Guidance on managing applications for medical device licences: Appendices
On this page
Appendix A – Application management process maps
The following process maps show the key steps and timelines (in calendar days) for managing medical device licence applications. The timelines for the Medical Devices Directorate are target timelines.
Figure 1: Process map for administrative screening of medical device licence applications
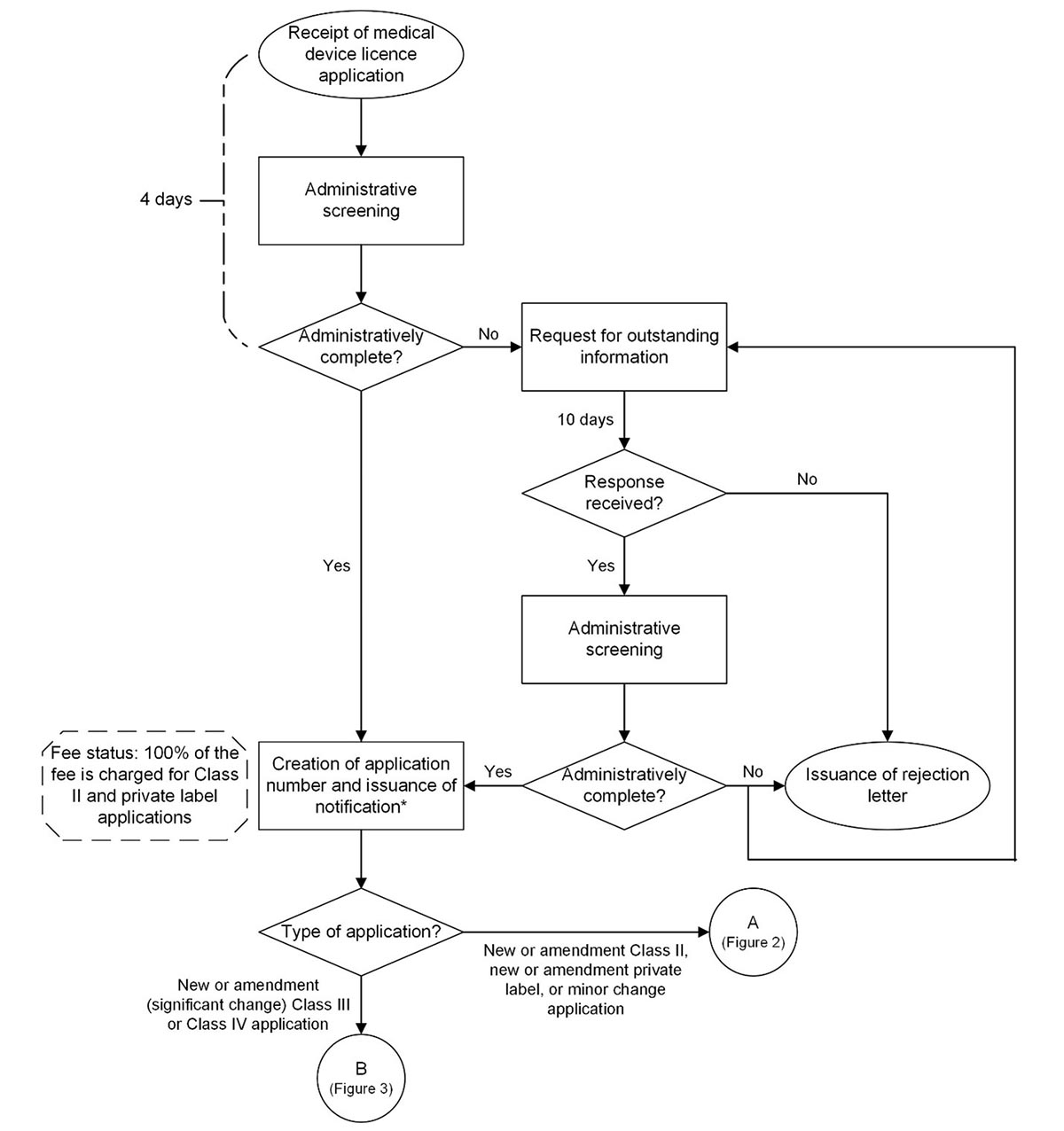
Figure 1: Text description
Steps
- A medical device licence application is received.
- The application undergoes administrative screening (4 days).
- Is the application administratively complete?
- If no, proceed to step 4.
- If yes, proceed to step 10.
- A request for outstanding information is issued.
- Is a response received within 10 days?
- If no, proceed to step 6.
- If yes, proceed to step 7.
- A rejection letter is issued. Process ends here.
- The application undergoes administrative screening.
- Is the application administratively complete?
- If no, proceed to step 9.
- If yes, proceed to step 10.
- A request for outstanding information is issued. Proceed to step 5. OR
A rejection letter is issued. Process ends here. - An application number is created and a notification* is issued. Fee status: 100% of the fee is charged for Class II and private label applications.
- What is the application type?
- If it is a new or amendment Class II, new or amendment private label, or minor change application, proceed to connector “A” in Figure 2.
- If it is a new or amendment (significant change) Class III or Class IV application, proceed to connector “B” in Figure 3.
* Notification applies to new and amendment Class II applications, new private label applications, and new and amendment (significant change) Class III and IV applications.
Figure 2: Process map of the screening period for new and amendment Class II*, new and amendment private label, and minor change medical device licence applications
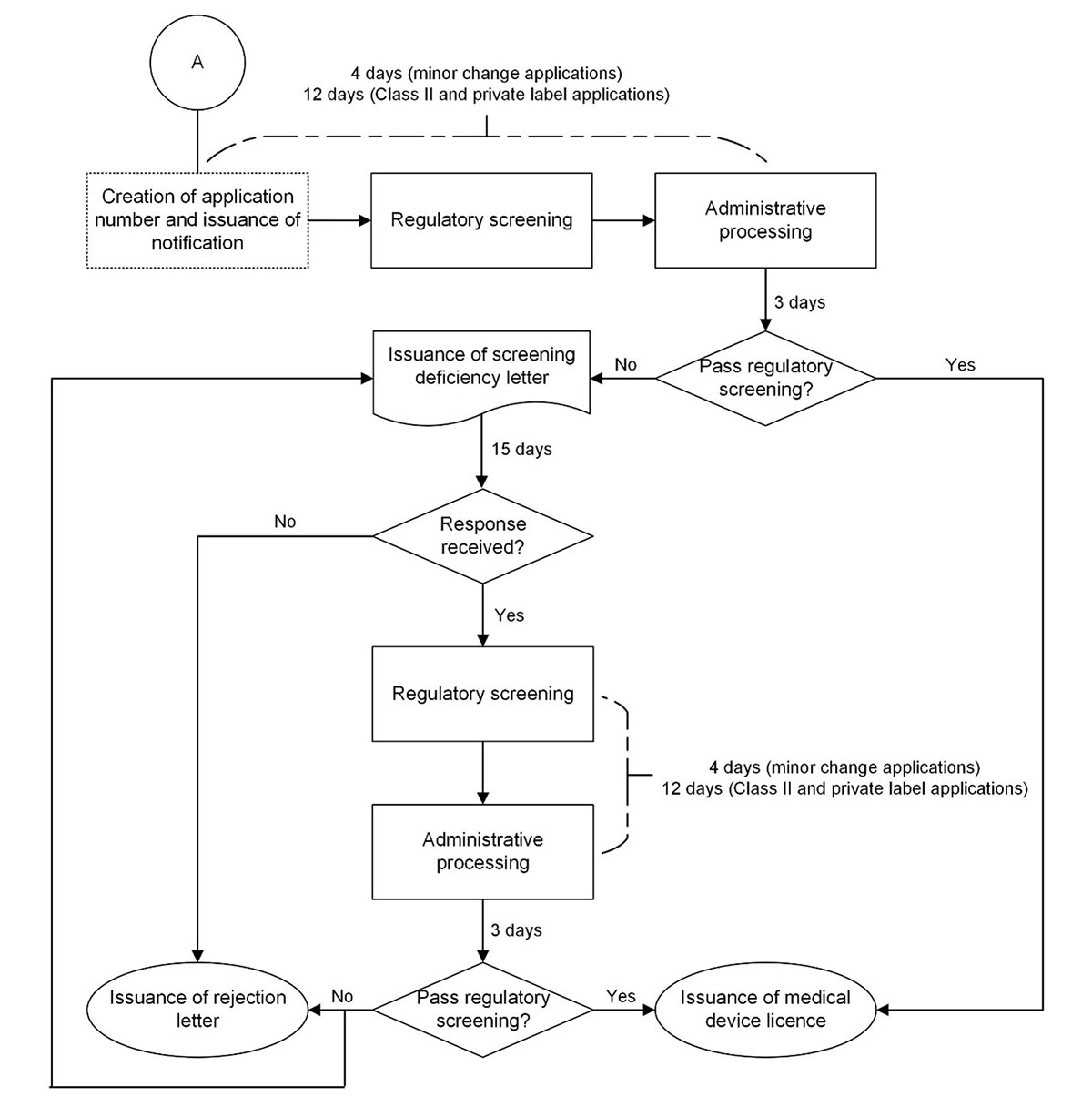
Figure 2: Text description
Steps
- The application undergoes regulatory screening (4 days for minor change applications and 12 days for Class II and private label applications).
- The application undergoes administrative processing (3 days).
- Does the application pass regulatory screening?
- If no, proceed to step 4.
- If yes, proceed to step 11.
- A screening deficiency letter is issued.
- Is a response received within 15 days?
- If no, proceed to step 6.
- If yes, proceed to step 7.
- A rejection letter is issued. Process ends here.
- The application undergoes regulatory screening (4 days for minor change applications and 12 days for Class II and private label applications).
- The application undergoes administrative processing (3 days).
- Does the application pass regulatory screening?
- If no, proceed to step 10.
- If yes, proceed to step 11.
- A screening deficiency letter is issued. Proceed to step 5. OR
A rejection letter is issued. Process ends here. - A medical device licence is issued. Process ends here.
* In certain cases, at the discretion of Health Canada, a Class II application may also undergo a review before a decision is made.
Figure 3: Process map of the screening period for new and amendment (significant change) Class III and Class IV medical device licence applications
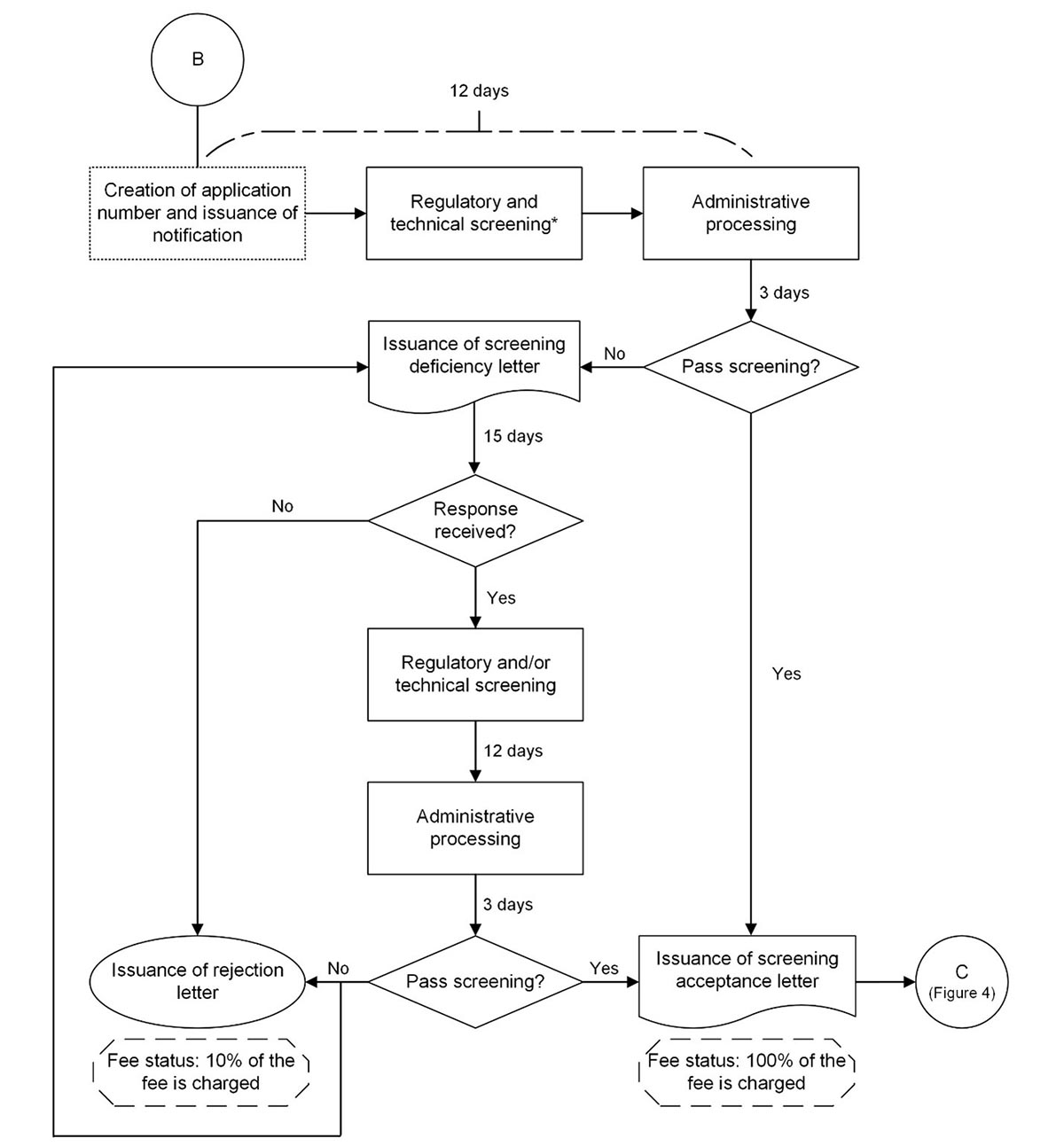
Figure 3: Text description
Steps
- The application undergoes regulatory and technical screening* (12 days).
