Canadian Biosafety Guideline - Dual-Use in Life Science Research
Notice to the reader:
The online consultation is now closed.

Download the alternative format
(PDF format, 1.12 MB, 48 pages)
Organization: Public Health Agency of Canada
Date published: 2018-06-29
June 29, 2018
Table of contents
- Preface
- Abbreviations and acronyms
- Chapter 1 - Introduction
- Chapter 2 - Governance of research with dual-use potential in Canada
- Chapter 3 - Risk assessment of research with dual-use potential
- Chapter 4 - International oversight of research with dual-use potential
- Chapter 5 - Glossary
- Chapter 6 - References and resources
Preface
In Canada, facilities where Risk Group 2, 3, and 4 human pathogens or toxins are handled and stored are regulated by the Public Health Agency of Canada (PHAC) under the Human Pathogens and Toxins Act (HPTA) and the Human Pathogens and Toxins Regulations (HPTR). The importation of animal pathogens, infected animals, animal products or by-products (e.g., tissue, serum), or other substances that may carry an animal pathogen or part of one (e.g., toxin) are regulated by the PHAC or the Canadian Food Inspection Agency (CFIA) under the Health of Animals Act (HAA) and Health of Animals Regulations (HAR).
The following figure depicts the document hierarchy used by the PHAC and CFIA to oversee biosafety and biosecurity operations. Each tier of the pyramid corresponds to a document type, with documents increasing in order of precedence moving upwards. Acts and regulations are found at the top of the pyramid as they are the documents that convey the PHAC’s and CFIA’s legal authorities. Guidance material and technical pieces are found at the bottom of the pyramid, as they are intended to summarize recommendations and scientific information only.
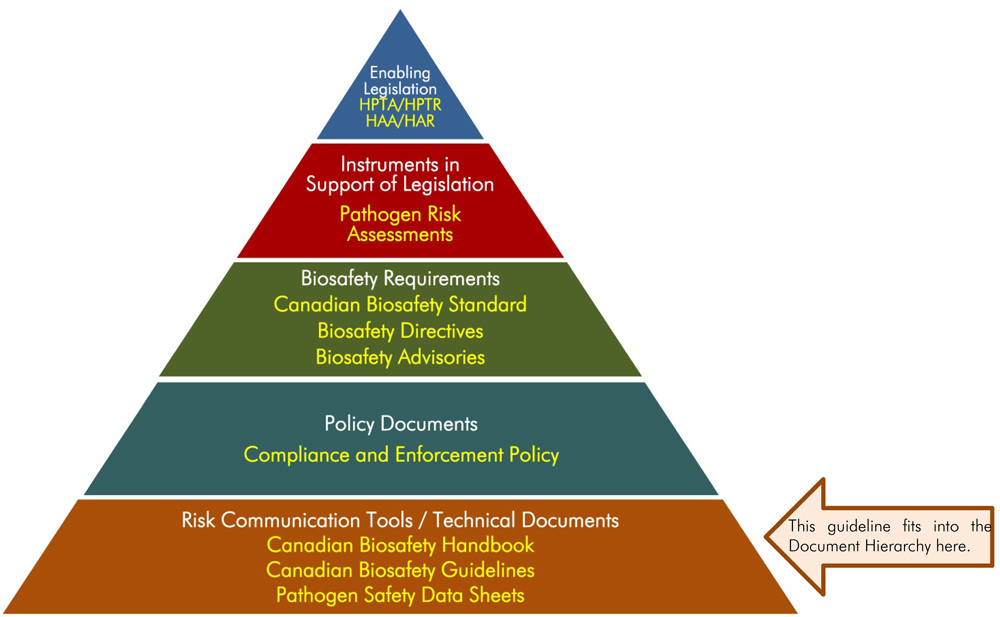
Figure 1 - Text Equivalent
Figure in the form of a pyramid depicting the document hierarchy used by the PHAC to oversee biosafety and biosecurity operations. Each of the five tiers of the pyramid corresponds to a document type, with documents increasing in order of precedence moving upwards.
At the top sits the Enabling Legislation, that is, the HPTA, HPTR, HAA, and HAR, that convey the PHAC’s legal authorities. Below the acts and regulations sit Instrument in Support of Legislation, which are the Pathogen Risk Assessments. In the next tier down are the Biosafety Requirements, which include the Canadian Biosafety Standard, Biosafety Directives, and Biosafety Advisories. In the second lowest tier are the Policy Documents, the Compliance and Enforcement Policy. Guidance material and technical pieces are found at the bottom of the pyramid, under the Risk Communication Tools and Technical Documents heading, as they are only intended to summarize recommendations and scientific information. These include the Canadian Biosafety Handbook, Canadian Biosafety Guidelines, and Pathogen Safety Data Sheets.
Dual-Use in Life Science Research was developed by the PHAC and the CFIA as part of a series of electronic publications that expand upon the biosafety and biosecurity concepts discussed in the current edition of the Canadian Biosafety Handbook (CBH), the companion document to the Canadian Biosafety Standard(CBS). This guideline provides guidance on how to identify and mitigate dual-use potential in research involving pathogens, toxins, or other infectious material. In addition, the governance of research with dual-use potential in Canada and other international jurisdictions is discussed. This guideline is intended to assist regulated parties in meeting the requirements specified in the CBS, but should not be interpreted as requirements. Regulated parties may choose alternate approaches to meet the requirements specified in the CBS.
This guideline is continuously evolving and subject to ongoing improvement. The PHAC and the CFIA welcome comments, clarifications, and suggestions for incorporation into future versions. Please send this information (with references, where applicable) to:
PHAC e-mail: PHAC.pathogens-pathogenes.ASPC@canada.ca
Abbreviations and acronyms
- BSO
- Biological safety officer
- CBH
- Canadian Biosafety Handbook
- CBS
- Canadian Biosafety Standard
- CFIA
- Canadian Food Inspection Agency
- DURC
- Dual-use research of concern
- HAA
- Health of Animals Act
- HAR
- Health of Animals Regulations
- HPTA
- Human Pathogens and Toxins Act
- HPTR
- Human Pathogens and Toxins Regulations
- IBC
- Institutional biosafety committee
- PAO
- Plan for Administrative Oversight
- PHAC
- Public Health Agency of Canada
- RG
- Risk Group (i.e., RG1, RG2, RG3, RG4)
- SSBAs
- Security sensitive biological agents
- WHO
- World Health Organization
Chapter 1 - Introduction
The words in bold type are defined in the glossary found in Chapter 4.
Over the past few decades, research in life science and biotechnology have led to tremendous improvements to public health, animal health, the environment, and the food supply. However, advances in some areas such as infectious diseases, genetic modification (including genetically modified organisms and foods), genomics, synthetic biology, and stem-cell research have raised a number of significant social, legal, and ethical issues.Footnote 1,Footnote 2 The concern is that certain types of organisms, research, and technologies may have the potential for detrimental consequences to public health and safety, the animal population, the environment, or national security. As a result of concerns for these consequences, governments around the world have been debating, and in many instances implementing, formal oversight of dual-use research.
