Archived - NACI Statement on Seasonal Influenza Vaccine for 2017–2018

 Download this article as a PDF (1.5 MB - 8 pages)
Download this article as a PDF (1.5 MB - 8 pages) Published by: The Public Health Agency of Canada
Issue: Volume 43-5: Implementation science
Date published: May 4, 2017
ISSN: 1481-8531
Submit a manuscript
About CCDR
Browse
Volume 43-5, May 4, 2017: Implementation science
Advisory Committee Statement
Summary of the NACI Statement on Seasonal Influenza Vaccine for 2017–2018
W Vaudry1, R Stirling2 on behalf of the National Advisory Committee on Immunization (NACI)*
Affiliations
1 NACI Influenza Working Group Chair, University of Alberta, Edmonton, AB
2 Centre for Immunization and Respiratory Infectious Diseases, Public Health Agency of Canada, Ottawa, ON
Correspondence
Suggested citation
Vaudry W, Stirling R on behalf of the National Advisory Committee on Immunization (NACI). Summary of the NACI Statement on Seasonal Influenza Vaccine for 2017–2018. Can Commun Dis Rep. 2017;43(5):96-103. https://doi.org/10.14745/ccdr.v43i05a03
Abstract
Background: Influenza is a respiratory infection caused primarily by influenza A and B viruses. Vaccination is the most effective way to prevent influenza and its complications. The National Advisory Committee on Immunization (NACI) provides recommendations regarding seasonal influenza vaccines annually to the Public Health Agency of Canada (PHAC).
Objective: To summarize the NACI recommendations regarding the use of seasonal influenza vaccines for the 2017–2018 influenza season.
Methods: Annual influenza vaccine recommendations are developed by NACI’s Influenza Working Group for consideration and approval by NACI, based on NACI’s evidence-based process for developing recommendations. The recommendations include a consideration of the burden of influenza illness and the target populations for vaccination; efficacy and effectiveness, immunogenicity and safety of influenza vaccines; vaccine schedules; and other aspects of influenza immunization. These recommendations are published annually on the Agency’s website in the NACI Advisory Committee Statement: Canadian Immunization Guide Chapter on Influenza and Statement on Seasonal Influenza Vaccine (the Statement).
Results: The annual statement has been updated for the 2017–2018 influenza season to incorporate recommendations for the use of live attenuated influenza vaccine (LAIV) that were contained in two addenda published after the 2016–2017 statement. These recommendations were 1) that egg-allergic individuals may be vaccinated against influenza using the low ovalbumin-containing LAIV licensed for use in Canada and 2) to continue to recommend the use of LAIV in children and adolescents 2–17 years of age, but to remove the preferential recommendation for its use.
Conclusion: NACI continues to recommend annual influenza vaccination for all individuals aged six months and older, with particular focus on people at high risk of influenza-related complications or hospitalization, people capable of transmitting influenza to those at high risk, and others as indicated.
Introduction
Influenza and pneumonia is ranked among the top 10 leading causes of death in Canada Footnote 1. Although the burden of influenza can vary from year to year, it is estimated that in a given year, there are an average of 12,200 hospitalizations related to influenza Footnote 2 and approximately 3,500 deaths attributable to influenza Footnote 3. The National Advisory Committee on Immunization (NACI) provides recommendations regarding seasonal influenza vaccines annually to the Public Health Agency of Canada (PHAC). The objective of this article is to summarize the NACI recommendations for the use of seasonal influenza vaccine for the 2017–2018 influenza season. Complete details can be found in the Statement on Seasonal Influenza Vaccine for 2017–2018 Footnote 4.
Methods
In the preparation of the 2017–2018 seasonal influenza vaccine recommendations, NACI’s Influenza Working Group (IWG) identified and reviewed evidence regarding the administration of live attenuated influenza vaccine (LAIV) in egg-allergic individuals and vaccine effectiveness of LAIV and inactivated influenza vaccine (IIV) in children and adolescents 2–17 years of age. Following the review and analysis of this information, the IWG proposed updated recommendations for vaccine use to NACI, based on NACI’s evidence-based process for developing recommendations Footnote 5. NACI critically appraised the available evidence and approved the specific recommendations brought forward. Complete details of the literature review, rationale and relevant considerations for the updated recommendations can be found in the Addendum – LAIV Use in Egg Allergic Individuals Footnote 6 the Addendum – LAIV Use in Children and Adolescents Footnote 7 and the Canadian Immunization Guide Chapter on Influenza and Statement on Seasonal Influenza Vaccine for 2017–2018 Footnote 4.
