Meds Pipeline Monitor 2020

ISSN: 2562-3834
Catalogue number: H79-5E-PDF
Table of contents
Contact Information
Patented Medicine Prices Review Board
Standard Life Centre
Box L40
333 Laurier Avenue West
Suite 1400
Ottawa, ON K1P 1C1
Tel.: 1-877-861-2350
TTY 613-288-9654
Email: PMPRB.Information-Renseignements.CEPMB@pmprb-cepmb.gc.ca
Acknowledgements
This report was prepared by the Patented Medicine Prices Review Board (PMPRB) as part of the National Prescription Drug Utilization Information System (NPDUIS) initiative.
The PMPRB wishes to acknowledge the members of the NPDUIS Advisory Committee for their expert oversight and guidance in the preparation of this report. Please note that the statements and findings for this report do not necessarily reflect those of the members or their organizations.
We gratefully acknowledge Patricia Carruthers-Czyzewski, BScPhm, MSc, Sintera Inc. for providing pharmaceutical expertise and for her contribution to the scientific analysis.
Appreciation goes to Allison Carey and Caroline Peterson for leading this project; and Tanya Potashnik and Jeffrey Menzies for their oversight in the development of the report. The PMPRB also wishes to acknowledge Nevzeta Bosnic for providing direction in the development of the analysis; and the contribution of the editorial staff Sarah Parker and Shirin Paynter.
Disclaimer
NPDUIS operates independently of the regulatory activities of the Board of the PMPRB. The research priorities, data, statements, and opinions expressed or reflected in NPDUIS reports do not represent the position of the PMPRB with respect to any regulatory matter. NPDUIS reports do not contain information that is confidential or privileged under sections 87 and 88 of the Patent Act, and the mention of a medicine in a NPDUIS report is not and should not be understood as an admission or denial that the medicine is subject to filings under sections 80, 81, or 82 of the Patent Act or that its price is or is not excessive under section 85 of the Patent Act.
Although based in part on data obtained under license from GlobalData and the IQVIA MIDAS® Database, the statements, findings, conclusions, views, and opinions expressed in this report are exclusively those of the PMPRB and are not attributable to either GlobalData or IQVIA.
Executive summary
Meds Pipeline Monitor (MPM) is a horizon scanning report that features a selection of new medicines in the late stages of clinical evaluation that may have a significant impact on future clinical practice and drug spending in Canada.
Medicines in Phase III clinical trials or pre-registration are considered as candidates if they have the potential to address an unmet therapeutic need, offer a novel mechanism of action or therapeutic benefit over existing therapies, or treat a serious condition. The final selection features medicines that treat a broad range of therapeutic areas. In addition to identifying new medicines for inclusion in the list, medicines featured in the 2019 Meds Pipeline Monitor are also reviewed to report on changes to their status in the pipeline. A section focused on Canada highlights potentially significant medicines currently under review by Health Canada.
This edition of the report includes a new section on COVID-19, which provides an overview of medicines undergoing Phase I, II, and III clinical trials or in pre-registration for the treatment and prevention of the novel coronavirus disease.
The report collects data from two main sources: GlobalData’s Healthcare database is used to identify medicines currently undergoing clinical evaluation, while Health Canada’s Drug and Health Product Submissions Under Review list provides information on new medicines under review in Canada.
Together with its companion publication Meds Entry Watch, this report series monitors the continuum of new and emerging medicines in Canada and internationally, providing key information to decision makers, researchers, patients, and clinicians, among other stakeholders.
Highlights of the Meds Pipeline 2020
- In 2020, the pipeline contained 6,946 new medicines in various stages of evaluation, of which 13% were in Phase III clinical trials and pre-registration, representing a wide range of therapeutic areas.
- Oncology continued to dominate the therapeutic mix in 2020, with cancer treatments representing one third (35%) of medicines in all phases of clinical trials. Treatments for infectious diseases held the second largest share of the pipeline, at 13%, and are expected to grow in importance in response to the COVID-19 pandemic.
- Over one third of medicines in Phase III clinical trials or pre-registration had an early orphan designation approved through the FDA or EMA, which is consistent with the increasing trend in the prevalence of orphan-designated medicines entering the pharmaceutical market.
- Sixteen late-stage new medicines, including five gene therapies, were selected for addition to the 2020 MPM based on their potential impact on the Canadian healthcare system. Some of these medicines may offer breakthroughs in treating previously unmet needs or may have the potential to treat large patient populations.
- Of the 24 new medicines featured in the 2019 edition of the MPM, 11 were retained on the list as they continued to satisfy the selection criteria.
- The report also highlights six of the new medicines currently under review by Health Canada. Four of these medicines have forecasted global revenues of over US $1 billion annually by 2026.
- As of November 2020, 427 vaccines and therapies were undergoing evaluation for the prevention and treatment of COVID-19.
List of Terms
For the purpose of this report, the following terms and associated definitions apply.
- Clinical efficacy
- The maximum response achievable from a medicine in research settings and the capacity for sufficient therapeutic effect in clinical settings.Footnote i
- Gene therapy
- A technique for the treatment of genetic disease in which a gene that is absent or defective is replaced by a healthy gene, as defined by Health Canada.Footnote ii
- Market authorization
- The process of approval for a medicine to be marketed in a given country. In Canada, market approval is granted following a substantive scientific evaluation of a product's safety, efficacy, and quality, as required by the Food and Drugs Act and Regulations.Footnote iii
- Medicinal ingredient
- A chemical or biological substance responsible for the claimed pharmacologic effect of a drug product. Sometimes referred to as a molecule, active substance, or active ingredient.Footnote iv
- Medicine
- A broad term encompassing both the final drug product and medicinal ingredient(s); this encompasses chemically manufactured active substances and biologics, including gene therapies. Medicines are reported at the medicinal ingredient level and can refer to a single ingredient or a unique combination of ingredients.
- Medicine pipeline
- A set of new medicine candidates under active research and development by biotechnology and pharmaceutical companies.
- New medicine
- A medicinal ingredient that has not previously received market authorization by a regulator.Footnote iv
- Orphan medicine
- A medicine used to treat a rare disease. For the purposes of this study, orphan medicines are defined as having an orphan designation granted by the US Food and Drug Administration (FDA) or the European Medicines Agency (EMA) for the relevant indication.
- Phase I
- These trials test an experimental medicine on a small group of people for the first time. The purpose is to look at the medicine's safety, determine a safe dosage range, and monitor if there are any side effects.
- Phase II
- In this phase, the medicine is given to a larger group of people (usually 100 or more) to gather data on how well the medicine works to treat a disease or condition, check its safety on a wider range of people, and determine the best dose.Footnote v
- Phase III
- These controlled or uncontrolled trials are conducted after preliminary evidence suggesting efficacy of the medicine has been demonstrated. They are intended to gather additional and confirmatory information about the clinical efficacy and safety of the medicine under the proposed conditions of use.Footnote ii Phase III trials are usually randomized with double-blind testing in several hundred to several thousand patients.
- Pre-registration
- A medicine is in the pre-registration phase once all the necessary clinical trials have been completed and it is waiting for registration or approval for use by a governing body.Footnote vi
Phases of clinical trials
Introduction
This tenth edition of the Meds Pipeline Monitor (MPM) features a selection of medicines in Phase III clinical trials or pre-registration in 2020 that have the potential to significantly impact clinical practice and drug spending in Canada.
The methodology, which is detailed in the next section, uses a specific set of criteria to identify a list of pipeline candidates from the GlobalData Healthcare database, as well as a list of candidates currently under review in Canada from Health Canada’s Drug and Health Product Submissions Under Review (SUR) lists. Medicines reported in the previous edition are also reviewed for this edition, including those that continue to qualify for the list of candidates as well as those that have since received market authorization. Likewise, the new medicines featured in this report will be monitored in future editions of the MPM to identify candidates that successfully enter the market.
To provide context for the selection of medicines, the MPM includes a snapshot of the entire pipeline, with an emphasis on the therapeutic breakdown of each phase of clinical evaluation. This edition of the report also highlights select vaccines and other medicines undergoing evaluation for the treatment and prevention of COVID-19, in global markets as well as in Canada. The medicines assessed for this portion of the analysis include new therapies as well as previously marketed treatments that have been repurposed.
Meds Pipeline Monitor is a companion publication to Meds Entry Watch, which analyzes the market dynamics of newly approved medicines in Canada and internationally. Together, these two PMPRB reports monitor the market continuum of late-stage pipeline medicines and new approvals, providing decision makers, researchers, patients, clinicians, and other stakeholders with information on the emerging medicines and evolving cost pressures.
Methodology
Snapshot of the Pipeline
The snapshot of the pipeline captures the composition of medicines in various phases of clinical evaluation at a single point in time. For the purpose of this analysis, a full list of pipeline medicines was retrieved from GlobalData’s Healthcare database in July 2020.
New medicinal ingredients are identified as those with no prior approvals through the US Food and Administration (FDA), the European Medicines Agency (EMA), or Health Canada.
The distribution of new medicines by therapeutic area corresponds to the indication under evaluation, as reported by GlobalData. Note that a single new medicine may be undergoing multiple clinical studies for separate indications.
The list of medicines used for the analysis of orphan medicines in the pipeline was retrieved in November 2020. For the purposes of this analysis, orphan medicines were defined as new medicines that had been granted an orphan designation by the FDA or the EMA.
Meds Pipeline Monitor
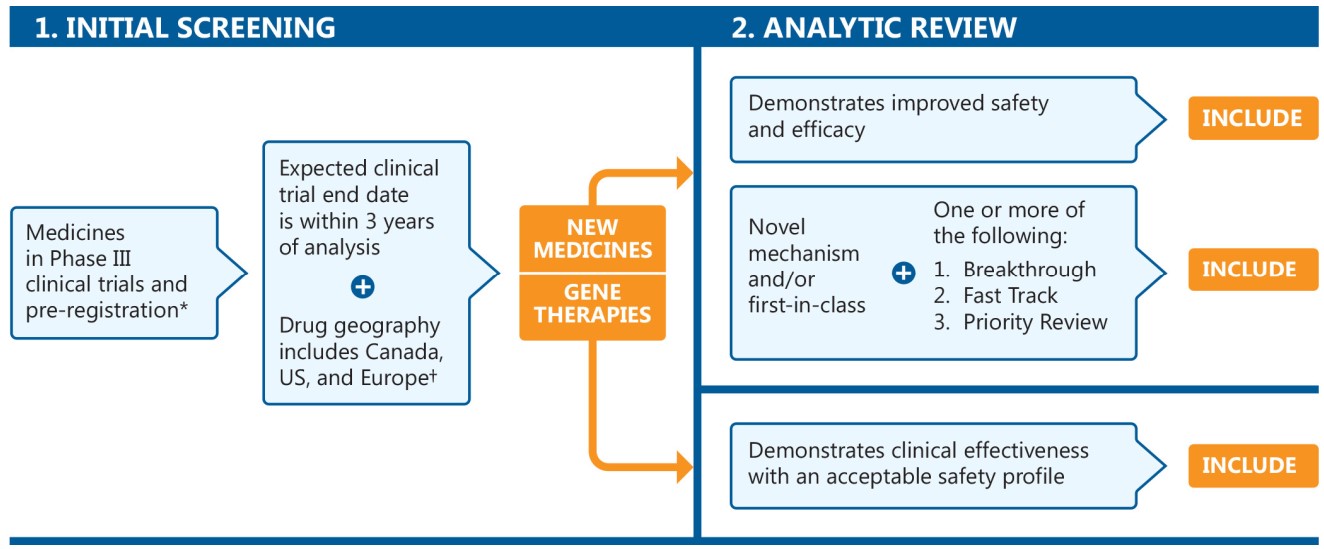
The MPM focuses on new medicines in Phase III clinical trials or pre-registration in Canada, the United States, and Europe. Pipeline medicines are selected for inclusion using a two-stage process (Figure 1). The initial screening stage selects medicines in the late phases of clinical evaluation, while the analytic review stage involves a more rigorous appraisal of each potential candidate to identify medicines that may have a significant clinical and budgetary impact. The second stage considers a specific set of criteria, in addition to the results of a thorough review of clinical evidence and scientific literature.
This methodology is reviewed annually and refined as required.
* In pre-registration with the US Food and Drug Administration (FDA).
† Has Phase III clinical trials in Canada, United States, or geographic Europe (excluding Russia and Turkey).
Figure description
This is a flowchart describing the process used to select the listed medicines. The chart consists of two steps:
1. Initial Screening
This step begins with all medicines in Phase III clinical trials or in pre-registration with the US Food and Drug Administration. Of these medicines, the next step includes only those with expected clinical trial end dates within three years of the analysis and drug geography including Canada, the US, and Europe. To qualify for the drug geography, a medicine must have Phase III clinical trials in Canada, the US, and/or geographic Europe (excluding Russia and Turkey).
2. Analytic Review
The analytical review step of the process is divided into two parts: one path for new medicines and the other for gene therapies.
New medicines must meet at least one of the following requirements to be included in the list:
Demonstrates improved safety and efficacy
Novel mechanism and/or first-in-class, with the addition of one or more of Breakthrough, Fast Track, and Priority Review designations
Gene therapies must demonstrate clinical effectiveness with an acceptable safety profile to be included in the list.
Stage 1. Initial screening
GlobalData’s Healthcare database is used to identify a list of medicines undergoing Phase III clinical trials or in pre-registration. These medicines serve as the basis for the initial screening stage.
