ARCHIVED Guidance Document - Fees for the Right to Sell Drugs
Notice to Reader:
This is an archived document. Please refer to the most current version of this document
Date Adopted: 1998/05
Revised Date: 2018/06/13
Effective Date: 2018/06/13
| Date | Change | Location (section, paragraph) | Nature of and/or Reason for Change |
|---|---|---|---|
| 2018/06/13 | Amended Scope and Application | S1.3 | Regulations Amending the Food and Drug Regulations (DIN Requirements for Drugs Listed in Schedule C to the Food and Drug Act that are in Dosage Form) and Regulations Amending the Fees in Respect of Drugs and Medical Devices Regulations (DIN Requirements for Drugs Listed in Schedule C to the Food and Drug Act that are in Dosage Form) |
| 2018/06/13 | Amended Dormant DIN information and Removal of Footnotes | S.2.2.4, Footnotes | Regulations Amending the Food and Drug Regulations (DIN Requirements for Drugs Listed in Schedule C to the Food and Drug Act that are in Dosage Form) |
| 2017/03/14 | Amended definition of Annual Drug Notification Form | S1.5 | To improve clarity |
| 2017/03/14 | Amended section to add additional details to Annual Notification process | S2.2.3, S2.2.5 | To specify additional process requirements to reflect regulatory amendment Regulations Amending the Food and Drug Regulations (Shortages of Drugs and Discontinuation of Sale of Drugs) |
| 2017/03/14 | Amended physical address for submission of Payment of Invoice | S2.3.2 | To reflect address change for Accounts Receivable |
| 2017/03/14 | Amended When to Pay Fees (first bullet) | S2.3.4 | To improve clarity |
| 2017/03/14 | Clarifications regarding the Dormant DINs and the fee remissions process for these products have been added in the document. | S2.2.4, S2.5.2, S2.5.4 | To specify additional process requirements to reflect regulatory amendment Regulations Amending the Food and Drug Regulations (Shortages of Drugs and Discontinuation of Sale of Drugs) |
| 2013/06/10 | Scope was changed to exclude Emergency Use New Drug (EUND) submissions from this guidance document. | S.1.3 | Regulatory amendment to the Fees in Respect of Drugs and Medical Devices Regulations (Fee Regulations) to exclude EUND submissions (NDS and ANDS) |
| 2013/06/10 | Submission and Information Policy Division changed to Office of Submissions and Intellectual Property | S.2.1 | To reflect the office name change |
| 2013/03/02 | Misleading sentence was removed | Last paragraph in S.2.3.4 | To improve clarity |
| 2013/03/02 | Complete name Right to Sell Drugs Fee Remission Request and Attestation Form was inserted | Throughout the document | To improve clarity |
| 2011/04/01 | Significant changes to this document include changes to the fee structure and in the remission and fee deferral process. | Whole document | The guidance document was rewritten to reflect the new Fee Regulations. |
Foreword
Guidance documents are meant to provide assistance to industry and health care professionals on how to comply with governing statutes and regulations. Guidance documents also provide assistance to staff on how Health Canada mandates and objectives should be implemented in a manner that is fair, consistent and effective.
Guidance documents are administrative instruments not having force of law and, as such, allow for flexibility in approach. Alternate approaches to the principles and practices described in this document may be acceptable provided they are supported by adequate justification. Alternate approaches should be discussed in advance with the relevant program area to avoid the possible finding that applicable statutory or regulatory requirements have not been met.
As a corollary to the above, it is equally important to note that Health Canada reserves the right to request information or material, or define conditions not specifically described in this document, in order to allow the Department to adequately assess the safety, efficacy or quality of a therapeutic product. Health Canada is committed to ensuring that such requests are justifiable and that decisions are clearly documented.
Table of Contents
- 1 Introduction
- 2 Guidance for implementation
1 Introduction
This document provides guidance on the interpretation of the Fees in Respect of Drugs and Medical Devices Regulations (Fee Regulations) with a focus on how the Fees for the Right to Sell Drugs, contained in Part 2, Division 4 of these regulations, will be administered.
1.1 Policy objectives
To ensure that the cost recovery system to defray the cost to government of applying the principles of risk assessment and risk management in the regulation of drugs reflects the current costs associated with the marketing of drugs, excluding natural health products and drugs for veterinary use only.
1.2 Policy statements
A manufacturer that holds a drug identification number (DIN) assigned to a drug under section C.01.014.2 of the Food and Drug Regulations will be charged a fee for the right to sell that drug each year that the drug is on the market in Canada.