For the review of LAIV use in egg-allergic individuals, data were obtained from three prospective cohort studies in the United Kingdom (UK) and Canada Footnote 8Footnote 9Footnote 10. Post-licensure safety data from the Canadian Adverse Events Following Immunization Surveillance System (CAEFISS) was analyzed to seek reports of adverse events in influenza vaccine recipients who describe a history of allergy to eggs.
Data on LAIV vaccine effectiveness in children and adolescents were obtained primarily from American studies using the test-negative design: the United States Influenza Vaccine Effectiveness Network (US Flu VE Network) (2010–2016) Footnote 11Footnote 12Footnote 13Footnote 14 the Influenza Clinical Investigation for Children (ICICLE) study (2013–2014 through 2015–2016 influenza seasons) Footnote 15Footnote 16Footnote 17 and the US Department of Defense (DoD) (2013–2014 and 2015–2016 influenza seasons) Footnote 13Footnote 18. The American Household Influenza Vaccine Effectiveness (HIVE) study derived vaccine effectiveness data using an alternative household cohort design (2012–2013 and 2013–2014 seasons) Footnote 19Footnote 20. Data on LAIV vaccine effectiveness from outside of the United States of America came from the Canadian Sentinel Practitioner Surveillance Network (SPSN) (2013–2014 and 2015–2016 seasons) Footnote 21Footnote 22 Germany (2012–2013 season) Footnote 23 the UK sentinel surveillance network (2013–2014 through 2015–2016 seasons) Footnote 24Footnote 25Footnote 26 and Finland (2015–2016 season) Footnote 27. These studies used the test-negative design Footnote 21Footnote 22Footnote 23Footnote 24Footnote 25Footnote 26 with one prospective cohort study Footnote 27 and two cluster randomized trials Footnote 28Footnote 29.
This article also presents information not provided in the published addenda or statement: figures summarizing the LAIV vaccine effectiveness data from the cited studies, by influenza season and influenza strain, as well as LAIV vaccine effectiveness data used to inform NACI’s decision that were not publicly available when the Addendum was finalized, but have subsequently been published Footnote 30Footnote 31.
Results
New for the 2017–2018 influenza season
There were two changes in NACI recommendations for the use of seasonal influenza vaccine for the 2017–2018 influenza season. Both changes related to updated recommendations on the use of LAIV.
LAIV is safe for egg-allergic individuals
All influenza vaccine products authorized for use in Canada are manufactured from influenza virus grown in chicken eggs, which may result in the vaccines containing trace amounts of residual egg protein. The formulation of LAIV licensed for use in Canada contains a low amount of residual ovalbumin (less than 0.24 µg/dose) (written communication from AstraZeneca), which is comparable to the amounts in IIVs available in Canada.
At the time of publication of the Canadian Immunization Guide Chapter on Influenza and Statement on Seasonal Influenza Vaccine for 2016–2017 Footnote 32 NACI did not recommend LAIV use in egg-allergic individuals due to a lack of data available to support this practice.
However, the safety of LAIV in egg-allergic individuals has now been studied in more than 1,100 children and adolescents (2–18 years of age) in the UK and Canada Footnote 8Footnote 9Footnote 10. After careful review of recently published studies, NACI concludes that egg-allergic individuals may be vaccinated against influenza using the low ovalbumin-containing LAIV licensed for use in Canada. The full dose of LAIV may be used without prior vaccine skin test and in any settings where vaccines are routinely administered. LAIV also appears to be well tolerated in individuals with a history of stable asthma or recurrent wheeze; however, it remains contraindicated for individuals with severe asthma (defined as currently on oral or high-dose inhaled glucocorticosteroids or active wheezing) or for those with medically attended wheezing in the seven days prior to immunization. The use of LAIV in egg-allergic individuals is a change from previous NACI statements.
