FluWatch report: December 21, 2014 to January 3, 2015 (Weeks 52–53)
Overall summary
- The percent positive for laboratory detections of influenza increased in week 52 but remained stable in week 53; perhaps indicating that we are nearing the peak in laboratory detections for the season. The majority of laboratory detections continued to be reported in AB, ON and QC; but with increasing activity in BC and MB.
- A(H3N2) continues to be the most common type of influenza affecting Canadians. In both laboratory detections, hospitalizations and deaths, the majority of cases have been among seniors ≥65 years of age.
- There were a large number of newly-reported laboratory-confirmed outbreaks of influenza over the two-week period (n=309). In week 53, there were 166 influenza outbreaks in 8 provinces, of which 122 were in long-term care facilities (LTCF).
- To date, the NML has found that the majority of A(H3N2) influenza specimens are not optimally matched to the vaccine strain. This may result in reduced vaccine effectiveness against the A(H3N2) virus. However, the vaccine can still provide some protection against A(H3N2) influenza illness and can offer protection against other influenza strains such as A(H1N1) and B.
Are you a primary health care practitioner (General Practitioner, Nurse Practitioner or Registered Nurse) interested in becoming a FluWatch sentinel for the 2015-16 influenza season? Contact us at FluWatch@phac-aspc.gc.ca
On this page
- Influenza/ILI Activity (geographic spread)
- Influenza and Other Respiratory Virus Detections
- Antiviral Resistance
- Influenza Strain Characterizations
- Influenza-like Illness (ILI) Consultation Rate
- Influenza Outbreak Surveillance
- Pharmacy surveillance
- Sentinel Hospital Influenza Surveillance
- Provincial/Territorial Influenza Hospitalizations and Deaths
- Emerging Respiratory Pathogens
- International Influenza Reports

Download the alternative format
(PDF format, 307 KB, 10 pages)
Related Topics
Influenza/ILI Activity (geographic spread)
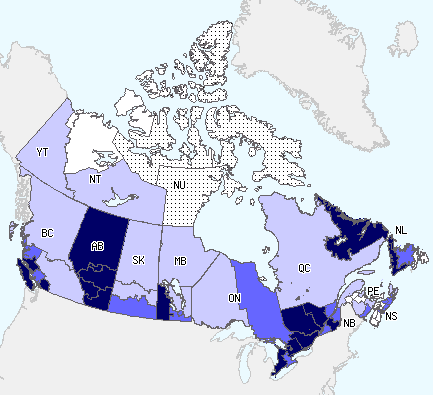
In week 52, 12 regions reported widespread activity and increased to 16 regions in week 53: in BC(2), AB(5), MB(1), ON(4), QC(2) and NL(2). The same five regions in Alberta reported widespread activity for both weeks. In week 53, 14 regions reported localized activity (down from 18, the previous week): BC, SK, MB(2), ON(2), QC(3), NB, NS(3) and NL. Fourteen regions reported sporadic activity in week 53 (up from 11 the previous week): BC(2), SK(2), MB(2), ON, QC, NB(3), NS, NT, and YK. No data was reported in NU for weeks 52 and 53 (Figure 1).
Figure 1. Map of overall influenza/ILI activity level by province and territory, Canada, Week 53
Note: Influenza/ILI activity levels, as represented on this map, are assigned and reported by Provincial and Territorial Ministries of Health, based on laboratory confirmations, sentinel ILI rates and reported outbreaks. Please refer to detailed definitions at the end of the report. Maps from previous weeks, including any retrospective updates, are available on the Flu Activity website.
Figure 1 Map of overall influenza/ILI activity level by province and territory, Canada, Week 30 - Text Description
Influenza and Other Respiratory Virus Detections
The number of positive tests increased during weeks 52 and 53. In week 53, the number of positive influenza tests increased to 5,550 influenza detections from 3,723 in week 52. The percent positive for influenza A detections rose to 34.6% in week 52 and stayed relatively stable in week 53 at 34.2% (Figure 2). To date, 98% of influenza detections have been influenza A, and 99.8% of those subtyped have been A(H3) (Table 1) The timing of the season and predominant A(H3N2) subtype is similar to the pattern observed during the 2012-13 influenza season when percent positive for influenza peaked in week 52 (35%). To date, among the cases of influenza with reported age, the largest proportion was in adults ≥65 years of age (62%) (Table 2).
Figure 2. Number of positive influenza tests and percentage of tests positive, by type, subtype and report week, Canada, 2014-15
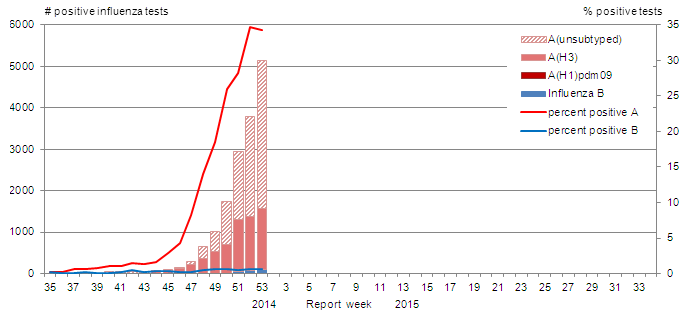
Figure 2 Number of positive influenza tests and percentage of tests positive, by type, subtype and report week, Canada, 2014-15 - Text Description
The number of positive tests increased during weeks 52 and 53. In week 53, the number of positive influenza tests increased to 5,550 influenza detections from 3,723 in week 52.
In week 53, the number of positive RSV tests increased to 807 RSV detections up from 764 RSV detections in week 52. RSV remains the second most frequently detected virus after influenza. Detections of RSV since week 38 have been higher than in the previous season while detections of parainfluenza and adenovirus continue to follow their seasonal patterns of broad winter circulation (figure 3).
For more details, see the weekly Respiratory Virus Detections in Canada Report.
Figure 3. Number of positive laboratory tests for other respiratory viruses by report week, Canada, 2014-15
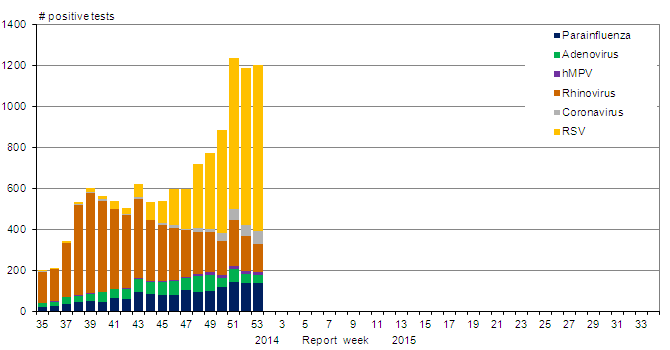
RSV: Respiratory syncytial virus; hMPV: Human metapneumovirus
Figure 3 Number of positive laboratory tests for other respiratory viruses by report week, Canada, 2014-15 - Text Description
| Reporting provincesFootnote 1 | Weekly (December 28, 2014 to January 3, 2015) | Cumulative (August 24 to January 3, 2015) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Influenza A | B | Influenza A | B | |||||||
| A Total | A(H1)pdm09 | A(H3) | A Footnote (Uns) | B Total | A Total | A(H1)pdm09 | A(H3) | A(UnS) | B Total | |
| BC | 118 | 0 | 84 | 34 | 3 | 380 | 2 | 302 | 76 | 16 |
| AB | 556 | 0 | 367 | 189 | 16 | 2006 | 0 | 1774 | 232 | 68 |
| SK | 124 | 0 | 95 | 29 | 0 | 247 | 0 | 142 | 105 | 3 |
| MB | 44 | 0 | 42 | 2 | 2 | 80 | 0 | 70 | 10 | 4 |
| ON | 591 | 0 | 375 | 216 | 4 | 1232 | 4 | 862 | 366 | 29 |
| QC | 1238 | 0 | 0 | 1238 | 30 | 2995 | 0 | 0 | 2995 | 101 |
| NB | 4 | 0 | 1 | 3 | 0 | 10 | 0 | 4 | 6 | 0 |
| NS | 12 | 0 | 10 | 2 | 0 | 23 | 0 | 17 | 6 | 3 |
| PE | 1 | 0 | 1 | 0 | 0 | 7 | 0 | 5 | 2 | 1 |
| NL | 52 | 0 | 0 | 52 | 0 | 73 | 0 | 16 | 57 | 1 |
| Canada | 2740 | 0 | 975 | 1765 | 55 | 7053 | 6 | 3192 | 3855 | 226 |
| Percentage Footnote 2 | 98.0% | 0.0% | 35.6% | 64.4% | 2.0% | 96.9% | 0.1% | 45.3% | 54.7% | 3.1% |
| Age groups (years) | Weekly (December 28, 2014 to to January 3, 2015) |
Cumulative (August 24, 2014 to January 3, 2015) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Influenza A | B | Influenza A | B | Influenza A and B | ||||||||
| A Total | A(H1) pdm09 | A(H3) | A Footnote (Uns) | Total | A Total | A(H1) pdm09 | A(H3) | A (UnS) | Total | # | % | |
| <5 | 169 | 0 | 33 | 136 | 3 | 976 | 4 | 468 | 504 | 42 | 1018 | 6.9% |
| 5-19 | 72 | 0 | 23 | 49 | 5 | 954 | 0 | 568 | 386 | 51 | 1005 | 6.8% |
| 20-44 | 278 | 0 | 57 | 221 | 13 | 1727 | 0 | 834 | 893 | 55 | 1782 | 12.1% |
| 45-64 | 379 | 1 | 77 | 301 | 15 | 1722 | 1 | 698 | 1023 | 68 | 1790 | 12.2% |
| 65+ | 2115 | 2 | 430 | 1683 | 48 | 8916 | 4 | 3034 | 5878 | 141 | 9057 | 61.7% |
| Unknown | 2 | 0 | 0 | 2 | 0 | 26 | 0 | 15 | 11 | 0 | 26 | 0.2% |
| Total | 3015 | 3 | 620 | 2392 | 84 | 14321 | 9 | 5617 | 8695 | 357 | 14678 | 100.0% |
| PercentageFootnote 2 | 97.3% | 0.1% | 20.6% | 79.3% | 2.7% | 97.6% | 0.1% | 39.2% | 60.7% | 2.4% | ||
Antiviral Resistance
During the 2014-2015 influenza season, NML has tested 198 influenza viruses for resistance to oseltamivir and 196 influenza viruses for resistance to zanamivir and all were sensitive to both agents. A total of 298 (99.7%) of influenza A viruses tested for amantadine resistance were resistant (Table 3).
| Virus type and subtype | Oseltamivir | Zanamivir | Amantadine | |||
|---|---|---|---|---|---|---|
| # tested | # resistant (%) | # tested | # resistant (%) | # tested | # resistant (%) | |
| A (H3N2) | 175 | 0 | 173 | 0 | 297 | 296 (997%) |
| A (H1N1) | 2 | 0 | 2 | 0 | 2 | 2 (100%) |
| B | 21 | 0 | 21 | 0 | NATable 3 - Footnote * | NA Table 3 - Footnote * |
| TOTAL | 198 | 0 | 196 | 0 | 299 | 298 |
Influenza Strain Characterizations
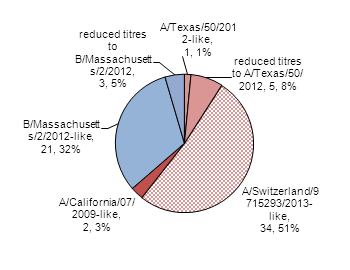
During the 2014-2015 influenza season, the National Microbiology Laboratory (NML) has characterized 66 influenza viruses [40 A(H3N2), 2 A(H1N1) and 24 influenza B]. The majority of circulating influenza B and A(H1N1) viruses have been antigenically similar (good match) to the recommended strains for the 2014-15 seasonal influenza vaccine, while the majority of A(H3N2) viruses have shown evidence of an antigenic drift (sub-optimal match) from the vaccine strain
Influenza A (H3N2): When tested by hemagglutination inhibition (HI) assay (n=40), one virus was antigenically similar to A/Texas/50/2012, five showed reduced titers to A/Texas/50/2012 and 34 were antigenically similar to A/Switzerland/9715293/2013, which is the influenza A(H3N2) component recommended for the 2015 Southern Hemisphere influenza vaccine. Additionally, 120 A(H3N2) viruses were unable to be tested by HI assay; however, sequence analysis showed that 119 belonged to a genetic group that typically shows reduced titers to A/Texas/50/2012.
Influenza A(H1N1): Two A(H1N1) viruses characterized were antigenically similar to A/California/7/2009.
Influenza B: Of the 24 influenza B viruses characterized, 21 viruses were antigenically similar to B/Massachusetts/2/2012, and three viruses showed reduced titers (Figure 4).
Figure 4. Influenza strain characterizations, Canada, 2014-2015, N = 66
The NML receives a proportion of the number of influenza positive specimens from provincial laboratories for strain characterization and antiviral resistance testing. Characterization data reflect the results of haemagglutination inhibition (HAI) testing compared to the reference influenza strains recommended by WHO.
The recommended components for the 2014-2015 northern hemisphere trivalent influenza vaccine include: an A/California/7/2009(H1N1)pdm09-like virus, an A/Texas/50/2012 (H3N2)-like virus, and a B/Massachusetts/2/2012-like virus (Yamagata lineage). For quadrivalent vaccines, the addition of a B/Brisbane/60/2008-like virus is recommended.
Figure 4 - Text Description
| Strain | Number of specimens | Percentage |
|---|---|---|
| A/Texas/50/2012-like | 1 | 1% |
| reduced titres to A/Texas/50/2012 | 5 | 8% |
| A/California/07/2009-like | 2 | 3% |
| A/Switzerland/97 15293/2013-like | 34 | 51% |
| B/Massachusetts/2/2012-like | 21 | 32% |
| reduced titres to B/Massachusetts/2/2012 | 3 | 5% |
| B/Brisbane/60/2008-like | 0 | 0% |
Influenza-like Illness (ILI) Consultation Rate
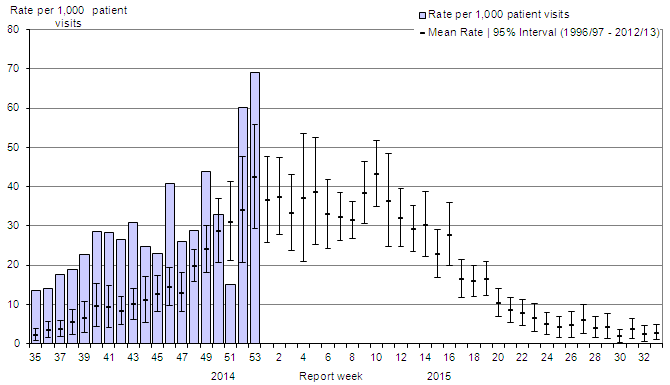
The national influenza-like-illness (ILI) consultation increased in week 52 and 53 to 69.1 consultations per 1,000, which is above expected levels for week 53 (Figure 5). In week 52, the rates were highest among the 20 to 64 years of age group (76.0 consultations per 1,000) and in week 53, the rates were highest among the adults ≥65 years of age (216.3 consultations per 1,000).
Figure 5. Influenza-like-illness (ILI) consultation rates by report week, compared to the 1996-97 through to 2012-13 seasons (with pandemic data suppressed), Canada, 2014-2015
No data available for mean rate for weeks 19 to 39 for the 1996-1997 through 2002-2003 seasons. Delays in the reporting of data may cause data to change retrospectively. The calculation of the average ILI consultation rate over 17 seasons was aligned with influenza activity in each season. In BC, AB, and SK, data is compiled by a provincial sentinel surveillance program for reporting to FluWatch. Not all sentinel physicians report every week.
Figure 5 - Text Description
The national influenza-like-illness (ILI) consultation increased in week 52 and 53 to 69.1 consultations per 1,000, which is above expected levels for week 53.
Influenza Outbreak Surveillance
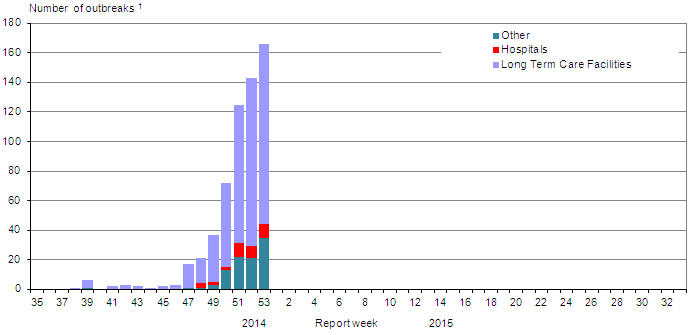
The number of outbreaks increased during weeks 52 and 53. In week 53, 166 new outbreaks of influenza were reported (up from 143 outbreaks in week 52): 122 in long-term care facilities (LTCF), nine in hospitals and 35 in institutional or community settings. Among the outbreaks in which the influenza subtype was known, three LTCF outbreaks and one institutional or community setting outbreak were associated with A(H3N2). To date this season, 471 outbreaks in LTCFs have been reported. The number of outbreaks reported since week 47 is above those of previous seasons and is similar to the numbers reported during the 2012-13 influenza season when influenza A(H3N2) also predominated (Figure 6).
Figure 6: Overall number of new laboratory-confirmed influenza outbreaks by report week, Canada, 2014-2015
1 All provinces and territories except NU report outbreaks in long-term care facilities. All provinces and territories with the exception of NU and QC report outbreaks in hospitals. Outbreaks of influenza or influenza-like-illness in other facilities are reported to FluWatch but reporting varies between jurisdictions. Outbreak definitions are included at the end of the report.
Figure 6 - Text Description
| Report week | Hospitals | Long Term Care Facilities | Other |
|---|---|---|---|
| 35 | 0 | 0 | 0 |
| 36 | 0 | 0 | 0 |
| 37 | 0 | 0 | 0 |
| 38 | 0 | 1 | 0 |
| 39 | 0 | 5 | 1 |
| 40 | 0 | 0 | 0 |
| 41 | 0 | 2 | 0 |
| 42 | 0 | 3 | 0 |
| 43 | 0 | 2 | 0 |
| 44 | 0 | 1 | 0 |
| 45 | 0 | 2 | 0 |
| 46 | 0 | 3 | 0 |
| 47 | 0 | 16 | 1 |
| 48 | 3 | 17 | 1 |
| 49 | 2 | 32 | 3 |
| 50 | 2 | 57 | 13 |
| 51 | 9 | 94 | 22 |
| 52 | 8 | 94 | 21 |
| 53 | 9 | 122 | 35 |
Pharmacy surveillance
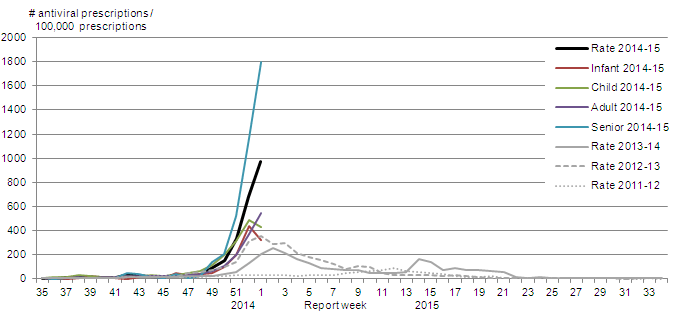
Among the outbreaks in which the influenza subtype was known, three LTCF outbreaks and one institutional or community setting outbreak were associated with A(H3N2). To date this season, 471 outbreaks in LTCFs have been reported. The number of outbreaks reported since week 47 is above those of previous seasons and is similar to the numbers reported during the 2012-13 influenza season when influenza A(H3N2) also predominated (Figure 7). The rate in infants and children decreased from week 52 to week 53 while the rates in adults and seniors increased. The antiviral prescription rate was highest amongst seniors and was 1,798 per 100,000 total prescriptions in week 53.
Figure 7. Proportion of prescription sales for influenza antivirals by age-group and week, Canada, 2014-15
Note: Pharmacy sales data are provided to the Public Health Agency of Canada by Rx Canada Inc. and sourced from major retail drug chains representing over 2,500 stores nationwide (excluding Nunavut) in 85% of Health Regions. Data provided include the number of new antiviral prescriptions (for Tamiflu and Relenza) and the total number of new prescriptions dispensed by Province/Territory and age group. Age-groups: Infant: 0-2y, Child: 2-18y; Adult: 19-64y, Senior: ≥65y
Figure 7 - Text Description
Proportion of antiviral prescriptions per 100,000 total prescriptions in week 53 for the current season compared to previous seasons:
2014-15: 974.9; 2013-14: 202.9; 2012-13: 352.2; 2011-12: 35.0
Proportion of antiviral prescriptions by age-group in week 53 for the 2014-15 season:
Infant: 325.1; child: 430.5; adult: 547.3; senior: 1798.0
Sentinel Hospital Influenza Surveillance
Paediatric Influenza Hospitalizations and Deaths (IMPACT)
The number of new laboratory-confirmed influenza-associated paediatric (≤16 years of age) hospitalizations reported by the Immunization Monitoring Program Active (IMPACT) network increased from 55 in week 51 to 67 in week 52 and decreased to 46 in week 53. Among these 113 cases (reported in weeks 52 and 53), 110 (97%) had influenza A (Figure 8a). Forty-one (36%) were <2 years of age, 59 (52%) were 2 to 9 years of age and 13 (12%) were 10-16 years of age. Twelve cases were admitted to the ICU. To date this season, 308 hospitalizations have been reported by the IMPACT network, 291 (95%) of which were cases of influenza A. Among cases for which the influenza A subtype was reported, 98% (121/123) were A(H3N2). Children <5 years of age represented 60% of cases (Table 4).To date, 30 cases were admitted to the ICU, of which 17 (57%) were 2 to 9 years of age (Figure 9a).
Note: The number of hospitalizations reported through IMPACT represents a subset of all influenza-associated paediatric hospitalizations in Canada. Delays in the reporting of data may cause data to change retrospectively.
Adult Influenza Hospitalizations and Deaths (PCIRN)
The number of new laboratory-confirmed influenza-associated adult (≥16 years of age) hospitalizations reported by the PHAC/CIHR Influenza Research Network (PCIRN) Serious Outcomes Surveillance (SOS) network increased to 139 in week 52 (from 73 in week 51), and decreased to 92 in week 53. Seventy-five cases (82%) were in adults over the age of 65. Ninety cases (98%) had influenza A (Figure 8b). To date this season, 500 cases have been reported; 493 (99%) with influenza A. The majority of cases (83%) were among adults ≥65 years of age (Table 5). Twenty-one ICU admissions have been reported and the majority of cases (81%) were adults ≥65 years of age with underlying conditions or comorbidities. Sixteen deaths have been reported, all adults >65 years of age (Figure 9b).
Note: The number of hospitalizations reported through CIRN represents a subset of all influenza-associated adult hospitalizations in Canada. Delays in the reporting of data may cause data to change retrospectively.
| Age groups | Cumulative (Aug. 24, 2014 to January 3, 2015) | |||||
|---|---|---|---|---|---|---|
| Influenza A | B | Influenza A and B | ||||
| A Total | A(H1) pdm09 | A(H3) | AFootnote (Uns) | Total | # (%) | |
| 0-5m | 33 | 0 | 12 | 21 | 2 | 35 (11.4%) |
| 6-23m | 64 | 1 | 23 | 40 | 5 | 69 (22.4%) |
| 2-4y | 77 | 1 | 34 | 42 | 4 | 81 (26.3%) |
| 5-9y | 75 | 0 | 34 | 41 | 4 | 79 (25.6%) |
| 10-16y | 42 | 0 | 18 | 24 | 2 | 44 (14.3%) |
| Total | 291 | 2 | 121 | 168 | 17 | 308 |
| % Footnote 1 | 94.5% | 0.7% | 41.6% | 57.7% | 5.5% | 100.0% |
| Age groups | Cumulative (December 14, 2014 to December 20, 2015) | |||||
|---|---|---|---|---|---|---|
| Influenza A | B | Influenza A and B | ||||
| A Total | A(H1) pdm09 | A(H3) | AFootnote (Uns) | Total | # (%) | |
| 16-20 | 2 | 0 | 0 | 2 | 0 | 2 (%) |
| 20-44 | 31 | 0 | 10 | 21 | 0 | 31 (6%) |
| 45-64 | 50 | 0 | 12 | 38 | 0 | 50 (10%) |
| 65+ | 410 | 2 | 67 | 341 | 7 | 417 (83%) |
| Total | 493 | 2 | 89 | 402 | 7 | 500 |
| % Footnote 1 | 99% | 0% | 18% | 82% | 1% | 100% |
Figure 8 - Number of cases of influenza reported by sentinel hospital networks, by week, Canada, 2014-15
A) Paediatric hospitalizations (≤16 years of age, IMPACT)
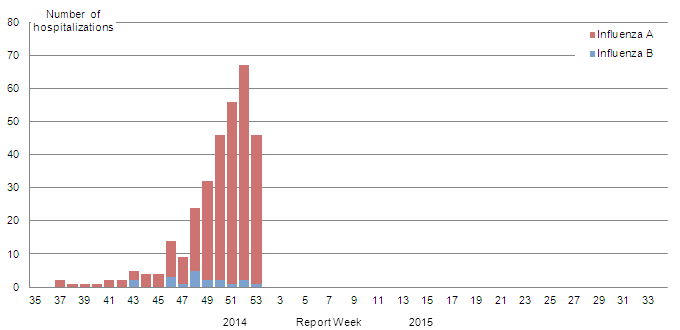
Figure 8A - Text Description
| Report week | Influenza A | Influenza B |
|---|---|---|
| 35 | 0 | 0 |
| 36 | 0 | 0 |
| 37 | 2 | 0 |
| 38 | 1 | 1 |
| 39 | 1 | 0 |
| 40 | 1 | 0 |
| 41 | 2 | 0 |
| 42 | 1 | 0 |
| 43 | 2 | 1 |
| 44 | 4 | 0 |
| 45 | 4 | 0 |
| 46 | 8 | 2 |
| 47 | 8 | 1 |
| 48 | 19 | 5 |
| 49 | 30 | 2 |
| 50 | 37 | 1 |
| 51 | 55 | 1 |
| 52 | 65 | 2 |
| 53 | 45 | 1 |
Figure 8B - Number of cases of influenza reported by sentinel hospital networks, by week, Canada, 2014-15
B) Adult hospitalizations (≥16 year of age, PCIRN-SOS)
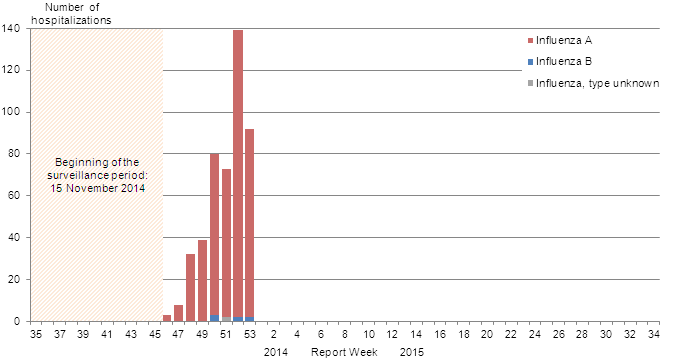
Figure 8B - Text Description
| Semaine de déclaration | Influenza A | Influenza B | Influenza de type inconnu |
|---|---|---|---|
| 35 | n/a | n/a | n/a |
| 36 | n/a | n/a | n/a |
| 37 | n/a | n/a | n/a |
| 38 | n/a | n/a | n/a |
| 39 | n/a | n/a | n/a |
| 40 | n/a | n/a | n/a |
| 41 | n/a | n/a | n/a |
| 42 | n/a | n/a | n/a |
| 43 | n/a | n/a | n/a |
| 44 | n/a | n/a | n/a |
| 45 | n/a | n/a | n/a |
| 46 | 3 | 0 | 0 |
| 47 | 8 | 0 | 0 |
| 48 | 32 | 0 | 0 |
| 49 | 39 | 0 | 0 |
| 50 | 77 | 3 | 0 |
| 51 | 101 | 0 | 1 |
| 52 | 137 | 2 | 1 |
| 53 | 90 | 2 | 0 |
Figure 9 - Percentage of hospitalizations, ICU admissions and deaths with influenza reported by age-group, Canada, 2014-15
A) Paediatric hospitalizations (≤16 years of age, IMPACT)
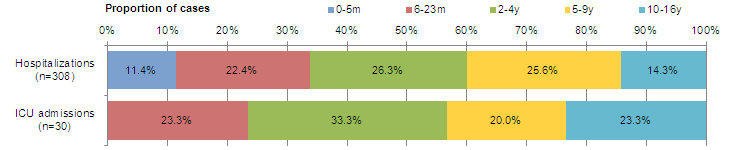
Figure 9A - Text Description
| Age-group (years) | Hospitalizations (n=308) | ICU admissions (n=30) |
|---|---|---|
| 0-5m | 11.4% | 0.0% |
| 6-23m | 22.4% | 23.3% |
| 2-4y | 26.3% | 33.3% |
| 5-9y | 25.6% | 20.0% |
| 10-16y | 14.3% | 23.3% |
Figure 9 - Percentage of hospitalizations, ICU admissions and deaths with influenza reported by age-group, Canada, 2014-15
Adult hospitalizations (≥16 year of age, PCIRN-SOS)
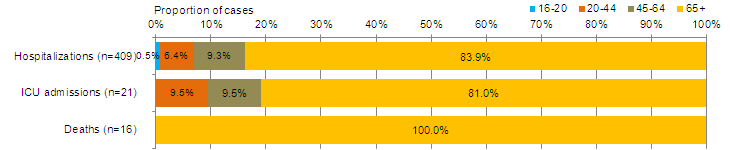
Figure 9B - Text Description
| Age-group (years) | Hospitalizations (n=235) | ICU admissions(n=18) | Deaths (n=8) |
|---|---|---|---|
| 16-20 | 0.5% | 0.0% | 0.0% |
| 20-44 | 6.4% | 9.5% | 0.0% |
| 45-64 | 9.3% | 9.5% | 0.0% |
| 65+ | 83.9% | 81.0% | 100% |
Provincial/Territorial Influenza Hospitalizations and Deaths
| Age groups | Cumulative (24 August, 2014 to 3 January, 2015) | |||||
|---|---|---|---|---|---|---|
| Influenza A | B | Influenza A and B | ||||
| A Total | A(H1) pdm09 | A(H3) | AFootnote (Uns) | Total | # (%) | |
| 0-4 years | 78 | 1 | 52 | 25 | 2 | 80 (10%) |
| 5-19 years | 67 | 0 | 46 | 21 | 2 | 69 (9%) |
| 20-44 years | 54 | 1 | 40 | 13 | 4 | 58 (7%) |
| 45-64 years | 86 | 0 | 69 | 17 | 2 | 88 (11%) |
| 65+ years | 462 | 0 | 314 | 148 | 9 | 471 (60%) |
| Unknown | 15 | 0 | 15 | 0 | 1 | 16 (2%) |
| Total | 762 | 2 | 536 | 224 | 20 | 782 |
| Percentage Footnote 1 | 97.4% | 0.3% | 70.3% | 29.4% | 2.6% | 100.0% |
See additional data on Reported Influenza Hospitalizations and Deaths in Canada: 2009-10 to 2014-15 on the Public Health Agency of Canada website.
Emerging Respiratory Pathogens
Human Avian Influenza
Influenza A(H7N9): Since the last FluWatch report, no new laboratory-confirmed case of human infection with avian influenza A(H7N9) virus have been reported by the World Health Organization. Globally to January 8, 2015, the WHO has been informed of a total of 470 laboratory-confirmed human cases with avian influenza A(H7N9) virus, including 182 deaths.
Documents related to the public health risk of influenza A(H7N9), as well as guidance for health professionals and advice for the public is updated regularly on the following websites:
PHAC - Avian influenza A(H7N9)
WHO - Avian Influenza A(H7N9)
Influenza A(H5N6): Since the last FluWatch report, no new cases of human infection with avian influenza A (H5N6) virus from China has been reported by the World Health Organization. Globally to January 8, 2015, the WHO has been informed of a total of two cases of avian influenza A (H5N6) virus, including one death.
Middle East Respiratory Syndrome Coronavirus (MERS-CoV)
Since the last FluWatch report, four laboratory-confirmed cases and one death of MERS-CoV have been reported by the World Health Organization. Globally, from September 2012 to January 8, 2015, the WHO has been informed of a total of 945 laboratory-confirmed cases of infection with MERS-CoV, including 348 deaths. All cases have either occurred in the Middle East or have had direct links to a primary case infected in the Middle East. The public health risk posed by MERS-CoV in Canada remains low (see the PHAC Assessment of Public Health Risk).
Documents related to the public health risk of MERS-CoV, as well as guidance for health professionals and advice for the public is updated regularly on the following websites:
Avian Influenza A(H5)
The Canadian Food Inspection Agency (CFIA) is continuing its investigation into an outbreak of highly pathogenic avian influenza H5N2 virus in British Columbia's Fraser Valley. To date, there have been 11 commercial infected premises and one non-commercial infected premise. As part of regular investigation activities, CFIA is fully tracing movements in and out of these sites. This may lead to further premises being identified and depopulated, which would not be unexpected. While there are no reports of H5N2 related illness in humans, as a precautionary measure public health officials are monitoring workers who are exposed to affected poultry. Avian influenza viruses do not pose risks to food safety when poultry and poultry products are properly handled and cooked. Avian influenza rarely affects humans that do not have consistent contact with infected birds. Further information on the outbreak is provided on the following CFIA website.
Enterovirus D68 (EV-D68)
BCCDC reported a death associated with EV-D68 in a young child <5 years of age which occurred earlier in the fall of 2014. Additional information is provided in the following report:
Information related to enterovirus D68, as well as guidance for health professionals and advice for the public is updated regularly:
International Influenza Reports
- World Health Organization influenza update
- World Health Organization FluNet
- WHO Influenza at the human-animal interface
- Centers for Disease Control and Prevention seasonal influenza report
- European Centre for Disease Prevention and Control - epidemiological data
- South Africa Influenza surveillance report
- New Zealand Public Health Surveillance
- Australia Influenza Report
- Pan-American Health Organization Influenza Situation Report
FluWatch definitions for the 2014-2015 season
Abbreviations: Newfoundland/Labrador (NL), Prince Edward Island (PE), New Brunswick (NB), Nova Scotia (NS), Quebec (QC), Ontario (ON), Manitoba (MB), Saskatchewan (SK), Alberta (AB), British Columbia (BC), Yukon (YT), Northwest Territories (NT), Nunavut (NU).
Influenza-like-illness (ILI): Acute onset of respiratory illness with fever and cough and with one or more of the following - sore throat, arthralgia, myalgia, or prostration which is likely due to influenza. In children under 5, gastrointestinal symptoms may also be present. In patients under 5 or 65 and older, fever may not be prominent.
ILI/Influenza outbreaks
- Schools:
-
Greater than 10% absenteeism (or absenteeism that is higher (e.g. >5-10%) than expected level as determined by school or public health authority) which is likely due to ILI.
Note: it is recommended that ILI school outbreaks be laboratory confirmed at the beginning of influenza season as it may be the first indication of community transmission in an area. - Hospitals and residential institutions:
- two or more cases of ILI within a seven-day period, including at least one laboratory confirmed case. Institutional outbreaks should be reported within 24 hours of identification. Residential institutions include but not limited to long-term care facilities ( LTCF) and prisons.
- Workplace:
- Greater than 10% absenteeism on any day which is most likely due to ILI.
- Other settings:
- two or more cases of ILI within a seven-day period, including at least one laboratory confirmed case; i.e. closed communities.
Note that reporting of outbreaks of influenza/ILI from different types of facilities differs between jurisdictions.
Influenza/ILI activity level
1 = No activity: no laboratory-confirmed influenza detections in the reporting week, however, sporadically occurring ILI may be reported
3 = Localized:
- evidence of increased ILIFootnote * and
- lab confirmed influenza detection(s) together with
- outbreaks in schools, hospitals, residential institutions and/or other types of facilities occurring in less than 50% of the influenza surveillance regionFootnote †
4 = Widespread:
- evidence of increased ILIFootnote * and
- lab confirmed influenza detection(s) together with
- outbreaks in schools, hospitals, residential institutions and/or other types of facilities occurring in greater than or equal to 50% of the influenza surveillance regionFootnote †
Note: ILI data may be reported through sentinel physicians, emergency room visits or health line telephone calls.
We would like to thank all the Fluwatch surveillance partners who are participating in this year's influenza surveillance program.