Pest Management Regulatory Agency Annual Report 2019–2020

Download the alternative format
(PDF format, 2.30 MB, 45 pages)
Organization: Health Canada
Date published: 2021-03-18
Message from the Executive Director
I am pleased to present to you the Pest Management Regulatory Agency's (PMRA) Annual Report for 2019–2020.
April 1, 2020, marked 25 years since the PMRA was established, bringing together scientists and regulatory experts from various federal departments into one cohesive organization, dedicated to making timely science-based decisions to support the safe and sustainable use of pesticide products in Canada.
This year also marked the onset of the COVID-19 pandemic, leading to the abrupt dispersal of our workforce to the safety of their homes. Despite having to be apart, PMRA never stopped working as a team.
A key focus of the PMRA in the last 2 years has been a major transformation of our pesticides program to address workload and sustainability issues. In 2019, we engaged broadly with our stakeholders about the pain points and challenges in our processes. This input inspired a shift in focus from changes to re-evaluation, to the development of a pesticide lifecycle model based on timely and transparent information sharing between PMRA and stakeholders over the entire duration of a pesticide's registration.
In this report, you will see many other examples of how PMRA is able to effect, respond to, and embrace change. For example, in 2019–2020 our scientists worked collaboratively with international partners on examining new and emerging technologies such as RNAi pesticides and drones. While these technologies may have many benefits, PMRA scientists work to carefully identify and assess their potentially unique health and environmental risks, and their regulatory impacts.
I am proud of the important work that PMRA continues to do under exceptional circumstances, and I look forward to seeing the positive impacts of PMRA's 2019–2020 accomplishments in the years to come.
Peter Brander
Executive Director
Pest Management Regulatory Agency
Table of Contents
- 2019-2020 PMRA performance highlights
- About the Pest Management Regulatory Agency
- What are pesticides?
- New pesticide registrations
- Regulation of pesticides on the market
- Keeping pace with change
- Program renewal
- Evaluating new technologies
- Reducing product exposure and misuse
- Remote piloted aircraft systems (drones) for pesticide application
- Water Monitoring
- Vegetative filter strips
- International scientific and regulatory cooperation
- Regulatory modernization
- Labelling amendments
- Formulants and contaminants of health and environmental concern
- Targeted regulatory review of the agri-food and aquaculture sector
- Annual Regulatory Modernization Bill (ARMB)
- Targeted regulatory review of international standards, digitalization and technology neutrality, and clean technology
- Pest Control Products Regulations review
- Stakeholder relations and outreach communications
- Financial profile
- Appendix
- Appendix Table 1. Product submission categories and service standards for pre-market applications
- Appendix Table 2. New active ingredients registered in 2019–2020
- Appendix Figure 1. Number of new active ingredients registered by PMRA from April 1, 2010, to March 31, 2020
- Appendix Figure 2. Performance against review timelines for category A, B, C, D, E, F, L and P submissions completed from April 1, 2017, to March 31, 2020
- Appendix Table 3. Re-evaluation/special review documents published in 2019–2020
2019-2020 PMRA performance highlights
- Registration of 12 new active ingredients, 25 new end-use products, 62 generic products, 299 new minor uses and 7 emergency registrations
- 4 joint reviews
- 16 re-evaluations completed and 10 proposed decisions for re-evaluations completed
- 1 Special Review completed and 3 proposed decisions for Special Reviews
- Received 1672 pesticide incident reports and 42 scientific studies
- 1526 inspections, 1695 violations, 1606 enforcement actions addressing the violations, 257 compliance promotion activities
About the Pest Management Regulatory Agency
The Pest Management Regulatory Agency (PMRA) is the branch of Health Canada responsible for regulating pesticides under the authority of the Pest Control Products Act. PMRA's primary mandate is to prevent unacceptable risks to Canadians and the environment from the use of these products.
PMRA applies current, evidence-based scientific approaches to assess whether the health and environmental risks of pesticides proposed for registration are acceptable, and if the products have value.
This same approach is used to regularly and systematically review whether pesticides already on the Canadian market continue to meet modern scientific standards.
Health Canada's Regulatory Operations and Enforcement Branch collaborates with PMRA on promoting, monitoring and enforcing compliance with the Pest Control Products Act across Canada. Health Canada is committed to doing this in a collaborative, open and transparent manner.
This work is carried out by a highly skilled workforce, the majority of whom are scientists, with additional expertise in areas such as regulatory and policy development, stakeholder engagement, international collaboration, and information management.
Vision
Canadians are confident that Canada's pesticide regulatory system protects their health and the environment.
Mission
To protect the health and environment of Canadians by using modern, evidence-based, scientific approaches to pesticide regulation, in an open and transparent manner.
Our people
Effective pesticide regulation requires an experienced workforce with a diversity of expertise. There are approximately 385 full-time employees at PMRA; 73% are scientists, including biologists, toxicologists, epidemiologists, environmental scientists and chemists; 80% of PMRA employees have more than 10 years of federal government experience.
What are pesticides?
Pesticides are toxic chemicals intentionally released into the environment to control pests on crops, in homes and workplaces, and in industrial processes. These can include personal insect repellents, wood preservatives, and pool sanitizers.
There are more than 600 registered active ingredients in almost 8000 registered pesticide products in Canada.
New pesticide registrations
Pesticides are regulated in Canada by Health Canada, reflecting the importance placed on human health and environmental protection in the regulation of these products. The Pest Control Products Act governs how pesticides are regulated based on scientific risk assessment and risk management, before and after they are registered for use.
Before a pesticide can be registered for sale in Canada, pesticide applicants are required to provide PMRA with extensive scientific data to show that their product does not pose unacceptable risks to health and the environment, and that the product has value. These data are reviewed by PMRA scientists to determine whether a product is acceptable for registration in Canada.
PMRA's science-based risk assessment includes the following:
- an examination of all sources and routes (oral, dermal or inhalation) of potential exposure to a given pesticide, including exposure through diet, from drinking water and from contact with treated areas like lawns and gardens
- an estimation of the amount of pesticides that people, including children, may come in contact with, both during and after a pesticide application
- a human health risk assessment with a particular focus on vulnerable populations, including pregnant women, infants, children, women, and seniors; this considers the potential for a pesticide to cause adverse health effects such as cancer, birth defects and endocrine effects, and allows registration only for those pesticides with exposures well below levels that cause adverse effects
- an environmental risk assessment that considers the fate (movement, persistence and transformation), toxicity, and risks to plants, birds, mammals, beneficial insects, and aquatic organisms
- a value assessment that considers the contribution of the product to pest management, as well as its health, safety and environmental benefits, and social and economic impact
For some currently registered pesticides, registrants may request changes to the use pattern. For these types of registrations, PMRA may also assess:
- additional environmental data, such as levels of pesticides detected through monitoring of pesticide concentrations in water across Canada or the United States
- any incident reports from Canada or other jurisdictions where the pesticide is already registered
- any other information needed to evaluate the health and environmental risks and the value of the pest control product
Various factors determine which studies are required to be submitted by applicants for registration, such as the nature of the product, the intended use, and the type of registration (for an overview of product submission types, see Appendix Table 1). PMRA follows established service standards, or defined timelines, for these evaluations as outlined in the Management of Submissions Policy (Regulatory Directive DIR2017-01).
The number and type of submissions reviewed by PMRA can vary significantly by year, as shown in Appendix Figure 1. Despite these shifts, PMRA continues to work to meet review timelines consistently across all submission categories (Appendix Figure 2).
New active ingredients and products registered in 2019–2020
In 2019–2020, 12 new active ingredients (the substance with the pesticidal effect) were registered for use in Canada, resulting in the registration of 25 new related end-use products (different formulations of products containing the active ingredient). Of the 12 new active ingredients, seven were biopesticides (derived from natural sources such as bacteria, fungi, viruses, plants, animals and minerals) and five were conventional chemical pesticides. Please see Appendix Table 2 for a full list of new active ingredients registered.
Some examples of end-use products registered in 2019–2020 include:
- products to protect field food crops, specialty crops and turf
- products that have a new mode of action for nuisance pests (including bed bugs) in commercial, industrial and residential structures
- first pet products for dogs and puppies to protect against walking dandruff mites
- biopesticides to protect greenhouse and field crops, stored potatoes, trees and shrubs, outdoor and greenhouse ornamental crops and non-agricultural and industrial sites
- a biopesticide to control varroa mites in honeybee hives
In the last decade, the total number of active ingredients registered for use in Canada has increased from just over 500 at the end of 2009 to 610 at the end of 2019. In the same 10-year period, the number of registered products increased from approximately 5700 to 7600. A number of products were removed from the market, either at the manufacturer's request or as a result of re-evaluation decisions.
PMRA continued to meet its performance targets on some pre-market evaluations, while for some categories of submissions, due to an increasingly complex workload, performance targets were not met. For category A and B, the target was missed mainly due to workload pressures related to re-evaluation and the writ period of the fall 2019 election. PMRA also responded to a high number of requests for pre-submission consultations or Subject to Regulation enquiries, including those for application of pesticides using drones and pesticides for use on cannabis or within cannabis production facilities.
Joint reviews
Joint reviews are pesticide assessments conducted in cooperation with other jurisdictions. In the last two decades, Canada has progressed from developing pilot pesticide joint review approaches with the United States, to conducting joint reviews as a primary course of business for pre-market reviews. Registrants must apply to register their product in each participating jurisdiction at the same time for a joint review to be conducted.
In 2019–2020, of the 12 active ingredients registered, four were joint reviews. PMRA is continuing to pilot new joint review approaches with the United States Environmental Protection Agency to increase efficiencies of the review process. The pilot approaches have been shared with international partners with the aim of increasing international interest in joint reviews, potentially leading to more global joint reviews in the future.
Generic registrations
When a new pesticide is developed, the innovator invests substantial funds into the studies required to show that the product works as intended, and poses no unacceptable health and environmental risks. The data supporting a new innovative product in Canada (that is, a new active ingredient) receives exclusive use protection for a period of time, to prevent it from being used for the benefit of a competitor without the innovator's approval. Data subsequently used to amend or maintain a registration or register a new product are given compensable protection.
This practice allows the innovator the opportunity to recover their investment, but also encourages further innovation by allowing competition on the market after a period of time. Allowing timely introduction of equivalent products by generic manufacturers following the exclusive period can enhance market competition to the benefit of users, including growers. These regulations are important to innovators, generic companies and to growers.
In 2019–2020, the PMRA received 234 applications to register generic products. The number of generic applications received continues to remain higher than previously anticipated by the PMRA. There were 62 generic products (27 technical and 35 end-use products) registered in 2019–2020. The PMRA continues to seek ways to improve the data protection program for innovator and generic companies.
Minor uses
A minor use is a use of a pest control product for which the anticipated volume of sales is not sufficient to persuade a manufacturer to register and sell the product in Canada. The definition emphasizes that it is the projected sales of the pest control product that is minor and not necessarily the size of the crop. A minor use may be registered on a major crop because the use may be needed only occasionally or is limited to a small percentage of the total area of the crop.
To help resolve these pesticide access issues for Canadian growers, PMRA works with Agriculture and Agri-Food Canada's Pest Management Centre who provide regulatory advice that supports growers and grower associations in identifying priorities for new minor use registrations in Canada. PMRA also works directly with the provinces to assist in addressing regional minor use needs.
In 2019–2020, PMRA reviewed minor use submissions from Agriculture and Agri-Food Canada and the provinces, and made 80 regulatory decisions, of which five were joint reviews or workshares with the United States Environmental Protection Agency. Final label reviews resulted in the registration of 299 new minor uses.
Emergency registrations
A pest control product can be registered for up to one year for the emergency control of seriously detrimental pest infestations, for example, following the introduction of an invasive species. The product must have acceptable value and the human health and environmental risks must be acceptable.
The number of emergency registration submissions that the PMRA receives can vary from year to year, depending on pest outbreaks, environmental conditions, and the availability of alternative products and control methods. In 2019–2020, the PMRA granted seven emergency registrations.
Maximum residue limits
A Maximum Residue Limit (MRL) is the maximum amount of residue that is expected to remain on food products when a pesticide is used according to label directions. These are set at levels well below the amount that could pose a health concern, and are established for each combination of pesticide and treated food crops.
Health Canada sets science-based MRLs to ensure the food Canadians eat is safe. As of December 2019, Canada had approximately 22,774 pesticide MRLs set (Figure 1). Typically, an MRL applies to the identified raw agricultural food commodity as well as to any processed food product derived from these raw commodities. If it is determined that an unacceptable risk exists based upon how the pesticide is intended to be used, the pesticide will not be permitted for sale or use in Canada.
The Canadian Food Inspection Agency (CFIA) is responsible for monitoring MRL compliance in foods in the Canadian marketplace. In two of their most recent reports from 2018-2019 surveys, the overall compliance rates for pesticide MRLs were 100% in products sampled for the Children's Food Project, and 99.3% of samples in CFIA's Pesticides and Metals in Selected Foods. The compliance rate from previous reports along with these recent surveys continues to indicate that the vast majority of food on the market meets Canadian pesticide standards.
Differences in MRLs between countries can lead to trade barriers. If an importing country's MRL for a given commodity is set lower than Canada's, this can lead to the importing country refusing entry to the Canadian commodity, despite the fact that the difference does not reflect a health risk.
International differences in MRLs can occur as a result of differences in both use patterns and data available to regulators at the time of MRL establishment, as well as other factors. Aligning MRLs globally has become increasingly important to reduce barriers to the movement of treated agricultural food products around the world. Domestic and international collaboration is critical in resolving these issues, which are of high importance to registrants, growers, and the Canadian economy.
PMRA continued work with its international partners under the North American Free Trade Agreement (now the Canada-United States-Mexico Agreement, or CUSMA), the Organisation for Economic Co-operation and Development (OECD) and Codex, on science policies relevant to establishing MRLs internationally.
The absence of an MRL for a particular pesticide-crop combination in an export market (sometimes called a "missing MRL") can also be a challenge for agricultural exporters. PMRA supports Agriculture and Agri-food Canada in efforts to address this challenge.
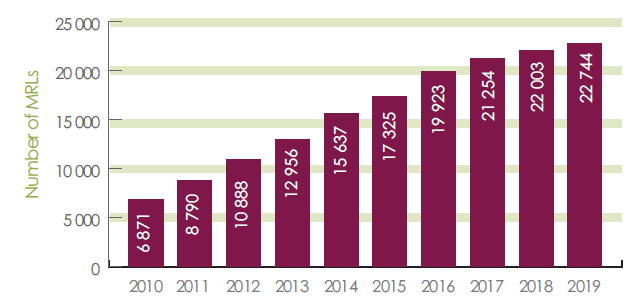
Figure 1. Total number of Canadian MRLs over time - Text description
- 2010 = 6871
- 2011 = 8790
- 2012 = 10888
- 2013 = 12956
- 2014 = 15637
- 2015 = 17325
- 2016 = 19923
- 2017 = 21254
- 2018 = 22003
- 2019 = 22744
Regulation of pesticides on the market
Once a pesticide has been granted registration status, it becomes subject to a system of post-market risk management controls under the Pest Control Products Act. This includes re-evaluations and special reviews of registered pesticides, compliance and enforcement activities, and reporting of health and environmental incidents.
Over the 10-year period between 2009 and 2019, the increase of the number of registered pest control products from 5700 to 7600 (Figure 2) also represents an increase in post-market activity workload.
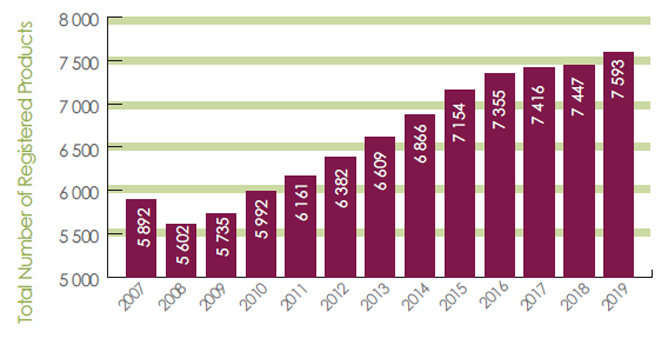
Figure 2. Increase in the number of registered pesticides over time - Text description
- 2007 = 5892
- 2008 = 5602
- 2009 = 5735
- 2010 = 5992
- 2011 = 6161
- 2012 = 6382
- 2013 = 6609
- 2014 = 6866
- 2015 = 7154
- 2016 = 7355
- 2017 = 7416
- 2018 = 7447
- 2019 = 7593
Note: "Registered Pest Control Products" includes registered Technical Grade Active Ingredients, End-Use Products and Manufacturing Concentrates.
These totals include products registered for all or part of each calendar year.
Re-evaluation and special review programs
Under the Pest Control Products Act, registered pesticides currently available on the market are subject to re-evaluations, which are initiated 15 years after the most recent registration decision, at the latest. Pesticides registered after 1995 are referred to in the re-evaluation context as 'cyclical pesticides'.
Pesticides registered prior to 1995 are referred to as 'older pesticides', and when the re-evaluation program was established, there were 401 of these older pesticides.
As of March 31, 2020, 387 of the original 401 were complete, with public consultations on the remaining 14 complete or imminent. The re-evaluation of older pesticides was scheduled to be completed by the end of 2020; however, delays due to the COVID-19 pandemic may affect the timing of some final decisions. In general, these remaining older pesticides are complex re-evaluations based on their large use patterns, and require large volumes of scientific data, and in some cases data that may be complex to generate.
Under the re-evaluation program, new methodologies, data, and scientific approaches are incorporated into the assessments to ensure that registered pesticides continue to meet modern standards for health and environmental protection, and have value.
Special reviews are another mechanism used under the Pest Control Products Act to determine the continued acceptability of registered pesticides. Unlike a re-evaluation, the intent of a special review is to address the specifically identified aspect(s) of concern, and may be triggered when:
- there are reasonable grounds to believe that the health or environmental risks of the product are, or its value is, unacceptable; or
- an OECD member country prohibits all uses of an active ingredient for health or environmental reasons.
The scope of a special review is narrower than a re-evaluation and it only evaluates the aspect(s) of concern that triggered the special review. In 2019, the Pest Control Products Act was amended to clarify that an identified aspect(s) of concern that would otherwise prompt a new special review can also be addressed through an ongoing re-evaluation or special review, reducing the need to duplicate work that is already being done.
Five-year re-evaluation and special review work plan
As part of its commitment to improve transparency, PMRA is publishing interim updates to its five-year Pest Management Regulatory Agency Re-evaluation and Special Review Work Plan 2019–2024 (Re-evaluation Note REV2019-05 and later). This work plan includes the target timelines to publish proposed and final decisions for ongoing re-evaluations and special reviews, as well as the list of anticipated re-evaluation initiations in the next five years.
In 2019–2020, PMRA made good progress on the re-evaluation of older pesticides, supported by additional temporary resources. Completing these large and complex re-evaluations will continue to be a priority, acknowledging that workload continues to increase as new re-evaluations and special reviews are initiated every year. As of March 31, 2020, 118 re-evaluations and special reviews are underway with a requirement to initiate 36 new re-evaluations later in 2020–2021.
Over the past five years, PMRA has completed an average of 25 final decisions per year for re-evaluations and special reviews. Though this is an improvement over previous years, workload continues to increase significantly as new re-evaluations and special reviews are initiated. Based on the projected number of re-evaluation initiations for the next five years, along with the average number of final decisions made per year, work on hand will grow significantly. The Program Renewal section in this Annual Report describes how PMRA is working to address this challenge.
Outreach and stakeholder engagement in re-evaluation and special review programs
PMRA has increased outreach efforts with global regulators such as the United States Environmental Protection Agency, the Australian Pesticides and Veterinary Medicines Authority and the European Food Safety Authority, to build awareness and potential opportunities for post-market collaboration.
PMRA continued its work to provide stakeholders with an opportunity for improved collaboration during re-evaluations. A unit has been dedicated to promote understanding of PMRA's re-evaluation process and risk assessments. To this end, the unit has been giving presentations to stakeholders and responding to information requests. In addition, work has begun on exploring options to obtain pesticide use information with a goal of collecting the information needed before a re-evaluation begins.
Pest control product sales information reporting
Since 2007, PMRA's Pest Control Product Sales Information Reporting Program has been collecting sales information, in the form of total quantity (by volume or mass), for all registered products available for sale. These data are reported by calendar year (January 1 to December 31). The purpose of the program is to collect sales data to be used by PMRA to better understand pesticide use in Canada.
Sales data are considered in risk assessments of pesticides, in policy decisions, in identifying trends in pesticide use, and in providing guidance for risk-reduction strategies.
For example, sales data are used in the re-evaluation of pesticides to help understand the presence and scale of use of the pesticide in the Canadian marketplace, as well as the potential impacts if changes are made to the registration status of the pesticide. Sales data are also used to inform the Pesticide Incident Reporting Program on the market share of particular pesticides to help identify potential risks that may require attention.
Erratum: In the 2018–2019 Pest Management Regulatory Agency Annual Report, sales information for the 2017 rather than 2016 calendar year was reported. However, in the 2018–2019 fiscal year, the PMRA published the annual sales report for the 2016 calendar year. Sales data for the 2016 calendar year are presented below, followed by a repeat of the 2017 calendar year sales data, for ease of comparison.
Note that due to differences between fiscal year and calendar year reporting, as well as the time required to collect and verify sales information, the most recent sales reports are not for the same year as the current annual report.
Sales of pest control products in Canada increased from 92.9 million kilograms of active ingredients (kg a.i.) in 2012 to 120.1 million kg a.i. in 2016 (Figure 3).
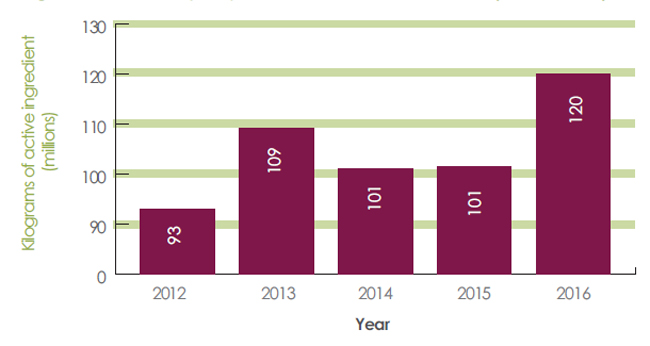
Figure 3. Quantity of pesticides sold in Canada 2012–2016 - Text description
- 2012 = 92 900 000 kilograms of active ingredients
- 2013 = 109 100 000 kilograms of active ingredients
- 2014 = 101 100 000 kilograms of active ingredients
- 2015 = 101 400 000 kilograms of active ingredients
- 2016 = 120 100 000 kilograms of active ingredients
In 2016, 74.7% of pesticide sales in Canada were agricultural sector products (Figure 4), whereas 20.1% were non-agricultural sector products, and 5.2% were domestic sector products.

Figure 4. Quantity of pesticides sold in 2016, by sector - Text description
- Non-agricultural = 20.1%, 24 100 000 kilograms of active ingredients
- Domestic = 5.2%, 6 200 000 kilograms of active ingredients
- Agricultural = 74.7%, 89 700 000 kilograms of active ingredients
Glyphosate remained the top active ingredient sold in Canada in 2016 (Table 1). Seven of the top 10 active ingredients sold in 2016 had been among the top 10 selling active ingredients since 2012. These top 10 active ingredients accounted for 68.7% of all pesticides sold in Canada in 2016.
| Active Ingredient | Product Type |
|---|---|
| Glyphosate | Herbicide |
| Available chlorine, present as sodium hypochlorite | Antimicrobial |
| Surfactant blend | Other |
| Creosote | Antimicrobial |
| 2,4-D | Herbicide |
| Glufosinate ammonium | Herbicide |
| MCPA | Herbicide |
| Mineral oil | Insecticide/Fungicide/Other |
| Borates | Insecticides/Fungicides/Antimicrobial |
| Corn gluten meal | Herbicide |
In 2019–2020, PMRA published the annual sales report for the 2017 calendar year.
Sales of pest control products in Canada have increased from 109.1 million kg of active ingredients (kg a.i.) in 2013 to 132.1 million kg a.i. in 2017 (Figure 5).

Figure 5. Quantity of pesticides sold in Canada 2013–2017 - Text description
- 2013 = 109 100 000 kilograms of active ingredients
- 2014 = 101 100 000 kilograms of active ingredients
- 2015 = 101 400 000 kilograms of active ingredients
- 2016 = 120 100 000 kilograms of active ingredients
- 2017 = 132 100 000 kilograms of active ingredients
In 2017, 73.4% of pesticide sales in Canada were agricultural sector products (Figure 6), whereas 21.4% were non-agricultural sector products, and 5.2% were domestic sector products.
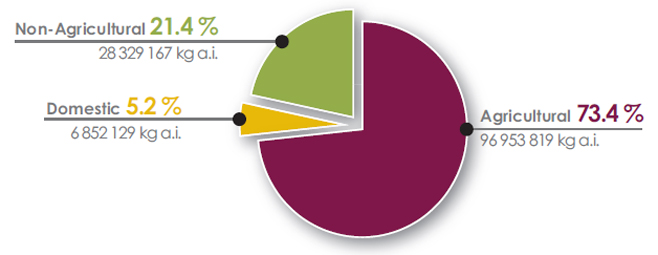
Figure 6. Quantity of pesticides sold in Canada in 2017 by sector - Text description
- Non-agricultural = 21.4%, 28 329 167 kilograms of active ingredients
- Domestic = 5.2%, 6 852 129 kilograms of active ingredients
- Agricultural = 73.4%, 96 953 819 kilograms of active ingredients
Glyphosate remained the top active ingredient sold in Canada in 2017 (Table 2). Seven of the top 10 active ingredients sold in 2017 had been among the top 10 selling active ingredients since 2013. These top 10 active ingredients accounted for 71% of all pesticides sold in Canada in 2017.
| Active Ingredient | Product Type |
|---|---|
| Glyphosate | Herbicide |
| Available chlorine, present as sodium hypochlorite | Antimicrobial |
| Creosote | Antimicrobial |
| Surfactant blend | Other |
| Glufosinate ammonium | Herbicide |
| Borates | Insecticides/Fungicides/Antimicrobial |
| 2,4-D | Herbicide |
| Mineral oil | Insecticide/Fungicide/Other |
| Available chlorine, present as trichloro-s-triazinetrione | Antimicrobial |
| Mancozeb | Fungicide |
Incident reporting
A pesticide incident is a negative effect to humans, animals or the environment that can result from being exposed to a pesticide. Pesticide registrants are required by law to report all incidents related to their products to Health Canada. Canadians may also report pesticide incidents to registrants, or directly to Health Canada's PMRA via the Public Engagement Portal Voluntary Incident Reporting Form.
PMRA uses incident reports to identify and characterize potential risks to humans, domestic animals and the environment from the use of pesticides, which were not evident during the initial registration of a pesticide. A pesticide incident is a negative effect (adverse reaction) on humans, animals (pets or livestock) or the environment (plants or wildlife) that can result from exposure to a pesticide.
Incident report assessments are prioritized based on the type of incident. Serious adverse effects such as death or life-threatening effects are evaluated immediately and mitigation measures are put into place, if warranted. If a potential risk is identified, it is investigated and protective action may be taken, such as changes to how a pesticide is manufactured, packaged, labelled, or used.
Incident reports also inform risk assessments for new registrations and re-evaluations. New scientific studies must also be submitted as an incident report to PMRA by registrants of a registered pesticide if the study demonstrates any new hazard, any risk that may be greater than the risk determined at the time of registration, or the presence of a previously undetected component or derivative of a pest control product.
Monitoring incidents for unanticipated effects is an ongoing process that includes re-assessing previous conclusions, as necessary. In cases where mitigation strategies have been adopted, PMRA also monitors incident reports to determine if the actions were effective in managing the identified risk.
In the 2019–2020 fiscal year, 1672 pesticide incident reports and 42 scientific studies were submitted to PMRA. Details of these reports can be found through the Pesticide Incident Reporting Database, by visiting Canada.ca/pesticides and selecting the link for the "Pesticide product information database".
Below is an overview of the incidents reported in 2019–2020:
- Domestic animal incidents were reported most frequently, followed by human and environmental incidents.
- The majority of reported domestic animal incidents involved spot-on pesticides used for flea, tick and mosquito control, and the reported health effects were mostly minor in nature.
- 1201 incidents occurred in Canada and 429 incidents relevant to Canadian products occurred in the United States.
- Overall, Canadian incidents involved approximately 190 different pesticide products
- The majority of products in reported incidents were domestic class pesticides, followed by commercial class pesticides.
- Only a very small number of products classified as restricted class or manufacturing class were reported in incidents.
Below are some examples of actions taken in 2019–2020 by PMRA following evaluation of incident reports:
- Label improvements were proposed to reduce the likelihood of exposure of children and animals to certain seed treatment products.
- Selected label amendments outlined in the PMRA Guidance Document: Structural Pest Control Products: Label Updates were recommended for certain products to inform stakeholders of standard definitions for different types of structural applications as well as human health precautionary label statements for structural pest control products.
To address the high number of domestic animal incident reports related to spot-on products, the PMRA implemented changes including new label statements for spot-on products, and updated data requirements for future registrations of companion animal products such as spot-on products, shampoos and impregnated collars.
The goal of these changes is to better inform consumers of the possible effects pets may experience following spot-on product use, and to improve the current animal safety testing strategy for pesticide products used on companion animals. See PMRA Guidance Document, Label Improvements for Spot-on Pesticides Used on Companion Animals and Consultation on Proposed Regulatory Changes for Pesticide Products Used on Companion Animals.
Label amendments similar to those outlined in the above guidance document were required for a new spot-on product approved for use in Canada, based on a review of incident reports from the United States involving the same product.
To report a pesticide incident, visit Canada.ca/pesticides and select the link for "Report a pesticide incident".
Compliance and enforcement
Health Canada's Pesticide Compliance Program is responsible for promoting, monitoring and enforcing compliance with the Pest Control Products Act and its Regulations. The primary objective of this legislation is to prevent unacceptable risks to the health and safety of Canadians and the environment from the use of pest control products. Since July 2019, all compliance and enforcement functions and accountability have been integrated into, and are now managed by, Health Canada's Regulatory Operations and Enforcement Branch.
The Pesticide Compliance Program has oversight on all parties regulated by the Pest Control Products Act, including pesticide registrants, manufacturers, importers, retailers, and users, and conducts a variety of compliance verification and compliance promotion activities within all sectors. Compliance verification includes the conduct of inspections and collection of samples to assess compliance. When required, enforcement action is taken against regulated parties to address non-compliance with the Pest Control Products Act.
Health Canada uses a range of enforcement tools including warning letters, compliance orders, notices of violation with warning or monetary penalty, prosecution, seizure, and partnering with the Canada Border Services Agency (CBSA) to refuse entry of unregistered pesticides into Canada.
Compliance promotion includes presentations, exhibits at trade shows, written articles, and the development and distribution of publications such as fact sheets and information packages. These activities increase the reach of the Pesticide Compliance Program and support overall levels of compliance by providing important information to regulated parties to foster compliance with the Pest Control Products Act and Regulations.
The delivery of compliance activities is prioritized based on risk. Criteria used in the selection of priority areas for compliance activities include potential risks to human health and the environment, compliance history, and outcomes of PMRA re-evaluation decisions. Considerations to assess risk include observations from the field, information from PMRA and provincial regulators, and data analysis.
2019–2020 Key statistics
The following is a summary of compliance and enforcement activities for the 2019–2020 fiscal year.
A total of 1526 inspections were conducted as a result of both planned and reactive activities (such as complaints), resulting in the following:
- 1695 violations were identified
- the most common violation types noted were importation of unregistered products (32%), possession of unregistered products (29%) and use of pest control products contrary to the approved label (23%)
A total of 1606 enforcement actions addressing single or multiple violations were issued to non-compliant parties, including:
- 988 warning letters
- 12 compliance orders
- in partnership with the Canadian Border Services Agency, 512 importations containing unauthorized products (including 1182 devices and more than 565 kg of pest control products) were refused entry into Canada
- 59 seizures of non-compliant pest control products
- 35 notices of violation with penalty under the Agriculture and Agri-Food Administrative Monetary Penalties Act, with a total value of $147,600 in penalties issued in 2019-2020
Finally, 257 compliance promotion activities were conducted.
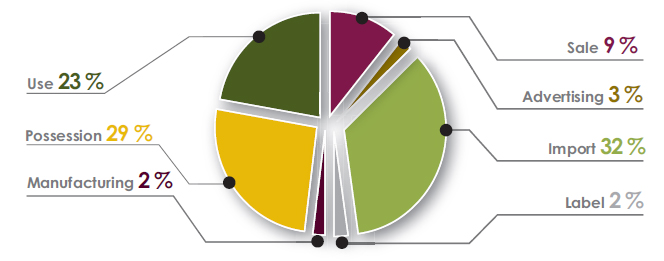
Figure 7. Contraventions under the Pest Control Products Act by type for 2019–2020 - Text description
- Sale - 9%
- Advertising - 3%
- Import - 32%
- Label - 2%
- Manufacturing - 2%
- Possession - 29%
- Use - 23%
Keeping pace with change
Globalization, rapid technological advances, evolving science, economic pressures and various other challenges and opportunities require a pesticide regulatory system that is flexible and responsive to change. PMRA is continuously modernizing risk assessment and risk management approaches, refining business practices to help ensure the needs of all stakeholders are met, and responding to major scientific and environmental developments, in Canada and abroad, with the goal of improving health and environmental protection.
Program renewal
In 2018, PMRA launched a two-year project to review its post-market pesticide program with the goal of achieving a more modern, efficient and sustainable re-evaluation program. Although the initial focus of the project was on the re-evaluation program, upon further analysis, it was apparent that changes across the full pesticide program were necessary to achieve the goals.
A new program model for an integrated approach to pesticide reviews was developed. This proposal is expected to improve health and environmental protection by transforming to a proactive, integrated risk-management approach across a pesticide's entire lifecycle, as well as address stakeholder concerns with the pesticide re-evaluation program.
This approach also supports the mandate issued to the Minister of Health in December 2019: "[...] to ensure that the Pest Management Regulatory Agency makes timely science-based decisions to support the safe and sustainable use of effective pesticide products in Canada."
In 2019–2020, PMRA began both internal and external consultations on the design of the integrated approach with a wide range of stakeholders across Canada, receiving broad support. With completion of this extensive engagement, PMRA is moving forward with Program Renewal and is implementing the new model over the next several years.
The integrated approach will allow PMRA to be more proactive throughout the pesticide lifecycle in updating assessments, identifying data needs earlier, and implementing protective measures when necessary. The integrated model incorporates expanded engagement and transparency measures in order to maintain up-to-date data and information, including improved environmental monitoring and use data, over the full lifecycle of the pesticide.
Evaluating new technologies
In addition to assessing the potential health and environmental risks of chemical and biological pesticides, PMRA scientists monitor new developments such as machine learning, robotics and drones. While these technologies may have many benefits (for example, for precision agriculture), their potentially unique health and environmental risks must be carefully identified and assessed. PMRA is working with manufacturers, other regulatory authorities globally, and international organizations such as the OECD, to understand and assess new technologies and equipment that support modern agricultural practices.
PMRA also seeks opportunities to reduce the need for animal testing wherever possible, while continuing to ensure scientifically robust approaches are in place for assessing risk. Integrated Approaches to Testing and Assessment or New Approach Methods (IATA/NAM) involve using data from existing laboratory animal studies, in vitro high-throughput screening assays, predictive models, mechanistic studies and other data in order to refine, reduce and in some cases even replace laboratory animal studies for human health and environmental assessment of pesticides.
PMRA collaborates with North American and OECD partners, and through other multi-stakeholder initiatives that are examining new approaches to current study types. This includes alternative approaches to acute studies, the developmental neurotoxicity study, and the internationally developed Risk21 tool, which allows users to calculate and manage risk in a new and efficient way.
Reducing product exposure and misuse
To minimize risks posed by exposure and misuse, PMRA works with various stakeholders to improve labelling, and to evaluate and mitigate risks related to how products are used. Examples from 2019–2020 include:
- guidance on label updates for structural pest control products
- revisions to data requirements for pesticide products used on companion animals
- label improvements for spot-on pesticides used on companion animals
PMRA works with stakeholders on minimizing worker exposure to pesticides after they are applied. In 2019–2020, PMRA completed a multi-year project with Agriculture and Agri-Food Canada's Pest Management Centre to measure how pesticide residues decrease in greenhouse crops following application. This information is used to determine the label instructions around re-entry following pesticide use that are necessary to protect worker health.
Remote piloted aircraft systems (drones) for pesticide application
PMRA has received a number of inquiries related to the application of pesticides by Remote Piloted Aircraft Systems (RPAS, or drones). RPASs are increasingly used internationally in agriculture for scouting-related activities (for example, sensing, mapping, and tracking), as well as for vehicles for the delivery of pesticides.
The use of RPASs for pesticide spraying is established in some jurisdictions including China and Japan, and is at the initial stages in others, such as the United States, Switzerland, New Zealand, and Australia. In each jurisdiction, both the aviation and the pesticide regulatory authorities have specific criteria that must be met to allow for the application of pesticides by RPASs.
In Canada, there can be no pesticide application by remote piloted aircraft until there are conditions of use approved by PMRA, including any limitations for use of these kinds of aircraft on pesticide labels (PMRA Information Notice Regarding the Use of Drones when Applying Pesticides; published on May 8, 2018). Parties interested in adding the use of RPASs are encouraged to work with registrants using the PMRA Pre-Submission process to determine the kinds of data necessary to assess this new application technology.
PMRA is currently participating in two international working groups to coordinate information sharing on the health and environmental safety of this new application method, in support of regulatory reviews:
- The OECD Working Group on Pesticides Drone Sub group is comprised of several OECD pesticide regulators and crop protection industry stakeholders, and is focussed on identifying information required for completing regulatory risk assessments.
- The North American Remotely Piloted Aerial Application Systems working group has regulatory participation from the PMRA, United States Environmental Protection Agency and Transport Canada, members from the crop protection industry, as well as pesticide spray drift researchers, pesticide applicators and RPAS equipment manufacturers. The primary focus of this group is in identifying and potentially generating information to support regulatory efforts for RPASs.
Due to the variety of RPAS delivery systems on the market and a lack of equipment standards internationally, there is a need to coordinate the review of available data for assessing the safety of these aircraft with other jurisdictions.
Water Monitoring
During the assessment of pesticides, PMRA determines the potential risk to humans to levels of pesticides in drinking water sources (ground water and surface water), and to aquatic life, from pesticides that may be present in water such as lakes, rivers and streams.
While computer modelling may be used to estimate residue levels of pesticides in drinking and ambient water sources, PMRA has been increasingly using monitoring data from federal, provincial and municipal departments, agencies and researchers to provide real-world information of the potential for exposure to pesticides to inform its regulatory decisions about pesticides.
PMRA continued working with other federal departments, provinces, and stakeholder associations on addressing challenges with obtaining robust datasets on pesticides for use in regulatory decision making. This initiative includes making use of tools such as PMRA's list of pesticides where water-monitoring data should be prioritized, as well as the development of a database of existing water monitoring data made available to PMRA.
PMRA collaborated with Health Canada's Healthy Environments and Consumer Safety Branch's Water and Air Quality Bureau in developing a proactive approach for sharing Human Health Reference Values (HHRVs) for pesticides with each other, and with members of the Canadian Federal-Provincial-Territorial Committee on Drinking Water. PMRA is also now an active member of the committee.
As of December 2019, PMRA has compiled and shared Aquatic Life Reference Values and Human Health Reference Values for approximately 250 pesticides that have the potential to reach surface, ground and drinking water. These reference values can be used to interpret the levels of pesticides detected in water and to identify and prioritize sites and pesticides that may require further investigation or remedial action.
Vegetative filter strips
PMRA continued to examine the use of vegetative filter strips (VFSs) as a risk mitigation tool. A VFS is a permanent strip of dense, perennial vegetation situated on the downslope border of the treated area (such as an agricultural field, plantation or woodlot), along the edge of the water body into which the area drains. The vegetation within a VFS contains grasses, but may also contain other vegetation, such as shrubs and trees. The VFS reduces the velocity of water runoff to allow soil and pollutants, such as pesticides, to settle out before entering the water.
PMRA is working to incorporate VFS modelling into environmental risk assessments using a computer model called VFSMOD. In the short term, modelling may help support the implementation of a mandatory 10-metre VFS for pesticides with a high tendency to bind to soil particles, without the need for field trials. PMRA is exploring the effectiveness of VFSs for pesticides that dissolve in water using VFS modelling.
Future work includes investigating ways to incorporate site-specific considerations (such as slope) to determine the most optimal width for a VFS.
International scientific and regulatory cooperation
Canada's internationally respected regulatory model has allowed Canada to form strong partnerships with other regulators, and to play a significant role in developing collaborative approaches to joint pesticide reviews, promoting international regulatory alignment, and addressing barriers to agricultural innovation and trade.
Stockholm Convention
The Stockholm Convention is a legally binding international treaty with a focus on the elimination or restriction of the production and use of persistent organic pollutants (POPs). PMRA is the responsible federal authority for meeting the obligations and for ongoing participation at the Stockholm Convention as it pertains to pesticides.
PMRA collaborates with other federal partners by providing scientific experts to work with the Persistent Organic Pollutants Review Committee (POPRC) and the Conference of the Parties (COP) of the Stockholm Convention, and in the development of Canadian positions and submissions.
- At POPRC, PMRA actively participates in the review of the scientific justification for identifying substances as POPs and making recommendations on how these substances can be managed globally.
- At the COP, PMRA provides experts to negotiate international decisionson the restrictions and ultimately the elimination of each POP at the global level.
In 2019–2020, the following agreements on pesticide-related substances were reached through COP:
- dicofol to be listed for global elimination
- pentadecafluorooctanoic acid (PFOA), plus its salts and PFOA-related compounds, a former pesticide formulant, to be listed for restriction with certain specific exemptions
- remaining uses of perfluorooctane sulfonic acid (PFOS), its salts and perfluorooctane sulfonyl fluoride to be banned
- adoption of a revised framework for evaluating effectiveness
In 2019–2020, the following agreements on pesticide-related substances were reached through POPRC:
- the pesticide methoxychlor met the screening criteria for considerationas a POP, thereby advancing it to the next review stage
Rotterdam Convention
The Rotterdam Convention promotes information exchange and informed consent in the international trade of chemicals, with the aim of protecting human health and the environment. The Convention is a multilateral treaty to promote shared responsibilities in relation to importation of hazardous chemicals.
The Convention calls on exporters of hazardous chemicals to use proper labelling, include directions on safe handling, and inform purchasers of any known restrictions or bans.
PMRA collaborated with other federal partners by providing scientific experts to work with the Chemical Review Committee (CRC) and the COP of the Rotterdam Convention, and in the development of Canadian positions and submissions.
For CRC, PMRA actively reviews submissions to the Rotterdam Convention against established Convention criteria. At the COP, PMRA provides experts to negotiate international decisions for each substance at the global level.
In 2019–2020, pesticide-related activities of the COP included:
- agreeing to add the pesticide phorate to Annex III of the Convention, thereby subjecting future imports of phorate to the Convention's prior informed consent provisions
- adopting procedures and mechanisms on compliance that were jointly developed and proposed by Canada and 11 other countries
Organisation for Economic Co-operation and Development
PMRA is involved with several OECD initiatives, including various OECD task forces and expert group projects. PMRA routinely participates in meetings of both the OECD Working Group on Pesticides (WGP) as well as the OECD Working Group on Biocides (WGB). Both working groups function as vehicles for global cooperation, information exchange and alignment of approaches with respect to pesticides assessment.
