developed a formal ongoing priority-setting framework that enhances the way new information from multiple sources is acquired and evaluated to determine if further action may be necessary. These enhancements are outlined in the Approach for identification of chemicals and polymers as risk assessment priorities under Part 5 of CEPA 1999, which provides for IRAP (see Figure 2).
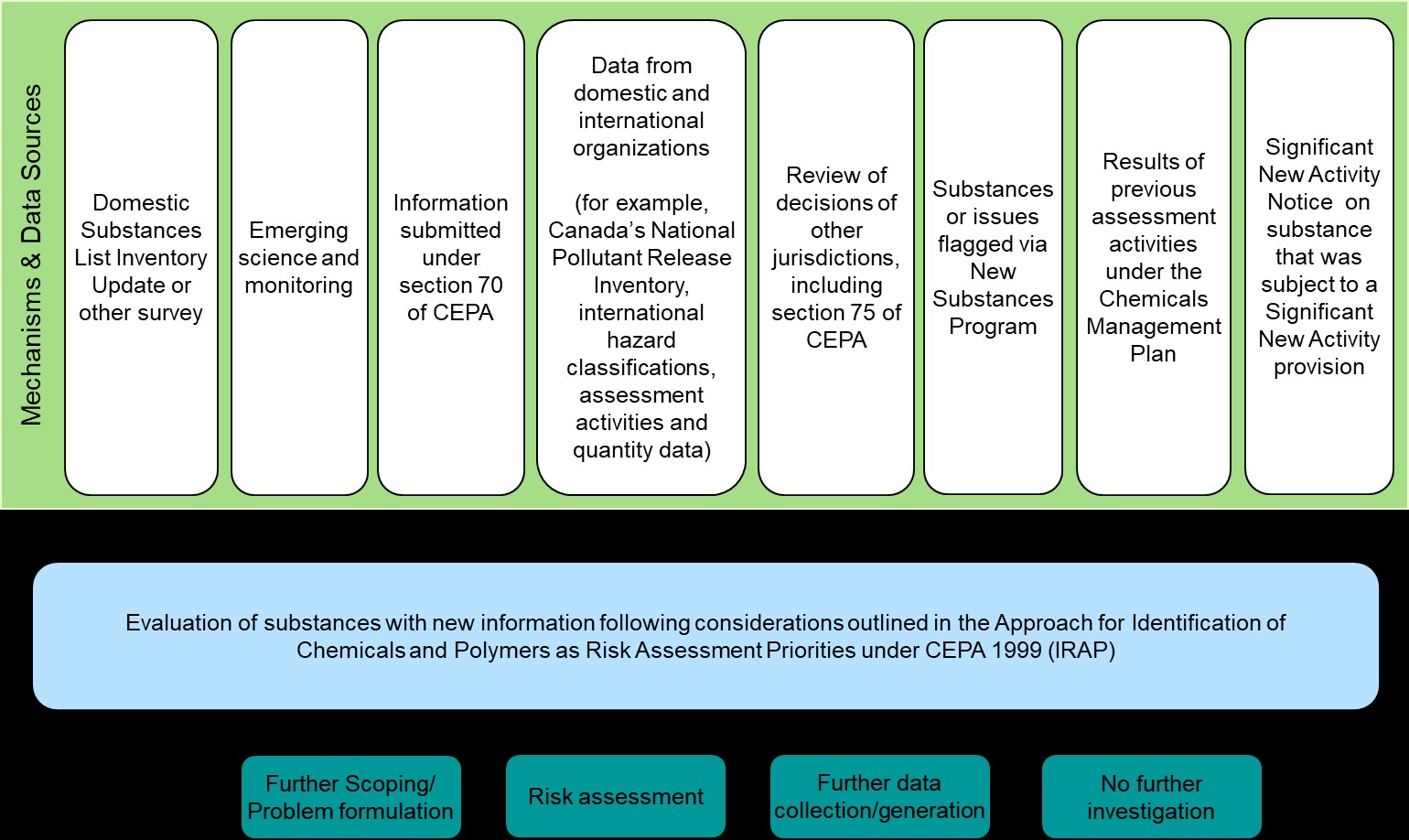
Figure 2: Text equivalent
Addition to DSL inventory update or other survey, emerging science and monitoring, information submitted under section 70 of CEPA 1999, data from domestic and international organizations, review of decisions of other jurisdictions including section 75 of CEPA 1999, issues flagged via New Substances Program, results of previous/current CMP assessment activities, and Significant New Activity Notice (SNAN) on substance that was subject to a Significant New Activity (SNAc) provision are the 8 mechanisms used to inform the annual application of the Approach for Identification of Chemicals and Polymers as Risk Assessment priorities under CEPA 1999 (IRAP). The IRAP process may lead to risk assessment, further data collection/generation, or no further work at this time.
Rather than prescriptive criteria, this approach is based on a set of guiding principles and a series of considerations and diverse sources of information on hazard and exposure indicators. The IRAP process is a cyclical systematic compilation and review of information from a large number of sources enabling the Government of Canada to be well-positioned to recognize concerns, to track emerging issues, and to identify and prioritize substances requiring further work. It provides for consideration of data received through the notification of new substances, such as information on potential chemistries of concern. Similarly, information and decisions from other domestic and international chemicals programs are considered, along with new research and monitoring data. If a substance is identified as a candidate for further work through the IRAP process, there are a range of options, including, since 2017/2018, further scoping/problem formulation (see discussion of further scoping/problem formulation in the Information gathering section).
Since 2015, IRAP reviews have been conducted 4 times, with the most recent iteration published in December 2020. Recommendations coming out of the IRAP reviews over these 4 cycles are shown in Figure 3. This illustrates the ongoing attention being given to IRAP within the Departments.Footnote 3

Figure 3: Text equivalent
Figure 3 is a table summarizing the results of the IRAP cycles, broken down by IRAP review year and the number of substances identified per recommended outcome in that review, as well as totals across all review cycles to date. Since 2015, there have been 4 IRAP reviews completed have been conducted 4 times, with the most recent iteration conducted in 2019 and published in December 2020. IRAP results include a number of recommendations for substances requiring further attention as listed in the far left column. These include data gathering, follow up on international activity, risk assessment and further scoping or problem formulation.
The 2015 review identified 194 substances for data gathering, 27 requiring follow up on international activity and 28 for risk assessment.
The 2016 review identified 184 substances for data gathering, 65 substances for follow up on international activity and 10 for risk assessment. Note that in the 2016 review, 51 substances were identified for both data gathering and follow up on international activity.
The 2017-18 review identified 60 substances for data gathering, 77 for follow up on international activity, 1 for risk assessment and 1,094 for further scoping or problem formulation. The 1,094 substances represent 13 individuals or groups of substances. The recommended outcome of further scoping or problem formulation was introduced as a new outcome starting in the 2017-18 review.
The 2019 review identified 443 substances for data gathering, 101 requiring follow up on international activity and 85 for further scoping or problem formulation. The 85 substances represent 25 individuals or groups of substances.
In total, across all four cycles of IRAP review conducted to date, 881 have been identified for data gathering, 270 require follow up on international activity, 39 for risk assessment and 1179 for further scoping or problem formulation. The 1179 substances represent 38 individuals or groups of substances.
The IRAP process is flexible. Moving forward, it is expected that there will be ongoing integration of new sources of information, including the use of emerging science, new data types and methodologies. For example, estrogen receptor actives [based on the high-throughput in vitro data generated by the United States (U.S.) Environmental Protection Agency's (EPA) Toxicity Forecaster (ToxCast) program and used by the U.S. EPA Endocrine Disruptor Screening Program (EDSP)] were considered as hazard indicators in the 2017/2018 IRAP exercise. In the 2019 IRAP exercise, again based on U.S. EPA ToxCast data, androgen receptor actives and positive results for steroidogenesis were identified as hazard indicators.
The IRAP process is responsive and, depending upon program direction, the scope of IRAP reviews can be expanded to encompass specific themes, such as vulnerable populations. An area to strengthen, as identified by stakeholders, is a clearly articulated process for public engagement and for external nomination of new priorities.
Information gathering: From generic mandatory surveys to a tiered approach
Throughout the CMP, information and data to inform risk assessments has been obtained from a number of sources, including peer-reviewed scientific literature, stakeholder submissions, departmental research, monitoring and surveillance data, information submitted to other HC and ECCC regulatory programs, and assessments from other jurisdictions, as well as outputs from predictive and modelling tools.
Although there are prescribed data requirements for new substances under the New Substances Notification Regulations of CEPA 1999, statutory requirements are not established for existing substances. There are no requirements for manufacturers or importers of substances on the DSL to update the Government on changes in commercial status. For substances of interest, though, mandatory surveys under section 71 of CEPA have been key for capturing up-to-date information on current activities, uses and quantities of substances in commerce in Canada. Together with information submitted by industry to meet other reporting requirements (for example, Cosmetic Notification System, National Pollutant Release Inventory), this information can be critical for characterizing exposure and risk.
Standardized surveys capturing all activities with generic questions were used for substances in the Ministerial challenge to industry and other stakeholders (the Challenge), an early risk assessment initiative under the first phase of the CMP. A targeted approach to mandatory surveys was introduced in the second phase of the CMP, with custom surveys focused on specific information needs. A tiered approach to information gathering was adopted for the third phase of the CMP, with voluntary approaches followed by mandatory approaches, as needed. In this phase, several industry associations liaised with the Departments to establish proactive approaches to information gathering.
From 2007 to 2020, 44 mandatory surveys, including 6 inventory updates collecting commercial data on approximately 6,600 substances were published (additionally, prior to the launch of the CMP, 6 surveys were published between 2000 and 2006, collecting information on approximately 500 other substances). These surveys gathered information retroactively and provided a snapshot in time of the substances' commercial status. Advances were made to make non-confidential data collected publicly available through the Government of Canada Open Data Portal.
A key challenge to robust information gathering identified by stakeholders in 2018 was the difficulty in obtaining information along the supply chain, in particular as it relates to imported products and manufactured items (approximately 80% of products used in Canada are imported). As well, requests for confidentiality on information submitted by distributors created challenges for government looking to engage downstream users.
With industry generating data to support the European Union (EU) Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) Regulation registration packages, non-confidential data summaries for thousands of substances became available on a public European Chemicals Agency (ECHA) database. This has been an important source of information for the Departments; however, the summaries vary in level of detail, and accessing the primary studies and confidential data has been a challenge. Sometimes, access necessitated development of data-sharing agreements with REACH industry consortia, which are time consuming to put in place.Footnote 4 It is encouraging that, over the last 2 years, the Departments have obtained unpublished studies from EU REACH registration packages from approximately 25 international companies without the need for data-sharing agreements.
