2023-24 Departmental Plan: Patented Medicine Prices Review Board
Catalogue No: H79-11E-PDF
ISSN 2371-7807
Patented Medicine Prices Review Board
The Honourable Jean-Yves Duclos
Minister of Health
Table of contents
- From the Board
- Plans at a glance
- Core responsibilities: planned results and resources, and key risks
- Internal services: planned results
- Planned spending and human resources
- Corporate information
- Supporting information on the program inventory
- Supplementary information tables
- Federal tax expenditures
- Organizational contact information
- Appendix: definitions
- Endnotes
From the Board
On behalf of the PMPRB’s Board, I am pleased to present the 2023–24 Departmental Plan for the Patented Medicine Prices Review Board (PMPRB).
The PMPRB is an independent quasi-judicial body established by Parliament in 1987 under the Patent Act (the “Act”). The PMPRB is a consumer protection agency with a dual regulatory and reporting mandate. Its regulatory mandate is to ensure that the prices of patented medicines sold in Canada are not excessive. Its reporting mandate is to provide stakeholders with pharmaceutical trends information to help them make informed choices.
The PMPRB enters in the 2023-24 fiscal year with the recent appointment of a new Chairperson, effective February 1, 2023. With the addition of this new leadership to the Board table, the PMPRB will pick up the project of conducting consultations on proposed new Guidelines to support and operationalize the coming-into-force of July 2022 amendments to the Patented Medicines Regulations (“Regulations”). The Board’s renewed efforts to adopting a meaningful and sustainable approach to new Guidelines will explore potential avenues for a more synergistic relationship between the reforms to the PMPRB’s regulatory framework and the federal government’s higher-level objectives in pharmaceutical policy.
Internally, the coming year will see the PMPRB round out a number of recent initiatives to ensure that our workplace is healthy, safe, and reflective of the Canadian population we serve. This includes the roll out of its new Accessibility PlanFootnote i, a comprehensive return to the workplace wellness plan courtesy of our recently appointed Wellness Coordinator, the implementation of action items from the Chairperson’s letter to the Clerk of the Privy CouncilFootnote ii in response to the Call to Action on Anti-Racism, Equity, and Inclusion in the Public Service, and new employee Mentorship and Sponsorship programs. Together, these initiatives will support our employees through the future of work transition, advance the PMPRB’s efforts to be an employer of choice that attracts top talent from both the public and private sector, and reify the principles of equity, diversity, and inclusion that are core tenets of a modern and progressive public service.
Douglas Clark
Executive Director on behalf of the Board
Plans at a glance
Priority 1 – Implement new pricing framework and begin evaluating its impact
In 2023–24, under the leadership of a new Chairperson and in light of recent jurisprudence, the PMPRB will renew its engagement and consultation on the development of new Guidelines to support the implementation of the amendments to the Patented Medicines Regulations (“Regulations”), which came into force on July 1, 2022.
The direction and approach for this priority will be informed in part by the upcoming consultations and ongoing work to explore all potential avenues for the Guidelines and to ensure that the operationalization of the amended Regulations allow for a more synergistic relationship between the regulatory reform and the federal government’s higher-level objectives in pharmaceutical policy.
To ensure that this process provides for a meaningful engagement and a smooth transition to the final framework, the PMPRB will maximize its outreach strategy to both consult on the Guidelines and to keep all stakeholders informed of new filing requirements, comparator countries, and priorities for investigation.
The PMPRB will also work to lay the ground for appropriate and effective implementation and measurement of the impact of the new Regulations and the proposed Guidelines. To do so, work on the Guidelines Monitoring and Evaluation Plan (GMEP) will be advanced by identifying and quantifying the appropriate benchmarks to be used for comparison purposes to assess the new regime.
The amended Regulations modernize the PMPRB’s approach to ensuring that prices of patented medicines are not excessive. Implementation of the PMPRB’s new Guidelines is the final step in a multi-year effort to give effect to the Government of Canada’s 2017 commitment to improve access to prescription medications, lower drug prices, and support appropriate prescribing.
Priority 2 – Support the Government’s high-level priorities for the future of pharmaceutical management in Canada
The PMPRB is engaged in ongoing work to better align and integrate the roles, responsibilities, and processes of the various participants in the pan-Canadian pharmaceutical pricing and reimbursement system. Together with its health system partners, this work will help address concerns related to the price, accessibility, and appropriate prescribing of medicines in Canada and will support the potential establishment of a Canadian Drug Agency, a national formulary, and a strategy for drugs for rare diseases. To help advance the national conversation on these initiatives, the PMPRB will continue to:
- Work with federal/provincial/territorial (F/P/T) health partners to align and optimize our respective processes in the context of its new regulatory framework and other recent or ongoing reforms that impact pricing and reimbursement;
- Provide analytical support and expertise to F/P/T health partners, as appropriate, in efforts to advance policy work in priority areas such as drugs for rare diseases, common formularies, and other pan-Canadian initiatives to improve the pricing and reimbursement of pharmaceuticals in Canada; and
- Focus reporting efforts on key areas for achieving greater savings for the Canadian healthcare systems in an era where very high-cost medicines pose an increasing threat to sustainability of public and private drug plans.