PMRA also contributes input (via the Canadian Delegation) to the OECD Joint Meeting of the Chemicals Committee and the Working Party on Chemicals, Pesticides and Biotechnology as required. PMRA routinely provides experts to participate in the OECD WGP Expert Groups on Residue Chemistry, Pollinator Safety, Bio-pesticides, and Electronic Exchange of Pesticide Data.
Some examples of OECD WGP initiatives include:
- development of a common approach to regulating novel pest control products, such as RNAi-pesticides
- implementation of technical guidelines (for example, those that provide guidance on alternative approaches to animal testing)
- identification of residues, metabolites and degradation products
- identification of relevant data requirements for regulating bacteriophages
- ongoing dialogue related to integrated pest management/pollinator protection
- aligning risk assessment of new digital and mechanical technologies for applying pesticides such as innovative drone technology
PMRA also plays a lead role on the OECD WGP e-label project to identify commonalities in pesticide labels that would support development of e-label solutions. PMRA also actively contributes to the Expert Group on Claims Development for Treated Articles.
In support of the OECD WGP's objectives, PMRA has led discussions with global manufacturers of pesticides regarding new chemistries to broaden collaboration and promote global joint reviews and alignment between international regulatory partners. PMRA has also initiated discussion with OECD partners on post-market review challenges and the potential benefit of having a greater collaboration in this area.
Codex
PMRA plays an active role in the World Health Organization/Food and Agriculture Organization Codex Committee on Pesticide Residues, which is responsible for setting international food standards. Codex participation enables PMRA to:
- enhance Canada's influence on Codex deliberations and outcomes
- promote the development of science-based standards that will result infair practices in food trade (for example, establishment of MRLs)
- promote more effective work-planning by the committee (help ensure priorities include Canadian stakeholders' interests)
- promote the timely development of standards (for example, continue to explore opportunities for parallel reviews with the Joint Meeting on Pesticide Residues)
Regulatory modernization
In 2019–2020, PMRA continued to take steps to modernize its legislative framework.
Labelling amendments
In June 2019, PMRA amended the Pest Control Products Regulations to clarify what information is required to be displayed on railway tank cars, transport truck trailer-tankers, and outer packaging that contains pest control products. These amendments modernized labelling requirements across a variety of different pest control product container types, and provided clarity by eliminating the term "bulk container".
Formulants and contaminants of health and environmental concern
In 2005, PMRA established a list of formulants and contaminants that the Minister considers to be of health or environmental concern. In July 2019, PMRA consulted on proposed changes to this formulants list.
The following formulants and contaminants were proposed for removal:
- those that are no longer found in a pest control product registered in Canada
- those that are no longer considered to adversely affect human health or the environment due to specific or approved use patterns
The following formulants and contaminants were proposed for addition:
- those known to cause anaphylactic-type reactions
- those designated as being of health or environmental concern under relevant domestic or international agreements
In July 2019, PMRA also consulted Canadians on the proposed approach and criteria for maintaining the formulants list, and how it would be used.
Targeted regulatory review of the agri-food and aquaculture sector
The Government of Canada announced in Budget 2018 that it would fund, over three years, "targeted reviews of regulatory requirements and practices that are bottlenecks to economic growth and innovation."
As part of this initiative, in 2018, PMRA participated in the targeted regulatory review of the agri-food and aquaculture sector. A central feature of the review was to invite input from businesses, Canadians, academia and other stakeholders, on ways to make regulations more agile, transparent, and responsive.
An Agri-food and Aquaculture Regulatory Review Roadmap was developed that lays out a regulatory modernization plan in support of innovation and economic growth in the agri-food and aquaculture sector. This roadmap was released in July 2019. As part of implementing the Roadmap:
- In 2019–2020, PMRA published final amendments to the Pest Control Products Incident Reporting Regulations and related provisions of the Agriculture and Agri-Food Administrative Monetary Penalties Regulations Respecting the Pest Control Products Act and Regulations. These amendments continue to uphold the requirements for protecting health and the environment while reducing regulatory and administrative burden.
- PMRA also updated the negotiation and arbitration rules to help innovators and generic producers to reach compensation agreements more easily.
In 2019–2020, PMRA continued work on other regulatory modernization initiatives on the Roadmap, including those related to the post-market review process, labelling, data protection, and the authorization of pesticides not requiring registration.
Annual Regulatory Modernization Bill (ARMB)
The Regulatory Modernization Bill, announced in the Fall Economic Statement 2018, is a new annual mechanism designed to remove outdated and redundant regulatory requirements and to allow for the updating of regulations. This annual exercise will help keep regulations current, to better reflect public policy and business realities, challenges, and opportunities.
- In June 2019, as part of the ARMB and the implementation of the Agri-food and Aquaculture Regulatory Review Roadmap, PMRA amended section 17 of the Pest Control Products Act to allow the Minister greater discretion with respect to initiating special reviews, thereby reducing regulatory burden for industry, while maintaining health and environmental protection.
- In 2019–2020, as part of the implementation the Agri-food and Aquaculture Regulatory Review Roadmap, PMRA continued work on proposed statutory changes to:
- broaden the Minister's powers to make risk-based authorizations and exercise appropriate post-market oversight over authorized products; and,
- broaden the Minister's power to amend pest control product labels without an application in certain situations (for example, to clarify wording of an existing health or environmental protection requirement).
Targeted regulatory review of international standards, digitalization and technology neutrality, and clean technology
In 2019, the Government of Canada announced the next round of targeted regulatory reviews that included a review of international standards, digitalization and technology neutrality, and clean technology. In 2019–2020, PMRA participated in the process that would identify projects to be included in the roadmaps for these targeted regulatory reviews.
Pest Control Products Regulations review
Prior to the launch of the regulatory reviews, the departments and agencies responsible for regulating the agri-food and aquaculture sector, including PMRA, each had an ambitious regulatory modernization agenda that extended over several years.
In 2019–2020, PMRA continued its comprehensive review of the Pest Control Products Regulations, the first such review since they were established in 2006. The review is aimed at ensuring the regulations continue to meet program objectives (for example, of health and environmental protection) in an effective and efficient manner, while attempting to minimize regulatory burden on regulatory parties.
In 2019–2020, the review included public and industry engagement on issues such as product exemptions.
Stakeholder relations and outreach communications
PMRA recognizes that the transparency and openness of its work is critical to strengthening trust in its regulatory decisions.
Public opinion research
In January 2020, the second public opinion research survey, "Awareness and Confidence in Canada's Pesticide Regulatory System", was completed. The results, when compared with the 2016 baseline survey results, will help PMRA determine which of its current activities support the goals of helping Canadians understand the pesticide regulatory process, how they can get involved, and be aware of decisions that affect them. A key finding reveals that, despite low levels of knowledge about the pesticide regulatory process, Canadians exhibit an increased confidence and trust in Health Canada's PMRA decision making in the protection of health and the environment.
Educational videos about PMRA
Building on the success of the 2018 animated video "Pesticides: What do Health Canada Scientists Do?" two new educational videos were produced and made available in the Pesticides in Canada section of Canada.ca, and on YouTube:
- Pesticides: Health Canada assesses health risks
- Pesticides: Health Canada assesses risks to the environment
Infographics covering a range of risk assessment topics were also created:
- Pesticides and Human Health
- Consideration of Sex and Gender in Pesticide Risk Assessment
- Pesticides and the Environment
- Health Canada Cares About Bees
- Health Canada Protects Bees from Pesticides
To publicize the availability of this information, PMRA has become more active on social media, through planned social media campaigns and by participating in and contributing to departmental campaigns with subject matter crossover.
Stakeholder webinars
In May and December of 2019, PMRA continued to host a bilingual and now bi-annual webinar series aimed at providing a diverse group of stakeholders with updates on pesticide regulation, as well as an opportunity to ask questions. While the first webinar in 2017 had 95 participants, the two 2019 sessions averaged 165 participants each. Participant feedback following the events was positive, with stakeholders reporting that they appreciated the information shared, and the question and answer portions of the sessions.
Pest Management Advisory Council
The new membership of the Minister of Health's Pest Management Advisory Council (PMAC) met in May 2019 and February 2020. Members currently include representatives from pesticide manufacturers, user groups, health and environment non-government organizations, and academia/research institutes. At the February 2020 meeting, PMRA provided an update on progress and sought the Council's advice on the Program Renewal Project.
The Council was supportive of the direction of the new integrated approach, recommending that PMRA be provided with increased funding to proceed with its implementation. The Council also concluded that the Pest Control Products Act provides a strong legislative basis for Canada's pesticide regulatory system and should be retained, with only minor amendments as outlined in the Agri-food and Aquaculture Roadmap.
