Archived - Increased risk of tick-borne diseases with climate change

 Download this article as a PDF
Download this article as a PDFPublished by: The Public Health Agency of Canada
Issue: Volume 45-4: Climate change and infectious diseases: The challenges
Date published: April 4, 2019
ISSN: 1481-8531
Submit a manuscript
About CCDR
Browse
Volume 45-4, April 4, 2019: Climate change and infectious diseases: The challenges
Overview
Increased risk of tick-borne diseases with climate and environmental changes
C Bouchard1,2, A Dibernardo3, J Koffi2,4, H Wood3, PA Leighton2, LR Lindsay3
Affiliations
1 Public Health Risk Sciences Division, National Microbiology Laboratory, Public Health Agency of Canada, St. Hyacinthe, QC
2 Groupe de recherche en épidémiologie des zoonoses et santé publique (GREZOSP), Faculté de médecine vétérinaire (FMV), Université de Montréal, St. Hyacinthe, QC
3 Zoonotic Diseases and Special Pathogens, National Microbiology Laboratory, Public Health Agency of Canada, Winnipeg, MB
4 Centre for Food-borne, Environmental and Zoonotic Infectious Diseases, Public Health Agency of Canada, St. Hyacinthe, QC
Correspondence
Suggested citation
Bouchard C, Dibernardo A, Koffi J, Wood H, Leighton PA, Lindsay LR. Increased risk of tick-borne diseases with climate and environmental changes. Can Commun Dis Rep 2019; 45(4):83–9. https://doi.org/10.14745/ccdr.v45i04a02
Keywords: climate change, tick-borne disease, Anaplasmosis, Babesiosis, Anaplasma phagocytophilum, Babesia microti, Powassan virus, and Borrelia miyamotoi
Visual Abstract
Visual Abstract
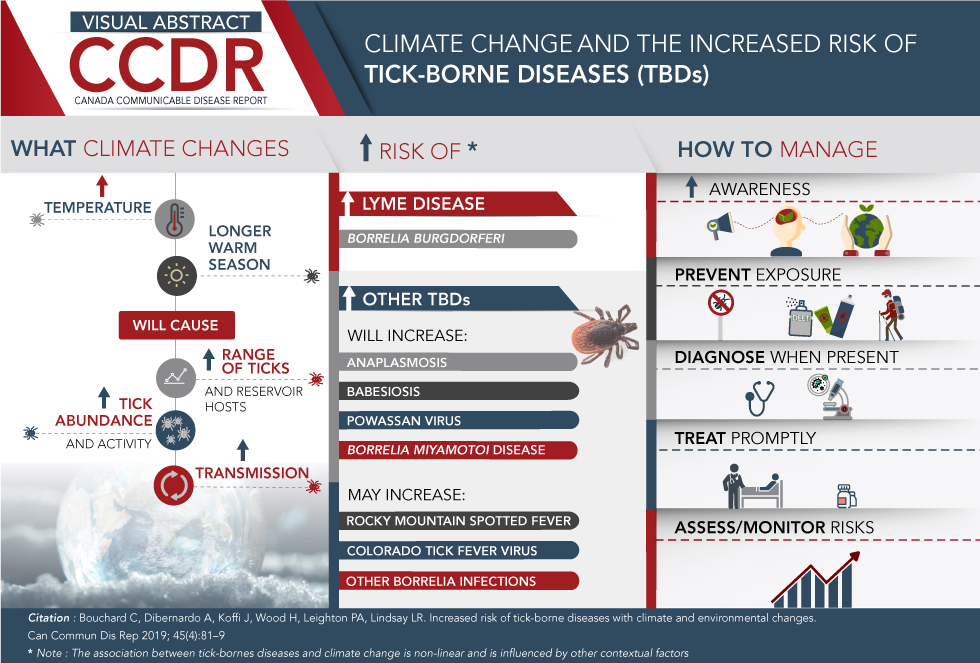
Click here to save the visual abstract
Description for Visual Abstract
This image is a visual abstract that illustrates the increase of Tick Borne diseases (TBDs) as a consequence of climate change.
The visual abstract is divided into three column sections:
First section discusses “What climate changes”
This section highlights how the increased temperature and longer warm season will cause the increase of tick abundance and activity as well as increase of tick and reservoir host range and increase tick transmission
Second section highlights “Increased risk of”:
- Increase in Lyme Disease: Borrelia burgdorferi virus
- Increase in other TBD’s that will increase with climate change such as:
- Anaplasmosis
- Babesiosis
- Powassan virus
- Borrelia miyamotoi disease
- Diseases that may increase with climate change such as:
- Rocky Mountain spotted fever
- Colorado tick fever virus
- Other Borrelia infections
Third section discusses 5 ways on how to manage the Tick-borne diseases:
- Increase awareness
- Prevent exposure
- Diagnose when present
- Treat promptly
- Assess/Monitor risks
Abstract
Climate warming and other environmental changes have contributed to the expansion of the range of several tick species into higher latitudes in North America. As temperatures increase in Canada, the environment becomes more suitable for ticks and the season suitable for tick activity lengthens, so tick-borne diseases are likely to become more common in Canada. In addition to Lyme disease, four other tick-borne diseases (TBDs) have started to emerge and are likely to increase: Anaplasmosis; Babesiosis; Powassan virus; and Borrelia miyamotoi disease. Increased temperature increases the survival and activity period of ticks, increases the range of both reservoir and tick hosts (e.g. mice and deer) and increases the duration of the season when people may be exposed to ticks. Other ticks and TBDs may spread into Canada as the climate changes. The public health strategies to mitigate the impact of all TBDs include surveillance to detect current and emerging TBDs, and public health actions to prevent infections by modifying environmental and social-behavioral risk factors through increasing public awareness. Clinical care strategies include patient education, early detection, laboratory testing, and treatment.
Introduction
Ticks transmit a wide diversity of bacterial, viral and protozoan pathogens in many tropical and temperate regions of the worldFootnote 1. Of particular concern in North America are the blacklegged ticks that transmit Borrelia burgdorferi, the bacterium that causes Lyme disease (LD) in southern parts of central and eastern CanadaFootnote 2. It is now widely acknowledged that the increase in temperature associated with climate change has contributed to a general increase in the number, types, level of activity and geographical distribution of ticks in North AmericaFootnote 1Footnote 2Footnote 3Footnote 4Footnote 5Footnote 6Footnote 7Footnote 8Footnote 9Footnote 10Footnote 11 and has directly contributed to the northward spread of blacklegged ticks and LD into CanadaFootnote 12. As a result, LD has emerged in Canada and the number of reported cases of Lyme disease continues to riseFootnote 13Footnote 14.
The purpose of this overview is to summarize the climate and other environmental changes affecting the risk of ticks and tick-borne diseases (TBDs), identify the ticks and TBDs that are occurring or that may spread into Canada and describe the public health and clinical strategies for the management of ticks and TBDs.
The effect of climate and other environmental changes
Climate and other environmental changes are expected to increase the risk of ticks and TBDs in a number of ways. The prevalence, activity and range of a variety of ticks and the pathogens they carry are expected to increase. This is due to changing weather that also causes an increase in the range of animal reproductive and reservoir hosts. Humans are also expected to change their behaviours as the climate changes; bringing both animal hosts and humans into annual contact with ticks over a longer seasonFootnote 15Footnote 16. Tick and host habitats can also be affected by factors other than climate.
Increase in number, activity and range of ticks in Canada
In Canada, there has been a documented increase in temperature, changes in rainfall patterns and extreme weather events (extreme heat and rainfall) associated with climate changeFootnote 17. The key climate change effect that has influenced ticks and tick-borne pathogens in Canada, however, is increasing temperatureFootnote 5. Rising temperature has led to improved conditions for survival and reproduction of ticks and faster development leading to an acceleration of the tick lifecycleFootnote 5 that has:
- Increased tick abundance, where tick populations already occurFootnote 8
- Enabled tick populations to spread to higher latitudesFootnote 18Footnote 19Footnote 20Footnote 21Footnote 22Footnote 23
- Increased tick activity and questing behavior resulting in longer seasonal activityFootnote 5Footnote 24
Prolonged extremes values of temperature (high or low), low humidity and intense rainfall could adversely affect tick development by reducing their activity and increasing their mortality rateFootnote 5. These changes in temperature are expected to have less of an effect on ticks than on mosquitoes because of the tick’s ability to find refuge in their woodland habitatsFootnote 5.
Increase in number, activity and range of animal hosts
Animals that are reservoir and reproduction hosts are crucial for the transmission cycle of tick-borne pathogens and the tick lifecycle, respectively. The reservoir host is the source of the pathogen for the immature stages of the ticksFootnote 25. For most TBDs, the main reservoir hosts are wild rodents, including mice. The reproduction hosts are the source of blood-meals essential for adult female ticks to reproduce. In contrast, the most common reproduction host are deerFootnote 26Footnote 27. Climate change affects both the reproduction hosts and the reservoir hosts involved in the tick lifecycle and spread of TBDs, respectively (Figure 1). Increasing temperatures will expand the distribution range of both rodents and deerFootnote 28Footnote 29 as well as their abundance and activityFootnote 3Footnote 29.
Figure 1: Weather and climate drivers that favor ticks’ lifecycle and increase risk to humans
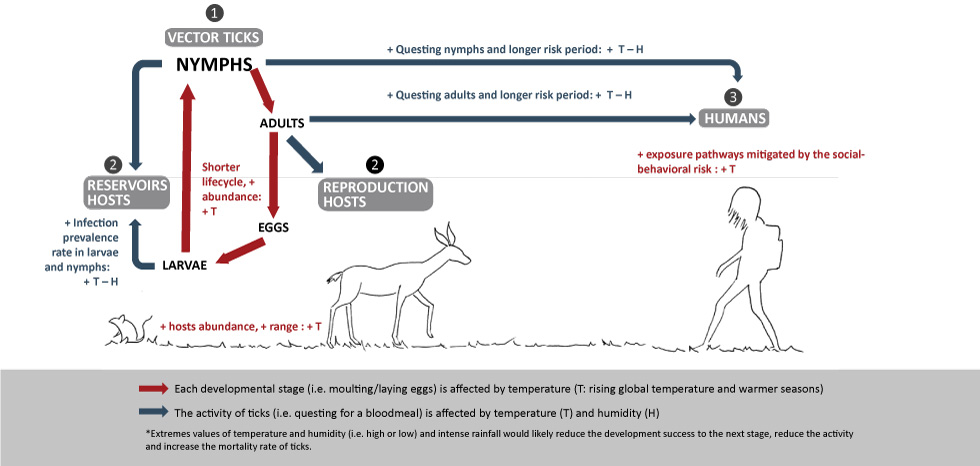
Text description: Figure 1
Figure 1: Weather and climate drivers that favor ticks’ lifecycle and increase risk to humans
This figure is a graphical illustration that shows the Key climate change drivers that are favoring the 1) vector ticks (i.e. development, activity, duration, and seasonality), 2) reservoirs and reproduction hosts, and 3) the likelihood of TBDs in humans depending on the social-behavioral risk.
Each developmental stage such as moulting/laying eggs is affected by the rising global temperature and warmer seasons. The activity of ticks (questing for bloodmeal) is affected by temperature (T) and humidity (H).
Increase in human exposure to ticks
Most ticks are active from the time that the snow melts in the spring until the reappearance of the snow cover in the fall. Typically, questing for a host begins when ambient air temperatures are 4–10°C. As a result of climate change, people may resume outdoor activity earlier in the spring and maintain it longer in the fall. With the increase in length of exposure to tick habitat, combined with an extended season of tick activity, there is an increased likelihood of tick exposure. In contrast, during consecutive hot and dry summer days (heat waves), both outdoor (human) activity and tick activity would likely be reduced. Overall, the risk of climate change on human exposure is more closely related to shorter winters, rather than extreme heat summer weather events.
The main risk groups for TBDs are those:
- Who are engaged in recreational or occupational outdoor activities (e.g. hunting, fishing, hiking, camping, gardening, mushroom or berries picking, dog walking, forestry and farming) in or near endemic areas
- Whose primary or secondary residence is located in or near endemic areas
- Who are either very young (5–9 years of age) or older (55 years of age and older)Footnote 30
Impact of other environmental changes
All tick species have preferred/optimal biomes and environmental conditions that, in part, determine their geographic distribution and consequently the areas of risk for humansFootnote 31. Microhabitat features, such as soil characteristics, are critical for tick survival and the successful establishment of new tick populationsFootnote 32Footnote 33Footnote 34. Modifications in habitat characteristics, in parallel with climate change, such as habitat fragmentation, loss of biodiversity, resource availability and land use, affect the dynamics of ticks, their animal hosts and the exposure of ticks to humansFootnote 29Footnote 35. As an historical example, LD emerged in the United States (US) in the 1970s as a consequence of the reforestation of farmland and the consequent increase in the deer populations, which allowed the expansion of the Ixodes scapularis tick populations that were carrying B. burgdorferiFootnote 3.
Increased tick-borne diseases in Canada
Lyme disease is the most common and well-known tick-borne disease in Canada. At least four other (non-LD) TBDs are emerging in Canada and these are anticipated to increase due to the effects of climate change: Anaplasmosis; Babesiosis; Powassan virus; and Borrelia miyamotoi disease.
Lyme disease
Lyme disease is caused by B. burgdorferi, which can infect I. scapularis ticks in central and eastern Canada and I. pacificus ticks in British Columbia. It has been reported in every province from British Columbia to Prince Edward Island (PEI) and it is well-known that LD is on the riseFootnote 13. Lyme disease typically presents with an erythema migrans rash and non-specific symptoms such as fatigue, fever, headache and muscle and joint pains and, if left untreated, can become a multisystem disease. Lyme disease is rarely fatal but deaths linked to Lyme carditis have recently been reportedFootnote 36.
Anaplasmosis
Anaplasmosis is caused by the bacterium Anaplasma phagocytophilum, which is spread by I. scapularis in eastern and central CanadaFootnote 37 and I. pacificus in British Columbia, and human or animal cases have been reported in most provinces where the ticks occurFootnote 38. Clinically, people can have asymptomatic A. phagocytophilum infections, but most frequently have non-specific symptoms (e.g. fever, headache and muscle aches). The case fatality rate is less than 1%Footnote 39.
Babesiosis
Babesiosis is caused by a malaria-like protozoan Babesia microti, which causes a Lyme-like disease. To date, human cases have been reported only in ManitobaFootnote 9 but the pathogen has been detected in I. scapularis ticks in Manitoba, Ontario, Quebec and New BrunswickFootnote 40. The case fatality rate in the US is 2%–5%Footnote 39.
Powassan virus
Powassan virus was first detected in Powassan, Ontario and can be found in a number of different tick species. Presentation of Powassan infection can vary greatly, from asymptomatic infections to fatal encephalitis cases (case fatality rate of 10%)Footnote 41. Although many of the tick-associated pathogens require an extended period of tick feeding prior to transmission, Powassan virus can be transmitted within 15–30 minutes of tick attachmentFootnote 42. Two lineages have been identified in vector ticks: Lineage I identified in Ixodes in Ontario, Quebec, New Brunswick and PEI; and Lineage II identified in I. scapularis from Manitoba, Ontario and Nova ScotiaFootnote 7.
Borrelia miyamotoi disease
Borrelia miyamotoi was first identified in 2013 in Canada and this pathogen has been found in I. scapularis and I. pacificus ticksFootnote 10. Borrelia miyamotoi disease is similar to LD signs but without a rash. Rarely, it can cause meningoencephalitis.
Rarer tick-borne diseases that may emerge
Dermacentor spp. ticks are common and can transmit the bacterium Rickettsia rickettsii, which causes Rocky Mountain spotted fever. Typically, fever, severe headache, myalgia, nausea and rash can occur 5 to 10 days after the infection. The estimated case fatality rate is around 5%–10%Footnote 39Footnote 43. Other spotted fever group rickettsial species may also be transmitted by Dermacentor ticks in Canada.
Colorado tick fever virus is currently found in some of the Western US states, and there have been a few reported cases in Saskatchewan and Alberta. It is spread by a tick common in western Canada: Dermacentor andersoni (or Rocky Mountain wood tick). Other Borrelia species have been found in the upper western and Midwestern states that have spread to a few cases in British Columbia and Ontario. Some Ehrlichia species have been found in the Southeastern and South Central US but there have been no human cases detected in Canada.
Table 1 summarizes the human pathogens associated with various tick species in Canada and those in the US that may spread north into Canada with climate change, identifies when a pathogens was first identified as a cause of TBD, its principal reservoir host species, current or historical geographic distribution and whether it has been detected in ticks, humans or other animals.
| Pathogen | Year of ID | Principal tick vector(s) | Principal reservoir host species | Geographic distributionFootnote a of Table 1 | Nationally notifiable | Detection in Canada | |||
|---|---|---|---|---|---|---|---|---|---|
| Canada | US | Tick | Human | Animal | |||||
| Anaplasma phagocytophilum | 1994 | Ixodes scapularis, Ixodes pacificus | Rodents | BC, AB, SK, MB, ON, QC, NB, NL, NS, PEI | Upper MW and NE states | No | Yes | Yes | Yes |
| Babesia microti | 1970 | Ixodes scapularis | Mice | MB, ON, QC, NB, NS | NE and upper MW states | No | Yes | Yes | Yes |
| Borrelia burgdorferi | 1982 | Ixodes scapularis, Ixodes pacificus | Rodents | BC, AB, SK, MB, ON, QC, NB, NS, NL, PEI | NE and upper MW states | Yes | Yes | Yes | Yes |
| Borrelia hermsii | 1935 | Ornithodoros hermsi | Rodents and rabbits | BC | Western states | No | - | Yes | - |
| Borrelia mayonii/ Borrelia mayonii-like | 2014 | Ixodes scapularis/Ixodes angustus | Rodents | ON, BC | Upper MW states: Minnesota and Wisconsin | No | Yes | - | Yes |
| Borrelia miyamotoi | 2013 | Ixodes scapularis, Ixodes pacificus | Mice | BC, AB, MB, ON, QC, NB, NS, NL, PEI | Upper MW, NE, and the Mid-Atlantic states | No | Yes | No | - |
| Colorado tick fever virus | 1946 | Dermacentor andersoni | Golden mantled squirrels, deer mice and rabbits | SK, AB | Western states: Colorado, Utah, Montana, Wyoming | No | No | Yes | - |
| Ehrlichia chaffeensis | 1987 | Amblyomma americanum | White-tailed deer | - | Southeastern and South Central states | No | No | No | - |
| Ehrlichia ewingii | 1999 | Amblyomma americanum | White-tailed deer | - | Southeastern and South Central states | No | - | - | - |
| Ehrlichia muris-like agent | 2011 | Ixodes scapularis/ Ixodes muris | Mice | MB | Upper MW states | No | Yes | - | - |
| Francisella tularensis | 1924 | Dermacentor variabilis, Dermacentor andersoni, Amblyomma americanum | Rabbits, hares, and rodents | Canada wide | All states | Yes | Yes | Yes | Yes |
| Heartland virus | 2012 | Amblyomma americanum | White-tailed deer | - | MW and South states | No | No | - | - |
| Lineage I Powassan virus | 1963 | Ixodes cookei, Ixodes marxi, Ixodes spinipalpis | Small and medium-sized woodland mammals (woodchucks) | ON, QC, NB, PEI | NE states and Great Lakes region | No | Yes | Yes | Yes |
| Lineage II Powassan virus | 2001 | Ixodes scapularis, Dermacentor andersoni | Mice | MB, ON, NS | NE and upper MW states | No | Yes | - | - |
| Rickettsia rickettsii | 1909 | Dermacentor variabilis, Dermacentor andersoni, Rhipicephalus sanguineus | Variety of wild mammals including rodents | BC, AB, SK, ON, NS | Eastern, Central, Western and Southwestern states | No | YesFootnote b of Table 1 | YesFootnote b of Table 1 | Yes |
Public health and clinical strategies
A key public health activity to address TBDs is surveillance. Detection of ticks, reporting of human cases and maintenance of accurate information on the overall risk of human exposure to ticks and their associated tick-borne pathogens are necessary to inform clinical care and public health actionFootnote 45. Currently, active and passive tick surveillance programs in Canada are focused mainly on I. scapularis, the main vector of LD. Surveillance efforts are centered on areas where LD did not previously exist or areas where it may not be recognized. As other TBDs emerge, the need for broader surveillance will follow.
Once the risk areas are identified geographically, education and awareness of risk and prevention strategies targeted to people who are at high risk of exposure is central to effective disease prevention. Due to the recent changing range of ticks, and hence the pathogens that they carry, this is especially important in newly-identified at-risk populations because knowledge and perception of risk are currently lowFootnote 46Footnote 47Footnote 48.
Health care providers play an important role in prevention education by informing their patients about the ways to reduce their exposure to vector ticks when travelling, both within Canada and abroad. Risk prevention strategies include applying personal protective measures and making tick checks routine after exposure in high risk areas. Clinicians are also critical for the early detection, obtaining laboratory confirmation and management of these illnesses. Public health efforts include surveillance, environmental modifications and management strategies for ticks and host animals.
Building capacity and awareness is important. For most TBD, early diagnosis and treatment are the most effective ways to reduce serious clinical outcomes. It is suspected that not all cases are currently being detected, reported and/or confirmed. TBDs have substantial clinical overlap (such as fever, headaches, myalgia, and arthralgia). In anticipation of an increase in the types of TBDs in Canada, clinicians need to be aware that if a patient presents with LD-like symptoms and has a negative test for LD, he/she could still have a TBD and further laboratory testing may be indicated. The absence of a rash should not rule out a TBD.
Discussion
In Canada, an ongoing process of emergence and spread of ticks and TBDs is anticipated in localities where climate, weather and habitat favor ticks and transmission cycles of tick-borne pathogens. It is important to note, however, that the relationship between tick-borne diseases and climate is not linear. There are modifiable risk factors that will affect the incidence of TBDs in Canada. These modifiable risk factors include both environmental and human factors. Environmental modification is not minor: one study indicated that removal of leaf litter (detritus and dead leaves) led to a 72%–100% reduction in ticksFootnote 49. Knowledge and risk perception about LD have been associated with the degree of adoption of personal tick bite preventive behaviors in CanadaFootnote 47Footnote 48; however, other factors need to be considered. Human population growth, movement and behavior, economics and politics have also been associated with differential rates of human exposure to ticks and the riskof transmission of TBDsFootnote 15Footnote 16Footnote 46. Rapid changes in socio-economic factors concurrent with the climate and other environmental changes underscore the importance of viewing the rising incidence and spread of TBDs as a complex socio-ecological problem, not driven just by climate or other environmental changes, and the need to quantify their relative contributions to the overall burden of disease.
The key climate change drivers and the social-behavioral factors that interact and determine the health outcomes from TBDs are noted in Figure 2. One of the challenges going forward will be to appreciate that the rising incidence and geographical spread of TBDs is a complex socio-ecological problem. This also provides opportunities for new intervention strategies. A few studies have addressed the human social-behavioral risk factors associated with TBDs in the context of adaptation to climate changeFootnote 46Footnote 47Footnote 48Footnote 50. More sociological studies are needed. Psycho-behavioral studies are also needed to assess how the knowledge that climate change will increase ticks and associated TBDs may be a motivating factor. Finally, it will be important to look for resilience factors or the adaptive capacity of the individuals or communities at risk to minimize the risk of TBDs.
Figure 2: Key climate changes, ecological factors and social-behavioral risks that affect the acquisition of tick-borne diseases
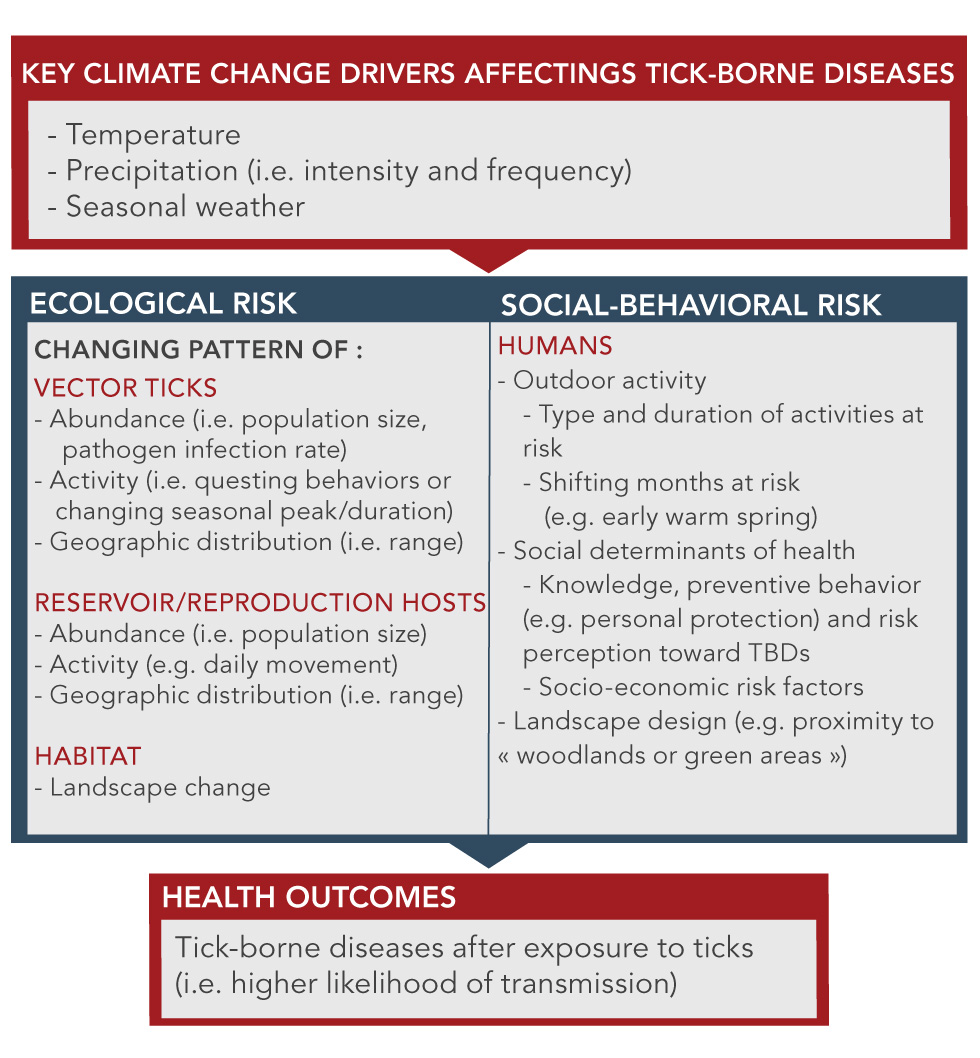
Text description: Figure 2
Figure 2: Key climate changes, ecological factors and social-behavioral risks that affect the acquisition of tick-borne diseases
This figure is a diagram that shows the key climate changes, ecological factors and social-behavioral risks that affect the acquisition of tick-borne diseases. It is made up of 4 boxes. The first box at the top identifies the key climate change drivers as: temperature, humidity, precipitation (including intensity and frequency) and seasonal weather. There is an arrow pointing from the bottom of the top box to two side-by-side boxes, identified as changing patterns of risk factors: Ecological risk and Socio-Behaviour risk.
In the ECOLOGICAL RISK box there are 3 categories with bullets under each:
VECTOR TICKS
- Abundance (i.e. population size, pathogen infection rate)
- Activity (i.e. questing behaviors or changing seasonal peak/duration)
- Geographic distribution (i.e. range)
RESERVOIR/REPRODUCTION HOSTS
- Abundance (i.e. population size)
- Activity (e.g. daily movement)
- Geographic distribution (i.e. range)
HABITAT
- Landscape change
In the SOCIAL-BEHAVIORAL RISK box there is just one category – HUMANS – and under this there are three bullets with sub-headings under each:
- Outdoor activity
- Type and duration of activities at risk
- Shifting months at risk (e.g. early warm spring)
- Social determinants of health
- Knowledge, preventive behavior (e.g. personal protection) and risk perception toward TBDs
- Socio-economic risk factors
- Landscape design (e.g. proximity to « woodlands or green areas »)
There is an arrow pointing from the bottom of the two side-by-side boxes to the lowest box, identified as HEALTH OUTCOMES. There is one health outcome noted: Tick-borne diseases after exposure to ticks (i.e. higher likelihood of transmission) thus illustrating that climate changes are mediated through both ecological and socio-behavioural risk factors to impact on the incidence of tick-borne diseases.
Conclusion
The expanding geographic range of tick vector species, and the diseases they carry, creates a moving target for clinicians and public health authorities. The clear link with climate change is an opportunity to increase the motivation to address current and emerging TBDs in Canada. While work on addressing climate change will continue more broadly, there is an opportunity to work on other modifiable risk factors that affect TBDs in Canada, appreciating that this is a complex socio-ecological challenge.
Authors’ statement
The authors would like to thank the anonymous reviewers and editorial staff of the Canada Communicable Disease Report who provided helpful suggestions that greatly improved the quality of this manuscript.
- CB — Conceptualization, writing: original draft, review and editing
- AD — Writing: original draft, review and editing
- JK — Writing: original draft, review and editing
- HW — Writing: original draft, review and editing
- PL — Writing: original draft, review and editing
- LR — Writing: original draft, review and editing
Conflict of interest
None.
Funding
This work was supported by the Public Health Agency of Canada.