Fees are charged for the right to sell a drug in the upcoming year beginning October 1 and ending September 30 of the following year.
DIN holders that have not completed their first calendar year of selling a drug in Canada will have their fee deferred to the end of their first completed calendar year.
DIN holders are eligible for a remission of a portion of the right to sell fee when the fee exceeds 1.5% of the actual gross revenue (AGR) for that drug during the previous calendar year.
The fee is to be increased annually by 2%, rounded up to the nearest dollar, beginning on April 1, 2012.
1.3 Scope and application
This guidance document applies to all drugs for which a DIN is assigned and the sale of the drug has commenced in Canada except where the drug is:
- an Emergency Use New Drug (EUND) that is filed as a new drug submission (NDS) or an abbreviated new drug submission (ANDS) under C.08.002.01 or C.08.002.1 respectively, of the Food and Drug Regulations
- a natural health product (NHP)
- for veterinary use only. Drugs for veterinary use must comply with the Authority to Sell Veterinary Drugs Fees Regulations
- a Schedule C drug. Note: The Schedule C drug exemption is a temporary measure as Health Canada is working to consult on, and revise its fees, including an expansion of the scope of the fees related to possession of a DIN to encompass Schedule C drugs.
1.4 Background
In the early 1990s, Health Canada was given the authority under the Financial Administration Act to charge industry user fees in order to recover some of the costs related to service delivery for drugs, including the costs associated with post-approval safety surveillance. However, the cost of service delivery has increased substantially since that time due to increasing volume of submissions, along with costs of inflation and other costs of doing business.
The fees for the right to sell drugs in the Fee Regulations aim to provide sufficient funding for Health Canada to meet service standards; to keep current with the assessment of signals and safety trends; and to produce risk communications concerning all regulated marketed health products. They also address costs associated with inflation.
1.5 Definitions
- Actual Gross Revenue
- The amount earned by the DIN holder from the sales in Canada of all drug products with the same DIN during the previous calendar year.
- Annual Drug Notification Form
- This form is a print-out of the information contained in the Drug Product Database listing all the drug products owned by the manufacturer, named in the form, as of the date printed on the form. This form is used to facilitate the annual renewal process.
- Calendar Year
- A period of twelve consecutive months commencing on January 1.
- Drug Notification Form
- This form is provided to the manufacturer upon the issuance of a DIN by Health Canada. It is to be used by the manufacturer to fulfil their obligation to notify Health Canada within 30 days of the commencement of the sale of the drug product on the Canadian market.
- First Completed Calendar Year
- The completion of a period of twelve consecutive months commencing on January 1 following the date on which the drug product was first marketed in Canada.
- Manufacturer
- For the purpose of this document, manufacturer is a DIN holder.
1.6 Acronyms
- AGR
- Actual Gross Revenue
- ADNF
- Annual Drug Notification Form
- DIN
- Drug Identification Number
- DNF
- Drug Notification Form
- EUND
- Emergency Use New Drug
- OSIP
- Office of Submissions and Intellectual Property
2 Guidance for implementation
2.1 General contact information
For questions regarding your invoice payment or your account balance, contact Accounts Receivable by phone at 613-957-1052 or 1-800-815-0506; by fax at 613-957-3495; or by email at AR-CR@hc-sc.gc.ca. Please have your customer account or invoice number available.
For questions related to the interpretation of the Fees for the Right to Sell Drugs, contact the Office of Submissions and Intellectual Property (OSIP formerly SIPD) by phone at 613-946-1151, by fax at 613-954-3067 or by email at hc.annual-annuelle.sc@canada.ca.
2.2 Regulatory requirements related to the Fees for the Right to Sell Drugs
2.2.1 Issuance of a Drug Identification Number (DIN)
Before a drug is authorized for sale in Canada, it must be issued a DIN in accordance with section C.01.014.2 or C.08.002 of the Food and Drug Regulations.
2.2.2 Drug Notification Form (DNF)
Upon issuance of a DIN, the manufacturer is provided with a DNF which must be completed and returned in accordance with section C.01.014.3 of the Food and Drug Regulations within 30 days after commencing sale of the drug in Canada.
2.2.3 Annual notification
In accordance with section C.01.014.5 of the Food and Drug Regulations, a manufacturer of a drug must annually, before October 1, provide Health Canada with a signed notification confirming that all the information previously supplied by the manufacturer with respect to that drug is correct.