Complete details of the literature review, rationale and relevant considerations for the updated recommendations can be found in the Addendum – LAIV Use in Egg Allergic Individuals Footnote 6 and the Canadian Immunization Guide Chapter on Influenza and Statement on Seasonal Influenza Vaccine for 2017–2018 Footnote 4.
Current evidence supports the continued use of LAIV in children and adolescents 2–17 years of age but does not support its preferential use
At the time of publication of the Canadian Immunization Guide Chapter on Influenza and Statement on Seasonal Influenza Vaccine for 2016–2017 Footnote 32 NACI recommended the preferential use of LAIV in children and adolescents 2–17 years of age who did not have contraindications to the vaccine. This recommendation was based upon randomized placebo controlled studies and post-marketing safety data that showed LAIV to be safe, efficacious and immunogenic in children and to provide better protection against influenza than trivalent IIV, especially in young children (less than six years of age), with weaker evidence of superior efficacy in older children Footnote 33.
The adjusted vaccine effectiveness estimates for LAIV and IIV against any influenza in children and adolescents (2–17 years of age) are summarized by study for the 2010–2011 through 2014–2015 (Appendix Figure 1) and 2015–2016 (Appendix Figure 2) influenza seasons. Summaries of adjusted vaccine effectiveness estimates by study and vaccine type are also provided for influenza A(H1N1)pdm09 (Appendix Figure 3), influenza A(H3N2) (Appendix Figure 4) and influenza B (Appendix Figure 5) for these same influenza seasons (Note: in some influenza seasons, sample sizes were too small to derive vaccine effectiveness estimates for all influenza strains).
Based upon the US Flu VE Network data showing that LAIV provided no protective benefit during the influenza A(H1N1) dominant 2015–2016 influenza season and no evidence of effectiveness against the dominant circulating strains in the two prior influenza seasons (2013–2014 and 2014–2015), the American Advisory Committee on Immunization Practices (ACIP) recommended during its June 2016 meeting that LAIV should not be used during the 2016–2017 influenza season Footnote 34. LAIV continued to be recommended for use in children in the UK and Finland for the 2016–2017 season Footnote 35. Studies conducted in both of these countries and in Canada found a statistically significant overall protective effect of LAIV in children for 2015–2016, although sample sizes limited the precision of those estimates Footnote 22Footnote 24Footnote 27. The United States Food and Drug Administration (US FDA) has also determined that specific regulatory action for LAIV was not necessary at the time, following a review of manufacturing and clinical data supporting licensure and the totality of evidence presented at the June 2016 ACIP meeting, and continues to find that the benefits of quadrivalent LAIV outweigh any potential risks Footnote 36. Quadrivalent LAIV remains licensed for use in the US. The FDA’s determination was made taking into account the limitations of observational studies in estimating vaccine effectiveness and the seasonal variability of influenza vaccine effectiveness.
After careful review of available studies from the last several influenza seasons, NACI concludes that the current evidence is consistent with LAIVs providing comparable protection against influenza to that afforded by IIV in various jurisdictions and has revised its recommendations on the use of influenza vaccine in children and adolescents 2–17 years of age:
- 1. In children and adolescents without contraindications to the vaccine, any of the following vaccines can be used: quadrivalent LAIV, quadrivalent inactivated influenza vaccine (QIV) or trivalent inactivated influenza vaccine (TIV).
- 2. The current evidence does not support a recommendation for the preferential use of LAIV in children and adolescents 2–17 years of age.
Given the burden of influenza B disease in children and the potential for lineage mismatch between the predominant circulating strain of influenza B and the strain in a trivalent vaccine, NACI continues to recommend that a quadrivalent formulation of influenza vaccine be used in children and adolescents 2–17 years of age. If a quadrivalent vaccine is not available, TIV should be used.
The observational study data reviewed highlight the challenge in interpreting the vaccine effectiveness of LAIV and IIV when point estimates by influenza subtype are derived based on small sample sizes associated with wide confidence intervals. Therefore, in making its recommendations, NACI recognizes the need to continue to closely monitor the data on the vaccine effectiveness of LAIV by influenza subtype and the relative effectiveness of LAIV compared to IIV. NACI has also identified the need for further research to address current knowledge gaps:
- 3. NACI strongly encourages further multidisciplinary (e.g. epidemiology, immunology, virology) research to investigate the reasons for the discordant 2015–2016 vaccine effectiveness estimates between studies and explanations for poor LAIV effectiveness against A(H1N1)pdm09 reported in some studies.
