Supplemental Statement - Afluria Tetra® - An Advisory Committee Statement (ACS)

Download the alternative format
(PDF format, 1.3 MB, 26 pages)
Organization: Public Health Agency of Canada
Published: 2018-XX-XX
Preamble
The National Advisory Committee on Immunization (NACI) provides the Public Health Agency of Canada (hereafter referred to as PHAC) with ongoing and timely medical, scientific, and public health advice relating to immunization.
In addition to burden of disease and vaccine characteristics, PHAC has expanded the mandate of NACI to include the consideration of programmatic factors in developing evidence-based recommendations to facilitate timely decision-making for publicly funded vaccine programs at provincial and territorial levels.
The additional factors to be considered by NACI include: economics, ethics, equity, feasibility, and acceptability. Over the coming years NACI will be refining methodological approaches to include these factors. Not all NACI Statements will require in-depth analyses of all programmatic factors. As NACI works towards full implementation of the expanded mandate, select Statements will include varying degrees of programmatic analyses for public health programs.
PHAC acknowledges that the advice and recommendations set out in this statement are based upon the best current available scientific knowledge and is disseminating this document for information purposes. People administering the vaccine should also be aware of the contents of the relevant product monograph(s). Recommendations for use and other information set out herein may differ from that set out in the product monograph(s) of the Canadian manufacturer(s) of the vaccine(s). Manufacturer(s) have sought approval of the vaccine(s) and provided evidence as to its safety and efficacy only when it is used in accordance with the product monographs. NACI members and liaison members conduct themselves within the context of PHAC’s Policy on Conflict of Interest, including yearly declaration of potential conflict of interest.
Table of contents
- Summary of the information contained in this NACI Supplemental Statement
- I. Introduction
- II. Methods
- III. Vaccine
- IV. Recommendations
- Tables
- List of abbreviations
- Acknowledgments
- References
- Appendix A: Prisma Flow Diagram
Summary of the information contained in this NACI Supplemental Statement
The following highlights key information for immunization providers. Please refer to the remainder of this supplemental statement for details.
1. What
Afluria® Tetra is a split virus quadrivalent inactivated influenza vaccine that has recently been authorized for use in Canada.
2. Who
This supplemental statement addresses the annual influenza vaccination of adults and children who do not have contraindications for the influenza vaccine.
3. How
Afluria® Tetra may be considered among the quadrivalent influenza vaccines offered to adults and children ≥5 years of age for their annual influenza vaccination.
4. Why
Afluria® Tetra is considered safe and immunogenic in adults and children ≥5 years of age, and has a comparable safety and immunogenicity profile to already licensed influenza vaccines.
I. Introduction
Influenza is a viral infection that is estimated to cause 12,200 hospitalizationsFootnote 1 and 3,500 deathsFootnote 2 in Canada annually. Each year, NACI publishes a statement on seasonal influenza vaccines, which contains recommendations on the use of influenza vaccines for the upcoming influenza season. Influenza in humans is caused by two main types of influenza virus: A, which is classified into subtypes based on haemagglutinin (HA) and neuraminidase surface proteins, and B, which consists of two antigenically distinct lineages, B/Yamagata and B/Victoria. Seasonal influenza vaccines are either trivalent or quadrivalent formulations. Trivalent influenza vaccines contain two influenza A and one influenza B strain, and quadrivalent influenza vaccines contain the three strains included in trivalent vaccines and an additional influenza B strain from the other lineage of influenza B.
Afluria® Tetra (Seqirus Pty Ltd) is a split virus quadrivalent inactivated influenza vaccine (QIV) that was authorized for use in Canada in adults and children 5 years of age and older in February 2018. This authorization triggered the need for a supplemental statement as NACI has not previously made a recommendation on the use of this vaccine in any population. Afluria® Tetra is the quadrivalent formulation of Afluria® (Seqirus Pty Ltd), a trivalent split virus inactivated influenza vaccine (TIV). The manufacturer of Afluria® (trivalent) has not sought approval for use in Canada at the time of publication of this supplemental statement.
Guidance objective:
The objective of this advisory committee supplemental statement is to review the efficacy, effectiveness, immunogenicity, and safety evidence available for Afluria® Tetra and to provide guidance on its use in adults and children.
II. Methods
The NACI Influenza Working Group (IWG) decided on a review protocol a priori that included review questions, search strategy, inclusion and exclusion criteria, and quality assessment. The IWG developed the following research question and accompanying PICO to guide the review:
What are the vaccine efficacy, effectiveness, immunogenicity, and safety of Afluria® Tetra in adults and children?
- P (Population): Adults and children (≥6 months)
- I (intervention): Afluria® Tetra or Afluria® (1.5% sodium taurodeoxycholate [TDOC] formulation)
- C (comparator): Comparator TIV, comparator QIV, placebo, or no comparator
- O (outcome): Efficacy, effectiveness, immunogenicity, safety
Since Afluria® Tetra is a new vaccine, no post-marketing studies have been completed to date. To supplement the evidence for Afluria® Tetra, the IWG decided to include any studies that assessed the efficacy, effectiveness, immunogenicity, and safety of Afluria® (the trivalent formulation) that is manufactured using the same manufacturing process as Afluria® Tetra. The manufacturing process for Afluria® Tetra involves the use of 1.5% TDOC as a splitting agent. Prior to the 2017–2018 Northern Hemisphere season, the manufacturing process for Afluria® used ≤1.5% TDOC. The use of 1.5% TDOC as a splitting agent was incorporated in the manufacturing process for Afluria® after a safety signal in the 2010 Southern Hemisphere influenza season in Australia showed that Afluria® made with ≤1.5% of TDOC was associated with an increased rate of fever and febrile seizures in children <5 years of ageFootnote 3. This issue is further discussed in Section III.4.2 of this document.
In addition, the IWG decided to include studies completed in children 6 months of age and older to capture the entirety of available evidence in children, despite the manufacturer’s not seeking an indication in this age group at the time of publication of this supplemental statement.
The search strategy was developed based on the research question and PICO in conjunction with a librarian from the Health Library of Health Canada and PHAC (search strategy available upon request). Two reviewers independently screened the titles and abstracts of records retrieved from the search and eligible full-texts for inclusion. Two reviewers also independently extracted data and appraised study quality using the criteria outlined by Harris et al.Footnote 4. Any disagreements or discrepancies were resolved by discussion and consensus. The knowledge synthesis was performed by KY, LZ, and KM and was supervised by the IWG. Following critical appraisal of individual studies, summary tables with ratings of the quality of the evidence using NACI's methodological hierarchy (Table 4 and 5) were prepared, and proposed recommendations for vaccine use developed. The IWG Chair and the Public Health Agency of Canada (PHAC) technical advisor presented the evidence and proposed recommendations to NACI on June 7th, 2018. Following thorough review of the evidence and consultation at the NACI meetings of June 6th–7th, 2018, NACI voted on specific recommendations. The description of relevant considerations, rationale for specific decisions, and knowledge gaps are described in the following sections.
III. Vaccine
III.1 Afluria® Tetra influenza vaccine preparation authorized for use in Canada
Afluria® Tetra is a split virus QIV. It is authorized for intramuscular injection and is available as a single-dose pre-filled syringe without a needle, and as a 5mL multi-dose vial. For more information on Afluria® Tetra, refer to the product monographFootnote 5.
| Route of administration | Dosage | Non-medicinal ingredients |
|---|---|---|
Intramuscular |
Each 0.5 mL dose contains 15 μg of haemagglutinin (HA) of each influenza virus strain |
Calcium chloride, dibasic sodium phosphate (anhydrous), monobasic potassium phosphate, monobasic sodium phosphate, potassium chloride, sodium chloride, thimerosal (multi-dose vial only) and water for injection. Each dose may also contain sodium taurodeoxycholate (TDOC), ovalbumin (egg proteins) and trace amounts of betapropiolactone, neomycin sulfate, polymyxin B sulfate and sucrose. |
III.2 Vaccine efficacy and effectiveness
No studies on the efficacy or effectiveness of Afluria® Tetra or Afluria® (1.5% TDOC formulation) were identified in this review.
III.3 Immunogenicity
Regulators in Canada, the United States (US), and Europe accept non-inferiority immunogenicity trials that compare the haemagglutination inhibition (HI) antibody response of the new vaccine to that of an existing licensed vaccine, or placebo-controlled immunogenicity trials that assess the HI antibody response to the new vaccine. Non-inferiority and placebo-controlled immunogenicity trials are often considered sufficient by regulatory authorities when there are bridging data to correlate immunogenicity outcomes to clinical protection, or when the new vaccines are very similar to vaccines already authorized. Serological assessments based on the geometric mean titres (GMT) of HI antibody that are used by regulators are: GMT ratio, seroprotection rate, and seroconversion rate. The US Food and Drug Administration (FDA) has published definitions for these serological assessments and criteria for immunogenicity data necessary for influenza vaccine licensureFootnote 6. These definitions and currently used criteria are shown in Table 2. Correlates of protection that are not based on HI antibody titres have not been well established.
III.3.1 Immunogenicity in adults
Two studies were identified that assessed the immunogenicity of Afluria® Tetra or Afluria® (1.5% TDOC formulation) in adultsFootnote 7Footnote 8. Both studies looked at the seroprotection rate and seroconversion rate of haemagglutinin (HA) at 21 days post-vaccination. Only one of the studies assessed GMT ratio. The comparator vaccines for these two studies included TIVs manufactured by Seqirus containing an influenza B/Victoria or an influenza B/Yamagata lineage strainFootnote 7 or no comparatorFootnote 8. Additional details on the immunogenicity findings in adults can be found in Table 6.
Afluria® Tetra was non-inferior to the comparator TIVs based on GMT ratios and differences in seroconversion rate for adults 18–64 years of age and ≥65 years of age at 21 days post-vaccination(Footnote 7Footnote 8. Afluria® Tetra also exceeded the thresholds for seroprotection rate for both of these age groups for all strains except for a B/Yamagata lineage strain in adults ≥65 years of ageFootnote 7Footnote 8.
III.3.2 Immunogenicity in children
Only one study that assessed the immunogenicity of Afluria® Tetra in childrenFootnote 9 was found. This study compared the immunogenicity of Afluria® Tetra to that of a comparator QIV (Fluarix® Quadrivalent, GlaxoSmithKline, not authorized for use in Canada). The study compared the GMT ratio, seroprotection rate, and seroconversion rate in the control and intervention groups at 28 days post-vaccination. Additional details on the immunogenicity findings in children are shown in Table 6.
Afluria® Tetra demonstrated non-inferiority to Fluarix® Quadrivalent in children 5–17 years of age, based on GMT ratios and differences in seroconversion rates for all influenza strainsFootnote 9. Differences in seroconversion rates were not calculated for the subgroups of children 5–8 years of age and 9–17 years of age; however, seroconversion rates appeared similar between the two groups, as they had widely overlapping confidence intervals (CIs). The vaccine also met the threshold for seroprotection for all strains except for a B/Yamagata lineage strain in the subgroup of children 5–8 years of ageFootnote 9.
III.4 Adverse events
III.4.1 Adverse events in adults
This review found two studies, one peer-reviewed and one not peer-reviewed, that assessed the safety of Afluria® Tetra or Afluria® (1.5% TDOC formulation) in adultsFootnote 7Footnote 8. The peer-reviewed study made direct comparisons between Afluria® Tetra and TIVs manufactured by Seqirus containing an influenza B/Victoria or an influenza B/Yamagata lineage strainFootnote 7. The study that was not peer-reviewed only reported safety for Enzira®, which is the trade name for Afluria® in the United Kingdom, and did not have a comparatorFootnote 8. Further details on the safety evidence for Afluria® Tetra in adults are shown in Table 7.
Among adults who received Afluria® Tetra or Afluria® (1.5% TDOC formulation), 37.4–52.5% experienced solicited local adverse events (AEs), 19.2–28.9% experienced solicited systemic AEs, and 20.5%–44.2% experienced unsolicited AEsFootnote 7Footnote 8. The most common AE was pain and specifically pain at the injection site. Overall, there was no difference in the proportion of people who experienced local or systematic AEs between groups that received Afluria® Tetra and groups that received comparable TIVsFootnote 7. The only significant difference reported between the two groups was that participants who received Afluria® Tetra were more likely to experience a headache compared to participants that received a TIV containing a B/Yamagata lineageFootnote 7.
The proportion of participants that received Afluria® Tetra or Afluria® and experienced any serious or severe adverse event (SAE) was 0–2.3% and the proportion that died during the course of the study was 0–0.3%Footnote 7Footnote 8. Participants that received TIVs experienced similar proportions of SAEsFootnote 7Footnote 8. The investigator of the Treanor et al. study considered four SAEs (one asthma event, one acute pancreatitis event, one hypoxia event, and one pneumonia event) and one death (pneumonia in adult ≥65) as related to Afluria® TetraFootnote 7. These four SAEs and the one death were not considered vaccine-related by the study sponsor or by the Canadian regulator.
III.4.2 Adverse events in children
Two studies were identified that assessed the safety of Afluria® Tetra or Afluria® (1.5% TDOC formulation) in childrenFootnote 9Footnote 10. One peer-reviewed study compared the safety of Afluria® Tetra to Fluarix® Quadrivalent (GlaxoSmithKline, not authorized for use in Canada)Footnote 9, and one study, not peer-reviewed, compared the safety of Afluria® (1.5% TDOC formulation) to Fluzone® Quadrivalent (Sanofi Pasteur)Footnote 10. These studies assessed safety in children 5–17 years of age. No safety data were identified for children <5 years of age. Additional details on the safety evidence for Afluria® Tetra in children are shown in Table 7.
Of children 5–8 years of age that received Afluria® Tetra or Afluria®, 57.2–70.2% experienced local AEs, 27.6–40.8% experienced systemic AEs, and 14.0% experienced unsolicited AEsFootnote 9Footnote 10. A comparatively smaller proportion of children 9–17 years of age experienced AEs, with 54.9% having experienced local AEs and 34.1% having experienced systemic AEsFootnote 9. The most common local AE experienced by children aged 5–8Footnote 9Footnote 10 and 9–17Footnote 9 was mild pain, and the most common systemic AE experienced by children aged 5–8Footnote 9Footnote 10 and 9–17Footnote 9 was headache. Overall, there was no difference in the proportion of children that experienced local or systemic AEs between groups that received Afluria® Tetra or Afluria® and groups that received a comparator QIV. The only notable differences were that the proportion of children with moderate solicited systemic AEs and the proportion that experienced swelling appeared lower in one study for children that received Afluria® compared to a QIVFootnote 10, and the proportion of children that experienced myalgia was significantly higher in one study for children who received Afluria® Tetra compared to children who received Fluarix® QuadrivalentFootnote 9.
The overall proportion of children that experienced SAEs was low, with 0.47% of children 5–17 years of ageFootnote 9 and 0.003% of children 5–8 years of ageFootnote 10 experiencing any SAE. Participants that received a comparator QIV had comparable proportions of SAEs.
Fever and febrile seizures
During Western Australia’s 2010 Southern Hemisphere influenza season surveillance, a safety signal was detected for the use of Afluria® (trivalent formulation) in childrenFootnote 3. The trivalent formulation of Afluria® was found to be associated with an increased rate of fever and febrile seizures in children <5 years of ageFootnote 11. An investigation by the manufacturer into this event revealed that the manufacturing process for Afluria® resulted in degraded ribonucleic acid (RNA) fragments being delivered by residual lipids, which increased the release of proinflammatory cytokinesFootnote 12. Further studies demonstrated that the release of proinflammatory cytokines was attenuated by an increased concentration of the splitting agent TDOC; therefore, the manufacturing process for Afluria® was modified to use 1.5% weight/volume TDOC as opposed to 0.9% for A(H1N1), 1.5% for A(H3N2), and 0.5% for B, which were the concentrations used previouslyFootnote 12. This modified manufacturing process is used for Afluria® Tetra.
In the two studies identified in this review, both reported on the proportion of participants who experienced fever or febrile seizures. The evidence shows no statistically significant difference in the proportion of children that experienced any, mild, moderate, or severe feverFootnote 9Footnote 10 between groups that received Afluria® Tetra or Afluria® (1.5% TDOC formulation) and groups that received a comparator QIV. There also appeared to be no difference in the proportion that experienced any vaccine-related fever eventFootnote 10; however, statistical significance was not reported for this outcome. No seizure or febrile seizure events were reported in either of the studies for any of the vaccines investigatedFootnote 9Footnote 10.
IV. Recommendations
1. NACI recommends that Afluria® Tetra may be considered among the QIVs offered to adults and children ≥5 years of age (Discretionary NACI Recommendation)
NACI concludes that there is fair evidence to recommend vaccination of adults and children ≥5 years of age (Grade B Evidence)
There is good evidence that Afluria® Tetra is safe and has non-inferior immunogenicity to comparable vaccines based on direct evidence in adults and children ≥5 years of age. The evidence is considered Grade B as there is no direct evidence on the efficacy or effectiveness of Afluria® Tetra. There is no evidence on the efficacy, effectiveness, immunogenicity, or safety for the use of Afluria® Tetra in children <5 years of age, and Afluria® Tetra is not authorized for use in this age group in Canada.
Tables
| Serological assay | Definition | Threshold |
|---|---|---|
GMT ratio |
Ratio of GMT post-vaccination of licensed vaccine to GMT post-vaccination of new vaccine |
Non-inferiority: The upper bound of the two-sided 95% CI on the ratio of the GMTs should not exceed 1.5. |
Seroprotection |
Proportion of subjects achieving an HI titre of ≥1:40 post-vaccination |
Placebo-controlled: Lower limit of the two-sided 95% CI for the percent of subjects achieving seroprotection should meet or exceed 70% (for adults <65 and children) or 60% (for adults ≥65) |
Seroconversion |
Proportion of subjects achieving an increase from ≤1:10 HI titre pre-vaccination to ≥1:40 post-vaccination or achieving at least four-fold rise in HI titres |
Non-inferiority: Upper limit of the two-sided 95% CI on the difference between the seroconversion rates (rate of licensed vaccine – rate of new vaccine) should not exceed 10 percentage points. Placebo-controlled: Lower limit of the two-sided 95% CI for the percent of subjects achieving seroprotection should meet or exceed 40% (for adults <65 and children) or 30% (for adults ≥65) |
| Abbreviations: CI: confidence interval, GMT: geometric mean titre, HI: haemagglutination inhibition | ||
| Strength of NACI recommendation | Grade of evidence |
|---|---|
Based on factors not isolated to strength of evidence (e.g. public health need) |
Based on assessment of the body of evidence |
Strong “should/should not be offered”
|
A - good evidence to recommend |
B – fair evidence to recommend |
|
C – conflicting evidence, however other factors may influence decision-making |
|
D – fair evidence to recommend against |
|
E – good evidence to recommend against |
|
I – insufficient evidence (in quality or quantity), however other factors may influence decision-making |
|
Discretionary “may be considered”
|
A - good evidence to recommend |
B – fair evidence to recommend |
|
C – conflicting evidence, however other factors may influence decision-making |
|
D – fair evidence to recommend against |
|
E – good evidence to recommend against |
|
I – insufficient evidence (in quality or quantity), however other factors may influence decision-making |
| Level | Description |
|---|---|
| I | Evidence from randomized controlled trial(s). |
| II-1 | Evidence from controlled trial(s) without randomization. |
| II-2 | Evidence from cohort or case-control analytic studies, preferably from more than one centre or research group using clinical outcome measures of vaccine efficacy. |
| II-3 | Evidence obtained from multiple time series with or without the intervention. Dramatic results in uncontrolled experiments (such as the results of the introduction of penicillin treatment in the 1940s) could also be regarded as this type of evidence. |
| III | Opinions of respected authorities, based on clinical experience, descriptive studies and case reports, or reports of expert committees. |
| Quality rating | Description |
|---|---|
| Good | A study (including meta-analyses or systematic reviews) that meets all design- specific criteriaTable 5 Footnote * well. |
| Fair | A study (including meta-analyses or systematic reviews) that does not meet (or it is not clear that it meets) at least one design-specific criterionTable 5 Footnote * but has no known "fatal flaw". |
| Poor | A study (including meta-analyses or systematic reviews) that has at least one design-specificTable 5 Footnote * "fatal flaw", or an accumulation of lesser flaws to the extent that the results of the study are not deemed able to inform recommendations. |
|
|
| Study Details | Summary | |||||
|---|---|---|---|---|---|---|
| Study | Vaccine | Study Design | Participants | Summary of Key Findings | Level of Evidence | Quality |
Airey J, Albano FR, Sawlwin DC, Jones AG, Fromica N, Matassa V, Leong J. Immunogenicity and safety of a quadrivalent inactivated influenza virus vaccine compared with a comparator quadrivalent inactivated influenza vaccine in a pediatric population: A phase 3, randomized noninferiority study. Vaccine, 2017;35(20)Table 6 Footnote 1 |
Afluria® Tetra |
RCT US 2015–2016 influenza season Funded by Seqirus |
Healthy children 5–17 years of age 47.9% female Group 1: 1709 children vaccinated with Afluria® Tetra Group 2: 569 children vaccinated with Fluarix® Quadrivalent |
GMT ratio 28 days post-vaccination (Group 2/Group 1): Age 5–17, estimate (95% CI)
Difference in seroconversion rate 28 days post-vaccination (Group 2 – Group 1): Age 5–17, estimate (95% CI)
Seroconversion rate 28 days post-vaccination: Age 5–17, Group 1 (95% CI)
Age 5–8, Group 1 (95% CI)
Age 9–17, Group 1 (95% CI)
Age 5–17, Group 2 (95% CI)
Age 5–8, Group 2 (95% CI)
Age 9–17, Group 2 (95% CI)
Seroprotection rate 28 days post-vaccination (Group 1): Age 5–17, estimate (95% CI)
Age 5–8, estimate (95% CI)
Age 9–17, estimate (95% CI)
There were no potentially important differences in GMFR between the two groups. |
I |
Good |
Treanor JT, Albano FR, Sawlwin DC, Jones AG, Airey J, Formica N, Matassa V, Leong J. Immunogenicity and safety of a quadrivalent inactivated influenza vaccine compared with two trivalent inactivated influenza vaccines containing alternate B strains in adults: A phase 3, randomized noninferiority study, Vaccine, 2017;35(15)Table 6 Footnote 2 |
Afluria® Tetra |
RCT US 2014–2015 influenza season Funded by Seqirus |
Healthy adults ≥18 years of age 57.2% female Group 1: Group 2: |
GMT ratio 21 days post-vaccination (Group 2/Group 1): Age ≥18, estimate (95% CI)
Age 18-64, estimate (95% CI)
Age ≥65, estimate (95% CI)
Difference in seroconversion rate 21 days post-vaccination (Group 2 - Group 1): Age ≥18, estimate (95% CI)
Age 18-64, estimate (95% CI)
Age ≥65, estimate (95% CI)
Seroprotection rate 21 days post-vaccination (Group 1): Age 18-64, estimate (95% CI)
Age ≥65, estimate (95% CI)
GMFR was also reported and showed no concerns; however, the difference in GMFR was not reported. |
I |
Good |
ClinicalTrials.gov |
Enzira® (Afluria® in other countries) |
RCT England 2013–2014 influenza season Sponsored by Seqirus |
Healthy adults 18–59 years of age 52.5% female Group 1: 120 adults vaccinated with Enzira® (Afluria® in other jurisdictions) No control arm |
Seroconversion rate 21 days post-vaccination (Group 1): Age 18–59, estimate (95% CI)
Seroprotection rate 21 days post-vaccination (Group 1): Age 18–59, estimate (95% CI)
There were no potentially important differences in GMFR between the two groups. |
I |
n/a Phase IV study that has not been peer-reviewed and did not contain a control group. |
Abbreviations: CI: confidence interval; GMFR: geometric mean fold rise; GMT: geometric mean titre; n/a: not applicable; RCT: randomized controlled trial; TIV: trivalent inactivated influenza vaccine; US: United States
|
||||||
| Study Details | Summary | |||||
|---|---|---|---|---|---|---|
| Study | Vaccine | Study Design | Participants | Summary of Key Findings | Level of Evidence | Quality |
Airey J, Albano FR, Sawlwin DC, Jones AG, Fromica N, Matassa V, Leong J. Immunogenicity and safety of a quadrivalent inactivated influenza virus vaccine compared with a comparator quadrivalent inactivated influenza vaccine in a pediatric population: A phase 3, randomized noninferiority study. Vaccine, 2017;35(20)Table 7 Footnote 1 |
Afluria® Tetra |
RCT US 2015–2016 influenza season Funded by Seqirus |
Healthy children 5–17 years of age 47.9% female Group 1: Group 2: |
Proportion of children 5–17 years of age experiencing AE and SAE: Age 5–17, Group 1
Age 5–17, Group 2
RR of AE (Group 1/Group 2): Age 5-8, estimate (95% CI)
Age 9–17, estimate (95% CI)
RR of fever (Group 1/Group 2):
The most common local AE for children 5–17 years of age was injection site pain, and the most common systemic AEs were headache and myalgia. There were no seizures or febrile seizures reported in either group. A greater proportion of children in Group 1 experienced myalgia than Group 2. |
I |
Good |
Treanor JT, Albano FR, Sawlwin DC, Jones AG, Airey J, Formica N, Matassa V, Leong J. Immunogenicity and safety of a quadrivalent inactivated influenza vaccine compared with two trivalent inactivated influenza vaccines containing alternate B strains in adults: A phase 3, randomized noninferiority study, Vaccine, 2017;35(15)Table 7 Footnote 2 |
Afluria® Tetra |
RCT US 2014–2015 influenza season Funded by Seqirus |
Healthy adults ≥18 years of age 57.2% female Group 1: Group 2: |
Proportion of adults ≥18 years of age experiencing AE and SAE: Group 1
Group 2 (B/Yam)
Group 2 (B/Vic)
RR of solicited AE (Group 1/Group 2 vaccinated with B/Yamagata TIV only): Age ≥18, estimate (95% CI)
Age 18–64, estimate (95% CI)
Age ≥65, estimate (95% CI)
RR of solicited AE (Group 1/Group 2 vaccinated with B/Victoria TIV only): Age ≥18, estimate (95% CI)
Age 18–64, estimate (95% CI)
Age ≥65, estimate (95% CI)
The most common local AE for adults 18–64 and ≥65 years of age was pain, and the most common systemic AEs were headache and myalgia. Participants in Group 1 were more likely to experience a headache compared to participants in Group 2 that received a TIV containing B/Yamagata. |
I |
Good |
ClinicalTrials.gov |
Afluria® |
RCT US 2015–2016 influenza season Sponsored by Seqirus |
Healthy children 5–8 years of age 48.0% female Group 1: 302 children vaccinated with Afluria® Group 2: 100 children vaccinated with Fluzone® Quadrivalent |
Proportion of children 5–8 years of age experiencing AE and SAE: Group 1
Group 2
Proportion of children 5–8 years of age experiencing fever: Group 1
Group 2
Proportion of children 5–8 years of age experiencing vaccine-related fever: Group 1
Group 2
The most common local AE was pain. It was not specified whether this pain was associated with the injection site or not. The most common systemic AE was myalgia. A smaller proportion of children in Group 1 appeared to have moderate solicited systemic AE and swelling than children in Group 2. |
I |
Good Phase IV study that has not been peer-reviewed. |
ClinicalTrials.gov |
Enzira® (Afluria® in other jurisdictions) |
RCT England 2013–2014 influenza season Sponsored by Seqirus |
Healthy adults 18–59 years of age 52.5% female Group 1: 120 adults vaccinated with Enzira® (licensed as Afluria® in other jurisdictions) No control arm |
Proportion of adults 18–59 years of age experiencing AE and SAE:
The most common local AE for adults were pain and injection site pain, and the most common systemic AE was headache. |
I |
n/a Phase IV study that has not been peer-reviewed and did not contain a control group. |
Abbreviations: AE: adverse event; CI: confidence interval; n/a: not applicable; RCT: randomized controlled trial; RR: relative risk; SAE: serious or severe adverse event; TIV: trivalent inactivated influenza vaccine; US: United States
|
||||||
List of abbreviations
- AE
- Adverse event
- CI
- Confidence interval
- FDA
- Food and Drug Administration (United States)
- GMFR
- Geometric mean fold rise
- GMT
- Geometric mean titre
- HA
- Haemagglutinin
- HI
- Haemagglutination inhibition
- IWG
- Influenza Working Group
- n/a
- Not applicable
- NACI
- National Advisory Committee on Immunization
- PHAC
- Public Health Agency of Canada
- QIV
- Quadrivalent inactivated influenza vaccine
- RCT
- Randomized controlled trial
- RNA
- Ribonucleic acid
- RR
- Relative risk
- SAE
- Serious or severe adverse event
- TDOC
- Sodium taurodeoxycholate
- TIV
- Trivalent inactivated influenza vaccine
- US
- United States
Acknowledgments
This supplemental statement was prepared by: Ms. K. Young, Dr. L. Zhao, Dr. I. Gemmill, and approved by NACI.
Influenza Working Group Members: Dr. I. Gemmill (Chair), Dr. C. Bancej (CIRID, PHAC), Ms. L. Cochrane, Dr. N. Dayneka, Dr. L. Grohskopf (Centers for Disease Control and Prevention [CDC], United States), Ms. A. Lebans (First Nations and Inuit Health Branch [FNIHB], Indigenous Services Canada [ISC]), Dr. D. Kumar, Dr. J. Langley, Dr. M. Lavoie, Dr. J. McElhaney, Dr. A. McGeer, Dr. D. Moore, Dr. B. Warshawsky, Dr. J. Xiong (Biologics and Genetic Therapies Directorate [BGTD], Health Canada [HC]).
NACI Members: Dr. C. Quach (Chair), Dr. W. Vaudry (Vice-Chair), Dr. N. Dayneka, Dr. S. Deeks, Dr. P. De Wals, Dr. V. Dubey, Dr. R. Harrison, Dr. M. Lavoie, Dr. S. Marchant-Short, Dr. M. Salvadori, Dr. B. Sander, Dr. N. Sicard, Dr. C. Rotstein.
Former NACI Members: Dr. R. Warrington
Liaison Representatives: Dr. J. Brophy (Canadian Association for Immunization Research and Evaluation), Dr. E. Castillo (Society of Obstetricians and Gynaecologist of Canada), Dr. A. Cohn (CDC, United States), Ms. T. Cole (Canadian Immunization Committee), Dr. J. Emili (College of Family Physicians of Canada), Dr. K. Klein (Council of Chief Medical Officers of Health), Dr. C. Mah (Canadian Public Health Association), Dr. D. Moore (Canadian Paediatric Society), Dr. A. Pham-Huy (Association of Medical Microbiology and Infectious Disease Canada).
Ex-Officio Representatives: Dr. (LCdr) K. Barnes (National Defence and the Canadian Armed Forces), Ms. G. Charos (CIRID, PHAC), Dr. R. Pless (BGTD, HC), Dr. J. Gallivan (Marketed Health Products Directorate [MHPD], HC), Mr. G. Poliquin (National Microbiology Laboratory [NML], PHAC), Ms. J. Pennock (CIRID, PHAC), Dr. T. Wong (FNIHB, ISC).
NACI gratefully acknowledges the contribution of Ms. L. Glandon (Healthy Library, HC), Ms. A. House (CIRID, PHAC), Ms. M. Laplante (CIRID, PHAC), and Mr. K. Moncion (CIRID, PHAC).
References
- Footnote 1
-
Schanzer DL, Allison M, Kathleen M. Statistical estimates of respiratory admissions attributable to seasonal and pandemic influenza for Canada. Influenza and Other Respiratory Viruses. 2013;7(5):799-808.
- Footnote 2
-
Schanzer DL, Sevenhuysen C, Winchester B, Mersereau T. Estimating influenza deaths in Canada, 1992–2009. PLOS ONE. 2013;8(11):e80481.
- Footnote 3
-
Therapeutic Goods Administration (Australian Government). Seasonal Flu Vaccine: Investigation into febrile reactions in young children following 2010 seasonal trivalent influenza vaccination [Internet]. 2010. Available from: https://www.tga.gov.au/alert/seasonal-flu-vaccine-investigation-febrile-reactions-young-children-following-2010-seasonal-trivalent-influenza-vaccination.
- Footnote 4
-
Harris RP, Helfand M, Woolf SH, Lohr KN, Mulrow CD, Teutsch SM, Atkins D. Current methods of the US Preventive Services Task Force: A review of the process. Am J Prev Med. 2001;20(3):21-35.
- Footnote 5
-
Seqirus Pty Ltd. Product monograph: AFLURIA® TETRA: Quadrivalent inactivated influenza vaccine (split virion). [Internet]. 2017. Available from: https://pdf.hres.ca/dpd_pm/00043998.PDF.
- Footnote 6
-
US Food and Drug Administration. Guidance for industry: Clinical data needed to support the licensure of seasonal inactivated influenza vaccines. [Internet]. 2007. Available from:https://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Vaccines/ucm091990.pdf.
- Footnote 7
-
Treanor JT, Albano FR, Sawlwin DC, Jones AG, Airey J, Formica N, Matassa V, Leong J. Immunogenicity and safety of a quadrivalent inactivated influenza vaccine compared with two trivalent inactivated influenza vaccines containing alternate B strains in adults: A phase 3, randomized noninferiority study. Vaccine. 2017;35(15):1856-64.
- Footnote 8
-
ClinicalTrials.gov. A study to assess the immunogenicity and safety of a trivalent influenza vaccine containing the 2013/2014 formulation of Enzira vaccine in healthy volunteers. Identifier NCT01863433. [Internet]. 2014. Available from: https://clinicaltrials.gov/ct2/show/NCT01863433?term=NCT01863433&rank=1
- Footnote 9
-
Airey J, Albano FR, Sawlwin DC, Jones AG, Formica N, Matassa V, Leong J. Immunogenicity and safety of a quadrivalent inactivated influenza virus vaccine compared with a comparator quadrivalent inactivated influenza vaccine in a pediatric population: A phase 3, randomized noninferiority study. Vaccine. 2017;35(20):2745-52.
- Footnote 10
-
ClinicalTrials.gov. A study to evaluate the safety and tolerability of trivalent influenza virus vaccine in children aged 5 years to < 9 years. Identifier NCT02212106. [Internet]. 2015. Available from: https://clinicaltrials.gov/ct2/show/NCT02212106?term=NCT02212106&rank=1.
- Footnote 11
-
Armstrong PK, Dowse GK, Effler PV, Carcione D, Blyth CC, Richmond PC, Geelhoed GC, Mascaro F, Scully M, Weeramanthri TS. Epidemiological study of severe febrile reactions in young children in Western Australia caused by a 2010 trivalent inactivated influenza vaccine. BMJ Open. 2011;1(1):e000016.
- Footnote 12
-
Rockman S, Becher D, Dyson A, Koernig S, Morelli AB, Barnden M, Camuglia S, Soupourmas P, Pearse M, Maraskovsky E. Role of viral RNA and lipid in the adverse events associated with the 2010 Southern Hemisphere trivalent influenza vaccine. Vaccine. 2014;32(30):3869-76.
Appendix A: PRISMA flow diagram
Efficacy, effectiveness, immunogenicity, and safety of Afluria® Tetra. August 22, 2017
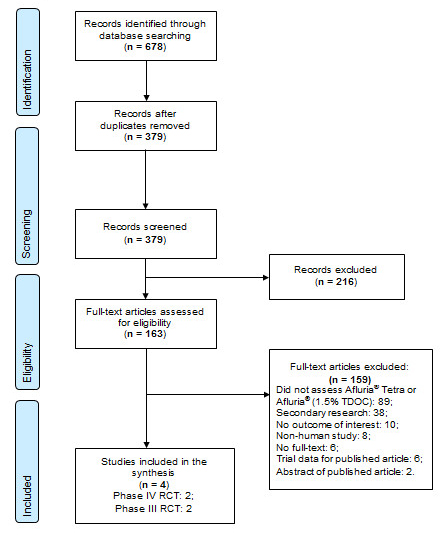
Text Description
In the identification stage, 678 records were identified through database searching. 379 records remained after duplicates were removed. The 379 records were then screened during the screening stage; 216 records were excluded and 163 full-text articles were assessed for eligibility. Of the 163 full-text articles, 159 were excluded: 89 did not assess Afluria® Tetra or Afluria®, 38 were secondary research, 10 had no outcome of interest, 8 were non-human studies, 6 had no full-text, 6 were trial data for published articles, and 2 were abstracts for published articles. 4 studies were included in the final synthesis: 2 Phase IV RCTs and 2 Phase III RCTs.