1.1 What is dual-use in life science research?
While there are various definitions of dual-use, the Public Health Agency of Canada (PHAC) defines dual-use potential as the qualities of a pathogen or toxin that allow it to be either used for legitimate scientific applications (e.g., commercial, medical, or research purposes), or intentionally misused as a biological weapon to cause harm (e.g., bioterrorism).
The definition of dual-use potential also encompasses any asset related to a biological agent that could be used for nefarious purposes, including knowledge, technologies, or products that contribute to the weaponization of a pathogen or toxin. Examples include the creation of a high risk pathogen or toxin, the development of a dispersal method, or the increase in risk of an existing pathogen or toxin. Another example is the knowledge gained through research on drug resistant microorganisms. While a better understanding of the resistance mechanisms could lead to improved treatment, the same information could also be used to develop organisms capable of evading drugs.
In Canada, the pathogens and toxins that have been identified as having dual-use potential are referred to as security sensitive biological agents (SSBAs) and are described as “prescribed human pathogens and toxins” in the Human Pathogens and Toxins Regulations (HPTR).Footnote 3
1.1.1 The history of dual-use in life science research
The field of molecular biology has expanded very rapidly since the late 1970s, with the discovery of cloning vectors, restriction endonucleases and ligases, the introduction of Sanger dideoxy sequencing, and polymerase chain reaction (PCR). Likewise, it has become much easier to analyse, modify, synthesize, and combine nucleic acids. Whole genomes can be sequenced or synthesized quite rapidly, and targeted genetic modifications can be made (e.g., using CRISPR technology). The rise of such technologies and the ability to create or modify living organisms has also led to concerns about their potential for dual-use.
The need for greater governance over research with biological agents was brought to the forefront in 2001 after letters containing Bacillus anthracis spores (the causative agent of anthrax) were mailed to two United States Senators and several news media offices, killing five people and infecting 17 others. A subsequent investigation revealed that the spores originated from a biodefense centre and that the person accused of sending the letters was a scientist who had access to the spores and the skills necessary to produce them.Footnote 4
Over subsequent years, advancements in science have also sparked concern over research with dual-use potential. Table 1-1 presents examples of such research that highlight the concern over intentional misuse of pathogens and toxins or technologies and have led to a global discussion on the oversight of dual-use research.
| Year | Research | Dual-use potential |
|---|---|---|
| 2001 | Creation of a genetically engineered mousepox virus as a form of pest control unexpectedly resulted in a much more virulent mousepox virus.Footnote 5 | A similar procedure could increase the virulence of smallpox or other poxvirus that infects humans and result in a vaccine-resistant virus. |
| 2002 | Synthesis of infectious poliovirus using in vitro biochemical manipulation and the published structure and nucleotide sequence of the poliovirus genome.Footnote 6 | A virus could be resurrected through assembly of oligonucleotides based on genomic information. |
| 2002 | Molecular engineering of a Variola virus protein to study the virulence of Variola and vaccinia virus resulted in a vaccinia virus with an increased virulence.Footnote 7 | The research information could be used to either increase the virulence of Vaccinia virus or reduce the effectiveness of vaccines against the smallpox virus. |
| 2005 | Reconstruction of the influenza A (H1N1) virus responsible for the 1918 Spanish flu pandemic to better understand the virulence of the influenza virus in order to evaluate current and future public health interventions.Footnote 8 | The reconstructed virus and the research information could be used to inflict harm. |
| 2011 | Creation of highly pathogenic strains of A/H5N1 avian influenza virus with enhanced transmissibility in mammals so that mammal-to-mammal airborne transmissibility could be studied, to raise awareness of the significant threat of H5N1 to public health, and to assist in the development of influenza vaccines.Footnote 9,Footnote 10 | The increased risk of an accidental release of the pathogenic virus from a laboratory and the information in the manuscripts could be misused to endanger public health or national security. |
1.2 Scope
The Dual-Use in Life Science Research guideline provides comprehensive guidance on how to identify research with dual-use potential and how to mitigate the risks. In Canada, all persons handling and storing human pathogens and toxins have a responsibility to be cognisant of the potential biosafety and biosecurity risks inherent in their activities, and are required to take all reasonable precautions to mitigate the risks associated with the human pathogens and toxins handled or stored (HPTA 6).Footnote 11 This guideline is meant to increase awareness on dual-use and to promote the responsible conduct of research among scientists, educators, senior management, biological safety officers (BSOs), funding organizations, policy and decision makers, and the public. It is to be used in conjunction with the Canadian Biosafety Standard(CBS) and the Canadian Biosafety Handbook (CBH).Footnote 12,Footnote 13
The information and recommendations provided in the Dual-Use in Life Science Research guideline are intended to be guidance and are not to be interpreted as requirements.
1.3 How to use the Canadian Biosafety Guideline: Dual-Use in Life Science Research
A detailed list of all abbreviations and acronyms used throughout this guideline is located at the beginning of this document. Each word or term is spelled out upon first use, with the abbreviation or acronym immediately following in brackets. After its initial definition, the abbreviation is used exclusively throughout the remainder of the document. A comprehensive glossary of definitions for technical terms is located in Chapter 5 of this document. Terms defined in the glossary appear in bold type upon first use in the guideline. Where the guidance relates to a requirement from the legislation (i.e., HPTA or HPTR), the specific section and subsection(s) will be referenced (e.g., HPTA 33). A list of references and other resources is provided in Chapter 6.
Chapter 2 - Governance of research with dual-use potential in Canada
The potential risks posed by dual-use research can be mitigated through biosafety and biosecurity programs. Biosafety describes the containment principles, technologies, and operational practices that are implemented to prevent unintentional exposure to pathogens or toxins, or their accidental release. In comparison, biosecurity refers to the security measures designed to prevent the loss, theft, misuse, diversion, or intentional unauthorized release of infectious material, toxins, and related assets (e.g., personnel, equipment, non-infectious material, and animals). These concepts are not mutually exclusive and are inherently complementary, as the implementation of good biosafety practices serves to strengthen the biosecurity program and vice versa.
With an increased international focus on dual-use research, there has been a call to action to review the appropriateness and effectiveness of existing biosafety and biosecurity measures in safeguarding against the risks posed by dual-use research. A number of countries have put mechanisms in place for the governance of dual-use research in order to balance the need to support scientific research while also protecting against potential harm to domestic and global health and security. This chapter provides a summary of oversight mechanisms in Canada, opportunities for oversight throughout the research continuum, as well as ethical considerations that should be addressed when evaluating research with dual-use potential.