2.2.4 Dormant DIN
For all marketed products, which have been issued a DIN under subsection C.01.014.2(1) of the Food and Drug Regulations, the manufacturer must indicate on the ADNF if the product has not been sold on the Canadian market for a period of 12 consecutive months. This product is considered dormant. Depending on the product type, there may be additional requirements outside of the ADNF. Please refer to section 6.3 of the Guidance Document: Cancellation of a Drug Identification Number (DIN) and Notification of the Discontinuation of Sales.
2.2.5 Discontinued sale notification
In accordance with C.01.014.7 of the Regulations Amending the Food and Drug Regulations (Shortages of Drugs and Discontinuation of Sale of Drugs), if the sale of a drug in Canada is discontinued, the manufacturer must notify Health Canada within 30 days after the day on which they discontinue the sale of the drug. The notification must include the following information:
- the drug identification number assigned for the drug under the subsection C.01.014.2 (1);
- the date on which the manufacturer discontinued the sale of the drug; and
- the latest expiration date of the drug that the manufacturer sold and the lot number of that drug.
Failure to Notify Health Canada of Discontinued Sale
If the sale of a product has been discontinued but no written notice has been received by Health Canada before October 1, the manufacturer is still liable for payment of the right to sell fee.
If the ownership of a product has been transferred to another company and the new owner has marketed the product with a new DIN, the previous DIN holder must notify Health Canada if the sale of their product is discontinued. Otherwise an annual right to sell fee will be applied to both the old DIN and the new DIN since both are on Health Canada records as being on the market.
2.3 Fee payment
Instructions on the payment of fees are contained in the document How to Pay Fees.
2.3.1 Who is responsible for payment?
The person on record as owning the DIN when the invoice is issued is responsible for payment. The invoice and any subsequent monthly statements are prepared in the name of the DIN holder and are sent to the regulatory affairs section of the company or address identified as the billing address for the DIN holder on the returned ADNF.
2.3.2 Where to submit payment of invoice
Health Canada
Accounts Receivable, Address Locator: 1918B
18th Floor, Room 1804B, Jeanne-Mance Building
161 Goldenrod Driveway, Tunney’s Pasture
Ottawa, Ontario
KIA 0K9
Canada
2.3.3 Annual fee
The annual fee is paid in advance of the year that it covers. Therefore, fees paid on October 1, 2011 cover the right to sell from October 1, 2011 to September 30, 2012. The only exception occurs when there is a fee deferral for a drug that has not completed its first calendar year on the market (see section 2.4 for further information on deferrals).
Adjustment of Fees
The right to sell fee is increased annually by 2%, rounded up to the nearest dollar, beginning April 1, 2012. An annual adjustment factor is necessary to ensure that service standards continue to be met. Each year, a Notice of Intent will be published in Canada Gazette, Part I setting out the revised fees. The Notice of Intent and the fee documents with the revised fees are found at Fees in Respect of Human Drugs and Medical Devices.
2.3.4 When to pay fees
Fees are charged for the right to sell a product in the upcoming year beginning October 1 and ending September 30 of the following year. Invoices for the annual fee for the right to sell are sent on October 1 each year and must be paid within 30 days.
Do not send payment with the completed ADNF in August.
In order to remind manufacturers of the annual renewal process, Health Canada sends an Annual Renewal Package in June to each manufacturer. The package contains the following:
- The ADNF which contains a list of drugs with DINs that, according to Health Canada records as of the date of printing, have been notified as being offered for sale in Canada, are approved for sale in Canada, or are in dormant status (have not been on the Canadian market for sale for at least 12 consecutive months), and for which discontinued notifications have not been received by Health Canada;
- Instructions for the completion of the ADNF;
- A Right to Sell Drugs Fee Remission Request and Attestation Form;
- Sale Discontinuation of Drug Form
- Any other information deemed necessary.
The completed ADNF and, when applicable, a Right to Sell Drugs Fee Remission Request and Attestation Form, must be submitted to Health Canada no later than mid-August (see section 2.5 for further information on fee remissions). This allows time for fee remission requests to be validated and reflected on the invoice.
2.4 Deferral of fees and timing of deferred payment
Section 35. (4) of the Fee Regulations states that if the manufacturer has not completed its first calendar year of selling a drug, the payment of the fee is deferred to the end of the first complete calendar year that the drug is on the market (that is, is payable on January 1 following completion of that year). This deferral provides an opportunity for manufacturers to accumulate actual revenue data from the sale of the new product in its first calendar year to determine if the manufacturer is eligible for a fee remission (see section 2.5.3 below for further information on remission of deferred fees).