Financial profile
| 2019-2020 funding and revenue (in millions of dollars) | Total |
|---|---|
| A-Base | 26.3 |
| Revenue - Application fees $5.4 and Annual charge $8.1 | 13.5 |
| Growing Forward | 3.3 |
| Chemicals Management Plan | 5 |
| Departmental pressure funding | 4.5 |
| Total PMRA fiscal year 2019-2020 | $52.60 |
- Financial profile includes employee benefit plan.
- A portion of revenues paid by regulated parties is allocated to support employee benefits plans (non-respendable revenue) and internal services.
- Departmental pressure funding of $4.5 million was not included in PMRA main estimates. (Funding received as in-year funding.)
- PMRA received $3.3 million through the Growing Forward initiative to support the registration of minor use products. As a result, newer, more environmentally sustainable, and more modern products have been made available to Canadian producers, which helps sustain Canada's competitive position globally.
- Through Canada's Chemicals Management Plan, PMRA received $5 million to re-evaluate older pesticides, improve risk management approaches through Incident Reporting and Sales Reporting regulations, and contribute to the development of scientific and regulatory approaches with other jurisdictions on high-priority issues. For more information, please consult the Chemicals Management Plan webpage.
Service Fees Act
In 2019–2020, PMRA completed drafting its Remission Policy for Missed Service Standards. This policy describes the scenarios under which a portion of pre-market application fees will be returned to the applicant when service standards are not met. Originally, this policy was to take effect on April 1, 2020; however, the Treasury Board of Canada Secretariat delayed its implementation for one year until April 1, 2021, due to the COVID-19 pandemic.
Appendix
| Submission category | Service standard in days |
|---|---|
| Category A New active ingredients or integrated system products, their related end-use products and manufacturing-use products; major new use of registered pest control products; maximum residue limits for an unregistered active ingredient; and user requested minor use registrations (URMUR). |
|
| Conventional chemicals and import MRLs for an unregistered active ingredient | 665 |
| Reduced risk, other biopesticides, non-conventionals, non-straight chain lepidopteran pheromone (NSCLP) | 555 |
| Microbials, and URMUR for all pesticide types (conventional chemical, reduced risk, microbial, other biopesticides, non-conventionals, NSCLP) | 470 |
| Straight Chain Lepidopteran Pheromone (SCLP), including URMUR | 285 |
| Applications with atypical timelines (joint reviews, tailgaiters, renegotiated timelines, synchronized timelines, coordination with re-evaluation | Variable |
| Category B New pest control products containing registered active ingredients; an amendment to existing pest con- trol products (for example, product chemistry, labelling); emergency registration; the addition of import MRLs for previously assessed active ingredients. |
|
| Conventional chemicals (including emergency use) and new import MRL for previously assessed active ingredient | 425 |
| Reduced risk, other biopesticides, non-conventionals, NSCLP (including emergency use) | 360 |
| Microbials and SCLP (including emergency use) | 240 |
| Streamlined applications (application rate changes, tank mixes, new pests, or changes to level of control) | 158 |
| Applications with atypical timelines (joint reviews, tailgaiters, renegotiated timelines, synchronized timelines, coordination with re-evaluation) | Variable |
| Category C Product registrations and amendments with no data requirements. These applications involve minor label or formulation reviews, such as product registration based on registered precedent products. |
|
| New/changes to product labels; addition of approved minor use; similar product | 240 |
| New/changes to technical grade active ingredient, integrated system product, manufacturing concentrate or end-use product chemistry; administrative changes; administrative re-instatement | 180 |
| Applications with atypical timelines (tailgaters, renegotiated/ synchronized timelines, coordination with re-evaluation) | Variable |
| Category D Submissions within particular programs. |
|
| Registration renewal | 247 |
| Registration/amendment to registration of active ingredient to be used in pest control product manufactured for export only | 46 |
| Master copies | 42 |
| Private labels | 10 |
| Own Use Import Equivalency and PermitsFootnote * | 70 (Equivalency) |
| 30 (Permits) | |
| Grower Requested Own Use Equivalency and PermitsFootnote * | TBD (Equivalency) |
| 30 (Permits) | |
| DiscontinuationsFootnote * | 45 |
| Category E Authorizations and notifications for research in Canada. |
|
| Research authorization for new technical grade active ingredients | 159 |
| Research authorization for new uses of registered active ingredients | 69 |
| Research notification for research carried out in Canada | 30 |
| Category F Notification. |
|
| Registration and amendments to registered pest control products via notification | 45 |
| Category L Submissions to register or amend products where the applicant wishes to use or rely upon data provided by another registrant. |
|
| Equivalency and data compensation assessment of end-use product and manufacturing concentrate with partial data package (conventional chemical) | 425 |
| Equivalency and data compensation assessment of active ingredient, end-use product and manufacturing concentrate with no data (all product types) | 365 |
| Equivalency and data compensation assessment of end-use prod- uct and manufacturing concentrate with partial data package (reduced risk, other biopesticide, non-conventional, NSCLP) | 360 |
| Equivalency and data compensation assessment of end-use product and manufacturing concentrate with partial data package (microbial and SCLP) | 240 |
| Applications with atypical timelines (tailgaters, renegotiated/ synchronized timelines, coordination with re-evaluation) | Variable |
| Regulatory DecisionFootnote * | 45 |
| Requests to extend the exclusive use protection period based upon minor usesFootnote * | 240 |
| Category P Pre-submission consultations. |
|
| Pre-submission consultations excluding those for joint reviews and subject to registration inquiriesFootnote * | 80 |
|
|
| New active ingredient | End-use product(s) | Product type | Product category | |
|---|---|---|---|---|
| 1 | 1-Octanol | 1,4Zap(R) | Plant Growth Regulator | Biopesticide |
| 2 | Bixafen | F9651-2 Fungicide | Fungicide | Conventional Chemical |
| 3 | Caprylic Acid | BioLink Herbicide EC | Herbicide | Biopesticide |
| 4 | Capric Acid | |||
| 5 | Dinotefuran | Vectra 3D For Dogs Weighing 25.1 to 43 kg | Insecticide | Conventional Chemical |
| Vectra 3D For Dogs and Puppies Over 7 Weeks of Age Weighing 9.1 to 25 kg | Insecticide | Conventional Chemical | ||
| Vectra 3D For Dogs and Puppies Over 7 Weeks of Age Weighing 4.6 to 9.0 kg | Insecticide | Conventional Chemical | ||
| Seclira Pressurized Insecticide | Insecticide | Conventional Chemical | ||
| Seclira Dust Insecticide | Insecticide | Conventional Chemical | ||
| Seclira Cockroach GeL Bait Reservoir | Insecticide | Conventional Chemical | ||
| 6 | Hop Beta Acids (Present as Potassium Salts) | HopGuard®Liquid | Acaricide | Biopesticide |
| HopGuard®II | Acaricide | Biopesticide | ||
| 7 | Mefentrifluconazole | BAS 752 RC | Fungicide | Conventional Chemical |
| Belyan | Fungicide | Conventional Chemical | ||
| Lenvyor | Fungicide | Conventional Chemical | ||
| Cevya | Fungicide | Conventional Chemical | ||
| Maxtima | Fungicide | Conventional Chemical | ||
| Relenya | Fungicide | Conventional Chemical | ||
| 8 | Mild Pepino mosaic virus isolate VC1 | V10 | Non-Parasitic Plant Disease Control | Biopesticide |
| 9 | Mild Pepino mosaic virus isolate VX1 | |||
| 10 | Pelargonic Acid | Beloukha Herbicide | Herbicide | Biopesticide |
| Beloukha Agricultural Herbicide | Herbicide | Biopesticide | ||
| 11 | Pethoxamid | Pethoxamid 480EC | Herbicide | Conventional Chemical |
| 12 | Tetraniliprole | Vayego 200SC Insecticide | Insecticide | Conventional Chemical |
| Reatis 480 FS | Insecticide | Conventional Chemical | ||
| Tetrino | Insecticide | Conventional Chemical | ||
| Tetraniliprole 200SC Turf Insecticide | Insecticide | Conventional Chemical |
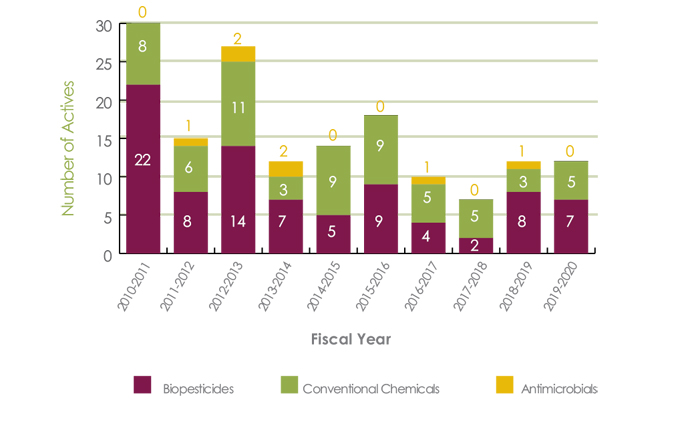
Appendix Figure 1. Number of new active ingredients registered by PMRA from April 1, 2010, to March 31, 2020 - Text description
- 2010-2011 - Biopesticides 22, Conventional Chemicals 8, Antimicrobials 0
- 2011-2012 - Biopesticides 8, Conventional Chemicals 6, Antimicrobials 1
- 2012-2013 - Biopesticides 14, Conventional Chemicals 11, Antimicrobials 2
- 2013-2014 - Biopesticides 7, Conventional Chemicals 3, Antimicrobials 2
- 2014-2015 - Biopesticides 5, Conventional Chemicals 9, Antimicrobials 0
- 2015-2016 - Biopesticides 9, Conventional Chemicals 9, Antimicrobials 0
- 2016-2017 - Biopesticides 4, Conventional Chemicals 5, Antimicrobials 1
- 2017-2018 - Biopesticides 2, Conventional Chemicals 5, Antimicrobials 0
- 2018-2019 - Biopesticides 8, Conventional Chemicals 3, Antimicrobials 1
- 2019-2020 - Biopesticides 7, Conventional Chemicals 5, Antimicrobials 0
This figure provides the number of new active ingredients registered over the course of the last 10 fiscal years. It represents active ingredients that have been registered for use in Canada, and excludes any new active ingredients for which only a maximum residue limit on imported food was established.
