2021 Compliance Monitoring Project: Review of ethanol-based hand sanitizers
On this page
- Why we did this project
- What we did
- What we found
- What enforcement actions we took
- What we will do with the results
- How to report a complaint
- List of products reviewed
Why we did this project
At the start of the COVID-19 pandemic, there was an urgent need for products used to limit the spread of COVID-19, including hand sanitizers.
Health Canada put in place a temporary interim measure. This measure allowed hand sanitizer products that did not fully meet regulatory requirements, but did not compromise the safety of the public, to be imported and sold in Canada. Additionally, we expedited the review of licences to increase the market supply.
Learn more about the interim measure and expedited licencing approach:
- Disinfectants and hand sanitizers accepted under COVID-19 interim measure
- Changes to process related to expiry of hand sanitizer interim measure (Drug Establishment Licensing Bulletin #74, February 16, 2023)
- Licensing approach to produce and distribute alcohol-based hand sanitizers: guidance document
In 2021, we looked at several ethanol-based hand sanitizers sold in Canada to verify if they posed a risk to health. We chose natural health products (NHPs) that were either licensed through our expedited licensing approach or accepted under the interim measure since the start of the pandemic.
Licensed NHP hand sanitizers sold in Canada must follow the requirements of both the:
NHP hand sanitizers accepted for sale under an interim measure were required to follow the specific criteria under which they were accepted.
What we did
We obtained 40 products directly from market authorization holders (MAHs). We chose the products based on various risk criteria, such as whether they were sold to vulnerable sub-populations (for example, schools or hospitals) and/or in high volume.
We tested and reviewed the products to assess their quality, safety and efficacy. We verified the following:
- Impurities: The levels of methanol, benzene, acetaldehyde, acetal acetate and ethyl acetate in the product were below the established safety limits.
- Ethanol:
- The concentration was between 60% and 80% (v/v) for efficacy and within the product's specifications for quality.
- The type of ethanol was appropriate for use in topical hand sanitizers.
- Package and label: Products were labelled according to their licence requirements (for example, the label listed the correct ingredients and brand name). For products with unconventional containers, such as beverage or food containers, they had:
- an appropriate closure
- specific warning statements and graphic requirements.
What we found
We tested 40 products and found that:
- 15 products were compliant or only had minor issues that did not pose a risk to health
- 25 products were not compliant and posed a risk to health
Although we found compliance issues for most products, not all issues posed a risk to health. Issues identified included product labels missing information or having incorrect information or products having ethanol concentration outside the allowed limits. The types of issues we found are described in Figure 1.
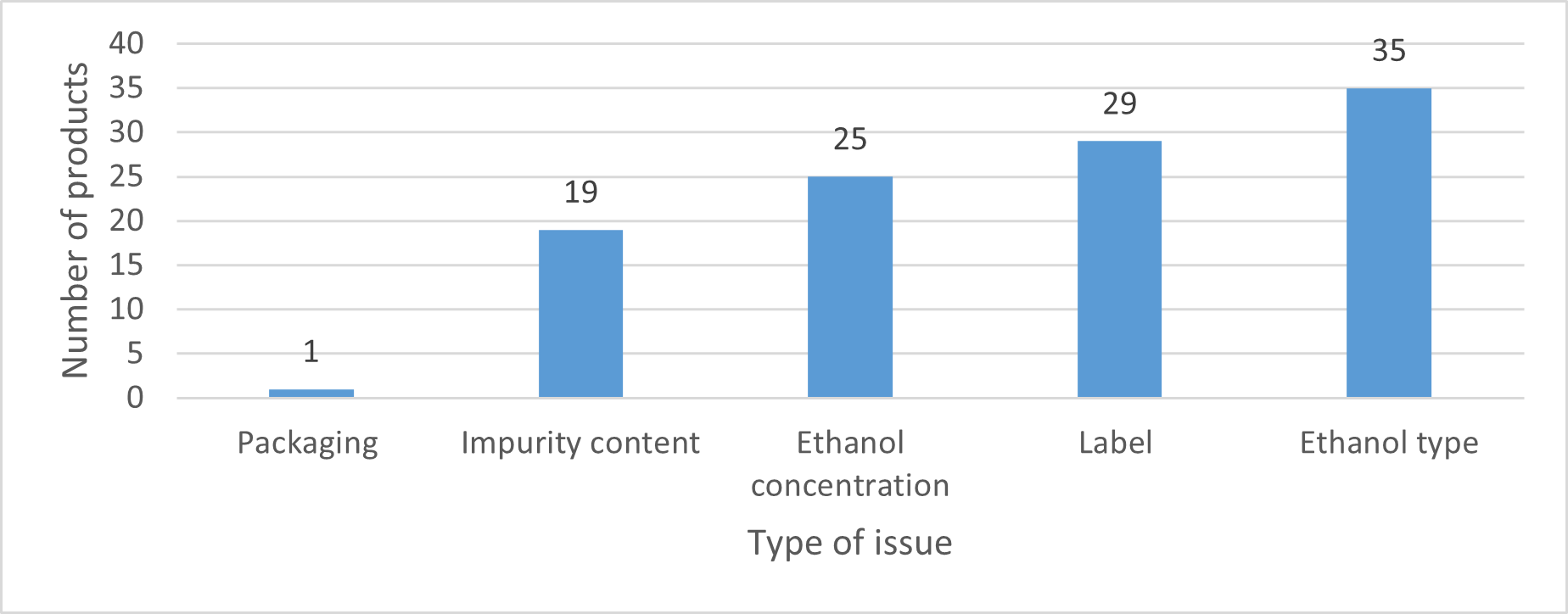
Note: We identified more than 1 issue for some products.
Figure 1 - Text description
This figure is a bar chart. It shows the number of products that were found to have deficiencies related to each requirement reviewed during the project.
Packaging: 1 product
Impurity content: 19 products
Ethanol concentration: 25 products
Label: 29 products
Ethanol type: 35 products
What enforcement actions we took
When taking enforcement actions, Health Canada:
- considers the nature and extent of the non-compliance
- takes action appropriate to the risk identified to protect the health and safety of the Canadian population
We worked with the companies to resolve the non-compliances. We asked companies to recall and/or stop selling products that posed a risk to health.
Learn more about hand sanitizers that have been recalled:
We also sent regulatory notices to companies for higher-risk issues such as impurities detected at high levels. We informed companies of the intended or immediate actions we were taking on their product licence, COVID-19 site licence or regular site licence, such as suspension, cancellation or revocation.
Some companies decided to discontinue their licences after receiving these notices. The number and type of actions that Health Canada took are described in Figure 2.
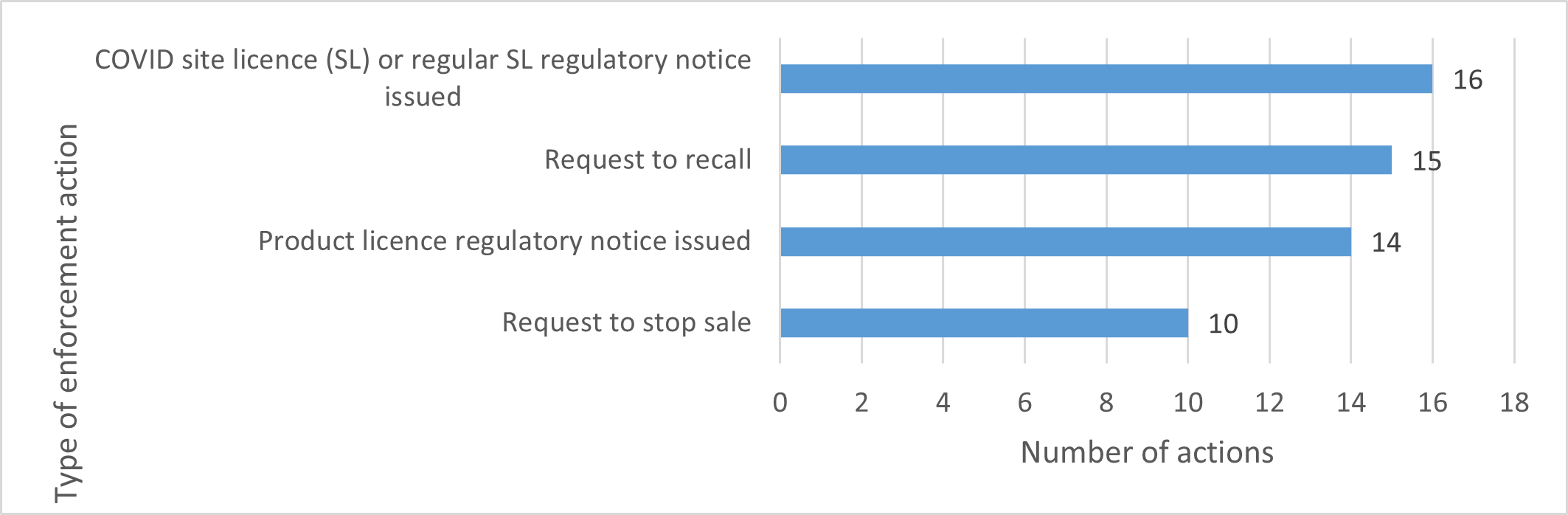
Note: We took more than 1 action for some products.
Figure 2 - Text description
This figure is a bar chart. It shows the number of enforcement actions taken by Health Canada during the project.
COVID-19 site licence or regular site licence regulatory notice: 16 notices
Request to recall: 15 requests
Product licence regulatory notice: 14 notices
Request to stop sale: 10 requests
You can find the status of a product or site licence in the:
What we will do with the results
Health Canada uses the results of compliance monitoring projects to:
- ensure the Canadian population has access to NHPs that are safe, effective and meet quality standards
- help us improve our regulatory oversight of NHPs
- provide guidance to the NHP industry to help them meet regulatory requirements.
This project also helps to enhance our response to future public health emergencies by providing a better understanding of gaps and potential solutions when putting in place emergency measures.
We will continue to inform the Canadian population about our proactive monitoring activities.
How to report a complaint
We want to hear from you. To submit a complaint about a health product or a company that handles health products:
To report a side effect for a health product:
List of products reviewed
| Product name |
Company | Natural product number (NPN) or foreign identifier | Recalls |
|---|---|---|---|
| 70% Alcohol Hand Sanitizer; 70% Alcohol Hand Sanitizer Unscented  |
Walter Surface Technologies Inc. | 80101551 | Not applicable |
Absolute Sanitizer; Gely |
Canada Direct Imports | 80099745 | Request to recall |
All Good Unscented Hand Sanitizer |
Quality Import Solutions | NDC 76150-234-41 | Not applicable |
Avalon Laboratories - Ethanol Hand Sanitizer 80%; Securol Hand Sanitizer |
Avalon Laboratories | 80101239 | Request for recall |
Beroia Hand Sanitizer |
Beroia Inc. | 80099277 | Not applicable |
BiOSS |
Espace Béton Inc. / BiOSS Canada Inc. | 80098279 | Not applicable |
Bleu Lavande Désinfectant pour les mains - Hand Sanitizer |
Bleu Lavande | 80083179 | Not applicable |
Boo Bamboo Hand Sanitizer Gel |
Hush Brands Inc. | 80100160 | Not applicable |
Cyber Clean; Power Gel |
The Orb Factory Limited | 80098826 | Not applicable |
Defenz |
Shiny Star Canada Ltd. | 80100296 appears on the label, which is the wrong NPN. 80100294 is the correct NPN. |
Request to recall |
Ecological Hand Sanitizer 70% |
Hunter Amenities International Limited | 80099539 | Not applicable |
Eco Sanitizer - Hand Sanitizer Gel |
Eco Sanitizer | 80100135 | Not applicable |
Embassy Ingredients - Hand Sanitizer |
Embassy Ingredients | 80097955 | Request to recall |
Gel Hand Sanitizer |
Ecolab Inc. | NDC 47593-487-31 | Not applicable |
Gel Hydroalcoolique au Thym Rouge |
Essences Zen Inc. | 80103989 | Not applicable |
Get Clean Moisturizing Hand Sanitizer |
Shaklee Canada Inc. | NDC 59899-050-01 | Not applicable |
Grace & Stella INC |
Grace & Stella INC | 80099233 | Not applicable |
Hand San 510D |
DuBois Chemicals Canada, Inc. | 80098648 | Request to recall |
Hand Sanitizer |
Latitude 55 Distilling Co. | 80099859 | Request to recall |
Hands Free |
AG Hair Ltd. (Vancouver Hand Made Product Co. Ltd.) | 80097837 | Not applicable |
iMed Hand Sanitizer; Yinba |
MTI-Mobiltech International Inc. | 80098244 | Request to recall |
Insignia Instant Hand Sanitizer |
Groupe Nayla Import | NDC 75410-003-03 | Not applicable |
Insta Safe Guard; AM Innovative Consulting Inc. - Ethanol sanitizer 67% |
AM Innovative Consulting Inc. | 80101932 | Not applicable |
Instant Hand Sanitizer |
KUUS INC. | 80098206 | Not applicable |
Luxe Health Hand Sanitizer Gel (Ethyl alcohol 74%) |
Luxe Decor Sales Ltd. | 80098844 | Request to recall |
MediCare Foaming Hand Sanitizer |
Dollarama L.P. | 80100289 | Request for recall |
Nudestix Hand Sanitizing Gel; Hand Sanitizing Gel |
Nudestix Inc. | 80098230 | Not applicable |
Peak Processing Solutions - Ethanol sanitizer 80% |
2682130 Ontario Limited (o/a Peak Processing Solutions) | 80098093 | Request to recall |
PURE75 |
Haywick Industries | 80098346 | Request for recall |
Puricia |
Pharmaberg Inc. | 80100723 | Request to recall |
Pur-Vie |
Literies Universelles Paga Inc. | 80101600 | Request for recall |
Quik-Care Foam Hand Sanitizer |
Ecolab Inc. | 47593-491-55 | Not applicable |
Safeguard Alcohol Hand Sanitizer |
Procter & Gamble Inc. | NDC 69423-467-59 | Not applicable |
Safetec Instant Hand Sanitizer |
Safecross First Aid Ltd. | NDC 61010-1111-7 | Not applicable |
Sanirell |
Wafting International Inc. | 80100324 | Not applicable |
Soft Hands AB Hand Sanitizer |
Epsilon Chemicals Ltd. | 80099708 | Not applicable |
TerraPure Hand Sanitizer |
TerraPure Sanitizer Inc. | 80103052 | Request to recall |
Touchland Power Mist Moisturizing Hand Sanitizer (Aloe Vera) |
Debut Group Inc. | NDC 72033-001-00 | Not applicable |
Wayward Distillery WHO Formula Hand Rub |
Wayward Distillery | 80098231 | Request to recall |
Zep Hand Sanitizer Gel |
Zep Manufacturing Company of Canada | 80105731 | Not applicable |
Related links
Products in Canada
- List of recalled hand sanitizers in Canada
- Licensing approach to produce and distribute alcohol-based hand sanitizers: Guidance document