A laboratory checklist for infectious disease outbreaks

 Download this article as a PDF
Download this article as a PDFPublished by: The Public Health Agency of Canada
Issue: Volume 46–10: Laboratory Biosafety
Date published: October 1, 2020
ISSN: 1481-8531
Submit a manuscript
About CCDR
Browse
Volume 46–10, October 1, 2020: Laboratory Biosafety
Implementation science
Laboratory response checklist for infectious disease outbreaks—preparedness and response considerations for emerging threats
Tracie EisBrenner1, Graham Tipples2,3, Theodore Kuschak1,3,4, Matthew Gilmour1,3,4
Affiliations
1 National Microbiology Laboratory, Winnipeg, MB
2 Alberta Provincial Laboratory for Public Health, Alberta Precision Laboratories, Edmonton, AB
3 Canadian Public Health Laboratory Network
4 Global Health Security Action Group Laboratory Network
Correspondence
Suggested citation
EisBrenner T, Tipples G, Kuschak T, Gilmour M. Laboratory response checklist for infectious disease outbreaks—preparedness and response considerations for emerging threats. Can Commun Dis Rep 2020:46(10):311–21. https://doi.org/10.14745/ccdr.v46i10a01
Keywords: checklist, COVID-19, Ebola, emerging infectious disease, emergency management, high consequence pathogens, infectious disease, laboratory response, outbreak preparedness, outbreak response, public health laboratory, SARS, SARS-CoV-2, Zika
Abstract
The purpose of the Laboratory Response Checklist for Infectious Disease Outbreaks (the Checklist) is to provide public health laboratories and laboratory networks operating at multiple jurisdictional levels with a useful, adaptable tool to help rapidly identify important outbreak response considerations, particularly when investigating a previously unknown infectious disease threat. The Checklist was developed by the National Microbiology Laboratory of Canada in collaboration with provincial/territorial, national and international laboratory experts, including the Canadian Public Health Laboratory Network, and the Global Health Security Action Group Laboratory Network. While the Checklist was initially designed to reflect lessons learned through National Microbiology Laboratory participation in extended national and international outbreak responses (e.g. Zika virus epidemic [2015–2016], Ebola virus epidemic, West Africa [2014–2016]), the importance of optimizing laboratory response coordination has only been underscored by the ongoing challenges presented by the coronavirus disease 2019 (COVID-19) pandemic response requirements. The Checklist identifies five highly interdependent laboratory response themes, each of which encompasses multiple considerations that may be critical to a coordinated, strategic outbreak response. As such, the comprehensive review of Checklist considerations by responding laboratory organizations may provide a valuable opportunity to quickly detect key response considerations and interdependencies, and mitigate risks with the potential to impact public health action.
Introduction
Infectious disease outbreak response poses unique challenges and considerations for laboratories, particularly when investigating a previously unknown or newly defined infectious diseaseFootnote 1. Through extensive National Microbiology Laboratory (NML) participation in national and international outbreak and pandemic responses, a number of key considerations and lessons learned have been highlighted. These include the potential need for responding laboratories to 1) rapidly develop and deploy novel pathogen-specific diagnostic testing methods, 2) participate in collaborative, iterative development of case definitions and testing criteria to reflect evolving scientific evidence as an outbreak progresses, 3) strategically engage public health partners to optimize response capacity and coordination, and 4) establish information sharing processes and procedures that support timely public health laboratory (PHL) investigation, surveillance, research, public health messaging and actionFootnote 2. Continually evolving public health genomics and other "omics" approaches present additional challenges, and provide valuable opportunities to further enhance infectious disease response capacityFootnote 3Footnote 4. Given the complex, outbreak-specific nature of laboratory response considerations, timely and effective coordination can prove challenging in the absence of a strategic and structured approach.
The potential usefulness of a checklist approach to strengthen laboratory preparedness and response coordination was most recently emphasized by extended NML engagement in national and international response efforts, in particular the Ebola virus epidemic in West Africa (2014–2016) and the Zika virus epidemic (2015–2016)Footnote 5Footnote 6Footnote 7Footnote 8. Within this context, development of a checklist tool was also considered in alignment with desired outcomes articulated in the Canadian Public Health Laboratory Network Strategic Plan, 2016–2020; including “Priority 2: Strengthen coordinated response capacity to address established, emerging and re-emerging infectious disease pathogens and public health threats”Footnote 9.
A review of the literature at the time, however, yielded few publicly available references with checklist content related to emerging and high-consequence infectious disease response. Notably, these references either lacked a strong focus on the laboratory component of public health resilience, or did not describe laboratory response considerations independently of organizational, jurisdictional or infectious disease-specific contextsFootnote 2Footnote 10Footnote 11. The recent emergence of the novel coronavirus infectious disease (coronavirus disease 2019, COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has only served to underscore the critical importance of timely and coordinated laboratory response to support public health actionFootnote 12.
The Laboratory Response Checklist for Infectious Disease Outbreaks (the Checklist) was developed to provide laboratory organizations with a useful, adaptable tool to rapidly identify key outbreak response considerations using a systematic approach, particularly when investigating a previously unknown or a newly defined infectious disease threat. To encompass considerations associated with larger scale, protracted laboratory responses, the Checklist was significantly informed by lessons learned through prior NML response efforts to Ebola, Zika, Severe Acute Respiratory Syndrome (SARS) in 2003, and by continuing COVID-19 response effortsFootnote 5Footnote 6Footnote 7Footnote 8Footnote 13Footnote 14. As laboratory-related response roles are expected to differ between organizations and jurisdictional levels, the Checklist content is not intended to be prescriptive. Rather, the Checklist was designed to broadly capture the scope of response considerations that may be relevant at all levels, while supporting content customization to reflect setting-specific requirements. As such, the Checklist is envisioned as a complementary tool to existing laboratory preparedness and response plans and protocols.
Methods
An NML working group (WG) was convened to inform development of the Checklist tool to support timely coordination of laboratory response efforts. The WG composition included multiple program representatives with prior experience fulfilling NML Emergency Operations Centre (EOC) roles during outbreak response activationsFootnote 15. The WG members identified requirements and challenges encountered when responding to infectious disease outbreaks and emerging threats both domestically and internationally. Information gathered was used to draft response considerations for a preliminary version of the Checklist. To support implementation by laboratory organizations operating at various jurisdictional levels, the Checklist development was guided by the need to incorporate the following key attributes: adaptability; acceptability; scalability; and ease of useFootnote 16.
To enhance adaptability of the Checklist, response considerations were described at a high level using generic, context-neutral terminology wherever possible. This approach was taken to allow users to readily modify content by incorporating preferred, organization and jurisdiction-specific terminology and operational requirements, thereby expanding the potential scope of implementing laboratory organizations and networks at the regional, provincial/territorial/state, national and international levels. The content of proposed response considerations was assessed, and five laboratory response themes were identified to support ease of use. Content within each theme was developed to be scalable, allowing users to expand or edit the scope of the Checklist items in alignment with response activities relevant to the role of the implementing laboratory organization. The resulting Checklist Review Table (Supplemental I) is intended to be replicable and adaptable using preferred spreadsheet or database software, offering the ability to flexibly select, sort and monitor the status of flagged items following user. To further support ease of use, a number of approaches to classify, customize and prioritize the Checklist items of interest were developed for user consideration (Supplemental II).
The draft Checklist was piloted using a scenario-based tabletop review exercise engaging NML EOC personnel and interjurisdictional laboratory liaison staff. Exercise input was used to refine content and to consider how to best operationalize the Checklist to enhance emergency response protocols. A supplementary NML Response Toolkit capturing laboratory contacts, references and resources specific to the Canadian context was concurrently developed to support NML implementation, and as a model adaptable by others (available upon request). To further verify content acceptability and validity, the Checklist and ‘NML Response Toolkit’ were distributed for review by federal and provincial/territorial laboratory experts (NML, Canadian Public Health Laboratory Network [CPHLN]), and internationally by the Global Health Security Action Group Laboratory Network. The Checklist was originally distributed in two forms: as a sortable ‘Checklist Review Table’ (Microsoft Excel 2010) (Supplemental I), and as a conventional ‘checkbox’ formatted ‘Checklist’ document (Microsoft Word 2010) (Supplemental III). External review input was addressed to produce a final reference version of the Checklist, which was then circulated to NML, CPHLN and Global Health Security Action Group Laboratory Network stakeholders in 2018. Interjurisdictional review indicated that the Checklist was well aligned with previously identified core PHL functions, and with priorities outlined in the Canadian Public Health Laboratory Network Strategic Plan, 2016–2020Footnote 9Footnote 17Footnote 18. Content was subsequently updated to produce the current version, which includes COVID-19 laboratory response context.
The Checklist
The Checklist encompasses five central themes: laboratory investigation; laboratory response capacity and training; laboratory surveillance and data management; interjurisdictional engagement and communication; and research and ethics (Supplementals I and III). Each theme includes multiple considerations identified as having the potential to impact a strategic laboratory response.
1. Laboratory investigation
When investigating a new or emerging pathogen, early response considerations include developing, validating, sharing and implementing evidence-based testing protocols and recommendations in close collaboration with interjurisdictional laboratory partners. Establishing standardized laboratory-based case confirmation criteria may also be prioritized to support consistent case reporting and surveillance across impacted jurisdictionsFootnote 2. The rapid development of testing capacity was a critical consideration from the outset of Canada’s national COVID-19 response. In anticipation of the arrival of COVID-19 in Canada, NML worked to develop molecular diagnostic testing methods that were used to successfully confirm the first presumptive-positive COVID-19 case in January 2020. NML continued to provide confirmatory reference testing and quality assurance support to responding provincial and territorial PHL partners to ensure the ongoing accuracy of national case detectionFootnote 19.
Response efforts may also demand sustained, high-volume front-line screening and/or confirmatory testing in excess of routinely available laboratory capacity. Under these circumstances, implementing testing criteria and triage protocols based upon known risk factors may become a consideration to prioritize laboratory investigations appropriately and to manage limited resources. This was a key NML consideration during its initial domestic response to the Zika epidemic, which posed significant capacity challenges as demand for Zika testing persisted at elevated levels well beyond the initial 2015–2016 response periodFootnote 6. During early COVID-19 response, Canadian provinces and territories similarly established testing criteria to prioritize testing within existing laboratory capacities. This approach was taken as overwhelming global demands on international supply chains significantly impacted the ability to rapidly secure necessary laboratory supplies, equipment and reagents; including personal protective equipment (PPE), specimen collection swabs, viral transport media, test kits, reagents and testing platformsFootnote 20Footnote 21.
