Scientific Review
Table of Contents
Purpose
The purpose of the Scientific Review is to determine which medicines are in the same therapeutic class as the medicine under In-Depth Review, what the comparable doses of these medicines are, and to what degree they are comparable to the patented medicine under In-Depth Review. This process is called the Therapeutic Class Comparison (TCC).
Method
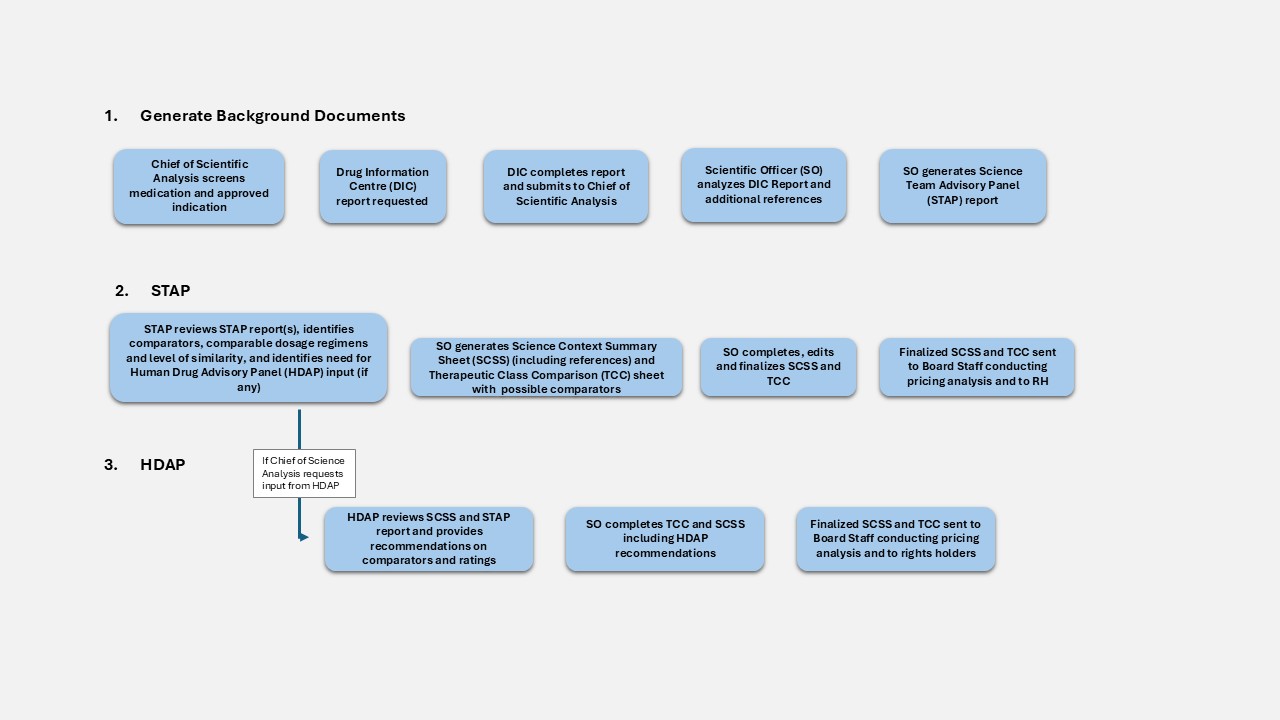
Figure description
A visual to show the overall process for the Scientific Review. The Chief of Science Analysis screens the medication under review and all approved indication(s). A Drug Information Centre (DIC) report is requested, and it is analyzed by the Scientific Officer (SO) along with additional references. The SO generates a Science Team Advisory Panel (STAP) report which is used to identify comparators, comparable dosage regimens, level of similarity, and identifies the need for Human Drug Advisory Panel (HDAP) consideration if required. The SO generates a Science Context Summary Sheet (SCSS) and Therapeutic Class Comparison (TCC) sheet for possible comparators. The finalized SCSS and TCC are then sent to Board Staff conducting the pricing analysis and the Rights Holder.
The Human Drug Advisory Panel (HDAP) may be consulted at the discretion of Staff on a case-by-case basis.
Comparators are identified for each approved indication or use of the patented medicine under In-Depth Review at the time the Review is initiated. If relevant, other indications/uses could potentially be considered in the context of a hearing.
TCC assessments are conducted for each indication from a population therapeutics perspective and are not intended to address all possible unique patient circumstances or needs. TCC assessments are confidential and will be shared only with the Rights Holder concerned, unless required by law.
Each comparator is assigned a level of similarity to the medicine under review in accordance with a two-step framework. Comparability is assigned for each comparator identified for each indication. Comparable dosage forms are also established at this stage. The most appropriate strength of the drug product is chosen for a particular dosage regimen. Generally, a dosage regimen based on a course of treatment is applicable to acute indications, while a per-day regimen (based on a maintenance dose) is applicable for chronic indications.
