A new diagnostic algorithm for Lyme disease in Canada

 Download this article as a PDF
Download this article as a PDFPublished by: The Public Health Agency of Canada
Issue: Volume 46–5: Nosocomial infection surveillance
Date published: May 7, 2020
ISSN: 1481-8531
Submit a manuscript
About CCDR
Browse
Volume 46–5, May 7, 2020: Nosocomial infection surveillance
Overview
Modified two-tiered testing algorithm for Lyme disease serology: the Canadian context
Todd Hatchette1, Robbin Lindsay2 on behalf of the Lyme Disease Diagnostics Working Group
Affiliations
1 Department of Pathology and Laboratory Medicine, Dalhousie University, Halifax, NS
2 National Microbiology Laboratory, Public Health Agency of Canada, Winnipeg, MB
Correspondence
Suggested citation
Hatchette TF, Lindsay LR on behalf of the Lyme Disease Diagnostics Working Group. Modified two-tiered testing algorithm for Lyme disease serology: the Canadian context. Can Commun Dis Rep 2020;46(5):125–31. https://doi.org/10.14745/ccdr.v46i05a05
Keywords: Borrelia burgdorferi, Lyme disease, serology, standard two-tiered testing, enzyme immunoassays, immunoblots, diagnostics
Abstract
Background: Lyme disease (LD) is emerging in many parts of central and eastern Canada. Serological testing is most commonly used to support laboratory diagnosis of LD. Standard two-tiered testing (STTT) for LD involves detection of Borrelia burgdorferi antibodies using an enzyme immunoassay (EIA) followed by IgM and/or IgG immunoblots. However, improved sensitivity has been demonstrated using a modified two-tiered testing (MTTT) approach, in which a second EIA instead of the traditional immunoblot is used. This article summarises the evidence supporting the MTTT versus STTT for laboratory diagnosis of LD in Canada.
Methods: Peer reviewed literature on the sensitivity and specificity of different EIAs were compared by Canadian experts in LD diagnostic for MTTT vs STTT in patients with clinical history of LD residing in LD endemic areas or in samples from the LD serum repository.
Results: The MTTT approach consistently demonstrated improved sensitivity to detect early infections with B. burgdorferi and also maintained high specificity vs STTT.
Conclusion: Diagnostic improvements in sensitivity of LD testing without significant loss of specificity have been consistently reported when MTTT is compared with STTT in studies conducted in highly LD endemic regions. Our working group agrees with the recommendation by the United States Centers for Disease Control that serological testing for LD using MTTT is an acceptable alternative to STTT. This recommendation is contingent on development and implementation of comprehensive validation studies on the performance of MTTT vs STTT within the Canadian context, including evaluation of the test performance in areas of low endemicity for LD.
Introduction
Lyme disease (LD) is an emerging tick-borne infection caused by spirochetes belonging to the Borrelia burgdorferi sensu lato species complex, which are transmitted to humans by infected ticksFootnote 1. The principal tick vectors are the blacklegged tick (Ixodes scapularis) and the western blacklegged tick (Ixodes pacificus) in eastern/central Canada and British Columbia, respectivelyFootnote 2. In Canada, infected blacklegged tick populations are endemic in parts of British Columbia, Manitoba, Ontario, Quebec, New Brunswick and Nova ScotiaFootnote 3. The number of Canadians with LD has risen since it became nationally reportable, from 144 cases in 2009 to 2,025 in 2017, which is likely an under-representation of the true numbersFootnote 1Footnote 2Footnote 4. As the geographic range of blacklegged ticks continues to expand, more Canadians will be at risk for acquiring LDFootnote 5. It is estimated that more than 300,000 cases of LD occur in the United States (US) each yearFootnote 6. The volume of diagnostic tests for LD performed in the US is much greater compared with CanadaFootnote 7. In part, this has driven efforts to improve testing efficiencies for LD, including the development and approval of the modified two-tiered testing (MTTT)Footnote 8. The objective of this document is to summarise the evidence supporting the improved performance of the MTTT approach compared to the currently used diagnostic algorithm for LD.
Intervention
The current reference method most commonly used for laboratory diagnosis of LD is serology, which detects antibodies to B. burgdorferi using standard two-tiered testing (STTT), using an enzyme immunoassay (EIA) as the first tier test followed by IgM and/or IgG immunoblots as a supplemental test (Figure 1). Most provincial public health or hospital laboratories perform the EIA testing locally while immunoblot testing is performed independently at provincial public health labs in British Columbia, Ontario (and shortly in Quebec) or at the National Microbiology Laboratory (NML). NML performs immunoblot testing for all provinces when LD is suspected in patients who travelled outside of North America (Figure 1). Regardless of the type of testing, results are reviewed by laboratory staff and reported to the requesting physician and positive results are also reported to local provincial public health.
Figure 1: Schematic depicting steps in standard two-tiered testing and modified two-tiered testing for Lyme diseaseFigure 1 footnote a
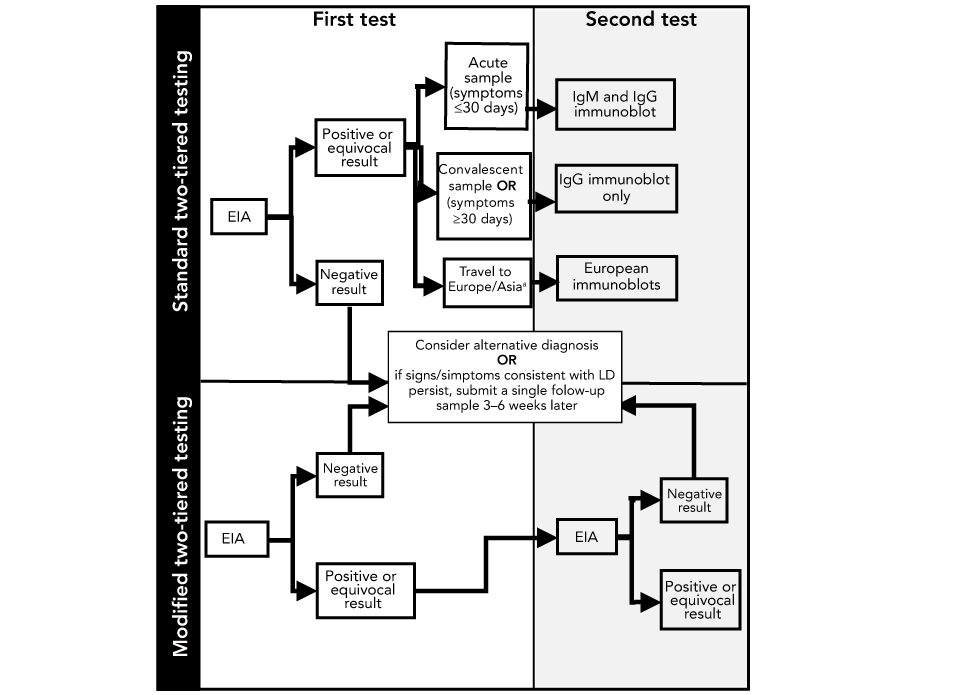
Text description: Figure 1
Figure 1: Schematic depicting steps in standard two-tiered testing and modified two-tiered testing for Lyme diseaseFigure 1 footnote a
Flow charts of first and second tests for standard two-tiered testing (STTT) and modified two-tiered testing (MTTT) are depicted in figure 1. Firstly, an enzyme immunoassay (EIA) is done. If the result is negative, consider alternative diagnosis OR if signs/symptoms consistent with Lyme disease (LD) persist, submit a single follow-up sample 3–6 weeks later. If the results of the EIA are positive or equivocal and the sample is acute (≤30 days), then the second test to be performed is an immunoglobulin M (IgM) and immunoglobulin G (IgG) immunoblot. If the sample is convalescent OR symptoms persisted for ≥30 days, an IgG immunoblot is done as the second test. If the patient has traveled to Europe/Asia, European immunoblots are the second test.
For MTTT, an EIA is done as the first test. If the result is negative, consider alternative diagnosis OR if signs/symptoms consistent with LD persist, submit a single follow-up sample 3–6 weeks later. If there is a positive or equivocal result, the second test is another EIA. The second EIA may have a positive or equivocal result or a negative result. If there is a negative result, consider alternative diagnosis OR if signs/symptoms consistent with LD persist, submit a single follow-up sample 3–6 weeks later.
A number of different EIAs are available for the first tier in the STTT including those composed of whole cell sonicates (WCS) of the laboratory strain of B. burgdorferi B31. More recently, EIAs based on synthetic peptides that contain regions conserved among multiple B. burgdorferi strains, such as the surface lipoprotein variable major protein-like sequence, expressed (VlsE), C6 (the invariable region 6 of VlsE) or C10 peptide (the conserved amino-terminal portion of outer surface protein C), have been developedFootnote 8Footnote 9. While the specificity of the newer assays is better than WCS, they are still not sufficiently specific to be used as a standalone assay. As a result, supplemental testing with immunoblots is recommendedFootnote 9Footnote 10Footnote 11Footnote 12. The STTT does have a number of technical limitations, including that immunoblots are more laborious to perform than EIAs and the scoring of the immunoblots can be subjective, which may lead to inter and intralaboratory variabilityFootnote 11. In addition, immunoblot testing is performed in relatively few reference diagnostic laboratories in the USFootnote 7 and Canada so turnaround times are typically longer than for EIAs aloneFootnote 8Footnote 11.
The performance characteristics of the STTT algorithm also depend on the stage of infection. A recent systematic review has shown that the sensitivity of STTT for LD is poor in early localized infection (less than 50%) but in late stages of infection the sensitivity approaches 100%Footnote 13. As such, diagnosis and treatment of early localized LD is based on clinical symptoms alone in patients who have exposure history in blacklegged tick endemic areasFootnote 10. However, the diagnosis of early LD can be challenging since some patients with early localized B. burgdorferi infections do not present with an erythema migrans rash and may have symptoms that overlap with those of other diseasesFootnote 9Footnote 14. Thus, improving the sensitivity of testing in early localized infections is important in identifying patients with LD, allowing for early treatment and potentially preventing infection from disseminating and causing severe disease.
Outcomes
Modified two-tiered testing for serologic diagnosis of Lyme disease
There have been a number of studies evaluating the use of a MTTT approach in which a second EIA is performed instead of the traditional immunoblots (Figure 1). A number of different combinations of EIAs have been used in this so-called “two EIA approach” including WCS EIA followed by C6 EIA, VlsE EIA followed by C6 EIA, C6 EIA followed by VlsE and VlsE/C10 followed by WCSFootnote 15Footnote 16Footnote 17Footnote 18Footnote 19Footnote 20. Samples for these evaluations have been drawn from smaller cohorts of patients with acute LDFootnote 15Footnote 18 or comparisons were made using well-characterised samples from the Centers for Disease Control (CDC) LD serum repositoryFootnote 16Footnote 19Footnote 21. Studies were performed on samples from childrenFootnote 17Footnote 20 as well as adultsFootnote 16Footnote 19Footnote 21. With few exceptionsFootnote 22, these evaluations have only been performed on patients from the US and the MTTT has not been fully validated for use on patients with exposure in Europe or Asia.
Although different combinations of EIAs were used in the MTTT algorithms, the MTTT was consistently more sensitive in detecting B. burgdorferi infections, particularly in early localized LD compared with STTT. Importantly, these MTTT had equivalent sensitivity for detecting late infections and comparable specificities to STTT regardless of the combinations of EIAs used in the MTTT (see summaries in Table 1 and Table 2). Recently, the US Food and Drug Administration approved a MTTT algorithm for the laboratory confirmation of LD acquired in North AmericaFootnote 23. This alternative testing algorithm has been endorsed by the US CDC that states that it is an acceptable alternative to the STTT because “the new Lyme disease assays indicates that test performance has been evaluated and is substantially equivalent to or better than a legally marketed predicate test”Footnote 24. It is unknown whether the MTTT approach will be validated in the US for patients who potentially acquired LD outside of North America; however, in Canada the STTT algorithm will be maintained using European-specific assays on Canadians with suspect LD acquired outside of North America (Figure 1).
| Sample size | Reference | Disease manifestations | EIAs combinations usedFootnote a of Table 1 | MTTT sensitivity % (CI or range) | STTT sensitivity % (CI or range)Footnote b of Table 1 |
|---|---|---|---|---|---|
| 140 | Footnote 15 | EM, ENB, LC | WCS f/b C6 | 61 (CI 53–69) | 48 (CI 40–56) |
| 318 | Footnote 11 | EM, ENB | WCS f/b C6 | 60 (CI 55–66) | 41 (CI 36–46) |
| 55 | Footnote 18 | Acute EM | WCS f/b C6; WCS f/b VlsE CFLIA; VlsE FLIA f/b C6 | 42.7(R 38.0–54.0) | 32 (R 25–36) |
| 47 | Footnote 18 | Convalescent EM | WCS f/b C6; WCS f/b VlsE CFLIA; VlsE FLIA f/b C6 | 70 (R 66–72) | 57.3 (R 55.0–60.0) |
| 95 | Footnote 16 | EM, ENB, LC | Vidas f/b C6 or VlsEFootnote c of Table 1 | 66.8 (R 65.2–68.4) | 60.2 (R 56.8–64.2) |
| 114 | Footnote 17 | All disease stages combined | WCS f/b C6 | 79.8 (CI 71.1–86.5) | 81.6 (CI 73.0–88.0) |
| 40 | Footnote 19 | Acute EM | VlsE f/b C6; WCS f/b C6; WCS f/b VlsE | 54.3 (R 50.0–58.0) | 45.3 (R 43.0–50.0) |
| 38 | Footnote 19 | Convalescent EM | VlsE f/b C6; WCS f/b C6; WCS f/b VlsE | 77 (R 76–79) | 61; 61; 63 |
| 124 | Footnote 19 | All disease stages combined | VlsE f/b C6; WCS f/b C6; WCS f/b VlsE | 76.7 (R 75.0–78.0) | 66, 67; 71 |
| 30 | Footnote 25 | Acute EM | VlsE/pepC10 f/b WCS | 73.3 | 50 |
| 30 | Footnote 25 | Convalescent EM | VlsE/pepC10 f/b WCS | 83.3 | 76.7 |
| 56 | Footnote 25 | Early disseminated disease-stageFootnote a of Table 1 | VlsE/pepC10 f/b WCS | 66.1 | 60.7 |
| 29 | Footnote 15 | LA, LNB | WCS f/b C6 | 100 (CI 86–100) | 100 (CI 86–100) |
| 122 | Footnote 11 | LA, LNB | WCS f/b C6 | 98 (CI 93–99) | 96 (CI 91–98) |
| 29 | Footnote 16 | LA | Vidas f/b C6 or VlsE | 100 | 98.9 (R 97–100) |
| 50 | Footnote 25 | Late disseminated disease-stageFootnote c of Table 1 | VlsE/pepC10 f/b WCS | 100 | 100 |
| Sample size | Reference | Patient cohortFootnote a of Table 2 | EIAs combinations usedFootnote b of Table 2 | MTTT sensitivity % (CI or range) | STTT sensitivity % (CI or range)Footnote c of Table 2 |
|---|---|---|---|---|---|
| Overall controls | |||||
| 1,300 | Footnote 15 | Healthy and symptomatic controls | WCS f/b C6 | 99.5 (CI 98.9–99.8) | 99.5 (CI 98.9–99.8) |
| 2,208 | Footnote 11 | Healthy controls & patients with other diseases | WCS f/b C6 | 99.5 (CI 99.1–99.8) | 99.5 (CI 99.1–99.7) |
| 347 | Footnote 16 | Healthy controls & patients with other diseases | Vidas f/b C6 or VlsEFootnote c of Table 2 | 98.3 (CI 96.2–99.3) | 98.3 (CI 96.2–99.3) |
| 931 | Footnote 17 | Healthy and symptomatic controls | WCS f/b C6 | 96.6 (R 94.6–97.6) | 98.7 (R 96.6–100.0) |
| 347 | Footnote 19 | Healthy controls & patients with other diseases | VlsE f/b C6; WCS f/b C6; WCS f/b VlsE | 98.6 (R 97.7–99.4) | 98.1 (R 95.7–99.7) |
| 190 | Footnote 25 | Healthy controls & patients with other diseases | VlsE/pepC10 f/b WCS | 98.9 (R 97.8–100.0) | 100 |
| Unhealthy controls | |||||
| 54 | Footnote 15 | Symptomatic controls | WCS f/b C6 | 100 | 100 |
| 50 | Footnote 18 | Patients with other diseases | WCS f/b C6; WCS f/b VlsE CLIA; VlsE CLIA f/b C6 | 99.3 (R 98.0–100.0) | 100 |
| 144 | Footnote 16 | Patients with other diseases | Vidas f/b C6 or VlsEFootnote d of Table 2 | 98.2 (R 96.5–100.0) | 97.1 (R 94.4–99.3) |
| 830 | Footnote 17 | Symptomatic controls | WCS f/b C6 | 96.5 (R 94.6–97.6) | 98.7 (R 96.6–100.0) |
| 144 | Footnote 19 | Patients with other diseases | VlsE f/b C6; WCS f/b C6; WCS f/b VlsE | 98.1 (R 96.5–100.0) | 97.4 (R 95.7–99.7) |
| 90 | Footnote 25 | Patients with other diseases | VlsE /PEPC10 f/b WCS | 97.8 | 100 |
Benefits and limitations of the modified two-tiered testing
In addition to greater sensitivity for the detection of early B. burgdorferi infections, the interpretation of the results of MTTT is less subjective than immunoblot testing (Table 3). The MTTT has also been shown to be more cost-effective than the STTTFootnote 26. The tests are also less labour-intensive and can be performed using automated instruments or platformsFootnote 8. As such, the MTTT does not require specialized testing (i.e. immunoblots) in a reference laboratory and can be performed by any laboratory that currently does serologic testing. These differences can lead to faster turnaround time for resultsFootnote 8Footnote 9.
| Advantages | Disadvantages |
|---|---|
|
|
The interpretation of the results of the MTTT diagnostic testing is either positive or negative, which is more straightforward than for STTT where IgM and IgG immunoblots can produce different outcomes, which can cause confusion for physiciansFootnote 11. Although more sensitive than STTT, the sensitivity of the MTTT is still less than 90%, so patients with early localized LD should continue to be treated based on their clinical presentation rather than serologic results. However, the rapid turnaround time for MTTT may be particularly useful in evaluating patients with a clinical suspicion of LD but without an erythema migrans rash, or in those who present with signs that overlap with other infections (e.g. Bell’s palsy or arthritis) where serologic results will help establish the diagnosisFootnote 8. The most recent evidence-based guidelines from the United Kingdom suggested that "if LD is still suspected in people with a negative ELISA who were tested within four weeks from symptom onset, repeat the ELISA 4–6 weeks after the first ELISA test”Footnote 12. Currently if the convalescent EIA is positive, it would still require further supplemental testing with an immunoblot in the STTT. Given the anticipated faster turnaround time for the MTTT, clinicians may be more inclined to follow the National Institute for Health and Care Excellence recommendation and consider acute and convalescent testing, which increases diagnostic certainty of the testing on patients who do not present with erythema migrans rash. This is a particularly important consideration when the clinical suspicion is not high, such as for patients without know tick exposure in LD risk areas.
Despite the numerous advantages of the MTTT, there are associated limitations. Since antibodies to B. burgdorferi can persist for months to years after initial infectionFootnote 27, the MTTT algorithm (and the STTT) cannot differentiate between active versus past infections, which further confounds serological diagnosis of reinfection with B. burgdorferi. In addition, it is possible that the MTTT algorithm may generate false positives based on the IgM component of the polyclonal EIAs used, since false positive IgM immunoblots are known to occur in healthy patients or in those with long-standing symptomsFootnote 28Footnote 29Footnote 30Footnote 31. The excellent performance characteristics of STTT in late stage LD may be difficult to match in the MTTT format, especially when polyvalent EIAs (containing epitopes for IgM) are used and it is likely that immunoblots will still need to be used in evaluating difficult LD casesFootnote 8. As such, the use of immunoblots may still have value in patients with manifestations of late stage LD such as Lyme arthritis or in suspect false positive cases where serologic results do not fit with the clinical presentation. In these circumstances, it is reasonable to consider performing an IgG immunoblot as patients with late stage LD have high IgG antibody responses and the immunoblot may allow for the evaluation of the response to specific Borrelial proteins, which some clinicians may find helpfulFootnote 32Footnote 33. Finally, most of the evaluations of the MTTT algorithm have been conducted in areas of high LD endemicity and testing has been restricted to primarily adult patients. Evaluations of the performance of the MTTT in areas of lower risk of LD and in pediatric populations are knowledge gaps that should be filled over timeFootnote 20.
Discussion
The Canadian Public Health Laboratory Network agrees with the CDC recommendationFootnote 24 that serologic assays for LD that utilize a MTTT approach (i.e. substitute a second EIA for the immunoblot in the second tier of testing) are acceptable alternatives to STTT. This recommendation assumes that the MTTT approach has been validated and shown to have comparable performance characteristics to the STTT in regions of Canada where incidence of LD is high, as well as in low incidence jurisdictions. At present, only Nova Scotia has data validating the MTTT approach for LD diagnostics. Based on 447 samples from LD patients in that province, a MTTT consisting of an EIA based on a WCS of B. burgdorferi followed by a C6 EIA, detected 25% more cases of early localized infection compared to the STTT and had a specificity of 99.5%Footnote 34. These results are consistent with previously published data from studies conducted in highly LD endemic areas in the USFootnote 11Footnote 15 and support the use of the MTTT in this province. However, this validation study was conducted in the province with the highest incidence of LD in CanadaFootnote 35. Further validation studies of the MTTT will need to be conducted in regions of Canada where LD incidence is lower, as it will be critical to document the performance characteristics of the MTTT in populations with a lower pre-test probability of infectionFootnote 15Footnote 36. Small reductions in specificity can reduce the predictive value of the test (Table 4), which has led to the recommendation that LD testing should not be considered when the pre-test probability is less than 20%Footnote 37. Given the strain variation within B. burgdorferi populations observed across CanadaFootnote 38, and the possible impact that this strain variability may have on LD diagnostic assaysFootnote 39, it seems prudent to verify that the improved sensitivity of MTTT reported in the literature will be maintained when applied within different jurisdictions in Canada that host diverse and varied strains of B. burgdorferi.
| Estimated prevalence (%) | MTTT positive predictive value (%) | MTTT negative predictive value (%) | ||
|---|---|---|---|---|
| Early localized LDFootnote b of undefined | Late LDFootnote c of undefined | Early localized LDFootnote a of undefined | Late LDFootnote b of undefined | |
| 5 | 84.8 | 91.3 | 97.6 | 100 |
| 3 | 76.6 | 86.0 | 98.6 | 100 |
| 1 | 51.7 | 66.8 | 99.5 | 100 |
| 0.1 | 9.6 | 16.6 | 100 | 100 |
The Lyme Disease Diagnostic Working Group of the Canadian Public Health Laboratory Network is working with provincial laboratories to develop validation plans for the MTTT. The goals of the validation will be to define the performance characteristics of the MTTT in areas with different incidences of LD (and possibly different strains of B. burgdorferi) and to evaluate which combination of the different EIAs available in Canada provide the data necessary to ensure that the benefits of the new MTTT algorithms are realized and specificity of LD serological testing is maintained. A second report will be publicly available once these validation studies are completed.
Conclusion
The US Food and Drug Administration has recently approved a MTTT diagnostic algorithm for LD serology and the US CDC has recommended this new approach as an acceptable alternative to STTT. There are a growing number of scientific publications, using patients from the US, that report improved sensitivity in detection of early localized LD infection, while maintaining high specificity, when MTTT algorithms are compared to STTT. Recent data from Nova Scotia, generated using MTTT, draws similar conclusions. The improved sensitivity of the MTTT and shorter turnaround times associated with this new approach warrant further validation studies and possible rollout of this new diagnostic algorithm for LD in Canada.
Authors’ statement
Lyme disease Diagnostics Working Group of the Canadian Public Health Laboratory Network comprises T Hatchette (co-chair), LR Lindsay (co-chair), K Bernat, G Desnoyers, A Dibernardo, K Fonseca, G German, A Lang, M Morshed, R Needle, S Patel, K Thivierge and P VanCaeseele.
Conflict of interest
Authors have no conflicts of interest to report.
Acknowledgements
The Canadian Public Health Laboratory Network kindly provided secretariat support that was extremely helpful during the development of this position statement.
Funding
No specific funding was provided for this project.
