Module 4: Health Canada’s review and communication of safety findings

Download the alternative format
(PowerPoint format, 2.2 MB, 33 pages)
Organization: Health Canada
Published: 2019-12-13
Module 4 - Learning Outcomes
Completion of Module 4 will enable you to:
- Provide an overview of health product vigilance in Canada
- Identify the stages of adverse reaction (AR) and medical device problem (MDP) report management
- Describe post-market surveillance activities, including signal detection, signal prioritization, signal assessment/safety review, and risk mitigation
- Describe risk communications from Health Canada
- Recognize the various resources provided by Health Canada to share AR and MDP data and findings
- Understand Health Canada's principles for the security and sharing of AR and MDP report data
Module 4 - Outline
- Health Product Vigilance
- Health Canada's AR and MDP Report Management
- Information Sharing from AR and MDP Reporting
- AR and MDP Online Databases
- Health Canada Safety Reviews
- Health Canada Recalls and Safety Alerts
- Health Product InfoWatch
- Drug and Health Product Register (DHPR)
- Data Security and Data Sharing from AR and MDP Reports
- Key Points to Remember
- Abbreviations
- Resources
Conceptual Model of Serious ADR and MDI Reporting by Hospitals
Module 4 describes Health Canada's review and communication of safety findings.
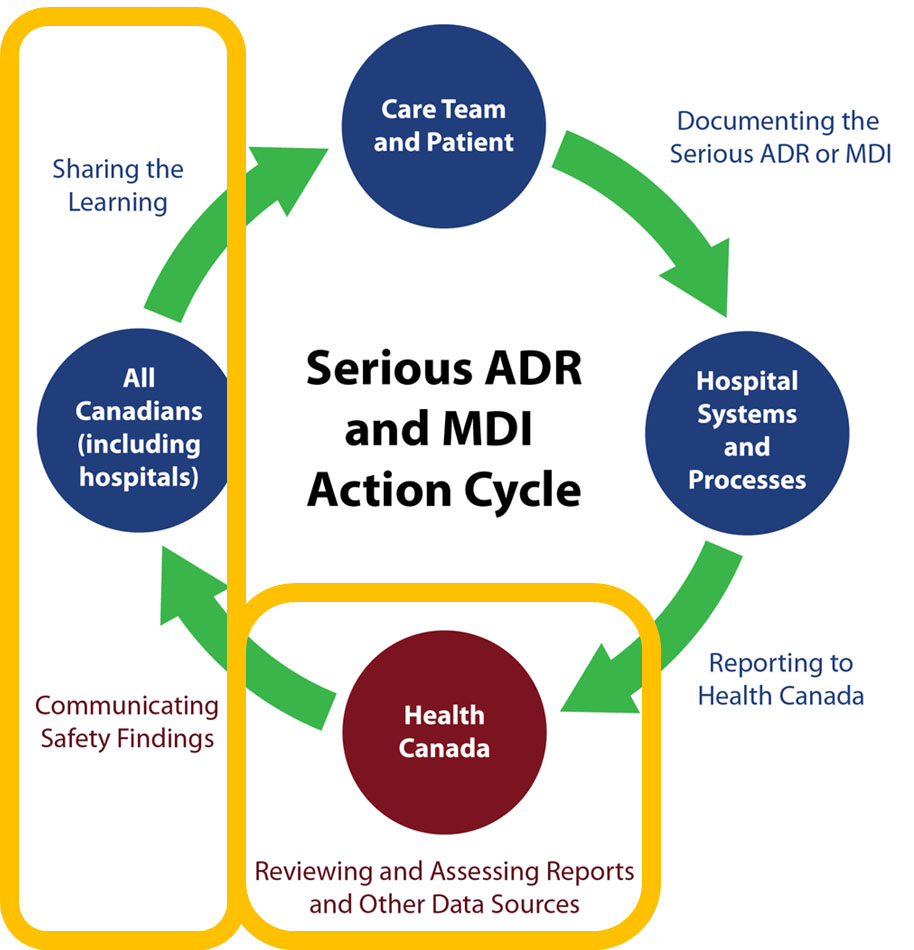
Source: Serious ADR and MDI Action Cycle. ISMP Canada, HSO, CPSI; 2019.
