Archived: Preliminary guidance on key populations for early COVID-19 immunization [2020-11-03]
Notice to reader
This is an archived version. Please refer to current COVID-19 vaccine pages:
On this page
- Preamble
- Summary of content included in this NACI statement
- Introduction
- Methods
- Vaccine(s)
- Ethics, equity, feasibility and acceptability considerations
- Recommendations
- Research priorities
- List of abbreviations
- Acknowledgments
- Appendix A: Algorithm outlining the process for applying the EEFA framework
- Appendix B: Core ethical dimensions filter applied to COVID-19
- Appendix C: Ethical procedural considerations filter applied to COVID-19
- Appendix D: Equity matrix applied to COVID-19 with evidence to date
- Appendix E: Feasibility matrix applied to COVID-19
- Appendix F: Acceptability matrix applied to COVID-19
- References
Preamble
The National Advisory Committee on Immunization (NACI) is an External Advisory Body that provides the Public Health Agency of Canada (PHAC) with independent, ongoing and timely medical, scientific, and public health advice in response to questions from PHAC relating to immunization.
In addition to burden of disease and vaccine characteristics, PHAC has expanded the mandate of NACI to include the systematic consideration of programmatic factors in developing evidence-based recommendations to facilitate timely decision-making for publicly funded vaccine programs at provincial and territorial levels.
The additional factors to be systematically considered by NACI include: economics, ethics, equity, feasibility, and acceptability. Not all NACI Statements will require in-depth analyses of all programmatic factors. While systematic consideration of programmatic factors will be conducted using evidence-informed tools to identify distinct issues that could impact decision-making for recommendation development, only distinct issues identified as being specific to the vaccine or vaccine-preventable disease will be included.
This statement contains NACI’s independent advice and recommendations, which are based upon the best current available scientific knowledge and is disseminating this document for information purposes. People administering the vaccine should also be aware of the contents of the relevant product monograph(s). Recommendations for use and other information set out herein may differ from that set out in the product monograph(s) of the Canadian manufacturer(s) of the vaccine(s). Manufacturer(s) have sought approval of the vaccine(s) and provided evidence as to its safety and efficacy only when it is used in accordance with the product monographs. NACI members and liaison members conduct themselves within the context of PHAC's Policy on Conflict of Interest, including yearly declaration of potential conflict of interest.
Summary of information contained in this NACI Statement
The objective of this advisory committee statement is to provide preliminary guidance for public health program level decision-making to plan for the efficient, effective, and equitable allocation of a novel coronavirus disease 2019 (COVID-19) vaccine once it is authorized for use in Canada when limited initial vaccine supply will necessitate the prioritization of immunization in some populations earlier than others. These recommendations aim to achieve Canada's pandemic response goal: "To minimize serious illness and overall deaths while minimizing societal disruption as a result of the COVID-19 pandemic." Due to anticipated constraints in supply, these National Advisory Committee on Immunization (NACI) recommendations apply to provincial/territorial publicly-funded immunization programs only and not for individuals wishing to prevent COVID-19 with vaccines not included in such programs.
The recommendations are informed by evidence available at the time of NACI deliberations, including the results of a rapid review of risk factors for severe COVID-19,Footnote 1 an expert stakeholder survey on the relative importance of pandemic immunization strategies,Footnote 2 and the systematic assessment of ethics, equity, feasibility and acceptability (EEFA) considerations with the peer-reviewed EEFA Framework.Footnote 3 NACI will continue to carefully monitor the evidence related to COVID-19 and COVID-19 vaccine(s) and will update recommendations as evidence evolves.
Recommendations for public health program level decision-making
Given arrival of vaccine supply is expected to be staggered over several months, NACI recommends that key populations in whom vaccine is deemed safe and effective based on clinical evidence available at the time of vaccine availability should be prioritized for COVID-19 immunization. These groups are not mutually exclusive and may overlap. A sequential approach cannot be determined until vaccine characteristics, results of clinical trials and the number of available doses are known. Key populations may change as the evidence base for COVID-19 (e.g., epidemiology, transmission dynamics) and vaccine characteristics (e.g., immunogenicity, safety, efficacy, effectiveness in preventing severe illness and interruption of transmission in different populations), as well as information on vaccine supply, evolves.
Sequencing of populations and sub-prioritization within these populations will be based on:
- A population-based risk-benefit analysis taking into consideration risk of exposure, risk of transmission to others, risk of severe illness and death, and the safety and effectiveness of vaccine(s) in key populations
- Vaccine supply (number of available vaccine types, number and timing of available doses, number of doses required)
- COVID-19 epidemic conditions when the vaccine(s) become(s) available
Key populations include:
Those at high risk of severe illness and death from COVID-19
- Advanced age
- Other high-risk conditions (to be defined as the evidence base evolves)
Those most likely to transmit COVID-19 to those at high risk of severe illness and death from COVID-19 and workers essential to maintaining the COVID-19 response
- Healthcare workers, personal care workers, and caregivers providing care in long-term care facilities, or other congregate care facilities for seniors
- Other workers most essential in managing the COVID-19 response or providing frontline care for COVID-19 patients
- Household contacts of those at high-risk of severe illness and death from COVID-19
Those contributing to the maintenance of other essential services for the functioning of society
- To be defined, prioritized and informed by federal/provincial/territorial (FPT) discussions
- Examples: those who cannot work virtually and have differential exposure to COVID-19 (e.g., police, firefighters, grocery store staff)
Those whose living or working conditions put them at elevated risk of infection and where infection could have disproportionate consequences, including Indigenous communities
- To be defined based on COVID-19 epidemiology and previous pandemic experience
- Examples: settings where physical distancing and other infection prevention and control measures are challenging, access to healthcare infrastructure is reduced, and infection could have disproportionate consequences
Other considerations for public health program level decision-making
- Efforts should be made to increase access to immunization services to reduce health inequities without further stigmatization or discrimination, and to engage systemically marginalized populations and racialized populations in immunization program planning.
- Jurisdictions should begin planning for the implementation of a COVID-19 immunization program, including close and rapid monitoring of safety, effectiveness, and coverage of the vaccine(s) in different key populations, as well as effective and efficient immunization of populations in remote and isolated communities.
- Efforts should be made to improve knowledge about the benefits of vaccines in general and of COVID-19 vaccine(s) specifically once available, address misinformation about immunization, and communicate transparently about COVID-19 vaccine allocation decisions.
Figure 1 summarizes NACI's interim recommendations on key populations for early COVID-19 immunization for public health program level decision-making.
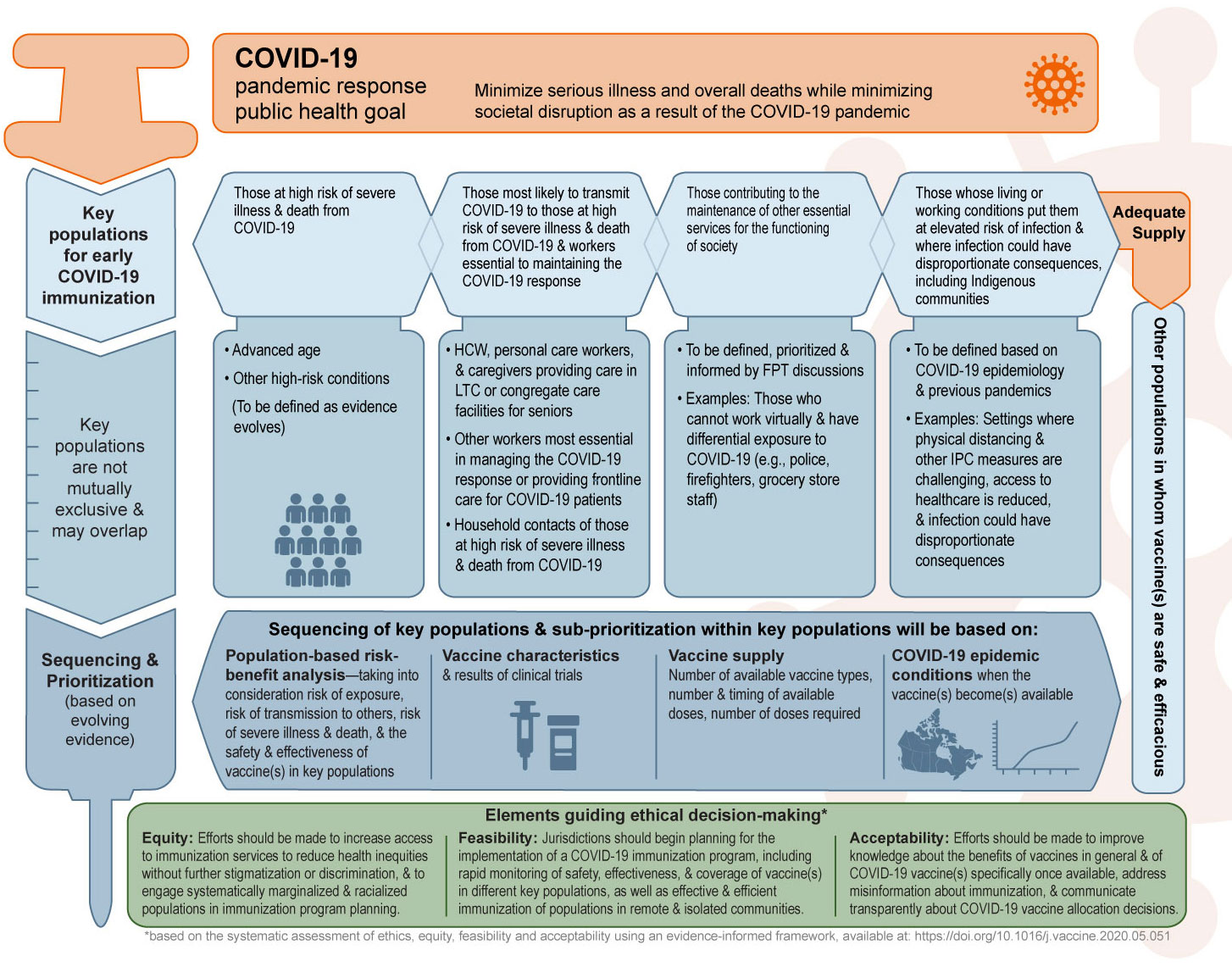
Figure 1 - Text description
The figure provides a visual summary of the preliminary National Advisory Committee on Immunization recommendations on key populations for early COVID-19 immunization.
The figure is organized into four sections. The first section states the COVID-19 pandemic response public health goal. The second section presents the key populations for early COVID-19 immunization. The third section presents considerations for sequencing and prioritization, which will be based on evolving evidence. Finally, the underlying principles guiding decision-making are presented.
The COVID-19 pandemic response public health goal is to minimize serious illness and overall deaths while minimizing societal disruption as a result of the COVID-19 pandemic.
Key populations for early COVID-19 immunization when COVID-19 vaccine supply is limited include the following:
- Those at high risk of severe illness and death from COVID-19 (to be defined as evidence evolves)
- Advanced age
- Other high-risk conditions
- Those most likely to transmit COVID-19 to those at high risk of severe illness and death from COVID-19 and workers essential to maintaining the COVID-19 response
- Healthcare workers, personal care workers, and caregivers providing care in long-term care or congregate care facilities for seniors
- Other workers most essential in managing the COVID-19 response or providing frontline care for COVID-19 patients
- Household contacts of those at high risk of severe illness and death from COVID-19
- Those contributing to the maintenance of other essential services for the functioning of society
- To be defined and informed by Federal/Provincial/Territorial discussions
- Examples: Those who cannot work virtually and have differential exposure to COVID-19 (e.g., police, firefighters, grocery store staff)
- Those whose living or working conditions put them at elevated risk of infection and where infection could have disproportionate consequences, including Indigenous communities
- To be defined based on COVID-19 epidemiology and previous pandemics
- Examples: Settings where physical distancing and other infection prevention and control measures are challenging, access to healthcare is reduced, and infection could have disproportionate consequences
Other populations in whom vaccine(s) are safe and efficacious can be considered for COVID-19 immunization when vaccine supply is adequate.
Key populations for early COVID-19 immunization are not mutually exclusive. Sequencing of key populations and sub-prioritization within key populations will be based on:
- Population-based risk-benefit analysis: taking into consideration risk of exposure, risk of transmission to others, risk of severe illness and death, and the safety and effectiveness of vaccine(s) in key populations
- Vaccine characteristics and results of clinical trials
- Vaccine supply: number of available vaccine types, number and timing of available doses, and number of doses required
- COVID-19 epidemic conditions when the vaccine(s) become(s) available
The underlying principles guiding decision-making include the following:
- Equity: Efforts should be made to increase access to immunization services to reduce health inequities without further stigmatization or discrimination, and to engage systematically marginalized and racialized populations in immunization program planning.
- Feasibility: Jurisdictions should begin planning for the implementation of a COVID-19 immunization program, including rapid monitoring of safety, effectiveness, and coverage of vaccine(s) in different key populations, as well as effective and efficient immunization of populations in remote and isolated communities.
- Acceptability: Efforts should be made to improve knowledge about the benefits of vaccines in general and of COVID-19 vaccine(s) specifically once available, address misinformation about immunization, and communicate transparently about COVID-19 vaccine allocation decisions.
Abbreviations: LTC (long-term care), FPT (federal, provincial and territorial), IPC (infection prevention and control)
Introduction
The novel coronavirus disease 2019 (COVID-19) pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is ongoing. It was declared a pandemic by the World Health Organization (WHO) on March 11, 2020. The pandemic has caused significant morbidity and mortality, as well as social and economic disruption worldwide.
The goal of Canada's pandemic response is to minimize serious illness and overall deaths while minimizing societal disruption as a result of the COVID-19 pandemic.
Clinical trials of candidate COVID-19 vaccines are currently underway. The National Advisory Committee on Immunization (NACI) has developed guidance on the research priorities for these clinical trials to support public health decisions.Footnote 4 Initial supplies of prospective COVID-19 vaccines are not expected to be sufficient to meet the demand for immunization. Therefore, recommendations are needed on key populations for early immunization.
Guidance objective
The objective of this advisory committee statement is to provide preliminary guidance to plan for the efficient, effective, and equitable allocation of an eventual COVID-19 vaccine when limited initial vaccine supply will necessitate the immunization of some populations earlier than others.
In order to support this objective, the advisory committee statement will:
- Summarize the current COVID-19 vaccine landscape;
- Present the results of a rapid review on risk factors for severe COVID-19;Footnote 1
- Present the results of an expert stakeholder survey ranking the relative importance of COVID-19 pandemic immunization strategies for different pandemic scenarios;Footnote 2
- Apply the evidence-informed tools (Ethics Integrated Filters, Equity Matrix, Feasibility Matrix, Acceptability Matrix) of NACI's Ethics, Equity, Feasibility and Acceptability (EEFA) Framework,Footnote 3 and finally;
- Present key populations prioritized for early COVID-19 immunization to best achieve Canada's pandemic response public health goal when initial vaccine supply is limited, based on the above.
NACI acknowledges that at the time of the development of this statement many uncertainties remain about COVID-19, potential COVID-19 vaccine(s), and therapeutics for COVID-19, as well as what the epidemiology and vaccine supply will be at the time of initial COVID-19 vaccine availability. This guidance is based on the evidence available at the time of NACI deliberations, along with expert and stakeholder opinion. NACI will provide further guidance when additional information on vaccine-specific characteristics (e.g., efficacy, immunogenicity, safety, effectiveness to prevent severe disease or interrupt transmission) in different populations (e.g., age groups, those with underlying medical conditions) becomes available. NACI will continue to monitor the evidence as it evolves.
Methods
This advisory committee statement will aim to address the following policy question:
- Assuming constrained early supply for COVID-19 vaccine(s) in Canada, which populations should be prioritized for early doses of vaccine?
To develop these recommendations over the summer of 2020, NACI reviewed available epidemiological summaries from national analyses of federal/provincial/territorial (FPT) surveillance data reported to the Public Health Agency of Canada (PHAC);Footnote 5 summaries of the COVID-19 vaccine product landscape from clinical trial registry data; and the Vaccine Annex of the Canadian Pandemic Influenza Preparedness: Planning Guidance for the Health Sector.Footnote 6 In addition:
- The NACI Secretariat conducted an environmental scan of international guidance on prioritization of key populations for initial COVID-19 immunization.
- The NACI Secretariat conducted a national survey of expert stakeholders between July 22 and August 14, 2020 to establish a comprehensive perspective on the relative importance of pandemic immunization strategies under four different pandemic scenarios at the time of initial COVID-19 vaccine availability. Full methodological details and results can be found in the preprint.Footnote 2
- The Alberta Research Centre for Health Evidence (ARCHE) was commissioned to conduct a rapid review of risk factors for severe COVID-19 (literature search carried out on June 15, 2020). Full methodological details and results can be found in the preprint.Footnote 1 An updated evidence synthesis will be carried out at a later date.
- The NACI Secretariat applied NACI's EEFA FrameworkFootnote 3 with accompanying evidence-informed tools (Ethics Integrated Filters, Equity Matrix, Feasibility Matrix, Acceptability Matrix) to systematically consider these programmatic factors for the development of clear, comprehensive, appropriate recommendations for timely, transparent decision-making. For details on the development and application of NACI's EEFA Framework and evidence-informed tools (including the Ethics Integrated Filters, Equity Matrix, Feasibility Matrix, and Acceptability Matrix), please see A framework for the systematic consideration of ethics, equity, feasibility, and acceptability in vaccine program recommendations .
As per the published process for the application of this Framework (see Appendix A), experts and stakeholder groups (e.g., Public Health Ethics Consultative Group, First Nations and Inuit Health Branch [FNIHB], Indigenous Services Canada, and other NACI liaison and ex-officio organisations ) were consulted. The following FPT committees were consulted during the development of this statement: Canadian Immunization Committee (CIC), the Pan-Canadian Public Health Network's Special Advisory Committee on COVID-19 (SAC), and SAC's Technical Advisory Committee. The Sex and Gender Based Analysis Plus (SGBA+) network with the Social Determinants of Health Division at PHAC also reviewed this guidance. The CIC, FNIHB, and PHAC's vaccine supply manager discussed the feasibility of proposed immunization strategies and validated the Feasibility Matrix. Emerging acceptability data related to COVID-19 vaccines were sought and included in the Acceptability Matrix. See Section IV for further details.
Available evidence was presented to the NACI High Consequence Infectious Disease Working Group (HCID WG) on July 24, 2020, and to NACI on August 13, 2020. NACI deliberated on the cumulative evidence and proposed recommendations on August 20, 2020, provided feedback through to September 1, 2020, and approved the revised recommendations on September 16, 2020.
Further information on NACI's process and procedures can be found elsewhere.Footnote 7
Vaccine(s)
Current COVID-19 vaccine landscape
Global efforts are underway to develop a COVID-19 vaccine and work is progressing at an unprecedented pace. The Government of Canada is reviewing regulatory pathways to help expedite access to safe and effective vaccine for Canadians.
As of August 5, 2020, there are over 130 candidate COVID-19 vaccines at different stages of development by academia and industry. Many candidates have shown promise to enter into human clinical trials, 15 Phase 1 clinical trials have been registered, 23 Phase 2 clinical trials have been registered, and five Phase 3 clinical trials are underway or planned to start. Phase 3 clinical trials have been started in the United Kingdom (UK), the United States (US), Brazil, and the United Arab Emirates with results expected in late 2020 or in early 2021 (e.g., University of Oxford/AstraZeneca, Moderna, Sinopharm, Sinovac). More than one vaccine developer plans to start clinical trials in Canada. As of September 4, 2020, one vaccine developer has already begun Phase 1 clinical trials and several others are expected to advance to that stage in the coming months.
The current landscape of candidate COVID-19 vaccines in clinical evaluation can be found at: Draft landscape of COVID-19 candidate vaccines
Summary of vaccine clinical development
Most of the trials in Phase 1 of clinical development are assessing the safety of vaccines in healthy adults 18 to 49 years of age without underlying health conditions. Due to the high burden of COVID-19 in older adult populations, some Phase 1 studies have expanded age ranges to include older adult populations. In later stages of clinical development (Phases 2 and 3), some vulnerable and at-risk populations (including people who are HIV+ and adults over 65 years of age) are included in study recruitment. Both established and experimental vaccine technologies, including nucleic acid (mRNA and DNA), vectored (viral and bacterial vectors), subunit (protein and glycan), particles (nanoparticles and virus-like-particles), inactivated virus vaccines, and prophylactic immune products (antibodies and sera) are being explored. Vaccine candidates are administered via different routes of administration, including intramuscular, intradermal (and electroporation), oral, and subcutaneous. Schedules of candidate vaccines range between one and three doses.
Canadian vaccine access
The unprecedented nature of this pandemic and vaccine development efforts has led to a highly competitive global landscape of vaccine access. As of October 27th, 2020, seven suppliers of promising vaccine candidates have committed to supply Canada with vaccine to cover a portion of or the entire Canadian population. It is still to be determined if these vaccines will be safe, efficacious, authorized in Canada, and be recommended by NACI.
Ethics, equity, feasibility and acceptability considerations
The EEFA FrameworkFootnote 3 facilitates the systematic consideration of factors critical for comprehensive immunization program decision-making and successful implementation of recommendations. The use of the EEFA Framework empowers the committee to review and balance all of the available evidence and transparently summarize their rationale for appropriate, timely recommendations. The evidence informed tools associated with the framework (Ethics Integrated Filters, Equity Matrix, Feasibility Matrix, Acceptability Matrix; see Appendices B-F) ensure that issues related to EEFA of expert committee guidance are adequately integrated. NACI followed the process outlined in the algorithm for applying the EEFA Framework (Appendix A) to this guidance.
Ethics considerations
NACI used the Public health ethics framework: A guide for use in response to the COVID-19 pandemic in CanadaFootnote 8 to support ethics deliberation and decision-making. NACI's Ethics Integrated Filters for core ethical dimensions (respect for persons and communities, beneficence and non-maleficence, justice, trust) and procedural ethical dimensions (accountability, inclusiveness, responsibility, responsiveness, transparency) were applied to this guidance document, and are summarized in Appendices B and C. An in-depth ethics analysis was conducted to analyse and weigh relative considerations and assess options for prioritization of COVID-19 immunization in the face of an uncertain pandemic vaccine supply.
NACI upheld the following core ethical principles:
1. Respect for persons and communities
In order to respect the right to exercise informed choice, NACI has reviewed the evidence to date and summarized it for stakeholders throughout this guidance document. The values and preferences of persons and communities have been considered through the national expert stakeholder survey ranking the relative importance of pandemic immunization strategies,Footnote 2 as well as evidence available to date on the acceptability of COVID-19 vaccines in various populations in Canada (summarized in Section IV.4). Extensive consultations were conducted in the development of this guidance (summarized in Section II).
2. Beneficence and non-maleficence
NACI considered evidence for minimizing the risk of harm and maximizing benefits for all potential key populations in their deliberations. As information about vaccine characteristics (including safety and efficacy in different populations) becomes available, the principles of proportionality (measures should be proportionate to the level of risk and benefits gained), effectiveness (reasonable likelihood that the action will achieve the goals and will be feasible), and precaution (take prudent action in the face of scientific uncertainty) will be applied. NACI has previously recommended that individuals with potential biological, social, and occupational vulnerabilities to COVID-19 be included in vaccine clinical trial groups4. However, in the absence of direct data in populations at high risk of severe illness and death due to COVID-19 (e.g., due to age or underlying medical conditions), NACI will consider the principles of proportionality, effectiveness, and precaution, and conduct an in-depth ethics analysis before making specific recommendations. Immunization strategies aimed at protecting healthcare capacity and other services essential for the functioning of society uphold the principle of reciprocity, as they aim to minimize the disproportionate burden faced by those taking on additional risks to protect the public. The public also benefits from the ongoing work of those who provide these services and could potentially benefit from reduced transmission from frontline workers, which aligns with the principle of beneficence.
3. Justice
Treating people and groups with equal concern and respect entails setting and applying prioritization criteria fairly, considering the needs of those most at risk of exposure or of severe disease, and weighing risks of furthering inequities, stigmatization, and discrimination. NACI reviewed special considerations for vulnerability of those most at risk through its application of the Equity Matrix (Appendix D), a rapid review of risk factors for severe COVID-19,Footnote 1 as well as a review of disease epidemiology in Canada.Footnote 5 NACI also considered evidence concerning other factors, such as systemic marginalization of groups with differential disease severity and differential access to healthcare, as well as the demonstrated potential for reduced exposure among healthcare workers with increased training in and access to personal protective equipment (PPE) and other infection prevention and control (IPC) measures.Footnote 9Footnote 10 In the face of uncertainties about the disease and vaccine with resulting challenges in program planning for an eventual COVID-19 vaccine, and in consultation with jurisdictions and vaccine supply experts, NACI considered the principle of distributive justice with the application of the Feasibility Matrix (Appendix E). The systematic assessment of all of these factors informed NACI's recommendations for the fair and equitable allocation of limited COVID-19 vaccine supply.
4. Trust
Reliability and integrity of guidance must be maintained for trust in this and other immunization programs. Expedited regulatory reviews of COVID-19 vaccines and evolving evidence about the disease may have an impact on the trust of the public in this immunization program and their perception of risks associated with these vaccines. NACI followed its established methodology, standard operating procedures (SOP), and conflict of interest guidelinesFootnote 7 in the context of this expedited review to ensure a robust analysis of evidence and to maintain stakeholder trust. This guidance is based on the best, current evidence available for all groups at risk of COVID-19, with transparency about knowns and unknowns, as well as certainty of evidence. NACI will monitor the evidence with revision of guidance as necessary. To maintain trust among stakeholders, NACI has upheld ethical procedural considerations (accountability, inclusiveness, responsibility, responsiveness, and transparency) with the tools and procedures as summarized in Appendix C.
NACI acknowledges the risk that there could be a perception of conflict of interest, and a resulting loss of trust, when expert groups of healthcare professionals are making recommendations on the prioritization of immunization strategies involving health care workers and personnel. NACI also acknowledges that trust may be eroded if healthcare professionals are not included in a priority group for earlier immunization with a novel vaccine. To maintain trust, reliability, integrity, and a mutually fair relationship with individuals and communities, NACI applied the Ethics Integrated Filters described above (Appendices B and C), made recommendations based on the evidence transparently summarized in this document, and considered a wide range of stakeholder views (including patient and community advocates) through an expert stakeholder survey,Footnote 2 as well as the views of the general public through public opinion researchFootnote 11 when developing this guidance.
Equity considerations
NACI reviewed the epidemiology of COVID-19 in CanadaFootnote 5 and the results of the rapid review of risk factors for severe COVID-191 (summarized in Section IV.2.1) to identify distinct inequities associated with COVID-19, potential reasons for these inequities, and suggested interventions to reduce inequities and improve access to vaccine when it becomes available. The results of this analysis are summarized in the Equity Matrix (Appendix D).
Factors contributing to severe COVID-19: results of a rapid review of risk factors
A rapid review was conducted by ARCHE to examine the magnitude of association between factors that may contribute to health inequity (summarized by the acronym "P2ROGRESS And Other Factors" in NACI's Equity Matrix, Appendix D) and severe COVID-19. The HCID WG decided on the primary outcomes, settings, and populations of interest and the protocol was registered in PROSPERO (CRD42020198001). Full details of the rapid review methodology and findings can be found in the preprint.Footnote 1
Literature searches were conducted on June 15, 2020 in the following bibliographic databases: Medline, Epistimonikos COVID-19 in LOVE Platform, and McMaster COVID-19 Evidence Alerts. The following inclusion criteria were used: studies from the Organisation for Economic Co-operation and Development (OECD) countries, published (vs. preprints), and using multivariate analysis to report on the independent contribution of each risk factor while accounting for confounders such as age, sex, race/ethnicity, socioeconomic status, and comorbidities. Populations included a general/community sample, people with confirmed COVID-19, and people hospitalized with COVID-19 (studies were excluded if all patients were in the intensive care unit (ICU) or were in a treatment study). Outcomes of interest included hospitalization and length of stay, severe disease (as defined by study authors) and mortality. The risk of bias/quality of each study was assessed, accounting for the extent of adjustment, follow-up duration/extent of censorship for length of stay and mortality, and inappropriate or large exclusions from the study and/or analysis.
No meta-analysis was conducted. For each risk factor and outcome, findings were assessed across studies in terms of the estimated magnitude of associations (i.e., "not important/not large" [e.g., odds ratio (OR) <1.7], moderate [OR ≥1.7 to 1.99], important/large [OR ≥2.0], very large [OR ≥5.0], and the review team's confidence in the magnitude of association based on the number, size and consistency between studies and the risk of bias. Varying confidence in the associations is referred to using the terms "uncertain" (no/very low), "may" (low/some), and "probably" (moderate).
A total of 34 published studies were included, although three from the UK had overlapping populations and another UK study is likely to overlap but the degree of overlap is unknown. Studies were conducted in the US (n=17), Italy (n=9), the UK (n=7 using 5 populations), and across multiple countries (n=1). Sample sizes ranged from 44 to 418,794 (median 596) and mean age in most studies was 54 to 71 years. The majority of studies (n=19; 56%) were rated low risk of bias.
None of the associations had a high level of certainty of evidence. There was low or moderate certainty of evidence for large/important associations (OR or risk ratio [RR]) with increased risk of hospitalization in people having confirmed COVID-19 for the following risk factors: obesity class III (body mass index [BMI] ≥40 kg/m2), heart failure, diabetes, chronic kidney disease, dementia, age (particularly over 70 years vs. 45 years or younger), male sex, Black race/ethnicity (vs. non-Hispanic white), homelessness, and low income (<25th vs. >50th percentile). Evidence that age over 70 years may be associated with important increases in the rate of severe disease was considered to be of moderate certainty. Risk factors that have large/important and very large important associations with hospitalization and mortality are presented in Table 1.
| Risk factor | Outcome of interest | Magnitude of riskFootnote 1 (confidence in association)Footnote 2 |
|---|---|---|
| Age | ||
| >80 vs. ≤ 45 years | Hospitalization | +++ (low) |
| Mortality | +++ (low) | |
| >70 vs. ≤ 45 years | Hospitalization | +++ (moderate) |
| Mortality | +++ (moderate) | |
| >60 vs. ≤ 45 years | Hospitalization | ++/+++ (moderate/low) |
| Mortality | ++/+++ (moderate/low) | |
| 50-64 vs. ≤ 45 years | Hospitalization | ++ (moderate) |
| Mortality | ++ (moderate) | |
| 45-54 vs. ≤ 45 years | Hospitalization | ++ (moderate) |
| Mortality | ++ (low) | |
| Pre-existing conditions | ||
| Obesity (BMI ≥40) | Hospitalization | ++ (low) |
| Heart failure | Hospitalization | ++ (low) |
| Diabetes | Hospitalization | ++ (low) |
| Liver disease | Mortality | ++ (low) |
| Chronic kidney disease | Hospitalization | ++ (low) |
| Alzheimer disease or dementia | Hospitalization | ++ (low) |
| Sex | ||
| Male vs. female | Hospitalization | ++ (moderate) |
| Race/ethnicity | ||
| Black vs. non-Hispanic white | Hospitalization | ++ (low) |
| Asian (Bangladeshi) vs. British white | Mortality | ++ (low) |
| Place of residence | ||
| Homeless vs. has a home | Hospitalization | ++ (low) |
| Socioeconomic status | ||
| Income ≤25th vs. >50th or 75th percentile | Hospitalization | ++ (low) |
Table 1 - footnotes
|
||
For mortality, important associations with increased risk may exist for liver disease, Bangladeshi ethnicity (vs. British white), and age particularly if over 70 years (vs. <45 years). The data were somewhat inconsistent for sex, with most studies showing moderate certainty of no important effect. One study directly compared subgroups of older adults, showing that compared to those aged 65-69 years, there may be no important increased risk of mortality among hospitalized adults aged 70-79 years, but risk may increase about 2-fold for those 80 years and older. Studies treating age on a continuum or across small increments consistently found that risks for hospitalization and mortality increased with increasing age (e.g., approximately 2-6% and 5-10% relative increase in risk per year).
Moderate association with low level of confidence may exist for increased risk of hospitalization with obesity (BMI ≥30 or 40 kg/m2); severe disease with heart failure; mortality with hematological malignancy; and hospitalization with social deprivation (lowest vs. highest quintile).
There was moderate certainty evidence for no important increase in risk of hospitalization with chronic respiratory conditions, cardiovascular disease (i.e., coronary artery disease, hypertension, hyperlipidaemia) apart from heart failure, non-specific cancer, Asian race/ethnicity other than Bangladeshi (vs. non-Hispanic white), and current or former smoking. Additionally, there was moderate certainty evidence for no important increase in severe disease (as defined by each study) with chronic respiratory conditions, chronic kidney disease, nonspecific cancer, and Black race/ethnicity (vs. non-Hispanic white) and no important increase in risk of mortality with obesity (BMI ≥30 kg/m2), chronic respiratory conditions, diabetes, chronic kidney disease, nonspecific cancer, male sex, Black or Asian race/ethnicity (vs. non-Hispanic white), and social deprivation (lowest vs. highest quintile).
In the rapid review, data for immunocompromised patients (specifically rheumatic disease and HIV) were limited by small studies and no conclusions could be drawn about the magnitude and certainty of the associations. Further, no studies on pregnancy met the publication date and eligibility criteria.
Generalization of findings from other countries to Canada should be made with caution, as high-risk groups may differ by population. Furthermore, because of differences in methodology, the list of important risk factors identified in this rapid review may differ from other sources. Updated evidence syntheses will inform future NACI decisions.
Feasibility considerations
NACI recognizes that there are a number of challenges to the feasible implementation of a COVID-19 immunization program due, in part, to the uncertainties around vaccine characteristics (e.g., indications, adverse events) and supply, as well as the novel nature of the disease and vaccine(s). Issues around the vaccine and the immunization program with respect to resources (e.g., vaccine and immunization supplies including storage and dissemination of new vaccine technologies in different vaccine delivery venues; human resources for administration of vaccine, communication, training, data entry, screening for COVID-19, operational planning, etc.) as well as integration with existing programs (e.g., registries, surveillance, adverse event following immunization (AEFI) reporting) abound. Close and rapid monitoring of safety, effectiveness, and coverage of the vaccine(s) in potentially different key populations will be critical.
To assist jurisdictions with the planning of a potential COVID-19 immunization program, jurisdictions may refer to the Feasibility Matrix (Appendix E), summarizing potential issues with implementing a COVID-19 immunization program. These issues apply to immunization in any population. Jurisdictions may also wish to refer to the Interim guidance on continuity of immunization programs during the COVID-19 pandemicFootnote 12 to minimize disruption to existing immunization programs.
The feasibility of immunizing different populations will vary with the size of the population to be immunized, vaccine characteristics in the population, and vaccine supply, among other considerations. Sub-prioritization or sequencing within key populations may be necessary, either initially and/or gradually.
In some Indigenous communities, crowded multi-generational living makes segregation of at-risk groups challenging, and precarious supply chain, infrastructure, and health systems are vulnerable to critical disruption. In these cases, there may be value in implementing multiple strategies concurrently and completely immunizing entire communities where relatively small quantities of vaccine are needed to achieve the pandemic response goal.
Acceptability considerations
In alignment with the ethical principle of respect for persons and communities, the values and preferences of a range of stakeholders, including experts, patient/community advocates, and the general public were considered. Factors influencing acceptability of a COVID-19 vaccine are summarized using the Acceptability Matrix (Appendix E).
Results of an expert stakeholder survey on COVID-19 immunization strategies
A PHAC-led survey was conducted to establish a preliminary expert stakeholder perspective on the relative importance of pandemic immunization strategies for different COVID-19 pandemic scenarios at the time of initial COVID-19 vaccine availability. These pandemic scenarios are visualized along a hypothetical pandemic curve in Figure 2. Scenario 4 was further delineated in the survey into two sub-scenarios where the vaccine or previous infection provides or does not provide long-term protection against COVID-19. Full methodology and results of the survey can be found in the preprint.Footnote 2
The survey was comprised of five questions that asked the respondent to rank, in order of importance with a rank of "1" being the most important, four COVID-19 pandemic immunization strategies (proposed by PHAC staff with input from the HCID WG) plus an optional respondent-specified strategy for each pandemic scenario. The respondents were asked to assume that the COVID-19 vaccine is in limited supply for each scenario and that the COVID-19 vaccine is safe and efficacious for all populations for the purposes of the survey.
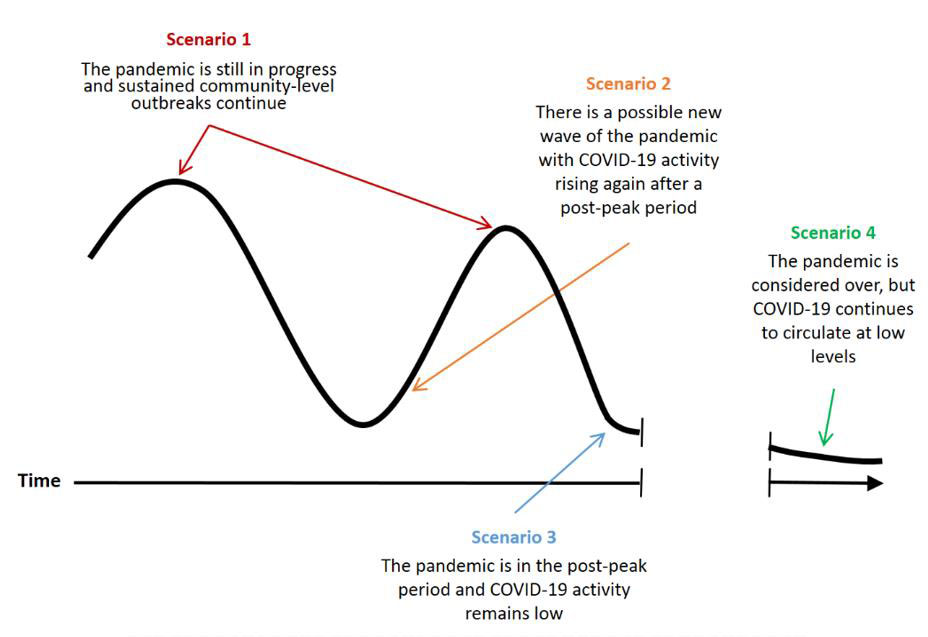
Figure 2 - Text description
The figure shows four pandemic scenarios along a hypothetical pandemic curve with the x-axis representing elapsed time. The pandemic curve includes a first peak, a first valley, a second peak, and a second valley. There is a break in the x-axis in the second valley. After the break in the x-axis, the pandemic curve continues to decrease.
- Scenario 1 points to the first and second peaks of the pandemic curve and represents the scenario where the pandemic is still in progress and sustained community-level outbreaks continue.
- Scenario 2 points to just after the first valley and before the second peak of the pandemic curve and represents the scenario where there is a possible new wave of the pandemic with COVID-19 activity rising again after a post-peak period.
- Scenario 3 points to just after the second peak of the pandemic curve and represents the scenario where the pandemic is in the post-peak period and COVID-19 activity remains low.
- Scenario 4 points to the pandemic curve after the break in the x-axis and represents the scenario where the pandemic is considered over, but COVID-19 continues to circulate at low levels.
Expert stakeholders were identified through consultations within PHAC and with the HCID WG. These stakeholders included members of clinical and public health expert groups involved with PHAC, members of provincial and territorial committees and representatives from national Indigenous groups, patient and community advocacy representatives and experts from the CanCOVID network, executives of Canadian health professional associations, and representatives of other federal government departments. An invitation to complete the survey, which was provided as a Word document in English and French, was sent by email to stakeholders in a format that facilitated shared review and discussion within their respective organisations. Members of expert groups (e.g., NACI) each provided individual expert responses, whereas organisational or provincial/territorial representatives each provided a single response on behalf of their organisation or jurisdiction.
The survey was conducted between July 22 and August 14, 2020. Survey results were analyzed using descriptive statistics across all respondents to identify overall trends and by stakeholder group to assess for any differences in prioritization among stakeholder groups. Trends in the rankings for each pandemic scenario were assessed by descriptive analysis in two ways: taking the average (mean, median, and mode) ranking and comparing the percentage of each ranking contributing to the total for COVID-19 pandemic immunization strategies for different pandemic scenarios at the time of initial COVID-19 vaccine availability.
Of 156 stakeholders contacted, 74 surveys were completed for a participation rate of 47.4%. A total of 22 (29.7%) respondents were members of clinical or public health expert groups involved with PHAC, 19 (25.7%) were patient or community advocacy representatives or experts from the CanCOVID network, 16 (21.6%) were executives of Canadian health professional associations, nine (12.2%) were members of provincial and territorial committees or national Indigenous groups, and eight (10.8%) were representatives of federal government departments.
For all pandemic scenarios, both descriptive analysis approaches showed that stakeholders generally ranked the strategies in the following order from most to least important:
- Protect those who are most vulnerable to severe illness and death from COVID-19
- Protect healthcare capacity
- Minimize transmission of COVID-19
- Protect critical infrastructure
In subgroup analysis by stakeholder group, the trends were less clear due to smaller sample sizes, but the strategy to protect those who are most vulnerable to severe illness and death from COVID-19 remained the most important in all groups and scenarios.
Results of surveys of the general Canadian public on COVID-19 immunization
Canada's COVID-19 Snapshot Monitoring Study (COSMO Canada) is a longitudinal study led by Impact Canada in collaboration with the Public Opinion Research Team within the Privy Council Office that began in April 2020 and continued through September 2020 in eight waves.Footnote 11 This online survey leverages a tool developed by the WHO to monitor knowledge, risk perceptions, and behaviour related to COVID-19, is adapted wave-to-wave in consultation with an external academic advisory committee, and collects data from a representative sample of approximately 2000 Canadians. NACI considered the most up-to-date results of this survey (up to wave 7, conducted between August 13 and 17, 2020)Footnote 13Footnote 14 and populated the Acceptability Matrix (Appendix F) to inform its guidance.
NACI acknowledges that survey data on intent to receive a vaccine can fluctuate, especially in the context of uncertainty about possible COVID-19 vaccines. NACI will continue to monitor data from the remaining wave of the COSMO study, as well as another study assessing acceptability of COVID-19 and other vaccines among Canadians, planned in the coming months.
Which immunization strategies do Canadians prioritize if COVID-19 vaccine supply is limited?
Wave 7 of the COSMO Canada survey asked respondents to prioritize the same immunization strategies that expert stakeholders were asked to rank in order of importance in the context of limited initial COVID-19 vaccine supply (see Section IV.4.1). A majority of these respondents prioritized protecting those most vulnerable (51%), followed by protecting healthcare capacity (28%), minimizing the spread (15%), and protecting critical infrastructure (5%).Footnote 14 The opinions of respondents from the general public on priority immunization strategies are consistent with those of expert stakeholders surveyed.
Which populations do Canadians prioritize to receive COVID-19 vaccine if supply is limited?
Three-quarters of COSMO Canada respondents (76% in wave 6 and 78% in wave 7) agree that specific groups should be first to get a safe and effective COVID-19 vaccine in the context of limited initial supplies.Footnote 13Footnote 14 In response to an open ended question in wave 6 asking which group should get the vaccine first, the most commonly identified populations for priority immunization included those with underlying medical conditions (57%), the elderly (53%), healthcare workers (22%), and frontline/essential workersFootnote a (18%). Although endorsed less frequently, respondents also identified children (9%), long-term care/nursing homes (3%), and hospitalized/ill individuals (2%), as other populations that should receive the vaccine first should there be a shortage.Footnote 13 When asked to rank a pre-determined list of groups to be prioritized to receive a COVID-19 vaccine before others in wave 7 of the survey, the most commonly identified group for priority immunization was healthcare workers (40%), followed by individuals with high risk medical conditions (19%), frontline workers (16%), seniors (12%), long-term care/nursing homes (10%), and children (2%).Footnote 14
The majority of respondents (87%) to an Angus Reid Institute online survey about COVID-19 in July 2020 of a representative sample of 1,519 Canadian adults agreed that "it is vital that people with chronic health conditions, such as asthma or diabetes, get vaccinated".Footnote 15
Does a high level of acceptability for a COVID-19 vaccine exist?
Almost two-thirds of COSMO Canada respondents (61%) in August 2020 were willing to get an effective recommended COVID-19 vaccine.Footnote 14 This has decreased from 71% in April. While willingness to get an effective recommended vaccine has decreased among all age groups surveyed, older Canadian respondents (aged 55 years of age and older) are significantly more willing to get immunized than younger Canadian respondents (72% in >54 year olds vs. 57% in 35-54 year olds and 51% in 18-34 year olds; p<0.05). Respondents 35 years and older with a "serious, long-term illness" are somewhat more willing to get an effective recommended vaccine compared to those without (68% vs 62%). This was not observed in younger individuals 18 to 34 years old with a "serious, long-term illness" compared to those without (43% vs. 57%). Non-visible minorities are more willing than visible minorities or Indigenous respondents to get an effective recommended vaccine (63% vs. 53% vs. 43%, respectively; p<0.05). About half of workers in frontline occupations such as grocery and gas station staff (51% vs. 63% for non-frontline workers; p<0.05) and healthcare providers (55% vs. 62% for non-healthcare workers; p>0.05) would get an effective recommended vaccine. Willingness to receive an effective COVID-19 vaccine does not seem to be significantly influenced by socioeconomic status or sex.Footnote 14
The findings from the COSMO Canada study are similar to results from other Canadian surveys. The Canadian Perspectives Survey Series 3 (CPSS3) in June 2020 (survey sample is representative of the Canadian population living in the ten provinces) found that approximately three-quarters of Canadians were either very likely (57.5%) or somewhat likely (19.0%) to get a COVID-19 vaccine when it becomes available.Footnote 16 The largest differences in intent to get a COVID-19 vaccine were observed across age groups and education levels. Significantly more Canadians 65 years and older reported being very likely to get a COVID-19 vaccine than those aged 15-64 (70% vs. 52-58%; p<0.05). Respondents with a university degree higher than a bachelor's degree were significantly more likely to report being very likely to get a COVID-19 vaccine (73% vs. 53% of those with a trades or university certificate below a bachelor's degree; p<0.05). The Angus Reid Institute online survey in July 2020 found that 46% of respondents would get a coronavirus vaccine as soon as one became available, but 32% would wait a while first.Footnote 15
What factors affect acceptability of immunization with a COVID-19 vaccine?
The most reported reasons for unwillingness to get a COVID-19 vaccine in wave 7 of the COSMO Canada survey included insufficient research or testing of the vaccine (32%), concerns about vaccine safety or effectiveness (26%), and a lack of trust in the newness of the vaccine (13%).Footnote 14 The top two reasons selected by respondents to the CPSS3 survey who were hesitant to get a COVID-19 vaccine also related to concerns about safety (54% cited a lack of confidence in the safety of the vaccine and 52% were concerned about its risks and side effects; respondents could select more than one response).Footnote 16 Similarly, the majority of respondents to the Angus Reid Institute survey who would wait to get a COVID-19 vaccine reported that they are worried about side effects (76%), and 61% of respondents overall shared concerns about vaccine safety.Footnote 15
Results from the Angus Reid Institute survey reveal that key factors for those willing to be immunized include trust in doctors (84% agree that "we should listen to doctors who recommend vaccines"), and a desire to protect their families (82% agree that they would "get a vaccine to protect my family").Footnote 15 Only 23% were concerned about getting infected with the coronavirus from a vaccine and only 23% believe a coronavirus vaccine will not be effective. Only a small minority (9%) agree that they will not have time to get a COVID-19 vaccine.
According to this poll, the majority of Canadians support mandatory immunization for healthcare workers (76%), extended care homes (76%), schools (63%), and workplaces such as offices and restaurants (52%).Footnote 15
What factors affect acceptability of immunization in general?
Research conducted to validate NACI's Acceptability Matrix prior to the COVID-19 pandemic revealed multiple factors that affect the acceptability of immunization in general.Footnote 3 Consistent with what has been found in recent surveys on a potential COVID-19 vaccine, concern about vaccine safety is the main reason for reluctance to vaccinate among Canadians.Footnote 17 However, vaccine effectiveness has the largest influence on choice of vaccine between two provided options.Footnote 18 Likewise, among high risk groups, perceived safety and effectiveness of a vaccine are linked to increased vaccine acceptability.Footnote 17 Perceived risk of disease and a desire to protect oneself and others are also linked to increased vaccine acceptability, as are low cost of vaccine, low time required to be vaccinated, and access to vaccine or a vaccine provider.Footnote 17Footnote 18
In general, receiving a recommendation from, or being in contact with, a healthcare provider is linked to increased vaccine acceptability.Footnote 17 However, a lack of familiarity with a vaccine plays a role in reluctance by healthcare providers to recommend it.Footnote 17Footnote 18 A recommendation by an expert committee is a notable factor for healthcare providers to recommend a vaccine.Footnote 18
Recommendations
The following recommendations are meant to help plan for the efficient, effective, and equitable allocation of an eventual COVID-19 vaccine(s) when limited initial vaccine supply will necessitate recommendations for the immunization of certain groups earlier than others. These recommendations aim to achieve Canada's pandemic response goal: "To minimize serious illness and overall deaths while minimizing societal disruption as a result of the COVID-19 pandemic." Due to anticipated constraints in supply, these NACI recommendations apply to provincial/territorial publicly-funded immunization programs only and not for individuals wishing to prevent COVID-19 with vaccines not included in immunization programs.
The recommendations are informed by evidence available at the time of NACI deliberations summarized in this document, including the results of a rapid review of risk factors for severe COVID-19,Footnote 1 an expert stakeholder survey on the relative importance of pandemic immunization strategies,Footnote 2 and the systematic assessment of EEFA considerations with the peer-reviewed EEFA Framework.Footnote 3
NACI has reviewed the available international guidance on priority groups for COVID-19 immunization at the time of deliberation. The key populations for early COVID-19 immunization identified by NACI have been similarly identified by other national immunization technical advisory groupsFootnote 19Footnote 20 and academic groupsFootnote 21Footnote 22 in their vaccine prioritization frameworks.
NACI will continue to carefully monitor the evidence related to COVID-19 and COVID-19 vaccine(s) and will update recommendations as evidence evolves.
Recommendations for public health program level decision-making
Due to anticipated challenges with sufficient supply to meet vaccine demand initially, NACI recommends that key populations in whom vaccine is deemed safe and effective based on evidence available at the time of vaccine availability should be prioritized for COVID-19 immunization. These groups are not mutually exclusive and may overlap. A sequential approach cannot be determined until vaccine characteristics, results of clinical trials, and the number of available doses are known. Key populations may change as the evidence base for COVID-19 and vaccine characteristics, as well as information on vaccine supply, evolves.
Sequencing of key populations and sub-prioritization within key populations will be based on:
- A population-based risk-benefit analysis taking into consideration risk of exposure, risk of transmission to others, risk of severe illness and death, and the safety and effectiveness of vaccine(s) in key populations
- Vaccine supply (number of available vaccine types, number and timing of available doses, number of doses required)
- COVID-19 epidemic conditions when the vaccine(s) become(s) available
Key populations include:
Those at high risk of severe illness and death from COVID-19
- Advanced age
- Other high-risk conditions (to be determined as the evidence base evolves)
Summary and rationale with current evidence:
- There are large/important independent associations of severe COVID-19 with increasing age and for certain high-risk health conditions.Footnote 1 There is moderate certainty of evidence for a very large/important association of hospitalization and mortality particularly in those over 70 years of age (vs. <45 years of age) and low certainty of evidence for a large/important association of hospitalization or mortality with certain high risk conditions (see Table 1).
- Current surveillance data in Canada have shown that hospitalization, ICU admission, and death rates from COVID-19 in Canada increase with age, and that persons with certain underlying health conditions are at highest risk of developing more severe illness from COVID-19.Footnote 5
- Expert stakeholder groups and patient/community advocates,Footnote 2 as well as the general Canadian public,Footnote 14 rank the relative importance of an immunization strategy to protect those who are most vulnerable to severe illness and death from COVID-19 as #1 in the context of limited vaccine supply.
- Canadians prioritize those with underlying medical conditions (57%) and the elderly (53%) for early immunization if supply of COVID-19 vaccine is limited.Footnote 13
- Older Canadians are significantly more willing than younger Canadians to get an effective recommended COVID-19 vaccine,Footnote 13Footnote 14Footnote 16 and those 35 years and older with a "serious long-term illness" are somewhat more willing to get an effective recommended COVID-19 vaccine.Footnote 13Footnote 14
Those most likely to transmit COVID-19 to those at high risk of severe illness and death from COVID-19 and workers essential to maintaining the COVID-19 response
- Healthcare workers, personal care workers, and caregivers providing care in long-term care facilities or other congregate care facilities for seniors
- Other workers most essential in managing the COVID-19 response or providing frontline care for COVID-19 patients
- Household contacts of those at high-risk of severe illness and death from COVID-19
Summary and rationale with current evidence:
- In Canada, long-term care facilities have experienced a large number of outbreaks associated with a high number of fatalities.Footnote 5
- Immunizing healthcare, personal care, and other workers providing frontline care directly protects them from acquisition of COVID-19 and could indirectly protect their patients and healthcare capacity.
- Though frontline healthcare workers and other workers functioning in a healthcare capacity (e.g., providing medical first response) have differential exposure to COVID-19 with potential transmission to high risk individuals, they may have more access to and training in the use of personal protective equipment and other infection prevention and control measures, and so exposure risk could be significantly reduced compared to other groups. Protection against infection with SARS-CoV-2 has been demonstrated in healthcare workers with the use of personal protective equipment.Footnote 9Footnote 10
- Immunizing healthcare workers and other workers functioning in a healthcare capacity minimizes the disproportionate burden of those taking on additional risks to protect the public.
- Absenteeism due to illness or perceived risk of illness from COVID-19 among healthcare workers and other workers most essential in managing the COVID-19 response (e.g. outbreak management, lab testing, immunization) may compromise healthcare capacity and the management of the COVID-19 response.
- Expert stakeholder groups and patient/community advocates,Footnote 2 as well as the general Canadian public,Footnote 14 rank the relative importance of an immunization strategy to protect healthcare capacity as #2 and an immunization strategy to minimize transmission of COVID-19 as #3 in the context of limited vaccine supply.
- 22% of Canadians identify "healthcare workers" as a key population for priority immunization in the case of COVID-19 vaccine shortage.Footnote 13
- Immunizing those able to transmit COVID-19 to those at high risk of severe illness and death could indirectly protect those at high risk (if vaccine is effective in interrupting transmission), which could be particularly important if vaccine characteristics are not favourable in these populations.
Those contributing to the maintenance of other essential services for the functioning of society
Summary and rationale with current evidence:
- Certain individuals who cannot work virtually may have differential exposure to COVID-19 (e.g., police, firefighters, grocery store staff).
- Designations of essential services in the context of the COVID-19 pandemic vary across jurisdictions within Canada. Guidance on essential services and functions in Canada during the COVID-19 pandemic , including lists published by provinces and territories, is available.
- Provinces and territories have expressed a desire for a harmonized approach to vaccine prioritization for essential services. The appropriate FPT health tables will be consulted to discuss prioritization for the purposes of immunization.
- Immunizing this population minimizes the disproportionate burden of those taking on additional risks to maintain services essential for the functioning of society.
- Absenteeism due to illness or perceived risk of illness from COVID-19 among some workers who cannot work virtually may compromise essential services.
- Expert stakeholder groups and patient/community advocates,Footnote 2 as well as the general Canadian public,Footnote 14 rank the relative importance of an immunization strategy to protect critical infrastructure as #4 in the context of limited vaccine supply.
- 18% of Canadians identify "frontline/essential workers" as a key population for priority immunization in the case of COVID-19 vaccine shortage.Footnote 13
Those whose living or working conditions put them at elevated risk of infection and where infection could have disproportionate consequences, including Indigenous communities
Summary and rationale with current evidence:
- In Canada, a high number of COVID-19 outbreaks/clusters in institutions (e.g., correctional facilities), work settings (e.g., agricultural or meat production/packing facilities), and congregate living settings (e.g., shelters, quarters for migrant workers) have occurred.Footnote 5
- The risk of transmission is high in these settings where physical distancing and other infection prevention and control measures are challenging and individuals may not be able to exercise sufficient personal actions to adequately protect themselves from infection. This increased risk may expand to other settings as they re-open.
- Remote or isolated populations or those in some congregate living settings may not have ready access to sufficient healthcare infrastructure. Therefore, their risk for death and societal disruption is proportionally greater since the response to any illness within the community might be sub-optimal.
- Indigenous communities have been disproportionately impacted by past pandemics (e.g., 2009 H1N1 influenza pandemic) and require special consideration of issues related to equity, feasibility and acceptability (see Section V.2).
Principles guiding public health program level decision-making
Efforts should be made to increase access to immunization services to reduce health inequities without further stigmatization or discrimination, and to engage systematically marginalized and racialized populations in immunization program planning (see Equity Matrix, Appendix D).
Summary and rationale with current evidence:
- Health inequities exist due, in part, to differential access to health care, as well as differential exposure, susceptibility, and severity of infectious diseases (see Equity Matrix, Appendix D). Interventions to reduce these inequities rather than potentiate them with further stigmatization or discrimination should be implemented as part of any immunization program.
- As with any immunization program, efforts should be made to ensure consideration of the needs of diverse population groups, based on health status, ethnicity/culture, ability, and other socioeconomic and demographic factors that may place individuals in vulnerable circumstances (e.g., occupational, social, economic, or biological vulnerabilities). These efforts should include integrating the values and preferences of these populations in vaccine program planning and building capacity to ensure access and convenience of immunization services.
- There is evidence of important/large independent associations of severe COVID-19 with race/ethnicity (low certainty of evidence of hospitalization or mortality), low socioeconomic status (low certainty of evidence for hospitalization), homelessness (low certainty of evidence for hospitalization), and male sex (moderate certainty of evidence for hospitalization).Footnote 1
- Outbreaks involving large numbers of reported cases have occurred in rural and remote communities in Canada.Footnote 5
- Outbreaks involving large numbers of reported cases have occurred in agricultural work settings, including those with congregate living for migrant workers.Footnote 5
- Visible minorities and Indigenous Canadians appear to be less willing than non-visible minorities to get an effective recommended COVID-19 vaccine.Footnote 13 Reasons for vaccine hesitancy are multifactorial.
- While significant differences in willingness to get vaccinated with a COVID-19 vaccine haven't been observed by sex or socioeconomic status,Footnote 13Footnote 14 immunization coverage rates have tended to be lower among men and those in lower socioeconomic groups for vaccine-preventable diseases where national data are available.Footnote 23
- Examples of interventions to engage communities and address barriers to accessing vaccine as summarized in the Equity Matrix (Appendix D) could help reduce inequities.
- This is aligned with the ethical principle of justice, where people and groups are treated with equal concern and respect.
- NACI continues to recommend (as outlined in its guidance on Research priorities for COVID-19 vaccines to support public health decisionsFootnote 4) that:
- Individuals with potential vulnerabilities to disease related to biological (e.g., increased age, pre-existing medical conditions), social (e.g., lower socioeconomic status, residence in long term care facilities or crowded/remote locations, homelessness, substance use disorder, race/ethnicity, immigration or refugee status), and occupational (e.g., healthcare workers, emergency workers, workers who have a high degree of social contact, international business travellers ) factors should be included in clinical trial groups as soon as possible.
- Vaccine outcome results should be disaggregated by potential factors contributing to inequities such as sex, age, race/ethnicity and health status. Trials should endeavour to power their groups to allow analyses by various sociodemographic variables.
Jurisdictions should begin planning for the implementation of a COVID-19 immunization program, including close and rapid monitoring of safety, effectiveness, and coverage of vaccine(s) in different key populations, as well as effective and efficient immunization of populations in remote and isolated communities (see Feasibility Matrix, Appendix E).
Summary and rationale with current evidence:
- Stakeholder reviews of feasibility identified multiple challenges requiring advanced planning and complex combinations of program administration through a variety of vaccine delivery models.
- Planning is required to address issues specific to a potential COVID-19 vaccine program (e.g., storage and dissemination of new vaccine technologies in different vaccine delivery venues; human resources for administration of vaccine, communication, training, data entry, screening for COVID-19, operational planning etc.), and integration with or enhancement of existing programs (e.g., registries, surveillance, adverse event following immunization reporting).
- Close and rapid monitoring of safety, effectiveness, and coverage of the vaccine(s) in potentially different key populations will be critical.
- The feasibility of sequential immunization of different key populations in remote and isolated communities is challenging. Deploying vaccine to entire communities may be more effective and efficient.
- Suggestions on planning for these issues are included in Appendix E.
Efforts should be made to improve knowledge about the benefits of vaccines in general, and of COVID-19 vaccine(s) specifically, once approved, address misinformation about immunization, and communicate transparently about COVID-19 vaccine allocation decisions (see Acceptability Matrix, Appendix F).
Summary and rationale with current evidence:
- Willingness to get a safe, effective COVID-19 vaccine has decreased in Canada (from 71% in April to 61% in August 2020).Footnote 14 The most reported reasons for unwillingness to get a COVID-19 vaccine across various Canadian surveys are concerns about safety and a lack of confidence and trust in a new COVID-19 vaccine.Footnote 13Footnote 14Footnote 15Footnote 16
- Deemed one of the top ten major global health threats by WHO in 2019, vaccine hesitancy (the reluctance or refusal to vaccinate despite the availability of vaccines) could lead to the resurgence of deadly, preventable diseases, and limit the success of a COVID-19 immunization program. Key reasons for vaccine hesitancy include complacency, inconvenience in accessing vaccines, and lack of confidence.Footnote 24
- Efforts should be made to reduce complacency, improve convenient access to vaccines, and improve confidence in and awareness of immunization in the general public, key populations for early COVID-19 immunization, and health care providers. Transparent, clear communication about vaccine allocation decisions is important to maintain trust, confidence, and improve access to vaccines for key populations.
- In general, receiving a recommendation from, or being in contact with a healthcare provider is linked to increased vaccine acceptability.Footnote 17 However, a lack of familiarity with a vaccine plays a role in reluctance by healthcare providers to recommend it.Footnote 17Footnote 18 A notable factor for healthcare providers to recommend a vaccine is a recommendation by an expert committee.Footnote 18
- Information on vaccine safety and pharmacovigilance can be found at: Vaccine safety and pharmacovigilance: Canadian Immunization Guide
See Figure 1 for a summary of the above recommendations on key populations for early COVID-19 immunization for public health program level decision-making.
Management options
Specific recommendations for COVID-19 vaccines in key populations will depend on yet unknown factors such as supply and characteristics (e.g., safety, immunogenicity, efficacy and effectiveness in preventing severe illness and interrupting transmission in different populations) of COVID-19 vaccine(s) available to Canadians, evolving evidence on COVID-19, as well as the epidemiological context at the time COVID-19 vaccine(s) become(s) available. When this information is available, NACI will provide additional guidance on management options for COVID-19 immunization.
Research priorities
NACI has recently published a focused statement on Research priorities for COVID-19 vaccines to support public health decisions,Footnote 4 including detailed discussion of clinical trial populations and outputs. In summary, research to address the following outstanding questions is encouraged:
New and emerging research priorities
- How will acceptability of prioritized key populations for early immunization with COVID-19 vaccine(s) evolve in different epidemiological contexts across the country?
- How can vaccine allocation decisions be communicated to individuals and communities in order to maintain trust in public health authorities?
- How will acceptability of COVID-19 vaccine(s), and vaccines in general, evolve in the public, key populations, marginalized populations, providers and policy-makers in different epidemiological contexts across the country? What factors will influence acceptability in these groups?
- What interventions will be effective in reducing health inequities and improving acceptability related to COVID-19 immunization?
Standing research priorities
- How safe and efficacious are candidate vaccines across diverse population groups (e.g., adults >60 years of age, those with high risk medical conditions, individuals with social or occupational vulnerabilities)?
- How effective will candidate vaccines be in preventing severe illness and death and interrupting transmission of COVID-19 across diverse population groups?
- Are IgA/IgG/IgM antibodies protective against SARS-CoV-2 and what is the correlate of protection?
- Is there a cell-mediated immunity correlate of protection against SARS-CoV-2?
- Is SARS-CoV-2 natural infection (symptomatic or asymptomatic) associated with protection against re-infection or severe disease? What is the duration of natural protection against re-infection or severe disease from SARS-CoV-2?
- What is the duration of vaccine protection against re-infection or severe disease from SARS-CoV-2?
- Does vaccination following prior SARS-CoV-2 infection or vaccination of SARS-CoV-2 naïve individuals elicit enhanced disease upon subsequent infection?
- Are any other vaccines (e.g., BCG) protective against COVID-19 through off-target effects?
- What is the epidemiological profile of COVID-19 (e.g., communicable period, all risk groups)?
- What is the disease distribution and spectrum of clinical illness for COVID-19, including burden of illness and risk by age, sex and other demographic variables associated with higher risk?
- What are the transmission dynamics of COVID-19, including degree of asymptomatic transmission, role of children in transmission, vertical transmissibility, onset and duration of viral shedding and communicable period, impact of changing weather conditions, and trends over time?
- What are the rates of COVID-19 co-infections with other respiratory pathogens and impact on pathogenesis and clinical outcomes?
- Is there cross-protection or interference from antibodies/exposure to human seasonal coronaviruses when exposed to SARS-CoV-2 or vaccinated against SARS-CoV-2?
- Are there any emerging safety signals with COVID-19 immunization that are not predicted by the current understanding of the safety profile of similar vaccines?
| Abbreviation | Term |
|---|---|
| AEFI | Adverse Events Following Immunization |
| ARCHE | Alberta Research Centre for Health Evidence |
| BMI | Body mass index |
| CIC | Canadian Immunization Committee |
| COSMO | Canada Canada's COVID-19 Snapshot Monitoring Study |
| COVID-19 | Novel coronavirus disease 2019 |
| CPSS3 | Canadian Perspectives Survey Series 3 |
| EEFA | Ethics, Equity, Feasibility and Acceptability |
| FNIHB | First Nations and Inuit Health Branch |
| FPT | Federal/Provincial/Territorial |
| GRADE | Grading of Recommendations, Assessment, Development and Evaluations |
| HCID | WG High Consequence Infectious Disease Working Group |
| ICU | Intensive Care Unit |
| IPC | Infection prevention and control |
| NACI | National Advisory Committee on Immunization |
| OECD | Organisation for Economic Co-operation and Development |
| OR | Odds ratio |
| PHAC | Public Health Agency of Canada |
| PPE | Personal protective equipment |
| P2ROGRESS | And Other Factors Pre-existing condition, Place of residence, Race/ethnicity/culture/language/immigration/refugee status, Occupation, Gender identity/sex, Religion/belief system, Education/literacy level, Socioeconomic status, Social capital, Age, Other factors |
| RR | Risk ratio |
| SAC | Special Advisory Committee |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| SGBA+ | Sex and Gender Based Analysis Plus |
| SOP | Standard Operating Procedures |
| UK | United Kingdom |
| US | United States |
| WHO | World Health Organization |
Acknowledgments
This statement was prepared by: Dr. SJ Ismail, Dr. L Zhao, Dr. MC Tunis, Dr. A Killikelly, Ms. P Doyon-Plourde, Dr. C Quach, and Dr. S Deeks on behalf of the NACI High Consequence Infectious Disease Vaccine Working Group and was approved by NACI.
NACI gratefully acknowledges the contribution of: Ms. MW Yeung, Dr. N Abraham, Ms. A Sinilaite, Dr. N Forbes, Ms. A House, Mr. M Patel, Mr. A Nam, Ms. M Matthieu-Higgins, and Ms. V Ferrante as well as the research team at the Alberta Research Centre for Health Evidence (ARCHE), including Ms. J Pillay, Ms. A Wingert, and Dr. L Hartling.
NACI High Consequence Infectious Disease Working Group
Members: Dr. C Quach (Chair), Dr. S Deeks (Vice-Chair), Dr. Y-G Bui, Dr. K Dooling, Dr. R Harrison, Dr. K Hildebrand, Dr. B Sander, Dr. M Murti, Dr. J Papenburg, Dr. R Pless, Dr. N Stall, and Dr. S Vaughan.
PHAC Participants: Dr. N Abraham, Ms. P Doyon-Plourde, Dr. N Forbes, Dr. SJ Ismail, Dr. A Killikelly, Ms. M Mattheiu-Higgins, Ms. V Ferrante, Dr. A Nam, Mr. M Patel, Ms. A Sinilaite, Dr. MC Tunis, Ms. MW Yeung, Dr. L Zhao.
NACI
Members: Dr. C Quach (Chair), Dr. S Deeks (Vice-Chair), Dr. J Bettinger, Dr. N Dayneka, Dr. P De Wals, Dr. E Dube, Dr. V Dubey, Dr. S Gantt, Dr. R Harrison, Dr. K Hildebrand, Dr. K Klein, Dr. J Papenburg, Dr. C Rotstein, Dr. B Sander, Ms. S Smith, and Dr. S Wilson.
Liaison representatives: Dr. LM Bucci (Canadian Public Health Association), Dr. E Castillo (Society of Obstetricians and Gynaecologists of Canada), Dr. A Cohn (Centers for Disease Control and Prevention, United States), Ms. L Dupuis (Canadian Nurses Association), Dr. J Emili (College of Family Physicians of Canada), Dr. D Fell (Canadian Association for Immunization Research and Evaluation), Dr. R Gustafson (Council of Chief Medical Officers of Health), Dr. D Moore (Canadian Paediatric Society), Dr. M Naus (Canadian Immunization Committee), and Dr. A Pham-Huy (Association of Medical Microbiology and Infectious Disease Canada).
Ex-officio representatives: Dr. D Danoff (Marketed Health Products Directorate, HC), Ms. E Henry (Centre for Immunization and Respiratory Infectious Diseases [CIRID], PHAC), Ms. M Lacroix (Public Health Ethics Consultative Group, PHAC), Ms. J Pennock (CIRID, PHAC), Dr. R Pless (Biologics and Genetic Therapies Directorate, Health Canada), Dr. G Poliquin (National Microbiology Laboratory, PHAC), Dr. V Beswick-Escanlar (National Defence and the Canadian Armed Forces), and Dr. T Wong (First Nations and Inuit Health Branch, Indigenous Services Canada).
Appendix A: Algorithm outlining the process for applying the EEFA framework
Appendix A: - Text description
The figure provides a visual summary of the algorithm outlining the process for applying the EEFA framework.
If immunization recommendation is needed, the following activities should be performed:
- Conduct reviews of scientific factors (burden of disease, vaccine characteristics)
- Conduct economic analyses as required
- Conduct systematic consideration of EEFA programmatic factors (by Technical Leads, as soon as possible, in advance of statement development):
- Fill out evidence-informed tools to identify distinct issues that could impact decision-making for recommendations development, consulting relevant groups/data as needed
Under activity c), consider the following:
- Have ethical concerns regarding implementation of the immunization program been adequately addressed?
- As needed: Consult Ethics Consultative Group
- Fill out the following tool: Ethics Integrated Filters (content, process)
- Is the program equitable in terms of accessibility of the vaccine for all target groups?
- As needed: Consult epi data, evidence synthesis, immunization surveys, input from stakeholder groups
- Fill out the following tool: Equity Matrix
- Is the program implementation feasible given existing resources?
- As needed: Consult regional immunization representatives, Vaccine Supply Managers
- Fill out the following tool: Feasibility Matrix
- Does a high level of demand or acceptability exist for the immunization program?
- As needed: Consult systematic lit review, immunization surveys
- Fill out the following tool: Acceptability Matrix
Technical Leads then present the filled out tools (Ethics Integrated Filters, Equity Matrix, Feasibility Matrix, Acceptability Matrix) to the Working Group (and the NITAG if deemed necessary by the Working Group) to inform the following decisions:
Are there distinct issues identified in the tools that:
- Are specific to this vaccine or vaccine preventable disease; AND
- Could have a significant change in/impact on the recommendation or implementation; AND
- Warrant additional time & resources to investigate?
If the response is "yes" to the above, conduct additional consultations/ lit review/ surveys as needed** before proceeding to the last step. If the response is "no" to the above, proceed directly to the last step.
Activities a), b), and c) all inform the last step of the algorithm.
For the last step of the algorithm, the following are included in the NACI Statement for jurisdictions to consider in their local contexts:
- Summarize distinct issues identified that are specific to the vaccine or vaccine preventable disease in: Recommendations Section (summary/rationale), Management Options table (& expand in Statement text if needed)
- Suggest evidence-based interventions to address these issues if applicable
- Link to full EEFA Framework and Tools in the Methods section
- Attach filled out tools as Appendices if deemed necessary by NITAG
Source: Ismail SJ, Hardy K, Tunis MC et al., A framework for the systematic consideration of ethics, equity, feasibility, and acceptability in vaccine program recommendations, Vaccine, https://doi.org/10.1016/j.vaccine.2020.05.051.
As acceptability is affected by contextual influences where different factors may weigh more or less heavily on vaccine decisions17, and the COVID-19 pandemic presents distinct issues that could significantly impact the implementation of recommendations, a reliance solely on historical perspectives of acceptability from other vaccine-preventable diseases or vaccine platforms is not sufficient. Therefore, emerging acceptability data related to COVID-19 vaccines was sought.
Appendix B: Core ethical dimensions filter applied to COVID-19
To ensure NACI's COVID-19 guidance upholds and integrates core ethical dimensions for public health
| Core Ethical Dimensions for Public Health | Description | Considerations for integration of core ethical dimensions | Tools to assist with integration of core ethical dimensions |
|---|---|---|---|
| Respect for persons and communities | Right to exercise informed choice based on all available evidence | Has all the evidence been presented in a comprehensive manner? Is the guidance accessible to stakeholders? |
|
| Have the values and preferences of persons and communities been considered? Does a high level of demand or acceptability exist for the immunization program? |
|
||
| Beneficence and non-maleficence | Promotion of well-being, minimize risk of harm vs benefits | Have the recommendations considered risks and benefits, and do benefits outweigh risks? Has the principle of reciprocityFootnote 1 been considered to minimize harm, especially in epidemic contexts? |
|
| If major risks are identified, has a risk analysis been conducted? |
|
||
| Justice | Treat people and groups with equal concern and respect | Is the recommendation equitable in terms of accessibility of the vaccine for all target groups? Are there special considerations for vulnerability of those most at risk? |
|
| Distributive justice: Is implementation feasible given existing resources? Do the recommendations result in a fair distribution of resources? |
|
||
| Trust | Long term reliability, integrity, sustainable and mutually fair relationship with individuals and communities | Are the recommendations based on the best, current evidence available for all groups at risk of the vaccine-preventable disease (VPD)? |
|
| Have the Ethics Procedural Considerations been upheld? (see Ethics Procedural Considerations Filter) |
|
||
Table 2 - footnotes
|
|||
Source: Ismail SJ, Hardy K, Tunis MC et al., A framework for the systematic consideration of ethics, equity, feasibility, and acceptability in vaccine program recommendations, Vaccine, https://doi.org/10.1016/j.vaccine.2020.05.051.
Appendix C: Ethical procedural considerations filter applied to COVID-19
To ensure NACI's COVID-19 guidance processes uphold and integrate ethical procedural considerations
| Ethical Procedural Considerations | Considerations for integration of ethical procedural considerations | Tools/procedures to assist with integration of ethical procedural considerations |
|---|---|---|
| Accountability | Quality and completeness: Was an appropriate review completed on all relevant topics? |
|
| Inclusiveness | Were relevant stakeholders engaged? |
|
| Responsibility | Did NACI act independently to make decisions (without conflicts of interest, independence from Industry)? |
|
| Responsiveness | Did guidance respond to needs of stakeholders? |
|
| Transparency | Was there a clear and strong rationale for recommendation? |
|
Table 3 - footnotes
|
||
Source: Ismail SJ, Hardy K, Tunis MC et al., A framework for the systematic consideration of ethics, equity, feasibility, and acceptability in vaccine program recommendations, Vaccine, https://doi.org/10.1016/j.vaccine.2020.05.051.
Appendix D: Equity matrix applied to COVID-19 with evidence to date
To identify distinct inequities associated with COVID-19, potential reasons for inequities, and suggested interventions to reduce the inequity and improve access to vaccine
| Factors that may contribute to health inequity | Why inequity may exist (differential access or differential disease exposure, susceptibility, severity or consequences and their intersections with other factors)Footnote 1 | Examples of suggested interventions to reduce inequity and improve access |
|---|---|---|
| Pre-existing condition (e.g., chronic disease, immunocompromised, pregnancy, disability) |
|
|
| Place of residence (remote, overcrowding, homeless, institutionalization) |
|
|
| Race/ethnicity/ culture/language/ immigration/refugee status |
|
|
| Occupation |
|
|
| Gender Identity/Sex |
|
|
| Religion/Belief system |
|
|
| Education/Literacy level |
|
|
| Socioeconomic status (SES) (including income, and coverage of healthcare and healthcare interventions) |
|
|
| Social Capital (social support/networks, trust) |
|
|
| Age |
|
|
| Other factors (e.g., risk behaviours - substance use disorders, smoking, sex workers) |
|
|
Table 4 - footnotes
|
||
Source: Ismail SJ, Hardy K, Tunis MC et al., A framework for the systematic consideration of ethics, equity, feasibility, and acceptability in vaccine program recommendations, Vaccine, https://doi.org/10.1016/j.vaccine.2020.05.051.
Appendix E: Feasibility matrix applied to COVID-19
To identify potential, distinct issues with respect to potential COVID-19 vaccine(s) and a COVID-19 immunization program to address the feasibility of implementing recommendations
| - | Vaccine | Immunization Program | Potential Issues |
|---|---|---|---|
| Resources | Vaccine and Immunization Supplies (short & long term) |
Human resources (for administration of vaccine, communication, training, data entry, screening for COVID-19, operational planning, etc.) |
|
| Funding for vaccine purchase | Funding for human and administrative resources |
|
|
| Integration with Existing Program | Vaccine coverage | Communication of program (to public, target groups, providers, labs) |
|
| VPD (sero)types included | Surveillance of VPD |
|
|
| Degree of similarity to existing vaccines, co-admin with other vaccines, targeting VPDs with existing programs | Alignment with existing schedule (e.g. with other program targeting same group) |
|
|
| Adverse Events | Inclusion in immunization registry, Adverse Event Following Immunization (AEFI) reporting |
|
Source: Ismail SJ, Hardy K, Tunis MC et al., A framework for the systematic consideration of ethics, equity, feasibility, and acceptability in vaccine program recommendations, Vaccine. 2020 June 10. https://doi.org/10.1016/j.vaccine.2020.05.051.
Appendix F: Acceptability matrix applied to COVID-19
To identify potential distinct issues with the acceptability of a recommendation for COVID-19 immunization from the perspective of the public and providers (based on a representative sample of Canadians surveyed through Canada's COVID-19 Snapshot Monitoring Study (COSMO Canada).Footnote 11Footnote 13Footnote 14
| - | Public | Providers | |
|---|---|---|---|
| General Population | Key Populations at High Risk | ||
Vaccine (perceptions of): E.g., efficacy, number of injections, route of administration, safety concerns, perceived benefits |
Almost two thirds of Canadians surveyed (61%) in August 2020 were willing to get an effective recommended COVID-19 vaccine. This has decreased from 71% in April. Main reasons for vaccine hesitancy were: not enough testing or research has been done (32%), do not believe the vaccine will be safe and/or effective (26%), and lack of trust in the newness of the vaccine (13%). |
Older Canadian respondents (aged 55 years and older) are more willing to get vaccinated than younger respondents (72% vs 57% in 35-54 years old and 51% in 18-34 years old; p<.05). Respondents 35 years and older with a "serious, long-term illness" are somewhat more willing to get an effective recommended vaccine compared to those without (68% vs 62%; 35-54 years old: 62% vs 59%; 55 years and older: 77% vs 74%). This was not observed in younger individuals 18 to 34 years old with a "serious, long-term illness" (43% vs 57%). Perceived safety (50%) is linked to increased vaccine acceptability compared to vaccine effectiveness (43%) among younger adults with "serious, long-term illness". Non-visible minorities are more willing than visible minorities or Indigenous Canadians to get an effective recommended vaccine (63% vs 53% vs 43%; p<.05). About half of self-identified workers in frontline occupations such as grocery and gas station staff would get an effective recommended vaccine (51% vs 63%, p<.05). |
Just over half of respondents who self-identified as healthcare providers would get an effective recommended vaccine (55% vs 62% for non-healthcare providers, p>.05). Concern about lack of testing or research (39%) and vaccine safety and/or efficacy (22%) are the main factors for vaccine hesitancy among healthcare providers surveyed. Other reasons for hesitancy include newness of the vaccine (19%) and do not believe in vaccines (6%). |
Disease (perceptions of): E.g., burden of illness, groups at risk, personal risk, severity, outbreak |
More respondents believe the worst of the crisis is yet to come than believe that it is behind us (44% vs 33%). Younger respondents are more likely to agree that the worst of the crisis is behind us than older age groups (45% vs 32% and 26%; 18-34 year olds vs 35-54 year olds and 55 years and older, respectively; p<.05). |
Three quarters of respondents (78%) agree that specific groups should be first to get a safe and effective vaccine in case of a shortage. The most commonly identified group for priority immunization was healthcare workers (40%), followed by individuals with high-risk medical conditions (19%), frontline workers (16%), seniors (12%), long-term care homes (10%) and children (2%). Half of older adults believe the worst of the crisis is yet to come (50% vs 33% and 45%; 55 years and older vs 18-34 year olds and 35-54 year olds; p<.05). Non-visible minorities are more likely to agree that the worst of the crisis is yet to come than visible minorities (45% vs 39%, p<.05). People living in small rural areas (<30K population) are more likely to believe the worst is yet to come than those living in medium (30K-999K population) or major (>1 million population) metropolitan areas (47% vs 43% vs 41%, p<.05). |
The majority of healthcare providers surveyed (83%) agree that specific groups should be first to get a vaccine in case of a shortage. They most often identified themselves (33%) as the priority target group for immunization, followed by individuals with high-risk medical conditions (19%), seniors (13%), long-term care homes (13%), frontline workers (12%) and children (7%). Among healthcare providers surveyed, 43% believe the worst of the crisis is yet to come and 28% believe it is behind us. Healthcare providers are more likely than non-healthcare providers to believe we are currently experiencing the worst of the crisis (21% vs 11%, p<.05). |
Process to get vaccinated E.g., access to vaccine, (opportunity) cost, past vaccine experience |
Almost half of Canadians surveyed (45%) think that immunization should be mandatory when available. The majority of Canadians surveyed (51%) agreed that in the context of limited initial vaccine supply, the most important strategy should be to protect those who are most vulnerable to severe illness and death from COVID-19; followed by protecting healthcare capacity (28%), minimize spread of COVID-19 (15%) and protect critical infrastructure (5%). |
Older adults are more likely to agree to mandatory immunization for COVID-19 (53%) than younger age groups (41% of 18-34 year olds and 40% of 35-54 year olds, p<.05). White respondents are less likely to say that minimizing the spread of COVID-19 is the most important strategy in the face of limited initial vaccine supply compared to visible minority and Indigenous respondents (15% vs. 19% and 27% respectively, p<.05). A third of workers in frontline occupations like grocery and gas station staff believe immunization against COVID-19 should be mandatory (37% vs 47%, p<.05). Less than 10% of these workers believed that protecting critical infrastructure is the most important strategy in the face of limited initial vaccine supply (8% vs. 5%, p<.05). Older Canadians are more likely to say that protecting healthcare capacity is the most important strategy in the face of limited initial vaccine supply (33% vs 30% and 19%, p<.05). |
In August, 37% of healthcare providers surveyed believed immunization against COVID-19 should be mandatory. This has decreased from 44% in July. |
Individual factors E.g., beliefs and values, experiences, trust in healthcare providers and healthcare system, trust in vaccine experts and vaccine industry, social norms/pressures and media. |
Almost half of Canadians surveyed (49%) report that they would get vaccinated in order to return to work, travel or attend a large gathering. This has decreased slightly from 55% in July. Although reported less frequently, lack of belief in vaccines (9%) and lack of trust in the government (7%) were reported as main reason for the vaccine refusal. Nearly all Canadians (84%) think scientific progress in vaccine development is the most needed type of information. Adults less than 55 years old most frequently reported using social media as a source of information on COVID-19 than older Canadians (14% in 18-34 year olds and 10% in 35-54 year olds vs 5% in 55 years and older, p<.05). Women are more likely than men to trust government agencies in their reporting about COVID-19 (55% vs 50%, p<.05) and to use provincial public health leaders to stay informed about COVID-19 (38% vs 33%, p<.05). |
Respondents with a "serious, long-term illness" are not more likely to get vaccinated in order to return to work, travel or attend a large gathering (47% vs 50%). Older adults are more likely to report that they don't believe in vaccines as the main reason for hesitancy compared to younger age groups (17% vs 6% in 18-34 year olds and 8% in 35-54 year olds, p<.05) Visible minorities are more likely to rely on various resources to stay up to date on COVID-19 information (i.e., federal websites [29% vs 23%], conversation with family [21% vs 12%] and colleagues [10% vs 5%], local radio stations [17% vs 12%], weekly newspapers [12% vs 7%], and social media [15% vs 8%]; all p<.05) compared to non-visible minorities who more often use provincial public health officials as a primary source of information (31% visible minorities vs 37% non visible minorities, p<.05). People with smaller household incomes are more likely to think of social media as a trustworthy source of information compared to those with higher incomes (5% <$40K vs 3% $40-100K vs 1% >$100K, p<.05). Those with high household income rely more often on online news resources to stay up to date on the COVID-19 situation (23% >$100K vs 19% $40-100K vs 17% <$40K, p<.05). |
A small proportion of healthcare providers surveyed would refuse to get vaccinated mainly because they believe the virus will naturally disappear (9%), don't believe in vaccines (6%), fear needles (4%), or do not trust the government (1%). The majority of healthcare providers (76%) think scientific progress in vaccine development is the most needed type of information. |
Source: Ismail SJ, Hardy K, Tunis MC et al., A framework for the systematic consideration of ethics, equity, feasibility, and acceptability in vaccine program recommendations, Vaccine, https://doi.org/10.1016/j.vaccine.2020.05.051. Potential issues with acceptability in policymakers is not included in this matrix.
References
- Footnote 1
-
Wingert A, Pillay J, Gates M, et al. Risk factors for severe outcomes of COVID-19: a rapid review. medRxiv. 2020. DOI: 10.1101/2020.08.27.20183434
- Footnote 2
-
Zhao L, Ismail SJ, Tunis MC. Ranking the relative importance of immunization strategies for novel coronavirus disease 2019 (COVID-19): a rapid survey of stakeholders. medRxiv. 2020. DOI: 10.1101/2020.09.16.20196295
- Footnote 3
-
Ismail SJ, Hardy K, Tunis MC, Young K, Sicard N, Quach, C. A framework for the systematic consideration of ethics, equity, feasibility, and acceptability in vaccine program recommendations. [published online June 10, 2020]. Vaccine. DOI: 10.1016/j.vaccine.2020.05.051
- Footnote 4
-
NACI. Research priorities for COVID-19 vaccines to support public health decisions [Internet]. 2020. Available at: https://www.canada.ca/en/public-health/services/immunization/national-advisory-committee-on-immunization-naci/research-priorities-covid-19-vaccines.html
- Footnote 5
-
Government of Canada. Epidemiological summary of COVID-19 cases in Canada [Internet]. 2020. Available at: https://health-infobase.canada.ca/covid-19/epidemiological-summary-covid-19-cases.html
- Footnote 6
-
Public Health Agency of Canada. Vaccine annex: Canadian Pandemic Influenza Preparedness: Planning Guidance for the Health Sector [Internet]. 2017. Available at: https://www.canada.ca/en/public-health/services/flu-influenza/canadian-pandemic-influenza-preparedness-planning-guidance-health-sector/vaccine-annex.html
- Footnote 7
-
Ismail SJ, Langley JM, Harris TM, Warshawsky BF, Desai S, FarhangMehr M. Canada's National Advisory Committee on Immunization (NACI): Evidence-based decision-making on vaccines and immunization. Vaccine. 2010;28:A58-63. DOI: 10.1016/j.vaccine.2010.02.035
- Footnote 8
-
Public Health Agency of Canada. Public health ethics framework: A guide for use in response to the COVID-19 pandemic in Canada [Internet]. 2020. Available at: https://www.canada.ca/en/public-health/services/diseases/2019-novel-coronavirus-infection/canadas-reponse/ethics-framework-guide-use-response-covid-19-pandemic.html
- Footnote 9
-
Liu M, Cheng SZ, Xu KW, et al. Use of personal protective equipment against coronavirus disease 2019 by healthcare professionals in Wuhan, China: cross sectional study. BMJ. 2020;369.
- Footnote 10
-
Wang W, Min YZ, Yang CM, et al. Association of personal protective equipment use with successful protection against COVID-19 infection among health care workers. medRxiv. 2020.
- Footnote 11
-
Impact and Innovation Unit. COVID-19 Snapshot Monitoring (COSMO Canada). Impact Canada. Available at: https://impact.canada.ca/en/challenges/cosmo-canada
- Footnote 12
-
Public Health Agency of Canada. Interim guidance on continuity of immunization programs during the COVID-19 pandemic [Internet]. 2020. Available at: https://www.canada.ca/en/public-health/services/immunization/national-advisory-committee-on-immunization-naci/interim-guidance-immunization-programs-during-covid-19-pandemic.html
- Footnote 13
-
Impact and Innovation Unit. (2020, July 27; wave 6). COVID-19 Snapshot Monitoring (COSMO Canada). Impact Canada. Available at: https://impact.canada.ca/en/challenges/cosmo-canada/wave6
- Footnote 14
-
Impact and Innovation Unit. (2020, August 17; wave 7). COVID-19 Snapshot Monitoring (COSMO Canada). Impact Canada.
- Footnote 15
-
Angus Reid. COVID-19: Three-in-five worry about side-effects of a vaccine; many plan to take a 'wait and see' approach [Internet]. 2020. Available at: http://angusreid.org/wp-content/uploads/2020/08/2020.08.03_COVID-VACCINE.pdf
- Footnote 16
-
Frank K and Arim R. StatCan COVID19: Data to insights for a better Canada. Group differences and reasons for vaccine hesitancy [Internet]. 2020. Available at: https://www150.statcan.gc.ca/n1/pub/45-28-0001/2020001/article/00073-eng.htm
- Footnote 17
-
Gates A, Gates M, Rahman S, et al. A systematic review of factors that influence the acceptability of vaccines among Canadians. Submitted for publication.
- Footnote 18
-
Environics Research. Vaccine acceptability factors for the general public and health care professionals in Canada. Ottawa (ON): Health Canada; 2020. ISBN: 978-0-660-32602-3.
- Footnote 19
-
Joint Committee on Vaccination and Immunisation. Joint Committee on Vaccination and Immunisation: interim advice on priority groups for COVID-19 vaccination [Internet]. 2020. Available at: https://www.gov.uk/government/publications/priority-groups-for-coronavirus-covid-19-vaccination-advice-from-the-jcvi/interim-advice-on-priority-groups-for-covid-19-vaccination
- Footnote 20
-
Haute Autorité de Santé. Stratégie vaccinale contre la Covid-19 : stratégie de déploiement des vaccins disponibles [Internet]. 2020. Available at: https://www.has-sante.fr/upload/docs/application/pdf/2020-07/note_de_cadrage_strategie_vaccinale_contre_la_covid_19.pdf
- Footnote 21
-
Toner E, Barnill A, Krubiner C, et al. Interim Framework for COVID-19 Vaccine Allocation and Distribution in the United States. Baltimore, MD: Johns Hopkins Center for Health Security; 2020.
- Footnote 22
-
Committee on Equitable Allocation of Vaccine for the Novel Coronavirus. Discussion draft of the preliminary framework for equitable allocation of COVID-19 vaccine [Internet]. Available at: https://www.nationalacademies.org/news/2020/09/national-academies-release-draft-framework-for-equitable-allocation-of-a-covid-19-vaccine-seek-public-comment
- Footnote 23
-
Roy M, Sherrard L, Dubé È, Gilbert NL. Determinants of non-vaccination against seasonal influenza. Health Reports. 2018;29(10):12-22.
- Footnote 24
-
World Health Organization. Ten threats to global health in 2019 [Internet]. Available at: https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019