RSV vaccine products

 Download this article as a PDF
Download this article as a PDFPublished by: The Public Health Agency of Canada
Issue: Volume 46–4: Respiratory syncytial virus (RSV)
Date published: April 2, 2020
ISSN: 1481-8531
Submit a manuscript
About CCDR
Browse
Volume 46–4, April 2, 2020: Respiratory syncytial virus (RSV)
Infographic
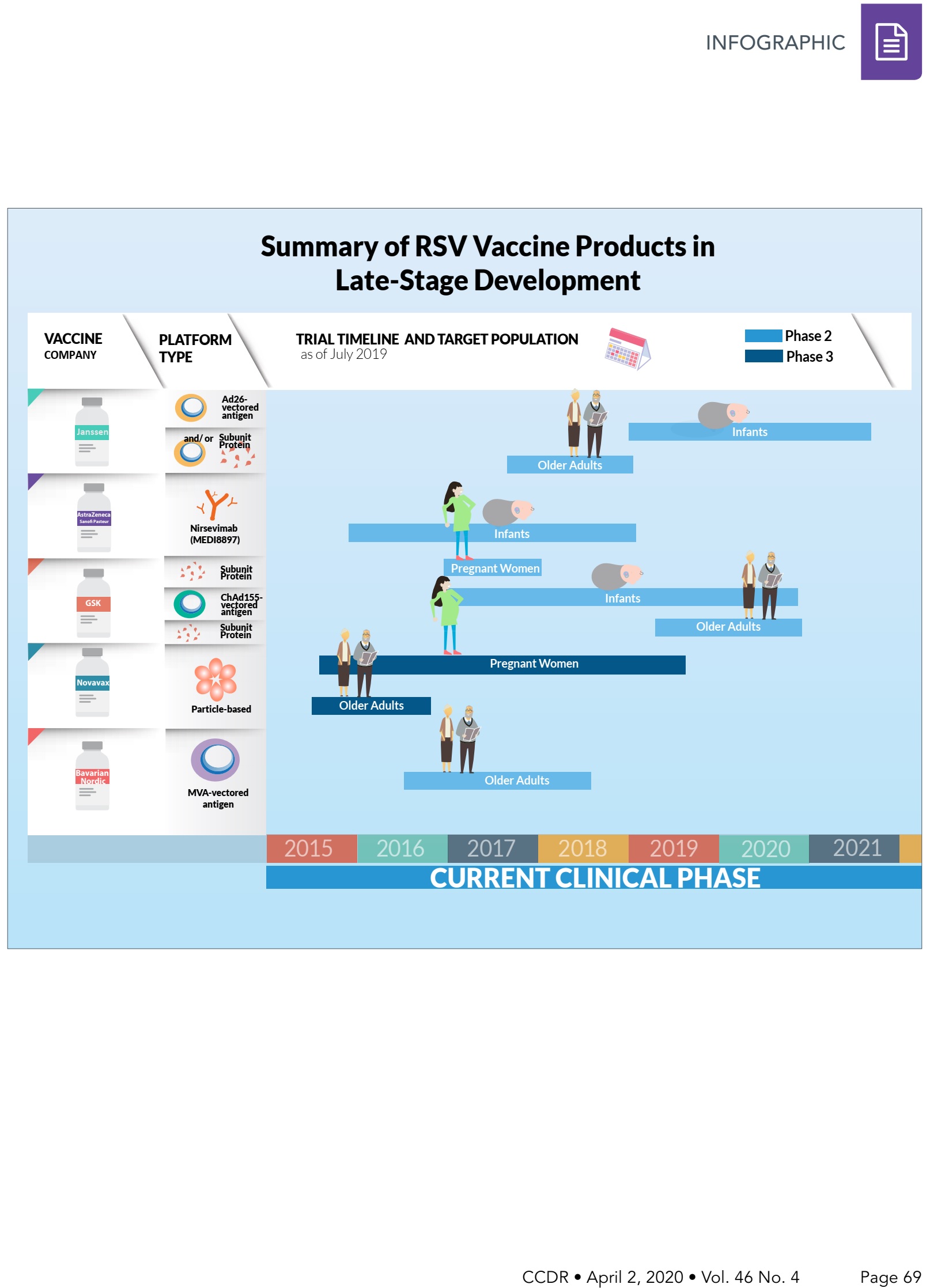
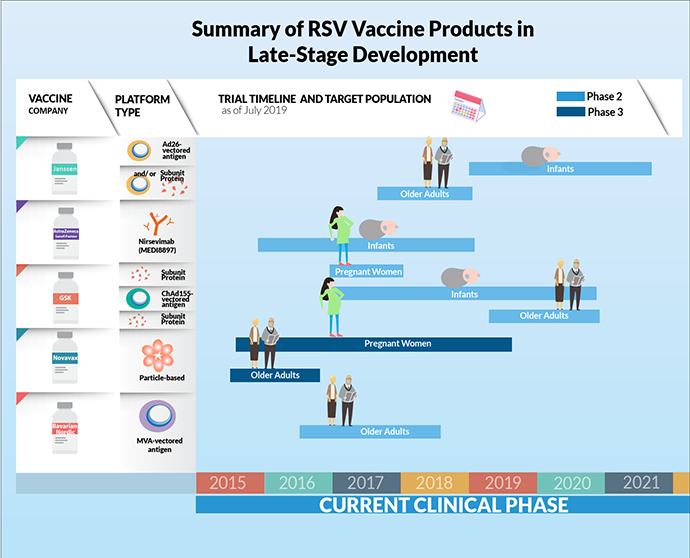
Summary of RSV vaccine products in late-stage development
Text description: Infographic
Summary of RSV vaccine products in late-stage development
This infographic provides a summary of respiratory syncytial virus (RSV) vaccine products in late-stage clinical development. It also shows the timeline of the clinical testing of these RSV products and their respective target populations, as of July 1, 2019.
Janssen is developing a vaccine program for infants and older adults. For infants, they are in Phase 2 clinical trials with a vectored antigen from Adenovirus 26 (Ad26). The Ad26 vectored antigen is also in Phase 2 clinical trials in older adults, potentially with a subunit protein.
AstraZeneca/Sanofi Pasteur is developing nirsevimab (MEDI8897) a monoclonal antibody targeted for infants. It is currently in Phase 2 clinical trials.
GSK is developing a vaccine program for pregnant women, infants and older adults. For pregnant women and older adults, they are developing a subunit protein vaccine. For infants, they are developing a Chimpanzee Adenovirus 155 (ChAd155)-vectored antigen. All the programs are in Phase 2 clinical trials.
Novavax is developing a particle-based vaccine for pregnant women and older adults. These programs are currently in Phase 3 clinical trials.
Bavarian Nordic is developing a modified vaccinia Ankara (MVA)-vectored vaccine targeted for older adults, currently in Phase 2 clinical trials.
Page details
- Date modified:
