Priority strategies to optimize self-testing in Canada
This is the fifth report of Canada’s COVID-19 Testing and Screening Expert Advisory Panel. It was released on August 12, 2021.
On this page
- Executive summary
- The Expert Advisory Panel and reports
- Background
- Evidence review of self-testing
- Testing hesitancy and behavioural science
- Opportunity costs
- Recommendations for self-testing
- Considerations for implementation
- Conclusion
- Annex A: Glossary of terms
- Annex B: Self-test studies
- Annex C: Self-test performance by brand and testing method
- Annex D: Reported RADT performance in symptomatic people by brand approved by Health Canada
Executive summary
In November 2020, the Minister of Health established the COVID-19 Testing and Screening Expert Advisory Panel. The Panel provides evidence-informed advice to the federal government on science and policy related to existing and innovative approaches to COVID-19 testing and screening.
The Panel has issued 4 reports since January 2021. This fifth report provides recommendations on the use of self-tests within Canada, including criteria for their application and potential cases for use. For the purpose of this report, the term “self-testing” refers to completely independent self-administered testing, from sample collection to reading results. This is distinct from “self-collection” of samples that are subsequently processed in a laboratory or at a point-of-care testing site.
The main objectives guiding recommendations for the use of self-testing for COVID-19 are to:
- reduce mortality and morbidity from COVID-19 by reducing community transmission of SARS-CoV-2
- support safer environments for more normal functioning of society and the economy
- maintain and, if possible, enhance surveillance of SARS-CoV-2 and its variants of concern (VoCs)
The Panel closed deliberations for this report on July 28, 2021 therefore the advice in this report may require revision due to the rapid evolution of the evidence, the availability of self-tests on the Canadian market and the epidemiological situation. The Panel is providing this advice as a third wave of COVID-19 has receded across Canada and vaccination rates are increasing. As of July 24, 2021, over 80% of eligible Canadians have received at least 1 dose of a vaccine. The expectation is that the percentage of the population receiving vaccines will continue to increase across the country. Approved vaccines have transformed COVID-19 from an infection with a high rate of severe disease and death in the elderly and people who are immunocompromised into an infection with a much lower mortality rate, highly concentrated among people who remain unvaccinated.
Evidence demonstrates that vaccination markedly reduces the risk of both symptomatic infections and severe disease. However, the Panel recognizes that not everyone is able or willing to be vaccinated. Self-testing provides an additional tool to allow people to rapidly identify infections and potentially mitigate transmission to others.
As vaccination rates increase across Canada and the incidence of COVID-19 decreases, demand for both diagnostic testing and test-based screening is expected to evolve. Dedicated specimen collection centres will not be as readily available as demand decreases. However, seasonal respiratory viruses, such as influenza, are expected to circulate along with COVID-19 in the upcoming months. This may trigger a renewed interest for testing people with symptoms who are vaccinated and unvaccinated.
Self-testing may have a role, particularly for those who are not vaccinated and those who have been hesitant to get tested if they exhibit COVID-19 symptoms. Self-testing may also play an important role should there be a marked resurgence of COVID-19 (for example, due to a vaccine-escape variant).
The Panel offers the following recommendations for the future use of self-tests as a complement to existing testing options:
Communication
- Self-tests should come with clear, concise messaging on how to use them, how to interpret the results, steps to take based on the result and how to dispose of the kits. There should also be a message about the importance of following public health measures, regardless of a negative self-test result.
Equity and affordability
- Where it is an effective use of public resources such as in the event of a COVID-19 resurgence, self-testing should be accessible at no cost and at various locations in communities.
Use of self-testing
- In the event of a COVID-19 resurgence, self-testing may be an effective tool for screening people who are asymptomatic and unvaccinated. It could also quickly identify potential infections in people with symptoms.
Implementation
- As self-test programs are deployed, they must be evaluated for test performance, accessibility, user acceptance, behavioural response and economic efficiency.
- Given the potential for outbreaks in the fall and winter, provinces and territories should maintain sufficient capacity for testing. They should not rely solely on self-testing to manage a potential resurgence of COVID-19.
The Expert Advisory Panel and reports
Mandate of the Panel
The COVID-19 Testing and Screening Expert Advisory Panel aims to provide timely and relevant guidance to the Minister of Health on COVID-19 testing and screening.
The Panel’s mandate is to complement, not replace, evolving regulatory and clinical guidance on testing and screening. Our reports reflect federal, provincial and territorial needs, as all governments seek opportunities to integrate new technologies and approaches into their COVID-19 response plans.
Plan for reports
The focus of the first Panel report included 4 immediate actions to optimize testing and screening:
- optimize diagnostic capacity with lab-based PCR testing
- accelerate the use of rapid tests, primarily for screening
- address equity considerations for testing and screening programs
- improve communications strategies to enhance testing and screening uptake
The second report focused on testing and screening strategies in the long-term care sector. The third report provided a perspective on how the recommendations from the first report can be applied to schools. The fourth report focused on testing and quarantine measures for Canada’s borders. This report provides recommendations on self-testing.
Consultation
The Panel consulted with more than 50 health and public policy experts in preparing this report. In addition, the Panel consulted with the Public Health Ethics Consultative Group (PHECG) regarding ethical considerations for self-testing. The Panel will continue to consult with a variety of stakeholders as we prepare further reports.