The total fee payable on January 1 includes the fee for each period of October 1 to September 30 that has commenced. Note that although the fee is payable January 1, Health Canada does not issue the invoice until February. The fee is due 30 days following issuance of the invoice.
Figure 1 shows an example of when payments would be due for a product commencing sale in May 2011. The fee payment for period A is deferred to the end of the first calendar year and is payable January 1, 2013. The fee payment for period B is also deferred to January 1, 2013. In February, 2013, the manufacturer will be invoiced as follows:
$1,020 (period A) + $1,020 (period B) + 2% x $1,020 (annual increase) = $2,061
Figure 1

Figure 1 - Description
This diagram shows the right to sell fee deferral time line for a drug that began sale in May 2011. Fees for the right to sell in the years 2011-2012 and 2012-2013 are deferred to the end of the product’s first calendar year on the market (2012) or as indicated January 2013. As shown the fee for right to sell in 2013-2014 is not deferred and is payable at the time of notification in October. The diagram also shows August as the expected time for the return of the annual market notification form.
2.5 Fee remission
2.5.1 Eligibility for fee remission
In order to be eligible for fee remission under section 35. (2) of the Fee Regulations, the right to sell fee must be greater than 1.5% of the manufacturer's AGR from the sale of the drug, in Canada, in the previous calendar year.
Manufacturers outside Canada
Manufacturers who own drug products and are outside Canada must determine their eligibility for remission by using their AGR received for total sales of all the drug products with the same DIN in Canada. Therefore, if the manufacturer sells the drug products with the same DIN through more than one importer in Canada, the remission is based on the AGR that the manufacturer receives from the sales of all the importers of the drug with the same DIN.
2.5.2 Documentation (Right to Sell Drugs Fee Remission Request and Attestation Form)
In order to be granted a fee remission, the manufacturer, in accordance with section 35. (2) of the Fee Regulations, must provide with the completed ADNF a statement certified by the individual responsible for the manufacturer's financial affairs that sets out the AGR from the sale of the drug in the previous calendar year i.e. The Right to Sell Drugs Fee Remission Request and Attestation Form.
It should be noted that a fee remission will automatically be applied to all dormant DINs as these products have not been sold on the Canadian market for a period of 12 consecutive months. Manufacturers are not required to complete the Right to Sell Drugs Fee Remission Request and Attestation Form for these products. These products will not be invoiced as part of the regular annual fees in October.
Please review the section 2.5.4 for the dormant DINs for which the Right to Sell fees were deferred.
Financial person responsible for several manufacturers
Persons who are responsible for the financial affairs of several manufacturers must submit a separate fee remission attestation form for each manufacturer that has been assigned a unique company code. The company code is comprised of a four or five digit number assigned by the Drug Products Database. It is used as the account number and is found on the ADNF above the DIN holder's name.
AGR questioned by Health Canada before invoice is sent
If it is determined, on the basis of any information available to Health Canada, that the statement submitted by the manufacturer or their financial affairs person is inaccurate or inadequate to determine the manufacturer's AGR in the previous calendar year, Health Canada may require the manufacturer to provide sales records that have been audited by a qualified independent auditor. These audited sales records will be used to determine the fees payable and an invoice reflecting the confirmed or corrected fee payable will be sent. If the manufacturer fails to provide the audited sales records as requested by Health Canada within 60 days of the date of the request, an invoice for the full applicable fee will be sent.
AGR questioned by Health Canada after invoice is sent
If it is determined, on the basis of any information available to Health Canada, that the statement submitted by the manufacturer or their financial affairs person is inaccurate or inadequate to determine the manufacturer's AGR in the previous calendar year, Health Canada may require the manufacturer to provide sales records that have been audited by a qualified independent auditor. If the sales records submitted by the manufacturer establish that the fee remission calculated and submitted by the manufacturer is correct, no further action will be taken. If the sales records establish that the fee calculated and submitted by the manufacturer is less than the amount owing under section 35 of the Fee Regulations, the difference is immediately payable. If the sales records submitted establish that the fee owing is less than the amount submitted by the manufacturer, the amount in excess will be remitted to the manufacturer.
2.5.3 Calculation of fee with remission
If the manufacturer meets the fee remission eligibility, the fee is calculated as follows: (Assuming the right to sell fee is more than 1.5% of the AGR)
AGR x 1.5% = the fee payable
For example: Based on an AGR of $58,000 and a right to sell fee of $1,020, the fee with remission payable would be $58,000 x 1.5% = $870. The manufacturer would be sent an invoice for $870.
