How to demonstrate foreign building compliance with drug good manufacturing practices (GUI-0080)

Download the alternative format
(PDF format, 568 KB, 36 pages)
Organization: Health Canada
Date published: 2018-01-18
Implementation: 2018-01-18
Replaces: Guidance on Evidence to Demonstrate Drug GMP Compliance of Foreign Sites (August 1, 2009)
Disclaimer
This document does not constitute part of the Food and Drugs Act (the Act) or its Regulations and in the event of any inconsistency or conflict between the Act or Regulations and this document, the Act or the Regulations take precedence. This document is an administrative document that is intended to facilitate compliance by the regulated party with the Act, the Regulations and the applicable administrative policies.
Table of contents
About this document
1. Purpose
This guide is for Canadian importers who want to list a foreign building on their drug establishment licence (DEL). It provides guidance on the type of information you should submit to support your DEL amendment application.
It will also help you understand how Part C, Division 2 (Good Manufacturing Practices) of the Food and Drug Regulations (FDR) applies to foreign buildings that supply Canadian importers with drugs (finished dosage forms (FDF) or active pharmaceutical ingredients (API), including any FDF and API intermediates) for import into Canada or import into Canada for future export.
How guidance documents work
Guidance documents like this one are meant to help industry and health care professionals understand how to comply with rules and regulations. They also provide guidance to Health Canada staff, so that the rules are enforced in a fair, consistent and effective way across Canada.
Guidance documents are administrative and do not have the force of law. Because of this, they allow for flexibility in approach. Use this guide to help you develop specific approaches that meet your specific needs.
Health Canada endeavours to provide timely guidance. These guidelines are not the only way GMP regulations can be interpreted, and are not intended to cover every possible case. However, in the case of a discrepancy between this guidance document and the regulations, the regulations will always take precedence. Other ways of complying with GMP regulations could be considered with proper scientific justification. Also, as new technologies emerge, different approaches may be required.
2. Scope
These guidelines apply to drugs for human and veterinary use imported from foreign building(s).
Information: The FDR have recently been amended to include APIs for veterinary use. The new regulations come into force on May 17, 2018. Importers of veterinary APIs will be permitted to continue importation without an establishment licence, provided that they have applied for one within 14 months after the coming into force of the applicable regulations. The information within this document will help you in submitting your application within the 14 month transition period that ends on July 17, 2019.
Foreign building licensable activities:
- fabrication
- packaging/labelling
- testing
Product category:
- pharmaceuticals
- finished dosage form (FDF)
- active pharmaceutical ingredient (API)
- biologicals
- finished dosage form (FDF)
- bulk process intermediates (BPI)
- medical gases
- radiopharmaceuticals
- vaccines
Information: The scope of this document does not include:
- atypical APIs
- drugs (FDF and API) used in clinical trials
- drugs (FDF and API) used for research and development
- excipients
- natural health products
3. Introduction
These guidelines interpret the good manufacturing practices (GMP) evidence regulatory requirements for importing drugs from foreign buildings into Canada. These guidelines were developed by Health Canada in consultation with stakeholders.
When a drug is fabricated, packaged/labelled, or tested outside of Canada, the foreign building where those activities occur must be listed on the Canadian importer's drug establishment licence (DEL). For the foreign building to be listed on the DEL, it must be deemed compliant with GMP requirements (as described in Part C, Division 2 of the FDR).
This guidance document outlines when you must submit evidence and what evidence must be submitted to support a foreign building's compliance with GMP requirements as a Canadian DEL holder or authorized representative. Health Canada will assess the evidence against the Food and Drugs Act (the Act) and its associated regulations.
It is ultimately your responsibility as a Canadian importer to ensure that drugs manufactured outside of Canada and imported into Canada are manufactured in accordance with GMP.
Important: As per C.01A.013, you must notify Health Canada within 15 days of any event that results in a foreign building contravening any of the applicable requirements of Division 2 of the FDR that may affect the quality, safety or efficacy of a drug.
Guidance
4. Listing foreign buildings
If you want to add a foreign building to your drug establishment licence (DEL), you are required to submit an application. Once the foreign building is added to your DEL, an application to maintain it may be required. The steps for meeting these requirements are outlined in this section.
Foreign buildings are listed on two different parts of a DEL, depending on the activities and the product type:
-
Foreign building annex (FB Annex)
Foreign buildings are listed on a FB Annex if they:
- fabricate, package/label or test finished dosage forms (FDF) and FDF intermediates
- fabricate, package/label or test sterile active pharmaceutical ingredients (API) and sterile API intermediates
- conduct release testing of non-sterile APIs, where the testing results are used to release the API for use in the fabrication of a FDF
- Active pharmaceutical ingredient foreign building annex (API FB Annex) or Table A
Foreign buildings are listed on a API FB Annex or Table A if they:
- fabricate, package/label non-sterile APIs and non-sterile API intermediates
- test non-sterile APIs and non-sterile API intermediates. This does not include release testing of non-sterile APIs, where the testing results are used to release the API for use in the fabrication of a FDF
New Evidence Required By Date (NERBY)
Until July 21, 2016, certain foreign buildings have been listed on the FB Annex of your DEL with an expiry date. However, the New Evidence Required By (NERBY) date has replaced the expiry date. You are no longer required to submit updated GMP evidence 250 days before the foreign building's expiry date. Instead, Health Canada's new policy that came into effect on July 21, 2016 is to submit an application to maintain the foreign building on the FB Annex of your DEL by the assigned NERBY date.
Health Canada assigns the NERBY date using a risk-based approach. The NERBY date is often four years, calculated from the start date of the regulatory/qualified authority inspection submitted to demonstrate GMP compliance. Health Canada may issue a shortened or extended NERBY date for a foreign building based on several factors, including (but not limited to):
- the compliance history of the building
- the category, type and dosage form of the drug (see Appendix A for definitions)
The assignment of a NERBY date is based on the location of the foreign building and the annex that it is listed on (see Table 1.0: Assignment of NERBY dates below).