Information is also obtained through CMP-funded research, monitoring and surveillance programs. An HC analysis has determined that, in the first phase of the CMP, data generated by HC informed less than 5% of the human health risk characterizations in screening assessment reports. In response, the need for targeted testing, research and monitoring/surveillance to support risk assessment was recognized and a formal process was put in place in 2016 to promote targeted work. Specifically, a portion of CMP-funded research was directed to short-term data needs to inform risk characterization of substances in the third phase of the CMP. The number of assessments incorporating data generated within HC went from less than 5% to approximately 40%. Key study types were dermal absorption, retrospective analyses of house dust, indoor air and biomonitoring samples for CMP substances, and toxicokinetics data to inform use of biomonitoring data. In ecological assessments, up to 25% of assessments have used data generated through CMP research and monitoring.
Given the aggressive timelines for publication of CMP assessments, a challenge with in-house data generation has been the inherent time lag between identification of a data need, and generation and receipt of the test data. Notably, development of analytical methods is often necessary before monitoring can be undertaken; this is especially true for novel chemistries. As well, a data need may only be definitively identified during the drafting phase of the screening assessment, such that time may not be available to undertake new research or monitoring. Another observation was that, while some government laboratories are focused on routine testing, most are geared to novel research, such that testing is not necessarily a good fit for those programs.
Data generated by other federal programs such as the Clean Air Regulatory Agenda (which generated indoor and outdoor air monitoring data, including for volatile and semi-volatile substances in homes across Canada) and the cyclical Canadian Total Diet Study (a food surveillance program that monitors the concentrations of chemical contaminants in foods that are typically consumed by Canadians) inform CMP assessments. The National Air Pollution Surveillance (NAPS) Program has also been an important source of ambient air quality data. The NAPS Program comprises nearly 260 stations in 150 rural and urban communities reporting to the Canada-Wide Air Quality Database. Ongoing generation of Canadian monitoring data will inform both prioritization and risk assessment moving forward.
As well, data maintained by agencies such as the Canada Border Services Agency can provide important commercial information to support exposure characterization.
Contributing to and using international data platforms, such as the Information Platform for Chemical Monitoring (IPCHEM) and Pollutant Release and Transfer Registers, provides access to exposure information.
The Departments are introducing a "further scoping/problem formulation" recommendation into the IRAP process. Preparation of a scoping/problem formulation would be recommended in instances where substances have both hazard and exposure indicators, but where more investigation is needed to determine the most appropriate next steps. This scoping would lay out the human health and ecological data landscape. Publication of the scoping/problem formulations would give stakeholders an early opportunity to engage and provide input. Based on the scoping and additional information received, a determination would be made as to whether risk assessment is the suitable direction to take or whether other options (such as no further work, data generation, application of significant new use provisions under CEPA 1999, or presentation of relative hazard to inform substitution) would be more appropriate. In instances where additional data is required prior to risk assessment, scoping/problem formulation can inform the timing and scheduling of risk assessment activity. In the CMP, substance profiles were published for the Challenge substances to indicate data landscape at that time and to similarly solicit early stakeholder input; this proved to be a useful mechanism to engage stakeholders early in the assessment process.
In terms of data capture, the Departments explored the use of the International Uniform Chemical Information Database (IUCLID) platformFootnote 5 to capture hazard data with mixed results. Early versions of IUCLID were used by both Departments to capture hazard data. The version made accessible to staff did not support customization of data entry, chemical structure similarity searching, or report generation. As a result of these limitations, the data entry was onerous and of limited utility. More recent releases of IUCLID have since addressed many of these limitations, including the ability to create custom web interfaces for data entry, and a report generator to extract IUCLID data. Moving forward, IUCLID is expected to facilitate data mining and data sharing with international partners who have also adopted the tool. This is important as, with advances in high-throughput data generation, the volume of data is growing exponentially.
Adoption of data capture approaches could also be important as the Departments look to incorporate elements of systematic review,Footnote 6 a best practice internationally. A challenge for the Existing Substances Program is that the volume and quality of data are often not sufficient to meet systematic review criteria per se. Nevertheless, systematic review-like processes would have merit moving forward and, to that end, more automated data collection and interpretation schemes with transparent criteria, as well as literature screening tools such as the U.S. EPA Abstract Sifter, are being explored. Moving forward, scheduling of risk assessments would need to take this additional step into consideration.
The Departments have identified a number of challenges and lessons learned pertaining to information gathering, including:
- Collecting robust information along the global supply chain, in particular as it relates to imported products and manufactured items, is a challenge. This information is important as use of consumer products and manufactured items is often the most significant source of exposure to the general population.
- Enhanced monitoring of market trends (rather than snapshots in time) is needed for a range of CMP activities, from prioritization to performance measurement.
- Accessing primary studies and confidential information for studies generated for EU REACH Regulation registration can be challenging and time-consuming.
- Increased early voluntary submission of information by industries would provide for more timely access to data.
- Statutory authorities to require generation of data under CEPA 1999 have not been exercised in the CMP. Developing a process/guidance on when this might be appropriate would be a first step in exploring the more routine use of such powers and could lead to the acquisition of critical data to reduce the use of conservative approaches/assumptions in risk assessments.
- More flexibility in scheduling of risk assessments would allow for data generation, as needed, to inform the assessments.
- In particular, for hazard information, the existing data capture tools and formats should be enhanced to encourage reuse of the data (for example, read-across) and to support systematic review-like approaches.
Assessment strategies: Evolution of a fit-for-purpose approach
Addressing the 4,363 priority substances identified through the categorization exercise necessitated development of new methodologies and approaches. Compared with the PSL Program, which assessed 69 substances (including individual chemicals, chemical classes, and complex effluents) over 10 years, 3,936 substances identified as priorities following categorization have been assessed under the CMP between December 2006 and December 31, 2020, with 330 found to be toxic as defined in section 64 of CEPA 1999.Footnote 7
Notwithstanding certain groups or classes assessed early in the CMP (such as PBDEs and PFOS-related substances), the assessment of substances identified as priorities through categorization has progressively evolved throughout the 3 phases of the CMP, from primarily a chemical-by-chemical approach (Challenge initiative of the first phase) to inclusion of assessments of groupings and classes of substances. Further efficiencies were gained through the development and implementation of streamlined risk-based science approaches that allowed for more rapid yet robust decision-making for many substances. Figure 4 illustrates the key assessment initiatives and approaches for each phase of the CMP.
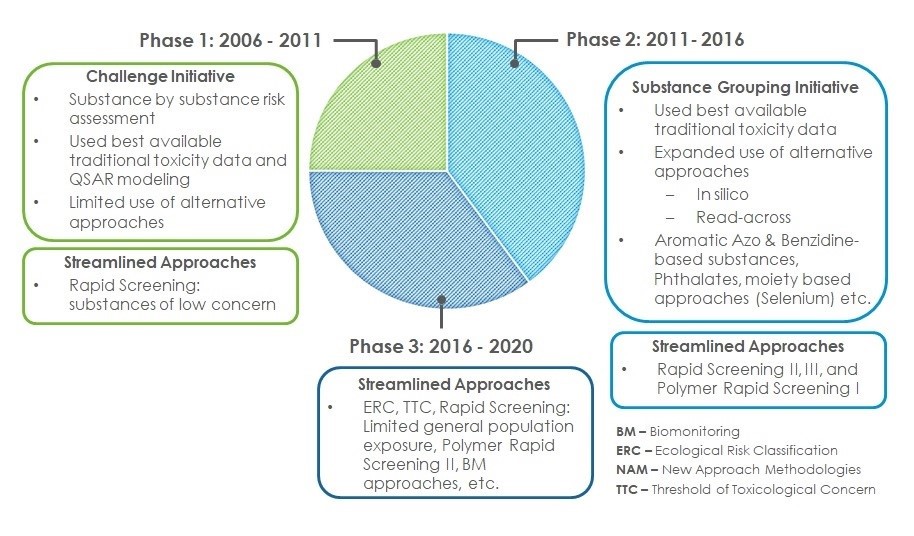
Figure 4: Text equivalent
Figure 4 is a three section pie chart with associated description boxes highlighting the assessment approaches that were applied over the 3 phases of the CMP from 2006 to 2020 to address the 4363 priority substances identified through the categorization exercise were addressed in the CMP over the 3 phases from 2006 to 2020.
The first section shows Phase 1, which progressed from 2006 to 2011 and addressed substances through the Challenge Initiative. The Challenge Initiative involved substance by substance risk assessment, using best available traditional toxicity data and QSAR modeling, with limited use of alternative approaches. Phase 1 also introduced streamlined risk-based approaches that allowed for more rapid yet robust decision-making for many substances. This included the rapid screening for substances of low concern conducted as part of Phase 1.
The second section shows Phase 2, which progressed from 2011 to 2016 and addressed substances through the Substance Grouping Initiative, as well as through the development and implementation of additional streamlined approaches. The Substance Grouping Initiative used the best available traditional toxicity data, and introduced the expanded use of alternative approaches, including in silico and read-across. Examples of substance groups addressed during Phase 2 of the CMP included the aromatic azo and benzidine-based substances, phthalates, and moiety based approaches such as selenium as an example. Rapid screening 2 and 3, and polymer rapid screening 1 were all streamlined approaches developed as part of Phase 2.
The third section shows Phase 3, which progressed from 2016 through 2020 and involved the development of a number of new streamlined approaches. Approaches developed included the Ecological Risk Classification Approach (ERC), the Threshold of Toxicological Concern (TTC)-based Approach, and biomonitoring approaches. Additional rapid screening approaches were also developed and implemented, including rapid screening based on limited general population exposure and a second polymer rapid screening approach.
A series of streamlined approaches, including rapid screening, were developed for substances that the Departments considered to be of low concern, which collectively addressed 2,599 of the 4,363 substances (60%). From a workload management perspective, use of these approaches meant that more risk assessment resources could be placed on substances of higher concern.
To assist the Departments in more efficiently addressing substances, a series of science approach documents (SciAD) were published between 2016 and 2020, namely:
- Ecological risk classification of organic substances (ERC) (2016)
- Biomonitoring-based approach 1 (2016)
- Threshold of toxicological concern (TTC)-based approach for certain substances (2016)
- Biomonitoring-based approach 2 (2016)
- Substances with low human health hazard potential (2019)
- Ecological risk classification of inorganic substances (ERC-I) (2020)
These science approaches to risk evaluation were key to the departments efficiently addressing large numbers of substances that may be of lower concern to either human health or the environment, and will have utility moving forward with further priority-setting exercises.