2.5.4 Calculating and requesting remission of fees where payment was deferred
Eligibility for fee remission and calculation of fee
In order to be eligible for fee remission under section 35. (2) of the Fee Regulations, the right to sell fee must be greater than 1.5% of the manufacturer's AGR from the sale of the drug, in Canada, in its first calendar year.
For the example in Figure 1 (assuming the manufacturer was eligible for a remission), the fee payable January 1, 2013 would be calculated as follows:
1.5% x AGR (first calendar year) x 2 (for periods A and B) = fee payable
Request for Remission of Deferred Fees
If the manufacturer is eligible for a remission of deferred fees, a Right to Sell Drugs Fee Remission Request and Attestation Form must be completed and submitted to Health Canada before January 20 following completion of the first complete calendar year that the product was on the market. The manufacturer must be prepared to submit, upon request, additional information concerning the determination of the AGR as outlined in Section 2.5.2. If January 20 falls on a weekend then the deadline is moved to the first Monday following January 20. This form includes a statement certified by the individual responsible for the manufacturer's financial affairs that sets out the AGR from the sale of the drug in its first calendar year. Health Canada will review the documentation and will issue an invoice in February with the appropriate fee for payment in 30 days.
Request for Remission of Deferred Fees for Dormant DINs
Manufacturers are required to provide the Right to Sell Drugs Fee Remission Request and Attestation Form for dormant deferred DINs if there were any sales of the product during the first calendar year. However, if the sale was stopped before the first completed calendar year begins; the fee remission will automatically be applied to these dormant DINs. Please see below the 2 examples provided to illustrate the two scenarios.
Scenario 1 - Sales within the first completed calendar year
Figure 2 shows an example of a deferred dormant DIN where the Right to Sell Drugs Fee Remission Request and Attestation Form should be provided if the right to sell fee is greater than 1.5% of the manufacturer’s AGR from the sale of the drug, in Canada.
Figure 2
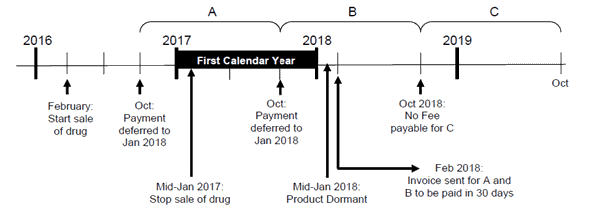
Figure 2 - Description
Figure 2 shows an example of a deferred dormant DIN where the Right to Sell Drugs Fee Remission Request and Attestation Form should be provided if the right to sell fee is greater than 1.5% of the manufacturer’s AGR from the sale of the drug, in Canada.
In this example, the product is market notified in February 2016, the sale stops in mid-January 2017 and after having no sale for 12 consecutive months, this DIN becomes dormant in mid-January 2018.
The Deferred Right to Sell Drugs fees for this product will be charged in February 2018 for the billing period A (October 2016) and the billing period B (October 2017). The manufacturer is required to submit the Right to Sell Drugs Fee Remission Request and Attestation Form based on the sales that occurred between January 2017 and December 2017 (the first completed calendar year) prior to the issuance of the invoice to be eligible for a fee remission.
Scenario 2 - No sales during the first completed calendar year
Figure 3 shows an example of a deferred dormant DIN where the Right to Sell Drugs Fee Remission Request and Attestation Form is not required.
Figure 3
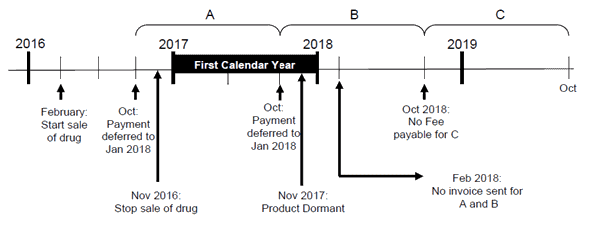
Figure 3 - Description
Figure 3 shows an example of a deferred dormant DIN where the Right to Sell Drugs Fee Remission Request and Attestation Form is not required.
In this example, the product is market notified in February 2016, the sale stops in November 2016 and after having no sale for 12 consecutive months, this DIN becomes dormant in November 2017.
In this scenario, there is no sale in the first completed calendar year (January 2017 to December 2017) therefore a fee remission will automatically be applied to this product. Manufacturers are not required to complete the Right to Sell Drugs Fee Remission Request and Attestation Form and this product will not be invoiced for the deferred fees in February.