NACI recommendations on the use of LAIV and HIV-infected individuals

 Download this article as a PDF
Download this article as a PDFPublished by: The Public Health Agency of Canada
Issue: Volume 46–9: Force Health Protection
Date published: September 3, 2020
ISSN: 1481-8531
Submit a manuscript
About CCDR
Browse
Volume 46–9, September 3, 2020: Force Health Protection
Advisory committee statement
Summary of the NACI systematic review and recommendation on the use of live attenuated influenza vaccine (LAIV) in HIV-infected individuals
Dorothy Moore1,2, Ian Gemmill3,4, Robyn Harrison5,6 on behalf of the National Advisory Committee on Immunization (NACI)
Affiliations
1 NACI Influenza Working Group Member
2 McGill University, Montréal, QC
3 NACI Influenza Working Group Chair
4 Queen’s University, Kingston, ON
5 NACI Influenza Working Group Vice Chair
6 University of Alberta, Alberta Health Services, Edmonton, AB
Correspondence
Suggested citation
Moore D, Gemmill I, Harrison R, on behalf of the National Advisory Committee on Immunization (NACI). Summary of the NACI systematic review and recommendation on the use of live attenuated influenza vaccine (LAIV) in HIV-infected individuals. Can Commun Dis Rep 2020;46(9):299–304. https://doi.org/10.14745/ccdr.v46i09a08
Keywords: National Advisory Committee on Immunization, NACI, HIV, live attenuated influenza vaccine, literature review
Abstract
Background: Annual influenza vaccination is recommended for all individuals six months of age and older, including those with HIV infection. Prior to this statement, the National Advisory Committee on Immunization (NACI) stated that live attenuated influenza vaccine (LAIV) was contraindicated for all individuals with HIV infection. The objective of this article is to update NACI’s guidance on the use of LAIV for HIV-infected individuals.
Methods: A systematic literature review of the use of LAIV in individuals with HIV was undertaken. The Canadian Adverse Events Following Immunization Surveillance System was searched for reports of adverse events following vaccination with LAIV in HIV-infected individuals. NACI approved the revised recommendations.
Results: NACI concluded that LAIV is immunogenic in children with HIV, and available data suggest that it is safe, although data were insufficient to detect possible uncommon adverse effects. LAIV may be considered as an option for vaccination of children 2–17 years old who meet the following criteria: 1) receiving highly active antiretroviral therapy for at least four months; 2) CD4 count of 500/µL or greater if age 2–5 years, or of 200/µL or greater if age 6–17 years; and 3) HIV plasma RNA less than 10,000 copies/mL. LAIV remains contraindicated for adults with HIV because of insufficient data. Intramuscular influenza vaccination is considered the standard for children living with HIV by NACI and the Canadian Paediatric & Perinatal HIV/AIDS Research Group, particularly for those without HIV viral load suppression (i.e. plasma HIV RNA is 40 copies/mL or greater). However, if intramuscular (IM) vaccination is not accepted by the patient or substitute decision-maker, LAIV would be reasonable for children meeting the criteria listed above.
Conclusion: LAIV may be considered as an option for annual vaccination of selected children with HIV.
Introduction
Annual vaccination against influenza is recommended for all individuals with HIV infectionFootnote 1 who are six months of age or older. Live vaccines are generally contraindicated in persons with immunodeficiency. Nevertheless, criteria have been established to permit vaccination with measles-mumps-rubella and varicella vaccines when immune function is not severely impaired. These vaccines have been shown to be safe and are recommended for persons with HIV if the HIV infection is controlled and immune function is satisfactory. The National Advisory Committee on Immunization’s (NACI) previous recommendation against live attenuated influenza vaccine (LAIV) use for individuals with immune compromising conditions including HIV was based on expert opinion and the small number of studies available (NACI Recommendation Grade D)Footnote 2. The product monograph states that LAIV administration to immunosuppressed individuals should be based on careful consideration of potential benefits and risksFootnote 3.
Immunization protocols state that LAIV is contraindicated for HIV-infected individuals in British Columbia, Alberta, Manitoba, Saskatchewan and New Brunswick, as well as in the United StatesFootnote 4Footnote 5Footnote 6Footnote 7Footnote 8Footnote 9Footnote 10. Some jurisdictions, such as Québec, the United Kingdom, and FranceFootnote 11Footnote 12Footnote 13 and professional organizations including the Infectious Diseases Society of America and the British Children’s HIV AssociationFootnote 14Footnote 15 state that LAIV may be given to individuals with HIV who meet specific criteria.
The objective of this advisory committee statement is to review the evidence for efficacy, effectiveness, immunogenicity and safety for LAIV use in HIV-infected individuals and to provide updated guidance on the use of LAIV in this population.
Methods
A systematic review of literature on the use of LAIV in HIV-infected individuals was performed. The systematic review’s methodology was specified a priori in a written protocol that included review questions, search strategy, inclusion and exclusion criteria and quality assessment. The NACI Influenza Working Group (IWG) reviewed and approved the protocol.
Six electronic databases (EMBASE, MEDLINE, Scopus, ProQuest Public Health, ClinicalTrials.gov and PROSPERO) were searched from inception to April 13, 2018 using search terms for LAIV and HIV. Searches were restricted to articles published in English and French. In addition, hand searching of included studies was performed by checking reference lists to identify additional relevant publications. Hand searching of reference lists was also performed for any relevant retrieved secondary research articles.
Two reviewers independently screened the titles and abstracts and eligible full-text articles.
Studies were included if they met the following criteria:
- The study population or subpopulation consisted of HIV-infected individuals
- The study assessed efficacy or effectiveness, immunogenicity, safety (including impact on markers of HIV infection), or vaccine virus shedding
Studies were excluded if they met one or more of the following criteria:
- The study did not present data on any of: efficacy and effectiveness, immunogenicity, safety or vaccine virus shedding outcomes for LAIV
- The study was in a language other than English or French
- The study was a non-human or in vitro study
- The article was an editorial, opinion, or news report
- The study presented only secondary research (e.g. literature review, systematic review, meta-analysis)
- The LAIV investigated was not a seasonal LAIV based on the Ann Arbor backbone
Data were extracted into evidence tables. One reviewer extracted data and appraised the methodological quality of the eligible studies. A second reviewer validated the data extraction and quality assessment. The Canadian Adverse Events Following Immunization Surveillance System (CAEFISS) was also searched for reports of adverse events (AE) following immunization (AEFI) with LAIV in HIV-infected individuals. A narrative synthesis of the extracted data was produced and a recommendation for LAIV use developed. NACI critically appraised the available evidence and approved the recommendation.
Results
The systematic review retrieved 220 unique articles, of which eight were retained for data extraction and analysis. These eight articles reported findings from five studies investigating the immunogenicity, safety or both of LAIV in HIV-infected individuals. Four studies were of good quality and one was fair according to ratings of Harris et al.Footnote 16. No studies investigating the efficacy or effectiveness of LAIV in this population were identified. A flow diagram of the study selection process is presented in Figure 1. Key study characteristics are summarized in Table 1.
Figure 1: Flow diagram of the study selection process for the systematic review on the efficacy, effectiveness, immunogenicity and safety of live attenuated influenza vaccine in HIV-infected individuals
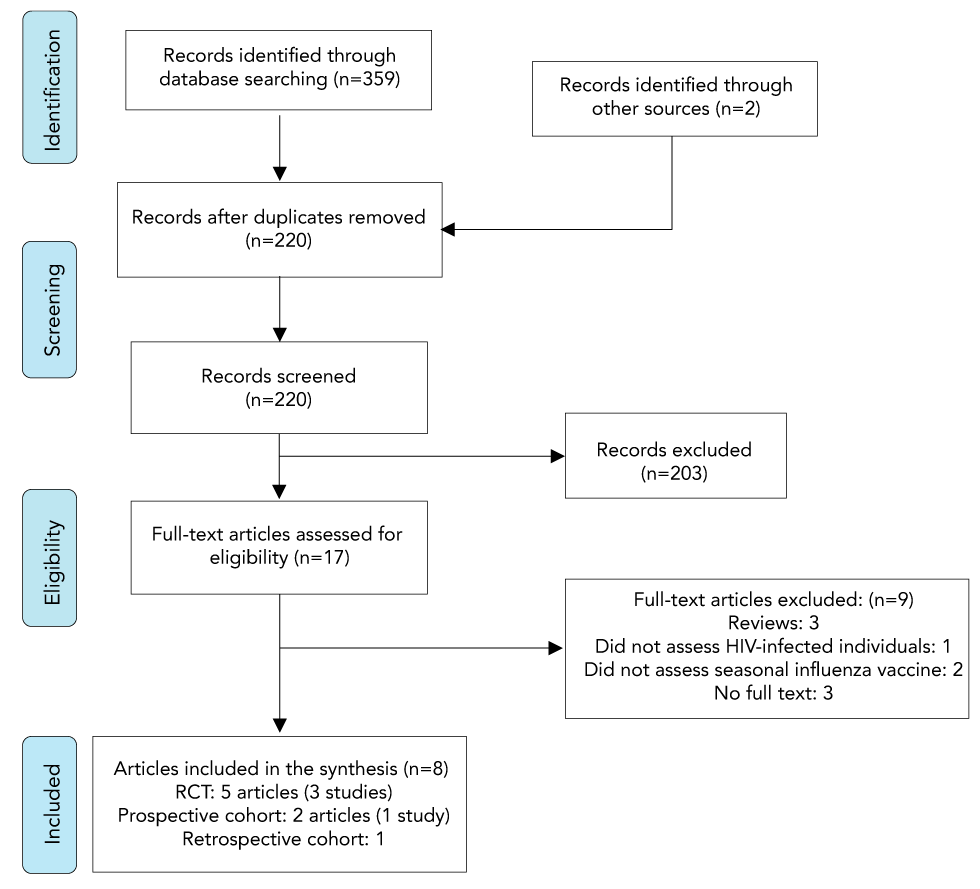
Text description: Figure 1
Figure 1: Flow diagram of the study selection process for the systematic review on the efficacy, effectiveness, immunogenicity and safety of live attenuated influenza vaccine in HIV-infected individuals
Figure 1 depicts a flow diagram of the study selection process for the systematic review. In the identification stage, 359 records were identified through database searching and an additional two records were identified through other sources. During the screening stage, 220 records remained after duplicates were removed. After the records were screened, 203 were excluded. In the eligibility stage, full-text articles were assessed and 17 articles were determined to be eligible. Nine full-text articles were excluded, including three reviews, one article that did not assess HIV-infected individuals, two that did not assess the seasonal influenza vaccine and three that had no full text. Eight articles were included in the synthesis, including five randomized controlled trails (three studies), two prospective cohort articles (one study), and one retrospective cohort.
| Author | Study design (vaccine administered) |
Study population | Outcomes |
|---|---|---|---|
| King et al., 2000Footnote 17 | RCT(LAIV3 versus placebo) | Adults 18–58 years of age with HIV (n=57 total; 28 received LAIV3) and without HIV (n=54 total; 27 received LAIV3)Eligibility criteria for HIV-infected subject: Immune class A1-2, plasma HIV RNA less than 10,000 copies/mL, and more than 200 CD4 cells/µL; if less than or equal to 500 CD4 cells/µL, on stable antiretroviral regimen) within three months prior to vaccination | HI antibody responseAE within 10 days of vaccinationEffect on HIV replication and CD4 cell countsVaccine virus shedding |
| King et al., 2001Footnote 18 | RCT(LAIV3) | Children younger than 8 years of age with HIV (n=24); without HIV (n=25)Eligibility criteria for HIV-infected subject: Immune class N1-2 or A1-2 and plasma HIV RNA less than 10,000 copies/mL within 100 days prior to enrolment | HI antibody responseAE within 10 days of vaccinationEffect on HIV replication and CD4 cell countsVaccine virus shedding |
| Levin et al., 2008Footnote 19Weinberg et al., 2010aFootnote 20Weinberg et al., 2010bFootnote 21 | RCT(LAIV3 vs. IIV3) | Children 5 to less than 18 years of age with HIV (n=243 total; 122 received LAIV3; 121 received IIV3)Eligibility criteria for HIV-infected subject: Stable HIV on HAART for more than or equal to 16 weeks and with HIV-1 plasma RNA fewer than 60,000 copies/mL within 60 days prior to vaccination. All subjects had received IIV3 in at least one of the prior two years | HI, MN antibody response Salivary mucosal IgA and IgG antibody response T cell response AE within 28 days of vaccination Effect on HIV replication and CD4 cell counts Vaccine virus shedding |
| Curtis et al., 2015Footnote 22 Weinberg et al., 2016Footnote 23 | Prospective cohort study (LAIV4) | Children and young adults 2–25 years of age with HIV (n=45) and without HIV (n=55) Eligibility criteria for HIV-infected subject: CD4 greater than 15% or more than 200 cells/µL on cART, or greater than 25% or more than 500 cells/µL if not on cART. All subjects had received influenza vaccine in one or more previous seasons | HI, MN antibody response Nasal mucosal IgA response IgA and IgG memory B cell response; T cell response AE within six weeks of vaccination Vaccine virus shedding |
| Menegay et al., 2017Footnote 24 | Retrospective cohort study(LAIV vs. IIV) | Adults—all active duty US Air Force members diagnosed with HIV (n=437) | Influenza-like illness within 30 days of vaccination |
Immunogenicity
Three studies investigated the immunogenicity of LAIV in a total of 191 HIV-infected children and young adults, 2–25 years of ageFootnote 18Footnote 19Footnote 20Footnote 21Footnote 22Footnote 23, and one study investigated the immunogenicity in 28 HIV-infected adults 18 years of age and olderFootnote 17. All four studies were of good quality according to the Harris et al. criteriaFootnote 16. Immunologic correlates of protection against influenza are relatively well established for hemagglutination inhibition (HI) antibodies for adults, but not for microneutralization (MN) antibodies for adults and not for any serological response for children.
There were no major differences in HI antibody responses following receipt of LAIV between individuals with and without HIVFootnote 17Footnote 18Footnote 22. In the study by Curtis et al.Footnote 22, HI response to influenza B/Yamagata was better in the group with HIV than in the HIV-negative control groupFootnote 22Footnote 23. The proportions of HIV-infected individuals with HI titres of at least 40 vaccinated with LAIV or inactivated influenza vaccine (IIV) were similar for influenza A(H1N1) and A(H3N2) but higher with IIV for influenza B and antibody titres were statistically significantly higher with IIV for influenza A(H3N2) and B vaccine strainsFootnote 19 and for mismatched strainsFootnote 20. A significant increase in MN titres was observed against mismatched, but not the vaccine A(H1N1) strain, in a study of HIV-infected children and young adultsFootnote 22Footnote 23. The proportion of HIV-infected individuals with MN titres greater than or equal to 1:40 was similar post-vaccination for LAIV and IIV, but the magnitude of response was higher for IIV than LAIVFootnote 20.
LAIV induces humoral and mucosal antibody responses as well as T and B cell-mediated responses. Correlates of protection have not been established for LAIV or for cell-mediated responses, and HI titre may underestimate protectionFootnote 25. Two studies looked at mucosal antibody responses. There was no important difference in nasal IgA antibody response to LAIV by HIV statusFootnote 22Footnote 23, or in salivary IgG antibody response to LAIV and IIV in HIV-infected individualsFootnote 19Footnote 20. One study investigated memory B cell and T cell responses. The IgG memory B cell responses did not differ significantly by HIV status for influenza A(H1N1) or A(H3N2); however, a lower absolute response to B/Yamagata post-vaccination was observed in the HIV-infected groupFootnote 22Footnote 23. The magnitude of the rise in T cell response did not differ by HIV statusFootnote 22Footnote 23.
Safety
Five studies reported AEFI with LAIV: three in a total of 191 HIV-infected children and young adultsFootnote 18Footnote 19Footnote 22, one in 28 adultsFootnote 17 and one in 437 adults investigated only for vaccine-associated influenza-like illness (ILI)Footnote 24. Four of the studies were of good quality and one was rated as fair.
In both children and adults with HIV, rates of AEFI with LAIV were comparable to rates observed in individuals without HIV receiving LAIV except for more muscle aches and decreased energy in those with HIVFootnote 17Footnote 18Footnote 22. Rates of AEFI in individuals with HIV receiving LAIV or IIV were also similar, with the exception of more frequent but expected nasopharyngeal symptoms (runny nose and nasal congestion) after LAIVFootnote 19. Reports of ILI after receiving LAIV were rareFootnote 24. No serious or severe AEFIs attributable to LAIV were reported in any study. There have been no reports to CAEFISS of AEFI with LAIV in HIV-infected individuals.
Effects of LAIV on HIV infection were assessed in two studies in childrenFootnote 18Footnote 19 and one in adultsFootnote 17. LAIV had no significant effect on HIV RNA viral load or CD4 count.
Four studies reported on the effect of HIV status on LAIV vaccine virus shedding: three in 191 HIV-infected children and young adultsFootnote 18Footnote 19Footnote 22 and one in 28 HIV-infected adultsFootnote 17. Vaccine virus shedding did not differ by HIV infection statusFootnote 17Footnote 18Footnote 19Footnote 22.
NACI recommendation for individual level decision-making
Following thorough review of the evidence, NACI made the following recommendation:
NACI recommends that LAIV may be considered as an option for children 2–17 years of age with stable HIV infection on highly active antiretroviral therapy (HAART) and with adequate immune function* (Discretionary NACI recommendation).
- NACI concludes that there is fair evidence based on immunogenicity data to recommend the use of LAIV vaccine as an option for children 2–17 years of age with stable HIV infection on HAART and with adequate immune function (Grade B Evidence)
- NACI concludes that, while LAIV appears to have a similar safety profile to IIV, there is insufficient evidence to detect uncommon AE related to the use of LAIV in HIV infected children (Grade I Evidence)
*LAIV should be considered only in children with HIV who meet the following criteria:
- Receiving HAART for at least four months
- Have a CD4 count greater than or equal to 500/µL if 2–5 years of age, or greater than or equal to 200/µL if 6–17 years of age (measured within 100 days before administration of LAIV)
- Have a level of HIV plasma RNA fewer than 10,000 copies/mL (measured within 100 days before administration of LAIV)
While intramuscular (IM) influenza vaccination is considered the standard for children living with HIV by NACI and the Canadian Paediatric and Perinatal HIV/AIDS Research Group, particularly for those without HIV viral load suppression (i.e. IM, plasma HIV RNA more than 40 copies/mL), LAIV would be reasonable for children meeting the criteria outlined above, if vaccination is not accepted by the patient or substitute decision-maker.
The decision to use LAIV in children with stable HIV should be made on a case-by-case basis. The evidence is considered Grade B as there is no direct evidence on the efficacy or effectiveness of LAIV in HIV-infected individuals and the sample size for the evidence base is small.
- There is evidence that LAIV is immunogenic in children 2–17 years of age with stable HIV infection on HAART and with adequate immune function
- LAIV appears to have a similar safety profile to IIV; however, the total number of subjects assessed is insufficient to effectively detect uncommon or rare AE
- Children with HIV receive all the routine childhood vaccines and additional parenteral vaccines warranted by their actual or potential immunocompromised state. Offering intranasal LAIV instead of IIV avoids one IM injection annually. A discussion on preference for route of administration should take place prior to vaccination, and may improve acceptance of the seasonal influenza vaccineFootnote 26Footnote 27
NACI concluded that the quantity of evidence available on the immunogenicity and safety of LAIV in adults with HIV is insufficient to justify a change in the current recommendation against the use of LAIV in this group. (Grade I Evidence). This recommendation is based on expert opinion.
The detailed findings of the literature review and additional information supporting this recommendation can be found in the NACI advisory committee statement: Recommendation on the Use of Live Attenuated Influenza Vaccine (LAIV) in HIV-Infected IndividualsFootnote 28.
Conclusion
LAIV is immunogenic in children with HIV and appears to have a similar safety profile to IIV, although uncommon or rare AE may not have been detected. NACI recommends that LAIV may be considered as an option for children 2–17 years of age with stable HIV infection HAART and with adequate immune function. Studies with sufficient sample size to detect uncommon or rare AE or to address efficacy or effectiveness of LAIV in children may not be feasible, given the limited numbers of children with HIV in high income countries where LAIV is used.
Authors’ statement
- DM — Writing, original draft, review, editing
- IG — Review, editing
- RH — Review, editing
The National Advisory Committee on Immunization (NACI) advisory committee statement: Recommendation on the Use of Live Attenuated Influenza Vaccine (LAIV) in HIV-Infected Individuals was prepared by D Moore, N Dayneka, L Zhao, A Sinilaite, K Young and I Gemmill, on behalf of the NACI Influenza Working Group and was approved by NACI.
Competing interests
None.
Acknowledgements
Influenza Working Group members: I Gemmill (Chair), R Harrison (Vice-Chair), C Bancej, L Cochrane, N Dayneka, L Grohskopf, D Kumar, J Langley, P Wolfe-Roberge, J McElhaney, A McGeer, D Moore, S Smith, B Warshawsky and J Xiong
NACI members: C Quach (Chair), S Deeks (Vice-Chair), N Dayneka, P De Wals, V Dubey, R Harrison, K Hildebrand, C Rotstein, M Salvadori, B Sander, N Sicard and S Smith
Liaison representatives: LM Bucci (Canadian Public Health Association), E Castillo (Society of Obstetricians and Gynaecologists of Canada), A Cohn (Centers for Disease Control and Prevention, United States), J Emili (College of Family Physicians of Canada), M Naus (Canadian Immunization Committee), D Moore (Canadian Paediatric Society) and A Pham-Huy (Association of Medical Microbiology and Infectious Disease Canada)
Ex-officio representatives: J Gallivan (Marketed Health Products Directorate, Health Canada [HC]), E Henry (Centre for Immunization and Respiratory Infectious Diseases [CIRID], Public Health Agency of Canada [PHAC]), M Lacroix (Public Health Ethics Consultative Group, PHAC), J Pennock (CIRID, PHAC), R Pless (Biologics and Genetic Therapies Directorate, HC), G Poliquin (National Microbiology Laboratory, PHAC) and T Wong (First Nations and Inuit Health Branch, Indigenous Services Canada)
The National Advisory Committee on Immunization (NACI) acknowledges and appreciates the contribution of A House, M Laplante, S Ismail, M Tunis, and the Canadian Paediatric & Perinatal HIV/AIDS Research Group to this statement.
Funding
The work of the National Advisory Committee on Immunization is supported by the Public Health Agency of Canada.