In 2015, a level of complexity-based Risk Assessment Toolbox was developed to formalize approaches being used to address existing substances (see Figure 5). Type 1 approaches address substances for which formal regulatory assessments under CEPA 1999 may not be required or optimal (for example, referring to another more appropriate program). Rapid screening and certain science approaches are examples of Type 2 approaches, broad-based approaches often useful for substances with lower potential for exposure. Type 3 approaches involved an increasing level of complexity, as warranted. Application of the Toolbox helped the Departments focus efforts on the substances of higher concern and engage stakeholders on substances as efficiently as possible. The Toolbox underwent consultation with stakeholders through a workshop held in May 2015 and was discussed at the June 2015 Committee meeting, where the Departments sought input on how to mitigate any potential challenges with the planned framework, as well as how to best operationalize this framework and its potential ongoing role post-2020.
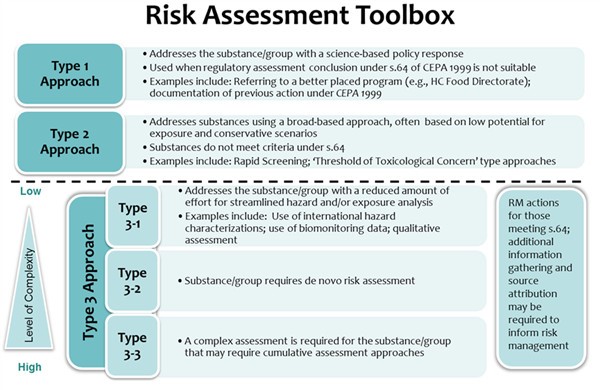
Figure 5: Text equivalent
Figure 5 illustrates the different types of risk assessment approaches that are used to address substances under CMP and the level of complexity for each.
Type 1 approaches are used to address substances or groups of substances with a science-based policy response. These approaches are used when it is considered that a formal conclusion under section 64 of CEPA 1999 is not appropriate at the time. Examples of Type 1 approaches include referral of the assessment to a better-placed federal risk assessment program, or documentation of a substance or group as having been previously addressed by an existing action or initiative under CEPA 1999.
Type 2 approaches are used to address substances using a broad-based approach. These approaches are typically applied to substances that have lower potential for exposure and risk. The assessments may use either qualitative or quantitative approaches to assess the substances, applying conservative (protective) assumptions. Assessments using this approach may or may not make a formal conclusion under section 64 of CEPA 1999. The Rapid Screening Approach and Polymer Rapid Screening Approach are past examples of Type 2 approaches. In upcoming assessments, the proposed approach for ecological risk classification of organic substances (ERC) and the health approach based on threshold of toxicological concern (TTC) are 2 other examples.
Type 3 approaches are used to address substances using a standard risk assessment approach that considers both hazard and exposure, for either the ecological assessment and/or health assessment, in more detail. Documents may have a similar structure to the typical screening assessments that have been completed under the CMP to date. This type of approach can be sub-divided into three levels representing a continuum of increasingly complex assessment approaches. They may include consideration of a combination of qualitative and quantitative lines of evidence in determining whether a substance or group of substances meet the criteria under section 64 of CEPA 1999. Assessments will be conducted according to a fit-for-purpose approach to focus efforts.
Type 3-1 approaches are streamlined to allow assessment of a substance or group of substances with a reduced effort on either, or both, the hazard or exposure characterization. Examples include adoption of existing hazard characterizations from international organizations, or use of Biological Equivalents (BE) for substances for which biomonitoring (exposure) data are available.
Type 3-2 approaches are those in which de novo exposure and hazard characterizations are undertaken.
Type 3-3 approaches are used to assess substances where a more in-depth consideration of exposure and/or hazard is required than in a type 3-2 assessment. Such assessments could include, for example, consideration of cumulative risk.
In the second phase of the CMP, there was an increased focus on group-based assessments. This initiative began with a notice of intent for the Aromatic Azo- and Benzidine-based Substance Grouping, published in 2010. That grouping included 358 aromatic azo- and benzidine-based substances with structural similarities and common functional uses and applications. In 2011, an announcement that applied to that grouping and 8 additional groupings of substances was published in the Canada Gazette. The initiative included the following substance groupings:
- Aromatic Azo- and Benzidine-based Substance Grouping
- Boron-containing Substances
- Certain Organic Flame Retardants Substance Grouping
- Cobalt-containing Substance Grouping
- Internationally Classified Substance Grouping
- Methylenediphenyl Diisocyanate and Diamine (MDI/MDA) Substance Grouping
- Phthalate Substance Grouping
- Selenium-containing Substance Grouping
- Substituted Diphenylamines Substance Grouping
Most of these substance groupings were selected based on structural or functional similarities with consideration given to assessment efficiencies, risk management efficiencies, characterization of cumulative risk and the ability to support informed substitution. Though group-based assessments had been conducted prior to the CMP, for example a series of metal moieties and organic compounds including polycyclic aromatic hydrocarbons, and polychlorinated dibenzodioxins and polychlorinated dibenzofurans under the PSL, the substance groupings initiative was broader in scope. Additional groupings were identified during the third phase of the CMP, including another flame retardants substance grouping.
Sector-based approach
A primary driver in the grouping of substances relevant to a specific industrial sector is to facilitate the engagement of implicated stakeholders; notably, information gathering can be streamlined and directed to the specific sector. As well, common assessment approaches can be developed and adopted, providing for greater assessment efficiencies, and assessors can develop a greater depth of understanding of the use, exposure and toxicity common to the substances across the group. For those substances that are determined to require risk management actions, a sector-based approach can also provide for early and ongoing engagement of risk managers and of the implicated sector.
In the first phase of the CMP, a sector-based assessment strategy was adopted for the petroleum sector, namely the Petroleum Sector Stream Approach. However, while the substances included in the approach had a link to the petroleum sector, they were a heterogeneous group, in terms of their chemical nature and complexity, their toxicity profiles, and the nature of human and ecological exposures, as well as the degree to which any given substance was of interest or relevance to the sector as a whole. As a result, the sectoral approach for the substances in this group did not provide the efficiencies that had been anticipated.
However, some of the group-based assessments noted above comprised substances with common functions/uses and this did provide efficiencies. For example, the Aromatic Azo and Benzidine-based Substance Grouping can be viewed as a function- / use-based approach as, in additional to structural similarities, substances in this grouping are used predominantly as colourants (that is, dyes, pigments). Thus, the success of sector/functional/use-based approaches requires ensuring that substances share sufficient commonalities.
Emerging priorities
Beyond the 4,363 substances, the Departments were able to take on a limited amount of additional assessment activity on high-priority existing substances. This included a state of the science report on lead to support provincial guideline-setting, science assessments on microbeads and plastic pollution, and a stakeholder technical consultation document on a group of bisphenols, as well as certain priorities identified through IRAP exercises, including parabens (see Priority-setting approaches section). Undertaking this additional activity was challenging given the assessment workload associated with the CMP priorities.
Through the CMP, the Departments have been developing novel science and policy approaches to deal with nanomaterials. Canada collaborated with the U.S. EPA on the Canada-U.S. Regulatory Cooperation Council (RCC) Nanotechnology Initiative, established to increase alignment in regulatory approaches for nanomaterials between the countries. Notably, HC, ECCC and the U.S. EPA shared and developed best practices for assessing and managing the risks of nanomaterials (RCC 2014). Domestically, an approach for addressing nanoscale forms of substances on the DSL and a consultation document on prioritization approaches for nanoscale forms of substances on the DSL were published in 2015 and 2016, respectively. The Risk Assessment Framework for Nanomaterials in Canada is undergoing science and policy review. Over 85 notified nanomaterials and substances with potential to be produced at the nanoscale have been assessed under the New Substances Program since 2010. Fifty-three substances that are listed on the DSL that may be produced at the nanoscale have been identified through a screening exercise for further follow-up.Footnote 8
Hazard characterization
A range of hazard data was available for CMP priorities, from data-rich to data-poor substances and groupings. Given that, in Canada and many other jurisdictions, commercial substances were not subject to premarket data requirements until the introduction of new substance notification provisions, the majority of substances on the DSL were "grandfathered in" and are data-poor. Given limited empirical toxicity data on most of the 4,363 substances, it was important that the Departments advance the use of computational toxicology and read-across approaches to assess these substances identified as priorities for action in order to meet the legislative requirements.
Computational tools
In silico approaches have been used by the Departments since the mid-1990s with the beginning of the New Substances Program and, to a more limited degree, the PSL Program for existing substances. There has been an evolution of in silico approaches from the 1990s through CMP for both priority-setting and risk assessment, with progressive adoption of computational and automated approaches for hazard (as well as exposure) prediction and read-across.
Prior to the categorization exercise, (quantitative) structure-activity relationship [(Q)SAR] and mechanistic mass-balance (for example, for bioaccumulation) modelling were used mainly for predicting physical-chemical properties and certain ecotoxicity endpoints. Models ranged from simple linear relationships to mathematically complex algorithms (for example, neural networks). There was limited experience and guidance on their use, and the range of applicability was often limited. Predictive tools were used extensively in the categorization exercise. Models were critical for the determination of whether substances met the criteria for persistence and bioaccumulation. With respect to human health effects, predictions of QSAR models (for example, TOPKAT, CASETOX), predictions of SAR models, information on chemical substructures of concern and metabolism prediction models (for example, MultiCASE) formed part of the qualitative preliminary weight-of-evidence component for determination of inherent toxicity to humans.
Through the CMP, Canada has continued to work with international partners to further develop in silico approaches and to facilitate their broader acceptance. Since 2005, Canada has worked with Organisation for Economic Co-operation and Development (OECD) partners in establishing OECD QSAR validation principles, in developing the OECD Guidance Document on the Validation of QSAR Models and in launching the OECD QSAR Toolbox. As well, Canada has advanced the use of consensus approaches (Kulkarni and Barton-Maclaren 2014) and continued to explore novel chemical space-based approaches to address model uncertainties and increasing complexity in molecular structure (Kulkarni et al. 2016). Canada was also a contributor in the development of the OECD Guidance on Grouping of Chemicals (OECD 2014).
In the second phase of the CMP, computational tools were used to address data gaps as well as to build chemical categories and read-across. Grouping approaches were also used in assessments. In November 2014, the Committee deliberated and provided advice on best practices for deriving a sufficient rationale for read-across, and identified challenges along with how best to address them. Three case studies illustrated current applications. Over all, input helped inform use of read-across in both Departments and resulted in improved guidance for risk assessors.
In the third phase of CMP, computational tools were a key resource for risk assessors, though many substances in the third phase had complex molecular structures and were part of mixtures or existed as UVCBs; such substances were not easily amenable to some predictive models. HC developed a Threshold of Toxicological Concern (TTC)-based Approach that made use of computational models (that is, OASIS TIMES, OECD QSAR Toolbox) to assign TTC values that were compared with exposure estimates to identify chemicals with a low potential for risk. Additionally, ECCC developed the ERC Approach to reprioritize 640 organic substances initially identified as priorities for assessment through categorization. The first version of ERC (ERC1) is an evidence-based computational system that allows evaluation of chemistries outside the domain of the 2006 categorization approach. In 2017, it was reviewed as part of the OECD Integrated Approaches for Testing and Assessment (IATA) case study project (OECD 2017). More recently, version 2 of ERC (ERC2) has been developed, as outlined in Part II of this background paper.