The Checklist identifies various response considerations with the potential to influence the timely coordination of laboratory investigation, ranging from requirements for specimen collection and transportation, to laboratory testing and result reporting. These considerations include 1) the ability to collect, store and transport specimens to meet specimen acceptance criteria for testing, 2) biosafety, biosecurity and infection control considerations, 3) legislative and regulatory requirements, 4) laboratory testing and case confirmation criteria and 5) triaging protocols for priority testing. Quality control and standardization of testing methods and result reporting processes were considered essential to all aspects of a coordinated laboratory response.
2. Laboratory response capacity and training
For responding public health laboratories, an early capacity consideration may be the availability of validated, pathogen-specific front-line screening and confirmatory diagnostic test methods with known performance characteristics. National reference laboratories may be expected to maintain the capability to rapidly develop and validate new test methods when external capacity either does not exist, or may not be reliably available under outbreak circumstancesFootnote 2. Public health laboratories may also be required to participate in coordinated, interjurisdictional sourcing and ongoing clinical validation of laboratory testing methods, equipment and supplies; particularly when dealing with multiple and/or changing vendors to manage supply chain continuity issues, as during the COVID-19 responseFootnote 21. Time-sensitive, resource-intense requirements of this nature can place considerable demands on responding scientific and technical staff, as there is a parallel need to maintain routine, mandated program activities.
Laboratory response capacity may be further challenged as personnel are impacted by government-mandated measures implemented to prevent community-level transmission of a newly emerging infectious disease (EID) threat, or by contracting EID-associated illness. As experienced during the COVID-19 response, such measures may include extended periods of self-isolation/quarantine due to confirmed disease, symptomatic illness or potential exposure (case-contact, travel history); as well as "remote work" and alternate work arrangements required to manage child and family-care issues resulting from school closures and other stay-at-home and physical distancing measuresFootnote 22Footnote 23.
Engagement and mobilization of personnel with response-essential skills and expertise may play a pivotal role in addressing surge capacity challenges both internal and external to a responding laboratory organization, as was demonstrated by the NML domestic responses to COVID-19 and Zika, and through the international deployment of mobile laboratory response teams to support on-site Ebola virus testing during the 2014–2016 Ebola epidemic in West AfricaFootnote 5. NML also deployed mobile laboratory capacity domestically to support testing of recently returned Canadian travellers at quarantine sites during the initial COVID-19 pandemic response.
Depending upon the magnitude and duration of response requirements, decentralization of diagnostic testing and other technology transfer activities may also be prioritized to expand interjurisdictional laboratory capabilities on a temporary or long term basis, and to improve access by remote or isolated populationsFootnote 24. During the Canadian domestic responses to Ebola, Zika and, most recently, COVID-19, NML and its provincial PHL counterparts worked collaboratively to support decentralization of front-line laboratory testing within select jurisdictions to improve the distribution of response capabilities where feasible, while maintaining centralized national capacity for reference and confirmatory testingFootnote 21Footnote 25.
The ability to flexibly address capacity and capability challenges specific to new or EID threats was deemed critical to response efforts. Related considerations include the collaborative identification of laboratory response capabilities with interjurisdictional public health partners, and the dynamic assessment of surge capacity and training requirements to support response-critical activities.
The Checklist highlights various considerations that may be explored if additional surge capacity is required. These include investigating alternate approaches to enhance laboratory test throughput and information sharing, engaging EOC site support for response coordination, and identifying surge capacity personnel with in-demand skillsets through response-focused personnel inventory processes. Surge positions may be cross-trained and mobilized to alternate laboratory sites within or external to the organization, or deployed to the field under the oversight of senior scientific staff as part of a mobile laboratory response team.
3. Laboratory surveillance and data management
Laboratory result data are well-recognized as a critical input to support infectious disease-related epidemiological investigations, surveillance and public health actionFootnote 26Footnote 27. NML response activities have emphasized that laboratory investigations are often similarly dependent on the timely availability of epidemiological data to inform test triaging processes, the selection of appropriate diagnostic and confirmatory testing algorithms, and appropriate interpretation of test results. During the domestic response to Zika, the ability to triage and route specimen testing using known risk factors relied on the provision of epidemiological and clinical data as part of the test requisition process (e.g. pregnancy status, travel history and onset date for symptomatic cases). Interjurisdictional linkage of NML test results with cases under investigation was similarly reliant on the provision of appropriate unique identifiersFootnote 28.
To support timely, integrated laboratory-based surveillance and data management, early response considerations may include rapid, iterative, consensus-based development of an infectious disease case definition that incorporates laboratory and epidemiological case confirmation criteria relevant within the current outbreak contextFootnote 24. Integral to this process is the identification of data elements required for laboratory investigation, case confirmation and surveillance efforts. Alignment of confirmation criteria and data element requirements between reporting jurisdictions may warrant consideration to ensure consistent case detection, reporting and surveillance; and comparability of subnational, national and international surveillance data wherever possible. An interrelated surveillance consideration is the ability to rapidly link laboratory results with cases under investigation, and to link confirmed cases with an outbreak or outbreak source. Linkage and data integration may prove challenging when laboratory and epidemiological data elements relevant to case investigations are generated or collected by separate public health jurisdictions.
Laboratory-based surveillance and response activities may be further enhanced by implementing standardized public health genomics approaches and other advanced molecular epidemiology tools to improve pathogen and outbreak detection, characterization, source attribution and transmission pattern identification. Use of whole genome sequencing methods to support real-time, laboratory-based surveillance of select pathogens also offers the promised advantage of unambiguous nomenclature for interjurisdictional comparison purposesFootnote 29.
The Checklist outlines a number of considerations that may impact laboratory-based surveillance and data management. A review of data flow requirements may be valuable at the outset of an EID threat response, including the need for standardized approaches to document, monitor and report laboratory investigation results and outbreak summary information to meet the intelligence requirements of various stakeholders (e.g. lab-confirmed case reporting by jurisdiction, percent positive tests versus total tests performed for target groups/populations). It may also be helpful to explore the potential of existing laboratory information management systems, web-based public health informatics and surveillance platforms, and other enabling tools to flexibly meet pathogen-specific surveillance and response requirements. As observed during NML participation in early COVID-19 response activities, desired functions may include timely and secure data collection, linkage and integration; laboratory test result and surveillance indicator reporting, public health alerting and predictive modelingFootnote 30Footnote 31Footnote 32. For laboratory partners implementing "omics" approaches to enhance outbreak detection and response capabilities, longer term considerations may include operational and infrastructure support requirements for data transfer pipelines and bioinformatics tools used to transmit, acquire, analyze, interpret and report whole genome sequencing and other "omics" resultsFootnote 27Footnote 32Footnote 33. Interjurisdictional sharing of laboratory-generated surveillance data may also require consideration within the context of relevant legislative and regulatory frameworks and information sharing agreements, in alignment with established jurisdictional and organization-specific public health rolesFootnote 33Footnote 34Footnote 35Footnote 36Footnote 37.
4. Interjurisdictional engagement and communication
To support evidence-based development of laboratory guidelines, clinical recommendations, public health surveillance and intervention strategies, it may be important to consider strengthening intelligence-sharing mechanisms with relevant public health partners and interjurisdictional networksFootnote 14Footnote 38. This may involve exploring alternative collaborative and multi-disciplinary approaches. Within Canada, CPHLN serves a critical function by providing an established, secretariat-supported network of national, provincial and territorial PHL experts to support timely consensus-based interjurisdictional development of response strategies, recommendations and guidelinesFootnote 9.
NML response efforts have also highlighted the importance of clearly identifying event-specific communication and reporting structures to support effective internal and external routing of requests and information. This includes consistent messaging using "single-window" points of contact for responding programs and task groups within the laboratory organization whenever possible. Such considerations are not unique to laboratory response; the need for clearly articulated leadership roles and communication structures was also identified in an assessment of broader public health resilience considerations related to community-level Ebola virus disease response in the United StatesFootnote 10. To support the routing of time-sensitive external requests, NML has had success with the provision of single-window access to laboratory support via a 24-hour emergency contact number, combined with site-based EOC support to centrally coordinate and direct requests within an incident command system contextFootnote 15. NML also maintains a public-facing, web-based Guide to Laboratory Services as a reference for external test requisitionsFootnote 28.
Increased frequency of time-sensitive interjurisdictional engagement and risk communication requirements may pose ongoing challenges to response coordination, particularly when a health event generates significant public concern, media interest and political attention over a prolonged time period. Laboratory subject matter experts responsible for organizational response may also be those most in-demand to address information requests from multiple sources.
As identified in the Checklist, a strategic communication strategy may become crucial to safeguard the valuable time of responding staff, facilitate coordinated stakeholder engagement, and ensure consistent messaging of public health intelligence to meet audience-specific needs. Use of social media tools and other web-based platforms may provide opportunities to improve the accessibility of laboratory guidance to public health professionals, the media and the public.
5. Research and ethics
In Canada, active engagement in public health-related research is considered a core PHL function, as the ongoing maintenance of this scientific capacity provides the required foundation for responsive public health actionFootnote 13Footnote 17. As demonstrated during the 2003 SARS coronavirus outbreak response, immediate laboratory-related public health research priorities may include rapid pathogen identification and characterization, including genomic sequencingFootnote 39. Launched in response to the 2020 COVID-19 coronavirus pandemic, the Canadian COVID Genomics Network initiative will, with public health laboratory engagement, create a “virus to patient” genomic database through large-scale sequencing of host and viral genomes to support national and international research into viral pathogenicity, evolution and health outcomes; as well as vaccines and therapeuticsFootnote 40.
Other immediate response priorities may include the development and validation of diagnostic testing methods, and applied biosafety research with a focus on timely knowledge translation. Pathogen transmission and vector competence studies may also be prioritized to help characterize risk and inform prevention strategies. As during the Zika response, this may be particularly relevant when the potential for introduction and sustained transmission of an emerging vector-borne disease has yet to be assessed for non-endemic settingsFootnote 41.
To reduce morbidity and mortality within at-risk populations, national research priorities may extend to collaborative development and implementation of public health interventions including vaccines and other medical countermeasures, as evidenced by the NML response to Ebola virus diseaseFootnote 42Footnote 43. Continuing COVID-19 pandemic response efforts have demonstrated the imperative need to understand the host immune response to infection, which is required to inform laboratory testing strategies to determine individual and population-level immune status (e.g. via seroprevalence studies), as well as therapeutic and vaccine development and implementation strategiesFootnote 44. In April 2020, the Government of Canada launched the COVID-19 Immunity Task Force, bringing together national experts in academia, hospitals and public health to help address outstanding questions related to COVID-19 immunity, including 1) immune status and duration of immunity post-infection and 2) the scope of population-level immunity to support national epidemic response effortsFootnote 45.
When faced with an EID threat, there may be an urgent need to strategically direct public health research activities to bridge important gaps in scientific knowledge and technical capabilities. Levels of research engagement may vary considerably amongst laboratory entities, contingent upon jurisdictional context and mandated public health responsibilities.
As outlined in the Checklist, an early response consideration may be to prioritize collaborative research activities within existing resources and capabilities. Research priorities may include pathogen identification and characterization studies, diagnostic and reference testing method development and validation studies, host and population-level immunity-related research, transmission and vector competency studies, vaccine and other medical countermeasure development and assessment, applied biosafety research and public health surveillance studies. To streamline response coordination, it may also be helpful to clearly differentiate between applied public health research and surveillance activities that are integral to routine laboratory response, and other targeted research activities that will require prior consent and completion of research ethics approval processes.
The EID response task forces and networks may also be convened to pursue high priority, time-sensitive research initiatives, engaging public health laboratory expertise along with other scientific experts in academia, hospitals and the private sector. When collaborative research involves interjurisdictional partners or multi-disciplinary teams, considerations may involve coordinating multiple research ethics board review processes, and addressing issues related to authorship and intellectual property in a manner that supports timely publication to inform public health decision-making.
Discussion
Interdependence of Public Health Laboratory functions
When assessing and prioritizing laboratory preparedness and response considerations, it is important to note that significant interdependencies exist between key PHL functions, which include the following: diagnostic and reference testing; infectious disease surveillance; outbreak preparedness and response; and basic and applied research (Figure 1). At the interface of these cyclic, adjacent PHL functions are highly interrelated EID response requirements. A robust public health response capable of meeting each of these mutually interdependent requirements is reliant upon the available capacity of the PHL system to fulfill each of its core functions.
Figure 1: Interdependencies of Public Health Laboratory response functions
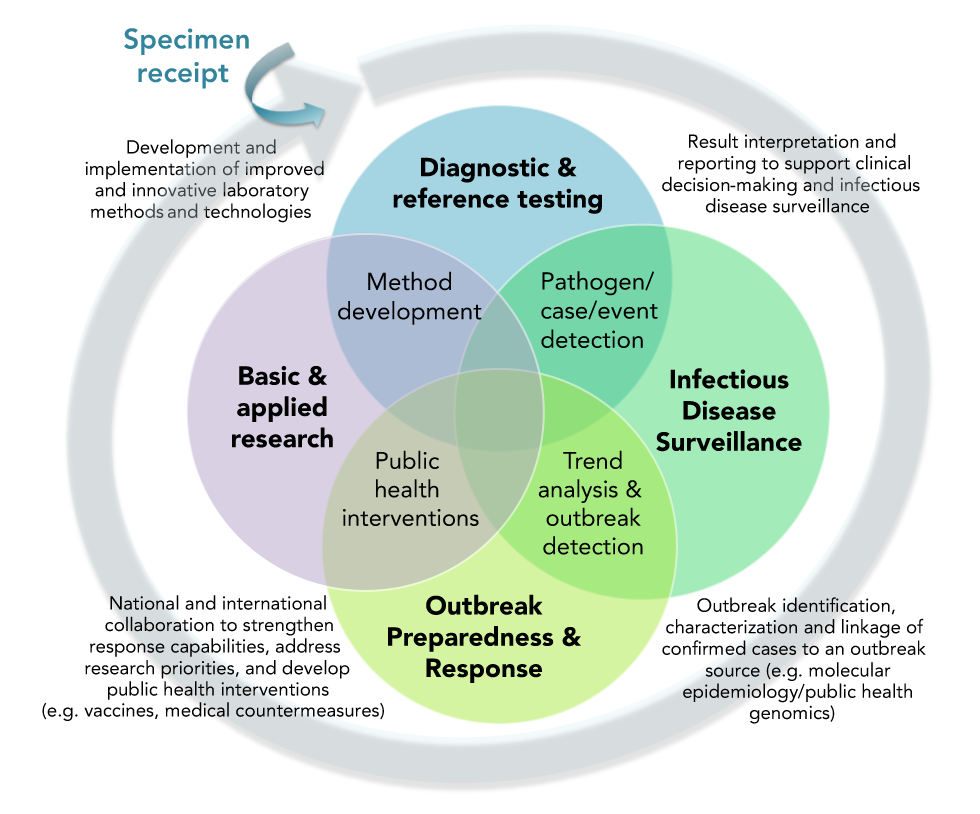
Text description: Figure 1
Figure 1: Interdependencies of Public Health Laboratory response functions
Figure 1 is a Venn diagram comprised of four overlapping circles, with each circle representing a distinct public health laboratory function, and each area of overlap indicating its interdependency with the immediately adjacent function. The Venn diagram is circumscribed by a circular, clockwise-oriented arrow to indicate the cyclical, ordinal nature of the four functions, beginning with laboratory receipt of a specimen for testing, which is depicted as an external input to the Venn diagram. Interdependencies indicated by areas of overlap between the four public health laboratory functions include laboratory capabilities for the following:
- Pathogen, case and event detection, including the interpretation and reporting of test results to support both clinical decision-making and infectious disease surveillance activities
- Trend analysis and outbreak detection, including disease detection and monitoring activities to support timely outbreak identification, characterization and the linkage of confirmed cases to an outbreak source (e.g. using molecular epidemiology/public health genomics methods)
- Public health interventions, including national and international collaboration to strengthen response capabilities and advance public health intervention research, including the development of medical countermeasures such as vaccines and other therapeutic approaches
- Method development, including development and implementation of improved and innovative laboratory methods and technologies
Figure 1 is a Venn diagram comprised of four overlapping circles, with each circle representing a distinct PHL function, and each area of overlap indicating its interdependency with the immediately adjacent function. The Venn diagram is circumscribed by a circular, clockwise-oriented arrow to indicate the cyclical, ordinal nature of the four functions, beginning with laboratory receipt of a specimen for testing, which is depicted as an external input to the Venn diagram. Interdependencies indicated by areas of overlap between the four public health laboratory functions include laboratory capabilities for the following:
- Pathogen, case and event detection, including the interpretation and reporting of test results to support both clinical decision-making and infectious disease surveillance activities
- Trend analysis and outbreak detection, including disease detection and monitoring activities to support timely outbreak identification, characterization and the linkage of confirmed cases to an outbreak source (e.g. using molecular epidemiology/public health genomics methods)
- Public health interventions, including national and international collaboration to strengthen response capabilities and advance public health intervention research, including the development of medical countermeasures such as vaccines and other therapeutic approaches
- Method development, including development and implementation of improved and innovative laboratory methods and technologies
Within the context of interdependent PHL functions, it becomes apparent that individual considerations identified within each of the five Checklist response themes may have important, far-reaching implications for interrelated response activities within the broader public health context. For example, the use of standardized laboratory investigation methods for case detection (Theme 1: Laboratory investigation) may facilitate reliable, well-integrated surveillance mechanisms which, in-turn, allow timely interjurisdictional case and outbreak detection and monitoring (Theme 3: Laboratory surveillance and data management). Public health intelligence generated through high-quality, purpose-based surveillance activities and shared in a timely manner via collaborative expert networks (Theme 4: Interjurisdictional engagement and communication) may then enable the identification of research priorities to inform short and long term preparedness and response efforts (Theme 5: Research and ethics). Strategic mobilization and training of surge capacity positions may be required to support priority activities across the response continuum (Theme 2: Laboratory response capacity and training).
When operating within resource-constrained environments and under significant time pressures, oversights during the initial planning phase have the potential to impact overall response effectiveness in a variety of ways. Impacts may include a lack of resource allocation to support overlooked laboratory response priorities, delays or disruptions to the sharing of scientific intelligence or the acquisition and distribution of materiel and biologicals, the absence of appropriate laboratory representation at key decision-making tables and other downstream challenges. The importance of rapid funding mechanisms was highlighted by the World Health Organization following the response to Ebola in West Africa, as “disease outbreaks often move faster than the money allocated to respond to them”Footnote 46.
A particular challenge may be the ability to maintain routinely mandated activities in parallel with the demands of evolving laboratory response efforts, and may require careful assessment of workforce surge capacity and prioritization of activities. For example, internal reallocation of highly-trained staff from existing programs to meet immediate surge response requirements may create significant operational gaps throughout the organization that must also be addressed to maintain overall public health response capacity. On a long term basis, gaps in expertise may cumulatively impact laboratory capacity over the course of multiple, sequential and/or concurrent response efforts. Such capacity shortfalls may not easily be remedied using short term approaches in the face of an emerging threat. This effect was observed by NML shortly after the commencement of COVID-19 response activities, and has been an ongoing consideration associated with prior extended infectious disease responses. While first identified as an issue in the 2003 Naylor Report which detailed lessons learned in the wake of Canada’s public health response to the SARS pandemic, the potential impacts of more recent, post-2014 shortfalls in public health expertise and resources on overall public health response capacity in Canada were again emphasized by Dr. David Butler-Jones on February 3, 2020; just days after the World Health Organization declared the novel 2019–nCoV coronavirus outbreak to be a Public Health Emergency of International Concern on January 30, 2020Footnote 13Footnote 47Footnote 48.
Checklist implementatio—additional considerations
The multifaceted, interdependent nature of laboratory response considerations poses various challenges to high-level coordination efforts. In the absence of a strategic approach, there is a real risk that not all considerations relevant to the response context will be identified for action in a timely manner.
In the Canadian setting, as in others, numerous guidance documents exist to provide in-depth, pathogen and disease-specific response recommendations. These range from the standardized testing methods, operating procedures and outbreak response protocols used within laboratory environments, to emergency planning and response guidelines used both organizationally and by interjurisdictional laboratory networksFootnote 28Footnote 49Footnote 50Footnote 51. Jurisdiction-specific legislative and regulatory requirements define the parameters within which laboratory response activities are conducted to support biosafety, biosecurity and privacy of information; while other regulations and multi-lateral information sharing agreements (e.g. Multi-Lateral Information Sharing Agreement [MLISA]) set-out disease-specific requirements and principles for interjurisdictional information sharing to support timely surveillance and outbreak responseFootnote 34Footnote 35Footnote 52Footnote 53.
Operationally, the purpose of the Checklist is to serve a complementary function relative to other, more prescriptive, context-specific laboratory guidance documents. Implementation of a flexible, non-prescriptive Checklist tool is proposed as a means to facilitate overall response coordination by quickly identifying and prioritizing relevant considerations across multiple response themes.
While the Checklist was designed for ease of use in its current reference form, it is recognized that not all Checklist considerations may be relevant or within the normal scope of response activities for a given laboratory entity (e.g. targeted research, development of medical counter-measures). As such, additional customization of Checklist content and terminology to reflect site-specific laboratory roles and responsibilities may optimize overall usefulness. Functionality may be further enhanced by developing supplementary appendices to capture important jurisdiction-specific references and resources, and to help direct further action regarding any considerations identified as relevant during a given Checklist review process.
Implementing organizations and networks may also wish to consider preferred approaches to engage participants in the Checklist review process, including the balance of subject matter expert and working-level representation needed to reflect the scope of potential response activities. It may be helpful to identify operational triggers that might prompt a formal Checklist review, recognizing that such triggers may vary under outbreak and inter-outbreak circumstances, and may be internal or external to the responding organization. For example, comprehensive Checklist review may be considered an immediate organizational priority when responding to infectious disease threats for which response protocols have yet to be developed. Alternately, review may be initiated in response to a relevant external trigger, such as the identification of a potentially high-consequence EID threat, or the formal declaration of a Public Health Emergency of International Concern by the World Health OrganizationFootnote 36.
Checklist implementation may be considered operationally within the context of existing Emergency Management Program planning tools to enhance mitigation, preparedness, response and recovery functions in alignment with the four phases of emergency managementFootnote 15. For example, a Checklist review process may be initiated in the event that response efforts require centrally coordinated surge support, including formal activation of an EOC associated with the laboratory organization. Review by a subject matter expert group representative of programs engaged in, or potentially impacted by, response activities may be initiated and coordinated by laboratory management or via the EOC. High-level considerations, gaps and proposed action items relevant to the current response may be documented using the electronic version of the Checklist tool, then distributed for follow-up in alignment with the incident command system currently in effectFootnote 15. Operationally, a quality-controlled, evergreen Checklist document may be centrally maintained for use as a planning reference during outbreak and inter-outbreak periods.
Whereas each Checklist review process may identify numerous relevant considerations, it may only be feasible to act on a subset of time-sensitive considerations in the midst of a response, while deferring others for future action during an inter-outbreak period. It should also be considered that once the initial response to an EID threat concludes, mandated laboratory responsibilities may not return to their preoutbreak baseline on an immediate or long term basis. As new tests are incorporated into routine laboratory test menus, and testing levels remain elevated to support ongoing case detection and surveillance, each subsequent EID response has the potential to cumulatively impact baseline laboratory activities such that additional resources are required to sustain inter-outbreak activities at "new normal" levels. Periodic review of Checklist considerations during inter-outbreak periods may help to identify resulting operational gaps and inform longer term efforts to strengthen laboratory response capabilities.
Limitations
The Checklist content primarily reflects the perspectives and experiences of Canadian and international PHL stakeholders directly involved in the development and review process. While considerations for laboratory organizations operating at local and regional levels (e.g. hospital laboratories and front-line diagnostic laboratories) may differ in focus and scope, they remain highly relevant to the overall responsiveness of the PHL ecosystem. An overarching limitation associated with the Checklist initiative is the extent to which the Checklist will be implemented and used as intended by laboratory organizations and networks involved in public health response.
Conclusion
When faced with an EID threat, effective laboratory response requires the time-sensitive coordination of multiple interdependent activities to support public health action. Preliminary input suggests that the Checklist may serve as a useful tool to rapidly and systematically identify key response considerations; highlight operational requirements and gaps and inform strategic planning, prioritization and decision-making to mitigate risk.
A primary objective of the Checklist initiative was to ensure the broad availability of a reference version that can be used in its current form, or adapted by implementing laboratory entities to enhance setting-specific relevance. Post-implementation, the Checklist is intended to serve as a "living document" that can be updated to reflect evolving roles, considerations and lessons learned through future laboratory response efforts.
Checklist implementation, customization, routine review and updating within the context of existing emergency management frameworks may provide an opportunity to further strengthen laboratory outbreak preparedness and response capabilities, and inform the development of long term public health resilience. Going forward, any future assessment of Checklist usefulness across implementing organizations and jurisdictions will need to take these factors into account.
Authors’ statement
Authorship contributions are as follows:
- TE — Conceptualization, content development (Checklist tool, Appendices, Figure illustration), writing, review and editing
- GT — Conceptualization, review and editing
- TK — Conceptualization, review and editing
- MG — Conceptualization, review and editing
Competing interests
The authors have no competing interests.
Acknowledgements
All individuals, organizations and networks involved in preliminary technical discussion, development and review of the Checklist document are gratefully acknowledged for their contributions, including the following:
National Microbiology Laboratory (NML): S Beddome, Dr. C Corbett, K Gordon, S Guercio, C Jorowski, L Kearney, K Keith, C Ouellette, S Radons Arneson, C Yoshida, R Cole (Laboratory Liaison Technical Officer [LLTO], Manitoba [MB]), K Cronin (LLTO, Ontario [ON]), R DePaulo (LLTO, Saskatchewan [SK]), LA Jalbert (LLTO, New Brunswick [NB]), K Macdonald (LLTO, British Columbia [BC]), C Phillips (LLTO, Nova Scotia [NS]), L Wong (LLTO, Alberta [AB])
Canadian Public Health Laboratory Network (CPHLN): Dr. M Gilmour (Federal Co-Chair, NML), Dr. P Van Caeseele (Provincial Co-Chair, Cadham Provincial Laboratory, Winnipeg, MB), Dr. T Kuschak (NML, CPHLN Secretariat), Dr. M Krajden (BC Centre for Disease Control Public Health Laboratory, Vancouver, BC), Y Chang (BC Centre for Disease Control Public Health Laboratory, Vancouver, BC), Dr. G Tipples (Alberta Provincial Laboratory for Public Health, Alberta Precision Laboratories, Edmonton, AB), Dr. P Levett (Roy Romanow Provincial Laboratory, Regina, SK), Dr. F Jamieson (Public Health Ontario Laboratories, Toronto, ON), Dr. J Longtin (Laboratoire de santé publique du Québec, Ste-Anne-de-Bellevue, Québec [QC]), Dr. G German (Health Prince Edward Island [PEI], Charlottetown, PEI), Dr. D Haldane (Queen Elizabeth II Health Science Centre, Halifax, NS), Dr. G Zahariadis (Newfoundland and Labrador [NL] Public Health Laboratory, St. John’s, NL), Dr. R Garceau (Centre hospitalier universitaire Dr-Georges-L.-Dumont, Moncton, NB)
Global Health Security Action Group (GHSAG) Laboratory Network: Dr. M Gilmour (Co-Chair, Winnipeg, MB, Canada), Dr. JA Diaz Quiñonez (Co-Chair, Mexico City, Mexico), Dr. T Kuschak (Secretariat Lead, Winnipeg, MB, Canada), Dr. A Nitsche (Berlin, Germany), A Roberts (London, United Kingdom), Dr. A Di Caro (Rome, Italy), C Chadwick (Washington DC, United States), Dr. M Saijo (Tokyo, Japan), Dr. MW Shaw (Atlanta, United States)
Funding
Support for this work was provided by the Public Health Agency of Canada (PHAC).
Supplemental
Supplemental I: Laboratory response checklist for infectious disease outbreaks
| ACTION REQUIRED | STATUS | PRIORITY | CHECKLIST ITEM |
|---|---|---|---|
| 1. LABORATORY INVESTIGATION | |||
| 1.1. Specimen Collection and Transportation: To support timely and coordinated laboratory investigation; identify and document specimen collection and transportation needs in advance, including: | |||
| 1.1.1. Appropriate specimen types, volumes and amounts; to be reassessed as required | |||
| 1.1.2. Appropriate specimen container(s) for transportation as per Risk Group classification | |||
| 1.1.3. Detailed specimen container packaging and labeling instructions | |||
| 1.1.4. Cold chain management requirements, i.e. temperature and timelines for shipping/arrival | |||
| 1.1.5. Transportation of Dangerous Goods (TDG) documentation requirements | |||
| 1.1.6. Preferred courier service recommendations in alignment with pathogen risk classification | |||
| 1.1.7. Geographic considerations impacting the transportation of specimens and essential testing equipment and supplies for remote/isolated populations | |||
| 1.1.8. Biosafety, biosecurity and infection control guidelines, protocols, equipment and supplies required to support specimen handling and processing within clinical, laboratory and field response settings, including: | |||
| 1.1.8.1. Personal protective equipment (PPE), specimen collection and transport materials (e.g. swabs, viral transport medium (VTM)) | |||
| 1.1.8.2. Containment measures (e.g. biological safety cabinets) and decontamination methods | |||
| 1.1.8.3. Specimen retention, storage and disposal requirements | |||
| 1.1.9. Additional documentation requirements and considerations within existing legislative and regulatory frameworks, including: | |||
| 1.1.9.1. Import and export permits | |||
| 1.1.9.2. Material transfer agreements between client and laboratory entities (e.g. to support distribution of reagents, controls, validation/proficiency panels) | |||
| 1.1.9.3. Alignment of specimen collection requirements with existing data sharing agreements | |||
| 1.1.9.4. Laboratory guideline, licensing and certification requirements relevant to the investigation of a new/emerging infectious agent (e.g. FDA, HC, HPTA, HPTR requirements) | |||
| 1.1.9.5. Emergency response assistance plans and protocols for transport (e.g. ERAP for Risk Group 4 pathogens, and engagement of Regional Response Coordinators) | |||
| 1.1.10. Drafting of a specimen flow chart to guide collection and transport requirements | |||
| 1.2. Laboratory Testing: To support laboratory investigation efforts; review, develop, document and communicate evidence-based ID testing recommendations and protocols in collaboration with public health partners, with consideration of: | |||
| 1.2.1. Diagnostic algorithm(s) to be used for laboratory confirmation of case investigations, to inform and/or align with the current ID case definition (which may be outbreak-specific) | |||
| 1.2.2. Testing criteria and triaging protocols for high volume testing, using available knowledge of exposure history (e.g. close contact, travel history) and other risk factor information (e.g. pregnancy, hospitalization status) | |||
| 1.2.3. Test Requisition Form (TRF) data field requirements, in alignment with the ID case definition to support timely and effective case management and public health response | |||
| 1.2.4. Specimen acceptance and rejection criteria | |||
| 1.2.5. Specimen retention and storage protocols (for diagnostic, surveillance and research purposes) | |||
| 1.2.6. Quality control and standardization of laboratory testing methods and reporting processes within the context of a quality management system, including: | |||
| 1.2.6.1. Validation, verification and comparison of diagnostic laboratory test performance specifications; including sensitivity, specificity, accuracy and precision | |||
| 1.2.6.2. Implementation and use of Standard Operating Procedures (SOPs) | |||
| 1.2.6.3. Monitoring and assessment of laboratory test turn-around times (TATs), upon specimen receipt | |||
| 1.2.7. Test result reporting requirements, including: | |||
| 1.2.7.1. Use of standardized test result communication processes, report forms, and terminology | |||
| 1.2.7.2. Possible need to report ‘preliminary’ results prior to issuance of a final report | |||
| 1.2.7.3. Ability to support result reporting for submitted specimens vs. case investigations | |||
| 1.2.8. Alignment and standardization of test methods and interpretation criteria across decentralized testing sites/jurisdictions to support accurate, consistent case reporting and surveillance efforts | |||
| 1.2.9. Review and updating of testing-related processes and protocols to support evolving outbreak response requirements in keeping with current scientific evidence | |||
| 2. LABORATORY RESPONSE CAPACITY AND TRAINING | |||
| 2.1. Laboratory Response Capability: To address laboratory response challenges specific to the ID threat, collaboratively assess the following with public health laboratory partners on an ongoing basis: | |||
| 2.1.1. Laboratory capabilities for pathogen identification and characterization, including front-line screening and confirmatory testing methods at all jurisdictional levels (i.e. hospital, regional, provincial/territorial (P/T), state, national and international) | |||
| 2.1.2. Availability of validated diagnostic testing methods (e.g. serological, molecular, subtyping, genomic, proteomic), including commercially available kits and lab-developed tests (LDTs) | |||
| 2.1.3. Performance characteristics of currently used diagnostic test methods, including sensitivity, specificity, turn-around times, and throughput using current platforms | |||
| 2.1.4. Requirement to develop and validate LDTs where external capacity does not exist, or is not reliably accessible during an outbreak | |||
| 2.1.5. Decentralized diagnostic testing capabilities and potential for technology transfer to improve national laboratory response surge capacity, dependent upon the magnitude and anticipated duration of response | |||
| 2.1.6. Access to testing by at-risk and under-served populations, including the need to pursue alternate testing strategies to support geographically remote and/or isolated populations (e.g. use of point-of-care testing approaches, enhanced community engagement models) | |||
| 2.1.7. Ongoing availability of response-critical laboratory equipment, supplies, reagents and controls via established vendors and supply chains | |||
| 2.1.8. Availability of essential laboratory supplies via local, regional and national emergency stockpiles (e.g. PPE, swabs, reagents) | |||
| 2.1.9. Requirement to coordinate the sourcing of laboratory supplies via multiple vendors, with a particular focus on domestically produced supplies (e.g. specimen collection devices and containers, laboratory disposables, chemicals/reagents) | |||
| 2.1.10. Capacity to support ongoing clinical validation activities as needed to verify the quality of all laboratory supplies, particularly when dealing with multiple and/or frequently changing vendors due to supply chain continuity issues | |||
| 2.1.11. Ability to acquire specimens from the international community to support the development of diagnostic tools to detect novel pathogens emerging in international settings | |||
| 2.2 Surge Capacity and Training: To support flexible, scalable and timely laboratory response to ID threats, dynamically assess requirements related to the following surge capacity considerations: | |||
| 2.2.1. ID-related scientific expertise and technical response capacity amongst current staff | |||
| 2.2.2. Availability of laboratory space, biocontainment facilities, testing equipment and consumables (e.g. biological safety cabinets, autoclaves, dedicated PCR workstations, testing platforms, reagents, extraction kits, PPE, swabs, VTM) | |||
| 2.2.3. Identification of ‘surge capacity’ positions that can be internally mobilized and/or cross-trained to mitigate workload issues across various laboratory response functions (e.g. via organization-wide, response-oriented personnel inventory and surge mobilization processes) | |||
| 2.2.4. Cross-training needs and priorities (e.g. core competencies, testing proficiencies, data entry/management skills to support high volume reporting) | |||
| 2.2.5. Standardized approaches for training and documentation of personnel proficiencies (e.g. testing methods; data collection, analysis and interpretation) | |||
| 2.2.6. Engagement of dedicated Emergency Operations Centre (EOC) support to enhance response capacity within an Incident Command System (ICS) | |||
| 2.2.7. Identification of funding constraints, and opportunities to access emergency funding mechanisms | |||
| 2.2.8. Alternative approaches to work-shift scheduling to increase available person-hours during heightened response periods | |||
| 2.2.9. Explore options for strategic short- and long-term staffing of positions with in-demand skill sets (scientific, technical and program support) to address capacity shortfalls due to increased workloads, alternate work arrangements, and anticipated EID illness/self-isolation requirements | |||
| 2.2.10. Mobile laboratory capacity to support ID outbreak field response requirements, both domestically and internationally | |||
| 2.2.11. Laboratory test throughput assessment, including the identification of any bottlenecks | |||
| 2.2.12. Available technologies with the potential to facilitate surge capacity development, including: | |||
| 2.2.12.1. Alternate testing platforms to increase testing throughput | |||
| 2.2.12.2. Training videos, e.g. to demonstrate specimen collection requirements | |||
| 2.2.12.3. Web-based information exchange and data sharing tools and platforms | |||
| 2.2.13. Risk mitigation planning to identify and address operational vulnerabilities observed during all phases of the laboratory response effort | |||
| 2.2.14. Participation in preparedness assessment exercises (including joint simulations) with interjurisdictional, interdisciplinary public health partners involved in coordinated ID response (e.g. epidemiologists, physicians, field investigators); typically during inter-outbreak periods | |||
| 3. LABORATORY SURVEILLANCE AND DATA MANAGEMENT | |||
| 3.1. Laboratory-based Surveillance and Data Management: To support timely and integrated laboratory-based ID surveillance activities, key considerations are as follows: | |||
| 3.1.1. Assess the current surveillance status of the ID at all jurisdictional levels including the existence of, or requirement for: | |||
| 3.1.1.1. An established case definition to support case investigation and confirmation using laboratory and epidemiological criteria within the current outbreak context | |||
| 3.1.1.2. Alignment of case confirmation criteria between reporting jurisdictions to ensure national consistency of case counts, reporting, surveillance and response efforts | |||
| 3.1.1.3. Reporting/notification processes for suspected and/or confirmed cases at each jurisdictional level (subnational, national, international (e.g. IHR reporting obligations)) | |||
| 3.1.1.4. Dedicated, site-based laboratory liaison personnel to support real-time reporting of laboratory-generated intelligence and surveillance data, to inform public health response activities (e.g. daily, national lab-confirmed case reporting by jurisdiction; % positive tests for populations of interest; changes to laboratory testing algorithms and specimen acceptance criteria) | |||
| 3.1.1.5. Surveillance systems/platforms capable of supporting timely ID detection, data integration, reporting, outbreak monitoring and development of risk models within the current response context | |||
| 3.1.1.6. Information management and information technology (IM/IT) support for data transfer pipelines and bioinformatics tools needed to acquire and analyze genomic (e.g. whole genome sequencing (WGS)) and other ‘omics’ data. | |||
| 3.1.2. Identify laboratory-based surveillance considerations related to ID case/outbreak detection, confirmation, characterization, monitoring and reporting, including: | |||
| 3.1.2.1. Current knowledge of ID epidemiology, natural history and ecology to inform laboratory testing strategies and triage recommendations | |||
| 3.1.2.2. Monitoring and classification of laboratory investigations in alignment with established case definition criteria (e.g. suspect case under investigation, probable positive case, laboratory-confirmed case, not a case/discarded) | |||
| 3.1.2.3. Key laboratory and epidemiological data elements necessary to inform laboratory investigation processes, including triaging of specimens, diagnostic algorithm selection, result interpretation, data linkage, and case classification and confirmation efforts (e.g. unique case identifiers, test history, symptom onset date, travel history, other risk factors, etc.) | |||
| 3.1.2.4. Data linkage and integration requirements to support timely surveillance and response, e.g.: | |||
| • linkage of specimens and associated test result(s) with the case under investigation | |||
| • linkage of laboratory and epidemiological case data held by separate public health jurisdictions | |||
| • linkage of confirmed cases with a given outbreak, or outbreak source | |||
| 3.1.2.5. Laboratory-confirmed case review, verification, monitoring and reporting processes | |||
| 3.1.2.6. Molecular epidemiology/public health genomics approaches to support ID strain surveillance, outbreak characterization and source attribution | |||
| 3.1.2.7. Feasibility of using web-based tools and public health informatics platforms to support timely, secure information sharing and linkage of case investigation data from disparate public health sources | |||
| 3.1.2.8. Design and development of surveillance reports to support ongoing laboratory-based monitoring, assessment and reporting of ID outbreak information, e.g.: | |||
| • Monitoring the status of laboratory investigation processes (real-time) | |||
| • Laboratory-confirmed case counts (e.g. cumulative totals, new cases/unit time) | |||
| • Final classification of all laboratory investigations across target populations (e.g. % positive tests vs. total tests performed, % laboratory-confirmed) | |||
| • Distribution of laboratory-confirmed cases using available data (i.e. by age, sex, geography, exposure history, other risk categories (e.g. health care workers (HCW), race/ethnicity)) | |||
| • Molecular epidemiology of the outbreak (e.g. case linkage, source attribution) | |||
| • Estimation of lab-based indicators to monitor and assess surveillance performance (e.g. timeliness, laboratory-based ID investigation rates, data quality and completeness) | |||
| 3.1.2.9. Legislative context and agreements relevant to interjurisdictional sharing of information and data to support ID laboratory investigation activities (e.g. MLISA, IHR) | |||
| 3.1.3. Align laboratory test requisition form (TRF) data fields with the ID case definition to support effective surveillance and response, with consideration of the following key data field types: | |||
| 3.1.3.1. Submitting laboratory/client contact information to support result reporting and other communication to inform clinical decision-making | |||
| 3.1.3.2. Unique identifiers to enable non-nominal, interjurisdictional linkage of laboratory result(s) with the case under investigation to support accurate case classification, reporting, clinical decision-making, surveillance and response | |||
| 3.1.3.3. Geolocator information for the case under investigation, as jurisdictionally relevant (e.g. city, reporting health region, province/territory/state, country, postal code, forward sortation area (i.e. three-digit postal code)) | |||
| 3.1.3.4. Clinical, epidemiological and laboratory fields needed to support triaging of high priority specimens, appropriate test algorithm selection and result interpretation; as well as case confirmation, targeted surveillance and reporting (e.g. test history, travel/exposure history, immunization history, pregnancy, hospitalization status, symptomatic vs. asymptomatic, other enhanced risk group information) | |||
| 3.1.3.5. Date fields needed to support diagnostic algorithm selection, test result interpretation, and to estimate laboratory-based surveillance indicators (i.e. symptom onset, specimen collection, specimen receipt, test result and case reporting dates) | |||
| 3.1.4. Assess laboratory information management system (LIMS) data flow requirements, and implement LIMS processes to support ID investigation, surveillance and response, including: | |||
| 3.1.4.1. Standardized approaches to the documentation and monitoring of test requisition, specimen tracking, data entry/review, data linkage and result reporting processes | |||
| 3.1.4.2. Terminology standardization, mapping of data flows, and definition of data retention/life cycle requirements | |||
| 3.1.4.3. ‘Chain of custody’ documentation requirements for high-profile laboratory investigation and result reporting (e.g. microbial forensics) | |||
| 3.1.4.4. Development of customized queries to support ongoing surveillance and response | |||
| 3.1.4.5. Summary report generation to facilitate monitoring, assessment and reporting of laboratory-based ID surveillance and outbreak response activities | |||
| 3.1.4.6. Use of web-based tools and platforms to support test requisition and reporting, as well as timely interjurisdictional linkage of case investigation data | |||
| 3.1.5. Dynamically assess and address operational vulnerabilities impacting overall response and reporting efforts, including: | |||
| 3.1.5.1. External communication and interjurisdictional reporting challenges | |||
| 3.1.5.2. Interjurisdictional differences in laboratory testing algorithms and result interpretation | |||
| 3.1.5.3. Changes to interjurisdictional confirmed case definitions that may impact surveillance | |||
| 3.1.5.4. Data linkage issues impacting accuracy and timeliness of case counting and reporting | |||
| 3.1.5.5. Challenges in the collection of enhanced ‘risk group’ data to support timely laboratory investigation, as well as epidemiological analysis of ID impacts specific to at-risk populations | |||
| 4. INTERJURISDICTIONAL ENGAGEMENT AND COMMUNICATION | |||
| 4.1. Interjurisdictional Engagement and Communication: To support coordinated public health stakeholder engagement and consistent messaging of laboratory information, develop a strategic communication strategy incorporating the following considerations: | |||
| 4.1.1. Accessibility of current ID laboratory response information to the public and to public health professionals via: | |||
| 4.1.1.1. Web-based content and social media tools | |||
| 4.1.1.2. A 24-hour emergency contact number that provides urgent access to laboratory support services | |||
| 4.1.1.3. A public-facing ‘Guide to Laboratory Services’, including specimen and test requisition requirements | |||
| 4.1.1.4. Laboratory testing recommendations and guidance documents that support decision-making by clinical and public health partners | |||
| 4.1.1.5. Alerting and notification processes that simultaneously disseminate time-sensitive information to interjurisdictional public health decision-makers | |||
| 4.1.2. Engagement of external laboratory partners, public health networks and interjurisdictional working groups involved in the coordination of ID public health response, including: | |||
| 4.1.2.1. Physician networks and committees responsible for clinical guideline development | |||
| 4.1.2.2. Public health laboratory and epidemiology partners, and interjurisdictional networks responsible for developing consensus case definitions and coordinating public health surveillance and intervention activities | |||
| 4.1.2.3. Biosafety networks and partners involved in the development of biosafety guidelines | |||
| 4.1.2.4. Policy makers responsible for evidence-based policy development and implementation to support public health action | |||
| 4.1.3. Strengthened mechanisms for collaboration and information sharing with public health partners and networks to enable: | |||
| 4.1.3.1. Evidence-based development of laboratory and clinical recommendations and guidelines, and public health surveillance and intervention strategies | |||
| 4.1.3.2. Consistent public health messaging of specimen collection and testing guidelines and recommendations for identified at-risk groups | |||
| 4.1.3.3. Alignment of ID case definitions used at multiple jurisdictional levels, including international case definitions where feasible | |||
| 4.1.3.4. Consensus regarding laboratory-confirmed case inclusion and exclusion criteria | |||
| 4.1.3.5. Accurate and consistent case classification, counting and reporting in fulfillment of P/T, state, national and international surveillance requirements | |||
| 4.1.3.6. Updating of publicly available web-based content to support coordinated laboratory response efforts and evidence-based public health decision-making | |||
| 4.1.4. Review and revision of external communication and reporting processes to address: | |||
| 4.1.4.1. Challenges impacting the timeliness of external reporting of ID cases and test results to inform clinical decision-making and public health action | |||
| 4.1.4.2. Inconsistencies and interjurisdictional differences in testing algorithms, result interpretation, case confirmation and surveillance processes | |||
| 4.1.4.3. Required changes to test reporting algorithms and information sharing methods | |||
| 4.1.4.4. Data sharing and linkage issues impacting timeliness of confirmed case reporting | |||
| 4.1.5. Event-specific internal communication and reporting structures and mechanisms needed to support consistent, effective information sharing and public health messaging, including: | |||
| 4.1.5.1. Identification of a lead subject matter expert (SME) responsible for scientific oversight of ID-specific laboratory response efforts (e.g. field deployment activities, participation in targeted research and surveillance studies) | |||
| 4.1.5.2. A ’single-window’ point of contact responsible for coordinating the receipt and distribution of event-specific information within the organization (e.g. EOC support) | |||
| 4.1.5.3. Appropriate routing mechanisms for external requests received within the organization (e.g. public health stakeholder requests for information, outbreak support, media-related communications, etc.) | |||
| 5. RESEARCH AND ETHICS | |||
| 5.1. Research Requirements and Ethical Considerations: To advance scientific research in support of public health action, consider the following laboratory-related issues in collaboration with public health partners: | |||
| 5.1.1. ID-specific applied and basic research requirements and priorities, e.g.: | |||
| 5.1.1.1. Pathogen identification and characterization studies (phenotypic, genomic, proteomic) | |||
| 5.1.1.2. Diagnostic and confirmatory reference test method development | |||
| 5.1.1.3. Participation in the rapid assessment/validation of critical testing supplies, methods and platforms, e.g. via public health/private industry collaborations | |||
| 5.1.1.4. Immunity-related ID research, including host immune response (nature and duration) and population-level seroprevalence studies | |||
| 5.1.1.5. Transmission studies to identify and characterize primary routes of infection, with particular consideration of at-risk groups and populations | |||
| 5.1.1.6. Development of risk models using the best available evidence, including predictive models to inform public-health decision-making | |||
| 5.1.1.7. Field studies, vector competency studies | |||
| 5.1.1.8. Vaccine and other medical countermeasure development and assessment | |||
| 5.1.1.9. Applied biosafety research with a focus on timely knowledge translation | |||
| 5.1.1.10. Public health surveillance studies requiring research ethics approval | |||
| 5.1.2. Clear differentiation between applied public health research projects that will require prior research ethics approval, and routine laboratory- based pathogen investigation, characterization and surveillance activities that are integral to timely public health response | |||
| 5.1.3. Prioritization of research-associated testing activities within the context of available laboratory capacity and resources to support broader public health response (e.g.: participation in EID response task forces, networks and other multi-disciplinary research initiatives) | |||
| 5.1.4. Ethical, safety and environmental requirements relevant to investigation of the ID agent using the proposed methods and study design(s) | |||
| 5.1.5. Patient consent requirements having the potential to impact laboratory testing for public health research purposes | |||
| 5.1.6. Available mechanisms to address consent-related concerns (e.g. development/updating of patient consent and test requisition forms) | |||
| 5.1.7. Procurement requirements and availability of relevant animal models and vectors | |||
| 5.1.8. Research Ethics Board (REB) approval requirements (human and animal), including coordination of multiple REB approval processes to support interjurisdictional collaboration | |||
| 5.1.9. Authorship and intellectual property considerations associated with interjurisdictional collaboration and publication | |||
| 5.1.10. Requirements for timely analysis and publication of research findings to inform public health decision-making | |||
| 5.1.11. Ongoing review and re-assessment of ID research priorities (applied and basic) within the context of current scientific evidence and evolving ID response requirements | |||
| 5.1.12. Identification of strategic partnerships and funding opportunities to advance research objectives | |||
| 5.1.13. Maintenance of REB approval status for ongoing research projects |
Supplemental II: Laboratory response checklist for infectious disease outbreaks—use recommendations
CHECKLIST USE
Implementation of an electronic version* of the Checklist Table can facilitate collaborative, real-time review, identification and prioritization of Checklist Items to support follow-up action. The following content includes basic suggestions to help users customize and adapt the reference version of the Checklist Table to meet organization and jurisdiction-specific requirements and preferences.
*Note: As the Checklist Table was created using Microsoft Excel 2010, any software-specific functions described below may not operate similarly using other spreadsheet software.
CHECKLIST ITEM CLASSIFICATION
Using the three ‘classification’ columns provided to the left of the Checklist Item column, reviewers can indicate for each item the review process:
- If there is ‘Action Required’* – this can be indicated by simply placing an ‘x’ in the corresponding cell; cells are left blank if action is not required to support current response efforts.
- The action ‘Status’ – this can be indicated for actionable items as preferred, e.g. by using simple, user-defined status designations such as Pending (P), Ongoing (O), Deferred (D), or Complete (C).
- ‘Priority’ – ranks items for which action is required according to user-defined priority levels, e.g.: Urgent (U), High (H), Medium (M), Low (L), and Not Applicable (NA)
*Note: When an item requiring action is marked ‘x’ (e.g. ‘1.1.7.2. Specimen retention, storage and disposal’), it is important to also mark all hierarchical items within the Table Section with an ‘x’ (e.g. items 1, 1.1, and 1.1.7). This ensures that the full context of the action item of interest is returned when the table is sorted/filtered using spreadsheet software (e.g. Excel).
CHECKLIST TABLE CUSTOMIZATION
Users can customize the Checklist Table to expand its functionality, or edit its content in a number of potential ways:
- Addition of new Checklist Items (rows) to capture additional, organization or jurisdiction-specific requirements that are not reflected in the published reference version.
- To insert a new row (Excel): In the left hand margin, click on the row above which the new row is to be inserted. On the ‘Home’ tab, click ‘Insert’ to add a row to the Table.
- Removal of Checklist Items that are not relevant within the user’s organization or jurisdiction, e.g.:
‘Item 5.1.1.5. Vaccine and other medical countermeasure development and assessment’- To remove a row (Excel): In the left hand margin, click on the row to be deleted.
On the ‘Home’ tab, click ‘Delete’ to remove the selected row.
- To remove a row (Excel): In the left hand margin, click on the row to be deleted.
- Addition of new Checklist Table data fields* (columns) to capture additional information relevant to Checklist Item review and follow-up action. Additional data fields that may be useful include, e.g.:
‘Comments’ – captures detailed information regarding the specific actions required to address relevant Checklist Items
‘Assigned’ – captures the individual/laboratory program responsible for follow-up action within an organization
‘Completion Date’ – records the date of completion for actionable Checklist Items- To add a new Table column (Excel), click on the column to the right of the intended location of the new column. On the ‘Home’ tab, click ‘Insert’ to add the column to the Table.
*Note: The reference version of the Checklist Table has been formatted to allow the full Table width to be legibly printed in ‘landscape’ orientation on 8.5”x11” paper (Excel); if the Table is further customized by adding additional columns, reconfiguration of the current print settings may be required to ensure that all Table data are easily viewed.
ITEM PRIORITIZATION
Once all Checklist Items have been reviewed and classified, the Checklist Table can be easily ‘sorted’ using spreadsheet software to display only the specific items of interest, e.g.: only items for which there is ‘Action Required’.
In Excel, the user can ‘sort’ the Checklist Table according to user-defined item classification as follows:
- Click on the ‘downward arrow’ button displayed at the bottom right of any column header, e.g.: ‘Action Required’, ‘Action Status’, ‘Priority’.
- In the ‘pop-up’ menu, place a ‘check’ in each box that corresponds with a classification category to be displayed; multiple categories can be displayed simultaneously, e.g.:
- Under the header ‘Action Required’ with the following ‘sort’ options:
- (Select All)
- x
- (Blanks);
- Under the header ‘Priority’ with the following ‘sort’ options:
- (Select All)
- U
- H
- M
- L
- NA
- (Blanks);
- Note: Checking the ‘Select All’ box will remove the ‘sort’ filter from the selected column, returning all data in the Table.
- Under the header ‘Action Required’ with the following ‘sort’ options:
Once sorted/filtered, the condensed Checklist Table displays only the items of interest, allowing more focused planning discussion, prioritization and tasking of all action items to support response efforts. It is recommended that users experiment with various table views to optimize its functionality to suit response-specific needs.
Supplemental III: Laboratory response checklist for infectious disease outbreaks
1. Laboratory investigation
1.1. Specimen collection and transportation: To support timely, coordinated laboratory investigation, identify and document specimen collection and transportation needs in advance, including:
- 1.1.1. Appropriate specimen types, volumes and amounts; to be reassessed as required
- 1.1.2. Appropriate specimen container(s) for transportation as per Risk Group classification
- 1.1.3. Detailed specimen container packaging and labeling instructions
- 1.1.4. Cold chain management requirements, i.e. temperature and timelines for shipping/arrival
- 1.1.5. Transportation of Dangerous Goods (TDG) documentation requirements
- 1.1.6. Preferred courier service recommendations in alignment with pathogen risk classification
- 1.1.7. Geographic considerations impacting the transportation of specimens and essential testing equipment and supplies for remote and isolated populations
- 1.1.8. Biosafety, biosecurity and infection control guidelines, protocols, equipment and supplies required to support specimen handling and processing within clinical, laboratory and field response settings, including:
- 1.1.8.1. Personal protective equipment (PPE), specimen collection and transport materials (e.g. swabs, viral transport medium (VTM))
- 1.1.8.2. Containment measures (e.g. biological safety cabinets) and decontamination methods
- 1.1.8.3. Specimen retention, storage and disposal requirements
- 1.1.9. Additional documentation requirements and considerations within existing legislative and regulatory frameworks, including:
- 1.1.9.1. Import and export permits
- 1.1.9.2 Material transfer agreements between client and laboratory entities (e.g. to support distribution of reagents, controls, validation/proficiency panels)
- 1.1.9.3. Alignment of specimen collection requirements with existing data sharing agreements
- 1.1.9.4. Laboratory guideline, licensing and certification requirements relevant to the investigation of a new/emerging infectious agent (e.g. FDA, HC, HPTA, HPTR requirements)
- 1.1.9.5. Emergency response assistance plans and protocols for transport (e.g. ERAP for Risk Group 4 pathogens, and engagement of Regional Response Coordinators)
- 1.1.10. Drafting of a specimen flow chart to guide collection and transport requirements
1.2. Laboratory Testing: To support laboratory investigation efforts; review, develop, document and communicate evidence-based ID testing recommendations and protocols in collaboration with public health partners, with consideration of:
- 1.2.1. Diagnostic algorithm(s) to be used for laboratory confirmation of case investigations, to inform and/or align with the current ID case definition (which may be outbreak-specific)
- 1.2.2. Testing criteria and triaging protocols for high volume testing, using available knowledge of exposure history (e.g. close contact, travel history) and other risk factor information (e.g. pregnancy, hospitalization status)
- 1.2.3. Test Requisition Form (TRF) data field requirements, in alignment with the ID case definition to support timely and effective case management and public health response
- 1.2.4. Specimen acceptance and rejection criteria
- 1.2.5. Specimen retention and storage protocols (for diagnostic, surveillance and research purposes)
- 1.2.6. Quality control and standardization of laboratory testing methods and reporting processes within the context of a quality management system, including:
- 1.2.6.1. Validation, verification and comparison of diagnostic laboratory test performance specifications; including sensitivity, specificity, accuracy and precision
- 1.2.6.2. Implementation and use of Standard Operating Procedures (SOPs)
- 1.2.6.3. Monitoring and assessment of laboratory test turn-around times (upon specimen receipt)
- 1.2.7. Test result reporting requirements, including:
- 1.2.7.1. Use of standardized test result communication processes, report forms, and terminology
- 1.2.7.2. Possible need to report ‘preliminary’ results prior to issuance of a final report
- 1.2.7.3. Ability to support result reporting for submitted specimens vs. case investigations
- 1.2.8. Alignment and standardization of test methods and interpretation criteria across decentralized testing sites/jurisdictions to support accurate, consistent case reporting and surveillance efforts
- 1.2.9. Review and updating of testing-related processes and protocols to support evolving outbreak response requirements in keeping with current scientific evidence
- REVIEW COMPLETE - LABORATORY INVESTIGATION
2. Laboratory response capacity and training
2.1. Laboratory response capability: To address laboratory response challenges specific to the ID threat, collaboratively assess the following with public health laboratory partners on an ongoing basis:
- 2.1.1. Laboratory capabilities for pathogen identification and characterization, including front-line screening and confirmatory testing methods at all jurisdictional levels (i.e. hospital, regional, provincial/territorial (P/T), state, national and international)
- 2.1.2. Availability of validated diagnostic testing methods (e.g. serological, molecular, subtyping, genomic, proteomic), including commercially available kits and lab-developed tests (LDTs)
- 2.1.3 Performance characteristics of currently used diagnostic test methods, including sensitivity, specificity, turn-around times, and throughput using current platforms
- 2.1.4. Requirement to develop and validate LDTs where external capacity does not exist, or is not reliably accessible during an outbreak
- 2.1.5. Decentralized diagnostic testing capabilities and potential for technology transfer to improve national laboratory response capacity, dependent upon magnitude and duration of response
- 2.1.6. Access to testing by at-risk and under-served populations, including the need to pursue alternate testing strategies to support geographically remote and/or isolated populations (e.g. use of point-of-care testing approaches, enhanced community engagement models)
- 2.1.7. Ongoing availability of critical equipment and reagents via established vendors and supply chains
- 2.1.8. Availability of essential laboratory supplies via local, regional and national emergency stockpiles (e.g. PPE, swabs, reagents)
- 2.1.9. Requirement to coordinate the sourcing of laboratory supplies via multiple vendors, with a particular focus on domestically produced supplies (e.g. specimen collection devices and containers, laboratory disposables, chemicals/reagents)
- 2.1.10. Capacity to support ongoing clinical validation activities as needed to verify the quality of all laboratory supplies, particularly when dealing with multiple and/or frequently changing vendors due to supply chain continuity issues
- 2.1.11. Ability to acquire specimens from the international community to support the development of diagnostic tools to detect novel pathogens emerging in international settings
2.2 Surge capacity and training: To support flexible, scalable and timely laboratory response to ID threats, dynamically assess requirements related to the following surge capacity considerations:
- 2.2.1. ID-related scientific expertise and technical response capacity amongst current staff
- 2.2.2. Availability of laboratory space, biocontainment facilities, testing equipment and consumables (e.g. biological safety cabinets, autoclaves, dedicated PCR workstations, testing platforms, reagents, extraction kits, PPE, swabs, VTM)
- 2.2.3. Identification of available ‘surge capacity’ positions that can be internally mobilized and/or cross-trained to mitigate workload issues across various laboratory response functions (e.g. via organization-wide, response-oriented personnel inventory and surge mobilization processes)
- 2.2.4. Cross-training needs and priorities (e.g. core competencies, testing and equipment proficiencies, data entry/management skills to support high volume reporting)
- 2.2.5. Standardized approaches for training and documentation of personnel proficiencies (e.g. testing methods; data collection, analysis and interpretation)
- 2.2.6. Engagement of dedicated Emergency Operations Centre (EOC) support to enhance response capacity within an Incident Command System (ICS)
- 2.2.7. Identification of funding constraints, and opportunities to access emergency funding mechanisms
- 2.2.8. Alternative approaches to work-shift scheduling to increase available person-hours during heightened response periods
- 2.2.9. Explore options for strategic short and long-term staffing of positions with in-demand skill sets (scientific, technical and program support) to address capacity shortfalls due to increased workloads, alternate work arrangements, and anticipated EID illness/self-isolation requirements
- 2.2.10. Mobile laboratory capacity to support ID outbreak field response requirements, both domestically and internationally
- 2.2.11. Laboratory test throughput assessment, including the identification of any bottlenecks
- 2.2.12. Available technologies with the potential to facilitate surge capacity development, including:
- 2.2.12.1. Alternate testing platforms to increase testing throughput
- 2.2.12.2. Training videos, e.g. to demonstrate specimen collection requirements
- 2.2.12.3. Web-based information exchange and data sharing tools and platforms
- 2.2.13. Risk mitigation planning to address operational vulnerabilities observed during all phases of the laboratory response effort
- 2.2.14. Participation in preparedness assessment exercises (including joint simulations) with interjurisdictional, interdisciplinary public health partners involved in coordinated ID response (e.g. epidemiologists, physicians, field investigators); typically during inter-outbreak periods
- REVIEW COMPLETE - LABORATORY RESPONSE CAPACITY AND TRAINING
3. Laboratory surveillance and data management
3.1. Laboratory-based Surveillance and Data Management: To support timely and integrated laboratory-based ID surveillance activities, key considerations are as follows:
- 3.1.1. Assess the current surveillance status of the ID at all jurisdictional levels including the existence of, or requirement for:
- 3.1.1.1. An established case definition to support case investigation and confirmation using laboratory and epidemiological criteria within the current outbreak context
- 3.1.1.2. Alignment of case confirmation criteria between reporting jurisdictions to ensure national consistency of case counts, reporting, surveillance and response efforts
- 3.1.1.3. Reporting/notification processes for suspected and/or confirmed cases at each jurisdictional level (subnational, national, international (e.g. IHR reporting obligations))
- 3.1.1.4. Dedicated, site-based laboratory liaison personnel to support real-time reporting of laboratory-generated intelligence and surveillance data, to inform public health response activities (e.g. daily, national lab-confirmed case reporting by jurisdiction; % positive tests for populations of interest; changes to laboratory testing algorithms, specimen acceptance criteria)
- 3.1.1.5. Surveillance systems/platforms capable of supporting timely ID detection, reporting, outbreak monitoring and development of risk models within the current response context
- 3.1.1.6. Information management and information technology (IM/IT) support for data transfer pipelines and bioinformatics tools needed to acquire and analyze genomic (e.g. whole genome sequencing (WGS)) and other ‘omics’ data
- 3.1.2. Identify laboratory-based surveillance considerations related to ID case/outbreak detection, confirmation, characterization, monitoring and reporting, including:
- 3.1.2.1. Current knowledge of ID epidemiology, natural history and ecology to inform laboratory testing strategies and triage recommendations
- 3.1.2.2. Monitoring and classification of laboratory investigations in alignment with established case definition criteria (e.g. suspect case under investigation, probable positive case, laboratory-confirmed case, not a case/discarded)
- 3.1.2.3. Key laboratory and epidemiological data elements necessary to inform laboratory investigation processes, including triaging of specimens, diagnostic algorithm selection, result interpretation, data linkage, and case classification and confirmation efforts (e.g. unique case identifiers, test history, symptom onset date, travel history, other risk factors, etc.)
- 3.1.2.4. Data linkage and integration requirements to support timely surveillance and response, e.g.:
- linkage of specimens and associated test result(s) with the case under investigation
- linkage of laboratory and epidemiological case data held by separate public health jurisdictions
- linkage of confirmed cases with a given outbreak, or outbreak source
- 3.1.2.5. Laboratory-confirmed case review, verification, monitoring and reporting processes
- 3.1.2.6. Molecular epidemiology/public health genomics approaches to support ID strain surveillance, outbreak characterization and source attribution
- 3.1.2.7. Feasibility of using web-based tools and public health informatics platforms to support timely, secure information sharing and linkage of case investigation data from disparate public health sources
- 3.1.2.8. Design and development of surveillance reports to support ongoing laboratory-based monitoring, assessment and reporting of ID outbreak information, e.g.:
- Monitoring the status of laboratory investigation processes (real-time)
- Laboratory-confirmed case counts (e.g. cumulative totals, new cases/unit time)
- Final classification of laboratory investigations across target populations
(e.g. % positive tests vs. total tests performed, % laboratory-confirmed) - Distribution of laboratory-confirmed cases using available data (i.e. by age, sex, geography, exposure history, other risk categories (e.g. healthcare workers (HCW), race/ethnicity))
- Molecular epidemiology of the outbreak (e.g. case linkage, source attribution)
- Estimation of lab-based indicators to monitor and assess surveillance performance (e.g. timeliness, laboratory-based ID investigation rates, data quality and completeness)
- 3.1.2.9. Legislative context and agreements relevant to interjurisdictional sharing of information and data to support ID laboratory investigation activities (e.g. MLISA, IHR)
- 3.1.3. Align laboratory test requisition form TRF data fields with the ID case definition to support effective surveillance and response, with consideration of the following key data field types:
- 3.1.3.1. Submitting laboratory/client contact information to support result reporting and other communication to inform clinical decision-making
- 3.1.3.2. Unique identifiers to enable non-nominal, interjurisdictional linkage of laboratory result(s) with the case under investigation to support accurate case classification, reporting, clinical decision-making, surveillance and response
- 3.1.3.3. Geolocator information for the case under investigation, as jurisdictionally relevant (e.g. city, reporting health region, province/territory/state, country, postal code, forward sortation area (i.e. three-digit postal code))
- 3.1.3.4. Clinical, epidemiological and laboratory fields needed to support triaging of high priority specimens, appropriate test algorithm selection and result interpretation; as well as case confirmation, targeted surveillance and reporting (e.g. test history, travel/exposure history, immunization history, pregnancy, hospitalization status, symptomatic vs. asymptomatic, other enhanced risk group information)
- 3.1.3.5. Date fields needed to support diagnostic algorithm selection, test result interpretation, and to estimate laboratory-based surveillance indicators (i.e. symptom onset, specimen collection, specimen receipt, test result and case reporting dates)
- 3.1.4. Assess laboratory information management system (LIMS) data flow requirements, and implement LIMS processes to support ID investigation, surveillance and response, including:
- 3.1.4.1. Standardized approaches to the documentation and monitoring of test requisition, specimen tracking, data entry/review, data linkage and result reporting processes
- 3.1.4.2. Terminology standardization, mapping of data flows, and definition of data retention/life cycle requirements
- 3.1.4.3. ‘Chain of custody’ documentation requirements for high-profile laboratory investigation and result reporting (e.g. microbial forensics)
- 3.1.4.4. Development of customized queries to support ongoing surveillance and response
- 3.1.4.5. Summary report generation to facilitate monitoring, assessment and reporting of laboratory-based ID surveillance and outbreak response activities
- 3.1.4.6. Use of web-based tools and platforms to support test requisition and reporting, as well as timely interjurisdictional linkage of case investigation data
- 3.1.5. Dynamically assess and address operational vulnerabilities impacting overall response and reporting efforts, including:
- 3.1.5.1. External communication and interjurisdictional reporting challenges
- 3.1.5.2. Interjurisdictional differences in laboratory testing algorithms and result interpretation
- 3.1.5.3. Changes to interjurisdictional confirmed case definitions that may impact surveillance
- 3.1.5.4. Data linkage issues impacting accuracy and timeliness of case counting and reporting
- 3.1.5.5. Challenges in the collection of enhanced ‘risk group’ data to support timely laboratory investigation, as well as epidemiological analysis of ID impacts specific to at-risk populations
- REVIEW COMPLETE – LABORATORY RESPONSE CAPACITY AND TRAINING
4. Interjurisdictional engagement and communication
4.1. Interjurisdictional Engagement and Communication: To support coordinated public health stakeholder engagement and consistent messaging of laboratory information, develop a strategic communication strategy incorporating the following considerations:
- 4.1.1. Accessibility of current ID laboratory response information to the public and to public health professionals via:
- 4.1.1.1. Web-based content and social media tools
- 4.1.1.2. A 24-hour emergency contact number that provides urgent access to laboratory support services
- 4.1.1.3. A public-facing ‘Guide to Laboratory Services’, including specimen and test requisition requirements
- 4.1.1.4. Laboratory testing recommendations and guidance documents to support decision-making by clinical and public health partners
- 4.1.1.5. Alerting and notification processes that simultaneously disseminate time-sensitive information to interjurisdictional public health decision-makers
- 4.1.2. Engagement of external laboratory partners, public health networks and interjurisdictional working groups involved in the coordination of ID public health response, including:
- 4.1.2.1. Physician networks and committees responsible for clinical guideline development
- 4.1.2.2. Public health laboratory and epidemiology partners, and interjurisdictional networks responsible for developing consensus case definitions, and coordinating public health surveillance and intervention activities
- 4.1.2.3. Biosafety networks and partners involved in the development of biosafety guidelines
- 4.1.2.4. Policy makers responsible for evidence-based policy development and implementation to support public health action
- 4.1.3. Strengthened mechanisms for collaboration and information sharing with public health partners and networks to enable:
- 4.1.3.1. Evidence-based development of laboratory and clinical recommendations and guidelines, and public health surveillance and intervention strategies
- 4.1.3.2. Consistent public health messaging of specimen collection and testing guidelines and recommendations for identified at-risk groups
- 4.1.3.3. Alignment of ID case definitions used at multiple jurisdictional levels, including international case definitions where feasible
- 4.1.3.4. Consensus regarding laboratory-confirmed case inclusion and exclusion criteria
- 4.1.3.5. Accurate and consistent case classification, counting and reporting in fulfillment of P/T, state, national and international surveillance requirements
- 4.1.3.6. Updating of publicly available web-based content to support coordinated laboratory response efforts and evidence-based public health decision-making
- 4.1.4. Review and revision of external communication and reporting processes to address:
- 4.1.4.1. Challenges impacting the timeliness of external reporting of ID cases and test results to inform clinical decision-making and public health action
- 4.1.4.2. Inconsistencies and interjurisdictional differences in testing algorithms, result interpretation, case confirmation and surveillance processes
- 4.1.4.3. Required changes to test reporting algorithms and information sharing methods
- 4.1.4.4. Data sharing and linkage issues impacting timeliness of confirmed case reporting
- 4.1.5. Event-specific internal communication and reporting structures and mechanisms needed to support coherent, effective information sharing and public health messaging, including:
- 4.1.5.1. Identification of a lead subject matter expert (SME) responsible for scientific oversight of ID-specific laboratory response efforts (e.g. field deployment activities, participation in targeted research and surveillance studies)
- 4.1.5.2. A ‘single-window’ point of contact responsible for coordinating the receipt and distribution of event-specific information within the organization (e.g. EOC support)
- 4.1.5.3. Appropriate routing mechanisms for external requests received within the organization (e.g. public health stakeholder requests for information, outbreak support, media-related communications, etc.)
- REVIEW COMPLETE – INTERJURISDICTIONAL ENGAGEMENT AND COMMUNICATION
5. Research and ethics
5.1. Research Requirements and Ethical Considerations: To advance scientific research in support of public health action, consider the following laboratory-related issues in collaboration with public health partners:
- 5.1.1. ID-specific applied and basic research requirements and priorities, e.g.:
- 5.1.1.1. Pathogen identification and characterization studies (phenotypic, genomic, proteomic)
- 5.1.1.2. Diagnostic and confirmatory reference test method development
- 5.1.1.3. Participation in the rapid assessment/validation of critical testing supplies, methods and platforms, e.g. via public health/private industry collaborations
- 5.1.1.4. Immunity-related ID research, including host immune response (nature and duration) and population-level seroprevalence studies
- 5.1.1.5. Transmission studies to identify and characterize primary routes of infection, with particular consideration of at-risk groups and populations
- 5.1.1.6. Development of risk models using the best available evidence, including predictive models to inform public-health decision-making
- 5.1.1.7. Field studies, vector competency studies
- 5.1.1.8. Vaccine and other medical countermeasure development and assessment
- 5.1.1.9. Applied biosafety research with a focus on timely knowledge translation
- 5.1.1.10. Public health surveillance studies requiring research ethics approval
- 5.1.2. Clear differentiation between applied public health research projects that will require research ethics approval, and routine laboratory-based pathogen investigation, characterization and surveillance activities that are integral to timely public health response
- 5.1.3. Prioritization of research-associated testing activities within the context of available laboratory capacity and resources to support broader public health response (e.g.: participation in EID response task forces, networks and other multi-disciplinary research initiatives)
- 5.1.4. Ethical, safety and environmental requirements relevant to investigation of the ID agent using the proposed methods and study design(s)
- 5.1.5. Patient consent requirements having the potential to impact laboratory testing for public health research purposes
- 5.1.6. Available mechanisms to address consent-related concerns (e.g. development/updating of patient consent and test requisition forms)
- 5.1.7. Procurement requirements and availability of relevant animal models and vectors
- 5.1.8. Research Ethics Board (REB) approval requirements (human and animal), including coordination of multiple REB approval processes to support interjurisdictional collaboration
- 5.1.9. Authorship and intellectual property considerations associated with interjurisdictional collaboration and publication
- 5.1.10. Requirements for timely analysis and publication of research findings to inform public health decision-making
- 5.1.11. Ongoing review and re-assessment of ID research priorities (applied and basic) within the context of current scientific evidence and evolving ID response requirements
- 5.1.12. Identification of strategic partnerships and funding opportunities to advance research objectives
- 5.1.13. Maintenance of REB approval status for ongoing research projects
- REVIEW COMPLETE – RESEARCH AND ETHICS
