Archived - Laboratory exposures to human pathogens and toxins in Canada

 Download this article as a PDF (308 KB - 8 pages)
Download this article as a PDF (308 KB - 8 pages) Published by: The Public Health Agency of Canada
Issue: Volume 43-11: Antimicrobial resistance and One Health
Date published: November 2, 2017
ISSN: 1481-8531
Submit a manuscript
About CCDR
Browse
Volume 43-11, November 2, 2017: Antimicrobial resistance and One Health
Surveillance
Surveillance of laboratory exposures to human pathogens and toxins: Canada 2016
A Bienek1, M Heisz1, M Su1*
Affiliation
1 Centre for Biosecurity, Public Health Agency of Canada, Ottawa, ON
Correspondence
Suggested citation
Bienek A, Heisz M, Su M. Surveillance of laboratory exposures to human pathogens and toxins: Canada 2016. Can Commun Dis Rep. 2017;43(11):228-35. https://doi.org/10.14745/ccdr.v43i11a04
Abstract
Background: Canada recently enacted legislation to authorize the collection of data on laboratory incidents involving a biological agent. This is done by the Public Health Agency of Canada (PHAC) as part of a comprehensive national program that protects Canadians from the health and safety risks posed by human and terrestrial animal pathogens and toxins.
Objective: To describe the first year of data on laboratory exposure incidents and/or laboratory-acquired infections in Canada since the Human Pathogens and Toxins Regulations came into effect.
Methods: Incidents that occurred between January 1 and December 31, 2016 were self-reported by federally-regulated parties across Canada using a standardized form from the Laboratory Incident Notification Canada (LINC) surveillance system. Exposure incidents were described by sector, frequency of occurrence, timeliness of reporting, number of affected persons, human pathogens and toxins involved, causes and corrective actions taken. Microsoft Excel 2010 was used for basic descriptive analyses.
Results: In 2016, 46 exposure incidents were reported by holders of 835 active licences in Canada representing 1,352 physical areas approved for work involving a biological agent, for an overall incidence of 3.4%. The number of incidents was highest in the academic (n=16; 34.8%) and hospital (n=12; 26.1%) sectors, while the number of reported incidents was relatively low in the private industry sector. An average of four to five incidents occurred each month; the month of September presented as an outlier with 10 incidents. A total of 100 people were exposed, with no reports of secondary exposure. Four incidents led to suspected (n=3) or confirmed (n=1) cases of laboratory-acquired infection. Most incidents involved pathogens classified at a risk group 2 level that were manipulated in a containment level 2 laboratory (91.3%). Over 22 different species of human pathogens and toxins were implicated, with bacteria the most frequent (34.8%), followed by viruses (26.1%). Eleven (23.9%) incidents involved a security sensitive biologic agent. Procedure breaches (n=15) and sharps-related incidents (n=14) were the most common antecedents to an exposure. In 10 (21.7%) cases, inadvertent possession (i.e., isolation of an unexpected biological agent during routine work) played a role. Possible improvements to standard operating procedures were cited in 71.7% of incidents. Improvements were also indicated for communication (26.1%) and management (23.9%).
Conclusions: The Laboratory Incident Notification Canada is one of the first surveillance systems in the world to gather comprehensive data on laboratory incidents involving human pathogens and toxins. Exposure incidents reported in the first year were relatively rare, occurring in less than 4% of containment zones within laboratory settings.
Introduction
The study of biological agents in academic, veterinary, industry, and government laboratory settings has many benefits; it also poses an inherent risk of exposure due to the nature of the work and the pathogens and toxins involved. Internationally, this risk to human biosafety and biosecurity has led to injury, with accidents reported in the literature and by governmentsFootnote 1 Footnote 2 Footnote 3 Footnote 4. Albeit rare, deaths have also occurred Footnote 5 Footnote 6.
Currently, there are limited and variable international requirements governing the reporting of laboratory incidents involving biological agents. In Great Britain, as part of a larger reporting system, the Health and Safety Executive enforces the mandatory reporting of incidents that involve disease caused by biological agents in a wide range of workplaces (including academic, hospital and central and local government facilities)Footnote 7. In England, Wales and Northern Ireland, an active surveillance system was developed to capture occupational exposures, but only to specific blood-borne virusesFootnote 8. Otherwise, most reporting of laboratory-acquired infection incidents is voluntary in nature or captured through surveysFootnote 9 Footnote 10 Footnote 11.
Canada has one of the first comprehensive national surveillance systems, which gathers data from reports submitted in close to real time on incidents pertaining to a wide range of human and terrestrial animal pathogens and toxins used in laboratory-specific settings. The Laboratory Incident Notification Canada (LINC) surveillance system was officially launched in December 2015 in response to the assent of the Human Pathogens and Toxins Act (HPT Act) in 2009 and the enactment of the HPT Regulations in 2015, and as part of a larger comprehensive national biosafety and biosecurity program that protects the Canadian public from the health and safety risks posed by human pathogens and toxins (HPTs)Footnote 12 Footnote 13. An overview of the scope, licensing requirements for laboratories and mandate of the Public Health Agency of Canada (PHAC) in the regulation and monitoring of HPT use can be found elsewhereFootnote 14. See the Appendix for the definition of some commonly used terms.
Under Canada's HPT Act, pathogens (including bacteria, viruses, fungi, protozoa and prions) and toxins are classified into four risk groups based on the risk level presented to an individual (e.g., laboratory staff) and the community (i.e., the Canadian public)Footnote 15. Factors considered include the pathogenicity of the HPT, route of infection, mode of transmission, availability of treatment and/or preventive measures, host range, natural distribution and impact of release into the environmentFootnote 16. Work with risk group 1 pathogens is of lowest risk and is unregulated in Canada. Of the work under federal regulation, the majority is performed with risk group 2 pathogens (92.8%). These pathogens pose a moderate risk to individuals but low risk to public health, because they can cause serious disease in humans but are unlikely to do so. Work with risk group 3 pathogens currently represents 6.6% of all regulated work. These pathogens pose a high risk to individuals but a low risk to public health, because they are likely to cause serious disease but unlikely to spread. The remaining category (risk group 4, 0.2%), as well as a specialized category of security sensitive biological agents above a trigger quantity (0.5%), constitutes only a small proportion of the work in Canada using HPTs, but are of highest risk to health at both the individual- and population-level.
The Centre for Biosecurity at PHAC is mandated to oversee the ongoing surveillance of laboratory incidents involving HPTs. The data in the LINC surveillance system are provided by regulated parties across Canada who recognize that an incident has occurred and is reportable as per the HPT RegulationsFootnote 12 Footnote 13 Footnote 14 Footnote 15. Currently, four types of incidents are reportable:
- exposures and laboratory-acquired infections;
- inadvertent possession, production and/or release of an HPT;
- missing, lost, or stolen HPT, including a security sensitive biological agent not being received within 24 hours of expected arrival; and
- changes affecting biocontainment.
When an incident occurs, the licence holder must inform PHAC in a timely manner to ensure that the situation is managed appropriatelyFootnote 12 Footnote 13 Footnote 14 Footnote 15. For incidents involving an exposure and/or laboratory-acquired infection, the initial notification report is submitted 'without delay' to observe requirements for notification identified in the HPT Act.
The initial report provides only the immediate, essential elements related to the incident, including key dates, cause of exposure, affected persons and HPT(s) involved. A follow-up report is then expected within 15 days after the first notification for incidents involving security sensitive biological agents, or within 30 days after the first notification for all other exposures and/or laboratory-acquired infections. The aim of the follow-up report is to provide information on the investigation outcomes, including the treatment and monitoring of the affected person(s), root causes and corrective actions that aim to reduce the risk of future incidents. The licence holder or local Biological Safety Officer leads the response to the incident, with support from PHAC when required, until a satisfactory resolution is reached and the file is closed.
Standardized and systematic reporting documents exposures in a way that permits comparison between incidents and over time. Collective and active analysis of reported incidents allow for the identification of patterns or trends that highlight common or emerging issues at the national level. Using the data collected and housed in the LINC surveillance system, this study provides a descriptive summary and interpretation of the first full year of data collected relating to exposures and/or laboratory-acquired infections in Canada between January 1 and December 31, 2016.
Methods
The LINC surveillance system is the single window for electronic incident reporting. The system is housed within a customized Microsoft Dynamics Customer Relations Management system maintained and secured by in-house information technology support at PHAC. Most data fields are mandatory, and high specificity is obtained through the use of a standardized form. Data inputs in this surveillance system are self-reported; accuracy is validated through the ongoing investigatory process involving both PHAC and the reporter. If, during the course of the investigatory process, an incident is deemed to be outside the scope of requirements defined within the HPT Act, the incident is ruled out and is excluded from analysis.
Data on laboratory incidents involving exposure and/or laboratory-acquired infection (classified as 'exposures', 'suspected laboratory-acquired infection' or 'confirmed laboratory-acquired infection') that occurred in 2016 were extracted from the system. Data elements include licence information (number of licences, number of containment zones), sector (academic, hospital, private industry/business, public health, veterinary/animal health, environmental), key dates (incident date, initial notification date, follow-up report date), affected persons (number of primary affected, number of secondary affected), implicated HPTs (type, risk group level), cause of incident (procedure, sharps, personal protective equipment, animal, spill, insect, equipment, loss of containment) and areas for improvement (standard operating procedures, training, communications, management and oversight, equipment, human interaction). Microsoft Excel 2010 was used for basic descriptive analyses on categorical variables (counts, proportions) and continuous variables (mean, range). Because the breadth of information collected allows for the identification of the licenced facility, identifiable characteristics were suppressed when necessary. All data were reported, except in instances where there was a risk of identifying a specific incident and/or laboratory.
Results
In the 2016 calendar year, there were 835 active licences permitting the use of HPTs across Canada, representing 1,352 containment zones. A containment zone is a physical area that meets requirements for a specific containment level required for work with particular HPTs. One laboratory can contain several containment zones (see Appendix for full definition).
A total of 50 incidents involving a potential exposure were extracted from the database, including four incidents that were reported in 2017 but that occurred in 2016. During the investigation process, it was determined that an exposure did not occur in four incidents; these were ruled out and removed from analysis, leaving a total of 46 incidents. The sample included nine incidents for which reporting was delayed until licence issuance; these were retained for analysis but were excluded from calculations related to timeliness of notification.
Exposure and/or laboratory-acquired infection incidents occurred in 3.4% of all regulated containment zones. The majority of reported incidents involved exposure only (n=42; 91.3%), while four incidents led to a suspected (n=3; 6.5%) or confirmed (n=1; 2.2%) laboratory-acquired infection. Most incidents involved HPTs classified at a risk group 2 level that were manipulated in a biosafety containment level 2 laboratory (91.3%). Three incidents occurred in a containment level 3 designated facility and one incident occurred in a containment level 4 designated facility.
Distribution of incidents by sector
The highest number of reported incidents occurred in the academic (n=16; 34.8%) and hospital (n=12; 26.1%) sectors, which was proportionate to the distribution of containment zones by sector (Table 1). Private industry represented 32.2% of all containment zones, but only 17.4% of reported exposure incidents.
| Sector | Number of active licences | Number of containment zones | Number of exposure incidents | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Academic | 168 | 20.1 | 436 | 32.2 | 16 | 34.8 |
| Hospital | 186 | 22.3 | 290 | 21.4 | 12 | 26.1 |
| Private industry/business | 376 | 45 | 436 | 32.2 | 8 | 17.4 |
| Public health (government) | 25 | 3 | 64 | 4.7 | 4 | 8.7 |
| Veterinary/animal health (government) | 18 | 2.2 | 38 | 2.8 | 4 | 8.7 |
| Environmental (government) | 32 | 3.8 | 37 | 2.7 | 0 | 0 |
| Other government | 30 | 3.6 | 51 | 3.8 | 2 | 4.3 |
| TOTAL | 835 | 100 | 1,352 | 100 | 46 | 100 |
Incident frequency and timeliness of reporting
Typically, four to five incidents occurred each month, with lower numbers seen in the summer (Figure 1). The month of September presented as an outlier, with 10 exposure incidents reported to PHAC. Upon examination, all September incidents were unrelated in terms of location, licence holder or implicated HPT. In addition, the characteristics of the incidents occurring in September were similar to that of all incidents when analysed by containment level, sector and pathogen type.
Figure 1: Reported human pathogen or toxin exposure incidents by month of incident, Canada 2016
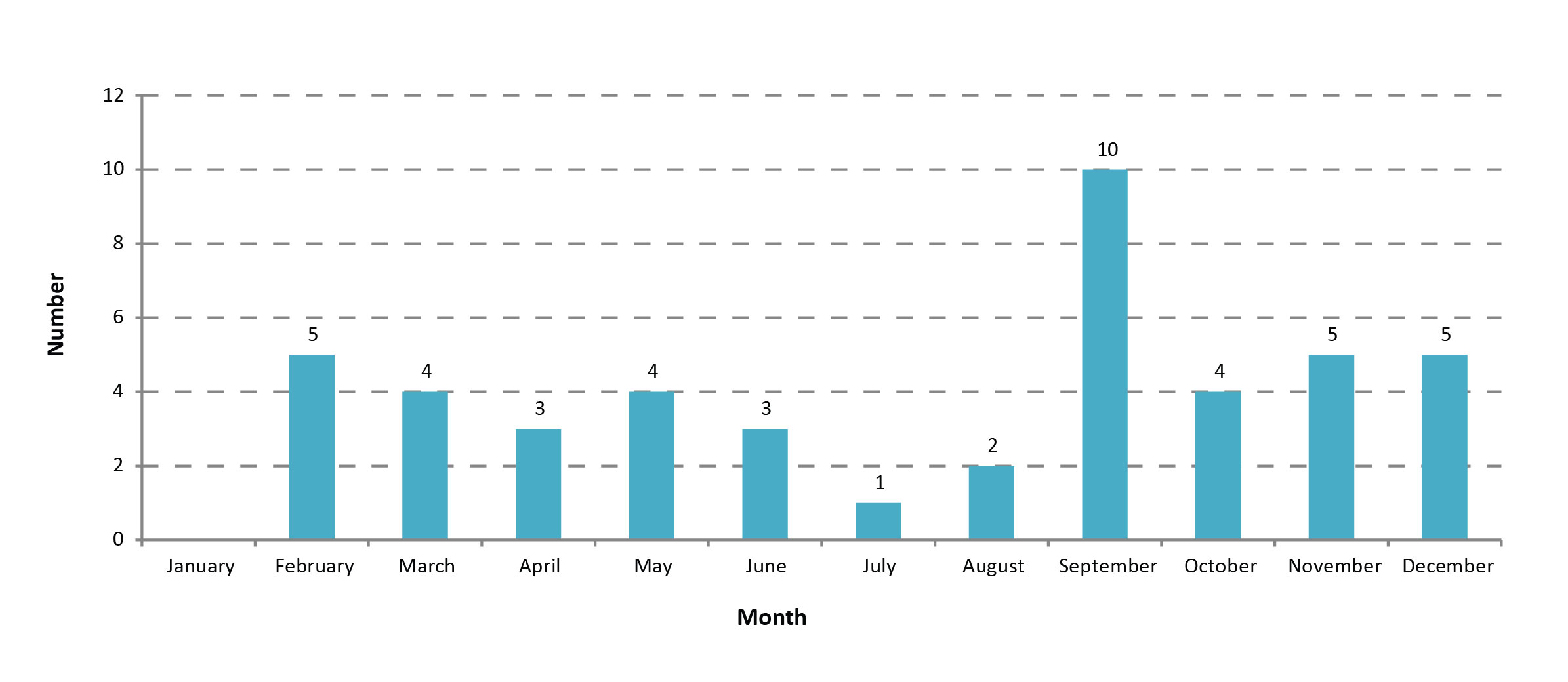
Text description: Figure 1
Figure 1: Reported human pathogen or toxin exposure incidents by month of incident, Canada 2016
| Month of incident | Number of incidents |
|---|---|
| January | 0 |
| February | 5 |
| March | 4 |
| April | 3 |
| May | 4 |
| June | 3 |
| July | 1 |
| August | 2 |
| September | 10 |
| October | 4 |
| November | 5 |
| December | 5 |
For incidents not involving a security sensitive biological agent, the number of days between incident occurrence and initial notification to PHAC ranged from 1 to 119 days, with an average lag of 23.5 days (based on calendar days and including non-business days) (Table 2). Although not shown in Table 2, half of incidents were first reported to PHAC within approximately one week of occurrence, while nine incidents were reported more than one month after occurrence. Reasons for delay included a lack of awareness regarding reporting requirements (n=4) and the need for assistance in report submission (n=3). On average, follow-up reports were submitted 18.4 days after the initial report, with 89.3% of reports meeting the target deadline of 30 days.
For incidents involving a security sensitive biological agent, the number of days between incident occurrence and initial notification to PHAC ranged from 0 to 65 days, with an average lag of 17.1 days (based on calendar days and including non-business days) (Table 2). The deadline for submission of a follow-up report after initial notification was 15 days; 77.4% of follow-up reports met this deadline. For the two incidents submitted past the target deadline, no clear reasons were available for the reporting delay. The observed delays in reporting are not uncommon with new regulatory systems, as there is a lag period while regulated parties become increasingly aware of their reporting obligations.
| Incident type | Time interval | Target interval | Actual interval | Number of incidents submitted before deadline | Number of incidents submitted past deadline | ||||
|---|---|---|---|---|---|---|---|---|---|
| From | To | Number of days | Range of days | Average number of days | n | % | n | % | |
| Not involving a security sensitive biological agentTable 2 - Footnote * | Incident occurrence | Initial notification | Without delay | 1 – 119 | 23.5 | N/A | N/A | N/A | N/A |
| Initial notificationTable 2 - Footnote † | Follow-up report | 30 | 0 – 39 | 18.4 | 25 | 89.3 | 3 | 10.7 | |
| Involving a security sensitive biological agent | Incident occurrence | Initial notification | Without delay | 0 – 65 | 17.1 | N/A | N/A | N/A | N/A |
| Initial notificationTable 2 - Footnote † | Follow-up report | 15 | 0 – 39 | 15.9 | 7 | 77.4 | 2 | 22.2 | |
Number of affected persons
As a result of 46 incidents, 100 people were exposed to an HPT. In the majority (84.8%) of incidents, a single person was exposed; in two incidents, two people were exposed and in five incidents, three or more people were exposed. All incidents involving two or more exposed individuals occurred in a hospital or diagnostic setting. Of the 100 people affected, four were diagnosed with a suspected or confirmed laboratory-acquired infection. No secondary exposures were reported.
Human pathogens and toxins involved
With over 22 different species of HPTs implicated in the incidents, bacteria were the most frequently involved, with 16 (34.8%) incidents involving a bacterium at either the risk group 2 (n=14) or risk group 3 (n=2) level, excluding bacteria classified as a security sensitive biological agent (Table 3). A total of 11 (23.9%) incidents involved a security sensitive biological agent at the risk group 3 (n=10) or risk group 4 (n=1) level. The most commonly reported HPT (n=5) was the bacterial species Brucella spp., which is classified as a risk group 3 security sensitive biological agent.
| Biological agent type | Risk group 2 | Risk group 3 | Risk group 4 | Unknown | Total | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | |
| Bacterium | 14 | 51.9 | 2 | 15.4 | 0 | 0 | 0 | 0 | 16 | 34.8 |
| Virus | 11 | 40.7 | 1 | 7.7 | 0 | 0 | 0 | 0 | 12 | 26.1 |
| Fungus | 1 | 3.7 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2.2 |
| Parasite | 1 | 3.7 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2.2 |
| Security sensitive biological agent | 0 | 0 | 10 | 76.9 | 1 | 100 | 0 | 0 | 11 | 23.9 |
| Unknown | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 100 | 5 | 10.9 |
| TOTAL | 27 | 100 | 13 | 100 | 1 | 100 | 5 | 100 | 46 | 100 |
Causes of incidents
The most common occurrences leading to an incident were procedure- (n=15) and sharps-related (n=14) (Figure 2); however, issues related to personal protective equipment, animal handling, spills, equipment and loss of containment were also cited. Upon review, the seven cases reported under the 'other' category were likely better classified within one or more of the existing categories. Notably, in 10 (21.7%) cases, the inadvertent possession or isolation of a biological agent during the course of routine work played a role in exposure (data not shown). As risk group 2 licence holders are only licensed to work with risk group 2 human pathogens and/or toxins below trigger quantity, any HPT that a licence holder may come across that is at a risk group 3 or risk group 4 level or toxins above trigger quantity would result in an inadvertent possession, and may pose an increased risk to staff.
Figure 2: Reported causes of human pathogen or toxin exposure incidents, Canada 2016
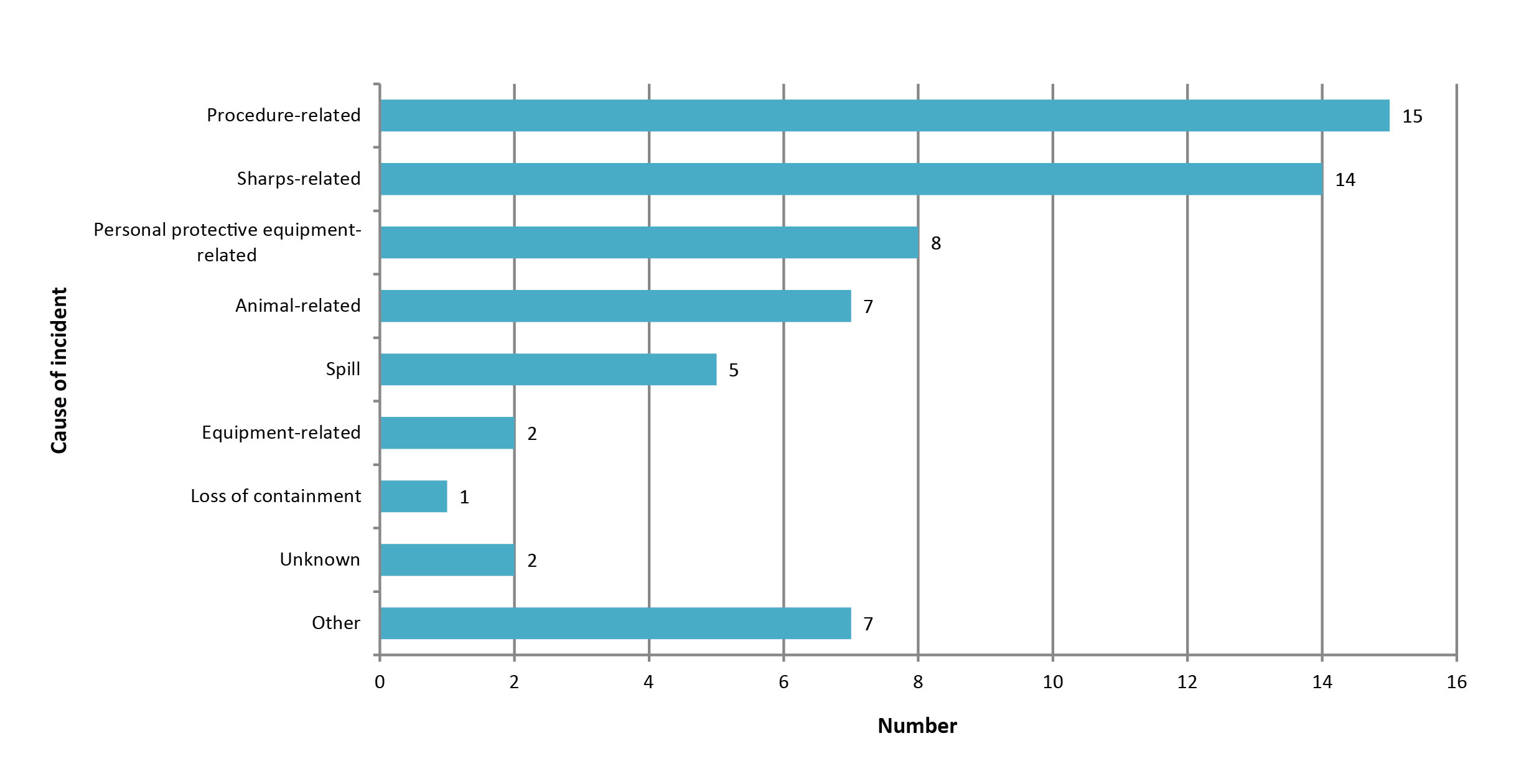
Text description: Figure 2
Figure 2: Reported causes of human pathogen or toxin exposure incidents, Canada 2016
| Cause of incident | Number of incidents |
|---|---|
| Other | 7 |
| Unknown | 2 |
| Loss of containment | 1 |
| Equipment-related | 2 |
| Spill | 5 |
| Animal-related | 7 |
| Personal protective equipment-related | 8 |
| Sharps-related | 14 |
| Procedure-related | 15 |
Corrective actions to improve safety in laboratory settings
As a result of the investigatory process, most reporters were able to identify root causes or areas for improvement in existing systems and processes that could avert a future similar incident. The most cited area for improvement centred on standards/standard operating procedures, policies, rules or electronic procedures (71.7%) (Table 4). Issues in communication were recognized in a quarter (26.1%) of incidents and issues in management or oversight were cited in 11 (23.9%) incidents. Upon analysis, the 15 'other' root causes could have been categorized within existing categories, as they included issues with communication, equipment and human error.
| Root cause | Areas of concern | Citations | Proportion of incidents citing root cause |
|---|---|---|---|
| n | % | ||
| Standard operating procedure |
|
33 | 71.7 |
| Training |
|
7 | 15.2 |
| Communication |
|
12 | 26.1 |
| Management and oversight |
|
11 | 23.9 |
| Equipment |
|
8 | 17.4 |
| Human interaction |
|
8 | 17.4 |
| Other | 15 | 32.6 | |
Discussion
This is the initial report of the first comprehensive national surveillance system on laboratory exposures to HPTs. Overall, exposures to HPTs from laboratory incidents were low. In the first year of data based on regulations requiring mandatory reporting of incidents, 46 exposure incidents were reported. One hundred workers were exposed to an HPT, which resulted in four suspected or confirmed laboratory-acquired infections. There were no reports of secondary exposure beyond the laboratory setting. These findings, including the peak in the number of incidents that occurred in September as well as a higher number of incidents in academic laboratories, will need to be further assessed with future years of data. Many of the key findings reinforce what has already been reported in the literature; for example, implicated biological agents were mainly bacteriaFootnote 1 Footnote 17 Footnote 18 with Brucella spp. being a frequently reported cause of laboratory-acquired infectionFootnote 2 Footnote 19 Footnote 20. In addition, common causes of exposure were the mishandling of sharps or the inadvertent possession of an HPT; these causes have also been commonly described elsewhereFootnote 21 Footnote 22 Footnote 23 Footnote 24 Footnote 25.
The strength of this research is that it is based on a mandatory reporting system with standardized and often mandatory reporting fields; however, there are some limitations that should also be considered. Data for 2016 are unlikely to include all reportable incidents due to several factors. First, the system was still in its infancy with licence issuance ongoing throughout the year of data collection. Data may also be incomplete due to self-selection or non-response bias resulting in incidents that are not reported, which may include undetected incidents, incidents not reported due to a lack of awareness or understanding of the regulatory requirements or reluctance to report incidents due to the negative connotation associated with 'accidents' and 'incidents'. Of the reported data, certain biases may exist. Self-reported data can be influenced by many factors, including recall bias, mode of data collection, experience of the reporter/staff and proxy respondent bias. Recall bias would be particularly notable in situations where new information or symptoms occur, forcing reporters to work backwards to identify the incident that likely precipitated the outcome. Changes are continually being made to the LINC system to improve clarity for reporters, with the aim of improving timeliness in reporting and standardization of data.
The information derived from these data can be used as a reference point to inform researchers, regulated parties and the public about the current landscape of laboratory biosafety in Canada and the performance of the LINC system to date. Findings related to data quality can be used to inform the development of similar surveillance systems elsewhere, while the data can be used internally by PHAC to enforce safety standards, improve prevention strategies and promote best practices. Based on these generalized findings, PHAC has already implemented outreach initiatives to improve awareness of commonly occurring incidents, including a notice sent to stakeholders regarding sharps injuries associated with the use of disposable scalpel blades (Biosafety and Biosecurity for Pathogens and Toxins Newsletter, Are You Using Scalpels with Disposable Blades?, May 2017, unpublished newsletter), as well as an advisory regarding an increasing trend of inadvertent isolations of Coccidioides spp., perhaps due to travellers returning to Canada from southwestern United States, northern Mexico and areas of Central and South America (Biosafety and Biosecurity for Pathogens and Toxins Newsletter, Laboratory Incident Notification Canada (LINC) Feature Report: Coccidioides, September 2016, unpublished newsletter).
Conclusion
In Canada, the HPT Act and Regulations require mandatory reporting of laboratory exposures to human pathogens and toxins in close to real time. Mandatory reporting requirements support comprehensive, timely and standardized data collection. Reporting incidents to a federal agency serves a wider purpose of strengthening the biosafety and biosecurity of Canadian laboratories through the understanding of potential risks experienced in practice that can lead to systematic change to benefit all regulated parties.
Authors' statement
AB, MH, and MS participate in laboratory incident monitoring. All authors worked on the conceptualization together; AB prepared the original draft and AB, MH, MS contributed to multiple draft review and editing and sign off on the final version. MS and MH also played a supervisory role.
Conflict of interest
None.
Acknowledgements
We would like to thank Ken Turcotte, Ismahan Hussein, Cindy Evans, Craig Brooks, Marnie Fiebig and Jennifer Mihowich at the Centre for Biosecurity for their expertise and provision of supplementary data. We would also like to extend our appreciation to all the licence holders and biological safety officers across Canada for providing high quality reports.
Funding
This work was supported by Public Health Agency of Canada as part of its core mandate.
Appendix: Definitions relating to the Human Pathogens and Toxins Act
- Term
- Definition
- Biological safety officer (BSO):
- An individual designated for overseeing the facility’s biosafety and biosecurity practices.
- Containment level (CL):
- Minimum physical containment and operational practice requirements for handling human pathogens or toxins safely in laboratory environments. There are four containment levels, ranging from a basic to the highest level of containment (1 to 4).
- Containment zone:
- A physical area that meets the requirements for a specified containment level. A containment zone can be a single room, a series of co-located rooms or several adjoining rooms. Dedicated support areas, including anterooms (with showers and ‘clean’ and ‘dirty’ change areas, where required), are considered to be part of the containment zone.
- Exposure:
- Contact with, or close proximity to, human pathogens or toxins that may result in infection or intoxication, respectively. Routes of exposure include inhalation, ingestion, inoculation and absorption.
- Exposure follow-up report:
- A tool used to report and document incident occurrence and investigation information for an exposure incident previously notified to the Public Health Agency of Canada.
- Exposure notification report:
- A tool used to notify and document preliminary information to the Public Health Agency of Canada of an exposure incident.
- Incident:
- An event or occurrence with the potential of causing injury, harm, infection, intoxication, disease or damage. Incidents can involve infectious material, infected animals or toxins, including a spill, exposure, release of human pathogens or toxins, animal escape, personnel injury or illness, missing human pathogens or toxins, unauthorized entry into the containment zone, power failure, fire, explosion, flood or other crisis situations (e.g., earthquake, hurricane). Incidents include accidents and near misses.
- Laboratory:
- An area within a facility or the facility itself where biological material is handled for scientific or medical purposes.
- Licence:
- An authorization to conduct one or more controlled activities with human pathogens or toxins issued by the Public Health Agency of Canada under Section 18 of the Human Pathogens and Toxins Act. One licence can cover many containment zones.
- Risk group (RG):
- The classification of biological material based on its inherent characteristics, including pathogenicity, virulence, risk of spread and availability of effective prophylactic or therapeutic treatments, that describes the risk to the health of individuals and the public as well as the health of animals and the animal population.
- Security sensitive biological agents (SSBAs):
- The subset of human pathogens and toxins that have been determined to pose an increased biosecurity risk due to their potential for use as a biological weapon. Security sensitive biological agents are identified as prescribed human pathogens and toxins by Section 10 of the Human Pathogens and Toxins Regulations. This includes all risk group 3 and 4 human pathogens that are in the List of Human and Animal Pathogens for Export Control, published by the Australia Group, as amended from time to time, with the exception of Duvenhage virus, Rabies virus and all other members of the Lyssavirus genus, Vesicular stomatitis virus, and Lymphocytic choriomeningitis virus. This also includes all toxins listed in Schedule 1 of the Human Pathogens and Toxins Act that are listed on the List of Human and Animal Pathogens for Export Control when in a quantity greater than that specified in Section 10(2) of the Human Pathogens and Toxins Regulations.
For more definitions, please see the Canadian Biosafety Standard, Second Edition Footnote 16.