Text description
A circle diagram depicts the action cycle for serious ADR and MDI reporting by hospitals. In summary, the figure illustrates that the care team and patients document the serious ADR or MDI. Reporting a serious ADR or MDI to Health Canada is supported by hospital systems and processes. Health Canada reviews and assesses reports and other data sources. Health Canada communicates safety findings to all Canadians, including hospitals, who are shown to share the learning. This concludes the action cycle.
The diagram superimposes two yellow rectangle shape around specific parts of the action cycle.
The first yellow rectangle is placed around:
- Health Canada, reviewing and assessing reports and other data sources
The second yellow rectangle is placed around:
- All Canadians (including hospitals), that learn from the safety findings Health Canada communicates and shares them.
Health Product Vigilance
- Health Canada builds post-market safety knowledge, which is integral to effective clinical use, from several data sources, including serious adverse drug reaction (serious ADR) and medical device incident (MDI) reports.
- In addition to serious ADR and MDI reports, a variety of other data sources contribute to therapeutic product safety monitoring, including:
- mandatory reports from regulated parties,
- voluntary reports from health care professionals and consumers,
- foreign data such as manufacturer assessment of worldwide safety data,
- information sharing with foreign regulatory agencies,
- medical literature, and
- information generated from the Drug Safety and Effectiveness Network (DSEN).
- This module reflects the broad scope of Health Canada's product vigilance activities beyond mandatory reporting by hospitals (e.g., serious ADRs and MDIs); which is reflected in the use of the AR and MDP report terminology.
Health Canada's Health Product Vigilance Life Cycle
- Market authorization submission
- Product submission (pharmaceuticals, natural health products, biologics and biosimilars, radiopharmaceuticals, disinfectants and sanitizers with disinfectant claims, and medical devices) by market authorization holder (MAH)
- Pre-market review
- Reviews product submissions to assess for safety, efficacy and quality
- Review risk management plans with MAH to mitigate potential risks (as applicable)
- Assesses product name and label (depending on class for medical devices)
- Post-market surveillance
- Monitors safety and effectiveness of health products by identifying and assessing potential safety signals through multiple sources including spontaneous reporting of adverse reactions to health products and medical device problems reports, literature review, annual safety summaries, DSEN, liaising with other regulators, etc.
- Risk mitigation
- Continuing observation
- Labelling changes
- Risk communications
- Product recall
- Educational activities
- Market withdrawal
- Other
- Compliance and enforcement
- Monitors quality of adverse reaction/ medical device problem reports through compliance promotion and enforcement (e.g., inspections)
- Enforce regulations
AR Reporting Is Essential to Post-market Surveillance
- Many safety issues are ONLY detected after market approval due to use of the health product in larger populations.