2.1 Canada
The oversight of dual-use pathogens and toxins, goods, and technology in Canada is shared by various departments and agencies, including:
- Public Health Agency of Canada;
- Canadian Food Inspection Agency;
- Global Affairs Canada; and
- Canada Border Services Agency.
2.1.1 Pathogen and toxin regulation in Canada
In Canada, facilities that conduct controlled activities with human pathogens, including zoonotic pathogens (i.e., that infect both humans and animals), or toxins are regulated by the PHAC under the HPTA and HPTR, unless they meet the exclusion criteria specified in Section 4 of the HPTA. Facilities that are not excluded from the HPTA or exempted from the HPTR, require a Pathogen and Toxin Licence to conduct controlled activities with a human pathogen or toxin, and must comply with the applicable requirements specified in the CBS.Footnote 12 The importation of animal pathogens is regulated by the PHAC or the Canadian Food Inspection Agency (CFIA) under the Health of Animals Act (HAA) and the Health of Animals Regulations (HAR).Footnote 14,Footnote 15
The HPTA, HPTR, and CBS have specific requirements for SSBAs and dual-use research involving human pathogens and toxins.Footnote 3,Footnote 11,Footnote 12 Non-indigenous animal pathogens (i.e., pathogens causing foreign animal diseases) and pathogens causing emerging animal diseases also have specific requirements under the CBS, HAA, and HAR.
Some of the key elements of the HPTA and HPTR intended to mitigate the risks of dual-use include the following:
- Requirement for an HPTA Security Clearance to work with, or have access to SSBAs (HPTR 10[1]).
- Listed trigger amounts of SSBA toxins (HPTR 10[2]).
- Mandatory reporting to the PHAC of inadvertent production or release of pathogens and toxins, and if they are stolen or missing, and if an SSBA is not received within 24 hours of when it was expected (HPTR 4[2], 9[1]).
- Prohibition on the handling and storing of certain human pathogens and toxins (HPTA 8).
- Mandatory reporting to the license holder and the BSO of any activities that could result in the creation of a human pathogen with increased virulence, pathogenicity, or communicability, that is resistant to preventative or therapeutic treatments, or produces a toxin with increased toxicity (HPTR 5). This requirement also applies to work that is not considered dual-use. Should the modification result in a pathogen of a higher risk group (e.g., a modified Risk Group 2 [RG2] pathogen is reclassified as RG3), it may require notification of the PHAC as a case of inadvertent possession, or an amendment to the licence may have to be submitted to the PHAC.
- Requirement for Pathogen and Toxin Licence applicants who intend to carry out scientific research to submit a Plan for Administrative Oversight for Pathogens and Toxins in a Research Setting (PAO) with their license application (HPTR 3).Footnote 16 Ten common administrative elements are required in a PAO and include the following elements specific to dual-use:
- Overview of how biosafety and biosecurity risks, including those from research with dual-use potential, are identified at the institution/organization;
- Overview of how biosafety and biosecurity risks, including those from research with dual-use potential, are assessed once they have been identified at the institution/organization; and
- Overview of how biosafety and biosecurity risks, including those from research with dual-use potential, are managed/controlled at an institutional/organizational level.
The PAO provides an overview of how biosafety and biosecurity risks, including those from research with dual-use potential, are assessed and mitigated once they have been identified at an institutional/organizational level. The purpose of the PAO is to demonstrate that organizations have internal accountability structures in place for the oversight of research involving pathogens and toxins. These include measures for managing and controlling biosafety and biosecurity risks, as well as for identifying, assessing, and managing research activities with dual-use potential.Footnote 16
2.1.2 Export from Canada
The Export and Import Permits Act (EIPA) implements export controls through the Export Control List (ECL) and is administered by Global Affairs Canada and the Canada Border Services Agency.Footnote 17,Footnote 18 Residents of Canada wishing to export any materials included on the ECL or to a country included in the Area Control List must first receive a Permit to Export from the Global Affairs Canada Export Controls Division.Footnote 19 The ECL lists under the Export and Import Permits Act include:
- Dual-use list (goods and technology), which fulfills Canada’s agreement under the Wassenaar Arrangement; and
- Chemical and Biological Weapons Non-Proliferation List, which fulfills Canada’s agreement in the Australia Group.
Canada also implemented controls that cover the export of items not listed elsewhere on the ECL. The ECL imposes a permit requirement on any item destined to an end-use or end-user involved in the development, production, handling, operation, maintenance, storage, detection, identification, or dissemination of chemical or biological weapons, nuclear explosive or radiological dispersal devices, or their missile delivery systems. Before exporting any items, exporters must be satisfied that their exports are not being transferred, directly or indirectly, to such an end-use or end-user.
2.2 Opportunities for oversight throughout the research continuum
There are opportunities throughout the research continuum to increase awareness about dual-use, identify research with dual-use potential, assess the risk, and when applicable, apply mitigation measures. The stages of the research continuum identified in Figure 2-1 are not exhaustive, but are examples of instances where guidance, oversight, and control of life science research may be applied.

Figure 2-1 - Text Equivalent
Figure depicting six gear wheels, each representing opportunities for oversight throughout the research continuum, that progresses from left to right. The first wheel on the left represents Education and Development, more specifically Education (such as high schools, colleges and universities); training of researchers (such as co-ops and internships) and science and technology competitions (such as iGEM). This Education and Development wheel turns clockwise into the second wheel that represents Capacity and Infrastructure. The Capacity and Infrastructure includes established research program and institution, funding streams, professional networks, laboratory infrastructure development, laboratory protocols and procedures development, and codes of conduct. This wheel turns counter-clockwise into the third wheel that represents Planning. Planning encompasses project concepts, study design and review, the initial review for dual-use potential, and discussion with collaborators. The Planning wheel turns clockwise into the fourth wheel that represents Approval and Funding, including the review and approval of research proposals. The Approval and Funding wheel turns counter-clockwise into the fifth wheel that represents the Research. Research includes on-going research, training of laboratory personnel, risk management strategies, periodic review and adjustment of study design, institutional oversight, and presentation at seminars and conferences. The Research wheel turns clockwise into the sixth and final wheel of the figure representing Results. Results include manuscripts for peer review, publications, patents, new products, and new research.
2.2.1 Education and development
In research, dual-use potential exists from the moment a scientific hypothesis is conceived, right until the experimental results are published. Safe and responsible conduct of research can be promoted at many instances throughout this process, but opportunities exist earlier on. Gaining the skills needed to assess the risks and benefits of research, and the ethical implications of the work at the earliest stage possible, will help individuals put the knowledge to use in their research plans. Given the converging nature of research and innovation, extending outreach beyond the life science fields (e.g., to engineers, computer scientists, biochemists, and social scientists) and outside conventional research settings (e.g., Do-It-Yourself Biologists) will help create awareness among others whose work may lead to potential dual-use applications. For example, awareness can be promoted through high schools, colleges and universities, and in science and engineering competitions, thereby shaping future scientists to be ethical and judicious at the earliest stages of their careers.
Among scientists, responsible conduct of science and professional standards can be learned during undergraduate and graduate studies, or informally through mentorship by senior researchers. Organizations should also decide if additional dual-use training or awareness is required for their personnel, based on their overarching risk assessment and training needs assessment (Matrix 4.1 of the CBS).Footnote 12
2.2.2 Capacity and infrastructure
Within an organization, there are opportunities to establish internal mechanisms for dual-use oversight (e.g., internal processes to obtain approval to work with biological agents that may be linked with the release research funds). Approaches can vary and may include the development and implementation of training programs, codes of conduct, and strategic risk communication plans. Institutional committees trained in risk management, as well as research ethics committees, could offer guidance to researchers, and provide an additional layer of oversight.
2.2.3 Planning
Before research is conducted, the project design should be evaluated for the possibility of dual-use potential and the means to balance the risk of misuse against the benefits of research. It is during this phase wherein the overall design of the research proposal is reviewed for potential adverse impact and consideration given to adding mitigation measures or, when possible, modifying the proposed research to lower the risk. Discussions among principal investigators, research and laboratory staff, collaborators, senior management, institutional biosafety committees (IBC), applicable regulatory bodies, and funders are encouraged and should be iterative to facilitate the development of an appropriate risk management plan.
2.2.4 Approval and funding
Policies and guidelines that govern the administration, award, and use of grants vary from complete oversight of funded projects to self-governance by the funded institution or organization. Nevertheless, organizations have the ability to control the work performed under their purview, whether through research ethics or biosafety review, control of funding, or other mechanisms (e.g., participation in mandatory training and meeting a particular standard). Whatever the approach, risk mitigation is most effective when all impacted stakeholders are engaged, and having all the parties participate in the dialogue will avoid unnecessarily impeding responsibly conducted research. These stakeholders may include funders, senior management, researchers, IBC, and regulating bodies.
2.3 Ethical considerations
Ethics in life science research has historically focused on the protection of human and animal research subjects, rather than biosafety and biosecurity issues. Many funding agencies have implemented policies on scientific integrity, without the consideration of ethical issues such as research with dual-use potential. The history of dual-use demonstrates the importance of a thorough ethical review of any proposed research conducted within an organization.
An ethical review of scientific research should involve many players within an organization. Researchers have the most knowledge of their work and are in a key position to weigh the potential harms and benefits of their project(s), but the ethical review should not be left to the researcher alone. Additional input may be provided by the BSO, IBC, research collaborators, funding agencies, relevant government departments and/or regulators, and when appropriate, the public. The ethical review should consider both the benefits of the work, such as the progress of innovative scientific research, as well as the protection of public health, safety, and security. The goal of the review is to reach an agreement on a risk profile that is acceptable to the organization, given the potential benefit to science.
The World Health Organization (WHO), in their Responsible Life Sciences Research For Global Health Security guidance, lists several key ethical questions to be considered when evaluating such research.Footnote 20 These questions may be used by organizations as guiding principles when conducting an ethical review of research:
- How are the potential benefits of research weighed against the risks for misuse? On what criteria should this assessment be based?
- How are the individual interests of researchers weighed against the common good of public health? Who should make these decisions? How can tensions between individual researchers and institutions or society best be managed?
- How are the risks associated with research best managed without hindering its beneficial application to public health?
- What are the responsibilities of individual researchers and of the scientific community as a whole?
Chapter 3 - Risk assessment of research with dual-use potential
In research, a balance must be achieved between the pursuit of innovation, and the protection of the public health through regulation. In Canada, a risk-based approach is used to support innovative research with human pathogens and toxins, while protecting the health and safety of Canadians. Facilities where scientific research is conducted are required to submit a plan (i.e., the PAO) that describes how they administratively manage and control biosafety and biosecurity risks, including risks from research with dual-use potential. Footnote 16 This includes the following types of risk assessments:
- Overarching risk assessment: Broad assessment of the program intent and planned activities at the organization or facility level. It informs the development of the biosafety program, which may include biosafety and biosecurity policies, risk management plan, personnel management, Biosafety Manual, medical surveillance program, emergency response plan, facility maintenance, and training program.
- Pathogen risk assessment: Assessment of the intrinsic properties of the biological agent or toxin. It can be used to evaluate proposed modifications and potential impacts on the parental organism.
- Local risk assessment: Site-specific risk assessment used to identify hazards based on the infectious material or toxins in use and the activities being performed.
- Biosecurity risk assessment: Assessment of risks associated with the loss, theft, diversion, or intentional unauthorized release of pathogens, toxins, and related assets.
While risk assessments and the development of risk mitigation strategies can include contributions of a broad range of individuals (e.g., a multidisciplinary team that may include the BSO, principle investigator, IBC, and personnel), it is the principal investigators who are the most knowledgeable about their work and should be the first to recognize dual-use potential from the planning stage through to completion. At the same time, oversight by the organization, funding agencies, and even peers, can play a role. If an IBC is involved, members should have knowledge, skills, and expertise in a wide range of fields to ensure that a thorough risk assessment can be performed without unduly restricting the research.
This chapter describes considerations for the identification and assessment of research with dual-use potential and the mitigation measures that may be implemented to protect the safety of personnel, the public, the animal population, and the environment.
3.1 Identify research with dual-use potential
A key component to mitigating risk is the early identification of research activities that could be misused to the detriment of humans, the animal population, the environment, or national security. Every research project should be reviewed for dual-use potential during the planning stages, throughout the course of the project (including when there are unexpected results), and prior to the use or dissemination of the results (including publication). The on-going review process should be a shared responsibility between individuals involved in the design and conduct of research, as well as members of the organization that oversee the facility’s biosafety and biosecurity practices.
Identification includes an evaluation of the proposed research and the organisms. The pathogens handled and work performed are evaluated from the early planning stages until the work is complete and the results analyzed, as dual-use potential can appear at any time, often inadvertently. There are several considerations that can help guide decisions on whether work has a dual-use potential. Questions related to the organism or toxin handled include asking:
- Is the organism or toxin an SSBA?
- What microorganisms, toxins, or parts thereof (including nucleic acids) are involved in the work? Are they harmful to humans, animals, or the environment?
- Are parts of different pathogens or organisms being combined?
- Is the pathogen or toxin novel?
- Has the pathogen been eradicated or is it extinct?
Questions related to the work planned include:
- Is a pathogen or toxin being modified and may this modification result in a higher risk pathogen or toxin?
- Is a pathogen being created or re-created and may the resulting pathogen result in a higher risk than the components?
- Are unexpected consequences possible if the pathogen or toxin is released from containment?
- Does the research contain or produce novel information that could be used to threaten public health, animal health, or the environment, does it evaluate modes of delivery (e.g., aerosolization and transmissibility), or does it highlight a vulnerability in public health or public safety preparedness or point out a gap in the regulatory oversight regime?
- Is there a potential for misuse of the knowledge or technology? How easy would it be for someone who intended to use the information from the proposed (or completed) research to do harm?
Keeping in mind considerations related to the organism or toxin handled as well as to the work planned, the decision tree presented in Figure 3-1 can serve as a guide to identify research with dual-use potential.

Figure 3-1 - Text Equivalent
Figure is a decision tree consisting of three steps (i.e., questions) leading to two boxes at the bottom of the figure concluding either dual-use or no dual-use potential. Step 1 of this decision tree, represented at the top of the figure, is the question: Are you creating, re-creating, or modifying a new or existing pathogen? If the answer is Yes, an arrow points down towards a second step on the left (step 2a), and if the answer is No, another arrow points down towards a second step on the right (step 2b). At step 2a, the question is: Will the pathogen(s) acquire any of the following potential hazards: Increase in virulence, Production of novel toxin, Enhance communicability or transmissibility, Alteration of host range, Interfere, by-pass or diminish the effectiveness of diagnostic tools and therapeutic or prophylactic antimicrobial or antiviral treatments, and Enhance capacity for spreading or for easy release or making them “weapons-grade”? The answer Yes to this question leads to an arrow pointing down to step 3, while the answer No results in following an arrow pointing to the right to step 2b. The question at step 2b is: Is there a potential for research knowledge (e.g., data, methodology, results), technologies, and intermediate or final products (e.g., toxins) to be misused? The answer No to this question results in following an arrow to a box at the bottom right of the figure with the finding that there is No Dual-Use; however, the answer Yes leads to an arrow pointing to step 3. At step 3, the question is: If released, will the pathogen or research information pose a threat to Humans, Terrestrial animals, invertebrates or plants, Aquatic animals, invertebrates or plants, and/or Public safety? The answer No to this question results in following an arrow pointing to the green box concluding No Dual-Use. Answering Yes results in following an arrow to a red box on the bottom left of the figure concluding there is a potential for Dual-Use.
Step 1: Will a new, existing, or extinct pathogen be created, re-created, or modified?
The first step is to determine whether the research is creating, re-creating, or modifying a new or existing pathogen. This can include adding genetic information (e.g., drug resistance plasmid), modifying the genome of existing organisms, combining genetic information from two different organisms, or assembling genetic information into a genome (e.g., combining synthetically produced oligonucleotides).
Step 2a: Will the pathogen acquire any new potential hazards?
The second step allows individuals to critically consider the potential impact of the experimental procedures and manipulations on the characteristics of a pathogen or toxin to be generated. A review of the scientific literature can be performed to verify if any unexpected results have been observed in similar studies. The following factors should be considered:
- Increased transmissibility (i.e., the ability of a pathogen to be transmitted from one host to another);
- Increased pathogenicity (i.e., the ability of a pathogen to cause disease);
- Increased ecological fitness of a pathogen;
- Ability to evade the immune system;
- Production of a new toxin or an increase in toxicity;
- A change in the host species that the pathogen is able to infect;
- Resistance or increased resistance to existing antimicrobial or antiviral treatments;
- A change in the effectiveness of prophylactic (i.e., preventive) treatments;
- Diminished ability for a pathogen to be detected using standard diagnostic tools; and
- Enhanced capacity to be spread, transmitted, or easily released (i.e., the ease with which it can be used as a weapon).
The intrinsic properties of the biological agent or toxin, or the components used, are evaluated in a pathogen risk assessment that takes into account the proposed modifications and potential impacts on the parental organism. Surrogate information from genetically-related pathogens may be used to assess the risk of novel pathogens with unknown pathogenicity. Similarly for novel toxins with unknown toxicity, surrogate information from a structurally similar toxin can be used.
Step 2b: Is there a potential for research knowledge, technologies, and intermediate or final products to be misused?
In the case where no creation, re-creation, or modification of a new or existing pathogen is expected, the potential for research knowledge (e.g., data, methodology, results), technology, and intermediate and final products (e.g., toxins) to be misused should be considered. These may include nucleic acid sequences of SSBA pathogens or key virulence genes, techniques or technologies for delivery (e.g., aerosolization, transmissibility studies), facility engineering designs and specifications, the Biosafety Manual and standard operating procedures, novel techniques (e.g., for dispersal and delivery of biological agents, or to alter drug susceptibility), and bioinformatics (e.g., to manipulate drug resistance or pathogenicity).
Step 3: What would be the threatened if the pathogen or research information were released?
The final step evaluates the threat to human and animal health, the environment, and public safety, should the pathogens, toxins, or knowledge be released. It is meant to consider if the research results can be misused to cause rapid or widespread infection, illness, or death, or to hinder disease surveillance, detection, diagnosis, and containment of the pathogen. It is also meant to evaluate the potential for the research results to be intentionally used to alter ecosystems, displace species, or adversely affect the sustainable use of biological diversity, particularly in resource sectors such as agriculture, fisheries, and forestry. Such considerations include the potential for the research to pose a threat to national security resulting from the direct or indirect misuse of published scientific information related to the pathogens or toxins or the use of pathogens or toxins to plan an attack against humans, the animal population, or the environment.
Another aspect that should be considered is public reaction if the research is completed and published, whether “as-is” or in abridged form. Could it lead to widespread concern or anxiety about public health, animal health, or other public safety and security concerns? Is there the possibility of public misunderstanding and, if so, what are the implications of such misunderstanding? Is there a possibility for sensationalism (e.g., exaggeration of the potential benefits, risks, or impacts) on the part of the authors or media?
3.2 Risk assessment of research with dual-use potential
When research with dual-use potential is identified, it is necessary to assess the risk associated with the research. The determination of risk takes into consideration the hazards identified, the likelihood of an event, and the consequences should an event occur. A proper risk assessment will guide the selection of appropriate mitigation strategies to protect the research materials, tools, and information against loss, theft, misuse, diversion, or intentional unauthorized release.
Some of the factors that may influence the likelihood of an incident resulting from dual-use research include the following:
- Laboratory conditions: What laboratory conditions are required to repeat or scale-up the experiment? This assesses whether the work can only be performed in a fully equipped academic laboratory, or if it can be repeated in a makeshift laboratory.
- Technical skills: What technical skills are required to repeat or scale-up the experiment? This assesses whether the work could only be repeated by a highly trained individual or if it could be reproduced by a person lacking the technical skills.
- Ease to acquire resources: How easy is it to acquire materials, tools, and equipment to repeat or scale-up the experiment? Are expensive or highly specialized materials and equipment needed?
- Timeframe: How quickly could the work be used to cause harm (e.g., immediate, near future)? This assesses whether the research could be used right away to cause harm, or if more work is needed to make the results of the research harmful.
- History of occurrence: Has a similar event of misuse of a pathogen, information, or technology occurred previously within the organization, the region, the country, or elsewhere globally? If there are known incidences, how often did they occur?
- Knowledge of insider or outsider threats: Is there evidence that a group or individual is interested in maliciously obtaining the pathogen, information, or technology?
The second factor to consider when assessing risk is the consequence of an incident, should it occur. This is the potential impact to the environment, public health, the animal population, the economy, or national security if the pathogen, toxin, equipment, or information is released. The severity of harmful impact should be evaluated (e.g., local or widespread, mild or moderate illness, death, impact on economy, public fear, and minor interruption).
The result of a dual-use assessment, or of any risk assessment, is the determination of the risk and if it needs to be mitigated. The risk is a grouping into a risk category, which can range from “very low” to “very high”. Organizations can decide on the scale that best suits their situation. Examples include a scale of three (e.g., low, moderate, high), five (e.g., very low, low, moderate, high, very high), and a numerical scale (e.g., 1 to 10, 1 to 100) where lower numbers indicate a lower risk.
3.2.1 Risk-benefit analysis
A final element of the risk assessment process is an evaluation of the potential benefits of the research. This will include the potential benefits to public health, animal health, and safety from the research, as well as the scope of the possible benefits and the time frame (e.g., immediate, near future or years away) the benefits could be seen. Research that offers a potential solution to an identified vulnerability for public health, animals, agriculture, plants, the environment, or material may be seen as a significant benefit. An essential element of the communications surrounding research with dual‑use potential is a discussion of the benefits of the research results. If the risks associated with the research concerned are deemed to be too high with respect to the anticipated benefit, the project may need to be modified or cancelled.
3.3 Risk mitigation
Risk mitigation is a process by which specific measures are put into place to minimize the likelihood of an incident or its consequences should it occur. Appropriate mitigation measures are commensurate to the level of risk and include physical, operational, and security measures that should be implemented, monitored, and enforced by organizations (e.g., BSOs, senior management, IBC, funders, and regulators).
It is important to keep in mind that a risk mitigation strategy cannot reduce risks to zero, unless the work is avoided altogether. The goal should be to adequately and appropriately manage the identified risks to a level that is acceptable (i.e., the risk tolerance threshold, which is determined by senior management). Research with risks that cannot be mitigated to acceptable levels may have to be modified, or the work terminated or moved to a facility with an appropriate level of mitigation controls.
Basic mitigation strategies for research with dual-use potential should include:
- Adherence to the applicable physical containment requirements, operational practice requirements, and performance and verification testing requirements specified in the CBS, and any national or international agreements, laws, or regulations pertaining to dual-use biological agents and technology (e.g., biosafety and biosecurity requirements, export controls, and biological weapons proliferation treaties).Footnote 12
- Development of a comprehensive biosecurity plan to address potential concerns related to access to pathogens or toxins, knowledge, information, technology, or products.
In facilities that are regulated under the HPTA, principal investigators and research and laboratory staff are encouraged to discuss their specific project plans with the BSO or licence holder to assess the adequacy of the existing risk management plan.Footnote 11
Consideration should be given to using a modified experimental design or method, or an alternative pathogen or toxin to reduce risk, if such a change would yield equivalent information. For example, an attenuated strain of a pathogen could be used, or a strain with reduced ability to proliferate outside of the laboratory environment or within different hosts (e.g., humans). If there are no existing countermeasures (i.e., prophylactics, therapeutic treatments) available for the pathogen or strain, consideration can be given to whether or not the overall research aims can still be met by using a strain or toxin that is susceptible to available countermeasures.
3.3.1 Biosafety and biosecurity measures
Following the identification of research as having dual-use potential, a risk assessment will evaluate whether or not the existing biosafety and biosecurity mitigation measures are adequate. It may be determined that additional measures are necessary to effectively manage the identified risk(s) associated with the dual-use research. This may include specific additional precautions such as administrative controls (e.g., use of additional personal protective equipment, more stringent personnel screening) or physical requirements (e.g., conducting the work at a higher containment level or with increased physical security). In cases where the potential for increasing the risks associated with the microorganism are uncertain, it may be prudent to work at a higher containment level with increased security, until it has been determined that the organism can be safely and securely handled at a lower containment level. Discussions with collaborators, senior management, IBC, applicable regulatory bodies, and funders can help in the development of appropriate risk mitigation strategies.
3.3.2 Training and awareness
Training is a cornerstone of biosafety and biosecurity, and is required for all regulated facilities where pathogens and toxins are handled and stored. In terms of dual-use potential, training provides all personnel, including principal investigators, technicians, students, members of the IBC, and senior management with an understanding of what constitutes dual-use potential and how to identify it. Awareness among these individuals makes it possible to identify dual-use potential throughout the research continuum so that the associated risks can be brought to the attention of senior management, and that mitigation strategies can be developed and implemented as needed.
Within an organization, training provides an opportunity to establish a mechanism for dual-use oversight that is enforceable (e.g., access to facility is not authorized until training is complete). Organizations can develop their own dual-use and biosecurity training programs or use external training materials. The PHAC’s e-learning portal has many free online training courses including Introduction to Dual-Use in Life Science Research and Introducing Biosecurity. The University of Bradford, the American Biological Safety Association, the National Science Advisory Board for Biosecurity, and the Federation of American Scientists, also have training materials available.
3.3.3 Research
During research, risk mitigation measures should include a periodic review of the research and preliminary data for dual-use potential. If identified, a risk assessment should be conducted taking into account the new information. It is at this stage of the research continuum where it is important to re-evaluate and, if warranted, modify or stop the research and adjust the existing communication plans and risk mitigation measures. For example, depending on the risk identified, enhancement of laboratory biosafety and biosecurity measures corresponding to the level of risk might be required. Discussions with key stakeholders (e.g., BSOs, senior management, IBC) are also important to re-assess the risk and benefit of the research and determine if it should continue.Footnote 20
3.3.4 Communication plan
An integral element to mitigating risk from research with dual-use potential is an organizational plan on what information assets (e.g., research data and experimental protocols) are to be disseminated, and how security-sensitive information is communicated. In some cases, there may be the perception that the science can lead to the release of dangerous pathogens, which may be sensationalized in the media. Since public trust is essential for the continuation of meaningful life sciences research, the potential for public concern, misunderstanding, or sensationalism, should be considered. It is up to scientists to foster a better understanding of their science by regularly participating in outreach that raises awareness on the importance of the research and its responsible management. A communication plan can help guide individuals on when or how to appropriately communicate data or information.
