Advice for the Use of the Multicomponent Meningococcal Serogroup B Vaccine
An Advisory Committee Statement (ACS) National Advisory Committee on Immunization (NACI)Footnote †
Preamble
The National Advisory Committee on Immunization (NACI) provides the Public Health Agency of Canada (hereafter referred to as the Agency) with ongoing and timely medical, scientific, and public health advice relating to immunization. The Agency acknowledges that the advice and recommendations set out in this statement are based upon the best current available scientific knowledge and is disseminating this document for information purposes. People administering the vaccine should also be aware of the contents of the relevant product monograph(s). Recommendations for use and other information set out herein may differ from that set out in the product monograph(s) of the Canadian manufacturer(s) of the vaccine(s). Manufacturer(s) have sought approval of the vaccine(s) and provided evidence as to its safety and efficacy only when it is used in accordance with the product monographs. NACI members and liaison members conduct themselves within the context of the Agency's Policy on Conflict of Interest, including yearly declaration of potential conflict of interest.
Table of Contents
- Summary of information contained in this NACI statement
- I. Introduction
- II. Methods
- III. Epidemiology
- IV. Vaccine
- V. Recommendations
- VI. Research priorities and surveillance issues
- List of abbreviations
- Acknowledgements
- References
Summary of information contained in this NACI statement
The following table highlights key information for immunization providers. Please refer to the remainder of the Statement for details.

Download the alternative format
(PDF format, 1.10 MB, 43 pages)
Cat.: HP40-104/2014E-PDF
ISBN: 978-1-100-23516-5
Pub.: 140012
Organization: Public Health Agency of Canada
Date published: April 2014
Related Topics
1. What
What is invasive meningococcal disease?
Invasive meningococcal disease (IMD) usually presents as an acute febrile illness with rapid onset and features of meningitis or septicemia (meningococcemia), or both, and a characteristic non-blanching rash. Overall case fatality is approximately 10%, and up to a third of survivors may have long term sequelae, which include hearing loss, neurologic disabilities, and digit or limb amputations. In 2011, 108 of 175 (62%) reported cases of IMD in Canada were due to serogroup B; 18.5% of serogroup B IMD cases were infants, the majority less than 6 months of age. The rates of IMD from other serogroups have been decreasing since the introduction of routine vaccination programs. Additional information about IMD is available on the Public Health Agency of Canada (the Agency) web site.
What is the multicomponent meningococcal serogroup B vaccine (4CMenB)?
The multicomponent meningococcal serogroup B (4CMenB) vaccine is the first available vaccine against serogroup B IMD in Canada. The vaccine is protective against strains that express antigens contained in the vaccine at sufficient levels. The 4CMenB vaccine is an immunogenic vaccine, though its effectiveness, impact on carriage and the duration of protection remains unknown. Further research, evaluation and surveillance will be required to determine the duration of protection, the efficacy or effectiveness of 4CMenB vaccine, its ability to induce herd protection, and the risk of adverse events with widespread use.
2. Who
Whom to immunize?
Individuals greater than or equal to two months of age:
- who are at high risk of meningococcal disease caused by serogroup B Neisseria meningitidis
- that have been in close contact with a case of IMD caused by serogroup B N. meningitidis
- who may be at risk during IMD outbreaks caused by serogroup B N. meningitidis or the emergence of hyperendemic and/or hypervirulent N. meningitidis strains that are predicted to be susceptible to the vaccine based on Meningococcal Antigen Typing System (MATS) testing
- who are without contraindications to the vaccine and who wish to be immunized
3. How
Dose, schedule and precautions, and co-administration
Vaccine schedules vary with age at administration. Because of unknown duration of protection after immunization, the need for a booster dose is yet to be determined.
4CMenB vaccine has been given simultaneously with a hexavalent tetanus diphtheria containing infant vaccine, heptavalent pneumococcal conjugate vaccine (PCV7), serogroup C meningococcal vaccine and MMRV. High rates of fever have been observed, particularly with simultaneous administration of 4CMenB vaccine and routine infant vaccines. The immune response to routine infant vaccines and the 4CMenB vaccine does not appear to be affected when these vaccines are administered simultaneously.
4CMenB vaccine is contraindicated in persons with a serious allergy to any vaccine component or previous dose. There are no studies of 4CMenB vaccine in pregnant or lactating women, persons less than 2 months and over 55 years of age, persons with a chronic medical condition and those with previous meningococcal infection.
4CMenB should be stored at +2 to +8°C and should not be frozen.
4. Why
Why immunize?
To prevent IMD caused by serogroup B meningococcal strains against which the vaccine may be effective.
I. Introduction
Bexsero®(Novartis Vaccines) is a novel multicomponent meningococcal serogroup B (4CMenB) vaccine. The 4CMenB is the first vaccine that has been created through a process of reverse vaccinology. Through this process potential vaccine targets (i.e. antigens) are identified and developed by sequencing the meningococcal serogroup B genome.Footnote 1Footnote 3 The vaccine is therefore protective only against the strains that express antigens contained in the vaccine at sufficient levels. A significant proportion, but not all, of serogroup B strains express vaccine containing antigens. In addition, antigens contained in the vaccine are not unique to serogroup B and may be expressed by other meningococcal serogroups. A detailed description of vaccine antigens and the process of vaccine development are described in the literature review.
Previously NACI recommended the use of available capsule polysaccharide based vaccines: three monovalent meningococcal conjugate vaccines for serogroup C (Menjugate®, Neis Vac-C® and MeningitecTM), two quadrivalent meningococcal conjugate vaccines for serogroups A, C, Y and W-135 (Menactra® and MenveoTM) and one quadrivalent polysaccharide meningococcal ACYW-135 vaccine (Menomune®) for the prevention of IMD serogroup A, C, W135 and Y. In March 2013, Health Canada issued a notice of compliance for a new quadrivalent meningococcal conjugate vaccine for serogroups A, C, Y and W-135 (Nimenrix TM).
This statement:
- Updates the epidemiology of IMD in Canada;
- Provides available vaccine efficacy, effectiveness, immunogenicity and safety information on 4CMenB vaccine;
- Identifies evidence gaps and ongoing research, evaluation and surveillance needs;
- Provides recommendations for use of the 4CMenB vaccine in Canada.
II. Methods
A comprehensive literature search and review was completed to identify relevant evidence on the 4CMenB vaccine including safety, immunogenicity, efficacy and effectiveness of the vaccine; vaccine schedules; target populations; and other aspects of the overall immunization strategy. In addition, the burden of illness due to IMD in Canada was reviewed. In anticipation that there would be no efficacy or effectiveness data available on the novel 4CMenB vaccine, an analogous process was taken for the NZ-OMV vaccine (MeNZBTM, Novartis Vaccines, formerly Chiron), a component of 4CMenB vaccine for which effectiveness data is available. The knowledge synthesis was performed by Public Health Ontario and supervised by the Meningococcal B Pilot Project Task Group (MBPPTG), composed of NACI representatives, Canadian Immunization Committee representatives, in addition to Canadian experts in IMD. Following critical appraisal of individual studies, summary tables with ratings of the quality of the evidence were prepared using NACI's methodological hierarchy. After a thorough review of the evidence and consultations, MBPPTG proposed provisional recommendations at the meeting of March 15, 2013 and presented them to NACI on March 28, 2013. NACI voted on the recommendations at the meeting of April 29, 2013. The description of relevant considerations, rationale for specific decisions, and knowledge gaps are described in the text. The full knowledge synthesis and review is maintained by the Agency.
III. Epidemiology
N. meningitidis (meningococcus) is a potentially serious pathogen that can cause IMD. It colonizes up to 10% of healthy individuals without causing harm. Meningococci can be classified based on the immunologic reactivity of the polysaccharide capsule into 12 different serogroups, of which five (A, B, C, W-135 and Y) are associated most frequently with IMD around the globe. Further classification into serotypes and serosubtypes can be made based on the immunologic reactivity of meningococcal outer membrane proteins (OMP). Characterization using nucleotide sequence-based methods such as genetic sequencing of porA and porB genes is used to substitute or supplement serology-based classifications.
IMD usually presents as an acute febrile illness with rapid onset and features of meningitis or septicemia (meningococcemia), or both, and a characteristic non-blanching rash. Overall case fatality is approximately 10%, and up to a third of survivors may have long term sequelae, which can include hearing loss, neurologic disabilities, and digit or limb amputations.Footnote 4Footnote 5
IMD is a reportable communicable disease in all provinces and territories (P/Ts). All probable and confirmed IMD cases are reported to P/T public health authorities and PHAC's Enhanced IMD Surveillance System. P/T public health and/or hospital laboratories send all meningococcal isolates to PHAC's National Microbiology Laboratory (NML) for strain characterization, including confirmation of serogroup and determination of serotype, serosubtype and sequence type/clonal complex.
Although IMD is reported year round, there is considerable variation in geographical and temporal incidence, with the majority of cases occurring between November and March. As depicted in Figure 1, the overall annual incidence of IMD in Canada has ranged from 0.45 to 1.18 cases per 100,000 population from 1995 to 2011. Between 2007 and 2011, an average of 192 cases of IMD was reported annually in Canada, with an overall average incidence of 0.57 cases per 100,000 population per year.
Figure 1. Incidence of IMD (per 100,000 population) in Canada by serogroup and year, 1995 to 2011.
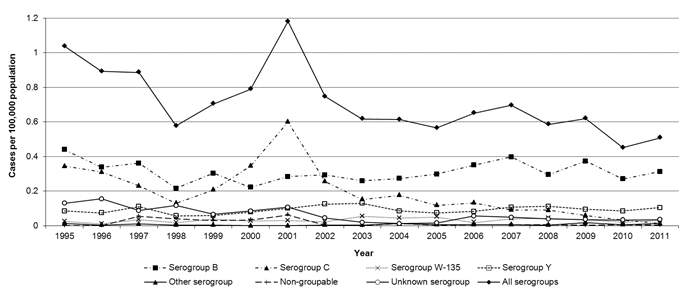
Figure 1 - Long Description
Figure 1. Incidence of IMD (per 100 000 population) in Canada by serogroup and year, 1995 to 2011
Figure 1 shows a line graph depicting the annual incidence rate of reported IMD cases in Canada per 100,000 population, by serogroup and year from 1995 to 2011. The x-axis contains the years from 1995 to 2011. The y-axis on the left displays the reported cases per 100,000 population, starting from 0 at the bottom to 1.2 at the top. The annual incidence rates of serogroups B, C, W-135, Y, other serogroup, non-groupable, unknown serogroup and all serogroups are each depicted by a line.
The incidence of IMD for all serogroups declined from 1.04 to 0.58 cases per 100,000 population from 1995 to 1998, then increased to a peak of 1.18 cases per 100,000 population in 2001. Thereafter, the overall incidence rate decreased slightly from 0.75 to 0.51 cases per 100,000 population from 2002 to 2011. For the majority of years, serogroup B had the highest incidence rates, ranging from 0.22 to 0.44 cases per 100,000 population. The incidence of serogroup C decreased from 0.35 to 0.13 cases per 100,000 population from 1995 to 1998, then increased to a peak of 0.60 cases per 100,000 population in 2001, and then decreased over the next ten years to a low of 0.01 cases per 100,000 population in 2011. The incidence of serogroups W-135 and Y were relatively stable, ranging from 0.01 to 0.05 cases per 100,000 population for W-135 and from 0.06 to 0.13 cases per 100,000 population for Y. From 1995 to 2011, IMD due to other serogroups ranged from 0 to 0.02 cases per 100,000 population. During the same period, the incidence of non-groupable ranged from 0 to 0.06 cases per 100,000 population and the incidence for unknown serogroups ranged from 0.01 to 0.16 cases per 100,000 population.
In Canada, serogroups B, C, W-135 and Y are responsible for the majority of IMD. Following the occurrence of multi-focal serogroup C outbreaks in the late 1990s and early 2000s, conjugate serogroup C vaccination programs were implemented in all Canadian P/Ts between 2002 and early 2007 (Table 1), resulting in significant decreases in serogroup C incidence in all age groups and regions.Footnote 5-26 With the declining incidence of serogroup C, serogroup B now makes up the greatest proportion of reported IMD cases in Canada (62% due to serogroup B versus 2% due to serogroup C in 2011). From 2007 to 2011, serogroup B incidence has fluctuated slightly between 0.27 and 0.40 cases per 100,000 per year.
| Province/ Territory | Year of initial implementation of routine meningococcal C conjugate program | Current infant schedule using meningococcal C conjugate | Current adolescent schedule using meningococcal C conjugate (C) or ACYW-135 conjugate (Q)Footnote * |
|---|---|---|---|
| BC | 2003 | 2, 12 months (since 2005) | (C) Grade 6 (since 2003) |
| AB | 2002 | 2, 4, 12 months (since 2007) | (Q) Grade 9 (since 2011) |
| SK | 2003 | 12 months (since 2004) | (Q) Grade 6 (since 2011) |
| MB | 2004 | 12 months (since 2009) | (C) Grade 4 (since 2004) |
| ON | 2004 | 12 months (since 2004) | (Q) Grade 7 (since 2009) |
| QC | 2002 | 12 months (since 2002) | (C) Grade 6 (since 2003) |
| NL | 2005 | 12 months (since 2005) | (Q) Grade 4 (since 2007) |
| NB | 2004 | 12 months (since 2004) | (Q) Grade 9 (since 2007) |
| NS | 2005 | 12 months (since 2005) | (C) Grade 7 (since 2010) |
| PE | 2003 | 12 months (since 2003) | (Q) Grade 9 (since 2006) |
| YK | 2005 | 2, 12 months (since 2009) | (C) Grade 6 (since 2006) |
| NT | 2004 | 2, 12 months (since 2004) | (C) Grade 9 (since 2008) |
| NU | 2007 | 12 months (since 2007) | (C) Grade 9 (since 2006) |
|
|||
Table 2 presents the number of reported cases and incidence of IMD by serogroup in 2011 as well as the mean number of cases for 2007 to 2011. It also indicates the median age and case fatality ratio (CFR) of IMD by serogroup from 2007 to 2011.
| Serogroup | 2011 | 2007 to 2011 | ||||
|---|---|---|---|---|---|---|
| Number of cases | Incidence (cases per 100,000 population) | Average annual number of cases (range) | Average annual incidence (cases per 100,000 population) | Median age (years) | Case fatality ratio | |
| A | 0 | 0 | 0.2 (0 to 1) | 0 | 16 | 0.0% |
| B | 108 | 0.31 | 111 (92 to 131) | 0.33 | 16 | 6.0% |
| C | 4 | 0.01 | 19 (4 to 30) | 0.06 | 44.5 | 15.3% |
| W-135 | 10 | 0.03 | 11.2 (7 to 14) | 0.03 | 38 | 8.5% |
| Y | 36 | 0.10 | 33.8 (29 to 37) | 0.10 | 47 | 12.1% |
| Other | 4 | 0.01 | 3 (1 to 6) | 0.01 | 34 | 0% |
| Non-groupable | 1 | 0 | 1.6 (1 to 2) | 0 | 28 | 10.0% |
| Unknown | 12 | 0.04 | 12.8 (11 to 16) | 0.04 | 16.5 | 8.2% |
| All serogroups | 175 | 0.51 | 192.4 (154 to 229) | 0.57 | 20 | 8.2% |
As demonstrated in Figure 2, geographic differences in the serogroup distribution of IMD exist across Canada. The highest incidence of IMD and serogroup B-specific IMD occurred in Québec where, on average, 77% of cases were due to serogroup B from 2007 to 2011. Among remaining provinces, the serogroup distribution varied, with serogroup B making up between 25% and 77% of cases on average from 2007 to 2011, depending on the region.
Very few cases were reported in the three territories and Prince Edward Island (zero to two cases per year) from 2007 to 2011, occasionally resulting in high proportions that should be interpreted with caution.
Figure 2. Average reported cases of IMD in Canada by serogroup and province/territory from 2007 to 2011

Figure 2 - Long Description
Figure 2. Average reported cases of IMD in Canada by serogroup and province/territory from 2007 to 2011
Figure 2 shows a stacked bar graph depicting the average number of reported cases of IMD which occurred in Canada between 2007 and 2011 by serogroup and province/territory. The x-axis contains each province and territory, from British Columbia to Nunavut. The y-axis on the left represents the average number of reported cases during that five year period of 2007 to 2011. The y-axis starts with 0 at the bottom and reaches 90 cases at the top. Each stacked bar depicts the average number of cases due to serogroups B, C, W-135, Y, non-groupable, other and unknown serogroups for each province or territory.
In Canada, serogroup B accounts for the largest proportion of the average number of reported cases overall.
Very few cases of IMD were reported in average in the three territories and Prince Edward Island (zero to two cases per year) from 2007 to 2011. In the Yukon Territory, the only case reported was of serogroup C, while in Northwest Territories and Nunavut the cases were of serogroup B. During that period, an average of 0.6 cases annually was reported in Prince Edward Island. In contrast, Quebec reported the highest average number of cases in comparison with all Canadian jurisdictions with 76.6 cases reported in average (serogroup distribution: 59.2 cases B, 4 cases of C, 3.4 cases W-135, 3.8 cases of Y, 1.2 cases other and 5 cases unknown). Ontario reported the second highest average number of cases with 52 cases (serogroup distribution: 23.2 cases B, 6.8 cases C, 2 cases W-135, 16 cases Y, 0.8 cases non-groupable, 0.4 case other and 2.8 cases with unknown).
Among the remaining provinces, 20 cases on average were reported in British Columbia from 2007 to 2011 (serogroup distribution: 8 cases B, 3.8 cases C, 1.2 cases W-135, 5.2 cases Y, 0.2 case non-groupable, 0.8 case other, 0.8 case unknown), 14.8 cases on average in Alberta (serogroup distribution: 6.2 cases B, 0.6 case C, 2 cases W-135, 4 cases Y, 0.4 case non-groupable, 0.4 case other and 1.2 cases unknown), 7.2 cases in Saskatchewan (serogroup distribution: 3 cases B, 1 case C, 0.6 case W-135 and 2.6 cases Y), 6.2 cases in Manitoba (serogroup distribution: 2 cases B, 0.8 case C, 1 case W-135, 1.2 cases Y, 0.2 case non-groupable, 0.2 case other and 0.8 case unknown), 4.2 cases in Newfoundland and Labrador (serogroup distribution: 2.8 cases B, 0.2 case C, 0.2 case W-135, 0.2 case Y and 0.8 case unknown), 5.6 cases in New Brunswick (serogroup distribution: 3.6 cases B, 0.4 case C, 0.6 case W-135, 0.4 case Y and 0.6 case unknown) and 4.6 cases in Nova Scotia (serogroup distribution: 2.4 cases B, 1.2 cases C, 0.4 case Y and 0.6 case unknown).
The serogroup distribution of IMD also differs by age, with serogroup Y cases having the highest median age from 2007 to 2011 (47 years), followed by C (44.5 years) and W-135 (38 years). As seen in Figure 3, the proportion of cases due to serogroup B decreases with age while conversely, the proportion of cases due to serogroups C and Y tends to increase with age.
Figure 3. Average reported cases of IMD in Canada by serogroup and age group from 2007 to 2011

Figure 3 - Long Description
Figure 3. Average reported cases of IMD in Canada by serogroup and age group from 2007 to 2011
Figure 3 shows a stacked bar graph depicting the average number of reported cases of IMD in Canada by serogroup and age group from 2007 and 2011. The x-axis contains the age groups and includes infants less than one year of age, one to four year olds, five to nine year olds, ten to 14 year olds, 15 to 19 year olds, 20 to 24 year olds, 25 to 29 year olds, 30 to 59 year olds and adults 60 years of age and older. The y-axis on the left represents the average number of reported cases during that five year period of 2007 to 2011. The y-axis starts with 0 at the bottom and reaches 50 cases at the top. Each stacked bar depicts the distribution of cases due to serogroups B, C, W-135, Y, non-groupable, other and unknown serogroups for each age group.
Serogroup B accounts for the largest average number of reported cases in all age groups. The proportion of cases due to serogroups C and Y tends to increase with age.
The average number of cases was of 27.2 for less than one year olds (serogroup distribution: 21.6 cases B, 1 case C, 0.6 case W-135, 1.8 cases Y, 2.2 unknown), 28 for one to four year olds (serogroup distribution: 20.6 cases B, 0.8 case C, 3 cases W-135, 1.2 cases Y, 0.2 case other, 2.2 cases unknown), 6.6 for five to nine year olds (serogroup distribution: 4.2 cases B, 1 case C, 0 case W-135, 0.4 case Y, 0.4 case other, 0.6 case unknown), 7 for ten to 14 year olds (serogroup distribution: 4.6 cases B, 0.2 case C, 0.2 case W-135, 1.6 cases Y, 0.4 cases unknown), 25 for 15 to 19 year olds (serogroup distribution: 16.4 cases B, 0.8 case C, 0.8 case W-135, 4.6 cases Y, 0.4 case non-groupable, 0.6 case other, 1.4 cases unknown), 15.6 for 20 to 24 year olds (serogroup distribution: 10 cases B, 1.6 cases C, 0.4 case W-135, 2.2 cases Y, 0.4 case non-groupable, 1 case unknown), 7.6 for 25 to 29 year olds (serogroup distribution: 4.8 cases B, 1.4 case C, 0.2 case W-135, 0.6 case Y, 0.2 case other, 0.4 unknown), 43.2 for 30 to 59 year olds (serogroup distribution: 18.2 cases B, 7.6 cases C, 2.8 cases W-135, 10.8 cases Y, 0.6 case non-groupable, 0.6 case other, 2.6 unknown), 31.8 for those 60 years of age and older (serogroup distribution: 10.6 cases B, 4.6 cases C, 3.2 cases W-135, 10.4 cases Y, 0.2 case non-groupable, 1 case other, 1.8 cases unknown).
The incidence of serogroup B is low and remains highest in infants less than one year of age with an age-specific incidence rate of 5.8 cases per 100,000 in 2011, followed by one to four year olds (1.4 cases per 100,000) and 15 to 19 year olds (0.7 cases per 100,000). As seen in Table 3, although serogroup B incidence rates follow similar trends across all P/Ts, the incidence of serogroup B among 15 to 19 year olds has been particularly high in Québec compared to other regions (2.6 cases per 100,000 in 2011).
| P/T | Less than 1 | 1 to 4 | 5 to 9 | 10 to 14 | 15 to 19 | 20 to 24 | 25 to 29 | 30 to 59 | 60 and greater | All ages |
|---|---|---|---|---|---|---|---|---|---|---|
| BC | 5.02 | 0.35 | 0.18 | 0 | 0.21 | 0.33 | 0.13 | 0.10 | 0.09 | 0.18 |
| AB | 3.61 | 0.65 | 0.10 | 0.09 | 0.08 | 0.28 | 0.06 | 0.10 | 0 | 0.17 |
| SK | 10.37 | 1.19 | 0.94 | 0 | 0 | 0 | 0 | 0.05 | 0.10 | 0.29 |
| MB | 0 | 1.34 | 0 | 0 | 0.22 | 0.22 | 0.48 | 0.08 | 0 | 0.16 |
| ON | 3.02 | 0.78 | 0.06 | 0.10 | 0.23 | 0.29 | 0.09 | 0.10 | 0.10 | 0.18 |
| QC | 11.95 | 3.05 | 0.57 | 0.71 | 2.57 | 1.01 | 0.53 | 0.20 | 0.36 | 0.76 |
| NL | 16.38 | 6.28 | 0 | 0 | 0.60 | 0 | 0.70 | 0.09 | 0.17 | 0.55 |
| NB | 5.38 | 4.16 | 0.53 | 0.50 | 0.42 | 0.43 | 0 | 0.24 | 0.24 | 0.48 |
| NS | 2.30 | 0.58 | 0.42 | 0.76 | 0.34 | 0.30 | 0 | 0.15 | 0.19 | 0.26 |
| PE | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.65 | 0.14 |
| YK | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| NT | 0 | 7.51 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.45 |
| NU | 24.66 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.60 |
| Canada | 5.78 | 1.40 | 0.23 | 0.23 | 0.73 | 0.43 | 0.21 | 0.12 | 0.16 | 0.33 |
| P/T | Less than 1 | 1 to 4 | 5 to 9 | 10 to 14 | 15 to 19 | 20 to 24 | 25 to 29 | 30 to 59 | 60 and greater | All ages |
|---|---|---|---|---|---|---|---|---|---|---|
| BC | 2.2 | 0.6 | 0.4 | 0 | 0.6 | 1 | 0.4 | 2 | 0.8 | 8 |
| AB | 1.8 | 1.2 | 0.2 | 0.2 | 0.2 | 0.8 | 0.2 | 1.6 | 0 | 6.2 |
| SK | 1.4 | 0.6 | 0.6 | 0 | 0 | 0 | 0 | 0.2 | 0.2 | 3 |
| MB | 0 | 0.8 | 0 | 0 | 0.2 | 0.2 | 0.4 | 0.4 | 0 | 2 |
| ON | 4.2 | 4.4 | 0.4 | 0.8 | 2 | 2.6 | 0.8 | 5.6 | 2.4 | 23.2 |
| QC | 10.4 | 10.2 | 2.2 | 3 | 12.8 | 5 | 2.8 | 6.8 | 6 | 59.2 |
| NL | 0.8 | 1.2 | 0 | 0 | 0.2 | 0 | 0.2 | 0.2 | 0.2 | 2.8 |
| NB | 0.4 | 1 | 0.2 | 0 | 0.2 | 0.2 | 0 | 0.8 | 0.4 | 3.6 |
| NS | 0.2 | 0.2 | 0.2 | 0.4 | 0.2 | 0 | 0 | 0.6 | 0.4 | 2.4 |
| PE | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.2 | 0.2 |
| YK | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| NT | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.2 |
| NU | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.2 |
| Canada | 21.6 | 20.6 | 4.2 | 4.6 | 16.4 | 10 | 4.8 | 18.2 | 10.6 | 111 |
Figure 4. Age distribution of reported invasive meningococcal disease cases among infants less than one year of age in Canada from 2005 to 2011 by serogroup and age in months

Figure 4 - Long Description
Figure 4. Age distribution of reported invasive meningococcal disease cases among infants less than one year of age in Canada from 2005 to 2011 by serogroup and age in months
Figure 4 shows a bar graph depicting the age distribution of reported IMD cases among infants less than one year of age in Canada from 2005 to 2011 by serogroup and age in months. The x-axis contains the age in months from 0 to 11 months. The y-axis on the left represents the number of reported cases during that seven year period of 2005 to 2011. The y-axis starts with 0 at the bottom and reaches 25 at the top. For each month, there are 2 bars which depict the number of reported cases for serogroup B and the number of reported cases for the other serogroups.
Serogroup B accounted for the largest number of cases among infants less than 1 year of age and especially among infants less than 6 months old. From 2005 to 2011, a total of 84 cases of serogroup B were reported in children less than 6 months old and 15 cases were reported with other serogroup. During that same period, 54 cases of serogroup B and 22 cases of other serogroup were reported in children aged between 6 and 11 months.
The total number of cases for children aged less than 1 month was 9 (7 cases of serogroup B, 2 others), 17 for children aged one month old (15 cases of serogroup B, 2 others), 12 for children aged 2 month old (12 cases of serogroup B), 17 cases for children aged 3 month old (15 cases of serogroup B, 2 others), 21 cases for children aged 4 month old (20 cases of serogroup B, 1 other), 23 cases for children aged 5 month old (15 cases of serogroup B, 8 others), 10 cases for children aged 6 month old (6 cases serogroup B), 17 cases for children aged 7 month old (11 cases serogroup B, 6 others ), 15 cases for children aged 8 month old (10 cases serogroup B, 5 others), 9 cases for children aged 9 month old (8 serogroup B, 1 other), 9 cases for children aged 10 months old (9 cases serogroup B) and 16 cases for children aged 11 month old (10 cases serogroup B, 6 others).
Antigenic and Genetic Characterization of serogroup B IMD by current routine methods
From 2007 to 2011, among cases known to be confirmed by culture or PCR, 85% of cases were confirmed by culture, 10% were confirmed by PCR, and 5% were confirmed by both (method of confirmation not stated for 3% of the cases). MenB isolates in Canada are characterized by serotyping and serosubtyping using monoclonal antibodies, Footnote 27 PorA genotype determination; Footnote 28Footnote 29 and Multilocus Sequence Typing (MLST) classification into sequence type (ST) and clonal complex (cc) according to methods described in the Neisseria.org website.
Analysis of serogroup B isolates from 2001 to 2011 has revealed extensive heterogeneity in the antigenic and genetic characteristics of circulating strains across the country, with the exception of Québec and New Brunswick.Footnote 30Footnote 31 In the province of New Brunswick, an increase in IMD in 2008 to 2011 was due to an ST-154 clone of MenB characterized as B:4:P1.4, PorA genotype P1.7-2,4,37 (member of the ST-41/44 cc). Outside of New Brunswick, this clone has been uncommon, e.g. accounting for only 5% of all invasive MenB isolates in Ontario between 2001-2010.(30) In Québec, the majority (76%) of the serogroup B isolates belonged to a highly homogeneous strain in the ST-269 cc with 92% being ST-269 and 86% expressing the PorA genotype of P1.19-1, 15-11, 36.Footnote 21Footnote 31 In contrast, in Ontario, of the 20 case isolates that belong to the ST-269 cc collected between 2001 and 2010, seven different sequence types and 11 different PorA genotypes were identified.Footnote 30
Relative to the porin A (PorA) P1.4 antigen contained in the 4CMenB vaccine, among serogroup B cases from 2007 to 2011, 8.5% were due to strains expressing this antigen.
There were differences seen across P/Ts, with P1.4 most commonly reported in the Maritimes (33% to 83% of cases depending on the province), but rarely in other provinces such as Québec (1%) and not at all reported in Manitoba and the Territories. Differences also occurred across age groups, with P1.4 most commonly reported in children aged one to four years of age (14% of serogroup B cases) and least commonly reported in adolescents aged 15 to 19 years (4% of serogroup B cases).
Serogroup B IMD Outcomes
From 2007 to 2011, 8.2% of nationally reported IMD cases died. Case fatality ratios (CFRs) differed by serogroup, with serogroup C having the highest CFR at 15.3% and B having the lowest at 6.0% (Table 2).
A study conducted by the Immunization Monitoring Program Active (IMPACT) reported outcomes of 413 laboratory confirmed Canadian serogroup B cases that were hospitalized between 2002 and 2011.Footnote 4 The mean length of hospital stay in this study was 11.2 days and 60.5% of cases required care in the intensive care unit. Among cases admitted to the ICU, 45% required assisted ventilation and 36% required blood pressure support using inotropes. Of 391 survivors, 19% had at least one sequelae due to their infection at or shortly after discharge, with 23% requiring inpatient rehabilitation. Most commonly reported sequelae included deafness (7.2%), skin scarring (6.4%), amputation (3.8%), neurologic sequelae (3.6%), seizures (2.6%), and renal dysfunction (2.0%). Long term outcomes were not reported in this study. However, a case-control study of 245 serogroup B meningococcal disease survivors in the UK, reported major disabling deficits in one tenth, and one or more deficits in physical, cognitive, and psychological functioning, with the additional burden of memory deficits and executive function problems in approximately a third of survivors.Footnote 4Footnote 5
International Burden
IMD is endemic in many countries around the world with regional differences in the serogroup distribution.(32) In Europe, Australia, and New Zealand, the most commonly reported serogroup is B, followed by C, although recent increases in Y have been reported in some areas of Europe.Footnote 32 In the United States, serogroups B and C are most commonly reported, followed closely by Y. There is variation in the serogroup distribution across South America, and although serogroup B followed by C is predominant in many countries, W-135 and Y make up a large proportion in others.Footnote 32 Little is known about endemic epidemiology in Asia.Footnote 32 Africa's most affected region, an area of sub-Saharan Africa known as the "meningitis belt" that stretches from Senegal to Ethiopia, is affected by large serogroup A outbreaks each year, although W-135 has also been predominant in recent years.Footnote 32Footnote 33Footnote 34 In 2011, several countries belonging to the "meningitis belt" reported historically low incidence rates of confirmed IMD cases following the introduction of national serogroup A conjugate vaccine programs.Footnote 32Footnote 35
In the last few decades, serogroup B outbreaks have been reported in regions around the world including the United States (Oregon), New Zealand, Norway, Chile, Cuba, France, Uruguay, Spain, Japan and Brazil, among others.Footnote 13Footnote 32Footnote 36-40 In response to specific outbreaks, several tailor-made, outer membrane vesicle (OMV) serogroup B vaccines have been produced and used in various serogroup B outbreaks with good effectiveness, including VA-MENGOC-BC® in Cuba during the 1980s and Uruguay in 2001, MenBvac® in Norway during the 1970s and 1980s and France from 2006 to 2009, and MeNZBTM in New Zealand from 2004 to 2008. Due to the nature of IMD epidemiology, associated mortality and morbidity, the World Health Organization has advised on the use of enhanced IMD surveillance for timely and appropriate prevention and management of IMD outbreaks and emerging N. meningitidis strains.Footnote 32Footnote 40
IV. Vaccine
IV.1 Preparations authorized for use in Canada
While the polysaccharide capsule provided the basis for previously approved meningococcal vaccines against serogroups A, C, W-135 and Y, the serogroup B capsular polysaccharide has significant similarity to the human neural cell adhesion molecule (nCAM) and cannot be used for vaccine development, primarily due to concerns about creating auto-antibodies. For this reason efforts to develop a serogroup B vaccine have focused on OMVs and other surface exposed protein antigens. Single component serogroup B OMV vaccines have been used in meningococcal serogroup B outbreak settings and appear to be safe and effective.Footnote 41-45
The multicomponent meningococcal vaccine (4CMenB) Bexsero® (Novartis Vaccines), authorized for use on December 6, 2013, is the first serogroup B-specific vaccine available in Canada. The vaccine contains 25 μ of detoxified OMV containing PorA P1.4 from the New Zealand MeNZBTM vaccine, plus three purified N. meningitidis serogroup B protein antigens identified by reverse vaccinology: 50 μ of factor H binding protein (fHbp, sub-variant 1.1) fused to genome-derived neisserial antigen 2091 (GNA2091), 50 μ of Neisseria heparin binding antigen (NHBA peptide 2) fused to genome-derived neisserial antigen 1030 (GNA1030), and 50 μ of single Neisserial adhesion A (NadA, subvariant 3.1).Footnote 26Footnote 46 Antigens contained in the vaccine are adsorbed on 1.5 mg of aluminum hydroxide which corresponds to 0.5 mg of elemental aluminum per vaccine dose. 4CMenB vaccine has been authorized for use in persons from 2 months through 17 years of age.
A detailed review of all vaccine 4CMenB vaccine components can be found in the Literature review on serogroup B invasive meningococcal disease: epidemiology, multicomponent meningococcal B vaccine characteristics and other factors for consideration.
IV.2 Efficacy and effectiveness
The conducted literature search did not identify any published studies on the efficacy or effectiveness of the 4CMenB vaccine. It should be noted that in non-epidemic settings pre-licensure efficacy studies for meningococcal vaccines are not considered feasible due to significant challenges in conducting such studies (i.e. relative rarity of endemic IMD), and other conjugate meningococcal vaccines have been licensed based on immunogenicity.
NZ-OMV monovalent vaccine effectiveness has been estimated between 33-84%, depending on the age cohort, number of doses, modeling methods and time from vaccination (i.e. waning immunity). No studies explicitly describe herd effects of the NZ-OMV monovalent vaccine, but in a declining outbreak setting a drop in IMD rates has been observed with its introduction. It is not known if the decrease in serogroup B IMD in New Zealand was due to secular trends, the immunization program, both, or any other factors.Footnote 40-42Footnote 47-49 It is not clear yet whether the NZ-OMV component in combination with the other antigens in 4CMenB will have the same protective effectiveness as the monovalent vaccine. At the same time, findings from phase II trials comparing the immunogenicity of 4CMenB vaccine to the candidate vaccine without NZ-OMV suggest that, in addition to inducing specific antibodies to the PorA P1.4 antigen, the NZ-OMV component may have an adjuvant effect on the immunogenicity of other 4CMenB vaccine components.Footnote 46Footnote 50Footnote 51
Herd immunity
Since 4CMenB vaccine has not yet been used at a population level, it is not known if it will confer herd immunity. Preliminary data obtained from an oral presentation submitted for the 31st Annual Meeting of the European Society for Pediatric Infectious Disease indicate, in the primary analysis, no reduction in nasopharyngeal carriage following immunization of 932 university students with two doses of 4CMenB vaccine. Other vaccines that eliminate carriage, including serogroup C meningococcal conjugate vaccine, have conferred herd immunity. For example, in a comparison of one year (July 1998-June 1999) prior to the introduction of serogroup C meningococcal conjugate vaccine to the routine childhood immunization schedule in the UK to a one year (July 2001-June 2002) period after the program began, a 35% (95% CI: 20%, 49%) decrease in the incidence of serogroup C IMD was observed among adults greater than 25 years old. In this vaccine ineligible group, the rate of serogroup C IMD went from 0.53/100 000 to 0.34/100 000.Footnote 52 Ongoing unpublished studies examining the effect of 4CMenB vaccine on nasopharyngeal carriage of meningococci are expected to provide additional information about its potential to affect herd immunity and to confer population-level benefits.
IV.3 Immunogenicity
Immunogenicity outcomes most commonly used and approved by regulators to determine susceptibility and short-term immunity to IMD are human complement serum bactericidal activity (hSBA) levels and enzyme linked immunosorbent assays (ELISA). In previously conducted trials of OMV vaccines for serogroup B IMD, the proportions of vaccinees with ≥ 4-fold rises in hSBA pre- to post-vaccination or hSBA titres of ≥1:4 have been correlated with clinical efficacy.Footnote 53
Immunogenicity of 4CMenB vaccine was measured and reported in ten trials including approximately 5800 healthy participants, of whom 4000 were children aged 2 to 24 months, 84 were children 40-43 months and 1738 were adolescents or adults aged 11 to 55 years. These trials assessed the post-vaccination immune response to each vaccine antigen independently, using a combination of hSBA titres (of ≥1:4 or ≥1:5) against selected reference strains H44/76 (fHbp Novartis sub-variant 1.1), 5/99 (NadA sub-variant 2.2) and NZ98/254 (PorA P1.7-2,4). Studies that were conducted prior to the identification of a reference strain that primarily expresses NHBA vaccine antigen peptide 10 (M10713) measured the quantity of antigen-specific IgG. Only one publication by Vesikariet al(2013) reported the percentage of participants with hSBA titres against reference strain M10713 in infants aged ≤12 months.Footnote 54Footnote 55Footnote 74
In infants aged ≤12 months, 4CMenB vaccine was found to be immunogenic after at least two doses, and an anamnestic response to a booster dose, given at 12 months of age, was also evident. The infant vaccination schedules assessed include: three doses given at 2, 3 and 4 months of age; three doses given at 2, 4 and 6 months of age with or without a booster at 12 months of age; and three doses given at 6 to 8 months of age, 60 days later and 12 months of age. In the group that received a booster dose at 12 months of age, hSBA titres waned prior to the booster dose, with only between 34% and 89% of infants meeting the antibody threshold, depending on the antigen.Footnote 46 Further, 12 months after the booster dose, at age 24 months, hSBA titres were low, especially against strain NZ98/254.Footnote 56 Non-inferiority was also demonstrated when the 4CMenB was administered with concomitant vaccines (Infanrix-hexa®and Prevenar®) compared to when it was administered alone; the exception was strain NZ98/254 where a higher proportion of infants obtained hSBA titres ≥1:5 when these vaccines were given on separate occasions, suggesting that the NZ-OMV component may be impacted by schedule.Footnote 5 In a different trial, similar proportions of infants reached the hSBA threshold after a booster dose, with or without concomitant Priorix-TetraTM.Footnote 55
In children aged 12 to 24 months, 4CMenB vaccine was found to be immunogenic against strains H44/76, 5/99 and NZ98/254 after two doses (given at either 12 and 14, or 13 and 15 months of age), Footnote 58 but not after a single dose given at 12 months of age.Footnote 46 Geometric mean titres (GMTs) were between 32 and 627 one month after the second dose of 4CMenB vaccine, compared to between 1.0 and 1.2 at baseline. However, hSBA titres waned after 9 to 10 months (when measured at age 24 months) and were lowest against strain NZ98/254.Footnote 56 A third dose of 4CMenB vaccine given at 24 months of age stimulated hSBA titres of ≥1:5 against strains H44/76, 5/99 and NZ98/254 in all participants.
For the 84 children who received two doses of 4CMenB vaccine at 40 and 42 months of life, seroprotection was achieved one month after the second dose for each of the reference strains by 70-100% of participants, depending on reference strain. The proportion with seroprotective titres was lowest against strain M10713 which measures response to the NHBA antigen.Footnote 59-62
In adolescents and adults, 4CMenB vaccine was found to be immunogenic against strains H44/76, 5/99 and NZ98/254 after at least one dose, although higher GMTs were seen after two compared to one dose of the vaccine; at 6 months, at least 91% of adolescents had hSBA titres of ≥1:4 for each of the three reference strains after two or three doses, compared to 73-76% after one dose.Footnote 63 In adults, four months after the second dose, 96% and 100% had hSBA titres of ≥1:4 against strains H44/76 and 5/99, respectively, compared to 67% against strain NZ98/254.Footnote 64
Overall, compared to the other selected reference strains, immune responses were generally lowest to strain NZ98/254, which expresses multiple antigens found in the 4CMenB vaccine including identical PorA (P1.4) and NHBA (peptide 2), as well as the cross-reactive fHbp variant 1.Footnote 65 It has been suggested that the low response of vaccinated sera with this strain may be attributable in part to the low level of expression of these antigens by NZ98/254.Footnote 46
Findings from phase II trials comparing the immunogenicity of 4CMenB vaccine to that of a candidate recombinant meningococcal B (rMenB) vaccine without the OMV component, suggest an adjuvant effect of the OMV component.Footnote 46Footnote 50 Studies of the immunogenicity of NZ-OMV vaccine among infants and children in New Zealand showed a beneficial effect of a third dose.Footnote 66 However, similar to 4CMenB vaccine, a fairly rapid decline of bactericidal antibodies was seen after three doses.Footnote 67 A fourth dose of NZ-OMV given at 10 months of age (5 months after the third dose) elicited a booster response, increasing the percentage of infants achieving the hSBA threshold from 48% after dose three to 69% after dose four.Footnote 67 Post-licensure NZ-OMV studies estimated the vaccine effectiveness to be between 53.3% and 84%.
The longest period in which studies to date have measured immunogenicity of 4CMenB vaccine was at 40 months of age, 28 months after the completion of 3+1 infant schedule.Footnote 56 In toddlers immunogenicity was measured 12 months after the last dose of a 2-dose series,Footnote 56 in adolescents 24 months after the last dose of a one-, two-, or three-dose schedule,Footnote 63 and in adults one month after the third dose.Footnote 64 Preliminary evidence indicates waning immunity to the PorA antigen. Because beyond these short periods there are no data regarding circulating antibody levels, the duration of protection will need to be addressed in future studies, particularly as it appears that high titres of circulating anti-meningococcal antibodies are required to prevent disease after exposure.Footnote 68
A detailed review of 4CMenB vaccine immunogenicity can be found in the Literature review on serogroup B invasive meningococcal disease: epidemiology, multicomponent meningococcal B vaccine characteristics and other factors for consideration.
Meningococcal Antigen Typing System (MATS)
The MATS assay developed by Novartis uses antigen-specific ELISA to measure the immunologic cross-reactivity and quantity of NHBA, NadA and fHbp antigens in a meningococcal isolate to predict the level of vaccine protection against a specific strain. In addition to the MATS assay, PorA genotyping information from the tested meningococcal strains is used for predicting the immune response. MATS is an in vitro prediction of how well 4CMenB vaccine will protect against currently circulating serogroup B meningococcal strains. At the time of the literature review, this prediction is based on the correlation of MATS and hSBA that was reported in only one published study.Footnote 69Footnote 70 Using pooled sera from 13-month-olds who had received 4CMenB (3+1) schedule, 89% of tested strains that were above the positive bactericidal threshold for one or more antigens were "killed" by the hSBA. Seventy-seven percent of tested strains that were below the positive bacterial threshold were also "not killed" by the hSBA. This means that 11% were falsely positive on MATS (predicted to have been killed but were not) and 23% were falsely negative (predicted to not be killed but were). Possible reasons for the potential underestimation of effectiveness include immunogenicity against other antigens present in OMV that are not captured in MATS, lack of assay ability to capture the synergistic action of antibodies to different antigens and the repression ofNadA expression in vitro. Potential reasons for the overestimation of effectiveness include over-expression of target antigens in vitro.
IMPACT investigators have looked for presence of vaccine antigen surface proteins on IMPACT derived Canadian strains using the MATS assay.Footnote 71Footnote 72 Susceptibility was assessed for 157 serogroup B meningococcal strains obtained in 12 Canadian cities through population-based catchment-area surveillance of over 17 million adults and children (just over 50% of the Canadian population) from 2006-2009. Overall, the 4CMenB vaccine MATS predicted strain coverage in Canada was 66% (95% CI: 46%, 78%), with 26% of strains covered by one, 29% covered by two and 11% covered by three vaccine antigens. Coverage by antigen was as follows: NHBA 51% (95% CI: 21%, 71%), NadA 1% (95% CI: 0.6%, 3%), fHBP 52% (95% CI: 40%, 59%), and PorA 13% (95% CI: 8%, 18%). Of the 6 isolates from fatal cases, 4 (67%) were predicted covered, as were 23 of the 34 (68%) isolates from cases that resulted in sequelae. The authors considered 4CMenB vaccine to protect against a strain if the strain possessed PorA P1.4 or had a relative potency above the positive bactericidal threshold for fHbp, NHBA or NadA. For isolates from children <1 year old, 49% (95% CI: 29%, 71%) were covered by the vaccine, whereas 74% (95% CI: 61%, 90%) of isolates from those aged 1-4 years and 81% (95% CI: 59%, 84%) from those aged 5-19 years were covered. Sixty five percent (95% CI: 39%, 72%) of isolates from adults aged 20 years and older were covered by the vaccine. By province, the predicted coverage of 4CMenB ranged from 43% to 100% and reflected the strains circulating within each region and the level of antigen expression within each isolate. A very large proportion (95%) of the 37 ST-269 isolates matched the vaccine. ST-269 was the most frequent clonal complex in Québec.Footnote 72Footnote 73
IV.4 Adverse events
Across nine 4CMenB vaccine trials reporting safety, outcomes were measured and reported in approximately 4,800 infants less than 12 months of age, 1600 children aged 12 to 24 months, 84 children 40-43 months of age and 1738 adolescents or adults aged 11 to 55 years. In these trials, solicited local and systemic reactions were recorded during a seven-day period following vaccination and serious and other adverse events were reported up to six months after the last dose of 4CMenB vaccine. The literature search did not identify any studies of the safety and reactogenicity of 4CMenB vaccine in children ages 4 to 10 or adults over the age of 55 years.
Among infants and children up to 12 months of age, most commonly reported local and systemic adverse events following vaccination with 4CMenB vaccine included erythema, induration, fever and sleepiness or irritability. Among infants, similar proportions of local reactions at the 4CMenB injection site were observed when 4CMenB vaccine and routine infant vaccines were given on separate occasions versus together, except for pain which was higher following concomitant administration.Footnote 57 Higher proportions of infants with solicited systemic reactions, including fever, were observed when 4CMenB vaccine was given together with Infanrix-hexa® and Prevenar®. When given concomitantly, temperature ≥38°C was reported in up to 61% children, compared to 38% when the 4CMenB vaccine was given alone and 33% when only routine vaccines were given. The fever was more common after the first or second dose of 4CMenB vaccine than the third dose and occurred mostly within the first six hours after vaccine administration, with very few fevers persisting beyond 2 days following vaccination.Footnote 74Footnote 75Footnote 57 In the only infant study that used Pediacel®Footnote 46 as the DTaP-IPV-Hib vaccine, the proportion that experienced fever following concomitant administration with 4CMenB vaccine was comparatively lower (9.2% all doses, 18% after the first dose). However, this study only included 46 4CMenB vaccine recipients and is too small to allow any conclusions to be drawn regarding the impact of differences in formulation of routine infant vaccines on fever after simultaneous administration of 4CMenB vaccine.
Among children 12-24 months old, the solicited local and systemic reactions were common and included tenderness, induration, fever, sleepiness or irritability. Systemic reactions were generally higher among children that received 4CMenB vaccine with Priorix-TetraTM. A higher proportion of children experienced temperatures of ≥38°C when 4CMenB vaccine was given concomitantly with Priorix-TetraTM, primarily due to two risk periods for fever occurring at 1-4 days (4CMenB vaccine) and 5-28 days (Priorix-TetraTM). In children who had previously received the 4CMenB vaccine at 2, 4 and 6 months of age, a booster dose of 4CMenB increased the reported rate of fever when given concomitantly with Priorix-TetraTM (48%) compared to when given separately (40%).Footnote 55Footnote 58Footnote 74
The 4CMenB vaccine was provided to only 84 children aged 40 to 42 months. In these children, up to 18% experienced fever and 7 participants experienced severe transient arthralgia, 2 of whom reported arthralgia after both first and second vaccination. Local reactions were very common in this group and included pain (up to 92%), erythema (up to 98%), induration (up to 50%) and swelling (up to 70%).Footnote 59Footnote 76
Among adolescents, proportions of local reactions after 4CMenB vaccine were somewhat similar after each dose, with a slight decrease in percentages after the second and third dose compared to after the first dose. Solicited local reactions were reported from 39% (swelling) up to 86% (pain) of 4CMenB vaccine recipients, while systemic reactions were reported in from 4% (fever ≥38°C) up to 51% (malaise) of 4CMenB vaccine doses (all doses combined).Footnote 63 Fever was significantly higher following 4CMenB vaccine compared to an alum-containing control (4% vs. 2%, p<0.01), as was the proportion of 4CMenB vaccine recipients that reported using antipyretic drugs (4% vs. 2%, p<0.02). In two adult studies, solicited local reactions were reported by 47% (erythema) up to 98% (pain) of 4CMenB vaccine recipients, while solicited systemic reactions were reported by 2.6% (fever) up to 38.1% (malaise) (all doses combined). Twelve percent of adolescents and 9% of adults reported staying home as a result of 4CMenB vaccination.
According to the authors, no increase in febrile seizures was seen in the initial reports from trials of 4CMenB vaccine.Footnote 77-79 Based on the Vesikari et al(2013) study, 4 seizures (all of which were accompanied by fever but two of which were reported as febrile seizures) occurred among 2478 infants < 12 months old within 24 hours of receipt of 4CMenB vaccine and routine vaccines.Footnote 80 In a group of 84 children given a two-dose primary series of 4CMenB vaccine at 40 and 42 months of age, only one febrile seizure was reported eight hours after the receipt of a second dose.Footnote 59Footnote 61Footnote 76
In addition, a total of 7 cases of suspected Kawasaki Disease (KD) were reported in phase 2 and phase 3 clinical studies (6 cases were reported in vaccine recipients and one in a control subject). This is a relatively high number when compared to the very low background incidence of KD. No definitive causal relationship has been determined by the study's authors.
Aluminum containing placebo
In the only placebo-controlled trial of 4CMenB vaccine, by Santolayaet al (2012), Footnote 63 there was comparable reactogenicity between 4CMenB vaccine and an aluminum hydroxide control. Rather than an inert, non-reactive placebo, the authors used a placebo containing aluminium, an adjuvant, as their control since 4CMenB vaccine also contains 1.5 mg of aluminium hydroxide. When interpreting the safety data from this trial, potential inflation of the adverse events profile of the reactogenic placebo and the consequent artificial increase of the study vaccine's safety profile should be taken into consideration.
A detailed review of studies concerning 4CMenB related vaccine safety can be found in the Literature review on serogroup B invasive meningococcal disease: epidemiology, multicomponent meningococcal B vaccine characteristics and other factors for consideration.
IV.5 Vaccine administration and schedule
4CMenB vaccine is supplied in packs of one or ten 0.5 mL pre-filled syringes with or without needles. The tip cap of the syringe may contain natural rubber latex. There is no reconstitution or dilution required prior to administration. It must be shaken before use to ensure a homogenous suspension. 4CMenB vaccine should be administered through intramuscular injection into the deltoid or anterolateral thigh, depending on the age of recipient.
Vaccine schedule varies with age at administration. The manufacturer suggests for infants who begin primary 4CMenB immunization between the ages of 2 months and 5 months, that three doses should be given, with an interval of at least one month between doses. This series is to be followed by a fourth dose (booster dose) administered between 12 and 23 months of age. The manufacturer advises a three dose schedule for infants who begin the series between ages 6 and 11 months. The first two doses should be separated by an interval of two months (rather than one month as used in the accelerated option for younger infants) and a third dose is recommended between 12 and 23 months of age, no less than two months after the second dose.
When primary immunization is initiated in children aged 12 months to 10 years, the manufacturer suggests two doses of 4CMenB vaccine separated by a two month interval.
For persons aged 11 through 17 years of age, the manufacturer recommends two doses given at least one month apart.
Although the manufacturer currently does not provide an adult schedule, in clinical trials of individuals from 18 to 55 years of age, two doses given at least one month apart have shown to be immunogenic and safe.
The duration of protection after primary immunization with 4CMenB vaccine is unknown. Therefore, the need for a booster dose, after any of the recommended immunization schedules, is yet to be determined.
IV.6 Storage requirements
4CMenB vaccine should be stored in the original package, to protect the vaccine from light, in a refrigerator at +2 to +8°C and should not be frozen.
IV.7 Simultaneous administration with other vaccines
4CMenB vaccine has been given simultaneously with a hexavalent tetanus-diphtheria containing infant vaccine, heptavalent pneumococcal conjugate vaccine (PCV7), serogroup C meningococcal vaccine and MMRV. The only study that compared simultaneous administration of 4CMenB vaccine with other vaccines to separate administration schedules was a multicenter phase IIB trial conducted at 60 sites in six European countries and involving 1571 infants. In this study, titres to some of the 4CMenB vaccine test strains were lower when the vaccine was given simultaneously with Prevenar® and Infanrix-hexa® but statistical non-inferiority criteria were met for all but the comparison of the separate schedule versus concomitant 2, 4, 6 month schedule against strain NZ98/254, suggesting that concomitant administration with DTaP-IPV-Hib-HepB and PCV7 does not significantly alter the immunogenicity of 4CMenB vaccine.Footnote 57 However, as described in the safety section, higher rates of fever were observed with simultaneous administration of 4CMenB vaccine and routine infant vaccines (DTaP-IPV-Hib-HepB and PCV7) versus when they were separated. This observation needs to be considered in the Canadian context, as some jurisdictions use all of these vaccines as a part of their current publicly funded programs.
Regarding the effect of 4CMenB vaccine on the immunogenicity of other vaccines, in the Gossger et al(2010) Footnote 57 trial comparing the immunogenicity of three different 4CMenB vaccination schedules, pre-specified non-inferiority criteria of routine vaccine responses when Infanrix-hexa® and Prevenar® were given concomitantly with 4CMenB vaccine at 2, 3 and 4 months of age to routine vaccines alone was met for all routine vaccine antigens with the exception of pertussis' pertactin and pneumococcal serotype 6B. The clinical significance of this finding is unknown.
In the Vesikari et al(2010) Footnote 54Footnote 74 trial comparing different 4CMenB vaccine lots given with concomitant Infanrix-hexa® and Prevenar®, at 2, 4 and 6 months of age, pre-specified non-inferiority criteria of routine vaccination responses were met for all vaccine antigens with the exception of polio 2 when 4CMenB vaccine was given concomitantly with Infanrix-hexa® and Prevenar® compared to Infanrix-hexa® and Prevenar® given alone.
In the Vesikariet et al(2011) Footnote 55Footnote 74 extension study, nearly all participants (97-100%) had immune responses to the four components of Priorix-TetraTM; responses were not significantly different when Priorix-TetraTM was given with or without 4CMenB vaccine.
A detailed review of evidence concerning concomitant use of 4CMenB with other vaccines can be found in the Literature review on serogroup B invasive meningococcal disease: epidemiology, multicomponent meningococcal B vaccine characteristics and other factors for consideration.
IV.8 Contraindications and precautions
4CMenB vaccine is contraindicated in persons with a serious allergy to any vaccine component or previous dose. There are no studies of 4CMenB vaccine in pregnant or lactating women, or in persons less than two months and over 55 years of age.
Immunogenicity and safety studies to date have excluded persons with chronic medical conditions, including those with increased risk of IMD such as terminal complement deficiencies. As such, it is unknown if there are any contraindications to or precautions for the use of 4CMenB vaccine in these groups.
Some 4CMenB vaccine studies excluded persons with history of previous serogroup B IMD Footnote 46Footnote 50Footnote 57 and others excluded persons with any IMD in the past, regardless of serogroup. Thus, it is unknown if there are any contraindications to or precautions for the use of 4CMenB vaccine in those with previous meningococcal infection.
IV.9 Other considerations
Implications of acetaminophen
Prymulaet al (2011) Footnote 81 assessed the impact of prophylactic acetaminophen on the immunogenicity and safety of routine vaccines (Infanrix-hexa® and Prevenar®) when given concomitantly with 4CMenB vaccine at 2, 3 and 4 months of age. There were no significant differences in the immunogenicity of 4CMenB vaccine against reference strains H44/76-SL, 5/99 and NZ98/254 when co-administered with routine vaccines with or without prophylactic acetaminophen. It is not clear whether parental administration of acetaminophen, independent of the study, was included in usual care in the non- acetaminophen group, or if this group was instructed not to take acetaminophen. Prophylactic acetaminophen was found to reduce febrile events after vaccinations. The proportion of infants with temperature ≥38.5°C was nearly 50% lower in infants who received acetaminophen than those who did not (51% vs. 25%). Although temperature ≥ 39.5°C was uncommon in both groups, a smaller proportion of infants had fever (≥ 39.5°C) when given acetaminophen (1% vs. 5%). Additionally, the proportion of infants with fever (≥ 38.5°C and ≥ 39.5°C) decreased with each successive dose of 4CMenB vaccine. The immunogenicity of the 4CMenB vaccine was not affected by the use of acetaminophen.
Interestingly, when parents of the open-label subset were informed of potential fever events after vaccination in an on-going phase III study, Footnote 74 the probability of medically attended fever among infants who received 4CMenB vaccine concomitantly with routine vaccines (Infanrix-hexa® and Prevenar®) was lower in the open-label subset than the observer-blind subset where parents were not informed of the potential for fever (1.42% vs. 5.27%). Although 93% of the parents had reported using analgesics or antipyretics after one of the 2, 4, or 6-month doses, details on whether they were counseled to prophylactically administer medication remains unclear.
The results of Prymulaet al (2011)Note de bas de page 81 imply that routine prophylactic administration of acetaminophen may be an appropriate strategy to counter high rates of fever among infants vaccinated with 4CMenB vaccine. A practice such as this would stray from current practice. Although there are no recommendations in the Canadian Immunization Guide regarding prophylactic use of antipyretics at the time of immunization, parental administration of anti-pyretic drugs, such as acetaminophen or ibuprofen, is generally recommended by health care providers for treatment of the self-limited fever that occurs after vaccination. There is not a typical practice among health care providers regarding prophylactic administration of antipyretics to prevent vaccine-related fever; some may recommend to do so and others may not. As well, there are no safety data on a practice whereby anti-pyretics are routinely given accompanying each dose of a given vaccine, as the Prymula et al study seems to suggest.
Cross-reactivity with other meningococcal serogroups
Sub-capsular proteins found in 4CMenB vaccine may be expressed in all meningococcal serogroups and there are data to indicate that 4CMenB could potentially confer protection against other IMD causing strains.Footnote 82-84. Prevalence and genetic diversity of 4CMenB vaccine containing antigens in strains beyond serogroup B will need to be further investigated in Canada to determine their susceptibility and potential impact on existing vaccination programs.
V. Recommendations
In developing the recommendations, NACI and MBPPTG considered the burden of illness from IMD, the safety and immunogenicity of the newly authorized 4CMenB vaccine, as well as other aspects of overall immunization strategies. However, due to the lack of evidence and the range of uncertainty of the underlying assumptions, particularly those concerning the vaccine's coverage of circulating strains, herd immunity, effectiveness and potential adverse effects of vaccination at the population level. These recommendations will be updated at the time new data becomes available. Epidemiological, economic and other local programmatic/operational factors will be considered by P/Ts when deciding on the inclusion of the following recommendations in publicly funded immunization programs (additional information is provided in the Common Guidance Statement.
Recommendation 1
Multi-component meningococcal serogroup B (4CMenB) vaccine may be considered on an individual basis, for persons greater than or equal to two months of age, to protect against invasive meningococcal disease caused by relevant strains of serogroup B Neisseria meningitidis. (NACI Recommendation Grade B)
For the individual, there is sufficient preliminary evidence that 4CmenB vaccine is immunogenic, and may offer protection against strains expressing antigens covered by the vaccine, when given according to the schedules used in clinical trials. It has an acceptable safety profile with variable rates of adverse effects, as outlined above.
In Canada, 4CMenB vaccine has been authorized for use in individuals from two months through 17 years of age. However, data reported in clinical trials indicates that 4CMenB vaccine is immunogenic and safe when given to adults up to 55 years of age using a two dose schedule with an interval of at least one month between doses. When advising on immunization with 4CMenB vaccine, individual preferences, regional serogroup B IMD incidence and strain susceptibility based on MATS testing should be considered. In circumstances in which the potential benefits of 4CMenB vaccine appear to outweigh the risks of adverse events following immunization, the use of 4CMenB vaccine should be considered. When giving the vaccine, vaccine recipients or parents/caregivers should be informed about anticipated local and systemic reactions and provided instructions for their optimal management. Common adverse events include pain and fever.
Recommendation 2
There is insufficient evidence for the use of multi-component meningococcal serogroup B (4CMenB) vaccine in routine immunization programs for Canadian infants, children, adolescents and adults. (NACI recommendation Grade I)
Serogroup B is the most common IMD causing strain in Canada. From 2007 to 2011, on average there were 22 cases of meningococcal B IMD reported in Canada in children less than one year of age and 21 cases in children one to four years of age. The majority of serogroup B cases have occurred in one province in children under four years of age.
There are no available effectiveness studies at the population level for this vaccine, and the only evaluation of strain susceptibility (i.e. strain characterization) in Canada comes from a single IMPACT study that used the MATS assay, whose validity in the field has not yet been assessed. Based on the MATS assay, 66% of the overall proportion of Canadian serogroup B meningococcal strains are predicted to be susceptible to the 4CMenB vaccine. Given this information and the fact that cases occur too early in life to be vaccine preventable, an infant vaccination in Canada that is 100% effective, with 100% population coverage and that protects until age four years would theoretically, prevent up to 11 cases in infants under one year of age and 16 cases in children from one to four years of age per year. A total of up to two deaths per year would be prevented in these age groups.
The risks of introducing the vaccine to the Canadian population as a whole remain unknown. There are concerns about high rates of fever reported in clinical trials (particularly when administered to infants simultaneously with other recommended vaccines) and other observed adverse events (i.e. febrile seizures, arthralgia, Kawasaki Disease) that may translate to high frequencies of adverse events should this vaccine be used widely in the general population.
On a population level, there is insufficient evidence to support the use of 4CmenB vaccine in routine immunization programs in Canada given the following: currently available information on burden of disease; predicted level of strain susceptibility and vaccine safety; uncertainty regarding the duration of protection; and the lack of data on the effects of 4CMenB vaccine on meningococcal carriage as well as its impact on herd immunity. However, in circumstances in which the potential benefits of 4CMenB vaccine may outweigh the uncertainty of using the 4CMenB vaccine at the population level, regional serogroup B IMD incidence and strain susceptibility based on MATS testing should be considered as part of decision making.
A detailed discussion of all outlined considerations is presented in Section IV of the Statement as well as in the Common Guidance Statement.
Recommendation 3
Multi-component meningococcal serogroup B (4CMenB) vaccine should be considered for active immunization of individuals greater than or equal to two months of age who are at high risk of meningococcal disease to prevent invasive meningococcal disease caused by serogroup B N. meningitidis. (NACI Recommendation Grade I)
NACI identifies the following groups as being at higher risk of meningococcal disease than the general population:
- Individuals with specific underlying medical conditions:
- persons with anatomic or functional asplenia (including sickle cell disease)
- persons with congenital complement, properdin, factor D or primary antibody deficiencies
- persons with acquired complement deficiencies (e.g. those receiving eculizumab)
NACI has previously stated that meningococcal vaccines could be considered for individuals with HIV.Footnote 85
- Individuals who are at an ongoing risk of exposure:
- research, industrial and clinical laboratory personnel who are routinely exposed to N. meningitidis
- military personnel during recruit training (military personnel may be at increased risk when accommodated in close quarters)
- see Recommendation 8 below regarding travellers
This recommendation is consistent with NACI recommendations for other meningococcal vaccines and is based on expert opinion. NACI was unable to provide a stronger recommendation due to insufficient evidence regarding the safety and immunogenicity of 4CMenB vaccine in individuals at higher risk of IMD. 4CMenB vaccine has only been studied in a small number of laboratory workers but not in any of the other high risk groups mentioned above.
Recommendation 4
Multi-component meningococcal serogroup B (4CMenB) vaccine should be considered, in addition to chemoprophylaxis, for protection of individuals 2 months of age or older having close contact with a case of invasive meningococcal disease caused by serogroup B N. meningitidis. (NACI Recommendation Grade I)
Close contacts of individuals with meningococcal infections have an increased risk of developing IMD and should receive vaccination (immunoprophylaxis) in addition to chemoprophylaxis. This risk is greatest for household contacts and may persist for up to 1 year after disease in the index case. Vaccination of close contacts of a case of serogroup B IMD should be carried out independent of MATS assay result or other tests of strain susceptibility to the vaccine to ensure there are no delays in contact management. The following individuals should be considered for immunoprophylaxis:
- Household contacts of a case of IMD
- Persons who share sleeping arrangements with a case of IMD
- Persons who have direct nose or mouth contamination with oral or nasal secretions of a case of IMD (e.g., kissing on the mouth, shared cigarettes, shared drinking bottles)
- Children and staff in contact with a case of IMD in child care or nursery school facilities
This recommendation is consistent with NACI recommendations for other meningococcal vaccines and is based on expert opinion. NACI was unable to provide a stronger recommendation due to insufficient evidence regarding the effectiveness of 4CmenB vaccine.
Recommendation 5
During invasive meningococcal disease outbreaks caused by serogroup B N. meningitidis or the emergence of hyperendemic and/or hypervirulent N. meningitidis strains that are predicted to be susceptible to the vaccine based on MATS testing, immunization with the multi-component meningococcal serogroup B (4CMenB) vaccine is recommended for individuals greater than or equal to two months of age. (NACI Recommendation Grade I)
Previous widespread use of conjugate serogroup C and serogroup B OMV vaccines against emerging hyperendemic and/or hypervirulent strains expressing homologous antigens as those present in a vaccine has been demonstrated to be an effective public health strategy for managing clonal IMD outbreaks. This recommendation is consistent with the public health management approach taken for other meningococcal serogroups, in Canada and internationally, and is recommended on the basis of expert opinion.
Consultation with public health officials and/or experts in communicable disease is required for optimal management of meningococcal disease outbreaks.
Recommendation 6
Routine prophylactic administration of acetaminophen and/or separating 4CMenB vaccination from routine vaccination schedule may be considered for preventing fever in infants and children up to three years of age. (NACI Recommendation Grade I)
As high rates of fever observed in the clinical trials represent an important adverse event, different strategies for reducing this risk should be considered in discussions with vaccine recipients and caregivers. High rates of fever have been reported in the first four days (up to 63% of children under 12 months of age and 48% of children 12-24 months of age) when the vaccine was administered concomitantly with routine infant vaccines. Preliminary safety data have demonstrated that the use of acetaminophen immediately prior to and following vaccination can reduce fever rates up to 50% after the first dose without altering the immunogenicity of the vaccine; however, while it may be presumed that fewer fevers should lead to fewer febrile convulsions, there is no evidence that prophylactic use of acetaminophen prevents febrile seizures in children. Prophylactic use of acetaminophen is not recommended for other vaccines. The effect of ibuprofen on fever and immunogenicity of 4CMenB vaccine has not been evaluated.
Recommendation 7
It is recommended that a comprehensive surveillance and vaccine evaluation program be implemented to monitor and evaluate the effects of immunization with 4CMenB vaccine, whether for routine use, outbreaks or for high risk groups/settings. (NACI Recommendation Grade A)
4CMenB vaccine is novel and uncertainty remains with respect to both potential benefits and potential risks of population-wide immunization. Although pre-marketing studies to date have not demonstrated an increased risk of many clinically serious significant adverse events, they were of relatively small sample size and short duration of follow-up (maximum length of follow-up to-date is 39 months following initial vaccination with 4CMenB vaccine at 2 months of age). Similarly, there are currently no data on the efficacy and effectiveness of the 4CMenB vaccine, particularly its potential to protect against Canadian meningococcal strains. Consequently, it will be important to conduct effectiveness and post-marketing safety studies following the introduction of the 4CMenB vaccine in Canada (i.e. monitoring for increased rates of KD and febrile seizures).
Validation of the MATS assay, comprehensive microbiological and enhanced epidemiological surveillance, and other program related issues including the potential effects of systematic prophylactic use of acetaminophen, the impact of 4CMenB vaccination on coverage of other routine infant immunization programs, duration of protection following vaccination, effects on herd immunity and carriage, effect on serogroups other than B and the vaccine's impact on the control of outbreaks and population groups that have not been studied in clinical trials require further surveillance, research and evaluation (see Section VI below).
Recommendation 8
Travellers do not need to receive 4CMenB vaccine unless they are travelling to an area with a hyperendemic strain or an outbreak that is known to be caused by aN. meningitidis serotype B that can be prevented by the vaccine. (NACI Recommendation Grade I)
Data concerning the duration of protection, strain match of the vaccine to circulating strains in different geographic areas and the use of 4CMenB in short or long-term travellers are currently inadequate or lacking. Long-term travellers and those who will be in close contact with the local population through accommodation, public transport, or work are at likely the same risk of IMD as the local population. If the local population is at increased risk due to a hyperendemic strain or an outbreak is occurring that is known to be caused by a N. meningitidis serotype that can be prevented by the vaccine, then the traveler should be vaccinated. Since severe adverse reactions to the vaccine are uncommon, and the disease is one that can have a fatal outcome within a very short period, it may be prudent to proceed with vaccination when the traveller is uncertain about the exact nature of their potential exposures to the local population.
VI. Research priorities and surveillance issues
Multiple research and surveillance priorities remain for all of the issues presented in this statement and are outlined below. Of note, "surveillance" and "research" are intertwined and a recommendation suggested in one area could also be addressed through work in the other. Evidence gaps have been grouped based on issues NACI must address to make a vaccine decision and include: potential of 4CMenB vaccine to protect against Canadian meningococcal B strains and other meningococcal serogroups; vaccine safety; vaccine effectiveness; duration of protection; herd immunity; special populations; and surveillance needs. The number of uncertainties about 4CMenB vaccine makes high quality post-marketing surveillance and research imperative to evaluate the impact of the vaccine and guide future decision making. An integrated funded protocol that addresses surveillance, program evaluation and research needs to be a pre-requisite for the population-wide use of this vaccine in any jurisdiction. The rarity of IMD also means that a co-ordinated multi-province approach would be required for such evaluation.
| Domain | Specific concern | Recommendation |
|---|---|---|
| Surveillance | Meningococcal epidemiology | Enhanced meningococcal surveillance both prior to and after vaccine implementation. Impact of vaccine on serogroup B and non-serogroup B strains among vaccine recipients (direct effects) and persons not receiving the vaccine (indirect/herd effects). |
| Microbiologic characteristics | Laboratories, and in particular reference laboratories, must have capacity to examine traditional microbiologic characteristics of meningococci as well as determine NHBA, NadA, and fHBP types of all isolates using standard (i.e. hSBA) and novel (i.e. MATS) testing methods. | |
| Adverse events following immunization (AEFI) | Enhanced AEFI surveillance such as that of New Zealand "Intensive Vaccines Monitoring Program" for NZ-OMV. As well, ensure baseline data regarding anticipated adverse events (e.g. febrile seizure, KD) is collected. | |
| Vaccine uptake | Universal immunization registries in all Canadian provinces. | |
| Direct vaccine effectiveness (VE) | Vaccination status of all cases. Enhanced surveillance of vaccine failures including microbiologic characteristics of serogroup B IMD in vaccinated and unvaccinated individuals. Sero-epidemiologic studies. Calculation of VE using methods such as case control studies or "screening method" if immunization registry or other source of good quality coverage data present. Measurement of expression of 4CMenB vaccine containing antigens by Canadian Meningococcal strains using MATS (i.e. estimation of potential vaccine effectiveness in Canada). |
|
| Potential Indirect/herd effects | Impact of immunization on disease incidence in unvaccinated cohorts. | |
| Research | Potential Indirect/herd effects | Studies of nasopharyngeal carriage of meningococci prior to and after vaccine implementation. |
| Duration of protection | Sero-epidemiologic studies of immunized persons to look for waning immunity. | |
| Molecular biology of the meningococcus | Microbiology research describing changes in serogroup, clonal complexes, surface-protein characteristics before and after vaccine implementation. | |
| Acceptability of vaccine to the general public | Research exploring risk tolerance/ acceptability of the adverse event profile of 4CMenB vaccine. Impact on coverage of other recommended antigens. |
| Level | Description |
|---|---|
| I | Evidence from randomized controlled trial(s). |
| II-1 | Evidence from controlled trial(s) without randomization. |
| II-2 | Evidence from cohort or case-control analytic studies, preferably from more than one centre or research group using clinical outcome measures of vaccine efficacy. |
| II-3 | Evidence obtained from multiple time series with or without the intervention. Dramatic results in uncontrolled experiments (such as the results of the introduction of penicillin treatment in the 1940s) could also be regarded as this type of evidence. |
| III | Opinions of respected authorities, based on clinical experience, descriptive studies and case reports, or reports of expert committees. |
| Quality Rating | Description |
|---|---|
| Good | A study (including meta-analyses or systematic reviews) that meets all design-specific criteriaFootnote * well. |
| Fair | A study (including meta-analyses or systematic reviews) that does not meet (or it is not clear that it meets) at least one design-specific criterionFootnote * but has no known "fatal flaw". |
| Poor | A study (including meta-analyses or systematic reviews) that has at least one design-specificFootnote * "fatal flaw", or an accumulation of lesser flaws to the extent that the results of the study are not deemed able to inform recommendations. |
|
|
| Grade | Recommendation |
|---|---|
| A | NACI concludes that there is good evidence to recommend immunization. |
| B | NACI concludes that there is fair evidence to recommend immunization. |
| C | NACI concludes that the existing evidence is conflicting and does not allow making a recommendation for or against immunization; however other factors may influence decision-making. |
| D | NACI concludes that there is fair evidence to recommend against immunization. |
| E | NACI concludes that there is good evidence to recommend against immunization. |
| F | NACI concludes that there is insufficient evidence (in either quantity and/or quality) to make a recommendation, however other factors may influence decision-making. |
List of abbreviations
- 4CMenB
- Multicomponent meningococcus serogroup B
- Agency
- Public Health Agency of Canada
- CFR
- Case fatality ratio
- CIRID
- Centre for Immunization and Respiratory Infectious Diseases
- CI
- Confidence interval
- CIC
- Canadian Immunization Committee
- DPTPHib
- Diphtheria, tetanus, acellular pertussis, Haemophilus influenzae type b and inactivated poliovirus
- DTap-HBV-IPV/Hib
- Diphtheria, tetanus, acellular pertussis, inactivated polio, Haemophilus influenzae type b and hepatitis B
- ELISA
- Enzyme-linked immunosorbent assay
- fHbp
- Factor H binding protein
- GMC
- Geometric mean concentration
- GMT
- Geometric mean titre
- GNA
- Genome-derived neisserial antigen
- hSBA
- Human complement serum bactericidal activity
- IgG
- Immunoglobulin G
- IM
- Intramuscular
- IMD
- Invasive meningococcal disease
- IMPACT
- Immunization Monitoring Program, ACTives
- KD
- Kawasaki Disease
- LL
- Lower limit
- MCCV-Hib
- Meningococcal serogroup C and Hib conjugate vaccine
- MenACWY-CRM
- Meningococcal serogroups A, C, W-135 and Y conjugate vaccin
- MMR
- Measles, mumps, rubella vaccine
- MMR
- Measles, mumps, rubella vaccine
- MMRV
- Measles, mumps, rubella and varicella vaccine
- N. Meningitidis
- Neisseria Meningitidis
- NACI
- National Advisory Committee on Immunization
- NHBA
- Neisseria heparin-binding antigen
- NadA
- Neisserial adhesion A
- NZ
- New Zealand
- nCAM
- neural cell adhesion molecule
- MATS
- Meningococcal Antigen Typing System
- MBPPTG
- Meningococcal B Pilot Project Task Group
- MLST
- Multilocus sequence typing
- OMP
- Outer membrane proteins
- OMV
- Outer membrane vesicle
- PorA
- Porin A
- P/Ts
- Provinces and Territories
- RCT
- Randomized controlled trial
- rMenB
- Recombinant meningococcal B
- SAE
- Serious adverse event
- SD
- Standard deviation
- ST
- sequence type
Acknowledgements
Former NACI Members: Dr. N. Crowcroft, Dr. S. McNeil
Liaison Representatives: Dr. J. Blake (Society of Obstetricians and Gynaecologists of Canada), Dr. S. Deeks (Canadian Public Health Association), Dr. A. Mawle (Centers for Disease Control and Prevention, United States), Dr. D. Moore (Canadian Paediatric Society), Dr. A. Pham-Huy (Canadian Association for Immunization Research and Evaluation).
Former Liaison Representatives: Dr. A Corriveau, (Council of Chief Medical Officers of Health). Dr. H. Morrison (Council of Chief Medical Officers of Health), Dr. A. Opavsky (Association of Medical Microbiology and Infectious Disease Canada), Dr. S. Rechner (College of Family Physicians of Canada).
Ex-Officio Representatives: Dr. (Lt.-Col.) P. Eagan (Canadian Forces Health Service Group, National Defence and the Canadian Armed Forces), Dr. A. Klein (Biologics and Genetic Therapies Directorate, Health Canada), Dr. B. Law (Centre for Immunization and Respiratory Infectious Diseases, Public Health Agency of Canada), Dr. B. Raymond (Centre for Immunization and Respiratory Infectious Diseases, Public Health Agency of Canada/Canadian Immunization Committee), Dr. E. Taylor (Marketed Health Products Directorate, Health Canada), Ms. M. St-Laurent (Centre for Immunization and Respiratory Infectious Diseases, Public Health Agency of Canada).
Former Ex-Officio Representatives: Dr. M. Carew (First Nations and Inuit Health Branch, Health Canada), Dr. C. Légaré (Marketed Biologicals, Biotechnology and Natural Health Products Bureau, Health Canada).
This statement was prepared by: Dr. A. Wormsbecker, Dr. N. Crowcroft, Ms. L. Strifler, Dr. O. Baclic, Dr. S. Desai, Ms. J. Lourenco, and approved by NACI.
NACI gratefully acknowledges the contribution of the Meningococcal B Project Pilot Task Group: Dr. N. Crowcroft (Co-Chair), Dr. P. De Wals (Co-Chair), Ms. H. Deehan, Dr. S. Deeks, Dr. S. Desai, Dr. S. Halperin, Dr. C. Kennedy, Dr. M. Landry, Dr. M. Naus, Dr. R. Tsang, Dr. W. Vaudry.
NACI also gratefully acknowledges the contribution of Ms. V. Dang, Dr. J. Laroche, Dr. B. Sander.