Mutual Recognition Agreements (MRA)
Health Canada is a partner in several mutual recognition agreements (MRAs) covering GMP compliance programs for drug/medicinal products. MRAs are established following a joint evaluation of the regulatory frameworks in place in each partner's jurisdiction for the purpose of establishing equivalency. The equivalency of the MRA partners' GMP compliance programs has been determined; therefore Health Canada will consider the Certificate of Compliance (CoC) as evidence to support the GMP compliance of a foreign building.
Information: APIs are temporarily excluded from MRAs since not all MRA partners have an API GMP compliance program that has been assessed to meet the requirements of the MRA. As the scope of MRAs evolves to include APIs, the list of countries with the updated MRAs (including APIs) will be available online.
Table 1.0 summarizes whether a NERBY date is assigned or not based on the location of the foreign building and the annex that it is listed on. If a NERBY date is not assigned to a foreign building, an application to provide more recent GMP evidence to maintain the foreign building on your DEL is not required.
| DEL annex (Foreign Building Annex or Active Pharmaceutical Foreign Building Annex) |
Location of Foreign Building | NERBY assigned (Yes/No) |
|---|---|---|
| FB Annex |
|
No |
| FB Annex |
|
Yes |
| API FB Annex/
Table A |
|
No |
4.1 Foreign building annex (FB Annex)
For a building to be listed on your FB Annex, the location of the foreign building dictates what to include in your application and when it needs to be filed with Health Canada. Follow the appropriate instructions for buildings:
- located in an MRA country (for product categories/activities covered under the MRA) (see section 4.1.1)
- located in an MRA country (for product categories/activities not covered under the MRA) (see section 4.1.2)
- not located in an MRA country (see section 4.1.2)
Information: To find out whether the foreign building you want to add to your FB Annex is located in an MRA country (and whether the product categories and activities are covered under that MRA), please see: Mutual Recognition Agreements.
4.1.1 Foreign buildings located in an MRA country (for product categories /activities covered under the MRA)
To add a foreign building to your DEL where the foreign building is located in an MRA country (for product categories or activities covered by the MRA), include the following information in your application:
- cover letter (see section 5.1: cover letter)
- all applicable sections of the Drug Establishment Licence Application: Forms and Instructions (FRM-0033)
Tip: All applications must be submitted to ELapplicationLE@hc-sc.gc.ca
Health Canada assessment
Once your application is received, Health Canada will request the certificate of compliance (CoC) directly from the applicable MRA partner. There are several possible outcomes resulting from Health Canada's request for a CoC:
-
The MRA partner provides Health Canada with the CoC(s). The information in the CoC(s) is compared to the scope indicated in the application. This includes verifying the product category, dosage forms and activities (fabricate, package/label or test) conducted by the foreign building. The foreign building will be added to the FB Annex of your DEL and you will receive an email including a supplement to the FB Annex, or an amended FB Annex for all activities and categories of drugs (and sterility) requested that are covered by the CoC(s).
For any activities and dosage forms requested that are not covered by the CoC(s), refer to section 4.1.2 of this document for information on filing an application when a foreign building is located in an MRA country but the product categories or activities are not covered under the MRA.
- The MRA partner informs Health Canada that a CoC cannot be issued due to the foreign building no longer holding a valid permit, licence or other authorization. Health Canada will notify you of this and your application will not be processed further.
Since NERBY dates are not assigned to foreign buildings located in an MRA country for activities and product categories covered under the MRA, you do not need to submit an application to maintain the foreign building on your DEL. You may continue to import drugs from the foreign building unless we inform you otherwise. Health Canada will continue to request CoCs from MRA partners to confirm that the foreign building(s) continue to hold a valid CoC.
If the scope of the CoC(s) no longer covers all your activities and dosage forms, the foreign building will be maintained on the FB Annex of your DEL and you will receive an email including an amended FB Annex for the activities and categories of drugs (and sterility) still covered by the CoC(s).
If the foreign building no longer holds a valid permit, licence or other authorization, you will receive an email and an amended FB Annex with the foreign building removed.
Important: As per C.01A.015 of the FDR, "an importer of a drug that is fabricated, packaged/labelled or tested in an MRA country at a recognized building shall immediately notify the Minister if the fabricator, packager/labeller or tester indicated in the importers establishment licence no longer holds a valid permit, licence or other authorization issued by the regulatory authority that recognized that building."
See Appendix D for a summary of the Health Canada process for adding foreign buildings to a DEL.
4.1.2 Foreign buildings located in an MRA country (for product categories or activities not covered under the MRA) or foreign buildings not located in an MRA country
To add or maintain a foreign building on your DEL where the foreign building is located in an MRA country (for product categories or activities not covered by the MRA), or the foreign building is not located in an MRA country, you must submit an application (cover letter and FRM-0033), along with recommended GMP evidence (see section 5: recommended GMP evidence for details on how to file the recommended GMP evidence).
Health Canada assessment
When adding or maintaining a foreign building under the circumstances noted above, your application (cover letter and FRM-0033) will be verified for accuracy and completeness, followed by screening and an assessment of the GMP evidence.
The evidence submitted is verified during GMP screening as per the requirements outlined in section 5 of this document. If any deficiency is identified during screening, Health Canada will send you a screening deficiency letter outlining the missing information. You must address all the deficiencies within the time period specified in the request. If you fail to provide all requested information within the specified time period, or the submitted information is incomplete or deficient, Health Canada will send you a screening rejection letter that outlines the reason(s) the application was rejected. You may submit a new application once you have obtained all the required information.
If no deficiency is identified or once all identified deficiencies are resolved, Health Canada will accept your application and GMP evidence for assessment and will send you a screening acceptance notice.
Health Canada will conduct an assessment to verify that the GMP evidence submitted demonstrates the foreign building's compliance with Division 2 of the FDR. We may issue a request for other evidence (see section 5.6 for more information) during the assessment. You will be required to provide the additional evidence within the time period specified in the request. If you fail to provide a complete response, or the response you provide is deemed insufficient, we may issue a rejection letter that outlines the reason(s) the application was rejected. You may submit a new application once you have obtained all the required information.
After Health Canada's assessment of the GMP evidence, a rating will be assigned to the foreign building:
- Compliant - Issued when the evidence is deemed acceptable and demonstrates GMP compliance with Division 2 of the FDR. The foreign building will be added or maintained on the FB Annex of your DEL.