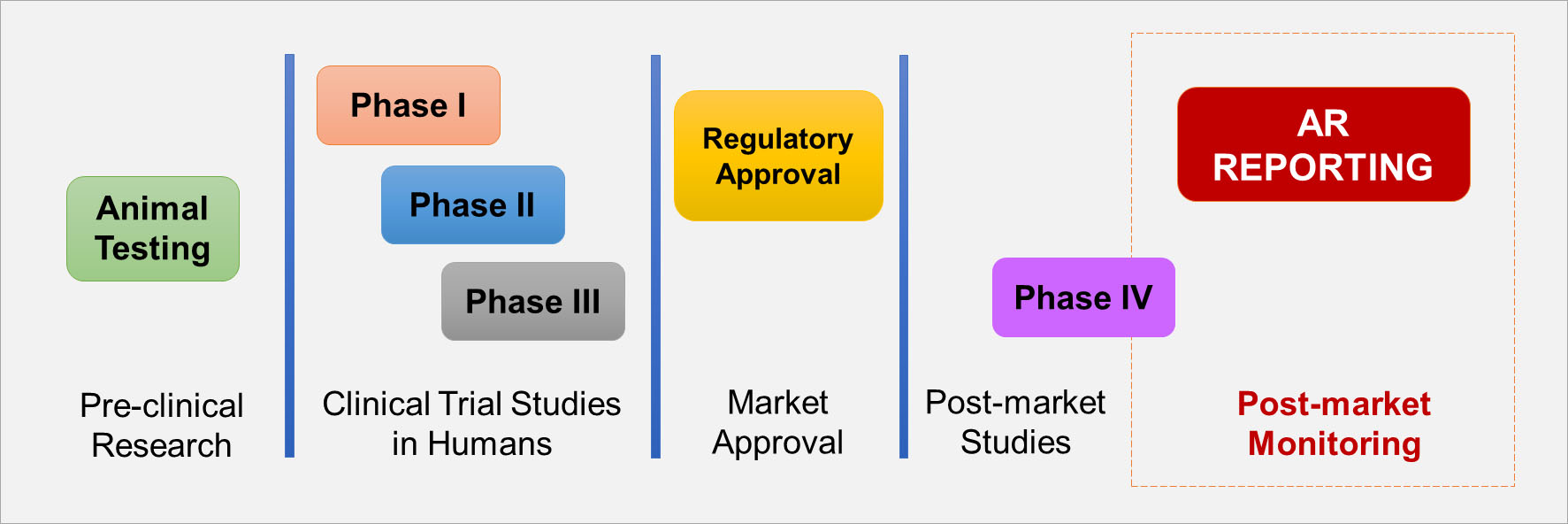
Text description
This diagram depicts the movement of drugs across five stages.
Stage 1: Pre-clinical Research
- Animal Testing
Stage 2: Clinical Trial Studies in Humans
- Phase One
- Phase Two
- Phase Three
Stage 3: Market Approval
- Regulatory Approval
Stage 4: Post-market Studies
- Phase Four
Stage 5: Post-market Monitoring
- Reporting
MDP Reporting Is Essential to Post-market Surveillance
- Many harms from medical devices are ONLY detected after market approval due to use of the device in larger populations.
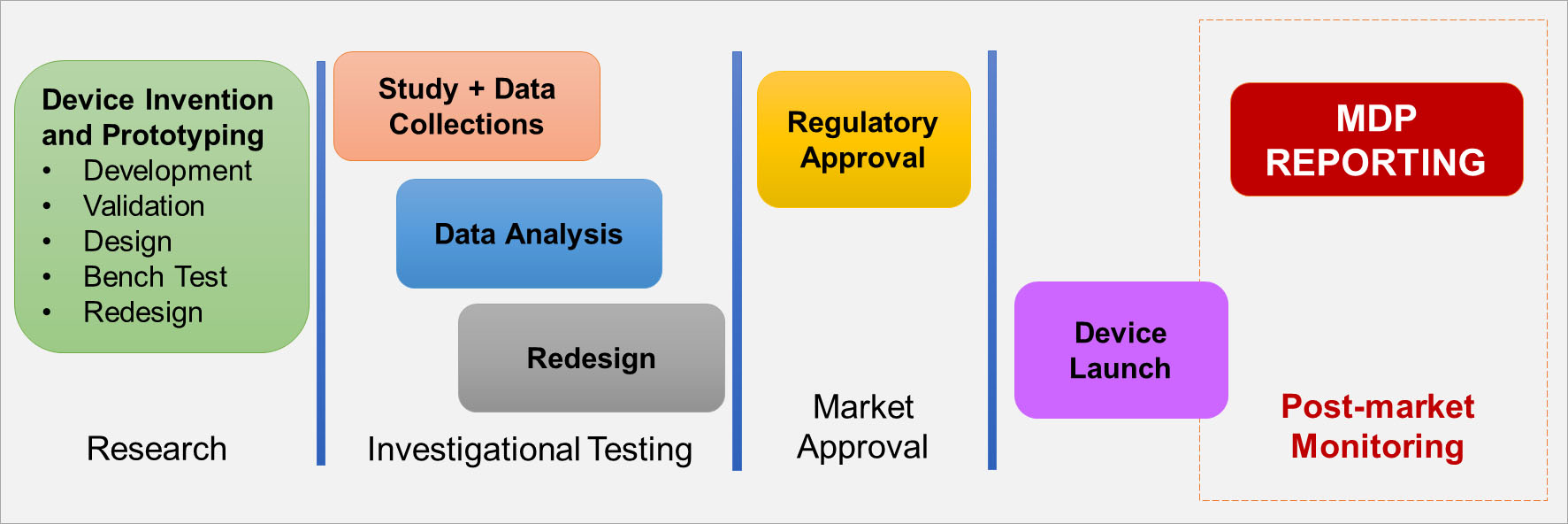
Text description
This diagram depicts the movement of medical devices across five stages.
Stage 1: Research
- Device Invention and Prototyping, which is further broken down into:
- Development
- Validation
- Design
- Bench Test
- Redesign
Stage 2: Investigational Testing
- Study and Data Collections
- Data Analysis
- Redesign
Stage 3: Market Approval
- Regulatory Approval
Stage 4: Device Launch
Stage 5: Post-market Monitoring
- MDP Reporting
| Clinical Trials / Investigational Testing Have Limited Scope |
Post-market Surveillance Identifies Emerging Safety Issues |
|---|---|
| Highly controlled environment | Real world use |
| Limited number of patients | Varied and large population |
| Short trial duration | Long term use |
| Highly selected patients | Off-label use in different patient groups |
| Selected cases and diseases | Patients with multiple co-morbidities |
| May not identify rare events | Rare events can be detected |
Health Canada's AR and MDP Report Management
Stages of AR and MDP Report Management

Text description
A diagram depicts the seven stages of AR and MDP report management.
- AR or MDP Report Received by Health Canada
- AR or MDP Report Processing
- Signal Detection
- Signal Prioritization
- Signal Assessment/Safety Review
- Risk Mitigation
- Possible Risk Communication
A green arrow called "Dissemination of Findings" is placed under steps five through seven.
Signal Detection and Assessment
- Safety signals (preliminary indications of product-related safety issues) are identified through data scanning, including review of AR and MDP reports.
- Potential signals are reviewed by an internal committee of scientists, pharmacists and physicians to determine if a signal assessment will be completed.
- Assessment from all data sources is used to consider possible risk mitigation activities.
- Risk considerations include strength of evidence, manageability of risk, dissemination of information, and communication targets.
- Following the completion of a signal assessment, recommendations are made and can include changing labels, including indication, recalling or withdrawing a product from the market, and communicating risks to stakeholders.
Protecting Canadians from Unsafe Drugs Act (Vanessa's Law) improves Health Canada's ability to collect post-market safety information and take appropriate action when a serious risk to health is identified.Footnote 1
Risk Communications
Target Audience: Health Care Professionals / Hospitals
- Health Product Risk Communication
- Ad hoc communication about safety issues
- Broad dissemination (web posting, RSS feed, MedEffect™ e-Notice)
- Targeted dissemination by the Market Authorization Holder or by Health Canada (fax, email, mail)
- Health Product InfoWatch
- Monthly publication to raise awareness of safety issues and stimulate reporting of the same
- Each publication includes a monthly recap of health product advisories and summary safety reviews, as well as a growing selection of new health product safety information.
- Broad dissemination (web posting, Twitter, RSS feed, MedEffect™ e-Notice)
Target Audience: General Public
- Recall Notice
- Written and distributed by industry; an "extract" of the information posted by Health Canada
- Posted at regular intervals on Health Canada's Recalls and Safety Alerts database
- Public Advisory
- Written by Health Canada for urgent, high risk issues
- Broad dissemination (Newswire, Twitter, RSS feed, MedEffect™ e-Notice)
- Targeted distribution to stakeholders as needed
- Information Update
- Written by Health Canada for less urgent, lower risk issues (e.g., labelling updates)
- Broad dissemination (Newswire, Twitter, RSS feed, MedEffect™ e-Notice)
- Foreign Product Alerts
- Health Canada communicates information as needed about unauthorized products from other countries which may have been brought into the country by travellers or purchased online
Information Sharing from AR and MDP Reporting
Information Sharing with Health System Partners
- Health Canada makes AR and MDP data available online, produces an annual trend report and publishes risk communications to health care stakeholders through a number of forums.