In the course of carrying out its regulatory and reporting mandate, the PMPRB has developed considerable policy and analytical capacity and is frequently used as a resource to support broader efforts by the federal Health Portfolio and pan-Canadian partners to foster a modern and sustainable health system. At a time of unprecedented change in the Canadian pharmaceutical ecosystem, the PMPRB will continue to leverage its resources and expertise to protect consumers from excessive prices and optimize its value to F/P/T health partners and the health system as a whole.
For more information on the PMPRB’s plans, see the “Core responsibilities: planned results and resources, and key risks” section of this plan.
Core responsibilities: planned results and resources, and key risks
This section contains information on the department’s planned results and resources for each of its core responsibilities. It also contains information on key risks related to achieving those results.
Regulate Patented Medicine Prices
Description
The PMPRB regulates the prices of patented medicines by setting non-excessive price ceilings and taking enforcement action before the Board in the event of non-compliance.
Planning highlights
After some delay owing to the COVID-19 pandemic, the amended Patented Medicines Regulations (the “Regulations”) came into force on July 1, 2022, and new Guidelines are expected to be issued in 2023–24. Together, these two instruments strengthen and modernize Canada’s pricing framework for patented medicines so that the PMPRB can continue to fulfill its statutory mandate to protect Canadian consumers against excessive prices. The PMPRB has worked hard to make the transition to the new filing requirements a smooth one.
The PMPRB’s draft new Guidelines provide general direction on Staff's approach to opening and prioritizing pricing investigations and potential further action, including hearings. To ensure the Guidelines are fair, functional, and fit for their purpose, the PMPRB is developing a comprehensive GMEP to assess changes in trends following implementation of the new Guidelines and inform future adjustments as necessary to ensure they are working as intended.
As currently envisaged, the GMEP will focus on four key trendlines:
- Changes in prices - This will include both list and net prices for patented medicines, as well as any changes in the prices of medicines not directly affected by the reforms.
- Changes in access - This will consider the access continuum, from the development of medicines (clinical trials) to medicine approval and availability, Health Technology Assessment (HTA), pan-Canadian Pharmaceutical Alliance (pCPA) negotiations, and formulary listings.
- Changes in the ecosystem - This will focus on research and development, economic footprint, drug spending, and the supply chain.
- Changes in processes - This will look at the operational aspects of the price assessment, scientific review of medicines, administrative burden, and outreach activities (specifically the number of engagement activities the PMPRB is undertaking to assist rights holders in understanding the Guidelines and their application).
Each area of focus will be monitored and evaluated by comparing trends prior to and post implementation of the PMPRB’s new regulatory framework. In 2023–24, the PMPRB will focus its efforts on finalizing the GMEP and generating baseline results which will serve as a benchmark against which to assess the impact of the new regulatory framework and to identify key trends relevant for continued relevance of the PMPRB Guidelines. The trends under the new Guidelines will be monitored on an ongoing basis and compared against the established benchmarks starting in 2023–24.
The PMPRB’s Compliance Information Management System (CIMS) is a web-based database application used to assist the review and analysis of data filed by rights holders. The PMPRB has made enhancements to CIMS so that it could accept and process the additional information rights holders must provide under the new Regulations, and will monitor and evaluate how well the system is functioning and make any necessary adjustments.
As directed by the Minister pursuant to s. 90 of the Act, under the National Prescription Drug Utilization Information System (NPDUIS) reporting mandate, the PMPRB provides decision-makers with critical information and intelligence on price, utilization, and cost trends so that Canada’s healthcare system has more comprehensive and accurate information on how medicines are being used and on sources of cost pressures. In 2023–24, the PMPRB plans to publish the results of NPDUIS analyses as annual publications, report series, and chartbooks.
In addition to regularly consulting with the NPDUIS Advisory Committee, participating in conferences and seminars, and organizing public information sessions on the results of its analytical studies, the PMPRB will support and strengthen its NPDUIS engagement activities by collaborating with stakeholders on complementary initiatives, renewing data holdings, and working to build visibility and responsiveness of its reporting.
Gender-based analysis plus
The PMPRB recognizes that sex and gender differences, race, ethnicity, age, and mental or physical disability are factors to consider in the accessibility, affordability, and appropriate use of prescription medicines and medical devices. Differences in sex and gender+ roles, income, and utilization of health care services can affect access to medicines and health insurance, prescribing patterns, and medicine use, and may have important repercussions for health and well-being.