Guiding principles
Public health initiatives should strive to:
- maximize benefit and minimize harm
- promote equity
- respect individual autonomy
- offer a reasonable expectation of privacy
- increase transparency and accountability
Where these goals come into conflict with other, trade-offs need to be made. Panel discussions and engagement with stakeholders highlighted a number of key principles to consider in its guidance, including equity, feasibility and acceptability. The Panel applied these principles in framing its guidance and aimed to be transparent in describing trade-offs.
This report contains the Panel’s independent advice and recommendations, which were based on available information at the time of writing the report. The Panel examined scientific journal articles, modeling studies, grey literature and news articles to inform its recommendations.
Terms
“Self-testing” (or “self-tests”) refers to independent, self-administered testing throughout the entire testing process, from start (sampling) to finish (results) according to the instructions provided by the test manufacturer. Some self-test kits may connect to a smartphone app and automatically upload results to a database for reporting purposes. Other self-test kits provide results without automatic reporting.
This report uses “self-collection” to refer to a process that enables individuals to independently collect their own samples for testing. Self-collection is performed by the person being tested. The sample processing and analysis is done by a professional in a laboratory or point-of-care testing site.
Some terms used in the report may not be familiar to all readers. See Annex A for a glossary of terms.
Case study
United Kingdom: The U.K. prioritized self-testing at no charge to the public to expand national testing capacity. The U.K. is sending self-tests by post to reach those who cannot collect them. In addition, personal care attendants and home care workers who support people with disabilities are testing themselves twice a week, regardless of their vaccination status, using rapid antigen detection test (RADT) self-tests. Individuals receive a box of 7 tests by mail every 21 days so that they can also test themselves.
Acknowledgements
The Panel expresses its appreciation to the ex officio members of the Panel and to officials at Health Canada who have been working tirelessly to support the Panel. In addition, the Panel received expert advice from leaders in government, academia and industry. The Panel also acknowledges the contributions of the "shadow panel" on testing and screening, a group of students and young scientists who provided expert research and analytical assistance. Shadow panel members include Matthew Downer, Jane Cooper, Michael Liu, Jason Morgenstern, Sara Rotenberg and Tingting Yan.
- Sue Paish, Co-Chair
- Dr. Irfan Dhalla, Co-Chair
Panel members:
- Dr. Isaac Bogoch
- Dr. Mel Krajden
- Dr. Jean Longtin
- Dr. Kwame McKenzie
- Dr. Kieran Moore
- Dr. David Naylor
- Mr. Domenic Pilla
- Dr. Udo Schüklenk
- Dr. Brenda Wilson
- Dr. Verna Yiu
- Dr. Jennifer Zelmer
Background
Status of self-testing and self-collection in Canada
As of July 5, 2021, there are 74 testing devices for COVID-19 that are authorized for use in Canada. For many of these tests, self-collection is under review or is being performed as a clinical trial.
As of July 5, 2021, the Lucira “Check It” COVID-19 Test Kit is the only self-test kit approved by Health Canada. It is used as an over-the-counter self-test in people aged 14 and older.
“Check It” is a nucleic acid amplification self-test that works with self-collected nasal samples. Results are provided in 30 minutes. The sensitivity of “Check It” self-tests compared to lab-based PCR tests is reported to be 92% for people with COVID-19 symptoms.
Off-label use of rapid antigen tests as self-tests are also occurring in some jurisdictions across Canada. Currently, there are no self-tests available for purchase in Canada, either with or without a prescription.
Health Canada is expecting additional applications for authorization of self-tests in the near future, including RADTs, which are generally less expensive than molecular tests. However, the availability of other self-tests on the market is uncertain. In the United States and in other countries, RADT self-test kits use a sample collected from the nose, throat or saliva and are available either with or without a prescription (for example, at retail stores, pharmacies).
Rationale for self-testing
As vaccination campaigns proceed across Canada, testing needs are decreasing. However, there remains a role for testing as the economy and public services re-open. There are also some Canadians who are ineligible, unable or unwilling to get vaccinated. Used properly, self-tests can quickly identify those who are infected and allow people to take measures to protect their household and their community.
There are benefits and considerations to weigh when determining how to deploy self-testing. In conventional testing, specimens are obtained using a nasopharyngeal (NP) swab at an assessment centre and processed at a laboratory. The potential benefits of self-tests include:
- privacy
- rapid results
- easier accessibility
- more acceptable (for instance, may use less invasive sampling methods and can be completed at a location of choice)
- minimal training or oversight required to administer the test (counsellors may be useful in some contexts)
- usability in a variety of settings such as schools, workplaces and remote communities and before large events such as concerts, sports and weddings
The potential drawbacks of self-tests include:
- inferior accuracy (more frequent false negatives and false positives)
- uncertainty on the performance of self-tests in a vaccinated population
- reduced opportunities for advice or guidance from a health care professional
- risk that negative test results may lead to high-risk behaviour due to false confidence
- risk that positive test results are not acted on or communicated to public health
In the event of a COVID-19 resurgence, self-testing may be used as a tool to enable rapid screening for infection and thereby help reduce transmission in the community. While self-tests can detect the presence of COVID-19 infection, they cannot currently distinguish whether the infection is from a variant of concern.
