Long-term care facilities: Antibiotic prescribing and antimicrobial stewardship

 Download this article as a PDF
Download this article as a PDFPublished by: The Public Health Agency of Canada
Issue: Volume 48-11/12, November/December 2022: Antimicrobial Use and Stewardship
Date published: November/December 2022
ISSN: 1481-8531
Submit a manuscript
About CCDR
Browse
Volume 48-11/12, November/December 2022: Antimicrobial Use and Stewardship
Overview
Antibiotic prescribing and antimicrobial stewardship in long-term care facilities: Past interventions and implementation challenges
Niyati Vyas1, Tyler Good2, Jorida Cila2, Mark Morrissey2, Denise Gravel Tropper1
Affiliations
1 Antimicrobial Resistance Task Force, Infectious Disease Prevention and Control Branch, Public Health Agency of Canada, Ottawa, ON
2 Office of Behavioural Science, Corporate Data and Surveillance Branch, Public Health Agency of Canada, Ottawa, ON
Correspondence
Suggested citation
Vyas N, Good T, Cila J, Morrissey M, Gravel Tropper D. Antibiotic prescribing and antimicrobial stewardship in long-term care facilities: Past interventions and implementation challenges. Can Commun Dis Rep 2022;48(11/12):512–21. https://doi.org/10.14745/ccdr.v48i1112a04
Keywords: antimicrobial stewardship, antibiotic stewardship, antibiotic prescribing, long-term care, long-term care facilities, nursing homes, antimicrobial resistance
Abstract
Background: The threat of antimicrobial resistance (AMR) is rising, leading to increased illness, death and healthcare costs. In long-term care facilities (LTCFs), high rates of infection coupled with high antibiotic use create a selective pressure for antimicrobial-resistant organisms that pose a risk to residents and staff as well as surrounding hospitals and communities. Antimicrobial stewardship (AMS) is paramount in the fight against AMR, but its adoption in LTCFs has been limited.
Methods: This article summarizes factors influencing antibiotic prescribing decisions in LTCFs and the effectiveness of past AMS interventions that have been put in place in an attempt to support those decisions. The emphasis of this literature review is the Canadian LTCF landscape; however, due to the limited literature in this area, the scope was broadened to include international studies.
Results: Prescribing decisions are influenced by the context of the individual patient, their caregivers, the clinical environment, the healthcare system and surrounding culture. Antimicrobial stewardship interventions were found to be successful in LTCFs, though there was considerable heterogeneity in the literature.
Conclusion: This article highlights the need for more well-designed studies that explore innovative and multifaceted solutions to AMS in LTCFs.
Introduction
Antimicrobial resistance (AMR) is a global health emergency with rising human and financial costs Footnote 1 The threat is especially pertinent in long-term care facilities (LTCFs), which provide a range of healthcare options to older adults unable to live independently in the community, ranging from resident and long-term care to post-acute rehabilitation care Footnote 2 Older adults living in LTCFs are often clinically frail and at high risk of infection and subsequent antibiotic use Footnote 3 Footnote 4 The leading indications for antibiotic use in LTCFs were urinary tract infections (UTIs), lower respiratory tract infections (LRTIs) and skin and soft tissue infections (SSTIs) Footnote 5. Of these, suspected UTIs provided the greatest challenge to antimicrobial stewardship (AMS), with up 70.5% of antibiotic prescriptions being considered clinically unnecessary, compared with 55.7% of prescriptions for LRTI and 22.0% for SSTI Footnote 5. While antibiotics are indispensable tools for combatting serious infections, inappropriate use, in terms of initiation, duration or dose, increases the possibility of selecting antimicrobial-resistant organisms (AROs) Footnote 3 Footnote 6. Long-term care facilities can become reservoirs for AROs, threatening the well-being of LTCF residents and staff as well as the surrounding hospital and community Footnote 7 Footnote 8 Footnote 9.
Methods
Antimicrobial stewardship programs have been implemented in some LTCFs, often leading to reduced prevalence of AROs and improved resident outcomes Footnote 10. However, there was a paucity of reviews from a Canadian perspective examining these AMS programs. This article describes factors influencing antibiotic prescribing decisions and the effectiveness of AMS interventions that have attempted to support those decisions. The emphasis of this literature review is on the Canadian LTCF landscape; however, due to the limited number of studies performed in Canada, we included international studies as well. The Embase, Medline and Global Health databases were searched to identify relevant articles published prior to April 2022 (see Appendix for a complete list of search terms). This search resulted in 26 primary research articles examining factors affecting antibiotic prescribing (seven Canadian) Footnote 6 Footnote 11 Footnote 12 Footnote 13 Footnote 14 Footnote 15 Footnote 16 and 22 articles assessing the success of AMS interventions in LTCFs (four Canadian) Footnote 17 Footnote 18 Footnote 19 Footnote 20. The overwhelming majority of these studies occurred in LTCFs or nursing homes, though one of the studies examining factors affecting antibiotic prescribing queried staff in assisted living facilities Footnote 21 and another included a sample of five nursing homes and two residential care facilities Footnote 22. Of the AMS intervention studies we assessed, two were implemented in skilled nursing facilities Footnote 23 Footnote 24, while another studied assisted living facilities Footnote 25.
Factors influencing antibiotic prescribing in long-term care facilities
Prescribing decisions are influenced by the context of the individual patient, their caregivers, the clinical environment, the healthcare system and the society that surrounds the prescriber. Figure 1 summarizes the evidence for barriers to AMS in LTCFs that operate at each level.
