Analysis of association of COVID-19 vaccination and incidence of cases in Canada

 Download this article as a PDF
Download this article as a PDFPublished by: The Public Health Agency of Canada
Issue: Volume 49-4, April 2023: Children's Health and COVID-19
Date published: April 2023
ISSN: 1481-8531
Submit a manuscript
About CCDR
Browse
Volume 49-4, April 2023: Children's Health and COVID-19
Surveillance
National epidemiological analysis of the association of COVID-19 vaccination and incidence of COVID-19 cases in Canada, January to August 2021
Public Health Agency of Canada, COVID-19 Surveillance, Vaccine Coverage and Information System, Vaccine Effectiveness Surveillance Program, and Public Health Risk Science/National Microbiology Laboratory Teams1
Affiliation
1 Public Health Agency of Canada, Ottawa, ON
Correspondence
Suggested citation
Public Health Agency of Canada COVID-19 Surveillance, Vaccine Coverage and Information System, Vaccine Effectiveness Surveillance Program and Public Health Risk Science/National Microbiology Laboratory Modelling Teams. National epidemiological analysis of the association of COVID-19 vaccination and incidence of COVID-19 cases in Canada, January to August 2021. Can Commun Dis Rep 2023;49(4):145–54. https://doi.org/10.14745/ccdr.v49i04a07
Keywords: COVID-19, Canada, epidemiology, surveillance, incidence, cases, vaccination
Abstract
Background: In December 2020, Canada began its coronavirus disease 2019 (COVID-19) vaccine rollout campaign. Canadians were vaccinated with differing time intervals between doses, vaccine products and vaccine schedules, based on age, timing of vaccination and jurisdiction. The objective of this study is to describe the epidemiology and association between the incidence of COVID-19 cases following vaccination, time since completion of primary series, time between doses and/or product combination and probability of developing severe outcomes.
Methods: The national COVID-19 case data and vaccination coverage data were extracted from the National COVID-19 Surveillance System, and the Canadian COVID-19 Vaccination Coverage Surveillance System. Population estimates from Statistics Canada were used as denominators for rates and for number of people "not fully vaccinated". Two binomial generalized linear models were constructed for analysis.
Results: Within the analysis period, fully vaccinated (i.e. completed primary series) cases (n=17,206) were more commonly female and older, and had fewer reported severe outcomes relative to not fully vaccinated cases (n=615,999). Episode date of fully vaccinated cases most frequently occurred two months after receiving their second dose, and time-between doses of 29–49 and 50–77 days were most common. The probability of becoming a detected COVID-19 case in not fully vaccinated individuals was higher than those fully vaccinated. Those receiving two doses of AstraZeneca and those with shortest time intervals between doses had higher probabilities of becoming COVID-19 cases.
Conclusion: Findings from Canada's national surveillance systems support that being fully vaccinated against COVID-19, having a longer time interval between doses and receiving a messenger ribonucleic acid (mRNA) COVID-19 vaccine schedule compared to other vaccines reduce the probability of becoming a case, using data from January to August 2021.
Introduction
On January 30, 2020, the World Health Organization (WHO) announced the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease 2019; COVID-19) outbreak as a Public Health Emergency of International Concern, and a pandemic was declared on March 11, 2020. Since then, the pandemic has resulted in significant morbidity, mortality, and threat to the overall well-being of Canadians Footnote 1 Footnote 2. Individual and collective actions were heavily relied on, while safe and effective vaccines were under development.
On December 14, 2020, Canada began its COVID-19 vaccine rollout campaign against SARS-CoV-2 infection following the approval of Pfizer-BioNTech Comirnaty, Moderna-Spikevax, AstraZeneca Vaxzevria and Janssen Jcovden (Johnson & Johnson) COVID-19 vaccines Footnote 3. Due to the anticipated constraints on vaccine supply, initial doses of COVID-19 vaccination were prioritized for key populations. The vaccination campaign began with residents in long-term care facilities, health workers and adults residing in the territories or living in remote and isolated communities. As vaccine supply changed, Canada's National Advisory Committee on Immunization (NACI) COVID-19 vaccination program's recommendations evolved. At the time of interest of this article (January 1 to August 31, 2021, i.e., during the Alpha, Gamma and Delta variants' waves) receiving a second dose of a two-dose vaccine schedule (completing the primary series) was recommended to provide better and longer-term protection against COVID-19 Footnote 4. The recommended time interval between doses was extended to four months by March 2021, from the initial recommendation of 21 to 28 days; however, intervals differed across jurisdictions based on their vaccination strategies. Vaccine eligibility expanded and, along with recommendations for extending the time interval between doses, all Canadian residents 12 years of age and older were eligible for a first dose by May 2021 Footnote 5 Footnote 6 Footnote 7. In June 2021, NACI recommended interchangeability of available vaccines and preferential use of messenger ribonucleic acid (mRNA) vaccines to complete primary vaccination series due to the safety concerns that arose with AstraZeneca use (thrombosis with thrombocytopenia) (6). As a result, Canadian residents were vaccinated with differing time intervals between doses, vaccine products and vaccine schedules, based on age, timing of vaccination and Province/Territory (P/T) of residence. A timeline of national recommendations is available in Supplemental material, Figure S1.
The COVID-19 vaccines have demonstrated high effectiveness against severe outcomes such as hospitalization and death. Assessing the variations in vaccine series and their impact on transmission dynamics within Canada contributes to the growing body of evidence to better define the long-term COVID-19 vaccine performance and future vaccine development policies Footnote 8 Footnote 9 Footnote 10 Footnote 11 Footnote 12 Footnote 13 Footnote 14 Footnote 15 Footnote 16 Footnote 17 Footnote 18.
The objective of this article is to describe the epidemiology and explore the association between the incidence of COVID-19 cases following vaccination and time since completion of primary series. The analysis includes a descriptive summary of vaccination coverage and cases following vaccination, and an analysis model of cases following vaccination to investigate whether time since last dose, time between doses, and/or product combination are associated with becoming a COVID-19 case or developing severe outcomes, following adjustments for relevant covariates.
Methods
Definitions
Based on NACI recommendations during the period of analysis (January 1 to August 31, 2021), vaccination programs prioritized delivery of primary vaccination series, whereby individuals with a completed primary vaccination series were denoted as "fully vaccinated". As Canadian vaccination programs evolved in the latter half of 2021, with the rollout of additional and booster doses, the following definitions reflect the vaccine rollout up to August 2021 focusing on primary series completion.
For this analysis, vaccination status of laboratory-confirmed cases was only based on approved vaccines for use by Health Canada for the period of analysis [Pfizer-BioNTech Comirnaty (Pfizer); Moderna Spikevax (Moderna); AstraZeneca Vaxzevria or COVISHIELD (AstraZeneca)] and was defined in the following categories:
- "Fully vaccinated" cases (i.e. case with a complete primary series): episode date 14 days or more after receipt of the second dose in a two-dose series. For this analysis, only those with a complete primary series are included, as the period of analysis predates the Canadian rollout of additional and booster doses.
- "Not fully vaccinated" cases included the following:
- Unvaccinated cases included those who were unvaccinated at the time of their episode date.
- Cases not yet protected from vaccination included those whose episode date occurred less than 14 days after their first vaccine dose.
- Partially vaccinated cases included those whose episode date occurred 14 days or more after their first vaccine dose or less than 14 days after their second vaccine dose.
Month of last dose refers to the month the second dose of a two-dose COVID-19 vaccine was administered. It was used to calculate the number of months since last dose to infection (based on episode date), referred to in this analysis as "months since last dose".
Episode date was used to temporally classify confirmed cases and refers to symptom onset date. When symptom onset date was unavailable or the case was asymptomatic, episode date refers to either laboratory specimen collection date or laboratory testing date.
Data sources
Vaccination coverage data were obtained from P/T immunization registries through the Canadian COVID-19 Vaccination Coverage Surveillance System. Data aggregated by 10-year age group, sex, month of last dose, vaccine product received (i.e. Pfizer-Pfizer, Moderna-Moderna, AstraZeneca-AstraZeneca, mixed mRNA, AstraZeneca-mRNA) and time interval between doses, which reflected the varying recommendations on dose intervals throughout the period of interest (see Supplemental material, Figure S1) (0–28 days, 29–49 days, 50–77 days, 78 or more days) were only available for individuals fully vaccinated as of August 14, 2021.
The national COVID-19 case data was extracted from the National COVID-19 Case dataset, which includes detailed case-level information received from all P/Ts and is maintained by the Public Health Agency of Canada (PHAC). For this analysis, COVID-19 case data included basic demographic data, episode dates, severe outcomes, vaccine products received and date of vaccination for each dose administered. Twelve out of 13 P/Ts (excluding Québec) reported case-level vaccination data for the analysis period (January 1, 2021, to August 31, 2021), with data extracted from the COVID-19 case dataset on February 18, 2022.
The July 1, 2021, population estimates provided by Statistics Canada for Provinces and Nunavut, and population estimates provided by the Yukon and Northwest Territories governments, were used as the denominator for the vaccination coverage rate and to calculate the number of people not fully vaccinated Footnote 19.
Analysis
Laboratory-confirmed cases 12 years and older, with episode dates between January 1, 2021, and August 31, 2021, were included in the analyses Footnote 20.
For statistical modelling analyses, cases were aggregated by P/T, age group, sex, vaccination status, and month of episode date. To establish denominators, the coverage data and population estimates were used to determine the number of individuals in each P/T, age group, sex and vaccination status group eligible to become a case each month. Counts of cases were linked to coverage denominators to calculate proportion of individuals that became a COVID-19 case in each aggregate group. Coverage data contained discrete counts of fully vaccinated individuals, meaning vaccination status was classified as either fully vaccinated or not fully vaccinated. Counts of not fully vaccinated individuals were derived by subtracting the number of fully vaccinated from Statistics Canada's population estimates. For modelling analyses of fully vaccinated individuals, cases were stratified and aggregated by vaccine product series, time interval between doses, and number of months since last dose. In the coverage data, individuals were considered fully vaccinated on the month they completed their primary vaccination series. With respect to number of months since last dose, fully vaccinated individuals were assigned a value of zero months during this month of series completion.
Cases with missing or invalid vaccination date or product, demographic data (age and sex), or episode date were excluded from the analysis (fewer than 0.5% of cases). To align COVID-19 case data with the coverage dataset, cases that received more than two doses of a COVID-19 vaccine, the Janssen COVID-19 vaccine, or a non-Health Canada authorized COVID-19 vaccine were excluded, along with fully vaccinated cases that completed their primary series after August 14, 2021.
Statistical models
Two binomial generalized linear models were constructed to assess associations between COVID-19 vaccination, vaccination characteristics (i.e. vaccine products received, time interval between doses and time since last dose), and COVID-19 case incidence. In each model, the main response variable was the proportion of individuals that became COVID-19 cases in a month, among their respective cohort. The total number of individuals in each aggregate group was included as weights in the models, and all predictor variables were included as categorical variables. Atlantic Provinces (Newfoundland and Labrador, New Brunswick, Prince Edward Island and Nova Scotia) were grouped as an "Atlantic" jurisdiction, while all Territories were excluded due to low case counts.
A main model (model 1) was fit to data aggregated by jurisdiction, age group, sex, month of episode date and vaccination status. A second model (model 2) was fit to only fully vaccinated groups, aggregated by jurisdiction, age group, sex, month of episode date, vaccine product series, time interval between doses and month of last dose. The variables used to aggregate data were included as predictor variables in each model.
Using both fit models, predicted effects of each level of each variable were calculated. When calculating the effect of a level, all other variable levels were controlled at their average value. For each level, point estimates and 95% confidence intervals were generated for the probability of an individual becoming a detected COVID-19 case. Variable-level p values were calculated to assess statistical significance of all predictor variables.
Data cleaning, manipulation, and visualizations were performed in Excel MS Office and SAS V9.4. Statistical analyses were performed in R version 4.0.2.
Results
As of August 14, 2021, 72% (n=24,209,666) of individuals 12 years of age and older were fully vaccinated in Canada. Of these, 9% completed their primary series between January and May 2021, and 84% completed their primary series between June and July 2021, given the vaccine rollout in Canada. Among those 50 years of age and older who were fully vaccinated, most completed their series in June 2021 (ranging from 44% in those aged 50–59 years to 67% in those aged 70–79 years). The majority of younger age groups who completed their primary series did so in July 2021 (ranging from 50% in those aged 40–49 years to 67% in those aged 12–17 years) (Figure 1).
(A)
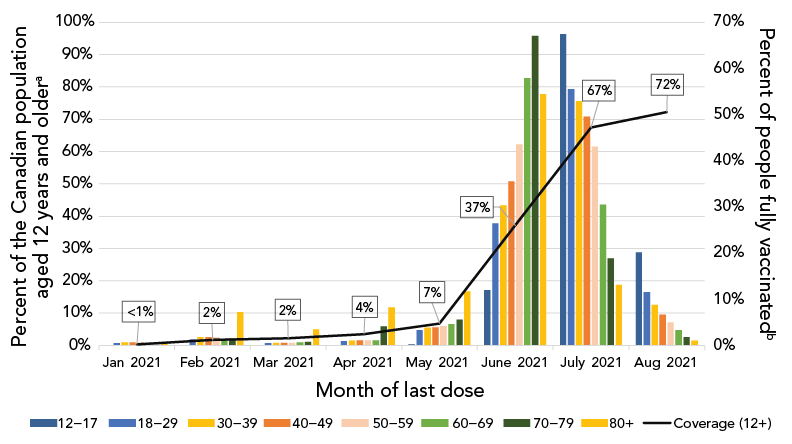
Figure 1A - Text description
| Month of last dose | 12–17 | 18–29 | 30–39 | 40–49 | 50–59 | 60–69 | 70–79 | 80+ | Coverage (12+) |
|---|---|---|---|---|---|---|---|---|---|
| Jan 2021 | 0.02% | 0.49% | 0.70% | 0.73% | 0.67% | 0.38% | 0.18% | 0.56% | 0% |
| Feb 2021 | 0.05% | 1.31% | 1.79% | 1.83% | 1.73% | 1.30% | 1.50% | 7.26% | 2% |
| Mar 2021 | 0.02% | 0.47% | 0.56% | 0.55% | 0.59% | 0.70% | 0.79% | 3.51% | 2% |
| Apr 2021 | 0.06% | 0.92% | 1.05% | 1.08% | 1.16% | 1.11% | 4.09% | 8.26% | 4% |
| May 2021 | 0.27% | 3.35% | 3.83% | 3.97% | 4.19% | 4.69% | 5.64% | 11.70% | 7% |
| June 2021 | 11.94% | 26.47% | 30.33% | 35.55% | 43.62% | 57.90% | 67.08% | 54.49% | 37% |
| July 2021 | 67.43% | 55.47% | 52.93% | 49.62% | 43.02% | 30.55% | 18.88% | 13.15% | 67% |
| Aug 2021 | 20.22% | 11.51% | 8.81% | 6.67% | 5.02% | 3.37% | 1.84% | 1.07% | 72% |
(B)

Figure 1B - Text description
| Month of episode | Not fully vaccinated cases | Fully vaccinated cases |
|---|---|---|
| January 2021 | 109,387 | 6 |
| February 2021 | 54,899 | 87 |
| March 2021 | 94,244 | 319 |
| April 2021 | 183,085 | 1,022 |
| May 2021 | 98,459 | 1,119 |
| June 2021 | 18,782 | 574 |
| July 2021 | 11,411 | 1,437 |
| August 2021 | 45,752 | 12,642 |
Across jurisdictions, those in the Territories completed their series earlier than those in the Provinces. Among the Territories, 62% to 75% of those aged 12 years and older completed their primary series by April 2021, compared to 2% to 11% among the Provinces (Supplemental material, Figure S2).
There were 633,205 confirmed cases of COVID-19 reported to the national COVID-19 case dataset with episode dates between January 1, 2021, and August 31, 2021, that met analysis inclusion criteria. Of these, 17,206 (2.8%) cases were fully vaccinated at episode date, compared to 615,999 (97.2%) not fully vaccinated at episode date (Table 1). See Supplemental material Figure S3 for description of case population for analysis. As vaccine campaigns prioritized key populations, including long-term care facility residents, healthcare workers and older adults by decreasing age over time, fully vaccinated cases were older (median age of 45 years compared to 37 years among not fully vaccinated cases) and more commonly female than for the not fully vaccinated cases. The majority of not fully vaccinated cases occurred in April 2021 (n=183,085; 29.7%), while most fully vaccinated cases occurred in August 2021 (n=12,642; 73.5%; Figure 1). There were fewer hospitalizations and deaths reported among fully vaccinated cases compared to not fully vaccinated cases during the period of analysis (Table 1). See Supplemental material Table S1 for number and percent of those 12 years of age and older who were fully vaccinated by jurisdiction, age group and sex in Canada.
| Characteristics | Total (n=633,205) | |||||
|---|---|---|---|---|---|---|
| "Not fully vaccinated" cases (n=615,999) | "Fully vaccinated" cases (n=17,206) | Total cases, n | ||||
| n | % | n | % | |||
| Age group (years) | ||||||
| 12–17 | 49,733 | 8.1% | 275 | 1.6% | 50,008 | |
| 18–29 | 171,734 | 27.9% | 3,533 | 20.5% | 175,267 | |
| 30–39 | 121,332 | 19.7% | 3,194 | 18.6% | 124,526 | |
| 40–49 | 98,259 | 16.0% | 2,829 | 16.4% | 101,088 | |
| 50–59 | 85,091 | 13.8% | 2,504 | 14.6% | 87,595 | |
| 60–69 | 50,873 | 8.3% | 2,136 | 12.4% | 53,009 | |
| 70–79 | 23,039 | 3.7% | 1,220 | 7.1% | 24,259 | |
| 80 and older | 15,938 | 2.6% | 1,515 | 8.8% | 17,453 | |
| Sex | ||||||
| Male | 314,935 | 51.1% | 7,185 | 41.8% | 322,120 | |
| Female | 301,064 | 48.9% | 10,021 | 58.2% | 311,085 | |
| Month of episode | ||||||
| January | 109,387 | 17.8% | 6 | 0.0% | 109,393 | |
| February | 54,899 | 8.9% | 87 | 0.5% | 54,986 | |
| March | 94,244 | 15.3% | 319 | 1.8% | 94,543 | |
| April | 183,085 | 29.7% | 1,022 | 5.9% | 184,107 | |
| May | 98,459 | 16.0% | 1,119 | 6.5% | 99,578 | |
| June | 18,782 | 3.0% | 574 | 3.3% | 19,356 | |
| July | 11,411 | 1.8% | 1,437 | 8.4% | 12,848 | |
| August | 45,752 | 7.4% | 12,642 | 73.5% | 58,394 | |
| Province/Territory | ||||||
| British Columbia | 98,201 | 15.9% | 3,575 | 20.8% | 101,776 | |
| Alberta | 125,909 | 20.4% | 5,425 | 31.5% | 131,334 | |
| Saskatchewan | 32,072 | 5.2% | 1,330 | 7.7% | 33,402 | |
| Manitoba | 26,296 | 4.3% | 607 | 3.5% | 26,903 | |
| Ontario | 326,645 | 53.0% | 5,923 | 34.4% | 332,568 | |
| Atlantic | 6,009 | 1.0% | 142 | 0.8% | 6,151 | |
| New Brunswick | 1,115 | 0.2% | 37 | 0.2% | 1,152 | |
| Nova Scotia | 3,783 | 0.6% | 72 | 0.4% | 3,855 | |
| Newfoundland and Labrador | 993 | 0.2% | 25 | 0.1% | 1,018 | |
| Prince Edward Island | 118 | 0.0% | 8 | 0.0% | 126 | |
| Territories | 867 | 0.1% | 204 | 1.2% | 1,071 | |
| Yukon | 441 | 0.1% | 88 | 0.5% | 529 | |
| Northwest Territories | 213 | 0.0% | 111 | 0.6% | 324 | |
| Nunavut | 213 | 0.0% | 5 | 0.0% | 218 | |
| Severe outcome | ||||||
| Hospitalization | 33,523 | 5.4% | 809 | 4.7% | 34,332 | |
| Mortality | 6,353 | 1.0% | 272 | 1.6% | 6,625 | |
| Months since last doseFootnote a | ||||||
| 0 months | N/A | N/A | 449 | 2.6% | 449 | |
| 1 month | N/A | N/A | 4,564 | 26.5% | 4,564 | |
| 2 months | N/A | N/A | 7,002 | 40.7% | 7,002 | |
| 3 months | N/A | N/A | 1,994 | 11.6% | 1,994 | |
| 4 months | N/A | N/A | 1,173 | 6.8% | 1,173 | |
| 5 months | N/A | N/A | 623 | 3.6% | 623 | |
| 6 months | N/A | N/A | 1,190 | 6.9% | 1,19 | |
| 7 months | N/A | N/A | 211 | 1.2% | 211 | |
| Time interval between dosesFootnote a | ||||||
| 0–28 days | N/A | N/A | 3,192 | 18.6% | 3,192 | |
| 29–49 days | N/A | N/A | 6,313 | 36.7% | 6,313 | |
| 50–77 days | N/A | N/A | 5,967 | 34.7% | 5,967 | |
| 78 or more days | N/A | N/A | 1,734 | 10.1% | 1,734 | |
|
||||||
The most common vaccine schedule to complete primary series was the Pfizer-Pfizer (63.2%), followed by the Moderna-Moderna (17.5%). Among fully vaccinated cases, the majority received two doses of Pfizer-Pfizer vaccine (n=11,608; 67.5%). Episode date of fully vaccinated cases was most frequent two months after receiving their second dose to complete primary vaccination series (n=7,002; 40.7%), and time-between doses of 29–49 days (n=6,313; 36.7%) and 50–77 days (n=5,967; 34.7%) were most common. See Supplemental material Table S2 for number and percent of those 12 years of age and older who were fully vaccinated, by dose interval, month of last dose and vaccine schedule in Canada, as of August 14, 2021. From model 1, variable-level p values indicated statistical significance at p<0.001 for all predictor variables except sex (p=0.18; Supplemental material Table S3; see Supplemental material Figure S4 for predicted effects of sex). Predicted effects demonstrated that the probability of becoming a detected COVID-19 case was higher among not fully vaccinated individuals than fully vaccinated individuals, after adjustment for P/T, age, sex and month of episode date (Figure 2). The probability of becoming a detected COVID-19 case of a not fully vaccinated individual was estimated to be 0.204% (95% CI, 0.203–0.206; Supplemental material Table S3). Comparatively, the estimated probability for a fully vaccinated individual was 0.023% (95% CI, 0.023–0.024).
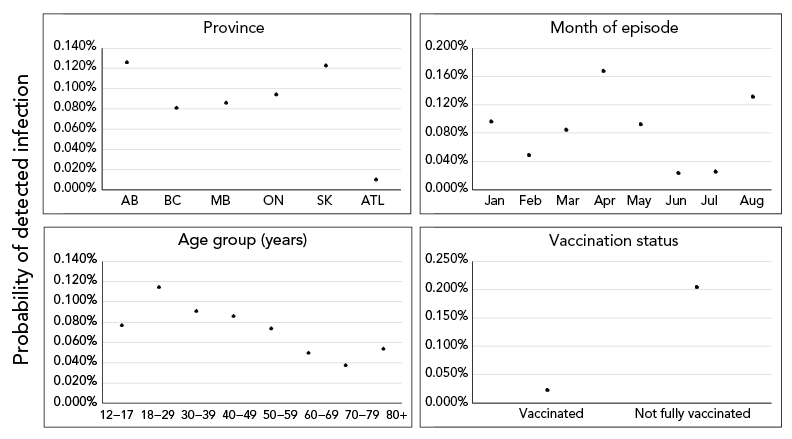
Figure 2 - Text description
The effect of covariates included in model 1 on predicted probability of an individual becoming a detected COVID-19 case. Solid points and vertical solid lines show the effect and 95% prediction interval, where visible. Covariates where effects did not differ significantly among levels (only sex) not shown.
From model 2, variable-level p values indicated statistical significance at p<0.001 for all predictor variables except sex (p=0.22; Supplemental material Table S4). Predicted effects demonstrated that, after controlling for predictor variables, fully vaccinated individuals receiving two doses of AstraZeneca and fully vaccinated individuals with the shortest time interval between doses (0–28 days) had higher probabilities of becoming a detected COVID-19 case, compared to individuals receiving other vaccine series and time interval between doses, respectively (Figure 3). Overall, there is a clear signal of increasing probability in becoming a detected case as time since primary series completion increased from zero to six months (Figure 3).
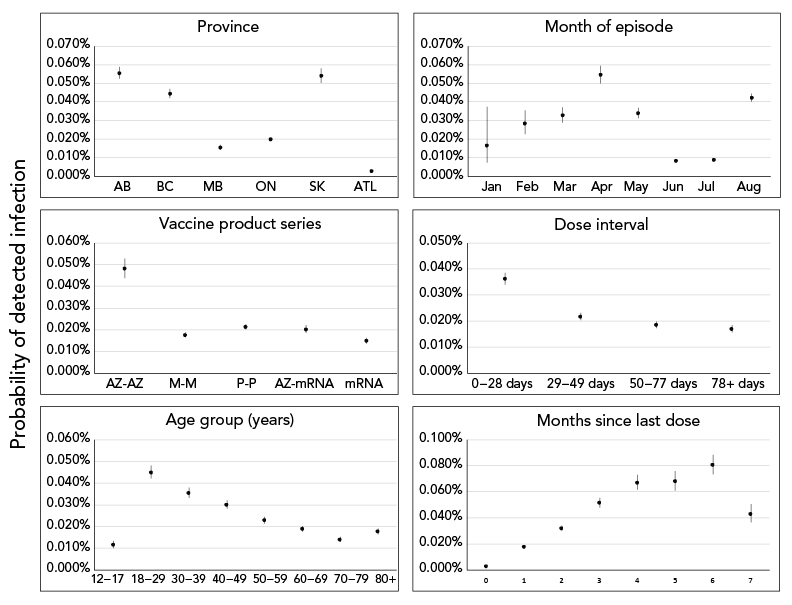
Figure 3 - Text description
The effect of covariates included in model 2 on predicted probability of fully vaccinated individuals becoming a detected COVID-19 case. Solid points and vertical solid lines show the effect and 95% prediction interval, where visible. Covariates where effects did not differ significantly among levels (only sex) not shown.
Discussion
Key results
At the national level, the results demonstrate that the majority of fully vaccinated 12-year-olds and older completed their series between June and July 2021, with most 50-year-olds and older completing their series in June and those 12–49-year-olds in July. Additionally, cases residing in the Territories completed their series earlier than those residing in Provinces. These observations coincide with the Canada's vaccine rollout program, which initially targeted the oldest age demographics, high-risk populations and adults residing in the Territories or living in remote and isolated communities. As vaccine eligibility expanded over time with decreasing age, Canadian residents 12 years of age and older were eligible for a first dose by May 2021 Footnote 5. These results highlighted that the majority of not fully vaccinated cases occurred in April 2021. This is consistent with the timing of the third wave of COVID-19 cases driven by the Alpha variant, as well as the lower vaccination coverage for those 12-year-olds and older (only 4% of this age group was fully vaccinated at the time). The first vaccine dose was being rolled out and eligibility was in the midst of expanding, resulting in lower number of individuals protected against COVID-19 at this time Footnote 21 Footnote 22 Footnote 23. Of the cases that met analysis criteria, not fully vaccinated individuals had higher case probability compared to fully vaccinated, consistent with international analyses Footnote 15 Footnote 24 Footnote 25. Fully vaccinated cases were more likely to be female and older than not fully vaccinated, as vaccine eligibility initially targeted older populations and people working in healthcare settings—where females are outnumbered Footnote 26 Footnote 27.
This analysis further investigated vaccine programmatic factors and whether time since last dose, time interval between doses and/or product combination are associated with becoming a COVID-19 case. Among fully vaccinated individuals, those with a shorter time interval between doses (0–28 days), those receiving two doses of AstraZeneca and those with increased time since last dose from zero to six months, had an elevated probability of becoming a COVID-19 case. Individuals who were vaccinated seven months after their last dose had a lower probability of becoming a case; however, results must be interpreted with caution as the cohort of individuals eligible to become a case seven months after series completion was small (n=211, August 2021), due to the length of the analytic period.
Comparison
Studies suggest that age Footnote 6 Footnote 9 Footnote 10 Footnote 11 Footnote 12 Footnote 13 Footnote 14 Footnote 16 Footnote 24 Footnote 25 Footnote 28Footnote 29 Footnote 30 Footnote 31, type of vaccine products used Footnote 6 Footnote 10 Footnote 12 Footnote 14 Footnote 16 Footnote 24 Footnote 25 Footnote 29 Footnote 31 Footnote 32, time interval between doses, time since last dose Footnote 6 Footnote 9 Footnote 11 Footnote 12 Footnote 13 Footnote 14 Footnote 15 Footnote 16 Footnote 18 Footnote 25 Footnote 28 Footnote 29 Footnote 30 Footnote 31 Footnote 32 Footnote 33 Footnote 34, vaccination status Footnote 8 Footnote 12 Footnote 24 Footnote 25 Footnote 29 Footnote 31 and predominance of emerging and evolving variants Footnote 9 Footnote 15 Footnote 25 Footnote 31 Footnote 33 may impact the duration of protection against COVID-19 and its severity Footnote 6 Footnote 8 Footnote 9 Footnote 10 Footnote 11 Footnote 12 Footnote 13 Footnote 14 Footnote 15 Footnote 16. This national analysis is consistent with analyses performed interprovincially and internationally, supporting that vaccine effectiveness against infection decreased across all age groups one to six months after full vaccination Footnote 12. Results suggest that decreases in vaccine effectiveness may be in part due to waning immunity, as demonstrated by a study conducted in Israel, where those who completed their vaccination series in January and February 2021 were at a 2.26-fold increased risk of developing COVID-19 compared to those who completed their series in March and April 2021 Footnote 18. A similar trend was observed in a study conducted in England, where greater waning in vaccine effectiveness was observed among older adults, specifically those 65 years and older, and in those who were clinically vulnerable Footnote 6. Canadian studies conducted in Ontario, British Columbia and Québec highlighted vaccine effectiveness against infection was greater with an mRNA containing schedule compared to two doses of AstraZeneca Vaxzevria vaccine Footnote 31 Footnote 35. As for the time interval between doses, studies conducted in the United States and Israel found that time elapsed between the last dose and becoming a case was significantly longer among those with a longer time interval between doses than those with a time interval less than 90 days, with attenuation increasing by month Footnote 28 Footnote 33.
Strengths and limitations
Given Canada's unique vaccine rollout, this is the first analysis to nationally assess the associations between COVID-19 vaccination status, vaccination characteristics (i.e. vaccine products received, time interval between doses and time since last dose) and case incidence based on these factors. This analysis has high representation across Canada, with 12 of 13 P/Ts providing vaccine information on COVID-19 cases and all P/Ts providing vaccination coverage information; however, variability in reporting across P/Ts may impact interpretation. There are several limitations to acknowledge in this analysis, one of which is that vaccination data were not available for cases from all P/Ts, which may result in an overgeneralization of national results. The expanding vaccine eligibility criteria in Canada over the analysis period (January to August 2021) also presented additional contextual challenges for the interpretation of results, specifically with varying representativeness of Canadian population through analysis period.
Second, this analysis was notably limited by the vaccination coverage dataset providing only counts of fully vaccinated individuals by month of last dose. As a result, only two vaccination status categories could be analyzed—fully vaccinated and not fully vaccinated individuals—as granularity was lost in this latter unconventional classification group. As partial vaccination has demonstrated to reduce the risk of SARS-CoV-2 infection, the inclusion of partially vaccinated individuals in the not fully vaccinated group may impact the estimated effects when comparing by vaccination status Footnote 36 Footnote 37 Footnote 38. Counts by month of last dose also prevented analyses from precisely accounting for the 14 days to achieve full-vaccination protection in the coverage dataset. Individuals were considered fully vaccinated the month they received their last dose, inflating fully vaccinated coverage estimates for this month (zero months since last dose) and to a lesser extent the following month.
Additionally, this analysis included only cases from the eight-month period following the beginning of vaccine rollout in Canada, limiting the generalizability and size of fully vaccinated coverage groups with longer months since last dose. The limited period of analysis also prevented assessment of severe outcomes, as hospitalizations among fully vaccinated cases were infrequent (n=809) and insufficient for the stratification required for model building.
Lastly, this analysis did not explicitly investigate impacts by public health measures and variants due to limited cases with whole genome sequencing. The period of analysis was performed during the Alpha and Delta waves, which are not explicit effect modifiers on vaccine breakthrough and vaccine effectiveness, as several studies have suggested that vaccine effectiveness and viral burden were reduced with Delta circulation Footnote 6 Footnote 11 Footnote 25 Footnote 30.
Interpretation and generalizability
The jurisdictions included in the generalized linear models represent 77% of the Canadian population (9/13 P/Ts) and the jurisdictions included in the descriptive analysis represent 78% of the Canadian population (12/13 P/Ts). Vaccination program rollout and vaccine availability varied across P/Ts and over time; therefore, interpretation at the national level should be done with caution.
Conclusion
Findings from Canada's national surveillance systems support that being fully vaccinated against COVID-19, a longer time interval between doses and mRNA COVID-19 vaccines schedule reduce the probability of becoming a COVID-19 case following vaccination. National analyses inform guidance on booster doses and contribute to the growing body of evidence on COVID-19 vaccine performance and vaccine recommendations. Further national analysis on variants, severe outcomes and public health measures may strengthen vaccine effectiveness research and recommendations.
Authors' statement
SB — Model analysis lead, data curation, formal analysis, review and editing
BHMF — Formal analysis of vaccination coverage datasets, review and editing
JR — Conceptualization and analysis of vaccination coverage datasets and review
SM — Formal analysis of case dataset, review and editing
FB — Formal analysis of case dataset
JM — Original draft, review and editing
NL — Manuscript lead, review and editing
ML — Methodological guidance, review and editing
BC — Literature review
AF — Review and editing
LW — Review and editing
Competing interests
None.
Acknowledgements
Authors wish to thank provincial and territorial surveillance partners, the Vaccine Coverage and Information System section, Vaccine Effectiveness Surveillance Program, Public Health Risk Science/National Microbiology Laboratory, the Data Integration Team and the COVID-19 Surveillance Team at the Public Health Agency of Canada. PHAC included the following authors by team:
COVID-19 Surveillance Team, Centre for Immunization and Respiratory Infectious Diseases: F Bang, S Buckrell, N Lapczak, S Merali, J Mielczarek, and L Whitmore.
Public Health Risk Science/National Microbiology Laboratory: M Li
Vaccine Coverage and Information System Team, Centre for Immunization Surveillance: B Ho M Fane, D Joy-Baysac, V Lavergne, A Maquiling and J Rotondo.
Vaccine Effectiveness Surveillance Program (VESPa), Centre for Immunization Surveillance: A Fall and B Cheng.
Funding
This work was supported by the Public Health Agency of Canada.
Supplemental material
These documents can be accessed on the Supplemental material file.
Figure S1: Timeline of relevant vaccine program in Canada for those 12 years of age and older, December 2020 to August 2021
Figure S2: Cumulative percent distribution of "fully vaccinated" individuals by month of last dose and Province/Territory, January 1, 2021 to August 14, 2021
Figure S3: Description of case population for analysis
Table S1: Number and percent of those 12 years of age and older who were "fully vaccinated" by jurisdiction, age group and sex in Canada, as of August 14, 2021
Table S2: Number and percent of those 12 years of age and older who were "fully vaccinated" by dose interval, month of last dose and vaccine schedule in Canada, as of August 14, 2021
Table S3: Predicted effects of variables included in model 1 on probability of becoming a detected COVID-19 case, after controlling for all other variables
Figure S4: Effect of sex on the predicted probability of "fully vaccinated" individuals becoming a detected COVID-19 case in the main model 1, and the model 2 (conditional on full-vaccination status)
Table S4: Predicted effects of variables included in model 2 on probability of becoming a detected COVID-19 case, after controlling for all other variables
References
- Footnote 1
-
Public Health Agency of Canada. Chief Public Health Officer of Canada's Report on the State of Public Health in Canada 2020. From risk to resilience: An equity approach to COVID-19. Ottawa, ON: PHAC; 2020. [Accessed 2022 June 17]. https://www.canada.ca/en/public-health/corporate/publications/chief-public-health-officer-reports-state-public-health-canada/from-risk-resilience-equity-approach-covid-19.html
- Footnote 2
-
World Health Organization. Statement on the second meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV). Geneva (CH): WHO; January 30, 2020. [Accessed 2022 June 17]. https://web.archive.org/web/20210815071616/https://www.who.int/news/item/30-01-2020-statement-on-the-second-meeting-of-the-international-health-regulations-%282005%29-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-%282019-ncov%29
- Footnote 3
-
Public Health Agency of Canada. Canada’s COVID-19 Immunization Plan: Saving Lives and Livelihoods. Ottawa, ON: PHAC; 2020. [Accessed 2022 June 17]. https://www.canada.ca/en/public-health/services/diseases/2019-novel-coronavirus-infection/canadas-reponse/canadas-covid-19-immunization-plan
- Footnote 4
-
Public Health Agency of Canada. National Advisory Committee on Immunization (NACI). Summary of National Advisory Committee on Immunization statement of June 17, 2021. Ottawa, ON: PHAC; 2021. [Accessed 2022 Sept 28]. https://www.canada.ca/en/public-health/services/immunization/national-advisory-committee-on-immunization-naci/recommendations-use-covid-19-vaccines/summary-statement-june-17-2021.html
- Footnote 5
-
Public Health Agency of Canada. Vaccines for COVID-19: How to get vaccinated. Ottawa, ON: PHAC; 2022. [Accessed 2022 June 17]. https://www.canada.ca/en/public-health/services/diseases/coronavirus-disease-covid-19/vaccines/how-vaccinated
- Footnote 6
-
Andrews N, Tessier E, Stowe J, Gower C, Kirsebom F, Simmons R, Gallagher E, Thelwall S, Groves N, Dabrera G, Myers R, Campbell CN, Amirthalingam G, Edmunds M, Zambon M, Brown K, Hopkins S, Chand M, Ladhani SN, Ramsay M, Lopez Bernal J. Duration of protection against mild and severe disease by covid-19 vaccines. N Engl J Med 2022;386(4):340–50. https://doi.org/10.1056/NEJMoa2115481
- Footnote 7
-
Public Health Agency of Canada. COVID-19 vaccination in Canada. Ottawa, ON: PHAC; 2023. [Accessed 2022 June 17]. https://health-infobase.canada.ca/covid-19/vaccine-distribution/
- Footnote 8
-
Harris JE. COVID-19 Incidence and hospitalization during the delta surge were inversely related to vaccination coverage among the most populous U.S. Counties. Health Policy Technol 2022;11(2):100583. https://doi.org/10.1016/j.hlpt.2021.100583
- Footnote 9
-
Chemaitelly H, Tang P, Hasan MR, AlMukdad S, Yassine HM, Benslimane FM, Al Khatib HA, Coyle P, Ayoub HH, Al Kanaani Z, Al Kuwari E, Jeremijenko A, Kaleeckal AH, Latif AN, Shaik RM, Abdul Rahim HF, Nasrallah GK, Al Kuwari MG, Al Romaihi HE, Butt AA, Al-Thani MH, Al Khal A, Bertollini R, Abu-Raddad LJ. Waning of BNT162b2 Vaccine Protection against SARS-CoV-2 Infection in Qatar. N Engl J Med 2021;385(24):e83. https://doi.org/10.1056/NEJMoa2114114
- Footnote 10
-
Cerqueira-Silva T, Oliveira VA, Boaventura VS, Pescarini JM, Júnior JB, Machado TM, Flores-Ortiz R, Penna GO, Ichihara MY, de Barros JV, Barreto ML, Werneck GL, Barral-Netto M. Influence of age on the effectiveness and duration of protection of Vaxzevria and CoronaVac vaccines: A population-based study. Lancet Reg Health Am 2022;6:100154. https://doi.org/10.1016/j.lana.2021.100154
- Footnote 11
-
Goldberg Y, Mandel M, Bar-On YM, Bodenheimer O, Freedman L, Haas EJ, Milo R, Alroy-Preis S, Ash N, Huppert A. Waning immunity after the BNT162B2 vaccine in Israel. N Engl J Med 2021;385(24):e85. https://doi.org/10.1056/NEJMoa2114228
- Footnote 12
-
Feikin DR, Higdon MM, Abu-Raddad LJ, Andrews N, Araos R, Goldberg Y, Groome MJ, Huppert A, O’Brien KL, Smith PG, Wilder-Smith A, Zeger S, Deloria Knoll M, Patel MK. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet 2022;399(10328):924–44. https://doi.org/10.1016/S0140-6736(22)00152-0
- Footnote 13
-
Glatman-Freedman A, Bromberg M, Dichtiar R, Hershkovitz Y, Keinan-Boker L. The BNT162b2 vaccine effectiveness against new COVID-19 cases and complications of breakthrough cases: A nation-wide retrospective longitudinal multiple cohort analysis using individualised data. EBioMedicine 2021;72:103574. https://doi.org/10.1016/j.ebiom.2021.103574
- Footnote 14
-
Andrews N, Stowe J, Kirsebom F, Toffa S, Sachdeva R, Gower C, Ramsay M, Lopez Bernal J. Effectiveness of COVID-19 booster vaccines against COVID-19-related symptoms, hospitalization and death in England. Nat Med 2022;28(4):831–7. https://doi.org/10.1038/s41591-022-01699-1
- Footnote 15
-
Lauring AS, Tenforde MW, Chappell JD, Gaglani M, Ginde AA, McNeal T, Ghamande S, Douin DJ, Talbot HK, Casey JD, Mohr NM, Zepeski A, Shapiro NI, Gibbs KW, Files DC, Hager DN, Shehu A, Prekker ME, Erickson HL, Exline MC, Gong MN, Mohamed A, Johnson NJ, Srinivasan V, Steingrub JS, Peltan ID, Brown SM, Martin ET, Monto AS, Khan A, Hough CL, Busse LW, Ten Lohuis CC, Duggal A, Wilson JG, Gordon AJ, Qadir N, Chang SY, Mallow C, Rivas C, Babcock HM, Kwon JH, Halasa N, Grijalva CG, Rice TW, Stubblefield WB, Baughman A, Womack KN, Rhoads JP, Lindsell CJ, Hart KW, Zhu Y, Adams K, Schrag SJ, Olson SM, Kobayashi M, Verani JR, Patel MM, Self WH. Influenza and Other Viruses in the Acutely Ill (IVY) Network. Clinical severity and mRNA effectiveness for Omicron, Delta, and Alpha Sars-COV-2 variants in the United States: prospective observational study. BMJ 2022;376:e069761. https://doi.org/10.1136/bmj-2021-069761
- Footnote 16
-
Tan SH, Pung R, Wang LF, Lye DC, Ong B, Cook AR, Tan KB. Association of homologous and heterologous vaccine boosters with COVID-19 incidence and severity in Singapore. JAMA 2022;327(12):1181–2. https://doi.org/10.1001/jama.2022.1922
- Footnote 17
-
Public Health Agency of Canada. COVID-19 epidemiology update: Cases following vaccination. Ottawa, ON: PHAC; 2022. [Accessed 2022 June 17]. https://health-infobase.canada.ca/covid-19/cases-following-vaccination.html
- Footnote 18
-
Mizrahi B, Lotan R, Kalkstein N, Peretz A, Perez G, Ben-Tov A, Chodick G, Gazit S, Patalon T. Correlation of SARS-CoV-2-breakthrough infections to time-from-vaccine. Nat Commun 2021;12(1):6379. https://doi.org/10.1038/s41467-021-26672-3
- Footnote 19
-
Statistics Canada. Quarterly Demographic Estimates (QDE). Ottawa, ON: StatCan; June 2022. [Accessed 2022 June 17]. https://www23.statcan.gc.ca/imdb/p2SV.pl?Function=getSurvey&SDDS=3601
- Footnote 20
-
Public Health Agency of Canada. National case definition: Coronavirus disease (COVID-19). Ottawa, ON: PHAC; 2022 [Accessed 2022 June 17]. https://www.canada.ca/en/public-health/services/diseases/2019-novel-coronavirus-infection/health-professionals/national-case-definition.html#conf
- Footnote 21
-
Public Health Agency of Canada. COVID-19 epidemiology update: Key updates. Ottawa, ON: PHAC; Jan 2023. [Accessed 2022 Sept 28]. https://health-infobase.canada.ca/covid-19/
- Footnote 22
-
Public Health Agency of Canada. National Advisory Committee on Immunization (NACI). National Advisory Committee on Immunization (NACI): Summary of extended dose interval statement of April 7, 2021. Ottawa, ON: PHAC; April 2021. [Accessed 2022 Sept 28]. https://www.canada.ca/en/public-health/services/immunization/national-advisory-committee-on-immunization-naci/covid-19-summary-extended-dose-interval.html
- Footnote 23
-
Detsky AS, Bogoch II. COVID-19 in Canada: Experience and Response to Waves 2 and 3. JAMA 2021;326(12):1145–6. https://doi.org/10.1001/jama.2021.14797
- Footnote 24
-
Shah SA, Robertson C, Rudan I, Murray JL, McCowan C, Grange Z, Buelo A, Sullivan C, Simpson CR, Ritchie LD, Sheikh A. BNT162b2 and ChAdOx1 nCoV-19 vaccinations, incidence of SARS-CoV-2 infections and COVID-19 hospitalisations in Scotland in the Delta era. J Glob Health 2022;12:05008. https://doi.org/10.7189/jogh.12.05008
- Footnote 25
-
Pouwels KB, Pritchard E, Matthews PC, Stoesser N, Eyre DW, Vihta KD, House T, Hay J, Bell JI, Newton JN, Farrar J, Crook D, Cook D, Rourke E, Studley R, Peto TE, Diamond I, Walker AS. Effect of Delta variant on viral burden and vaccine effectiveness against new SARS-CoV-2 infections in the UK. Nat Med 2021;27(12):2127–35. https://doi.org/10.1038/s41591-021-01548-7
- Footnote 26
-
Public Health Agency of Canada. COVID-19 infections among healthcare workers and other people working in healthcare settings. Ottawa, ON: PHAC; March 2022. [Accessed 2022 June 17]. https://www.canada.ca/en/public-health/services/diseases/coronavirus-disease-covid-19/epidemiological-economic-research-data/infections-healthcare-workers-other-people-working-healthcare-settings.html
- Footnote 27
-
Sriharan A, Ratnapalan S, Tricco AC, Lupea D, Ayala AP, Pang H, Lee DD. Occupational Stress, Burnout, and Depression in Women in Healthcare During COVID-19 Pandemic: Rapid Scoping Review. Front Glob Womens Health 2020;1:596690. https://doi.org/10.3389/fgwh.2020.596690
- Footnote 28
-
Israel A, Merzon E, Schäffer AA, Shenhar Y, Green I, Golan-Cohen A, Ruppin E, Magen E, Vinker S. Elapsed time since BNT162b2 vaccine and risk of SARS-CoV-2 infection: test negative design study. BMJ 2021;375:e067873. https://doi.org/10.1136/bmj-2021-067873
- Footnote 29
-
Naleway AL, Groom HC, Crawford PM, Salas SB, Henninger ML, Donald JL, Smith N, Thompson MG, Blanton LH, Bozio CH, Azziz-Baumgartner E. Incidence of SARS-CoV-2 Infection, Emergency Department Visits, and Hospitalizations Because of COVID-19 Among Persons Aged ≥12 Years, by COVID-19 Vaccination Status - Oregon and Washington, July 4-September 25, 2021. MMWR Morb Mortal Wkly Rep 2021;70(46):1608–12. https://doi.org/10.15585/mmwr.mm7046a4
- Footnote 30
-
Fabiani M, Puopolo M, Filia A, Sacco C, Mateo-Urdiales A, Spila Alegiani S, Del Manso M, D’Ancona F, Vescio F, Bressi M, Petrone D, Spuri M, Rota MC, Massari M, Da Cas R, Morciano C, Stefanelli P, Bella A, Tallon M, Proietti V, Siddu A, Battilomo S, Palamara AT, Popoli P, Brusaferro S, Rezza G, Riccardo F, Menniti Ippolito F, Pezzotti P. Effectiveness of an mRNA vaccine booster dose against SARS-CoV-2 infection and severe COVID-19 in persons aged ≥60 years and other high-risk groups during predominant circulation of the delta variant in Italy, 19 July to 12 December 2021. Expert Rev Vaccines 2022;21(7):975–82. https://doi.org/10.1080/14760584.2022.2064280
- Footnote 31
-
Chung H, He S, Nasreen S, Sundaram ME, Buchan SA, Wilson SE, Chen B, Calzavara A, Fell DB, Austin PC, Wilson K, Schwartz KL, Brown KA, Gubbay JB, Basta NE, Mahmud SM, Righolt CH, Svenson LW, MacDonald SE, Janjua NZ, Tadrous M, Kwong JC; Canadian Immunization Research Network (CIRN) Provincial Collaborative Network (PCN) Investigators. Effectiveness of BNT162b2 and mRNA-1273 covid-19 vaccines against symptomatic SARS-CoV-2 infection and severe covid-19 outcomes in Ontario, Canada: test negative design study. BMJ 2021;374:n1943. https://doi.org/10.1136/bmj.n1943
- Footnote 32
-
Heftdal LD, Schultz M, Lange T, Knudsen AD, Fogh K, Hasselbalch RB, Linander CB, Kallemose T, Bundgaard H, Grønbæk K, Valentiner-Branth P, Iversen K, Nielsen SD. Incidence of Positive Severe Acute Respiratory Syndrome Coronavirus Polymerase Chain Reaction After Coronavirus Disease 2019 Vaccination With up to 8 Months of Follow-up: Real-life Data From the Capital Region of Denmark. Clin Infect Dis 2022;75(1):e675–82. https://doi.org/10.1093/cid/ciac012
- Footnote 33
-
Britton A, Fleming-Dutra KE, Shang N, Smith ZR, Dorji T, Derado G, Accorsi EK, Ajani UA, Miller J, Schrag SJ, Verani JR. Association of COVID-19 Vaccination With Symptomatic SARS-CoV-2 Infection by Time Since Vaccination and Delta Variant Predominance. JAMA 2022;327(11):1032–41. https://doi.org/10.1001/jama.2022.2068
- Footnote 34
-
de Michelena P, Torres I, Albert E, Bracho A, González-Candelas F, Navarro D. Impact of time elapsed since full vaccination on SARS-CoV-2 RNA load in Delta-variant breakthrough COVID-19. J Infect 2022;84(4):579–613. https://doi.org/10.1016/j.jinf.2022.01.006
- Footnote 35
-
Skowronski DM, Febriani Y, Ouakki M, Setayeshgar S, El Adam S, Zou M, Talbot D, Prystajecky N, Tyson JR, Gilca R, Brousseau N, Deceuninck G, Galanis E, Fjell CD, Sbihi H, Fortin E, Barkati S, Sauvageau C, Naus M, Patrick DM, Henry B, Hoang LM, De Wals P, Garenc C, Carignan A, Drolet M, Jassem AN, Sadarangani M, Brisson M, Krajden M, De Serres G. Two-dose severe acute respiratory syndrome coronavirus 2 vaccine effectiveness with mixed schedules and extended dosing intervals: test-negative design studies from British Columbia and Quebec, Canada. Clin Infect Dis 2022;75(11):1980–92. https://doi.org/10.1093/cid/ciac290
- Footnote 36
-
Centers for Disease Control and Prevention. CDC COVID-19 Study Shows mRNA Vaccines Reduce Risk of Infection by 91 Percent for Fully Vaccinated People. Atlanta, GA; CDC; June 2021 [Accessed 2022 Oct 4]. https://www.cdc.gov/media/releases/2021/p0607-mrna-reduce-risks.html#:~:text=Once%20fully%20vaccinated%2C%20participants'%20risk,included%20symptomatic%20and%20asymptomatic%20infections
- Footnote 37
-
Britton A, Jacobs Slifka KM, Edens C, Nanduri SA, Bart SM, Shang N, Harizaj A, Armstrong J, Xu K, Ehrlich HY, Soda E, Derado G, Verani JR, Schrag SJ, Jernigan JA, Leung VH, Parikh S. Effectiveness of the Pfizer-BioNtech Covid-19 Vaccine among Residents of Two Skilled Nursing Facilities Experiencing Covid-19 Outbreaks - Connecticut, December 2020–February 2021. MMWR Morbid Mortal Wkly Rep. 2021;70(11):396-401. https://www.cdc.gov/mmwr/volumes/70/wr/mm7011e3.htm
- Footnote 38
-
European Centre for Disease Prevention and Control. Partial COVID-19 vaccination, vaccination following SARS-COV-2 infection and heterologous vaccination schedule: Summary of evidence. Solna (SE); ECDC; July 2021. [Accessed 2022 Oct 4]. https://www.ecdc.europa.eu/en/publications-data/partial-covid-19-vaccination-summary
