Review Process
Table of Contents
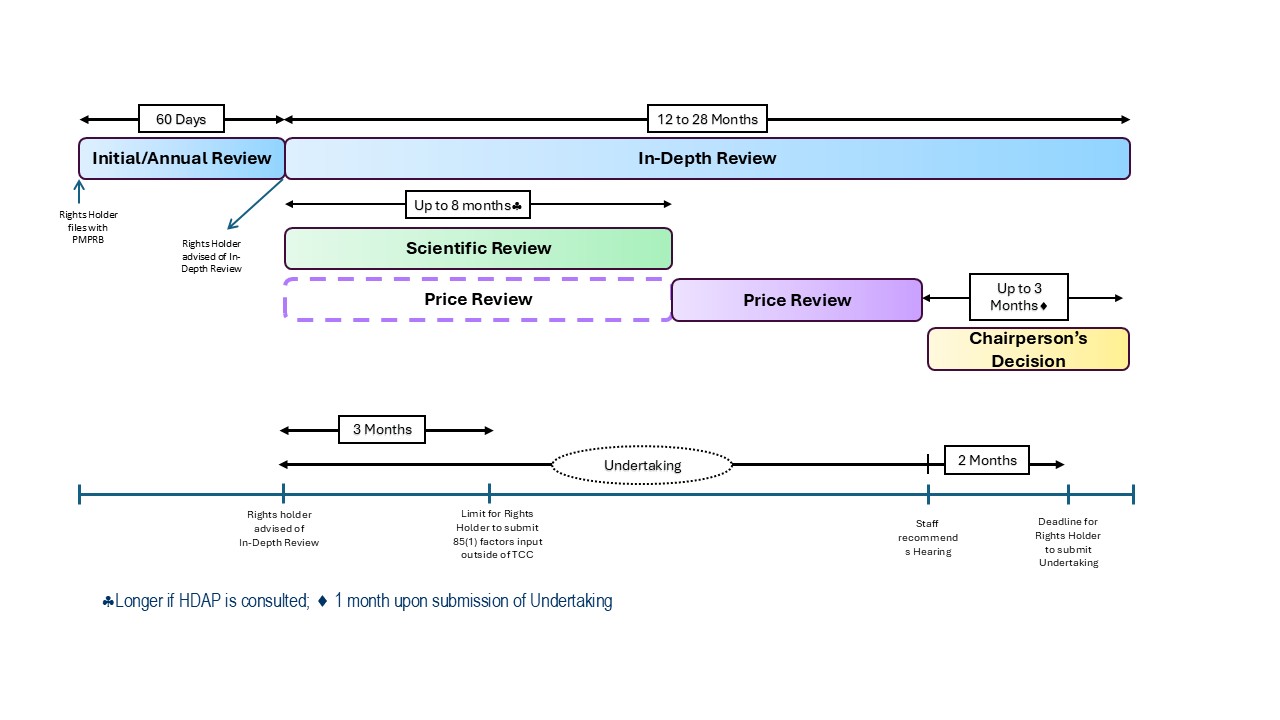
Figure description
A timeline visual depicts relevant service standards for the Initial/Annual Review (60 days), In-Depth Review (12-28 months), Scientific Review (up to 8 months but may be longer if HDAP is consulted, starts concurrently with the In-Depth Review), Price Review (starts concurrently with the In-Depth Review but the main analysis begins after the Scientific Review is completed). Rights Holders have up to 3 months after an In-Depth Review notice is received to submit any relevant information regarding the 85(1) factors they wish to be considered by Staff in their analysis. In addition, the Rights Holder may submit an undertaking for the Chairperson’s consideration up to 2 months after Staff provides their recommendation to the Chairperson. The Chairperson will decide whether to close the review or to issue a Notice of Hearing up to 3 months after receiving Staff’s recommendation. If a Notice of Hearing is issued by the Chairperson, the Rights Holder may no longer provide an undertaking. Instead, the Rights Holder may make a settlement proposal to the Hearing Panel.
The PMPRB monitors the prices charged by Rights Holders for patented medicines on an ongoing basis. Under the Patent Act (the Act) and the Patented Medicines Regulations (the Regulations), Rights Holders are required to file price and sales information about their patented medicines within 30 days after their date of first sale in Canada. Price and sales information must then be filed twice a year for each strength of each dosage form.
The role of the PMPRB’s Guidelines is to provide a general procedural framework for a review process that will help PMPRB Staff (Staff) to determine whether a recommendation for a Hearing should be made to the Chairperson regarding the price of a medicine. While directed at Staff, these Guidelines provide Rights Holders with a transparent, predictable, and procedurally fair price review process that will be applied in most circumstances.
“Existing medicines” are defined as patented medicines first sold before July 1, 2022, while “new medicines” are defined as patented medicines first sold on or after July 1, 2022. The review process begins once a Rights Holder files price and sales information to the PMPRB.
- For more details, please consult the Guidelines
Initial Review
The Initial Review uses the first price filing of a patented medicine and compares the highest Canadian list price against the Highest International Price (HIP) among the 11 countries found in the PMPRB11 Schedule Countries. If the highest Canadian list price for a patented medicine is above the HIP, it becomes subject to an In-Depth Review. Associated Drug Identification Numbers (DINs) are considered as part of the In-Depth Review. Associated DINs are defined as all DINs to which the same patented invention(s) pertain(s), according to the filings made by the Rights Holder, such as different dosage forms of the same medicine.
Rights Holders may expect to receive the results of the Initial Review (either “reviewed” or “subject to in-depth review”) within the 60-day service standard following the filing deadline.
For example, if a patented medicine is first sold on September 1, 2025, the first filing to the PMPRB will be for the period between July to December 2025 and submitted no later than January 30, 2026. The Initial Review would take place right after this filing and the results provided to the Rights Holder by March 31, 2026.
Annual Review
The Annual Review is conducted for each patented medicine at the beginning of each year, after all information from the previous year has been filed by the Rights Holders, to see whether they should be subject to an In-Depth Review. For example, the Annual Review for 2028 prices would start in early February 2029, after the last filing of 2028 is received. The Annual Review first compares the highest Canadian list price of a patented medicine against the Highest International Price (HIP) of the PMPRB11 Schedule Countries, as filed by the Rights Holder. It also compares the price change of the highest Canadian list price for each patented medicine against changes in the Consumer Price Index (CPI). Just like the Initial Review, associated DINs are considered as part of the In-Depth Review.
“New medicines” and “Existing medicines” are subject to the same Annual Review process. As of the Guidelines coming into effect January 1, 2026, the following will apply:
New medicines:
- The first Annual Review for new medicines which will have received an Initial Review in 2026 will begin in February 2027.
- If a patented medicine price is above the 1-year CPI, the 2-year CPI will be considered (if applicable).
Existing medicines:
- The first Annual Review for existing medicines will begin in February 2028, but only the HIP criterion will be applied for that year.
Rights Holders may expect to receive the results of the Annual Review within the 60-day service standard following the filing deadline of January 30th for price and sales information for the second half of the previous year.
Complaints
The receipt of a complaint from an approved individual or organization who believes that the price of a patented medicine may be excessive in any market in Canada will automatically lead to an In-Depth Review. This applies even if the Initial Review or Annual Review criteria have not otherwise been met.
- More information on the complaints process
In-Depth Review
An In-Depth Review is the process by which Staff analyses and balances information related to identified factors of the Patent Act to prepare a recommendation to the Chairperson on whether a matter should be brought to a Hearing. This includes a Scientific Review and a Price Review.
The criteria used during the Initial and Annual Reviews consider only the medicine’s international prices and the change in the domestic price relative to CPI. The In-Depth Review considers all factors, including the therapeutic class (both domestically and internationally) identified during the Scientific Review. Rights Holders are expected to receive the results of the In-Depth Review within 12 to 28 months, depending on its complexity.
Scientific Review
The Scientific Review is a key component of the In-Depth Review process. It is conducted by the scientific team, which is composed of health care professionals with significant education, background, and experience in a variety of areas of clinical practice, drug evaluation, and drug utilization. A committee of experts, known as the Human Drug Advisory Panel (HDAP), may also assist with scientific evaluations on an ad-hoc basis, if Staff determine circumstances warrant such assistance.
The Scientific Review will identify comparators that will then be used to perform a Therapeutic Class Comparison (TCC) analysis. For each approved indication of the medicine subject to In-Depth Review, comparators are identified, and each comparator is assigned a comparability score, as well as a comparable dosage regimen.
- More information on the Scientific Review process
- More information on the Human Drug Advisory Panel (HDAP)
Price Review
The Price Review includes a more comprehensive analysis of the medicine’s price in Canada and across the PMPRB11 Schedule Countries, the pricing history of the medicine, and any other information that Staff deem relevant for the analysis. This includes price and sales data reported by the Rights Holder, as well as publicly available information collected by Staff that relates to the section 85(1) factors of the Act. This analysis is updated throughout the duration of the In-Depth Review.
When Staff are provided with the results of the Scientific Review, they use the TCC to do a domestic and international analysis and supplement the preliminary price analysis with this information.
Staff weigh and balance the analysis made under the different factors to make a recommendation to the Chairperson. This recommendation could be for the Chairperson to close the review (which may involve consideration of any undertakings proposed by the Rights Holder) or for the Chairperson to issue a Notice of Hearing.
- More information on the In-Depth Review process
The anticipated timeline for the entire In-Depth Review process is between 12 and 28 months.
Undertakings and Settlement Proposals
Rights Holders are informed when Staff begin an In-Depth Review. Rights Holders may choose to provide a written commitment to the PMPRB to lower the price of a medicine under review and/or to repay revenues earned from selling the medicine at a higher price. The Chairperson may consider any undertakings made by Rights Holders when deciding whether to issue a Notice of Hearing or, alternatively, to close a price review. However, if a Notice of Hearing has been issued by the Chairperson, Rights Holders can no longer propose an undertaking for consideration by the Chairperson. Instead, they may make a formal settlement proposal to the Hearing Panel.
- More information on undertakings and settlement proposals
Recommendation to the Chairperson and Hearing Process
At the conclusion of the In-Depth Review, Staff make a recommendation to the Chairperson. This recommendation could be for the Chairperson to close the review (which may involve consideration of any undertakings proposed by the Rights Holder) or for the Chairperson to issue a Notice of Hearing. The Chairperson will render their decision no more than three months after receiving Staff’s recommendation.
- More information on the hearing process
- More information on the status of ongoing proceedings