Risk assessment modernization: Integrated approaches to testing and assessment, and use of NAMs
Modernization of risk assessment, based on emerging approaches (such as high-throughput in vitro screening methods), was envisioned by the U.S. National Research Council in 2007 in the report Toxicity Testing in the 21st Century: A Vision and a Strategy (U.S. National Research Council 2007). In 2012, the Council of Canadian Academies articulated a similar future direction for chemical risk assessment in the report Integrating Emerging Technologies into Chemical Safety Assessment (Council of Canadian Academies 2012). Canada's contributions in this area have focused on regulatory application. Two significant initiatives that the Departments have been involved in since mid-CMP are:
- OECD IATA Case Studies Project. In 2013, the OECD's Working Party on Hazard Assessment (WPHA) within the OECD Cooperative Chemicals Assessment Programme pivoted from a focus on chemical-specific hazard assessment to a focus on methodologies, including IATA. In 2015, the WPHA launched the IATA Case Studies Project, and the Departments have contributed case studies and have been active in reviewing case studies contributed by others.
- Accelerating the Pace of Chemical Risk Assessment (APCRA) Initiative. The focus of this initiative has been on working government-to-government to advance case studies to build confidence in approaches to accelerate the pace of risk assessment activity. This initiative is co-led by U.S. EPA, ECHA and HC, and HC and ECCC lead, or are participants in, several case studies. Progress is documented by Kavlock et al. (2018) and in a series of Accelerating the Pace of Chemical Risk Assessment (APCRA) reports.
Krewski et al. (2020) noted that increased emphasis on case studies by the OECD and other international bodies, international efforts to develop best practices / guidance, and continued interaction and discussion between the research and regulatory communities are necessary to facilitate regulatory application.
ECCC continues to collaborate with academia and regulators in developing scientific approaches for understanding and classifying modes and mechanisms of action (Sapounidou et al. 2020, Kienzler et al. 2019, Armitage et al. 2018, Connors et al. 2019), and in developing and enhancing tools and approaches for ecological receptors, such as eco-TTC and EnviroTox database tools, the 2017 OECD project group on uncoupling of oxidative phosphorylation (part of work of the OECD QSAR Toolbox Management group), and classification schemes for grouping chemicals (Sapounidou et al. 2020). This work has also resulted in advancing mode-of-action-based assessment factors used in predicted no-effect concentration (PNEC) calculations for risk assessments (Okonski et al. 2020).
Substances with endocrine-disrupting properties
In 2012, an analysis of assessments of existing substances from 2001 to 2012 was carried out by the Departments in order to document the extent to which potential endocrine-related effects were considered within the Existing Substances Program. The analysis showed that within the hazard characterizations, 20% of substances were considered to have endocrine-related effects. Further, of those determined to meet one or more criteria for being toxic under section 64 of CEPA 1999, endocrine-related effects were often a line of evidence in the risk assessment. Although this analysis is dated, it did illustrate the extent to which endocrine-related effects can be an important driver in risk assessments (see Enhanced consideration of endocrine-disrupting effects section).
Similarly, substances with endocrine disrupting potential accounted for about 25% of the 195 substances classified as being of high hazard according to information considered in ERC1.
At the Committee meeting on endocrine disrupting chemicals (EDCs) in July 2018, the Departments sought input as they work to advance and expand approaches for EDCs and potential EDCs, in particular for priority-setting and regulatory decision-making.
The Departments have identified a number of challenges and lessons learned pertaining to hazard characterization, including:
- Limited data availability/accessibility resulted in higher uncertainty in read-across as source chemicals with robust data were often difficult to identify.
- Requiring data generation to address areas of uncertainty could refine hazard and risk characterization.
- The Departments have only limited bioinformatics and cheminformatics tools to support the development of innovative new scientific tools and techniques (IT capacity).
Fate and exposure characterization
As with hazard characterization, there was a range of exposure data available for the CMP's existing substance priorities. Over all, only a small number of substances were data-rich, with biomonitoring and/or environmental monitoring information. As such, it was important that the Departments advance the use of predictive models to characterize exposure. The importance of predictive computational approaches to improve exposure science for risk-based assessment was recognized by the U.S. National Academies of Sciences, Engineering, and Medicine (NAS), in their 2017 publication, Using 21st Century Science to Improve Risk-Related Evaluations.
Multimedia fate and exposure modelling has been a critical data source for the assessment of new and existing substances since the late 1990s. Canada has been active in promoting, developing and supporting fate and exposure science globally, for example via the Canadian Environmental Modelling Centre (CEMC) at Trent University. Canada was a founding member of the OECD Multimedia Modelling Expert Group in 2002, which led to the development of the OECD guidance on the application of multimedia models for chemical evaluation (OECD 2004) and the OECD POPs Tool (Wegmann et al. 2009), a key in silico tool used for evaluating persistence and long-range transport and deposition of organic chemicals. More recently, Bonnell et al. (2018) provided a reflection on fate and exposure modelling in risk assessment, illustrated with experiences from the CMP. The recommendations from that paper have also helped inform future project development for the OECD's Working Party on Exposure Assessment (WPEA).
Environmental media
Measured levels of substances in environmental media are available for a very limited number of substances; when available, these are of limited temporal and spatial scope. Most assessments therefore rely on predictive models for estimating exposures in environmental media. Information on the total volumes manufactured, imported and used in Canada and on the nature and rates of releases is critical to support predictive exposure modelling. In the absence of specific data on Canadian emissions, OECD Emission Scenario Documents have often been used to predict potential releases in Canada.
For ecological assessments, environmental concentrations are generally derived based on local-scale scenarios as well as far-field scenarios via long-range transport and deposition modelling. Regional-scale scenarios are most typically considered for human health risk assessment, typically using the multimedia fugacity model ChemCAN and the air-quality dispersion model SCREEN3. More recently, in-house models have been developed to take into account the substance profile, including river dilution and consumer product releases of down-the-drain models.
Over all, there has been limited consideration of how emission rate, fate and transport quantitatively affect exposures to humans and non-human targets beyond the local scale; this was a topic at a recent Committee meeting on new approaches for integrating chemical fate and spatial and temporal scales in exposure assessment. The Committee considered that assessments of ecological exposure as well as far-field human exposure could be improved by the development, use and curating of up-to-date models and provided a road map for doing so. The Departments are considering this input, with a current focus on integration of fate and exposure modelling in ecological prioritization approaches (ERC1 and ERC2). The Departments recognize the need for continued refinement in deriving more realistic exposure scenarios, especially when characterizing combined exposure to multiple chemicals. Currently, for example, the PetroTox model developed by the Conservation of Clean Air and Water in Europe (CONCAWE) is used in the assessment of petroleum substances to calculate the removal of hydrocarbons during wastewater treatment and the resulting post-treatment releases. ECCC is using more probabilistic approaches in ecological exposure analyses. For example, the triarylmethanes assessment presents ranges and distributions for many of the input parameters, resulting in ranges in predicted environmental concentrations and risk quotients, as well as percentages of iterations that result in a risk quotient (RQ) of greater than one.
Consumer products
Assessment outcomes indicate that general population exposure is frequently higher from use of products available to consumers than from levels of substances in environmental media. As such, as the CMP evolved, approaches for deriving exposure estimates for these scenarios were strengthened.
The primary tool used by Canada for derivation of exposure estimates from use of consumer products is the ConsExpo model, developed by RIVM (the National Institute for Public Health and the Environment) in the Netherlands. Canada has worked closely with the Netherlands to enhance this model over time, with a particular focus on refining input parameters relevant to Canada. Using ConsExpo, HC can derive estimates of general population exposure for a large number of scenarios, such as the use of paint, cosmetics, cleaning products, and children toys, by both the dermal and inhalation routes of exposure. The most recent version of ConsExpo is a web version and was updated from the original model by RIVM in consultation with the Supervisory Committee of which HC is a member.
Additionally, over the course of the CMP, HC has developed exposure algorithms for many personal care product scenarios and has identified frequency of use and amount of product used parameters for approximately 76 exposure scenarios. As assessment outcomes showed that the predominant route of exposure was often dermal, in the absence of empirical data, approaches to predict dermal absorption were developed, such as the use of dermal absorption flux. HC contributed to the World Health Organization (WHO) International Programme on Chemical Safety (IPCS) Environmental Health Criteria on Dermal Exposure, which provides a comprehensive overview of analytical approaches to estimating dermal exposure, as well as models and tools.
Biomonitoring data - integrated exposure from multiple sources
In Canada, multiple biomonitoring initiatives have taken place during the last 15 years,Footnote 9 and use of biomonitoring data in risk assessments has evolved through the CMP, with biomonitoring-based risk assessment approaches outlined in 2 SciADs (see Assessment strategies section).
Additionally, biomonitoring results have:
- provided evidence of co-occurrence in cumulative risk assessment (Phthalate Substance Grouping)
- allowed for examination of exposure trends and patterns, for example by subpopulation for example, Selenium and its compounds assessment) or gender (for example, Triclosan assessment)
- supported examination of potential association with health outcomes from cross-sectional health surveys, prospective or retrospective (for example, lead and neurodevelopmental effects); this type of analysis supports consideration of a public health-based approach to chemicals management, as was deliberated upon at the November 2018 Committee meeting
The use of human biomonitoring data in risk assessments conducted under the CMP has been summarized by Zidek et al. (2017).
The Departments have identified a number of challenges and lessons learned pertaining to exposure characterization, including the following:
- Given the paucity of environmental monitoring data, it is critical that work continue to refine predictive approaches to estimate releases, fate and environmental exposures, including the development of probabilistic approaches in exposure assessments. There is a need for more refined, more realistic exposure scenarios that consider fate and spatial and temporal scales, including for drinking water.
- Notifications to the Departments on presence or concentration of substances in products (for example, cosmetics, natural health products, food additives, food packaging) and release to the environment (NPRI) are key sources of information for identifying potential risks. Identifying concentrations in other products and manufactured items (for example, textiles, toys, crafts, building materials and cookware)-most of which are imported-continues to be a challenge and can be among the greatest areas of uncertainty in characterizing exposures to consumers.
- The volumes of substances manufactured or imported can be a poor predictor for exposure to the general population (Bonnell et al. 2018), as use of consumer products drive exposure and low volumes may still result in high consumer product exposure.
- Estimated exposures to the general population from use of consumer products are typically higher than exposures from environmental media, with cosmetics, children's products, do-it-yourself products, textiles/foam, cleaning products and paints being top categories,Footnote 10 and there is a continued need to strengthen consumer exposure models.