Since the price of a patented medicine does not vary for the sex or gender+ of the user, the PMPRB’s price review process does not take explicit account of the diversity of user groups or their economic situation. Lower patented medicine prices, and associated savings for all payers, will benefit all populations directly through lower out-of-pocket costs and indirectly through health system reinvestments and improved access to better care. In addition, the very high-cost patented medicines, which are the focus of the PMPRB’s new risk-based regulatory framework, often treat rare diseases that can impact certain minority ethnic groups disproportionately.
United Nations 2030 Agenda for Sustainable Development and the UN Sustainable Development Goals
The PMPRB is responsible for contributing to the United Nations 2030 Agenda for Sustainable Development and will work to advance the UN’s Sustainable Development Goals (SDGs) in 2023–24.
While the majority of this work will be conducted at an Internal Services level, SDG 10: Reduce Inequality Within and Among Countries is addressed from a program perspective in much the same terms as the gender-based analysis plus initiative. Although the PMPRB’s regulatory mandate does not target specific consumers, the price review process under the new regulatory regime contributes to a more equitable access to pharmaceuticals for all Canadians by prioritizing investigations of medicines with the greatest risk of excessive pricing. Non-excessive prices for medicines directly benefit those who cannot otherwise afford their prescriptions because they do not have access to adequate insurance coverage, and savings from any resultant price reductions can enable reinvestments in the health system.
Health impacts and inequalities resulting from the effects of climate change will require flexibility in health system spending for payers in coming years. The PMPRB is an essential part of the response to SDG 13: Take Urgent Action to Combat Climate Change and its Impacts as it contributes to the development of a sustainable healthcare system that will allow payers to be responsive to the needs of Canadians and furnishes analytic reports that support informed policy development for healthcare initiatives.
Innovation
The PMPRB has no high-impact innovation planned for 2023–24. Over the past several years, the primary focus of the organization has been to reform and modernize its regulatory framework. In the coming year, the PMPRB will be invested in the successful transition to this new framework, as well as in the monitoring and assessment of its impact.
Key risk(s)
The PMPRB has identified three key risks to the achievement of results for its Core Responsibility.
The first risk is that in the transition to the new regulatory framework, rights holders may be confused about their filing responsibilities (what data to file and how and when that data will be used for price review purposes). To mitigate this risk, the PMPRB has implemented an outreach strategy to help rights holders understand the new Guidelines and developed tools to support the transition. In addition, under subsection 98(4) of the Act, the Board may provide advance guidance to a rights holder on a medicine’s price if there is enough information to do so.
The second risk is that the new regulatory framework may have unintended consequences on patient access to innovative new medicines, clinical trial activity, or pharmaceutical distribution and supply in Canada. The PMPRB will implement a comprehensive plan for monitoring and evaluating the effect new Regulations and Guidelines might have on prices, access, research and development, and internal to PMPRB processes so that remedial action can be taken quickly if warranted.
Lastly, although the PMPRB has significant in-house expertise for the benefit of Canadians, low public awareness of the PMPRB’s role in the health system and lack of integration with its health system partners may prevent these resources from being fully optimized within the Health Portfolio. To close the gap posed by this third risk, the PMPRB will continue to seek opportunities to collaborate and find efficiencies with like-minded federal organizations, open doors for meaningful engagement with its stakeholders, identify research topics that are timely and pertinent to the work of Canadian payers, and effectively communicate the results of its reporting.
Planned results for Regulate Patented Medicine Prices
The following table shows, for Regulate Patented Medicine Prices, the planned results, the result indicators, the targets and the target dates for 2023–24, and the actual results for the three most recent fiscal years for which actual results are available.
| Departmental result | Departmental result indicator | Target | Date to achieve target | 2019–20 actual result | 2020–21 actual result | 2021–22 actual result |
|---|---|---|---|---|---|---|
Affordable patented drug prices |
% of patented drug prices in Canada below the median of the PMPRB’s comparator countries(a) |
50%(b) |
March 31, 2024 |
56.9% |
58.2% |
59.5%(c) |
% of patented drug prices in Canada within the thresholds set out in the PMPRB’s Guidelines |
95%(d) |
March 31, 2024 |
88.4% |
86.3% |
84.6%(e) |
Note: Results and targets identified in this section are not a factor in the Board’s discretion in the exercise of its quasi-judicial powers.
(a)As of July 1, 2022, the Patented Medicine Regulations stipulate the use of a group of 11 schedule countries as comparators (“PMPRB11”): Australia, Belgium, France, Germany, Italy, Japan, the Netherlands, Norway, Spain, Sweden, and the United Kingdom. Prior to this change, a group of seven comparator countries was used (“PMPRB7”), including the United States and Switzerland, which have some of the highest prices for patented medicines in the Organisation for Economic Co-operation and Development (OECD). As such, results for this indicator are expected to vary from 2022-23 onward and may not be directly comparable to previous years.