The drug geography, defined as the geographical region or country in which the medicine is either marketed or in pipeline development, is restricted to Canada and other countries with similar regulatory and approval processes: the US and geographic Europe (excluding Russia and Turkey). Only new medicinal ingredients that have adequate data that supports increased efficacy and safety from clinical trials are considered as candidates for inclusion.
Medicines approved or sold in Canada, the US, or Europe for any other indication or in any other strength or formulation are excluded during the selection process, as are medicines whose clinical trials are inactive, suspended, withdrawn, or terminated.
The selection process groups pipeline candidates into two categories: (a) new medicines and (b) new gene therapies. As illustrated in Figure 1, the initial screening process for both groups is the same, but the analytic review stage is slightly different, as the available data for gene therapies is limited.
Stage 2: Analytic screening
Selection criteria
Following the initial screening, the second stage of the process considers a number of selection criteria to determine the final list of pipeline candidates. These criteria are detailed in Table 1.
Gene therapies are selected using a broader approach, as the clinical evidence available for this group is relatively limited. A gene therapy is retained on the list if the preliminary (or completed) results from Phase III trials suggest that there is evidence of clinical effectiveness with an acceptable safety profile.
Table 1. Selection criteria for the Meds Pipeline Monitor
| Selection criteria |
|---|
| Improved safety and efficacy shown in clinical trials: a medicine that demonstrates increased safety, new outcome measures, or increased life expectancy or quality of life |
| Novel mechanism / First-in-class: a medicine that uses a new mechanism of biochemical interaction to produce a medical effect, or a medicine that is the first in its therapeutic class In addition, the medicine must fall into one or more of the three following FDA designations for expedited development and review:
|
Additional descriptive information
A profile of each successful pipeline candidate is provided, including a brief outline of the indication and mechanism of action, as well as a summary of the applicable published outcomes from clinical trials. Specific attributes that may influence the potential uptake or cost of each medicine are also identified. Table 2 provides a detailed description of these key attributes.
Table 2. Key attributes of new medicines selected for the Meds Pipeline Monitor
| Attribute | Relevance | Data sources |
|---|---|---|
| Phase III clinical trials in Canada | Medicines tested in Canada are likely to be of interest to Canadians | GlobalData Healthcare; Health Canada Clinical Trials Database; Health Canada Drug and Health Product Submissions Under Review; National Institutes of Health (NIH) Clinical Trial Registry |
| Rare or orphan designation | Medicines used to treat rare diseases or conditions that generally have high treatment costs and may result in substantial spending | GlobalData Healthcare |
| Biologic medicine | These complex molecules produced by living organisms are expected to have high costs, resulting in substantial spending | |
| Add-on therapy | Medicines designed to be used in conjunction with existing medicines may increase the treatment cost and contribute to higher spending |
The profile also provides details of potential cost implications, if available, which includes the forecasted global revenues reported by GlobalData.
The indications and therapeutic areas of the featured medicines correspond to their Phase III clinical trial or pre-registration stage. A single clinical trial may assess multiple indications within the same therapeutic area. These medicines may also have additional indications at various phases of clinical evaluation that are not mentioned in this report. The scientific description and Key Attributes provided are focused on the specified indication(s) for the selected medicines.
Spotlight on Canada
Health Canada’s Drug and Health Product Submissions Under Review (SUR) are assessed using a modified approach to the selection criteria to establish a list of medicines that may have the potential to significantly affect Canadian drug spending.
Medicines listed in the SUR include new drug submissions containing medicinal ingredients that have not been approved in Canada for any indication, in any strength or form. Unlike the selection of medicines identified in the pipeline lists, these medicines may have previously received market authorization through the FDA or the EMA.
Selection Criteria
Following this initial screening, the medicine must demonstrate at least one of three selection criteria to qualify for inclusion in the report. These criteria are listed in Table 3.
Table 3. Selection criteria for the list of medicines currently under review by Health Canada
| Selection Criteria |
|---|
| Improved safety and efficacy shown in clinical trials: a medicine that demonstrates increased safety, new outcome measures, or increased life expectancy or quality of life |
| Novel mechanism / First-in-class: a medicine that uses a new mechanism of biochemical interaction to produce a medical effect, or a medicine that is the first in its therapeutic class |
| Gene therapy: a technique for the treatment of genetic disease in which a gene that is absent or defective is replaced by a healthy gene |
Additional descriptive information
As in the pipeline lists, the profile of each medicine under review includes the key attributes listed in Table 2, as well as a brief outline of the indication and mechanism of action, and a summary of the applicable published outcomes from clinical trials. Specific attributes that may influence the potential uptake or cost of each medicine are also identified, as well as potential cost implications, if available, which includes the forecasted global revenues reported by GlobalData.
Although FDA designations for expedited development or review are not a selection criteria for this list, relevant Breakthrough, Fast Track, and Priority Review designations are indicated where available. For a description of these designations, see Table 1.
Indications and therapeutic areas correspond to the information provided by GlobalData. The scientific description and Key Attributes provided are focused on the specified indication(s) for the selected medicine. For medicines under review for multiple indications, the primary indication is used.
Emerging COVID-19 Therapies
Vaccines and medicines under development worldwide with an indication for COVID-19 were extracted for this section of the report, based on a development stage of Phase I, II, and III clinical trials or pre-registration. All such medicines were assessed for this analysis, both new and existing. New medicines were identified as those that have not yet been marketed for any indication, while existing medicines include previously marketed therapies undergoing evaluation for new indications related to the treatment of COVID-19.
In light of the rapid development of the medicines in this category of the pipeline, the data for this analysis was extracted in November 2020.
Data Sources
The GlobalData Healthcare database is the primary data source for the identification of pipeline medicines and their corresponding clinical information, including the clinical trial end date. GlobalData Healthcare tracks medicines from pre-clinical discovery, through clinical trials, to market launch and subsequent sales. The database is a comprehensive resource of medicines under various stages of clinical development. Search capabilities allow for controlled selection of specific attributes, including but not limited to the following: phase of clinical development, therapeutic area, molecule type, indication, drug geography, mechanism of action, and regulatory designations.
The Health Canada Drug and Health Product Submissions Under Review (SUR) lists are used to determine the featured selection of new medicines currently undergoing review by Health Canada. The SUR is a publicly available set of lists that identify pharmaceutical and biologic drug submissions containing new medicinal ingredients not previously approved in Canada that have been accepted for review. This applies to submissions accepted on or after April 1, 2015.
As this selection is restricted to new medicines, additional sources of information are cross-referenced to confirm that the candidates have not previously been approved or sold. These include recorded sales data from the IQVIA MIDAS® Database (all rights reserved); regulatory approval records from the National Institutes of Health (NIH), US FDA, the EMA, and Health Canada; and information in Health Canada’s Clinical Trials database and ClinicalTrials.org.
Limitations
This analysis captures a snapshot of the pipeline over a specific time period. Although it is assumed to be representative of the composition of medicines over the entire year, the pipeline is fairly dynamic and the share of medicines in any particular therapeutic area will vary.
This assessment is restricted to medicines under development for market in Canada and other countries with similar regulatory and approval processes: the US and Europe (excluding Russia and Turkey). Medicines that have not yet received market authorization in these countries were considered as potential pipeline candidates, even if they have been approved elsewhere in the world.
Some of the selected medicines may be undergoing clinical trials for additional indications; this analysis only reports on indications in the late stages of development, that is, in Phase III clinical trials or pre-registration with the US FDA, that satisfy the selection criteria set out in the methodology.
For each selected pipeline medicine, the primary manufacturer(s) and trade name, if available, are given along with the indication. In some cases, additional manufacturers, including subsidiaries, may also be involved in the development of the medicine with the primary companies, or other manufacturers may be developing the same medicine for other indications.
Although this report attempts to identify the most important pipeline medicines, the selection is not exhaustive and some medicines that are not included in this selection may have a significant impact on future clinical practice and drug spending in Canada.
Unless otherwise specified, the featured lists capture the composition of the pipeline as of July 2020 and are validated as of the end of November 2020. Due to the unpredictability and fast-moving nature of pipeline medicines entering the market, some of the medicines listed in this edition may have been approved or marketed in Canada, US, or Europe following this date. Pipeline medicines that have not been included in this report due to the timing of the selection may presently meet the selection criteria and these, along with the rest of the drug pipeline will be considered for the next edition of the report.
Snapshot of the 2020 Pipeline
Pharmaceutical innovation is transforming the development and application of medical treatments worldwide. Nearly 7,000 new medicines were in clinical evaluation or in pre-registration in 2020.
Figure 2 provides a snapshot of the pipeline in 2020, including the number of new medicinal ingredients in each phase of clinical evaluation. Of the 6,946 new medicines, 935 (13%) were in Phase III clinical trials or in pre-registration.
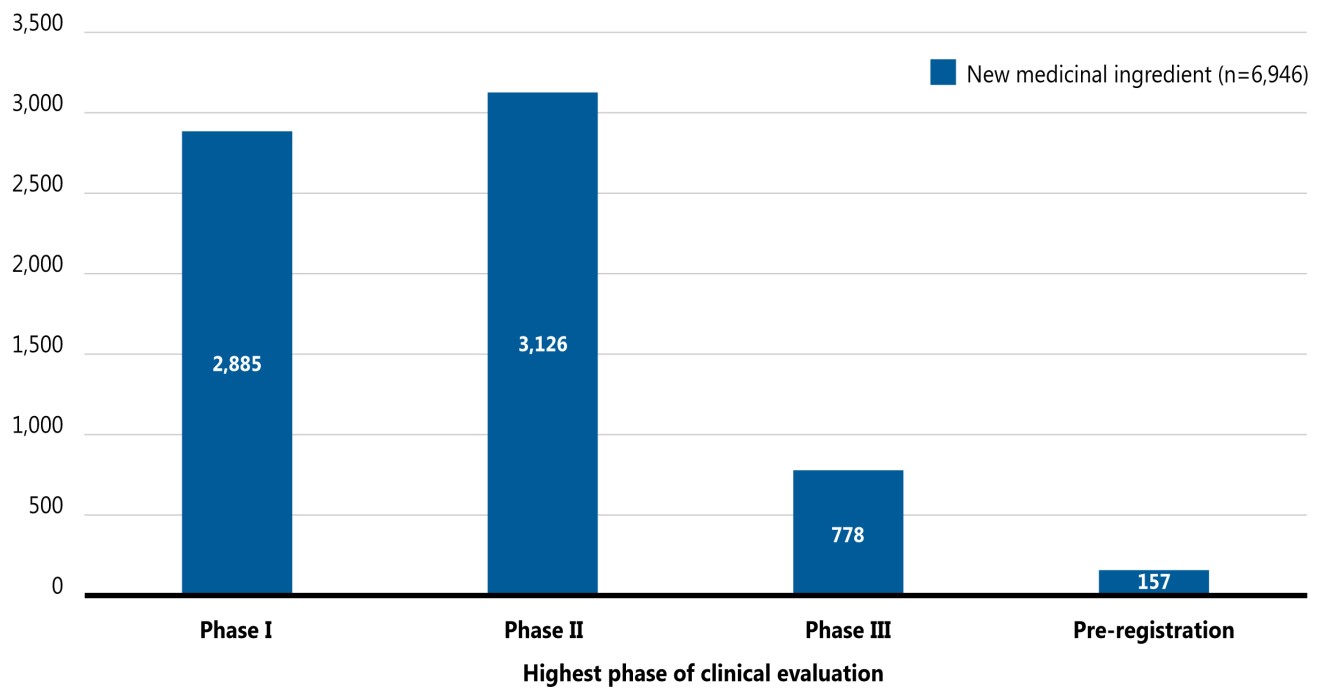
Figure description
This bar graph illustrates the number of new medicinal ingredients in the pipeline by their highest phase of clinical evaluation in July 2020.
There were 6,946 new medicines across the pipeline, of which 2,885 were in Phase I clinical trials, 3,126 were in Phase II, 778 were in Phase III, and 157 were in pre-registration.
Figure 3 illustrates the distribution of new medicines by therapeutic area from Phase I through pre-registration. Although the findings show that pipeline medicines represented a wide range of therapeutic areas in 2020, cancer treatments dominated the therapeutic mix across the pipeline, accounting for over one third (35%) of medicines in all phases of clinical evaluation. Other important pipeline therapies include those for infectious diseases (such as COVID-19) and central nervous system therapies.
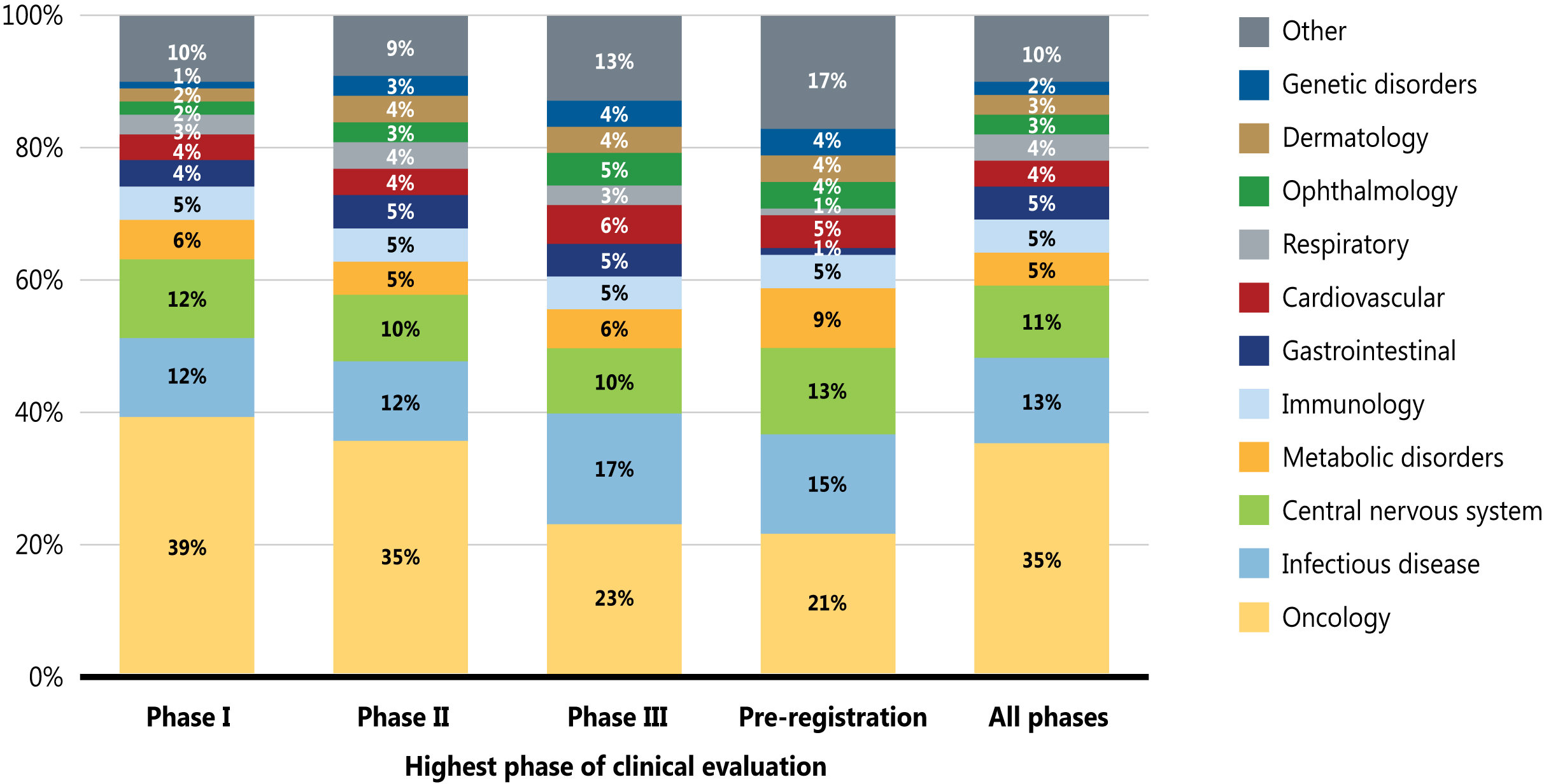
Figure description
A stacked bar graph shows the distribution of new medicines in the pipeline by their therapeutic area. The distribution is given as a percentage of all new medicines in each phase of development, as well as a total share for all phases.| Therapeutic area | Phase I | Phase II | Phase III | Pre-registration | All phases |
|---|---|---|---|---|---|
| Oncology | 39% | 35% | 23% | 21% | 35% |
| Infectious Disease | 12% | 12% | 17% | 15% | 13% |
| Central Nervous System | 12% | 10% | 10% | 13% | 11% |
| Metabolic Disorders | 6% | 5% | 6% | 9% | 5% |
| Immunology | 5% | 5% | 5% | 5% | 5% |
| Gastrointestinal | 4% | 5% | 5% | 1% | 5% |
| Cardiovascular | 4% | 4% | 6% | 5% | 4% |
| Respiratory | 3% | 4% | 3% | 1% | 4% |
| Ophthalmology | 2% | 3% | 5% | 4% | 3% |
| Dermatology | 2% | 4% | 4% | 4% | 3% |
| Genetic Disorders | 1% | 3% | 4% | 4% | 2% |
| Other | 10% | 9% | 13% | 17% | 10% |
Orphan medicines, as designated by the US Food and Drug Administration (FDA) or the European Medicines Agency (EMA), accounted for a notable proportion of the total medicine pipeline in 2020. Figure 4 provides the shares of orphan and other medicines in the pipeline from Phase I to pre-registration. Orphan medicines made up a greater share of medicines in the latter stages of clinical evaluation, accounting for 8% of pipeline medicines in Phase I clinical trials and 40% of those in pre-registration. A distribution of orphan medicines by highest phase of clinical evaluation indicates that, compared to non-orphan medicines, rare disease treatments are more highly concentrated in the advanced phases of evaluation.
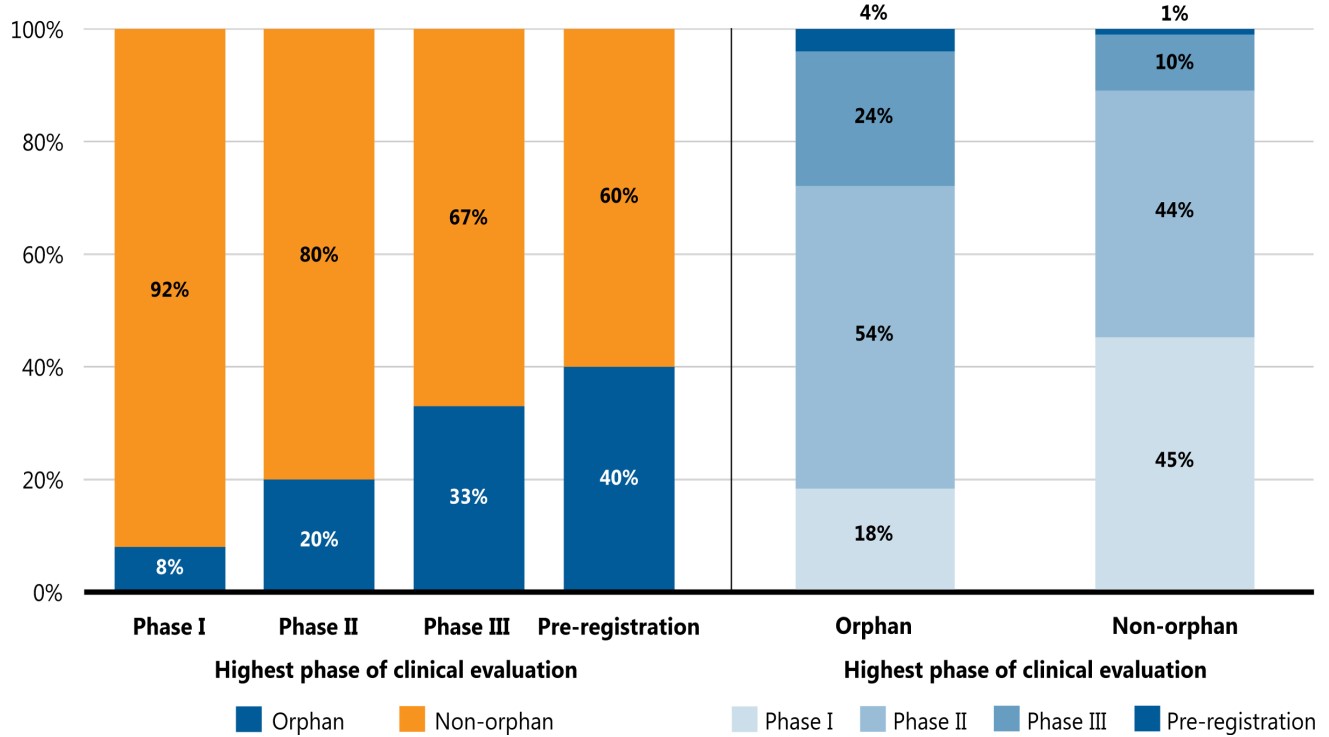
Figure description
Two stacked bar graphs give the distribution of orphan and non-orphan medicines in the pipeline. The first graph shows the share of orphan medicines in each phase of development (Phase I, Phase II, Phase III, and pre-registration) as of November 2020, while the second examines the distribution all orphan pipeline medicines by their highest phase of evaluation, as compared to the distribution of non-orphan pipeline medicines. Orphan medicines were defined as pipeline medicines that have been granted an orphan designation by the US Food and Drug Administration (FDA) or the European Medicines Agency (EMA).
a) Share of orphan versus non-orphan medicines in each phase of clinical evaluation
| Phase I | Phase II | Phase III | Pre-registration | |
|---|---|---|---|---|
| Orphan | 8% | 20% | 33% | 40% |
| Non-orphan | 92% | 80% | 67% | 60% |
b) Distribution of orphan and non-orphan medicines in the pipeline by phase of clinical evaluation
| Orphan | Non-orphan | |
| Phase I | 18% | 45% |
| Phase II | 54% | 44% |
| Phase III | 24% | 10% |
| Pre-registration | 4% | 1% |
Note: Includes all pipeline medicines with a highest development stage of Phase I to pre-registration that are being developed for market in Canada, the United States, or geographic Europe (excluding Russia and Turkey). Orphan medicines were defined as pipeline medicines that have been granted an orphan designation by the US Food and Drug Administration (FDA) or the European Medicines Agency (EMA).
Data source: GlobalData Healthcare database (accessed November 2020).
Meds Pipeline Monitor 2020
The following tables list the selection of new pipeline medicines in 2020, those retained from earlier editions of the Meds Pipeline Monitor, as well as medicines featured in previous editions that have since gained market authorization. These pipeline medicines will continue to be monitored in future editions of this report.
Applying the screening criteria described in the Methodology section, 16 of the 935 pipeline medicines in late stages of clinical evaluation, including 5 gene therapies, were selected for inclusion in the 2020 new medicines list ("Selected new medicines for 2020"). Likewise, 12 late-stage medicines were retained from the 2019 list as they continued to satisfy the same criteria ("Pipeline medicines retained from the 2019 Meds Pipeline Monitor").
Three new medicines featured in the 2019 edition of the MPM had received market authorization in the US, Europe, or Canada as of November 2020. These medicines are listed under "Pipeline medicines from the 2019 Meds Pipeline Monitor that have gained market authorization".
Selected new medicines for 2020
| Medicine (Trade name) Company | Indication(s) | Description and Key Attributes |
|---|---|---|
Inclisiran
|
Atherosclerosis |
|
* Consensus forecasts for global revenue data were collected from GlobalData, Q4-2020, and are given in US dollars.
Data source: GlobalData Healthcare database.
| Medicine (Trade name) Company | Indication(s) | Description and Key Attributes |
|---|---|---|
| Aducanumab Biogen Inc.
|
Alzheimer’s disease |
|
* Consensus forecasts for global revenue data were collected from GlobalData, Q4-2020, and are given in US dollars.
Data source: GlobalData Healthcare database.
| Medicine (Trade name) Company | Indication(s) | Description and Key Attributes |
|---|---|---|
| Pegunigalsidase alfa Chiesi Farmaceutici SpA
|
Fabry disease (FD) |
|
* Consensus forecasts for global revenue data were collected from GlobalData, Q4-2020, and are given in US dollars.
Data source: GlobalData Healthcare database.
| Medicine (Trade name) Company | Indication(s) | Description and Key Attributes |
|---|---|---|
| Etranacogene dezaparvovec UniQure NV
|
Hemophilia B (factor IX deficiency) |
|
| Fidanacogene elaparvovec (previously SPK-9001) Pfizer Inc
|
Hemophilia B (factor IX deficiency) |
|
* Consensus forecasts for global revenue data were collected from GlobalData, Q4-2020, and are given in US dollars.
Data source: GlobalData Healthcare database.
| Medicine (Trade name) Company | Indication(s) | Description and Key Attributes |
|---|---|---|
| Anifrolumab AstraZeneca Plc
|
Systemic lupus erythematosus |
|
| Mirikizumab Eli Lilly and Co.
|
Plaque psoriasis (psoriasis vulgaris) |
|
* Consensus forecasts for global revenue data were collected from GlobalData, Q4-2020, and are given in US dollars.
Data source: GlobalData Healthcare database.
| Medicine (Trade name) Company | Indication(s) | Description and Key Attributes |
|---|---|---|
| Ibrexafungerp Scynexis Inc.
|
Aspergillosis; Blastomycosis; Candidiasis; Coccidioidomycosis; Histoplasmosis; Systemic candidiasis; Vulvovaginal candidiasis |
|
* Consensus forecasts for global revenue data were collected from GlobalData, Q4-2020, and are given in US dollars.
Data source: GlobalData Healthcare database.
| Medicine (Trade name) Company | Indication(s) | Description and Key Attributes |
|---|---|---|
| Dasiglucagon Zealand Pharma AS
|
Hypoglycemia |
|
| Teplizumab Provention Bio Inc.
|
Type 1 diabetes (juvenile diabetes) |
|
* Consensus forecasts for global revenue data were collected from GlobalData, Q4-2020, and are given in US dollars.
Data source: GlobalData Healthcare database.
| Medicine (Trade name) Company | Indication(s) | Description and Key Attributes |
|---|---|---|
| Idecabtagene vicleucel (bb2121) Bristol-Myers Squibb Co.
|
Refractory multiple myeloma; Relapsed multiple myeloma |
|
| Lisocabtagene maraleucel Juno Therapeutics Inc.
|
Diffuse large B-cell lymphoma; Follicular lymphoma; Non-Hodgkin lymphoma; Primary CNS lymphoma; Primary mediastinal B-cell lymphoma |
|
| Ofranergene obadenovec Vascular Biogenics Ltd
|
Epithelial ovarian cancer |
|
| Pevonedistat Millennium Pharmaceuticals Inc.; Takeda
|
Myelodysplastic syndromes (MDS) |
|
| Umbralisib tosylate TG Therapeutics Inc
|
Extranodal marginal zone B-cell lymphoma (mucosa-associated lymphoid tissue or MALT-lymphoma); Follicular lymphoma (FL); Nodal marginal zone B-cell lymphoma; Splenic marginal zone B-cell lymphoma |
|
* Consensus forecasts for global revenue data were collected from GlobalData, Q4-2020, and are given in US dollars.
Data source: GlobalData Healthcare database.
| Medicine (Trade name) Company | Indication(s) | Description and Key Attributes |
|---|---|---|
| Tezepelumab Amgen Inc.
|
Asthma |
|
* Consensus forecasts for global revenue data were collected from GlobalData, Q4-2020, and are given in US dollars.
Data source: GlobalData Healthcare database.
Pipeline medicines retained from the 2019 Meds Pipeline Monitor
| Medicine (Trade name) Company |
Indication(s) | Description and Key Attributes |
|---|---|---|
| Gantenerumab Hoffmann-La Roche Ltd
|
Alzheimer’s disease |
|
* Consensus forecasts for global revenue data were collected from GlobalData, Q4-2020, and are given in US dollars.
Data source: GlobalData Healthcare database. The database search for new medicines added to the MPM was performed in July 2020.
| Medicine (Trade name) Company |
Indication(s) | Description and Key Attributes |
|---|---|---|
| Cenicriviroc Allergan PLC
|
Liver fibrosis; Non-alcoholic steatohepatitis (NASH) |
|
* Consensus forecasts for global revenue data were collected from GlobalData, Q4-2020, and are given in US dollars.
Data source: GlobalData Healthcare database. The database search for new medicines added to the MPM was performed in July 2020.
| Medicine (Trade name) Company |
Indication(s) | Description and Key Attributes |
|---|---|---|
Elivaldogene tavalentivec (Lenti-D)
bluebird bio, Inc.
|
Adrenoleuko-dystrophy (ADL) |
|
* Consensus forecasts for global revenue data were collected from GlobalData, Q4-2020, and are given in US dollars.
Data source: GlobalData Healthcare database. The database search for new medicines added to the MPM was performed in July 2020.
| Medicine (Trade name) Company |
Indication(s) | Description and Key Attributes |
|---|---|---|
| Fitusiran Alnylam Pharmaceuticals Inc.; Genzyme, a Sanofi Co.
|
Hemophilia A; Hemophilia B |
|
| Vadadustat Otsuka Holdings Co., Ltd; Akebia Therapeutics, Inc.
|
Anemia in chronic kidney disease (CKD; renal anemia) |
|
* Consensus forecasts for global revenue data were collected from GlobalData, Q4-2020, and are given in US dollars.
Data source: GlobalData Healthcare database. The database search for new medicines added to the MPM was performed in July 2020.
| Medicine (Trade name) Company |
Indication(s) | Description and Key Attributes |
|---|---|---|
| Ipatasertib Genentech, Inc.
|
Metastatic hormone refractory (castration-resistant, androgen-independent) prostate cancer |
|
| Melphalan flufenamide hydrochloride (Melflufen, Ygalo) Oncopeptides AB
|
Refractory multiple myeloma; Relapsed multiple myeloma (MM) |
|
| Tavokinogene telseplasmid (ImmunoPulse, Tavo) OncoSec Medical Inc.
|
Metastatic melanoma |
|
| Ublituximab (Utuxin) TG Therapeutics, Inc.; LFB S.A.
|
Refractory chronic lymphocytic leukemia (CLL); Relapsed chronic lymphocytic leukemia (CLL); Relapsing multiple sclerosis (RMS) |
|
* Consensus forecasts for global revenue data were collected from GlobalData, Q4-2020, and are given in US dollars.
Data source: GlobalData Healthcare database. The database search for new medicines added to the MPM was performed in July 2020.
| Medicine (Trade name) Company |
Indication(s) | Description and Key Attributes |
|---|---|---|
| Timrepigene emparvovec [AAV2-REP1, NSR-REP-1] Biogen Inc.
|
Choroideremia |
|
| Zuretinol acetate Retinagenix LLC
|
Leber congenital amaurosis (LCA); Retinitis |
|
* Consensus forecasts for global revenue data were collected from GlobalData, Q4-2020, and are given in US dollars.
Data source: GlobalData Healthcare database. The database search for new medicines added to the MPM was performed in July 2020.
Pipeline medicines from the 2019 Meds Pipeline Monitor that have gained market authorization
| Medicine (Trade name) Company |
Indication(s) | Description and Key Attributes |
|---|---|---|
| Bempedoic acid (Nexletol) Esperion Therapeutics Inc.
|
Hyperlipidemia atherosclerosis |
|
* Consensus forecasts for global revenue data were collected from GlobalData, Q4-2020, and are given in US dollars.
Data source: GlobalData Healthcare database.
| Medicine (Trade name) Company |
Indication(s) | Description and Key Attributes |
|---|---|---|
| Setmelanotide (Imcifree) Rhythm Pharmaceuticals, Inc.
|
Genetic disorders; Obesity |
|
* Consensus forecasts for global revenue data were collected from GlobalData, Q4-2020, and are given in US dollars.
Data source: GlobalData Healthcare database.
| Medicine (Trade name) Company |
Indication(s) | Description and Key Attributes |
|---|---|---|
| Imlifidase (Idefirix) Hansa Biopharma AB
|
Kidney transplant rejection |
|
* Consensus forecasts for global revenue data were collected from GlobalData, Q4-2020, and are given in US dollars.
Data source: GlobalData Healthcare database.
| Medicine (Trade name) Company |
Indication(s) | Description and Key Attributes |
|---|---|---|
| Cabotegravir (Cabenuva, Vocabria) ViiV Healthcare UK Ltd
|
HIV infections (AIDS) |
|
| Fostemsavir tromethamine (Rukobia [US]) ViiV Healthcare UK Ltd; Bristol-Myers Squibb Co.; GlaxoSmithKline PLC
|
HIV infections (AIDS) |
|
* Consensus forecasts for global revenue data were collected from GlobalData, Q4-2020, and are given in US dollars.
Data source: GlobalData Healthcare database.
Spotlight on Canada
This section includes a list of select medicines currently under review by Health Canada that may have a significant impact on future clinical practice and drug spending. Medicines included on this list are new to Canada but may have been approved in other jurisdictions.
“Selected new medicines currently under review by Health Canada, 2020” highlights six new medicines currently on Health Canada’s Drug and Health Product Submissions Under Review (SUR) list that have a novel mechanism of action or demonstrated improved safety and efficacy in clinical trials. Of the nine medicines reported in the 2019 edition, seven have since received market authorization from Health Canada while two were retained for the current list.
The SUR is a publicly available source that identifies pharmaceutical and biologic drug submissions with new medicinal ingredients that have been accepted for review in Canada.
Selected new medicines currently under review by Health Canada, 2020
| Medicine (Trade name) Company |
Indication(s) | Description and Key Attributes |
|---|---|---|
| Inclisiran Novartis AG
|
Atherosclerosis |
|
* Consensus forecasts for global revenue data were collected from GlobalData, Q4-2020, and are given in US dollars.
Data source: GlobalData Healthcare database.
| Medicine (Trade name) Company |
Indication(s) | Description and Key Attributes |
|---|---|---|
Roxadustat (Ai Ruizhuo)
AstraZeneca Canada Inc.
|
Anemia in chronic kidney disease (CKD) |
|
* Consensus forecasts for global revenue data were collected from GlobalData, Q4-2020, and are given in US dollars.
Data source: GlobalData Healthcare database.
| Medicine (Trade name) Company |
Indication(s) | Description and Key Attributes |
|---|---|---|
| Tildrakizumab (Ilumetri) Sun Pharma Global FZE
|
Plaque psoriasis |
|
* Consensus forecasts for global revenue data were collected from GlobalData, Q4-2020, and are given in US dollars.
Data source: GlobalData Healthcare database.
| Medicine (Trade name) Company |
Indication(s) | Description and Key Attributes |
|---|---|---|
| Binimetinib (Mektovi); Encorafenib (Braftovi) Pfizer Canada ULC
|
For unresectable or metastatic melanoma |
|
| Idecabtagene vicleucel (bb2121) Bristol-Myers Squibb Co.
|
Refractory multiple myeloma; Relapsed multiple myeloma |
|
* Consensus forecasts for global revenue data were collected from GlobalData, Q4-2020, and are given in US dollars.
Data source: GlobalData Healthcare database.
| Medicine (Trade name) Company |
Indication(s) | Description and Key Attributes |
|---|---|---|
| Ospemifene (Osphena, Senshio) Duchesnay Inc.
|
Dyspareunia |
|
* Consensus forecasts for global revenue data were collected from GlobalData, Q4-2020, and are given in US dollars.
Data source: GlobalData Healthcare database.
EMERGING COVID-19 THERAPIES
This new section of the Meds Pipeline Monitor includes an overview of new and existing pipeline medicines that are under evaluation for indications related to the prevention and treatment of COVID-19. An analysis of global markets provides information on COVID-19 medicines in all phases of clinical trials and pre-registration. In addition, a review of processes for the expedited development of medicines for COVID-19 in Canada demonstrates the potential impact of this fast-moving pipeline on the Canadian market.
Global markets
There is significant uncertainty in the COVID-19 drug pipeline worldwide. Published information to confirm safety and efficacy of the various treatments for COVID-19 is limited, and the available information is continuously evolving.146
In addition to the wide variety of vaccines under development, many novel and repurposed medicines are currently being evaluated in clinical trials for their potential benefits in the treatment of COVID-19. These include antivirals, interferons, antimalarials, antiparasitics, biologics (monoclonal antibodies and interleukin receptor antagonists), cellular therapies (mesenchymal stem cells and natural killer cells), convalescent plasma, and cytokine adsorbers.147
A breakdown of COVID-19 pipeline vaccines and treatments by phase of clinical evaluation is given in Figure 5. For this snapshot, data was extracted for medicines indicated for the treatment of COVID-19 with a development stage of Phase I, II, III, or pre-registration. These medicines are presented in three categories: vaccines, which are used to prevent infection of the novel coronavirus; COVID-19 treatments (new), which are new medicines used for the prevention or reduction of some of the complications associated with COVID-19 (e.g., pneumonia or respiratory complications, cytokine storm, and hyperinflammation); and COVID-19 treatments (existing), which are previously marketed medicines that have been repurposed to treat COVID-19 or its symptoms.
Brief Insights
The pipeline for COVID-19 medicines is growing rapidly, with clinical investigations of novel and existing drugs:
- Gilead's remdesivir received FDA approval in October 2020, and is now approved in the EU, Japan, India, Singapore, Australia, and South Korea for treatment of hospitalized patients.
- The corticosteroid dexamethasone has been approved in the UK, Japan, and Taiwan.
- The FDA granted an emergency use authorization (EUA) for convalescent plasma to treat hospitalized COVID-19 patients.
- The FDA granted an EUA for Eli Lilly's monoclonal antibody bamlanivimab (LY-CoV555) to treat mild to moderate COVID-19. Health Canada has also granted authorization (with conditions).
- The FDA issued an EUA for Eli Lilly’s Olumiant (baricitinib) in combination with remdesivir for the treatment of hospitalized COVID-19 patients requiring supplemental oxygen.
- The FDA also granted an EUA for Regeneron's REGN-COV2 antibody for treatment of mild to moderate COVID-19 for patients with positive SARS-CoV-2 viral testing who are at high risk for progressing to severe COVID-19 and/or hospitalization.
Source: Pharma COVID-19 Bulletin, GlobalData (November 30, 2020); Health Canada (November 2020).
Figure 6 breaks down the COVID-19 candidate vaccines by category and highest development phase.Footnote vii Vaccines fall into various categories based on their mechanism of action; for example, while live attenuated vaccines target the whole virus, subunit and recombinant vaccines target one specific part of the virus.
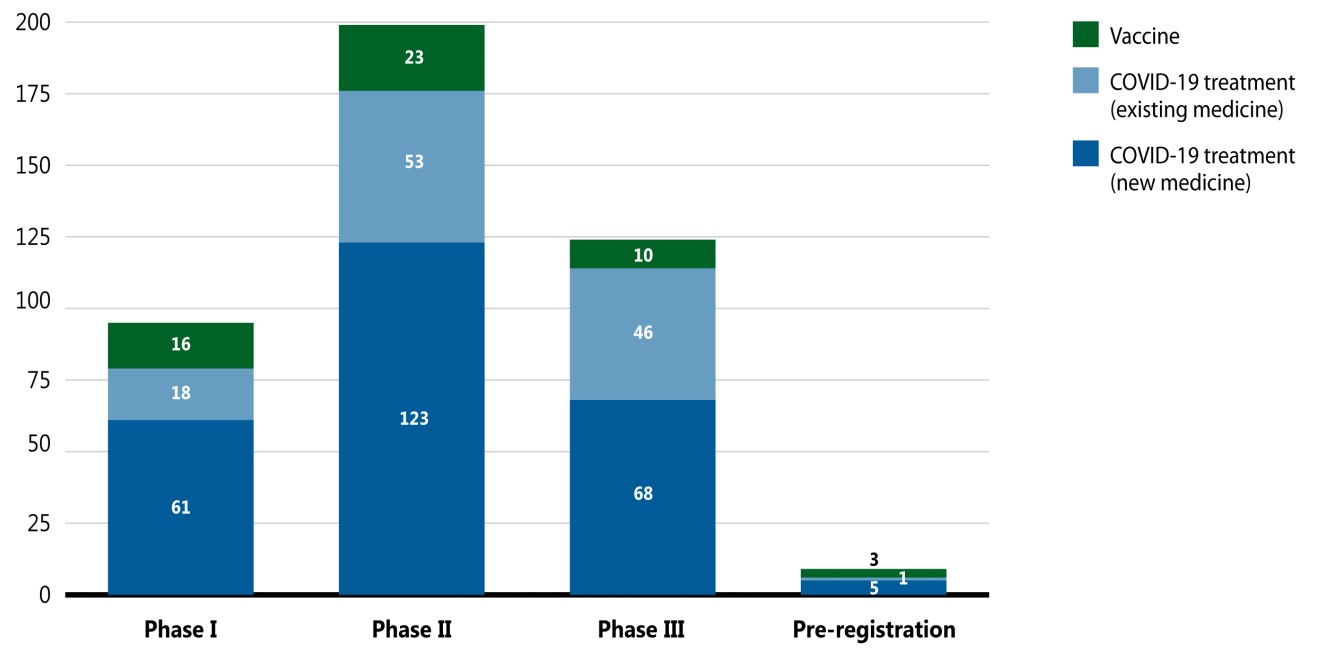
Figure description
This stacked bar graph illustrates the number of COVID-19 treatments and vaccine in the pipeline by their highest phase of clinical evaluation in November 2020. Totals are given for vaccines and COVID-19 treatments, with separate totals for new and existing medicines.
| Phase I | Phase II | Phase III | Pre-registration | |
|---|---|---|---|---|
| Vaccine | 16 | 23 | 10 | 3 |
| COVID-19 treatment, existing medicine | 18 | 53 | 46 | 1 |
| COVID-19 treatment, new medicine | 61 | 123 | 68 | 5 |
| Total | 95 | 199 | 124 | 9 |
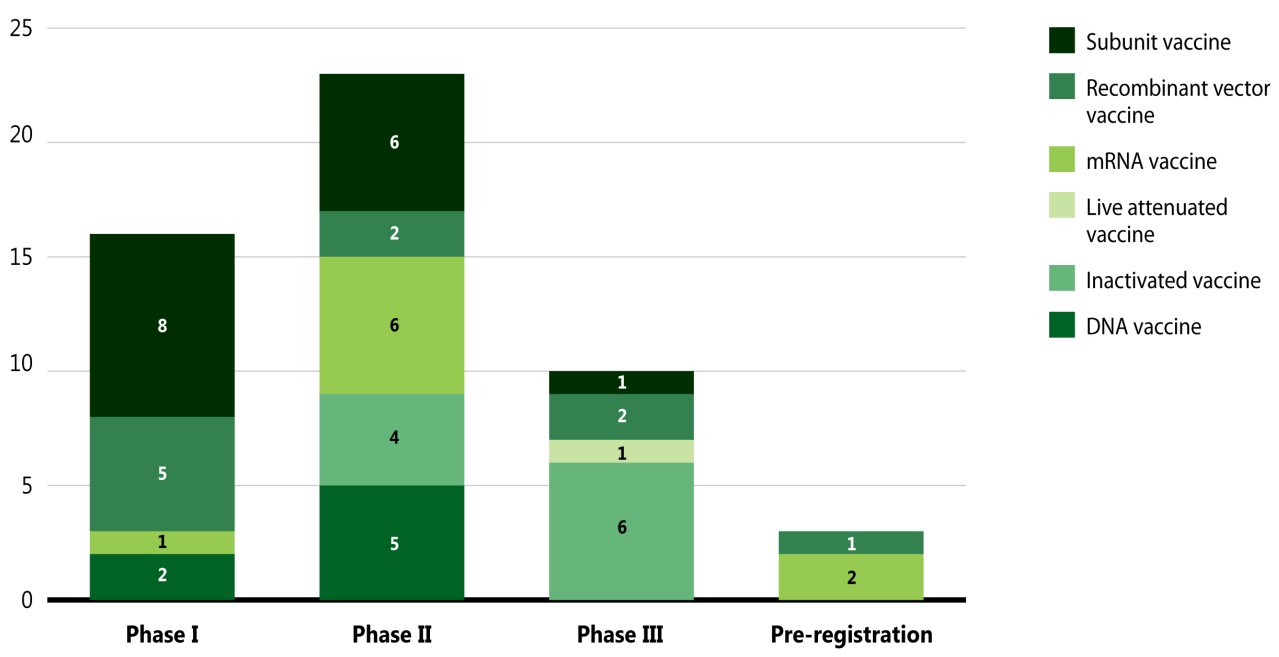
Figure description
A stacked bar graph gives the distribution of coronavirus vaccines in each phase of clinical evaluation by vaccine type, as of November 2020.
| DNA Vaccine | Inactivated Vaccine | Live Attenuated Vaccine | mRNA Vaccine | Recombinant Vector Vaccine | Subunit Vaccine | Grand Total | |
|---|---|---|---|---|---|---|---|
| Phase I | 2 | - | - | 1 | 5 | 8 | 16 |
| Phase II | 5 | 4 | - | 6 | 2 | 6 | 23 |
| Phase III | - | 6 | 1 | - | 2 | 1 | 10 |
| Pre-Registration | - | - | - | 2 | 1 | 1 | 3 |
| Grand Total | 7 | 10 | 1 | 9 | 10 | 12 | 52 |
Canada
Canada has issued specific processes and guidance to expedite access to medicines during the COVID-19 pandemic. On August 25, 2020, the Public Health Agency of Canada released the federal/provincial/territorial public health response plan for ongoing management of COVID-19 to help guide decision-making in order to respond to future waves of the pandemic.148 As of November 2020, five interim orders have been issued by the Minister of Health in response to COVID-19.149, 150, 151, 152, 153 For example, the Interim Order Respecting the Importation, Sale and Advertising of Drugs for Use in Relation to COVID-19,154 signed by the Minister of Health on September 16, 2020, allows for the issuance of an expedited authorization for the importation, sale, and advertising of drugs used in relation to COVID-19, and the Interim Order Respecting Clinical Trials for Medical Devices and Drugs Relating to COVID-19, approved on May 23, 2020, introduced an alternate pathway to facilitate clinical trials for potential COVID-19 drugs and medical devices, while upholding strong patient safety requirements and validity of trial data.155
As of November 2020, Health Canada had received five applications for drug and vaccine authorizations under the interim order (Table 8). Two of the five medicines—remdesivir and bamlanivimab (LY-CoV555)—had received conditional authorizations, while the remaining three applications were pending. In addition, 71 clinical trials for COVID-19 medicines and vaccines had been authorized by Health Canada at the time of the analysis,156 with GlobalData reporting more than 90 planned and ongoing clinical trials in Canada.
Canada has joined the COVAX facility, a global initiative organized by the WHO, Gavi, the Vaccine Alliance, and the Coalition for Epidemic Preparedness Innovations (CEPI), formed to accelerate the development and manufacture of COVID-19 vaccines, as well as diagnostics and treatments, and to guarantee rapid, fair, and equitable access for people in all countries.157
Table 8. COVID-19 treatment and vaccine applications received by Health Canada, 2020
| Applicant | Medicinal ingredient(s) | Therapeutic area | Date application was received | Outcome of application | Date of decision/outcome |
|---|---|---|---|---|---|
| Eli Lilly Canada, Inc. | Bamlanivimab (LY-CoV555) | Immune sera and immunoglobulins, for human use | 2020-10-12 | Authorized (with terms and conditions) | 2020-11-20 |
| Moderna Therapeutics, Inc. | mRNA-1273 vaccine | Vaccines, for human use | 2020-10-12 | Pending | N/A |
| Pfizer Canada ULC/BioNTech SE | mRNA vaccine BNT162b2 | Vaccines, for human use | 2020-10-09 | Pending | N/A |
| AstraZeneca Canada, Inc. | Adenovirus vaccine vector (ChAdOx1) | Vaccines, for human use | 2020-10-01 | Pending | N/A |
| Gilead Sciences Canada, Inc. | Remdesivir | Antivirals for systemic use, for human use | 2020-06-19 | Authorized (with conditions) | 2020-07-27 |
Data source: Heath Canada, Drug and vaccine authorizations for COVID-19: List of applications received (accessed November 2020): https://www.canada.ca/en/health-canada/services/drugs-health-products/covid19-industry/drugs-vaccines-treatments/authorization/applications.html
References
- Klinovski M, Boucher M, Perras C, Grobelna A. 2019. Inclisiran: A Small Interfering RNA Molecule for Treating Hypercholesterolemia. Ottawa: CADTH. Available: https://cadth.ca/inclisiran-small-interfering-rna-molecule-treating-hypercholesterolemia
- Brandts J, Ray KK. 2020. Small interfering RNA to proprotein convertase subtilisin/kexin type 9: transforming LDL-cholesterol-lowering strategies. Curr Opin Lipidol. (4):182-6. doi: 10.1097/MOL.0000000000000691.
- Brandts J, Ray KK. 2020. Small interfering RNA to proprotein convertase subtilisin/kexin type 9: transforming LDL-cholesterol-lowering strategies. Curr Opin Lipidol. (4):182-6. doi: 10.1097/MOL.0000000000000691.
- Klinovski M, Boucher M, Perras C, Grobelna A. 2019. Inclisiran: A Small Interfering RNA Molecule for Treating Hypercholesterolemia. Ottawa: CADTH. Available: https://cadth.ca/inclisiran-small-interfering-rna-molecule-treating-hypercholesterolemia
- Klinovski M, Boucher M, Perras C, Grobelna A. 2019. Inclisiran: A Small Interfering RNA Molecule for Treating Hypercholesterolemia. Ottawa: CADTH. Available: https://cadth.ca/inclisiran-small-interfering-rna-molecule-treating-hypercholesterolemia
- GlobalData. 2020. FDA approval of Biogen’s Aducanumab could change the Alzheimer’s treatment landscape. July 10, 2020. Available: https://www.clinicaltrialsarena.com/comment/biogen-aducanumab-fda-approval-alzheimers/
- Tolar M, Abushakra S, Sabbagh M. 2020. The path forward in Alzhimer’s disease therapeutics: Re evaluating the amyloid cascade hypothesis. J Alzheimers Dis. doi: 10.1016/j.jalz.2019.09.075.
- Schiffmann R, Goker-Alpan O, Holida M, et al. 2019. Pegunigalsidase alfa, a novel PEGylated enzyme replacement therapy for Fabry disease, provides sustained plasma concentrations and favorable pharmacodynamics: A 1-year Phase 1/2 clinical trial. J Inherit Metab Dis. 42(3):534-44. doi: 10.1002/jimd.12080.
- Protalix BioTherapeutics, Inc. 2020. Press Release: Protalix BioTherapeutics Presents Clinical Data of Pegunigalsidase Alfa for the Treatment of Fabry Disease at the 16th Annual WORLDSymposium 2020. February 10, 2020. CISION. Available: https://www.prnewswire.com/news-releases/protalix-biotherapeutics-presents-key-clinical-data-of-pegunigalsidase-alfa-for-the-treatment-of-fabry-disease-at-the-16th-annual-worldsymposium-2020-301002142.html
- Schiffmann R, Goker-Alpan O, Holida M, et al. 2019. Pegunigalsidase alfa, a novel PEGylated enzyme replacement therapy for Fabry disease, provides sustained plasma concentrations and favorable pharmacodynamics: A 1-year Phase 1/2 clinical trial. J Inherit Metab Dis. 42(3):534-44. doi: 10.1002/jimd.12080.
- Von Drygalski A, Giermasz A, Castaman G, et al. 2019. Etranacogene dezaparvovec (AMT-061 phase 2b): normal/near normal FIX activity and bleed cessation in hemophilia B. Blood Adv. 3(21):3241-7. doi: 10.1182/bloodadvances.2019000811.
- Von Drygalski A, Giermasz A, Castaman G, et al. 2019. Etranacogene dezaparvovec (AMT-061 phase 2b): normal/near normal FIX activity and bleed cessation in hemophilia B. Blood Adv. 3(21):3241-7. doi: 10.1182/bloodadvances.2019000811.
- Von Drygalski A, Giermasz A, Castaman G, et al. 2019. Etranacogene dezaparvovec (AMT-061 phase 2b): normal/near normal FIX activity and bleed cessation in hemophilia B. Blood Adv. 3(21):3241-7. doi: 10.1182/bloodadvances.2019000811.
- George LA, Sullivan SK, Rasko JEJ, et al. 2019. Efficacy and Safety in 15 Hemophilia B Patients Treated with the AAV Gene Therapy Vector Fidanacogene Elaparvovec and Followed for at Least 1 Year. Am Soc Hematol. 134(Supplement 1):3347. doi: 10.1182/blood-2019-124091.
- George LA, Sullivan SK, Rasko JEJ, et al. 2019. Efficacy and Safety in 15 Hemophilia B Patients Treated with the AAV Gene Therapy Vector Fidanacogene Elaparvovec and Followed for at Least 1 Year. Am Soc Hematol. 134(Supplement 1):3347. doi: 10.1182/blood-2019-124091.
- Spark Therapeutics. 2018. Press Release: Pfizer Initiates Pivotal Phase 3 Program for Investigational Hemophilia B Gene Therapy. July 16, 2018. Available: https://sparktx.com/press_releases/pfizer-initiates-pivotal-phase-3-program-for-investigational-hemophilia-b-gene-therapy/
- Tanaka Y. 2020. State-of-the-art treatment of systemic lupus erythematosus. Int J Rheum Dis. 23(4):465-71. doi: 10.1111/1756-185X.13817.
- Shi H, Gudjonsson JE, Kahlenberg JM. 2020. Treatment of cutaneous lupus erythematosus: current approaches and future strategies. Curr Opin Rheumatol. 32(3):208-14. doi: 10.1097/BOR.0000000000000704.
- Shi H, Gudjonsson JE, Kahlenberg JM. 2020. Treatment of cutaneous lupus erythematosus: current approaches and future strategies. Curr Opin Rheumatol. 32(3):208-14. doi: 10.1097/BOR.0000000000000704.
- Klavdianou K, Lazarini A, Fanouriakis A. 2020. Targeted Biologic Therapy for Systemic Lupus Erythematosus: Emerging Pathways and Drug Pipeline. BioDrugs. 34(2):133-47. doi: 10.1007/s40259-020-00405-2.
- Morand EF, Furie R, Tanaka Y, et al. 2020. Trial of Anifrolumab in Active Systemic Lupus Erythematosus. N Engl J Med. 382:211-21. doi: 10.1056/NEJMoa1912196.
- Salimi S, Yamauchi PS, Thakur R, et al. Interleukin 23p19 inhibitors in chronic plaque psoriasis with focus on mirikizumab: A narrative review. Dermatology Therapy. 33(4). doi: 10.1111/dth.13800.
- Lilly Investors. 2020. Press Release: Lilly's Mirikizumab Superior to Cosentyx® (secukinumab) in a Phase 3 Study for Patients with Moderate to Severe Plaque Psoriasis. July 17, 2020. Eli Lilly and Co. Available: https://investor.lilly.com/news-releases/news-release-details/lillys-mirikizumab-superior-cosentyxr-secukinumab-phase-3-study
- Petraitis V, Petraitiene R, Katragkou A, et al. 2020. Combination Therapy with Ibrexafungerp (Formerly SCY-078), a First-in-Class Triterpenoid Inhibitor of (1 →3)-β-d-Glucan Synthesis, and Isavuconazole for Treatment of Experimental Invasive Pulmonary Aspergillosis. Antimicrob Agents Chemother. 64(6):e02429-19. doi: 10.1128/AAC.02429-19.
- Lorenz J. 2020. Oral Ibrexafungerp Effective & Well-Tolerated in FURI Phase 3 Trail. May 19, 2020. Contagion Live. Available: https://www.contagionlive.com/news/oral-ibrexafungerp-effective--welltolerated--furi-phase-3-trial
- Lorenz J. 2020. Oral Ibrexafungerp Effective & Well-Tolerated in FURI Phase 3 Trail. May 19, 2020. Contagion Live. Available: https://www.contagionlive.com/news/oral-ibrexafungerp-effective--welltolerated--furi-phase-3-trial
- Hawkes CP, De Leon DD, Rickels MR. 2019. Novel Preparations of Glucagon for the Prevention and Treatment of Hypoglycemia. Current Diabetes Report. 19(10):97. doi: 10.1007/s11892-019-1216-4.
- A Trial to Confirm the Efficacy and Safety of Dasiglucagon in the Treatment of Hypoglycemia in Type 1 Diabetes Subjects. ClinicalTrials.gov: NCT03378635. https://clinicaltrials.gov/ct2/show/NCT03378635
- Herold KC, Bundy BN, Long A, et al. 2019. An Anti-CD3 Antibody, Teplizumab, in Relatives at Risk for Type 1 Diabetes. N Engl J Med. 381(7):603-13. doi: 10.1056/NEJMoa1902226.
- Herold KC, Bundy BN, Long A, et al. 2019. An Anti-CD3 Antibody, Teplizumab, in Relatives at Risk for Type 1 Diabetes. N Engl J Med. 381(7):603-13. doi: 10.1056/NEJMoa1902226.
- Hippensteele A. 2020. Study: Teplizumab Delays Progression to Type 1 Diabetes by 3 Years in High-risk Patients. 22 June 2020. Pharmacy Times. Available: https://www.pharmacytimes.com/news/study-teplizumab-delays-progression-to-type-1-diabetes-by-3-years-in-high-risk-patients
- Bristol Myers Squibb. 2020. Press Release: Bristol Myers Squibb and bluebird bio Provide Regulatory Update on Idecabtagene Vicleucel (ide-cel, bb2121) for the Treatment of Patients with Multiple Myeloma. May 13, 2020. Bristol Myers Squibb. Available: https://news.bms.com/press-release/corporatefinancial-news/bristol-myers-squibb-and-bluebird-bio-provide-regulatory-updat
- Abramson H. 2020. B-Cell Maturation Antigen (BCMA) as a Target for New Drug Development in Relapsed and/or Refractory Multiple Myeloma. Int J Mol Sci. 21(15):e5192. doi: 10.3390/ijms21155192. Available: https://pubmed.ncbi.nlm.nih.gov/32707894/
- Abramson H. 2020. B-Cell Maturation Antigen (BCMA) as a Target for New Drug Development in Relapsed and/or Refractory Multiple Myeloma. Int J Mol Sci. 21(15):e5192. doi: 10.3390/ijms21155192. Available: https://pubmed.ncbi.nlm.nih.gov/32707894/
- Efficacy and Safety Study of bb2121 Versus Standard Triplet Regimens in Subjects With Relapsed and Refractory Multiple Myeloma (RRMM) (KarMMa-3). Clinical Trials.gov: NCT03651128. https://clinicaltrials.gov/ct2/show/NCT03651128?term=bb2121&draw=2&rank=5
- Bristol Myers Squibb. 2020. Press Release: European Medicines Agency Validates Bristol Myers Squibb’s Applications for Idecabtagene Vicleucel (Ide-cel, bb2121) and CC-486. May 22, 2020. Bristol Myers Squibb. Available: https://news.bms.com/press-release/corporatefinancial-news/european-medicines-agency-validates-bristol-myers-squibbs-ap-0
- Kersten MJ, Spanjaart AM, Thieblemont C. 2020. CD19-directed CAR T-cell therapy in B-cell NHL. Curr Opin Oncol. 32(5):408-17. doi: 10.1097/CCO.0000000000000668.
- Kersten MJ, Spanjaart AM, Thieblemont C. 2020. CD19-directed CAR T-cell therapy in B-cell NHL. Curr Opin Oncol. 32(5):408-17. doi: 10.1097/CCO.0000000000000668.
- Byrne M, Oluwole OO, Savani B, Majhail NS, Hill BT, Locke FL. 2019. Understanding and Managing Large B Cell Lymphoma Relapses after Chimeric Antigen Receptor T Cell Therapy. Biol Blood Marrow Transplant. 25(11):e344-51. doi: 10.1016/j.bbmt.2019.06.036.
- Helwick C. 2020. Strong Activity Shown for Lisocabtagene Maraleucel CAR T-Cell Therapy in Aggressive Large B-Cell Lymphoma. February 25, 2020. The ASCO Post. Available: https://www.ascopost.com/issues/february-25-2020/strong-activity-shown-for-lisocabtagene-maraleucel-car-t-cell-therapy-in-aggressive-large-b-cell-lymphoma/
- Arend RC, Beer HM, Cohen YC, et al. 2020. Ofranergene obadenovec (VB-111) in platinum-resistant ovarian cancer; favorable response rates in a phase I/II study are associated with an immunotherapeutic effect. Gynecol Oncol. 157(3):578-84. doi: 10.1016/j.ygyno.2020.02.034.
- Arend RC, Beer HM, Cohen YC, et al. 2020. Ofranergene obadenovec (VB-111) in platinum-resistant ovarian cancer; favorable response rates in a phase I/II study are associated with an immunotherapeutic effect. Gynecol Oncol. 157(3):578-84. doi: 10.1016/j.ygyno.2020.02.034.
- Wei LY, Wu ZX, Yang Y, et al. 2020. Overexpression of ABCG2 confers resistance to pevonedistat, an NAE inhibitor. ExpCell Res. 388(2). doi: 10.1016/j.yexcr.2020.111858.
- Best S, Lam V, Liu T, et al. 2020. Immunomodulatory effects of pevonedistat, a NEDD8-activating enzyme inhibitor, in chronic lymphocytic leukemia-derived T cells. Leukemia. doi: 10.1038/s41375-020-0794-0.
- Demko S. 2020. Pevonedistat plus azacitidine may increase survival for myelodysplastic syndrome, AML. June 15, 2020. HemOnctoday. Available: https://www.healio.com/news/hematology-oncology/20200615/pevonedistat-plus-azacitidine-may-increase-survival-for-myelodysplastic-syndrome-aml
- Demko S. 2020. Pevonedistat plus azacitidine may increase survival for myelodysplastic syndrome, AML. June 15, 2020. HemOnctoday. Available: https://www.healio.com/news/hematology-oncology/20200615/pevonedistat-plus-azacitidine-may-increase-survival-for-myelodysplastic-syndrome-aml
- Davids MS, Kim HT, Nicotra A, et al. 2019. Umbralisib in combination with ibrutinib in patients with relapsed or refractory chronic lymphocytic leukaemia or mantle cell lymphoma: a multicentre phase 1-1b study. Lancet Haematol. 6(1): e38-e47. doi: 10.1016/S2352-3026(18)30196-0.
- Burris HA, Flinn IW, Patel MR, et al. 2018. Umbralisib, a novel PI3Kδ and casein kinase-1ε inhibitor, in relapsed or refractory chronic lymphocytic leukaemia and lymphoma: an open-label, phase 1, dose-escalation, first-in-human study. Lancet Oncol. 19(4):486-96. doi: 10.1016/S1470-2045(18)30082-2.
- Slater H. 2020. FDA Grants Orphan Drug Designation to Umbralisib for Patients with Follicular Lymphoma. 10 March 2020. Cancer Network. Available: https://www.cancernetwork.com/view/fda-grants-orphan-drug-designation-umbralisib-patients-follicular-lymphoma
- Maharaj K, Powers JJ, Achille A, et al. 2020. The dual PI3Kδ/CK1ε inhibitor umbralisib exhibits unique immunomodulatory effects on CLL T cells. Blood Adv. 4(13):3072-84. doi: 10.1182/bloodadvances.2020001800.
- Matera MG, Rogliani P, Calzetta L, Cazzola M. 2020. TSLP Inhibitors for Asthma: Current Status and Future Prospects. Drugs. 80(5):449-58. doi: 10.1007/s40265-020-01273-4.
- Marone G, Spadaro G, Barile M, et al. 2019. Tezepelumab: a novel biological therapy for the treatment of severe uncontrolled asthma. Expert Opin Investig Drugs. 28(11): 931-40. doi: 10.1080/13543784.2019.1672657.
- Corren J, Parnes JR, Wang L, et al. 2017. Tezepelumab in Adults with Uncontrolled Asthma. N Engl J Med. 377:936-46. doi: 10.1056/NEJMoa1704064.
- Safety and Efficacy Study of Gantenerumab in Participants With Early Alzheimer's Disease (AD). ClinicalTrials.gov: NCT03443973. https://www.clinicaltrials.gov/ct2/show/NCT03443973?term=Gantenerumab&rank=2
- Efficacy and Safety Study of Gantenerumab in Participants With Early Alzheimer's Disease (AD). ClinicalTrial.gov: NCT03444870. https://www.clinicaltrials.gov/ct2/show/NCT03444870?term=Gantenerumab&rank=3
- A Study of Gantenerumab in Participants With Mild Alzheimer Disease. ClinicalTrial.gov: NCT02051608. https://www.clinicaltrials.gov/ct2/show/NCT02051608?term=Gantenerumab&rank=6
- A Study of Gantenerumab in Participants With Prodromal Alzheimer's Disease. ClinicalTrial.gov: NCT01224106. https://www.clinicaltrials.gov/ct2/show/NCT01224106?term=Gantenerumab&rank=8
- Dominantly Inherited Alzheimer Network Trial: An Opportunity to Prevent Dementia. A Study of Potential Disease Modifying Treatments in Individuals at Risk for or With a Type of Early Onset Alzheimer's Disease Caused by a Genetic Mutation. Master Protocol DIAN-TU001 (DIAN-TU). ClinicalTrial.gov: NCT01760005. https://www.clinicaltrials.gov/ct2/show/NCT01760005?term=Gantenerumab&rank=12
- A Phase II/III Randomized, Double-Blind, Placebo-Controlled Multi-Center Study of 2 Potential Disease Modifying Therapies in Individuals at Risk for and with Dominantly Inherited Alzheimer's Disease (ADAD). Washington University. Health Canada Clinical Trials Database: 168399. https://www.health-products.canada.ca/ctdb-bdec/info.do
- A Phase III, Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group, Efficacy, and Safety Study of Gantenerumab in Patients with Early (Prodromal to Mild) Alzheimer’s Disease. Hoffmann La Roche Limited. Health Canada Clinical Trials Database: 216415. https://health-products.canada.ca/ctdb-bdec/info.do
- A Phase III, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group, Multicenter, Efficacy, and Safety Study of Gantenerumab in Patients with Mild Alzheimer's Disease. Hoffmann La Roche Limited. Health Canada Clinical Trials Database: 171314. https://health-products.canada.ca/ctdb-bdec/info.do
- DʼAntoni ML, Paul RH, Mitchell BI, et al. 2018. Improved Cognitive Performance and Reduced Monocyte Activation in Virally Suppressed Chronic HIV After Dual CCR2 and CCR5 Antagonism. J Acquir Immune Defic Syndr. 79(1):108-16. doi:10.1097/QAI.0000000000001752.
- Friedman SL, Ratziu V, Harrison SA, et al. 2018. A randomized, placebo-controlled trial of cenicriviroc for treatment of nonalcoholic steatohepatitis with fibrosis. Hepatology. 67(5):1754-67. doi: 10.1002/hep.29477.
- Friedman SL, Ratziu V, Harrison SA, et al. 2018. A randomized, placebo-controlled trial of cenicriviroc for treatment of nonalcoholic steatohepatitis with fibrosis. Hepatology. 67(5):1754-1767. doi: 10.1002/hep.29477
- Tacke F. 2018. Cenicriviroc for the treatment of non-alcoholic steatohepatitis and liver fibrosis. Expert Opin Investig Drugs. 27(3):301-11. doi:10.1080/13543784.2018.1442436.
- DʼAntoni ML, Paul RH, Mitchell BI, et al. 2018. Improved Cognitive Performance and Reduced Monocyte Activation in Virally Suppressed Chronic HIV After Dual CCR2 and CCR5 Antagonism. J Acquir Immune Defic Syndr. 79(1):108-116. doi:10.1097/QAI.0000000000001752.
- Bowler S, Siriwardhana C, Mitchell BI, et al. 2019. Cenicriviroc, a dual CCR2 and CCR5 antagonist leads to a reduction in plasma fibrotic biomarkers in persons living with HIV on antiretroviral therapy. HIV Res Clin Pract. 20(4-5):123-9. doi: 10.1080/25787489.2020.1719319.
- AURORA: A Phase 3 Study to Evaluate the Efficacy and Safety of Cenicriviroc for the Treatment of Liver Fibrosis in Adult Subjects With Nonalcoholic Steatohepatitis. ClinicalTrials.gov: NCT03028740. https://www.clinicaltrials.gov/ct2/show/NCT03028740?term=Cenicriviroc&draw=2&rank=12
- Eichler F, Duncan C, Musolino PL, et al. 2017. Hematopoietic Stem-Cell Gene Therapy for Cerebral Adrenoleukodystrophy. N Engl J Med. 377(17):1630-8.
- A Phase 3 Study of Lenti-D Drug Product After Myeloablative Conditioning Using Busulfan and Fludarabine in Subjects ≤17 Years of Age With Cerebral Adrenoleukodystrophy (CALD). ClinicalTrials.gov: NCT03852498. https://clinicaltrials.gov/ct2/show/NCT03852498?term=Elivaldogene&draw=2&rank=1
- Pasi KJ, Rangarajan S, Georgiev P, et al. 2017. Targeting of Antithrombin in Hemophilia A or B with RNAi Therapy. N Engl J Med. 377(9):819-828. doi: 10.1056/NEJMoa1616569.
- ATLAS-PPX: An Open-label, Multinational, Switching Study to Describe the Efficacy and Safety of Fitusiran Prophylaxis in Patients With Hemophilia A and B Previously Receiving Factor or Bypassing Agent Prophylaxis. ClinicalTrials.gov: NCT03549871. https://www.clinicaltrials.gov/ct2/show/NCT03549871?term=fitusiran&draw=2&rank=1
- An Open-label, Long-term Safety and Efficacy Study of Fitusiran in Patients With Hemophilia A or B, With or Without Inhibitory Antibodies to Factor VIII or IX. ClinicalTrials.gov: NCT03754790. https://www.clinicaltrials.gov/ct2/show/NCT03754790?term=fitusiran&draw=2&rank=3
- ATLAS-INH: A Phase 3 Study to Evaluate the Efficacy and Safety of Fitusiran in Patients With Hemophilia A or B, With Inhibitory Antibodies to Factor VIII or IX. ClinicalTrials.gov: NCT03417102. https://www.clinicaltrials.gov/ct2/show/NCT03417102?term=fitusiran&draw=2&rank=5
- ATLAS-A/B: A Phase 3 Study to Evaluate the Efficacy and Safety of Fitusiran in Patients With Hemophilia A or B, Without Inhibitory Antibodies to Factor VIII or IX. ClinicalTrials.gov: NCT03417245. https://www.clinicaltrials.gov/ct2/show/NCT03417245?term=fitusiran&draw=2&rank=6
- Martin ER, Smith MT, Maroni BJ, Zuraw QC, deGoma EM. 2017. Clinical Trial of Vadadustat in Patients With Anemia Secondary to Stage 3 or 4 Chronic Kidney Disease. Am J Nephrol. 45(5):380-388. doi: 10.1159/000464476.
- Haase VH, Chertow GM, Block GA, et al. 2018. Effects of vadadustat on hemoglobin concentrations in patients receiving hemodialysis previously treated with erythropoiesis-stimulating agents. Nephrol Dial Transplant. doi: 10.1093/ndt/gfy055.
- Trial Evaluating the Efficacy and Safety of Oral Vadadustat Once Daily (QD) and Three Times Weekly (TIW) for the Maintenance Treatment of Anemia in Hemodialysis Subjects Converting From Erythropoiesis-Stimulating Agents (ESAs) Clinicaltrials.gov: NCT04313153. https://www.clinicaltrials.gov/ct2/show/NCT02865850?term=vadadustat&recrs=d&phase=2&draw=2&rank=1
- de Bono JS, De Giorgi U, Nava Rodrigues D, et al. 2018. Randomized Phase II Study of Akt Blockade With or Without Ipatasertib in Abiraterone-Treated Patients With Metastatic Prostate Cancer With and Without PTEN Loss. Clin Cancer Res. doi:10.1158/1078-0432.CCR-18-0981.
- Costa RLB, Han HS, Gradishar WJ. 2018. Targeting the PI3K/AKT/mTOR pathway in triple-negative breast cancer: a review. Breast Cancer Res Treat. 169(3):397-406. doi: 10.1007/s10549-018-4697-y.
- Kim SB, Dent R, Im SA, et al. 2017. Ipatasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (LOTUS): a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 18(10):1360-1372. doi: 10.1016/S1470-2045(17)30450-3.
- A Phase Ib/III Study of Ipatasertib Plus Palbociclib and Fulvestrant Versus Placebo Plus Palbociclib and Fulvestrant in Hormone Receptor Positive and HER2 Negative Locally Advanced Unresectable or Metastatic Breast Cancer. ClinicalTrials.gov: NCT04060862. https://www.clinicaltrials.gov/ct2/show/NCT04060862?term=Ipatasertib&draw=2&rank=15
- A Phase III, Double-blind, Placebo-controlled, Randomized Study Of Ipatasertib in Combination With Atezolizumab and Paclitaxel as a Treatment for Participants With Locally Advanced Unresectable or Metastatic Triple-Negative Breast Cancer. ClinicalTrials.gov: NCT04177108. https://www.clinicaltrials.gov/ct2/show/NCT03072238?term=Ipatasertib&draw=2&rank=9
- A Phase III, Randomized, Double-Blind, Placebo-Controlled, Multicenter Trial Testing Ipatasertib Plus Abiraterone Plus Prednisone/Prednisolone, Relative to Placebo Plus Abiraterone Plus Prednisone/Prednisolone in Adult Male Patients With Asymptomatic or Mildly Symptomatic, Previously Untreated, Metastatic Castrate-Resistant Prostate Cancer. ClinicalTrials.gov: NCT03072238. https://www.clinicaltrials.gov/ct2/show/NCT03072238?term=Ipatasertib&draw=2&rank=9
- The Roche Group. 2020. Press Release: Roche’s IPATential150 study evaluating ipatasertib in combination with abiraterone and prednisone/prednisolone met one of its co-primary endpoints. June 19, 2020. Hoffman-La Roche Ltd. Available: https://www.roche.com/media/releases/med-cor-2020-06-19.htm
- Schjesvold F, Robak P, Pour L, Aschan J, Sonneveld P. 2020. OCEAN: a randomized Phase III study of melflufen + dexamethasone to treat relapsed refractory multiple myeloma. Future Oncol. 16(11):631-41. doi: 10.2217/fon-2020-0024.
- Chauhan D, Ray A, Viktorsson K, et al. 2013. In vitro and in vivo antitumor activity of a novel alkylating agent, melphalan-flufenamide, against multiple myeloma cells. Clin Cancer Res. 19(11):3019-31. doi: 10.1158/1078-0432.CCR-12-3752.
- A Randomized, Controlled, Open-label, Phase 3 Study of Melflufen/Dexamethasone Compared With Pomalidomide/Dexamethasone for Patients With Relapsed Refractory Multiple Myeloma Who Are Refractory to Lenalidomide. ClinicalTrials.gov: NCT03151811. https://www.clinicaltrials.gov/ct2/show/NCT03151811?term=melflufen&draw=2&rank=4
- A Multicenter Phase 2, Open Label Study of Intratumoral Tavo Plus Electroporation in Combination With Intravenous Pembrolizumab in Patients With Stage III/IV Melanoma Who Are Progressing on Either Pembrolizumab or Nivolumab Treatment. ClinicalTrials.gov: NCT03132675. https://clinicaltrials.gov/ct2/show/NCT03132675
- Burkart C, Mukhopadhyay A, Shirley SA, et al. 2018. Improving therapeutic efficacy of IL-12 intratumoral gene electrotransfer through novel plasmid design and modified parameters. Gene Ther. 25(2):93-103.
- Babiker HM, Glode AE, Cooke LS, Mahadevan D. 2018. Ublituximab for the treatment of CD20 positive B-cell malignancies. Expert Opin Investig Drugs. 27(4):407-412. doi:10.1080/13543784.2018.1459560.
- Pithadia DJ, Reynolds KA, Lee EB, et al. 2019. Tildrakizumab in the treatment of psoriasis: latest evidence and place in therapy. Ther Adv Chronic Dis. 10:2040622319865658. doi:10.1177/2040622319865658.
- Sharman JP, Farber CM, Mahadevan D, et al. 2017. Ublituximab (TG-1101), a novel glycoengineered anti-CD20 antibody, in combination with ibrutinib is safe and highly active in patients with relapsed and/or refractory chronic lymphocytic leukaemia: results of a phase 2 trial. Br J Haematol. 176(3):412-420. doi: 10.1111/bjh.14447.
- UbLiTuximab in Multiple Sclerosis Treatment Effects (ULTIMATE I STUDY). ClinicalTrials.gov: NCT03277261. https://www.clinicaltrials.gov/ct2/show/NCT03277261?term=Ublituximab&draw=2&rank=5
- Mitsios A, Dubis AM, Moosajee M. 2018. Choroideremia: from genetic and clinical phenotyping to gene therapy and future treatments. Ther Adv Ophthalmol. 10:1-18.
- Ong T, Pennesi ME, Birch DG, Lam BL, Tsang SH. 2019. Adeno-Associated Viral Gene Therapy for Inherited Retinal Disease. Pharm Res. 36(2):34.
- Fischer MD, Ochakovski GA, Beier B, et al. 2019. Efficacy and Safety of Retinal Gene Therapy Using Adeno-Associated Virus Vector for Patients With Choroideremia: A Randomized Clinical Trial. JAMA Ophthalmol. doi: 10.1001/jamaophthalmol.2019.3278. [Epub ahead of print].
- A Randomised, Open Label, Outcomes-Assessor Masked, Prospective, Parallel Controlled Group, Phase 3 Clinical Trial Of Retinal Gene Therapy For Choroideremia Using An Adeno-Associated Viral Vector (AAV2) Encoding Rab Escort Protein 1 (REP1). ClinicalTrials.gov: NCT03496012. https://www.clinicaltrials.gov/ct2/show/NCT03496012?term=NSR-REP1&rank=3
- A Single-Masked, Randomized, Controlled, Parallel Group, Phase 3 Clinical Trial Of Retinal Gene Therapy for Choroideremia Using an Adeno-Associated Viral Vector (AAV2) Encoding Rab Escort Protein 1 (REP1). Health Canada Clinical Trials Database: 193958. https://health-products.canada.ca/ctdb-bdec/info.do
- Bilen O, Ballantyne CM. 2016. Bempedoic Acid (ETC-1002): an Investigational Inhibitor of ATP Citrate Lyase. Curr Atheroscler Rep. 18(10):61. doi: 10.1007/s11883-016-0611-4.
- Bilen O, Ballantyne CM. 2016. Bempedoic Acid (ETC-1002): an Investigational Inhibitor of ATP Citrate Lyase. Curr Atheroscler Rep. 18(10):61.
- Ballantyne CM, Laufs U, Ray KK, et al. 2019. Bempedoic acid plus ezetimibe fixed-dose combination in patients with hypercholesterolemia and high CVD risk treated with maximally tolerated statin therapy. Eur J Prev Cardiol. 29:2047487319864671. doi: 10.1177/2047487319864671. [Epub ahead of print].
- Laufs U, Banach M, Mancini GBJ, et al. 2019. Efficacy and Safety of Bempedoic Acid in Patients With Hypercholesterolemia and Statin Intolerance. J Am Heart Assoc. 8(7):e011662. doi: 10.1161/JAHA.118.011662.
- Penson P, McGowan M, Banach M. 2017. Evaluating bempedoic acid for the treatment of hyperlipidaemia. Expert Opin Investig Drugs. 26(2):251-9. doi: 10.1080/13543784.2017.1280458.
- Ray KK, Bays HE, Catapano AL, et al. 2019. Safety and Efficacy of Bempedoic Acid to Reduce LDL Cholesterol. N Engl J Med. 380(11):1022-32. doi: 10.1056/NEJMoa1803917.
- Collet TH, Dubern B, Mokrosinski J, et al. 2017. Evaluation of a melanocortin-4 receptor (MC4R) agonist (Setmelanotide) in MC4R deficiency. Mol Metab. 6(10):1321-1329. doi: 10.1016/j.molmet.2017.06.015.
- A Phase 3 Trial of Setmelanotide (RM-493), a Melanocortin-4 Receptor (MC4R) Agonist, in Bardet-Biedl Syndrome (BBS) and Alström Syndrome (AS) Patients With Moderate to Severe Obesity. ClinicalTrials.gov: NCT03746522. https://www.clinicaltrials.gov/ct2/show/NCT03746522?term=Setmelanotide&draw=2&rank=3
- An Open Label, 1-Year Trial, Including a Double-Blind Placebo-Controlled Withdrawal Period, of Setmelanotide (RM-493), a Melanocortin 4 Receptor (MC4R) Agonist, in Early Onset Leptin Receptor (LEPR) Deficiency Obesity Due to Bi-Allelic Loss-of-Function LEPR Genetic Mutation. ClinicalTrials.gov: NCT03287960. https://www.clinicaltrials.gov/ct2/show/NCT03287960?term=Setmelanotide&draw=2&rank=4
- Lonze BE, Tatapudi VS, Weldon EP, et al. 2018. IdeS (Imlifidase): A Novel Agent That Cleaves Human IgG and Permits Successful Kidney Transplantation Across High-strength Donor-specific Antibody. Ann Surg. 268(3):488-96. doi: 10.1097/SLA.0000000000002924.
- Lonze BE, Tatapudi VS, Weldon EP, et al. 2018. IdeS (Imlifidase): A Novel Agent That Cleaves Human IgG and Permits Successful Kidney Transplantation Across High-strength Donor-specific Antibody. Ann Surg. 268(3):488-496. doi: 10.1097/SLA.0000000000002924.
- Whitfield T, Torkington A, van Halsema C. 2016. Profile of cabotegravir and its potential in the treatment and prevention of HIV-1 infection: evidence to date. HIV AIDS (Auckl). 8:157-164.
- Whitfield T, Torkington A, van Halsema C. 2016. Profile of cabotegravir and its potential in the treatment and prevention of HIV-1 infection: evidence to date. HIV AIDS (Auckl). 8:157-164.
- Trezza C, Ford SL, Spreen W, Pan R, Piscitelli S. 2015. Formulation and pharmacology of long-acting cabotegravir. Curr Opin HIV AIDS. 10(4):239-45. doi:10.1097/COH.0000000000000168.
- Margolis DA, Brinson CC, Smith GHR, et al. 2015. Cabotegravir plus rilpivirine, once a day, after induction with cabotegravir plus nucleoside reverse transcriptase inhibitors in antiretroviral-naive adults with HIV-1 infection (LATTE): a randomised, phase 2b, dose-ranging trial. Lancet Infect Dis. 15(10):1145-1155. doi: 10.1016/S1473-3099(15)00152-8.
- Andrews CD, Heneine W. 2015. Cabotegravir long-acting for HIV-1 prevention. Curr Opin HIV AIDS. 10(4):258-263. doi:10.1097/COH.0000000000000161.
- Cahn P, Fink V, Patterson P. 2018. Fostemsavir: a new CD4 attachment inhibitor. Curr Opin HIV AIDS. 13(4):341-345. doi: 10.1097/COH.0000000000000469.
- [No authors listed]. 2019. Darolutamide Slows Metastasis in Prostate Cancer. Cancer Discov. 9(4):OF6. doi: 10.1158/2159-8290.CD-NB2019-029.
- Kozal M, Aberg J, Pialoux G, et al. 2020. Fostemsavir in Adults with Multidrug-Resistant HIV-1 Infection. N Engl J Med. 382(13):1232-43. doi: 10.1056/NEJMoa1902493.
- Klinovski M, Boucher M, Perras C, Grobelna A. 2019. Inclisiran: A Small Interfering RNA Molecule for Treating Hypercholesterolemia. Ottawa: CADTH. Available: https://cadth.ca/inclisiran-small-interfering-rna-molecule-treating-hypercholesterolemia
- Brandts J, Ray KK. 2020. Small interfering RNA to proprotein convertase subtilisin/kexin type 9: transforming LDL-cholesterol-lowering strategies. Curr Opin Lipidol. (4):182-6. doi: 10.1097/MOL.0000000000000691.
- Brandts J, Ray KK. 2020. Small interfering RNA to proprotein convertase subtilisin/kexin type 9: transforming LDL-cholesterol-lowering strategies. Curr Opin Lipidol. (4):182-6. doi: 10.1097/MOL.0000000000000691.
- Klinovski M, Boucher M, Perras C, Grobelna A. 2019. Inclisiran: A Small Interfering RNA Molecule for Treating Hypercholesterolemia. Ottawa: CADTH. Available: https://cadth.ca/inclisiran-small-interfering-rna-molecule-treating-hypercholesterolemia
- Klinovski M, Boucher M, Perras C, Grobelna A. 2019. Inclisiran: A Small Interfering RNA Molecule for Treating Hypercholesterolemia. Ottawa: CADTH. Available: https://cadth.ca/inclisiran-small-interfering-rna-molecule-treating-hypercholesterolemia
- Liu A. 2020. 4. Roxadustat. February 3, 2020. Fiercepharma. Available: https://www.fiercepharma.com/special-report/4-roxadustat
- Liu A. 2020. 4. Roxadustat. February 3, 2020. Fiercepharma. Available: https://www.fiercepharma.com/special-report/4-roxadustat
- Yan Z, Xu G. A. 2020. A Novel Choice to Correct Inflammation-Induced Anemia in CKD: Oral Hypoxia-Inducible Factor Prolyl Hydroxylase Inhibitor Roxadustat. Front Med (Lausanne). 7:393. doi: 10.3389/fmed.2020.00393.
- Li ZL, Tu Y, Liu BC. 2020. Treatment of Renal Anemia with Roxadustat: Advantages and Achievement. Kidney Dis. 6:65-73. doi: 10.1159/000504850.
- Reich K, Papp KA, Blauvelt A, et al. 2017. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (esurface 1 and esurface 2): results from two randomised controlled, phase 3 trials. Lancet. 390(10091):276-88. doi: 10.1016/S0140-6736(17)31279-5.
- Bangert C, Kopp T. 2018. Tildrakizumab for the treatment of psoriasis. Immunotherapy. 10(13):1105-22. doi: 10.2217/imt-2018-0028.
- Pithadia DJ, Reynolds KA, Lee EB, et al. 2019. Tildrakizumab in the treatment of psoriasis: latest evidence and place in therapy. Ther Adv Chronic Dis. 10:2040622319865658. doi: 10.1177/2040622319865658.
- Reich K, Papp KA, Blauvelt A, et al. 2017. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet. 390(10091):276-288. doi: 10.1016/S0140-6736(17)31279-5.
- Bangert C, Kopp T. 2018. Tildrakizumab for the treatment of psoriasis. Immunotherapy. 10(13):1105-22. doi: 10.2217/imt-2018-0028.
- Bangert C, Kopp T. 2018. Tildrakizumab for the treatment of psoriasis. Immunotherapy. 10(13):1105-22. doi: 10.2217/imt-2018-0028.
- Tran B, Cohen MS. 2020. The discovery and development of binimetinib for the treatment of melanoma. Expert Opin Drug Discov. 15(7):745-54. doi: 10.1080/17460441.2020.1746265. [Epub ahead of print]
- Indini A, Mandalà M. 2020. Safety and efficacy evaluation of encorafenib plus binimetinib for the treatment of advanced BRAF-mutant melanoma patients. Expert Opin Drug Saf. 19(10):1229-36. doi: 10.1080/14740338.2020.1817376. [Epub ahead of print].
- Bristol Myers Squibb. 2020. Press Release: Bristol Myers Squibb and bluebird bio Provide Regulatory Update on Idecabtagene Vicleucel (ide-cel, bb2121) for the Treatment of Patients with Multiple Myeloma. May 13, 2020. Bristol Myers Squibb. Available: https://news.bms.com/press-release/corporatefinancial-news/bristol-myers-squibb-and-bluebird-bio-provide-regulatory-updat
- Abramson H. 2020. B-Cell Maturation Antigen (BCMA) as a Target for New Drug Development in Relapsed and/or Refractory Multiple Myeloma. Int J Mol Sci. 21(15):e5192. doi: 10.3390/ijms21155192. Available: https://pubmed.ncbi.nlm.nih.gov/32707894/
- Abramson H. 2020. B-Cell Maturation Antigen (BCMA) as a Target for New Drug Development in Relapsed and/or Refractory Multiple Myeloma. Int J Mol Sci. 21(15):e5192. doi: 10.3390/ijms21155192. Available: https://pubmed.ncbi.nlm.nih.gov/32707894/
- Efficacy and Safety Study of bb2121 Versus Standard Triplet Regimens in Subjects With Relapsed and Refractory Multiple Myeloma (RRMM) (KarMMa-3). Clinical Trials.gov: NCT03651128. https://clinicaltrials.gov/ct2/show/NCT03651128?term=bb2121&draw=2&rank=5
- Bristol Myers Squibb. 2020. Press Release: European Medicines Agency Validates Bristol Myers Squibb’s Applications for Idecabtagene Vicleucel (Ide-cel, bb2121) and CC-486. May 22, 2020. Bristol Myers Squibb. Available: https://news.bms.com/press-release/corporatefinancial-news/european-medicines-agency-validates-bristol-myers-squibbs-ap-0
- Simon JA, Altomare C, Cort S, Jiang W, Pinkerton JV. 2018. Overall Safety of Ospemifene in Postmenopausal Women from Placebo-Controlled Phase 2 and 3 Trials. J Womens Health (Larchmt). 27(1):14-23. doi: 10.1089/jwh.2017.6385.
- Shin JJ, Kim SK, Lee JR, Suh CS. 2017. Ospemifene: A Novel Option for the Treatment of Vulvovaginal Atrophy. J Menopausal Med. 23(2):79-84. doi: 10.6118/jmm.2017.23.2.79.
- Shin JJ, Kim SK, Lee JR, Suh CS. 2017. Ospemifene: A Novel Option for the Treatment of Vulvovaginal Atrophy. J Menopausal Med. 23(2):79-84. doi: 10.6118/jmm.2017.23.2.79.
- Bruyniks N, Biglia N, Palacios S, Mueck AO. 2017. Systematic indirect comparison of ospemifene versus local estrogens for vulvar and vaginal atrophy. Climacteric. 20(3):195-204. doi: 10.1080/13697137.2017.1284780.
- Faught BM, Soulban G, Yeaw J, et al. 2019. Ospemifene versus local estrogen: adherence and costs in postmenopausal dyspareunia. J Comp Eff Res. doi: 10.2217/cer-2019-0091. [Epub ahead of print].
- Bai C, Chotirmall SH, Rello J, et al. 2020. Updated guidance on the management of COVID-19: from an American Thoracic Society/European Respiratory Society coordinated International Task Force (29 July 2020). Eur Respir Rev. 29(157):200287. doi: 10.1183/16000617.0287-2020.
- Samaddar A, Grover M, Nag VL. 2020. Pathophysiology and Potential Therapeutic Candidates for COVID-19: A Poorly Understood Arena. Front Pharmacol. 11:585888. doi: 10.3389/fphar.2020.585888.
- Public Health Agency of Canada. 2020. Federal/provincial/territorial public health response plan for ongoing management of COVID-19. August 25, 2020. Available: https://www.canada.ca/en/public-health/services/diseases/2019-novel-coronavirus-infection/guidance-documents/federal-provincial-territorial-public-health-response-plan-ongoing-management-covid-19.html
- Health Canada. 2020. Interim Order respecting the prevention and alleviation of shortages of drugs in relation to COVID-19. October 16, 2020. Available: https://www.canada.ca/en/health-canada/services/drugs-health-products/covid19-industry/drugs-vaccines-treatments/interim-order-drug-shortages.html
- Health Canada. 2020. Interim Order Respecting the Importation and Sale of Medical Devices for Use in Relation to COVID-19. March 18, 2020. Available: https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/announcements/interim-order-importation-sale-medical-devices-covid-19.html
- Health Canada. 2020. Interim Order Respecting Drugs, Medical Devices and Foods for a Special Dietary Purpose in relation to COVID-19. March 30, 2020. Available: https://www.canada.ca/en/health-canada/services/drugs-health-products/compliance-enforcement/covid19-interim-order-drugs-medical-devices-special-foods.html
- Health Canada. 2020. Interim Order Respecting Clinical Trials for Medical Devices and Drugs Relating to COVID-19. May 23, 2020. Available: https://www.canada.ca/en/health-canada/services/drugs-health-products/covid19-industry/interim-order-respecting-clinical-trials-medical-devices-drugs/notice-interim-order.html
- Health Canada. 2020. Interim Order Respecting the Importation, Sale and Advertising of Drugs for Use in Relation to COVID-19. September 16, 2020. Available : https://www.canada.ca/en/health-canada/services/drugs-health-products/covid19-industry/drugs-vaccines-treatments/interim-order-import-sale-advertising-drugs.html
- Health Canada. 2020. Interim Order Respecting the Importation, Sale and Advertising of Drugs for Use in Relation to COVID-19. September 16, 2020. Available: https://www.canada.ca/en/health-canada/services/drugs-health-products/covid19-industry/drugs-vaccines-treatments/interim-order-import-sale-advertising-drugs.html
- Health Canada. 2020. Interim Order Respecting Clinical Trials for Medical Devices and Drugs Relating to COVID-19. May 23, 2020. Available: https://www.canada.ca/en/health-canada/services/drugs-health-products/covid19-industry/interim-order-respecting-clinical-trials-medical-devices-drugs/notice-interim-order.html
- Health Canada. Drugs and vaccines for COVID-19: List of authorized clinical trials. Available: https://www.canada.ca/en/health-canada/services/drugs-health-products/covid19-industry/drugs-vaccines-treatments/list-authorized-trials.html
- Berkley S. 2020. COVAX explained. September 3, 2020. The Vaccine Alliance. Available: https://www.gavi.org/vaccineswork/covax-explained