Industry and some jurisdictions who were consulted for this report indicated that various forms of screening will be needed in the short to medium term to reduce the risk of outbreaks. Especially at risk are:
- workplaces such as food processing facilities where people are working indoors and in close proximity
- long-term care homes and similar facilities where people are working with a vulnerable population
Similarly, jurisdictions aiming to minimize community transmission may continue to use testing for surveillance. In this scenario, self-testing may offer a lower-cost option compared to other methods.
Screening programs are of greater value if protective behaviour is maintained. Public health measures should not be disregarded due to a negative test result. In addition, positive self-tests should be confirmed with laboratory-based PCR.
Evidence review of self-testing
The available evidence on the effectiveness of self-testing in terms of reducing community transmission is limited.
For this report, the Panel relied on research and evidence related to both self-testing and self-collection, as well as case studies from other countries. New evidence may emerge over the coming months that may influence the recommendations below.
Test acceptability
Self-tests rely on samples collected (typically nasal) by the layperson (collecting a sample on themselves or their children). In contrast, nasopharyngeal swabs (the most common and reliable sampling technique for lab-based PCR tests) are collected by a health care professional. Previous studies (Valentine-Graves and others, Goldfarb and others, Siegler and others) suggest that populations generally accept and tolerate self-collection of samples when less invasive methods are used, particularly saliva and nasal swabs.
Recent research indicates that self-testing is feasible within the general population. For example, 81% of primarily young and educated participants in 1 study stated that the self-test was easy to use. Some participants suggested a number of improvements would facilitate self-testing:
- illustrations
- video formats
- multiple languages
- marks on swabs to guide insertion depth
- instructions with precise or simple language
Despite reported confidence and comfort using self-tests, self-test administration can result in user error, which can decrease the sensitivity of self-tests.
Test performance
Scientific studies generally compare COVID-19 self-test performance with lab-based PCR tests using NP swabs collected by health care providers. This report uses these comparisons for test sensitivity and specificity, unless otherwise specified. However, current estimates of sensitivity and specificity for self-tests are imprecise because performance characteristics reported by manufacturers are based on small studies. Examining the 95% confidence intervals (95% CI) can give some indication of the level of certainty, with wider confidence intervals indicating less certainty.
Overall, the performance of RADT and nucleic acid self-collected tests is lower than lab-based PCR tests using samples collected by health care providers (see Annex B). Other smaller studies (Lindner and others, Goldfarb and others, Hanson and others, McCullough and others, Braz-Silva and others, Frediani and others) found sensitivities of self-collected anterior nasal swabs, saline gargle and saliva between 77% and 98% compared to nasopharyngeal swab samples collected by health care providers using the same test kit. A study found that older age, lower viral load and self-reported difficulty with sampling are associated with reduced self-collection performance.
There is some variation in the performance of different brands of self-tests available in the U.S. and the United Kingdom. Overall, both nucleic acid tests and RADTs have high specificity. RADTs are less sensitive than nucleic acid tests (Annex C and Annex D).
The performance of RADTs, which are commonly used for self-testing, varies based on symptom status and viral load. A recent Cochrane review found that RADTs conducted in people with symptoms were 72% sensitive compared to 58% in people without symptoms. Furthermore, sensitivity was 95% in those with high viral loads compared to 41% in those with lower viral loads. Sensitivity across RADT brands ranged from 34% to 88%, while specificity for all tests considered was high (~99%).
Given evidence of higher transmissibility (Alberta Health, Chian Kohn and others, Buitrago-Garcia and others, Byambasuren and others) in those who have symptoms and/or higher viral loads, the impact of lower sensitivity of RADTs in people without symptoms and/or lower viral load cases is unclear. One study found high concordance with PCR test results when viral load was high (Ct counts below 25) but less concordance with higher Ct counts.
Current evidence suggests that self-testing may be an effective tool to reduce SARS-CoV-2 transmission in communities when incidence is high. A modelling study from the U.S. found that self-testing with RADTs could reduce COVID-19 transmission if tests are conducted frequently.
Asymptomatic testing criteria
Self-tests work best when the prevalence of infection is high. The proportion of false positives is related to the sensitivity and specificity of the test and the pre-test probability of a positive result. For asymptomatic screening, the pre-test probability is the prevalence of COVID-19 in the population undergoing screening. This may be an over-estimation because excluding symptomatic people lowers the pre-test probability.
One study shows that the predictive value of positive test results drops greatly when prevalence is low. A prevalence threshold can be calculated for any pre-determined minimum acceptable positive predictive value.
Thus far, there is little direct evidence related to the effects of large-scale screening programs using self-tests on community transmission. There is also little direct evidence on the potential negative consequences (for example, loss of income from a false positive). The proportion of false positives is related to the sensitivity and specificity of the test and the pre-test probability. For asymptomatic screening, the pre-test probability is the prevalence of COVID-19 in the population. As prevalence decreases, the proportion of positive results that are false positives increases. For example, for a test with 90% sensitivity and 99.9% specificity, the proportion of false positives will be about 53% when the prevalence is 0.1%, but 92% when prevalence is 0.01%. Figure 1 provides an example of performance of a test in a setting where the prevalence is low.