- Given the number of dermal exposure scenarios, there is a need for higher-tier dermal exposure models and dermal absorption data.
- For products, children are typically the most highly exposed population when estimates are derived for them.
- For food, there is a need for models that can predict exposures from substances that may bioaccumulate in the food chain and for approaches to address additional sources of exposure (for example, crops, fish, meat, milk) in the absence of monitoring data.
- There is a need for models that can offer higher-tier inhalation exposure estimates from niche product uses, such as do-it-yourself products and cleaning products, as well as indoor air models.
- It is important to anticipate emerging sources of exposures to the environment and general population.
Risk characterization
Both qualitative and quantitative (for example, ecological risk quotients and human health margins of exposure) approaches for risk characterization are used in the CMP.
Qualitative approaches have been used for some streamlined approaches, including rapid screening. Additionally, in the Challenge Initiative, the Ministers considered that "where there is evidence that a substance for which the critical health effect is assumed to have no threshold-that is a mutagenic carcinogen-it is assumed that there is a probability of harm to human health at any level of exposure, and therefore indicates that the substance meets the criterion in paragraph 64(c)." (Canada 2006). Similarly, the Ministers considered that "evidence that a substance is both persistent and bioaccumulative (according to the Persistence and Bioaccumulation Regulations), when combined with evidence of toxicity and release into the environment can lead to harmful ecological impacts. This indicates that the substance meets the criterion in paragraph 64(a)." In such instances, the risk characterization was qualitative.
Throughout the CMP, as required under CEPA 1999, weight of evidence and precaution have been applied in risk characterization and decision-making. For the Challenge Initiative, the external advisory body, the CMP Challenge Advisory Panel (CAP), advised on the adequacy of application of weight of evidence and precaution for each assessment. In a report submitted to the Departments by the Industry Coordinating Group for CEPA in 2013, stakeholders noted the importance of application of an appropriate level of precaution in screening assessments, emphasizing, for example, the use of reasonable worst case over worst case as an exposure metric and suggested use of probabilistic models in predicting environmental exposure. They also recommended that assessments for high-hazard endpoints be informed by science to the extent possible.
Generally, both qualitative and quantitative lines of evidence support the risk characterization. Conclusions are based on consideration of multiple lines of evidence. For example, rather than a simple pass-fail decision based on risk quotients, ecological conclusions consider, when such information is available: temporal trends and spatial variations in manufacturing, use and release of a substance, which impact the extent and duration of exposure; persistence, which can lead to chronic exposure or increases in exposure over time; bioaccumulation and biomagnification potential, which can result in food-chain transfers and impacts on consumers; potential for long-range transport, which can result in presence in remote or sensitive habitats and exposure of sentinel species; and the nature and extent of potential toxic effects at all levels, from cell and tissue to individuals, populations and ecosystems. Ongoing developments will presumably lead to increasing quantification and integration of such factors.
Cumulative risk was not routinely considered in the CMP. A cumulative risk assessment was undertaken for the Phthalate Substance Grouping in the second phase of the CMP as scientific information showed that certain phthalates had a common mode of action and that there was a likelihood of co-occurrence of exposure to some phthalates. Additionally, the Departments consider assessments of metals to be cumulative assessments when the focus is on a common moiety.
Certain vulnerable populations were frequently considered in risk assessments conducted under the CMP, in particular infants, children and pregnant women; to some extent those living in the vicinity of industrial facilities; and, where available, data specific to Indigenous populations were incorporated into exposure characterizations.
Mid-point in the CMP, the Departments introduced a mechanism for flagging substances with high hazard characteristics that, though they were not of concern at the evaluated levels of exposure, could become a concern if levels of exposure to the environment or the general population were to increase.
Capturing and communicating uncertainty was the first topic of the Committee, and was raised in the context of the specific topics in most of the subsequent meetings. As a result of the Committee's suggestions, new tools were developed, and improvements have been made during the course of CMP. For example, transparency in the risk characterization section of assessments was improved with ECCC implementing a weight-of-evidence table to document how the various lines of evidence contributed to the assessment conclusion, and HC implementing a table to describe key uncertainties and their impacts on the conclusion. Work will continue to develop best practices in communicating conclusions and associated uncertainties. An example of this is ECCC's leadership, with ECHA, in development of the OECD document, Guiding Principles and Key Elements for Establishing a Weight of Evidence for Chemical Assessment, published in 2019.
Governance and engagement
Cross-functional, inter-departmental teams
Given the horizontal nature of the CMP across HC and ECCC, cross-functional inter-departmental teams, across regulatory programs, were established for assessment and management of substances. This ensured that assessments addressed exposures from multiple sources (for example, air, water, food, consumer products). As such, there was broad input to, and review of, screening assessments within and across Departments. These integrated teams helped with regulatory alignment and supported decision making under the Food and Drugs Act and the Canada Consumer Product Safety Act, as appropriate. Over all, the CMP had considerable success with the "one assessment, many uses" approach.
This cross-functional intra- and inter-departmental collaboration supported a more integrated risk assessment/risk management interface in order to act on substances of concern in a timely manner. Under the second phase of the PSL, environmental resource groups, which included experts from government, academia and industry, as well as program partners, were formed to support ecological assessments for each substance and helped promote integration and transition to risk management. More effective transition to risk management was further strengthened in the CMP as risk management consultation on substances of potential concern began with the release of the draft screening assessment (with publication of a risk management scope), followed by publication of the proposed risk management approach concurrent with release of the final screening assessment. These closer ties also supported engagement on cross-cutting issues, such as informed substitution and approaches for substances with high hazard characteristics.
External science input
Science advisory bodies have been a key source of external science input. At the outset of the CMP, the CAP was created to advise on the application of weight of evidence and precaution in assessments conducted as part of the Challenge Initiative. Moving into subsequent phases of the CMP, the Departments saw value in having a broader expertise represented on a science advisory body and established the CMP Science Committee-established in 2013, renewed in 2017, then extended by 6 months to 2021.
The Departments also receive external science input through external peer review and peer consultation on risk assessments and SciADs. In 2017, HC undertook a review of the external peer review process and updated certain elements to ensure best practices were in place, such as updated standard charge questions and addition of a mechanism for risk assessors to provide feedback on the value of the peer review input.
Technical input was also received through the public consultation periods for draft screening assessments and SciADs. Additionally, ad hoc technical workshops also played an important role in complex undertakings, as warranted (for example, azo grouping, phthalates grouping).
As well, the Departments bring forward science issues for input to various external fora, including the WHO, the OECD's Cooperative Chemicals Assessment Programme, Society of Environmental Toxicology and Chemistry (SETAC) global interest groups, committees of the Health and Environmental Sciences Institute (HESI), European Centre for Ecotoxicology and Toxicology of Chemicals (ECETOC) workshops, and American Chemistry Council (ACC) and European Chemical Industry Council (CEFIC) working groups.
Stakeholder engagement
The CMP Stakeholder Advisory Council (SAC) has been in place since 2008 and is a multi-stakeholder group created to provide stakeholders the opportunity to offer advice and input to the Departments on the implementation of the CMP, and to foster dialogue on issues pertaining to the CMP between stakeholders and government, and among different stakeholder groups. Additionally, throughout the CMP, the Departments have held many multi-stakeholder information sessions and meetings to obtain input at specific stages of the CMP.
Knowledge translation
To communicate technical information to a non-technical audience, at the outset of the CMP, substance-specific public summaries were prepared for each assessment, including information on risk management, as appropriate. A consultation on the content of the public summaries, which sought internal and external input, between 2014 and 2015, led to a rebranding of the document as an "information sheet" and the addition of more content, in particular the addition of a new "effects" section.
Beginning in 2015, the Departments introduced a series of risk assessment fact sheets, which provided information on risk assessment approaches and particular issues of interest to stakeholders and the general population (such as a fact sheet on the assessment of substances with endocrine-related properties). These documents, as well as the information sheets noted above, are written for an informed non-technical audience.
International
At an international policy level, the Strategic Approach to International Chemicals Management (SAICM), overseen by a multi-sectoral group, was adopted in February 2006 at the International Conference on Chemicals Management at the World Summit on Sustainable Development. SAICM set the goal of sound management of chemicals by 2020. The CMP was established, in part, to address Canada's commitments under SAICM.
The OECD's Cooperative Chemicals Assessment Programme has played an important role throughout the CMP, initially as a resource for hundreds of hazard assessments. Screening Information Data Set (SIDS) Initial Assessment Reports were available for approximately 220 CMP substances. Additionally, the OECD has coordinated the development of methodologies, technical approaches and guidance. Probably most significant for the CMP assessments, and for the application of NAM data for prioritization and risk assessment, has been the OECD QSAR Tool Box and Guidance on Grouping of Chemicals, as well as the ECCC/ECHA-authored OECD document on weight of evidence, which accommodates the use of NAM data for chemical evaluation (Canada Gazette 2006).
Both the WPHA and the Working Party on Exposure Assessment (WPEA) have been important fora for discussion of technical issues throughout the CMP. The WPHA has been an important forum for advancing collaboration on new and emerging approaches for hazard characterization throughout the CMP. Key activities have included the development of considerations for assessing the risks of combined exposures to multiple chemicals, identification of international best practices for identification of priorities within chemicals management systems, and the overview of concept and available guidance related to IATA. The focus of the WPEA evolved over the CMP from being predominantly ecologically focused to a balanced focus on ecological and human health exposure science. Key WPEA activities, which informed CMP assessments, included development of guidance on inclusion of children in risk assessments and, specifically, characterizing their exposure from mouthing products and manufactured items.
Canada is also an active participant in the OECD Working Party on Manufactured Nanomaterials (WPMN) and chairs its Steering Group on Risk Assessment and Regulatory Programme (SGAP), fora that are important in the development of common or compatible science and policy approaches to dealing with manufactured nanomaterials.
Enhanced partnerships over time with other risk assessment jurisdictions have also been important. Under the auspices of the Canada-U.S. RCC, the countries established the RCC U.S.-Canada Assessment Collaboration Framework, implemented through a rolling work plan, which has resulted in work sharing on common chemical priorities and methods. Another example is the collaboration with the Netherlands on consumer exposure modelling (see Exposure characterization section).
With ECHA and U.S. EPA, HC launched the Accelerating the Pace of Chemical Risk Assessment (APCRA) Initiative, an international effort focused on identifying and overcoming barriers to regulatory acceptance of new approach methodologies through collaborative government-to-government case studies (see Hazard characterization section).