- The application undergoes administrative processing (3 days).
- Does the application pass screening?
- If no, proceed to step 4.
- If yes, proceed to step 11.
- A screening deficiency letter is issued.
- Is a response received within 15 days?
- If no, proceed to step 6.
- If yes, proceed to step 7.
- A rejection letter is issued. Fee status: 10% of the fee is charged. Process ends here.
- The application undergoes regulatory and/or technical screening (12 days).
- The application undergoes administrative processing (3 days).
- Does the application pass screening?
- If no, proceed to step 10.
- If yes, proceed to step 11.
- A screening deficiency letter is issued. Proceed to step 5. OR
A rejection letter is issued. Fee status: 10% of the fee is charged. Process ends here. - A screening acceptance letter is issued. Fee status: 100% of the fee is charged.
- Proceed to connector “C” in Figure 4.
* In certain cases where an application has significant regulatory deficiencies, a screening deficiency letter may be issued before technical screening.
Figure 4: Process map of the review period for new and amendment (significant change) Class III and Class IV medical device licence applications

Figure 4: Text description
Steps
- The application undergoes Review 1* (57 days for Class III applications and 72 days for Class IV applications).
- The application undergoes administrative processing (3 days).
- Does the application pass review?
- If no, proceed to step 4.
- If yes, proceed to step 11.
- An additional information letter is issued.
- Is a response received within 10 or 60 days†?
- If no, proceed to step 6.
- If yes, proceed to step 7.
- A refusal letter is issued. Process ends here.
- The application undergoes Review 2‡ (42 days).
- The application undergoes administrative processing (3 days).
- Does the application pass review?
- If no, proceed to step 10.
- If yes, proceed to step 11.
- An additional information letter is issued. Proceed to step 5. OR
A refusal letter is issued. Process ends here. - A medical device licence is issued. Process ends here.
* Review 1 – Period from the date an application is accepted for review (date of screening acceptance letter) to the date of first decision. Excludes 3 days for administrative processing and clock pauses.
† 10 days for additional information – noncompliance letter and 60 days for additional information – deficiency letter.
‡ Review 2 – Period from the date a response to an additional information letter is received to the date of subsequent decision. Excludes 3 days for administrative processing and clock pauses. Usually, no more than 2 additional information letters are issued, but additional letters may be issued in certain situations.
Appendix B – Reconsideration process map
The following map shows the key steps and timelines (in calendar days) in the reconsideration process. The timelines for the Medical Devices Directorate are target timelines.
Figure 5: Process map for the reconsideration of medical device licence applications

Figure 5: Text description
Steps
- Medical Devices Directorate (MDD) issues a rejection or refusal decision.
- Manufacturer files an RR within 30 days.
- Is the request eligible for reconsideration?
- If no, MDD issues a letter of ineligibility within 15 days of receiving the RR. Process ends here.
- If yes, what is the reconsideration process pathway selected by the DG?
- If an internal review pathway is selected, proceed to step 4.
- If an external panel review pathway is selected, proceed to step 10.
Internal review
- MDD issues a letter of eligibility within 15 days of receiving the RR.
- Independent internal reconsideration reviewer is identified within 10 days.
- Is a reconsideration meeting requested?
- If yes, proceed to step 7.
- If no, procced to step 8.
- Reconsideration meeting is held within 35 days from the date the internal reconsideration reviewer is identified.
- Designated independent reconsideration advisor submits their recommendations to the DG within 20 days from the date the internal reconsideration reviewer is identified or from the date of the meeting (if requested).
- DG issues a reconsideration decision within 5 days. Process ends here.
External panel review
- MDD issues a letter of eligibility within 15 days of receiving the RR.
- Manufacturer and original bureau submit panel nominations and questions within 10 days.
- External panel members are selected within 15 days.
- Reconsideration meeting is held within 70 days.
- External panel submits a report containing recommendations within 15 days.
- Designated independent reconsideration advisor submits their recommendations to the DG within 10 days.
- DG issues a reconsideration decision within 5 days. Process ends here.
- RR
- request for reconsideration
- DG
- Director General or delegate