- 4. NACI strongly recommends that sufficient resources be provided to enhance influenza-related research and sentinel surveillance systems in Canada to improve the evaluation of influenza vaccine efficacy and effectiveness to provide the best possible evidence for Canadian influenza vaccination programs and recommendations.
Complete details of the literature review, rationale and relevant considerations for the updated recommendations can be found in the Addendum – LAIV Use in Children and Adolescents Footnote 7 and the Canadian Immunization Guide Chapter on Influenza and Statement on Seasonal Influenza Vaccine for 2017–2018 Footnote 4.
Summary of NACI recommendations for the use of influenza vaccines for the 2017–2018 influenza season
NACI continues to recommend influenza vaccination for all individuals aged six months and older who do not have contraindications to the vaccine, with particular focus on people at high risk of influenza-related complications or hospitalization, people capable of transmitting influenza to those at high risk of complications, and others as indicated in Table 1.
People at high risk of influenza-related complications or hospitalization
People capable of transmitting influenza to those at high risk
Others
|
Recommended influenza vaccine options by specific age and risk groups and by dosage and route of administration by age are summarized in Table 2 and Table 3, respectively.
| Recipient by age group | Vaccine types available for use | Comments |
|---|---|---|
Children 6–23 months of age |
|
TIV, QIV and ATIV are authorized for this age group. NACI recommends that, given the burden of influenza B disease, QIV should be used. If QIV is not available, either unadjuvanted or adjuvanted TIV should be used. |
Children 2–17 years of age |
|
In children without contraindications to the vaccine, any of the following vaccines can be used: LAIV, QIV or TIV. The current evidence does not support a recommendation for the preferential use of LAIV in children and adolescents 2–17 years of age. Given the burden of influenza B disease in children and the potential for lineage mismatch between the predominant circulating strain of influenza B and the strain in a trivalent vaccine, NACI continues to recommend that a quadrivalent formulation of influenza vaccine be used in children and adolescents 2–17 years of age. If a quadrivalent vaccine is not available, TIV should be used. LAIV is not recommended for children with immune compromising conditions. LAIV, TIV or QIV can be used in children with chronic health conditions and without contraindications (see full statement for more details) Footnote 4. |
Adults 18–59 years of age |
|
TIV and QIV are the recommended products for adults with chronic health conditions. TIV and QIV, instead of LAIV, are recommended for health care workers. LAIV is not recommended for adults with immune compromising conditions. |
Adults 60–64 years of age |
|
TIV and QIV are authorized for use in this age group. |
Adults 65 years of age and older |
|
Given the burden of Influenza A(H3N2) disease and evidence of better efficacy in this age group, it is expected that high-dose TIV should provide superior protection compared with the standard-dose intramuscular vaccine for older adults. |
Pregnant women |
|
LAIV is not recommended because of the theoretical risk to the fetus from administering a live virus vaccine. |
Age group |
TIV without adjuvantTable 3 - Footnote 1 Intramuscular |
QIV without adjuvantTable 3 - Footnote 2 Intramuscular |
TIV without adjuvant, high- dose (Fluzone® High-Dose) Intramuscular |
MF59-adjuvanted TIV (Fluad Pediatric® or Fluad®) Intramuscular |
LAIV (FluMist® Quadrivalent) Intranasal |
Number of doses required |
|---|---|---|---|---|---|---|
| 6–23 months | 0.5 mLTable 3 - Footnote 3 | 0.5 mLTable 3 - Footnote 3 | N/A | 0.25 mL | N/A | 1 or 2Table 3 - Footnote 4 |
| 2–8 years | 0.5 mL | 0.5 mL | N/A | N/A | 0.2 mL (0.1 mL per nostril) | 1 or 2Table 3 - Footnote 4 |
| 9–17 years | 0.5 mL | 0.5 mL | N/A | N/A | 0.2 mL (0.1 mL per nostril) | 1 |
| 18–59 years | 0.5 mL | 0.5 mL | N/A | N/A | 0.2 mL (0.1 mL per nostril) | 1 |
| 60–64 years | 0.5 mL | 0.5 mL | N/A | N/A | N/A | 1 |
| 65 years and older | 0.5 mL | 0.5 mL | 0.5 mL | 0.5 mL | N/A | 1 |
Conclusion
NACI continues to recommend annual influenza vaccination for all individuals aged six months and older (noting product-specific age indications and contraindications), with particular focus on people at high risk of influenza-related complications or hospitalization, including all pregnant women; people capable of transmitting influenza to those at high risk; and others as indicated. For the 2017–2018 influenza season, NACI has also updated LAIV use recommendations: 1) egg-allergic individuals may be vaccinated against influenza using the low ovalbumin-containing LAIV licensed for use in Canada, and 2) LAIV continues to be recommended for use in children and adolescents 2–17 years of age, but is no longer recommended preferentially.
Authors’ statement
This statement was prepared by the Influenza Working Group: Vaudry W (Chair), Grohskopf L, Henry E, Kumar D, Langley J, Lavoie M, McElhaney J, McGeer A, Moore D, Vinh D, Warshawsky B, Xiong J
Conflict of Interest
None.
Acknowledgements
NACI acknowledges and appreciates the contribution of Christina Bancej, Gina Charos, Althea House, Vanessa Meikle, Robert Stirling and Linlu Zhao to the statement.
NACI members: Gemmill I (Chair), Quach C (Vice-Chair), Dayneka N, Deeks S, Henry B, Marchant-Short S, Salvadori M, Sicard N, Vaudry W, Vinh D, Warrington R
Liaison representatives: Blake J (Society of Obstetricians and Gynaecologists of Canada), Brophy J (Canadian Association for Immunization Research and Evaluation), Cohn A (Centers for Disease Control and Prevention, United States), Emili J (College of Family Physicians of Canada), Lavoie M (Council of Chief Medical Officers of Health), Mah C (Canadian Public Health Association), Moore D (Canadian Paediatric Society), Pham-Huy A (Association of Medical Microbiology and Infectious Disease Canada), Sartison E (Canadian Immunization Committee)
Ex-officio representatives: Barnes K (National Defence and the Canadian Armed Forces), Charos G (Centre for Immunization and Respiratory Infectious Diseases [CIRID], Public Health Agency of Canada [PHAC]), Coleman G (Biologics and Genetic Therapies Directorate, Health Canada [HC]), Gallivan J (Marketed Health Products Directorate, HC), Pennock J (CIRID, PHAC), Pless R (CIRID, PHAC), Wong T (First Nations and Inuit Health Branch, HC)
Funding
The work of NACI is supported by the Public Health Agency of Canada.
Appendix
Figure 1: Adjusted vaccine effectiveness estimates against any influenza by study and vaccine type for the 2010–2011 through 2014–2015 influenza seasons in children and adolescents 2–17 years of age Figure 1 footnote 1
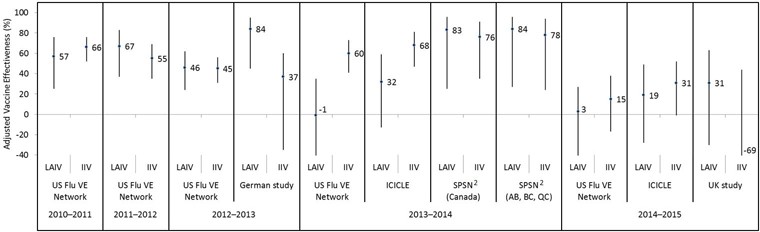
Text description: Figure 1
Figure 1: Adjusted vaccine effectiveness estimates against any influenza by study and vaccine type for the 2010–2011 through 2014–2015 influenza seasons in children and adolescents 2–17 years of age Figure 1 footnote 1
| Influenza season | Study | Vaccine type | Adjusted vaccine effectiveness point estimate (%) | Lower 95% confidence interval limit (%) | Upper 95% confidence interval limit (%) |
|---|---|---|---|---|---|
| 2010–2011 | US Flu VE Network | LAIV | 57 | 25 | 76 |
| 2010–2011 | US Flu VE Network | IIV | 66 | 52 | 76 |
| 2011–2012 | US Flu VE Network | LAIV | 67 | 37 | 83 |
| 2011–2012 | US Flu VE Network | IIV | 55 | 35 | 69 |
| 2012–2013 | US Flu VE Network | LAIV | 46 | 24 | 62 |
| 2012–2013 | US Flu VE Network | IIV | 45 | 31 | 56 |
| 2012–2013 | German study | LAIV | 84 | 45 | 95 |
| 2012–2013 | German study | IIV | 37 | -35 | 60 |
| 2013–2014 | US Flu VE Network | LAIV | -1 | -57 | 35 |
| 2013–2014 | US Flu VE Network | IIV | 60 | 41 | 73 |
| 2013–2014 | ICICLE | LAIV | 32 | -13 | 59 |
| 2013–2014 | ICICLE | IIV | 68 | 47 | 81 |
| 2013–2014 | SPSN (Canada) | LAIV | 83 | 25 | 96 |
| 2013–2014 | SPSN (Canada) | IIV | 76 | 35 | 91 |
| 2013–2014 | SPSN (AB, BC, QC) | LAIV | 84 | 27 | 96 |
| 2013–2014 | SPSN (AB, BC, QC) | IIV | 78 | 24 | 94 |
| 2014–2015 | US Flu VE Network | LAIV | 3 | -50 | 27 |
| 2014–2015 | US Flu VE Network | IIV | 15 | -17 | 38 |
| 2014–2015 | ICICLE | LAIV | 19 | -28 | 49 |
| 2014–2015 | ICICLE | IIV | 31 | -1 | 52 |
| 2014–2015 | UK study | LAIV | 31 | -30 | 63 |
| 2014–2015 | UK study | IIV | -69 | -409 | 44 |
Figure 2: Adjusted vaccine effectiveness estimates against any influenza by study and vaccine type for the 2015–2016 influenza season in children and adolescents 2–17 years of age Figure 2 footnote 1
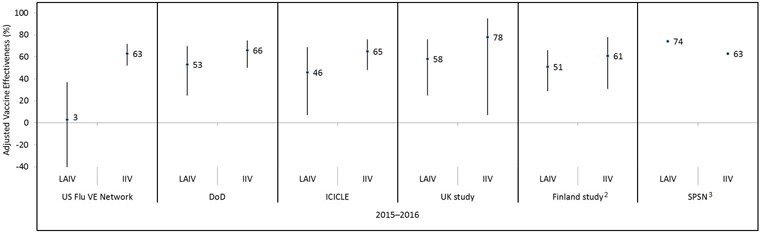
Text description: Figure 2
Figure 2: Adjusted vaccine effectiveness estimates against any influenza by study and vaccine type for the 2015–2016 influenza season in children and adolescents 2–17 years of age Figure 2 footnote 1
| Influenza season | Study | Vaccine type | Adjusted vaccine effectiveness point estimate (%) | Lower 95% confidence interval limit (%) | Upper 95% confidence interval limit (%) |
|---|---|---|---|---|---|
| 2015–2016 | US Flu VE Network | LAIV | 3 | -49 | 37 |
| 2015–2016 | US Flu VE Network | IIV | 63 | 52 | 72 |
| 2015–2016 | DoD | LAIV | 53 | 25 | 70 |
| 2015–2016 | DoD | IIV | 66 | 50 | 75 |
| 2015–2016 | ICICLE | LAIV | 46 | 7 | 69 |
| 2015–2016 | ICICLE | IIV | 65 | 48 | 76 |
| 2015–2016 | UK study | LAIV | 58 | 25 | 76 |
| 2015–2016 | UK study | IIV | 78 | 7 | 95 |
| 2015–2016 | Finland study | LAIV | 51 | 29 | 66 |
| 2015–2016 | Finland study | IIV | 61 | 31 | 78 |
| 2015–2016 | SPSN | LAIV | 74 | Not available | Not available |
| 2015–2016 | SPSN | IIV | 63 | Not available | Not available |
Figure 3: Adjusted vaccine effectiveness estimates against influenza A(H1N1)pdm09 by influenza season, study and vaccine type in children and adolescents 2–17 years of age for A(H1N1)pdm09-dominant seasons since 2009 Figure 3 footnote 1
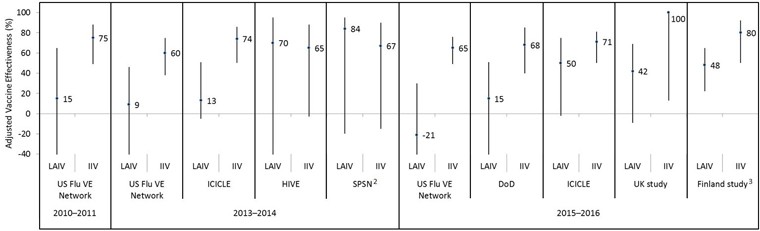
Text description: Figure 3
Figure 3: Adjusted vaccine effectiveness estimates against influenza A(H1N1)pdm09 by influenza season, study and vaccine type in children and adolescents 2–17 years of age for A(H1N1)pdm09-dominant seasons since 2009 Figure 3 footnote 1
| Influenza season | Study | Vaccine type | Adjusted vaccine effectiveness point estimate (%) | Lower 95% confidence interval limit (%) | Upper 95% confidence interval limit (%) |
|---|---|---|---|---|---|
| 2010–2011 | US Flu VE Network | LAIV | 15 | -110 | 65 |
| 2010–2011 | US Flu VE Network | IIV | 75 | 49 | 88 |
| 2013–2014 | US Flu VE Network | LAIV | 9 | -53 | 46 |
| 2013–2014 | US Flu VE Network | IIV | 60 | 38 | 75 |
| 2013–2014 | ICICLE | LAIV | 13 | -5 | 51 |
| 2013–2014 | ICICLE | IIV | 74 | 50 | 86 |
| 2013–2014 | HIVE | LAIV | 70 | -55 | 95 |
| 2013–2014 | HIVE | IIV | 65 | -3 | 88 |
| 2013–2014 | SPSN | LAIV | 84 | -20 | 95 |
| 2013–2014 | SPSN | IIV | 67 | -15 | 90 |
| 2015–2016 | US Flu VE Network | LAIV | -21 | -108 | 30 |
| 2015–2016 | US Flu VE Network | IIV | 65 | 49 | 76 |
| 2015–2016 | DoD | LAIV | 15 | -48 | 51 |
| 2015–2016 | DoD | IIV | 68 | 40 | 85 |
| 2015–2016 | ICICLE | LAIV | 50 | -2 | 75 |
| 2015–2016 | ICICLE | IIV | 71 | 50 | 81 |
| 2015–2016 | UK study | LAIV | 42 | -9 | 69 |
| 2015–2016 | UK study | IIV | 100 | 13 | 100 |
| 2015–2016 | Finland study | LAIV | 48 | 22 | 65 |
| 2015–2016 | Finland study | IIV | 80 | 50 | 92 |
Figure 4: Adjusted vaccine effectiveness estimates against influenza A(H3N2) by influenza season, study and vaccine type in children and adolescents 2–17 years of age for A(H3N2)-dominant seasons since 2009 Figure 4 footnote 1
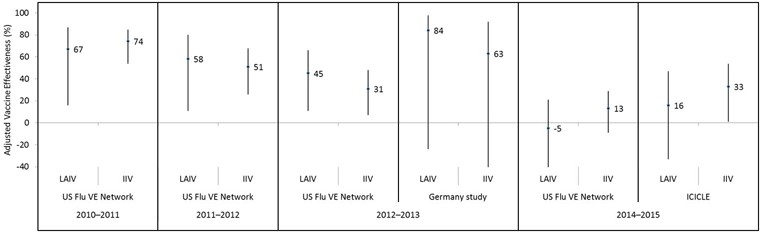
Text description: Figure 4
Figure 4: Adjusted vaccine effectiveness estimates against influenza A(H3N2) by influenza season, study and vaccine type in children and adolescents 2–17 years of age for A(H3N2)-dominant seasons since 2009 Figure 4 footnote 1
| Influenza season | Study | Vaccine type | Adjusted vaccine effectiveness point estimate (%) | Lower 95% confidence interval limit (%) | Upper 95% confidence interval limit (%) |
|---|---|---|---|---|---|
| 2010–2011 | US Flu VE Network | LAIV | 67 | 16 | 87 |
| 2010–2011 | US Flu VE Network | IIV | 74 | 54 | 85 |
| 2011–2012 | US Flu VE Network | LAIV | 58 | 11 | 80 |
| 2011–2012 | US Flu VE Network | IIV | 51 | 26 | 68 |
| 2012–2013 | US Flu VE Network | LAIV | 45 | 11 | 66 |
| 2012–2013 | US Flu VE Network | IIV | 31 | 7 | 48 |
| 2012–2013 | Germany study | LAIV | 84 | -24 | 98 |
| 2012–2013 | Germany study | IIV | 63 | -67 | 92 |
| 2014–2015 | US Flu VE Network | LAIV | -5 | -50 | 21 |
| 2014–2015 | US Flu VE Network | IIV | 13 | -9 | 29 |
| 2014–2015 | ICICLE | LAIV | 16 | -33 | 47 |
| 2014–2015 | ICICLE | IIV | 33 | 1 | 54 |
Figure 5: Adjusted vaccine effectiveness estimates against influenza B since 2009 by influenza season, study and vaccine type in children and adolescents 2–17 years of age Figure 5 footnote 1
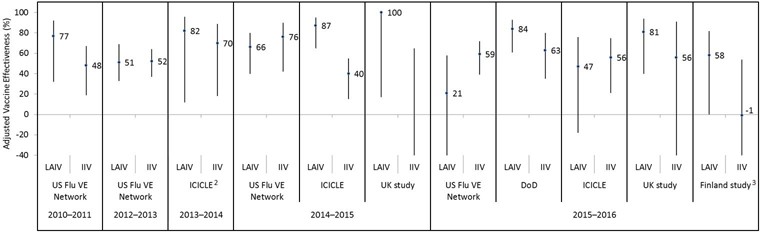
Text description: Figure 5
Figure 5: Adjusted vaccine effectiveness estimates against influenza B since 2009 by influenza season, study and vaccine type in children and adolescents 2–17 years of age Figure 5 footnote 1
| Influenza season | Study | Vaccine type | Adjusted vaccine effectiveness point estimate (%) | Lower 95% confidence interval limit (%) | Upper 95% confidence interval limit (%) |
|---|---|---|---|---|---|
| 2010–2011 | US Flu VE Network | LAIV | 77 | 32 | 92 |
| 2010–2011 | US Flu VE Network | IIV | 48 | 19 | 67 |
| 2012–2013 | US Flu VE Network | LAIV | 51 | 33 | 69 |
| 2012–2013 | US Flu VE Network | IIV | 52 | 37 | 64 |
| 2013–2014 | ICICLE | LAIV | 82 | 12 | 96 |
| 2013–2014 | ICICLE | IIV | 70 | 18 | 89 |
| 2014–2015 | US Flu VE Network | LAIV | 66 | 40 | 80 |
| 2014–2015 | US Flu VE Network | IIV | 76 | 42 | 90 |
| 2014–2015 | ICICLE | LAIV | 87 | 65 | 95 |
| 2014–2015 | ICICLE | IIV | 40 | 15 | 55 |
| 2014–2015 | UK study | LAIV | 100 | 17 | 100 |
| 2014–2015 | UK study | IIV | -124 | -1343 | 65 |
| 2015–2016 | US Flu VE Network | LAIV | 21 | -46 | 58 |
| 2015–2016 | US Flu VE Network | IIV | 59 | 39 | 72 |
| 2015–2016 | DoD | LAIV | 84 | 61 | 93 |
| 2015–2016 | DoD | IIV | 63 | 35 | 80 |
| 2015–2016 | ICICLE | LAIV | 47 | -18 | 76 |
| 2015–2016 | ICICLE | IIV | 56 | 21 | 75 |
| 2015–2016 | UK study | LAIV | 81 | 40 | 94 |
| 2015–2016 | UK study | IIV | 56 | -122 | 91 |
| 2015–2016 | Finland study | LAIV | 58 | 0 | 82 |
| 2015–2016 | Finland study | IIV | -1 | -123 | 54 |