Part II: Moving Forward: Considerations
"While Canada was one of the first countries to systematically start addressing the risks of legacy chemicals, the priority-setting exercise is now almost a decade old. It is essential for Canada to take into consideration new scientific information regarding chemicals and to support the continued development of modernized and harmonized approaches for the assessment and management of chemicals, ensuring a sustainable chemicals management programme beyond the 2020 goal." (Promoting Green and Inclusive Growth in Canada: Better Policies Series, OECD 2016)
From an international policy perspective, ongoing discussions to develop a renewed voluntary framework to replace SAICM have identified a series of aspirational objectives for chemicals management beyond 2020, including recognition that there is a need to identify, select and address "issues of concern," and that "comprehensive and sufficient knowledge, data, information and awareness are generated, available and accessible to all to enable informed decisions and actions."
Global trends outlined in the United Nations Environment Programme (UNEP) Global Chemicals Outlook II provide a broad context for chemicals management regimes. These trends include ongoing and significant growth in the number of new chemicals being identified yearly, increasingly complex global supply chain and an increase in chemical-intensive industry sectors.
In addition to the challenges and lessons learned identified through the evolution of CMP from 2006 to 2020 (see Part I), a number of external inputs are key to informing future directions of the program. These include ongoing input from the 2 CMP advisory bodies, a series of multi-stakeholder public consultations, as well as the recommendations from the House of Commons Standing Committee on Environment and Sustainable Development on CEPA 1999 (ENVI). Additionally, evolution of risk assessment programs in other jurisdictions (EU, U.S.) and collaborations at the supranational level (especially those led by the OECD's Environment Directorate), as well as government-to-government initiatives (such as the APCRA Initiative), are informative. Technically, a series of foundational publications on risk assessment modernization are also key (see Risk assessment modernization section).
Several key considerations moving forward are summarized below.
Ecological public health approach: A different orientation
"While recognizing that the approach must evolve rather than being adopted as a single change, an opportunity exists in Canada to explore such an approach given the existing expertise in ecosystem and human health population studies, particularly in academia and government, as well as ongoing communication and partnerships between these communities." (CMP Science Committee Report - Public Health Approach November 28-29, 2018)
With advances in science, an ecological public health-based approach to chemicals management is closer to being possible. A number of jurisdictions and organizations are currently exploring public health approaches to chemicals (for example, "One Health" and similar concepts). The Committee saw merit in this more holistic approach based on multiple determinants of health, though outlined many challenges, including in coordinating work across multiple groups and identifying appropriate long-term cohort studies. The Committee saw value in recognizing the transdisciplinary opportunity that exists in Canada to explore such an approach, given the existing expertise in ecosystem and human health population studies. The Committee endorsed a number of existing and developing elements that would help to realize an ecological public health approach to chemicals management. They provided their views on foundational elements that would be required to develop a roadmap to advance such an approach. The Committee provided workflows and key elements of a roadmap, as well as criteria for selecting case studies. This input was a driver in the establishment of a new Office of Environmental Health at Health Canada formed partly to help move this initiative forward.
A public health approach aligns with key areas of focus that the Government of Canada has made a commitment to in its follow-up report to ENVI. Specifically, an ecological public health approach could provide a lens from which to focus efforts, as required, on priority areas such as vulnerable populations, hot spots and cumulative risk.
At a more technical level, an ecological public health approach is aligned with understanding toxicity pathways for chemicals and adverse outcome pathways, as well as integrating exposure and toxicological sciences using the aggregate exposure pathways concept (for example, Teeguarden et al. 2016; Tan et al. 2018), key areas of risk assessment modernization. This would include approaches for substances that affect the endocrine system and for substances that cause genetic damage, the effects of which can become transmissible in human and ecological populations, which is thought to contribute to certain disease outcomes.
Risk assessment modernization
"In advancing its vision for chemicals management post-2020, the Government of Canada continues working closely with the international regulatory and research communities to build confidence and harmonize the expanded application of emerging technologies in chemical risk assessment." (Krewski et al. 2020)
Internationally, the broader context for risk assessment modernization is set forth in a series of foundational publications, including U.S. National Research Council (NRC) (2007), Council of Canadian Academies (2012), U.S. NRC (2012) and U.S. NAS (2017). Recently, Krewski et al. (2020) provided an update on progress and future perspectives. A driver internationally, as well as in Canada,Footnote 11 is the reduction or elimination of animal testing.
Consistent with this, the Departments are continuing to focus on a fit-for-purpose approach to risk assessment, with progressive integration of NAMs. The Committee has noted that the Risk Assessment Toolbox (see Figure 5) appeared to be sufficiently robust in concept and that, provided the associated governance and learning processes remain built into it, it will serve Canada well in the post-2020 period. A focus is expected on continuing to advance broad-based approaches to prioritization and assessment (Type 2 assessments) and on supporting hazard and risk characterization for substances and groupings (Type 3 assessments).
It is also recognized that a flexible, adaptive and innovative approach to risk assessment needs to be explored for certain groups of substances (for example, pharmaceuticals in the environment) that do not necessarily need to follow the traditional CEPA 1999 cycle (namely, risk assessment followed by risk management).
The following are 2 examples of domestic modernization initiatives that are illustrative of the Departments' applied focus.
Firstly, as noted previously, ECCC has developed version 2 of ERC (Bonnell et al. 2018, Bonnell 2020), which is expected to become an important component for post-2020 ecological prioritization and scoping/problem formulation of organic substances, as well as supporting assessment. ERC2 represents a considerable expansion of several concepts first introduced in ERC1 and considered OECD IATA case study feedback on ERC1. The ERC2 system was developed to identify "chemicals of concern" from approximately 12,000 organic chemicals on the DSL not categorized as ecological priorities in 2006. ERC2 was built using the adverse outcome pathway (AOP) concept for gathering and relating toxicological evidence, while also evaluating the likelihood of multimedia exposure (including biota) at varying temporal and spatial scales. ERC2 is built on an IATA consensus approach integrating in silico, in chemico, in vitro and in vivo data in hazard and exposure profiles to provide weighted evidence (via confidence scoring) for prioritizing the risk of chemicals in the environment. The system contains approximately 10 million to 20 million data points for numerous endpoints and properties used in ecological risk assessment. ERC2's main outputs include hazard, exposure and risk classifications, and confidence and severity scores for these classifications. ERC2 can provide information to help answer questions posed post-2020, such as EDC potential, cumulative risk, chemicals of global concern, alternatives, and priorities for monitoring and research. Elements of ERC2 were presented to the Committee at previous meetings (for example, June 2019 meeting on endocrine disrupting chemicals).
Secondly, HC is developing the use of the Bioactivity Exposure Ratio, an approach that considers high-throughput in vitro bioactivity data together with high-throughput toxicokinetic modelling to derive an in vitro-based point of departure. This bioactivity-based point of departure can serve as a protective surrogate in the absence of traditional hazard data. When compared with exposure estimates to establish a bioactivity exposure ratio, it is envisioned that the approach could have applications in both priority-setting and assessment. The foundational proof-of-concept analysis demonstrating the utility of in vitro bioactivity as a lower-bound estimate of in vivo adverse effect levels was conducted through an international collaborative case study under the APCRA Initiative. This showed that, in approximately 90% of cases, for the oral route of exposure, NAMs can provide a conservative point of departure as protective or more compared with classical in vivo data (Friedman et al. 2020).
The Departments plan to publish both approaches for public comment in 2021.
As noted in Part I, the Departments, with partners internationally, continue to advance risk assessment modernization, in part through case studies, as part of the APCRA Initiative and the OECD IATA Case Studies Project.
One longer-term APCRA case study that the Departments are advancing collaboratively involves various levels of data generation and data analyses to address the question of whether a NAM battery can be used to derive a health protective point of departure and also be used to identify qualitative hazard flags that are comparable with the outcomes from in vivo repeat-dose toxicity studies used in traditional hazard characterization. Using a test battery that includes in silico consensus QSAR models, targeted in vitro high-throughput screening assays, and multi-omics approaches, the goal is to evaluate a set of chemicals derived from multiple national inventories that have limited toxicological data and significant potential for exposure to test the hypothesis that NAM may be used with confidence for hazard identification and POD derivation.
Internationally, the U.S. EPA in 2018 published the Strategic Plan to Promote the Development and Implementation of Alternative Test Methods with the TSCA Program. The EPA's long-term goal is to move towards making decisions under the Toxic Substances Control Act with NAMs, in order to reduce, refine or replace vertebrate animal testing. In 2020, the U.S. EPA released the NAM Work Plan. The work plan describes an agency-wide roadmap to reduce animal testing by 30% by 2025 and eliminate animal testing by 2035. The document lays out the roadmap and objectives, including establishing scientific confidence in NAMs and demonstrating application to regulatory decisions. As well, Thomas et al. (2020) provides The Next Generation Blueprint of Computational Toxicology at the U.S. EPA.
The European Commission, in its Chemicals Strategy for Sustainability Towards a Toxic-Free Environment (2020), commits to innovation in chemical risk assessment - advanced tools, methods and models, and data analysis capacities. ECHA (2020), in its most recent report to the European Commission on the use of alternatives to testing on animals for the REACH Regulation, shows progress on use of alternatives to testing on animals under the REACH Regulation.
Moving forward, a close partnership between the research and risk assessment communities across the 2 Departments will be key, as integration of new approach methodologies into risk assessment is a joint endeavour.
Priority-setting
Post-categorization, it is important that the priority-setting mechanism functions in an efficient, timely and proactive manner. A plan outlining risk assessment and other priorities is key to moving forward.
As outlined in Part I, the IRAP approach provides a flexible, responsive mechanism to identify priorities; one that can encompass a broad array of hazard and exposure indicators, as distinct from the more prescriptive criteria of categorization. Although the properties of persistence, bioaccumulation and inherent toxicity remain important, earlier indicators of potential concern, including NAM results, are expected to support identification of potential priorities.
In the follow-Up report to ENVI, the Government committed to continuing to prioritize risk assessment activity in accordance with the IRAP approach, including consideration of feeders such as emerging science, international activities, data-gathering and research, and to provide the results of the approach in the annual CEPA report to Parliament.
While assessment work under the CMP has focused on substances on the DSL, future assessment activity could encompass classes, mixtures and complex effluents and emissions. Some such substances have previously been assessed under the PSL (for example, chlorinated wastewater effluents, releases from primary and secondary copper smelters and copper refineries) and merit consideration moving forward.
The need for a reassessment mechanism has been noted in various fora (including at the 2016 Committee meeting) and in audits and evaluations, as well as in the ENVI report. Reassessment, as well as performance measurement, would factor into future priority-setting exercises.
At the November 2016 meeting, the Committee concluded that, although NAMs are still evolving, they are mature enough, in many cases, to begin to have an application in priority-setting and endorsed their use for that purpose. At the same meeting, the Committee also supported the use of ECCC's ERC approach for organic substances and HC's TTC in priority-setting.
The addition of a further scoping/problem formulation step to priority-setting (as noted in Part I) provides a mechanism through which computational tools and NAMs could be used to define substance groupings, describe the data landscape, consider emerging concerns such as EDCs and provide a forum to more effectively characterize exposures of potential concern (including vulnerable populations and cumulative risk). Importantly, this scoping/problem formulation step would also provide an opportunity to engage research scientists and stakeholders early in identifying and addressing data needs, and would inform scheduling of risk assessment activity. Conversely, a scoping/problem formulation step could also allow for de-prioritization of substances as warranted and could allow for identification of more suitable approaches to address a substance.
In 2018, the U.S. EPA published its approach for identifying potential candidate chemicals for prioritization. The U.S. EPA proposes a longer-term approach, whereby chemicals on the Toxic Substances Control Act (TSCA) active inventory are "binned" into pools that can inform potential prioritization based on risk-based data and information availability. The approach would include computational and novel data (for example, ToxCast data), emerging approaches and tools, such as the BER, and new approach methodologies to carry out binning. In this approach, susceptible subpopulations are considered in derivation of a binning score, specifically the potential for exposure to children. Canada could use many of the same emerging data and tools.
A survey of prioritization approaches in regulatory jurisdictions, including best practices for identification of priorities within various chemicals management systems, has recently been published by the OECD. These best practices are being considered by the Departments.
Lastly, traditional approaches for prioritization have relied on manual curation of chemical lists in order to identify emerging priority substances. In order to increase efficiencies in this aspect, it is proposed that a greater emphasis be placed on the use of computational tools and predictive models for the initial screening of the chemical space, including use of approaches outlined in a series of SciADs (see Assessment strategies section). It is thought that the combination of computational screening followed by expert manual curation would be a streamlined way to produce a comprehensive and accurate list of emerging existing priority substances. The importance of automated data collection was emphasized by the Committee at the November 2016 meeting.
Information gathering
Enhanced curation of the DSL to better track the commercial status of the chemicals landscape in Canada will be important to robust priority-setting and focusing resources on priorities for action. Enabling the program to monitor commercial status, volume and uses of substances of potential concern over time (rather than simply a snapshot in time) will also be important to future priority-setting mechanisms as well as performance measurement activities.
More generally, introduction of a further scoping/problem formulation step in the priority-setting cycle will highlight key uncertainties and data needs associated with planned risk assessment initiatives. These gaps could be addressed through earlier engagement of industry stakeholders, continuing with the tiered approach to information gathering, from voluntary to mandatory.
Enhanced information gathering will need to be sufficiently flexible to collect information related to emerging areas of focus, including vulnerable populations and cumulative risk. For instance, although some mandatory surveys under CEPA 1999 include a flag for children's products, data collection on uses by other vulnerable populations could be incorporated. As another example, refinement of application codes (that is, product uses) of interest could aid in determining potential combined exposures to multiple chemicals.
As well, the Departments will need to continue to gather information on substances identified in CMP assessments as having high hazard characteristics of concern if exposures were to increase.
Enhanced partnerships, collaborations and data sharing with other government departments (for example, Canada Border Services Agency, Statistics Canada) could also support exposure characterization and identification of emerging issues. CMP SAC members noted the strategic value in establishing global partnerships that could help close data gaps and unpack supply chain transparency.
Vulnerable populations, including workers
"[. . .]We had as the purpose of this meeting to begin to look at chemicals management through a vulnerabilities lens. In particular, if we can look at chemicals management through the lens of the most vulnerable segments of our population, I think we can develop a much stronger chemicals management plan for Canada." (Joshua McNeely, member, CMP SAC when introducing the Vulnerable Populations Panel at the May 2018 CMP SAC meeting.)
The Departments are looking to strengthen protection of vulnerable populations. Although CMP screening assessments have routinely addressed infants, children and pregnant women; to some extent, those living in the vicinity of industrial facilities; and, where available, data specific to Indigenous populations. Those not specifically addressed include people living in substandard housing, new Canadians, people with pre-existing conditions and workers.
In 2018, the Departments launched an online consultation on a proposed definition of vulnerable populations in the context of federal chemicals management activities and comments have been compiled. This was considered a first step towards a policy framework focusing on enhancing the protection of vulnerable populations post-2020.
At the May 2018 meeting of the CMP SAC, a special ad hoc panel was convened with the objective of informing CMP SAC members on how chemical exposures may affect vulnerable populations, with a view to strengthening protection. Points raised included the need for a broader definition of vulnerable populations so that it reflects the full spectrum of vulnerabilities (biological but also important socio-economic factors), as well as the need for multidisciplinary approaches that include science but also lived experiences and other considerations. Additional discussions took place at the November 2018 CMP SAC meeting.
As noted in the government follow-up report to ENVI, the Departments are committed to continuously improving the consideration of vulnerable populations in the assessment and management of chemicals. Several additional ENVI recommendations intersect with vulnerable populations, including those on EDCs and those on cumulative risk.
There is an opportunity to enhance biomonitoring programs to better encompass vulnerable populations. Small, more targeted biomonitoring studies are envisioned in order to examine specific populations with a view to strengthening protection of all Canadians from the harmful effect of chemicals.
Internationally, the European Commission, in its Chemicals Strategy for Sustainability Towards a Toxic-Free Environment (2020), identifies vulnerable population groups-such as children, pregnant women and elderly people-as particularly sensitive to certain chemicals, and are advancing a preventative approach focused on the most harmful chemicals. Canada looks to benefit from experiences of other jurisdictions as it elaborates its approach for vulnerable populations.
One area that has frequently been raised in the context of vulnerable populations is workers. HC is working with provincial and territorial governments to determine if there is greater role HC could take in supporting provinces and territories in their mandates related to workplace exposures to chemicals. In 2019, a consultation on an integrated strategy for the protection of Canadian workers from exposure to chemicals took place and feedback has been compiled. A focus on occupational exposures to chemicals was welcomed by the CMP SAC at the November 2018 meeting, and the Committee provided advice on this topic at its February 2020 meeting (committee report currently being finalized). Federal chemicals management programs in the U.S. and the EU already have responsibilities with respect to workplace chemicals exposures.
Finally, the vulnerable populations policy is closely aligned with the Government of Canada's commitments for Gender-based Analysis Plus (GBA+). Efforts are under way to develop more systematic tools for chemicals management work and to develop tools and training to enhance GBA+. The program will also start to explore ways to disaggregate data so that GBA+ can be undertaken.
Cumulative risk
"The Committee continues to be concerned that the cumulative effects of exposure to substances are not being adequately accounted for in assessments." (ENVI Committee Report. Healthy Environment, Healthy Canadians, Healthy Economy: Strengthening the CEPA, 1999. House of Commons, Canada)
Within the Existing Substances Risk Assessment Program, cumulative risk is defined as the risk associated with the combined exposure to multiple chemicals. Assessment of mixtures has been an outstanding challenge in the field of regulatory toxicology for many years. In real-world scenarios, all environmental exposures to chemicals involve complex mixtures and individuals are, similarly, exposed to an array of chemicals through their lifetimes, from sources including environmental media, food, consumer products and manufactured items. The sheer number of combinations of chemicals has been daunting. The scientific tools and approaches for tackling the complexity of chemical mixtures and associated effects have evolved since the inception of the CMP. A challenge remains as to how to holistically manage mixtures in a regulatory context.
Several ENVI recommendations touch on cumulative risk and, in its response, the Government has recognized the benefit of better assessing the risks from real-life exposures to a range of chemicals and acknowledged the complexity of the issue.
In 2015, the Departments sought advice on cumulative risk assessment from the Committee. The Committee emphasized the importance of an initial and carefully conducted problem formulation step and noted other considerations, such as circumstances in which dose addition is not appropriate.
Characterizing cumulative risk will be a challenge for the Departments moving forward, and advancements will need to proceed in a step-wise manner. Through pilot projects, ECCC plans to examine the environmental impacts from chemical mixtures in selected areas of Canada. A scientific approach is proposed where mixtures will progress from their identification, prioritization, assessment (for example, investigation of toxicity drivers) and management (for example, investigation of solutions) to abatement. Real-world harmful effects observed in living organisms will guide the identification and characterization of mixtures as well as the measure of success of implemented solutions.
Environmental and biological monitoring is also a key tool for identifying co-occurrence to multiple chemicals and will be an important resource for identifying candidates for cumulative risk assessment. As well, rather than specifically targeting specific chemicals in environmental samples, consideration is being given to using instrumentation (such as Orbitrap mass spectrometers) for non-targeted screening of substances in environmental samples to help identify possible future priorities for assessment, including mixtures.
The EC, in its Chemicals Strategy for Sustainability Towards a Toxic-Free Environment, have identified actions to protect people and the environment from the combination effects of chemicals. Specific actions under the REACH Regulation relate to introduction of a mixture assessment factor and, in other relevant legislation, relate to introducing or reinforcing provisions to take account of the combination effects of chemicals. The strategy notes that a scientific consensus is emerging that the effects of chemical mixtures need to be taken into account and integrated more generally into chemical risk assessments.
It will be important to work collaboratively with international jurisdictions facing the challenge of assessing cumulative risk. To that end, the Departments recently played a leadership role in the development of an OECD guidance document on the considerations for assessment of combined exposure to multiple chemicals. This was informed by HC's Pest Management Regulatory Agency's Cumulative Risk Assessment Framework.
Enhanced consideration of endocrine-disrupting effects
"In the post-2020 timeframe, the SC suggests a future evaluation of the entire approach and a review of "lessons" learned from EDC (and other) experiences. This "big and bold" thinking is especially needed to address the challenges faced [by] EDCs (and other mechanisms of toxicity of concern) from multidisciplinary dimensions, including uncertainties, exposure to chemical mixtures, vulnerable populations, and risk communication. Taking a big and bold approach would enable the evolution from a chemical-by-chemical approach (which has been the focus up to now) towards more disease-based scenarios that are more indicative of real-world exposures". (CMP Science Committee Report, July 18-19, 2018 Meeting)
The research, monitoring/surveillance and risk assessment communities across the 2 Departments continue to commit significant resources to EDCs. Regulators and researchers are active collaborators in the international community, which is working to advance and adapt novel approaches for testing and assessment of endocrine-related effects. In the Government follow-up to the ENVI report, the Government committed to improving its ability to consider endocrine-disrupting effects in its risk assessments and is committed to continuously improving its ability to keep pace with the latest scientific developments.
From a risk assessment perspective, 3 examples of work in this area are:
- HC is expanding upon an OECD IATA Project case study carried out with the U.S. EPA (Webster et al. 2019), by using available data from across the levels of the OECD Conceptual Framework to identify clusters of substances with the potential for estrogen receptor activity. This approach could be used in the future to identify priorities for assessment based on other known endocrine modalities.