- Health Canada plans to continually improve its AR and MDP data analytics, ensuring health system partners have timely access to key information.
- Data analytics:
- Invest in information technology to support the timely analysis of the AR and MDP data and streamline the identification of potential safety signals
- Invest in the optimization of the existing AR/MDP searchable databases
- Sharing of information with partners, including:
- Health Canada's annual AR and MDP report
- Outreach and education activities on reporting and post-market surveillance
- Data analytics:
Examples of AR and MDP Safety Information Sharing
Health Canada disseminates findings to health care providers and the public to alert and educate them about identified health risks related to health products.
Multiple sources of safety information are available to provide up-to-date information on ARs and MDPs:
- Adverse Reaction Online Database
- Medical Device Incidents Database
- Annual AR/MDP Trends Report
- Health Canada Safety Reviews
- Health Canada Recalls and Safety Alerts
- Health Product InfoWatch
- Drug and Health Product Register (DHPR)
Adverse Reaction Online Database
Canada Vigilance Adverse Reaction Online Database
- Searchable database that contains information from post-market AR reports since 1965
- Contains suspected adverse reactions to health products
- Files can be exported and saved in various formats
AR reports can be searched by:
- report date, seriousness and source
- patient information (gender, age and outcomes)
- suspect health product by brand name and
- active ingredient
- adverse reaction term or by system organ class
Source: Canada Vigilance adverse reaction online database
Medical Devices Online Database
Medical Device Incident Database
- Searchable database that contains information from post-market MDP reports since 1980
- Includes devices approved for the Canadian market
- Downloadable full extract available
MDP reports are searched by free text and the following data is returned:
- incident ID
- receipt date
- device name
- device type
- company name
- hazard severity
- description
- code types assigned
Source: Medical Devices
Annual Trends Report
The annual trends report provides a descriptive analysis of adverse reaction case reports of health products and medical device problem incidents that have been submitted to Health Canada between 2008 and 2017.
Health Canada Safety Reviews
Health Canada regularly publishes summaries of post-market signal assessment.
Summary Safety Reviews (SSRs) provide a more complete understanding of:
- What was assessed
- What was found
- What action was taken
These summaries can help Canadians make informed decisions about their medication choices and medical devices.
Source: Safety Reviews
Recalls and Safety Alerts
The recalls and safety alerts database provides centralized access to recalls and safety alerts from:
- Health Canada
- The Canadian Food Inspection Agency
- Transport Canada
Source : Recalls and safety alerts
Health Product InfoWatch
- A monthly publication primarily intended for health care providers
- Provides clinically relevant safety information on
- pharmaceuticals,
- biologics,
- medical devices, and
- natural health products.
- Each publication includes:
- recap of health product advisories,
- recap of summary of safety reviews,
- new health product safety information, and
- product monograph updates.
SUBSCRIBE NOW
Drug and Health Product Register (DHPR)
The DHPR provides safety information on health products available to Canadians.
The public is able to:
- Access plain language overviews of regulatory decisions:
- Summary Safety Review
- Summary Basis of Decision
- Regulatory Decision Summary
- Search for AR and MDP information
- Search reported adverse reactions, medical device problems and summary reports of safety information
- Report adverse reactions about health products
Source: The Drug and Health Product Register
Health Canada's Post-market Publication Portal
MedEffect Canada provides health care professionals and consumers with access to safety information (advisories, alerts, recalls, etc.) generated by Health Canada following post-market monitoring and assessment activities.
This portal can be used to access additional resources for health products.
Source: MedEffect Canada
Data Security and Data Sharing from AR and MDP Reports
Use of Collected Data - Security and Privacy
Health Canada:
- Stores AR and MDP reports in a confidential database.Footnote 2
- Follows protocols to ensure that identifying patient and reporter information is protected under the federal Privacy Act.Footnote 2
- Ensures AR and MDP reports are de-identified before sharing.
- Sends AR data to the World Health Organization (WHO) Global Pharmacovigilance Database.
- Commits to ensure that data are used and shared in a scientifically and socially responsible way.
International Collaboration
- Supports the monitoring and identification of new safety issues caused by health products
- Facilitates identification of safety signals by providing a larger pool of data
- Enhances patient safety by allowing for consistent communication around health product risks
- Includes such regulatory agencies as: USA's FDA, EU's EMA, UK's MHRA, Australia's TGA, Japan's PMDA
- Advances worldwide pharmacovigilance standards, systems and learning with organizations such as IMDRF, ICH, ISoP, ICMRA
Key Points to Remember
- AR and MDP reporting is essential because many safety issues are detected after market approval.
- The stages for management of AR and MDP reports are:
- AR or MDP report received by Health Canada;
- AR or MDP report processing;
- signal detection;
- signal prioritization;
- signal assessment/safety review;
- risk mitigation; and
- possible risk communication.
- Health Canada has multiple mechanisms to share learning from reported ARs and MDPs such as the Drug and Health Product Register (online searchable databases, summary safety reviews and access to reporting) and MedEffect Canada.
Abbreviations
- ADR:
- Adverse Drug Reaction
- AR:
- Adverse Reaction
- DHPR:
- Drug and Health Product Register
- DSEN:
- Drug Safety and Effectiveness Network
- EMA:
- European Medicines Agency
- EU:
- European Union
- FDA:
- Food and Drug Administration
- ICH:
- International Conference on Harmonization of Technical Requirements for Pharmaceuticals for Human Use
- ICMRA:
- International Coalition of Medicines Regulatory Authorities
- IMDRF:
- International Medical Device Regulators Forum
- ISoP:
- International Society of Pharmacovigilance
- MAH:
- Market Authorization Holder
- MDI:
- Medical Device Incident
- MDP:
- Medical Device Problem (any type of medical device issue; not necessarily MDI)
- MHRA:
- Medicines & Healthcare Products Regulatory Agency
- PMDA:
- Pharmaceuticals and Medical Devices Agency
- SSRs:
- Summary Safety Reviews
- TGA:
- Therapeutic Goods Administration
- UK:
- United Kingdom
- USA:
- United States of America
- WHO:
- World Health Organization
Resources
- Adverse Reaction Database
- Annual ADR/MDP Trends Report
- Drug and Health Product Register (DHPR)
- Health Canada Recalls and Safety Alerts
- Health Canada Safety Reviews
- Health Product InfoWatch
- Mandatory reporting of serious adverse drug reactions and medical device incidents by hospitals - Guidance document
- MedEffect Canada
- Medical Devices Incident Database
- Guidance on releasing information from adverse reaction and medical device incident reports to the public
- Protecting Canadians from Unsafe Drugs Act (Vanessa's Law) Amendments to the Food and Drugs Act (Bill C-17)
- Regulations Amending the Food and Drug Regulations (Serious Adverse Drug Reaction Reporting - Hospitals): SOR/2019-190
- Regulations Amending the Medical Devices Regulations (Medical Device Incident Reporting - Hospitals): SOR/2019-191
- The Privacy Act
- World Health Organization (WHO) Global Pharmacovigilance Database
For additional information, please contact the Canada Vigilance Program at:
Email: hc.canada.vigilance.sc@canada.ca
Telephone: 1-866-234-2345
Acknowledgments
- All materials were developed by the collaborating parties: Health Canada, Institute for Safe Medication Practices Canada (ISMP Canada), Health Standards Organization (HSO), and the Canadian Patient Safety Institute (CPSI).
- Any stakeholder interested in using the materials should acknowledge Health Canada as the owner and source: Educational Support for Mandatory Reporting. Health Canada; 2019.
- Footnote 1
-
Protecting Canadians from Unsafe Drugs Act (Vanessa's Law) Amendments to the Food and Drugs Act (Bill C-17)
- Footnote 2
-
Reported Side Effects - Disclaimers
Page details
- Date modified: