NACI literature review on the effects of palivizumab prophylaxis on reducing the complications associated with respiratory syncytial virus in infants
Download in PDF format
(1.26 MB, 133 pages)
Organization: Public Health Agency of Canada
Date published: 2023-02-01
Published : February 1, 2023
On this page
- Executive summary
- Introduction
- Methods
- Results
- Discussion/Summary
- Evidence Gaps
- Conclusion
- List of Abbreviations
- Acknowledgement
- Appendix A: Search strategy and results
- Appendix B: Level of evidence based on research design and quality (internal validity) rating of evidence
- Appendix C: Amstar quality assessment of original inesss systematic literature review
- Appendix D: PRISMAflow diagram
- Appendix E: Summary of evidence from updated INESSS literature review related to efficacy/effectiveness of PVZ prophylaxis in infants and children
- Appendix F: Summary of evidence from original INESSS literature review related to efficacy/effectiveness of PVZ prophylaxis in infants and children
- Appendix G: NNT with PVZ to avoid one RSVH or intensive care admission, or recurrent wheezing
- References
Preamble
The National Advisory Committee on Immunization (NACI) provides the Public Health Agency of Canada (PHAC) with ongoing and timely medical, scientific, and public health advice relating to immunization.
In addition to burden of disease and vaccine characteristics, PHAC has expanded the mandate of NACI to include the systematic consideration of programmatic factors in developing evidence-based recommendations to facilitate timely decision-making for publicly funded vaccine programs at provincial and territorial levels.
The additional factors to be systematically considered by NACI include economics, ethics, equity, feasibility, and acceptability. Not all NACI Statements will require in-depth analyses of all programmatic factors. While systematic consideration of programmatic factors will be conducted using evidence-informed tools to identify distinct issues that could impact decision-making for recommendation development, only distinct issues identified as being specific to the vaccine or vaccine-preventable disease will be included.
PHAC acknowledges that the advice and recommendations set out in this statement are based upon the best current available scientific knowledge and is disseminating this document for information purposes. People administering the vaccine should also be aware of the contents of the relevant product monograph(s). Recommendations for use and other information set out herein may differ from that set out in the product monograph(s) of the Canadian manufacturer(s) of the vaccine(s). Manufacturer(s) have sought approval of the vaccine(s) and provided evidence as to its safety and efficacy only when it is used in accordance with the product monographs. NACI members and liaison members conduct themselves within the context of PHAC's Policy on Conflict of Interest, including yearly declaration of potential conflict of interest.
Executive summary
Since the publication of the NACI statement in 2003, there have been a series of updated palivizumab (PVZ) guidance documents published by expert committees such as the American Academy of Pediatrics (2014) Footnote 1 Footnote 2 and the Canadian Paediatric Society (2015) Footnote 3, which have made PVZ prophylaxis recommendations that significantly differ from current NACI guidance and highlighted the need to reassess NACI's recommendations. In 2016, the Quebec Institut national d'excellence en santé et en services sociaux (INESSS) conducted a systematic literature search to December 22, 2015 to assess the effectiveness of PVZ prophylaxis in reducing the risk of respiratory syncytial virus (RSV)-associated complications in children in order to inform use criteria for PVZ in the province of Québec Footnote 4. The research question investigated in the INESSS review was directly aligned with the needs of the NACI RSV Working Group (RSV WG). The purpose of the present systematic literature review is to update the systematic review published in 2016 by INESSS and synthesize new findings with those of the original review to inform NACI's updated recommendations on the use of PVZ prophylaxis in infants.
The literature review examined the evidence on the effectiveness of PVZ prophylaxis compared to the administration of placebo or to no prophylaxis in reducing the complications associated with RSV infection in children. The outcomes of interest included hospitalization, length of hospital stay, stay in intensive care, length of stay (LOS) in intensive care, use of oxygen therapy, length of oxygen therapy use, use of mechanical ventilation (MV), length of MV use, long-term sequelae (e.g., wheezing, asthma), and mortality.
A search strategy using the INESSS methodology, devised in conjunction with a Health Canada librarian, was used to search multiple electronic databases from December 2015 to February 2017 to identify articles of interest published in English or French. In 2019 and 2020, repeat searches using the same strategy identified publications from February 2017 to July 29, 2020. Two reviewers independently screened the records captured by each search and assessed the quality of the included studies. One of the reviewers abstracted relevant data from the identified studies with the accuracy of data abstraction verified by the second reviewer. A narrative summary of the results of the included studies was then created and the findings compiled with those of the original INESSS literature review.
The updated literature review on the efficacy/effectiveness of PVZ prophylaxis identified three systematic reviews of good (2) and average (1) quality, two randomized control trials (RCTs) of good and fair quality, 17 observational cohort studies of either fair (13), good (2) or poor (2) quality and two case control studies of fair quality.
The updated review identified studies in mixed populations of infants, in children born prematurely without chronic lung disease of prematurity (CLD), in infants with CLD, in infants with hemodynamically significant congenital heart disease (hsCHD), in children with cystic fibrosis (CF) and in infants with Down syndrome. The review did not identify any new studies involving children living in remote communities.
As in the original INESSS literature review, the updated review did not identify any studies on the effectiveness of PVZ prophylaxis compared to placebo or no intervention in children with serious neuromuscular disorders affecting respiratory function, upper airway anomalies affecting respiratory function, chronic lung disease other than that associated with prematurity or CF, immunosuppression, or metabolic disease, or in healthy infants of multiple births with a twin or triplet eligible to receive PVZ.
The findings of the updated literature review were generally consistent with those of the INESSS literature review. Differences in study design and quality make it difficult to directly compare the studies' findings and draw firm conclusions.
The outcome most investigated was RSV-associated hospitalizations. PVZ prophylaxis was associated with reduction in the risk of hospitalization in mixed populations of children at risk of severe RSV infection and in premature children without CLD, although the level of prematurity at which PVZ is most effective is not clear. PVZ was effective in infants of 29–33 weeks Gestational age (wGA), but data on more premature infants and those over 33 wGA were not definitive.
Although an early RCT showed evidence of reduction in risk of hospitalization for RSV in infants with CLD, subsequent observational studies have had conflicting results. PVZ was effective in two larger studies of infants with hsCHD, but one showed protection with acyanotic heart disease only while the other showed an effect only with cyanotic disease, and two smaller studies showed no effect.
PVZ prophylaxis significantly reduced hospitalization risk in premature Inuit children living in Nunavut and in Alaska. PVZ had no effect on hospitalization for RSV infection in most studies (5 of 6 studies) of infants with CF.
Three studies of children with Down syndrome suggested that PVZ may not benefit children who do not have other high risk conditions that warrant PVZ administration, but the number investigated was small. The number needed to treat (NNT) to prevent one RSV related hospitalization varied widely and was influenced by patient population, location, number of participants and RSV hospitalization rate (RSVH) in controls. NNT appeared to be lowest in infants with CLD and highest in those with hsCHD.
The evidence base for the effectiveness of PVZ prophylaxis on other outcomes such as length of hospital stay, admission to and duration of stay in intensive care, use of MV) is limited. PVZ did not appear to influence severity of illness when breakthrough RSV infections requiring hospitalization occurred, but studies may have been underpowered to detect a protective effect.
There are conflicting results on the effect of PVZ on all-cause mortality as the sample size in most studies is too low to analyze this rare outcome. In premature infants PVZ may have an effect in infants born at ≤32 wGA, but not in more mature infants. No effect was observed in infants with CLD or hsCHD. However, these findings are based upon few studies which may have been underpowered to detect an effect.
Studies of long term sequelae of RSV infection suggest that PVZ prophylaxis reduces the risk of recurrent wheezing in the first few years of life, but may not have a significant impact on persistence of recurrent wheezing in older children. PVZ also had no significant effectiveness on long term sequelae in children with CF.
Introduction
I.1 Background
RSV is the most common cause of lower respiratory tract illness in young children worldwide and infects almost all infants by 2 years of age Footnote 5 Footnote 6. In Canada, RSV causes yearly epidemics every winter with the RSV season typically beginning in October or November and lasting until April or May and with most cases occurring in December to March Footnote 7 Footnote 8.
The most common diagnoses in young children requiring RSV hospitalization (RSVH) are bronchiolitis and pneumonia Footnote 9. Hospitalization rates are highest in children < 1 year of age and especially in the first few months of life Footnote 5. Hospitalization rates per 1000 children per year in high income countries are reported as 26.3 (95% confidence interval (CI) 22.8-30.2), 11.3 (95% CI: 6.1 to 20.9) and 1.4 (95% CI: 0.9 to 2.0) for age groups 0-5 months, 6-11 months and 12-59 months respectively Footnote 6. In Canada, similar rates of 20, 10.2, and 4.8 per 1000 per year are reported for children aged < 6 months Footnote 9, < 1 year, and 1-3 years Footnote 10.
Most hospitalized children < 2 years of age have no co-morbidities Footnote 5 Footnote 10, but higher rates and durations of hospitalization and more intensive care unit (ICU) admissions have been found in premature infants and in children with CLD or hsCHD Footnote 3 Footnote 5 Footnote 10 Footnote 11. Children with other lung diseases not associated with prematurity (e.g., cystic fibrosis (CF)), other chronic conditions and Indigenous children may also be at increased risk of severe RSV disease Footnote 3. In high income countries mortality from RSV is uncommon and usually occurs in children with significant co-morbidities Footnote 12.
In June 2002, Health Canada approved palivizumab (Synagis TM , Abbott Laboratories, Ltd., Saint-Laurent, Quebec), a monoclonal anti-RSV antibody, for the prevention of serious lower respiratory tract disease caused by RSV in infants at high risk of serious RSV disease. In 2003, the National Advisory Committee on Immunization (NACI) published recommendations on the use of PVZ for the prevention of RSV disease Footnote 13.
At that time, NACI recommended PVZ be used during the RSV season for premature infants (≤32 weeks gestational age (wGA)) who would be <6 months of chronological age during the RSV season, children <24 months of age with CLD requiring oxygen and/or medical therapy in the previous six months or other pulmonary disorders requiring oxygen therapy, and children <24 months of age with hemodynamically significant CHD. PVZ prophylaxis could also be considered for children born at <35 wGA who are less than 6 months of age during the RSV season and who live in remote northern communities Footnote 13.
Since the 2003 statement, NACI recommendations have been modified in the Canadian Immunization Guide (CIG) but no new Statement has been issued. From 2013, in addition to the above recommendations, the CIG states that PVZ prophylaxis may benefit selected infants between 33 and 35 wGA who are <6 months of age at the start of the RSV season and may be considered for infants in this gestational age (GA) group who live in rural or remote communities according to an assessment of access to medical care (e.g., requirement for air transportation to hospital facilities) and other factors known to increase risk. In addition, PVZ prophylaxis should be considered for all Inuit children in northern remote communities who are younger than 6 months of age at the start of RSV season, regardless of GA.
Since the publication of the NACI statement in 2003, there have been a series of updated PVZ guidance documents published by expert committees such as the American Academy of Pediatrics (2014) Footnote 1 Footnote 2 and the Canadian Paediatric Society (2015) Footnote 3, which have made PVZ prophylaxis recommendations that differ significantly from the NACI-CIG guidance and highlighted the need to reassess NACI's recommendations.
In 2016, the Quebec Institut national d'excellence en santé et en services sociaux (INESSS) conducted a systematic literature review to assess the effectiveness of PVZ prophylaxis in reducing the risk of RSV-associated complications in children in order to inform use criteria for PVZ in the province of Québec Footnote 4. The research question investigated in the INESSS review was directly aligned with the needs of the NACI RSV Working Group (RSV WG).
I.2 Purpose and objectives
The purpose of this systematic literature review is to update the search used in the 2016 INESSS systematic literature review and synthesize new findings with those of the original INESSS literature review. These data will be used to inform NACI's updated recommendations on the use of PVZ prophylaxis in infants.
Methods
II.1 Assessment of original INESSS systematic literature review
Prior to undertaking an update of the original INESSS systematic literature review, the RSV WG assessed the quality of the original review to determine whether it would provide an appropriate evidence base to inform NACI recommendations. The original INESSS systematic literature review was assessed independently by two reviewers using A Measurement Tool to Assess Systematic Reviews (AMSTAR) Footnote 14 and the results presented to the RSV WG.
In addition, an assessment was made as to whether the Critical Appraisal Skills Programme (CASP) Footnote 15 quality appraisal methodology used to evaluate individual studies in the original INESSS review was comparable to NACI's quality appraisal methodology.
II.2 Updated literature review: Research question
This updated systematic review examines the evidence on the effectiveness of PVZ prophylaxis compared to the administration of placebo or to no prophylaxis on reducing the complications associated with RSV infection in children.
- P – Population:
- Infants and children (<18 years of age)
- I – Intervention:
- PVZ prophylaxis
- C – Comparison:
- Placebo or no prophylaxis
- O – Outcomes: RSV-specific complications, such as hospitalizations, length of hospital stay, stay in intensive care, LOS in intensive care, use of oxygen therapy, length of oxygen therapy, use of mechanical ventilation (MV), length of MV, long-term sequelae (e.g., wheezing, asthma), and mortality
II.3 Search strategy
MEDLINE, the Cochrane Database of Systematic Reviews and Health Technology Assessment (which is included in MEDLINE) and EMBASE electronic databases were searched from December 1, 2015 until February 17, 2017 using search strategies adapted by a Health Canada library specialist from the previously conducted INESSS literature review Footnote 4.
The searches were restricted to articles published in the English and French languages. The search was repeated, using the same strategy, for publications from February 1, 2017 to April 12, 2019 and again for publications from April 2019 to July 29, 2020. The full electronic search strategies are presented in Appendix A.
II.4 Identification of eligible studies
Studies retrieved from the database searches were loaded into RefWorks (ProQuest LLC, Ann Arbor, MI) with duplicate records removed. Record screening and eligibility assessment were performed independently by two reviewers. Records returned by the database searches were initially screened by title and abstract for potential eligibility.
The full text of studies deemed potentially eligible after title and abstract screening, or for which insufficient information was available to determine eligibility (e.g., no abstract), were obtained and further reviewed for eligibility. Inclusion and exclusion criteria for the literature identification and selection process were adapted from the criteria used in the original INESSS literature review Footnote 4.
Studies were included if they met the criteria outlined in Section II.2 above and they were primary research studies (randomized controlled trials (RTC), observational studies [cohort, case-control]) or systematic reviews of primary studies with or without meta-analysis.
Articles were excluded from review if they met one or more of the following criteria:
- The study did not contain any of the outcomes of interest
- Articles representing doctoral or masters theses, case series, case study, conference summaries, economic study, clinical practice guidelines, consensus conference, health technology assessment report; or
- Non-English and non-French language publication.
Handsearching of the reference lists of included articles was performed by one reviewer to identify additional relevant publications. Potential articles identified through handsearching were then subjected to eligibility screening by two reviewers as described above.
II.5 Data extraction
One reviewer extracted data from the studies included for review into an evidence table using a piloted data abstraction template designed to capture information on study design, population and outcomes of interest. A second reviewer independently validated the abstracted data with any disagreements or discrepancies resolved by discussion and consensus.
II.6 Methodological quality assessment
The methodological quality of included observational studies was assessed independently by two reviewers using the design-specific criteria by Harris et al. adopted by NACI for rating the internal validity of individual studies (Appendix B) Footnote 16. For included systematic reviews with or without meta-analysis, study quality was assessed using AMSTAR Footnote 14.
II.7 Qualitative synthesis
Narrative synthesis of the information extracted from the included studies was used to explore the efficacy and effectiveness of PVZ prophylaxis for the outcomes of interest, including summaries of the direction, size and statistical significance of reported effect estimates for various study-defined outcomes.
These outcomes were then combined with the findings from the original INESSS review in the Discussion section of this report. The extracted data and quality assessment for each of these studies are presented in the evidence table in Appendix E. The outcomes from the studies identified in the original INESSS review are summarized in Appendix F.
Results
The assessment of the quality of the original INESSS systematic literature review, as well as an assessment of the comparability of the CASP and NACI methodologies for assessing study quality is summarized in Section III.1.
The study identification and selection process used in the updated systematic literature review, including study details and an assessment of methodological quality, are summarized in Section III.2.
The evidence related to the efficacy and effectiveness of PVZ prophylaxis for various RSV-related outcomes identified in the updated literature review are summarized in Section III.3 by the populations included in the original INESSS literature review:
- mixed population (prematurity, CLD, congenital heart disease (CHD)) (Section III.3.1), premature infants without CLD (Section III.3.2), premature infants with CLD (Section III.3.3), children with hsCHD (Section III.3.4),
- children residing in remote communities (Section III.3.5), children with CF (Section III.3.6), and children with Down syndrome (Section III.3.7).
III.1 Assessment of original INESSS systematic literature review
The original INESSS systematic literature review received an AMSTAR score of 7 out of 11 (Appendix C) and was considered to be of an acceptable quality by the RSV WG. In addition, the CASP quality appraisal methodology was determined to be comparable to NACI's quality appraisal methodology,
So the quality of studies included in the original INESSS review was not re-assessed. Twenty-six studies were included – 7 systematic reviews (4 with meta-analysis); 5 RCT, 13 observational cohort studies and one case-control study.
The extracted data and quality assessment for each of the studies identified in the original INESSS literature review has been summarized in an evidence table in Appendix F.
III.2 Updated literature review: Study inclusion and characteristics
The study identification, screening and eligibility assessment process is summarized visually in Appendix D. Following removal of duplicates, database searches and subsequent hand-searches yielded a total of 277 records retained for title and abstract screening. A total of 118 articles were subject to full-text screening, resulting in 24 studies eligible for inclusion in the qualitative synthesis. All included studies were English language publications.
III.2.1 Systematic reviews
A systematic review by Robinson et al. Footnote 17 was an updated review of RCTs of the efficacy and safety of PVZ in infants and children with CF. The systematic review retained only a single study but was assessed of good quality. A later systematic review of efficacy and safety of PVZ in children with CF Footnote 18 that had 5 relevant studies was assessed of good quality.
A third systematic review of PVZ efficacy and safety in various patient groups at risk for severe RSV infection had 18 relevant studies and was rated as average Footnote 19. Extracted data from these studies as well as quality ratings are presented in the evidence tables in Appendices E and F.
III.2.2 Individual Studies
The 17 observational cohort studies included in this updated review included infants and children from single tertiary care institutions in the United States (US) Footnote 20, Israel Footnote 21, Taiwan Footnote 22, and Spain Footnote 23; multicenter cohorts of CF patients in Northern Ireland Footnote 24 Alberta Footnote 25 and the US Footnote 26; a cohort of preterm infants from Japan, whose initial outcomes had been previously published Footnote 27, cohorts of preterm infants from Europe and Canada Footnote 28; preterm infants from nine Medicaid managed care programs in the US Footnote 29; multicenter cohorts of preterm infants from Hong Kong Footnote 30, Spain Footnote 31 and Italy Footnote 32, multicenter cohorts of children with hsCHD in Taiwan Footnote 33 and Argentina Footnote 34; and multicenter cohorts with Down syndrome in Spain Footnote 35 and Japan Footnote 36.
Observational cohort studies received a fair (13), good (2) or poor (2) rating of study methodology, based on the assessment of internal validity using the parameters outlined by Harris et al. Footnote 16. Two RCT were identified. One, rated as good Footnote 37, presented post-hoc analysis of data on preterm infants of various wGA without CLD from a multinational study that had previously published data on these preterm infants as a group Footnote 38.
The second was multicenter study from the Netherlands assessing the efficacy of PVZ in prevention of asthma that was rated as fair Footnote 39. There were two case-control studies, both rated as fair, a study of a mixed population from Canada and the US Footnote 40 and a study of children with CF from two centers in France Footnote 41.
The extracted data and quality assessment for each of these studies are presented in the evidence table in Appendix E.
III.3 Efficacy and effectiveness of PVZ prophylaxis by population
III.3.1 Mixed population
The updated literature review identified five observational cohort studies, all rated as fair quality, which compared PVZ effectiveness (PE) in cohorts separated in time. Blake et al. studied infants born at 29 to <32 wGA. The infants were not described in sufficient detail to rule out the presence of comorbidities, such as CLD and hsCHD; therefore, the study is included in the mixed population Footnote 20.
The study by Prais et al. included children who were born extremely prematurely (<29 weeks GA). The study excluded children with cardiac disease, but some members of the cohort had bronchopulmonary dysplasia(BPD) Footnote 21. In a study in Taiwan, where RSV is not seasonal, PVZ was given to infants ≤28 wGA with or without CLD and to infants ≤35 wGA with CLD as 6 monthly doses starting at the time of initial discharge from hospital.
Outcomes were assessed at 6 months from the time of first PVZ dose, and again at 12 months from the time of first PVZ dose, to determine if the 6 month schedule was appropriate. The control cohort was from an earlier time but matched by propensity scoring Footnote 22. In a study of infants born at <29 wGA with or without CLD in Hong Kong, the control cohort was from the time before a PVZ program had been started Footnote 30. Priante et al. studied infants born at 29-35 wGA, some of whom had CLD or hsCHD, before and after a change in criteria for use of PVZ Footnote 32.
Lacaze-Masmonteil in an observational cohort study rated as fair, reported on infants born at <33 wGA with or without CLD during a single RSV season Footnote 31. In a study of infants admitted to hospital with bronchiolitis, methodology rated as poor, the proportions of RSV positive infants in the PVZ and control groups were determined Footnote 23.
A test-negative case control study, methodology rated as fair, evaluated children born at ≤ 35 wGA and ≤ 12 months of age or <24 months of age with hsCHD or CLD hospitalized for acute lower respiratory tract infection. PVZ administration in the 30 days prior to admission in RSV positive cases and RSV negative controls was determined. PE was adjusted using an inverse propensity score weight. In addition, to control for potential biases, PE against human metapneumovirus (HMPV; a respiratory virus for which PVZ offers no protection) was also assessed Footnote 40.
III.3.1.1 RSV-associated hospitalizations
In the overall population of the Blake et al. study, infants born at 29 to <32 wGA and not prescribed PVZ tended toward more admissions to hospital with a positive RSV test compared to infants who received PVZ; however, the finding was based on a small number of RSV-positive hospitalizations (three infants in the pre-PVZ introduction cohort and one in the post-PVZ introduction cohort) and was not significant (p=0.09) Footnote 20.
The study of Priante et al. reported a non-significant increase in hospitalization due to RSV (RSVH) after restriction of PVZ prophylaxis, but the numbers admitted were small and there was a higher proportion of infants with CLD or hsCHD in the post-restriction group Footnote 32. In the study by Prais et al., receipt of PVZ in children born at <29 wGA resulted in a statistically significant reduction in RSV-positive hospital admissions compared to non-recipients (20.0%, n=2 versus 59.3%, n=19, p=0.033) Footnote 21.
In the Taiwan study, hospitalization within 6 months of the first dose of PVZ occurred in 1.6% of recipients, versus 10.2% of controls (p=0.002), a reduction of 86% (95% CI:36 to 97). Within 12 months, rates in the PVZ and control groups were 3.9% versus 15.7% (p=0.004), a reduction of 78% (95% CI: 40 to 92%). For infants ≤28 wGA the reduction was 89% (95% CI: 36 to 99) (p=0.007) at 6 months and 80% (95%CI: 38, 93) p=0.005 at 12 months.
The authors concluded that in an area where RSV infection is not seasonal, 6 months of protection from the time of initial discharge from hospital could be considered Footnote 22. Hospitalization for RSV occurred in 7.4% of PVZ recipients and 5.1% in non-recipients (p=0.13) in the study of Lacaze-Masmonteil et al. PVZ recipients were of lower GA and more had CLD than non-recipients and results were not adjusted for these confounders Footnote 31.
Lee et al. reported hospitalization for RSV in 5% in PVZ recipients and 15% in non-recipients (p=0.096). Among those of <27 wGA, the difference was significant (8.7% vs. 33.3%, p=0.046). Birth weight and GA were lower and the proportion with CLD higher in the PVZ recipients than non-recipients, and were not controlled for, and the total number of RSVHs was small (2 with PVZ and 15 without) Footnote 30. In the study of infants admitted to hospital with bronchiolitis the proportion of infants who were RSV positive was 35.5% in those who received PVZ and 57.3% in those who did not (p=0.006).
PVZ recipients were of lower gestational at birth and higher chronological age than those who did not receive PVZ Footnote 23. In the case-control study, the adjusted PVZ effectiveness (PE) to prevent hospitalization for RSV was 58.0% (95% CI: 43.1% to 69.0%). PVZ had no significant effectiveness against hospitalization in the HMPV control analysis (34.7, 95% CI: −12.9% to 62.2%) Footnote 40.
III.3.1.2 Length of hospital stay due to RSV
In the Blake et al. study, of the four infants born at 29 to <32 wGA admitted to hospital with a positive RSV test, infants not prescribed PVZ prophylaxis (n=3) tended to have longer LOS in hospital than the infant (n=1) prescribed PVZ, although this did not reach statistical significance (14.9 days vs. 2 days, p=0.08).
In the studies by Prais et al. and Lacaze-Masmonteil et al., LOS of children hospitalized with RSV was not reported. In the study of Chi et al., there was no significant difference in LOS for hospitalizations occurring in the first 6 months after discharge for those who received PVZ (n=2) and those who did not (n= 13), median 7.0 days (IQR3.5-10.5) versus 13.0 days (IQR 8.0-21.0) respectively (p=0.31) or in hospitalizations occurring within 12 months, median 7.0 days (IQR 3.5-10.5) versus 9.5 days (IQR 6.3-18.0) respectively (p=0.19) Footnote 22.
Lee et al. also reported no significant difference in LOS between PVZ recipients (n=2) and controls (n=15) (mean ± standard deviation (SD) 0.7 ± 3.7 days for PVZ recipients versus 1.1 ± 1.1 days for controls (p=0.52) Footnote 30. Narbona-Lopez reported a longer LOS in those who received PVZ (mean ± SD = 9.2 ± 4.2 days) than in the total cohort (7.1 ± 4.1 days) (p = 0.006); infants in the PVZ group were of lower wGA.
III.3.1.3 Admission to ICU due to RSV
In the study by Chi et al., admission to an ICU for RSV within the first 6 months after initial dose of PVZ occurred in 0.8% of PVZ recipients and 7.1% of controls (p=0.024); at 12 months the rates were 0.8% and 7.9% of PVZ recipients and controls respectively (p=0.014). Footnote 22. The proportions of ICU admissions in patients hospitalized for RSV were calculated from the data presented.
For hospitalizations in the first 6 months, one of two PVZ recipients and 9 of 13 controls were admitted to an ICU, and by 12 months the proportions were 1 of 5 and 10 of 20 (no significant differences). Lee et al. reported ICU admission rates of 2.5% for PVZ recipients and 7.4% for controls (p=0.436) Footnote 30.
One of two PVZ recipients hospitalized for RSV versus 7 of 15 hospitalized controls were admitted to ICU. In the case control study, the adjusted PE to prevent ICU admission was 62.1% (95% CI: 35.1% to 77.9%) Footnote 40.
III.3.1.4 Length of ICU stay due to RSV
In the study by Chi et al., there was no significant difference in ICU LOS between PVZ recipients and controls at 6 months (median 8.0 days (IQR 8.0, 8.0-8.0) versus 10.0 days, (IQR 4.5-13.0, p=1.0) or at 12 months (median 8.0 days IQR 8.0-8.0) versus 9.0 days, IQR 4.0-13.0 days, p=1.0).
III.3.1.5 Mechanical ventilation
In the first 6 months after enrollment, MV was required by none of the PVZ recipients and 3.1% of the controls (p=0.13) in the study by Chi et al. At 12 months there were no additional infants requiring MV Footnote 22. The proportions of hospitalized infants requiring MV were 0 of 5 for PVZ recipients versus 4 of 20 for controls.
Lacaze-Masmonteil et al. reported MV in 0.5% of PVZ recipients and 0.4% of controls Footnote 31. Two of 23 hospitalized PVZ recipients and 9 of 17 hospitalized controls required MV (not significantly different). In the case-control study, PE was not observed for MV (adjusted PE 31.5% (95% CI: −41.2% to 66.8%)) Footnote 40.
III.3.1.6 Mortality
In one study there were 2 RSV deaths among the 2370 who did not receive PVZ and none in the 376 who did Footnote 31. Two other studies reported no deaths Footnote 22 Footnote 30.
III.3.1.7 Long-term sequelae (wheezing, asthma)
The study by Prais et al. investigated the effects of PVZ prophylaxis on respiratory morbidity in the first two years of life and pulmonary function and bronchial responsiveness at school age (7–10 years of age) of children born at <29 wGA. These outcomes were analyzed by whether or not the children had received PVZ and not by whether or not they had a documented prior history of admission for RSV infection.
Based on parental responses to a questionnaire, the proportion of children with wheezing episodes in the first two years of life was significantly lower in those receiving PVZ prophylaxis compared to children who did not receive prophylaxis (26.7% versus 69.7%, p=0.008). In contrast, there were no significant differences found between these two groups in the proportion of children experiencing wheezing episodes at school age (4 of 30,13% versus 6 of 33, 18%, p=0.73) or using bronchodilators and inhaled corticosteroids (p=0.71) in the year prior to pulmonary function testing or in lung function parameters or bronchial responsiveness at school age. The lung function results were similar when the analysis was restricted to children born at <26 wGA, with BPD, or with and without a family or personal history of eczema or allergic rhinitis.
III.3.2 Premature infants without infantile chronic lung disease
There were seven studies identified in the updated literature review that compared the effectiveness of PVZ prophylaxis to placebo or no intervention in premature infants without CLD. Notario et al. published data from the 1996 IMpact RCT (methodology rated as good) on outcomes in premature infants without CLD by GA categories Footnote 37 Footnote 38 Footnote 38. In another RCT, rated as fair, premature infants who received PVZ or placebo were assessed for asthma at school age Footnote 39.There were four observational cohort studiesFootnote 22 Footnote 27 Footnote 28 Footnote 29
The study by Farber et al., rated of fair quality, examined the effect of PVZ prophylaxis on RSVHs in a cohort of premature infants from nine Medicaid managed care programs in Texas and analyzed the results by wGA of infants (29–32 wGA and 33–36 wGA) Footnote 29. The study of Chi et al., rated as fair, reported on infants ≤ 28 wGA assessed at 6 and 12 months after initial dose of a 6 month course of PVZ.
The control cohort was from an earlier time but matched by propensity scoring Footnote 22. In the study by Simoes et al., rated as a good quality study, the impact of PVZ prophylaxis on the subsequent development of physician-diagnosed recurrent wheezing at 24-month follow-up was examined in children born prematurely (<36 wGA) from multiple centres in Europe and Canada. Post hoc subgroups were determined based on family history of asthma and atopy Footnote 28. And finally, the Mochizuki et al. study, which was rated of fair quality, examined the effect of PVZ prophylaxis on the incidence of atopic asthma and growth in children at 6 years of age, as well as physician-diagnosed wheezing and respiratory outpatient visits and hospitalizations in the first 6 years of life.
The study followed a cohort of children born at 33–35 wGA at one of 52 medical centres in Japan. For the purposes of analysis, the study population was divided into three subpopulations: an intention-to-treat population of all children, a per-protocol population who completed the 6-year follow-up and an atopic asthma subpopulation who had blood collection for determination of immunoglobin E (IgE) at 6-year follow-up. Outcomes were analyzed by whether or not children had received PVZ and not specifically for children with and without a documented prior history of RSV infection Footnote 27. Simoes et al. compared outcomes in PVZ recipients, none of whom had been hospitalized for RSV, a control group without PVZ of which 33% were hospitalized for RSV and a subgroup of the controls that had no RSVHs Footnote 28. In a test-negative case control study, children born at ≤ 35 wGA and ≤12 months of age were studied. PE was adjusted using an inverse propensity score weight Footnote 40.
III.3.2.1 Hospitalizations due to RSV
In the RCT, significant reduction in hospitalization rate with PVZ was found for infants of 28-31 wGA: 6.7% vs. 1.8%, relative risk reduction (RRR) 73.0 (95% CI: 7.7 to 95.1); 29-32 wGA: 7.7% vs. 1.6%, RRR 79.7 (95% CI: 35.7, 96.9); 29-33 wGA: 9.1% vs. 1.8%, RRR 79.8 (95% CI: 49.0 to 94.2); 32-34 wGA: 10.8% vs. 2.0% RRR 81.8 (95% CI: 45.4 to 96.5); 32-35 wGA: 10.1% vs. 1.8%, RRR 82.1(95% CI: 45.9 to 96.6).There was no significant reduction in the <29 wGA or the 33-34 or 33-35 wGA groups Footnote 37.
The study by Farber et al. found there were fewer RSVHs in infants 29–32 wGA who received PVZ compared to infants who had not received prophylaxis (3.1% versus 5.0%, p=0.04). Most of this difference was accounted for by infants who received 80–100% of recommended PVZ doses (adjusted odds ratio [aOR] = 0.30, 95% CI: 0.12 to 0.78). Analyzing the data by four adherence groups (0–25%, 30–50%, 60–75%, 80–100%) there was a statistically significant dose-response between adherence and reduction in hospitalization for RSV (p for trend=0.009).
In contrast, there was no statistically significant difference in RSVH in infants 33–36 weeks GA who did and did not receive PVZ prophylaxis (4.2% vs. 4.5%, p=0.70). In the study of Chi et al., there was no significant reduction in hospitalization rate in infants ≤ 28 wGA without CLD (reduction 70%, 95% CI: -18 to 99) at 6 months and 70 %, 95% CI: -204 to 97) at 12 months Footnote 22. In the case control study, the adjusted PE was 74.1% (95% CI: 56.2% to 84.7%) in premature infants 29–35 wGA aged <6 months. PE was not observed for infants <29 wGA but numbers were small (n=33) Footnote 40.
III.3.2.2 Long-term sequelae (atopic asthma, physician-diagnosed recurrent wheezing, growth parameters)
In the study by Mochizuki et al., the prevalence of atopic asthma (defined as high serum total or specific IgE level and recurrent expiratory wheezing) at 6 years of age in children born at 33–35 weeks' GA was similar in children who had (31/202, 15.3%) and had not (12/66, 18.2%) received PVZ prophylaxis (RR=0.82, 95%CI: 0.39–1.70, p=0.57). In multivariable logistic regression, the findings were unchanged when comparing children with and without a family history of allergy Footnote 27.
Multivariate analysis in the Mochizuki et al. study also found that compared to no intervention, receipt of PVZ was associated with reduced rates of physician-diagnosed recurrent wheezing during the first six years of life in all three study subpopulations, but only in the subgroups of children with a family history of allergy (intention to treat, aOR=0.48, 95%CI: 0.26–0.90; per protocol, aOR=0.28, 0.13–0.60; atopic asthma, aOR=0.54, 0.11–0.27) Footnote 27.
In contrast, the study by Simoes et al., in which post-hoc subgroups were determined based on family history of asthma and atopy, found that it was only in premature (<36 weeks' GA) children without a family history of asthma (aOR=0.32, 95%CI: 0.14–0.75) or atopy (aOR=0.20, 95%CI: 0.07–0.59) in which PVZ prophylaxis in a previous respiratory season decreased the incidence of physician-diagnosed wheezing at 24-months follow-up after study enrollment.
In children without a family history of asthma or atopy, multiple logistic regression analysis also found increased birth weight (aOR=0.35, 95% CI: 0.15 to 0.81) and/or increasing GA (aOR=0.82, 95% CI: 0.68 to 0.98) were associated with a reduced risk of physician-diagnosed wheezing. The Simoes et al. study also used proportional hazard regression analysis to examine the time to third physician-diagnosed wheezing episode.
In this analysis, PVZ prophylaxis significantly increased the time to third physician-diagnosed wheezing episode compared to children receiving no intervention (adjusted hazard ratio (aHR)=0.33, 95% CI: 0.51 to 0.74 and aHR=0.21, 95% CI: 0.08 to 0.59, respectively), but again only in children without a family history of asthma or atopy. In these children, greater wGA was also associated with a longer time to third episode of physician-diagnosed wheezing Footnote 28.
The Mochizuki et al. study also found that only in the overall, intention to treat population receipt of PVZ resulted in significantly fewer outpatient respiratory visits during the first six years of life compared to children who received no intervention (19.0 vs. 23.9 visits/person, p=0.018). However, the etiology underlying these respiratory visits was not determined. There was no significant difference between these two groups in the number of hospitalizations due to respiratory disease in this same time period Footnote 27.
The study by Mochizuki et al. also found no significant differences in weight (19.4 ± 3.46 kg vs. 19.5 ± 2.66 kg, p=0.83), height (112.0 ± 4.0 cm vs. 112.7 ± 5.76 cm, p=0.33), or body mass index (15.4 ± 1.85 vs. 15.3 ± 1.26, p=0.75) between children who received PVZ prophylaxis compared to children who did not receive prophylaxis when assessed at 6 years of age Footnote 27.
Scheltema et al. assessed otherwise healthy infants born at 32-35 wGA, who had received either PVZ or placebo in their first RSV season, for asthma at age 6 years. Parents reported asthma, defined as wheeze or the use of asthma medication in the past 12 months, in 14.1% of PVZ recipients and 24.0% of placebo recipients (absolute risk reduction (aRR) 9.9%, 95% CI: 2.2 to 17.6). However, the difference was significant only for those with infrequent wheeze (1-3 episodes per year). There was no significant difference in the use of asthma medication (9.0% vs. 12.8%, aRR 3.5% (95% CI: -2.4 to 9.9), nor in physician diagnosed asthma in the previous 12 months (10.3% vs. 9.9%, ARR -0.4%, 95% CI: -6.5 to 5.8). Pulmonary function at 6 years of age did not differ between the groups. Mean (SD) forced expiratory volume in 0.5 seconds were 89.1 (10.6) with PVZ and 90.1 (11.1) with placebo; several other measurements as well as results after administration of a bronchodilator were all similar in the two groups Footnote 39.
III.3.3 Premature infants with infantile chronic lung disease
The updated literature review identified one observational cohort study rated Footnote 22 and one case-control study Footnote 40, both rated as fair, that examined PVZ prophylaxis in premature infants with CLD. In the study by Chi et al., infants ≤ 35 wGA with CLD were assessed at 6 and 12 months after the initial dose of a 6 month course of PVZ. The control cohort was from an earlier time but matched by propensity scoring Footnote 22. The test-negative case control study evaluated children born at ≤ 35 wGA and < 24 months of age with CLD Footnote 40.
III.3.3.1 Hospitalizations due to RSV
In the study by Chi et al., RSVH rate was reduced by 86% (95% CI: 13, 96, p= 0.039) in infants of ≤ 35 wGA in the first 6 months after initial discharge and 79% (95% CI: 36, 93, p 0.006) at 12 months. When analyzed by GA, the reduction in rate was significant in those of ≤ 28 wGA (89%, 95% CI: 8, 99; p =0.038) at 6 months and 82%, 95% CI: 34, 95; p=0.010 at 12 months.
Reduction was not significant in those 29-35 wGA (47%, 95% CI: -534 to 96; p=0.61 at 6 months and 67%, 95% CI: -252 to 97; p=0.67 at 12 months) but numbers in the latter group were small (19 PVZ recipients and 21 controls) Footnote 22. In the case control study, PVZ effectiveness was not observed in infants with CLD (33.8%, 95% CI: -31.1 to 66.6 at age <12 months and 63.8%, 95% CI: -9.3 to 88) age 12-24 months) Footnote 40.
III.3.4 Children with hemodynamically significant congenital heart disease
The updated literature review identified two observational cohort studies and one case-control study, all rated as fair, that examined PVZ effectiveness in children with hsCHD. Both observational cohort studies were of infants with hsCHD aged <1 year and the PVZ and control cohorts were from different time periods. In one, PVZ and control groups were matched by a propensity score Footnote 33. In the other, available only as an extensive abstract, cases and controls did not differ by wGA, birth weight or sex, but the control group had fewer infants with more severe forms of hsCHD Footnote 34. The case control study included infants with hsCHD aged <24 months Footnote 40.
III.3.4.1 Hospitalizations due to RSV
In the study by Chiu, hospitalization incidence rates per 1000 person-days, after matching by propensity score, were 0.076 for PVZ recipients and 0.145 for controls. Hospitalization was decreased by 49% in the PVZ recipients (rate ratio 0.51, 95% CI: 0.28 to 0.93, p <0.05). The difference was significant for those with cyanotic hsCHD with a rate ratio of 0.35 (95% CI: 0.14 to 0.90, p <0.05), but not for those with acyanotic hsCHD with a rate ratio of 0.65 (95% CI: 0.29 to 1.44) Footnote 33.
Soraiz et al. reported hospitalization rates of 6% in PVZ recipients and 20% in the control group, with a risk ratio (RR) of 0.28 (95% CI: 0.08 to 0.97), p=0.04 Footnote 34. The number needed to treat (NNT) to prevent one hospitalization was reported as seven. In the case control study, PVZ effectiveness was not observed at age <12 months (15.5%, 95% CI: -141 to 44.6) or at age 12-24 months (69.2%, 95% CI: -101.6 to 95.3) Footnote 40.
III.3.4.2 Length of hospital stay due to RSV
One study looked at length of hospital stay Footnote 33. There was no significant difference in hospital LOS, either for all patients (risk 0.640 vs. 1.573 days per 1000 person-days for PVZ recipients and controls respectively, rate ratio 0.396, 95% CI: 0.137 to 1.146) or for the subgroups with acyanotic or cyanotic hsCHD.
III.3.4.3 Admission to ICU due to RSV
The study by Chiu et al. reported no significant difference in admission to ICU, either for all patients (0.030 and 0.064 per 1000 person-days for PVZ recipients and controls respectively, rate ratio 0.426, 95% CI: 0.167 to 1.038) or for subgroups with acyanotic or cyanotic hsCHD Footnote 33. Of those hospitalized for RSV, the proportions admitted to the ICU were 7 of 18 PVZ recipients vs. for 15 of 34 controls (no significant difference).
III.3.5 Children residing in remote communities
The updated literature review did not identify any studies that examined PVZ prophylaxis efficacy/effectiveness in children living in remote communities.
III.3.6 Children with CF
The updated literature review identified one good quality systematic review Footnote 17, two fair quality retrospective observational cohort studies Footnote 24 Footnote 25, one retrospective observational cohort study of poor quality Footnote 26 and one case control study of fair quality Footnote 41 that examined the effect of PVZ prophylaxis compared to no intervention or placebo in children with CF.
The systematic review by Robinson et al. is an update to a previous Cochrane Database systematic review captured in the initial INESSS literature review Footnote 42. As with the previous review, the updated review identified the same single multicentre RCT by Cohen et al. which could not be assessed for quality as it was published as a conference abstract and poster, but not as a complete article. The study examined the efficacy and safety of PVZ prophylaxis versus placebo in preventing RSVHs and mortality, as well as a number of secondary outcomes (adverse events related to PVZ, nutritional status at 12-month follow-up, P. aeruginosa colonization) in children with CF Footnote 43.
The observational study by Groves et al. examined the effect of PVZ prophylaxis on RSVH rates and long-term outcomes (lung function, growth parameters, bacterial colonization) in small historical cohorts of CF patients in Ireland. The observational cohort by Bjornson et al. assessed children with CF <24 months of age in Alberta who did or did not receive PVZ Footnote 25.
The case-control study reported on long term outcome of children with CF aged ≤ 36 months in a center in France that used PVZ systematically and a center that did not use PVZ. PVZ recipients were matched with 3 controls for year and month of birth, gender and CF genotype. All were followed to age 3 years Footnote 41. The observational study of Fink et al. reported all-cause mortality in the first two years of life and long term outcomes Footnote 26.
Groves et al. reported on pulmonary function test results at 6 years of age in those who were hospitalized for RSV and those who were not Footnote 24. Other long term outcomes in that study and all long term outcomes in the other four studies were analyzed by whether or not children had received PVZ prophylaxis and not specifically for children with and without a documented prior history of RSVH.
III.3.6.1 Hospitalizations due to RSV
In the multicentre RCT study by Cohen et al., there were 13 (14.1%) children with CF (mean age 12.8 months) who received PVZ prophylaxis and were hospitalized compared to 14 (14.9%) hospitalizations in the placebo group, but only one child in each group was identified as being hospitalized due to RSV (based on a positive RSV antigen test). The calculated RR found no significant difference between the children who did and did not receive PVZ prophylaxis in the risk for RSVH (RR=1.02, 95%CI: 0.06–16.09) Footnote 43.
In contrast, in the study by Groves et al., the historical cohort of children who did not receive PVZ prophylaxis (n=47) were found to be at increased risk of hospitalization for RSV infection (RR=4.78, 1.1–20.7) and to be significantly more likely to have a RSV-related hospitalization for lower respiratory tract infection compared to the cohort of children (n=45) who received PVZ prophylaxis (10/47 vs. 2/45, p=0.027) Footnote 24.
In the study by Bjornson et al., the rate of hospitalization for RSV was 2.7% in the PVZ group (n=183) and 6.0% in the control group (n=84) (p=0.20). PVZ recipients had lower wGA at birth and lower birth weight, were less likely to have siblings and more likely to be born during RSV season. After adjustment for these confounders, the PVZ group again did not have a decreased odds of hospitalization for RSV (Exp(B) = 0.43 [0.10–1.80], p=0.25). The PVZ group did have a significantly lower rate of hospitalization for respiratory illness (Exp(B) = 0.23 [0.11–0.49], p<0.0005) and it was noted that the overall rate of testing for RSV was low at 53% Footnote 25. In the case control study there was no significant difference in hospitalization rate between PVZ recipients (2 of 40, 5%) and controls (4 of 140, 2.9%; p=0.634) Footnote 41.
III.3.6.2 Length of hospital stay due to RSV
The LOS was shorter for PVZ recipients (mean ± SD 5.66 ±2.41 days) than for controls (47.00 ±39.32 days; p=0.048) in the study of Bjornson et al. Footnote 25.
III.3.6.3 Admission to ICU due to RSV
In the study by Bjornson et al., none of the 5 admitted patients in the PVZ group and 2 of the 5 admitted patients in the control group required ICU admission (p=0.11). Of the total cohorts, 0/183 and 2/84 (2.4%) required ICU admission (p >0.05) Footnote 25.
III.3.6.4 Length of ICU stay due to RSV
In the study of Bjornson et al., the mean ± SD LOS in ICU was 5 ± 5.66 days in the control group. No PVZ recipients were admitted to the ICU Footnote 25.
III.3.6.5 Use of oxygen therapy due to RSV
In the Cohen et al. study, one participant in the PVZ prophylaxis group and no participants in the placebo intervention group required oxygen therapy, resulting in no significant difference between the groups in the need for oxygen therapy Footnote 43. In the Bjornson et al. study, increased respiratory support, either MV or supplemental oxygen, was required by 4 (80% of admitted patients) in the PVZ group and 1 (20% of admitted patients) in the control group (p=0.06). Of the total cohorts, 2.2% of the PVZ group and 1.2% of the control group required respiratory support (p=0.58) Footnote 25. No admitted patients required supplemental oxygen or MV in the study by Buchs et al. Footnote 41.
III.3.6.6 Mortality
Cohen et al. study examined this outcome and reported no deaths in either the PVZ or placebo groups during the 6 months of study follow-up Footnote 43. In the study of Fink et al. there were no differences in all-cause mortality before age 2 years between those who did or did not receive PVZ, whether throughout the year or when restricted to the RSV seasons Footnote 26.
III.3.6.7 Long-term sequelae (lung function, growth parameters, P. aeruginosa or S. aureus colonization)
The study by Groves et al. assessed the lung function of children with CF at 6 years of age. The study assessed lung function by measurement of the percent of predicted forced expiratory volume in one second, (FEV 1 ), and found no significant differences between PVZ recipients and non-recipients (97.1% vs. 97.5%, p=0.92) or between those who had been hospitalized for RSV and those who had not Footnote 24. Fink et al. reported no difference in percent of predicted FEV 1 at age 7 years between children who had or had not received PVZ (98.2%, 95% CI: 96.9-99.5 vs. 97.3%, 95% CI: 96.1 to 98.5, respectively) Footnote 26.
Robinson et al. obtained additional information on the nutritional status (weight gain and weight to height ratio) of the children with CF in the Cohen et al. study at 12-month follow-up. There were no significant differences between the PVZ and placebo groups with respect to weight gain (2.7 kg, range: 1.1–6.3 kg vs. 2.7 kg, range: 0.3–6.9 kg) or weight to height ratio (data not provided) Footnote 17.
The Groves et al. study also found no significant differences between children who did and did not receive PVZ prophylaxis in weight (22.1 kg vs. 21.8 kg, p=0.63), height (117.2 cm vs. 116.6 cm, p=0.60), or body mass index (16.0 vs. 16.0, p=0.95) at 6 years of age Footnote 24. The study of Buchs et al. also found no significant difference in growth parameters (weight Z scores) at 1, 2 or 3 years of age between children who did or did not receive PVZ Footnote 41.
The authors of the Robinson et al. systematic review also obtained additional data from the Cohen et al. authors on the number of children colonized with P. aeruginosa in the study. There were similar numbers with P. aeruginosa colonization in the PVZ prophylaxis (14, 15.2%) and placebo (12, 12.8%) groups (RR=1.19, 95%CI: 0.58–2.44) at 12 month follow-up Footnote 17. Similarly, the study by Groves et al. found no significant difference in P. aeruginosa colonization rates between PVZ recipients and non-recipients at 6 years of age.
However, the median time to a first isolate of P. aeruginosa was significantly shorter in PVZ recipients than in non-recipients (57 months versus 96 months, p=0.025) as was the RR of a first P. aeruginosa isolate during the study period (RR=2.5, 1.44–4.2, p=0.001) Footnote 24. Buchs et al. assessed age at first colonization with S. aureus and with P. aeruginosa and the percentage of infants colonized with these organisms by 3 years of age.
There were no significant differences between PVZ recipients and controls in age at first colonization with either organism, or in the proportion colonized with P. aeruginosa by age 3 years. The proportion colonized with S. aureus by age 3 years was significantly higher in the PVZ group than in the control group (97% vs. 85%, p=0.001).
The authors speculated that PVZ recipients may have had more exposure to S. aureus during monthly clinic visits for PVZ, or that background S. aureus colonization rates may have been different in the two separate towns where the PVZ recipients and controls lived Footnote 41. Fink et al. reported no difference in time to first P. aeruginosa colonization between those who did or did not receive PVZ (unadjusted hazard ratio 1.1 (95% CI: 1.0 to 1.2); after propensity score adjustment 1.1 (95% CI: 0.96 to 1.2) Footnote 26.
III.3.7 Children with Down syndrome
The updated literature review identified two observational studies that examined PVZ prophylaxis effectiveness in children with Down syndrome. The first, rated as fair, studied term infants age <1 year with no CLD or hsCHD. Cases were compared to a control cohort of term infants without Down syndrome with no CLD or hsCHD, matched by sex and date of birth Footnote 35. The second study, rated as good, reported on children age <2 years with Down syndrome with or without co-morbidities Footnote 36.
III.3.7.1 Hospitalizations due to RSV
In the study of Sanchez-Luna et al., RSVH rate was higher in infants with Down syndrome than in the control group Footnote 35. Of those with Down syndrome, 1 of 33 (3%) of PVZ recipients and 9/60 (15%) without PVZ were hospitalized (p=0.075). PVZ prophylaxis was not an independent predictor for RSVH. Kimura et al. reported a decrease in overall RSVH after PVZ prophylaxis was approved for all children with Down syndrome but there was no difference in RSVH in those without additional risk factors for RSV. For all children with Down syndrome aOR for those receiving PVZ was 0.41 (95% CI: 0.18 to 0.92, p=0.03); for the group without hsCHD, aOR was 0.43, 95% (CI: 0.04, 4.26, p=0.47) and for those without any additional risk factors for RSVH aOR was 0.68 (95% CI: 0.06, 7.73, p= 0.75) Footnote 36.
Discussion/summary
The updated literature review on the efficacy/effectiveness of PVZ prophylaxis in reducing complications associated with RSV infections in infants identified three systematic reviews, two RCT and 19 observational studies. The evidence related to the efficacy/effectiveness of PVZ prophylaxis for various RSV-related outcomes are discussed below in relation to the findings from the original INESSS literature review and are summarized by the high risk populations included in the studies: mixed population (Section IV.1), premature infants without CLD (Section IV.2), premature infants with CLD (Section IV.3), children with hemodynamically significant CHD (Section IV.4), children residing in remote communities (Section IV.5) children with CF (Section IV.6) and children with Down syndrome (Section IV.7). The data from the updated literature review and the original INESSS literature review are summarized in table format in Appendix E and Appendix F, respectively.
IV.1 Mixed population
IV.1.1 RSV-associated hospitalizations
The updated literature review identified five observational cohort studies and one test-negative case control study, all rated as fair, that presented data on mixed populations. An observational cohort study by Prais et al. found PVZ prophylaxis resulted in a significant reduction of 40% in RSV-positive hospital admissions in the first two years of life in children <29 wGA, some with CLD Footnote 21. In another observational cohort study, including infants of ≤ 28 wGA and of ≤ 35 wGA with CLD, PVZ reduced the RSVH by 86% Footnote 22.
Two other observational cohort studies of premature infants, some with CLD, did not find a statistically significant reduction in RSVH with PVZ, but the PVZ groups had more high risk patients than the control groups and rates were not adjusted for these confounders Footnote 30 Footnote 31. The fifth study reported a non-significant increase in RSVH in the untreated group but there were few admissions and more high risk infants were untreated Footnote 32. A test-negative case-control study, of fair methodology, reported a significant PVZ effectiveness to prevent hospitalization for RSV of 58% Footnote 40.
In addition, a fair quality study found PVZ recipients to be less likely to have a positive RSV test at admission to hospital for respiratory disease compared to non-recipients in a population of infants born at 29 to <32 wGA. However, this finding did not reach statistical significance, perhaps because very few infants (one PVZ recipient and three non-recipients) had a positive RSV test) and the study may have been underpowered to detect such a difference Footnote 20. In another study, of poor quality, infants admitted with bronchiolitis who had received PVZ were significantly less likely to have a positive RSV test than non-recipients Footnote 23.
The original INESSS literature review found PVZ prophylaxis associated with reductions in the risk of RSV-associated hospital admissions of between 50–65%, based on findings from two systematic reviews and meta-analyses of average to good quality involving children ≤35 weeks GA, some with CLD or hsCHD Footnote 44 Footnote 45. Similarly, a RCT of good quality involving children born at ≤35 wGA or children ≤24 months of age with CLD, found PVZ prophylaxis associated with a 55% reduction in the risk of RSV-associated hospital admissions Footnote 38.
An observational study of poor quality involving either children born at <33 wGA or 33–35 wGA with CLD or requiring home oxygen found PVZ prophylaxis associated with a 60% reduction in RSV-associated hospitalizations Footnote 46. And finally a very poor quality observational study involving children born at either ≤28 wGA or at 29–32 wGA, some with CLD, found PVZ prophylaxis associated with a 74% and 46% reduction in RSV-associated hospitalizations, respectively Footnote 47.
The findings of the updated literature review appear consistent with the conclusions reached in the original INESSS literature review that PVZ prophylaxis is associated with reductions in the risk of RSV-associated hospital admissions in mixed populations of infants at risk of severe RSV infection. Differences in the health conditions of the mixed populations, study design and study quality preclude definitive conclusions about relative benefits for different patient groups.
IV.1.2. Additional hospital outcomes due to RSV
IV.1.2.1 Length of hospital stay due to RSV
In the study by Blake et al., RSV-positive infants who had received PVZ prophylaxis tended to have shorter LOS compared to infants who had not received prophylaxis (2.0 vs. 14.9 days), but the finding did not reach statistical significance. Likewise, LOS was not significantly shorter in PVZ recipients than in non-recipients in the studies of Chi et al. (7 vs. 13 days) and Lee et al. (0.7 vs. 1.1 days). The number of hospitalizations in each of these studies was low. In the study of Narbona-Lopez, the LOS for PVZ recipients was significantly longer than in the total cohort of infants admitted with RSV bronchiolitis (9 vs. 7 days), however the infants who received PVZ were of lower GA Footnote 23.
In the original INESSS review, PVZ prophylaxis was associated with a significantly lower total number of days of RSV-associated hospitalization vs. placebo in a single RCT involving children born at ≤35 wGA or ≤24 months of age with CLD (36.4 vs. 62.6 days per 100 children). As numbers of subjects and the admission rates differed between two groups, the study does not provide direct information on the LOS in those who were admitted. Actual LOS calculated from data in the publication was 7.58 days for the PVZ group and 5.91 for the placebo group (not significantly different) Footnote 38. There was a small but significantly decreased duration of hospitalization versus no intervention in one observational study of poor quality of children born at either ≤28 weeks' GA or at 29–32 weeks' GA, some with CLD (6 days vs. 8 days) Footnote 47.
The findings do not provide an indication that PVZ has an important impact on LOS for infants hospitalized for RSV, but may be underpowered to detect such an effect.
IV.1.2.2 Admission to and LOS in an ICU due to RSV
The updated literature review identified three studies that examined the effect of PVZ prophylaxis on admission to ICU or ICU LOS due to RSV. Admission to ICU occurred in 0.8% of PVZ recipients and 7.1% of controls (89% reduction, p=0.024) in the study of Chi et al. and in 2.5% of PVZ recipients and 7.4% of controls (p=0.436) in the study of Lee et al. In the case-control study, PVZ effectiveness to prevent ICU admission was significant at 62% Footnote 40. Of those hospitalized for RSV, the proportions admitted to ICU were one of two and 9 of 13 in the PVZ and control groups respectively Footnote 22 and one of two and 7 of 15 respectively Footnote 30.
The original INESSS literature review identified three studies that examined ICU admission rates: an RCT of good quality Footnote 38, a historical cohort study of very poor quality Footnote 47, and two of the studies rated high quality identified in a systematic review and meta-analysis of good quality Footnote 44. The meta-analysis and the RCT found PVZ prophylaxis associated with significant 50% (RR=0.50, 95% CI: 0.30 to 0.81) and 57% (RR=0.43, 95% CI: 0.21 to 0.90) reductions, respectively in the risk of admission to an ICU due to RSV in PVZ recipients compared to placebo Footnote 38 Footnote 44. In the IMpact study, the proportion of children hospitalized with RSV who were admitted to ICU was 27% in the PVZ group and 28% in the placebo group. The observational cohort study found no significant difference in the proportion of children admitted to the ICU due to RSV between children who received PVZ prophylaxis (9/71, 13%) and children who received no intervention (33/161, 20%) (RR=0.62, 95% CI: 0.31 to 1.22) Footnote 47.
The updated literature review identified one observational cohort that reported ICU LOS. There was no significant difference in duration between PVZ recipients (8 days, n=1) and controls (10 days n=9) Footnote 22. The original INESSS literature review did not identify studies that reported length ICU stay. The IMpact study reported the total number of ICU days per 100 children in PVZ and placebo recipients. PVZ recipients had a significantly higher number of ICU days per 100 children compared to placebo recipients (13.3 vs.12.7 p=0.023)Footnote 38.
IV.1.2.3. Use of and duration of use of MV due to RSV
The updated literature review identified three studies that examined the effect of PVZ prophylaxis on use of MV due to RSV. In two observational studies there were no significant differences between PVZ recipients and controls in the proportions of the total groups or the proportions of patients hospitalized for RSV infection that required MV, but numbers in both studies were small Footnote 22 Footnote 31. In the case-control study, PVZ was not effective in preventing need for MV Footnote 40.
The original INESSS literature review identified three studies that examined the use of MV due to RSV: two of the studies of high quality identified in the systematic review and meta-analysis by Andabaka et al., the IMpact RCT, and the historical cohort study by Pedraz et al. Footnote 38 Footnote 44 Footnote 47. The results from all three analyses found no significant difference in the use of MV due to RSV in children who received PVZ prophylaxis compared to children who received placebo or no intervention.
There were no studies in the updated review or the original INESSS literature review that addressed duration of MV. One study in the original INESSS review, the IMpact RCT, found children receiving PVZ prophylaxis had a total of 8.4 days of MV /100 children compared to 1.7 days/100 children in placebo recipients, but the difference was not statistically significant (p=0.210).
There is no evidence that PVZ has an effect on need for MV in high risk children with RSV infection, but studies may have been underpowered to detect this outcome.
IV.1.2.4 Duration of oxygen therapy due to RSV
There were no studies identified in the updated literature review that examined the effect of PVZ prophylaxis on the duration of oxygen therapy due to RSV.
The only study identified in the original INESSS literature review that examined need for oxygen therapy was again the IMpact RCT involving children born at ≤35 wGA or ≤24 months of age with CLD. This study found children receiving PVZ prophylaxis required significantly fewer total days of oxygen therapy per 100 children than placebo recipients (30.3 versus 50.6, p<0.001).
These results suggest that PVZ has a significant effect in reducing the overall rate of ICU admissions by 50-60% in high risk populations. Although numbers are small, it appears that for breakthrough RSV infections in PVZ recipients that require hospitalization, severity of illness, as manifested by need for ICU admission, ICU LOS and need for MV is not impacted by PVZ.
IV.1.3 Mortality
Three studies identified in the updated literature review reported on mortality. In one study there were 2 RSV-related deaths in 2370 patients in the control group and none in 376 patients who received PVZ Footnote 31, The other two studies reported no deaths Footnote 22 Footnote 30.
The original INESSS literature review identified four studies that examined all-cause mortality that come to conflicting results: the systematic review and meta-analysis by Andabaka et al. that included data from three RCT Footnote 44; the systematic review and meta-analysis by Checchia et al. Footnote 45, involving children ≤35 wGA, some with CLD or CHD that included data from the same 3 RCT plus 4 cohort studies; the RCT involving children born at ≤35 wGA or ≤24 months of age with BPD Footnote 38; and the historical cohort study involving children born at ≤32 wGA, some with CLD Footnote 47. In the historical cohort study, all-cause deaths were 6 in 1919 PVZ recipients and 22 in 1583 non-recipients (p<0.001) Footnote 47.
Of the other three analyses, only the meta-analysis of Checchia et al. found PVZ prophylaxis to be associated with a significant reduction in all-cause mortality compared to children who received placebo or no intervention (OR=0.30, 95% CI: 0.17 to 0.55, p<0.001). In contrast, the meta-analysis from Andabaka and the RCT found PVZ prophylaxis to be associated with non-significant RR reductions in all-cause mortality of 0.69 (95% CI: 0.42 to 1.15) and 0.40 (95% CI: 0.11 to 1.48), respectively.
Checchia et al. also reported on RSV-related deaths: 3 of 6358 in the PVZ group and 2 of 6162 in the no prophylaxis group (OR, 1.22; 95% CI: 0.20 to 7.38). In the RCT, two deaths in the PVZ group and none of in the placebo group occurred during hospitalization for RSV Footnote 38. In the historical cohort study there were no RSV-related deaths in PVZ recipients and one RSV-related death in the control group Footnote 47.
These studies indicate conflicting results on the effect of PVZ on all cause mortality in high risk infants with RSV. RSV-related deaths were rare in both PVZ recipients and those who did not receive PVZ.
IV.1.4 Long-term sequelae
The updated review identified a single study of fair quality that suggests PVZ prophylaxis may reduce wheezing in the short term (first two years of life), but may not have a significant impact on longer term outcomes Footnote 21. The study found that significantly fewer children born at <29 wGA who received PVZ prophylaxis had wheezing episodes during the first two years of life compared to similar children who did not receive prophylaxis.
In contrast, by the time these children had reached school age (7–10 years of age), there were no significant differences between these two groups in the proportion of children experiencing wheezing episodes, or using bronchodilators and inhaled corticosteroids at school age, or in lung function parameters or bronchial responsiveness at school age. The lung function results were similar when the analysis was restricted to children born at <26 wGA, with CLD, or with and without a family or personal history of eczema or allergic rhinitis.
The original INESSS literature review did not identify any studies that examined this outcome in a mixed population. Therefore, the evidence on the effect of PVZ prophylaxis on long-term sequelae of RSV infection in a mixed population is very limited. No clear conclusions can be drawn.
IV.2 Premature infants without infantile chronic lung disease
IV.2.1 RSV-associated hospitalizations
The updated review identified four studies that examined this outcome. A retrospective cohort study of fair quality by Farber et al. found fewer RSVHs in the first RSV season in infants 29–32 wGA who received PVZ compared to infants receiving no prophylaxis, with additional analysis suggesting most of the effect was accounted for by infants with higher adherence to prophylaxis (receipt of 80–100% of recommended doses).
The same study found no statistically significant difference in RSVH in infants 33–36 wGA who did and did not receive PVZ prophylaxis. However, use of and adherence to PVZ prophylaxis in infants born at 33–36 wGA was quite low Footnote 26. Notario et al. analyzed data from the IMpact RCT (rated as good) for premature infants without CLD and reported that PVZ resulted in significant reductions in hospitalization rates for RSV for infants of 28-31 wGA (73%), 29-32 wGA (80%), 32-34 wGA (82%), and 32-35 wGA (82%), but not for those <29 wGA or 33-35 wGA Footnote 37.
In an observational study rated fair, PVZ prophylaxis did not significantly reduce RSVH rate for infants of ≤ 28 wGA Footnote 22. In a case-control study rated as good, PVZ effectiveness for prevention of RSVH was 74% in premature infants of 29-35 wGA. Effectiveness was not observed in those <29 wGA but the numbers were small Footnote 40.
The original INESSS literature review identified seven studies that examined the effect of PVZ on RSVH in premature infants without CLD: a systematic review and meta-analysis of average quality Footnote 45, three RCTs of good Footnote 38 to average quality Footnote 48 Footnote 49, and three cohort studies of either good Footnote 50, average Footnote 51, or poor quality Footnote 52.
The systematic review and meta-analysis found that compared to no prophylaxis, PVZ use was associated with 72% fewer RSVHs in infants born at ≤32 wGA and 74% fewer in infants born at 32–35 wGA. A similar significant protective effect of PVZ prophylaxis was found in the three RCTs of infants born at ≤32 wGA (74, 47%) Footnote 38 Footnote 48, 32–35 wGA (72%) Footnote 38, and 33–35 wGA (82%) Footnote 49.
The historical cohort studies cited in the original INESSS review had conflicting results about the impact of PVZ prophylaxis on RSVHs in premature infants. PVZ prophylaxis was found to significantly reduce RSVHs, in cohorts of children born at ≤30 wGA (1.1% vs. 13.6% Footnote 52 and 32–34 wGA (55% reduction) Footnote 51 but was not significantly effective in other cohorts of children born at 32–34 wGA Footnote 51 and 32–35 wGA Footnote 50.
The finding of significant PVZ effectiveness in reducing RSV associated hospitalizations in infants 29–33 wGA in the studies identified in the updated review is consistent with the findings from the systematic review and RCTs identified in the original INESSS literature review. Three studies suggested lack of effect in infants of <29 wGA but this may be the result of small numbers of infants without CLD in this very premature group Footnote 22 Footnote 37 Footnote 40.
The conflicting results for infants over 33 wGA are difficult to interpret, but may in part be due to differences in study design and methodology. However, in general, it appears there is evidence in support of the effectiveness of PVZ in reducing RSV associated hospitalizations in children born prematurely, although the level of prematurity at which PVZ is most effective is not entirely clear from the present findings.
IV.2.2 Mortality
There were no studies identified in the updated literature review that examined the effect of PVZ prophylaxis on mortality in this population.
The only study identified in the original INESSS literature review that examined all-cause mortality in this population was the systematic review and meta-analysis of average quality by Checchia et al. Footnote 45. In each group of premature infants, the meta-analysis consisted of data from three studies (two of the studies were common to both wGA groups). In infants born at ≤32 wGA, PVZ recipients had a significantly reduced risk of all-cause mortality (OR=0.25, 95% CI: 0.13 to 0.49, p<0.001) compared to recipients of placebo or no intervention, while in infants born at 32–35 wGA, the difference was not significant (OR=0.22, 95% CI: 0.03 to 1.89, p=0.085). RSV-related mortality was not determined.
Therefore, it is possible that PVZ prophylaxis may have an effect on all-cause mortality in infants born at ≤32 wGA, but not at prematurity of 32–35 wGA. However, these findings are based upon few studies which may have been underpowered to detect difference in mortality in the less premature infants.
IV.2.3 Long-term sequelae
IV.2.3.1 Recurrent wheezing and atopic asthma
The updated literature review identified one RCT Footnote 39 of fair quality and two cohort studies of good Footnote 28 and fair Footnote 27 quality that examined rates of physician-diagnosed wheezing in children who had and had not previously received PVZ prophylaxis. The RCT was a follow-up to the study by Blanken et al. referred to below. Otherwise healthy premature infants of 32-35 wGA received PVZ or placebo in their first RSV season. At 6 years of age there were no differences in receipt of medications for asthma or physician diagnosed asthma in the preceding year and no difference in results of pulmonary function tests.
Significantly more parent-reported wheezing occurred in the placebo group than the PVZ group but this was only significant in those reporting infrequent wheezing (1-3 episodes per year) Footnote 39. In the study by Simoes et al., children born at <36 wGA and ≤36 months of age at enrollment who received PVZ prophylaxis in a previous respiratory season had a significantly decreased incidence of physician-diagnosed wheezing at 24-months after study enrollment and a significantly longer time to a third physician-diagnosed wheezing episode compared to children receiving no intervention, but only in children without a family history of asthma or atopy.
There was no significant difference in these outcomes in children with a family history of asthma or atopy Footnote 28. The study by Mochizuki et al. of children born at 33–35 wGA found that children who had received PVZ prophylaxis had reduced rates of physician-diagnosed recurrent wheezing during the first 6 years of life compared to children who had not received prophylaxis. However, this association was found only in the subgroups of children with a family history of allergy. The study also found the prevalence of atopic asthma (defined as recurrent wheezing plus a high total or specific level of IgE) at age 6 years to be similar in children who had received PVZ compared to those who received no intervention, regardless of family history of allergy Footnote 27.
The original INESSS literature review identified three studies that examined the effect of PVZ prophylaxis on the risk of wheezing in the first year of life: an average quality RCT Footnote 49 and two cohort studies of poor Footnote 53 to average Footnote 54 quality. The Yoshihara study was an earlier report on the cohort described in the publication by Mochizuki et al. referred to above. One study (Blanken) investigated parent-reported wheezing only, while the other two investigated physician-diagnosed wheezing.
All three studies found that PVZ prophylaxis in otherwise healthy premature infants born at 33–35 wGA Footnote 49 Footnote 53 or ≤35 wGA Footnote 54 resulted in a significant reduction (46-66%) in the risk of recurrent wheezing in children in the first one Footnote 49, two Footnote 53 or three Footnote 54 years of life. In the study of Blanken et al. the actual proportion of days with wheeze in the first year of life was 1.8 % with PVZ vs. 4.5% with placebo Footnote 49.
Therefore, it appears PVZ prophylaxis may have an impact in reducing the incidence of recurrent wheezing in young children in the first few years of life, but this effect may not persist. The findings are contradictory as to the relative impact of PVZ prophylaxis versus a family history of atopy on persistence of recurrent wheezing in older children. It also is not clear whether PVZ may be more effective in having a long term impact in infants with more extreme prematurity, as no data were found.
IV.2.3.2 Growth parameters
The updated review identified only one fair quality study that assessed parameters of growth (weight, height, body mass index) at 6 years of age in children born at 33–35 wGA Footnote 27. The study found no significant differences in these outcomes between children who received PVZ compared to children who received no intervention. The INESSS literature review did not report on this outcome.
IV.3 Premature infants with infantile chronic lung disease
IV.3.1 RSV-associated hospitalizations
The updated literature review identified one observational cohort study Footnote 22 and one case control study Footnote 40, both rated as fair, that examined the effect of PVZ prophylaxis on RSVH in premature infants with CLD. In the study of Chi, rate of RSVH was reduced 86% in infants born at ≤35 wGA in the first 6 months after initial hospital discharge. By GA, reduction was significant for those of ≤28 wGA (89%) and not those 29-35 wGA, but numbers in the latter group were small Footnote 22. Significant reduction was not observed in the case-control study of infants ≤35 wGA and <12 months or 12-24 months of age Footnote 40.
The INESSS literature review identified three studies examining this outcome: a good quality RCT Footnote 38 and two observational studies of poor Footnote 55 and very poor Footnote 47 quality. The RCT involving children born at ≤35 wGA and ≤24 months of age found PVZ recipients had a reduced risk of RSV-associated hospitalization compared to infants who received placebo (RR=0.60, 95% CI: 0.40 to 0.95) Footnote 38. Similarly, the observational studies of lower quality involving children born at ≤32 wGA both found PVZ prophylaxis to be associated with a reduced risk of RSV-associated hospitalization (RR=0.15, 95% CI: 0.05 to 0.49, p<0.01) Footnote 55 and (RR=0.28, 95% CI: 0.14 to 0.58, p<0.007) Footnote 47.
The results suggest that PVZ prophylaxis may provide a reduction in the risk of RSV-associated hospital admissions in this population, but the evidence is inconsistent and does not clearly identify a level of prematurity which would derive benefit.
IV.3.2 Mortality
There were no studies identified in the updated literature review that examined the effect of PVZ prophylaxis on mortality in premature infants with CLD.
In the INESSS review, the meta-analysis of average quality by Checchia et al. showed no observed effect of PVZ vs. no intervention/placebo (0.22% vs. 0.34%; Peto OR, 0.83; 95% CI: 0.13 to 5.25), on all-cause mortality but there were only 3 events in the prophylaxis group and 2 events in the placebo/no intervention group. RSV-related mortality was not determined for this group Footnote 45.
IV.4 Children with hemodynamically significant congenital heart disease
IV.4.1 RSV-associated hospitalizations
Three studies were identified in the updated literature review that examined the effectiveness of PVZ prophylaxis in children with hsCHD, two observational cohort studies of fair quality of infants <1year of age Footnote 33 Footnote 34 and one case control study of fair quality of infants age <24 months Footnote 40. Chiu et al. observed significant reductions of 49% for all cases and 65% for the subgroup with cyanotic hsCHD but a nonsignificant reduction of 35% for those with acyanotic disease Footnote 33. A significant RR of 0.28 (72% reduction) in hospitalization for all cases of hsCHD was reported in a small study by Soraiz Footnote 34. In the case-control study, significant PVZ effectiveness was not observed, either in the first or the second year of life Footnote 40.
The original INESSS literature review identified one good quality RCT Footnote 56 and one poor quality cohort study Footnote 57 that examined this outcome. In the RCT, children with hsCHD and ≤24 months of age at the start of the RSV season who received PVZ prophylaxis had a significant relative decrease (RD) in hospitalizations due to RSV compared to children receiving placebo (RD=45%, p=0.003).
This significant relative decrease in hospitalizations was also seen in children with acyanotic CHD (RD=58%, p=0.003), but not in children with cyanotic CHD (RD=29%, p=0.285). The cohort study by Harris et al. did not find PVZ prophylaxis to result in a significant reduction in RSV-associated hospitalizations compared to no intervention in children with CHD who were born at ≤36 w GA and ≤24 months of age at the start of the RSV season (RR=0.58, 95% CI: 0.21 to 1.65), but the rate of hospitalization for RSV in the control population was very low (2.9%) Footnote 57.
These studies show conflicting results on the protective effect of PVZ on hospitalization for RSV in infants with hsCHD. The two studies that did not show a significant effect Footnote 40 Footnote 57 had smaller numbers of participants than two larger studies that showed 45-49% risk reduction Footnote 33 Footnote 56. One of these studies showed significant protection in children with cyanotic heart disease but not in those with acyanotic heart disease Footnote 33, but the other showed the opposite Footnote 56. The reasons for these discrepancies are not evident.
IV.4.2 Additional hospital outcomes due to RSV
IV.4.2.1 Length of hospital stay due to RSV
In the study of Chiu et al., the LOS was not significantly different in patients who did or did not receive PVZ, either for the total group or for those with cyanotic or acyanotic hsCHD Footnote 33.
In the original INESSS review, the RCT involving children with hsCHD and ≤24 months of age at the start of the RSV season found PVZ recipients to have a significant relative decrease in the total number of days/100 children in hospital due to RSV compared to placebo recipients (RD=56%, p=0.003) Footnote 56. LOS for those admitted was not reported. Mean LOS calculated from the data provided was 10.8 days for PVZ recipients and 13.3 days for placebo, not significantly different.
IV.4.2.2 Admission to and LOS in ICU due to RSV
Chiu et al. reported no significance differences in rates of admission to ICU in those who received PVZ and those who did not, either for the total group or for those with cyanotic or acyanotic hsCHD Footnote 33. The proportions of those hospitalized for RSV who required ICU admission were also not significantly different.
In the original INESSS review, the RCT by Feltes et al. and the cohort study by Harris et al. examined ICU admission rates. In children with hsCHD aged ≤24 months, the RCT found that compared to placebo recipients, PVZ recipients had a relative decrease in the number of admissions to ICU but the reduction was not significant (RD=46%, p=0.094).
Harris et al. also found that compared to no intervention, children with CHD who were born at ≤36 wGA and ≤24 months of age who received PVZ had a relative decrease in admissions to an ICU due to RSV (RD=86%) but the reduction was not significant Footnote 57. In both studies, the proportions of hospitalized infants admitted to ICU were not significantly different in the groups that received PVZ and those that did not.
In the RCT there was a relative decrease in the total number of days/100 children in an ICU due to RSV; however, the reduction was not significant (RD=78%, p=0.80) Footnote 56. In the cohort study the mean ICU LOS was decreased from 14.9 to 10 days but difference was not significant Footnote 57.
IV.4.2.3 Use of MV due to RSV
The updated review found no new studies that addressed this outcome.
In the original INESSS review, the RCT by Feltes et al. found no significant difference in the use of MV, reported as total days/100 children, between children with hsCHD and ≤24 months of age at the start of the RSV season who received PVZ compared to placebo recipients (RD=41%, p=0.282) Footnote 56.
IV.4.2.4 Duration of oxygen therapy due to RSV
The updated review found no new studies that addressed this outcome.
In the original INESSS review, the RCT by Feltes et al. found that compared to placebo, children with hsCHD and ≤24 months of age at the start of the RSV season who received PVZ prophylaxis had significantly fewer total days/100 children on oxygen therapy (RD=73%, p=0.014) Footnote 56.
These results suggest that for children with hsCHD who are hospitalized with RSV infection, having received PVZ does not affect the severity of illness, as manifested by hospital LOS, ICU admission, ICU LOS, or need for MV, although the number of studies is small.
IV.4.3 Mortality
The updated review found no new studies that addressed this outcome.
In the original INESSS review, both the RCT by Feltes et al. and the cohort study by Harris et al. examined all-cause mortality in this population Footnote 56 Footnote 57. In the RCT, there was no significant difference in all-cause mortality between children with hsCHD and ≤24 months of age at the start of the RSV season who received PVZ compared to placebo recipients (RR=0.79, 95% CI: 0.45 to 1.38). In children with CHD who were born at ≤36 weeks GA and ≤24 months of age at the start of the RSV season, the Harris et al. study reported one death in the no intervention group and no deaths in PVZ prophylaxis group. The RCT reported deaths from RSV in 2 of 639 PVZ recipients and 4 of 648 controls (p=0.46) Footnote 56.
IV.5 Children residing in remote communities
IV.5.1 RSV-associated hospitalizations
There were no studies identified in the updated literature review that examined the efficacy/effectiveness of PVZ prophylaxis in children living in remote communities.
The original INESSS literature review identified two cohort studies of poor quality that examined this outcome Footnote 58 Footnote 59. The Banerji et al. study included Inuit children from Nunavut, Canada who were born at either <36 wGA and/or had significant cardiac or respiratory disease and were <6 months of age at the start of the RSV season. Children who received PVZ had significantly fewer RSV-associated hospitalizations (2/91, 2.2%) compared to PVZ eligible children receiving no intervention (5/10, 50%) (OR=0.04, 95% CI: 0.008 to 0.26, p=0.0005) Footnote 58.
As not all PVZ eligible infants were identified, the actual reduction rate is likely to be less than that reported. In the study by Singleton et al., RSV-associated hospitalizations were assessed in Alaskan Aboriginal children before and after introduction of a PVZ program for high risk infants. There was a significant reduction in RSV-associated hospitalizations in infants born at ≤36 wGA (RR=0.34, 95% CI: 0.17 to 0.68, p<0.001).
After the PVZ program introduction, among high-risk infants the rate of first RSVH was 0.55 per 1000 PVZ protected days and 1.07 per 1000 unprotected days (relative rate, 0.52; 95% CI, 0.28 to 0.93). The NNT to prevent one RSV-related hospitalization was reported as 3.4.
Although Inuit children residing in remote northern communities are known to be at high risk of hospitalization for RSV Footnote 59 Footnote 60, data on PVZ effectiveness to prevent hospitalization in this group is very limited, with there being no published data in healthy term infants.
IV.6 Children with CF
IV.6.1 RSV-associated hospitalizations
The updated literature review identified a systematic review of good quality Footnote 17, which included a single multicentre RCT Footnote 43, and three retrospective observational studies of fair quality Footnote 24 Footnote 25 Footnote 41. The study by Cohen et al. found no significant difference in RSV-related hospitalizations in children with CF who received either PVZ prophylaxis or placebo Footnote 43. However there were few hospitalizations in either group (PVZ n=13; placebo n=14) and only one child in each group was hospitalized due to RSV.
In contrast, the study by Groves et al. found that children who did not receive PVZ prophylaxis were at increased risk of RSV infection and significantly more likely to have a RSV-related hospitalization compared to children who received PVZ prophylaxis (21.3% vs. 4.4%) Footnote 24. In the study by Bjornson et al. hospitalization rate was 2.7% for PVZ recipients and 6.0 % for controls (p=0.20).
After adjustment for confounding factors, the hospitalization rate for RSV was not significantly less in children who received PVZ than in those who did not. However there was a significantly reduced rate of hospitalization for respiratory illness in the PVZ recipients, and overall testing rate for RSV was low at 53% Footnote 25. Buchs et al. in a case-control study, found no significant reduction in hospitalization for RSV between the PVZ (5%) and control (2.9%) groups Footnote 41.
The original INESSS literature review identified three studies that examined this outcome. A systematic review by Robinson et al. rated of good quality, identified the same multicentre RCT by Cohen et al., that was identified in the updated systematic review of Robinson in 2016 Footnote 42. The other two studies were observational cohorts of poor quality Footnote 61 Footnote 62. These observational studies found non-significant benefits of PVZ prophylaxis compared to no intervention on subsequent RSV-related hospitalizations.
No firm conclusions on the effectiveness of PVZ prophylaxis in reducing the risk of RSV-associated hospitalizations in children with CF can be drawn from the findings of these studies. Only the observational study of Groves et al. found a significant preventive effect of PVZ prophylaxis on RSV-associated hospitalizations. The rate of RSVH in the control group in that study was very high and the number of participants was small.
IV.6.2 Additional hospital outcomes due to RSV
IV.6.2.1 Length of hospital stay due to RSV
One study identified in the updated literature review examined the effect of PVZ on the duration of hospitalization due to RSV in children with CF. The mean duration of hospitalization was significantly less in the PVZ recipients (5.7± 2.4 days) than in the controls (47 ± 39 days), p=0.048 Footnote 25.
The original INESSS literature review identified one study of poor quality that examined this outcome Footnote 61. The small (n=35–40 participants per group) historical cohort study of children with CF did not find a significant difference in the median number of days of hospitalization due to RSV in PVZ recipients (11, interquartile range: 3–14) compared to children receiving placebo (13, interquartile range: 2–14) (OR=0.46, 95% CI: 0.16 to 1.31 Footnote 61.
IV.6.2.2. Admission to ICU due to RSV
In the updated literature review, one study assessed this outcome. None of 183 PVZ recipients and 2 of 84 controls were admitted to ICU because of RSV. Of patients hospitalized for RSV, 2 of 5 patients in the control group required ICU admission Footnote 25.
IV.6.2.3 Use of oxygen therapy or MV
The study by Cohen et al. identified by both the original INESSS literature review and the updated literature review, examined the effect of PVZ prophylaxis on the use of oxygen therapy due to RSV in children with CF. No significant difference between the groups was found in the need for oxygen therapy; however, the number of outcomes was small (PVZ prophylaxis group, n=1; placebo intervention group, n=0)Footnote 17 . In the study of Bjornson, increased respiratory support, either MV or oxygen therapy, was required by 2.2 % of PVZ recipients and 1.2% of the control group (p=0.58)Footnote 25 . In the study by Buchs, no patients required supplemental oxygen or MV41.
IV.6.3 All-cause mortality
Both the original INESSS literature review and the updated review identified the same, single multicentre RCT study that examined the effectiveness of PVZ in reducing all-cause mortality in children with CF Footnote 43. There were no deaths identified in either group during the 6 months of follow-up during the study. Another study found no differences in all-cause mortality in the first 2 years of life among children who did or did not receive PVZ Footnote 26.
IV.6.4 Long-term sequelae
IV.6.4.1 Lung function
The updated literature review identified two studies that looked at lung function in children with CF. In the small historical cohort study of Groves et al., no significant difference in lung function (as assessed by measurement of FEV 1 ) between children with CF who had and had not received PVZ was found on follow-up assessments at 6 years of age Footnote 24. A second study found no differences in FEV 1 at age 7 years Footnote 26. The original INESSS literature review did not identify any studies examining this outcome.
IV.6.4.2 Growth parameters
The Groves et al. study found no significant differences in growth parameters (weight, height, body mass index) at 6 years of age between children who did and did not receive PVZ prophylaxis Footnote 24. This is consistent with the findings from the Cohen et al. study identified in both the original and updated literature reviews in which there were no significant differences between the PVZ and placebo groups at 12 month follow-up with respect to weight gain or weight to height ratio Footnote 43. The case control study of Buchs et al. also found no significant difference between PVZ recipients and controls in growth in the first 3 years of life.
IV.6.4.3 P. aeruginosa and S. aureus colonization
In the updated literature review, the study by Cohen et al. found no statistically significant differences in the numbers of children with P. aeruginosa airway colonization in children receiving PVZ compared to those receiving placebo at 12 months follow-up Footnote 43. In the study by Groves et al., the median time to a first isolate of P. aeruginosa was significantly shorter in PVZ recipients than in non-recipients and the RR of a first isolate during the study period was also significantly increased in PVZ recipients.
However, at follow-up at 6 years of age there was no significant difference in chronic P. aeruginosa colonization rates between the two groups Footnote 24. Buchs et al. reported that PVZ prophylaxis had no significant effect on age at first colonization with P. aeruginosa or S. aureus or in the proportion of children colonized with P. aeruginosa by age 3 years. The proportion of infants colonized with S. aureus by age 3 years was significantly increased in the PVZ recipients (97%) in comparison to controls (85%). Fink et al. reported no difference in age at first P. aeruginosa colonization in children who did or did not receive PVZ Footnote 26.
The results from these studies appear consistent with no significant differences in the longer term sequelae examined between children with CF who have and have not received PVZ prophylaxis. However, the number of children studied is small.
IV.7 Children with Down syndrome
IV.7.1 RSV-associated hospitalizations
The updated literature review identified two studies of PVZ prophylaxis in children with Down syndrome. A small observational cohort study of fair quality examined the effect of PVZ prophylaxis in reducing RSV-associated hospitalizations in infants with Down syndrome and without other criteria for PVZ administration.
Hospitalization rate was not significantly different in those who received PVZ and those who did not, but the numbers of patients in each group were small (PVZ n=33; control n=60) Footnote 35. The second study, of good quality, reported on children with Down syndrome with and without co-morbidities and found no significant effect of PVZ in children without co-morbidities Footnote 36.
The original INESSS literature review identified a single cohort study of poor quality that examined the effectiveness of PVZ prophylaxis in reducing RSV-associated hospitalizations Footnote 63. In this study, children <24 months of age and diagnosed with Down syndrome were identified from a Canadian PVZ registry and compared to a cohort of children of the same age and diagnosed with Down syndrome identified from a Dutch birth registry (no receipt of PVZ).
After adjusting for hsCHD, insignificant CHD, GA, and birth weight, the analysis found that compared to no intervention receipt of PVZ was associated with a statistically significantly 72% reduction in RSV-associated hospitalizations (incidence rate ratio [IRR]=3.63 95% CI: 1.52 to 8.67, p=0.002). Significant reduction in hospitalization was also found when the analysis was restricted to children with at least one standard risk criteria for RSV prophylaxis (hsCHD, born at ≤35 wGA, CLD) (IRR 3.39 (1.02–11.25).
However, when the analysis was restricted to children with no standard RSV risk criteria, the difference in RSV-associated hospitalizations between children receiving PVZ prophylaxis and children receiving no intervention was not significant (IRR=6.57 95% CI: 0.70 to 62.16).
IV.7.2 Additional hospital outcomes due to RSV
IV.7.2.1 Duration of hospital stay due to RSV
The Yi et al. study found that there was no significant difference in average number of days of hospital stay due to RSV in PVZ recipients compared to children receiving no intervention (6.4 versus 12.4 days, p=0.48) Footnote 63.
IV.7.2.2 Admission to and duration of stay in ICU due to RSV
In the Yi et al. study, none of the 532 children who received PVZ prophylaxis were admitted to an ICU, while in the 233 without PVZ there were 4 admissions to an ICU for an average of 10.3 days. The authors were unable to determine whether there was a significant difference in the risk of ICU admission and duration of stay between these children and children who received no intervention.
IV.7.2.3 Use and duration of MV due to RSV
The Yi et al. study also had no children who received PVZ prophylaxis requiring MV, while in the group without PVZ there were 4 children who required MV for an average of 10.3 days. No determination could be made of the risk of the use and duration of MV between these children and children who received no intervention.
IV.7.2.4 Use and duration of oxygen therapy due to RSV
The Yi et al. study found that children who received PVZ prophylaxis had significantly less use of supplemental oxygen therapy (2/532, 0.004% versus 19/233, 0.08%, p<0.001) and fewer average number of days of use of oxygen therapy (4 versus 13.7 days, p=0.046) compared to children who did not receive PVZ.
The significance of the results from these studies, one of poor quality and the other involving very few children, is unclear, but suggests that PVZ may not benefit children with Down syndrome who do not have other conditions that may warrant PVZ administration. Further studies are required before conclusions can be drawn on the benefit of PVZ in this population.
IV.8 Number needed to treat
An important consideration in evaluating studies of the effectiveness of PVZ is the number of infants that would need to be treated in order to avoid one hospitalization, ICU admission or death. The NNT to prevent hospitalization was reported in only three studiesFootnote 34 Footnote 52 Footnote 59. NNT was calculated for all studies that provided sufficient data to do so if there was a significant protective effect from PVZ. The NNT to prevent hospitalization, ICU admission, or persistent wheeze for various patient populations in the studies reported above are shown graphically in Figure 1 below and in table form in Appendix G.
IV.8.1 NNT to prevent hospitalization
In mixed populations, NNT to prevent hospitalization ranged from 2 to 24 in 8 estimates. In the one RCT, a 1996 study of infants ≤ 6 months of age with prematurity or <24 months with CLD, NNT was 18 (95% CI: 11.3 to 35.7) Footnote 38.
Four estimates were ≤ 10 and three were ≤4. NNT was 3 in a study of infants ≤ 35 wGA or with CLD in Alaska Footnote 59, 2 in a study of infants <29 WGA with or without CLD in Israel Footnote 21, and 4 in a study of infants <27 wGA with or without CLD in Hong Kong Footnote 30. All three were small studies with very high rates of RSV infection in the control groups.
NNT for premature infants without CLD ranged from 5 to 54 in 11 estimates, with 7 being ≤ 17. Data from the IMpact RCT resulted in NNT of 21 for infants of 28-31 wGA, 17 for 29-32 wGA, 14 for 29-33 wGA, 12 for 32-34 wGA, and 13 for 32-35 wGA Footnote 37. In a 2009-11 RCT from Turkey of infants ≤ 28 wGA aged <12 months or 29-32 wGA aged <6 months, NNT was 5.
Again, this was a small study with a high rate of RSV in controls Footnote 48. NNT was 9 in a 2000-2004 study of infants ≤ 30 wGA in France Footnote 52. In contrast, NNT was 54 in a 2012-14 study of infants of 29-32 wGA from Texas that used data from Medicaid databases Footnote 29.
In children with CLD, NNT in 5 estimates ranged from 3 to 21. NNT was 21 in the IMpact RCT Footnote 38. All other NNT were ≤ 13, including NNT of 3 in a small 1999-2002 study of infants ≤ 32 wGA aged <6 months from France Footnote 55.
NNT for children with hsCHD aged <24 months was 23 for all cases and 15 for those with non-cyanotic CHD in the 1998-2002 RCT by Feltes et al. Footnote 56. In contrast in a 2010-16 cohort study of infants aged <1 year from Taiwan, NNT was 45 for all hsCHD and 31 for cyanotic CHD. NNT was 7 in a study of infants <1 year old with hsCHD from Argentina with a high RSV rate in controls Footnote 34.
In a study of infants with Down syndrome with or without other comorbidities such as hsCHD, a NNT was 12 Footnote 63. In the only study showing that PVZ was associated with reduced hospitalization for RSV in infants with CF, a 1997-2007 study from Ireland, NNT was 6. RSV infection rate in the control group was very high.
Checchia et al. calculated NNT in a systematic review and meta-analysis of data published to 2007 Footnote 45 . The number needed to be treated with PVZ to prevent one hospitalization for RSV was 11 (95% CI: 8 to 259) for infants with CLD; 16 (95% CI: 14 to 20) for all preterm infants; 14 (95% CI: 13 to 16) for those ≤ 32 wGA, 18 (95% CI: 15 to 35) for those 32-35 wGA and 24 (95% CI: 18 to 58) for those <35 wGA where further breakdown by GA was not possible. For infants with CHD, NNT was 23 (95% CI: 17 to 56).
IV.8.2 NNT to prevent intensive care admission
NNT to prevent one intensive care admission for RSV was calculated for 2 instances. In the IMpact RCT of infants ≤ 35 wGA and age ≤ 6 months or CLD aged <24 months, NNT was 59, with very wide 95% CI Footnote 38. In a study in Taiwan of infants ≤ 28 wGA or ≤ 35 wGA with CLD, NNT to prevent ICU admission by 6 months after initial discharge was 16 Footnote 22.
IV.8.3 NNT to prevent recurrent wheezing
To prevent one case of recurrent wheezing, NNT ranged from 3 to 15 in 10 estimates. NNT to prevent wheezing in the first year of life in otherwise healthy infants of 33-35 wGA aged <6 months was 11 in a 2008-2010 RCT Footnote 49.
NNT of 3 to prevent wheezing in the first 2 years of life was from the small study in Israel that had a high rate of wheezing in control group Footnote 21 NNT to prevent wheezing within 2 years of PVZ prophylaxis ranged from 8 to 15 in different wGA groups Footnote 54. In prematures of <36 wGA with no family history of asthma or of atopy, NNT to prevent wheezing at age 2-5 years were 14 and10 respectively Footnote 28. NNT to prevent wheezing at age 3 years and age 6 years were 8 and 7 respectively in a study from Japan Footnote 27 Footnote 53.
IV.8.3 NNT to prevent all-cause mortality
Data from the individual studies presented in this review do not permit calculation of NNT to prevent mortality. Checchia et al. in their meta-analysis calculated NNT to prevent one death (all-cause mortality). NNT was 270 (95% CI: 227 to 412) for all preterm infants and 136 (95% CI: 117 to 189) for those of ≤ 32 wGA. NNT for infants of 32-35 wGA, infants with CLD, and infants with CHD were 987, 1736 and 113 respectively, but 95% CI could not be calculated.
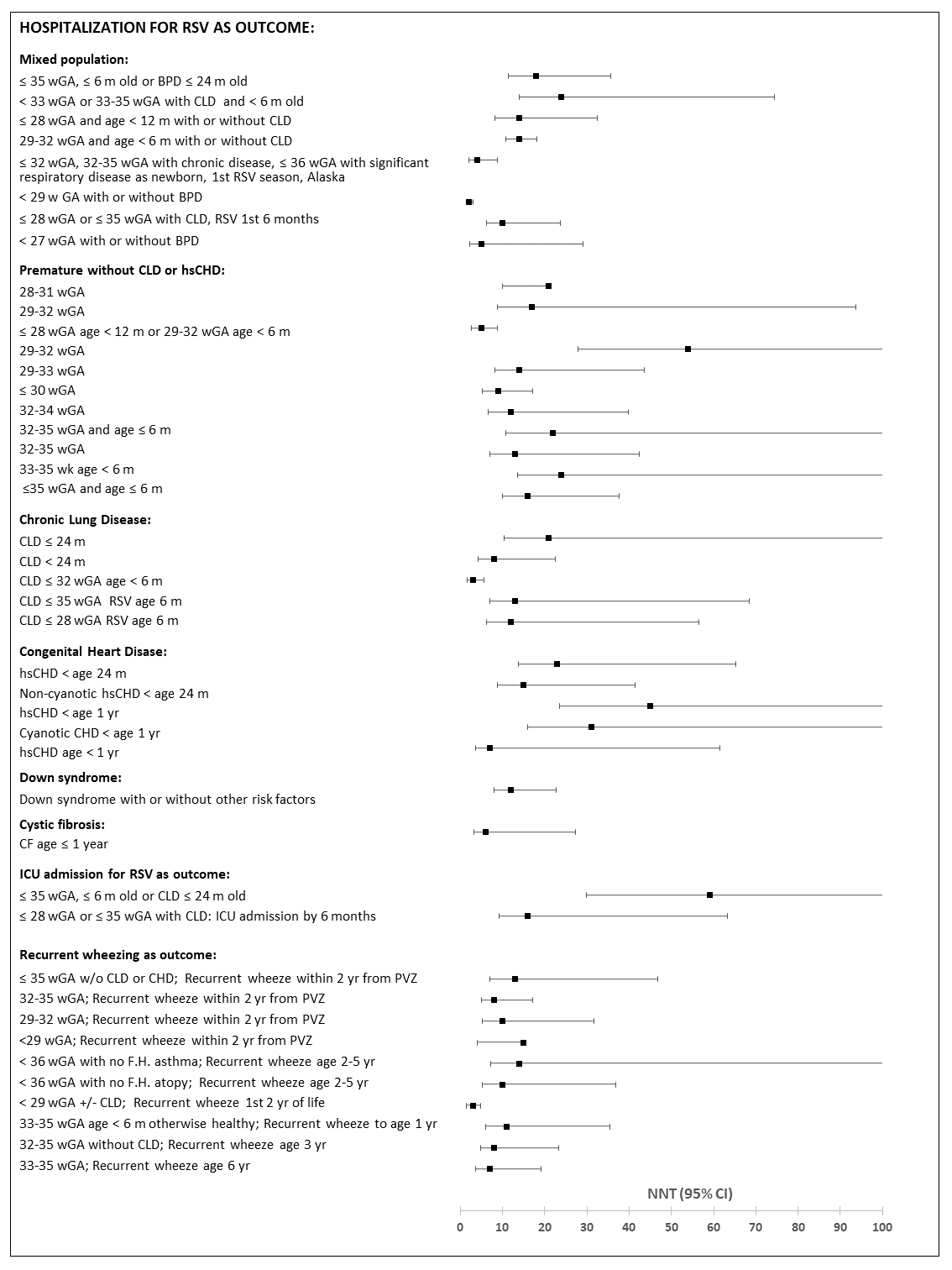
Figure 1 - Text description
This figure is a summative “box and whisker plot” graphically displaying variation in number needed to treat with PVZ to prevent hospitalization, ICU admission and recurrent wheezing for various populations. The information graphically depicts the number needed to treat and the 95% confidence interval.
For the outcome of prevention of hospitalization there are 31 studies presenting number needed to treat and 95% confidence intervals. These studies are grouped into 6 population groups: mixed population, premature without CLD or hsCHD, Chronic Lung Disease, Congenital Heart Disease, Down syndrome and Cystic Fibrosis.
For mixed populations, this includes: ≤ 35 wGA, age ≤ 6 m or BPD age ≤ 24 m (18, 11.3-35.7), < 33 wGA or 33-35 wGA with CLD or discharged on O2 and age <6 m (24, 13.9-74.4), ≤ 28 wGA age < 12 m without CLD (14, 8.3-32.4), 29-32 wGA < 6 m without CLD (14, 10.8-18.1), ≤ 32 wGA, 32-35 wGA with chronic disease, ≤ 36 wGA with significant respiratory disease in newborn period, 1st RSV season in Alaska (3, 2.1-8.9), < 29 wGA +/- BPD (2, 1.4-3.1), ≤ 28 wGA or ≤ 35 wGA with CLD, Matched by propensity score (10, 6.2-23.7) and < 27 w GA +/- BPD (4, 2.2-29).
For premature without CLD, this includes: 28-31 wGA (21), 29-32 wGA (17, 8.9-93.6), ≤ 28 wGA age < 12 m or 29-32 wGA age < 6 m, otherwise healthy (5, 2.7-8.9), 29-32 wGA w/o chronic illness (54, 27.9-547.6), 29-33 wGA (14, 8.3-43.6), ≤ 30 wGA w/o BPD (9, 5.3-17.1), 32-34 wGA (12, 6.6-39.8), 32-35 wGA and ≤ 6 m old w/o BPD (22, 10.8-1246.3), 32-35 wGA (13, 7-42.4), 33-35 wk < 6 m otherwise healthy (24, 13.5-103.4) and ≤35 wGA and ≤ 6 m old w/o BPD (16,10-37.6). For the 28-31 wGA population, the lower 95% CI is 10 and the 95% CI for absolute risk reduction extends from a negative number to a positive number.
For CLD, this includes: CLD ≤ 24 m (21, 10.4-385.2), CLD < 24 m (8, 4.2-22.5), CLD wGA ≤ 32 age < 6 m (3, 1.7-5.7), CLD ≤ 35 wGA RSV age 6 m (13, 7-68.4), and CLD ≤ 28 wGA RSV age 6 m (12, 6.3-56.6).
For CHD, this includes: CHD < age 24 m (23, 13.8-65.3), Non-cyanotic CHD < age 24 m (15, 8.9-41.3), hsCHD < age 1 yr (45, 23.6-327.2), Cyanotic CHD < age 1 yr (31, 16-260.1), hsCHD age < 1 yr (7, 3.7-61.5).
For Down Syndrome and Cystic Fibrosis there is only one study each: for Down Syndrome (12, 8.1-22.7) and for Cystic Fibrosis (6, 3.3-27.2).
For the outcome of prevention of ICU admission there are two studies presenting number needed to treat and 95% confidence intervals with the following populations: ≤ 35 wGA, ≤ 6 m old or BPD ≤ 24 m old (59, 29.8-1948.2) and ≤ 28 wGA or ≤ 35 wGA with CLD (16, 9.1-63.3).
For the outcome of prevention of recurrent wheeze there are ten studies presenting number needed to treat and 95% confidence intervals with the following populations: ≤ 35 wGA w/o CLD or CHD to prevent recurrent wheeze within 2 yrs from PVZ (13, 7-46.7), 32-35 wGA to prevent recurrent wheeze within 2 yrs from PVZ (8, 5-17.2), 29-32 wGA to prevent recurrent wheeze within 2 yrs from PVZ (10, 5.3-31.7), <29 wGA to prevent recurrent wheeze within 2 yrs from PVZ (15), < 36 wGA with no F.H. asthma to prevent recurrent wheeze within 2-5 yr from PVZ (14, 7.3-117.1), < 36 wGA with no F.H. atopy to prevent recurrent wheeze within 2-5 yr from PVZ (10, 5.2-36.9), < 29 wks +/- BPD to prevent recurrent wheeze 1st 2 yrs of life (3, 1.5-4.8), 33-35 wGA age < 6 m otherwise healthy to prevent recurrent wheeze in first year of life (11, 6-35.4), 33-35 wGA without CLD to prevent recurrent wheeze age 3 yr (8, 4.8-23.4), and 33-35 wGA without CLD to prevent recurrent wheeze age 6 yr (7, 3.6-19.2). For <29 wGA, the lower 95% CI is 4.1 and the 95% CI for absolute risk reduction extends from a negative number to a positive number.
For details of studies, see Appendix G - Table 3.
Evidence gaps
There are limitations to the data summarized in this report. Only 5 of the studies identified were RCT, and the largest of these were carried out in the late 1990s. RSV disease burden in high risk groups may have changed since then. Most studies were observational cohorts with historical controls, and outcomes may have been influenced by improvements in the management of prematurity and CHD over time.
Many studies had small numbers of participants and may have been underpowered to detect differences in certain outcomes, such as ICU admissions or death. Lastly, many studies were funded by the manufacturers of PVZ, which may have influenced the choice of populations investigated or the questions asked.
PVZ has been shown to be beneficial in premature infants of various GAs, but it has not been possible to determine whether there is differential effectiveness by level of prematurity.
The relative benefit of PVZ in preventing RSV infection in the child's first vs. second RSV season has not been studied.
The effectiveness of PVZ prophylaxis in children with serious neuromuscular disorders affecting respiratory function, upper airway anomalies affecting respiratory function, or chronic lung disease other than that related to prematurity or CF, or in immunocompromised children or those with metabolic diseases, or in healthy infants of multiple births with a twin or triplet eligible to receive PVZ is unknown.
The effectiveness of PVZ prophylaxis in preventing persistent asthma later in life is unknown, and the role of RSV in the development of asthma is unclear.
The effectiveness of PVZ prophylaxis using alternative dosing schedules (e.g. fewer doses, longer dosing intervals, higher doses) has not been rigorously investigated.
Conclusions
The original INESSS literature review and the NACI updated review identified studies in mixed populations of infants, in children born prematurely without CLD, in premature infants with CLD, in infants with hsCHD, in infants born in remote northern communities, in children with CF and in infants with Down syndrome. The outcome most investigated is RSVH.
- In mixed populations of infants at risk of severe RSV infection, PVZ prophylaxis is associated with reductions of 40-86% in the risk of RSV-associated hospital admissions. However such information is not useful in determining the specific risk groups for which PVZ prophylaxis might be warranted. The findings in mixed populations do not indicate that PVZ has an important impact on LOS for infants hospitalized for RSV, but may be underpowered to detect such an effect.
- Although numbers are small, it appears that for breakthrough RSV infections in PVZ recipients that require hospitalization, severity of illness, as manifested by need for ICU admission and ICU LOS and need for MV is not impacted by PVZ. There are conflicting results on the effect of PVZ on all-cause mortality. RSV-related deaths were rare in both PVZ recipients and those who did not receive PVZ. PVZ prophylaxis may reduce wheezing in the first few years of life, but may not have a significant impact on longer term outcomes.
- Evidence supports the effectiveness of PVZ in reducing RSVH in premature children without CLD, although the level of prematurity at which PVZ is most effective is not entirely clear. Data suggests effectiveness of 72-80% in infants of 29–33 wGA, but PVZ may not be effective in more premature infants and data on premature infants over 33 wGA are inconsistent. PVZ may have an effect on all-cause mortality in infants born at ≤32 wGA, but not at lesser levels of prematurity (32–35 wGA).
- However, these findings are based upon few studies which may have been underpowered to detect difference in mortality in the less premature infants. PVZ prophylaxis may have an impact in reducing the incidence of recurrent wheezing in young children in the first few years of life, but the findings are contradictory as to the relative impact of PVZ prophylaxis versus a family history of atopy on persistence of recurrent wheezing in older children.
- PVZ prophylaxis reduced the risk of hospitalization for RSV in infants with CLD by 40% in an early RCT, but evidence from subsequent observational studies is inconsistent and does not clearly identify a level of prematurity which would benefit. No effect was observed on all-cause mortality but the numbers of deaths were very low.
- Studies of infants with hsCHD show conflicting results on the effect of PVZ on hospitalization for RSV, with two larger studies showing an effect and two smaller studies showing no effect. One study showed significant protection in children with cyanotic heart disease only, while another showed effect only with acyanotic disease. For those admitted with RSV infection, PVZ did not affect hospital LOS, ICU admission, ICU LOS, or need for MV. All-cause mortality was not different in those who received or did not receive PVZ.
- PVZ prophylaxis significantly reduced hospitalization risk in premature Inuit children living in Nunavut and in Alaska, but study quality was poor and data are limited.
- Most studies showed no effect of PVZ prophylaxis on hospitalization for RSV infection in infants with CF. There were conflicting results on effect on LOS and no effect on ICU admission or use of MV or oxygen therapy but very few required these interventions. PVZ also had no significant effectiveness on long term outcomes of CF.
- Three studies of children with Down syndrome suggested that PVZ may not benefit children with Down syndrome who do not have other high risk conditions that warrant PVZ administration, but the number investigated was small.
- NNT to prevent one RSV related hospitalization varied widely, influenced by patient population, location, number of participants and RSVH rate in controls. NNT tended to be lowest in infants with CLD and highest in those with hsCHD. Among premature infants without CLD data from an RCT indicated higher NNT with increasing degree of prematurity while a meta-analysis showed the opposite.
- The reviews did not identify any studies on the effectiveness of PVZ prophylaxis compared to placebo or no intervention in children with other conditions that compromise respiratory function or that compromise immune function, or that may put them at increased risk for serious RSV infection. For more rare conditions, it is unlikely that studies of PVZ effectiveness will be feasible. Considerations for PVZ prophylaxis may have to be based on burden of RSV disease and extrapolation of possible benefit from studies in other populations.
List of abbreviations
- aHR
- Adjusted hazard ratio
- AMSTAR
- A Measurement Tool to Assess Systematic Reviews
- aOR
- Adjusted odds ratio
- aRR
- Absolute risk reduction
- BPD
- Bronchopulmonary dysplasia
- CASP
- Critical Appraisal Skills Programme
- CF
- Cystic fibrosis
- CHD
- Congenital heart disease
- CI
- Confidence interval
- CLD
- Chronic lung disease of prematurity
- FEVFootnote 1
- Forced expiratory volume in one second
- FVC
- Forced vital capacity
- GA
- Gestational age
- HMPV
- Human metapneumovirus
- hsCHD
- Hemodynamically significant congenital heart disease
- ICU
- Intensive care unit
- INESSS
- Institut national d'excellence en santé et en services sociaux
- IQR
- Interquartile range
- IRR
- Incidence rate ratio
- LOS
- Length of stay
- MV
- Mechanical ventilation
- NACI
- National Advisory Committee on Immunization
- NNT
- Number needed to treat
- OR
- Odds ratio
- PE
- palivizumab effectiveness
- PVZ
- palivizumab
- RCT
- Randomized controlled trial
- RD
- Relative decrease
- RR
- Relative risk (also known as risk ratio)
- RRR
- Relative risk reduction
- RSV
- Respiratory syncytial virus
- RSVH
- RSV hospitalization
- RSV WG
- RSV Working Group
- SD
- Standard deviation
- UK
- United Kingdom
- US
- United States
- wGA
- Gestational age in weeks
Acknowledgments
This statement was prepared by: D. Moore, R. Stirling, A Sinilaite, and approved by NACI.
NACI gratefully acknowledges the contribution of: P Doyon-Plourde, S Duschesne-Belanger, E Poirier, A House, SJ Ismail, A Sumner, C Tremblay, MC Tunis, V Mouajou Feujio, L Zhao, A Killikelly and N St-Pierre as well as the research team at the Alberta Research Centre for Health Evidence (ARCHE), including J Pillay, A Wingert, and L Hartling
NACI RSV Working Group
Members: D Moore (Chair), M Salvadori, V Dubey, J Papenburg, J Robinson
Former Members: S Gantt, W Vaudry
PHAC Participants: S Duschesne-Belanger, A Killikelly, R Pless, A Sinilaite, R Stirling , A Sumner, MC Tunis, MW Yeung, and L Zhao
NACI Members: S Deeks (Chair), R Harrison (Vice-Chair), J Bettinger, N Brousseau, P De Wals, E Dubé, V Dubey, K Hildebrand, K Klein, J Papenburg, C Rotstein, B Sander, S Smith, and S Wilson.
Former NACI Members: C Quach (Chair)
Liaison Representatives: L Bill / M Nowgesic (Canadian Indigenous Nurses Association), LM Bucci (Canadian Public Health Association), E Castillo (Society of Obstetricians and Gynaecologists of Canada), A Cohn (Centers for Disease Control and Prevention, United States), L Dupuis (Canadian Nurses Association), D Fell (Canadian Association for Immunization Research and Evaluation), S Funnell (Indigenous Physicians Association of Canada), J Hu (College of Family Physicians of Canada), M Lavoie (Council of Chief Medical Officers of Health), D Moore (Canadian Paediatric Society), M Naus (Canadian Immunization Committee), and A Ung (Canadian Pharmacists Association).
Former Liaison Representatives: N Dayneka, J Emili and A Pham-Huy
Ex-Officio Representatives: V Beswick-Escanlar (National Defence and the Canadian Armed Forces), E Henry (Centre for Immunization and Respiratory Infectious Diseases [CIRID], PHAC), M Lacroix (Public Health Ethics Consultative Group, PHAC), C Lourenco (Biologic and Radiopharmaceutical Drugs Directorate, Health Canada), D MacDonald (COVID-19 Epidemiology and Surveillance, PHAC), S Ogunnaike-Cooke (CIRID, PHAC), K Robinson (Marketed Health Products Directorate, HC), G Poliquin (National Microbiology Laboratory, PHAC), and T Wong (First Nations and Inuit Health Branch, Indigenous Services Canada).
Former Ex-Officio Representatives: D Danoff, J Pennock and R Pless
Appendix A: Search strategy and results
| # | Searches | Results |
|---|---|---|
1 |
exp Antibodies, Monoclonal/ or exp antivaral agents/ or exp immunoglobulins/ or (antibody protein or anti viral agent* or anti viral drug* or antiviral agent* or antiviral drug* or antiviral substance or antivirals or antivirus agent* or antivirus drug* or anti-RSV or clonal antibody or endobulin or flebogamma or flebogammadif or gamastan or gamimmune n or gamimune or gamma globulin* or gamma-globulin* or gammaglobulin* or gammar or gamulin or globuman or humanized antibody or humanized monoclonal antibody or hybridoma antibody or Ig or igam or igc or immune gamma globulin or immune globin or immune globulin* or immune serum globulin* or immuno gamma globulin* or immuno globulin* or immunogammaglobulin* or immunoglobin* or immunoglobulin* or immunoprotein* or intragam or intraglobin f or isiven or iveegam or ivega or mAbs or MEDI 493 or monoclonal antibodies or monoclonal antibody or PVZ or panglobulin* or passive immunization or sandoglobin* or sandoglobulin* or synagis or tegelin* or veinoglobulin* or venoglobulin* or viral inhibitor or virostatic agent* or virucidal agent* or virucide agent* or virustatic agent* or vivaglobin).tw,kw,nm. |
1003897 |
2 |
RSV infections/pc |
1305 |
3 |
RSV infections/ or (respiratory syncytial vir* or RSV*).tw. |
15925 |
4 |
prophylaxis/ or (control or health protection or immunoprophylaxis or prevention or preventive measures or preventive medication or preventive therapy or preventive treatment or prophylactic institution or prophylactic management or prophylactic medication or prophylactic therapy or prophylactic treatment or prophylaxis).tw. |
2563613 |
5 |
2 or (3 and 4) |
3821 |
6 |
and/1,5 |
1597 |
7 |
exp Infant/ or exp child/ or exp adolescent/ or exp minors/ or exp puberty/ or exp pediatrics/ or exp schools/ or (infant* or infancy or newborn* or baby* or babies or neonat* or preterm* or prematur* or postmatur* or child or children or schoolchild* or school age* or preschool* or kid or kids or toddler* or adoles* or teen* or boy or boys or girl* or minors* or pubert* or pubescen* or prepubescen* or pediatric* or paediatric* or peadiatric* or Nursery school* or kindergar* or primary school* or secondary school* or elementary school* or high school* or highschool*).tw. |
3841894 |
8 |
(exp guidelines as topic/ or observational study/ or comparative study/ or exp health planning guidelines/ or exp consensus/ or exp "Review literature as topic"/ or exp critical pathways/ or exp algorithms/ or exp meta-analysis as topic/ or exp meta-analysis/ or exp technology assessment,biomedical/ or (guideline* or guide line* or CPG or CPGs or guidance or practical guide* or practice parameter* or (best adj3 practice*) or evidence base* or consensus or algorithm* or (clinical adj3 pathway*) or (critical adj3 pathway*) or recommendation* or comparative stud* or comparison$ or non experimental stud* or nonexperimental stud* or observation* stud* or committee opinion* or policy statement* or position statement* or standard or standards or (systematic* adj3 (review* or overview* or literature or search* or research*)) or meta-analy* or metaanaly* or met analy* or metanaly* or HTA or HTAs or technology assessment* or technology overview* or technology appraisal*).tw.) not (case report/ or editorial/ or letter/) |
4046816 |
9 |
and/6-8 |
359 |
10 |
(2015121* or 2016* or 2017*).dc. |
1615239 |
11 |
9 and 10 |
32 |
12 |
limit 11 to (english or french) |
31 |
Database(s): Embase
Search Strategy:
| # | Searches | Results |
|---|---|---|
1 |
antivirus agent/ or immunoglobulin/ or monoclonal antibody/ or PVZ/ or (abbosynagis or antibody protein or anti viral agent* or anti viral drug* or antiviral agent* or antiviral drug* or antiviral substance or antivirals or antivirus agent* or antivirus drug* or anti-RSV or clonal antibody or endobulin or flebogamma or flebogammadif or gamastan or gamimmune n or gamimune or gamma globulin* or gamma immunoglobulin* or gamma-globulin* or gammagee or gammaglobulin* gammar or gammimune or gamulin or globuman or glovenin i or humanized antibody or humanized monoclonal antibody or hybridoma antibody or Ig or igam or igc or immune gamma globulin or immune globin* or immune globulin* or immune serum globulin* or immuno gamma globulin* or immuno globulin* or immunogammaglobulin* or immunoglobin* or immunoglobulin* or immunoprotein* or intragam or intraglobin* f or isiven or iveegam or ivega or mAbs or MEDI493 or MEDI 493 or monoclonal antibodies or monoclonal antibody or PVZ or panglobulin* or passive immunization or sandoglobin* or sandoglobulin* or synagis or synagys or tegelin* or veinoglobulin* or venoglobulin* or viral inhibitor or virostatic agent* or virucidal agent* or virucide agent* or virus repressor or virustatic agent* or vivaglobin).tw,kf. |
607418 |
2 |
RSV infection/pc |
605 |
3 |
RSV infection/ or (RSV infection* or RSV*).tw. |
15757 |
4 |
prophylaxis/ or (control or health protection or immunoprophylaxis or prevention or preventive measures or preventive medication or preventive therapy or preventive treatment or prophylactic institution or prophylactic management or prophylactic medication or prophylactic therapy or prophylactic treatment or prophylaxis).tw. |
3294079 |
5 |
2 or (3 and 4) |
4066 |
6 |
and/1,5 |
1602 |
7 |
exp infant/ or exp child/ or exp adolescent/ or exp minors/ or exp puberty/ or exp pediatrics/ or school/ or (infant* or infancy or newborn* or baby* or babies or neonat* or preterm* or prematur* or postmatur* or child or children or schoolchild* or school age* or preschool* or kid or kids or toddler* or preadoles* or adoles* or teen* or boy or boys or girl* or minors* or pubert* or pubescen* or prepubescen* or pediatric* or paediatric* or peadiatric* or nursery school* or kindergar* or primary school* or secondary school* or elementary school* or high school* or highschool*).mp. |
3972676 |
8 |
(exp practice guideline/ or health care planning/ or consensus/ or algorithm/ or systematic review/ or "systematic review (topic)"/ or meta-analysis/ or "meta analysis (topic)"/ or biomedical technology assessment/ or observational study/ or comparative study/ or (guideline* or guide line* or CPG or CPGs or guidance or practical guide* or practice parameter* or (best adj3 practice*) or evidence base* or consensus or algorithm* or (clinical adj3 pathway*) or (critical adj3 pathway*) or recommendation* or committee opinion* or policy statement* or position statement* or standard or standards or (systematic* adj3 (review* or overview* or literature or search* or research*)) or meta-analy* or metaanaly* or met analy* or metanaly* or HTA or HTAs or technology assessment* or technology overview* or technology appraisal* or "comparative stud*" or comparison or "non experimental stud*" or "nonexperimental stud*" or "observation* stud*").tw.) not (case report/ or editorial/ or letter/) |
4142791 |
9 |
and/6-8 |
466 |
10 |
(2015121* or 2016* or 2017*).dc. |
1992030 |
11 |
and/9-10 |
57 |
12 |
limit 11 to (english or french) |
56 |
Appendix B: Level of evidence based on research design and quality (internal validity) rating of evidence
| I | Evidence from randomized controlled trial(s). |
| II-1 | Evidence from controlled trial(s) without randomization. |
| II-2 | Evidence from cohort or case–control analytic studies, preferably from more than one centre or research group using clinical outcome measures of vaccine efficacy. |
| II-3 | Evidence obtained from multiple time series with or without the intervention. Dramatic results in uncontrolled experiments (such as the results of the introduction of penicillin treatment in the 1940s) could also be regarded as this type of evidence. |
| III | Opinions of respected authorities, based on clinical experience, descriptive studies and case reports, or reports of expert committees. |
Good |
A study (including meta-analyses or systematic reviews) that meets all design- specific criteria* well. |
Fair |
A study (including meta-analyses or systematic reviews) that does not meet (or it is not clear that it meets) at least one design-specific criterion but has no known "fatal flaw". |
Poor |
A study (including meta-analyses or systematic reviews) that has at least one design-specific "fatal flaw", or an accumulation of lesser flaws to the extent that the results of the study are not deemed able to inform recommendations. |
* General design specific criteria are outlined in Harris et al., 2001, with very minor modifications
Appendix C: Amstar quality assessment of original INESSS systematic literature review
| AMSTAR criteria | Yes | No | Can't answer | N/A |
|---|---|---|---|---|
1. Was an 'a priori' design provided? |
X |
|||
2. Was there duplicate study selection and data extraction? |
√ |
|||
3. Was a comprehensive literature search performed? |
√ |
|||
4. Was the status of publication (i.e. grey literature) used as an inclusion criterion? |
√ |
|||
5. Was a list of studies (included and excluded) provided? |
√ |
|||
6. Were the characteristics of the included studies provided? |
√ |
|||
7. Was the scientific quality of the included studies assessed and documented? |
√ |
|||
8. Was the scientific quality of the included studies used appropriately in formulating conclusions? |
√ |
|||
9. Were the methods used to combine the findings of studies appropriate? |
X |
|||
10. Was the likelihood of publication bias assessed? |
X |
|||
11. Was the conflict of interest included? |
X |
|||
Total (out of 10) |
7 |
Appendix D: PRISMAflow diagram
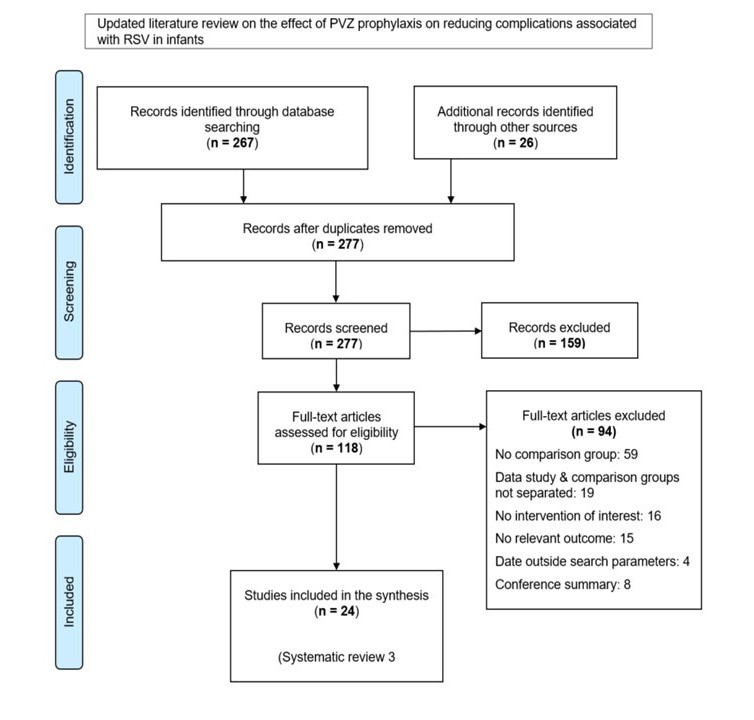
Appendix D: Text description
The PRISMA flow diagram describes the process by which articles were selected for the literature review on the effect of PVZ prophylaxis on reducing complications associated with RSV in infants. The process is broken down into four stages: Identification, Screening, Eligibility and Included.
Stage 1: Identification
- 267 records were identified through the February 1, 2017 to April 12, 2019 and again for publications from April 2019 to July 29, 2020 database search, 26 records were identified through hand-searching.
- 277 records remained after duplicates were removed.
Stage 2: Screening
- 277 records were then screened.
- Of these 277 records, 159 records were excluded.
Stage 3: Eligibility
- 118 full-text articles were assessed for eligibility.
- Of these 118 full-text articles, 94 were excluded. The exclusion breakdown is as follows: 59 had no comparison group, 19 the data study and comparison groups were not separated, 16 did not include the intervention of interest, 15 did not include relevant outcomes, 4 were outside the date range and 8 were conference summaries..
Stage 4: Included
- 24 articles were included.
3 systematic reviews.
Appendix E: Summary of evidence from updated INESSS literature review related to efficacy/effectiveness of PVZ prophylaxis in infants and children
Order of references: Systematic reviews, by quality, then alphabetic. Individual studies, by level of evidence, then quality, then alphabetic
| Study details | Summary | ||||
|---|---|---|---|---|---|
| Study | Study design | Participants | Summary of key findings | Level of evidence | Quality |
Kua and Lee 2017 Footnote 18 Not funded or sponsored |
Systematic review of controlled trials and observational studies to Jan 31, 2017 Efficacy of PVZ in reducing incidence of RSVH |
Infants and children ≤2 years of age with CF (3891 participants) |
For systematic reviews without meta-analysis, the individual studies meeting inclusion criteria for the updated literature review are presented separately in this table or in Appendix F, by author. Therefore, please see elsewhere in this table or in Appendix F for the findings from the following studies: Bjornson et al. 2015 (conference abstract; replaced with publication) Cohen et al. 2005 5 studies excluded as not meeting criteria (no comparator group or insufficient data) (data from systematic review not used) |
No rating under NACI methods |
Good (as assessed using AMSTAR) |
Robinson et al. 2016 Footnote 17 Funding: CF Foundation USA, NIHR UK |
Systematic review of randomized controlled trials (one RTC found) Multicentre United States (40 centers) 1998-2001 PVZ prophylaxis versus no intervention or placebo. Monthly injections of 15 mg/kg over 5 months of one RSV season Hospitalizations, mortality and adverse events assessed up to 6 months after start of study Nutritional status, number with P. aeruginosa colonization, and need for oxygen therapy assessed up to 12 months after start of study Method of RSV diagnosis not stated |
Children with CF aged ≤ 24 months (n=186) Mean age: 12.8 months (0.4–24.4) months PVZ: n = 92 Placebo: n = 94 |
Primary outcomes:
|
No rating under NACI methods |
Good (as assessed using AMSTAR) No explicit mention of a priori study design Only 1 unpublished study identified (conference presentation, abstract available), methods to combine results and assessment of publication bias not applicable |
Simoes et al. 2018 Footnote 19 Funded by AbbVie |
Systematic review of studies from Jan 1 1995 to Dec 31 2017 Effectiveness of prophylactic agents for RSV infection |
Infants and children ≤18 years of age PVZ prophylaxis: (15,407 participants) |
For systematic reviews without meta-analysis, the individual studies meeting inclusion criteria for the updated literature review are presented separately in this table or in Appendix F, by author. Therefore, please see elsewhere in this table or in Appendix F for the findings from the following studies: Anderson et al. 2017 (Data from systematic review not used) |
No rating under NACI methods |
Average (As assessed using AMSTAR) |
Notario et al. 2014 Footnote 37 Supported by AbbVie Inc. |
Randomized controlled trial Multinational Multicentre (Canada: 9, United Kingdom: 11, United States: 119) RSV season 1996–1997 PVZ versus placebo for prevention of RSVH Post-Hoc analysis of data from IMpact-RSV study 1998 Footnote 38 PVZ 15 mg/kg monthly x 5 doses RSV diagnosed by antigen test |
Premature infants born at ≤ 35 wk GA and ≤ 6 months of age without chronic lung disease PVZ: (n=494) Placebo: (n=230) |
Baseline characteristics similar for the two groups in all GA categories. Incidence of RSV-related hospitalization for PVZ vs. placebo treatment groups stratified by GA. Placebo (n=230) PVZ (n=494) RRR:(95% CI):67.9:(-969.4, 99.0) Placebo (n=230) PVZ (n=494) RRR:(95% CI):80.4:(-8.3, 97.4) Placebo (n=230) PVZ (n=494) RRR:(95% CI):73.0:(7.7–95.1) Placebo (n=230) PVZ (n=494) RRR:(95% CI):100.0:(-564.0, 100.0) Placebo (n=230) PVZ (n=494) RRR:(95% CI):64.5:(-64.0, 95.4) Placebo (n=230) PVZ (n=494) RRR:(95% CI):79.7:(35.7–96.9) Placebo (n=230) PVZ (n=494) RRR:(95% CI):79.8:(49.0–94.2) Placebo (n=230) %: (95% CI):10.8:(5.5–18.5) PVZ (n=494) RRR:(95% CI):81.8:(45.4–96.5) Placebo (n=230) PVZ (n=494) RRR:(95% CI):82.1:(45.9–96.6) Placebo (n=230) PVZ (n=494) RRR:(95% CI):72.3:(-14.1, 96.3) Placebo (n=230) %: (95% CI):8.2:(3.1–17.0) %:(95% CI) RRR:(95% CI):73.2:(-10.8, 96.4) n/N = number hospitalized/number in group. RRR, relative risk reduction with PVZ versus placebo * significant difference (p <0.05). Significant relative risk reduction in 28-31, 29-32, 29-33, 32-34, 32-35 wGA groups |
I |
Good |
Scheltema et al. 2018 Footnote 39 Funding: Abbvie |
RCT Multicenter Netherlands 15 sites Two RSV seasons (2008-2010) PVZ versus placebo (15 mg/kg every 30 days; total of 5 doses) Follow-up at 6 years for current asthma and pulmonary function. |
Children born at 32 1/7 –35 wGA and otherwise healthy, six months of age at start of the RSV season PVZ recipients (n=214) (follow-up at 6 years: n= 199) Placebo recipients (n=215) (follow-up at 6 years: n=196) |
Status at 6 years of age: Parent-reported current asthma* PVZ: 14.1% Status at 6 years of age: asthma medication PVZ: 9.0% Status at 6 years of age: wheeze PVZ: 11.6% Status at 6 years of age: infrequent wheeze (1-3 episodes per year) PVZ: 6.0% Status at 6 years of age: Physician diagnosed asthma in past 12 months PVZ: 10.3% FEV 0.5 % predicted † at 6 years of age 89.1 (10.6) PVZ: 90.1% ARR = absolute risk reduction * wheeze or use of asthma medication in the past 12 months FEV 0.5 = forced expiratory volume in 0.5 seconds. FEV 0.5, FEV 1 , FEF 25–75,, FEV 0.5 :forced vital capacity (FVC) ratio, FEV 1 :FVC ratios were similar in the PVZ and placebo groups, as were FEV 0·5 and other lung function results after administration of a bronchodilator PVZ had no major effect on current asthma or lung function at school age. |
1 |
Fair PVZ and placebo visually different at time of administration Parents un-blinded after 1 st year of follow-up. |
Kimura T et al. 2020 Footnote 36 Source of funding not reported. |
Retrospective cohort study Multicenter Japan; Medical claims from JMDC database (Tokyo, Japan) 1 period: 2007-2012 2 period: 2013-2015 PVZ ≥1 dose recorded; amount not stated versus no prophylaxis RSV diagnosis by ICD-10 codes |
Children aged £24 months with Down syndrome. Expanded criteria for PVZ eligibility 2013 First Period n=268 PVZ n=81 Median age (IQR), months: 6 (3-13) Male (46%) No prophylaxis n=187 Median age (IQR), months: 13 (6-18) Male (56%) Second Period n=364 PVZ n=303 Median age (IQR), months: 10 (4-16) Male (53%) No prophylaxis n=61 Median age (IQR), months: 16 (12-22) Male (57%) |
Proportion of patients with specific conditions receiving PVZ Target population: Whole population Target population: CHD Target population: Premature Target population: CLD Target population: Immunodeficiency Target population: Abnormalities of respiratory system Target population: Hematological malignancies or solid tumors Target population: History of hospitalization for respiratory infection RSVH among children with Down syndrome (DS) with or without PVZ Whole population Infants without CHD Infants without additional risk factors While analyzing the whole study population, the following factors were included in the models as fixed effects: preterm birth and congenital heart disease. While analyzing the infants with DS without CHD, the following factor was included in the models as a fixed effect: preterm birth. While analyzing the infants with DS without additional risk factors, only crude OR derived from mixed effects logistic regression models are reported because no infant in this population had a defined confounding condition (preterm birth and CHD). Conclusion: PVZ administration was associated with reduction in RSVH in children with Down syndrome but not in subgroups without hsCHD or without risk factors for severe RSV infection |
II-2 |
Good |
Simoes et al., 2010 Footnote 28 Funding source: Abbott |
Prospective Cohort Multicentre Europe (Spain, Germany, Netherlands, Poland, Sweden) and Canada ( 27 sites) 2001-2 PVZ prophylaxis versus no intervention PVZ dose not stated Treated group matched to combined untreated group by wGA (<28, 28–32, 29–35) and age (±3 months) Method of RSV diagnosis not stated |
Children ≤36 months of age at enrollment and born prematurely (<36 wGA) without CLD (n=420) Mean age: 19 (1–40) months Treated group : Children who had received PVZ in a previous respiratory season and were not hospitalized with RSV (n=190) Combined untreated group : Children who never received PVZ (n=230) No history of RSVH (n=154) Documented RSVH in year before enrollment (n=76) Exclusion criteria: Children on MV at time of potential enrollment, life expectancy <6 months, or known immunodeficiency |
Primary outcome (1) Incidence of physician-diagnosed recurrent wheeze** at 24-month follow-up (age 2-5 yr) Decreased incidence of recurrent wheeze in treated children compared to untreated children if no family history of asthma (adjusted † OR=0.32, 95% CI: 0.14 to 0.75) or atopy # (aOR=0.20, 95% CI: 0.07 to 0.59) No difference between treated and untreated children with a family history of asthma (unadjusted OR=0.56, 95% CI: 0.23 to 1.35) or atopy (unadjusted OR=0.69, 95% CI: 0.34 to 1.39) Secondary outcome (2) Time to third physician-diagnosed wheezing episode PVZ treated group had significantly longer time to third episode compared infants with no family history of asthma (aHR=0.33, 95% CI: 0.51 to 0.74) or atopy (aHR=0.21, 95% CI: 0.08 to 0.59) With family history of asthma or atopy, no difference between treated and untreated groups In infants with family history of atopy, greatest risk factor for time to third episode of physician-diagnosed wheezing is asthma (aHR=2.94, 95% CI: 1.14 to 7.61) † Covariates adjusted for in multiple logistic regression: age, sex, age at enrollment, GA at birth, birth weight, multiple birth status, baseline RSV-neutralizing antibody titers, daycare attendance, numbers of adults and siblings in the home, numbers of siblings in daycare, presence of a wood-burning stove in the home, and family history of asthma, atopic dermatitis, allergic rhinitis, or food allergies Definitions: **Physician-diagnosed recurrent wheezing: three or more episodes of wheezing in the last 12 months verified by a physician at a physician's visit, emergency department visit, or hospitalization (Episode of wheezing: one or more consecutive days of wheezing preceded and followed by a non-wheezing healthy period of at least one week) # Family history of atopy: history of asthma, atopic dermatitis, or allergic rhinitis |
Level II-2 |
Good Some infants could have primary outcome prior to enrollment in study, but would apply to both intervention and non-intervention groups Small number of infants per subgroup could limit ability to find statistically significant differences between groups |
Anderson et al. 2017 Footnote 40 Sponsored by MedImmune |
Test-negative case-control study Multinational Multicenter (USA, Canada) Number of sites not stated 2002-2006 Nov-Apr PZV effectiveness (PE) to prevent RSVH: Odds of PVZ administration in the 30 d prior to admission between RSV cases and controls RSV diagnosed by PCR |
Children with hsCHD or CLD <24 months old or born at ≤ 35 wk GA and age ≤ 12 months hospitalized for acute LRTI 849 enrolled: Cases 403 Controls 446 434 received PVZ |
Cases: 403 RSV positive. 150 received PVZ (37.2%) Controls: 446 RSV negative. 284 received PVZ (63,8%) Unadjusted PVZ effectiveness (PE) to prevent hospitalization: 43.1% (95% CI: 34.1 to 51.2) Inverse propensity score weight (IPSW) created using known risk factors for RSVH. IPSW multiple logistic regression model used to adjust PE. Adjusted PE to prevent hospitalization: 58.0% (95% CI: 43.1 to 69.0) Adjusted PE to prevention ICU admission: 62.1% (95% CI: 35.1 to 77.9). PE was not observed for MV: Adjusted PE 31.5% (95% CI: −41.2 to 66.8). Adjusted PE to prevent hospitalization Subgroup analysis Study group: Preterm infants 29–35 wGA without hsCHD or CLD and <6 months of chronological age Adjusted PE (95% CI): 74.1 (56.2-84.7) Study group: Preterm infants <29 wGA without hsCHD or CLD and <6 months of chronological age Adjusted PE (95% CI): 65.6 (-47.3 - 92.2) Study group: Preterm infants <6 months of chronological age without hsCHD or CLD Adjusted PE (95% CI): 66.3 (34.4-82.7) Study group: Preterm infants 3-<6 months of chronological age without hsCHD or CLD Adjusted PE (95% CI): 79.8 (57.5-90.4) Study group: Children with hsCHD (may include CLD) and <12 months of chronological age on Nov 1 Adjusted PE (95% CI): -15.5 (-141 to 44.6) Study group: Children with hsCHD (may include CLD) and 12 to <24 months of chronological age on Nov 1 Adjusted PE (95% CI): 69.2 (-101.6 to 95.3) Study group: Children with CLD (without hsCHD) and <12 months of chronological age on Nov 1 Adjusted PE (95% CI): 33.8 (-31.1 to 66.6) Study group: Children with CLD (without hsCHD) and 12 to <24 months of chronological age on Nov 1 Adjusted PE (95% CI): 63.8 (-9.3 to 88) PE not observed in group <29 wGA without CLD but numbers were small. PE not observed in the groups with CLD or hsCHD. PVZ had no significant effectiveness against hospitalization for HMPV (tested to control for study bias): PE 34.7% (95% CI: -12.9 to 62.2). |
II-2 |
Fair |
Bjornson et al. 2018 Footnote 25 Funded by AbbVie |
Retrospective observational comparative cohort Multicenter Alberta, province-wide (2 centers) 2000-2017 PVZ vs. no treatment to prevent RSVH (review of medical records) PVZ 15 mg/kg monthly RSV diagnosed by PCR, enzyme or immunofluorescent assay, or viral culture |
267 children <2 yrs old with CF 183 PVZ recipients 84 no intervention PVZ mean 4.4 ± 1.5 injections |
Hospitalization for RSV: Outcome: No. of patients admitted for RSV Outcome: Admissions for RSV Outcome: Total LOS (days) a Outcome: ICU admission (% of admissions) b Outcome: ICU LOS (days) a Outcome: Respiratory support (MV or supplemental oxygen) (% of admissions) c Outcome: Duration of respiratory support (days) a a mean ± SD b of the total cohorts, 0% of PVZ recipients and 2.4% of controls required ICU admission (p >0.05). c of the total cohorts, 2.2% of PVZ recipients and 1.2 % of controls required respiratory support. (p = 0.58) PVZ recipients were born at a significantly younger GA, had a lower birth weight, were less likely to have siblings, and more likely to be born in November to January. After adjustment for the above confounders the PVZ cohort did not have decreased odds of hospitalization for RSV (Exp(B) = 0.43 [0.10–1.80], p = 0.25 26 (14.2%) in the PVZ group and 29 (34.5%) in the control group had hospitalization for a respiratory illness. After adjusting for confounders, (Exp(B) = 0.23 [0.11–0.49], p<0.0005). |
II-2 |
Fair Low overall rate of testing for RSV (53% overall); testing rates in PVZ recipient and control groups not stated |
Blake et al., 2017 Footnote 20 Funding source: not stated |
Retrospective Cohort Single center North Carolina Level IV intensive care and level II special care nurseries in single institution 2012-2016 PVZ prophylaxis versus no intervention (in analysis of overall cohort) PVZ dose not stated RSV seasons determined using national-, regional- and state-level surveillance data Tested for RSV on admission with respiratory symptoms Method of RSV diagnosis not stated (refers to "RSV viral panels") |
Overall cohort: Infants 29 to <32 wGA and born October 2012–March 2016 PRE cohort (discharged Oct 1, 2012- Apr 30, 2013 or Oct 1, 2013-Apr 30, 2014 (n = 98) POST cohort (discharged Oct 1, 2014 - Apr 30, 2015 or Oct 1, 2015 - Mar 31, 2016) (n = 75) Exclusion criteria: Infants who died during initial admission; outcomes occurring after local RSV season; outcomes not associated with primary respiratory diagnosis |
Primary outcomes
|
Level II-2 |
Fair Co-morbidities (e.g., CLD, CHD, CF) with potential impact on RSV outcomes not described Analysis not controlled for potential con-founding factors PVZ compliance not assessed Advanced care hospitalized population; may be biased to a more severely affected premature infant population |
Buchs et al. 2017 Footnote 41 Funding not stated |
Retrospective case-control Single center (comparison between two centers PVZ used systematically in one center, not used in the other) France 2001-2012 Effect of PVZ on acquisition of S. aureus (Sa) and P. aeruginosa (Pa) PVZ 15 mg/kg monthly during RSV season RSV diagnosed by IFA, molecular diagnostic test or viral culture |
Children with CF born between Jan 1, 2001 and June 30, 2012 and ≤36 months of age Cases matched with 3 controls (for year and month of birth, gender and CF genotype) Followed to 3 years of age PVZ 40 Controls 140 |
Infants from both groups were similar in patient characteristics Microbiologic culture techniques similar at both centers. PVZ effect on microbial colonization and hospitalization for respiratory illness Outcome: Age at first Sa isolation, months, median ( range) PVZ (n=40): 6.4 (2.0–59.0) Outcome: Age at first Pa isolation (months, median (range) PVZ (n=40): 12.3 (3.8-32.6) Outcome: Sa isolation by age 3 yr (%) a,b PVZ (n=40): 97.5% Outcome: Pa isolation by age 3 yr (%) b PVZ (n=40): 40% Outcome: Hospitalization for LRTI PVZ (n=40): 7 (17.5%) Outcome: Hospitalization for RSV LRTI PVZ (n=40): 2 (5%) Sa = S. aureus Pa = P. aeruginosa No patients admitted with RSV required supplemental oxygen or MV. a higher rate of Sa acquisition with PVZ may be related to exposures during visits for PVZ or to different rates of Sa colonization in the two towns b for comparison, microbial colonization rates at 3 years of age from the French National CF Registry were 74% for Sa and 37% for Pa No difference between the groups in outpatient visits in the 1, 2 or 3 years No difference between the groups in growth parameters (weight Z scores at 1, 2 and 3 years of age) |
II-2 |
Fair Cases and controls from different centers; possible outcome bias |
Chi et al. 2014 Footnote 22 Supported by grants from Mackay Memorial Hospital, Taipei |
Prospective cohort with historical controls Single center Taiwan Apr 2011-Mar 2013 (no RSV seasonality) PVZ vs. no treatment to prevent RSV infection PVZ 15 mg/kg monthly x 6 doses starting at initial hospital discharge RSV diagnosed by IFA or viral culture Test for RSV if admitted |
Infants born at ≤ 28 wGA and infants born at ≤35 wGA with CLD PVZ: n=127 ≤ 28 wGA: 108 29-35 wGA with CLD: 19 Controls: (retrospective; born July 2000-June 2008) n = 347 ≤ 28 wGA: 284 29-35 wGA with CLD: 63 |
Follow-up to 12 months to determine overall effectiveness of 6 month prophylaxis. RSVHs in PVZ recipients and controls at 6 and 12 months following initial hospital discharge Within 6 months: Outcome: Hospitalization for RSV n (%) Outcome: ICU admission n (%) Outcome: MV n (%) Outcome: Total LOS (days, median, IQR) Outcome: ICU LOS (days, median, IQR Within 12 months Outcome: Hospitalization for RSV n (%) Outcome: ICU admission n (%) Outcome: MV n (%) Outcome: Total LOS (days, median, IQR) Outcome: ICU LOS (days, median, IQR PVZ recipients matched with controls by propensity score to reduce confounding bias a Reduction in RSVH at 6 months: 86% (95% CI: 36 to 97) b Reduction in RSVH at 12 months: 78% (95% CI: 40 to 92) ICU admission as proportion of RSVHs: at 6 months 1/2 with PVZ and 9/13 in control group; at 12 months 1/5 with PVZ and 10/ 20 in control group MV: at 6 months 0/2 with PVZ and 4/13 in control group; at 12 months 0/5 with PVZ and 4/20 in control group. Reduction in RSVH by subgroup: Within 6 months ≤28 wGA, CLD ≤28 wGA, no CLD ≤28 wGA all 29-35 wGA, CLD ≤ 35 wGA, CLD Within 12 months ≤28 wGA, CLD ≤28 wGA, no CLD ≤28 wGA all 29-35 wGA, CLD ≤ 35 wGA, CLD * n/N = number hospitalized for RSV / number in subgroup No deaths were reported during the study |
II-2 |
Fair Historical controls Minor discrepancies in numbers in text and table, data from text presented here |
Chiu et al. 2018 Footnote 33 Supported by the Taiwan Society of Pediatric Cardiology |
Prospective / retrospective cohort Multicenter Taiwan (4 sites) 2010-2016 PVZ reimbursed from 2013 RSVH with PVZ vs. no treatment. PVZ 15 mg/kg every 4 weeks from time of CHD diagnosis x 6 doses RSV diagnosed by antigen test or culture Routinely tested for virus if hospitalized with LRTI before age 1 yr |
Infants with hsCHD age <1 yr PVZ: n = 747 Controls: n = 809 Followed until 1 yr of age, PVZ recipients prospectively and controls retrospectively Matched with propensity scores. After matching, 705 in each group Mean PVZ doses 3.9 Jan 2010-June 2012: no PVZ Jul 2012-Dec 31, 2013 transition: 44.3% received PVZ Jan 2014-Dec 2015: 98.1% received PVZ |
Patient characteristics similar in the two groups Incidence rates of hospitalization for RSV for PVZ recipients and controls All cases Hospitalization ICU admission Days hospitalized Acyanotic CHD Hospitalization ICU admission Days hospitalized Cyanotic CHD Hospitalization ICU admission Days hospitalized * Incidence rates per 1000 person-days after matching by propensity score All cases Hospitalization ICU admission Days hospitalized Acyanotic CHD Hospitalization ICU admission Days hospitalized Cyanotic CHD Hospitalization ICU admission Days hospitalized PVZ prophylaxis effective in total group and in those with cyanotic CHD but not with acyanotic CHD During PVZ period: 12 RSV admissions. 5 did not get PVZ because of late CHD diagnosis; 3 did not get PVZ because of noncompliance and 3 did not fit criteria for PVZ. Only 1 PVZ failure. |
II-2 |
Fair Historical controls, may be biased |
Farber et al., 2016 Footnote 29 No external funding |
Retrospective Cohort Multicentre United States 9 Medicaid managed care programs 2012-2015 RSV season defined each year as October 1 –March 30 PVZ prophylaxis versus no intervention PVZ dose not stated (reported amounts of PVZ dispensed) RSV infection identified by ICD-9 codes |
Premature infants 29–36 wGA who were ≤6 months of age at start of RSV season in 2012, 2013, and 2014 without risk factors for severe RSV infection other than prematurity (n=14,097) Infants 29–32 wGA (n=2031) PVZ (n=843, 41.5%) No intervention (n=1188, 58.5%) Infants 33–36 wGA (n=12,066) PVZ (n=442, 3.7%) No intervention (n=11,624, 96.3%) Exclusion criteria: <29 wGA or >36 wGA; infants with CLD, hemodynamically significant CHD, pulmonary hypertension, hematopoietic stem cell or other transplantation, and severe genetic syndrome; infants with pharmacy claim for medications for heart failure, CLD, and pulmonary hypertension; and infants with <3 months of health plan eligibility during RSV season in first year of life |
|
Level II-2 |
Fair RSV diagnoses based on hospital discharge diagnoses; no access to laboratory data Possible that some hospitalizations for bronchiolitis without RSV were in infants not tested for RSV PVZ doses and amounts dispensed determined from outpatient pharmacy claims Medicaid recipients may not be representative of other populations. PVZ adherent and nonadherent recipients may have differed in unmeasured confounders |
Groves et al., 2016 Footnote 24 Funding source not stated |
Retrospective Cohort Single regional center Northern Ireland Regional Paediatric CF Centre patient registry 1997-2007 PVZ prophylaxis versus no intervention PVZ dose not stated RSV diagnosed using PCR |
Children diagnosed with CF on neonatal screening and born in Northern Ireland between 1997 and 2007 (n=92) Pre-PVZ initiation cohort (born 1997–2002) (n=47) Post-PVZ initiation cohort (born 2003–2007) (n=45) Exclusion: Children with CF born outside of Northern Ireland |
Primary outcome
# controlling for gender and CF genotype |
Level II-2 |
Fair Minimal adjustment for potential confounders in analysis Cohorts separated in time with possible changes in clinical and/or infection prevention and control practices Small numbers could have limited study power |
Lacaze-Masmonteil et al. 2004 Footnote 31 Funded by Abbott France |
Prospective cohort study Multicenter France 46 centers 2000 Risk factors for RSVH with and without PVZ RSV diagnosed by IFA |
Infants born at <33 wGA and born April – December 2000 followed to end of RSV season n= 2813 ≥ one dose of PVZ: 376 No PVZ: 2370 PVZ status uncertain: 67 |
Patients who received PVZ were significantly more premature, had a higher incidence of BPD, were more frequently initially discharged at onset of the RSV season, and more likely to be hospitalized for LRTI 2256 without BPD did not receive PVZ Outcome: Patients hospitalized at least once for LRTI Outcome: Hospitalizations for LRTI Outcome: Patients hospitalized at least once for RSV Outcome: Hospitalizations for RSV Outcome: MV * 4 had RSV before start of PVZ administration, therefore 19 with PVZ (5.1%) (p 0.13) No significant difference in RSVH rates between PVZ and control group but PVZ group had more LRTI admissions 2 deaths from RSV (no prophylaxis) |
II-2 |
Fair Differences reported in PVZ and no treatment groups but results not adjusted for confounders Minor discrepancies between numbers in tables and text, in which case data from tables used |
Lee et al. 2018 Footnote 30 Funding not reported |
Retrospective Multicenter Hong Kong (2 centers) 2010-2014 PVZ program onset 2012 Cost-effectiveness of PVZ program 15 mg/kg monthly for 5 doses RSV diagnosis by immunofluorescent assay or viral culture |
Infants born <29 wks GA PVZ: n = 40 No PVZ: n = 95 Infants followed for RSVH within 1 year after initial discharge. |
RSVHs in PVZ recipients and controls <29 wGA (all): Hospitalization for RSV <29 wGA (all): Length of stay (days) mean ±SD <29 wGA (all): PICU admission <29 wGA (all): PICU LOS (days) mean ±SD <29 wGA (all): RSV mortality <27 wGA: Hospitalization for RSV PVZ only effective for those <27 wk GA |
II-2 |
Fair Birthweight, GA, BPD differed between the two groups; confounding not adjusted for |
Mochizuki et al., 2017 Footnote 27 Funding: Ministry of Health, Labour and Welfare, Japan |
Prospective Cohort Multicentre Japan Number of centers not stated 6-year follow-up of previously published CREW study that examined outcomes at three years of age Recruitment during 2007–2008 RSV season PVZ prophylaxis versus no intervention PVZ dosage not stated RSV infection not an outcome |
Intention to treat population Preterm infants (33–35 wGA) without CLD (n=440) PVZ ≥3 doses (n=345) No intervention (n=95) Per protocol population Children completing 6-year follow-up (n=328) PVZ (n=249) No intervention(n=79) Atopic asthma sub-population Children with blood collected for IgE determination (n=268) PVZ (n=202) No intervention (n=66) Exclusion criteria: Small for GA, CLD, history of respiratory diseases requiring MV regardless of surfactant use, children who had received <3 doses of PVZ during the first 6 months of life |
Primary outcome
# Definitions Recurrent wheezing: occurrence of 3 or more episodes of physician-diagnosed expiratory wheezing in a 12-month period Atopy: high serum total (30 IU/mL or greater) or specific IgE (0.35 IU/mL or greater) Atopic asthma: presence of both recurrent expiratory wheeze and atopy † Analysis controlled for a number of potential confounders: GA, smokers in the home, family history of allergy |
Level II-2 |
Fair Details on representativeness and derivation of cohort available only in original CREW study Insufficient detail provided to determine if assessment blinded to child's prophylaxis status Some exposure/outcome data (e.g., recurrent wheeze, clinic/hospital visits) collected from parental self-reports Analysis controlled for only a subset of the potential confounders for which data collected |
Prais et al., 2016 Footnote 21 Funding source: Abbott |
Retrospective Cohort Israel Single tertiary care children's medical centre 2000-2003 PVZ prophylaxis versus no intervention PVZ dosage not stated Routine PVZ prophylaxis introduced in 2001 for infants born <29 weeks GA Method of RSV diagnosis not stated |
School age children (7–10 years) who had been born extremely premature (<29 wGA) between 2000–2003 (n=63) Some children had BPD Mean age: 8.9 (SD: 0.68) years Exposed: Children born in 2001–2003 who received PVZ (n=30) Unexposed: Children born in 2000–2001 who did not receive PVZ (n=33) Exclusion criteria: Subjects unable to undergo pulmonary function testing for any reason; subjects with severe systemic disease, including cardiac disease |
Short-term outcomes (within first two years of life)
# Pulmonary lung function tests performed: Percentage of predicted forced vital capacity (FVC), percentage of predicted forced expiratory volume in one second (FEV 1 ), number and proportion of subjects with FEV 1 <80% of predicted, FEV 1 /FVC ratio, percentage change in FEV 1 after bronchodilation, number and proportion of subjects with significant reversibility, percentage of predicted forced expiratory flow (FEF) at 50% of FVC, percentage of predicted FEF at 25–75% FVC, percentage change in FEF 25–75% after bronchodilation, median provocative concentration leading to a 20% fall in FEV 1 (PC 20 ), number and percentage of subjects with positive methacholine test, and number and percentage of subjects with PC 20 <1 mg/mL. |
Level II-2 |
Fair Subjects recruited from single tertiary care centre Episodes of wheezing in first two years of life dependent upon parental self-reports subject to recall bias Small number of infants in each group could reduce power of study Analysis controlled only for age and extreme prematurity (although groups found not to differ on a number of potential confounders) Results may not be generalizable to term infants or infants with severe systemic disease (e.g., cardiac disease) |
Priante E et al., 2019 Footnote 32 Source of funding not reported |
Retrospective cohort study Multicentre (3) Italy season 1: 2015-2016 season 2: 2016-2017. Change in eligibility criteria for PVZ 2016-17 PVZ 15 mg/kg monthly x 5 doses versus no prophylaxis RSV confirmed by EIA, IFA, PCR or viral culture |
Infants born at 29-35 wGA Season 1 (n=262) Mean wGA: 229±10.9 CLD 0% hsCHD 2.3% PVZ: n=95 No prophylaxis: n=167 Season 2 (n=274) Mean wGA (days): 228±11.6 CLD 4.4% hsCHD 2.92% PVZ: n=53 No prophylaxis: n=221) |
The proportion of infants not exposed to PVZ increased significantly from 63.7% in season 1 to 80.6% in season 2 (p-value <0.0001) Hospital admissions due to RSV over two consecutive seasons All infants (n/N) Season 1: 5/262 (1.91%) PVZ (n/N) No PVZ (n/N) *n/N = number hospitalized for RSV / number in subgroup Across the two seasons, 3 of 148 infants (2.03%) receiving PVZ and 16 of 388 infants (4.12%) without prophylaxis were hospitalized due to RSV. Risk of RSVH among infants receiving PVZ compared to those not receiving prophylaxis across the two seasons Season 1 Season 2 Overall Calculated using metabin function in meta package in R studio. Overall estimate was pooled using a fixed effects model (I Footnote 2 = 0%). Conclusion: The authors conclude that restricting eligibility for PVZ reimbursement led to a significant increase in RSVH. Using the raw data reported by the authors, we found that the risk of RSVH was reduced in those receiving PVZ compared to no prophylaxis but the result was not statistically significant [OR (95%CI); 0.57 (0.16-2.07)]. |
II-2 |
Fair Results not analyzed by presence or absence of CLD or hsCHD. More high risk infants in the group without PVZ in 2 nd season. Author's conclusion does not seem to be supported by the data (non-significant increase; also, increase in % RSVH in those without prophylaxis in period 2 vs. period 1 not addressed) |
Sanchez-Luna et al. 2017 Footnote 35 Funded by AbbVie |
Prospective observational study Multicenter Spain 50 sites Oct – Mar 2012-13 and 2013-4 RSVH rates in term infants with Down syndrome (DS) and infants without DS RSV dx by rapid antigen test (+ culture or molecular technique in some) |
Term infants with DS age <1 yr Control cohort: infants without DS matched by sex and date of birth. Excluded if hsCHD, BPD, <35 wGA. DS: n = 93 Non-DS: n = 68 |
Higher incidence of RSVH in DS group compared to the non-DS group. DS group had lower weight at baseline and lower GA at birth; higher percentage of infants previously hospitalized for RSV infection or for other reasons and higher percentage with concomitant disease and use of concomitant medications. Hospitalization for RSV: Down syndrome group: PVZ prophylaxis was not an independent predictor for RSVH |
II-2 |
Fair Small number of the DS group received PVZ. Potential confounders in PVZ treated and untreated groups not explored. |
Soraiz et al. 2017 Footnote 34 Funding not stated |
Prospective cohort with historical control Single center Argentina 2014-2016 (prospective) 2007-2009 (historic controls) Impact of PVZ vs. no treatment on hospitalization for RSV Method of RSV testing not stated |
hsCHD age <1 yr PVZ: n = 53 Control: n = 50 |
No significant difference in GA, birth weight, sex, age at hospitalization. Fewer with cyanotic heart disease, left ventricular hypoplasia and pulmonary hypertension and more with corrected CHD with minor residua in the control group. Hospitalization for respiratory tract infection (RTI) RTI Hospitalization RSV-RTI hospitalization MV No difference in respiratory tract infections or LOS for respiratory tract infections; no respiratory deaths |
II-2 |
Fair Historical controls; differences in types and severity of CHD between the PVZ and untreated groups but confounders not adjusted for Conference abstract |
Fink et al., 2019 Footnote 26 No external funding |
Retrospective cohort study USA Multicenter CF Foundation Patient Registry (CFFPR) 2008 -2016 PVZ prophylaxis versus no intervention Dose and number of doses of PVZ not stated RSV not diagnosed |
Infants diagnosed with CF (CF) at age <6 months and had ³1 yr of CFFPR data by age 2 yrs PVZ: n = 1,588 <32 wGA 1% 32-<37 wGA:10% No prophylaxis: n = 2,679 <32 wGA1%; 32 - <37 wGA: 6% |
Short-term mortality: all cause mortality before age 2 yrs total and specifically during the RSV season. Risk of death among children age <2 yrs who did or did not receive PVZ Before age 2 yr During RSV season Annual rate of hospitalizations among children who did or did not receive PVZ Age, yr : <1 Age, yr : 1 to <2 Age, yr : 2 to <3 Age, yr : 3 to <4 Age, yr : 4 to <5 Age, yr : 5 to <6 Age, yr : 6 to <7 Age, yr : 7 to <8 Footnote 1 Adjusted for propensity score. Variables included in the model; birth season, Hispanic, mutation class group, smoker in household, received influenza vaccine, length percentile, weight percentile, MRSA positive culture results, Dornase alfa treatment, hypertonic saline treatment, pancreatic enzyme replacement therapy. Footnote 2 HR: High-risk infants defined as weight or weight-for-length percentile of <5% or hospitalization for pulmonary exacerbation or other pulmonary complications before their 1st RSV season Long-term sequelae: pulmonary function impairment/deterioration: annualized percent FEV 1 predicted (average of the maximum measure from each quarter) during the 12 months before the seventh birthday. Mean percent FEV 1 (95% CI) predicted at 7 years of age All (n=1,323) High-risk (n=462) Time to 1 st P. aeruginosa culture: The unadjusted hazard ratio for acquisition of P. aeruginosa was 1.1 (95% CI: 1.0 to 1.2); after propensity score adjustment, the hazard ratio was 1.1 (95% CI: 0.96 to 1.2). Conclusion: The authors found no evidence of long-term benefits associated with PVZ in infants with CF |
II-2 |
Poor No documentation of RSV infection or RSVH |
Narbona-Lopez et al. 2018 Footnote 23 Funding not mentioned (authors state no conflict of interest with any financial organization) |
Retrospective observational study Single center Spain 2000-2012 Characterize patients admitted because of bronchiolitis and analyze separately those who did or did not receive PVZ PVZ monthly during RSV season, up to 5 doses RSV diagnosis by PCR |
All infants admitted with bronchiolitis (n = 952) All infants who received PVZ (n=641) (criteria for PVZ: <28 wGA and age <12 months at beginning of RSV season; 29-32 wGA and age <6 months at beginning of RSV season; age <2 yrs with BPD, neuromuscular disease, chromosomal abnormalities, or CHD) |
Overall: 531/900 tested (59%) of those admitted with bronchiolitis were RSV+. Mean GA 38.4 wks (range 25-43 wks). RSV+: mean age at admission 107±109 d; mean LOS 7.1 ± 4.1 d. (± not defined) PVZ recipients: 63/641 (9.8%) admitted with bronchiolitis. 22/62 tested (3.4% of total PVZ cohort and 35.5% of those admitted with bronchiolitis) were RSV+. RSV+: mean age at admission 121± 105 d; mean LOS 9.24 ±4.2 d. Mean age at admission for RSV older in PVZ group (p=0.042) RSV+ LOS significantly longer in PVZ group (p=0.006) Lower proportion of RSV infection in those admitted with bronchiolitis if PVZ received (35.5%) vs. all cases (59%)* but groups not matched for GA or actual age. * calculation for no PVZ group: 952-63 = 889; RSV in 531-22 = 509 (57.3%) vs. PVZ group (35.5%): p=0.0056 |
II-2 |
Poor Hospitalization rate stated only for PVZ group. PVZ and comparator groups have confounding factors but no adjustment made. |
For individual studies, Level of Evidence and Quality were assessed using the methods of Harris 2001.
Abbreviations: BPD: bronchopulmonary dysplasia; CI: confidence interval; CF: cystic fibrosis; GA: gestational age; DS: Down syndrome; hsCHD: hemodynamically significant congenital heart disease; ICU: intensive care unit; IFA: immunofluorescence assay; IQR: interquartile range; IPSW: inverse propensity score weight; LOS length of hospital stay; LRTI: lower respiratory tract infection; MV: mechanical ventilation; n/N: number hospitalized/number in group; NNT: number needed to treat; OR: odds ratio; PCR: polymerase chain reaction; PE: palivizumab effectiveness; PVZ: palivizumab; RCT: randomized controlled trial; RR: relative risk; RRR: relative risk reduction; RSVH: respiratory syncytial virus-related hospitalization; RTI: respiratory tract infection; RT-PCR: reverse transcription PCR; UK: United Kingdom; US: United States; wGA: weeks of gestational age.
Appendix F: Summary of evidence from original INESSS literature review related to efficacy/effectiveness of PVZ prophylaxis in infants and children
| Study details | Summary | ||||
|---|---|---|---|---|---|
| Study | Study design | Participants | Summary of key findings | Level of evidence | Quality |
Systematic reviews |
|||||
Andabaka et al., 2013 Footnote 44 Funding source not stated |
Systematic review with meta-analysis of RCT 1996–2012 PVZ prophylaxis versus placebo |
Children born at ≤35 wGA and aged ≤6 months of age at start of RSV season, or <24 months of age with CLD or hsCHD and under 24 months at start of the RSV season (N= 2831) |
Effectiveness of PVZ prophylaxis versus placebo, premature infants and infants with CLD or hsCHD
*The three studies included in the meta-analysis were Feltes et al., 2003; IMpact-RSV, 1998; and Subramanian et al., 1998 † The two studies included in the meta-analysis were Feltes et al., 2003 and IMpact-RSV, 1998 |
No rating under NACI methods |
Good (as assessed using R-AMSTAR) |
Robinson et al., 2014 Footnote 42 Funding: CF Foundation, USA. |
Systematic review of randomized controlled trials 1995-2014 PVZ prophylaxis versus placebo or no intervention |
Infants and children (up to 18 years of age) with a diagnosis of CF (N=186) |
For systematic reviews without meta-analysis, the individual studies meeting the inclusion criteria of the INESSS systematic literature review are presented separately in this table, by author. Therefore, please see elsewhere in this table for the findings from the following study: · Cohen et al., 2005 |
No rating under NACI methods |
Good (as assessed using R-AMSTAR) |
Checchia et al., 2011 Footnote 45 Funded by MedImmune |
Systematic review with meta-analysis of RCT and observational studies 1990–2007 PVZ prophylaxis versus placebo or no intervention |
Children born at ≤35 wGA or with CLD or hsCHD (N≈15,000) |
Effectiveness of PVZ prophylaxis versus placebo or no intervention:
*The eight studies included in the meta-analysis were Mitchell et al., 2006; Grimaldi et al., 2004; Henckel et al., 2004; Perez et al., 2004; Wegner et al., 2004; Pedraz et al., 2003; IMpact-RSV, 1998; and Subramanian et al., 1998 Premature infants without CLD: Effectiveness of PVZ prophylaxis compared to placebo or no intervention
**The three studies included in the meta-analysis were Henckel et al., 2004; Pedraz et al., 2003; and IMpact-RSV, 1998 † The two studies included in the meta-analysis were Wegner et al., 2004 and IMpact-RSV, 1998 ¥ The three studies included in the meta-analysis were Wegner et al., 2004; Pedraz et al., 2003; and IMpact-RSV, 1998 + The three studies included in the meta-analysis were Kasuda et al., 2006; Wegner et al., 2004; and IMpact-RSV, 1998 |
No rating under NACI methods |
Average (as assessed using R-AMSTAR) |
Homaira et al., 2014 Footnote 64 Funding not stated |
Systematic review of observational studies 1999-2013 PVZ prophylaxis versus placebo or no intervention |
Infants and children (≤2 years of age) at high risk for severe RSV disease due to prematurity and/or any chronic congenital conditions (N=89,469) |
For systematic reviews without meta-analysis, the individual studies meeting the inclusion criteria of the INESSS systematic literature review are presented separately in this table, by author. Therefore, please see elsewhere in this table for the findings from the following studies:
(data from the systematic review not used) |
No rating under NACI methods |
Average (as assessed using R-AMSTAR) |
Morris et al., 2009 Footnote 65 Funding not stated |
Systematic review with meta-analysis of RCT 1990 to 2009 PVZ prophylaxis versus placebo or no intervention; RSV immunoglobulin versus placebo or no intervention |
Children <48 months of age with elevated risk of severe RSV infection |
The three randomized controlled trials of PVZ versus placebo (Feltes, 2003; IMpact-RSV, 1998; Subramanian, 1998) identified in this systematic review were included in the meta-analysis of Andabaka et al. As the Andabaka et al. study was more recent and of higher methodological quality, only Andabaka was considered in the INESSS literature review. |
No rating under NACI methods |
Average (as assessed using R-AMSTAR) |
Pons et al., 2010 Footnote 66 Supported by the Spanish National Health System |
Systematic review with meta-analysis of RCT 1990-2009 PVZ prophylaxis and other immunoprophylaxes (immunoglobulin, motavizumab) vs. placebo |
Children presenting an elevated risk of contracting an RSV infection |
The meta-analysis combined results from trials of PVZ and other immunoprophylaxes (immunoglobulin, motavizumab) in deriving overall effect estimates for outcomes. Therefore, the results were not considered in the INESSS literature review. The three randomized controlled trials of PVZ versus placebo (Feltes, 2003; IMpact-RSV, 1998; Subramanian, 1998) were captured in the systematic review and meta-analysis of Andabaka et al. |
No rating under NACI methods |
Average (as assessed using R-AMSTAR) |
Wegzyn et al., 2014 Footnote 67 Funding source: AbbVie Inc |
Systematic review of RCT and prospective observational studies 1996-2013 PVZ prophylaxis versus placebo or no intervention |
Children born at ≤35 wGA with CLD or hsCHD (N ≈ 42,000) |
For systematic reviews without meta-analysis, the individual studies meeting the inclusion criteria of the INESSS systematic literature review are presented separately in this table, by author. Therefore, please see elsewhere in this table for the findings from the following studies:
(data from the systematic review not used) |
No rating under NACI methods |
Average (as assessed using R-AMSTAR) |
Individual studies |
|||||
Feltes et al., 2003 Footnote 56 Supported by MedImmune |
RCT Multinational multicenter Canada, US, Switzerland, Germany, Poland, France, UK (76 sites) Four RSV seasons (1998–2002) PVZ prophylaxis versus placebo PVZ 15 mg/kg every 30 days; total of 5 doses) RSV diagnosed by antigen detection |
Children with hsCHD and ≤24 months of age at the start of the RSV season PVZ recipients (n=639) Placebo recipients (n=648) |
Effectiveness of PVZ prophylaxis compared to placebo
|
Level I |
Good (as assessed using CASP) |
IMpact-RSV, 1998 Footnote 38 Contributions from MedImmune |
RCT (Phase III study) Multinational, multicenter Canada, UK, US Sites: Canada: 9, UK: 11, US: 119 One RSV season (1996–1997) PVZ prophylaxis versus placebo PVZ 15 mg/kg every 30 days; total of 5 doses) RSV diagnosed by antigen test |
Children born at ≤35 wGA AND who were ≤6 months of age at the start of the RSV season OR who were ≤24 months at start of the RSV season and had a diagnosis of BPD and who received steroids, bronchodilators, diuretics or supplementary oxygen in previous 6 months PVZ recipients (n=1002) Placebo recipients (n=500) |
Premature infants with or without CLD Effectiveness of PVZ versus placebo
Premature infants with CLD Effectiveness of PVZ versus placebo
Premature infants without CLD Effectiveness of PVZ versus placebo
Premature infants by GA (with or without BPD) Effectiveness of PVZ versus placebo
|
Level I |
Good (as assessed using CASP) |
Blanken et al., 2013 Footnote 49 Funding: Abbott Laboratories |
RCT Multicentre Netherlands 15 sites Two RSV seasons (2008–2010) PVZ versus placebo (15 mg/kg every 30 days; total of 5 doses) RSV diagnosis determined by RT-PCR |
Children born at 33–35 wGA without CLD, and six months of age at start of the RSV season PVZ recipients (n=214) Placebo recipients (n=215) |
Effectiveness of PVZ prophylaxis compared to placebo
|
Level I |
Average (as assessed using CASP) |
Tavsu et al., 2014 Footnote 48 Funding not stated |
RCT Single center Turkey Two RSV seasons (2009–2010, 2010–2011) PVZ prophylaxis compared to no intervention PVZ 15 mg/kg every 30 days; total of 5 doses) Nasal swabs tested by Respi-Strips |
Children born at ≤ 32 wGA without CLD or hsCHD (Born at <28 wGA and aged <12 months at the start of RSV season OR born at 29–32 wGA and aged <6 months at the start of RSV season) PVZ prophylaxis (recipients (n=39) No intervention recipients (n=41) |
Effectiveness of PVZ prophylaxis compared to no intervention RSV-associated hospitalizations
No difference in growth or development at age 18 months |
Level I |
Average (as assessed using CASP) |
Cohen et al., 2005 Footnote 43 Industry supported (source not specified) |
RCT Multicenter United States (40 sites) 1998-2001 PVZ prophylaxis versus placebo 15 mg/kg every 30 days; total of 5 doses Method of RSV testing not stated |
Infants and children aged ≤ 24 months with a diagnosis of CF (n=186) PVZ recipients (n=92) Placebo recipients (n=94) Mean age: 12.8 (0.4–24.4) months |
Effectiveness of PVZ prophylaxis compared to placebo
|
Level I |
Not assessed in the INESSS literature review* *The study was not assessed for quality as it was published only in abstract form |
Wegner et al., 2004 Footnote 50 Funding: AccessCare and the Agency for Healthcare Research and Quality's University of North Carolina Center for Education and Research on Therapeutics |
Historical cohort Multicenter North Carolina Medicaid Program cohort 2002- 2003 PVZ prophylaxis versus no intervention PVZ monthly injections, up to 6 doses during RSV season RSV diagnosed by rapid antigen test |
Children born at 32–35 wGA without CLD PVZ recipients (n=185) No intervention (n=182) |
Effectiveness of PVZ prophylaxis versus no intervention RSV-associated hospitalizations
*multivariate logistic regression controlled for possible confounding factors |
Level II-2 |
Good (as assessed using CASP) |
Simoes et al., 2007 Footnote 54 Funded by Abbott Laboratories |
Inception cohort study Multinational, multicenter Canada, Germany, Netherlands, Poland, Spain, Switzerland (27 sites) 2001-2002 PVZ prophylaxis versus no intervention PVZ dosing not stated Method of RSV diagnosis not stated (RSV infection not an outcome) |
Children born at ≤35 wGA without chronic lung disease or CHD PVZ recipients (n=191) No intervention (n=230) |
Effectiveness of PVZ prophylaxis versus no intervention Recurrent wheezing in the 24 months following enrollment
|
Level II-2 |
Average (as assessed using CASP) |
Winterstein et al., 2013 Footnote 51 Funding: Florida Agency of Healthcare Administration |
Historical cohort study Multicenter United States Medicaid billing data (Florida, Texas) 1999–2004 PVZ prophylaxis versus no intervention PVZ dosing not stated RSV infection diagnosed by ICD-9 codes |
Children born at 32–34 wGA with no CLD, hsCHD, CF or immunosuppression Florida PVZ prophylaxis recipients (n=461) No intervention (n=1853) Texas PVZ prophylaxis recipients (n=671) No intervention (n=3015) |
Effectiveness of PVZ prophylaxis compared to no intervention on RSV-associated hospitalizations
|
Level II-2 |
Average (as assessed using CASP) |
Banerji et al., 2014 Footnote 58 Funding not stated |
Cohort study Multicenter Nunavut, Canada (number of sites not stated) Two RSV seasons (2009, 2010) PVZ prophylaxis compared to no intervention PVZ 15 mg/kg, first two doses 3 weeks apart, then every 4 weeks for duration of RSV season RSV diagnosed by EIA or RT-PCR |
Children born at <36 wGA or with CLD or hsCHD and <6 months of age at the start of the RSV season PVZ prophylaxis recipients (n=91) No intervention (n=10) |
Effectiveness of PVZ prophylaxis compared to no intervention RSV-associated hospitalizations
|
Level II-2 |
Poor (as assessed using CASP) |
Giebels et al., 2008 Footnote 61 Funding not stated |
Historical cohort study Single centre Quebec Single, tertiary care centre CF clinic 1997–2005 PVZ prophylaxis compared to no intervention PVZ dosage not stated RSV diagnosed by ELISA or viral culture |
Children born between 1997 and 2005 and diagnosed with CF before 18 months of age PVZ recipients (n=35) No intervention (n=40) |
Effectiveness of PVZ prophylaxis versus no intervention
|
Level II-2 |
Poor (as assessed using CASP) Few admissions, limited testing for RSV: No intervention group: 7 hospitalized for respiratory illness, 4 tested for RSV, 3 positive. PVZ: 3 hospitalized, none tested for RSV |
Grimaldi et al., 2004 Footnote 55 Supported by the Burgundy Regional Hospitalization Agency |
Inception cohort (compared to historical cohort) Multicenter France (12 sites) Three RSV seasons (1999–2000, 2000–2001, 2001–2002) PVZ prophylaxis compared to no intervention PVZ 15 mg/kg per dose RSV diagnosed by ELISA or rapid IFA antigen test |
Children born at ≤32 wGA with BPD and ≤6 months of age at the start of the RSV season PVZ recipients (2000–2001 and 2001–2002 immunoprophylaxis program) (n=43) No intervention recipients (1999–2000, before immunoprophylaxis program) (n=26) |
Effectiveness of PVZ prophylaxis versus no intervention
|
Level II-2 |
Poor (as assessed using CASP) |
Grimaldi et al., 2007 Footnote 52 Supported by The Burgundy Regional Hospitalization Agency |
Inception cohort (compared to historical cohort) Multicenter France (12 sites) Five RSV seasons (1999–2000, 2000–2001, 2001–2002, 2002–2003, 2003–2004) PVZ prophylaxis compared to no intervention PVZ 15 mg/kg per dose RSV diagnosed by ELISA or rapid IFA antigen test |
Children born at ≤30 wGA without CLD PVZ recipients (2002–2003 and 2003–2004 immunoprophylaxis program) (n=88) No intervention recipients (1999–2000, 2000–2001, and 2001–2002, before immunoprophylaxis program) (n=118) |
Effectiveness of PVZ prophylaxis versus no intervention
|
Level II-2 |
Poor (as assessed using CASP) |
Harris et al., 2011 Footnote 57 Honourarium of <$1000 from Abbott Laboratories. |
Inception cohort (compared to historical cohort) Single center British Columbia 1998-2007 PVZ vs. no prophylaxis PVZ dosing not stated Method of RSV diagnosis not stated |
Children born at ≤36 wGA with hsCHD and <24 months of age at the start of the RSV season PVZ recipients (2003–2007) (n=292) No intervention (1998–2003) (n=412) |
Effectiveness of PVZ prophylaxis versus no intervention
|
Level II-2 |
Poor (as assessed using CASP) |
Mitchell et al., 2006 Footnote 46 Sponsor: Abbott Laboratories |
Historical cohort study Single center Calgary, Canada 1995–2002 PVZ prophylaxis versus no intervention PVZ dosing not stated RSV infection diagnosed by ICD-9 codes |
High-risk Children born at <33 wGA OR Children born at 33–35 wGA with CLD OR Children born at 33–35 wGA and requiring at-home oxygen therapy AND ≤6 months of age at the start of the RSV season PVZ recipients (1999–2002) (n=411) No intervention (1995–1998) (n=496) |
High risk (<33 wGA or 33-35 wGA with CLD Effectiveness of PVZ prophylaxis versus no intervention RSV-associated hospitalizations
|
Level II-2 |
Poor (as assessed using CASP) |
Singleton et al., 2003 Footnote 59 No funding reported |
Historical cohort study Multicenter Alaska, USA (Yukon-Kuskokwim -Delta region) 1993-2001 PVZ prophylaxis versus no intervention PVZ dosing not stated RSV diagnosed by enzyme immune assay or virus culture |
Children born <36 wGA and children >36 wGA and age <1 year Before PVZ prophylaxis program, 1993-1996: Births: <36 wGA n = 41 >36 wGA n =1740 After PVZ prophylaxis program, 1998–2001: Births: <36 wGA n = 60 >36 wGA n= 1805 |
Effectiveness of PVZ prophylaxis compared to no intervention RSV-associated hospitalizations
|
Level II-2 |
Poor (as assessed using CASP) |
Winterstein et al., 2013 Footnote 62 Funding: Florida Agency of Healthcare Administration |
Historical cohort Multicenter United States (27 states, Medicaid programs) 1999–2006 PVZ prophylaxis versus no intervention PVZ dosing not stated RSV infection diagnosed by ICD-9 codes |
Children under 24 months of age diagnosed with CF PVZ prophylaxis recipients (n=575) No intervention (n=2300) |
Effectiveness of PVZ prophylaxis versus no intervention RSV-associated hospitalizations
*Cox regression modelling adjusted for possible confounding variables |
Level II-2 |
Poor (as assessed using CASP) |
Yi et al., 2014 Footnote 63 No external funding |
Inception cohort (registry) Multicenter Canada 32 sites The Netherlands (number of sites not stated) 2003-2012 PVZ prophylaxis versus no intervention RSV diagnostic test: EIA, RT-PCR or antigen test |
Children <24 months of age with Down syndrome in CARESS registry (2005–2012) (PVZ recipients, n=552) Children <24 months of age with Down syndrome in Dutch birth cohort registry (2003–2005) (No intervention, n=233) |
Effectiveness of PVZ prophylaxis versus no intervention
* adjusted for hsCHD, benign heart disease, GA and birth weight ** Risk factors that involve standard indications for RSV prophylaxis: any combination of hsCHD, CLD, prematurity (≤35 wGA) |
Level II-2 |
Poor (as assessed using CASP) |
Yoshihara et al., 2013 Footnote 53 Funded by Abbott Japan Co, Ltd |
Prospective cohort study Multicenter Japan 52 sites 2007–2008 PVZ prophylaxis versus no intervention PVZ dosing not stated RSV infection not an outcome |
Children born at 33–35 wGA without CLD PVZ prophylaxis recipients (n=345) No intervention (n=95) |
Effectiveness of PVZ prophylaxis versus no intervention Wheezing in the first two years of life
|
Level II-2 |
Poor (as assessed using CASP) |
Pedraz et al., 2003 Footnote 47 Funding: Abbott Laboratories |
Historical cohort Multicenter Spain (14-21 sites) 1998–2002 PVZ prophylaxis versus no intervention PVZ dosing not stated RSV diagnostic test: ELISA or rapid immunofluorescence test |
Children born at ≤32 wGA, with or without CLD and ≤6 months of age at the start of the RSV season PVZ recipients (2000–2002) (n=1919) No intervention (1998–2000, before immunoprophylaxis program) (n=1583) |
Whole Cohort by GA: Effectiveness of PVZ prophylaxis versus no intervention
Premature infants with CLD Effectiveness of PVZ prophylaxis versus no intervention
|
Level II-2 |
Very Poor (as assessed using CASP) |
Abbreviations: BPD: bronchopulmonary dysplasia; CI: confidence interval; CF: cystic fibrosis; ELISA: enzyme-linked immunosorbent assay; GA: gestational age; hsCHD: hemodynamically significant congenital heart disease; ICU: intensive care unit; IFA: immunofluorescence assay; INESSS: Institut national d'excellence en santé et en services sociaux; OR: odds ratio; PCR: polymerase chain reaction; PVZ: palivizumab; RCT: randomized controlled trial; RR: relative risk; RRR: relative risk reduction; RTI: respiratory tract infection; RT-PCR: reverse transcription PCR; UK: United Kingdom; US: United States; wGA: weeks of gestational age
Appendix G: NNT with PVZ to avoid one RSVH or intensive care admission, or recurrent wheezing
| Reference | Years of study | Population | To prevent | NNT * | 95% CI |
|---|---|---|---|---|---|
Mixed population |
|||||
IMpact 1998 Footnote 38 RCT † |
1996 |
≤ 35 wGA, age ≤ 6 m or BPD age ≤ 24 m |
RSVH |
18 |
11.3 - 35.7 |
Mitchell et el 2006 Footnote 46 |
1999-2002 |
<33 wGA or 33-35 wGA with CLD or discharged on O2 and age <6 m |
RSVH |
24 |
13.9 - 74.4 |
Pedraz et al. 2003 Footnote 47 |
1998-2002 |
≤ 28 wGA age <12 m without CLD |
RSVH |
14 |
8.3 - 32.4 |
Pedraz et al. 2003 Footnote 47 |
1998-2002 |
29-32 wGA <6 m without CLD |
RSVH |
14 |
10.8 - 18.1 |
Singleton et al. 2003 Footnote 59 |
1993-2001 |
≤ 32 wGA, 32-35 wGA with chronic disease, ≤ 36 wGA with signif. respiratory disease in newborn period, 1 RSV season. Alaska |
RSVH |
2.1 - 8.9 |
|
Prais et al. 2016 Footnote 21 |
2000-2003 |
<29 wGA +/- BPD |
RSVH in first 2 years of life |
2 |
1.4 - 3.1 |
Chi et al. 2014 Footnote 22 |
2011-2013 |
≤ 28 wGA or ≤ 35 wGA with CLD, Matched by propensity score |
RSVH by 6 m |
10 |
6.2 - 23.7 |
Lee et al. 2018 Footnote 30 |
2010-2014 |
<27 w GA +/- BPD |
RSVH |
4 |
2.2 - 29 |
| 1. Premature without CLD | 2. | 3. | 4. | 5. 6. 7. | |
(Notario et al. 2014 Footnote 37) |
1996 |
28-31 wGA |
RSVH |
21 |
10 - ‡ |
(Notario Footnote 37) RCT |
1996 |
29-32 wGA |
RSVH |
17 |
8.9 - 93.6 |
Tavsu et al. 2014 Footnote 48 RCT |
2009-2011 |
≤ 28 wGA age <12 m or 29-32 wGA age <6 m, otherwise healthy |
RSVH |
5 |
2.7 - 8.9 |
Farber et al. 2016 Footnote 29 |
2012-2014 |
29-32 wGA w/o chronic illness |
RSVH |
54 |
27.9 - 547.6 |
(Notario Footnote 37) RCT |
1996 |
29-33 wGA |
RSVH |
14 |
8.3 - 43.6 |
Grimaldi et al. 2007 Footnote 52 |
2000-2004 |
≤ 30 wGA w/o BPD |
RSVH |
5.3 - 17.1 |
|
(Notario Footnote 37) RCT |
1996 |
32-34 wGA |
RSVH |
12 |
6.6 - 39.8 |
IMpact 1998 Footnote 38 RCT |
1996 |
32-35 wGA and ≤ 6 m old w/o BPD |
RSVH |
22 |
10.8 - 1246.3 |
(Notario Footnote 37) RCT |
1996 |
32-35 wGA |
RSVH |
13 |
7 - 42.4 |
Blanken et al. 2013 Footnote 49 RCT |
2008-2010 |
33-35 wk <6 m otherwise healthy |
RSVH |
24 |
13.5 - 103.4 |
IMpact 1998 Footnote 38 RCT |
1996 |
≤35 wGA and ≤ 6 m old w/o BPD |
RSVH |
16 |
10 - 37.6 |
CLD |
|||||
IMpact 1998 Footnote 38 RCT |
1996 |
CLD ≤ 24 m |
RSVH |
21 |
10.4 - 385.2 |
Pedraz et al. 2003 Footnote 47 |
1998-2002 |
CLD <24 m |
RSVH |
8 |
4.2 - 22.5 |
Grimaldi et al. 2004 Footnote 55 |
1999-2002 |
CLD wGA ≤ 32 age <6 m |
RSVH |
3 |
1.7 - 5.7 |
Chi et al. 2014 Footnote 22 |
2011-2013 |
CLD ≤ 35 wGA RSV age 6 m |
RSVH by 6 m |
13 |
7 - 68.4 |
Chi et al. 2014 Footnote 22 |
2011-2013 |
CLD ≤ 28 wGA RSV age 6 m |
RSVH by 6 m |
12 |
6.3 - 56.6 |
CHD |
|||||
Feltes et al. 2003 Footnote 56 RCT |
1998-2002 |
CHD <age 24 m |
RSVH |
23 |
13.8 - 65.3 |
Feltes et al. 2003 Footnote 56 RCT |
1998-2002 |
Non-cyanotic CHD < age 24 m |
RSVH |
15 |
8.9 - 41.3 |
Chiu et al. 2018 Footnote 33 |
2010-16 |
hsCHD < age 1 yr |
RSVH |
45 |
23.6 - 327.2 |
Chiu et al. 2018 Footnote 33 |
2010-16 |
Cyanotic CHD < age 1 yr |
RSVH |
31 |
16 - 260.1 |
Soraiz et al. 2017 Footnote 34 |
1997- 2016 |
hsCHD age <1 yr |
RSVH |
3.7 - 61.5 |
|
| Down Syndrome | |||||
Yi et al. 2014 Footnote 63 |
2003-2012 |
Down syndrome |
RSVH |
12 |
8.1 - 22.7 |
| CF | |||||
Groves et al. 2016 Footnote 24 |
1997-2007 |
CF (PVZ first yr of life) |
RSVH |
6 |
3.3 - 27.2 |
| OUTCOME: ICU ADMISSION | |||||
IMpact 1998 Footnote 38 RCT |
1996 |
≤ 35 wGA, ≤ 6 m old or BPD ≤ 24 m old |
ICU for RSV |
59 |
29.8 - 1948.2 |
Chi et al. 2014 Footnote 22 |
2011-2013 |
≤ 28 wGA or ≤ 35 wGA with CLD Matched by propensity score |
ICU for RSV by 6 m |
16 |
9.1 - 63.3 |
| OUTCOME: RECURRENT WHEEZE | |||||
Simoes et al. 2007 Footnote 54 |
2001-2002 |
≤ 35 wGA w/o CLD or CHD |
Recurrent wheeze within 2 yrs from PVZ |
13 |
7 - 46.7 |
Simoes et al. 2007 Footnote 54 |
2001-2002 |
32-35 wGA |
Recurrent wheeze within 2 yrs from PVZ |
8 |
5 - 17.2 |
Simoes et al. 2007 Footnote 54 |
2001-2002 |
29-32 wGA |
Recurrent wheeze within 2 yrs from PVZ |
10 |
5.3 - 31.7 |
Simoes et al. 2007 Footnote 54 |
2001-2002 |
<29 wGA |
Recurrent wheeze within 2 yrs from PVZ |
15 |
4.1 - ‡ |
Simoes et al. 2010 Footnote 28 |
Not stated |
<36 wGA with no F.H. asthma |
Recurrent wheeze age 2-5 yr |
14 |
7.3 - 117.1 |
Simoes et al. 2010 Footnote 28 |
Not stated |
<36 wGA with no F.H. atopy |
Recurrent wheeze age 2-5 yr |
10 |
5.2 - 36.9 |
Prais et al. 2016 Footnote 21 |
2000-2003 |
<29 wks +/- BPD |
Recurrent wheeze 1 2 yr of life |
3 |
1.5 - 4.8 |
Blanken et al. 2013 Footnote 49 RCT |
2008-2010 |
33-35 wGA age <6 m otherwise healthy |
Recurrent wheeze in first year of life |
11 |
6 - 35.4 |
Yoshihara et al. 2013 Footnote 53 |
2007-2008 |
33-35 wGA without CLD |
Recurrent wheeze age 3 yr |
8 |
4.8 - 23.4 |
Mochizuki et al. 2017 Footnote 27 |
2007-2008 |
33-35 wGA without CLD |
Recurrent wheeze age 6 yr |
7 |
3.6 - 19.2 |
* NNT = number needed to treat (aRR = current event rate – expected event rate; NNT = 1/ aRR x 100).
† RCT = randomized controlled trial. All not labelled RCT are observational cohort studies.
‡ 95% CI for aRR extends from a negative number to a positive number.
Footnote 1 Author's calculation: NNT = 3.4.
Footnote 2 Author's calculation: NNT = 6.
Footnote 3 Author's calculation: NNT = 7.
Studies or study groups where no significant PVZ effect was found are not included in this table.
References
- Footnote 1
-
American Academy of Pediatrics Committee on Infectious Diseases; American Academy of Pediatrics Bronchiolitis Guidelines Committee. Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics. 2014 Aug;134(2):415-20.
- Footnote 2
-
American Academy of Pediatrics Committee on Infectious Diseases; American Academy of Pediatrics Bronchiolitis Guidelines Committee. Technical report. Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics 2014 Aug;134(2):e620-38.
- Footnote 3
-
Robinson JL, Le Saux N, Canadian Paediatric Society Infectious Diseases and Immunization Committee. Preventing hospitalizations for respiratory syncytial virus. Paediatr Child Health 2015;20(6):321.26
- Footnote 4
-
Breton M, Rossignol M, Tardif M, et al. Effect of Palivizumab Prophylaxis on the Reduction of Complications Associated with Respiratory Syncytial Virus in Infants: Systematic Review. Institut national d'excellence en santé et en services sociaux (INESSS). 2017. 65p. https://www.inesss.qc.ca/fileadmin/doc/INESSS/Rapports/Medicaments/INESSS_Systematic_review_Synagis_EN.pdf
- Footnote 5
-
Bont L, Checchia PA, Fauroux B et al. Defining the epidemiology and burden of severe respiratory syncytial virus infection among infants and children in western countries. Infect Dis Ther (2016) 5:271–298
- Footnote 6
-
Shi T, McAllister DA, O'Brien KL et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet. 2017 Sep 2;390(10098):946-958
- Footnote 7
-
Government of Respiratory virus detections in canada.. 2014-2019:2020. https://www.canada.ca/en/public-health/services/surveillance/respiratory-virus-detections-canada.html
- Footnote 8
-
Butt ML, Symington M, Janes M, Elliott L, Steele S, Paes BA. The impact of prophylaxis on paediatric intensive care unit admissions for RSV infection: a retrospective, single-centre study. Eur J Pediatr (2011) 170:907–913.
- Footnote 9
-
Schanzer DL, Langley JM, Tam TWS. Hospitalization attributable to influenza and other viral respiratory illnesses in Canadian children Pediatr Infect Dis J 2006;25:795–800.
- Footnote 10
-
Pisesky A, Benchimol EI, Wong CA et al. Incidence of hospitalization for respiratory syncytial virus infection amongst children in Ontario, Canada: A population-based study using validated health administrative data. PLOS ONE | DOI:10.1371/journal.pone.0150416 March 9, 2016. http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0150416.
- Footnote 11
-
Scheltema NM, Gentile A, Lucion F et al. Global respiratory syncytial virus-associated mortality in young children (RSV GOLD): a retrospective case series. Lancet Glob Health. 2017 Oct;5(10):e984-e991.
- Footnote 12
-
Tam J. Papenburg J, Fanella S, Asner S,Barton, M, Bergeron C, Desai S et al. Pediatric Investigators Collaborative Network on Infections in Canada study of respiratory syncytial virus–associated deaths in pediatric patients in Canada, 2003–2013. Clin Infect Dis 2019;68(1):113–9.
- Footnote 13
-
National Advisory Committee on Immunization. Statement on the recommended use of monoclonal anti-RSV antibody (palivizumab). CCDR 2003;29.ACS-7,8.
- Footnote 14
-
Shea BJ, Grimshaw JM, Wells GA et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Medical Research Methodology 2007, 7:10 https://www.ncbi.nlm.nih.gov/pubmed/17302989 accessed Jan 2018.
- Footnote 15
-
Critical Appraisal Skills Programme. CASP checklists. http://www.casp-uk.net/#!casp-tools-checklists/c18f8.
- Footnote 16
-
Harris RP, Helfand M, Woolf SH, et al. Current methods of the US Preventive Services Task Force: a review of the process. Am J Prev Med. 2001;20(3S):21-35.
- Footnote 17
-
Robinson KA, Odelola OA, Saldanha IJ. Palivizumab for prophylaxis against respiratory syncytial virus infection in children with cystic fibrosis. Cochrane Database of Systematic Reviews 2016, Issue 7. Art. No.: CD007743. DOI: 10.1002/14651858.CD007743.pub6.
- Footnote 18
-
Kua KP, Lee SWH. Systematic review of the safety and efficacy of palivizumab among infants and young children with cystic fibrosis. Pharmacotherapy 2017;37(6):755–769.
- Footnote 19
-
Simoes EAF, Bont L, Manzoni P, et al. Past, present and future approaches to the prevention and treatment of respiratory syncytial virus infection in children. Infect Dis Ther 2018;7:87–120
- Footnote 20
-
Blake SM, Tanaka D, Bendz LM, et al. Evaluation of the financial and health burden of infants at risk for respiratory syncytial virus. Advances in Neonatal Care 2017;17(4):292-298.
- Footnote 21
-
Prais D, Kaplan E, Klinger G, et al. Short- and long-term pulmonary outcome of palivizumab in children born extremely prematurely. Chest 2016;149(3): 802-8.
- Footnote 22
-
Chi H, Hsu CH, Chang JH, et al. A novel six consecutive monthly doses of palivizumab prophylaxis protocol for the prevention of respiratory syncytial virus infection in high-risk preterm infants in Taiwan. PLOS ONE 2014; 9(6):e100981.
- Footnote 23
-
Narbona-Lopez E, Uberos J, Checa-Ros A, et al. Prevention of syncytial respiratory virus infection with palivizumab: descriptive and comparative analysis after 12 years of use. Minerva Pediatrica 2018;70(6):513-8.
- Footnote 24
-
Groves HE, Jenkins L, Macfarlane M, et al. Efficacy and long-term outcomes of palivizumab prophylaxis to prevent respiratory syncytial virus infection in infants with cystic fibrosis in Northern Ireland. Pediatr Pulmon 2016;51:379–385.
- Footnote 25
-
BjornsonC, Chan P, Li A, et al. Palivizumab prophylaxis for respiratory syncytial virus in infants with cystic fibrosis: is there a need? Eur J Clin Microbiol Infect Dis. 2018 Jun;37(6):1113-1118.
- Footnote 26
-
Fink AK, Graff G, Byington CL, et al. Palivizumab and long-term outcomes in cystic fibrosis. Pediatrics 2019;144(1):e20183495.
- Footnote 27
-
Mochizuki H, Kusuda S, Okada K, et al, on behalf of the Scientific Committee for Elucidation of Infantile Asthma. Palivizumab prophylaxis in preterm infants and subsequent recurrent wheezing. Six-year follow-up study. Am J Respir Crit Care Med 2017;196 (1):29–38
- Footnote 28
-
Simoes EAF, Carbonell-Estrany X, Rieger CHL, et al, on behalf of the Palivizumab Long-Term Respiratory Outcomes Study Group. The effect of respiratory syncytial virus on subsequent recurrent wheezing in atopic and nonatopic children. J Allergy Clin Immunol 2010;126:256-62.
- Footnote 29
-
Farber HJ, Buckwold FJ, Lachman B, et al. Observed effectiveness of palivizumab for 29–36-week gestation infants. 2016;138(2):e20160627.
Lee S-YR, Kwok KL, Ng DKK, Hon KL. Palivizumab for infants < 29 weeks in Hong Kong without a clear-cut season for respiratory syncytial virus infection—A cost-effectiveness analysis. J Trop Pediatr 2018;64:418–425.
- Footnote 30
-
Lee S-YR, Kwok KL, Ng DKK, Hon KL. Palivizumab for infants < 29 weeks in Hong Kong without a clear-cut season for respiratory syncytial virus infection—A cost-effectiveness analysis. J Trop Pediatr 2018;64:418–425.
- Footnote 31
-
Lacaze-Masmonteil T, Truffert P, Pinquier D, et al. Lower respiratory tract illness and RSV prophylaxis in very premature infants. Arch Dis Child 2004;89:562–567.
- Footnote 32
-
Priante E, Tavella E, Girardi E, et al. Restricted palivizumab recommendations and the impact on RSV hospitalizations among infants born at > 29 weeks of gestational age: An Italian multicenter study. Am J Perinatol 2019;36(suppl S2):S77–S82.
- Footnote 33
-
ChiuSN, Wang JN, Fu YC, et al. Efficacy of a novel palivizumab prophylaxis protocol for respiratory syncytial virus infection in congenital heart disease: A multicenter study. J Pediatr 2018;195:108-14.
- Footnote 34
-
Soraiz MG, Andrés SB, Castro SB, et al. Palivizumab in infants less than 1 year with hemodynamically significant congenital heart disease in Argentina. A comparative study with historical control group. Cardiology in the Young: 2017 Supplement 4, WCPCCS 2017 Abstract P2182 -S165-6
- Footnote 35
-
Sánchez-Luna M, Medrano C, Lirio J, on behalf of the RISK-21 Study Group. Down syndrome as risk factor for respiratory syncytial virus hospitalization: A prospective multicenter epidemiological study. Influenza Other Respi Viruses 2017; 11: 157–164.
- Footnote 36
-
Kimura T, Takeuchi M, Kawakami K. Utilization and efficacy of palivizumab for children with Down syndrome. Pediatrics International 2020; 62:677–682.
- Footnote 37
-
Notario G, Vo P, Gooch K, et al. Respiratory syncytial virus-related hospitalization in premature infants without bronchopulmonary dysplasia: subgroup efficacy analysis of the IMpact-RSV trial by gestational age group. Pediatr Health Med Therapeut 2014:5:43–48.
- Footnote 38
-
IMpact-RSV. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. The IMpact-RSV Study Group. Pediatrics 1998;102(3):531-7.
- Footnote 39
-
Scheltema NM, Nibbelke EE, Pouw J, et al. Respiratory syncytial virus prevention and asthma in healthy preterm infants: a randomised controlled trial Lancet Respir Med 2018; 6: 257–64.
- Footnote 40
-
Anderson EJ, Carosone-Link P, Yogev R, Yi J, Simoes EAF. Effectiveness of palivizumab in high-risk infants and children. A propensity score weighted regression analysis. Pediatr Infect Dis J 2017;36 (8):699-704.
- Footnote 41
-
Buchs C, Dalphin M-L, Sanchez S, et al. Palivizumab prophylaxis in infants with cystic fibrosis does not delay first isolation of Pseudomonas aeruginosa or Staphylococcus aureus. Eur J Pediatr 2017;176:891–897.
- Footnote 42
-
Robinson KA, Odelola OA, Saldanha IJ. Palivizumab for prophylaxis against respiratory syncytial virus infection in children with cystic fibrosis. Cochrane Database Syst Rev 2014;22(5):CD007743
- Footnote 43
-
Cohen AH, Boron ML, Dingivan C. A phase IV study of the safety of Synagis®(Palivizumab) for prophylaxis of respiratory syncytial virus disease in children with cystic fibrosis [Poster presented at the American Thoracic Society International Conference, May 20-25, 2005 in San Diego, CA]. 2005.
- Footnote 44
-
Andabaka T et Rojas-Reyes MX. Cochrane in context: Monoclonal antibody for reducing the risk of respiratory syncytial virus infection in children. Evid Based Child Health 2013;8(6):2377-9.
- Footnote 45
-
Checchia PA, Nalysnyk L, Fernandes AW, et al. Mortality and morbidity among infants at high risk for severe respiratory syncytial virus infection receiving prophylaxis with palivizumab: A systematic literature review and meta-analysis. Pediatr Crit Care Med 2011;12(5):580-8.
- Footnote 46
-
Mitchell I, Tough S, Gillis L, Majaesic C. Beyond randomized controlled trials: A « real life » experience of respiratory syncytial virus infection prevention in infancy with and without palivizumab. Pediatr Pulmonol 2006;41(12):1167-74.
- Footnote 47
-
Pedraz C, Carbonell-Estrany X, Figueras-Aloy J, Quero J. Effect of palivizumab prophylaxis in decreasing respiratory syncytial virus hospitalizations in premature infants. Pediatr Infect Dis J 2003;22(9):823-7.
- Footnote 48
-
Tavsu I, Gursoy T, Dirman S, Erbil N, Ovali F. Palivizumab prophylaxis: Does it have any influence on the growth and development of the infants? Am J Perinatol 2014;31(8):667-72.
- Footnote 49
-
Blanken MO, Rovers MM, Molenaar JM, et al. Respiratory syncytial virus and recurrent wheeze in healthy preterm infants. N Engl J Med 2013;368(19):1791-9.
- Footnote 50
-
Wegner S, Vann JJ, Liu G, et al. Direct cost analyses of palivizumab treatment in a cohort of at-risk children: Evidence from the North Carolina Medicaid Program. Pediatrics 2004;114(6):1612-9.
- Footnote 51
-
Winterstein AG, Knox CA, Kubilis P, Hampp C. Appropriateness of age thresholds for respiratory syncytial virus immunoprophylaxis in moderate-preterm infants: a cohort study. JAMA Pediatrics 2013;167 (12):1118-24.
- Footnote 52
-
Grimaldi M, Gouyon B, Sagot P, Quantin C, Huet F, Gouyon JB. Palivizumab efficacy in preterm infants with gestational age < or = 30 weeks without bronchopulmonary dysplasia. Pediatr Pulmonol 2007;42(3):189-92.
- Footnote 53
-
Yoshihara S, Kusuda S, Mochizuki H, Okada K, Nishima S, Simoes EA. Effect of palivizumab prophylaxis on subsequent recurrent wheezing in preterm infants. Pediatrics 2013;132(5):811-8.
- Footnote 54
-
Simoes EA, Groothuis JR, Carbonell-Estrany X, Rieger CH, Mitchell I, Fredrick LM, Kimpen JL. Palivizumab prophylaxis, respiratory syncytial virus, and subsequent recurrent wheezing. J Pediatr 2007;151(1):34-42, 42.e1
- Footnote 55
-
Grimaldi M, Gouyon B, Michaut F, Huet F, Gouyon JB. Severe respiratory syncytial virus bronchiolitis: epidemiologic variations associated with the initiation of palivizumab in severely premature infants with bronchopulmonary dysplasia. Pediatr Infect Dis J 2004;23(12):1081-5.
- Footnote 56
-
Feltes TF, Cabalka AK, Meissner HC, Piazza FM, Carlin DA, Top FH Jr, et al. Palivizumab prophylaxis reduces hospitalization due to respiratory syncytial virus in young children with hemodynamically significant congenital heart disease. J Pediatr 2003;143(4):532-40.
- Footnote 57
-
Harris KC, Anis AH, Crosby MC, Cender LM, Potts JE, Human DG. Economic evaluation of palivizumab in children with congenital heart disease: A Canadian perspective. Can J Cardiol 2011;27(4):523.e11-5.
- Footnote 58
-
Banerji A, Panzov V, Young M, Lee BE, Mamdani M, Giles BL, et al. The real-life effectiveness of palivizumab for reducing hospital admissions for respiratory syncytial virus in infants residing in Nunavut. Can Respir J 2014;21(3):185-9.
- Footnote 59
-
Singleton R, Dooley L, Bruden D, Raelson S, Butler JC. Impact of palivizumab prophylaxis on respiratory syncytial virus hospitalizations in high risk Alaska Native infants. Pediatr Infect Dis J 2003;22(6):540-5
- Footnote 60
-
Banerji A, Panzov V, Young M, Robinson J, Lee B. Moraes T, Mamdani M et al. Hospital admissions for lower respiratory tract infections among infants in the Canadian Arctic: a cohort study. CMAJ OPEN 2016: 4(4);E615-622. DOI:10.9778/cmajo.20150051
- Footnote 61
-
Giebels K, Marcotte JE, Podoba J, Rousseau C, Denis MH, Fauvel V, Laberge S. Prophylaxis against respiratory syncytial virus in young children with cystic fibrosis. Pediatr Pulmonol 2008;43(2):169-74.
- Footnote 62
-
Winterstein AG, Eworuke E, Xu D, Schuler P. Palivizumab immunoprophylaxis effectiveness in children with cystic fibrosis. Pediatr Pulmonol 2013;48(9):874-84.
- Footnote 63
-
Yi H, Lanctôt KL, Bont L, Bloemers BL, Weijerman M, Broers C, et al. Respiratory syncytial virus prophylaxis in Down syndrome: A prospective cohort study. Pediatrics 2014;133(6):1031-7
- Footnote 64
-
Homaira N, Rawlinson W, Snelling TL, Jaffe A. Effectiveness of palivizumab in preventing RSV hospitalization in high risk children: A real-world perspective. Int J Pediatr 2014;2014:571609.
- Footnote 65
-
Morris SK, Dzolganovski B, Beyene J, Sung L. A meta-analysis of the effect of antibody therapy for the prevention of severe respiratory syncytial virus infection. BMC Infect Dis 2009;9:106.
- Footnote 66
-
Pons JM, Tebe C, Paladio N, Garcia-Altes A, Danes I, Valls ISA. Meta-analysis of passive immunoprophylaxis in paediatric patients at risk of severe RSV infection. Acta Paediatr 2011;100(3):324-9.
- Footnote 67
-
Wegzyn C, Toh LK, Notario G, Biguenet S, Unnebrink K, Park C, et al. Safety and effectiveness of palivizumab in children at high risk of serious disease due to respiratory syncytial virus infection: A systematic review. Infect Dis Ther 2014;3(2):133-58.