- As part of the APCRA Initiative, Canada is active on a case study to develop an inventory of validated NAMs to identify endocrine disruptors.
- The approach for identifying endocrine potential has been considerably more developed in ERC2 than ERC1. For example, ERC2 includes consensus-driven confidence scoring of endocrine-disrupting potential in ecological receptors, which is of utility for both priority-setting and assessment.
The Committee meeting on EDCs in July 2018 focused on the scientific considerations needed to guide the advancement of a potential program of work on EDCs in Canada that builds on international and best practices and benefits from new and emerging methodologies and data. The Committee report included a number of recommendations regarding assays and methods of utility for the New and Existing Substances Programs, and offered a workflow consisting of a tiered testing and evaluation framework.
The Committee noted that non-EATS (estrogen, androgen, thyroid and steroidogenesis) pathways require additional development. Intersections with vulnerable populations and cumulative risk were noted. The Committee discussed using an adverse outcome pathway ramework and suggested developing an EDC-TTC for both human and ecological health. In response to a charge question, the Committee provided 6 recommendations for where future Government efforts would be best placed in areas of research, risk assessment and risk management for EDCs. This input is informing deliberations on future directions.
In the EU, the first substance listed as an endocrine disruptor was added to the REACH List of Substances of Very High Concern in 2011, and EU leadership in this area is recognized. In the EU strategy document, it is noted that EDCs require specific attention due to risk to human health and wildlife, and the document has a number of commitments regarding EDCs, including establishing legally binding hazard identification of endocrine disrupters, ensuring a ban in endocrine disruptors in consumer products, and accelerating the development and uptake of methods to generate information on endocrine disruptors through screening and testing of substances.
The U.S. EPA has had a robust program in place for EDCs-the Endocrine Disruptor Screening Program (EDSP)-for a number of years. Over time, the EDSP has developed and used validated methods for screening and testing chemicals to identify potential EDCs, determine adverse effects, dose-response, assess risk and ultimately manage risk under current laws. The U.S. EPA EDSP for the 21st Century (EDSP21) Work Plan lays out the work plan for the incorporation of in silico models and in vitro high-throughput assays in the EDSP for prioritization and screening.
Given significant activity in the EU and the U.S., Canada will benefit from collaboration with these regulators.
References
Armitage J, Arnot JA, Bonnell M. 2018. Comparing mode of action (MOA) classification using body residues, membrane concentrations and chemical activity for chemical prioritization. Abstracts, 39th Annual Meeting, Society of Environmental Toxicology and Chemistry Sacramento, CA, USA, November 4-8: 420-421.
Bonnell M. 2020. Collecting and Weighing Evidence for Identifying Chemicals of Concern Using Version 2.0 of the Ecological Risk Classification Approach. https://risk21.org/wp-content/uploads/2020/03/Bonnell-RISK21-Summit-2020.pdf
Bonnell M, Inglis C, Jagla C, Prindiville J, Shore B. 2018. A Computational Approach for the Ecological Prioritization of Organic Chemicals in Canada: ERC 2.0. Society of Environmental Toxicology and Chemistry 39th Annual Meeting. Sacramento, California. November. https://cdn.ymaws.com/www.setac.org/resource/resmgr/abstract_books/sacramento-abstract-book.pdf
Canada, Dept. of the Environment. 2006. Notice of intent to develop and implement measures to assess and manage the risks posed by certain substances to the health of Canadians and their environment. Canada Gazette Part I. December 9, 2006:4109-4116. https://gazette.gc.ca/rp-pr/p1/2006/2006-12-09/pdf/g1-14049.pdf
Connors KA, Beasley A, Barron MG, Belanger SE, Bonnell M, Brill JL, de Zwart D, Kienzler A, Krailler J, Otter R, et al. 2019. Creation of a curated aquatic toxicology database: EnviroTox. Environ. Toxicol Chem 38:1062-1073. https://pubmed.ncbi.nlm.nih.gov/30714190/
Environment Canada. 2003. Guidance manual for the categorization of organic and inorganic substances on Canada's Domestic Substances List. Determining persistence, bioaccumulation potential, and inherent toxicity to non-human organisms. Existing Substances Branch, Environment Canada, Gatineau, Quebec.
Environment Canada. 2005a. Approach for the ecological categorization of substances on the Domestic Substances List: Organometallics. Existing Substances Branch, Environment Canada, Gatineau, Quebec.
Environment Canada. 2005b. Approach for the ecological categorization of substances on the Domestic Substances List: Polymers. Existing Substances Branch, Environment Canada, Gatineau, Quebec.
Environment Canada. 2005c. Approach for the ecological categorization of substances on the Domestic Substances List: Unknown or variable composition complex reaction products and biological materials (UVCBs). Existing Substances Branch, Environment Canada, Gatineau, Quebec.
Friedman KP, Gagne M, Loo L, Karamertzanis P, Netzeva T, Sobanski T, Franzosa JA, Richard AM, Lougee RR, Gissi A, et al. 2020. Utility of In Vitro Bioactivity as a Lower Bound Estimate of In Vivo Adverse Effect Levels and in Risk-Based Prioritization, Toxicological Sciences, 173(1):202-225. https://doi.org/10.1093/toxsci/kfz201
Health Canada. 2009. Final Integrated Framework for the Health-related Components of Categorization of the DSL under CEPA.
Kavlock, RJ, Bahadori T, Barton-Maclaren TS, Gwinn, MR, Rasenberg M, Thomas RS. 2018. Accelerating the Pace of Chemical Risk Assessment. Chem Res Toxicol. 31(5):287-290. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6666390/
Kienzler A, Connors KA, Bonnell M, Barron MG, Beasley A, Inglis CG, Norberg‐King TJ, Martin T, Sanderson H, Vallotton N, Wilson P, Embry MR. 2019. Mode of Action Classifications in the EnviroTox Database: Development and Implementation of a Consensus MOA Classification. Environ Toxicol Chem, 38:2294-2304. https://doi.org/10.1002/etc.4531
Krewski D, Andersen ME, Tyshenko MG, Krishnan K, Hartung T, Boekelheide K, Wambaugh JF, Jones D, Whelan M, Thomas R, et al. 2020. Toxicity testing in the 21st century: progress in the past decade and future perspectives. Archives of Toxicology 94:1-58. https://doi.org/10.1007/s00204-019-02613-4
[OECD] Organisation for Economic Co-operation and Development. 2004. Guidance document on the use of multimedia models for estimating overall environmental persistence and long-range transport. ENV/JM/MONO (2004)5. http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=ENV/JM/MONO(2004)5&doclanguage=en
[OECD] Organisation for Economic Co-operation and Development. 2014. Guidance on Grouping of Chemicals: Second Edition. Environment Directorate (Series on Testing and Assessment No. 194). Report No.: ENV/JM/MONO (2014)4, JT03356214. Paris (FR) https://www.oecd-ilibrary.org/docserver/9789264274679-en.pdf?expires=1608490863&id=id&accname=guest&checksum=8BFD486AEAD3064FE0A75FA4CD2D9822
[OECD] Organisation for Economic Co-operation and Development. 2017. Prioritisation of chemicals using the integrated approaches for testing and assessment (IATA)-based ecological risk classification. OECD Environment, Health and Safety Publications Series on Testing and Assessment. No. 291. http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=ENV/JM/MONO(2018)27&docLanguage=En
Okonski A, MacDonald DB, Potter K, Bonnell M. 2020. Deriving predicted no-effect concentrations (PNECs) using a novel assessment factor method. Human and Environmental Risk Assessment. Accepted 15 Dec. 2020.
[RCC] Regulatory Cooperation Council Nanotechnology Initiative. 2014. Final Report of the Work Element 3: Risk Assessment / Risk Management.
Sapounidou M, Ebbrell DJ, Bonnell M, Campos B, Firman WB, Gutsell S, Hodges G, Roberts J, Cronin MTD. 2020. Development of an Enhanced Mechanistically-Driven Mode of Action Classification Scheme for Adverse Effects in Environmental Species. (Environ. Sci. and Technol. - Accepted Jan 8th 2021).
Tan YM, Leonard JA, Edwards S, Teeguarden J, Paini A, Egeghy P. 2018. Aggregate exposure pathways in support of risk assessment, Current Opinion in Toxicology, 9: 8-13, ISSN 2468-2020. https://www.sciencedirect.com/science/article/abs/pii/S2468202017301432
Teeguarden JG, Tan YM, Edwards SW, Leonard JA, Anderson KA, Corley RA, Kile ML, Simonich SM, Stone D, Tanguay RL, et al. 2016. Completing the link between exposure science and toxicology for improved environmental health decision making: The Aggregate Exposure Pathway framework, Environ Sci Technol 50:4579-4586. https://pubmed.ncbi.nlm.nih.gov/26759916/
U.S. National Research Council. 2007. Toxicity Testing in the 21st Century: A Vision and a Strategy. Washington, DC: The National Academies Press. https://www.nap.edu/catalog/11970/toxicity-testing-in-the-21st-century-a-vision-and-a
U.S. National Research Council. 2012. Exposure Science in the 21st Century: A Vision and a Strategy. https://www.nap.edu/read/13507/chapter/1
Webster F, Gagné M, Patlewicz G, Pradeep P, Trefiak N, Judson RS, Barton-Maclaren TS. 2019. Predicting estrogen receptor activation by a group of substituted phenols: An integrated approach to testing and assessment case study. Regulatory Toxicology and Pharmacology, 106:278-291. https://www.sciencedirect.com/science/article/pii/S027323001930145X
Wegmann F, Cavin L, MacLeod M, Scheringer M, Hungerbühler K. 2009. The OECD software tool for screening chemicals for persistence and long-range transport potential. Environmental Modelling & Software 24(2): 228-237. https://www.researchgate.net/publication/223584958_The_OECD_Software_Tool_for_Screening_Chemicals_for_Persistence_and_Long-Range_Transport_Potential
Zidek A, Griffiths A, Gutzman D. 2018. Fate and exposure modelling in regulatory chemical evaluation: new directions from retrospection. Environ Sci: Processes Impacts, 20:20-31 https://pubs.rsc.org/en/content/articlelanding/2018/em/c7em00510e#!divAbstract
Zidek A, Macey K, MacKinnon L, Patel M, Poddalgoda D, Zhang Y. 2017. A review of human biomonitoring data used in regulatory risk assessment under Canada's Chemicals Management Program. Int Journal of Hygiene and Env Health 220:167-178. https://www.sciencedirect.com/science/article/abs/pii/S1438463916302012
Appendix A: Charge questions
- What elements of our current risk assessment program are working well?
- What areas of enhancement should be considered for a modernized risk assessment program?
- To access external scientific expertise in the future, what engagement mechanisms are most suitable and for what types of topics?