Infection Prevention and Control Measures for Prehospital Care and Ground Transport of Persons Under Investigation for Ebola Disease or with Confirmed Ebola Disease
Related links
Table of contents
- Introduction
- Aim and scope of this guideline
- Guideline development methodology
- Target users
- Role of provinces and territories
- Application of hierarchy of controls to EBOD: Engineering controls, administrative controls and personal protective equipment
- Occupational health considerations
- Routine practices
- Prehospital call assessment/triage
- Additional precautions for Ebola disease management
- Notifications
- Appendix A: Acknowledgements
- Appendix B: Algorithm for screening and assessment for Ebola disease in persons presenting to healthcare settings
- Appendix C: Management of Ebola disease waste and environmental cleaning for prehospital care and ground transport
- References
Introduction
Preamble
This update of the Infection Prevention and Control Measures for Pre-hospital Care and Ground Transport of Patients with Suspected or Confirmed Ebola Virus Disease (2019) has updated recommendations for:
- Classification of a Person Under Investigation (PUI) for Ebola disease (EBOD)
- Personal protective equipment
- Nomenclature
Background
Ebola Disease (EBOD) is part of a group of illnesses called Viral Hemorrhagic Fevers (VHFs) that are caused by several distinct families of viruses. The infection prevention and control (IPC) advice in this document is developed for EBOD, however, the guiding principles and IPC measures are applicable for management of persons under investigation or with confirmed disease in healthcare settings associated with agents that cause VHFs (i.e., Lassa, Marburg, Crimean-Congo).
EBOD is a severe acute viral illness that begins with fever, often with malaise, myalgia, and headache, and is typically followed by progressive gastrointestinal symptoms that include anorexia, nausea, and abdominal discomfort, followed by vomiting and diarrhea. The diarrhea and vomiting are often profuse in later stages of the illness and lead to severe volume depletion, electrolyte abnormalities, and shock. While hemorrhage may occur, usually from the gastrointestinal tract, it is a late manifestation and occurs in a minority of patients. The incubation period of EBOD varies from 2 to 21 days. People with EBOD are not infectious during the incubation period.
Risk and transmission
EBOD is transmitted by direct contact (i.e., through non-intact skin or mucous membranes) with the blood or other body fluids (e.g., stool, emesis, urine, saliva, semen and sweat) of an infected individual and/or indirectly through contact with environmental surfaces and fomites contaminated with blood or other body fluids. The risk of transmission increases with the amount of infectious materials to which the individual is exposed.
Cases are not communicable before the onset of symptoms, but communicability increases with each subsequent stage of illness. Individuals with EBOD are most infectious in later stages of their illness when viral load rises and they experience copious fluid loss due to diarrhea, vomiting or hemorrhage. Cases remain communicable as long as blood or other body fluids contain the virus. This includes the convalescence period, before they have recovered, and the post-mortem period.
Investigations conducted to date, taking into account the thousands of EBOD cases in Africa and the very small number of EBOD cases in Europe and the US, have not demonstrated human-to-human transmission of EBOD in the absence of direct contact with an infected case. EBOD is not spread through the airborne route.
Public health case management relies on early identification of EBOD cases, patient isolation and care, diligent contact tracing, appropriate infection prevention and control measures, and safe burial. The following document contains additional information on case and contact management in community settings:
Aim and scope of this guideline
The purpose of this guideline is to provide infection prevention and control (IPC) guidance for safe prehospital care and ground transport of a PUI for EBOD or person with confirmed EBOD.
Prehospital care includes acute emergency patient assessment and care delivered in a variety of settings (e.g., street, home, LTC, mental health facility) at the beginning of the continuum of care. Clinical judgement remains essential, and this, along with jurisdictional policies, may result in decisions that differ from recommendations provided in this document.
This document has been developed based on the Canadian context and therefore may differ from guidance developed by other countries. Recommendations for non-healthcare-related interactions or settings are beyond the scope of this document.
Guideline development methodology
PHAC developed this guideline with technical expertise from the National Advisory Committee on Infection Prevention and Control (NAC-IPC) and subject matter experts. The recommendations are informed by a review of the evidence, expert opinion and core IPC principles as identified in PHAC's Routine Practices and Additional Precautions for Preventing the Transmission of Infection in Healthcare Settings. This advice is based on currently available scientific evidence and expert opinion and adopts a precautionary approach where the evidence is lacking or inconclusive. It is subject to review and change as new information becomes available.
Please refer to Appendix A for a list of members.
Target users
This document is intended for prehospital personnel including, but not limited to, medical first responders, paramedics, emergency ground transport personnel, firefighters, enforcement officers, and personnel within prehospital organizations responsible for education and training for occupational health and safety (OHS) and IPC. The term "prehospital personnel" in this document refers to these personnel. Prehospital organizations include but are not limited to emergency departments, rehabilitation hospitals, mental health hospitals and long-term care facilities.
Role of provinces and territories
The advice contained in this document should be read in conjunction with relevant federal, provincial, territorial and local legislation, regulations, and policies and adapted to local requirements as necessary. Recommended measures should not be regarded as rigid standards, but principles and recommendations which may be used to inform IPC practice.
Application of hierarchy of controls to EBOD: Engineering controls, administrative controls and personal protective equipment
The hierarchy of controls is a fundamental occupational health and safety framework, designed to optimize protection of the worker from exposures to hazards, including infectious hazards such as EBOD. Following the hierarchy of controls will produce safer systems and reduce potential illness or injury for healthcare workers (HCWs).
Elimination
While elimination may not be possible, to mitigate risk of transmission, systems and protocols should be in place to limit the care of persons under investigation for EBOD or confirmed patients with EBOD to centres designated to care for these patients, where available. Prehospital transport staff should be aware of designated centres for EBOD care in their province or territory.
Substitution
Substitution as part of the hierarchy of controls is not a feasible approach to preventing transmission of EBOD.
Engineering controls
Engineering controls are used to either remove the infectious agent or put a barrier between the HCW and the infectious agent. Engineering controls are those elements of the healthcare organization's physical plant/infrastructure that function to prevent exposure to and/or transmission of the infectious agent at the source, or along it's path.
Examples of engineering controls related to prehospital care and transporting of a PUI or confirmed patient with EBOD include the following:
- Use of designated emergency vehicles dedicated to a single patient; ensure thorough cleaning and disinfection following use. Refer to Environmental Cleaning section.
- Removal of nonessential equipment from a designated vehicle as part of vehicle preparation. Avoid contamination of reusable porous surfaces not designated for single use through replacement or impermeable covering.
- Covering the stretcher with an impermeable material. Use of a stretcher mattress and pillow with plastic or other covering that fluids cannot penetrate.
- Method of isolation measures to prevent blood and body fluid exposure for transport dependent upon point-of-care risk assessment and source control. Refer to Classification of Persons Under Investigation for EBOD and Source Control sections.
- Performing aerosol-generating medical procedures (AGMPs) only when absolutely necessary, controlling the situation as much as possible, i.e., performed prior to transport. Refer to Source Control section.
- Use of safety-engineered sharps or devices.
- Use of point-of-use sharps containers with safety closures.