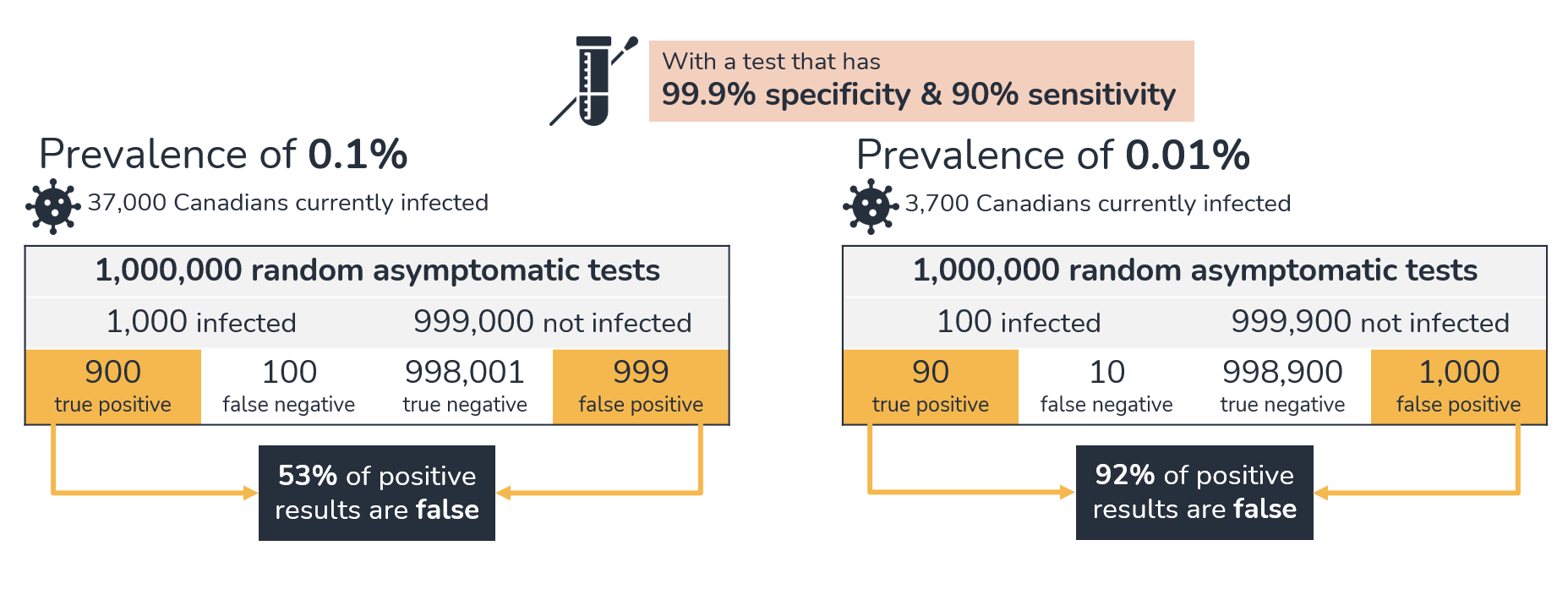
Figure 1 - Text description
This graphic highlights false positive results using a test with 99.9% specificity and 90% sensitivity, at 2 different levels of prevalence. At 0.1% prevalence, about 37,000 Canadians would be currently infected. One million random asymptomatic tests would attempt to identify about 1,000 infected and 999,000 non-infected individuals. There would be 900 true positive, 100 false negative, 998,001 true negative and 999 false positive results. Of the positive results, 53% would be false. At 0.01% prevalence, there would be about 3,700 Canadians currently infected. One million random asymptomatic tests would attempt to identify about 100 infected and 999,900 non-infected individuals. There would be 90 true positive, 10 false negative, 998,900 true negative and 1,000 false positive results. Of the positive results, 92% would be false.
Usefulness in vaccinated people
Using effective testing modalities to navigate the months ahead and avoid strict public health interventions (“lockdowns”) at high economic and social costs will be key.
While our understanding of the virus is growing, we still know little about the performance of self-tests in people who are partly or fully vaccinated. This is especially pertinent given emerging evidence of decreased viral loads after partial or full vaccination. People who are vaccinated will have a lower pre-test probability of infection, which increases the likelihood that a positive test result may be a false positive.
Testing hesitancy and behavioural science
There are many reasons for testing rates being lower among marginalized groups than would be expected given the rates of COVID-19. These include:
- mistrust of health systems
- inequitable access to testing
- concerns about the potential financial and social impacts of a positive test
Note that these reasons are downstream consequences of both systemic and interpersonal racism.
Effective deployment of self-tests may help improve testing equity and decrease community transmission by making it possible to test people who would not have been tested. Self-testing is part of a multi-pronged approach to developing a testing program that addresses equity and accessibility and reduces stigma for marginalized populations.
To encourage testing, tailored interventions that offer a lot of support and links to health care resources should reflect local issues and needs. Communities with positive or negative self-test results should be supported and encouraged to follow public health guidance. Positive self-tests should be confirmed with laboratory-based PCR test to allow for contact tracing, thereby reducing the risk of spread.
