Impurities found in certain angiotensin II receptor blocker (ARB) products, also known as sartans
Table of Contents
Overview
In the summer of 2018, several valsartan products were recalled in Canada and worldwide because of the impurity, N-nitrosodimethylamine (NDMA), found in the active ingredient manufactured by Zhejiang Huahai Pharmaceuticals in China.
Since that time, NDMA and other similar impurities, N-nitrosodiethylamine (NDEA), N-Nitrosodiisopropylamine (NDIPA) and N-Nitrosomethyl-n-butylamine (NMBA), have been found in valsartan or other drugs in the same class as valsartan (referred to as angiotensin II receptor blockers or ARBs) made by several different manufacturers in different countries and has prompted additional recalls in Canada and worldwide.
ARBs are used to treat patients with high blood pressure to help prevent heart attacks and stroke. NDEA, NDMA, NDIPA and NMBA are nitrosamines that are classified as probable or potential human carcinogens, which means that long-term exposure could increase the risk of cancer. Since the risk of cancer is with long-term exposure, there is no immediate health risk associated with the use of ARBs containing these impurities.
Health Canada recognizes the stress caused by this issue to Canadians who rely on these important medications. The Department has been working with companies and international regulatory partners to determine the root cause of the issue and to verify that appropriate actions are taken to prevent it from happening again.
Health Canada continues to hold manufacturers responsible for the safety and effectiveness of drugs sold on the Canadian market and has taken several actions to mitigate the risk to Canadians, including:
- Requested, confirmed and monitored the effectiveness of recalls due to this issue. A list of recalled products is provided below and will be updated as needed.
- Communicated the recalls, NDMA risk estimates, and Health Canada actions to Canadians on several occasions.
- Contacted all market authorization holders of all ARBs of concern in Canada and assessed the manufacturing processes they use to make products sold in Canada.
- Determined that the Chuannan site of Zhejiang Huahai Pharmaceuticals and Hetero Laboratories Limited, Unit 1 are Non-Compliant with Good Manufacturing Practices requirements. This means that no products can be imported from those sites, unless they are considered medically necessary.
- Tested samples of ARBs on the Canadian market. Test results are provided below and will be updated as additional test results become available.
- Requested market authorization holders of ARBs of concern test all products currently on the market and any new products not yet released for NDEA and NDMA. Companies were also requested to consider using manufacturing practices that avoid the generation and presence of all nitrosamine impurities. This added safeguard will provide greater assurance of the safety of ARBs in Canada.
Health Canada continues to work closely with international regulatory partners, including the US Food and Drug Administration and the European Medicines Agency, to share information and coordinate efforts on inspections, risk assessments and public communications. We will continue to take action and update Canadians should any new risks be identified for products on the Canadian market.
Recalls
Health Canada is publishing a complete list of angiotensin II receptor blocker (ARB) products recalled in Canada due to the presence of or the potential for nitrosamine impurities. This list will be updated if new products are recalled. Products not on this list have not been recalled in Canada due to this issue. It should be noted that recalls taking place in other countries may not impact Canadian products.
Patients taking recalled medications should:
- Continue taking your medication unless you have been advised to stop by your health care provider.
- Contact your health care provider to discuss treatment options if you have been using an affected product.
- Ask your pharmacist if you are unsure whether you are taking a recalled product.
- Contact your health care provider if you have taken a recalled product and you have concerns about your health.
| Product name/Active Pharmaceutical Ingredient | DIN | Strength | Lot # | Date Recalled | API Manufacturer |
|---|---|---|---|---|---|
| AURO-IRBESARTAN/HCT | 02447878 | 150/12.5 mg | IN1518001-A | April 17, 2019 | Aurobindo Pharma Limited, Unit-I |
| PRO DOC LIMITEE - IRBESARTAN | 02365200 | 150 mg | 604292 | March 11, 2019 | TEVA API India Ltd. |
| PRO DOC LIMITEE - IRBESARTAN | 02365219 | 300 mg | 601795 | March 11, 2019 | TEVA API India Ltd. |
| APO-LOSARTAN | 02379058 | 25 mg | NL1453 | March 8, 2019 | Hereto Laboratories Limited Unit 1 |
| APO-LOSARTAN | 02379058 | 25 mg | NL1452 | March 8, 2019 | Hereto Laboratories Limited Unit 1 |
| APO-LOSARTAN | 02353504 | 50 mg | NK1254 | March 8, 2019 | Hereto Laboratories Limited Unit 1 |
| APO-LOSARTAN | 02353504 | 50 mg | NK1253 | March 8, 2019 | Hereto Laboratories Limited Unit 1 |
| APO-LOSARTAN | 02353512 | 100 mg | NL1461 | March 8, 2019 | Hereto Laboratories Limited Unit 1 |
| APO-LOSARTAN | 02353512 | 100 mg | NG2092 | March 8, 2019 | Hereto Laboratories Limited Unit 1 |
| APO-LOSARTAN | 02353512 | 100 mg | NH5932 | March 8, 2019 | Hereto Laboratories Limited Unit 1 |
| APO-LOSARTAN | 02353512 | 100 mg | NH5933 | March 8, 2019 | Hereto Laboratories Limited Unit 1 |
| APO-LOSARTAN | 02353512 | 100 mg | NL1460 | March 8, 2019 | Hereto Laboratories Limited Unit 1 |
| APO-LOSARTAN | 02353512 | 100 mg | NH5934 | March 8, 2019 | Hereto Laboratories Limited Unit 1 |
| APO-LOSARTAN/HCTZ | 02371235 | 50/12.5 mg | NL1441 | March 8, 2019 | Hereto Laboratories Limited Unit 1 |
