Archived: Guidance on the prioritization of key populations for COVID-19 immunization [2021-02-12]
Notice to reader
This is an archived version. Please refer to current COVID-19 vaccine pages:
On this page
- Introduction
- Methods
- Recommendations
- Stages of increasing vaccine availability
- Underlining principles for sequencing and prioritization of key populations
- Foundational elements guiding ethical decision-making (for equitable, feasible, and acceptable recommendations) and implementation of COVID-19 immunization programs across all key populations
- Key populations prioritized for COVID-19 immunization
- Acknowledgments
- Appendix A: Factors contributing to severe COVID-19 – Preliminary results of an updated rapid review of risk factors
- References
Introduction
The goal of Canada's pandemic response is to minimize serious illness and death while minimizing societal disruption as a result of the COVID-19 pandemic. Safe and effective COVID-19 vaccines will help achieve this goal. Initial supplies of authorized COVID-19 vaccines are not expected to be sufficient to offer vaccination to all Canadians in whom they are authorized for use until the fall of 2021. Therefore, recommendations to prioritize key populations for early immunization are needed in order to meet the pandemic response goal as equitably, ethically and efficiently as possible.
The evidence on COVID-19 and COVID-19 vaccines is rapidly evolving. To date, the National Advisory Committee on Immunization (NACI) has developed the following evidence-informed guidance related to the prioritization of key populations in the context of limited vaccine supply to inform the planning of provincial and territorial publicly funded COVID-19 immunization programs:
- Preliminary guidance on key populations for early COVID-19 immunization (November 2020)Footnote 1: NACI developed this guidance in the absence of information on the results of vaccine clinical trials, vaccine characteristics, and vaccine supply. NACI conducted a comprehensive systematic assessment of ethics, equity, feasibility and acceptability (EEFA) considerations with the peer-reviewed EEFA FrameworkFootnote 2 in order to develop the recommendations. The guidance was informed by evidence available at the time of NACI deliberations including: the epidemiology of COVID-19, the results of a rapid review of risk factors for severe COVID-19Footnote 3, and stakeholder consultations (including surveys of experts, patient and community advocatesFootnote 4, and the Canadian publicFootnote 5Footnote 6Footnote 7Footnote 8Footnote 9). This guidance document provided a framework upon which further prioritization could be based as evidence evolved. This framework is summarized in Figure 1 of the evidence-informed guidance document.
- Guidance on the prioritization of initial doses of COVID-19 vaccines (December 2020): NACI developed this urgent guidance based on the framework above when preliminary evidence on the results of the Phase 3 clinical trials of the Pfizer-BioNTech and Moderna COVID-19 vaccines became available. These recommendations further sequenced the key populations identified in NACI’s preliminary guidance in anticipation of the arrival of initial doses of authorized COVID-19 vaccines.
Since the publication of the above guidance documents, COVID-19 vaccines have been authorized for use in Canada under an interim order. NACI released recommendations on the use of the first authorized COVID-19 vaccine on December 12, 2020, and has updated their guidance as additional vaccines have become authorized for use in Canada, and as evidence on these vaccines has evolved. Refer to NACI recommendations on the use of COVID-19 vaccines.
Guidance objective
The objective of this advisory committee statement is to provide guidance for the equitable, ethical, and efficient allocation of authorized COVID-19 vaccines in the context of staggered arrival of vaccine supply that will necessitate offering vaccines to some populations earlier than others. This guidance builds on the foundational framework of NACI’s preliminary guidance with updates informed by current evidence on COVID-19 and authorized COVID-19 vaccines.
Methods
Consultations with various stakeholders since the development of the initial guidance on key populations have included: Public Health Ethics Consultative Group, First Nations and Inuit Health Branch (FNIHB), Indigenous Services Canada, NACI liaison and ex-officio organisations, the Canadian Immunization Committee (CIC), the Pan-Canadian Public Health Network’s Special Advisory Committee on COVID-19 (SAC), SAC’s Technical Advisory Committee, the Sex and Gender Based Analysis Plus (SGBA+) network with the Social Determinants of Health Division at PHAC; and Immigration, Refugees and Citizenship Canada (IRCC). NACI considered the values and preferences of the general Canadian population, expert stakeholders, and specific key populations through the results of surveysFootnote 4Footnote 9Footnote 10Footnote 11, literature reviewsFootnote 12Footnote 13, and communication with patient and community advocates via the NACI Chair, NACI Secretariat and the Public Health Agency of Canada.
On January 19, 2021, NACI and the NACI High Consequence Infectious Disease Working Group (HCID WG) reviewed updated evidence on the epidemiology of COVID-19, Canadian data on the intersectionality between various biological and social risk factors for COVID-19, and issues related to the ethics, equity, feasibility and acceptability of immunization in different key populations. Members of NACI and the NACI HCID WG completed surveys on the prioritization of different key populations on January 24, 2021. NACI reviewed results from a rapid review of evidenceFootnote 3 on risk factors for severe outcomes of COVID-19 that informed NACI’s previous guidance, as well as preliminary results (see Appendix A) from an updated rapid review of biological risk factors (in studies from the Organisation for Economic Co-operation and Development (OECD) countries) and social risk factors (in studies from Canada) by the Alberta Research Centre for Health Evidence (ARCHE) on January 19 and 28, 2021. On January 28, 2021, NACI deliberated on the cumulative evidence and proposed recommendations. On February 5, 2021, NACI voted on and approved the revised recommendations.
Further information on NACI’s process and procedures is available elsewhereFootnote 14.
Recommendations
Figure 1 summarizes NACI’s recommendations for the equitable, ethical, and efficient allocation of authorized COVID-19 vaccines in the context of staggered arrival of vaccine supply. This figure updates the foundational framework from NACI’s Preliminary guidance on key populations for early COVID-19 immunization with current evidence on COVID-19 and COVID-19 vaccines.
NACI developed these evidence-informed recommendations to inform the planning of provincial and territorial publicly funded COVID-19 immunization programs with evidence available at the time of deliberations. NACI recognizes that logistical/operational and epidemiological contexts vary between provinces and territories across Canada, and this may affect the sequencing of, and sub-prioritization within, key populations identified in each stage. NACI encourages jurisdictions to align with these recommendations as much as possible to ensure the equitable, ethical, efficient and consistent allocation of COVID-19 vaccines in Canada, while considering their local contexts.
NACI also acknowledges that the epidemiology of COVID-19 (including the emergence of SARS-CoV-2 virus variants of concern) and the evidence on COVID-19 vaccines are rapidly evolving and will continue to monitor the evidence and update recommendations as needed.
Figure 1: Summary of NACI recommendations on the prioritization of key populations for COVID-19 immunization
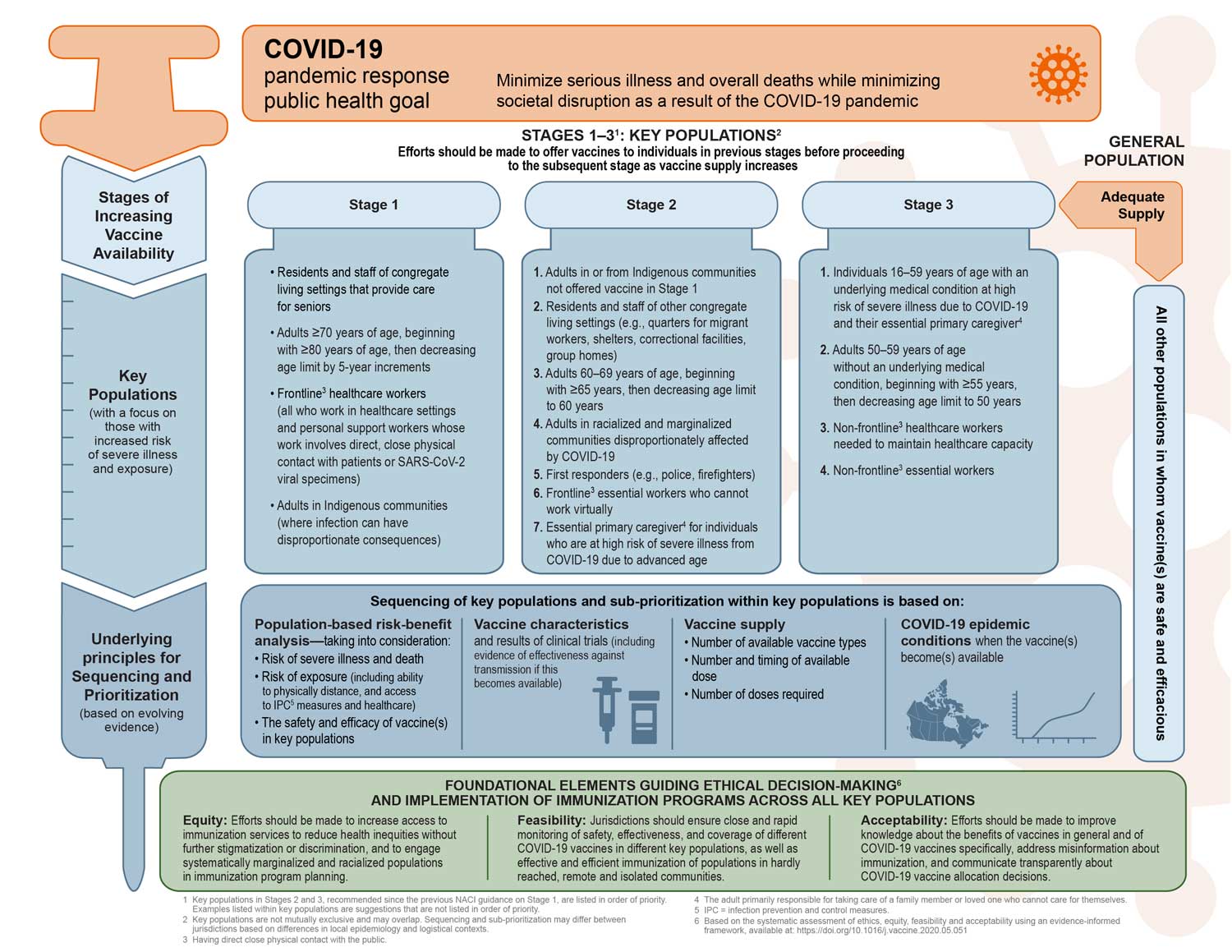
Figure 1 - Long description
The figure provides a visual summary of the National Advisory Committee on Immunization recommendations on the prioritization of key populations for COVID-19 vaccination.
The figure is organized into four sections. The first section states the COVID-19 pandemic response public health goal. The second section presents the key populations for 3 stages of increasing COVID-19 vaccine availability with a focus on those with increased risk of severe illness and exposure. The third section presents underlying principles for sequencing and prioritization, which will be based on evolving evidence. Finally, the foundational elements guiding ethical decision-making and implementation of immunization programs across all key populations is presented.
The COVID-19 pandemic response public health goal is to minimize serious illness and overall deaths while minimizing societal disruption as a result of the COVID-19 pandemic.
Efforts should be made to offer vaccines to individuals in previous stages before proceeding to the subsequent stage as vaccine supply increases. Key populations are not mutually exclusive and may overlap. Sequencing and sub-prioritization may differ between jurisdictions based on differences in local epidemiology and logistical contexts. Key populations prioritized for COVID-19 vaccination include the following:
Stage 1
- Residents and staff of congregate living settings that provide care for seniors
- Adults 70 years of age and older, beginning with adults 80 years of age and older, then decreasing the age limit by 5-year increments
- Health care workers (all who work in health care settings and personal support workers whose work involves direct contact with patients)
- Adults in Indigenous communities (where infection can have disproportionate consequences)
Stage 2 (listed in order of priority)
- Adults in or from Indigenous communities not offered vaccine in Stage 1
- Residents and staff of other congregate living settings (e.g., quarters for migrant workers, shelters, correctional facilities, group homes)
- Adults 60-69 years of age, beginning with ≥65 years, then decreasing age limit to 60 years
- Adults in racialized and marginalized communities disproportionately affected by COVID-19
- First responders (e.g., police, firefighters)
- Frontline essential workers who cannot work virtually (Frontline is defined as: “having direct close physical contact with the public”)
- Essential primary caregiver for individuals who are at high risk of severe illness from COVID-19 due to advanced age (≥ 60 years) (Primary caregiver is defined as: “The adult primarily responsible for taking care of a family member or loved one who cannot care for themselves”)
Stage 3 (listed in order of priority)
- Individuals 16-59 years of age with an underlying medical condition at high risk of severe illness due to COVID-19 and their essential primary caregiver
- Adults 50-59 years of age without an underlying medical condition, beginning with ≥ 55 years then decreasing age limit to 50 years
- Non-frontline healthcare workers needed to maintain healthcare capacity (Frontline is defined as: “having direct close physical contact with the public”)
- Non-frontline essential workers
Finally, when there is adequate vaccine supply, other populations in whom vaccine(s) are safe and efficacious can be considered for COVID-19 immunization.
Key populations focus on those with increased risk of severe illness and exposure. Underlying principles for sequencing and prioritization are based on evolving evidence on the following:
- Population-based risk-benefit analysis: taking into consideration risk of severe illness and death , risk of exposure (including ability to physically distance, and access to infection, prevention and control measures and healthcare), , and the safety and efficacy of vaccine(s) in key populations
- Vaccine characteristics and results of clinical trials (including evidence of effectiveness against transmission if this becomes available)
- Vaccine supply: number of available vaccine types, number and timing of available doses, and number of doses required
- COVID-19 epidemic conditions when the vaccine(s) become(s) available
The foundational elements guiding ethical decision-making and implementation of immunization programs across all key populations include the following:
- Equity: Efforts should be made to increase access to immunization services to reduce health inequities without further stigmatization or discrimination, and to engage systematically marginalized and racialized populations in immunization program planning.
- Feasibility: Jurisdictions should begin planning for the implementation of a COVID-19 immunization program, including rapid monitoring of safety, effectiveness, and coverage of vaccine(s) in different key populations, as well as effective and efficient immunization of populations in remote, hardly reached, and isolated communities.
- Acceptability: Efforts should be made to improve knowledge about the benefits of vaccines in general and of COVID-19 vaccine(s) specifically once available, address misinformation about immunization, and communicate transparently about COVID-19 vaccine allocation decisions.
Stages of increasing vaccine availability
Key populations are sequenced in three stages corresponding to increasing vaccine availability in each quarter of 2021. By the end of the third quarter of 2021, it is anticipated that sufficient vaccine supply will be available to offer vaccines to the general Canadian population. NACI recommends that efforts should be made to offer authorized COVID-19 vaccines to individuals in the key populations identified in each stage before proceeding to the subsequent stage as vaccine supply increases.
Underlying principles for sequencing and prioritization of key populations
NACI’s recommendations on the sequencing of key populations and sub-prioritization within key populations are based on evidence available on the following:
- Population-based risk-benefit analysis, taking into consideration:
- Risk of severe illness and death from COVID-19
- Risk of exposure to SARS-CoV-2 (including ability to physically distance, as well as access to other infection prevention and control measures and healthcare)
- Safety and efficacy of authorized vaccines in key populations
- Vaccine characteristics and results of clinical trials (recognizing that evidence of effectiveness against transmission of SARS-CoV-2 is evolving)
- Vaccine supply (number of available vaccine types, number and timing of available doses, number of doses required for each key population)
- COVID-19 epidemic conditions when the vaccines become available (recognizing that these may differ across Canadian jurisdictions)
NACI recommends that authorized COVID-19 vaccines be prioritized for individuals in the key populations sequenced in three stages of increasing vaccine supply, and sub-prioritized within each stage as outlined in Figure 1 until adequate supply is available to offer vaccines to the general population. NACI recognizes that sequencing and sub-prioritization within each stage may differ between jurisdictions depending on local COVID-19 epidemiology and logistical contexts. Sub-prioritization within key populations should be based on increased risk of severe illness and death from COVID-19 (e.g., age) and increased risk of exposure to SARS-CoV-2 (e.g., inability to physically distance). The key populations are not mutually exclusive and may overlap.
Foundational elements guiding ethical decision-making (for equitable, feasible, and acceptable recommendations) and implementation of COVID-19 immunization programs across all key populations
The foundational elements that guided ethical decision-making for these recommendations are consistent with those outlined in NACI’s EEFA framework. Evidence-informed tools were used to comprehensively assess issues related to ethics, equity, feasibility and acceptabilityFootnote 2 of COVID-19 vaccination in NACI’s Preliminary guidance on key populations for early COVID-19 immunization.
NACI endeavoured to make ethical, equitable and evidence-based recommendations. NACI acknowledges that some populations are at increased risk of exposure to the SARS-CoV-2 virus (e.g., due to living or occupational settings), and some populations are at increased risk of severe COVID-19 disease and outcomes (e.g., hospitalization and death) due to various biological (e.g., advanced age, pre-existing medical conditions) and social (e.g., low socioeconomic status, belonging to a racialized population)Footnote 3 factors that may intersect. Factors for risk of severe disease and risk of exposure may overlap, further increasing risk. Any combination of these factors, as well as varying access to health care services, has the potential for disproportionate consequences for specific populations characterized by increased rates of infection and disease, severe illness, hospitalizations, and deaths. NACI also acknowledges that evidence regarding risk factors for severe outcomes from COVID-19 continues to emerge. NACI will monitor the evidence and update its recommendations as needed.
Many of the populations at increased risk of severe disease or exposure face challenges accessing immunization. The COVID-19 pandemic has magnified social and biologic inequities and threatens to exacerbate them with the inequitable allocation of vaccinesFootnote 15. Please see the Equity Matrix, updated with evolving evidence and consultations, for a summary of inequities associated with COVID-19, potential reasons for and intersections between these inequities, and suggested interventions to reduce inequities and improve access to vaccines.
NACI also endeavoured to make recommendations that are acceptable to Canadians and feasible to implement, informed by stakeholder consultations and surveys of experts, the general Canadian population, and key populations.
Based on these foundational elements, as well as updated considerations on the spectrum of biological and social inequities and their intersectionsFootnote 15, NACI makes the following recommendations for the implementation of immunization programs across all populations:
- Efforts should be made to increase access to immunization services to reduce health inequities without further stigmatization or discrimination, and to engage systemically marginalized populations and racialized populations in immunization program planning.
- Examples of interventions to engage communities and address barriers to accessing vaccine, as summarized in the Equity MatrixFootnote 15, could help reduce inequities. For example, strategies should be implemented to increase availability and access to COVID-19 vaccines to migrant groups, who fall within the above key populations, but for whom vaccines are not typically provided under provincial or territorial health plans. Migrant groups can include: temporary residents (e.g., temporary foreign workers, international students, asylum seekers etc.) and undocumented migrants (i.e., individuals without status).
- Jurisdictions should ensure close and rapid monitoring of safety, effectiveness, and coverage of different COVID-19 vaccines in different key populations, as well as effective and efficient immunization of populations in remote, hardly reached, and isolated communities.
- For example, implementation plans for immunization programs should consider convenient, reachable locations for all populations, including those with challenges accessing traditional healthcare settings such as those experiencing homelessness, those with disabilities, or those without transportation. Plans to book appointments, for recall and reminder systems, and to monitor safety and effectiveness should consider how to reach individuals without phones or access to technology.
- Efforts should be made to improve knowledge about the benefits of vaccines in general and of COVID-19 vaccines specifically, address misinformation, and communicate transparently about COVID-19 vaccine allocation decisions.
- For example, engaging social influencers, Elders and leaders of cultural and faith-based groups with community-driven efforts for coordinated public health approaches and immunization program planning, and providing culturally sensitive educational materials in appropriate languages, literacy levels and media channels could combat misinformation and mistrust about vaccination in general, and COVID-19 vaccines specifically.
Key populations prioritized for COVID-19 vaccination
Based on the above principles for sequencing and prioritization of key populations, and the foundational elements guiding ethical decision-making, NACI recommends that the following key populations in Table 1, for whom authorized COVID-19 vaccines are recommended, be prioritized for COVID-19 immunization. Additional details on key populations, as well as the evidence and rationale for the prioritization of these key populations, are summarized in Table 2.
Table 1: Key populationsFootnote a prioritized for COVID-19 immunization in the context of staggered vaccine supply
| Stage 1 | Stage 2Footnote b | Stage 3Footnote b |
|---|---|---|
|
|
|
Footnotes
|
||
In situations where multiple authorized COVID-19 vaccines are available in Canada, it would be reasonable that a vaccine not authorized for use in a prioritized key population, or not expected to offer optimal protection in these populations, be made available to individuals in populations outside of the priority sequence who provide informed consent. Offering a less efficacious vaccine to key populations prioritized for early immunization may achieve some direct protection in the short-term if they could get access to the vaccine more quickly, but the long-term ramifications of less protection could perpetuate the unfair distribution of benefits and burdens associated with COVID-19 in these groups who are at increased risk of severe disease or exposure. When deciding which vaccine to offer to key populations, it is important to examine the potential for exacerbating inequities in populations who experience intersecting risk factors for severe disease (e.g., poverty, homelessness, underlying medical conditions) and exposure (e.g., multigenerational housing, over-representation in jobs providing essential services such as food and healthcare) such as racialized and marginalized populations who have been disproportionately affected by COVID-19, and that experience systemic barriers to accessing necessary supportive care for COVID-19. The benefits of earlier vaccination should outweigh the risks of vaccinating with a less efficacious vaccine. This will depend on an assessment of the local COVID-19 epidemic conditions and vaccine supply, as well as the risk of severe disease and exposure in a population. Please see NACI’s recommendations on the use of COVID-19 vaccines for additional guidance.
Table 2 provides additional details and examples of key populations, as well as a summary of the evidence and rationale for the recommendations on the prioritization sequence.
Table 2: Summary of evidence and rationale for the recommendations on the sequencing and sub-prioritization of key populations in three stagesFootnote a of increasing COVID-19 vaccine supply
| Recommended key population for early COVID-19 immunization | Summary of evidence and rationale for the recommendation |
|---|---|
Stage 1 |
|
Residents and staff of congregate living settings that provide care for seniors |
|
Adults >70 years of age, beginning with those >80 years, then decreasing age limit by 5 year increments |
|
FrontlineFootnote b healthcare workers
|
|
Adults in Indigenous communities (includes First Nations, Métis, and Inuit communities such as those living in remote or isolated areas where access to health care may be limited) |
|
Stage 2 |
|
1. Adults in or from Indigenous communities not offered vaccine in Stage 1 |
|
2. Residents and staff of other congregate living settings For example:
|
|
3. Adults 60-69 year of age, beginning with ≥65 years, then decreasing age limit to 60 years |
|
4. Adults in racialized and marginalized communities disproportionately affected by COVID-19 |
|
5. First responders For example:
|
|
6. FrontlineFootnote b essential workers who cannot work virtually For example:
|
|
Essential primary caregiverFootnote c for individuals who are at high risk of severe illness from COVID-19 due to advanced age |
|
Stage 3 |
|
Individuals 16-59 years of age with an underlying medical condition at high risk of severe illness due to COVID-19 and their essential primary caregiversFootnote c |
|
Adults 50-59 years of age without an underlying medical condition, beginning with ≥55 years, then decreasing age limit to 50 years |
|
Non-frontlineFootnote b healthcare workers needed to maintain healthcare capacity |
|
Non-frontlineFootnote b essential workers |
|
Footnotes
|
|
Acknowledgments
This statement was prepared by: Dr. S.J. Ismail, Dr. C. Quach, Dr. S. Deeks, Dr. M.C. Tunis, Ms. K. Farrah, Ms. K. Young, and Dr. B. Warshawsky
NACI gratefully acknowledges the contribution of: Ms. E. Tice, Ms. E. Wong, Ms. A. Gil, Dr. M. Salvadori, Ms. C. Sanmartin, Ms. J. Mader, Ms. E. Massicotte, Ms. M. Lacroix, Ms. V. Ferrante, the Public Health Ethics Consultative Group (PHECG), Dr. S. MacDonald, Ms. J. Vachon, Ms. J. Macri, the research team at the Alberta Research Centre for Health Evidence (ARCHE), including Ms. J. Pillay, Ms. A. Wingert, and Dr. L. Hartling, and the NACI Secretariat.
NACI
Members: Dr. C. Quach (Chair), Dr. S. Deeks (Vice-Chair), Dr. J. Bettinger, Dr. N. Dayneka, Dr. P. De Wals, Dr. E. Dubé, Dr. V. Dubey, Dr. S. Gantt, Dr. R. Harrison, Dr. K. Hildebrand, Dr. K. Klein, Dr. J. Papenburg, Dr. C. Rotstein, Dr. B. Sander, Ms. S. Smith, and Dr. S. Wilson.
Liaison representatives: Dr. L.M. Bucci (Canadian Public Health Association), Dr. E. Castillo (Society of Obstetricians and Gynaecologists of Canada), Dr. A. Cohn (Centers for Disease Control and Prevention, United States), Ms. L. Dupuis (Canadian Nurses Association), Dr. J. Emili (College of Family Physicians of Canada), Dr. D. Fell (Canadian Association for Immunization Research and Evaluation), Dr. M. Lavoie (Council of Chief Medical Officers of Health), Dr. D. Moore (Canadian Paediatric Society), Dr. M. Naus (Canadian Immunization Committee), and Dr. A. Pham-Huy (Association of Medical Microbiology and Infectious Disease Canada).
Ex-officio representatives: Dr. D. Danoff (Marketed Health Products Directorate, HC), Ms. E. Henry (Centre for Immunization and Respiratory Infectious Diseases [CIRID], PHAC), Ms. M. Lacroix (Public Health Ethics Consultative Group, PHAC), Ms. J. Pennock (CIRID, PHAC), Dr. R. Pless (Biologic and Radiopharmaceutical Drugs Directorate, Health Canada), Dr. G. Poliquin (National Microbiology Laboratory, PHAC), Dr. V. Beswick-Escanlar (National Defence and the Canadian Armed Forces), and Dr. T. Wong (First Nations and Inuit Health Branch, Indigenous Services Canada).
NACI High Consequence Infectious Disease Working Group
Members: Dr. C. Quach (Chair), Dr. S. Deeks (Vice-Chair), Dr. Y-G. Bui, Dr. K. Dooling, Dr. R. Harrison, Dr. K. Hildebrand, Dr. M. Murti, Dr. J. Papenburg, Dr. R. Pless, Dr. N. Stall, and Dr. S. Vaughan, Dr. M. Miller, Dr. S. Ramanathan.
PHAC Participants: Dr. N. Abraham, Dr. O. Baclic, Ms. Y-E. Chung, Ms. L. Coward, Ms. P. Doyon-Plourde, Ms. K. Farrah, Ms. V. Ferrante, Dr. N. Forbes, Dr. S.J. Ismail, Ms. C. Jensen, Dr. A. Killikelly, Dr. R. Krishnan, Dr. A. Nam, Mr. M. Patel, Dr. M. Salvadori, Ms. A. Sinilaite, Dr. R. Stirling, Ms. E. Tice, Dr. M.C. Tunis, Ms. E. Wong, Ms. MW. Yeung, Ms. K. Young, Dr. J. Zafack, and Dr. L. Zhao.
Appendix A: Factors contributing to severe COVID-19 – Preliminary results of an updated rapid review of risk factors
The Alberta Research Centre for Health Evidence is conducting an update of their rapid reviewNote de bas de page 3 examining the magnitude of association between factors that may contribute to health inequity (summarized by the acronym "P2ROGRESS And Other Factors" in NACI's Equity MatrixNote de bas de page 15) and severe outcomes of COVID-19. The full methodological details for the updated review are available in the registered protocol: PROSPERO (CRD42021230185). The original rapid review, published as a pre-print in September 2020,Note de bas de page 3 was limited to studies published to June 15, 2020, conducted in Organisation for Economic Co-operation and Development (OECD) countries, and adjusting their analysis for age and sex at a minimum. The review found that there was low or moderate certainty evidence for associations of a large magnitude (≥2-fold) with increased hospitalization in people having confirmed COVID-19 for the following risk factors: obesity class III (body mass index [BMI] ≥40 kg/m2), heart failure, diabetes, chronic kidney disease, dementia, age (particularly >70 vs. ≥45 years), male sex, Black race/ethnicity (vs. non-Hispanic white), homelessness, and low income (<25th vs. >50th percentile). It also found moderate certainty evidence that age over 70 versus 45 or younger may be associated with large increases in mortality. Studies treating age on a continuum or across small increments consistently found that risks for hospitalization and mortality increased with increasing age (e.g., approximately 2-6% and 5-10% relative increase in risk per year). These findings informed and are summarized in NACI’s previous guidance. The review update is ongoing, but has completed results for the following risk factors: age (60 to 69 years vs. <60 years); pre-existing conditions (1 or ≥2 vs. no pre-existing conditions); social factors (Canadian data); and occupational exposure (e.g., essential workers). This review update is informing NACI’s current guidance.
A research librarian conducted the literature search update. MEDLINE and Epistemonikos COVID-19 in L.OVE Platform were searched on December 2, 2020. The database search was supplemented by handsearching Canadian websites on January 6-7, 2021, including: Statistics Canada, Public Health Agency of Canada, Public Health Ontario ICES, Government of Canada’s First Nations and Inuit Health Branch. Studies were eligible if they were Canadian epidemiologic reports or prospective and retrospective cohort studies (from OECD countries) published in English or French since 1 January 2020, including preprints accepted for publication in a peer-reviewed journal, or government reports. Only higher quality studies were included, i.e., those that had a total sample size of >1000 participants to ensure sufficient adjustment for multiple variables. For social risk factors, only Canadian reports were included because these were considered by NACI as most relevant. Populations included any of the following: a general/community sample, people with confirmed COVID-19, people hospitalized with COVID-19, or people with severe COVID-19 (e.g., in ICU or mechanically ventilated). Outcomes of interest included hospitalization and length of stay, ICU admission and length of stay, mechanical ventilation, severe disease (as defined by study authors), and mortality.
No meta-analysis was conducted due to large heterogeneity in comparisons and measures of association. For each risk factor and outcome, findings were assessed across studies in terms of the estimated magnitude of association (i.e., "little to no difference" [e.g., odds ratio (OR) <2.0], “large” [OR 2.0 to 3.9], “very large” [OR ≥4.0]), and the review team's certainty in the magnitude of association based on the number, size and consistency between studies, the precision of the estimates, and the relevance of the setting and risk factors (e.g., type of healthcare system, uncertainty about risk factor clearly matching review criteria). Varying certainty in the associations is referred to using the terms "very uncertain" (very low certainty), "may be associated" (low certainty), "probably associated" (moderate certainty), and “is associated” (high certainty).
There were 43 studies included that reported data relevant to those aged 60-69 compared to those under 60 years; 22 studies that reported on pre-existing conditions (as a categorical outcome or on a comorbidity index); 4 studies on occupational exposure; and 9 Canadian reports relevant to social and other risk factors (none meeting the review criteria for multivariable adjustment). Risk factors found to have large or very large associations with any outcome in populations with COVID-19 are presented in Table 3.
There was low or moderate certainty evidence for a large association with increased hospitalization, mechanical ventilation, severe disease, and mortality in persons with COVID-19 aged 60-69 years compared to those under 60 years. For those hospitalized with COVID, there was high certainty in a large association with increased mortality for those 60-69 versus under 60 years. In persons with COVID-19 who have two or more pre-existing conditions, there was low or moderate certainty of evidence for a large association with increased hospitalization, ICU admission, mechanical ventilation, and mortality compared to those without pre-existing conditions. Findings were similar for associations with severe disease and mortality in those hospitalized with COVID-19. There was evidence for little to no association between having a single pre-existing condition (versus none) and hospitalization (moderate certainty) or mortality (low certainty) among patients with COVID-19; findings were similar for those hospitalized with COVID-19 in terms of severe disease and mortality. This rapid review will be updated with evolving evidence on the association between pre-existing medical conditions and severe outcomes from COVID-19. NACI will continue to monitor the evidence.
For healthcare workers with COVID-19, there may be association with a large reduction in hospitalization and ICU admission (low certainty) compared to the general population or non-healthcare workers. However, the relationship between patient facing versus non-patient facing healthcare workers and these outcomes is very uncertain. Results from two Canadian studies without adjusted analysis were similar to findings from other studies suggesting a reduction in hospitalization and ICU admission, and suggested a reduction in mortality. This finding could be related to increased testing among asymptomatic healthcare workers, or increased access to, and training in the use of personal protective equipment.
Table 3: Risk factors identified by an updated rapid review that have large (+) or very large (++) associations with severe COVID-19 outcomes and the corresponding level of certainty in the association (population: people with confirmed COVID-19)
| Risk factor | Outcome of interest | Magnitude of riskFootnote a (certainty in associationFootnote b) |
|---|---|---|
Age |
||
60-69 vs. <60 years |
Hospitalization |
+ (moderate) |
Mechanical ventilation |
+ (low) |
|
Severe disease |
+ /++ (low) |
|
Mortality |
+/++ (moderate/low) |
|
Pre-existing conditions |
||
≥2 vs. no pre-existing conditions |
Hospitalization |
+ (moderate) |
ICU admission |
+ (low) |
|
Mechanical ventilation |
+ (low) |
|
Mortality |
+ (moderate) |
|
Occupational exposure |
||
Healthcare workers (vs. non-healthcare workers) |
Hospitalization |
+ reduction (low) |
ICU admission |
+ reduction (low) |
|
Footnotes
|
||
Table 4 summarizes findings from Canadian data on social risk factors. Among people with COVID-19, there is probably a large association between living in long-term care and increased mortality, and the association may be very large for those ages 60-80 years. Living on a First Nations reserve may be associated with lower rates of hospitalization and mortality compared to those living off-reserve among people with COVID-19, although the evidence did not account for other covariates, such as age. Among the general population, there was uncertain evidence that being a member of a visible minority population may be associated with increased mortality. Evidence on homeless persons and homeless shelter workers was uncertain and no conclusions could be drawn about the magnitude of the associations.
Table 4: Risk factors identified by an updated rapid review of Canadian data that have may have associations with severe COVID-19 outcomes and the corresponding magnitude of association
| Equity risk factor | Association | Magnitude of association |
|---|---|---|
Long-term care vs. not (among people with COVID-19) |
Increase in mortality |
Large, highest for those in their 60s and 70s. |
General population (off-reserve) vs. First Nations on-reserve (among people with COVID-19) |
Increase in hospitalization |
Uncertain. Evidence did not account for other important covariates. |
Increase in mortality |
||
Race/ethnicity (general population) |
Increase in mortality |
Uncertain. Evidence relied on ecological data and did not account for other important covariates. |
Generalization of findings from other countries to Canada should be made with caution, as high-risk groups may differ by population. Furthermore, because of differences in methodology, the list of important risk factors identified in this rapid review may differ from other sources. Updated evidence syntheses on other risk factors, including various pre-existing medical conditions, will inform future NACI decisions.
References
Footnotes
- Footnote 1
-
Ismail SJ, Zhao L, Tunis MC, et al. Key populations for early COVID-19 immunization: preliminary guidance for policy. CMAJ. 2020;192:E1620-32. doi:10.1503/cmaj.202353.
- Footnote 2
-
Ismail SJ, Hardy K, Tunis MC, et al. A framework for the systematic consideration of ethics, equity, feasibility, and acceptability in vaccine program recommendations. Vaccine. 2020;38:5861-76. doi:10.1016/j.vaccine.2020.05.051.
- Footnote 3
-
Wingert A, Pillay J, Gates M, et al. Risk factors for severe outcomes of COVID-19: a rapid review. medRxiv. 2020 doi:10.1101/2020.08.27.201834344.
- Footnote 4
-
Zhao L, Ismail SJ, Tunis MC. Ranking the relative importance of immunization strategies for novel coronavirus disease 2019 (COVID-19): a rapid survey of stakeholders. medRxiv. 2020 doi:10.1101/2020.09.16.20196295.
- Footnote 5
-
Public Health Agency of Canada. Interim guidance on continuity of immunization programs during the COVID-19 pandemic [Internet]. 2020 May 13 [cited 2021 February 1]. Available from: https://www.canada.ca/en/public-health/services/immunization/national-advisory-committee-on-immunization-naci/interim-guidance-immunization-programs-during-covid-19-pandemic.html
- Footnote 6
-
Impact and Innovation Unit. COVID-19 Snapshot Monitoring (COSMO Canada) [Internet]. (2020, July 27; wave 6). [cited 2021 February 1]. Available from: https://impact.canada.ca/en/challenges/cosmo-canada/wave6
- Footnote 7
-
Impact and Innovation Unit. COVID-19 Snapshot Monitoring (COSMO Canada) [Internet]. (2020, August 17; wave 7). [cited 2021 February 1]. Available from: https://impact.canada.ca/en/challenges/cosmo-canada/wave7
- Footnote 8
-
Angus Reid Institute. COVID-19: Three-in-five worry about side-effects of a vaccine; many plan to take a ‘wait and see’ approach [Internet]. 2020 August 4 [cited February 1]. Available from: http://angusreid.org/coronavirus-vaccine/
- Footnote 9
-
Impact and Innovation Unit. COVID-19 Snapshot Monitoring (COSMO Canada) [Internet]. 2021 [cited 2021 February 1]. Available from: https://impact.canada.ca/en/challenges/cosmo-canada
- Footnote 10
-
EKOS Research Associates. Wave 11 Report. 2021 January 15.
- Footnote 11
-
MacDonald SE, Gagneur A, COVImm study team. COVID-19 vaccination perceptions and intentions among groups of special interest: A pan-Canadian survey, December 2020. Unpublished report from the Applied immunization (Aimm) research program (available upon request from aimm@ualberta.ca). 2021.
- Footnote 12
-
Gates A, Gates M, Rahman S, et al. A systematic review of factors that influence the acceptability of vaccines among Canadians. Vaccine. 2021;39:222-36. doi:S0264-410X(20)31332-3.
- Footnote 13
-
Corrin T. Evergreen rapid review on COVID-19 vaccine knowledge, attitudes, and behaviors – update 1. Ottawa (ON): Public Health Agency of Canada; 2020 December.
- Footnote 14
-
Ismail SJ, Langley JM, Harris TM, et al. Canada's National Advisory Committee on Immunization (NACI): Evidence-based decision-making on vaccines and immunization. Vaccine. 2010;28:A58-63. doi:10.1016/j.vaccine.2010.02.035.
- Footnote 15
-
Ismail SJ, Tunis MC, Zhao L, et al. Navigating inequities: a roadmap out of the pandemic. BMJ Glob Health. 2021 Jan;6(1):e004087. doi: 10.1136/bmjgh-2020-004087.
- Footnote 16
-
Public Health Agency of Canada. Canada COVID-19 weekly epidemiology report (10 January to 16 January 2021) [Internet]. 2021 January 22 [cited 2021 January 22]. Available from: https://impact.canada.ca/en/challenges/cosmo-canada
- Footnote 17
-
Public Health Agency of Canada. Health Portfolio Operations Centre (HPOC): Case-level data. 2021 January 16.
- Footnote 18
-
Alberta Research Centre for Health Evidence (ARCHE). Risk factors for severe outcomes of COVID-19: an updated rapid review. [Unpublished preliminary report]. Presented to: National Advisory Committee on Immunization Public Health Agency of Canada; 2021 January.
- Footnote 19
-
Statistics Canada. Custom data table (population with at least one underlying health condition), based on Canadian Community Health Survey, 2017-2018. Date received: 2021 January 18.
- Footnote 20
-
Statistics Canada. Custom data table (labour force, essential workers), based on 2016 Census of Population, 25% Data. Date received: 2021 January 19.
- Footnote 21
-
Richardson L, Crawford A. COVID-19 and the decolonization of Indigenous public health. CMAJ 2020;192:E1098-100. doi:10.1503/cmaj.200852.
- Footnote 22
-
Indigenous Services Canada, Communicable Disease Control Division. Reported COVID-19 cases. 2012 January 20.
- Footnote 23
-
Public Health Agency of Canada. Outbreak database. 2021 January 22.
- Footnote 24
-
Richard L, Booth R, Rayner J, et al. Testing, infection and complication rates of COVID-19 among people with a recent history of homelessness in Ontario, Canada: a retrospective cohort study. CMAJ Open. 2021;9:E1-9. doi:10.9778/cmajo.20200287.
- Footnote 25
-
Canadian Observatory on Homelessness. Racialized communities [Internet]. 2019 [cited 2021 January 26]. Available from: https://www.homelesshub.ca/about-homelessness/population-specific/racialized-communities.
- Footnote 26
-
Statistics Canada. Table 10: Admissions of youth to correctional services, by characteristics of the person admitted and type of supervision program, selected jurisdictions, 2017/2018 [Internet]. 2019 May 9 [cited 2021 January 26]. Available at: https://www150.statcan.gc.ca/n1/pub/85-002-x/2019001/article/00010/tbl/tbl10-eng.htm.
- Footnote 27
-
Frank K, Arim R. COVID-19: Data to Insights for a Better Canada. Canadians’ willingness to get a COVID-19 vaccine: Group differences and reasons for vaccine hesitancy [Internet]. Ottawa (ON): Statistics Canada; 2020 August 25 [cited 2021 January 26]. Available from: https://www150.statcan.gc.ca/n1/pub/45-28-0001/2020001/article/00073-eng.htm
- Footnote 28
-
Public Health Ontario. COVID-19 in Ontario – A focus on diversity [Internet]. 2021 May 14 [cited 2012 February 1]. Available from: https://www.publichealthontario.ca/-/media/documents/ncov/epi/2020/06/covid-19-epi-diversity.pdf?la=en
- Footnote 29
-
Subedi R, Greenberg L, Turcotte M. COVID-19 mortality rates in Canada’s ethno-cultural neighbourhoods [Internet]. Ottawa (ON): Statistics Canada; 2020 October 28 [cited 2021 February 1]. Available from: https://www150.statcan.gc.ca/n1/pub/45-28-0001/2020001/article/00079-eng.htm
- Footnote 30
-
Ottawa Public Health. COVID-19 and racial identity in Ottawa. February to August 2020 [Internet]. 2020 November [cited 2021 February 1]. Available from: https://www.ottawapublichealth.ca/en/reports-research-and-statistics/resources/Documents/covid-19/Special-Focus/Report---COVID-19-and-Racial-Identity-in-Ottawa-2020.pdf
- Footnote 31
-
Chung H, Fung K, Ferreira-Legere LE, et al. COVID-19 Laboratory testing in Ontario: Patterns of testing and characteristics of individuals tested, as of April 30, 2020 [Internet]. Toronto (ON): ICES; 2020 May [cited 2021 February 1]. Available from: https://www.ices.on.ca/Publications/Atlases-and-Reports/2020/COVID-19-Laboratory-Testing-in-Ontario
- Footnote 32
-
Toronto Public Health. COVID-19: Status of cases in Toronto [Internet]. Toronto (ON): City of Toronto; 2020 September 30 [cited 2020 September 30]. Available from: https://www.toronto.ca/home/covid-19/covid-19-latest-city-of-toronto-news/covid-19-status-of-cases-in-toronto/
- Footnote 33
-
Canadian Institute for Public Safety Research and Treatment (CIPSRT). Glossary of terms: First responder(s) [Internet]. 2020 [cited 2021 February 1]. Available from: https://www.cipsrt-icrtsp.ca/en/glossary/first-responders.
- Footnote 34
-
Government of Canada. Mandatory isolation or quarantine. Who is exempt from quarantine [Internet]. 2021 February 1 [cited 2021 February 1]. Available from: https://travel.gc.ca/travel-covid/travel-restrictions/isolation#exemptions.
- Footnote 35
-
Government of Canada. People who are at risk of more severe disease or outcomes from COVID-19 [Internet]. 2020 December 8 [cited 2021 February 1]. Available from: https://www.canada.ca/en/public-health/services/publications/diseases-conditions/people-high-risk-for-severe-illness-covid-19.html.