Both behavioural barriers (for example, not being able to access testing close to home) and financial barriers (for example, lack of access to paid sick leave and needing time off to get tested) can also promote testing hesitancy. Behavioural barriers that self-tests can address are outlined in Table 1.
| Barrier | Contribution to hesitancy | Self-test application |
|---|---|---|
| Time/ geography | Time investment for travel to and from testing sites, and turn-around time to obtain results | Results are available in 30 minutes or less Do not need to go to testing site Tests available where people already go (for example, supermarket, pharmacy) |
| Stigma | People are hesitant to reveal contacts to contact tracers | Self-tests can be anonymous and private Affected individuals may notify their own contacts |
| Social norms | The perception that peers do not get tested makes individuals less likely to get tested themselves | Widespread test availability makes testing more normal |
| Logistical frictions | Barriers that discourage testing include locating and getting to a testing site, language barriers, time and process to obtain results, requiring a health insurance card/number | Tests available where people already go (for example, supermarket, pharmacy) Results are available in 30 minutes or less |
| Procrastination | People tend to put off unpleasant tasks | Self-collection of samples is more pleasant Results are available in 30 minutes or less |
| Status quo bias | People dislike change in their routines and prefer more of the same once routines are established | Do not need to go to testing site Tests available where people already go (for example, supermarket, pharmacy) |
| Uncertainty | Mild symptoms or symptoms that overlap with other conditions (for example, allergies) may not trigger a decision to go to a testing site | Do not need to go to testing site |
In the U.S., the price of self-testing kits ranges from $12 to $55 USD (costs vary based on test type). RADT self-tests are less expensive, while nucleic acid self-tests are more accurate but also more expensive. RADT self-tests may be better suited for screening given their lower cost. (Note: Currently, there are no RADT self-tests available for purchase in Canada.)
Case study
Austria: As part of the Austrian Testing Strategy for SARS-CoV-2, the federal government is offering up to 5 free self-tests per month at pharmacies starting in March 2021. Additional tests can be bought for about €8. Positive self-tests need to be followed up with a PCR test and public health authorities are to be informed immediately. Lower Austria has launched a platform to register valid self-tests in order to visit restaurants and bars, as individuals are only allowed in if they have been tested, vaccinated or recovered from COVID-19. After submitting a picture with a negative result, the user receives a QR code for proof for entry.
Opportunity costs
Some countries have made free self-tests available on demand. Whether they will continue to do so in low-prevalence settings when the population is vaccinated is unclear. For instance, the daily number of RADTs conducted in the United Kingdom has been decreasing since May. The cost of an $8 test twice a week for 5 million people would be about $320 million per month. In low-prevalence settings in a vaccinated population, it will be very expensive to find an additional positive case, with minimal benefit if the population has high vaccination coverage. This is corroborated by a study that found serial screening using RADTs becomes less cost-effective as transmission rates drop.
Provincial and territorial governments are well placed to weigh the cost of distributing free or inexpensive self-tests for public health purposes.
Businesses and private enterprise are also well placed to weigh the cost of implementing their own self-test programs. The Government of Canada and some provinces have been working with industry associations, non-profits and other organizations to provide access to rapid testing in many sectors.
Recommendations for self-testing
The Panel’s self-testing recommendations are based on the evidence available when this report was written. The goal of the recommendations is to provide accessible testing and screening in order to identify positive cases, reduce community transmission of COVID-19 and facilitate re-opening in Canada. As additional data and evidence become available, the Panel may need to revisit these recommendations.
Communication
Recommendation 1
Self-testing means that an individual is responsible for independently performing the entire testing process. For this reason, self-tests should come with clear, concise messaging:
- how to use them
- how to interpret the results
- which steps to take if the result is positive or negative
- how to dispose of the kits
There should also be a message about the importance of following public health measures, regardless of a negative self-test result.
With self-tests available on the Canadian market, there will also be a need to provide guidance to Canadians on what tests are recommended, if any, for different scenarios. For example, Canadians will need to know that self-testing is not the preferred test for an individual who has been exposed to someone with COVID-19. Lab-based PCR is the preferred test in this context.
Clear, transparent, creative and accessible information about COVID-19 and self-testing must be available in multiple languages, not just French and English. As well, accessibility and multiple formats are especially important for people with disabilities, as many individuals in Canada have felt excluded from COVID-19 messaging. Health helplines should also be equipped to respond to questions on using self-tests.
All this information should be available when a user obtains the test and also included with the self-test package.
Communications tools such as websites or apps would be useful for reporting self-test results. Provinces and territories could consider offering tools for reporting self-test reports, where this is possible through their existing legislative and regulatory frameworks.
Equally important is the need to use strong messaging to inform people who are self-testing that they should continue to follow the relevant public health guidance.
Case study
Nova Scotia: Halifax’s campaign “Negative for the Night” has been an effective slogan to communicate the benefits and limitations of testing. A negative test is good for the night, but not subsequent days. People who participate in the rapid testing program receive messaging on mitigating risk, including the following:
- Remember a negative test still means you have to wear a mask, wash your hands, and social distance six feet.
- A negative test is only valid for the day. You could become positive after today. If you develop symptoms at any point or have a known COVID positive contact, you must call 811.
- Come out and get tested again soon.
Equity and affordability
Recommendation 2
Where it is an effective use of public resources, such as in the event of a COVID-19 resurgence, self-testing should be accessible at no cost and at various locations in communities.
If people are required to pay for self-tests, they will only be accessible to individuals who can afford them. This does not align with the goals of screening programs and the values that underlie the delivery of health care in Canada.
If one of the goals of deploying self-tests is to reduce testing hesitancy, it is important that self-tests be easily accessible to all Canadians, especially in high-incidence areas and/or for high-risk populations. High-risk populations include:
- older people
- essential workers
- people living in remote communities
- people living in high incidence communities
- people with disabilities or pre-existing health conditions
- racialized communities, including black and on- and off-reserve Indigenous communities
If there is a resurgence of COVID-19 cases, in high-incidence areas, self-tests should be available in high-incidence areas. They should be offered at no cost and at various locations in a community. These include:
- schools
- workplaces
- testing centres
- places of worship
- community centres
- Indigenous service organizations
In some cases, it may be desirable to mail self-tests. This option would complement making self-tests available for sale at retail locations such as pharmacies and grocery stores.
Case study
United States: The Centers for Disease Control (CDC) and National Institutes of Health (NIH) launched Rapid Acceleration of Diagnostics Underserved Populations (RADx-UP). This $500-million COVID-19 testing initiative aims to help disproportionately impacted communities across the country. CDC and NIH funded a pilot study in North Carolina and Tennessee with the Quidel QuickVue At-Home OTC COVID-19 Test to determine if community transmission is reduced by providing free self-tests and testing regularly. They also funded a randomized trial of home-based COVID-19 testing with American Indian and Latino communities in Montana and the Yakima Valley of Washington. This study investigates barriers to home-based testing, delivering tests by community health educators compared to mail and community-driven testing protocols.
Using self-tests
Recommendation 3
In the event of a COVID-19 resurgence, self-testing may be an effective tool for screening people who are asymptomatic and unvaccinated. It could also quickly identify potential infections in people with symptoms.
Evidence from scientific studies and modelling demonstrates acceptable sensitivity and specificity among self-tests (see Annex B and C) in unvaccinated individuals. This suggests that self-tests may have a role in testing asymptomatic unvaccinated people from time to time when there are high case counts. In the case of current screening programs, using self-tests can be less costly as they do not require dedicated staff for testing.
When case counts are low, many tests are needed to find a single case and false positives make up a larger proportion of positive results. In this case, screening programs are unlikely to be cost-effective. While rare, false positives can also cause harm (for example, loss of income due to isolation requirements after a false positive result).
The prevalence threshold and desired minimum positive predictive value for asymptomatic screening using a given test can be calculated. For example, for a 99.9% specific, 90% sensitive test, prevalence would be at least 1% to have an 80% positive predictive value.
The decision to implement a COVID-19 self-test screening program may be based on the following factors:
- low test cost
- high test specificity and sensitivity
- public support and desire for screening
- effective ability to isolate with positive results
- high COVID-19 prevalence for the jurisdiction
- population particularly vulnerable to COVID-19 due to:
- age
- high-risk groups
- low vaccination rates
- high variants of concern rates with potentially lower vaccine effectiveness
- lack of access to rapid PCR testing or limited testing personnel
- robust reporting of self-test results and contract tracing/quarantine capacity
- barriers to accessing other forms of testing (for example, testing available at limited times/places or testing hesitancy)
Case study
United Kingdom: The U.K. used a RADT self-test at a cost of approximately $8.50 CAD for distribution through the NHS Test and Trace program. The sensitivity of the test is 57.5% when used by self-trained members of the public and the specificity is 99.7%. There was no difference between samples collected by symptomatic and asymptomatic people. The U.K. recommended that everyone self-test twice a week. Tests are available at pharmacies and testing centres. In June 2021, the U.K. shifted its self-testing focus to people who are not vaccinated and those deemed to be highly vulnerable.
All secondary school students have been asked to take 2 tests every week since March as part of the school reopening program. From March 8 to April 4, 26,144,449 rapid self-tests were reported, with about 81% of these taking place in educational contexts. Of these, 30,904 were positive. Among the positive tests that had a confirmatory PCR test, 18% were identified as false positives. Over this period, the prevalence of COVID-19 in schoolchildren was estimated to be about 0.43%.
The U.K. program has been criticized for a lack of evidence around the testing recommendations, questionable impact and high cost (see Mahase, Raffle and Gill, Halliday).
As public health restrictions are relaxed, other respiratory viruses will once again begin to circulate. It may be difficult to distinguish between SARS-CoV-2, influenza, other respiratory viruses or co-infection. Multiplex testing is used to simultaneously identify if an individual is infected with the SARS-CoV-2 virus or other respiratory viruses (such as influenza or respiratory syncytial virus). Self-testing can also help people determine whether they are likely to have COVID-19 or be infected with another respiratory virus. People with respiratory symptoms should be encouraged to stay home and to follow public health guidance.
Considerations for implementation
Research and evaluation
Recommendation 4
As self-test programs are deployed, they must be evaluated for test performance, accessibility, user acceptance, behavioural response and economic efficiency.
Continuous quality improvement frameworks should be applied, with both process and outcome metrics to modify or scale back ineffective or suboptimal programs. Analyses should disaggregate for Indigenous populations, other ethnic and racial groups, income groups, rural and urban groups, and genders.
Evaluating self-testing should consider the following factors:
- its effectiveness, acceptability, feasibility, test performance and effects on COVID-19 transmission
- how the supply chain can respond to high demands
- how to report results, including how to address privacy concerns
- its effect on surveillance data, contact tracing and rate of follow-up PCR tests
- financial impacts and cost-effectiveness
- social impacts and effects on testing equity
- individual autonomy (for instance, in contexts where test results are required to access settings such as workplaces and educational institutions)
- the user experience, including qualitative information from people on the acceptability of various self-tests (sample collection, convenience, comfort, ease of access)
These factors will help inform future self-testing programs for COVID-19 or other pandemics.
Research is needed on the effectiveness of self-tests in vaccinated populations. There is also benefit to better understanding the behavioural response to a negative result and whether the result encourages high-risk behaviour.
Self-tests can be done in private without consulting a health care provider. It would be useful to know:
- about the types of people who would not go to a testing centre but would use a self-test
- if there are settings where people who are otherwise hesitant to be tested would use self-tests
Reporting, public good and privacy
Self-collected samples that are processed in a lab or at the point-of-care will have results automatically relayed to the public health authority. However, Health Canada has already authorized 1 self-test with no built-in reporting mechanism. The Panel respects the rights of Canadians to a reasonable expectation of privacy, including privacy of their health information.
The Panel also recognizes that mandated reporting for independently processed self-tests is likely not feasible. The lack of reporting creates challenges for contact tracing and quarantine compliance monitoring. Tools will be needed to encourage people to voluntarily report their self-test results.
People who voluntarily undergo self-testing may be more inclined to adjust their behaviour if they receive a positive result, whether or not they opt for a confirmatory PCR test.
The Panel suggests the following measures to encourage the voluntary reporting of self-test results:
- support and incentives for those who receive positive test results, such as paid sick-leave, to reduce any negative consequences for those who decide to report
- clear communication about the need for a confirmatory PCR if the self-test result is positive
- accessible communications outlining the importance of self-reporting and the community-wide benefits of contact tracing
- teaming up with community leaders, including health care and religious leaders, for communication campaigns may help increase uptake
- clear information on best practices, where the approach is on trusting people to self-isolate when sick
- less reliance on the public health system and enforcement
Recommendation 5
Given the potential for outbreaks in the fall and winter, provinces and territories should maintain sufficient capacity for testing. They should not rely solely on self-testing to manage a potential resurgence of COVID-19.
As vaccination rates increase across the country, it is expected that specimen collection sites will decrease capacity. Screening for COVID-19 in certain settings (such as workplaces) will also decrease over time, assuming case counts remain low.
As the demand for testing decreases, it may not be a reasonable use of public resources to maintain testing infrastructure, such as mass COVID-19 testing sites. The Panel recommends that provinces and territories take care when scaling down infrastructure. We can’t predict the infrastructure need for several months, especially since we have not yet had an influenza season during the pandemic.
Diagnostic testing will remain important as the pandemic subsides and the COVID-19 virus continues to circulate.
Use cases for self-testing
In addition to the recommendations outlined in this report, the Panel offers 3 potential use cases for self-testing to put the recommendations in context.
Homes for populations at risk of severe outcomes from COVID-19
The immune response of some vulnerable populations (for example, elderly or people with comorbidities) can be lower. They are more susceptible to COVID-19, particularly if they receive in-home care from an external provider, live in a congregate or multi-generational setting or live in a remote or isolated community.
In these settings, personal support workers, health care workers and family members should be given easily accessible and rapid self-testing tools to protect the vulnerable people they serve, especially if there are those who choose not to be vaccinated. Self-tests could be deployed to home care agencies for distribution to their employees.
Empowering safer socialization and travel
Throughout the pandemic, people were encouraged to stay home and avoid seeing family or friends to protect each other from the spread of COVID-19. In many jurisdictions, these restrictions are being lifted and people are once again visiting friends and family. However, many individuals may still worry about spreading COVID-19, particularly if they:
- must travel in close proximity to others (for example, by plane, bus, train)
- are not vaccinated or are visiting someone who is not vaccinated
- are vulnerable to COVID-19 or are visiting someone who is vulnerable (elderly, people with comorbidities who may not have full protection from the vaccine)
In these cases, a self-test could be taken right before the visit, and potentially also a few days after travel. This would add a layer of protection by screening for COVID-19.
Along with strong communication and ongoing public health measures, the self-test may have significant value to individuals, who will be empowered to test themselves. The risk is there may be false negatives or people may be less careful if they receive a negative result. More research is needed to better understand the behavioural responses to a negative self-test.
Schools
Currently, no COVID-19 vaccines have been approved for children under 12. Other respiratory illnesses will likely occur in the fall as restrictions loosen, particularly in congregate settings like schools.
Schools will need to ensure that low-barrier testing is available for students who have been exposed to SARS-CoV-2 and for students with symptoms. This is especially important, as school closures may have a wide-reaching effect on childhood development.
Self-tests could be distributed on a voluntary basis to students and staff at schools. They would be able to take the test quickly and in private. For students and staff who are high-risk, extra protective measures may be necessary.
Conclusion
Canadians have been living with the COVID-19 pandemic for more than a year. During this time, the testing and screening landscape has shifted dramatically and will continue to do so as we increase vaccination rates across the country.
Testing will continue to play an important role over the months and years to come. As part of the testing landscape, self-testing is an important tool that can be used to identify COVID-19 cases and potentially break the chains of transmission.