| APO-LOSARTAN/HCTZ | 02371235 | 50/12.5 mg | NZ8848 | March 8, 2019 | Hereto Laboratories Limited Unit 1 |
| APO-LOSARTAN/HCTZ | 02371235 | 50/12.5 mg | NL1445 | March 8, 2019 | Hereto Laboratories Limited Unit 1 |
| APO-LOSARTAN/HCTZ | 02371235 | 50/12.5 mg | NZ8849 | March 8, 2019 | Hereto Laboratories Limited Unit 1 |
| APO-LOSARTAN/HCTZ | 02371235 | 50/12.5 mg | NZ8860 | March 8, 2019 | Hereto Laboratories Limited Unit 1 |
| APO-LOSARTAN/HCTZ | 02371243 | 100/12.5 mg | NG2087 | March 8, 2019 | Hereto Laboratories Limited Unit 1 |
| APO-LOSARTAN/HCTZ | 02371243 | 100/12.5 mg | NL1421 | March 8, 2019 | Hereto Laboratories Limited Unit 1 |
| APO-LOSARTAN/HCTZ | 02371243 | 100/12.5 mg | NG2086 | March 8, 2019 | Hereto Laboratories Limited Unit 1 |
| APO-LOSARTAN/HCTZ | 02371243 | 100/12.5 mg | NL1422 | March 8, 2019 | Hereto Laboratories Limited Unit 1 |
| APO-LOSARTAN/HCTZ | 02371251 | 100/25 mg | NL1429 | March 8, 2019 | Hereto Laboratories Limited Unit 1 |
| APO-LOSARTAN/HCTZ | 02371251 | 100/25 mg | NZ8846 | March 8, 2019 | Hereto Laboratories Limited Unit 1 |
| APO-LOSARTAN/HCTZ | 02371251 | 100/25 mg | NZ8847 | March 8, 2019 | Hereto Laboratories Limited Unit 1 |
| APO-LOSARTAN/HCTZ | 02371251 | 100/25 mg | NZ8845 | March 8, 2019 | Hereto Laboratories Limited Unit 1 |
| PMS-LOSARTAN | 02309750 | 25 mg | 498294 | March 8, 2019 | Hereto Laboratories Limited Unit 1 |
| PMS-LOSARTAN | 02309750 | 25 mg | 605342 | March 8, 2019 | Hereto Laboratories Limited Unit 1 |
| PMS-LOSARTAN | 02309750 | 25 mg | 611944 | March 8, 2019 | Hereto Laboratories Limited Unit 1 |
| PMS-LOSARTAN | 02309769 | 50 mg | 498285 | March 8, 2019 | Hereto Laboratories Limited Unit 1 |
| PMS-LOSARTAN | 02309769 | 50 mg | 600047 | March 8, 2019 | Hereto Laboratories Limited Unit 1 |
| PMS-LOSARTAN | 02309769 | 50 mg | 600091 | March 8, 2019 | Hereto Laboratories Limited Unit 1 |
| PMS-LOSARTAN | 02309769 | 50 mg | 603894 | March 8, 2019 | Hereto Laboratories Limited Unit 1 |
| PMS-LOSARTAN | 02309769 | 50 mg | 612025 | March 8, 2019 | Hereto Laboratories Limited Unit 1 |
| PMS-LOSARTAN | 02309769 | 50 mg | 612031 | March 8, 2019 | Hereto Laboratories Limited Unit 1 |
| PMS-LOSARTAN | 02309769 | 50 mg | 612679 | March 8, 2019 | Hereto Laboratories Limited Unit 1 |
| PMS-LOSARTAN | 02309769 | 50 mg | 616743 | March 8, 2019 | Hereto Laboratories Limited Unit 1 |
| PMS-LOSARTAN | 02309777 | 100 mg | 498864 | March 8, 2019 | Hereto Laboratories Limited Unit 1 |
| PMS-LOSARTAN | 02309777 | 100 mg | 602668 | March 8, 2019 | Hereto Laboratories Limited Unit 1 |
| PMS-LOSARTAN | 02309777 | 100 mg | 603816 | March 8, 2019 | Hereto Laboratories Limited Unit 1 |
| PMS-LOSARTAN | 02309777 | 100 mg | 605298 | March 8, 2019 | Hereto Laboratories Limited Unit 1 |
| PMS-LOSARTAN | 02309777 | 100 mg | 605300 | March 8, 2019 | Hereto Laboratories Limited Unit 1 |
| PMS-LOSARTAN | 02309777 | 100 mg | 613935 | March 8, 2019 | Hereto Laboratories Limited Unit 1 |
| PMS-LOSARTAN | 02309777 | 100 mg | 613936 | March 8, 2019 | Hereto Laboratories Limited Unit 1 |
| LOSARTAN (PRO DOC LIMITEE) | 02394367 | 25 mg | 498292 | March 8, 2019 | Hereto Laboratories Limited Unit 1 |
| LOSARTAN (PRO DOC LIMITEE) | 02394367 | 25 mg | 605344 | March 8, 2019 | Hereto Laboratories Limited Unit 1 |
| LOSARTAN (PRO DOC LIMITEE) | 02394375 | 50 mg | 498779 | March 8, 2019 | Hereto Laboratories Limited Unit 1 |
| LOSARTAN (PRO DOC LIMITEE) | 02394375 | 50 mg | 600046 | March 8, 2019 | Hereto Laboratories Limited Unit 1 |
| LOSARTAN (PRO DOC LIMITEE) | 02394375 | 50 mg | 603903 | March 8, 2019 | Hereto Laboratories Limited Unit 1 |
| LOSARTAN (PRO DOC LIMITEE) | 02394375 | 50 mg | 498284 | March 8, 2019 | Hereto Laboratories Limited Unit 1 |
| LOSARTAN (PRO DOC LIMITEE) | 02394375 | 50 mg | 603895 | March 8, 2019 | Hereto Laboratories Limited Unit 1 |
| LOSARTAN (PRO DOC LIMITEE) | 02394383 | 100 mg | 499008 | March 8, 2019 | Hereto Laboratories Limited Unit 1 |
| LOSARTAN (PRO DOC LIMITEE) | 02394383 | 100 mg | 605299 | March 8, 2019 | Hereto Laboratories Limited Unit 1 |
| LOSARTAN (PRO DOC LIMITEE) | 02394383 | 100 mg | 605297 | March 8, 2019 | Hereto Laboratories Limited Unit 1 |
| TEVA-LOSARTAN/HCTZ | 02358263 | 50/12.5 mg | 35344801A | March 6, 2019 | Hereto Laboratories Limited Unit 1 |
| TEVA-LOSARTAN/HCTZ | 02358263 | 50/12.5mg | 35349397A | March 6, 2019 | Hereto Laboratories Limited Unit 1 |
| MYLAN-VALSARTAN | 02383527 | 40 mg | All lots | November 28, 2018 | Mylan Laboratories Limited |
| MYLAN-VALSARTAN | 02383535 | 80 mg | All lots | November 28, 2018 | Mylan Laboratories Limited |
| MYLAN-VALSARTAN | 02383543 | 160 mg | All lots | November 28, 2018 | Mylan Laboratories Limited |
| MYLAN-VALSARTAN | 02383551 | 320 mg | All lots | November 28, 2018 | Mylan Laboratories Limited |
| TEVA-VALSARTAN/HCTZ TABLETS | 02356996 | 80/12.5 mg | 35211136A | August 17, 2018 | Zhejiang Huahai Pharmaceuticals |
| TEVA-VALSARTAN/HCTZ TABLETS | 02357003 | 160/12.5 mg | 35211335A | August 17, 2018 | Zhejiang Huahai Pharmaceuticals |
| TEVA-VALSARTAN/HCTZ TABLETS | 02357003 | 160/12.5 mg | 35211844R | August 17, 2018 | Zhejiang Huahai Pharmaceuticals |
| TEVA-VALSARTAN/HCTZ TABLETS | 02357011 | 160/25 mg | 35210937R | August 17, 2018 | Zhejiang Huahai Pharmaceuticals |
| TEVA-VALSARTAN/HCTZ TABLETS | 02357011 | 160/25 mg | 35210938R | August 17, 2018 | Zhejiang Huahai Pharmaceuticals |
| TEVA-VALSARTAN/HCTZ TABLETS | 02357011 | 160/25 mg | 35210939R | August 17, 2018 | Zhejiang Huahai Pharmaceuticals |
| TEVA-VALSARTAN/HCTZ TABLETS | 02357011 | 160/25 mg | 35210940R | August 17, 2018 | Zhejiang Huahai Pharmaceuticals |
| TEVA-VALSARTAN/HCTZ TABLETS | 02357038 | 320/12.5 mg | 35211546R | August 17, 2018 | Zhejiang Huahai Pharmaceuticals |
| ACT-VALSARTAN 40MG FC TABLETS | 02337487 | 40 mg | K47338 | July 9, 2018 | Zhejiang Huahai Pharmaceuticals |
| ACT-VALSARTAN 80MG FC TABLETS | 02337495 | 80 mg | K45370 | July 9, 2018 | Zhejiang Huahai Pharmaceuticals |
| ACT-VALSARTAN 80MG FC TABLETS | 02337495 | 80 mg | K47652 | July 9, 2018 | Zhejiang Huahai Pharmaceuticals |
| ACT-VALSARTAN 80MG FC TABLETS | 02337495 | 80 mg | K47653 | July 9, 2018 | Zhejiang Huahai Pharmaceuticals |
| ACT-VALSARTAN 80MG FC TABLETS | 02337495 | 80mg | K47654 | July 9, 2018 | Zhejiang Huahai Pharmaceuticals |
| ACT-VALSARTAN 160MG FC TABLETS | 02337509 | 160 mg | K39691 | July 9, 2018 | Zhejiang Huahai Pharmaceuticals |
| ACT-VALSARTAN 160MG FC TABLETS | 02337509 | 160 mg | K44167 | July 9, 2018 | Zhejiang Huahai Pharmaceuticals |
| ACT-VALSARTAN 160MG FC TABLETS | 02337509 | 160 mg | K47657 | July 9, 2018 | Zhejiang Huahai Pharmaceuticals |
| ACT-VALSARTAN 160MG FC TABLETS | 02337509 | 160 mg | K47658 | July 9, 2018 | Zhejiang Huahai Pharmaceuticals |
| ACT-VALSARTAN 320MG FC TABLETS | 02337517 | 320 mg | K44166 | July 9, 2018 | Zhejiang Huahai Pharmaceuticals |
| ACT-VALSARTAN 320MG FC TABLETS | 02337517 | 320 mg | K45371 | July 9, 2018 | Zhejiang Huahai Pharmaceuticals |
| PRO DOC LIMITEE VALSARTAN 40 MG | 02367726 | 40 mg | All lots | July 9, 2018 | Zhejiang Huahai Pharmaceuticals |
| PRO DOC LIMITEE VALSARTAN 80 MG | 02367734 | 80 mg | All lots | July 9, 2018 | Zhejiang Huahai Pharmaceuticals |
| PRO DOC LIMITEE VALSARTAN 160 MG | 02367742 | 160 mg | All lots | July 9, 2018 | Zhejiang Huahai Pharmaceuticals |
| PRO DOC LIMITEE VALSARTAN 320 MG | 02367750 | 320 mg | All lots | July 9, 2018 | Zhejiang Huahai Pharmaceuticals |
| SANDOZ VALSARTAN 40 MG | 02356740 | 40 mg | All lots | July 9, 2018 | Zhejiang Huahai Pharmaceuticals |
| SANDOZ VALSARTAN 80 MG | 02356759 | 80 mg | All lots | July 9, 2018 | Zhejiang Huahai Pharmaceuticals |
| SANDOZ VALSARTAN 160 MG | 02356767 | 160 mg | All lots | July 9, 2018 | Zhejiang Huahai Pharmaceuticals |
| SANDOZ VALSARTAN 320 MG | 02356775 | 320 mg | All lots | July 9, 2018 | Zhejiang Huahai Pharmaceuticals |
| SANIS VALSARTAN 40 MG | 02366940 | 40 mg | All lots | July 9, 2018 | Zhejiang Huahai Pharmaceuticals |
| SANIS VALSARTAN 80 MG | 02366959 | 80 mg | All lots | July 9, 2018 | Zhejiang Huahai Pharmaceuticals |
| SANIS VALSARTAN 160 MG | 02366967 | 160 mg | All lots | July 9, 2018 | Zhejiang Huahai Pharmaceuticals |
| SANIS VALSARTAN 320 MG | 02366975 | 320 mg | All lots | July 9, 2018 | Zhejiang Huahai Pharmaceuticals |
| SIVEM PHARMACEUTICAL ULC VALSARTAN 40 MG | 02384523 | 40 mg | All lots | July 9, 2018 | Zhejiang Huahai Pharmaceuticals |
| SIVEM PHARMACEUTICAL ULC VALSARTAN 80 MG | 02384531 | 80 mg | All lots | July 9, 2018 | Zhejiang Huahai Pharmaceuticals |
| SIVEM PHARMACEUTICAL ULC VALSARTAN 160 MG | 02384558 | 160 mg | All lots | July 9, 2018 | Zhejiang Huahai Pharmaceuticals |
| SIVEM PHARMACEUTICAL ULC VALSARTAN 320 MG | 02384566 | 320 mg | All lots | July 9, 2018 | Zhejiang Huahai Pharmaceuticals |
| TEVA-VALSARTAN/HCTZ TABLETS PP 30s | 02357046 | 320/25 mg | 35212731R | July 9, 2018 | Zhejiang Huahai Pharmaceuticals |
Test Results
Health Canada has tested samples of ARBs on the Canadian market for NDMA and NDEA and the results are posted below. Health Canada will continue to assess new developments to determine whether additional testing is necessary.
The results indicate the NDMA or NDEA levels detected, whether they exceed acceptable limits, based on a lifetime exposure, and whether the product was recalled.
| Market Authorization Holder (Company) Name | Product Name | DIN | Strength (mg) |
Lot Number | API Manufacturer | Expiry Date | NDMA Result ng / tablet |
NDEA Result ng / tablet |
NDMA Limit (96 ng/day) Exceeded?Footnote * |
NDEA Limit (26.5 ng/day) Exceeded?Footnote * |
Recalled in Canada | Date Added |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Accel Pharma Inc. | ACCEL-CANDESARTAN | 02463784 | 32 | 1805003937 | Alembic Pharmaceuticals Limited (API Division - II) | 2020-02-29 | Not Detected | Not Detected | No | No | No | December 20, 2018 |
| Actavis Pharma Company | ACT VALSARTAN | 02337517 | 320 | K44166 | Zhejiang Huahai Pharmaceutical Co., Ltd. | 2018-07-31 | 15242.72 | 12.78 | Yes | No | Yes | December 20, 2018 |
| ACT VALSARTAN | 02337517 | 320 | K45371 | Zhejiang Huahai Pharmaceutical Co., Ltd. | 2018-10-31 | 10770.86 | 186.67 | Yes | Yes | Yes | December 20, 2018 | |
| ACT- OLMESARTAN | 02442205 | 40 | F83746 | Zhejiang Huahai Pharmaceutical Co., Ltd. | 2020-03-31 | Not Detected | Not Detected | No | No | No | December 20, 2018 | |
| Angita Pharma Inc. | AG-IRBESARTAN | 02474417 | 300 | IE317017AR | Jubilant Generics Limited | 2019-10-31 | Not Detected | Not Detected | No | No | No | December 20, 2018 |
| Apotex Inc. | APO-OLMESARTAN/HCTZ | 02453614 | 40/12.5 | NF7704 | Apotex Pharmachem India Pvt. Ltd. | 2019-02-28 | Not Detected | Not Detected | No | No | No | December 20, 2018 |
| APO-OLMESARTAN/HCTZ | 02453614 | 40/12.5 | NN7635 | Signa S.A. de C.V. | 2019-12-31 | Not Detected | Not Detected | No | No | No | December 20, 2018 | |
| APO-LOSARTAN/HCTZ | 02371235 | 50/12.5 | NL1445 | Hetero Labs Limited | 2019-08-31 | Not Detected | Not Detected | No | No | No | December 20, 2018 | |
| APO-LOSARTAN | 02353512 | 100 | NL1461 | Hetero Labs Limited | 2019-08-31 | Not Detected | Not Detected | No | No | No | December 20, 2018 | |
| AstraZeneca Inc. | ATACAND - CANDESARTAN | 02311658 | 32 | KL0275 | Takeda Pharmaceutical Company Limited | 2021-04-30 | Not Detected | Not Detected | No | No | No | December 20, 2018 |
| Auro Pharma Inc. | AURO-CANDESARTAN HCT | 02421046 | 32/12.5 | WKSA18004-A | Aurobindo Pharma Limited & Chromo Laboratories Limited | 2020-07-29 | Not Detected | Not Detected | No | No | No | December 20, 2018 |