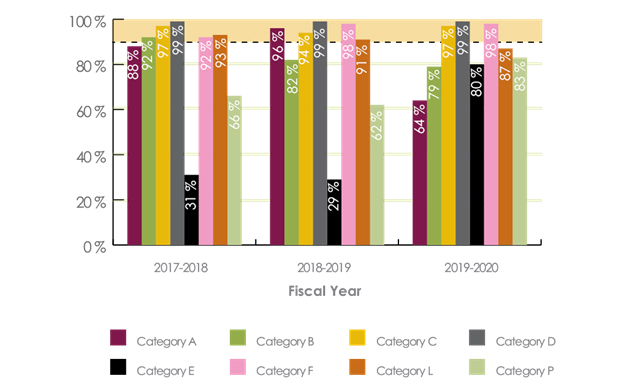
Appendix Figure 2. Performance against review timelines for category A, B, C, D, E, F, L and P submissions completed from April 1, 2017, to March 31, 2020 - Text description
- 2017-2018 - Category A - 88%, Category B - 92%, Category C - 97%, Category D - 99%, Category E - 31%, Category F - 92%, Category L - 93%, Category P 66%
- 2018-2019 - Category A - 96%, Category B - 82%, Category C - 94%, Category D - 99%, Category E - 29%, Category F - 98%, Category L - 91%, Category P 62%
- 2019-2020 - Category A - 64%, Category B - 79%, Category C - 97%, Category D - 99%, Category E - 80%, Category F - 98%, Category L - 87%, Category P - 83%
- Effective April 1, 2017, categories F, L and P were added to the Management of Submissions Policy.
- This figure shows the percentages of submissions by submission category that met the applicable review timelines outlined in the Management of Submissions Policy over the last three fiscal years.
- All categories of pre-market submissions have a performance standard of 90% against the established review timelines for the different submission categories.
- PMRA continued to meet its performance targets on some pre-market evaluations (C, D, F), while for some categories of submissions (A, B,E, L), due to an increasingly complex workload, performance targets were not met.
| Active ingredient | Document number | Summary of decision or proposed decision |
|---|---|---|
| Re-evaluation Decisions | ||
| Thiamethoxam: Pollinator Re-evaluation | RVD2019-04 | Acceptable for continued registration for certain uses. Mitigation includes new/revised label statements to further protect the environment. Cancellation of other uses due to environmental risk concerns. |
| Clothianidin: Pollinator Re-evaluation | RVD2019-05 | Acceptable for continued registration for certain uses. Mitigation includes new/revised label statements to further protect the environment. Cancellation of other uses due to environmental risk concerns. |
| Imidacloprid: Pollinator Re evaluation | RVD2019-06 | Acceptable for continued registration for certain uses. Mitigation includes new/revised label statements to further protect the environment. Cancellation of other uses due to environmental risk concerns. |
| Fomesafen | RVD2019-07 | Acceptable for continued registration. Mitigation includes new/revised label statements to further protect human health and the environment. |
| Fosetyl-aluminum | RVD2019-08 | Acceptable for continued registration. Mitigation includes new/revised label statements to further protect human health and the environment. |
| Copper (present as cuprous thiocyanate) | RVD2019-09 | Acceptable for continued registration. Mitigation includes new/revised label statements to further protect human health and the environment. |
| Octyl bicycloheptene dicarboximide (MGK-264) | RVD2019-10 | Acceptable for continued registration for certain uses. Mitigation includes new/revised label statements to further protect the environment. Cancellation of other uses due to health risk concerns. |
| Permethrin | RVD2019-11 | Acceptable for continued registration for certain uses. Mitigation includes new/revised label statements to further protect the environment. Cancellation of other uses due to health risk concerns. |
| Triforine | RVD2019-12 | Acceptable for continued registration. Mitigation includes new/revised label statements to further protect human health and the environment. |
| Iron (Present as Ferrous Sulfate Monohydrate and Ferrous Sulfate Heptahydrate) | RVD2019-13 | Acceptable for continued registration. Mitigation includes new/revised label statements to further protect human health and the environment. |
| Clodinafop-propargyl | RVD2020-01 | Acceptable for continued registration. Mitigation includes new/revised label statements to further protect human health and the environment. |
| Folpet (agricultural uses) | RVD2020-02 | Acceptable for continued registration. Mitigation includes new/revised label statements to further protect human health and the environment. |
| Uniconazole-P | RVD2020-03 | Acceptable for continued registration for certain uses. Mitigation includes new/revised label statements to further protect the human health. Cancellation of one use due to health risk concerns. |
| Streptomyces strain K61 | RVD2020-04 | Acceptable for continued registration. Mitigation includes new/revised label statements to further protect human health and the environment. |
| Dimethomorph | RVD2020-05 | Acceptable for continued registration. Mitigation includes new/revised label statements to further protect human health and the environment. |
| Strychnine (Richardson's ground squirrels) | RVD2020-06 | Cancellation of strychnine used to control Richardson's ground squirrels to environmental risk concerns. |
| Special Review Decisions | ||
| Bromoxynil | SRD2019-02 | Acceptable for continued registration. Mitigation includes new/revised label statements to further protect human health and the environment. |
| Proposed Re-evaluation Decisions | ||
| Dimethomorph | PRVD2019-03 | Acceptable for continued registration. Proposed mitigation includes new/revised label statements to further protect human health and the environment. |
| Acephate, Updated Environmental Risk Assessment | PRVD2019-04 | Acceptable for continued registration for certain uses. Proposed mitigation includes new/revised label statements to further protect the environment. Proposed cancellation of other uses due to environmental risk concerns. |
| Chlorpyrifos | PRVD2019-05 | Acceptable for continued registration for certain uses. Proposed mitigation includes new/revised label statements to further protect the environment. Proposed cancellation of other uses due to environmental risk concerns. |
| Tebufenozide | PRVD2019-06 | Acceptable for continued registration. Proposed mitigation includes new/revised label statements to further protect human health and the environment. |
| Thiophanate-Methyl | PRVD2019-07 | Acceptable for continued registration for certain uses. Proposed mitigation includes new/revised label statements to further protect human health and the environment. Proposed cancellation of other uses due to health risk concerns. |
| Streptomyces strain K61 | PRVD2019-08 | Acceptable for continued registration. No further mitigation measures beyond clarifying label amendments were proposed. |
| Uniconazole-P | PRVD2019-09 | Acceptable for continued registration for certain uses. Proposed mitigation includes new/revised label statements to further protect the human health. Proposed cancellation of one use due to health risk concerns. |
| Pyriproxyfen | PRVD2019-10 | Acceptable for continued registration. Proposed mitigation includes new/revised label statements to further protect human health. |
| Fenhexamid | PRVD2020-01 | Acceptable for continued registration. Proposed mitigation includes new/revised label statements to further protect human health and the environment. |
| Cyromazine | PRVD2020-02 | Acceptable for continued registration for certain uses. Proposed mitigation includes new/revised label statements to further protect the environment. Proposed cancellation of other uses due to health risk concerns. |
| Proposed Special Review Decisions | ||
| Naled (17(1)) | PSRD2019-02 | Proposed cancellation of all uses due to human health and environmental risk concerns. |
| Naled (17(2)) | PSRD2019-03 | Proposed cancellation of all uses due to human health and environmental risk concerns. |
| Tetrachlorvinphos | PSRD2019-04 | Acceptable for continued registration for certain uses. Proposed mitigation includes new/revised label statements to further protect human health and the environment. Proposed cancellation of other uses due to health risk concerns. |