- Ensuring sufficient IPC supplies (e.g., personal protective equipment (PPE), disinfectant, hand hygiene products including point-of-care alcohol based hand rub, separate dedicated plastic-lined biohazard waste and linen receptacles, blood and body fluid containment products such as disposable absorbent pads, continence products) and other essential supplies and equipment are available for the duration of the transport.
- Providing ready-to-use or commercially prepared disinfectant wipes with a broad spectrum virucide claim and a Health Canada drug identification number (DIN). Use for immediate cleaning and disinfecting of surfaces that become potentially contaminated during transport. Refer to Environmental Cleaning section.
Administrative controls
Administrative controls include policies, procedures, education, training and patient care practices intended to prevent exposure to and/or transmission of an infectious agent during the provision of care and transport. Each organization should develop comprehensive policies and procedures for putting on and removing PPE. To be effective in preventing transmission of EBOD and/or detecting cases of EBOD, administrative controls should be applied from the first encounter with a PUI for EBOD and continued until the patient is accepted into a receiving hospital.
Examples of administrative controls for prehospital care and transport of a PUI for EBOD or patient with confirmed EBOD include the following:
- Ongoing education and training about Routine Practices and Additional Precautions including but not limited to prehospital call assessment/triage, point-of-care risk assessment, use of PPE including additional PPE, notification/communication, source control, personnel recommendations, hand hygiene, patient movement, sharps safety, patient equipment, cleaning and disinfection of equipment and transport vehicle, and handling waste and linen.
- Protocols for prehospital care and safe transport of a PUI or confirmed patient with EBOD including:
- Screening for EBOD risk factors and EBOD compatible symptoms during prehospital call assessment
- Prompt initiation of isolation measures and appropriate selection and use of PPE, including additional PPE based on a clinical and point of care risk assessment, as outlined in the Agency's Infection Prevention and Control Measures for Ebola Disease in Acute Care Settings and organizational requirements
- Containing and disposing of body fluids, (e.g., urine, stool, emesis) including the use of disposable bedpan/urinal with the addition of a solidifier and disposed of as waste, during the transport of the patient. Refer to Ebola Virus Disease: Management of Waste and Environmental Cleaning for Prehospital Care and Ground Transport
- Addressing critical interventions and/or issues such as need to stop the vehicle to manage breach of PPE, personnel exposure, or personnel requirements for bathroom breaks during extended transports
- Addressing vehicle breakdown or incident during transport
- Determining personnel ability to provide direct care to a PUI or confirmed patient with EBOD and protocols for incident management (including breach in PPE, post exposure and first aid). Refer to Occupational Health Considerations section.
- Assigning a trained monitor to each HCW/visitor entering the patient's room to coach and observe the putting on and removing of PPE. (Refer to Table 1 for roles and responsibilities of the trained monitor)
- Specialized training including drills in the selection, application, use, removal and disposal of PPE.
- Respiratory protection programs (i.e., respirator fit-testing, training).
- For more information on respiratory protection programs, see PHAC's Routine Practices and Additional Precautions guidance document.
- Limiting the number of prehospital and transport personnel to the minimum required to safely provide care and transport. Monitoring and maintaining a log of all personnel involved in prehospital care and transport.
| N/A | Trained Monitor |
|---|---|
| Responsibility | To assist with and ensure adherence to entire PPE use and removal process by HCWs providing direct patient care, and visitors (if permitted). |
| Role |
|
| Number Needed | One Trained Monitor at all times for each individual entering the room. |
Occupational health considerations
- Personnel should consult with OHS (or delegate) or their primary physician if there are concerns related to the ability of personnel to provide direct care. For details/examples of such conditions, refer to the Agency's Infection Prevention and Control Measures for Ebola Disease in Acute Care Settings.
- Personnel should be aware when returning from countries with travel advisories for EBOD to self-monitor for signs and symptoms of EBOD for 21 days after travel.
- Personnel should:
- Self-monitor while caring for a PUI or confirmed EBOD
- Notify OHS (or delegate) and Public Health (PH) if symptoms arise within 21 days following last contact with a PUI or confirmed patient with EBOD
- Follow any directives regarding self-isolation
- Eating or drinking should not occur in areas where direct patient care is provided.
- Personnel should avoid touching the mucous membranes of their eyes, nose, and mouth to prevent self-contamination.
- Personnel should report potential occupational/community exposure to EBOD (i.e., direct exposure without appropriate PPE, a breach in safely removing PPE, percutaneous injuries) to immediate supervisor, OHS or delegate and PH.
- First aid should be performed immediately if there has been exposure to blood or other body fluids.
- The exposure should be reported immediately to the manager/supervisor and OHS or delegate and immediate medical attention should be obtained.
- The site of a percutaneous injury or exposed non-intact skin should be immediately and thoroughly rinsed with running water (e.g., using a water bottle) and any wound should be gently cleansed with soap and water.
- Exposed mucous membranes of the eyes, nose or mouth should be flushed with copious running water (e.g., using a water bottle) if contaminated with blood, body fluids, secretions or excretions.
- Exposed non-intact skin should be rinsed thoroughly with running water (e.g., using a water bottle) and gently cleansed with soap and water.
- All appropriate follow-up for blood-borne pathogens as per organizational policy should be initiated.
Routine practices
Routine Practices are the IPC measures that should be applied in the routine care of all patients, at all times, in all healthcare settings, including prehospital care. Routine Practices and Additional Precautions are covered in detail in PHAC's Routine Practices and Additional Precautions guidance document.
Routine practices outlined in this document include:
- Point of Care Risk Assessment (PCRA)
- Hand Hygiene (see PHAC's Hand Hygiene Practices in Healthcare Settings guidance document)
- Source control
- Patient Placement
- Respiratory hygiene
- Personal Protective Equipment (PPE)
- Injection and Medication Safety
- Cleaning and Disinfection Procedures
Due to the unique setting of prehospital transport, hand hygiene sinks may not always be available. The following measures are recommended for the use of hand wipes:
- Hand wipes, impregnated with soap, antimicrobials or alcohol, may be used as an alternative solution to soap and water when hands are visibly soiled. In this instance, ABHR should be used after the use of hand wipes and hands should be washed with soap and water once an acceptable sink/facility is available.
- An assessment should be conducted prior to every interaction to determine the infectious risk posed to themselves and others.
Prehospital call assessment/triage
When calls are assessed by a dispatcher from individuals concerned about Ebola, the following questions as per the Appendix B Algorithm for Screening and Assessment for Ebola Disease (EBOD) in Persons Presenting to Healthcare Settings should be asked to identify a PUI for EBOD:
- Within the previous 21 days, has the person lived in or travelled to a country with endemic Ebola disease OR a public health notice due to widespread Ebola transmission OR had contact with a PUI or confirmed case of EBOD OR contact with laboratory specimens from a PUI or confirmed case of EBOD OR contact with primates, bats or wild animal bush meat from affected countries/regions:
- If YES, enquire about EBOD compatible symptoms:
- Does the person have a fever: subjective or ≥38°C (if measured)
- OR
- Does the person have at least one of the other EBOD compatible symptoms: malaise, myalgia, headache, arthralgia, fatigue, loss of appetite, conjunctival redness, sore throat, chest pain, abdominal pain, nausea, vomiting, diarrhea that can be bloody, hemorrhage, erythematous maculopapular rash on the trunk
- If YES, enquire about EBOD compatible symptoms:
- If YES, to either enquiry about EBOD compatible symptoms (i.e., PUI) above, the dispatcher should inform prehospital personnel that an EBOD response is required.
- If NO to both symptom-based enquiries:
- Usual prehospital service/care should be provided.
Additional precautions for Ebola disease management
Classification of persons under investigation for EBOD
- A point-of-care risk assessment (PCRA) should be performed prior to every interaction with a PUI or patient with EBOD (or contact with their care environment) to protect personnel from exposure to the Ebola virus (i.e., contact with or sprays of blood or other body fluids, respiratory tract or other secretions or excretions and used needles and other sharps). For details refer to the Agency's Infection Prevention and Control Measures for Ebola Disease in Acute Care Settings.
- If the patient is under investigation for EBOD, PPE should be put on before entering the scene.
- Based on clinical presentation, patients will be classified as:
- Stable PUI,
- Unstable PUI, or
- Patient with confirmed EBOD
This classification will determine PPE requirements.
Recommendations for source control
- The transport vehicle should be dedicated to a single patient. The stretcher should be covered with impervious material.
- The patient should wear a mask, if tolerated, to contain droplets. If a mask is not tolerated, advise the patient to use tissues to contain respiratory secretions and to cover nose and mouth during coughing or sneezing, with prompt disposal into a plastic-lined biohazard waste receptacle.
- Patients should be instructed and/or assisted with performing hand hygiene after contact with blood or body fluids (e.g., after use of toilet, using tissues for respiratory secretions, vomiting).
- If an AGMP is absolutely necessary (e.g., endotracheal intubation), the following strategies to reduce aerosol generation are recommended:
- AGMP should be anticipated and performed prior to transport whenever possible.
- The number of personnel in the patient care/isolation area should be limited to those required to perform the AGMP and to those highly skilled in performing the required task.
- Household members should not be in the patient care/isolation area during an AGMP.
- Fit-tested, approved N95 respirator (or equivalent, or higher protection) should be worn by all personnel in the patient care/isolation area during an AGMP.
- Closed endotracheal suction systems should be used wherever possible.
- Provider performing AGMP should be experienced/ skilled in the AGMP technique
- If point-of-care risk assessment indicates the potential for blood and body fluid exposure during transport (i.e., patient is bleeding, vomiting, and/or has diarrhea) methods should be used to contain fluids (i.e., use continence products and place absorbent pads under the patient) to prevent exposure of personnel and contamination of emergency vehicle.
- For example, if a patient is vomiting, assist them by providing a single-use disposable or plastic-lined emesis basin.
- A designated patient care/isolation area should be established in the back of the transport vehicle and patient movement and patient care is to be restricted to this area.
- Personnel wearing PPE should remain in this area.
- Only essential personnel with appropriate PPE should enter the patient care/isolation area.
- A log should be maintained to monitor all persons entering and exiting the patient care/isolation area.
- Supplies and clean PPE should be stored in a designated area outside the patient care/ isolation area when possible, or covered with an impermeable barrier in a manner that prevents contamination. Plastic-lined biohazard waste receptacle should be placed inside the isolation area.
- Space in the patient care/isolation area should be provided to allow monitoring of personnel by a trained monitor during personnel-patient interaction.
- If a portable isolation unit is considered for use to isolate the patient during transport, the following measures are recommended:
- The patient should be assessed to determine their suitability for transport in a portable isolation unit (i.e., consider body morphology, potential for patient vomiting, having diarrhea, and/or bleeding, intubation/ventilation needs, and psychological safety and comfort)
- HCWs should wear appropriate PPE at all times as the unit is not a replacement for PPE
- Method for management of body fluids (e.g., emesis, feces, urine, blood) should be determined
- The portable isolation unit should be large enough for the patient to turn to the side to protect their airway if there is a possibility of the patient vomiting during transport. As portable isolation unit may use safety belts to secure a patient, assessment should include patient's ability to turn on own or with the safe assistance of personnel.
- Describe the purpose of the unit and provide the patient with physical, emotional and psychological comfort while in the portable isolation unit
- The portable isolation unit should provide re-sealable portals for allowing personnel to assist the patient as needed, including airway management (i.e., intubation/ventilation)
- Personnel should have specific education and training in assessing the patient's suitability for transport in a portable isolation unit and the appropriate use of portable isolation unit according to manufacturer's instructions including measures to reduce the risk of contaminating the unit, self and patient care area
- Used portable isolation units should be disposed of into EBOD waste following use rather than cleaning and disinfection for reuse due to high risk of exposure to personnel and the ineffectiveness of complete cleaning and disinfection related to the portable isolation unit construction
Recommendations for the use of Personal Protective Equipment
Principles of Personal Protective Equipment (PPE)
- For additional details on the principles, selection and use of PPE refer to Infection Prevention and Control Measures for Ebola Disease in Acute Care Settings.
- Personnel must be trained in the principles of safe and effective PPE use, including safely putting on and removing PPE.
- PPE must be put on correctly before entering the patient care/isolation area. Refer to Source Control section.
- PPE must remain on and be worn correctly for the duration of prehospital patient care, transport or when in contact with the patient's potentially contaminated care environment.
- PPE should not be adjusted during patient care.
- If a breach in PPE occurs, personnel should immediately stop patient care, initiate PPE removal process with the assistance of the monitor and remove themselves from the patient care/isolation area. Refer to Occupational Health Considerations.
- Removal of PPE presents a high risk for self-contamination if not done properly and requires a structured and monitored process that must be done slowly and deliberately
- PPE must be provided and put on outside the patient care/isolation area. PPE should be put on and removed in separate areas.
- A trained and tested monitor must be assigned to coach, observe and monitor appropriate selection, application, removal and disposal of PPE, to avoid potential contamination of personnel and the area outside of the patient care/isolation area.
- Those who do not wear PPE must not have contact with the patient or the patient care/isolation area.
- Those involved with moving the patient into and out of the transport vehicles must wear PPE.
- The driver of the transport vehicle should only wear PPE when in contact with the patient or their designated care/isolation area. If PPE is worn it should be removed (as outlined above) prior to operating the vehicle, in order to prevent contamination of the vehicle. The driver does not need to wear PPE to operate the vehicle.
PPE recommendations for EBOD
-
Prehospital PPE for a stable PUI (e.g., vital signs within normal limits, no hemorrhaging, formed stool, no vomiting) will require:
- fluid-resistant mask
- separate face shield (or eye goggles)
- gloves
- fluid-resistant or impermeable gown
If patient requires an AGMP, becomes unstable or is confirmed to have EBOD, HCW should follow recommendations for the unstable PUI or confirmed patient with EBOD.
- Prehospital PPE for an unstable PUI [e.g., signs and symptoms of shock (resp. distress, hypotension, neurological impairment), hemorrhaging, possibility of intubation or resuscitation, diarrhea, vomiting, clinical findings suggesting that patient may contaminate the environment] or patient with confirmed EBOD will require:
- fit-tested, N95 (or equivalent, or higher protection)
- face shield long enough to prevent splashing underneath
- double gloves
- fluid resistant or impermeable gown or hazardous material suit
- fluid-impermeable apron
- fluid resistant or impermeable body coverings including foot and leg coverings, head and neck coverings
*All exposed skin is protected
Moving patient in and out of transport vehicle
- Personnel involved with moving the patient in and out of the transport vehicle should wear appropriate PPE as outlined in section 3.
- Appropriate care should be taken to avoid dislodging or tearing PPE and subsequent possible contamination during transfer of patient from stretcher to stretcher, as transfer requires close contact and physical manipulation of the patient.
- Emergency department personnel should meet the ambulance with a prepared stretcher to limit prehospital personnel movement within the facility.
- The patient must be taken directly to the receiving area in the hospital via the most direct route secured and monitored to avoid exposure of other individuals (e.g., other patients, visitors) and HCWs who are not involved in the patient's care.
Environmental cleaning
Dedicated equipment
- Disposable equipment is preferred (i.e., bedpan/urinal with the use of a solidifier) and should be discarded into a plastic-lined biohazard waste receptacle after use.
- Non-critical reusable patient-care equipment should be dedicated to the patient for single-patient use. Immediately after use, equipment should be placed into biohazard bags and labeled for cleaning and disinfection according to manufacturer's instructions and organization's policy by trained personnel wearing correct PPE before reuse with another patient.
Cleaning and disinfection of transport vehicle
- Education, hands-on training, practice, and observation of ability to adhere to correct processes and procedures, and appropriate PPE should be provided to those responsible for environmental cleaning.
- Those responsible for cleaning and disinfection should wear the same level of protection, at a minimum, as personnel providing care to a PUI or confirmed patient with EBOD
- Responsibility and accountability for cleaning and disinfection of patient care/isolation area and vehicle should be assigned and monitored to ensure appropriate and consistent processes are followed.
- In selecting disinfectants that are expected to inactivate EBOV on non-critical hard surfaces and medical devices, Health Canada recommends products with the following approved criteria:
- Registered with a Health Canada Drug Identification Number (DIN);
- The label should have a "broad spectrum virucide" claim
- Following completed transfer of the patient to the emergency department stretcher or inpatient bed, cleaning and disinfection measures should be taken.
- The following surfaces, equipment and other items used during transport should be cleaned and disinfected using a disinfectant with a broad spectrum virucide claim with a Health Canada DIN and used according to the manufacturer's instructions:
- All surfaces or equipment the patient or their blood and other body fluids have potentially contacted (e.g., transport stretcher surfaces).
- All exposed surfaces in the transport vehicle.
- All non-contaminated areas of the vehicle as per regular protocol (i.e., areas where there was no cross contamination from equipment/items, personnel with PPE etc.).
- All equipment and reusable containers prior to their return to transport vehicle.
- Blood or other body fluid-contaminated seat cushions or webbed seats/seat straps should be removed and disposed of into plastic-lined biohazard waste receptacle, as disinfection is not appropriate for these items.
- Compressed air or sprayers for vehicle cleaning should not be used.
- For additional information on environmental cleaning and blood and other body fluid spills, refer to Ebola Virus Disease: Management of Waste and Environmental Cleaning for Prehospital Care and Ground Transport.
Handling waste and linen
Notifications
- The receiving hospital and local PH should be notified as soon as a need for patient transport is determined.
- Upon arrival to the receiving hospital, and prior to entry, transport personnel must ensure that the emergency department is ready for the patient.
- The receiving hospital should notify PH of the arrival of a PUI or confirmed EBOD case. Refer to Infection Prevention and Control Measures for Ebola Disease in Acute Care Settings.
- Prehospital personnel should inform household members who shared the residence to not have further contact with items contaminated with blood or body fluids of the patient, and to not leave the residence until PH has contacted them with further information and instruction.
Appendix A: Acknowledgements
National Advisory Committee on Infection Prevention and Control (NAC-IPC):
Joanne Embree, MD, MSc
Pediatric Infection Disease Specialist, Shared Health
Professor, University of Manitoba
Winnipeg, MB
Matthew P. Muller, MD, PhD, FRCPC
Associate Professor, University of Toronto
Medical Director, Infection Prevention & Control, Unity Health Toronto
Toronto, ON
Molly Blake, RN, BN, MHS CIC
Program Director, Infection Prevention & Control, Winnipeg Regional Health Authority (WRHA)
Acting Program Director, Medical Device Reprocessing, WRHA
Winnipeg, MB
Patsy Rawding, RN, BScN, CIC
Health Services Manager, Infection Prevention & Control
Western Zone, NSHA Lead Manager in LTC
Middleton, NS
Patrice Savard, MD, MSc, FRCPC
Clinical Associate Professor, University of Montreal
Clinical microbiologist and Infectious diseases specialist, CHUM
Medical director, nosocomial infection prevention and control unit, CHUM
Montréal, QC
Jennie Johnstone, MD, PhD, FRCPC
Associate Professor, University of Toronto
Medical Director Infection Prevention and Control, Sinai Health
Toronto, ON
Stephanie W. Smith, MD, MSc, FRCPC
Professor, Division of Infectious Diseases, Department of Medicine, University of Alberta
Director, Infection Prevention and Control, University of Alberta Hospital
Edmonton, AB
Suzanne Rhodenizer Rose, RN, BScN, MHS, CIC
Director, Clinical Planning, QEII New Generation Project
Project Infection Control Specialist
Halifax, NS
Anne Masters-Boyne, R.N., M.N.
Occupational Health Nurse, Employee Health Services, Horizon Health Network
Fredericton, NB
Jennifer Happe, BSc, MSc
Alberta Children's Hospital
Director, Infection Prevention and Control Canada
Infection Control Professional, Alberta Health Services
Calgary, AB
Nisha Thampi, MD, MSc, FRCPC
Medical Director, Infection Prevention and Control Program, Division of Infectious Diseases, Children's Hospital of Eastern Ontario
Associate Professor, Department of Pediatrics, University of Ottawa
Ottawa, ON
Susy Hota, MSc, MD, FRCPC
Medical Director, Infection Prevention and Control
Infectious Diseases Specialist, University Health Network
Associate Professor, Department of Medicine, Division of Infectious Diseases, University of Toronto
Toronto, ON
Brian Sagar, MSc, BA
Senior Director, Communicable Disease
British Columbia Ministry of Health
Victoria, BC
Healthcare-acquired Infection Prevention and Control Section:
Maureen McGrath, BScN., RN
Steven Ettles, MPH
Amanda Graham, MPH
Jennifer Selkirk, MSc., RN
Appendix B: Algorithm for screening and assessment for Ebola disease in persons presenting to healthcare settings
Download a printable PDF version of Appendix B.
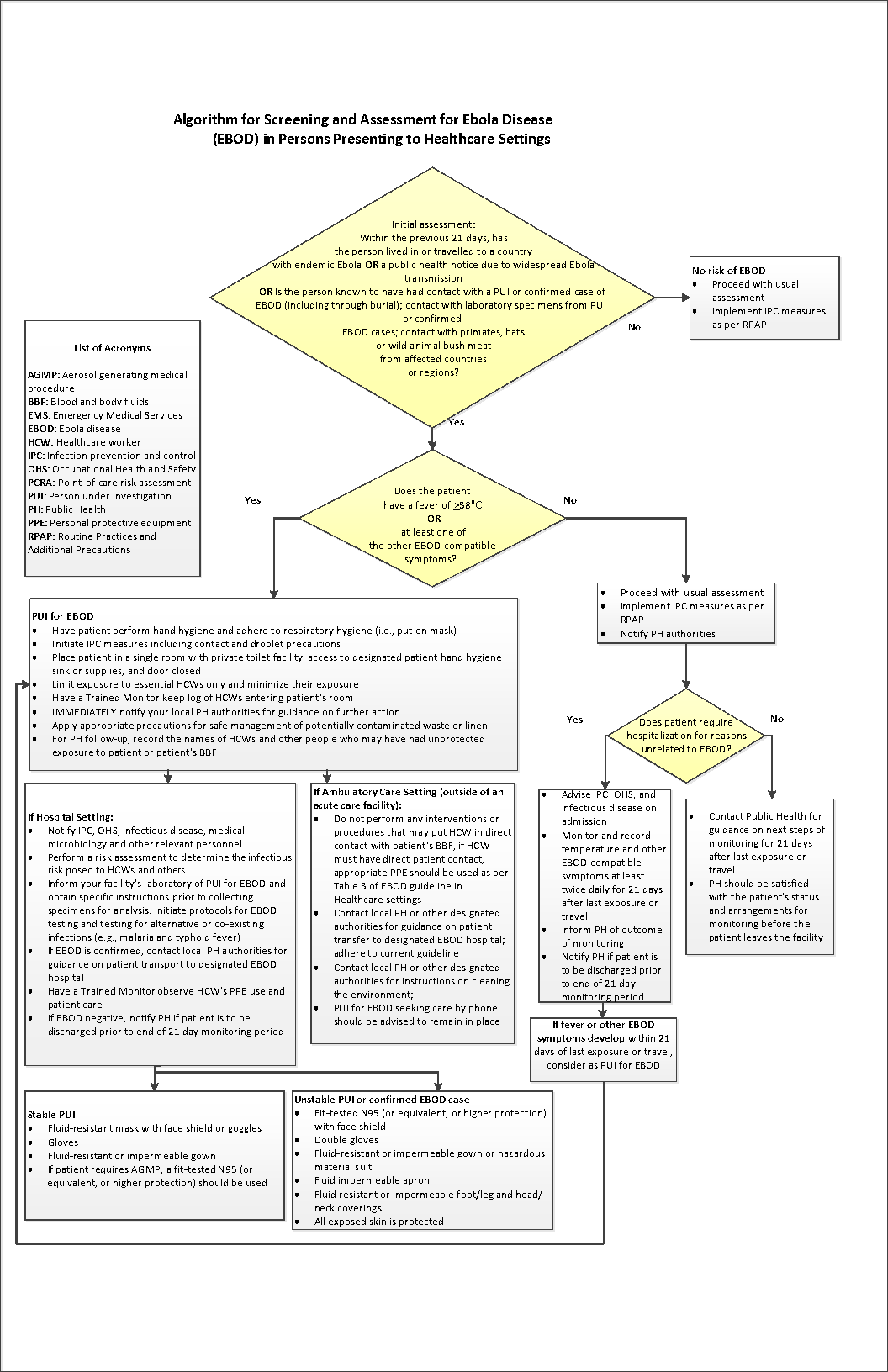
Long description: Appendix B – Algorithm for screening and assessment for Ebola disease in persons presenting to healthcare settings
An algorithm for the screening and assessment of patients presenting to a healthcare setting is used as a tool to assess risk of EBOD and to make decisions about the appropriate IPC measures to enact. This is based on a multi-stage assessment of a person’s travel history, possible contact with EBOD via a person(s) under investigation (PUI), confirmed patient(s) with EBOD, or laboratory specimens, and symptoms.
The algorithm begins with an initial assessment of the person presenting to the healthcare setting. Assess the following factors:
- Within the previous 21 days, has the person lived in or travelled to a country with endemic EBOD, or a public health notice due to widespread EBOD transmission?
- Within the previous 21 days, is the person known to have had contact with a PUI for EBOD or patient confirmed to have EBOD, including through burial?
- Within the previous 21 days, has the person had contact with laboratory specimens from a PUI or patient(s) confirmed to have EBOD?
- Within the previous 21 days, has the person had contact with primates, bats, or wild animal bush meat from EBOD-affected countries or regions?
If the answer to any of the above questions is NO, there is no risk of EBOD. As such, healthcare workers should proceed with usual assessment and implement IPC measures as per Routine Practices and Additional Precautions (RPAP). The EBOD-specific assessment ends at this point for this person.
If the answer to any of the above questions is YES, the assessment should continue with an assessment of the person’s symptoms. Assess the following:
- Does the person have a fever of greater than or equal to 38 degrees Celsius, or
- Does the person have at least one other EBOD-compatible symptom?
If the answer to these questions is NO, healthcare workers should proceed with usual assessment, implement IPC measures as per RPAP, and notify public health authorities.
Next, assess:
- Does the patient require hospitalization for reasons unrelated to EBOD?
If the answer is YES:
- Advise infection prevention and control (IPC), Occupational Health and Safety (OHS), and infectious disease on admission;
- Monitor and record temperature and other EBOD-compatible symptoms at least twice daily for 21 days after last exposure or travel;
- Inform public health of outcome of monitoring;
- Notify public health if patient is to be discharged prior to end of the 21 day monitoring period.
- If the patient develops fever or other EBOD-compatible symptoms within 21 days of last exposure or travel, consider as a PUI for EBOD. Refer to the “PUI for EBOD” section of this description.
If the answer is NO:
- Contact public health for guidance on next steps of monitoring for 21 days after the last exposure or travel;
- Public health should be satisfied with the patient’s status and arrangements for monitoring before the patient leaves the facility.
“PUI for EBOD”: If the answer to question (e) [Does the person have a fever of greater than or equal to 38 degrees Celsius?] or (f) [Does the person have at least one other EBOD-compatible symptom?] is YES, the person should be considered as a person under investigation (PUI) for EBOD. Initiate the following actions:
- Have the patient perform hand hygiene and adhere to respiratory hygiene (i.e., put on mask)
- Initiate IPC measures including contact and droplet precautions
- Place patient in a single room with private toilet facility, access to designated patient hand hygiene sink or supplies, and door closed
- Limit exposure to essential healthcare workers and minimize their exposure
- Have a Trained Monitor keep log of healthcare workers entering patient’s room
- Immediately notify local public health authorities for guidance on further action
- Apply appropriate precautions for safe management of potentially contaminated waste or linen
- For public health follow up, record the names of healthcare workers and other people who may have had unprotected exposure to patient or patient’s blood and bodily fluids (BBF)
If a PUI for EBOD is identified in an AMBULATORY CARE SETTING, i.e., outside of an acute care facility, complete the following actions:
- Do not perform any interventions or procedures that may put healthcare workers in direct contact with the patient’s BBF. If healthcare worker must have direct patient contact, appropriate PPE should be worn as per Table 3 of the guideline.
- Contact local public health or other designated authorities for guidance on patient transfer to designated EBOD hospital. Adhere to the current guideline.
- Contact local public health or other designated authorities for instructions on cleaning the environment.
- PUI for EBOD seeking care by phone should be advised to remain in place.
If a PUI for EBOD is identified in a HOSPITAL SETTING, complete the following actions:
- Notify IPC, OHS, infectious disease, medical microbiology and other relevant personnel
- Perform a risk assessment to determine the infectious risk posed to healthcare workers and others
- Inform your facility’s laboratory of PUI for EBOD and obtain specific instructions prior to collecting specimens for analysis. Initiate protocols for EBOD testing and testing for alternative or co-existing infections (e.g., malaria and typhoid fever)
- If EBOD is confirmed, contact local public health authorities for guidance on patient transport to designated EBOD hospital
- Have a Trained Monitor observe healthcare workers PPE use and patient care
- If EBOD negative, notify public health if patient is to be discharged prior to end of 21 day monitoring period
If the PUI for EBOD is stable, the following PPE should be used by healthcare workers:
- Fluid-resistant mask with separate face shield or goggles
- Gloves
- Fluid-resistant or impermeable gown
- If the patient requires an aerosol-generating medical procedure (AGMP), a fit-tested N95 (or equivalent, or higher protection) should be used.
If the PUI for EBOD is unstable, or if EBOD is confirmed, the following PPE should be used by healthcare workers:
- Fit-tested N95 (or equivalent, or higher protection) with separate face shield
- Double gloves
- Fluid-resistant or impermeable gown or hazardous material suit
- Fluid impermeable apron
- Fluid-resistant or impermeable foot/leg and head/neck coverings
- All exposed skin is protected
Appendix C: Management of Ebola disease waste and environmental cleaning for prehospital care and ground transport
The following guidance provides measures for the safe handling, containment, transport and disposal of waste (including linen and sharps) generated during prehospital care and ground transport from persons under investigation (PUI) and persons confirmed with Ebola disease (EBOD), along with measures for cleaning the environment contaminated, or potentially contaminated, with the Ebola virus (EBOV). Its use is intended for prehospital personnel including, but not limited to, medical first responders, paramedics, emergency ground transport personnel, firefighters, and enforcement officers, along with personnel within prehospital organizations responsible for education and training in occupational health and safety (OHS), infection prevention and control (IPC) and environmental services.
The guidance is based on currently available scientific evidence, standards and regulations, and adopts a precautionary approach where the evidence is lacking or inconclusive. It is subject to review and change as new information becomes available.
The guidance should be read in conjunction with relevant federal, provincial, territorial and local legislation, regulations, and policies, and adapted to local requirements as necessary.
EBOD-associated waste
EBOV is categorized as a Risk Group 4 agent, under the Public Health Agency of Canada's Human Pathogens and Toxins Act, as it is likely to cause serious disease, and effective treatment is not available. Waste contaminated with the EBOV requires special handling and disposal to prevent exposure to the virus.
All EBOD-associated waste is considered as regulated biohazardous waste and includes items (including linen) contaminated with human blood and body fluids (i.e., respiratory secretions, saliva, emesis, feces, and urine) that warrants special handling and disposal as it may in certain situations present a risk of disease transmission. EBOD-associated waste that has been appropriately incinerated or autoclaved is not infectious and does not pose a health risk.
Recommended measures for prehospital organizations
The following measures are recommended for the safe management of EBOD-associated waste and environmental cleaning during prehospital transport:
- Implement a biohazardous waste management and environmental cleaning program with the development of policies and procedures to include the following:
- Protocols for the management of waste, on-site spills, and environmental cleaning
- Protocols for adequate supplies of biohazard waste bags and containers/receptacles, cleaning supplies, disinfectants, hand hygiene products and personal protective equipment (PPE)
- Protocols for segregating, packaging, labelling, moving, storing and transporting EBOD-associated waste (both on- and off-site)
- Methods for keeping records of the quantities of EBOD-associated waste both generated and disposed of
- A list of all regulations and legislation concerning EBOD-associated waste that are applicable within the organization's jurisdiction
- Protocols for wearing PPE when handling EBOD-associated waste and/or providing cleaning measures that should include:
- All personnel handling EBOD-associated waste and/or providing cleaning services should wear appropriate PPE
- Regular, ongoing training and education of personnel on proper handling and potential hazards of EBOD-associated waste, type and quality of waste containers/receptacles, and PPE selection and use, including how to properly put on and remove PPE
- For information on PPE selection, use, and safely putting on and removing PPE, refer the PPE section of this document
- Provision for regular and ongoing education for Routine Practices, including Hand Hygiene as per PPE protocol, according to organization/company policy, with alcohol-based hand rub (60-90% alcohol) or washing with soap and water, if hands are visibly soiled
- Assign only personnel trained and educated in OHS, IPC practices and appropriate selection and use of PPE for the management of EBOD-associated waste and environmental cleaning
- Develop and implement a monitoring system, using a trained observer, for ensuring consistency in safely putting on and removing PPE when handling EBOD-associated waste and performing environmental cleaning
- Develop and implement protocols for the containment and storage of EBOD-associated waste as per the organization's biohazardous materials policies, and for off-site transport in accordance with applicable legislation, such as Transport Canada's Transportation of Dangerous Goods Regulations
- Determine capability for/availability of transporting EBOD-associated waste within their municipality/region and ensure waste is disposed of in accordance with local, municipal or regional requirements and regulations and/or bylaws for regulated biohazardous waste
- Provide education to personnel on steps to take when a breach in safe handling and containment occurs resulting in exposure or potential exposure to EBOV, during the management of EBOD-associated waste. This includes:
- Personnel to immediately stop work, safely remove PPE as per organization/company protocol and leave the area
- Rinse the affected skin surface with soap and water OR for mucous membrane splashes (e.g., conjunctiva), irrigate with copious amounts of water or eyewash solution, according to the organization's first aid protocol
- Report immediately according to the organization's exposure/injury protocol, including notification to Public Health authorities, and
- Adhere to organization's and PH's follow-up procedures
Recommended measures for EBOD-associated waste during ground transport
All prehospital transport staff handling EBOD-associated waste should wear appropriate PPE, including additional PPE based on a risk assessment, along with following guidance for safe removal of PPE, according to the organization's policy.
Examples of EBOD-associated waste:
- Human waste - blood and other body fluids, such as urine, feces, emesis, respiratory secretions and saliva
- Linen - bedding, towels, washcloths, gowns
- Other non-sharps waste - PPE, disposable bedpans, disposable linen, dressings, sponges, pads, procedure drapes, incontinent products/diapers, cleaning cloths/wipes, mop cloths/wipes, spills, intravenous/gastrointestinal/urine catheters and bags, suctioning equipment/tubing, non-fluid-impermeable pillows or mattresses
- Sharps waste - syringes, needles, razors, scalpels
Human waste
- Human waste should only be handled in the care area where it is generated and by personnel wearing appropriate PPE.
- Urine, feces and emesis may be disposed of through the normal sanitary sewer system, or in accordance with municipal/regional regulations.
- Where municipal regulatory restrictions exist on disposal through the normal sanitary sewer system, the use of a solidifier for liquid waste (with bedpans - disposable or with liners, and/or disposable emesis basins/receptacle) and disposed of as waste should be considered. (Refer to Other Non-Sharps Waste section).
- In settings where on-site septic sewage disposal systems are used (i.e., septic tanks), no specific measures are required providing the system is operating according to local regulations.
Linen
- Disposable linen should be used in a prehospital and ground transport setting.
- Handling and containing linen should occur in the care area by trained personnel wearing appropriate PPE.
- Consider all linen in the care area contaminated linen, regardless of whether it was used or not.
- The number of personnel handling linen should be limited.
- Linen should be folded inward and handled with a minimum of agitation and shaking to avoid contamination of air, surfaces and persons.
- For further information on managing and disposing of EBOD-associated linen, follow recommended measures under Other Non-Sharps Waste in the next section below.
Other non-sharps waste
- Handling, containing and removing waste should only occur in the care area by trained personnel wearing appropriate PPE.
- Consider all supplies taken into the care area to be contaminated, whether used or not.
- The number of personnel handling waste should be limited.
- The following measures are recommended for non-sharps waste:
- Contain waste at point of generation
- Place waste immediately into a sturdy and leak resistant container lined with a leak and tear resistance plastic biohazard bag
- Do not manually compact waste in the bags
- When the bag is two-thirds full, seal securely preventing tearing/puncturing the bag and ensuring no leaks
- Remove the bag from the container (note: this container should stay in the care area until patient discharge to receiving healthcare facility and relined with a new biohazard bag for next fill)
- Decontaminate the entire outside of the bag by wiping using a disinfectant with a broad spectrum virucide claim with a Health Canada DIN and used according to the manufacturer's instructions
- Place the decontaminated bag into a second biohazard bag and seal securely, preventing tearing/puncturing the second bag and ensuring no leaks
- Wipe the entire outside of the second bag using a disinfectant with a broad spectrum virucide claim with a Health Canada DIN used according to the manufacturer's instructions, immediately before removing it from the area
- To move the double-bagged waste from the care area, (i.e., ground transport vehicle) personnel should place the double bagged waste in a leak-proof/impervious, puncture-resistant plastic or metal single-use container. The waste container should be:
- Located at the periphery/outside of the area for taking off PPE to avoid risk of recontamination of the container during PPE removal
- Securely sealed, clearly labelled and identified as EBOD-associated biohazardous material, by a second person wearing appropriate clean PPE
- Decontaminated by wiping the entire outside of the container using a disinfectant with a broad spectrum virucide claim with a Health Canada DIN and used according to the manufacturer's instructions, immediately before removing the container from the area
- Not be re-opened once sealed
- For moving large or heavy containers, carts with guard rails or raised edges should be used and loaded in a manner that will prevent items from tipping.
- Carts should be disinfected after each use using a disinfectant with a broad spectrum virucide claim with a Health Canada DIN and used according to the manufacturer's instructions.
- Containers may be transferred to the receiving hospital if these arrangements have been made in advance OR taken immediately and directly to a designated locked holding area with restricted access and stored as per the organization's biohazardous material policy until ready for transport to disposal site.
- EBOD-associated waste storage areas should be clearly marked with a biohazard symbol and kept separate from other storage areas.
- Stored containers of waste should be packaged and transported separately in accordance with applicable legislation, such as Transport Canada's TDGR, and disposed of in accordance with local, municipal or regional requirements and regulations and/or bylaws for regulated biohazardous waste.
Sharps Waste
- Sharps waste should be segregated from other waste and discarded:
- At point of use
- Directly into single-use containers, that are leak-proof/impervious, puncture resistant, fitted with securely closed lids and specifically designed for sharps waste
- Sharps containers should not be filled beyond two-thirds full, to allow for safe closure.
- The following measures for sharps containers are recommended:
- When the container is two-thirds full, securely close the lid
- Wipe the outside of the container using a disinfectant with a broad spectrum virucide claim with a Health Canada DIN and used according to the manufacturer's instructions
- Place the sharps container into a second leak-proof/impervious, puncture resistant biohazard container
- Securely seal, clearly label and identify the second container as EBOD-biohazardous sharps material
- Wipe the outside of the second container using a disinfectant with a broad spectrum virucide claim with a Health Canada DIN and used according to the manufacturer's instructions
- Containers may be transferred to the receiving hospital if these arrangements have been made in advance OR taken immediately and directly to a designated locked holding area with restricted access and stored as per the organization's biohazardous material policies.
- EBOD-associated sharps waste storage areas should be clearly marked with a biohazard symbol and kept separate from other storage areas.
- Stored containers of EBOD-associated sharps waste should be packaged and transported separately off-site in accordance with applicable legislation, such as Transport Canada's TDGR, and disposed of in accordance with local, municipal or regional requirements and regulations and/or bylaws for regulated biohazardous waste.
Recommended measures for on- site spills and environmental cleaning related to EBOD-associated blood and other body fluids
- All personnel providing cleaning services should be managed by trained personnel wearing appropriate PPE.
- All major spill incidents should be documented, according to organization policy for any follow-up required.
- 'Spill kits' should be made available, according to organization policy, for use in designated areas.
- The spill area should be isolated from access to other individuals until cleaning and disinfection is completed.
- Special cleaning of upholstery and carpets is not indicated unless they are visibly soiled with blood or other body fluids.
- Items visibly soiled (e.g., upholstery, cloth chairs, cloth seats, seat covers or carpets covered in blood or other body fluids) that are difficult to clean should be removed and treated as waste.
- All surfaces, areas, items and objects visibly contaminated or potentially contaminated with blood or other body fluids should be cleaned and disinfected (e.g., walls, floors, locks, counters, door knobs, light switches, tables/work surfaces, other high contact surfaces and items/objects).
- Visible blood and other body fluids should be removed first with disposable damp cleaning cloths or wipes and regular detergent.
- The following cleaning and disinfection measures are recommended:
- Allow fluid and droplets to settle, fluids should not be permitted to fully dry
- Gently cover a spill with disposable absorbent paper towels, wipes or pads (a solidifier agent may be used); remove organic/bulk material, and place waste immediately into sturdy, leak and tear resistant biohazard plastic bag and securely seal (Refer to Other Non-Sharps Waste section for further details)
- With disposable cleaning cloths or wipes, apply a disinfectant with a broad spectrum virucide claim with a Health Canada DIN to the surface and allow sufficient contact time according to manufacturer's instructions
- Spraying disinfectant or using wet vacuum should not be done in order to avoid any splashes and splatter; compressed air, pressurized water or similar procedures which might create droplets, should not be used; dry sweeping and dusting with a broom or cloth should not be done
- Start at one end of the affected area and move in one direction until all surfaces have been disinfected and do not use a circular motion
- Use cleaning cloths, wipes, etc., only once and after use, discard all cleaning items immediately into a biohazard bag located within reach (Refer to Other Non-Sharps Waste section)
- Patient's personal items visibly soiled with blood or other body fluids should be cleaned and disinfected as per above. If items cannot be properly clean and disinfected, they should be treated as waste.
References
Public Health Agency of Canada:
- Ebola virus disease: Symptoms and treatment. https://www.canada.ca/en/public-health/services/diseases/Ebola.html
- Hand Hygiene Practices in Healthcare Settings (2012). https://www.canada.ca/en/public-health/services/infectious-diseases/nosocomial-occupational-infections/hand-hygiene-practices-healthcare-settings.html
- Public Health Agency of Canada. Pathogen Safety Data Sheets: Infectious Substances – Ebolavirus (2018). https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/pathogen-safety-data-sheets-risk-assessment/ebolavirus.html
- Public Health Agency of Canada. Environmental sanitation practices to control the spread of communicable disease in passenger conveyances and terminals (2019). https://www.canada.ca/en/public-health/services/emergency-preparedness-response/centre-emergency-preparedness-response/travelling-public-program/environmental-sanitation-practices-control-spread-communicable-disease-passenger-conveyances-terminals.html
- Public Health Agency of Canada Case Definition: Ebola virus disease outbreak (2018). https://www.canada.ca/en/public-health/services/infectious-diseases/viral-haemorrhagic-fevers/national-case-definition-Ebola-virus-disease.html
- Public Health Management of Cases and Contacts of Ebola Virus Disease in the Community Setting in Canada (2018). https://www.canada.ca/en/public-health/services/diseases/Ebola/health-professionals-Ebola/interim-guidance-public-health-management-cases-contacts-Ebola-community-setting-canada.html
- Pathogen Safety Data Sheets: Infectious Substances - Ebolavirus (2018). https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/pathogen-safety-data-sheets-risk-assessment/Ebolavirus.html
- Routine Practices and Additional Precautions for Preventing the Transmission of Infection in Healthcare Settings (2013). https://www.canada.ca/en/public-health/services/publications/diseases-conditions/routine-practices-precautions-healthcare-associated-infections.html
Canada:
- Canadian Critical Care Society, Canadian Association of Emergency Physicians, Association of Medical Microbiology and Infectious Diseases. Ebola Clinical Care Guidelines: A guide for clinicians in Canada, Report #2 (2014). http://www.canadiancriticalcare.org/resources/Pictures/Ebola%20Clinical%20Care%20Guidelines_ENG.pdf
Canadian Standards Association. Standard Z317.10:21. Handling of health care waste materials (2021).
Canadian Standards Association. Standard Z316.6:20. Sharps injury protection - Requirements and test methods - Sharps containers (2021).
- Government of Canada. Canadian Biosafety Standard, Second Edition (2015). https://www.canada.ca/en/public-health/services/canadian-biosafety-standards-guidelines/second-edition.html
- Transport Canada. Transportation of Dangerous Goods Regulations (2017). https://laws-lois.justice.gc.ca/eng/regulations/SOR-2001-286/index.html
Centers for Disease Control and Prevention (CDC), United States:
- Centers for Disease Control and Prevention. Ebola-Associated Waste Management (2022). http://www.cdc.gov/vhf/ebola/hcp/medical-waste-management.html
- Centers for Disease Control and Prevention. Frequently Asked Questions (FAQs) on Interim Guidance for Managers and Workers Handling Untreated Sewage from Suspected or Confirmed Individuals with Ebola in the U.S. (2014). http://www.cdc.gov/vhf/ebola/prevention/faq-untreated-sewage.html
- Centers for Disease Control and Prevention. Interim Guidance for Environmental Infection Control in Hospitals for Ebola Virus (2018). http://www.cdc.gov/vhf/ebola/hcp/environmental-infection-control-in-hospitals.html
- Centers for Disease Control and Prevention. Procedures for Safe Handling and Management of Ebola-Associated Waste (2014). http://www.cdc.gov/vhf/ebola/prevention/ebola-associated-waste.html
- Centers for Disease Control and Prevention. Recommendations for Safely Performing Acute Hemodialysis in Patients with Ebola Virus Disease (EVD) in U.S. Hospitals (2015). http://www.cdc.gov/vhf/ebola/hcp/guidance-dialysis.html
- Infection Prevention and Control Recommendations for Hospitalized Patients Under Investigation (PUIs) for Ebola Virus Disease (EVD) in U.S. Hospitals (2018). https://www.cdc.gov/vhf/Ebola/clinicians/evd/infection-control.html
- Morbidity and Mortality Weekly Report (MMWR). Surveillance and Preparedness for Ebola Virus Disease - New York City, 2014 (2014). http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6341a5.htm?s_cid=mm6341a5_w
Public Health Ontario:
- Provincial Infectious Disease Advisory Committee. Best Practices for Environmental Cleaning for Prevention and Control of Infections In All Health Care Settings (3rd edition) (2018). https://www.publichealthontario.ca/-/media/documents/bp-environmental-cleaning.pdf?la=en
- Provincial Infectious Disease Advisory Committee. Environmental Cleaning Toolkit (2010). https://www.publichealthontario.ca/en/health-topics/infection-prevention-control/environmental-cleaning/environmental-cleaning-toolkit
- Provincial Infectious Disease Advisory Committee. Infection Prevention and Control for Clinical Office Practice (1st revision) (2015). https://www.publichealthontario.ca/-/media/documents/bp-clinical-office-practice.pdf?la=en
- Provincial Infectious Disease Advisory Committee. Routine Practices and Additional Precautions in All Health Care Settings (3rd edition) (2012). https://www.publichealthontario.ca/-/media/documents/bp-rpap-healthcare-settings.pdf?la=en
World Health Organization:
- Ebola virus disease (2019). http://www.who.int/Ebola/en/
- Ebola virus disease (2019). https://www.who.int/en/news-room/fact-sheets/detail/Ebola-virus-disease
- Clinical management of patients with viral haemorrhagic fever: a pocket guide for the front-line health worker: interim emergency guidance - generic draft for West African adaptation (2014). https://apps.who.int/iris/handle/10665/130883
- World Health Organization. Ebola virus disease - Key questions and answers concerning health care waste (2021). https://www.who.int/publications/i/item/WHO-HEP-ECH-WSH-2021.2
- World Health Organization/UNICEF. Ebola Virus Disease (EVD): Key questions and answers concerning water, sanitation and hygiene (2021). https://www.who.int/publications/i/item/ebola-virus-disease-(evd)-key-questions-and-answers-concerning-water-sanitation-and-hygiene
- World Health Organization. Infection prevention and control (IPC) guidance summary: Ebola guidance package (2014). https://www.who.int/publications/i/item/WHO-HIS-SDS-2014.4-Rev.1
- World Health Organization. Safe management of wastes from health-care activities (2014). https://www.who.int/publications/i/item/9789241548564