| AURO-IRBESARTAN | 02406098 | 75 | IA7517001-A | Aurobindo Pharma Limited- Unit 1 | 2020-06-30 | Not Detected | 4.7 | No | No | No | April 29, 2019 | |
| AURO-IRBESARTAN | 02406098 | 75 | IA7517002-A | Aurobindo Pharma Limited- Unit 1 | 2020-11-05 | Not Detected | 4.44 | No | No | No | April 29, 2019 | |
| AURO-IRBESARTAN | 02406098 | 75 | IA7517003-A | Aurobindo Pharma Limited- Unit 1 | 2020-11-05 | Not Detected | 4.61 | No | No | No | April 29, 2019 | |
| AURO-IRBESARTAN | 02406101 | 150 | IA1517002-A | Aurobindo Pharma Limited- Unit 1 | 2020-10-31 | Not Detected | 9.77 | No | No | No | April 29, 2019 | |
| AURO-IRBESARTAN | 02406101 | 150 | IA1517001-A | Aurobindo Pharma Limited- Unit 1 | 2020-06-30 | Not Detected | 13.63 | No | Yes2 | No2 | April 29, 2019 | |
| AURO-IRBESARTAN | 02406101 | 150 | IA1517003-A | Aurobindo Pharma Limited- Unit 1 | 2020-10-31 | Not Detected | 8.97 | No | No | No | April 29, 2019 | |
| AURO-IRBESARTAN | 02406128 | 300 | IA3017001-A | Aurobindo Pharma Limited- Unit 1 | 2020-06-30 | Not Detected | 27.81 | No | Yes2 | No2 | April 29, 2019 | |
| AURO-IRBESARTAN | 02406128 | 300 | IA3017003-A | Aurobindo Pharma Limited- Unit 1 | 2020-10-31 | Not Detected | 17.47 | No | No | No | April 29, 2019 | |
| AURO-IRBESARTAN | 02406128 | 300 | IA3017002-A | Aurobindo Pharma Limited- Unit 1 | 2020-10-31 | Not Detected | 19.6 | No | No | No | April 29, 2019 | |
| AURO-IRBESARTAN HCT | 02447894 | 300/25 | IN3018001-A | Aurobindo Pharma Limited- Unit 1 | 2020-04-06 | Not Detected | 26.78 | No | Yes2 | No2 | April 29, 2019 | |
| AURO-IRBESARTAN HCT | 02447886 | 300/12.5 | IR3018001-A | Aurobindo Pharma Limited- Unit 1 | 2020-04-06 | Not Detected | 30.29 | No | No | This lot was never distributed | April 29, 2019 | |
| AURO-IRBESARTAN HCT | 02447878 | 150/12.5 | IN1518001-A | Aurobindo Pharma Limited- Unit 1 | 2020-04-06 | Not Detected | 15.82 | No | Yes | Yes | April 29, 2019 | |
| AURO-OLMESARTAN | 02443872 | 40 | WOSB18004-A | Aurobindo Pharma Unit-I | 2020-01-12 | Not Detected | Not Detected | No | No | No | December 20, 2018 | |
| AURO-LOSARTAN | 02403358 | 100 | WB1018003-A | Aurobindo Pharma Unit-I | 2021-03-15 | Not Detected | Not Detected | No | No | No | December 20, 2018 | |
| AURO-VALSARTAN | 02414201 | 40 | VWSA17003-A | Aurobindo Pharma Limited, Unit-XI | 2019-10-31 | Not Detected | 1.41 | No | No | No | January 16, 2019 | |
| AURO-VALSARTAN | 02414201 | 40 | VWSA18007-A | Aurobindo Pharma Limited, Unit-XI | 2020-08-24 | Not Detected | 1.07 | No | No | No | January 16, 2019 | |
| AURO-VALSARTAN | 02414201 | 40 | VWSA18003-A | Aurobindo Pharma Limited, Unit-XI | 2020-08-23 | Not Detected | 1.04 | No | No | No | January 16, 2019 | |
| AURO-VALSARTAN | 02414228 | 80 | VWSB17010-A | Aurobindo Pharma Limited, Unit-XI | 2019-07-31 | Not Detected | Not Detected | No | No | No | January 16, 2019 | |
| AURO-VALSARTAN | 02414228 | 80 | VWSB17011-A | Aurobindo Pharma Limited, Unit-XI | 2019-07-31 | Not Detected | Not Detected | No | No | No | January 16, 2019 | |
| AURO-VALSARTAN | 02414236 | 160 | VWSC17001-A | Aurobindo Pharma Limited, Unit-XI | 2018-12-31 | Not Detected | < 3.20 | No | No | No | January 16, 2019 | |
| AURO-VALSARTAN | 02414236 | 160 | VWSC17005-A | Aurobindo Pharma Limited, Unit-XI | 2019-05-31 | Not Detected | < 3.20 | No | No | No | January 16, 2019 | |
| AURO-VALSARTAN | 02414236 | 160 | VWSC18002-A | Aurobindo Pharma Limited, Unit-XI | 2020-01-19 | Not Detected | 4.58 | No | No | No | January 16, 2019 | |
| AURO-VALSARTAN | 02414236 | 160 | VWSC18001-A | Aurobindo Pharma Limited, Unit-XI | 2020-01-19 | < 6.40 | 4.39 | No | No | No | January 16, 2019 | |
| AURO-VALSARTAN | 02414236 | 160 | VWSC18016-A | Aurobindo Pharma Limited, Unit-XI | 2020-08-24 | Not Detected | 4.7 | No | No | No | January 16, 2019 | |
| AURO-VALSARTAN | 02414244 | 320 | VWSD17001-B | Aurobindo Pharma Limited, Unit-XI | 2019-05-31 | Not Detected | Not Detected | No | No | No | January 16, 2019 | |
| AURO-VALSARTAN | 02414244 | 320 | VWSD18005-A | Aurobindo Pharma Limited, Unit-XI | 2020-08-25 | Not Detected | 12.14 | No | No | No | January 16, 2019 | |
| AURO-VALSARTAN | 02414244 | 320 | VWSD18001-A | Aurobindo Pharma Limited, Unit-XI | 2020-04-15 | Not Detected | 8.56 | No | No | No | January 16, 2019 | |
| AURO-VALSARTAN | 02414244 | 320 | VWSD18001-A | Aurobindo Pharma Limited | 2020-04-15 | Not Detected | 8.61 | No | No | No | December 20, 2018 | |
| AURO-VALSARTAN HCT | 02408112 | 80/12.5 | HHSA18001-A | Aurobindo Pharma Limited, Unit-XI | 2021-08-02 | Not Detected | 1.88 | No | No | No | January 16, 2019 | |
| Jamp Pharma Corp | JAMP-OLMESARTAN | 02461668 | 40 | MC218002A | Glenmark Pharmaceuticals Limited | 2020-04-30 | Not Detected | Not Detected | No | No | No | December 20, 2018 |
| JAMP-LOSARTAN-HCTZ | 02408252 | 100/25 | LY218001A | Jubilant Generics Limited | 2019-06-30 | Not Detected | Not Detected | No | No | No | December 20, 2018 | |
| JAMP-IRBESARTAN | 02418215 | 300 | IE318005A | Jubilant Generics Limited | 2020-03-31 | Not Detected | Not Detected | No | No | No | December 20, 2018 | |
| Merck Canada Inc. | OLMETEC - OLMESARTAN | 02318679 | 40 | N021852 | Daiichi Sankyo Chemical Pharma Co., Ltd. Hiratsuka Plant | 2020-04-30 | Not Detected | Not Detected | No | No | No | December 20, 2018 |
| Mint Pharmaceuticals Inc. | MINT-LOSARTAN/HCTZ DS | 02389673 | 100/25 | 1805009844 | Alembic Pharmaceuticals Limited (API Division - II) | 2021-07-31 | Not Detected | Not Detected | No | No | No | December 20, 2018 |
| Mylan Pharmaceuticals ULC | MYLAN-VALSARTAN | 02383527 | 40 | 3048813 | Mylan Laboratories Limited, Unit 8, in Hyderabad, India | 2017-11-30 | < 1.60 | 8.32 | No | Yes | Yes | January 16, 2019 |
| MYLAN-VALSARTAN | 02383535 | 80 | 3056368 | Mylan Laboratories Limited, Unit 8, in Hyderabad, India | 2019-06-30 | < 3.20 | 22.43 | No | Yes | Yes | January 16, 2019 | |
| MYLAN-VALSARTAN | 02383543 | 160 | 3056371 | Mylan Laboratories Limited, Unit 8, in Hyderabad, India | 2019-06-30 | Not Detected | 62.76 | No | Yes | Yes | January 16, 2019 | |
| MYLAN-VALSARTAN | 02383551 | 320 | 3048815 | Mylan Laboratories Limited, Unit 8, in Hyderabad, India | 2017-11-30 | Not Detected | 132.77 | No | Yes | Yes | January 16, 2019 | |
| Novartis Pharmaceuticals Canada Inc. | DIOVAN | 02289504 | 320 | BEK32 | Novartis Pharma Schweizerhalle | 2021-07-31 | Not Detected | Not Detected | No | No | No | December 20, 2018 |
| Pharmascience Inc. | PMS-CANDESARTAN HCTZ | 02391295 | 16 | 615170 | Zhejiang Huahai Pharmaceutical Co., Ltd. | 2020-04-30 | Not Detected | Not Detected | No | No | No | December 20, 2018 |
| PMS-LOSARTAN | 02309777 | 100 | 613936 | Hetero Labs Limited | 2021-03-31 | Not Detected | 10.41 | No | No | No | December 20, 2018 | |
| PMS-LOSARTAN-HCTZ | 02392232 | 100/12.5 | 0803847 | Dr. Reddy's Laboratories Limited | 2020-06-30 | Not Detected | Not Detected | No | No | No | December 20, 2018 | |
| PMS-IRBESARTAN | 02317087 | 300 | 615908 | Zhejiang Tianyu Pharmaceutical Co., Ltd. | 2021-03-31 | Not Detected | Not Detected | No | No | No | December 20, 2018 | |
| PMS-IRBESARTAN-HCTZ | 02328526 | 300/12.5 | 606524 | Zhejiang Tianyu Pharmaceutical Co., Ltd., | 2018-11-30 | Not Detected | Not Detected | No | No | No | December 20, 2018 | |
| PMS-IRBESARTAN-HCTZ | 02328526 | 300/12.5 | 611016 | USV Private Limited | 2019-11-30 | Not Detected | Not Detected | No | No | No | December 20, 2018 | |
| Ranbaxy Pharmaceuticals Canada Inc. | RAN-VALSARTAN | 02363119 | 160 | 2884804 | Sun Pharmaceutical Industries Limited | 2019-05-31 | Not Detected | Not Detected | No | No | No | December 20, 2018 |
| Sandoz Canada Inc. | SANDOZ CANDESARTAN | 02417340 | 32 | JC3223 | Zhejiang Huahai Pharmaceutical Co., Ltd. | 2020-06-30 | Not Detected | Not Detected | No | No | No | December 20, 2018 |
| SANDOZ CANDESARTAN PLUS | 02420732 | 32 | HY8217 | Zhejiang Tianyu Pharmaceutical Co., Ltd. | 2020-04-30 | Not Detected | Not Detected | No | No | No | December 20, 2018 | |
| SANDOZ OLMESARTAN | 02443422 | 40 | HX2039 | Zhejiang Huahai Pharmaceutical Co., Ltd. | 2020-02-28 | Not Detected | Not Detected | No | No | No | December 20, 2018 | |
| SANDOZ LOSARTAN | 02313359 | 100 | JD6746 | Zhejiang Huahai Pharmaceutical Co., Ltd. | 2020-04-30 | Not Detected | Not Detected | No | No | No | December 20, 2018 | |
| SANDOZ VALSARTAN | 02356775 | 320 | HW4965 | Zhejiang Huahai Pharmaceutical Co., Ltd. | 2020-12-31 | 2703.76 | Not Detected | Yes | No | Yes | December 20, 2018 | |
| SANDOZ IRBESARTAN | 02328496 | 300 | HX4282 | Zhejiang Huahai Pharmaceutical Co., Ltd. | 2020-01-31 | Not Detected | Not Detected | No | No | No | December 20, 2018 | |
| SANDOZ IRBESARTAN | 02328496 | 300 | JD1818 | Zhejiang Tianyu Pharmaceutical Co., Ltd. | 2020-05-31 | Not Detected | Not Detected | No | No | No | December 20, 2018 | |
| Sanis Health Inc. | IRBESARTAN | 02372398 | 300 | 35213058A | TEVA API INDIA PVT. LTD. | 2019-12-31 | Not Detected | Not Detected | No | No | No | December 20, 2018 |
| Sanofi-Aventis Canada Inc. | AVAPRO - IRBESARTAN | 02237925 | 300 | KC001 | CHINOIN Pharmaceutical and Chemical Works Private Co. Ltd. | 2020-01-31 | Not Detected | Not Detected | No | No | No | December 20, 2018 |
| Septa Pharmaceuticals Inc. | SEPTA-LOSARTAN | 02424983 | 100 | LR318004A | Jubilant Generics Limited | 2020-03-31 | Not Detected | Not Detected | No | No | No | December 20, 2018 |
| Sivem Pharmaceuticals Inc. | LOSARTAN HCT | 02388987 | 100/25 | QX1018005-B | Zhejiang Tianyu Pharmaceutical Co., Ltd. | 2021-06-08 | Not Detected | Not Detected | No | No | No | December 20, 2018 |
| IRBESARTAN | 02385309 | 300 | HX7687 | Zhejiang Huahai Pharmaceutical Co., Ltd. | 2019-12-31 | Not Detected | Not Detected | No | No | No | December 20, 2018 | |
| IRBESARTAN HCT | 02385325 | 300/12.5 | HY7380 | Zhejiang Huahai Pharmaceutical Co., Ltd. | 2020-11-30 | Not Detected | Not Detected | No | No | No | December 20, 2018 | |
| Teva Canada Limited | TEVA-CANDESARTAN | 02366339 | 32 | 2537058 | Pliva Croatia Ltd. | 2020-05-31 | Not Detected | Not Detected | No | No | No | December 20, 2018 |
| TEVA-LOSARTAN | 02357976 | 100 | 2070318 | Teva API India Private Ltd | 2021-03-31 | Not Detected | Not Detected | No | No | No | December 20, 2018 | |
| TEVA-LOSARTAN/HCTZ | 02358263 | 50/12.5 | 35349397A | Hetero Labs Limited | 2020-09-30 | Not Detected | 10.3 | No | No | No | December 20, 2018 | |
| TEVA-IRBESARTAN | 02316412 | 300 | 35213086A | TEVA API INDIA LTD | 2019-12-31 | Not Detected | Not Detected | No | No | No | December 20, 2018 | |
| TEVA-VALSARTAN | 02356686 | 320 | 35211729R | Jubilant Generics Limited | 2019-03-31 | Not Detected | Not Detected | No | No | No | December 20, 2018 | |
| TEVA-VALSARTAN/HCTZ | 02357038 | 320/12.5 | 35212732 | Zhejiang Huahai Pharmaceutical Co., Ltd. | 2019-10-31 | 14538.35 | Not Detected | Yes | No | This lot was never distributed | December 20, 2018 | |
| TEVA-VALSARTAN/HCTZ | 02357038 | 320/12.5 | 35211546R | Zhejiang Huahai Pharmaceutical Co., Ltd. | 2019-01-31 | 258.19 | 1770.87 | Yes | Yes | Yes | December 20, 2018 | |
| TEVA-VALSARTAN/HCTZ | 02357046 | 320/25 | 35212731R | Zhejiang Huahai Pharmaceutical Co., Ltd. | 2019-11-30 | 13367.64 | Not Detected | Yes | No | Yes | December 20, 2018 | |
|
||||||||||||
Test Method
Health Canada is providing a method that has been developed to detect and quantify the nitrosamine impurities N-nitrosodimethylamine (NDMA) and N-nitrosodiethylamine (NDEA) in angiotensin II receptor blockers (ARBs).