- Non-Compliant - Issued when the evidence is deemed unacceptable and does not demonstrate GMP compliance with Division 2 of the FDR. The foreign building will not be added or will be removed from the FB Annex of your DEL.
Terms and conditions may be added to your DEL if other factors (such as the foreign building's compliance history, drug type, medical necessity, category, dosage form or activities conducted at the foreign building) require additional oversight.
Important: As per section C.01A.011, it is the importer's responsibility to comply with the terms and conditions listed on the DEL.
Health Canada will communicate the outcome of the assessment to you by email and issue a supplement to the FB Annex (or an amended FB Annex).
A NERBY date will be assigned as a result of this Health Canada assessment. It is your responsibility to submit an application along with updated recommended GMP Evidence to maintain the foreign building on the FB Annex of your DEL by the assigned NERBY date.
See Appendix D for a summary of the Health Canada process for adding foreign buildings to a DEL.
4.2 Active pharmaceutical ingredient foreign building annex (API FB Annex) or Table A
To add a foreign building to your DEL (API FB Annex or Table A); include the following information in your application:
- cover letter
- all applicable sections of the Drug Establishment Licence Application: Forms and Instructions (FRM-0033)
- a completed Table A (part of FRM-0033)
Important: You must not add a foreign building to your DEL (API FB Annex or Table A) if you do not have GMP evidence that supports the foreign building's compliance with Division 2 of the FDR. The GMP evidence you indicate on Table A must meet the evidence requirements outlined in section 5 of this document.
To maintain a foreign building on your DEL (API FB Annex or Table A) an application is not required since foreign buildings listed on your API FB Annex or Table A are not assigned a NERBY date.
Health Canada assessment
Health Canada will assess your application. The information listed in Table A (part of FRM-0033) will be verified for accuracy and completeness. If any deficiencies are identified, Health Canada will send you a screening deficiency notice outlining the missing information. You must address all the deficiencies within the time period specified in the request. If you fail to provide all requested information within the specified time period, or the submitted information is incomplete or deficient, Health Canada will issue you a rejection of application notice that outlines the reason(s) the application was rejected. You may submit a new application once you have obtained all the required information.
If no deficiency is identified or once all identified deficiencies are resolved, Health Canada will send you an acknowledgement of application acceptance along with an API FB Annex or an approved Table A.
Health Canada assesses information related to foreign buildings that fabricate, package/label or test non-sterile APIs on an ongoing basis. Health Canada uses a risk-based approach to select foreign buildings for the assessment of GMP evidence. This includes the consideration of factors such as: the type of GMP evidence (corporate audit or inspection by qualified or regulatory authority) available; and the compliance history of the foreign building. Health Canada may request, at any time, that you submit the evidence to demonstrate the foreign building's GMP compliance.
If you receive such a request, follow the instructions provided. Such a request will generally ask that you submit the following information:
Submit the information requested to: foreign_site_etranger@hc-sc.gc.ca account.
Health Canada will assess the evidence received as described in section 4.1.2. A NERBY date is not assigned to foreign buildings listed on the API FB Annex or Table A of your DEL. During the assessment of GMP evidence, you may continue to import drugs from the foreign building unless you are informed otherwise.
See Appendix D for a summary of the Health Canada process for adding foreign buildings to a DEL.
5. Recommended GMP evidence
This section outlines the recommended GMP evidence required to demonstrate the GMP compliance of foreign buildings that fabricate, package/label or test FDF and/or API on your behalf.
If the original information is not available in either of the official languages (English or French), you must provide a copy of the original information and a translated copy in English or French, along with an attestation of the accuracy of the translation, signed by the certified translator.
How to submit the evidence to Health Canada
All applications (cover letter and FRM-0033) must be submitted to Health Canada by the Canadian importer. The recommended GMP evidence to support an application should also be submitted by the importer. However, importers may be unable to submit some of the recommended evidence, in which case Health Canada will accept the evidence directly from the foreign building under the following conditions:
- The evidence must be submitted after the importer has filed an application (cover letter and FRM-0033) and received an application tracking number in the Acknowledgement of Application Acceptance from the Establishment Licence Unit.
- The evidence must be submitted to the Establishment Licence Unit (ELapplicationLE@hc-sc.gc.ca) and clearly reference the application tracking number.
Evidence submitted without an application tracking number or submitted to the wrong location will not be accepted and may lead to the rejection of the application. It is the importer's responsibility to ensure that Health Canada receives a complete application along with all GMP evidence.
Letter of authorization (LoA) - evidence already submitted to Health Canada
In certain instances, the GMP evidence required to support your application may have been submitted to Health Canada as part of an application by another importer or by the foreign building. In those cases, Health Canada will accept a Letter of Authorization (LoA) from the party (importer or the foreign building) that previously submitted these documents. This will allow you to reference the GMP evidence to support your application. The LoA must be submitted with your application (cover letter and FRM-0033) only under the following conditions:
- LoA issued by the foreign building to an importer directly (this is only applicable if the foreign building submitted the evidence directly to Health Canada)
- LoA issued by one importer to another importer (this is only applicable if the importer providing the LoA has submitted the evidence directly to Health Canada)
- LoA must reference an inspection report that covers the activities and dosage forms that you are requesting
If the conditions stated above are met, the LoA must meet the following requirements:
- provided on the letterhead of the issuing company
- clearly state that authorization is being provided to Health Canada
- clearly state the name and address of the foreign building
- specify which GMP evidence documents are being referenced (this must include the date of the inspection)
- signed and dated (with complete signature block including email address)
- demonstrate that the GMP evidence has been previously submitted to Health Canada (i.e. application tracking number, copy of acknowledgement of application acceptance, or screening acceptance notice)
Important: Although Health Canada may accept evidence directly from the foreign building, it is important for importers to continue to fulfill their requirements as outlined in the FDR. Health Canada strongly recommends written agreements between importers and foreign buildings include provisions to ensure importers are able to fulfill their regulatory obligations, including provisions that compel the foreign buildings to provide importers with sufficient information necessary to demonstrate compliance with Canadian regulatory requirements.
5.1 Cover letter
All applications should include a cover letter. The following information is required for all cover letters:
- reason for filing and what is included within the application
- a reference to correspondence with Health Canada, prior to filing, if applicable
- drug establishment licence (DEL) Number
5.2 FRM-0033
You must complete all applicable sections of the Drug Establishment Licence Application Form (FRM-0033) accurately before submitting your application to Health Canada.
5.3 Inspection report
Health Canada will accept the final and most recent (i.e. within the last 3 years), inspection report signed and issued by:
- Health Canada (Exit Notice)
- regulatory authority (refer to Appendix A for the definition)
- qualified authority (refer to Appendix A for the definition)
- World Health Organization (WHO) - applicable to foreign buildings listed on API FB Annex or Table A
- European Directorate for the Quality of Medicines (EDQM) - applicable to foreign buildings listed on API FB Annex or Table A
- corporate/consultant auditor (see section 5.3.1)
Tip: If you submit a cover letter along with a Health Canada assessment report (Exit Notice) as evidence of GMP compliance of a foreign building, none of the other recommended evidence should be submitted.
The inspection report should cover the activities and dosage forms that you are requesting. If there are multiple inspection reports available for a foreign building from different regulatory/qualified authorities, you must submit the most recent and applicable inspection report that covers the activities and dosage forms that you are requesting.
Information: An on-site evaluation (OSE), conducted by the Biologics and Genetic Therapeutics Directorate, is not considered sufficient on its own to demonstrate the GMP compliance of a foreign building as it is not intended to cover all applicable sections of Part C, Division 2 of the FDR.
5.3.1 Corporate/consultant audits
If no inspection reports by regulatory/qualified authorities are available, you may submit a corporate or consultant audit report to Health Canada to demonstrate GMP compliance of foreign buildings that manufacture pharmaceuticals (FDF or API) only for the following specific activities/ product types:
- fabricating, packaging/labeling or testing of over-the-counter (OTC) drugs
- fabricating, packaging/labeling or testing of medical gases
- fabricating, packaging/labeling or testing of ethical drugs
- sterilization of packaging materials for drugs that will be aseptically filled without terminal sterilization.
A Health Canada form titled "Good Manufacturing Practices - Audit Report Form (FRM-0211)" is available and should be used to ensure that all applicable Canadian GMP requirements are assessed. The use of this template is strongly recommended as it may facilitate Health Canada's assessment.
Important: A report of a self-audit conducted by the foreign building is not considered acceptable GMP evidence.
Ensure the following information accompanies the corporate or consultant audit report as part of your application:
- a justification for submitting a consultant or corporate audit report (include in your cover letter)
- the resume of the individual(s) performing the audit, which clearly demonstrates their qualifications and experience (the individual(s) must have experience in and knowledge of Canadian GMP- see regulatory requirements for personnel in section C.02.006 of the Good Manufacturing Practices (GMP) Guidelines (GUI-0001)
- any deficiencies, noted during the audit, are to be risk classified using the document: Risk Classification of GMP Observations (GUI-0023) (the classification of deficiencies will be reviewed by Health Canada)
- the audit report signed and dated by the auditor
- If an inspection report more than 3 years old from a Regulatory Authority or a Qualified Authority is available, the report should accompany the consultant or corporate report
Important: If the last two applications for a foreign building were based on a corporate/consultant audit, Health Canada may select the site for an on-site assessment. See section 7: Health Canada on-site assessment of a foreign building for information related to Health Canada on-site assessments of foreign buildings.
5.4 Corrective actions
Submit a copy of the corrective actions taken as a result of the inspection, signed by a responsible official of the foreign building.
In the case of a corporate/consultant audit, you must provide documentation indicating that the auditor has reviewed the company's corrective actions and found them acceptable.
5.5 Site master file
Submit a copy of the most recent, signed and dated site master file (SMF) or site reference file (SRF) for foreign buildings that fabricate, package/label or test FDFs or APIs. In the situation where a SMF or SRF is not available, you may submit an equivalent document(s) that fulfills the requirements outlined in the PIC/S Annex 1: Explanatory Notes for Industry on the Preparation of a Site Master File.
A quality manual may be submitted for foreign buildings that only conduct the activity of testing, secondary packaging or sterilization of packaging material.
5.6 Other evidence
Health Canada may ask you for other evidence during our assessment. You must provide this additional evidence within the time period specified in the request. If the evidence is not available, you must provide a written justification of why the evidence cannot be submitted.
Examples of other evidence may include but are not limited to: standard operating procedures (SOP), written quality agreements and annexes or appendices referred to in the corrective action response(s).
Important: It is the importer's responsibility to maintain all evidence of the foreign buildings' GMP compliance at their Canadian site (as outlined in sections C.02.012 and C.02.020-C.02.024 of the FDR).
6. Requesting an extension of the NERBY date
You may submit a request for an extension of the NERBY date in certain circumstances, for example:
- An inspection by a regulatory or qualified authority has taken place but there is a delay in the issuance of the inspection report.
- An inspection by a regulatory or qualified authority is expected or scheduled to take place.
Your extension request must be submitted before your assigned NERBY date. Extensions will not be granted for requests submitted after the NERBY date.
Your request should include the following:
- cover letter
- all applicable sections of the Drug Establishment Licence Application: Forms and Instructions (FRM-0033)
Tip: The request(s) must be submitted to ELapplicationLE@hc-sc.gc.ca.
Your cover letter for this request should also include:
- the reason for the extension request
- dates of all recent inspections (within the last 4 years) and information on any upcoming inspections by a recognized/qualified authority
- a statement attesting that the foreign building has not undergone any recent inspections resulting in critical observations or the issuance of a non-compliant or unacceptable rating
- a statement indicating whether there have been any changes in quality assurance management, equipment or manufacturing processes since the most recent inspection by a recognized/qualified authority (provide any relevant documentation)
Health Canada assessment
Health Canada will assess your request for extension of the NERBY date. As long as an application is being assessed or unless otherwise indicated, the foreign building will continue to be considered GMP compliant and you may continue to import drugs from the foreign building in accordance with your DEL and the FDR. Health Canada's assessment will take the following factors into consideration:
- compliance history of the foreign building
- most recent inspection report, including:
- date
- result
- repeat observations
- any major changes since last inspection to:
- quality assurance (QA) management
- manufacturing processes
- equipment
- drug type
- licensable activities conducted at the foreign building
Health Canada's assessment will result in one of two outcomes:
-
Extension granted - Health Canada determined that the information provided supports an extension of the NERBY date. This outcome will be communicated to you by email and a supplement to the FB Annex will be issued with the extended NERBY date. Therefore, you may continue to import from the foreign building for the period of the extension. However, you will be required to submit an application to Health Canada as soon as the inspection report becomes available, irrespective of what the revised NERBY date is.
-
Extension not granted - Health Canada determined that the information provided does not support an extension of the NERBY date. This outcome will be communicated to you by email and will include the reason(s) for not granting an extension. The foreign building may be removed from the FB Annex of your DEL in which case you are no longer authorized to import from this building.
Important: Health Canada may cancel any extension to a NERBY date previously granted, or may change a NERBY date previously assigned, if Health Canada has grounds to believe that the foreign building is not in compliance with GMP requirements. Health Canada will notify you and provide a grace period before an extension is cancelled unless circumstances are such that the health and safety of consumers are at risk and immediate action is necessary.
7. Health Canada on-site assessment of a foreign building
You may submit an application requesting that Health Canada conduct an on-site assessment of a foreign building. Your application should include:
- cover letter
- Good Manufacturing Practices - Request for assessment of a Foreign Building Form (FRM-0213)
Tip: Submit your application(s) to ELapplicationLE@hc-sc.gc.ca.
Health Canada will send you an acknowledgement by email within 1 month of receiving your application.
Health Canada assessment
Upon receipt of your application, Health Canada will assess the information provided in the cover letter and FRM-0213. Health Canada may issue a request for clarification during the assessment. Send your response directly to the person and/or account outlined in the request within the specified time period.
During the assessment, Health Canada will evaluate the following:
- all licensable activities conducted by the foreign building
- all categories of drug(s) and dosage forms manufactured by the foreign building
- information about the drug manufactured at the foreign building (product type, product category, product dosage form or medical necessity of product)
- compliance history of foreign building
- if other qualified or regulatory authorities are planning to inspect the foreign building
Health Canada's assessment is typically conducted within 1 month of the acknowledgement of receipt, but may be delayed if responses to clarification requests are not received promptly or due to other considerations.
Health Canada's assessment will result in one of two outcomes:
-
Decision to assess - Based on its assessment, Health Canada has determined that an on-site assessment will be conducted. Health Canada will send you an acceptance email informing you that an inspector will contact you to make scheduling arrangements and request preparatory documents, contract and logistical arrangements.
-
Decision not to assess - Based on its assessment, Health Canada has determined that an on-site assessment will not be conducted at this time. Health Canada will send you an email declining your request and providing you with further guidance.
Should an on-site assessment be conducted, there are two possible outcomes of the assessment:
- If the on-site assessment results in a compliant rating, the foreign building will be added or maintained on the FB or API FB Annex of the DEL.
- If the on-site assessment results in a non-compliant rating, the foreign building will not be added or will be removed on the FB or API FB Annex of the DEL.
Terms and conditions may be added to your DEL if other factors (such as the foreign building's compliance history, drug type, medical necessity, category, dosage form or activities conducted at the foreign building) require additional oversight.
Information: Health Canada assesses information related to foreign buildings on an ongoing basis and uses a risk-based approach to proactively select foreign buildings for a Health Canada on-site assessment including, but not limited to, consideration of the following:
- the type of GMP evidence available (corporate audit or inspection by qualified or regulatory authority)
- compliance history of the foreign building
- possibility of a joint inspection with other qualified or regulatory authority
- medical necessity of the drug product
- foreign buildings with no available GMP evidence and that are no longer eligible for a NERBY date extension
If the foreign building selected is listed on the FB or API FB Annex of your DEL, you will be contacted and notified of our intent to conduct an on-site GMP assessment and your acceptance of the assessment will be requested.
Appendices
Appendix A - Glossary
Acronyms
- API:
- Active pharmaceutical ingredient
- API FB Annex:
- Active pharmaceutical ingredient foreign building annex
- BPI:
- Bulk pharmaceutical ingredient
- C:
- Compliant
- CoC:
- Certificate of compliance
- DEL:
- Drug establishment licence
- DIN:
- Drug identification number
- EDQM:
- European directorate for the quality of medicines
- FB:
- Foreign building
- FB annex:
- Foreign building annex
- FDA:
- Food and Drug Act
- FDF:
- Finished dosage form
- FDR:
- Food and Drug Regulations
- GMP:
- Good manufacturing practices
- LoA:
- Letter of authorization
- MRA:
- Mutual recognition agreement
- NC:
- Non-compliant
- NERBY:
- New evidence required by
- OSE:
- On-site evaluation
- PIC/S:
- Pharmaceutical inspection cooperation scheme
- SMF:
- Site master file
- SOP:
- Standard operating procedure
- SRF:
- Site reference file
- WHO:
- World health organization
Terms
Information: These definitions explain how terms are used in this document. If there is a conflict with a definition in the Food and Drugs Act (FDA) or Food and Drug Regulations (FDR), the definition in the Act/Regulations prevails.
- Acknowledgement of Application Acceptance
- A document issued by Health Canada once an application is received and accepted.
- Active ingredient
- A drug that, when used as a raw material in the fabrication of a drug in dosage form, provides its intended effect (FDR C.01A.001).
- Active pharmaceutical ingredient (API)
- An active ingredient that is used in the fabrication of a pharmaceutical (FDR C.01A.001).
Note: For the purpose of these guidelines, this definition also includes: an active ingredient that is used in the fabrication of a drug that is of non-biological origin and that is listed in Schedule C to the Act.
- Active pharmaceutical ingredient foreign building annex (API FB Annex)
- A listing of foreign buildings that manufacture non-sterile APIs. This annex is part of the drug establishment licence (DEL).
- Active pharmaceutical ingredient (API) Intermediate
- A material (isolated or not) produced during steps of the processing of an API that undergoes further molecular change or purification before it becomes a final API.
- Atypical active pharmaceutical ingredient
- Active ingredients used in human pharmaceutical drugs, which are also currently used as pharmaceutical excipients, or as ingredients in natural health products (NHP), foods, cosmetics and that meet recognized standards other than GMPs.
- Bulk process intermediate (BPI)
- An active ingredient that is used in the fabrication of either a drug of biological origin that is listed in Schedule C to the FDA or a drug that is listed in Schedule D to the FDA (FDR C.01A.001).
- Certificate of compliance (CoC)
- A certificate issued by a regulatory authority attesting to the GMP compliance of a recognized building in that country. In Canada, a CoC is issued by Health Canada.
- Corrective action
- Steps taken by the regulated party to address the specified deficiencies (non-compliance with the law). Corrective action is taken to prevent a deficiency from happening again.
- Compliance history
- A foreign buildings history of conformity with good manufacturing practices as outlined by legislative or regulatory requirements.
- Compliant (C)
- At the time of the assessment, the foreign building has demonstrated that the activities it conducts comply with the FDA and its associated Regulations. A "C" rating does not mean that there are no observations or corrective actions required.
- Critical observation
- Observation of a critical deviation from the Food and Drug Regulations that describes a situation that may produce an immediate or latent health risk as a result of the absence of drug safety information. Observations that involve fraud, misrepresentation or falsification under the Food and Drugs Act and Regulations of data are also considered critical.
- Dosage form
- A drug that has been processed to the point to where it is now in a form that may be administered in individual doses (unless otherwise defined in the FDR).
- Drug
- Any substance or mixture of substances manufactured, sold or represented for use in;
- the diagnosis, treatment, mitigation or prevention of a disease, disorder or abnormal physical state, or its symptoms, in human beings or animals,
- restoring, correcting or modifying organic functions in human beings or animals, or
- disinfection in premises in which food is manufactured, prepared or kept
(Section 2 of the Food and Drugs Act)
In Division 1A and Division 2 of the Food and Drug Regulations, "drug" does not include a dilute drug premix, a medicated feed as defined in subsection 2(1) of the Feeds Regulations, 1983 or a drug that is used only for the purposes of an experimental study in accordance with a certificate issued under section C.08.015 (C.01A.001(2)).
- Drug establishment licence (DEL)
- A licence issued to a person in Canada to conduct licensable activities in a building which has been inspected and assessed as being in compliance with the requirements of Divisions 2 to 4 of the Food and Drug Regulations
- Drug identification number (DIN)
- A drug identification number (DIN) is a computer-generated eight-digit number assigned by Health Canada to a drug prior to being marketed in Canada. It uniquely identifies any drugs sold in a dosage form in Canada. It is located on the label of prescription and over-the-counter drugs that have been evaluated and authorized for sale in Canada. A DIN uniquely identifies the following drug characteristics: manufacturer, drug product name, active ingredient(s), strength(s) of active ingredient(s), pharmaceutical form, and, route of administration.
- Ethical drug
- A drug that-according to federal legislation-does not require a prescription, but is generally prescribed by a medical practitioner (e.g., nitroglycerine).
- Fabricate
- To prepare and preserve a drug for the purposes of sale (FDR C.01A.001). This definition applies to Divisions 1A, 2, 3 and 4 of the FDR.
- Finished Dosage Form (FDF) Intermediate
- Any physical mix, starting when any 2 ingredients (e.g., active ingredient, anti-oxidant, preservative, filler, binder, solvent, etc.) are first added to the drug lot being manufactured, and before it becomes a drug in dosage form. Partially processed drug product intermediate, in-process drugs or bulk drug are examples of drug in dosage form intermediates.
- Foreign building
- A building outside of Canada where the following licensable activities are conducted for drugs that are sold in Canada: fabrication, packaging/labelling, and/or testing.
- Foreign building annex
- A listing of foreign buildings that have been assessed by Health Canada as being compliant with the requirements of Divisions 2 to 4 of the FDR. This annex is part of the DEL.
- Letter of Authorization (LoA)
- A letter written and signed by the party who submitted GMP evidence documents permitting Health Canada access to the evidence on behalf of the importer.
- Medical gas
- "Any gas or mixture of gases manufactured, sold or represented for use as a drug" (FDR C.02.002). Refer to the Good Manufacturing Practices (GMP) for Medical Gases (GUI-0031) for information.
- MRA country
- A country that is a participant to a mutual recognition agreement with Canada (FDR C.01A.001).
- Mutual recognition agreement (MRA)
- "An international agreement that provides for the mutual recognition of compliance certification for Good Manufacturing Practices for drugs" (FDR C.01A.001).
- New evidence required by (NERBY)
- The date by which new evidence is required to be submitted to Health Canada as part of an application to maintain a foreign building on a DEL.
- Non-compliant (NC)
- At the time of the assessment, the foreign building has not demonstrated that the activities it conducts comply with the FDA and its associated regulations.
- Over-the-counter (OTC)
- A non-prescription drug which still requires a market authorization.
- Package/label
- To put a drug in its immediate container or to affix the inner or outer label to the drug (FDR C.01A.001). This includes the repackaging and relabeling of previously packaged and labelled drugs.
- Product category
- For the purpose of this guidance, includes pharmaceutical, active ingredient, vaccine, biologic, radiopharmaceutical, controlled drugs and narcotics, or any other product category designated by the Minister.
- Product type
- For the purpose of this guidance:
- Sterile: prescription, OTC, veterinary, category IV
- Non-sterile: prescription, OTC, medical gas, veterinary, category IV
- Qualified authority
- A member of the Pharmaceutical Inspection Cooperation/Scheme (PIC/S).
- Regulatory authority
- A government agency or other entity in an MRA country that has a legal right to control the use or sale of drugs within that country and that may take enforcement action to ensure that drugs marketed within its jurisdiction comply with legal requirements (FDR C.01A.001).
- Screening acceptance notice
- A document issued by Health Canada once GMP evidence submitted with an application has been determined acceptable for review as per the recommend GMP evidence requirements in GUI-0080.
- Test
- To perform any examinations, evaluations and assessments as specified under Division 2 of the FDR.
- Written agreement
- A formal document between parties (e.g., the Canadian DEL holder and the contractor), that defines the responsibilities and duties of both parties for all aspects of a drug's quality. For more guidance on the content of a written agreement refer to GUI-0001.
Appendix B - References
Laws and regulations
Forms
- Drug Establishment Licence Application Form (FRM-0033)
- Good Manufacturing Practices - Audit Report Form (FRM-0211)
- Good Manufacturing Practices - Request for Inspection of a Foreign Site Form (FRM-0213)
Good manufacturing practices
- Good Manufacturing Practices (GMP) for Active Pharmaceutical Ingredients (APIs) Guidelines (GUI-0104)
- Good Manufacturing Practices (GMP) Guidelines - 2009 Edition, Version 2 (GUI-0001)
- Risk Classification of Good Manufacturing Practices (GMP) Observations (GUI-0023)
Other related documents
- Compliance and Enforcement Policy (POL-0001)
- Guidance on Drug Establishment Licences and Drug Establishment Licensing Fees (GUI-0002)
- Health Canada Decision-Making Framework for Identifying, Assessing, and Managing Health Risks - August 1, 2000
- Mutual Recognition Agreements
International guidance documents
- Pharmaceutical Inspection Co-operation Scheme
- PIC/S Annex 1: Explanatory Notes for Industry on the Preparation of a Site Master File
- European Directorate for the Quality of Medicines & HealthCare
Appendix C - Questions and answers
Below is a select list of questions received from industry members, and Health Canada's answers.
-
What do I do if no new evidence is available by the NERBY date?
You may submit a request for a Health Canada on-site assessment. You may also submit a request to extend the NERBY date if an inspection by a regulatory or qualified authority has taken place but there is a delay in the issuance of the inspection report or if an inspection by a regulatory or qualified authority is scheduled to take place.
-
Is a certificate of GMP compliance (CoC) issued by an MRA country for a foreign building not located within their jurisdiction considered sufficient evidence for Health Canada to conduct their assessment?
No. A CoC issued by an MRA country for a foreign building located outside of their jurisdiction is not considered sufficient evidence. However, a copy of the CoC may be submitted along with the full inspection report.
-
Would Health Canada conduct an on-site assessment of a foreign building if asked by a sponsor, to support the GMP compliance for a new drug submission?
Yes. Health Canada may consider conducting an on-site assessment of a foreign building to demonstrate GMP compliance in support of a new drug submission. Health Canada will assess the need for an on-site assessment following the guidelines outlined in section 7 of this document.
-
Why are foreign buildings that manufacture sterile APIs listed on the FB Annex and not on the API FB Annex of the DEL?
The FB Annex of a DEL lists all the foreign buildings that manufacture finished dosage forms (FDF). Since the sterilization of APIs (not terminally sterilized) is considered as an FDF manufacturing step, sterile APIs are listed on the FB Annex.
-
Is GMP evidence only required for the foreign API sites imported from directly, or also for the API sites used by third party FDF fabrication/packaging/testing sites?
GMP evidence must be available for all sites involved in the fabrication of the API, not just the last processing step. The API foreign buildings used by a third party FDF fabricator should be listed in Table A and acceptable GMP compliance evidence must also be available for API buildings used by a third party FDF fabrication, packaging/labeling and testing sites.
As stated in section C.02.003 of the Food and Drug Regulations, no importer shall sell a drug unless it has been fabricated, packaged/labeled, tested and stored according to Division 2 of the Regulations (GMP requirements). Also, as stated in C.02.003.3, no person shall use an active ingredient in the fabrication of a drug unless it is fabricated, packaged/labeled, tested and stored according to GMP requirements.
-
Are there biological veterinary drugs?
Yes, there are veterinary drugs that are biological. The term "veterinary biologic" refers to veterinary vaccines and select classes of immunomodulators that fall within the scope of the Canadian Centre for Veterinary Biologics at the Canadian Food Inspection Agency. The term "biological veterinary drug" covers all other products that fall within the scope of this document.
-
Are the results of Health Canada's assessments of foreign building GMP compliance available publicly?
As a regulator, Health Canada plays an important role in protecting the health and safety of Canadians and is committed to greater transparency and openness to further strengthen trust in our regulatory decisions. As such, the latest results from Canada's drug and health product inspections are posted online on the Drug Health Product Inspection database.
Appendix D - Summary of Health Canada process
The diagram below shows Health Canada's process for adding or maintaining foreign buildings to drug establishment licences (DELs), depending on the situation
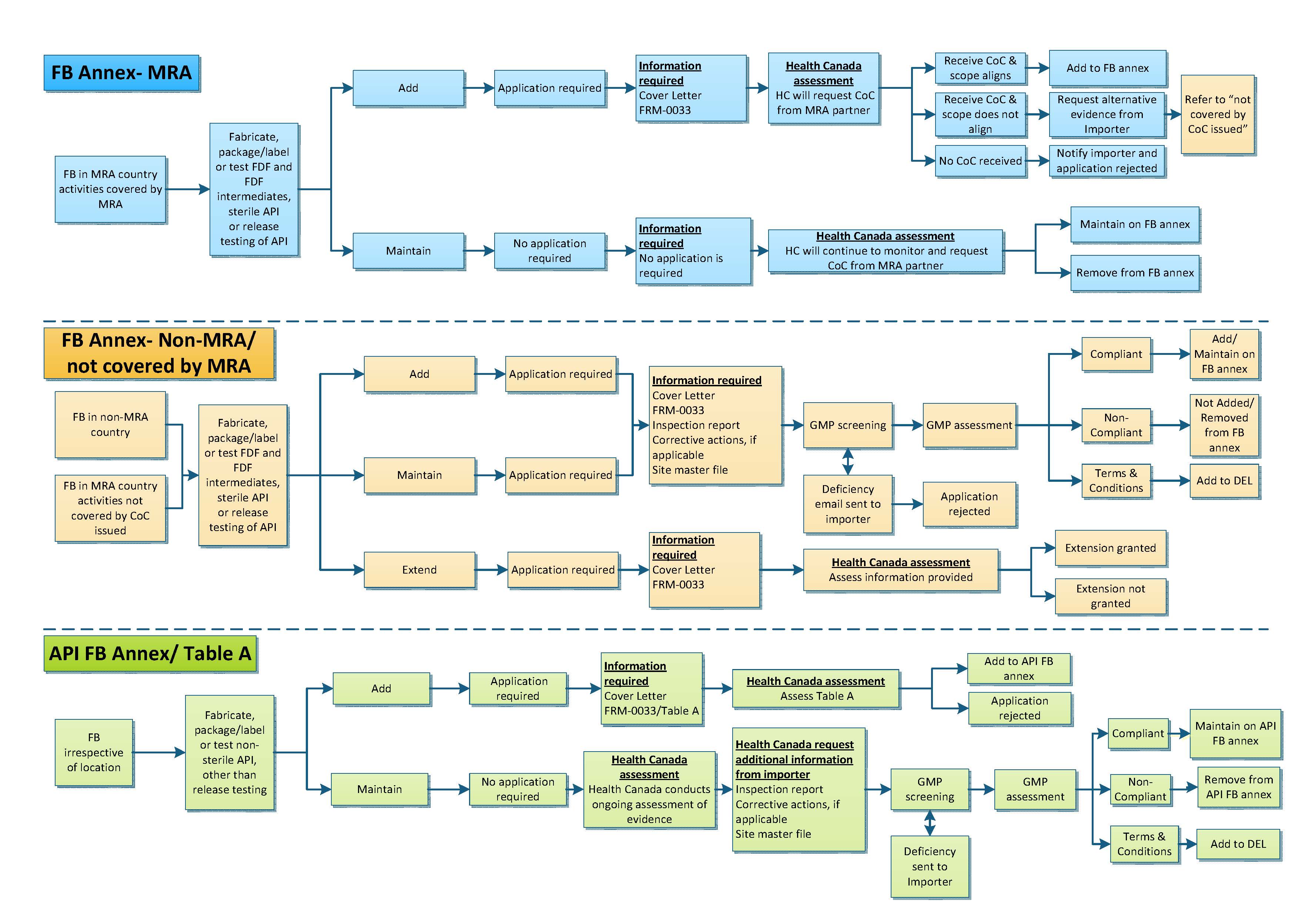
Figure 1 - Text description
The diagram shows three different flowcharts that describe what happens when an application to add or maintain a foreign building on a drug establishment license is submitted to Health Canada.
The first flowchart – FB Annex-MRA
The flowchart outlines the process for a foreign building located in an MRA country for activities covered by the MRA that should be listed on the foreign building annex of a Drug Establishment Licence. The foreign building can fabricate, package, label or test finished dosage forms, sterile active pharmaceutical ingredients or release testing of sterile and non-sterile APIs.
To add a foreign building to a drug establishment license an application is required. The application should include a cover letter and Form-0033. Once the application is received, Health Canada will request the certificate of compliance from the MRA partner in order to conduct an assessment. If the scope of the certificate of compliance aligns with the scope requested in the application, the foreign building is added to the foreign building annex. If the scope does not align a request is sent to the importer to provide alternate evidence. If no alternate evidence is provided the application is rejected and a new application is required. If the MRA partner informs Health Canada that there is no certificate of compliance for the foreign building, Health Canada will notify the importer and reject the application.
To maintain a foreign building to a drug establishment license no application is required. Health Canada will continue to monitor the status of the foreign building and request the certificate of compliance from the MRA partner. The assessment will result in the foreign building being maintained or removed from the foreign building annex.
The second flowchart – FB Annex – Non-MRA or not covered by MRA
The flowchart outlines the process for a foreign building located in an MRA country for activities not covered by the MRA or for foreign buildings located in a non-MRA country that should be listed on the foreign building annex of a Drug Establishment Licence. The foreign building can fabricate, package, label or test finished dosage forms, sterile active pharmaceutical ingredients or release testing of sterile and non-sterile active pharmaceutical ingredients.
To add or maintain a foreign building to a drug establishment license an application is required. The application should include a cover letter, Form-0033, inspection report, corrective actions, if applicable, and a site master file.
Once the application is received, it undergoes GMP screening. If any deficiencies are identified, a deficiency email will be sent to the importer. If a response is received the GMP screening resumes. If a response is not received or the response is not adequate the application is rejected.
Once all deficiencies are addressed or if there are no deficiencies identified the application undergoes a GMP assessment. The assessment will result in a compliant rating ora non-compliant rating. Terms and conditions may be added to the Drug Establishment Licence. If a compliant rating is assigned, the foreign building is added or maintained on the foreign building annex. If a non-compliant rating is assigned, the foreign building is not added or removed from the foreign building annex. If terms and conditions are proposed they are added to the drug establishment license.
To request an extension for a foreign building an application is required. The application should include a cover letter and Form-0033. Health Canada will assess the information provided and the assessment will result in one of two outcomes: an extension is granted or an extension is not granted.
The third flowchart – API FB Annex or Table A
The flowchart outlines the process for a foreign building listed on theactive pharmaceutical ingredient foreign building annex or Table A of a drug establishment licence irrespective of its location . The foreign building can fabricate, package, label or test non-sterile active pharmaceutical ingredients, other than release testing.
To add a foreign building to a drug establishment license an application is required. The application should include a cover letter and Form-0033. Once the application is received Health Canada will assess the information submitted. The outcome of the assessment is that the foreign building is either added to the active pharmaceutical ingredient foreign building annex or Table A or the application is rejected.
To maintain a foreign building to a drug establishment licence no application is required. Health Canada conducts an on-going assessment of evidence. Health Canada will request additional information from the importer. This information is the inspection report, corrective actions, if applicable, and site master file. The application then undergoes GMP screening. If any deficiencies are identified, a deficiency email will be sent to the importer. Once all deficiencies are addressed or if there are no deficiencies the application undergoes a GMP assessment. The GMP assessment can result in a compliant rating or a non-compliant rating. termsTerms and conditions may be added to the Drug Establishment Licence. If compliant the foreign building is maintained on the active pharmaceutical ingredient foreign building annex or Table A. If non-compliant the foreign building is removed from the active pharmaceutical ingredient foreign building annex or Table A. If terms and conditions are proposed they are added to the drug establishment license.