(b) This indicator was introduced in 2015-16. Operating under the premise that the PMPRB would continue to conduct its price reviews without significant changes in its regulatory framework, the PMPRB established a target of 50% of patented medicine prices being below the median price. Analysis in the PMPRB’s 2015 Annual Report indicated that the percentage of patented medicines priced below the median price of the PMPRB’s comparator countries was 51.8%, a decline from the previous two years. Based on these factors, it was determined that 50% would be a reasonable target.
(c) The 59.5% of patented medicine prices in Canada reported as being below the median international price includes a significant number of patented medicines being sold in fewer than five countries and therefore are not being compared to the actual median international price. Of the 1,158 patented medicines sold in Canada in 2021, only 700 were sold in five or more countries. Of this 700, only 343 patented medicines (49.0%) had a Canadian price below the median price. This is a significant difference from the reported 59.5%, in part because the median international prices for medicines with fewer than five comparator countries are typically heavily skewed towards prices in the United States.
(d) This percentage, based on the number of price reviews completed by March 31 of the fiscal year referred to, is calculated as follows: the sum of the number of price reviews found to be within the Guidelines, plus the number of price reviews that did not trigger an investigation, plus the number of Voluntary Compliance Undertakings; divided by the number of patented medicines for which the price review was completed by March 31 of the fiscal year.
(e) As of March 31, 2022, 44 patented medicines were still under review, 169 were under investigation, four were the subject of a hearing, and one was subject to a Stay Order.
The financial, human resources and performance information for the PMPRB’s program inventory is available on GC InfoBase.Footnote iii
Planned budgetary spending for Regulate Patented Medicine Prices
The following table shows, for Regulate Patented Medicine Prices, budgetary spending for 2023–24, as well as planned spending for that year and for each of the next two fiscal years.
| 2023–24 budgetary spending (as indicated in Main Estimates) | 2023–24 planned spending | 2024–25 planned spending | 2025–26 planned spending |
|---|---|---|---|
13,927,400 |
13,927,400 |
13,927,400 |
13,927,400 |
Budgetary spending in 2023–24 and planned spending for 2023–24 and beyond include funds for a Special Purpose Allotment (SPA) to conduct public hearings. The SPA can only be used to cover the costs of public hearings, such as external legal counsel and expert witnesses, etc. For the purposes of forecasting planned spending, the PMPRB assumes that the entire SPA funding will be spent. This is because these expenditures are dependent on the number of hearings and the length and complexity of the hearings held, which are difficult to predict. The annual SPA for 2023–24 and beyond is $4.5 million. Any unspent amount at the end of each fiscal year is returned to the Consolidated Revenue Fund.
Financial, human resources, and performance information for the PMPRB’s program inventory is available on GC InfoBase.Footnote iv
Planned human resources for Regulate Patented Medicine Prices
The following table shows, in full‑time equivalents, the human resources the department will need to fulfill this core responsibility for 2023–24 and for each of the next two fiscal years.
| 2023–24 planned full-time equivalents | 2024–25 planned full-time equivalents | 2025–26 planned full-time equivalents |
|---|---|---|
| 58 | 58 | 58 |
Financial, human resources and performance information for the PMPRB’s program inventory is available on GC InfoBase.Footnote v
Internal services: planned results
Description
Internal services are the services that are provided within a department so that it can meet its corporate obligations and deliver its programs. There are 10 categories of internal services:
- management and oversight services
- communications services
- legal services
- human resources management services
- financial management services
- information management services
- information technology services
- real property management services
- materiel management services
- acquisition management services
Planning highlights
The PMPRB is committed to implementing the Clerk of the Privy Council’s call to action on anti-racism, equity, and inclusion, in 2023–24 and will work to complete action items from the Chairperson’s letter to the Clerk of the Privy Council.Footnote vi
Further to the call to action, and in response to Part 7(1)(a) of The Accessible Canada Act,Footnote viithe PMPRB released its Accessibility PlanFootnote viii in December 2022 and will continue work on the identified objectives through 2023–24. The Plan was developed in consultation with employees with disabilities to incorporate best accessibility practices and to create a work environment that is welcoming and conducive to success, with leadership that models and reinforces accessibility-positive attitudes and practices. In its commitment to becoming an employer of choice in the public service for those with disabilities and an accessibility leader for work environment and services, the PMPRB will be working through 2023–24 and beyond to implement the requirements set out in the Government of Canada’s Accessibility StrategyFootnote ix and to build on this foundation to cultivate a pro-accessibility culture and operational standards.
To adapt to its new conditions of work under the revised regulatory framework, the PMPRB also intends to release a new Strategic Plan for 2023–2026 and will engage in a thorough review of its Departmental Results Framework to ensure that results indicators and targets accurately reflect the impact of its programs.
Planning for Contracts Awarded to Indigenous Businesses
The PMPRB intends to support the Government of Canada’s commitment to a mandatory minimum target 5.0% of the total value of annual contracts awarded to Indigenous businesses in 2023–24. To meet this target, Indigenous suppliers will be sourced for information technology (IT) equipment such as tablets and monitors as well as IT services, and graphic design and contracted web development services will be transitioned to Indigenous vendors. These expenses, estimated based on planned spending for the year, are expected to amount to 5.8% of total contracted spending in 2023–24 and will be monitored as part of the financial update cycle.
| 5% reporting field description | 2021–22 actual % achieved | 2022–23 forecasted % target | 2023–24 planned % target |
|---|---|---|---|
| Total percentage of contracts with Indigenous businesses | N/A | 5.7% | 5.8% |
Planned budgetary spending for internal services
The following table shows, for internal services, budgetary spending for 2023–24, as well as planned spending for that year and for each of the next two fiscal years.
| 2023–24 budgetary spending (as indicated in Main Estimates) | 2023–24 planned spending | 2024–25 planned spending | 2025–26 planned spending |
|---|---|---|---|
| 3,166,274 | 3,166,274 | 3,166,274 | 3,166,274 |
Planned human resources for internal services
The following table shows, in full‑time equivalents, the human resources the department will need to carry out its internal services for 2023–24 and for each of the next two fiscal years.
| 2023–24 planned full-time equivalents | 2024–25 planned full-time equivalents | 2025–26 planned full-time equivalents |
|---|---|---|
| 23 | 23 | 23 |
Planned spending and human resources
This section provides an overview of the department’s planned spending and human resources for the next three fiscal years and compares planned spending for 2023–24 with actual spending for the current year and the previous year.
Planned spending
Departmental spending 2020–21 to 2025–26
The following graph presents planned spending (voted and statutory expenditures) over time.
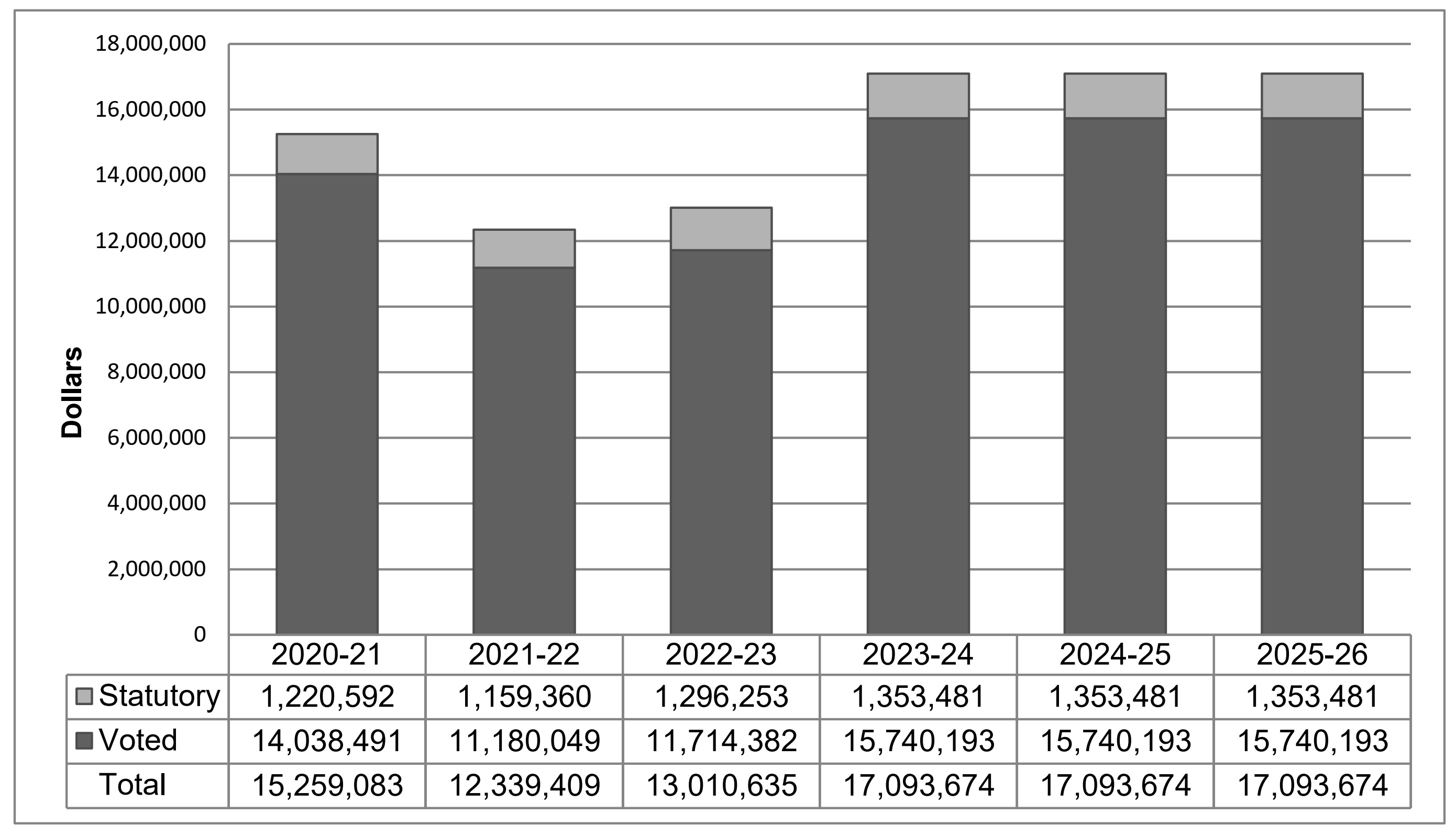
Long description
This stacked bar graph provides actual and planned spending amounts, in dollars, for the Patented Medicine Prices Review Board (PMPRB) from fiscal year 2020–21 to fiscal year 2025–26. Total expenditures are separated into statutory and voted amounts.
| 2020–21 | 2021–22 | 2022–23 | 2023–24 | 2024–25 | 2025–26 | |
|---|---|---|---|---|---|---|
Statutory |
$1,220,592 |
$1,159,360 |
$1,296,253 |
$1,353,481 |
$1,353,481 |
$1,353,481 |
Voted |
$14,038,491 |
$11,180,049 |
$11,714,382 |
$15,740,193 |
$15,740,193 |
$15,740,193 |
Total |
$15,259,083 |
$12,339,409 |
$13,010,635 |
$17,093,674 |
$17,093,674 |
$17,093,674 |
As announced in Budget 2017, the PMPRB received additional funding for future years: $6.7 million in 2020–21, $7.7 million in 2021–22, and $5.7 million in 2022–23 and ongoing, including Employee Benefit Package (EBP) and increased funding for the Special Purpose Allotment (SPA).
Spending in 2021–22 was lower than in 2020–21 because of the completion of hearing room construction. It is necessary to assume that SPA funding will be required in its entirety for planned spending in future years. However, forecast spending on the SPA in 2022–23 is only $1.2 million, with a projected lapse of $3.2 million.
Budgetary planning summary for core responsibilities and internal services (dollars)
The following table shows information on spending for each of the PMPRB’s core responsibilities and for its internal services for 2023–24 and other relevant fiscal years.
| Core responsibilities and internal services | 2020–21 actual expenditures | 2021–22 actual expenditures | 2022–23 forecast spending | 2023–24 budgetary spending (as indicated in Main Estimates) | 2023–24 planned spending | 2024–25 planned spending | 2025–26 planned spending |
|---|---|---|---|---|---|---|---|
Regulate Patented Medicine Prices |
10,858,873 |
8,999,721 |
9,630,322 |
13,927,400 |
13,927,400 |
13,927,400 |
13,927,400 |
Subtotal |
10,858,873 |
8,999,721 |
9,630,322 |
13,927,400 |
13,927,400 |
13,927,400 |
13,927,400 |
Internal services |
4,400,210 |
3,339,688 |
3,380,313 |
3,166,274 |
3,166,274 |
3,166,274 |
3,166,274 |
Total |
15,259,083 |
12,339,409 |
13,010,635 |
17,093,674 |
17,093,674 |
17,093,674 |
17,093,674 |
The forecast spending for 2022–23 is based on actual spending and anticipated spending to year end, which does not anticipate full use of the Special Purpose Allotment (SPA). At the time of preparing this report, forecast spending of the SPA amounted to approximately $1.2 million of the $4.5 million allotted. This and an anticipated surplus of $1.5 million in Vote 1 spending account for the variance in 2022–23 forecast spending and 2023–24 planned spending.
For purposes of forecasting planned spending for 2023–24 and future years, the PMPRB assumes the entire SPA funding for hearings will be spent. This is because these expenditures are dependent on the number of hearings and the length and complexity of the hearings held, which are difficult to predict.
Planned human resources
The following table shows information on human resources, in full-time equivalents (FTEs), for each of the PMPRB’s core responsibilities and for its internal services for 2023–24 and the other relevant years.
Human resources planning summary for core responsibilities and internal services
| Core responsibilities and internal services | 2020–21 actual full‑time equivalents | 2021–22 actual full‑time equivalents | 2022–23 forecast full‑time equivalents | 2023–24 planned full‑time equivalents | 2024–25 planned full‑time equivalents | 2025–26 planned full‑time equivalents |
|---|---|---|---|---|---|---|
Regulate Patented Medicines Prices |
57 |
55 |
55 |
58 |
58 |
58 |
Subtotal |
57 |
55 |
55 |
58 |
58 |
58 |
Internal services |
23 |
23 |
22 |
23 |
23 |
23 |
Total |
80 |
78 |
77 |
81 |
81 |
81 |
The slight increase in planned FTEs for 2023–24 and beyond is a result of backfilling departures and fulfilling postponed staffing actions.
Estimates by vote
Information on the PMPRB’s organizational appropriations is available in the 2023–24 Main Estimates.Footnote x
Future-oriented condensed statement of operations
The future‑oriented condensed statement of operations provides an overview of the PMPRB’s operations for 2022–23 to 2023–24.
The forecast and planned amounts in this statement of operations were prepared on an accrual basis. The forecast and planned amounts presented in other sections of the Departmental Plan were prepared on an expenditure basis. Amounts may therefore differ.
A more detailed future‑oriented statement of operations and associated notes, including a reconciliation of the net cost of operations with the requested authorities, are available on the PMPRB’s website.Footnote xi
Future‑oriented condensed statement of operations for the year ending March 31, 2024 (dollars)
| Financial information | 2022–23 forecast results | 2023–24 planned results | Difference (2023–24 planned results minus 2022–23 forecast results) |
|---|---|---|---|
Total expenses |
14,479,547 |
18,646,416 |
4,166,869 |
Total revenues |
202 |
- |
(202) |
Net cost of operations before government funding and transfers |
14,479,345 |
18,646,416 |
4,167,071 |
Note: The amounts included in this table differ from the information included in the other financial tables in this report because this table includes accrued information (e.g., amortization), as well as values for services provided to the PMPRB without charge. |
|||
The PMPRB is projecting $18.6 million in expenses based on 2023–24 Main Estimates and accrued information. This amount does not include future supplementary estimates. It represents an increase of $4.2 million from 2022–23 projections, primarily attributable to a lapse in SPA funding for hearings. The PMPRB assumes the entire SPA funding for hearings will be spent. This is because these expenditures are dependent on the number of hearings and the length and complexity of the hearings held, which are difficult to predict.
The 2023–24 planned expenses by core responsibility are as follows:
- Regulate Patented Medicine Prices: $15.1 million; and,
- Internal Services: $3.5 million.
The PMPRB receives most of its funding through annual Parliamentary appropriations.
Corporate information
Organizational profile
Appropriate minister(s): The Honourable Jean-Yves Duclos
Institutional head: Thomas J. Digby, Chairperson
Ministerial portfolio: Health
Enabling instrument(s): Patent ActFootnote xii and Patented Medicines RegulationsFootnote xiii
Year of incorporation / commencement: 1987
Raison d’être, mandate and role: who we are and what we do
Information on the PMPRB’s raison d’être, mandate and role is available on the PMPRB’s website.Footnote xiv.
Information on the PMPRB’s mandate letter commitments is available in the Minister’s mandate letter.Footnote xv.
Operating context
Information on the operating context is available on the PMPRB’s website.Footnote xvi
Reporting framework
The PMPRB’s approved departmental results framework and program inventory for 2023–24 are as follows.
| Departmental Results Framework | Core Responsibility: Regulate Patented Medicine Prices | Internal Services | |
| Departmental Results: Affordable patented drug prices | Indicator 1: % of patented drug prices in Canada below the median price of the PMPRB’s comparator countries | ||
| Indicator 2: % of patented drug prices in Canada within the threshold set out in the PMPRB’s Guidelines | |||
| Program Inventory | Patented Medicine Price Regulation Program | ||
| Pharmaceutical Trends Program | |||
Supporting information on the program inventory
Supporting information on planned expenditures, human resources, and results related to the PMPRB’s program inventory is available on GC InfoBaseFootnote xvii
Supplementary information tables
The following supplementary information tables are available on the PMPRB’s website:
- Gender-based analysis plusFootnote xviii
- United Nations 2030 Agenda for Sustainable Development and the Sustainable Development Goals Footnote xix
Federal tax expenditures
The PMPRB’s Departmental Plan does not include information on tax expenditures.
Tax expenditures are the responsibility of the Minister of Finance. The Department of Finance Canada publishes cost estimates and projections for government‑wide tax expenditures each year in the Report on Federal Tax Expenditures.Footnote xx This report provides detailed information on tax expenditures, including objectives, historical background, and references to related federal spending programs, as well as evaluations, research papers, and gender-based analysis plus.
Organizational contact information
Mailing address
The Patented Medicine Prices Review Board
Box L40
Standard Life Centre
333 Laurier Avenue West
Suite 1400
Ottawa, Ontario
K1P 1C1
Telephone: 1-877-861-2350
TTY: 613-288-9654
Fax: 613-288-9643
Email: PMPRB.Information-Renseignements.CEPMB@pmprb-cepmb.gc.ca
Website(s): https://www.canada.ca/en/patented-medicine-prices-review.htmlFootnote xxi
Appendix: definitions
appropriation (crédit)
Any authority of Parliament to pay money out of the Consolidated Revenue Fund.
budgetary expenditures (dépenses budgétaires)
Operating and capital expenditures; transfer payments to other levels of government, organizations or individuals; and payments to Crown corporations.
core responsibility (responsabilité essentielle)
An enduring function or role performed by a department. The intentions of the department with respect to a core responsibility are reflected in one or more related departmental results that the department seeks to contribute to or influence.
Departmental Plan (plan ministériel)
A document that sets out a department’s priorities, programs, expected results and associated resource requirements, covering a three‑year period beginning with the year indicated in the title of the report. Departmental Plans are tabled in Parliament each spring.
departmental result (résultat ministériel)
A change that a department seeks to influence. A departmental result is often outside departments’ immediate control, but it should be influenced by program-level outcomes.
departmental result indicator (indicateur de résultat ministériel)
A factor or variable that provides a valid and reliable means to measure or describe progress on a departmental result.
departmental results framework (cadre ministériel des résultats)
A framework that consists of the department’s core responsibilities, departmental results, and departmental result indicators.
Departmental Results Report (rapport sur les résultats ministériels)
A report on a department’s actual performance in a fiscal year against its plans, priorities and expected results set out in its Departmental Plan for that year. Departmental Results Reports are usually tabled in Parliament each fall.
full‑time equivalent (équivalent temps plein)
A measure of the extent to which an employee represents a full person‑year charge against a departmental budget. Full‑time equivalents are calculated as a ratio of assigned hours of work to scheduled hours of work. Scheduled hours of work are set out in collective agreements.
gender-based analysis plus (GBA Plus) (analyse comparative entre les sexes plus [ACS Plus])
An analytical tool used to support the development of responsive and inclusive policies, programs and other initiatives. GBA Plus is a process for understanding who is impacted by the issue or opportunity being addressed by the initiative; identifying how the initiative could be tailored to meet diverse needs of the people most impacted; and anticipating and mitigating any barriers to accessing or benefitting from the initiative. GBA Plus is an intersectional analysis that goes beyond biological (sex) and socio-cultural (gender) differences to consider other factors, such as age, disability, education, ethnicity, economic status, geography, language, race, religion, and sexual orientation.
government-wide priorities (priorités pangouvernementales)
For the purpose of the 2023–24 Departmental Plan, government-wide priorities are the high-level themes outlining the Government’s agenda in the 2021 Speech from the Throne: building a healthier today and tomorrow; growing a more resilient economy; bolder climate action; fighter harder for safer communities; standing up for diversity and inclusion; moving faster on the path to reconciliation; and fighting for a secure, just, and equitable world.
high impact innovation (innovation à impact élevé)
High impact innovation varies per organizational context. In some cases, it could mean trying something significantly new or different from the status quo. In other cases, it might mean making incremental improvements that relate to a high-spending area or addressing problems faced by a significant number of Canadians or public servants.
horizontal initiative (initiative horizontale)
An initiative in which two or more federal organizations are given funding to pursue a shared outcome, often linked to a government priority.
non‑budgetary expenditures (dépenses non budgétaires)
Net outlays and receipts related to loans, investments, and advances, which change the composition of the financial assets of the Government of Canada.
performance (rendement)
What an organization did with its resources to achieve its results, how well those results compare to what the organization intended to achieve, and how well lessons learned have been identified.
plan (plan)
The articulation of strategic choices, which provides information on how an organization intends to achieve its priorities and associated results. Generally, a plan will explain the logic behind the strategies chosen and tend to focus on actions that lead up to the expected result.
planned spending (dépenses prévues)
For Departmental Plans and Departmental Results Reports, planned spending refers to those amounts presented in the Main Estimates.
A department is expected to be aware of the authorities that it has sought and received. The determination of planned spending is a departmental responsibility, and departments must be able to defend the expenditure and accrual numbers presented in their Departmental Plans and Departmental Results Reports.
program (programme)
Individual or groups of services, activities, or combinations thereof that are managed together within a department and that focus on a specific set of outputs, outcomes, or service levels.
program inventory (répertoire des programmes)
An inventory of a department’s programs that describes how resources are organized to carry out the department’s core responsibilities and achieve its planned results.
result (résultat)
An external consequence attributed, in part, to an organization, policy, program or initiative. Results are not within the control of a single organization, policy, program, or initiative; instead, they are within the area of the organization’s influence.
statutory expenditures (dépenses législatives)
Expenditures that Parliament has approved through legislation other than appropriation acts. The legislation sets out the purpose of the expenditures and the terms and conditions under which they may be made.
target (cible)
A measurable performance or success level that an organization, program or initiative plans to achieve within a specified time period. Targets can be either quantitative or qualitative.
voted expenditures (dépenses votées)
Expenditures that Parliament approves annually through an Appropriation Act. The vote wording becomes the governing conditions under which these expenditures may be made.