Determination of N-Nitrosodimethylamine (NDMA) and N-Nitrosodiethylamine (NDEA) by GC-MS-MS (Direct Injection) in Sartan Finished Products and Drug Substances
1. Principle and Scope
The present method has been developed to detect and quantify the nitrosamine impurities N-nitrosodimethylamine (NDMA) and N-nitrosodiethylamine (NDEA) in Valsartan, Irbesartan and Losartan finished products. The method is performed by gas chromatography-tandem mass spectrometry (GC-MS-MS) using direct injection.
The method can also be used to detect and quantify NDMA and NDEA in candesartan and olmesartan finished products, and in sartan drug substances (e.g. valsartan, irbesartan, losartan, candesartan, and olmesartan). However, if interferences are observed, further validation may be required.
2. Safety
Laboratory safety precautions are followed to ensure a safe and healthy work environment, including the use of personal protective equipment (including but not limited to a lab coat, protective eyewear, and nitrile or butyl rubber gloves) and appropriate laboratory engineering controls (e.g. containment ventilation equipment).
The chemicals used in this method are hazardous. Analysts should carefully read the Material Safety Data Sheet (MSDS) for each chemical. Due to the toxic nature of nitrosamines, it is recommended that diluted reference standard solutions be purchased in order to reduce the extent of potential exposure.
3. Reagents and Reference Standards
- Methanol, HPLC grade (CAS #: 67-56-1)
- NDMA: N-Nitrosodimethylamine solution (5000 μg/mL in methanol; CAS #: 62-75-9)
- NDEA: N-Nitrosodiethylamine solution, (100 μg/mL in methanol; CAS #: 55-18-5)
- NDMA-d6 solution: N-nitrosodimethylamine-d6 (1000 μg/mL in methanol; CAS #: 17829-05-9)
4. Instrument/Equipment
- Agilent GC 7890A with MSMS 7000 with EI source or equivalent
- Agilent DB-624 25 m x 0.32 μm 1.8 μm or equivalent
- 2 mL amber screw-cap GC vials with caps (PTFE/Silicone)
- 20 x 125 mm screw cap round bottom glass tubes with caps
- Automatic pipettes, various volumes
- Top-loading balance suitable for ± 0.01 g and Analytical balance suitable for ± 0.0001 g readability
- Volumetric Flasks (class A), various volumes
- Pasteur pipettes and pipette bulbs
- Spatula
- 15 mL amber glass vials with caps
- 40 mL amber glass vials with caps
- Vortex mixer, single and multi-tube
- Vial racks and storage racks
- Kimwipes
- Mortar and pestle
- Ultrasonic water bath
- Centrifuge, Beckmann-Coulter Allegra X-15R or equivalent
5. Preparation of Solutions
Standard solutions
- Reference Standard Stock solutions (as purchased)
-
- NDMA standard solution in Methanol (5000 ppm)
- NDEA standard solution in Methanol (100 ppm)
- Internal Reference Standard Stock Solution (as purchased)
-
- NDMA-d6 standard solution in Methanol (1000 ppm)
Diluted standard solutions
- NDMA standard solution (200 ppm):
- Transfer 800 μl of NDMA reference solution (5000 ppm) into a 20 mL volumetric flask, dilute to volume with methanol.
- Diluted standard solution (NDMA: 40 ppm, NDEA: 20 ppm):
- Transfer 800 μl of NDMA standard solution (200 ppm) and 800 μl of NDEA reference solution (100 ppm) into a 4 mL volumetric flask, dilute to volume with methanol.
- Internal standard solution-1 (20 ppm):
- Transfer 500 μl of NDMA-d6 standard solution (1000 ppm) into a 25 mL volumetric flask, dilute to volume with methanol.
- Internal standard solution-2 (0.2 ppm):
- Transfer 5 ml of NDMA-d6 standard solution (20 ppm) into a 500 mL volumetric flask, dilute to volume with methanol.
Calibration solutions
- STD-12:
- Transfer 500 μl of Diluted standard solution (NDMA: 40 ppm, NDEA: 20 ppm) and 50 μl of Internal standard solution-1 (20 ppm) into a 5 mL volumetric flask, dilute to volume with methanol. Mix well.
- STD-7:
- Transfer 1000 μl of STD-12 into a 20 mL volumetric flask, dilute to volume with Internal standard solution-2 (0.2 ppm). Mix well. This solution will be used for the system suitability test and system drift check.
- STD-11:
- 1:1 dilution of STD-12 with Internal standard solution-2.
- STD-10:
- 1:1 dilution of STD-11 with Internal standard solution-2.
- STD-9:
- 1:1 dilution of STD-10 with Internal standard solution-2.
- STD-8:
- 3:2 dilution of STD-9 with Internal standard solution-2.
- STD-6:
- 1:1 dilution of STD-7 with Internal standard solution-2.
- STD-5:
- 1:1 dilution of STD-6 with Internal standard solution-2.
- STD-4:
- 2:3 dilution of STD-5 with Internal standard solution-2.
- STD-3:
- 1:1 dilution of STD-4 with Internal standard solution-2.
- STD-2:
- 1:1 dilution of STD-3 with Internal standard solution-2.
- STD-1:
- 2:3 dilution of STD-2 with Internal standard solution-2.
| Description | NDMA concentration (µg/mL) | NDEA concentration (µg/mL) | NDMA-d6 concentration (µg/mL) |
|---|---|---|---|
| STD-1 | 0.002 | 0.001 | 0.2 |
| STD-2 | 0.005 | 0.0025 | 0.2 |
| STD-3 | 0.01 | 0.005 | 0.2 |
| STD-4 | 0.02 | 0.01 | 0.2 |
| STD-5 | 0.05 | 0.025 | 0.2 |
| STD-6 | 0.1 | 0.05 | 0.2 |
| STD-7 | 0.2 | 0.1 | 0.2 |
| STD-8 | 0.3 | 0.15 | 0.2 |
| STD-9 | 0.5 | 0.25 | 0.2 |
| STD-10 | 1 | 0.5 | 0.2 |
| STD-11 | 2 | 1 | 0.2 |
| STD-12 | 4 | 2 | 0.2 |
For NDMA, STD-1 to STD-6 are used as working range from 0.002 - 0.1 µg/mL, STD-6 to STD-12 are used as working range from 0.1 - 4.0 µg/mL.
For NDEA, STD-1 to STD-5 are used as working range from 0.001 - 0.025 µg/mL, STD-5 to STD-11 are used as working range from 0.025 - 1.0 µg/mL.
The working ranges of NDMA and NDEA can be adjusted as needed.
Sample Preparation
For finished product:
Weigh NLT 20 tablets and calculate average tablet weight. Carefully grind NLT 20 tablets into fine powder using a mortar and pestle.
Prepare triplicate samples for each product. Accurately weigh an amount equivalent to 250 mg of drug substance of the homogenized sample powder into a screw cap round bottom glass tube.
Using an automatic pipette, add 5 mL of NDMA-d6 internal standard solution-2 to each sample tube. Tightly cap the tubes, sonicate for 5 min, and then vortex the rack of tubes on the multi-tube vortex mixer at 2000 rpm for five minutes. Centrifuge the tubes for at least five minutes at 1500 rpm. Carefully remove the tubes from the centrifuge. Use a Pasteur pipette to transfer an aliquot from each tube to a 2 mL GC vial and cap.
Note: Method accuracy was assessed by recovery studies. Valsartan, Irbesartan, and Losartan finished products were spiked with reference standard solution in the following way:
- For samples with NDMA above 0.3 ppm and/or NDEA above 0.08 ppm:
- Spike sample solution with the reference standard solution at concentration level close to sample concentration and calculate recovery.
- For samples with NDMA below 0.3 ppm and/or NDEA below 0.08 ppm:
- Spike sample solution with STD-3 (1:1) to get solution containing 0.05 ppm of NDMA and 0.025 ppm of NDEA. Check S/N and calculate the LOD and LOQ.
For drug substance:
Prepare triplicate samples for each substance. Accurately weigh 250 mg of the homogenized sample powder into a screw cap round bottom glass tube.
Continue as per the instructions above for finished product. Spiking to determine method accuracy is recommended.
6. Instrument Operating Parameters:
Suggested GC parameters:
Injector Settings:
- Injector Mode:
- Pulsed Splitless
- Injector temperature:
- 240 °C
- Flow rate:
- 1.8 mL/min
- Septum Purge Flow:
- 3 mL/min
- Purge Flow:
- 50 mL/min after 0.75 minutes
- Injection volume:
- 2.0 µL
Oven Program:
Initial Temp: 60°C
Hold: 2 min
| Ramp # | Rate (°C /min) | Final Temp (°C) | Hold Time (min) |
|---|---|---|---|
| 1 | 5 | 130 | 0 |
| 2 | 40 | 240 | 5 |
| Total Run Time: 24 min | |||
Suggested MS settings
- MS Transfer Line (Aux. Temp):
- 250 °C
- Ion Source:
- EI
- Source Temperature:
- 250 °C
- Solvent Delay:
- 6 min
- Stop time:
- 15 min
- Quench gas:
- Helium at 2.25 mL/min
- Collision gas:
- Nitrogen at 1.5 mL/min
| Analyte | Retention time (min) | Segment | Retention time window (min) | Precursor ion (m/z) | Product ion (m/z) | CE (V) |
Resolution | Dwell (ms) |
|---|---|---|---|---|---|---|---|---|
| NDMA-d6 (ISTD) | 7.8 | 1 | 7.5-8.1 | 80 | 50 | 5 | wide/wide | 100 |
| NDMA | 7.8 | 1 | 7.5-8.1 | 74 | 42 | 15 | wide/wide | 100 |
| 74 | 44 | 4 | 100 | |||||
| NDEA | 12.7 | 2 | 12.4-13.0 | 102 | 44 | 12 | wide/wide | 150 |
| 102 | 85 | 2 | 150 |
7. System Suitability
The coefficient of determination (R2) for each calibration curve is NLT 0.995.
The signal-to-noise of the STD-1 (NDMA = 0.002 µg/mL; NDEA = 0.001 µg/mL) solution should be NLT 10.
8. Calculation
Construct calibration curves for NDMA and NDEA by plotting the ratio of response factor (NDMA or NDEA peak area divided by internal standard peak area) against standard concentration (µg/mL). Using the slopes and intercepts of the calibration curves, determine the content of NDMA and NDEA in each sample using the following equations.
For finished product:
The results, in ppm relative to the declared amount of sartan drug substance in the product, are given by:
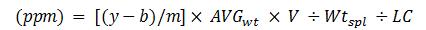
Where,
- y =
- Ratio of Peak Area of NDMA or NDEA to Peak Area of NDMA-d6
- b =
- intercept of the linear curve
- m =
- slope of the linear curve
- Wtspl =
- sample weight (g)
- AVGwt =
- average tablet weight (g)
- LC =
- label claim of sample (g)
- V =
- 5 mL (volume)
Equation 1 - Text Description
Parts per million equals y minus b over m, which is the difference between the ratio of the peak area of the impurity to peak area of internal standard minus the intercept of the linear curve over the slope of the linear curve. This value is multiplied by average tablet weight, then multiplied by the volume, divided by the sample weight and then divided by the label claim of the sample.
For drug substance:
The results, in ppm relative to the drug substance being tested, are given by:
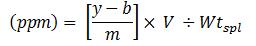
Where,
- y =
- Ratio of Peak Area of NDMA or NDEA to Peak Area of NDMA-d6
- b =
- intercept of the linear curve
- m =
- slope of the linear curve
- Wtspl =
- sample weight (g)
- V =
- 5 mL (volume)
Equation 2 - Text Description
Parts per million equals y minus b over m, which is the difference between the ratio of the peak area of the impurity to peak area of internal standard minus the intercept of the linear curve over the slope of the linear curve. This value is multiplied by the volume and then divided by the weight of the sample.
9. LOD and LOQ results
LOD/LOQ can be calculated using the S/N of the spiked sample solution (spiked with STD-3 at 1:1).
Theoretical LOD/LOQ:
If no spiked results are available, the theoretical LODs and LOQs can be calculated by using the S/N of STD-1 (NDMA: 0.002 µg/mL; NDEA 0.001 µg/mL).
For reference, the theoretical LOD/LOQ results at Health Canada are as follows:
| NDMA | NDEA | |||||||
|---|---|---|---|---|---|---|---|---|
| Drug substance conc. µg/mL | µg/mL | S/N | LOD (calc.) ppm | LOQ (calc.) ppm | µg/mL | S/N | LOD (calc.) ppm | LOQ (calc.) ppm |
| 50 | 0.002 | 74 | 0.002 | 0.0054 | 0.001 | 55 | 0.002 | 0.0073 |
10. Sample Chromatograms
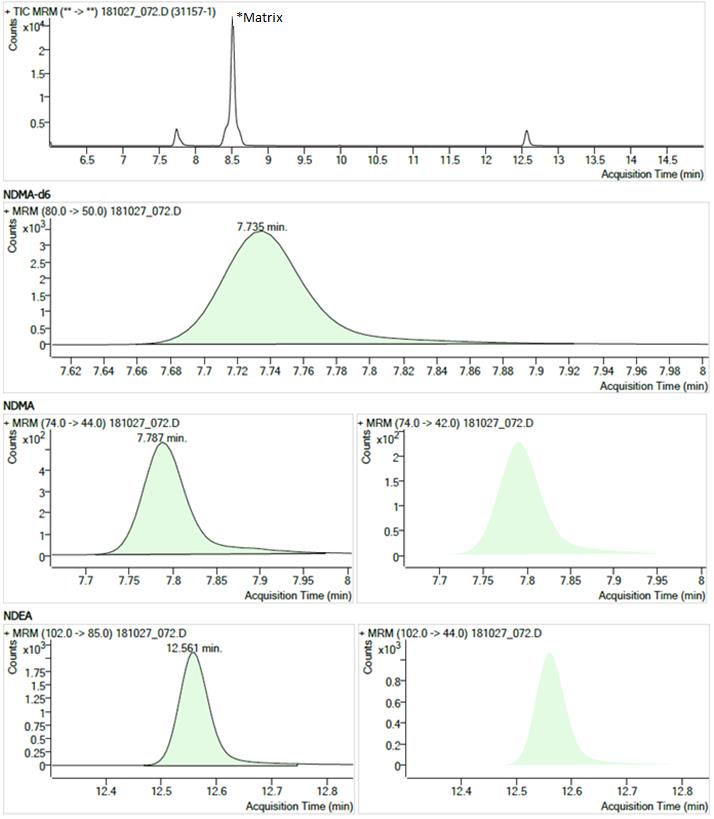
Figure 1 - Text Description
The figure shows chromatograms of a sample showing typical peaks for NDMA and NDEA. The chromatograms are arranged in four rows.
- The first row shows the Total Ion Current chromatogram from 6.0 to 15.0 minutes. There is a large peak at 8.5 minutes (labelled "matrix") and smaller peaks at 7.7 minutes (NDMA, NDMA-d6) and 12.6 minutes (NDEA).
- The second row shows a Multiple Reaction Monitoring chromatogram (m/z 80.0 → 50.0) with a single peak at 7.735 minutes for the NDMA-d6 internal standard.
- The third row shows two Multiple Reaction Monitoring chromatograms (m/z 74.0 → 44.0 and m/z 74.0 → 42.0) each with a single peak at 7.787 minutes for NDMA.
- The fourth row shows two Multiple Reaction Monitoring chromatograms (m/z 102.0 → 85.0 and m/z 102.0 → 44.0) each with a single peak at 12.561 minutes for NDEA.
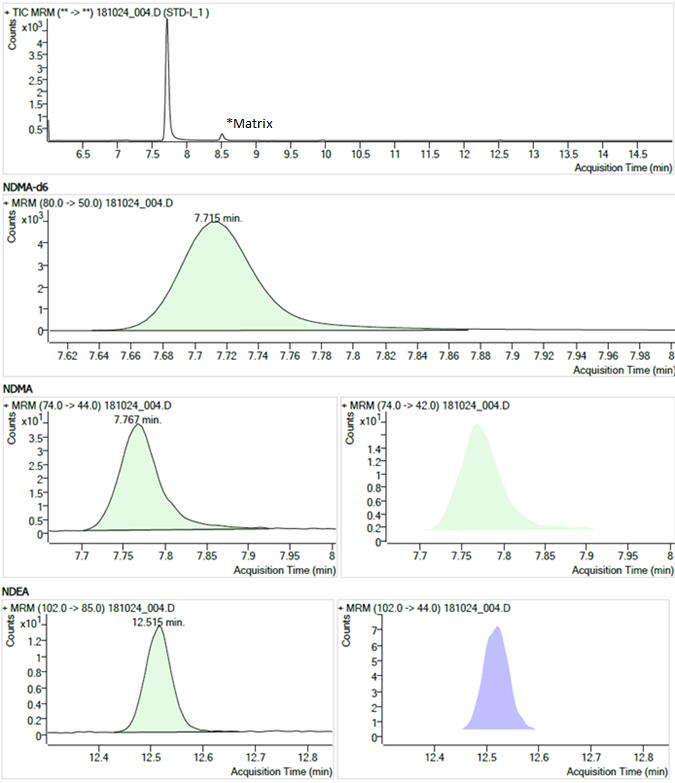
Figure 2 - Text Description
The figure shows chromatograms of the STD-1 standard solution. The chromatograms are arranged in four rows.
- The first row shows the Total Ion Current chromatogram from 6.0 to 15.0 minutes. There is a large peak at 7.7 minutes (NDMA, NDMA-d6) and a small peak at 8.5 minutes (labelled "matrix").
- The second row shows a Multiple Reaction Monitoring chromatogram (m/z 80.0 → 50.0) with a single peak at 7.715 minutes for the NDMA-d6 internal standard.
- The third row shows two Multiple Reaction Monitoring chromatograms (m/z 74.0 → 44.0 and m/z 74.0 → 42.0) each with a single peak at 7.767 minutes for NDMA.
- The fourth row shows two Multiple Reaction Monitoring chromatograms (m/z 102.0 → 85.0 and m/z 102.0 → 44.0) each with a single peak at 12.515 minutes for NDEA.
The United States Food and Drug Administration (FDA) and the European Directorate for the Quality of Medicines (EDQM) have also published methods to detect NDMA and NDEA:
Health Canada Communications
- Health Canada information update (2019-04-18): Auro Pharma Inc. voluntarily recalls one lot of Auro-Irbesartan HCT tablets because of nitrosamine impurity
- Health Canada information update (2019-03-14): Pro Doc Limitée voluntarily recalls two lots of irbesartan drugs because of nitrosamine impurity
- Health Canada information update (2019-03-09): Multiple Losartan-containing drugs voluntarily recalled because of potential for nitrosamine impurity
- Health Canada information update (2018-12-20): Health Canada releases test results of certain sartan drugs
- Health Canada information update (2018-11-28): Mylan-Valsartan medications voluntarily recalled as a precaution due to an impurity
- Health Canada information update (2018-10-02): Health Canada finds Zhejiang Huahai Pharmaceuticals site non-compliant with requirements for the manufacture of drug ingredients
- Health Canada information update (2018-09-13): Health Canada advises of a second impurity linked to recalled valsartan drugs
- Health Canada information update (2018-09-10): Health Canada updates Canadians on estimates of health risks for recalled valsartan drugs containing NDMA
- Health Canada information update (2018-08-18): Teva Canada expands recall of valsartan drugs to include additional lots, as a precaution
- Health Canada advisory (2018-07-09): Several drugs containing valsartan being recalled due to contamination with a potential carcinogen