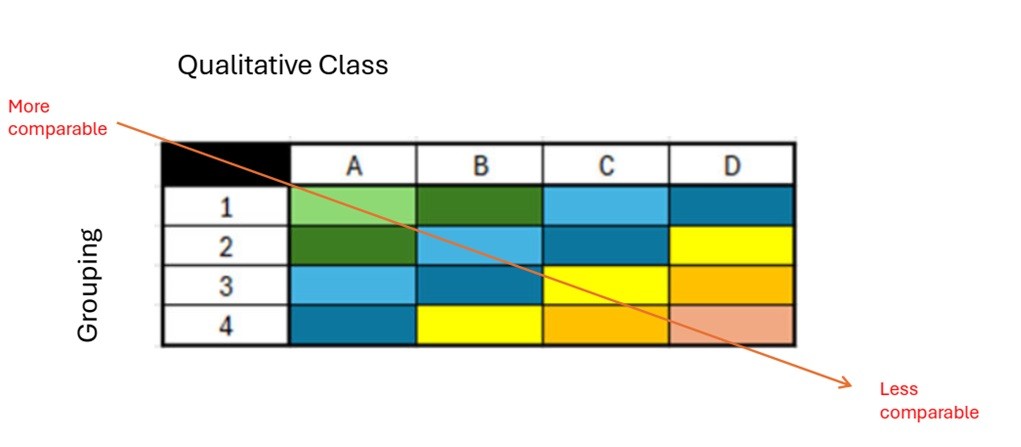
Figure description
This table illustrates the spectrum of comparability, ranging from more comparable (top-left) to less comparable (bottom-right). The horizontal axis represents the qualitative class (A, B, C, D), while the vertical axis indicates groupings (1, 2, 3, 4). The figure effectively shows how comparability decreases progressively across qualitative classes (left to right) and across groupings (top to bottom).
Comparability is visually distinguished by color:
- Green: High comparability (e.g., A1, A2 and B1)
- Blue: Medium comparability (e.g., A3, A4, B2, B3, C1, C2, D1)
- Yellow/Orange/Pink: Low comparability (e.g., B4, C3, C4, D2, D3 and D4)
Depending on the circumstances, Staff may choose to consult the Human Drug Advisory Panel (HDAP) or other experts on an ad hoc basis. Neither the HDAP nor any other experts perform any adjudicative functions. They are a potential resource that Staff may choose to utilize in certain situations to provide additional input into the analysis undertaken by Staff. Accordingly, Rights Holders cannot request a review of their patented medicine by the HDAP (or by other experts that may be consulted by Staff).
- More information on the Human Drug Advisory Panel (HDAP)
Information Sources
When assembling and assigning a comparability rating to medicines in the TCC, the Staff scientific team may consult the following sources:
- ATC classification;
- Approved indications or uses, or proposed indications, where applicable;
- Available medical literature;
- Clinical evaluations undertaken by domestic and international health technology assessment organizations (e.g. Canada’s Drug Agency, INESSS, EMA, NICE, etc.);
- Written input provided by the Rights Holder (if any);
- Research by a Drug Information Centre (DIC) – Staff may use the services of various drug information centres to obtain scientific information, such as clinical trial information, clinical practice guidelines, etc.;
- Research by Staff;
- HDAP – On an ad hoc basis, Staff may choose to consult with the HDAP in certain situations where Staff determines such assistance is required; and
- Guidelines or consensus statements from Canadian or foreign clinical groups pertaining to the treatment of the approved Health Canada indication of the patented medicine under review.
Rights Holder Input
Rights Holders may submit any relevant information they wish to be considered as soon as possible after being notified of an In-Depth Review. This information can be filed via our Online Filing Tool under “Scientific Submissions”. Notification that an online scientific submission has been filed should be sent by the Rights Holder by email to PMPRB.Filing-Depot.CEPMB@pmprb-cepmb.gc.ca.
Submissions which clearly explain the rationale behind the Rights Holder’s proposals for level of comparability, medicines for comparison purposes, and comparable dosage regimens are likely to be the most useful to the Staff scientific team.
For more information on details to include in the scientific submission, please visit Rights Holder Input Relating to the Scientific Review Process.
Timeline
Rights Holders are generally notified regarding the selection of comparators for each approved indication or use within 8 months of the initiation of the In-Depth Review.
Outputs
Rights Holders can expect to receive these documents following a scientific review:
- dTCC (and iTCC where applicable) assessments which include comparable dosage regimens
- Comparability score of each comparator
- Science Context Summary Sheet (SCSS) including a summary of the rationale used in the assessment of each comparator and relevant references