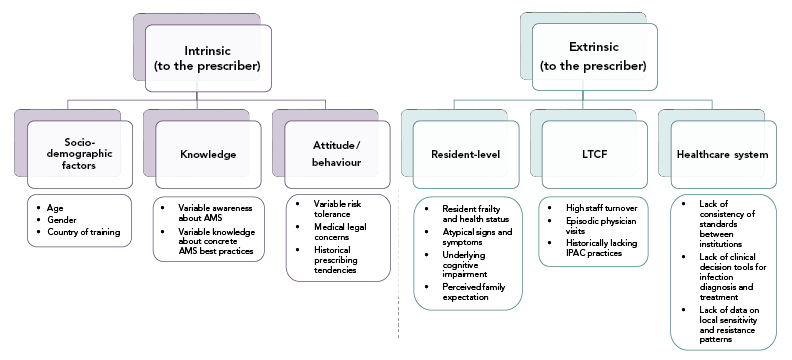
Figure 1 - Text description
The figure shows a hierarchy of barriers to antimicrobial stewardship in long-term care facilities. The highest level of the hierarchy show barriers are either intrinsic to the prescriber or extrinsic to the prescriber. The intrinsic barriers include socio-demographic factors (Age, gender, country of training), knowledge (variable awareness about AMS, variable knowledge about concrete AMS best practices), and attitude/behaviour (variable risk tolerance, medical legal concerns, historical prescribing tendencies). The extrinsic barriers include those at the resident-level (resident frailty and health status, atypical signs and symptoms, underlying cognitive impairment, perceived family expectation), LTCF-level (high staff turnover, episodic physician visits, historically lacking IPAC practices), and healthcare system-level (lack of consistency of standards between individual institutions, lack of clinical decision tools for infection diagnosis and treatment, lack of data on local sensitivity and resistance patterns).Prescriber factors
Antibiotic prescribing habits are highly variable among prescribers in LTCFs, and this variability is not accounted for by differences in resident characteristics Footnote 6 suggesting that individual prescribers have a role in driving antibiotic use and overuse. Past prescribing behaviour is a strong predictor of future prescribing Footnote 6, and being older, male and having completed medical school outside of Canada are associated with higher levels of antibiotic prescribing Footnote 6. Furthermore, tendency towards risk aversion (i.e. risk of delayed treatment and associated consequences) also influence antibiotic prescribing decisions Footnote 9 Footnote 14 Footnote 22 Footnote 26.
Research also suggests that knowledge about AMR is variable in physicians and nurses and that knowledge gaps are associated with inappropriate prescribing Footnote 16 Footnote 27 Footnote 28. The search did not identify articles examining AMR knowledge in non-regulated caregivers, who provide much of the primary care in LTCFs.
Resident population factors
Residents of LTCFs are an increasingly frail population with complex care needs Footnote 29 Footnote 30. Medical complaints from LTCF residents often present with non-specific or atypical symptoms that create diagnostic uncertainty, posing a challenge to confident antibiotic prescribing Footnote 13 Footnote 21 Footnote 22 Footnote 28 Footnote 31. Furthermore, a high proportion of residents have underlying cognitive impairment that limits their ability to communicate the specific symptoms and disease course that would inform diagnosis Footnote 13 Footnote 21 Footnote 22 Footnote 28 Footnote 31. Caregivers, who are important advocates for residents, may be perceived as having expectations that can influence antibiotic prescribing decisions Footnote 16 Footnote 32 Footnote 33 Footnote 34.
Long-term care facilities environmental factors
Staffing patterns also contribute to antibiotic prescribing practices in LTCFs. Physicians visit LTCFs episodically, causing reliance on asynchronous communication strategies (i.e. fax, email, calls), which may lead to care team members not having the information they need to prescribe judiciously Footnote 9 Footnote 27 Footnote 28 Footnote 31 Footnote 32 Footnote 35 Footnote 36. High nursing and personal support worker turnover are also a major barrier to AMS in the LTCFs Footnote 16, perpetuating knowledge gaps among staff from lack of stability. Moreover, effective infection prevention and control practices, which are recognized to limit the spread of AMR, have historically been lacking in LTCFs due to limited resources and training opportunities Footnote 8 Footnote 16 Footnote 37 Footnote 38 Footnote 39. Prescribers may also perceive pressure due to medical legal concerns associated with adverse patient outcomes following the decision not to initiate an antibiotic prescription Footnote 38.
Healthcare systems factors and surveillance
At the healthcare system level, lack of access to resident-relevant information and consistency of standards between different healthcare institutions are key factors impeding informed decision-making in antibiotic prescribing Footnote 16 Footnote 36 Footnote 38. While many hospitals have robust antibiogram programs, LTCFs lack data on local sensitivity or resistance patterns. In fact, most specimens collected from LTCFs are processed in private laboratories in Canada and antimicrobial susceptibility data from those sites are not always made available to prescribers, leaving them without local resistance determinants to inform prescribing (personal communication, RP Rennie). There is also a lack of specific guidelines or clinical decision tools regarding infection diagnosis and treatment for LTCF residents Footnote 14 Footnote 22 Footnote 28 Footnote 35 Footnote 38; these gaps impede informed antibiotic decision-making and ultimately increase the risk of selecting for AROs Footnote 22 Footnote 28. Lastly, there are limited antibiotic surveillance data from Canadian LTCFs and an absence of data on appropriate use, which represent a missing foundation for AMS programs in the sector.
Effectiveness of antimicrobial stewardship interventions in long-term care facilities
A variety of AMS intervention approaches in LTCF have been reported, with most articles testing multiple methods. Of the 22 articles reviewed, twelve used educational strategies and clinical practice guidelines Footnote 17 Footnote 18 Footnote 20 Footnote 22 Footnote 23 Footnote 35 Footnote 40 Footnote 41 Footnote 42 Footnote 43 Footnote 44 Footnote 45. Others used a range of strategies, including audit and feedback Footnote 18 Footnote 19 Footnote 44 Footnote 46 Footnote 47 Footnote 48, clinical care pathways Footnote 25 Footnote 41 Footnote 44, modified urine culture reporting Footnote 49, use of an infectious disease team Footnote 43 Footnote 47 Footnote 50 Footnote 51 and interventions tailored to local needs Footnote 18 Footnote 23 Footnote 42 Footnote 43. There was no single AMS intervention best practice; instead, articles have shown generally positive, but heterogeneous, results for many approaches. The AMS interventions most commonly targeted physicians Footnote 18 Footnote 19 Footnote 23 Footnote 42 Footnote 46 Footnote 47 Footnote 50 Footnote 51 or both physicians and nurses Footnote 17 Footnote 22 Footnote 24 Footnote 25 Footnote 35 Footnote 44 Footnote 48 Footnote 52. It was less common for AMS trials to focus solely on nurses Footnote 40 Footnote 41 Footnote 45 Footnote 53, pharmacists Footnote 22 Footnote 52, caregivers Footnote 25 Footnote 43 Footnote 44 or residents Footnote 44. Antimicrobial stewardship intervention approaches were reported only rarely in Canada; four of the 22 articles were implemented in Canadian LTCFs Footnote 17 Footnote 18 Footnote 19 Footnote 20.
In the following sections, the results from these 22 articles are summarized and organized by outcome measure.
Antibiotic prescribing
Available evidence suggests that AMS interventions have generally been effective in reducing antibiotic prescribing, with a recent meta-analysis finding interventions associated with a 14% overall reduction in antimicrobial use (AMU) Footnote 10. Primary research points to the positive effects of AMS interventions in reducing antibiotic prescriptions, especially for the treatment of UTIs Footnote 20 Footnote 45 Footnote 53. It should be noted that outcomes assessing the appropriateness of antibiotic prescriptions are a more precise measure of stewardship than AMU; however, collecting these data is more labour-intensive and fewer articles examined this outcome measure Footnote 18 Footnote 22 Footnote 23 Footnote 24 Footnote 41 Footnote 46 Footnote 52. Among the studies that did measure the appropriateness of antibiotic prescriptions, the evidence was mixed; with some showing statistically significant improvements Footnote 18 Footnote 41 Footnote 46 and others not Footnote 22 Footnote 23 Footnote 24 Footnote 52. Another important study outcome was the duration of therapy, where deprescribing interventions (i.e. the planned process of reducing or stopping medications that are no longer needed or may be causing harm) showed promise Footnote 54. Two articles showed reductions in the duration of antibiotic therapy following an AMS intervention Footnote 19 Footnote 48, but more research is needed in this area.
Balancing measures
A recent systematic review found AMS interventions did not increase hospital admissions or deaths, indicating that these programs did not lead to under-treatment of infections Footnote 55. There was still limited evidence in this area and a need for further study. Future AMS articles should continue to monitor the safety of interventions by tracking mortality and morbidity outcomes as well as appropriateness measures.
Special focus on urinary tract infection
Antibiotic prescribing for suspected UTIs is a primary focus of AMS in LTCF. At the core of this challenge is the diagnosis of asymptomatic bacteriuria, which has a remarkably high incidence among LTCF residents Footnote 3 Footnote 56. The judicious use of diagnostic tools for UTIs plays an important role in supporting UTI treatment decisions. The practice of routine dipstick analysis regardless of UTI symptoms increased the frequency of antibiotic use despite the known lack of utility of these tests among LTCF residents Footnote 22 Footnote 38. Dipstick analysis is generally not recommended for LTCF residents Footnote 57; however, the rate of de-adoption is unknown. Only one article examined this outcome and it did not show a decrease in the use of dipstick analysis following an AMS intervention that included the education of staff about new clinical practice guidelines through AMS program champions Footnote 40.
An upstream focus on the judicious use of urine cultures may be helpful in reducing unnecessary antibiotic prescriptions for UTIs given the high rates of asymptomatic bacteriuria in the LTCF population. Three articles have taken this approach, all showing a successful reduction in urine cultures, as well as, importantly, AMU Footnote 13 Footnote 20 Footnote 48. The timing of microbiology test results was also relevant, as delayed results increased the use of antibiotics, especially when coupled with increased risk aversion in the prescriber Footnote 16 Footnote 22 Footnote 31 Footnote 32 Footnote 38. Lastly, providing prescribers with local annual antibiograms may also be effective in reducing the rate of urine cultures and urinary antibiotics Footnote 58.
Discussion
Antimicrobial resistance is a public health threat with considerable health and economic burden Footnote 3 and a serious health-related issue for LTCF residents Footnote 7 Footnote 59. Available evidence points to multiple factors influencing antibiotic overprescribing in LTCFs operating at various levels. These range from 1) individual differences in health care workers' knowledge of AMS to 2) variability in risk tolerances in nurses and doctors to 3) lack of consistent clinical guidelines and to 4) established practices (e.g. dipstick analysis). A significant issue in the Canadian context is the lack of institutional surveillance on AMU and local resistance patterns, which is foundational to successful AMS programs. Published articles showed that the adoption of AMS interventions in LTCFs can be effective, albeit with significant variability in effect sizes. Meaningful, sustainable implementation of AMS programs in LTCFs will require multifaceted solutions that address barriers faced by different decision-makers in the system.
The most frequently used interventions in AMS programs were educational components and clinical practice guidelines; however, there was no consensus on one specific strategy for an effective stewardship program, as no single intervention generated sufficient, sustainable improvement in antibiotic prescribing Footnote 60 Footnote 61. Multifaceted AMS interventions at different levels could help reduce unnecessary or inappropriate AMU, ensure the optimal selection of antimicrobial therapies (i.e. dosage and duration) and help impede selective pressure for AROs Footnote 9 Footnote 10. Implementation of a multifaceted AMS intervention would require dedicated resources in LTCFs Footnote 9. The practice of behavioural science has at its core a focus on changing behaviour—a foundational pillar of AMS. In other sectors, including acute care hospitals and community, behavioural science trials have been successful in delivering impactful, low-cost components to AMS programs Footnote 62 Footnote 63. Heavier-handed solutions, like antibiotic restriction policies, may also play a role in enforcing stewardship, though their implementation must be carefully considered Footnote 64.
In the Canadian context, barriers to AMS partly reflect a historical and ongoing under-emphasis of vulnerable older adults, which manifests as poorly funded institutions with substandard working conditions, and a struggle to attract and retain a stable and qualified workforce—a situation only made more precarious during the coronavirus disease 2019 pandemic. A more thorough examination of social and cultural drivers of AMS in Canada has been conducted by other researchers Footnote 65.
The literature documents many barriers to AMS in LTCFs, with a particularly strong focus on factors that affect prescribers. This is crucial given the integral role these clinicians play in AMS; however, there is room for further study of the perspectives of non-prescribing healthcare providers on AMS, who provide most of the primary care in LTCFs (e.g. registered nurses, registered practical nurses, and personal support workers) and who are often the first to identify infections within the residents of LTCFs. A study of the diverse stakeholders in LTCFs may reveal novel opportunities for a broader set of individuals to participate in stewardship. Additionally, the relative importance and interconnectedness of barriers are unclear and further study is needed to parse the potential benefits of AMS interventions focused on each part of the system. A multifaceted problem warrants a multifaceted approach. Learning from the hospital sector Footnote 66, systems dynamics modelling may provide an important role on this front, as outcomes in non-linear systems like LTCFs are difficult to predict with conventional methods. Most of the articles assessing AMS effectiveness also rely on small sample sizes, limiting generalizability, which is particularly relevant given a heterogeneous LTCF landscape. Finally, we note that there is limited national-level surveillance data on AMU and AMR in Canadian LTCFs, which is necessary to inform future AMS efforts.
Conclusion
This article identified a wide range of barriers to judicious antibiotic prescribing in LTCFs and summarized evidence that indicates that AMS programs can be effective in this environment. While this article focused on LTCFs, its findings may also be relevant to assisted living facilities as the resident populations in these settings are similar. Future work should consider perspectives from a diverse group of stakeholders to help uncover how a larger group of actors can be supported as allies in AMS in LTCFs. The development of further high-quality trials is also needed, especially in Canada, to help understand which interventions retain effectiveness over time and across the heterogeneous LTCF landscape. Finally, strengthening the national surveillance system for AMU and AMR in LTCFs in Canada will be foundational to measure the impact of AMS strategies in this challenging setting.
Authors' statement
NV — Literature search, wrote the first draft
TG — Conceptualization, oversaw data collection, revisions
JC — Revisions
MM — Conceptualization, oversaw data collection, revisions
DGT — Conceptualization, oversaw data collection, revisions
Competing interests
The authors report no competing interests.
Contributors
Jerome A Leis (Sunnybrook Health Sciences Centre, Toronto, Ontario [ON]; Department of Medicine and Centre for Quality Improvement and Patient Safety, University of Toronto, Toronto, ON); Patrick Quail (Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada); Marianna Ofner (Vice President's Office, Infectious Disease Prevention and Control Branch, Public Health Agency of Canada, Ottawa, ON); Peter Daley (Discipline of Medicine, Memorial University of Newfoundland, St. John's, Newfoundland); Lauryn Conway (Impact and Innovation Unit, Privy Council Office).
Acknowledgements
Thank you to the Health Canada Library for facilitating the literature review.
Funding
This work was supported by the Public Health Agency of Canada.
References
- Footnote 1
-
Organisation for Economic Co-operation and Development. OECD Health Policy Studies. Stemming the Superbug Tide. Paris (FR): OECD; 2018. https://www.oecd-ilibrary.org/content/publication/9789264307599-en
- Footnote 2
-
Arieti F, Göpel S, Sibani M, Carrara E, Pezzani MD, Murri R, Mutters NT, Lòpez-Cerero L, Voss A, Cauda R, Tacconelli E. ARCH working group. White Paper: bridging the gap between surveillance data and antimicrobial stewardship in long-term care facilities—practical guidance from the JPIAMR ARCH and COMBACTE-MAGNET EPI-Net networks. J Antimicrob Chemother 2020;75 Suppl 2:ii42–51. https://doi.org/10.1093/jac/dkaa428
- Footnote 3
-
Organisation for Economic Co-operation and Development. OECD Health Working Papers. Antimicrobial resistance in long-term care facilities. Paris (FR): OECD; 2022. (accessed 2022-07-15). https://www.oecd-ilibrary.org/social-issues-migration-health/antimicrobial-resistance-in-long-term-care-facilities_e450a835-en
- Footnote 4
-
Nicolle LE. Antimicrobial stewardship in long term care facilities: what is effective? Antimicrob Resist Infect Control 2014;3(1):6. https://doi.org/10.1186/2047-2994-3-6
- Footnote 5
-
Penney CC, Boyd SE, Mansfield A, Dalton J, O'Keefe J, Daley PK. Antimicrobial use and suitability in long-term care facilities: A retrospective cross-sectional study. Off J Assoc Med Microbiol Infect Dis Can 2018;3(4):209–16. https://doi.org/10.3138/jammi.2018-0021
- Footnote 6
-
Daneman N, Campitelli MA, Giannakeas V, Morris AM, Bell CM, Maxwell CJ, Jeffs L, Austin PC, Bronskill SE. Influences on the start, selection and duration of treatment with antibiotics in long-term care facilities. CMAJ 2017;189(25):E851–60. https://doi.org/10.1503/cmaj.161437
- Footnote 7
-
Marra F, McCabe M, Sharma P, Zhao B, Mill C, Leung V, Chong M, Patrick DM. Utilization of Antibiotics in Long-Term Care Facilities in British Columbia, Canada. J Am Med Dir Assoc 2017;18(12):1098.e1–11. https://doi.org/10.1016/j.jamda.2017.09.018
- Footnote 8
-
Nucleo E, Caltagirone M, Marchetti VM, D'Angelo R, Fogato E, Confalonieri M, Reboli C, March A, Sleghel F, Soelva G, Pagani E, Aschbacher R, Migliavacca R, Pagani L; AMCLI – GLISTer Group; ESCMID Study Group Elderly Infections – ESGIE. Colonization of long-term care facility residents in three Italian Provinces by multidrug-resistant bacteria. Antimicrob Resist Infect Control 2018;7:33. https://doi.org/10.1186/s13756-018-0326-0
- Footnote 9
-
Ramly E, Tong M, Bondar S, Ford JH 2nd, Nace DA, Crnich CJ. Workflow Barriers and Strategies to Reduce Antibiotic Overuse in Nursing Homes. J Am Geriatr Soc 2020;68(10):2222–31. https://doi.org/10.1111/jgs.16632
- Footnote 10
-
Wu JH, Langford BJ, Daneman N, Friedrich JO, Garber G. Antimicrobial Stewardship Programs in Long-Term Care Settings: A Meta-Analysis and Systematic Review. J Am Geriatr Soc 2019;67(2):392–9. https://doi.org/10.1111/jgs.15675
- Footnote 11
-
Carusone SC, Loeb M, Lohfeld L. A clinical pathway for treating pneumonia in the nursing home: part I: the nursing perspective. J Am Med Dir Assoc 2006;7(5):271–8. https://doi.org/10.1016/j.jamda.2005.11.004
- Footnote 12
-
Carusone SC, Loeb M, Lohfeld L. A clinical pathway for treating pneumonia in the nursing home: part II: the administrators' perspective and how it differs from nurses' views. J Am Med Dir Assoc 2006;7(5):279–86. https://doi.org/10.1016/j.jamda.2005.11.005
- Footnote 13
-
Lohfeld L, Loeb M, Brazil K. Evidence-based clinical pathways to manage urinary tract infections in long-term care facilities: a qualitative case study describing administrator and nursing staff views. J Am Med Dir Assoc 2007;8(7):477–84. https://doi.org/10.1016/j.jamda.2007.05.006
- Footnote 14
-
Langford BJ, Quirk J, Carey S, Daneman N, Garber GE. Influencing duration of antibiotic therapy: A behavior change analysis in long-term care. Am J Infect Control 2019;47(12):1409–14. https://doi.org/10.1016/j.ajic.2019.05.020
- Footnote 15
-
Daneman N, Gruneir A, Bronskill SE, Newman A, Fischer HD, Rochon PA, Anderson GM, Bell CM. Prolonged antibiotic treatment in long-term care: role of the prescriber. JAMA Intern Med 2013;173(8):673–82. https://doi.org/10.1001/jamainternmed.2013.3029
- Footnote 16
-
Laur C, Sribaskaran T, Simeoni M, Desveaux L, Daneman N, Mulhall C, Lam J, Ivers NM. Improving antibiotic initiation and duration prescribing among nursing home physicians using an audit and feedback intervention: a theory-informed qualitative analysis. BMJ Open Qual 2021;10(1):e001088. https://doi.org/10.1136/bmjoq-2020-001088
- Footnote 17
-
Loeb M, Brazil K, Lohfeld L, McGeer A, Simor A, Stevenson K, Zoutman D, Smith S, Liu X, Walter SD. Effect of a multifaceted intervention on number of antimicrobial prescriptions for suspected urinary tract infections in residents of nursing homes: cluster randomised controlled trial. BMJ 2005;331(7518):669. https://doi.org/10.1136/bmj.38602.586343.55
- Footnote 18
-
Monette J, Miller MA, Monette M, Laurier C, Boivin JF, Sourial N, Le Cruguel JP, Vandal A, Cotton-Montpetit M. Effect of an educational intervention on optimizing antibiotic prescribing in long-term care facilities. J Am Geriatr Soc 2007;55(8):1231–5. https://doi.org/10.1111/j.1532-5415.2007.01250.x
- Footnote 19
-
Daneman N, Lee SM, Bai H, Bell CM, Bronskill SE, Campitelli MA, Dobell G, Fu L, Garber G, Ivers N, Lam JM, Langford BJ, Laur C, Morris A, Mulhall C, Pinto R, Saxena FE, Schwartz KL, Brown KA. Population-Wide Peer Comparison Audit and Feedback to Reduce Antibiotic Initiation and Duration in Long-Term Care Facilities with Embedded Randomized Controlled Trial. Clin Infect Dis 2021;73(6):e1296–304. https://doi.org/10.1093/cid/ciab256
- Footnote 20
-
Pasay DK, Guirguis MS, Shkrobot RC, Slobodan JP, Wagg AS, Sadowski CA, Conly JM, Saxinger LM, Bresee LC. Antimicrobial stewardship in rural nursing homes: impact of interprofessional education and clinical decision tool implementation on urinary tract infection treatment in a cluster randomized trial. Infect Control Hosp Epidemiol 2019;40(4):432–7. https://doi.org/10.1017/ice.2019.9
- Footnote 21
-
Kistler CE, Zimmerman S, Scales K, Ward K, Weber D, Reed D, McClester M, Sloane PD. The Antibiotic Prescribing Pathway for Presumed Urinary Tract Infections in Nursing Home Residents. J Am Geriatr Soc 2017;65(8):1719–25. https://doi.org/10.1111/jgs.14857
- Footnote 22
-
van Buul LW, van der Steen JT, Doncker SM, Achterberg WP, Schellevis FG, Veenhuizen RB, Hertogh CM. Factors influencing antibiotic prescribing in long-term care facilities: a qualitative in-depth study. BMC Geriatr 2014;14:136. https://doi.org/10.1186/1471-2318-14-136
- Footnote 23
-
Naughton BJ, Mylotte JM, Ramadan F, Karuza J, Priore RL. Antibiotic use, hospital admissions, and mortality before and after implementing guidelines for nursing home-acquired pneumonia. J Am Geriatr Soc 2001;49(8):1020–4. https://doi.org/10.1046/j.1532-5415.2001.49203.x
- Footnote 24
-
Furuno JP, Comer AC, Johnson JK, Rosenberg JH, Moore SL, MacKenzie TD, Hall KK, Hirshon JM. Using antibiograms to improve antibiotic prescribing in skilled nursing facilities. Infect Control Hosp Epidemiol 2014;35 Suppl 3:S56–61. https://doi.org/10.1086/677818
- Footnote 25
-
Sloane PD, Zimmerman S, Reed D, Beeber AS, Chisholm L, Kistler C, Khandelwal C, Weber DJ, Mitchell CM. Antibiotic prescribing in 4 assisted-living communities: incidence and potential for improvement. Infect Control Hosp Epidemiol 2014;35 Suppl 3:S62–8. https://doi.org/10.1086/677821
- Footnote 26
-
Feldstein D, Sloane PD, Weber D, Ward K, Reed D, Zimmerman S. Current Prescribing Practices for Skin and Soft Tissue Infections in Nursing Homes. J Am Med Dir Assoc 2017;18(3):265–70. https://doi.org/10.1016/j.jamda.2016.09.024
- Footnote 27
-
Fleming A, Bradley C, Cullinan S, Byrne S. Antibiotic prescribing in long-term care facilities: a meta-synthesis of qualitative research. Drugs Aging 2015;32(4):295–303. https://doi.org/10.1007/s40266-015-0252-2
- Footnote 28
-
Russell J, Gallen D. Influencing factors on antibiotic prescribing in nursing homes. Prim Health Care Res Dev 2003;4(1):69–75. https://doi.org/10.1191/1463423603pc132oa
- Footnote 29
-
Canadian Institute for Health Information. 1 in 9 new long-term care residents potentially could have been cared for at home. CIHI; 2020. (accessed 2022-07-26). https://www.cihi.ca/en/1-in-9-new-long-term-care-residents-potentially-could-have-been-cared-for-at-home
- Footnote 30
-
Australian Government. Department of Health and Aged Care. 2017–18 Report on the Operation of the Aged Care Act 1997 (ROACA). Canberra (AU); Australia Government; 2019. (accessed 2022-07-26). https://www.health.gov.au/resources/publications/2017-18-report-on-the-operation-of-the-aged-care-act-1997-roaca
- Footnote 31
-
Yogo N, Gahm G, Knepper BC, Burman WJ, Mehler PS, Jenkins TC. Clinical Characteristics, Diagnostic Evaluation, and Antibiotic Prescribing Patterns for Skin Infections in Nursing Homes. Front Med (Lausanne) 2016;3:30. https://doi.org/10.3389/fmed.2016.00030
- Footnote 32
-
Schweizer AK, Hughes CM, Macauley DC, O'Neill C. Managing urinary tract infections in nursing homes: a qualitative assessment. Pharm World Sci 2005;27(3):159–65. https://doi.org/10.1007/s11096-005-1191-5
- Footnote 33
-
Dowson L, Friedman ND, Marshall C, Stuart RL, Buising K, Rajkhowa A, Gotterson F, Kong DC. The role of nurses in antimicrobial stewardship near the end of life in aged-care homes: A qualitative study. Int J Nurs Stud 2020;104:103502. https://doi.org/10.1016/j.ijnurstu.2019.103502
- Footnote 34
-
Scales K, Zimmerman S, Reed D, Beeber AS, Kistler CE, Preisser JS, Weiner BJ, Ward K, Fann A, Sloane PD. Nurse and Medical Provider Perspectives on Antibiotic Stewardship in Nursing Homes. J Am Geriatr Soc 2017;65(1):165–71. https://doi.org/10.1111/jgs.14504
- Footnote 35
-
Pettersson E, Vernby A, Mölstad S, Lundborg CS. Infections and antibiotic prescribing in Swedish nursing homes: a cross-sectional study. Scand J Infect Dis 2008;40(5):393–8. https://doi.org/10.1080/00365540701745279
- Footnote 36
-
Helton MR, van der Steen JT, Daaleman TP, Gamble GR, Ribbe MW. A cross-cultural study of physician treatment decisions for demented nursing home patients who develop pneumonia. Ann Fam Med 2006;4(3):221–7. https://doi.org/10.1370/afm.536
- Footnote 37
-
Barney GR, Felsen CB, Dumyati GK. One-day point prevalence as a method for estimating antibiotic use in nursing homes. Infect Control Hosp Epidemiol 2019;40(2):221–3. https://doi.org/10.1017/ice.2018.309
- Footnote 38
-
Lim CJ, Kwong MW, Stuart RL, Buising KL, Friedman ND, Bennett NJ, Cheng AC, Peleg AY, Marshall C, Kong DC. Antibiotic prescribing practice in residential aged care facilities--health care providers' perspectives. Med J Aust 2014;201(2):101–105. https://doi.org/10.5694/j.1326-5377.2014.tb04232.x
- Footnote 39
-
Ackers L, Ackers-Johnson G, Welsh J, Kibombo D, Opio S. Infection Prevention Control (IPC) and Antimicrobial Resistance (AMR). In: Anti-Microbial Resistance in Global Perspective. Cham: Palgrave Macmillan; 2020. p. 53–80. https://doi.org/10.1007/978-3-030-62662-4_4
- Footnote 40
-
Cooper D, Titler M, Struble L, Redman R. A multifaceted, evidence-based program to reduce inappropriate antibiotic treatment of suspected urinary tract infections. Ann Longterm Care 2017;25(2):36–43. https://www.hmpgloballearningnetwork.com/site/altc/articles/multifaceted-evidence-based-program-reduce-inappropriate-antibiotic-treatment-suspected
- Footnote 41
-
Fleet E, Gopal Rao G, Patel B, Cookson B, Charlett A, Bowman C, Davey P. Impact of implementation of a novel antimicrobial stewardship tool on antibiotic use in nursing homes: a prospective cluster randomized control pilot study. J Antimicrob Chemother 2014;69(8):2265–73. https://doi.org/10.1093/jac/dku115
- Footnote 42
-
McMaughan DK, Nwaiwu O, Zhao H, Frentzel E, Mehr D, Imanpour S, Garfinkel S, Phillips CD. Impact of a decision-making aid for suspected urinary tract infections on antibiotic overuse in nursing homes. BMC Geriatr 2016;16:81. https://doi.org/10.1186/s12877-016-0255-9
- Footnote 43
-
Rahme CL, Jacoby JM, Avery LM. Impact of a Hospital's Antibiotic Stewardship Team on Fluoroquinolone Use at a Long- Term Care Facility. Ann Long-Term Care. 2016. https://www.hmpgloballearningnetwork.com/site/altc/articles/impact-hospitals-antibiotic-stewardship-team-fluoroquinolone-use-long-term-care-facility
- Footnote 44
-
Zimmerman S, Sloane PD, Bertrand R, Olsho LE, Beeber A, Kistler C, Hadden L, Edwards A, Weber DJ, Mitchell CM. Successfully reducing antibiotic prescribing in nursing homes. J Am Geriatr Soc 2014;62(5):907–12. https://doi.org/10.1111/jgs.12784
- Footnote 45
-
Nace DA, Hanlon JT, Crnich CJ, Drinka PJ, Schweon SJ, Anderson G, Perera S. A Multifaceted Antimicrobial Stewardship Program for the Treatment of Uncomplicated Cystitis in Nursing Home Residents. JAMA Intern Med 2020;180(7):944–51. https://doi.org/10.1001/jamainternmed.2020.1256
- Footnote 46
-
Gugkaeva Z, Franson M. Pharmacist-Led Model of Antibiotic Stewardship in a Long-Term Care Facility. Ann Long-Term Care. 2012. https://www.hmpgloballearningnetwork.com/site/altc/articles/pharmacist-led-model-antibiotic-stewardship-long-term-care-facility
- Footnote 47
-
Stuart RL, Orr E, Kotsanas D, Gillespie EE. A nurse-led antimicrobial stewardship intervention in two residential aged care facilities. Infect Dis Health 2015;20(1):4–6. https://doi.org/10.1071/HI14016
- Footnote 48
-
Zabarsky TF, Sethi AK, Donskey CJ. Sustained reduction in inappropriate treatment of asymptomatic bacteriuria in a long-term care facility through an educational intervention. Am J Infect Control 2008;36(7):476–80. https://doi.org/10.1016/j.ajic.2007.11.007
- Footnote 49
-
Rehan Z, Pratt C, Babb K, Filier B, Gilbert L, Wilson R Peter D. Modified reporting of positive urine cultures to reduce treatment of asymptomatic bacteriuria in long-term care facilities: a randomized controlled trial. JAC-Antimicrob Resist 2022;4(5):dlac109. https://doi.org/10.1093/jacamr/dlac109
- Footnote 50
-
Doernberg SB, Dudas V, Trivedi KK. Implementation of an antimicrobial stewardship program targeting residents with urinary tract infections in three community long-term care facilities: a quasi-experimental study using time-series analysis. Antimicrob Resist Infect Control 2015;4:54. https://doi.org/10.1186/s13756-015-0095-y
- Footnote 51
-
Jump RL, Olds DM, Seifi N, Kypriotakis G, Jury LA, Peron EP, Hirsch AA, Drawz PE, Watts B, Bonomo RA, Donskey CJ. Effective antimicrobial stewardship in a long-term care facility through an infectious disease consultation service: keeping a LID on antibiotic use. Infect Control Hosp Epidemiol 2012;33(12):1185–92. https://doi.org/10.1086/668429
- Footnote 52
-
Linnebur SA, Fish DN, Ruscin JM, Radcliff TA, Oman KS, Fink R, Van Dorsten B, Liebrecht D, Fish R, McNulty M, Hutt E. Impact of a multidisciplinary intervention on antibiotic use for nursing home-acquired pneumonia. Am J Geriatr Pharmacother 2011;9(6):442–450.e1. https://doi.org/10.1016/j.amjopharm.2011.09.009
- Footnote 53
-
Arnold SH, Nygaard Jensen J, Bjerrum L, Siersma V, Winther Bang C, Brostrøm Kousgaard M, Holm A. Effectiveness of a tailored intervention to reduce antibiotics for urinary tract infections in nursing home residents: a cluster, randomised controlled trial. Lancet Infect Dis 2021;21(11):1549–56. https://doi.org/10.1016/S1473-3099(21)00001-3
- Footnote 54
-
Kua CH, Mak VS, Huey Lee SW. Health Outcomes of Deprescribing Interventions Among Older Residents in Nursing Homes: A Systematic Review and Meta-analysis. J Am Med Dir Assoc 2019;20(3):362–372.e11. https://doi.org/10.1016/j.jamda.2018.10.026
- Footnote 55
-
Crespo-Rivas JC, Guisado-Gil AB, Peñalva G, Rodríguez-Villodres Á, Martín-Gandul C, Pachón-Ibáñez ME, Lepe JA, Cisneros JM. Are antimicrobial stewardship interventions effective and safe in long-term care facilities? A systematic review and meta-analysis. Clin Microbiol Infect 2021;27(10):1431–8. https://doi.org/10.1016/j.cmi.2021.06.003
- Footnote 56
-
Brown KA, Chambers A, MacFarlane S, Langford B, Leung V, Quirk J, Schwartz KL, Garber G. Reducing unnecessary urine culturing and antibiotic overprescribing in long-term care: a before-and-after analysis. CMAJ Open 2019;7(1):E174–81. https://doi.org/10.9778/cmajo.20180064
- Footnote 57
-
Choosing Wisely Canada. Using Antibiotics Wisely. Toronto, ON: CWC. (accessed 2022-07-24). https://choosingwiselycanada.org/long-term-care/antibiotics/
- Footnote 58
-
Rennie RP, Weiss S, Pasay D. Optimizing microbiology value in small, resource limited laboratories: Providing early diagnostic value to clinicians and their patients in regional settings. Canadian Clinical Microbiology Proficiency Testing Newsletter, Feb 13, 2018. https://cmpt.ca/optimizing-microbiology-value-in-small-resource-limited-laboratories/
- Footnote 59
-
Daneman N, Bronskill SE, Gruneir A, Newman AM, Fischer HD, Rochon PA, Anderson GM, Bell CM. Variability in Antibiotic Use Across Nursing Homes and the Risk of Antibiotic-Related Adverse Outcomes for Individual Residents. JAMA Intern Med 2015;175(8):1331–9. https://doi.org/10.1001/jamainternmed.2015.2770
- Footnote 60
-
Barlam TF, Cosgrove SE, Abbo LM, MacDougall C, Schuetz AN, Septimus EJ, Srinivasan A, Dellit TH, Falck-Ytter YT, Fishman NO, Hamilton CW, Jenkins TC, Lipsett PA, Malani PN, May LS, Moran GJ, Neuhauser MM, Newland JG, Ohl CA, Samore MH, Seo SK, Trivedi KK. Implementing an Antibiotic Stewardship Program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis 2016;62(10):e51–77. https://doi.org/10.1093/cid/ciw118
- Footnote 61
-
McElligott M, Welham G, Pop-Vicas A, Taylor L, Crnich CJ. Antibiotic Stewardship in Nursing Facilities. Infect Dis Clin North Am 2017;31(4):619–38. https://doi.org/10.1016/j.idc.2017.07.008
- Footnote 62
-
Hallsworth M, Chadborn T, Sallis A, Sanders M, Berry D, Greaves F, Clements L, Davies SC. Provision of social norm feedback to high prescribers of antibiotics in general practice: a pragmatic national randomised controlled trial. Lancet 2016;387(10029):1743–52. https://doi.org/10.1016/S0140-6736(16)00215-4
- Footnote 63
-
Australian Government. Department of the Prime Minister and Cabinet. Nudge vs Superbugs: a behavioural economics trial to reduce the overprescribing of antibiotics. Canberra (AU); Australia Government; 2018. (accessed 2022-08-05). https://behaviouraleconomics.pmc.gov.au/projects/nudge-vs-superbugs-behavioural-economics-trial-reduce-overprescribing-antibiotics
- Footnote 64
-
Garau J. Impact of antibiotic restrictions: the ethical perspective. Clin Microbiol Infect 2006;12 Suppl 5:16–24. https://doi.org/10.1111/j.1469-0691.2006.01527.x
- Footnote 65
-
Public Health Agency of Canada. Preserving antibiotics now and into the future. Ottawa, ON: PHAC; 2019. (accessed 2022-06-26). https://www.canada.ca/en/public-health/corporate/publications/chief-public-health-officer-reports-state-public-health-canada/preserving-antibiotics.html
- Footnote 66
-
Zhu NJ, Ahmad R, Holmes A, Robotham JV, Lebcir R, Atun R. System dynamics modelling to formulate policy interventions to optimise antibiotic prescribing in hospitals. J Oper Res Soc 2021;72(11):2490–502. https://doi.org/10.1080/01605682.2020.1796537
Appendix
Table A1: Embase, 1974 to April 1, 2022
Table A2: Ovid MEDLINE(R) ALL, 1946 to April 1, 2022
Table A3: Global Health, 1973 to April 1, 2022
Table A1: Embase, 1974 to April 1, 2022
| # | Search terms |
|---|---|
| 1 | *Antimicrobial Stewardship/ |
| 2 | (antimicrobial stewardship or amr).ti,kw. |
| 3 | ((stewardship* or "use" or misus* or abus* or overus* or therap* or prescrib*) and (antimicrobial* or antibiotic* or antibacterial* or antiviral* or antifungal* or anti microbial* or anti biotic* or anti bacterial* or anti viral* or anti fungal*)).ti,kw. |
| 4 | or/1-3 [AMR] |
| 5 | residential home/ or nursing home/ or assisted living facility/ |
| 6 | (long term care facilit* or convalascence home or convalascence facilit* or nursing home? or group home? or assisted living or seniors home? or seniors residence? or old age home? or old age residence? or aged care home? or aged care residence? or residential facilit* or residential institution?).tw,kw. |
| 7 | (elder* or older adult? or old age? or seniors or geriatric).tw,kw. |
| 8 | or/5-7 [long term care facilities] |
| 9 | exp Clinical Audit/ |
| 10 | (program* or intervention* or audit or feedback or prescriber education or pharmacist education).ti,kw. or (stewardship adj2 program*).ab. or (intervention* or audit or feedback or prescriber education).ab. /freq=2 |
| 11 | or/9-10 [interventions] |
| 12 | exp health personnel attitude/ |
| 13 | exp health care personnel/ or (doctor? or physician? or family practitioner? or clinician? or nurse? or nursing staff or personal support worker? or caregiver? or care giver? or health care professional? or Health Personnel or health care personnel or pharmacist?).tw,kw. |
| 14 | (perspective? or perception? or perceive? or believe? or belief? or view? or attitude? or opinion?).tw,kw. |
| 15 | and/13-14 |
| 16 | or/12,15 [attitude of health personell] |
| 17 | 4 and 8 and (11 or 16) |
| 18 | limit 17 to (english or french) |
Table A2: Ovid MEDLINE(R) ALL, 1946 to April 1, 2022
| # | Search terms |
|---|---|
| 1 | *Antimicrobial Stewardship/ |
| 2 | (antimicrobial stewardship or amr).ti,kw,kf. |
| 3 | ((stewardship* or "use" or misus* or abus* or overus* or therap* or prescrib*) and (antimicrobial* or antibiotic* or antibacterial* or antiviral* or antifungal* or anti microbial* or anti biotic* or anti bacterial* or anti viral* or anti fungal*)).ti,kw,kf. |
| 4 | or/1-3 [AMR] |
| 5 | exp Residential Facilities/ |
| 6 | (long term care facilit* or convalascence home or convalascence facilit* or nursing home? or group home? or assisted living or seniors home? or seniors residence? or old age home? or old age residence? or aged care home? or aged care residence? or residential facilit* or residential institution?).tw,kw,kf. |
| 7 | (elder* or older adult? or old age? or seniors or geriatric).tw,kw,kf. |
| 8 | or/5-7 [long term care facilities] |
| 9 | exp Clinical Audit/ |
| 10 | (program* or intervention* or audit or feedback or prescriber education or pharmacist education).ti,kw,kf. or (stewardship adj2 program*).ab. or (intervention* or audit or feedback or prescriber education).ab. /freq=2 |
| 11 | or/9-10 [interventions] |
| 12 | exp "attitude of health personnel"/ |
| 13 | exp Health Personnel/ or (doctor? or physician? or family practitioner? or clinician? or nurse? or nursing staff or personal support worker? or caregiver? or care giver? or health care professional? or pharmacist?).tw,kw,kf. |
| 14 | (perspective? or perception? or perceive? or believe? or belief? or view? or attitude? or opinion?).tw,kw,kf. |
| 15 | and/13-14 |
| 16 | or/12,15 [attitude of health personell] |
| 17 | 4 and 8 and (11 or 16) |
| 18 | limit 17 to (english or french) |
Table A3: Global Health, 1973 to April 1, 2022
| # | Search terms |
|---|---|
| 1 | (antimicrobial stewardship or amr).ti,hw. |
| 2 | ((stewardship* or "use" or misus* or abus* or overus* or therap* or prescrib*) and (antimicrobial* or antibiotic* or antibacterial* or antiviral* or antifungal* or anti microbial* or anti biotic* or anti bacterial* or anti viral* or anti fungal*)).ti,hw. |
| 3 | or/1-2 [AMR] |
| 4 | residential institutions/ or nursing home/ or long term care/ |
| 5 | (long term care facilit* or convalascence home or convalascence facilit* or nursing home? or group home? or assisted living or seniors home? or seniors residence? or old age home? or old age residence? or aged care home? or aged care residence? or residential facilit* or residential institution?).tw,hw. |
| 6 | (elder* or older adult? or old age? or seniors or geriatric).tw,hw. |
| 7 | or/4-6 [long term care facilities] |
| 8 | (program* or intervention* or audit or feedback or prescriber education or pharmacist education).ti,hw. or (stewardship adj2 program*).ab. or (intervention* or audit or feedback or prescriber education).ab. /freq=2 |
| 9 | exp health care workers/ or (doctor? or physician? or family practitioner? or clinician? or nurse? or nursing staff or personal support worker? or caregiver? or care giver? or health care professional? or Health Personnel or health care personnel or pharmacist?).tw,hw. |
| 10 | (perspective? or perception? or perceive? or believe? or belief? or view? or attitude? or opinion?).tw,hw. |
| 11 | and/9-10 |
| 12 | 3 and 7 and (8 or 11) |
| 13 | limit 12 to (english or french) |
