National Advisory Committee on Immunization (NACI) supplemental statement on recombinant influenza vaccines

 Download this article as a PDF
Download this article as a PDFPublished by: The Public Health Agency of Canada
Issue: Volume 48-9, September 2022: Invasive Diseases Surveillance in Canada
Date published: September 2022
ISSN: 1481-8531
Submit a manuscript
About CCDR
Browse
Volume 48-9, September 2022: Invasive Diseases Surveillance in Canada
Rapid Communication
Summary of the National Advisory Committee on Immunization (NACI) Supplemental Statement on Recombinant Influenza Vaccines
Anabel Gil1, Angela Sinilaite1, Jesse Papenburg2,3,4,5 on behalf of the National Advisory Committee on Immunization (NACI)
Affiliations
1 Centre for Immunization Readiness, Public Health Agency of Canada, Ottawa, ON
2 NACI Influenza Working Group Chair
3 Division of Pediatric Infectious Diseases, Department of Pediatrics, Montréal Children’s Hospital of the McGill University Health Centre, Montréal, QC
4 Division of Microbiology, Department of Clinical Laboratory Medicine, Optilab Montréal - McGill University Health Centre, Montréal, QC
5 Department of Epidemiology, Biostatistics, and Occupational Health, School of Population and Global Health, McGill University, Montréal, QC
Correspondence
Suggested citation
Gil A, Sinilaite A, Papenburg J, on behalf of the National Advisory Committee on Immunization (NACI). Summary of the National Advisory Committee on Immunization (NACI) Supplemental Statement on Recombinant Influenza Vaccines. Can Commun Dis Rep 2022;48(9):383–91. https://doi.org/10.14745/ccdr.v48i09a02
Keywords: National Advisory Committee on Immunization, NACI, recombinant vaccine, influenza, immunization
Abstract
Background: Recombinant protein technology is a novel platform for influenza vaccine manufacturing that differs significantly from existing egg-based and mammalian cell culture-based technologies. Supemtek™ is the first and, to date, the only recombinant quadrivalent influenza vaccine (RIV4) authorized for use in Canada in adults aged 18 years and older. The objective is to review the available evidence for efficacy, effectiveness, immunogenicity and safety of RIV4, and to summarize the National Advisory Committee on Immunization (NACI) recommendation regarding the use of Supemtek.
Methods: A systematic literature review and meta-analysis on the vaccine efficacy, effectiveness, immunogenicity and safety of RIV4 in adults was conducted according to methodology specified a priori in a written protocol. NACI evidence-based process was used to assess the available evidence and develop a recommendation regarding the use of Supemtek.
Results: Ten eligible studies were included in the evidence synthesis. One randomized controlled trial (RCT) in adults aged 50 years and older provided evidence that RIV4 may potentially offer improved protection against laboratory-confirmed influenza A infection compared to standard egg-based influenza vaccines. Data from eight RCTs assessing immunogenicity and five RCTs and one post-marketing surveillance study assessing safety indicated that Supemtek is a safe, well tolerated, and immunogenic alternative to conventional egg-based influenza vaccines for adults.
Conclusion: There is fair evidence that Supemtek is effective, safe, and has non-inferior immunogenicity to comparable vaccines, based on direct evidence in adults 18 years of age and older; thus, NACI recommends that Supemtek may be considered among the seasonal influenza vaccines offered to adults 18 years of age and older for their annual influenza vaccination.
Introduction
Recombinant protein technology is an established vaccine-manufacturing platform that has been used to produce vaccines approved for use in Canada against various vaccine-preventable diseasesFootnote 1. This platform is a new, alternative method for influenza vaccine production, which is significantly different from existing egg-based and mammalian cell culture-based technology. The production of recombinant influenza vaccine (RIV) involves the expression of recombinant hemagglutinin in a proprietary insect cell line using a baculovirus expression vector systemFootnote 1. This process does not rely on egg supply nor the availability of an avian or canine kidney cell substrate, as it does not require propagation of candidate vaccine virus in egg or mammalian cellFootnote 2, thus allowing for more rapid scale-up of vaccine production in the event of an epidemic, pandemic or egg shortage. The flexible and quick manufacturing process of RIV and the continued diversification of influenza vaccine platforms may be helpful in overcoming influenza supply vulnerabilities and improving vaccine-production capacity for a prompt response to rapid and emerging circulating seasonal influenza strains in a post-coronavirus disease 2019 (COVID-19) pandemic setting. Recombinant influenza vaccines may also offer other advantages related to vaccine quality compared to conventional platforms for influenza vaccine manufacturing, including high vaccine purity, three times higher hemagglutinin content than standard-dose vaccines, and reduced risk of a mismatch between vaccines and circulating viral strains because it is not subject to adaptive mutations acquired from growth in eggs or in cells Footnote 3Footnote 4Footnote 5Footnote 6.
Supemtek™ (Sanofi Pasteur, Ltd.) is the first and, to date, the only recombinant quadrivalent influenza vaccine (RIV4) licensed in Canada for use in adults 18 years of age and olderFootnote 1. The RIV4 (licensed in the United States under the trade name Flublok® Quadrivalent) builds on the clinical development of its trivalent predecessor, Flublok (RIV3), an inactivated, recombinant influenza vaccine developed by Protein Sciences, Inc. (currently operating as Sanofi Pasteur, Ltd.). The trivalent and quadrivalent RIV formulations have the same manufacturing process; however, the quadrivalent RIV formulation comprises proteins from four strains of influenza virus A (H1N1), A (H3N2), B/Victoria lineage, B/Yamagata lineage)Footnote 1Footnote 3.
The National Advisory Committee on Immunization (NACI) has not previously made a recommendation on recombinant influenza vaccines in any population; therefore, the objective of the advisory committee supplemental statement was to review the available evidence on the efficacy, effectiveness, immunogenicity and safety of RIV4, and to provide provincial and territorial health authorities and healthcare professionals with guidance on its use among adults in Canada. This article provides a concise summary of NACI’s recommendation for RIV4, supporting information and conclusions from the evidence review. Complete details can be found on the Public Health Agency of Canada website in the NACI Supplemental Statement – Recombinant Influenza Vaccines Footnote 7.
Methods
A systematic literature review and meta-analysis on the vaccine efficacy, effectiveness, immunogenicity and safety of RIV4 in adults 18 years of age and older was performed. The methodology was specified a priori in a written protocol that included the research questions, search strategy, inclusion and exclusion criteria, and quality assessment. The NACI’s Influenza Working Group reviewed and approved the protocol. A search strategy based on the objective was developed in consultation with a federal Reference Librarian from the Health Library of Health Canada and the Public Health Agency of Canada. Searches were restricted to primary research studies from peer-reviewed journals and case reports published in English or French. Evidence was retrieved from the EMBASE, MEDLINE, Cochrane Central, Scopus, ProQuest Public Health and ClinicalTrials.gov electronic databases. Registered clinical trials and grey literature from international public health authorities and National Immunization Technical Advisory Groups were also considered. The search spanned publications from January 1, 2000, to January 12, 2021, with an update to August 8, 2021. Two reviewers independently screened the titles, abstracts and eligible full-text articles.
Studies were included if they met the following criteria:
- Study population or sub-population consisted of adults 18 years of age and older
- Study assessed efficacy and effectiveness, immunogenicity, or safety of RIV4
- Primary research studies from peer-reviewed scientific literature
- Case reports and case series
- Registered clinical trials and grey literature from international public health authorities (Australian Technical Advisory Group on Immunisation; Centers for Disease Control and Prevention; clinicaltrials.gov; European Centre for Disease Prevention and Control; European Medicines Agency; Department of Health Services Research & Policy; International Clinical Trials Registry Platform; World Health Organization)
- Study was published in English or French
- Study was published in 2000 or later
Studies were excluded if they met one or more of the following criteria:
- Study did not present data on the efficacy, effectiveness, immunogenicity or safety of RIV4
- Study is in a language other than English or French
- Study is a non-human or in vitro study
- Article is not a primary research study
- Article is an editorial, opinion, commentary or news report
- Article is an economic study, clinical practice guideline, consensus conference, health technology assessment report
- Article was a doctoral dissertation, master’s thesis, conference summary
- Article is a duplicate
Data were extracted from the included studies into an evidence table using a piloted data abstraction template. The quality (internal validity) of included studies was assessed using Cochrane tools (RoB 2.022 for randomized trials and ROBINS-I23 for non-randomized studies of interventions). The Joanna Briggs Institute checklist was used to evaluate case reports or case series. Data extraction and quality assessment were completed by one reviewer and independently validated by a second reviewer.
Results from included studies were synthesized narratively and analyzed according to NACI evidence-based process to develop a new recommendation. The results of studies deemed to be clinically and methodologically similar were also pooled using random effects meta-analyses. Subgroup analyses were conducted by age group, vaccine strains, and influenza vaccine type. Forest plots illustrating the results of the meta-analyses are presented in the Appendix.
The Grading of Recommendations, Assessment, Development and Evaluation (GRADE) framework Footnote 8 was used to organize and analyze the quality of the body of evidence across studies in developing recommendations. The strength and certainty of evidence included in syntheses were assessed by two independent reviewers using the GRADE system. GRADE assessment was reserved for the following outcomes deemed to be critical for decision-making by the Influenza Working Group through a prioritization exercise:
- Serious adverse event (SAE): Any untoward medical occurrence that at any dose results in death requires inpatient hospitalization or prolongation of existing hospitalization, results in persistent or significant disability or incapacity, or is life-threatening
- Laboratory-confirmed influenza (LCI)-related mortality: A death during an influenza season resulting from a clinically compatible illness that was confirmed to be influenza by an appropriate laboratory test (e.g. reverse transcription polymerase chain reaction [RT-PCR], virus culture or antigen detection); all influenza (A and B)
- Laboratory-confirmed influenza (LCI): Symptoms of influenza with a positive laboratory diagnosis by RT-PCR, virus culture or antigen detection; all influenza (A and B)
- Solicited systemic adverse event (AE): Intentionally solicited systemic reactions including but not limited to fever, malaise, muscle pain, headache or loss of appetite
- Seroprotection: Proportion of subjects achieving a haemagglutination inhibition (HI) titre of at least 1:40 post-vaccination
- Seroconversion: Proportion of subjects achieving an increase from equal or less than 1:10 HI titre pre-vaccination to at least 1:40 post-vaccination or achieving at least a four-fold rise in HI titres
- Geometric mean titre ratio (GMTR): Ratio of geometric mean titre post-vaccination of licensed vaccine to geometric mean titre post-vaccination of new vaccine
NACI’s peer-reviewed framework and evidence-informed toolsFootnote 9 were also used to assess the implications of ethics, equity, feasibility and acceptability (EEFA) of the recommendation for the use of Supemtek (RIV4) for the prevention of influenza in adults aged 18 years and older in Canada.
Following a thorough review of the evidence according to NACI’s evidence-based process, NACI approved the recommendation.
Results
A total of 1,082 articles were retrieved after removing duplicates, of which ten were retained for data extraction and analysis; however, only three of the 10 studies could be pooled through a meta-analysis. One randomized controlled trial (RCT) that reported on the efficacy of RIV4 was identifiedFootnote 10. Eight RCTs investigated the immunogenicity of RIV4Footnote 10Footnote 11Footnote 12Footnote 13Footnote 14Footnote 15Footnote 16Footnote 17. Six studies assessed the safety of RIV4, including five RCTsFootnote 10Footnote 13Footnote 14Footnote 15Footnote 18 and one post-marketing surveillance studyFootnote 19. Studies reporting critical outcomes related to the effectiveness of RIV4 were not available at the time of this review. Notably, at the time of this Statement’s development, studies reporting on vaccination with RIV4 during pregnancy or during breastfeeding were not available. A flow diagram of the study selection process is presented in Figure 1 and key study characteristics are summarized in Table 1.
Figure 1: PRISMA flow diagram of the study selection process for the systematic review on the efficacy, effectiveness, immunogenicity and safety of Supemtek™
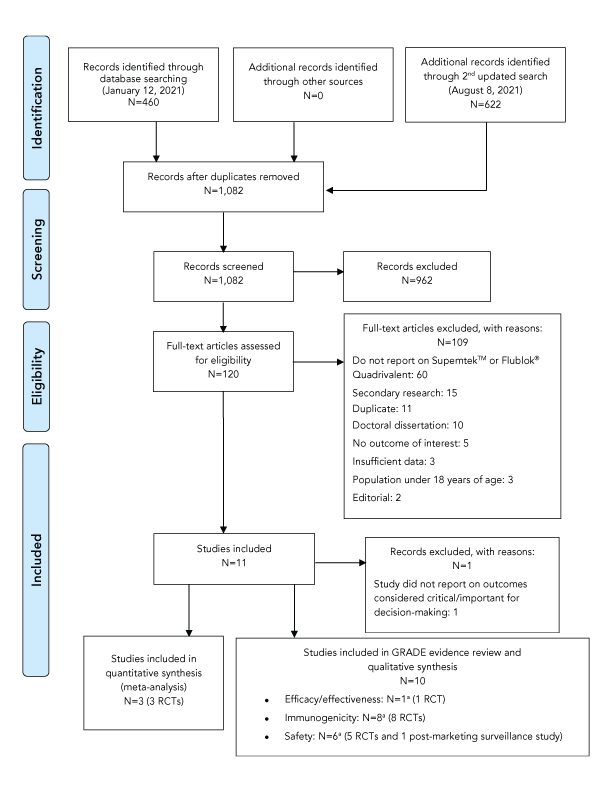
Text description: Figure 1
The PRISMA flow diagram describes the process by which articles were selected for the literature review. The process is broken down into four stages: Identification, Screening, Eligibility and Included.
Stage 1: Identification
- 460 records were identified through the January 12, 2021, database search, 0 records were identified through additional sources and 622 additional records were identified through the August 8, 2021, database search
- 1,082 records remained after duplicates were removed
Stage 2: Screening
- 1,082 records were then screened
- Of these 1,082 records, 962 records were excluded
Stage 3: Eligibility
- 120 full-text articles were assessed for eligibility
- Of these 120 full-text articles, 109 were excluded. The exclusion breakdown is as follows: 60 did not report on SupemtekTM or Flublok® Quadrivalent, 15 were secondary research, 11 were duplicates, 10 were doctoral dissertations, 5 did not report on outcomes of interest, 3 had insufficient data, 3 had a study population under 18 years of age and 2 were editorials
Stage 4: Included
- 11 articles were included
- Of these 11 articles, 1 was excluded as the study did not report on outcomes considered critical or important for decision-making
- Of these 11 articles, 3 RCTs were included in the quantitative synthesis (meta-analysis)
- Of these 11 articles, 10 were included in the GRADE evidence review and qualitative synthesis under the following outcome categories: 1 RCT reported on efficacy/effectiveness, 8 RCTs reported on immunogenicity and 6 studies (5 RCTs and 1 post-market surveillance study) reported on safety. Some studies fit into more than out outcome category
| Study | Design | Study | Intervention/Control | Outcomes |
|---|---|---|---|---|
| Dunkle et al.Footnote 10 NCT02285998 |
|
Adults 50 years of age or older | RIV4 (n=4,498) IIV4-SD (n=4,505) |
Efficacy
|
| Dawood et al.Footnote 17 NCT03722589 |
|
Adult healthcare personnel aged 18–64 years | RIV4 (n=202) IIV4-cc (n=283) Fluarix IIV4-SD (n=120) Fluzone IIV4-SD (n=122) |
Immunogenicity
|
| Belongia et al.Footnote 12 NCT02872311 |
|
Adults 65–74 years of age | RIV4 (n=30) IIV3-HD (n=29) IIV3-Adj (n=30) |
Immunogenicity
|
| Shinde et al.Footnote 14 NCT03658629 |
|
Adults 65 years of age or older | RIV4 (n=153) IIV3-HD (n=154) |
Immunogenicity
|
| Dunkle et al.Footnote 15 NCT02290509 |
|
Adults 18–49 years of age or older | RIV4 (n=1,011) IIV4-SD (n=339) |
Immunogenicity
|
| Wang et al.Footnote 11 NCT03734237 |
|
Adults 18–83 years of age | RIV4 (n=51) IIV4-SD (n=46) IIV4-cc (n=36) |
Immunogenicity
|
| Cowling et al.Footnote 13 NCT03330132 |
|
Community-dwelling adults 65–82 years of age | RIV4 (n=355) IIV4-SD (n=508) IIV3-Adj (n=508) IIV3-HD (n=510) |
Immunogenicity
|
| Cowling et al.Footnote 18 NCT03330132 |
|
Community-dwelling adults 65–82 years of age | RIV4 (n=355) IIV4-SD (n=508) IIV3-Adj (n=508) IIV3-HD (n=510) |
Safety
|
| Gouma et al.Footnote 16 NCT03068949 |
|
Adults 18–49 years of age | RIV4 (n=23) IIV4-SD (n=23) IIV3-HD (n=16) IIV4-cc (n=23) |
Immunogenicity
|
| Woo et al.Footnote 19 |
|
Persons vaccinated with RIV4 July 1, 2017–June 30, 2020 |
Reports on SAEs: N=39 Reports on systemic AEs: N=300 |
Safety
|
An overview of the key efficacy and effectiveness, immunogenicity, and safety findings for this review is provided below. Further details are available in the NACI Supplemental Statement on Recombinant Influenza Vaccines Footnote 7.
Vaccine efficacy
One RCT assessed the relative vaccine efficacy (rVE) of RIV4 compared to egg-based standard-dose quadrivalent inactivated influenza vaccines (IIV4) against LCI infection. The RCT was conducted indults 50 years of age and older during the 2014–2015 influenza season in the United States (US)Footnote 10. Data from this study demonstrated that RIV4 was statistically significantly more efficacious than egg-based IIV4 influenza vaccines in preventing LCI type A infection, but not LCI type B infection in older adults.
Overall, there was fair evidence (of low certainty) that the efficacy of RIV4 is non-inferior to traditional egg-based comparators, based on direct data in adults aged 50 years and older.
Immunogenicity
Eight RCTs reported on the immunogenicity of RIV4 compared to different influenza vaccines, including IIV3-HD, IIV3-Adj, IIV4-SD and IIV4-cc. Two studies were from the 2014–2015 influenza seasonFootnote 10Footnote 15, three from the 2017–2018 influenza seasonFootnote 12Footnote 13Footnote 16 and three from the 2018–2019 influenza seasonFootnote 11Footnote 14Footnote 17. For all immunogenicity outcomes, non-inferiority was assessed using the criteria specified by the US Food and Drug AdministrationFootnote 20, which are also used in Canada. Critical immunogenicity outcomes reported by these studies included seroconversion rates, seroprotection rates and GMTR.
Eight RCTs assessed seroconversion rates of RIV4 compared to IIV3-HD, IIV3-Adj, IIV4-SD and IIV4-cc in adults aged 18 years and olderFootnote 10Footnote 11Footnote 12Footnote 13Footnote 14Footnote 15Footnote 16Footnote 17. In fourFootnote 12Footnote 13Footnote 14Footnote 17 of the eight studies, seroprotection rates were similar among all vaccine groups against all influenza strains. The remaining four studies reported different results. In two studiesFootnote 10Footnote 15 RIV4 did not meet the non-inferiority threshold compared to IIV4-SD against the B/Victoria lineage in adults 18 to 64 years of age. Additionally, rates of seroconversion following RIV4 did not meet the non-inferiority threshold compared to IIV4-SD against influenza A(H1N1) in adults 64 and olderFootnote 10. Two RCTsFootnote 11Footnote 16 did not report confidence intervals and non-inferiority could not be assessed. Pooled seroconversion estimates from three RCTsFootnote 10Footnote 13Footnote 14 suggested that RIV4 induced similar antibody responses compared to IIV4-SD, IIV3-HD, and IIV3-Adj in adults 50 years of age and older (Figure A1).
Four RCTs examined seroprotection rates of RIV4 compared to IIV3-HD, IIV3-Adj, IIV4-SD and IIV4-cc in adults 18 years of age and olderFootnote 10Footnote 12Footnote 14Footnote 17. Similar seroprotection rates were observed among the five treatment groups. Across these studies, non-inferiority of the RIV4 vaccine was demonstrated for five of seven tested A (H3N2) strainsFootnote 10Footnote 12Footnote 14Footnote 17. In two of the four studies, RIV4 demonstrated non-inferiority for all influenza strainsFootnote 14Footnote 17. In one studyFootnote 12, RIV4 demonstrated lower rates of seroprotection for two of four tested A (H3N2) influenza strains in older adults aged 65–74 years. In the study by Dunkle et al.Footnote 10, non-inferiority of RIV4 seroprotection rate was demonstrated for influenza A (H1N1), A (H3N2) and B/Yamagata lineage, but not for the B/Victoria lineage in adults aged 50 years and older.
Three RCTs evaluated GMTR of RIV4 compared to IIV4-SD in adults aged 18 years and olderFootnote 10Footnote 15Footnote 17Footnote 21. In one study, RIV4 demonstrated non-inferiority for all influenza strainsFootnote 17. In the other two studiesFootnote 10Footnote 15Footnote 21, the GMTR against influenza A and B/Yamagata lineage were comparable in both vaccine groups. However, GMTR against B/Victoria lineage for IIV4-SD recipients compared to RIV4 recipients did not meet the non-inferiority criteria.
Overall, there was fair evidence (of moderate certainty) that the immunogenicity for RIV4 is non-inferior to traditional egg-based vaccines, based on data in adults aged 18 years and older.
Safety
Six studies reported on the safety of RIV4 compared to IIV3-HD, IIV3-Adj and IIV4-S in adults aged 18 years of age and older. Of the six studies, five were RCTs Footnote 10Footnote 13Footnote 14Footnote 15Footnote 18 and one study was a post-marketing surveillance studyFootnote 19. Of the included RCTs, two were conducted during the 2014–2015 influenza seasonFootnote 10Footnote 15, two were conducted during the 2017–2018 influenza seasonFootnote 13Footnote 18, and one was conducted during the 2018–2019 influenza seasonFootnote 14. The post-marketing surveillance study reported data from the US Vaccine Adverse Event Reporting System (VAERS) from July 1, 2017, through June 30, 2020Footnote 19. Limited safety data were available on the use of RIV4 during pregnancy. Critical safety outcomes reported by these studies included solicited systemic AEs and SAEs. Systemic reactions were transient, mild to moderate in intensity and similar in frequency between RIV4 and comparator vaccines. The SAEs reported across the RCTs were comparable between the study vaccines and were not considered to be vaccine-related by the investigators. Most AEs reported to VAERs were non-serious; 39 out of 849 AEs reports were SAEs reportsFootnote 19. Data from two RCTsFootnote 10Footnote 14 conducted among adults aged 50 years and older receiving RIV4, IIV3-HD and IIV4-SD vaccines, were pooled in a meta-analysis and there was no difference in the odds of experiencing a SAE between RIV4 and egg-based vaccine comparators (Figure A2).
Overall, there was fair evidence (of moderate certainty) that RIV4 is a safe and well-tolerated alternative to egg-based influenza vaccines, based on data in adults aged 18 years and older.
Discussion
The RIV4 is considered effective, immunogenic and safe in adults 18 years of age and older and has a comparable immunogenicity and safety profile to egg-based and cell-based vaccines already licensed in Canada. The immunogenicity evidence for RIV4 builds on the clinical development program of RIV3, which is a trivalent recombinant influenza vaccine that has been licensed in the US since 2013Footnote 22. Recombinant technology is a vaccine manufacturing process that is considerably different from traditional egg-based production and mammalian cell-culture-based technology. Recombinant technology can allow for faster production times, yields a highly pure product, and mitigates the risk of a mismatch between manufactured vaccines and circulating influenza strains.
There were no factors identified through the EEFA FrameworkFootnote 9 that could contribute to inequity or ethical issues related to the recommendation of RIV4; however, potential perceived risks and unknowns of a new influenza vaccine platform could influence people’s acceptance of RIV4. Additionally, barriers that may restrict feasibility include limited manufacturing infrastructure and higher cost of production of recombinant influenza vaccine compared to egg-based vaccines.
Given the novelty of recombinant influenza vaccines, there is sparse peer-reviewed literature on the use of RIV4 in pregnant individualsFootnote 23 and in other vulnerable populations; however, available data on the use of RIV3 in pregnant individualsFootnote 24 may be used to supplement the safety evidence base of recombinant vaccines as both trivalent and quadrivalent vaccine formulations have the same manufacturing process and overlapping compositions.
Seasonal influenza vaccination remains the best strategy for preventing influenza infection. Efforts to diversify influenza vaccine development, manufacturing and promotion of innovative technologies are critical for reducing and preventing future influenza epidemics and pandemics. Nevertheless, a more robust, comprehensive and consistent body of evidence is needed on influenza recombinant vaccines to further evaluate the effectiveness, efficacy, immunogenicity and safety of RIV4 compared with other seasonal influenza vaccines.
Limitations
There were limited peer-reviewed studies available at the time of the review that evaluated the relative efficacy and effectiveness of RIV4 compared to other injectable influenza vaccines. The study evaluating the rVE against LCI analyses identified in this review was conducted using data from a single influenza season in the US and in adults aged 50 years and older. As influenza seasons vary from year to year, interpretation of the data is limited and further data on multiple influenza seasons, and a wider age range that includes adults aged 18 and older, are needed. Moreover, no studies reporting on vaccine effectiveness against LCI were identified. Additionally, no data on the use of RIV4 in pregnancy were included in this review. A more robust, comprehensive and consistent body of evidence, including data on comorbidities, pregnant individuals, health status and other potential confounders, is needed to evaluate the efficacy, effectiveness, immunogenicity and safety of RIV4 compared to other licensed seasonal influenza vaccines.
NACI recommendation for individual level decision-making
Based on the review of the available evidence summarized above and the assessment of ethics, equity, feasibility and acceptability considerations with the EEFA Framework regarding the use of RIV4 in adults, NACI made the following recommendation, supplementing NACI’s overarching recommendation for influenza vaccination, which is available in the NACI Seasonal Influenza StatementFootnote 25:
NACI recommends that Supemtek may be considered among the seasonal influenza vaccines offered to adults 18 years of age and older (Discretionary NACI Recommendation)
- NACI concludes that there is fair evidence to recommend vaccination of adults 18 years of age and older with Supemtek (Grade B Evidence)
The complete details of this review, rationale, relevant considerations and additional information supporting this recommendation can be found in the NACI Supplemental Statement – Recombinant Influenza VaccinesFootnote 7.
Conclusion
There is fair evidence that RIV4 is effective, safe and has non-inferior immunogenicity to comparable vaccines, based on direct evidence in adults 18 years of age and older. NACI recommends that RIV4 may be considered among the seasonal influenza vaccines offered to adults 18 years of age and older for their annual influenza vaccination. NACI will continue to monitor the evidence on RIV and update the supplemental statement as needed and as data on the use of RIV4 from several different influenza seasons accumulate.
Authors’ statement
- AG — Writing, original draft, review, editing
- AS — Writing, review, editing
- JP — Review, editing
The NACI Supplemental Statement – Recombinant Influenza Vaccines was prepared by A Gil, A Sinilaite, M Xi, R Harrison and J Papenburg, on behalf of the NACI Influenza Working Group, and was approved by NACI.
Competing interests
J Papenburg reports grants to his institution from MedImmune, Sanofi Pasteur, Merck and AbbVie, and personal fees from AbbVie, AstraZeneca and Merck, which were all outside of the submitted work.
Acknowledgements
NACI Influenza Working Group Members: J Papenburg (Chair), P De Wals, D Fell, I Gemmill, R Harrison, J Langley, A McGeer, and D Moore
Former members: N Dayneka, K Klein, J McElhaney, D Kumar and S Smith
Liaison representatives: L Grohskopf (Centers for Disease Control and Prevention [CDC], United States)
Ex-officio representatives: C Bancej (Centre for Immunization and Respiratory Infectious Diseases [CIRID], Public Health Agency of Canada [PHAC]), J Reiter (First Nations and Inuit Health Branch [FNIHB], Indigenous Services Canada [ISC]), B Warshawsky (Vice President’s Office, Infectious Disease Prevention and Control Branch [IDPCB]), and J Xiong (Biologics and Genetic Therapies Directorate [BGTD], Health Canada [HC])
NACI Members: S Deeks (Chair), R Harrison (Vice-Chair), M Andrew, J Bettinger, N Brousseau, H Decaluwe, P De Wals, E Dubé, V Dubey, K Hildebrand, K Klein, J Papenburg, A Pham-Huy, B Sander, S Smith, and S Wilson
Former members: C Rotstein
Liaison representatives: L Bill/M Nowgesic (Canadian Indigenous Nurses Association), LM Bucci (Canadian Public Health Association), E Castillo (Society of Obstetricians and Gynaecologists of Canada), A Cohn (Centers for Disease Control and Prevention, United States), L Dupuis (Canadian Nurses Association), D Fell (Canadian Association for Immunization Research and Evaluation), S Funnell (Indigenous Physicians Association of Canada), J Hu/N Ivers (College of Family Physicians of Canada), M Lavoie (Council of Chief Medical Officers of Health), D Moore (Canadian Paediatric Society), M Naus (Canadian Immunization Committee), A Ung (Canadian Pharmacists Association)
Ex-officio representatives: V Beswick-Escanlar (National Defence and the Canadian Armed Forces), E Henry (CIRID, PHAC), M Lacroix (Public Health Ethics Consultative Group, PHAC), C Lourenco (Biologic and Radiopharmaceutical Drugs Directorate, HC), D MacDonald (COVID-19 Epidemiology and Surveillance, PHAC), S Ogunnaike-Cooke (CIRID, PHAC), K Robinson (Marketed Health Products Directorate, HC), G Poliquin (National Microbiology Laboratory, PHAC), and T Wong (First Nations and Inuit Health Branch, Indigenous Services Canada)
The National Advisory Committee on Immunization acknowledges and appreciates the contribution of P Doyon-Plourde (CIRID, PHAC), R Stirling (CIRID, PHAC), C Tremblay (CIRID, PHAC), K Young (CIRID, PHAC) M Tunis (CIRID, PHAC), and M Xi (CIRID, PHAC) to this statement.
Funding
The work of the National Advisory Committee on Immunization is supported by the Public Health Agency of Canada.
Appendix
Figure A1: Odds of seroconversion on days 28–30 post-vaccination between RIV4 and other seasonal influenza vaccine recipients 50 years and older
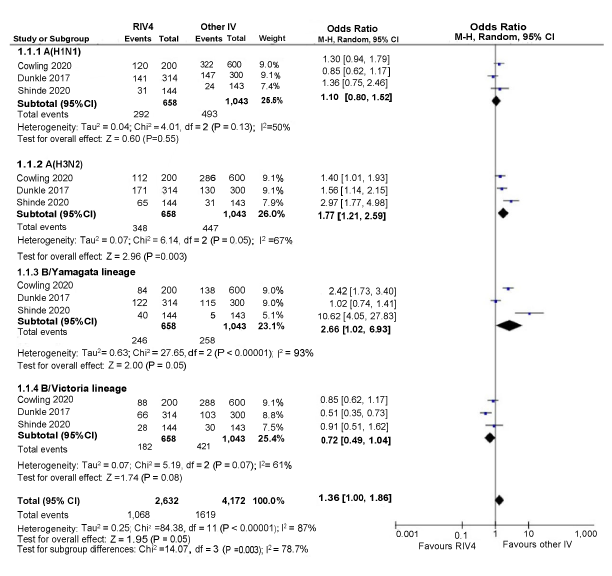
Text description: Figure A1
Figure A1 depicts a forest plot showing the results of a random-effects meta-analysis reporting the odds of seroconversion rate following immunization of quadrivalent recombinant influenza vaccine (RIV4) compared to other seasonal influenza vaccine (IV) in recipients aged 50 years and older. The leftmost column shows the identities of the three included studies by influenza strain. The included studies are represented by the last name of the first author and the year of publication. Next, to the right, data regarding the number of patients having the outcome of interest (events), the sample size of the intervention (RIV4) and comparison group (other IV) are presented. The x-axis representing OR estimates and 95% confidence interval (CI) ranges from 0.001 to 1,000. The vertical line of "no effect" appears at the value of 1 and separates outcomes that favor RIV4 (on the left) and other IV (on the right). Each horizontal line on the forest plot represents an individual study with the result plotted as a box and the 95% CI of the result displayed as the line. The size of the box surrounding each estimate represents the relative weight of that study in producing the pooled result. The black diamonds show the pooled results when the individual studies are combined together and averaged. The horizontal points of the diamonds are the limits of the 95% CI of each combined point estimate.
The following information is depicted in this figure:
| Author | Influenza strain | RIV4 events | RIV4 sample size | Other IV events | Other IV sample size | Weight | OR (95% CI) | |
|---|---|---|---|---|---|---|---|---|
| A(H1N1) | ||||||||
| Cowling 2020 | A(H1N1) | 120 | 200 | 322 | 600 | 9.0% | 1.30 (0.94, 1.79) | |
| Dunkle 2017 | A(H1N1) | 141 | 314 | 147 | 300 | 9.1% | 0.85 (0.62, 1.17) | |
| Shinde 2020 | A(H1N1) | 31 | 144 | 24 | 143 | 7.4% | 1.36 (0.75, 2.46) | |
| A(H3N2) | ||||||||
| Cowling 2020 | A(H3N2) | 112 | 200 | 286 | 600 | 9.1% | 1.40 (1.01, 1.93) | |
| Dunkle 2017 | A(H3N2) | 171 | 314 | 130 | 300 | 9.1% | 1.56 (1.14, 2.15) | |
| Shinde 2020 | A(H3N2) | 65 | 144 | 31 | 143 | 7.9% | 2.97 (1.77, 4.98) | |
| B/Yamagata lineage | ||||||||
| Cowling 2020 | B/Yamagata lineage | 84 | 200 | 138 | 600 | 9.0% | 2.42 (1.73, 3.40) | |
| Dunkle 2017 | B/Yamagata lineage | 122 | 314 | 115 | 300 | 9.0% | 1.02 (0.74, 1.41) | |
| Shinde 2020 | B/Yamagata lineage | 40 | 144 | 5 | 143 | 5.1% | 10.62 (4.05, 27.83) | |
| B/Victoria lineage | ||||||||
| Cowling 2020 | B/Victoria lineage | 88 | 200 | 288 | 600 | 9.1% | 0.85 (0.62, 1.17) | |
| Dunkle 2017 | B/Victoria lineage | 66 | 314 | 103 | 300 | 8.8% | 0.51 (0.35, 0.73) | |
| Shinde 2020 | B/Victoria lineage | 28 | 144 | 30 | 143 | 7.5% | 0.91 (0.51, 1.62) | |
| Statistical model | Influenza strain | RIV4 total events | RIV4 sample size | Other IV total events | Other IV sample size | Weight | Pooled OR (95% CI) |
Heterogeneity: Tau2, Chi2, df (p-value), I2 |
Test for overall effect: Z (p-value) |
Test for subgroup differences Chi2, df (p-value), I2 |
|---|---|---|---|---|---|---|---|---|---|---|
| Random effect model | A(H1N1) | 292 | 658 | 493 | 1,043 | 25.5% | 1.10 (0.80, 1.52) |
0.04; 4.01, 2 (p=0.13); 50% |
0.60 (p=0.55) |
- |
| Random effect model | A(H3N2) | 348 | 658 | 447 | 1,043 | 26.0% | 1.77 (1.21, 2.59) |
0.07; 6.14; 2 (p=0.05); 67% |
2.96 (p=0.003) |
- |
| Random effect model | B/Yamagata lineage | 246 | 658 | 258 | 1,043 | 23.1% | 2.66 (1.02, 6.93) |
0.63; 27.65; 2 (p<0.00001); 93% |
2.00 (p=0.05) |
- |
| Random effect model | B/Victoria lineage | 182 | 658 | 421 | 1,043 | 25.4% | 0.72 (0.49, 1.04) |
0.07; 5.19; 2 (p=0.07); 61% |
1.74 (p=0.08) |
- |
| Random effect model | All studies | 1,068 | 2,632 | 1,619 | 4,172 | 100.0% | 1.36 (1.00, 1.86) |
0.25; 84.38; 11 (p<0.00001); 87% |
1.95 (p=0.05) |
14.07; 3 (p=0.003); 78.7% |
Figure A2: Odds of experiencing a serious adverse event within 180 days of vaccination between RIV4 and other seasonal influenza vaccine recipients 50 years and older
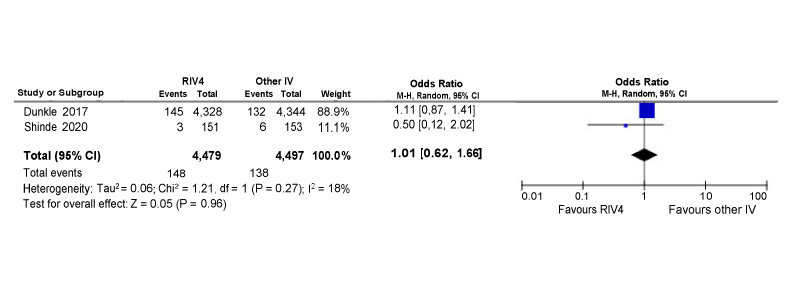
Text description: Figure A2
Figure A2 depicts a forest plot showing the results of a random-effects meta-analysis reporting the odds of experiencing a serious adverse event within 180 days of vaccination following immunization of quadrivalent recombinant influenza vaccine (RIV4) compared to other seasonal influenza vaccine (IV) in recipients aged 50 years and older. The leftmost column shows the identities of the two included studies. The included studies are represented by the last name of the first author and the year of publication. Next, to the right, data regarding the number of patients having the outcome of interest (events) and the sample size of the intervention (RIV4) and comparison group (other IV) are presented. The next two columns include the same information in numerical format that is contained in the diagram. The penultimate column to the right contains the year of the study publication. The diagram located in the rightmost column visually displaying the results. The x-axis representing OR estimates and 95% confidence interval (CI) ranges from 0.01 to 100. The vertical line of "no effect" appears at the value of 1 and separates outcomes that favor RIV4 (on the left) and other IV (on the right). Each horizontal line on the forest plot represents an individual study with the result plotted as a box and the 95% CI of the result displayed as the line. The size of the box surrounding each estimate represents the relative weight of that study in producing the pooled result. The black diamonds show the pooled results when the individual studies are combined together and averaged. The horizontal points of the diamonds are the limits of the 95% CI of each combined point estimate.
The following information is depicted in this figure:
| Author | RIV4 events | RIV4 Sample size | Other IV events | Other IV sample size | Weight | OR (95% CI) |
|---|---|---|---|---|---|---|
| Dunkle 2017 | 145 | 4328 | 132 | 4,344 | 88.9% | 1.11 (0.87, 1.41) |
| Shinde 2020 | 3 | 151 | 6 | 153 | 11.1% | 0.50 (0.12, 2.02) |
| Statistical model | RIV4 total events | RIV4 sample size | Other IV total events | Other IV sample size | Weight | Pooled OR (95% CI) | Heterogeneity: Tau2, Chi2, df (p-value), I2 |
Test for overall effect: Z (p-value) |
|---|---|---|---|---|---|---|---|---|
| Random effect model | 148 | 4479 | 138 | 4497 | 100% | 1.01 (0.62, 1.66) |
0.06; 1.21; 1 (p=0.27); 18% |
0.05 (p=0.96) |