Given the available evidence, the Panel recommends that self-tests be available to Canadians in the event of a COVID-19 resurgence and where costs are justified. The emphasis should be on affordable or no-cost access for people who are most vulnerable to COVID-19.
Annex A: Glossary of terms
Diagnostic testing: Used to identify if an individual who is suspected to have been infected with the SARS-CoV-2 virus has been infected.
Loop-mediated isothermal amplification (LAMP) test: A testing method that amplifies and detects genetic material in a sample to identify a specific organism or virus without temperature cycles. LAMP tests can be more readily deployed as rapid tests, but may not be as sensitive or specific as PCR tests.
Multiplex testing: Used to simultaneously identify if an individual is infected with the SARS-CoV-2 virus or other respiratory viruses (such as influenza or respiratory syncytial virus).
Polymerase chain reaction (PCR) test: A testing method that amplifies and detects genetic material in a sample to identify a specific organism or virus through cycling high and low temperatures. PCR tests can identify SARS-CoV-2 genetic material during an active infection and also dead virus for some time after the infection has resolved. PCR tests are considered the most reliable and accurate tests for COVID-19. They are usually processed in a lab but can also be performed as a rapid test.
Pre-test probability: The chance that a person has COVID-19, estimated before the test result is known and based on the probability of the suspected disease in that person given their symptoms, exposure history and epidemiology in the community.
Prevalence: The proportion of a population with COVID-19 at a given time.
Rapid antigen detection test (RADT): A testing method that identifies a specific organism or virus by detecting proteins in a sample. RADTs are a form of lateral flow test that is relatively cheap and easy to deploy in community settings. These tests are generally less sensitive than PCR and LAMP tests. They are most likely to be positive during the symptomatic phase of disease.
Screening test: Performed in people who are asymptomatic without known exposure to the SARS-CoV-2 virus. Screening can be used to detect asymptomatic or pre-symptomatic COVID-19 infections and prevent large outbreaks. This is especially important in settings where individuals have more contacts (for example, students and essential workers).
Self-collection: A process that enables people to collect their own sample for testing. Self-collection is performed by the person being tested, but the sample processing and analysis is done by a professional in a laboratory or point-of-care testing site.
Self-testing: A process that enables people to conduct a COVID-19 test from start to finish, thereby allowing them to assess and monitor their own infection status. Self-testing includes sample collection, processing and analysis.
Sensitivity: In a population of individuals who have a condition of interest, the proportion of people who test positive with a particular test.
Specificity: In a population of individuals who do not have a condition of interest, the proportion of people who test negative with a particular test.
Annex B: Self-test studies
| Study | Self-test/self-collection sensitivity (positive percent agreement) vs. lab-based PCR |
|---|---|
| Dutch study | RADT self-test: 78.0% (95% CI: 72.5% to 82.8%) |
| Canadian study | Saline gargle + PCR: 90% (95% CI: 86% to 94%) Oral + PCR: 82% (95% CI: 72% to 89%) Oral/anterior nasal swab + PCR: 87% (95% CI: 77% to 93%) |
| U.K. evaluation | RADT self-test: 57.5% (95% CI: 52.3% to 62.6%) RADT collected by trained health care worker: 73.0% (95% CI: 64.3% to 80.5%) |
Annex C: Self-test performance by brand and testing method
| Brand | Sensitivity (positive percent agreement) | Specificity (negative percent agreement) | Sample type | Turn around time |
|---|---|---|---|---|
| RADT | ||||
| Quidel Sofia |
(95% CI: 71.8% to 92.4%) |
99.1% (95% CI: 95.2% to 99.8%) |
Nasal | 15 minutes |
| Abbott BinaxNow | 84.6% (95% CI: 76.8% to 90.6%) |
98.5% (95% CI: 96.6% to 99.5%) |
Nasal | 15 minutes |
| Ellume | 95% (95% CI: 82% to 99%) |
97% (95% CI: 93% to 99%) |
Nasal | 20 minutes |
| Innova | 57.5% (95% CI: 52.3% to 62.6%) |
99.7%Footnote * | Nasal or throat | 20 minutes |
| LAMP | ||||
| Lucira Checkit COVID-19 Test Kit | 94.1% (95% CI: 85.5% to 98.4%) |
98% (95% CI: 89.4% to 99.9%) |
Nasal | 30 minutes |
|
||||
Annex D: Reported RADT performance in symptomatic people by brand approved by Health Canada
| Brand | Symptom status | Sensitivity | Specificity |
|---|---|---|---|
| Abbott Panbio | Symptomatic, any stage | 72.6% (95% CI: 64.5% to 79.9%)Footnote * |
100% (95% CI: 99.7% to 100%) |
| BD Veritor | Within 7 days of symptom onset | 76.3% (95% CI: 60.8% to 87.0%) |
99.5% (95% CI: 97.4% to 99.9%) |
| Quidel SofiaFootnote ** | Symptomatic, any stage |
(95% CI: 64.4% to 90.9%) |
98.9% (95% CI: 96.2% to 99.9%) |
| Roche SD Biosensor | Symptomatic, any stage |
(95% CI: 79.1% to 89.4%) |
99.5% (95% CI: 98.7% to 99.8%) |
|
|||
Page details
- Date modified:
