Infection prevention and control measures for Ebola disease in acute care settings
Table of contents
- Introduction
- Aim and scope of this guideline
- Guideline development methodology
- Target users
- Role of provinces and territories
- Application of hierarchy of controls to Ebola disease: engineering controls, administrative controls and personal protective equipment
- Occupational health considerations
- Routine practices
- Triage and screening
- Additional precautions for Ebola disease management
- Duration of precautions
- Notification
- Laboratory precautions
- Appendix A: Acknowledgements
- Appendix B: Algorithm for screening and assessment for Ebola disease in persons presenting to healthcare settings
- Appendix C: Management of Ebola disease-associated waste in Canadian healthcare settings
- References
Introduction
Preamble
This guidance document updates the previous Infection Prevention and Control Measures for Ebola Virus Disease (EVD) in Healthcare Settings (August 28, 2019). This version has updated recommendations for:
- Classification of person under investigation (PUI) for Ebola disease (EBOD)
- Personal protective equipment
- Duration of precautions
- Nomenclature
Background
EBOD is part of a group of illnesses called Viral Hemorrhagic Fevers (VHFs) that are caused by several distinct families of viruses. The infection prevention and control (IPC) advice in this document is developed for EBOD, however, the guiding principles and IPC measures are applicable for management of persons under investigation or confirmed disease in healthcare settings associated with other agents that cause VHFs (i.e., Lassa, Marburg, Crimean-Congo).
EBOD is a severe acute viral illness that begins with fever, often with malaise, myalgia, and headache, and is typically followed by progressive gastrointestinal symptoms that include anorexia, nausea, abdominal discomfort, and subsequent vomiting and diarrhea. Diarrhea and vomiting are often profuse in later stages of the illness and leads to severe volume depletion, electrolyte abnormalities, and shock. While hemorrhage may occur, usually from the gastrointestinal tract, it is a late manifestation and occurs in a minority of patients. The incubation period of EBOD varies from 2 to 21 days. People with EBOD are not infectious during the incubation period.
Risk and transmission
Ebola virus (EBOV) is transmitted by direct contact (i.e., through non-intact skin or mucous membranes) with the blood or other body fluids (e.g., stool, emesis, urine, saliva, semen and sweat) of an infected individual and/or indirectly through contact with environmental surfaces and fomites contaminated with blood or other body fluids. The risk of transmission increases with the amount of infectious material to which the individual is exposed.
Cases are not communicable before the onset of symptoms but communicability increases with each subsequent stage of illness. Individuals with EBOD are most infectious in later stages of their illness when viral load rises and they experience copious fluid loss due to diarrhea, vomiting or hemorrhage. Cases remain communicable as long as blood or other body fluids contain the virus. This includes the convalescence period, before they have recovered, and the post-mortem period. Further details on IPC precautions during these periods are provided in the sections on "Duration of precautions and handling bodies of deceased patients".
Investigations conducted to date have not demonstrated human-to-human transmission of EBOV in the absence of direct contact with an infected case. This takes into account the thousands of EBOD cases in Africa and the very small number of EBOD cases in Europe and the United States. EBOV is not spread through the airborne route.
Public health case management relies on early identification of EBOD cases, individual isolation and care, diligent contact tracing, appropriate IPC measures, and safe burial. The following document contains additional information on case and contact management in community settings:
Aim and scope of this guideline
The purpose of this document is to provide guidance on the minimum level of IPC measures in healthcare settings in the event that a person under investigation for EBOD or patient with EBOD is identified within a Canadian healthcare facility. Clinical judgement remains essential, and this, along with jurisdictional policies, may result in decisions that differ from recommendations provided in this document.
This document has been developed based on the Canadian context and therefore may differ from guidance developed by other countries.
Recommendations for non-acute healthcare settings are beyond the scope of this document.
Guideline development methodology
PHAC developed this guideline with technical expertise from the National Advisory Committee on Infection Prevention and Control (NAC-IPC) and subject matter experts. The recommendations are informed by a review of the evidence, expert opinion and core IPC principles as identified in PHAC's Routine Practices and Additional Precautions for Preventing the Transmission of Infection in Healthcare Settings. This advice is based on currently available scientific evidence and expert opinion and adopts a precautionary approach where the evidence is lacking or inconclusive. It is subject to review and change as new information becomes available.
Please refer to Appendix A for a list of members.
Target users
The target audiences for this document are IPC professionals, Occupational Health and Safety professionals, healthcare organizations, and healthcare providers responsible for educating healthcare workers (HCWs) on IPC. The advice is intended for acute healthcare settings where there may be potential for contact with a person under investigation (PUI) for EBOD or confirmed to have EBOD.
Role of provinces and territories
The advice contained in this document should be read in conjunction with relevant federal, provincial, territorial and local legislation, regulations, and policies. Recommended measures should not be regarded as rigid standards, but principles and recommendations which may be used to inform IPC practice.
Application of hierarchy of controls to EBOD: engineering controls, administrative controls and personal protective equipment
The hierarchy of controls is a fundamental occupational health and safety framework, designed to optimize protection of HCWs from exposures to hazards, including infectious hazards such as EBOD. Following the hierarchy of controls will produce safer systems and reduce potential illness or injury for HCWs.
Elimination
While elimination may not be possible, to mitigate risk of transmission in healthcare facilities, systems and protocols should be in place to limit the care of persons under investigation for EBOD or confirmed patients with EBOD to designated locations within a hospital (e.g., ER, ICU), or designated hospitals with dedicated personnel.
Substitution
Substitution as part of the hierarchy of controls is not a feasible approach to preventing transmission of EBOV.
Engineering controls
Engineering controls are used to either remove the hazard or put a barrier between the HCW and the hazard. Engineering controls are those elements of the healthcare organization's physical plant/infrastructure that function to prevent exposure to and/or transmission of the infectious agent at the source, or along the path of the hazard.
Examples of engineering controls in managing a patient with EBOD include:
- Use of partitions at triage
- Accommodation in a private single room with designated private toilet and patient sink, as well as a designated area to donn and doff personal protective equipment (PPE) safely
- Use of airborne infection isolation rooms for aerosol-generating medical procedures (AGMPs), if available
- Designated staff hand washing sinks with soap
- Point-of-care alcohol-based hand rub (ABHR)
- Point-of-use sharps containers
- Designated no touch biohazardous waste receptacles
- Safety-engineered sharps or devices
- Point-of-care laboratory testing
Administrative controls
Administrative controls include the policies, procedures, education, training and patient care practices intended to prevent exposure to and/or transmission of an infectious agent to a susceptible host during the provision of health care. Each healthcare organization should develop comprehensive policies and procedures for putting on and removing personal protective equipment. To be effective in preventing transmission of EBOD and/or detecting cases of EBOD, administrative controls should be applied from the first encounter with a PUI and continue until the patient leaves the healthcare setting, is no longer infectious, or is deceased (note, proper post-mortem care is required).
Examples of administrative controls in managing a patient with EBOD include:
- Development and maintenance of an up-to-date EBOD assessment policy
- Education and training of selected HCW at highest risk of needing to assess cases of suspected EBOD
- Ensuring that the facility maintains sufficient, non-expired and easily available PPE appropriate for the care of PUIs or confirmed EBOD patients and consistent with the PPE that staff have trained on
- Screening protocols for relevant travel history and symptoms at multiple points of entry
- Triage procedures and prompt initiation of isolation and appropriate PPE
- Case and contact tracing
- Initiation of Incident Management Structure
- Designated regional centres for care of patients
- Designated transport vehicles
- Designated trained care teams
- Trained Monitors to coach and monitor use when putting on and removing PPE. Refer to Table 2 for roles and responsibilities of the Trained Monitor
- Designated individual(s) to oversee safe and effective delivery of EBOD treatment with responsibility for all aspects of EBOD IPC in a facility, including:
- Oversee implementation of administrative and engineering controls
- Support care before, during and after HCW enters an isolation or treatment area (if these individuals are not on-site when the patient initially arrives, care can be provided by appropriately-trained staff until their arrival)
- Know and apply the facility EBOD exposure management plan in event of unintended breach in procedure
- Monitor and evaluate supplies
- Specialized training, including drills, in the selection, application, use, and removal and disposal of PPE
- Respiratory protection programs (i.e., respirator fit-testing, training)
- For more information on respiratory protection programs, see PHAC's Routine Practices and Additional Precautions guidance document
- Limiting the number of HCWs providing care to a patient with EBOD, and monitoring and maintaining a log of all persons entering and exiting the patient room
- Determining HCW fitness to provide care to a patient with EBOD
In addition to organizational practices, there are federal, provincial and territorial Occupational Health and Safety Acts and Regulations that require compliance. This is typically accomplished through implementation of policies, procedures, education and training. In many provinces and territories, Joint Health and Safety Committees are also legislated and are jointly chaired by a management and a HCW representative. Hospitals will also have internal responsibility systems (IRS), which is the underlying philosophy of the occupational health and safety legislation in all Canadian jurisdictions. The fundamental principle of the IRS is that everyone in the workplace - both employees and employers - is responsible for their own safety and for the safety of co-workers.
Personal protective equipment
Personal Protective Equipment (PPE) refers to all personal equipment and clothing recommended by the employer for use by the HCW. For the equipment to be effective, the other controls must be in place. Federal, provincial and territorial Occupational Health and Safety Acts define specific duties for the employer, supervisor and HCW regarding PPE. The employer must ensure that the appropriate PPE is available and in good working order and that there has been comprehensive instruction, training and supervision in its correct usage. The supervisor and HCW must know the hazards for any potential exposure to blood, other body fluids, or surfaces contaminated with EBOV. The supervisor is responsible to ensure that the HCW uses the PPE required by the employer and the HCW shall use the PPE required by the employer.
Healthcare organizations need to ensure an adequate supply of appropriate PPE to protect HCWs and that their HCWs are adept in the application, use and removal of their PPE.
Organizational risk assessment
A major responsibility of any healthcare organization is the evaluation of the components in the hierarchy of controls to minimize the risk of exposure to and transmission of microorganisms within healthcare settings, including EBOV. This organizational risk assessment (ORA) is central to any healthcare organization's preparation and planning to protect all individuals (e.g., HCW, patient, visitor, and contractor) from EBOD in all healthcare settings. Organizations have a responsibility to provide information and train HCWs regarding the organization's ORA and its impact on their practice. For example, the availability of functioning airborne infection isolation rooms (AIIRs) may affect when and where aerosol-generating medical procedures (AGMPs) are performed.
The ORA will characterize the organization's patient population, level and intensity of health care provided and resources available, including the various skilled workers required to provide the necessary care. Conducting an ORA will help the facility identify the effectiveness of present control measures and the breadth of the hierarchy of controls to prevent transmission of the EBOV.
The ORA as it relates to EBOD should be conducted on a regular basis. ORA frequency is determined by:
- Global epidemiological context of EBOD
- The organization's probability of receiving a PUI for EBOD and confirmed patient with EBOD
- The organization's ability to maintain readiness and competency for EBOD-related interventions and activities (i.e., EBOD-specific PPE training, EBOD-specific PPE stockpile, etc.)
Examples of aspects of an organizational risk assessment in managing a patient with EBOD include:
- Identifying where patients with EBOD are likely to present and receive care
- Identifying HCWs who will have a lead role in EBOD response and who may come into contact with patients with EBOD and/or their environment
- Ensuring a comprehensive PPE program is in place, including education, training, and repeated observed practice on specific PPE selected for HCW
Occupational health considerations
General guidelines for HCW's fitness to provide direct care to patients with EBOD
Fitness to work incorporates factors that relate to the individual HCW's ability to safely perform the duties of their job. The HCW should be encouraged to communicate any concerns regarding potential impact on their underlying health conditions through the usual reporting mechanism within their organization (e.g. supervisor, manager, Occupational Health Service). Any work restrictions/limitations can then be shared appropriately, protecting the personal health privacy of the individual.
Certain health conditions or pregnancy preclude some HCWs from providing direct care for patients with EBOD. The principles for identifying these conditions are based on the following considerations:
- Inability to sustain work times required while providing direct patient care in the recommended PPE.
- Demonstrated or expected higher EBOD mortality based on the underlying health condition(s)
Examples of conditions that should be assessed for when determining fitness to provide direct care to patients with EBOD include:
- Underlying medical conditions that could affect the HCW's ability to exit the room safely, or that may require another HCW to enter the room to provide urgent medical assistance to the HCW
- Inability to safely put on, use, or remove recommended PPE (e.g. N95 fit-testing failure, claustrophobia, body morphology, mobility issues)
- Skin integrity
- Immune competence
- History of heat stroke
- Pregnancy (due to a reported increased risk of maternal and fetal mortality)
Where necessary, the ability of a HCW to engage in work activities related to caring for a patient with EBOD should be assessed by an Occupational Health and Safety professional.
HCW awareness when caring for a patient with EBOD
These recommendations apply to all persons who have the potential for exposure to PUIs or patients with confirmed EBOD.
- Be aware of signs and symptoms of EBOD, appropriate control measures, and the need to self-monitor while caring for PUIs or confirmed cases of EBOD and for 21 days following last contact with a patient with EBOD.
- Potential occupational/community exposure to EBOD (e.g., direct exposure without appropriate PPE, percutaneous injuries) should be reported to immediate manager/supervisor and occupational health services or delegate as well as to Public Health, as above.
- First aid should be performed immediately if there has been exposure to blood or other body fluids.
- The exposure should be reported immediately to the manager/supervisor and occupational health services or delegate, and immediate medical attention should be obtained.
- The site of a percutaneous injury should be thoroughly rinsed with running water, and any wound should be gently cleansed with soap and water.
- Mucous membranes of the eyes, nose or mouth should be flushed with running water if contaminated with blood, body fluids, secretions or excretions.
- Non-intact skin should be rinsed thoroughly with running water if contaminated with blood, body fluids, secretions or excretions.
- Appropriate follow-up for blood-borne pathogens should be initiated.
Routine practices
Routine practices are the IPC measures used in the routine care of all patients, at all times, in all healthcare settings and are determined by the circumstances of the patient, the environment and the task to be performed. Routine practices and additional precautions are covered in detail in PHAC's Routine Practices and Additional Precautions guidance document.
Routine practices include:
- Point of Care Risk Assessment (PCRA)
- Hand Hygiene (See PHAC's Hand Hygiene Practices in Healthcare Settings guidance document for more information)
- Source Control
- Patient Placement
- Respiratory Hygiene
- Personal Protective Equipment (PPE)
- Injection and Medication Safety
- Cleaning and Disinfection Procedures
An assessment should be conducted prior to every interaction to determine the infectious risk posed to oneself and others.
Triage and screening
Acute healthcare settings should have the following triage measures in place:
- Ability to direct patients to an appropriate care setting and initiate necessary IPC measures prior to patient's arrival, if possible, or immediately upon arrival, if the patients have called the doctor's office, clinic or Emergency Department (ED) notifying that they are feeling feverish and/or have a fever or other symptoms compatible with EBOD and have:
- A travel or work history to an area or country with endemic Ebola or a public health notice due to widespread Ebola transmission
- Contact with a PUI or confirmed case of EBOD
- Contact with laboratory specimens from a PUI or confirmed case of EBOD
- Contact with primates, bats or wild animal bush meat from affected countries or regions
- A physical barrier (e.g., plastic partition at triage desk) located between infectious sources (e.g., patients with symptoms of EBOD) and susceptible hosts (e.g., other patients, HCWs)
- Supplies for emesis management and respiratory hygiene available (masks, tissues, basins, hand hygiene products, designated hand washing sinks and no-touch biohazardous waste receptacles)
- Patients with symptoms should be assessed in a timely manner for EBOD and for other alternative or co-existing potential infections (e.g., malaria, meningitis, dysentery, typhoid fever, tuberculosis, measles, gastroenteritis, or other VHFs)
Refer to Appendix B: Algorithm for screening and assessment for Ebola disease in persons presenting to healthcare settings for a quick reference guide for frontline healthcare workers involved in triage and screening.
Additional precautions for Ebola disease management
Additional precautions in addition to routine practices are required for the care of all PUIs and confirmed patients with EBOD. Please refer to Table 3. PPE recommended for care of a stable PUI, unstable PUI or confirmed case. Additional precautions are covered in detail in PHAC's Routine Practices and Additional Precautions guidance document.
Classification of persons under investigation for EBOD
EBOD cases will require hospitalization for diagnosis and supportive care during their acute illness. Based on clinical presentation and laboratory results, patients will be classified as:
- Stable PUI,
- Unstable PUI, or
- Patient with confirmed EBOD
| Stable PUI | Unstable PUI |
|---|---|
|
|
| Note: this list is not exhaustive and is to be used in concert with a Point of Care Risk Assessment (PCRA). | |
Recommendations for source control
- Ensure each PUI and patients with confirmed EBOD are placed in a private single room with dedicated toilet or commode; keep the door closed.
- Advise the patient to perform hand hygiene and adhere to respiratory hygiene.
- Only essential HCWs with appropriate PPE may enter the patient's room.
- Assign a Trained Monitor to coach and monitor appropriate use, removal, and disposal of PPE, to avoid contamination of the HCW and the environment outside of the patient's room (refer to Table 2 for roles and responsibilities of the Trained Monitor)
- Patients should be asked and assisted to perform hand hygiene after toileting and vomiting.
- AGMPs should be avoided. If necessary (e.g., intubation), implement the following strategies to reduce aerosol generation:
- AGMPs should be anticipated and planned for when possible
- The number of HCWs in the room should be limited to those required to perform the AGMP and to those highly skilled in performing the required task
- AGMPs should be performed in an AIIR (also referred to as negative pressure room)
- Private single rooms (with the door closed), should be used in settings where AIIRs are unavailable
- Fit-tested N95 respirator (or equivalent, or higher protection) should be worn by all HCWs in the room during an AGMP
- Closed endotracheal suction systems should be used wherever possible
Recommendations for the use of personal protective equipment
The effectiveness of PPE (e.g., gowns, aprons, hazardous material suits, foot and leg coverings, gloves, head and neck coverings, masks, face shields, eye goggles, and respirators) is highly dependent on prior training and experience of the HCW with the PPE. Prior to working with patients with EBOD, the HCW must have had comprehensive training and observed and ongoing practice with the PPE. They must be adept at its use and removal, including correct technique for putting on and removing, discarding into designated receptacles, and hand hygiene to minimize risk of transmission.
Important principles of personal protective equipment (PPE):
- PPE to be inspected for integrity (e.g., tears, fluid penetration)
- PPE must be large enough to allow unrestricted free movement of body and arms
- PPE must be intact and correctly in place before entering the patient care area
- Have a Trained Monitor coach and observe appropriate selection, application, removal and disposal of PPE and observe that the HCW does not self-contaminate (refer to Table 2 for roles and responsibilities of the Trained Monitor)
- PPE must be worn for the duration of exposure to potentially contaminated areas and should not be adjusted during patient care
- If a breach in PPE occurs, the HCW should stop patient care, initiate PPE removal process, and then leave the patient room
- Facial protection should not be donned, doffed, or adjusted without performing hand hygiene
- Removal of PPE presents a high risk for self-contamination if not done properly and requires a structured and monitored process that must be done slowly and deliberately
- A risk assessment for exposure must be conducted to determine the required PPE to be worn by the Trained Monitor for the Monitor to safely assist the HCW
- Clean and potentially contaminated areas need to be clearly demarcated and evident to all HCWs working in the area, and traffic flow should minimize the risk of contamination
- PPE should be put on in a clean area either outside the patient room or in the anteroom
- If the anteroom is used for removing soiled PPE, then it must not be considered a clean area and clean supplies, including PPE, should not be stored there
- The HCW should have sufficient and undisturbed time to put on and remove PPE correctly
Experience suggests that the greatest risk of contamination with EBOV comes from lapses in IPC techniques, especially when removing PPE. HCWs caring for a patient with EBOD should always be paired with a Trained Monitor who will coach, assist as necessary, and observe the HCW complete the following activities:
- put on PPE
- remove PPE
- provide care to the patient with EBOD
- recommend corrective action when necessary (e.g., if PPE becomes dislodged)
| N/A | Trained Monitor |
|---|---|
| Responsibility | To assist with and ensure adherence to entire PPE use and removal process by HCWs providing direct patient care, and visitors (if permitted). |
| Role |
|
Number Needed |
One Trained Monitor at all times for each individual entering the room. |
PPE recommendations for EBOD
For stable PUI, the recommended PPE includes:
- Fluid-resistant mask
- Separate face shield or goggles
- Gloves
- Fluid-resistant or impermeable gown
If patient requires an invasive medical procedure or AGMP, follow PPE for Unstable PUI or Confirmed case.
For unstable PUI or confirmed EBOD, the recommended PPE includes:
- Fit-tested N95 respirator (or equivalent, or higher protection)
- Separate face shield
- Double gloves
- Fluid-resistant or impermeable gown or hazardous material suit
- Fluid impermeable apron
- Fluid resistant or impermeable body coverings including foot/leg and head/neck coverings
Although EBOD is not an airborne disease, we recommend a fit-tested N95 respirator (or equivalent, or higher protection) for all unstable PUI or confirmed EBOD. This recommendation is based on:
- Level of care may change unexpectedly with little time to doff and don appropriate PPE safely (e.g. sudden need for AGMP)
- Risk of exposure to blood and body fluids/splashing/contamination of mucous membranes and ability of N95 respirators to maximize the distance between mask contamination and the user's mucous membranes
- High mortality, low infectious dose
- Documented risk of infection for HCWs
While some facilities have chosen to use powered air purifying respirators (PAPRs), these are not required for the care of patients with EBOD. Their removal poses a recognized potential risk for self-contamination if worn by HCWs who are not adept at their use and removal. Effective cleaning of reusable components of the equipment is challenging, requiring multiple steps.
There are two general types of body coverings that have been used by organizations that have experience providing care to patients with EBOD:
- Combination of gown, foot and leg coverings, neck and head coverings
- Hazardous material suit
Either is acceptable (when used with the other components of PPE), provided that it meets the criteria outlined in Table 3.
| Stable PUI | Unstable PUI or confirmed case |
|---|---|
|
|
| If patient requires an invasive medical procedure or AGMP, follow PPE for Unstable PUI or Confirmed case. | All exposed skin is protected |
Patient placement and patient transfers between or within facilities
Patient placement
- Private single room, with a private bathroom, and door to remain closed.
- AIIR to be used for AGMPs, if available.
- Place appropriate EBOD isolation signage on door.
- Only essential personnel with appropriate PPE to enter the patient room.
- Room that will allow monitoring of HCW by a trained monitor during HCW-patient interaction.
- Monitor and maintain a log of all persons entering and exiting the patient room.
- There is a clearly demarcated area in close proximity to the patient's room where HCWs can remove and discard their PPE.
- There is an area outside the patient's room where clean PPE is stored and where HCWs can put on PPE before entering the patient's room.
- Do not store potentially contaminated equipment, used PPE, or waste from the patient's room in this area.
- If waste must pass through this area, it must be properly contained.
Patient transfers between or within facilities
- Patient to remain in room unless medically necessary to leave.
- Transfer of patients within the facility should be avoided unless medically required.
- When transfer or movement in healthcare facilities is necessary, the patient should be provided with a clean gown, clean bedclothes and bedding and drainage should be contained.
- The patient should perform hand hygiene with assistance as necessary before leaving the room.
- Transport staff should put on the recommended PPE to enter the patient room.
- Soiled PPE should be removed prior to exiting the patient room or designated soiled area.
- Clean PPE should be put on after leaving the patient room to transport the patient to area in case there is a need to handle the patient during transport and at transport destination.
- The patient must be taken directly to the receiving area, free of other patients and HCWs who are not involved in the patient's care.
- Prior to transfer, the most direct route within the facility should be chosen and closed off to avoid exposure of other individuals (e.g. HCWs, visitors, etc.).
- Personnel in the area to which the patient is to be transported must be informed of precautions to follow and instructed to see the patient immediately to reduce time outside of the patient room.
Environmental cleaning
Provide education, hands-on training, practice, and observation of ability to adhere to correct processes and procedures, and appropriate PPE to those responsible for environmental cleaning.
Environmental cleaning staff should, at minimum, wear the same level of protection as HCWs providing care to the patient.
Assign responsibility and accountability for cleaning and disinfection of patient care environment; and monitor to ensure appropriate processes.
A disinfectant with a broad spectrum virucide claim with a Health Canada drug identification number (DIN) should be used according to the manufacturer's instruction.
Surfaces that are likely to be touched and/or used frequently should be cleaned and disinfected on a more frequent schedule. This includes surfaces that are in close proximity to the patient (e.g., bedrails, bedside/over-bed tables, call bells) and frequently touched surfaces in the patient care environment, such as door knobs and surfaces in the patient's bathroom.
When precautions are discontinued or the patient is moved or discharged, everything in the room that cannot be cleaned and disinfected should be discarded into a designated no-touch biohazardous waste receptacle.
Cleaning and reprocessing of medical equipment
Dedicated equipment
- Non-critical patient care equipment, such as bedpans and blood pressure cuffs, should be disposable when possible and discarded in a designated no-touch biohazardous waste receptacle after use.
- Non-critical reusable patient-care equipment (e.g., commode) should be dedicated to the use of one patient and cleaned and disinfected according to manufacturer/organizational policy before reuse with another patient.
Reprocessing (cleaning, disinfection and sterilization of medical equipment)
- Semi-critical and critical equipment is to be reprocessed according to usual organizational policies and procedures (no special measures are recommended).
- For proper handling and disposal of items contaminated with blood or other body fluids of an infected person refer to Appendix C: management of Ebola disease-associated waste in Canadian healthcare settings.
- In selecting disinfectants that are expected to inactivate EBOV on non-critical hard surfaces and medical devices, Health Canada recommends products with the following approved criteria:
- Registered in Canada with a Health Canada DIN
- Labeled as a "broad spectrum virucide" claim and/or acknowledged effective testing against VHFs/Ebolavirus
- Healthcare organizations should provide education, hands-on training, repeated practice, observation of ability to adhere to correct processes and procedures, and appropriate PPE to those responsible for reprocessing (decontamination, cleaning, disinfection and sterilization) reusable medical equipment.
- Healthcare organizations should assign responsibility and accountability for reprocessing non-critical patient care equipment.
- Non-critical patient care equipment should be cleaned and disinfected according to a regular schedule and when visibly soiled.
Waste, linen, and nutritional services
Waste and linen management
Patient bed linen should be changed regularly and when soiled, upon discontinuation of precautions and following patient discharge. Linen should:
- Be handled with minimum agitation to avoid contamination of air, surfaces and persons
- Not be sent to the laundry
- Be disposed of in a designated no-touch biohazardous waste receptacle at the point-of-use
Different jurisdictions have different requirements for disposal of human waste. For further information on management of waste, including the disposal of urine, stool and emesis, and linen refer to Appendix C: Management of Ebola disease-associated waste in Canadian healthcare settings.
Handling dishes and cutlery
Use disposable dishes/cutlery and dispose of in a designated biohazardous waste receptacle at the point-of-use.
Education of patients and visitors
Patients, their visitors, families and decision makers should be educated about the additional precautions being used, the duration of precautions, as well as the prevention of transmission of disease to others, with a particular focus on hand hygiene and respiratory hygiene.
Discharge planning (including but not limited to continuation of infection control precautions in the home setting) should be managed on a case-by-case basis in consultation with the IPC program, infectious disease specialists, and public health officials.
Visitor considerations
Avoid entry of visitors into the patient room. Exceptions may be considered on a case-by-case basis for those who are essential for the patient's wellbeing (e.g., caregiver of children). Visits should be scheduled and monitored to allow for:
- Screening of the visitor for EBOD (e.g., symptoms and risk factors) before entering or upon arrival to the hospital
- Evaluating risk to the health of the visitor and ability to comply with precautions
- Providing instruction before entry into the patient care area on hand hygiene, limiting surfaces touched, and use of PPE according to the current facility policy while in the patient's room
Visitors should be made aware of the risk of self-contamination when using their PPE, and movement within the facility should be restricted to the patient care area only.
Handling bodies of deceased patients
Post-mortem examinations (if necessary) and human remains handling should be done in accordance with respective federal and provincial or territorial regulations.
In addition to routine practices, PPE consistent with an unstable PUI or confirmed EBOD case should be used. This includes a fit-tested N95 respirator (or equivalent, or greater protection) with face shield, double gloves, fluid-resistant or impermeable gown or hazardous materials suit, fluid-impermeable apron, fluid-resistant or impermeable body coverings including foot/leg and head/neck coverings.
Medical devices (i.e., intravenous catheters, urinary catheter, or endotracheal tubes) should be left in place.
At the site of the death, the body should be wrapped in high quality puncture-resistant plastic shroud. Care should be taken to prevent the contamination of the exterior surface of the shroud. A leak-proof body bag should be used over the shroud. Once closed the body bag should not be re-opened. While wearing PPE, perform cleaning and disinfection of the outer bag with a broad spectrum virucide disinfectant registered with a Health Canada DIN, according to the manufacturer's instruction.
Handling of human remains should be kept to a minimum (e.g., no autopsies unless necessary, no embalming, and no post-mortem care) and direct contact with the human remains must only be done by trained HCWs.
Duration of precautions
The decision to discontinue precautions for PUIs or patients with confirmed EBOD should be made on a case-by-case basis, jointly in consultation with the IPC program, infectious disease specialists, and public health officials.
Patients should be advised that some body fluids remain positive for some time after the virus is no longer detectable in the blood and advised on the appropriate personal precautions to take with close contacts. This includes semen and breast milk. Refer to the Public Health Management of Cases and Contacts of Ebola Disease in the Community Setting in Canada for specific recommendations on case management of convalescent confirmed cases after discharge from hospital.
Notification
Refer to the Public Health Management of Cases and Contacts of Ebola Disease in the Community Setting in Canada for notification protocols.
A PUI or confirmed case should be reported immediately to the infection prevention and control program, laboratory and local public health officials as per jurisdictional protocols in the respective province or territory in Canada. Ensure a timely, person-to-person discussion of the case with relevant authorities.
Concurrent with a request for laboratory services for EBOD or other VHF, provinces and territories are requested to notify and provide a clinical history of the patient's illness to the Public Health Agency of Canada.
Laboratory precautions
Public health authorities should be involved in provision of information regarding laboratory testing requirements and specimen transport protocols. Prior to collecting specimen, contact facility laboratory to notify them of the possible diagnosis and for specific instructions before collecting or sending any specimens to the laboratory. The decision for specimen collection and testing should be predicated on the clinical status of the patient and based on an on-going risk assessment. No viral culture should be attempted outside of the Public Health Agency of Canada's National Microbiology (NML) Containment Level 4 laboratory.
For diagnostic or confirmatory services for EBOD, liaise with the provincial public health laboratory of your jurisdiction to coordinate with the National Microbiology Laboratory (NML) Operations Centre Director (OCD). The NML OCD will work with the requesting provincial jurisdiction to activate the Emergency Response Assistance Plan.
Laboratories receiving specimens from person under investigation (PUI) for EBOD must be aware that improper handling of these specimens may pose a risk to the health of laboratory personnel. Consult the "Biosafety Guidelines for Laboratories Handling Specimens from Patients under Investigation for Ebola Virus Disease" before any testing occurs.
Wipe specimen container using a disinfectant with a broad spectrum virucide claim with a Health Canada DIN, according to the manufacturer's instructions, in the patient room prior to placing into a secure container for transport. Consult the "Biosafety Guidelines for Laboratories Handling Specimens from Patients under Investigation for Ebola Virus Disease".
Do not send specimens in a pneumatic tube system.
Appendix A: Acknowledgements
National Advisory Committee on Infection Prevention and Control (NAC-IPC)
Joanne Embree, MD, MSc
Pediatric Infection Disease Specialist, Shared Health
Professor, University of Manitoba
Winnipeg, MB
Matthew P. Muller, MD, PhD, FRCPC
Associate Professor, University of Toronto
Medical Director, Infection Prevention & Control, Unity Health Toronto
Toronto, ON
Molly Blake RN BN MHS CIC
Program Director, Infection Prevention & Control, Winnipeg Regional Health Authority (WRHA)
Acting Program Director, Medical Device Reprocessing, WRHA
Winnipeg, MB
Patsy Rawding, RN, BScN, CIC
Health Services Manager, Infection Prevention & Control
Western Zone, NSHA Lead Manager in LTC
Middleton, NS
Patrice Savard, MD, MSc, FRCPC
Clinical Associate Professor, University of Montreal
Clinical microbiologist and Infectious diseases specialist, CHUM
Medical director, nosocomial infection prevention and control unit, CHUM
Montréal, QC
Jennie Johnstone, MD, PhD, FRCPC
Associate Professor, University of Toronto
Medical Director Infection Prevention and Control, Sinai Health
Toronto, ON
Stephanie W. Smith, MD, MSc, FRCPC
Professor, Division of Infectious Diseases, Department of Medicine, University of Alberta
Director, Infection Prevention and Control, University of Alberta Hospital
Edmonton, AB
Suzanne Rhodenizer Rose RN BScN MHS CIC
Director, Clinical Planning, QEII New Generation Project
Project Infection Control Specialist
Halifax, NS
Anne Masters-Boyne, R.N., M.N.
Occupational Health Nurse, Employee Health Services, Horizon Health Network
Fredericton, NB
Jennifer Happe, BSc, MSc
Alberta Children's Hospital
Director, Infection Prevention and Control Canada
Infection Control Professional, Alberta Health Services
Calgary, AB
Nisha Thampi, MD, MSc, FRCPC
Medical Director, Infection Prevention and Control Program, Division of Infectious Diseases, Children's Hospital of Eastern Ontario
Associate Professor, Department of Pediatrics, University of Ottawa
Ottawa, ON
Susy Hota MSc MD FRCPC
Medical Director, Infection Prevention and Control
Infectious Diseases Specialist, University Health Network
Associate Professor, Department of Medicine, Division of Infectious Diseases, University of Toronto
Toronto, ON
Brian Sagar, MSc, BA
Senior Director, Communicable Disease
British Columbia Ministry of Health
Victoria, BC
Healthcare-acquired Infection Prevention and Control Section
Maureen McGrath, BScN., RN
Steven Ettles, MPH
Amanda Graham, MPH
Jennifer Selkirk, MSc., RN
Appendix B: Algorithm for screening and assessment for Ebola disease in persons presenting to healthcare settings
Download a printable PDF version of Appendix B.
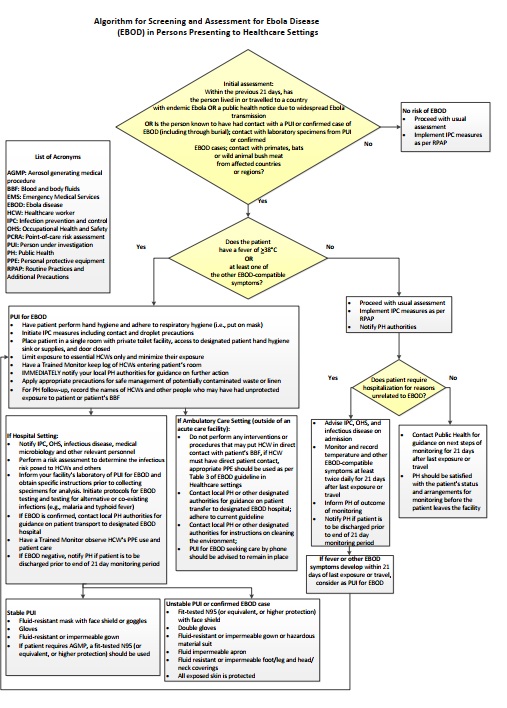
Figure - Text description
An algorithm for the screening and assessment of patients presenting to a healthcare setting is used as a tool to assess risk of EBOD and to make decisions about the appropriate IPC measures to enact. This is based on a multi-stage assessment of a person's travel history, possible contact with EBOD via a person(s) under investigation (PUI), confirmed patient(s) with EBOD, or laboratory specimens, and symptoms.
The algorithm begins with an initial assessment of the person presenting to the healthcare setting. Assess the following factors:
- a. Within the previous 21 days, has the person lived in or travelled to a country with endemic Ebola, or a public health notice due to widespread Ebola transmission?
- b. Within the previous 21 days, is the person known to have had contact with a PUI for EBOD or patient confirmed to have EBOD, including through burial?
- c. Within the previous 21 days, has the person had contact with laboratory specimens from PUI or patient(s) confirmed to have EBOD?
- d. Within the previous 21 days, has the person had contact with primates, bats, or wild animal bush meat from EBOD-affected countries or regions?
If the answer to any of the above questions is NO, there is no risk of EBOD. As such, healthcare workers should proceed with usual assessment and implement IPC measures as per Routine Practices and Additional Precautions (RPAP). The EBOD-specific assessment ends at this point for this person.
If the answer to any of the above questions is YES, the assessment should continue with an assessment of the person's symptoms. Assess the following:
- e. Does the person have a fever of greater than or equal to 38 degrees Celsius, or
- f. Does the person have at least one other EBOD-compatible symptom?
If the answer to these questions is NO, healthcare workers should proceed with usual assessment, implement IPC measures as per RPAP, and notify public health authorities.
Next, assess:
- g. Does the patient require hospitalization for reasons unrelated to EBOD?
If the answer is YES:
- Advise infection prevention and control (IPC), Occupational Health and Safety (OHS), and infectious disease on admission;
- Monitor and record temperature and other EVD-compatible symptoms at least twice daily for 21 days after last exposure or travel;
- Inform public health of outcome of monitoring;
- Notify public health if patient is to be discharged prior to end of the 21 day monitoring period.
- If the patient develops fever or other EBOD-compatible symptoms within 21 days of last exposure or travel, consider as a PUI for EBOD. Refer to the "PUI for EBOD" section of this description.
If the answer is NO:
- Contact public health for guidance on next steps of monitoring for 21 days after the last exposure or travel;
- Public health should be satisfied with the patient's status and arrangements for monitoring before the patient leaves the facility.
"PUI for EBOD": If the answer to question (e) [Does the person have a fever of greater than or equal to 38 degrees Celsius?] or (f) [Does the person have at least one other EBOD-compatible symptom?] is YES, the person should be considered as a person under investigation (PUI) for EBOD. Initiate the following actions:
- Have the patient perform hand hygiene and adhere to respiratory hygiene (i.e., put on mask);
- Initiate IPC measures including contact and droplet precautions;
- Place patient in a single room with private toilet facility, access to designated patient hand hygiene sink or supplies, and door closed;
- Limit exposure to essential healthcare workers and minimize their exposure;
- Have a Trained Monitor keep log of healthcare workers entering patient's room;
- Immediately notify local public health authorities for guidance on further action;
- Apply appropriate precautions for safe management of potentially contaminated waste or linen;
- For public health follow up, record the names of healthcare workers and other people who may have had unprotected exposure to patient or patient's blood and bodily fluids (BBF).
If a PUI for EBOD is identified in an AMBULATORY CARE SETTING, i.e., outside of an acute care facility, complete the following actions:
- Do not perform any interventions or procedures that may put healthcare workers in direct contact with the patient's BBF. If healthcare worker must have direct patient contact, appropriate PPE should be worn as per Table 3 of the guideline
- Contact local public health or other designated authorities for guidance on patient transfer to designated EBOD hospital. Adhere to the current guideline.
- Contact local public health or other designated authorities for instructions on cleaning the environment;
- PUI for EBOD seeking care by phone should be advised to remain in place.
If a PUI for EBOD is identified in a HOSPITAL SETTING, complete the following actions:
- Notify IPC, OHS, infectious disease, medical microbiology and other relevant personnel;
- Perform a risk assessment to determine the infectious risk posed to healthcare workers and others;
- Inform your facility's laboratory of PUI for EBOD and obtain specific instructions prior to collecting specimens for analysis. Initiate protocols for EBOD testing and testing for alternative or co-existing infections (e.g., malaria and typhoid fever);
- If EBOD is confirmed, contact local public health authorities for guidance on patient transport to designated EBOD hospital;
- Have a Trained Monitor observe healthcare workers PPE use and patient care;
- If EBOD negative, notify public health if patient is to be discharged prior to end of 21 day monitoring period.
If the PUI for EBOD is stable, the following PPE should be used by healthcare workers:
- Fluid-resistant mask with separate face shield or goggles
- Gloves
- Fluid-resistant or impermeable gown
- If the patient requires an aerosol-generating medical procedure (AGMP), a fit-tested N95 (or equivalent, or higher protection) should be used.
If the PUI for EBOD is unstable, or if EBOD is confirmed, the following PPE should be used by healthcare workers:
- Fit-tested N95 (or equivalent, or higher protection) with separate face shield
- Double gloves
- Fluid-resistant or impermeable gown or hazardous material suit
- Fluid impermeable apron
- Fluid-resistant or impermeable foot/leg and head/neck coverings
- All exposed skin is protected
Appendix C: Management of Ebola disease-associated waste in Canadian healthcare settings
This guidance addresses the safe management (handling, containment, transport and disposal) of waste generated in healthcare settings from persons under investigation and persons confirmed with EBOD.
Its use is intended for healthcare organizations, in particular, individuals responsible for facility and medical waste management, and those responsible for IPC and occupational health and safety training and education of healthcare workers (HCWs) and environmental services personnel who may be involved with EBOD-associated waste management.
The guidance is based on currently available scientific evidence, standards and regulations, and adopts a precautionary approach where the evidence is lacking or inconclusive. It is subject to review and change as new information becomes available.
The guidance should be read in conjunction with relevant federal, provincial, territorial and local legislation, regulations, and policies, and adapted to local requirements as necessary.
This guidance does not cover the management of waste in laboratory settings, guidance for cleaning, disinfection and sterilization of equipment or guidance on the management of the deceased body.
EBOD-associated waste
EBOV is categorized as a Risk Group 4 agent, is likely to cause serious disease and effective treatment is not available. Waste contaminated with EBOV requires special handling and disposal to prevent exposure to the virus.
All EBOD-associated waste is considered biohazardous (or infectious) waste and includes items (including linen and sharps) contaminated with human blood and body fluids (i.e., respiratory secretions, saliva, emesis, feces, urine, dialysate/effluent) that warrants special handling and disposal as they may in certain situations present a risk of disease transmission.
EBOD-associated waste that has been appropriately incinerated or autoclaved is not infectious and does not pose a health risk. The following guidance on the safe management of EBOD-associated waste is recommended for healthcare organizations that do not have on-site facilities or means to inactivate EBOD-associated waste by incineration or autoclaving.
Recommended measures for the healthcare organization
It is the responsibility of the healthcare organization to minimize the risk of exposure to and transmission of infectious diseases. The following measures are recommended for healthcare organizations for the safe management of EBOD-associated waste:
- Implement a biohazardous waste management program with the development of policies and procedures to include the following:
- Protocols for adequate supplies of biohazard waste bags and containers, hand hygiene products, cleaning supplies and disinfectants, and personal protective equipment (PPE)
- Protocols for segregating, packaging, labelling, moving, storing and transporting EBOD-associated waste (both on- and off-site)
- Methods for keeping records of the quantities of EBOD-associated waste generated and disposed of
- A list of all regulations and legislation concerning EBOD-associated waste that are applicable within the organization's jurisdiction
- A list of individuals responsible for managing EBOD-associated waste and in the event of an on-site accident or spill
- Protocols for regular, ongoing training and education of HCWs (i.e., nurses, doctors) and environmental services personnel on proper handling and potential hazards of EBOD-associated waste, type and quality of waste containers, PPE selection and use, including enhanced PPE based on a risk assessment
- Provision for regular and ongoing education on routine practices and hand hygiene performed as per PPE protocol, according to the organization's policy, with alcohol-based hand rub or washing with soap and water, if hands are visibly soiled
- Assign only primary/direct care HCWs (i.e., nurses, doctors) and environmental services personnel trained and educated in occupational health and safety, IPC practices and appropriate selection and use of PPE for the management of EBOD-associated waste
- Develop and implement a monitoring system, using a trained observer, for ensuring consistency in safely putting on and removing PPE when handling EBOD-associated waste
- Develop and implement protocols for the containment and storage of EBOD-associated waste as per organization's biohazardous materials policy, and for off-site transport in accordance with applicable legislation, for example, the Transport Canada's Transportation of Dangerous Goods Regulations (TDGR)
- Determine capability for/availability of EBOD-associated waste transport and disposal services within the municipality/region and ensure that waste is disposed of in accordance with local, municipal or regional requirements and regulations and/or bylaws for regulated biohazardous waste
- Provide education to personnel on steps to take if a breach in safe handling and containment occurs, resulting in exposure or potential exposure, during the management of EBOD-associated waste, which includes:
- Personnel to immediately stop work, safely remove PPE, as per organization's protocol, and leave the area
- Rinse the affected skin surface with soap and water OR for mucous membrane splashes (e.g., conjunctiva), irrigate with copious amounts of water or eyewash solution as per organization's first aid protocol
- Report immediately according to organization's exposure/injury protocol, including notification to Public Health (PH) authorities
- Adhere to organization's and PH follow-up procedures
Recommended management of EBOD-associated waste generated in healthcare settings
All HCWs (i.e., nurses, doctors) and environmental services personnel handling EBOD-associated waste should wear appropriate PPE, including enhanced PPE based on a risk assessment, along with following guidance for safe removal of PPE, according to the organization's policy.
Examples of waste:
- Human - blood and other body fluids, such as respiratory secretions, saliva, emesis, feces, urine, dialysate/effluent
- Linen - bedding, towels, washcloths, gowns, and curtains (privacy, shower, window)
- Other Non-sharps - PPE, disposable bedpans, disposable linen, disposable dishes/cutlery/trays, dressings, sponges, pads, procedure drapes, incontinent products/diapers, tissues, replaced string/cloth call bells and light cords, cleaning cloths/wipes, mop heads/cloths, spills, intravenous/gastrointestinal/urine catheters and bags, dialysis tubing, suctioning equipment/tubing, non-fluid-impermeable pillows and mattresses
- Sharps - syringes, needles, razors, scalpels
Human waste
- Human waste should only be handled in the patient-care room by trained HCWs (i.e., nurses, doctors) wearing appropriate PPE.
- Urine, feces, emesis and dialysate/effluent from dialysis may be disposed of through the normal sanitary sewer system, or in accordance with municipal/regional regulations.
- In settings where municipal regulatory restrictions exist on disposal through the normal sanitary sewer system, the use of a solidifier for liquid waste (with either disposable toilet hat inserts or bedpans/commode pans - disposable or with liners, and with disposable emesis basins) to be disposed of as waste should be considered.
- In settings where on-site septic sewage disposal systems are used (i.e., septic tanks), no specific measures are required providing the system is operating according to local regulations.
- The following measures for waste should be taken:
- Pour waste in a controlled manner; pour from a low level into the toilet to avoid splashing
- Close the lid, THEN flush the toilet
- Clean and disinfect flush handles, toilet seat and lid surfaces using a disinfectant with a broad spectrum virucide claim with a Health Canada Drug Identification Number (DIN) according to manufacturer's instructions and discard cleaning cloths in biohazard bags
Linen
- The number of personnel (i.e., HCW - nurses, doctors; environmental services) managing linen should be limited.
- Only personnel trained and wearing appropriate PPE should be managing linen (handling, containing and on-site transport).
- Handling and containing linen should only occur in the patient-care room and in the room/area where PPE is removed by trained HCWs (i.e., nurses, doctors) wearing appropriate PPE.
- The following measures should be taken:
- Consider all linen in the patient-care room contaminated, whether used or not
- Contain linen at point of use
- Fold linen inward and handle with minimal agitation and shaking to avoid contamination of air, surfaces and persons
- Place disposable linen into waste container
- Place reusable linen immediately into a sturdy, leak resistant container lined with a leak and tear resistant plastic biohazard bag
- Do not manually compact linen into the bags
- When the bag is two-thirds full, seal securely preventing tearing/puncturing the bag and ensuring no leaks
- Remove the bag from the container (Note: this container should stay in the patient's room until discharge and relined with a new biohazard bag for next fill)
- Clean and disinfect the entire outside of the bag by wiping using a disinfectant with a broad spectrum virucide claim with a Health Canada DIN and used according to the manufacturer's instructions
- Place the decontaminated bag into a second biohazard bag and seal securely, preventing tearing/puncturing the second bag and ensuring no leaks
- Wipe the entire outside of the second bag using a disinfectant with a broad spectrum virucide claim with a Health Canada DIN and used according to the manufacturer's instructions, immediately before removing it from the room
- To move the double-bagged linen from the patient-care room, personnel should place the double-bagged linen in a leak-proof/impervious, puncture-resistant plastic or metal single-use container.
- The linen container should be:
- Located at the periphery/outside of the area for taking off PPE to avoid risk of recontamination of the container during PPE removal
- Securely sealed, clearly labelled and identified as EBOD-associated biohazardous material
- Decontaminated by wiping the entire outside of the container using a disinfectant with a broad spectrum virucide claim with a Health Canada DIN and used according to the manufacturer's instructions, immediately before removing the container from the area
- Personnel removing the linen container from the area should only handle the outer container and transport carts.
- Containers should not be re-opened once sealed.
- For moving large or heavy containers, carts with guard rails or raised edges should be used and loaded in a manner that will prevent items from tipping.
- Carts should be disinfected after each use with a disinfectant with a broad spectrum virucide claim with a Health Canada DIN and used according to the manufacturer's instructions.
- The container should be moved immediately and directly to a designated locked holding area with restricted access and stored as per the organization's biohazardous materials policy until test results that confirm whether or not the patient has EBOD are available.
- EBOD-associated linen storage areas should be clearly marked with a biohazard symbol and kept separate from other storage areas.
If test result for EBOV is negative: No further special handling of the stored reusable linen required. Regular laundry process for stored reusable linen is appropriate and disposal of reusable linen into waste stream is not necessary.
If test result for EBOV is positive: Stored containers of linen should be packaged and transported separately off-site and disposed of in accordance with applicable legislation for regulated biohazardous waste.
Other non-sharps waste
- The number of personnel (i.e., HCW - nurses, doctors; environmental services) managing other non-sharps waste should be limited.
- Only personnel trained and wearing appropriate PPE should be managing waste (handling, containing and on-site transport).
- Handling and containing waste should only occur in the patient-care room and in the room/area where PPE is removed by trained HCWs (i.e., nurses, doctors) wearing appropriate PPE.
- The following measures should be taken:
- Consider all supplies taken into the patient-care room contaminated, whether used or not
- Contain waste at point of generation
- Place waste immediately into a sturdy and leak resistant container lined with a leak and tear resistance plastic biohazard bag
- Do not manually compact waste in the bags
- When the bag is two-thirds full, seal securely preventing tearing/puncturing the bag and ensuring no leaks
- Remove the bag from the container (Note: this container should stay in the patient's room until discharge and relined with a new biohazard bag for next fill)
- Clean and disinfect the entire outside of the bag by wiping using a disinfectant with a broad spectrum virucide claim with a Health Canada DIN and used according to the manufacturer's instructions
- Place the decontaminated bag into a second biohazard bag and seal securely, preventing tearing/puncturing the second bag and ensuring no leaks
- Wipe the entire outside of the second bag using a disinfectant with a broad spectrum virucide claim with a Health Canada DIN used according to the manufacturer's instructions, immediately before removing it from the room
- To move the double-bagged waste from the patient-care room, personnel should place the double-bagged waste into a leak-proof/impervious, puncture-resistant plastic or metal single-use container.
- The waste container should be:
- Located at the periphery/outside of the area for taking off PPE to avoid risk of recontamination of the container during PPE removal
- Securely sealed, clearly labelled and identified as EBOD-associated biohazardous material
- Decontaminated by wiping the entire outside of the container using a disinfectant with a broad spectrum virucide claim with a Health Canada DIN and used according to the manufacturer's instructions, immediately before removing the container from the area
- Personnel removing the waste container from the area should only handle the outer container and transport carts.
- Containers should not be re-opened once sealed.
- For moving large or heavy containers, carts with guard rails or raised edges should be used and loaded in a manner that will prevent items from tipping.
- Carts should be disinfected after each use with a disinfectant with a broad spectrum virucide claim with a Health Canada DIN and used according to the manufacturer's instructions.
- The container should be moved immediately and directly to a designated, locked holding area with restricted access and stored as per the organization's biohazardous materials policy until test results that confirm whether or not the patient has EBOD are available.
- EBOD-associated waste storage area should be clearly marked with a biohazard symbol and kept separate from other storage areas.
If test result for EBOV is negative: No further special handling of the stored waste is required. Regular waste disposal process as per organization's protocol for biohazardous waste is appropriate.
If test result for EBOV is positive: Stored containers of waste should be packaged and transported separately off-site and disposed of in accordance with applicable legislation for regulated biohazardous waste.
Sharps waste
- Sharps waste should be segregated from other waste and discarded:
- At point of use
- Directly into single-use containers, that are leak-proof/impervious, puncture resistant, fitted with securely closed lids and specifically designed for sharps waste
- Sharps containers should not be filled beyond two-thirds full, to allow for safe closure.
- The following measures for sharps containers should be taken:
- When the container is two-thirds full, securely close the lid
- Wipe the outside of the container using a disinfectant with a broad spectrum virucide claim with a Health Canada DIN and used according to the manufacturer's instructions
- Place the sharps container into a second leak-proof/impervious, puncture resistant biohazard container
- Securely seal, clearly label and identify the second container as EBOD-biohazardous sharps material
- Wipe the outside of the second container using a disinfectant with a broad spectrum virucide claim with a Health Canada DIN and used according to the manufacturer's instructions
- The container should be moved immediately and directly to a designated, locked holding area with restricted access and stored, awaiting transport off site, as per the organization's biohazardous materials policy.
- EBOD-associated sharps waste storage areas should be clearly marked with a biohazard symbol and kept separate from other storage areas.
- Stored containers of EBOD-associated sharps waste should be transported separately off-site and disposed of in accordance with applicable legislation for regulated biohazardous waste.
Recommended management of on-site spills related to EBOD-associated blood and other body fluids
- Spills containing EBOD-associated human waste (i.e., blood, emesis, urine, feces and dialysate/effluent) should be managed by trained HCWs (i.e., nurses, doctors) caring for the patient, and wearing appropriate PPE, including enhanced PPE based on a risk assessment, along with following guidance for safe removal of PPE, according to the organization's policy.
- 'Spill kits' should be made available, according to organization's policy, for use in designated assessment/care rooms/areas.
- The spill area should be isolated from access to other individuals until cleaning and disinfection is completed.
- All major spill incidents should be documented, according to organization's policy.
- The following cleaning and disinfection measures are recommended:
- Allow fluid and droplets to settle; fluids should not be permitted to fully dry
- Gently cover the spill with disposable absorbent paper towels, wipes or pads (a solidifier agent may be used); remove organic/bulk material, and place waste immediately into sturdy, leak and tear resistant biohazard plastic bag and securely seal
- With disposable cleaning cloths or wipes, apply a disinfectant with a broad spectrum virucide claim with a Health Canada DIN to the surface and allow sufficient contact time according to manufacturer's instructions
- Do not spray disinfectant or use wet vacuum in order to avoid any splashes and splatter
- Start at one end of the affected area and move in one direction until all surfaces have been disinfected and do not use a circular motion
- Use cleaning cloths, wipes, etc., only once and after use, discard all cleaning items immediately into a biohazard bag located within reach
References
Public Health Agency of Canada
- Ebola virus disease: Symptoms and treatment. https://www.canada.ca/en/public-health/services/diseases/Ebola.html
- Hand Hygiene Practices in Healthcare Settings (2012). https://www.canada.ca/en/public-health/services/infectious-diseases/nosocomial-occupational-infections/hand-hygiene-practices-healthcare-settings.html
- Public Health Agency of Canada. Pathogen Safety Data Sheets: Infectious Substances - Ebolavirus (2018). https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/pathogen-safety-data-sheets-risk-assessment/ebolavirus.html
- Public Health Agency of Canada. Environmental sanitation practices to control the spread of communicable disease in passenger conveyances and terminals (2019). https://www.canada.ca/en/public-health/services/emergency-preparedness-response/centre-emergency-preparedness-response/travelling-public-program/environmental-sanitation-practices-control-spread-communicable-disease-passenger-conveyances-terminals.html
- Public Health Agency of Canada Case Definition: EBOV disease outbreak (2018). https://www.canada.ca/en/public-health/services/infectious-diseases/viral-haemorrhagic-fevers/national-case-definition-Ebola-virus-disease.html
- Public Health Management of Cases and Contacts of Ebola Virus Disease in the Community Setting in Canada (2018). https://www.canada.ca/en/public-health/services/diseases/Ebola/health-professionals-Ebola/interim-guidance-public-health-management-cases-contacts-Ebola-community-setting-canada.html
- Pathogen Safety Data Sheets: Infectious Substances - Ebolavirus (2018). https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/pathogen-safety-data-sheets-risk-assessment/Ebolavirus.html
- Routine Practices and Additional Precautions for Preventing the Transmission of Infection in Healthcare Settings (2013). https://www.canada.ca/en/public-health/services/publications/diseases-conditions/routine-practices-precautions-healthcare-associated-infections.html
Canada
- Canadian Critical Care Society, Canadian Association of Emergency Physicians, Association of Medical Microbiology and Infectious Diseases. Ebola Clinical Care Guidelines: A guide for clinicians in Canada, Report #2 (2014). http://www.canadiancriticalcare.org/resources/Pictures/Ebola%20Clinical%20Care%20Guidelines_ENG.pdf
- Canadian Standards Association. Standard Z317.10:21. Handling of health care waste materials (2021).
- Canadian Standards Association. Standard Z316.6:20. Sharps injury protection - Requirements and test methods - Sharps containers (2021).
- Government of Canada. Canadian Biosafety Standard, Second Edition (2015). https://www.canada.ca/en/public-health/services/canadian-biosafety-standards-guidelines/second-edition.html
- Transport Canada. Transportation of Dangerous Goods Regulations (2017). https://laws-lois.justice.gc.ca/eng/regulations/SOR-2001-286/index.html
Centers for Disease Control and Prevention (CDC), United States
- Centers for Disease Control and Prevention. Ebola-Associated Waste Management (2022). http://www.cdc.gov/vhf/ebola/hcp/medical-waste-management.html
- Centers for Disease Control and Prevention. Frequently Asked Questions (FAQs) on Interim Guidance for Managers and Workers Handling Untreated Sewage from Suspected or Confirmed Individuals with Ebola in the U.S. (2014). http://www.cdc.gov/vhf/ebola/prevention/faq-untreated-sewage.html
- Centers for Disease Control and Prevention. Interim Guidance for Environmental Infection Control in Hospitals for Ebola Virus (2018). http://www.cdc.gov/vhf/ebola/hcp/environmental-infection-control-in-hospitals.html
- Centers for Disease Control and Prevention. Procedures for Safe Handling and Management of Ebola-Associated Waste (2014). http://www.cdc.gov/vhf/ebola/prevention/ebola-associated-waste.html
- Centers for Disease Control and Prevention. Recommendations for Safely Performing Acute Hemodialysis in Patients with Ebola Virus Disease (EVD) in U.S. Hospitals (2015). http://www.cdc.gov/vhf/ebola/hcp/guidance-dialysis.html
- Infection Prevention and Control Recommendations for Hospitalized Patients Under Investigation (PUIs) for Ebola Virus Disease (EVD) in U.S. Hospitals (2018). https://www.cdc.gov/vhf/Ebola/clinicians/evd/infection-control.html
- Morbidity and Mortality Weekly Report (MMWR). Surveillance and Preparedness for Ebola Virus Disease - New York City, 2014 (2014). http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6341a5.htm?s_cid=mm6341a5_w
Public Health Ontario
- Provincial Infectious Disease Advisory Committee. Best Practices for Environmental Cleaning for Prevention and Control of Infections In All Health Care Settings (3rd edition) (2018). https://www.publichealthontario.ca/-/media/documents/bp-environmental-cleaning.pdf?la=en
- Provincial Infectious Disease Advisory Committee. Environmental Cleaning Toolkit (2010). https://www.publichealthontario.ca/en/health-topics/infection-prevention-control/environmental-cleaning/environmental-cleaning-toolkit
- Provincial Infectious Disease Advisory Committee. Infection Prevention and Control for Clinical Office Practice (1st revision) (2015). https://www.publichealthontario.ca/-/media/documents/bp-clinical-office-practice.pdf?la=en
- Provincial Infectious Disease Advisory Committee. Routine Practices and Additional Precautions in All Health Care Settings (3rd edition) (2012). https://www.publichealthontario.ca/-/media/documents/bp-rpap-healthcare-settings.pdf?la=en
World Health Organization
- Ebola virus disease (2019). http://www.who.int/Ebola/en/
- Ebola virus disease (2019). https://www.who.int/en/news-room/fact-sheets/detail/Ebola-virus-disease
- Clinical management of patients with viral haemorrhagic fever: a pocket guide for the front-line health worker: interim emergency guidance - generic draft for West African adaptation (2014). https://apps.who.int/iris/handle/10665/130883
- World Health Organization. EBOV disease - Key questions and answers concerning health care waste (2021). https://www.who.int/publications/i/item/WHO-HEP-ECH-WSH-2021.2
- World Health Organization/UNICEF. Ebola Virus Disease (EVD): Key questions and answers concerning water, sanitation and hygiene (2021). https://www.who.int/publications/i/item/ebola-virus-disease-(evd)-key-questions-and-answers-concerning-water-sanitation-and-hygiene
- World Health Organization. Infection prevention and control (IPC) guidance summary: Ebola guidance package (2014). https://www.who.int/publications/i/item/WHO-HIS-SDS-2014.4-Rev.1
- World Health Organization. Safe management of wastes from health-care activities (2014). https://www.who.int/publications/i/item/9789241548564