Potential security considerations can impact how and when communication occurs, and what is communicated. Restriction of communication has the benefit of preventing adversaries from accessing the information, but carries the risk of slowing scientific progress and contributing to public fear of the science. Conversely, full and open communication allows for the rapid validation of findings and scientific progress, but has the disadvantage of allowing open access to the public, including adversaries. The decision to censor publication should never be taken lightly and the results of life science research should be communicated to the fullest extent possible.
Several items are important to remember in discussing communications of the results of dual‑use research. The first is that the decision to communicate is rarely a simple “Yes/No”. As well, research plans and findings are typically communicated at many points throughout the research process, including during: (1) project concept and design, (2) application for funding, (3) institutional approval, (4) current research, (5) development of publications or other communication of the research results, and (6) at the time of publication of the manuscript or other communication product. Responsible communication should be considered throughout the entire process.
3.3.4.1 Developing a communication plan
The development of a communication plan is essential and indicates what will be communicated, to whom it will be communicated, and how and when it will be communicated. A communication plan should be developed in consultation with senior management, IBC, funders, and relevant regulatory bodies, and should include the following elements and considerations:
- Content to communicate
- The physical and operational biosafety measures in place during research and compliance with national regulations and standards.
- The importance of the research (i.e., the benefits to public health and national security) and public reassurance that the research is being conducted responsibly. Benefits can include discussion of how the research could (or has) led to the development of countermeasures (e.g., vaccines, antibiotics, and antitoxins), or improved disease surveillance, preparedness, or response. Footnote 21
- Contextual information to minimize public concerns, misunderstanding, and sensationalism by highlighting the benefit, significance, and utility of the research for the scientific community and the public.
- Biosecurity considerations and compliance with federal legislation and international agreements.
- Target audience
- Is the target audience the scientific community or the general public? Will the information be widely distributed or kept confidential (e.g., limited to select individuals on a “need to know” basis)?
- The possibility that dual-use information may raise biosecurity concerns, not only within the scientific community but also within the general public, should be considered.
- Timing and communication method
- How will the research be presented in editorials, press releases, questions and answers, talking points, and other methods? How will the risk-benefit analysis be presented?
- How will responsible timing and level of detail be used when reporting the research content? Options could range from full and immediate communication, delayed and redacted communication, or restricted communication.
- A mechanism for pre-publication or pre-communication review within the organization should be developed.
3.3.4.2 The role of scientists and scientific journals
There have been many discussions about the role of scientists and scientific journals in the publication of research that could lead to malicious use of information. While there has been no consensus on an approach, there have been several positions on this issue.
In 2003, an editorial in the journal Nature, entitled the Statement on the Considerations of Biodefence and Biosecurity, recognized the special status of scientific information in peer-reviewed research journals.Footnote 22 Some of the key messages written in this statement were:
- The right balance of timely publication and strong models created to govern research design is needed to ensure that papers are effectively reviewed.
- If the potential harm of publication outweighs the potential social benefits, the paper should be modified or not published. The possibilities for other types of scientific communication are available to disseminate information which maximizes public benefits but minimizes risks of misuse.
The Committee on Publication Ethics (COPE) is a non-profit organization that supports editorial independence and is responsible for the development of a Code of Conduct for international editors and publishers.Footnote 23 According to COPE, editors should be responsible for everything published in their journals and they should strive to meet the needs of readers and authors, produce quality publications, champion freedom of expression, and maintain the integrity of the scientific record.
3.4 Monitoring and review
Risks can change over time based on new findings or technological developments. As with the pathogen and biosecurity risk assessments that are routinely reviewed and updated as needed, so too is the assessment of dual-use potential. The regular review of dual-use potential can help the organization, funding agency, PHAC, and CFIA confirm that the risks are being adequately and appropriately managed over time. If the potential for dual-use is discovered, the pathogen risk assessment and biosecurity risk assessment may have to be revisited. Similarly, if the risks associated with the work diminish (e.g., from the development of a vaccine), the mitigation measures may be proportionately reduced.
Chapter 4 - International oversight of research with dual-use potential
Canada is signatory to a number of international instruments, all of which are reflected in Canadian legislation, regulations, and policies. This chapter describes international initiatives that endeavour to identify and mitigate the risks associated with biological research, and provides the current global context on dual-use in life-science research.
4.1.1 Biological and toxin weapons convention
Internationally, deliberate development of microbial agents as a biological weapon has been prohibited since 1975 under the Convention on the Prohibition of the Development, Production and Stockpiling of Bacteriological (Biological) and Toxin Weapons and on their Destruction, specifically Article I which states: “Each State Party to this Convention undertakes never in any circumstances to develop, produce, stockpile or otherwise acquire to retain: (1) microbial or other biological agents, or toxins whatever their origin or method of production, of types and in quantities that have no justification for prophylaxis, protective or other purposes; (2) weapons, equipment or means of delivery designed to use such agents or toxins for hostile purposes or in armed conflict.”Footnote 24 The Convention is a key element in the international community’s efforts to address the proliferation of weapons of mass destruction. It has approximately 180 member states, including Canada.
4.1.2 The Wassenaar Arrangement
The Wassenaar Arrangement on Export Controls for Conventional Arms and Dual-Use Goods and Technologies, commonly known as the Wassenaar Arrangement, was established in 1996 in order to contribute to regional and international security and stability, by promoting voluntary transparency and greater responsibility in transfers of conventional arms and dual-use goods and technologies, thus preventing destabilising accumulations.Footnote 25 Participating States seek, through their national policies, to ensure that transfers of such items do not contribute to the development or enhancement of military capabilities that undermine the goals of the arrangement, and are not diverted to support such capabilities. Currently there are 41 participating states, including Canada.
4.1.3 The Australia Group
The Australia Group is an informal arrangement that aims to harmonize export controls to prevent exports from contributing to the development and proliferation of chemical and biological weapons. It was formed in 1985 and consists of 43 member countries, including Canada. In the 1990s, biological agents with the potential for dual-use were included in the export control lists. Canada implements the Australia Group’s List of Human and Animal Pathogens and Toxins for Export Control (i.e., common control list) through the Export and Import Permits Act, for dual-use biological equipment and related technology and software, human and animal pathogens and toxins, and plant pathogens.Footnote 17 In addition, the Australia Group’s common control list in incorporated into the HPTR as a criterion of prescribed human pathogens and toxins (i.e., SSBAs) in Canada.Footnote 26 In order for a human pathogen to be considered an SSBA, it must fall into RG3 or RG4 and be published on the common control list. Toxins found in Schedule 1 of the HPTA and on the common control list are also SSBAs.
4.1.4 United Nations Security Council Resolution 1540
The United Nations Security Council (UNSC) Resolution 1540 was adopted in 2004 to prevent the development of biological and other weapons and their related materials.Footnote 27 The resolution requires all States to adopt and enforce appropriate laws to this effect as well as other effective measures to prevent the proliferation of these weapons and their means of delivery to prevent non-State actors gaining access to them, in particular for terrorist purposes. Members of the United Nations, including Canada, must report on their compliance.
4.1.5 World Health Organization
In 2009, the WHO held a workshop on responsible life science research. The resulting publication summarized current information at the time, and put forth the concept of using three pillars of biorisk management to ensure control of life science research: (1) research excellence, (2) bioethics, and (3) biosafety and laboratory biosecurity. Footnote 20 In 2013, the WHO held an informal consultation on dual-use research of concern (DURC) to identify key issues relating to DURC and to explore possible measures to address gaps in existing management approaches for dealing with DURC.Footnote 28 While other international discussions on biological research have focused mainly on weaponization and security issues, the WHO has worked to raise awareness on the public health implications of dual-use research, while promoting responsible and innovative life sciences research on a global level.
4.1.6 United States
With the exception of select agents, oversight in the United States is limited to domestic or foreign institutions who receive funding from the United States Federal Government (e.g., National Institutes of Health [NIH]). Facilities funded by the NIH that intend on modifying nucleic acids or organisms are required to follow the NIH Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules.Footnote 29 Research that does not clearly fit the definitions of the guideline is referred to the NIH’s Recombinant DNA Advisory Committee. If the agent handled is a Select Agent, the NIH will defer to the appropriate Federal agency (i.e., United States Department of HHS or United States Department of Agriculture Select Agents Division).
The NIH Tool for the Identification, Assessment, Management, and Responsible Communication of DURC was intended to be a companion guide for institutions and principal investigators implementing the 2012 U.S Government Policy for Institutional Oversight of Life Sciences Dual Use Research of Concern.Footnote 30 These apply only to federally conducted or funded research but can also provide guidance to institutions not directly subject to the oversight mechanism.
Chapter 5 - Glossary
It is important to note that while some of the definitions provided in the glossary are universally accepted, many of them were developed specifically for the CBS or the CBH, and some have been modified to be applicable in the context of the Dual-Use in Life Science Research guideline.
- Biological safety officer (BSO)
- An individual designated for overseeing the facility’s biosafety and biosecurity practices.
- Biosafety
- Containment principles, technologies, and practices that are implemented to prevent unintentional exposure to infectious materials and toxins, or their accidental release.
- Biosecurity
- Security measures designed to prevent the loss, theft, misuse, diversion, or intentional release of pathogens, toxins, and other related assets (e.g., personnel, equipment, non-infectious material, and animals).
- Containment
- The combination of physical design parameters and operational practices that protect personnel, the immediate work environment, and the community from exposure to biological material. The term “biocontainment” is also used in this context.
- Containment level (CL)
- Minimum physical containment and operational practice requirements for handling infectious materials or toxins safely in laboratory, large scale production, and animal work environments. There are four containment levels ranging from a basic laboratory (containment level 1 [CL1]) to the highest level of containment (containment level 4 [CL4]).
- Dual-use potential
- Qualities of a pathogen or toxin that allow it to be either used for legitimate scientific applications (e.g., commercial, medical, or research purposes), or intentionally misused as a biological weapon to cause disease (e.g., bioterrorism).
- Exposure
- Contact with, or close proximity to, infectious material or toxins that may result in infection or intoxication, respectively. Routes of exposure include inhalation, ingestion, inoculation, and absorption.
- Incident
- An event or occurrence with the potential of causing injury, harm, infection, intoxication, disease, or damage. Incidents can involve infectious material, infected animals, or toxins, including a spill, exposure, release of infectious material or toxins, animal escape, personnel injury or illness, missing infectious material or toxins, unauthorized entry into the containment zone, power failure, fire, explosion, flood, or other crisis situations (e.g., earthquake, hurricane). Incidents include accidents and near misses.
- Microorganism
- A cellular or non-cellular microbiological entity, capable of replication or transferring genetic material and that cannot be reasonably detected by the naked human eye. Microorganisms include bacteria, fungi, viruses, and parasites, and may be pathogenic or non-pathogenic in nature.
- Pathogen
- A microorganism, nucleic acid, or protein capable of causing disease or infection in humans or animals. Examples of human pathogens are listed in Schedules 2 to 4 and in Part 2 of Schedule 5 of the Human Pathogens and Toxins Act, but these are not exhaustive lists. Examples of animal pathogens can be found through the Automated Import Reference System on the Canadian Food Inspection Agency website.
- Pathogen and Toxin License
- An authorization to conduct one or more controlled activities with human pathogens or toxins issued by the Public Health Agency of Canada under Section 18 of the Human Pathogens and Toxins Act.
- Pathogenicity
- The ability of a pathogen to cause disease in a human or animal host.
- Release
- The discharge of infectious material or toxins from a containment system.
- Risk
- The probability of an undesirable event (e.g., accident, incident, breach of containment) occurring and the consequences of that event.
- Risk group (RG)
- The classification of biological material based on its inherent characteristics, including pathogenicity, virulence, risk of spread, and availability of effective prophylactic or therapeutic treatments, that describes the risk to the health of individuals and the public as well as the health of animals and the animal population.
- Scientific research
- As defined in Section 1 of the Human Pathogens and Toxins Regulations: the following types of systematic investigation or research that are carried out in a field of science or technology by means of controlled activities:
- Basic research, where the controlled activities are conducted for the advancement of scientific knowledge without a specific practical application;
- Applied research, when the controlled activities are conducted for the advancement of scientific knowledge with a specific practical application;
- Experimental development, when the controlled activities are conducted to achieve scientific or technological advancement for the purpose of creating new – or improving existing- materials, products, processes, or devices.
- Security sensitive biological agents (SSBAs)
- The subset of human pathogens and toxins that have been determined to pose an increased biosecurity risk due to their potential for use as a biological weapon. SSBAs are identified as prescribed human pathogens and toxins by Section 10 of the Human Pathogens and Toxins Regulations. This means all Risk Group 3 and Risk Group 4 human pathogens that are in the List of Human and Animal Pathogens for Export Control, published by the Australia Group, as amended from time to time, with the exception of Duvenhage virus, Rabies virus and all other members of the Lyssavirus genus, and Vesicular stomatitis virus; as well as all toxins listed in Schedule 1 of the Human Pathogens and Toxins Act that are listed on the List of Human and Animal Pathogens for Export Control when in a quantity greater than that specified in Section 10(2) of the Human Pathogens and Toxins Regulations.
- (Microbial) Toxin
- A poisonous substance that is produced or derived from a microorganism and can lead to adverse health effects in humans or animals. Human toxins are listed in Schedule 1 and Part 1 of Schedule 5 in the Human Pathogens and Toxins Act.
- Virulence
- The degree or severity of a disease caused by a pathogen.
Chapter 6 - References and resources
Page details
- Date modified: