FluWatch report: January 6, 2019 to January 12, 2019 (week 02)
Download the alternative format
(PDF format, # MB, # pages)
Organization: Public Health Agency of Canada
Date published: 2019-01-18
Related Topics
Overall Summary
- In week 02, laboratory detections continued to decline sharply from the previous week confirming that the influenza season reached peak levels in the last week of December (week 52).
- Overall, the Central and Eastern regions are reporting higher levels of influenza activity than the rest of the country.
- Influenza A is the most common influenza virus circulating in Canada, and the majority of these viruses are A(H1N1)pdm09.
- The majority of lab confirmations and hospitalizations have been among individuals under the age of 65.
On this page
- Influenza/ILI Activity (geographic spread)
- Laboratory Confirmed Influenza Detections
- Syndromic/Influenza-like Illness Surveillance
- Participatory Syndromic Surveillance
- Influenza Outbreak Surveillance
- Severe Outcomes Influenza Surveillance
- Antiviral Resistance
- Provincial and International Influenza Reports
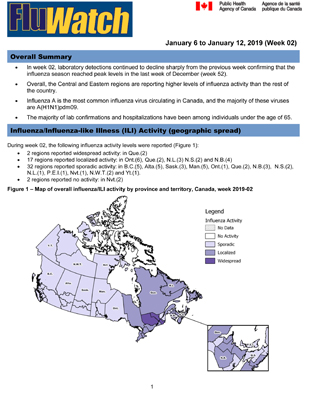
Influenza/Influenza-like Illness Activity (geographic spread)
During week 02, the following influenza activity levels were reported (Figure 1):
- 2 regions reported widespread activity: in Que.(2)
- 17 regions reported localized activity: in Ont.(6), Que.(2), N.L.(3) N.S.(2) and N.B.(4)
- 32 regions reported sporadic activity: in B.C.(5), Alta.(5), Sask.(3), Man.(5), Ont.(1), Que.(2), N.B.(3), N.S.(2), N.L.(1), P.E.I.(1), Nvt.(1), N.W.T.(2) and Yt.(1).
- 2 regions reported no activity: in Nvt.(2)
Figure 1 – Map of overall influenza/ILI activity by province and territory, Canada, week 2019-02

Figure 1 - Text description
| Province | Influenza Surveillance Region | Activity Level |
|---|---|---|
| N.L. | Eastern | Localized |
| N.L. | Labrador-Grenfell | Localized |
| N.L. | Central | Localized |
| N.L. | Western | Sporadic |
| P.E.I. | Prince Edward Island | Sporadic |
| N.S. | Zone 1 - Western | Localized |
| N.S. | Zone 2 - Northern | Sporadic |
| N.S. | Zone 3 - Eastern | Sporadic |
| N.S. | Zone 4 - Central | Localized |
| N.B. | Region 1 | Localized |
| N.B. | Region 2 | Sporadic |
| N.B. | Region 3 | Localized |
| N.B. | Region 4 | Sporadic |
| N.B. | Region 5 | Sporadic |
| N.B. | Region 6 | Localized |
| N.B. | Region 7 | Localized |
| Que. | Nord-est | Localized |
| Que. | Québec et Chaudieres-Appalaches | Sporadic |
| Que. | Centre-du-Québec | Widespread |
| Que. | Montréal et Laval | Sporadic |
| Que. | Ouest-du-Québec | Widespread |
| Que. | Montérégie | Localized |
| Ont. | Central East | Localized |
| Ont. | Central West | Localized |
| Ont. | Eastern | Localized |
| Ont. | North East | Localized |
| Ont. | North West | Sporadic |
| Ont. | South West | Localized |
| Ont. | Toronto | Localized |
| Man. | Northern Regional | Sporadic |
| Man. | Prairie Mountain | Sporadic |
| Man. | Interlake-Eastern | Sporadic |
| Man. | Winnipeg | Sporadic |
| Man. | Southern Health | Sporadic |
| Sask. | North | Sporadic |
| Sask. | Central | Sporadic |
| Sask. | South | Sporadic |
| Alta. | North Zone | Sporadic |
| Alta. | Edmonton | Sporadic |
| Alta. | Central Zone | Sporadic |
| Alta. | Calgary | Sporadic |
| Alta. | South Zone | Sporadic |
| B.C. | Interior | Sporadic |
| B.C. | Fraser | Sporadic |
| B.C. | Vancouver Coastal | Sporadic |
| B.C. | Vancouver Island | Sporadic |
| B.C. | Northern | Sporadic |
| Y.T. | Yukon | Sporadic |
| N.W.T. | North | Sporadic |
| N.W.T. | South | Sporadic |
| Nvt. | Qikiqtaaluk | No Activity |
| Nvt. | Kivalliq | Sporadic |
| Nvt. | Kitimeot | No Activity |
Laboratory Confirmed Influenza Detections
In week 02, the following results were reported from sentinel laboratories across Canada (Figure 2):
- The percentage of tests positive for influenza decreased to 20.5% in week 02.
- A total 2,486 laboratory detections of influenza were reported, of which 98% were influenza A.
To date this season 20,494 laboratory-confirmed influenza detections have been reported:
- 99% have been influenza A.
- Among the 7,523 influenza A viruses subtyped, 94% have been A(H1N1)pdm09.
- Provincial and territorial differences in influenza type/subtype distribution are observed (Figure 3).
To date this season, detailed information on age and type/subtype has been received for 17,295 laboratory-confirmed influenza cases (Table 1):
- 86% of all influenza A(H1N1)pdm09 detections have been reported in individuals younger than 65 years of age.
- 63% of all influenza A(H3N2) detections have been reported in adults 65 years of age and older.
For more detailed weekly and cumulative influenza data, see the text descriptions for Figures 2 and 3 or the Respiratory Virus Detections in Canada Report.
Figure 2 – Number of positive influenza tests and percentage of tests positive, by type, subtype and report week, Canada, weeks 2018-35 to 2019-02
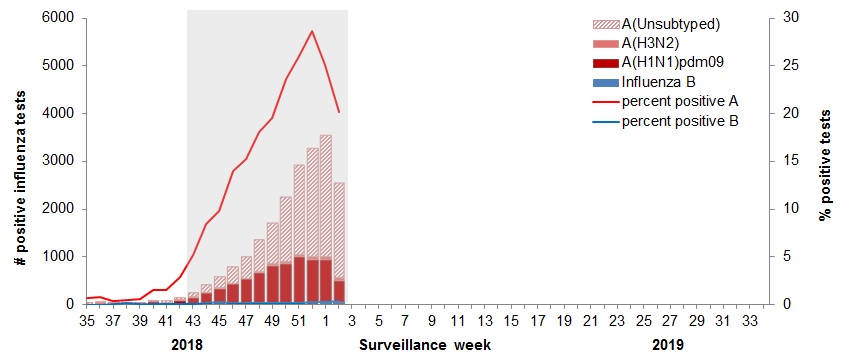
The shaded area indicates weeks where the positivity rate was at least 5% and a minimum of 15 positive tests were observed, signalling the period of seasonal influenza activity.
Figure 2 - Text description
| Surveillance Week | A(Unsubtyped) | A(H3) | A(H1)pdm09 | Influenza B |
|---|---|---|---|---|
| 35 | 3 | 2 | 7 | 0 |
| 36 | 4 | 7 | 4 | 0 |
| 37 | 3 | 2 | 3 | 1 |
| 38 | 6 | 3 | 2 | 3 |
| 39 | 11 | 5 | 1 | 3 |
| 40 | 16 | 7 | 29 | 3 |
| 41 | 27 | 6 | 21 | 3 |
| 42 | 40 | 19 | 55 | 2 |
| 43 | 83 | 23 | 128 | 4 |
| 44 | 169 | 13 | 214 | 6 |
| 45 | 244 | 18 | 295 | 15 |
| 46 | 346 | 10 | 404 | 9 |
| 47 | 449 | 17 | 507 | 8 |
| 48 | 679 | 29 | 632 | 10 |
| 49 | 851 | 35 | 785 | 16 |
| 50 | 1368 | 35 | 828 | 14 |
| 51 | 1890 | 54 | 953 | 21 |
| 52 | 2292 | 55 | 903 | 26 |
| 01 | 2563 | 58 | 893 | 31 |
| 02 | 1986 | 66 | 443 | 42 |
Figure 3 – Cumulative numbers of positive influenza specimens by type/subtype and province/territory, Canada, weeks 2018-35 to 2019-02
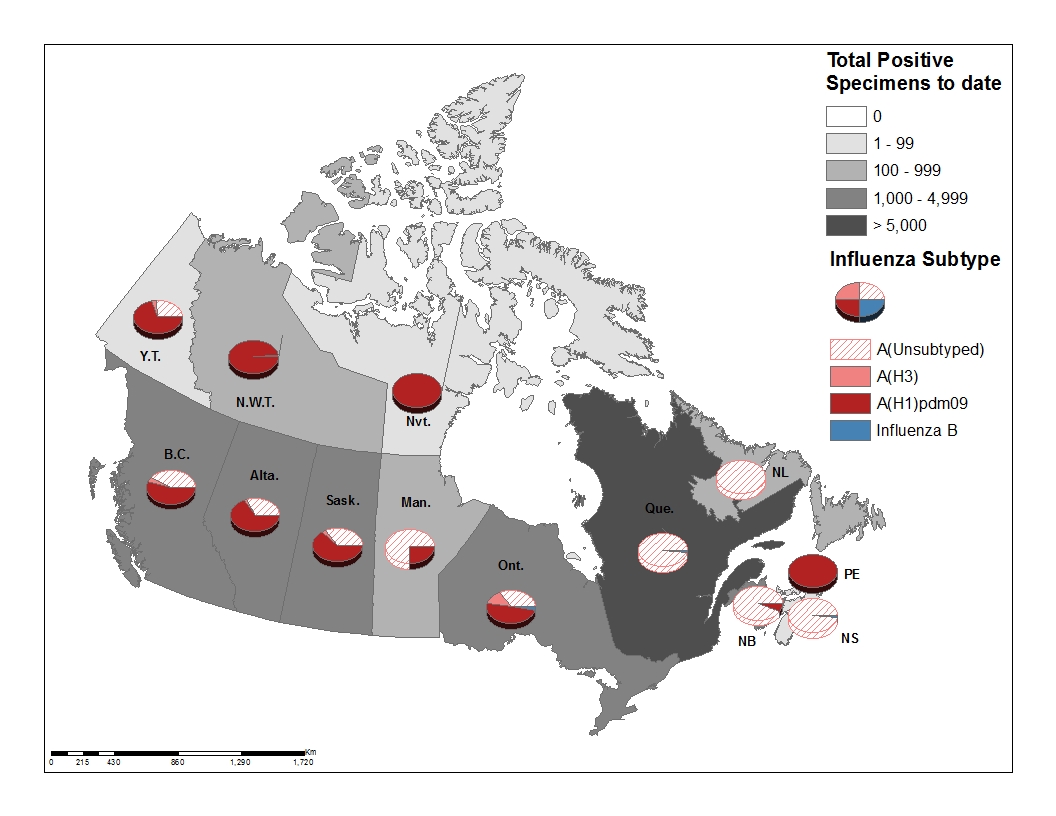
Figure 3 - Text description
| Reporting provincesTable Figure 3 - Footnote 1 |
Week (January 6, 2018 to Januray 12, 2019) | Cumulative (August 26, 2018 to January 12, 2019) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Influenza A | B | Influenza A | B | A & B Total |
|||||||
| A Total |
A (H1N1)pdm09 |
A (H3N2) |
A(UnS)Table Figure 3 - Footnote 3 | B Total |
A Total |
A (H1N1)pdm09 |
A (H3N2) |
A(UnS)Table Figure 3 - Footnote 3 | B Total |
||
| BC | 98 | 31 | 1 | 66 | 0 | 1958 | 1069 | 77 | 812 | 10 | 1968 |
| AB | 147 | 102 | 7 | 38 | 3 | 4915 | 3250 | 82 | 1583 | 33 | 4948 |
| SK | 26 | 14 | 0 | 12 | 0 | 2034 | 1266 | 54 | 714 | 1 | 2035 |
| MB | 89 | 15 | 0 | 74 | 0 | 830 | 211 | 3 | 616 | 3 | 833 |
| ON | 409 | 213 | 56 | 140 | 6 | 1893 | 977 | 236 | 680 | 48 | 1941 |
| QC | 1 334 | 0 | 0 | 1 334 | 32 | 7387 | 0 | 0 | 7387 | 115 | 7502 |
| NB | 267 | 26 | 2 | 239 | 0 | 1025 | 68 | 3 | 954 | 2 | 1027 |
| NS | 33 | 0 | 0 | 33 | 0 | 85 | 0 | 0 | 85 | 1 | 86 |
| PEI | 20 | 20 | 0 | 0 | 0 | 58 | 58 | 0 | 0 | 0 | 58 |
| NL | 21 | 0 | 0 | 21 | 0 | 135 | 1 | 0 | 134 | 1 | 136 |
| YT | 0 | 0 | 0 | 0 | 0 | 23 | 16 | 1 | 6 | 0 | 23 |
| N.W.T | 0 | 0 | 0 | 0 | 0 | 142 | 140 | 2 | 0 | 0 | 142 |
| NU | 1 | 1 | 0 | 0 | 0 | 9 | 9 | 0 | 0 | 0 | 9 |
| Canada | 2445 | 422 | 66 | 1957 | 41 | 20494 | 7065 | 458 | 12971 | 214 | 20708 |
| PercentageTable Figure 3 - Footnote 2 | 98% | 17% | 3% | 80% | 2% | 99% | 34% | 2% | 63% | 1% | 100% |
Discrepancies in values in Figures 2 and 3 may be attributable to differing data sources. Cumulative data includes updates to previous weeks. |
|||||||||||
| Age groups (years) | Cumulative (August 26, 2018 to January 12, 2019) | ||||||
|---|---|---|---|---|---|---|---|
| Influenza A | B | Influenza A and B | |||||
| A Total | A(H1) pdm09 | A(H3) | A (UnS)Footnote 1 | Total | # | % | |
| 0-4 | 3679 | 1351 | 18 | 2310 | 28 | 3707 | 21% |
| 5-19 | 2698 | 1168 | 17 | 1513 | 29 | 2727 | 16% |
| 20-44 | 3676 | 1412 | 62 | 2202 | 24 | 3700 | 21% |
| 45-64 | 3601 | 1253 | 76 | 2272 | 29 | 3630 | 21% |
| 65+ | 3464 | 816 | 294 | 2354 | 67 | 3531 | 20% |
| Total | 17118 | 6000 | 467 | 10651 | 177 | 17295 | 100% |
|
|||||||
Syndromic/Influenza-like Illness Surveillance
Healthcare Professionals Sentinel Syndromic Surveillance
In week 02, 2.5% of visits to healthcare professionals were due to influenza-like illness (ILI) (Figure 4). The percentage of visits for ILI is within expected levels.
Figure 4 – Percentage of visits for ILI reported by sentinels by report week, Canada, weeks 2018-35 to 2019-02
Number of Sentinels Reporting in Week 02: 97
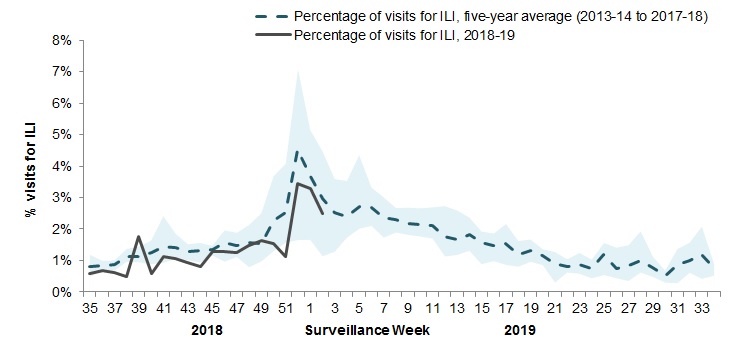
The shaded area represents the maximum and minimum percentage of visits for ILI reported by week from seasons 2013-14 to 2017-18
Figure 4 - Text description
| Report week | 2018-19 | Average | Min | Max |
|---|---|---|---|---|
| 35 | 0.6% | 0.6% | 0.4% | 0.9% |
| 36 | 0.7% | 0.8% | 0.5% | 1.0% |
| 37 | 0.6% | 0.9% | 0.7% | 1.1% |
| 38 | 0.5% | 1.0% | 0.7% | 1.4% |
| 39 | 1.8% | 1.1% | 0.8% | 1.5% |
| 40 | 0.6% | 1.6% | 0.9% | 3.4% |
| 41 | 1.1% | 1.6% | 1.1% | 2.1% |
| 42 | 1.0% | 1.4% | 0.8% | 1.8% |
| 43 | 0.9% | 1.4% | 0.7% | 1.8% |
| 44 | 0.8% | 1.4% | 1.1% | 1.6% |
| 45 | 1.3% | 1.5% | 1.2% | 1.8% |
| 46 | 1.3% | 1.7% | 1.1% | 2.2% |
| 47 | 1.3% | 1.6% | 1.1% | 2.2% |
| 48 | 1.6% | 1.8% | 1.0% | 2.8% |
| 49 | 1.8% | 1.5% | 1.1% | 1.8% |
| 50 | 1.6% | 2.1% | 1.4% | 2.7% |
| 51 | 1.2% | 2.3% | 1.6% | 3.2% |
| 52 | 3.4% | 3.6% | 1.9% | 5.0% |
| 01 | 3.3% | 3.8% | 1.8% | 5.6% |
| 02 | 2.5% | 2.6% | 1.3% | 3.9% |
Participatory Syndromic Surveillance
In week 02, 2,259 participants reported to FluWatchers, of which 64 (2.8%) reported symptoms of cough and fever (Figure 5).
Among the 64 participants who reported fever and cough:
- 19% consulted a healthcare professional;
- 72% reported days missed from work or school, resulting in a combined total of 142 missed days of work or school.
Figure 5 – Percentage of participants reporting cough and fever, Canada, weeks 2018-40 to 2019-02
Number of Participants Reporting in Week 02: 2,259
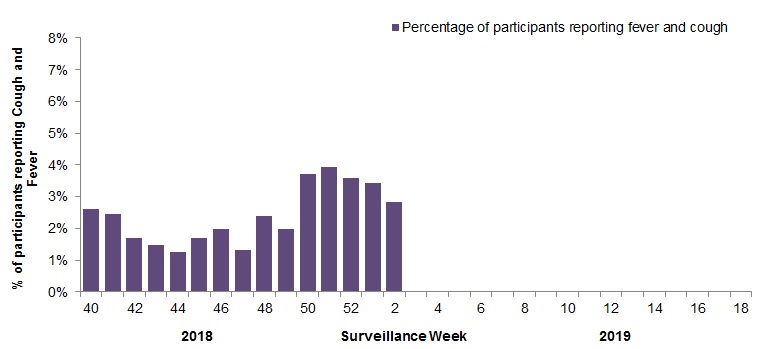
Figure 5 - Text description
| Surveillance week | % cough and fever |
|---|---|
| 40 | 2.6% |
| 41 | 2.5% |
| 42 | 1.7% |
| 43 | 1.5% |
| 44 | 1.3% |
| 45 | 1.7% |
| 46 | 2.0% |
| 47 | 1.3% |
| 48 | 2.4% |
| 49 | 2.0% |
| 50 | 3.7% |
| 51 | 3.9% |
| 52 | 3.6% |
| 01 | 3.4% |
| 02 | 2.8% |
Influenza Outbreak Surveillance
In week 02, 33 new laboratory-confirmed influenza outbreaks were reported: long-term care facilities (LTCF) (21), acute care facilities (4), and other settings (8). Two new ILI outbreaks in schools were also reported in week 02.
To date this season, 206 laboratory-confirmed influenza outbreaks have been reported (Figure 6):
- 116 outbreaks were in LTCF, 22 were in schools, 26 in acute care facilities, and 42 were in other settings.
- All of the 194 outbreaks for which the influenza type was available were associated with influenza A.
- Among the 93 outbreaks for which the influenza A subtype was available:
- 80% (74) were associated with influenza A(H1N1)pdm09;
- 20% (19) were associated with A(H3N2),
To date this season, 44 ILI outbreaks have been reported; 32 occurred in LTCF, nine in schools, and three in acute care facilities.
Figure 6 – Number of new outbreaks of laboratory-confirmed influenza by report week, Canada, weeks 2018-35 to 2019-02
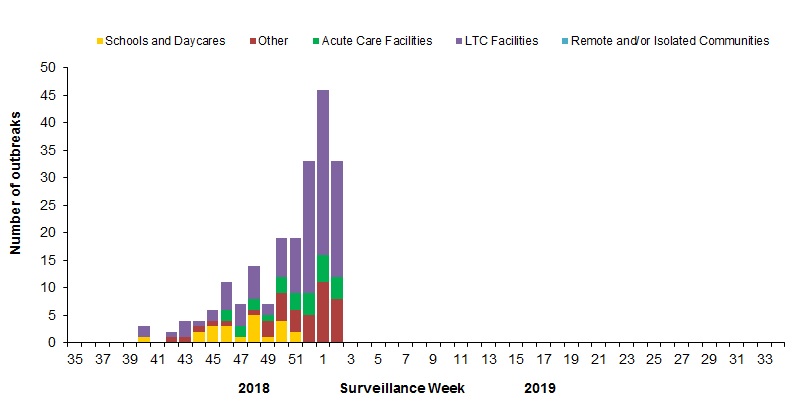
Figure 6 - Text description
| Surveillance Week | Acute Care Facilities | Long Term Care Facilities | Other | Schools and Daycares | Remote and/or Isolated Communities |
|---|---|---|---|---|---|
| 35 | 0 | 0 | 0 | 0 | 0 |
| 36 | 0 | 0 | 0 | 0 | 0 |
| 37 | 0 | 0 | 0 | 0 | 0 |
| 38 | 0 | 0 | 0 | 0 | 0 |
| 39 | 0 | 0 | 0 | 0 | 0 |
| 40 | 0 | 2 | 0 | 1 | 0 |
| 41 | 0 | 0 | 0 | 0 | 0 |
| 42 | 0 | 1 | 1 | 0 | 0 |
| 43 | 0 | 3 | 1 | 0 | 0 |
| 44 | 0 | 1 | 1 | 2 | 0 |
| 45 | 0 | 2 | 1 | 3 | 0 |
| 46 | 2 | 5 | 1 | 3 | 0 |
| 47 | 2 | 4 | 0 | 1 | 0 |
| 48 | 2 | 6 | 1 | 5 | 0 |
| 49 | 1 | 2 | 3 | 1 | 0 |
| 50 | 3 | 7 | 5 | 4 | 0 |
| 51 | 3 | 10 | 4 | 2 | 0 |
| 52 | 4 | 24 | 5 | 0 | 0 |
| 01 | 5 | 30 | 11 | 0 | 0 |
| 02 | 4 | 21 | 8 | 0 | 0 |
Severe Outcomes Influenza Surveillance
Provincial/Territorial Influenza Hospitalizations and Deaths
To date this season, 1,518 influenza-associated hospitalizations have been reported by participating provinces and territoriesFootnote 1.
Hospitalizations (Table 2):
- 99.6% (1,512) were associated with influenza A
- The highest estimated rate of hospitalization is among children under 5 years of age.
Intensive Care Unit (ICU) cases and deaths:
- To date this season 227 ICU admissions and 47 deaths have been reported.
- 42% (97) of reported ICU admissions were in adults aged 45-64 years.
- All reported deaths were associated with influenza A.
| Age Groups | Cumulative (August 26, 2018 to January 12, 2018) | ||
|---|---|---|---|
| Influenza A | Influenza B | Rate per 100,000 population | |
| 0-4 | 252 | 2 | 53,36 |
| 5-19 | 152 | 0 | 10,95 |
| 20-44 | 213 | 0 | 7,49 |
| 45-64 | 413 | 0 | 18,98 |
| 65+ | 482 | 4 | 39,95 |
| Total | 1512 | 6 | - |
| 99.6% | 0.4% | - | |
Pediatric Influenza Hospitalizations and Deaths
In week 02, 57 pediatric (≤16 years of age) hospitalizations with influenza have been reported by the Immunization Monitoring Program Active (IMPACT) network (Figure 7).
To date this season, 603 pediatric hospitalizations have been reported (Figure 8):
- 99% (598) of cases have been associated with influenza A.
- Among the 240 cases for which the influenza subtype was available, 234 (98%) were associated with A(H1N1)pdm09.
To date this season, 98 ICU admissions, and seven deaths have been reported.
- 85% (84) of ICU admissions were in children 6 months to 9 years of age
- 99% (97) of ICU admissions have been associated with influenza A.
- All deaths occurred in children under the age of 10
Figure 7 – Number of pediatric (≤16 years of age) hospitalizations reported by the IMPACT network, by week, Canada, weeks 2018-35 to 2019-02
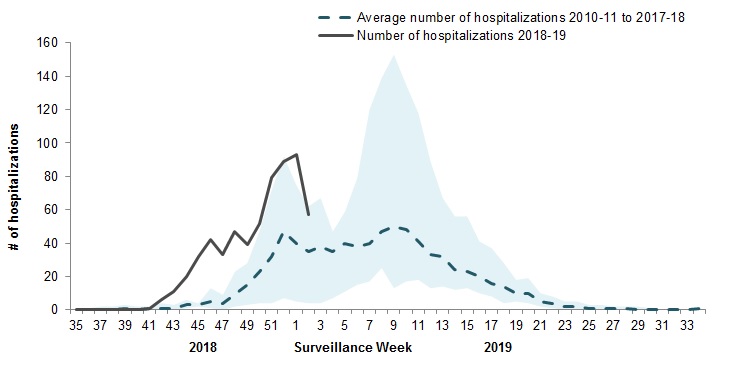
Figure 7 - Text description
| Surveillance week | 2018-19 | Average | Min | Max |
|---|---|---|---|---|
| 35 | 0 | 0 | 0 | 0 |
| 36 | 0 | 0 | 0 | 1 |
| 37 | 0 | 0 | 0 | 2 |
| 38 | 0 | 0 | 0 | 2 |
| 39 | 0 | 1 | 0 | 3 |
| 40 | 0 | 0 | 0 | 2 |
| 41 | 1 | 1 | 0 | 2 |
| 42 | 6 | 1 | 0 | 4 |
| 43 | 11 | 1 | 0 | 3 |
| 44 | 20 | 3 | 1 | 6 |
| 45 | 32 | 3 | 2 | 4 |
| 46 | 42 | 5 | 1 | 13 |
| 47 | 33 | 4 | 0 | 9 |
| 48 | 47 | 9 | 2 | 23 |
| 49 | 39 | 15 | 3 | 28 |
| 50 | 52 | 23 | 4 | 47 |
| 51 | 79 | 32 | 4 | 72 |
| 52 | 89 | 47 | 7 | 92 |
| 01 | 93 | 40 | 5 | 75 |
| 02 | 57 | 35 | 4 | 62 |
Figure 8 - Cumulative numbers of pediatric hospitalizations (≤16 years of age) with influenza by age-group reported by the IMPACT network, Canada, weeks 2018-35 to 2019-02
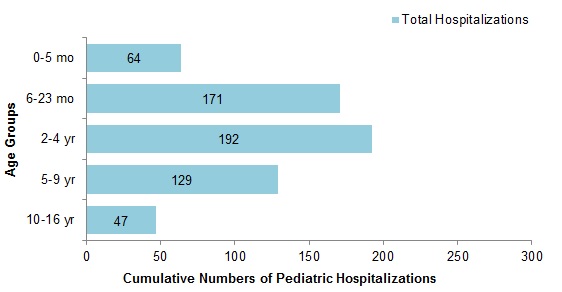
Figure 8 - Text description
| Age Group | Total |
|---|---|
| 0-5 mo | 64 |
| 6-23 mo | 171 |
| 2-4 yr | 192 |
| 5-9 yr | 129 |
| 10-16 yr | 47 |
Adult Influenza Hospitalizations and Deaths
Surveillance of laboratory-confirmed influenza-associated adult (≥16 years of age) hospitalizations by the Canadian Immunization Research Network (CIRN) Serious Outcomes Surveillance (SOS) network began on November 1st for the 2018-19 season.
To date this season, 278 hospitalizations have been reported (Figure 9):
- 262 (94%) were associated with influenza A.
- The distribution of cases among adults <65 years of age is similar to adults ≥65 years of age
- The most commonly reported comorbidity among hospitalized cases was endocrine disorders, which were reported in 70% of hospitalized cases.
Figure 9 - Cumulative numbers of adult hospitalizations (>20 years of age) with influenza by age-group reported by CIRN, Canada, 2018-19, weeks 2018-44 to 2019-02
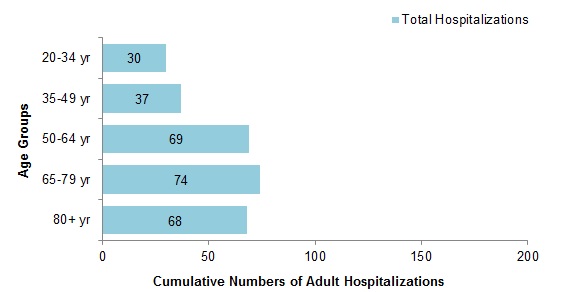
Figure 9 - Text description
| Age Group | Total |
|---|---|
| 20-34 yr | 29 |
| 35-49 yr | 37 |
| 50-64 yr | 69 |
| 65-79 yr | 74 |
| 80+ yr | 69 |
Influenza Strain Characterizations
Since September 1, 2018, the National Microbiology Laboratory (NML) has characterized 571 influenza viruses (49 A(H3N2), 506 A(H1N1) and 16 B) that were received from Canadian laboratories.
Genetic Characterization of Influenza A(H3N2):
39 influenza A(H3N2) viruses did not grow to sufficient hemagglutination titer for antigenic characterization by hemagglutination inhibition (HI) assay. Therefore, NML has performed genetic characterization to determine the genetic group identity of these viruses.
Sequence analysis of the HA gene of the viruses showed that:
- Six viruses belonged to genetic group 3C.2a.
- 32 viruses belonged to subclade 3C.2a1.
- One isolate could not be sequenced.
A/Singapore/INFIMH-16-0019/2016-like virus belongs to genetic group 3C.2a1 and is the influenza A(H3N2) component of the 2018-19 Northern Hemisphere influenza vaccine.
Antigenic Characterization:
Influenza A (H3N2):
- 17 influenza A(H3N2) viruses were antigenically characterized as A/Singapore/INFIMH-16-0019/2016-like by HI testing using antiserum raised against egg-propagated A/Singapore/INFIMH-16-0019/2016.
- A/Singapore/INFIMH-16-0019/2016-like virus is the influenza A(H3N2) component of the 2018-19 Northern Hemisphere influenza vaccine.
- 13 influenza A (H3N2) viruses characterized belonged to genetic group 3C.2a1. Two viruses belonged to genetic group 3C.2a and two to 3C.3a.
Influenza A(H1N1):
- 495 A(H1N1) viruses characterized were antigenically similar to A/Michigan/45/2015, which is the influenza A(H1N1) component of the 2018-19 Northern Hemisphere influenza vaccine.
- 11 viruses showed reduced titer with ferret antisera raised against cell culture-propagated A/Michigan/45/2015
Influenza B:
Influenza B viruses can be divided into two antigenically distinct lineages represented by B/Yamagata/16/88 and B/Victoria/2/87 viruses. The recommended influenza B components for the 2018-19 Northern Hemisphere influenza vaccine are B/Colorado/06/2017 (Victoria lineage) and B/Phuket/3073/2013 (Yamagata lineage).
- Two influenza B viruses were characterized as B/Colorado/06/2017, which belong to the Victoria lineage and are included as an influenza B component of the 2018-19 Northern Hemisphere influenza vaccine
- 14 influenza B viruses were characterized as B/Phuket/3073/2013-like, which belongs to the Yamagata lineage and is included as an influenza B component of the 2018-19 Northern Hemisphere quadrivalent influenza vaccine.
Antiviral Resistance
Antiviral Resistance – Amantadine:
290 influenza A (41 A(H3N2) and 249 A(H1N1)) viruses were tested for resistance to amantadine and it was found that:
- All 290 influenza A viruses were resistant to amantadine.
Antiviral Resistance – Oseltamivir:
467 influenza viruses (47 A(H3N2), 404 A(H1N1) and 16 B) were tested for resistance to oseltamivir and it was found that:
- All 467 influenza viruses were sensitive to oseltamivir
Antiviral Resistance – Zanamivir:
466 influenza viruses (47 A(H3N2), 403 H1N1 and 16 B) were tested for resistance to zanamivir and it was found that:
- All 466 influenza viruses were sensitive to zanamivir.