Comparison testing used by the CLRN’s SARS-CoV-2 Program

 Download this article as a PDF
Download this article as a PDFPublished by: The Public Health Agency of Canada
Issue: Volume 49-5, May 2023: Innovative Technologies in Public Health
Date published: May 2023
ISSN: 1481-8531
Submit a manuscript
About CCDR
Browse
Volume 49-5, May 2023: Innovative Technologies in Public Health
Implementation Science
Comparison of fifteen SARS-CoV-2 nucleic acid amplification test assays used during the Canadian Laboratory Response Network's National SARS-CoV-2 Proficiency Program, May 2020 to June 2021
Charlene Ranadheera1, Kym Antonation1, Cindi Corbett1
Affiliation
1 Health Security and Response Division, National Microbiology Laboratory, Public Health Agency of Canada, Winnipeg, MB
Correspondence
Suggested citation
Ranadheera C, Antonation K, Corbett C. Comparison of fifteen SARS-CoV-2 nucleic acid amplification test assays used during the Canadian Laboratory Response Network's National SARS-CoV-2 Proficiency Program, May 2020 to June 2021. Can Commun Dis Rep 2023;49(5):180–9. https://doi.org/10.14745/ccdr.v49i05a03
Keywords: PCR, SARS-CoV-2, nucleic acid amplification test, COVID-19
Abstract
Background: On March 11, 2020, the World Health Organization declared a pandemic caused by the recently emerged severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). This led to increased clinical testing and decentralizing of this testing from provincial health laboratories to regional and private facilities. Leveraging the results from the Canadian Laboratory Response Network's National SARS-CoV-2 Proficiency Test (PT) Program, this study compares multiple commercial and laboratory-developed nucleic acid amplification tests, assessing both sensitivity and specificity across multiple users.
Methods: Each panel consisted of six blinded, contrived-clinical samples. Panels were distributed to international, provincial and territorial laboratories and subsequently to partner facilities. Participating laboratories were asked to run these sample through their respective extraction/PCR workflows and submit results to the National Microbiology Laboratory, outlining the nucleic acid extraction platform and nucleic acid amplification test employed, as well as the viral gene target and Ct values or equivalent obtained. Data were compiled for each molecular platform and gene target used.
Results: The PT schemes were deployed in May 2020, November 2020 and June 2021, resulting in 683 data sets using 37 different nucleic acid amplification tests. Over the course of three PT schemes, the average score obtained was 99.3% by participants demonstrating consistent testing between laboratories and testing platforms.
Conclusion: This study confirmed the rapid and successful implementation of a Canadian PT Program and provided comparative analysis of the various emergency use authorized and laboratory developed tests employed for the detection of SARS-CoV-2 and demonstrated an overall 99.3% test concordance nationwide.
Introduction
In late 2019, a novel respiratory virus, severe acute respiratory coronavirus 2 (SARS-CoV-2), emerged in the Hubei province of China and subsequently caused the coronavirus disease 2019 (COVID-19) global pandemic. As the case numbers rapidly grew, it became necessary to decentralize testing to support testing at the federal, provincial/territorial and municipal levels, including private laboratories, hospitals and healthcare facilities.
The Canadian Laboratory Response Network (CLRN) at the National Microbiology Laboratory (NML) in Winnipeg, Canada provides high-consequence proficiency panels for biothreat agents to ensure that public health laboratories are ready to respond with high quality diagnostic testing. During the COVID-19 pandemic, the CLRN was leveraged to develop a Proficiency Test (PT) program to support facilities conducting SARS-CoV-2 clinical testing using molecular methods. Similar to other international efforts, the National SARS-CoV-2 PT Program supports the ability of public health testing facilities to establish competency and obtain or maintain accreditation to conduct SARS-CoV-2 clinical testing against a known reference standard to ensure consistency between testing platforms and laboratories across the country and across the globe Footnote 1 Footnote 2 Footnote 3. Nucleic acid amplification tests (NAAT) have been considered the gold standard method for the detection of active SARS-CoV-2 cases. Since the emergence of SARS-CoV-2 in December 2019, there have been a variety of NAATs developed, both laboratory-developed tests and commercial assays. This study provides a comparison of the various NAAT platforms employed within Canada over the course of three PT schemes from May 2020 to June 2021.
Materials and methods
Production, quality control and panel distribution
Irradiated viruses were diluted in a pooled, negative human nasal secretion as the background matrix at varying concentrations and immediately aliquoted into pre-labelled tubes. Each panel consisted of six blinded, contrived-clinical samples. Samples were sorted by site number, packaged appropriately for transport and stored at −80°C until distribution.
Prior to distribution, quality control measures were taken to ensure sample homogeneity and stability. In short, ten aliquots of each sample were removed from storage, nucleic acids were extracted as per manufacturer's instructions (MagMaxTM CORE Nucleic Acid Purification Kit, Applied BiosystemsTM, Ontario) and assayed by quantitative real-time polymerase chain reaction (qRT-PCR) (QuantiNova® Probe RT-PCR Kit, Qiagen®, Ontario) targeting the E gene of SARS-CoV-2 Footnote 4. Coefficient of variations were calculated for each set of panel samples using GraphPad® Prism's descriptive statistics. An average Ct value with a coefficient of variation less than 10% was necessary to pass sample homogeneity quality controls. Stability testing began day 1 post-production and continued at specified intervals for the duration of the PT scheme using the same approach outlined above. If quality controls passed for homogeneity and stability testing on day 1 and seven post-production, the panels were released for distribution. Stability testing continued for the duration of the test scheme.
Panels were packed on dry ice and distributed to the international, provincial and territorial laboratories, who subsequently distributed panels to their partner facilities within their jurisdiction. Cold chain was monitored and if not maintained, a new panel was shipped directly from NML.
Participant selection and intended use
Provincial and territorial members of the Canadian Public Health Laboratory Network (CPHLN) approached the NML to assist the pandemic response by producing and administering a SARS-CoV-2 PT Program, as one was not readily available at the time. The CPHLN provincial and territorial partners provided NML with a list of participants and were responsible for distribution of the test panels within their respective jurisdictions. Participants included provincial and territorial laboratories, public health laboratories, hospitals and healthcare facilities in both urban and rural communities. Specific metadata and details on individual site licensing and accreditation for SARS-CoV-2 were not made available to NML.
The PT panel was intended to be used as an internal validation of SARS-CoV-2 molecular processes, which are performed in conjunction with a nucleic acid extraction method. This panel was not intended to be used on platforms requiring fresh swab material, or the detection of viral antigens or virus-specific antibodies.
Test result submission and analysis
Participating laboratories submitted results to NML outlining the nucleic acid extraction platform and NAAT employed, as well as the viral gene target and Ct values or equivalent obtained. Data were compiled for each molecular platform and gene target used. Coefficient of variation for each gene target within a single platform was determined using GraphPad® Prism's descriptive statistics. Probit analysis using a 95% cut-off was used to determine limit of detection based on sample detection Footnote 5.
Results and discussion
The PT schemes were deployed in May 2020, November 2020 and June 2021, resulting in 683 data sets using 37 different NAAT (Table 1). Each PT scheme assessed assay sensitivity and specificity. The most commonly used platforms were fully automated low-throughput assays such as the DiaSorin SimplexaTM COVID-19 Direct Molecular Assay, Cepheid Xpert® Xpress SARS-CoV-2, Cepheid Xpert® Xpress SARS-CoV-2/Flu/RSV and BioFire® FilmArray RP2.1 Test Panel. These systems were employed mainly in hospital laboratories and in rural communities. Larger diagnostic centres, such as provincial laboratories and reference centres, generally employed high-throughput assays, including the Roche Cobas® SARS-CoV-2 Test (for Cobas 6800/8800), Seegene AllplexTM 2019 nCoV Assay, Thermo Fisher TaqPathTM COVID-19 Combo Kit and LDT targeting the E gene (Table 1).
| Nucleic acid amplification test platform | Proficiency test scheme, Number of sites/platform |
|||
|---|---|---|---|---|
| Manufacturer | Product name | May 2020 | Nov 2020 | June 2021 |
| AbbottTM | AlinityTM m SARS-CoV-2 AMP Kit | 0 | 5 | 16 |
| SARS-CoV-2 Real Time PCR | 1 | 3 | 3 | |
| Agena Bioscience | MassARRAY® SARS-CoV-2 Panel | 0 | 0 | 1 |
| Altona | AltoStar® SARS-CoV-2 RT-PCR Kit 1.5 | 1 | 1 | 2 |
| BD | SARS-CoV-2 Reagents for the BD MAXTM System | 2 | 9 | 4 |
| BGITM | Real Time Fluorescent RT-PCR Kit for detecting SARS-CoV-2 | 0 | 2 | 1 |
| BioFire® | Film Array® Respiratory 2.1 Panel | 0 | 20 | 49 |
| Biomeme | SARS-CoV-2 Go StripsTM | 0 | 1 | 1 |
Cepheid |
Xpert® Xpress SARS-CoV-2 | 34 | 36 | 52 |
| Xpert® Xpress SARS-CoV-2/Flu/RSV | 0 | 0 | 29 | |
| DiaSorin | SimplexaTM COVID-19 Direct Molecular Assay | 5 | 42 | 81 |
Hologic |
Panther Fusion® SARS-CoV-2 Assay | 0 | 2 | 2 |
| Aptima® SARS-CoV-2 Assay (Panther System) | 0 | 6 | 8 | |
| Hyris | Virus Finder COVID-19 bKitTM | 0 | 0 | 1 |
Laboratory-developed test |
3' UTR Target | 0 | 0 | 1 |
| 5' UTR Target | 0 | 2 | 4 | |
| CDC CoVPlex Real-Time PCR Assay | 0 | 0 | 1 | |
| E Gene Target | 12 | 27 | 49 | |
| N Gene Target | 1 | 1 | 10 | |
| ORF1a/b Gene Target (RdRp) | 5 | 5 | 8 | |
| S Gene Target | 0 | 1 | 0 | |
| E and N Gene Pooled Targets | 0 | 1 | 6 | |
| E and ORF1a/b Gene Pooled Targets | 0 | 0 | 1 | |
| N, ORF1a/b and S Gene Pooled Targets | 0 | 1 | 1 | |
Luminex |
Aries® SARS-CoV-2 Assay | 0 | 1 | 1 |
| NxTAG® Respiratory Pathogen Panel + SARS-CoV-2 | 0 | 1 | 1 | |
| LuminUltra | GeneCount® COVID-19 RT-qPCR Assay | 0 | 0 | 1 |
Quidel |
Lyra® SARS-CoV-2 Assay | 0 | 0 | 3 |
| Solana® SARS-CoV-2 Assay | 0 | 0 | 1 | |
| RIDA® Gene | SARS-CoV-2 Test | 0 | 2 | 1 |
Roche |
Cobas® SARS-CoV-2 Test (for Cobas 6800/8800) | 13 | 6 | 19 |
| Cobas® SARS-CoV-2 & Influenza A/B Test (for Cobas 6800/8800) | 0 | 0 | 1 | |
| Cobas® SARS-CoV-2 (for Liat®) | 0 | 0 | 1 | |
| Cobas® SARS-CoV-2 & Influenza A/B Assay (for Liat) | 0 | 0 | 9 | |
Seegene |
AllplexTM 2019 nCoV Assay | 4 | 19 | 19 |
| AllplexTM SARS-CoV-2/FluA/FluB/RSV Assay | 0 | 0 | 1 | |
| Thermo Fisher Scientific | TaqPathTM COVID-19 Combo Kit | 1 | 6 | 15 |
Total number of results submitted |
79 | 200 | 404 | |
|
||||
Panel results obtained using commercially available NAATs that have at least three datasets in any given test scheme are presented in Figure 1. Infrequently used platforms were not assessed further. Abbott produces two high-throughput, laboratory-based molecular assays for the detection of SARS-CoV-2: the Alinity m SARS-CoV-2 AMP Kit used with the Alinity m System; and SARS-CoV-2 RealTime PCR employing the m2000 RealTime System. Both systems obtained expected results for all samples across three test schemes. All sites demonstrated consistent results from November 2021 to June 2021 with coefficient of variations less than 10% (Figure 1).
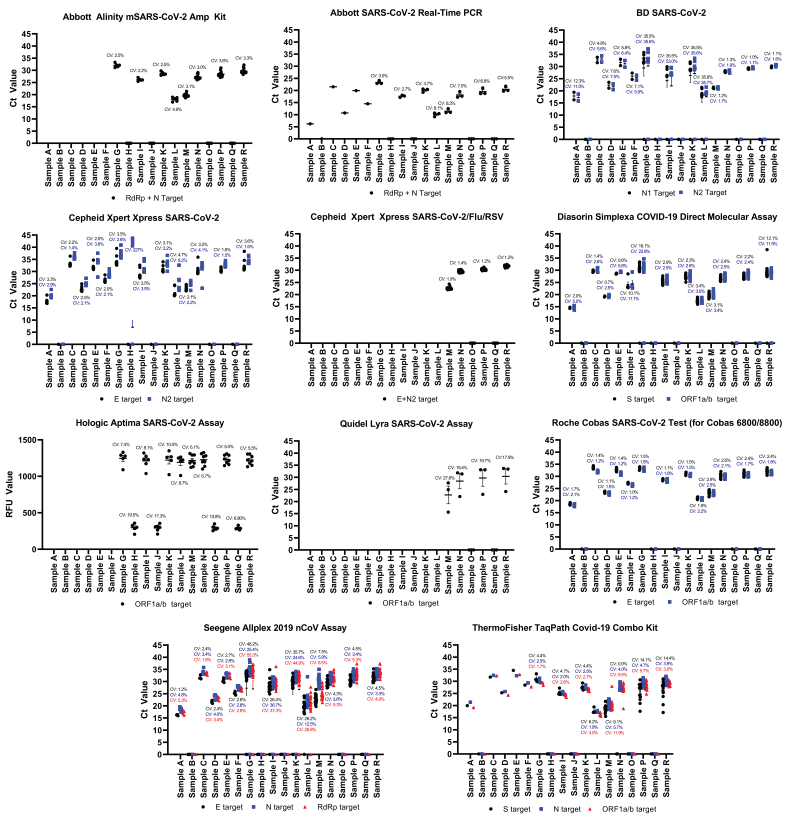
Figure 1 - Text description
This figure consists of 11 panels. Each panel represents one commercial nucleic acid amplification test for the detection of SARS-CoV-2. The data presented include the Ct values for each sample obtained during the Canadian Laboratory Response Network’s SARS-CoV-2 Proficiency Test Program from May 2020 to June 2021. The Ct values for each specific gene target within one commercial test is represented by a black circle, blue square or red triangle. Data points located on the x-axis indicate that there was no detectable SARS-CoV-2 RNA present in the sample. The text above the data point is the coefficient of variation (CV) for each respective gene target set using the same representative colour. Within one sample, typically the gene targets cluster together indicating similar detection of the viral RNA. In some cases, the gene targets are more scattered, which indicate some variation in detecting the viral RNA. Samples containing low amounts of virus had Ct values between 35 and 40, samples containing moderate amounts of virus had Ct values between 25 and 34, and samples containing high amounts of virus had Ct values less than 25.
The BD SARS-CoV-2 Reagents for the BD MAX System targeting the N gene were utilized for the detection of SARS-CoV-2. The BD MAX System is a fully automated system, allowing the user to run up to 24 samples at a time. Over the course of 13 months, the BD SARS-CoV-2 Reagents for the BD MAX System performed with variable accuracy. During the May 2020 test scheme, samples were accurately detected in all cases, but the coefficient of variation ranged from 4.8%–12.3%, indicating increased variation between users. Discordant results were observed during the November 2021 test scheme; 6/7 failures to detect SARS-CoV-2 were attributed to user error (Figure 1, Table 2); therefore, the data obtained for Sample G–L were skewed and the accuracy and consistency were negatively affected. Removing these data points would regain an overall 100% target accuracy for the N1 target and 99% accuracy for N2; the latter target failed to identify the presence of Sample I (Figure 1, Table 2). During the June 2021 test scheme, the BD SARS-CoV-2 Reagents for the BD MAX System performed with 100% accuracy. Ct values were consistent among all users denoted by a coefficient of variations of less than 5% (Figure 1).
| PCR platform | Assay target | PCR platform SARS-CoV-2 discordant results (%) | Sensitivity: 95% detectionFootnote a Footnote b (copies/ml) |
Specificity | ||
|---|---|---|---|---|---|---|
| Positive agreement (%)Footnote b | Negative agreement (%)Footnote b | |||||
| BD SARS-CoV-2 Reagents for the BD MAXTM System | N1 | 6/96 (6.25%) | 1,100 or fewer | 100 | 100 | |
| N2 | 7/96 (7.29%) | 1,100 or fewer | 100 | 100 | ||
| BioFire® Film Array® RP2.1 | M/S | 1/414 (0.24%) | 1,100 or fewer | 100 | 100 | |
| Cepheid Xpert® Xpress SARS-CoV-2 | E | 0/730 (0.00%) | 1,100 or fewer | 100 | 100 | |
| N | 6/730 (0.82%) | 1,100 or fewer | 100 | 97 | ||
| DiaSorin SimplexaTM COVID-19 Direct Molecular Assay | ORF1a/b | 3/768 (0.39%) | 1,100 or fewer | 99.7 | 100 | |
| S | 4/768 (0.52%) | 1,100 or fewer | 99.6 | 100 | ||
| NxTAG® Respiratory Pathogen Panel + SARS-CoV-2 | ORF1a/b | 0/12 (0.00%) | 1,100 or fewer | 100 | 100 | |
| M | 1/12 (8.33%) | 1,100 or fewer | 100 | 100 | ||
| RIDA® gene SARS-CoV-2 Test | E | 2/18 (11.11%) | 1,100 or fewer | 100 | 100 | |
| Seegene AllplexTM 2019 nCoV Assay | E | 11/252 (4.37%) | 1,100 or fewer | 100 | 100 | |
| RdRp | 14/252 (5.56%) | 1,358 | 99.1 | 100 | ||
| N | 9/252 (3.57%) | 1,100 or fewer | 100 | 100 | ||
|
||||||
The BioFire Film Array RP2.1 test kit uses a fully automated system to test for the presence of 22 different pathogens, including SARS-CoV-2. This assay has a nucleic acid extraction step followed by reverse transcription/nested PCR step coupled with deoxyribonucleic acid melt curve technology to identify the presence of target pathogens qualitatively. Out of 414 samples tested, it missed identifying the presence of SARS-CoV-2 in only one sample; demonstrating a 99.8 concordance rate (Table 3). One site was unable to detect SARS-CoV-2 in Sample P; however, it was determined that insufficient mixing of the test sample was likely responsible for the discrepant results. Furthermore, this site correctly identified the presence of other target pathogens, which were present in the samples such as rhinovirus (Sample M), respiratory syncytial virus (Sample K), influenza A virus (Sample H and O) and influenza B virus (Sample R) (Table 3).
| Platform | Sample ID | Sample G | Sample H | Sample I | Sample J | Sample K | Sample L | |
|---|---|---|---|---|---|---|---|---|
| BioFire® Film Array® Repiratory Panel 2.1 | Expected results | Detected SARS-CoV-2 | Detected Influenza A | Detected SARS-CoV-2 | No agent detected | Detected SARS-CoV-2 RSV | Detected SARS-CoV-2 | |
| Sample concordance | 100% (20/20) |
100% (20/20) |
100% (20/20) |
100% (20/20) |
100% (20/20) |
100% (20/20) |
||
| Sample ID | Sample M | Sample N | Sample O | Sample P | Sample Q | Sample R | ||
| Expected results | Detected SARS-CoV-2 rhinovirus | Detected SARS-CoV-2 | Detected Influenza A | Detected SARS-CoV-2 | No agent detected | Detected SARS-CoV-2 Influenza B |
||
| Sample concordance | 100% (n=49/49) |
100% (n=49/49) |
100% (n=49/49) |
98.6% (n=48/49) |
100% (n=49/49) |
100% (n=49/49) |
||
| Overall concordance | 99.8% (413/414) | |||||||
| Roche Cobas® SARS-CoV-2 & Influenza A/B Assay (for Liat®) | Sample ID | Sample M | Sample N | Sample O | Sample P | Sample Q | Sample R | |
| Expected results | Detected SARS-CoV-2 | Detected SARS-CoV-2 | Detected Influenza A | Detected SARS-CoV-2 | No agent detected | Detected SARS-CoV-2 Influenza B |
||
| Sample concordance | 100% (n=9/9) |
100% (n=9/9) |
100% (n=9/9) |
100% (n=9/9) |
100% (n=9/9) |
100% (n=9/9) |
||
| Overall concordance | 100% (n=54/54) | |||||||
|
||||||||
The Cepheid GeneXpert platform is readily used across Canada for the detection of SARS-CoV-2 employing the Xpert Xpress SARS-CoV-2 and Xpert Xpress SARS-CoV-2/Flu/RSV assays. The Xpert Xpress SARS-CoV-2 E assay performed with accuracy (100% detection rate) and consistency (coefficient of variation less than 5%) for all samples; however, discordant results were observed using the N target, specifically for Sample H. Sample H did not contain SARS-CoV-2 but did contain a moderate amount of influenza A virions (Ct 27); there were six instances where the SARS-CoV-2 N2 target produced a Ct greater than 40, which was deemed positive for SARS-CoV-2 by the GeneXpert software (Figure 1, Table 2). Apart from Sample H, the Ct values for the N target were consistent and had a coefficient of variation less than 10%, Figure 1. The recently developed Cepheid Xpert Xpress SARS-CoV-2/Flu/RSV assay was employed during the June 2021 test scheme and the result output for SARS-CoV-2 was combined for both E and N2 targets. The platform had a 100% accuracy and produced very consistent results with a coefficient of variation less than 2% among all users (Figure 1). The Xpert Xpress SARS-CoV-2/Flu/RSV assay also correctly identified the presence of influenza A and B in Samples O and R, respectively (data not shown).
The Diasorin Simplexa COVID-19 Direct Molecular Assay is a low throughput, automated system that can run up to eight samples at once. Its main distinction from other similar systems, such as the BioFire Film Array and Cepheid GeneXpert platforms, is that it eliminates the nucleic acid extraction/purification step. Discordant results were observed for Sample G and Sample R, the ORF1a/b target missed detecting SARS-CoV-2 n=2/768 times (0.26%), while the S target did not detect SARS-CoV-2 n=3/768 times (0.39%) (Figure 1, Table 2). According to the manufacturer, the S assay has a 95.8% detection rate of 500 copies/ml (2,000 copies/ml for 100% detection) and the ORF1a/b is detected 93.8% of the time at 1,000 copies/ml (2,000 copies/ml for 100% detection) Footnote 6. Similar observations were observed here: the S assay performed better than the ORF1a/b assay (Table 2). Sample G and R are approximately 1,100 and 3,500 copies/ml respectively, which is the range of the assay's limit of detection (LOD) for both targets, and is the likely cause for the discrepant results (Table 4). Furthermore, there was an additional discordant result for each target due to a software error that reported "no result" when Ct values were obtained for both targets (Table 2). For samples where all targets were correctly identified (Samples A–F and H–Q), coefficient of variations were 5% or less, except for Sample F which had coefficients of variations of 11.1% and 10.1% for the ORF1a/b and S targets, respectively (Figure 1).
| Sample | Identity | SARS-CoV-2 E approximate copies/ml |
Approximate Ct value (SARS-CoV-2 E target)Footnote a |
|---|---|---|---|
| CLRN's SARS-CoV-2 proficiency test scheme – May 2020 | |||
| A | SARS-CoV-2 wild type | 120,000,000 | 20 |
| B | Blank | 0 | 0 |
| C | SARS-CoV-2 wild type | 1,600 | 36 |
| D | SARS-CoV-2 wild type | 2,700,000 | 25 |
| E | SARS-CoV-2 wild type | 3,900 | 35 |
| F | SARS-CoV-2 wild type | 216,000 | 29 |
| CLRN's SARS-CoV-2 proficiency test scheme – November 2020 | |||
| G | SARS-CoV-2 wild type | 1,100 | 36 |
| H | Influenza A virus | 0 | 0 |
| I | SARS-CoV-2 wild type | 54,000 | 31 |
| J | Blank | 0 | 0 |
| K | SARS-CoV-2 wild type | 10,800 | 33 |
| Respiratory syncytial virus | 0 | ||
| L | SARS-CoV-2 wild type | 13,000,000 | 22 |
| CLRN's SARS-CoV-2 proficiency test scheme – June 2021 | |||
| M | SARS-CoV-2 B.1.351 | 280,000 | 28 |
| Rhinovirus | 0 | ||
| N | SARS-CoV-2 B.1.1.7 | 2,100 | 35 |
| O | Influenza A virus | 0 | 0 |
| P | SARS-CoV-2 P.1 | 1,600 | 36 |
| Q | Blank | 0 | 0 |
| R | SARS-CoV-2 wild type | 3,500 | 35 |
| Influenza B virus | 0 | ||
|
|||
Hologic produces two SARS-CoV-2 assays that were employed during the scope of the CLRN SARS-CoV-2 PT schemes: Panther Fusion SARS-CoV-2 assay and Aptima SARS-CoV-2 assay. The Panther Fusion SARS-CoV-2 assay was not presented here as only two sites employing this platform, while the Aptima SARS-CoV-2 assay was employed during the November 2020 and June 2021 test schemes with six and eight users respectively (Table 1). This platform demonstrated 100% concordance (n=90/90 samples); however, the Ct values obtained were quite variable, with coefficients of variation ranging from 5% to 19.5% across samples (Figure 1).
During the June 2021 CLRN PT scheme, the Quidel Lyra SARS-CoV-2 Assay targeting the ORF1a/b was employed for the first time by three participants (Table 1). This assay was able to correctly identify all test samples (n=18); however, the variability between Ct values was large, with a coefficient of variations ranging from 17.9 to 27.8 (Figure 1). This variation in Ct values is largely attributed to one set of test panel results, which provided substantially lower Ct values than the other participants, indicating differences in threshold settings between participants.
The Seegene Allplex 2019 nCoV Assay is a multiplex RT-PCR assay that detects the E, N and RdRp targets and can be automated for high volume testing. This test performed well during the May 2020 and June 2021 PT schemes demonstrating a 100% concordance and consistent results conveyed by a coefficient of variation less than 10% (Figure 1); however, a number of discordant results were observed during the November 2020 PT scheme, causing subsequent decreases in reproducibility and elevated coefficients of variation. Sample G was associated with n=3/19 E target failures, n=4/19 RdRp target failures and n=1/19 N target failures. While n=2/19 RdRp target failures were associated with the use of a nucleic extraction platform, the remaining failures were associated with a divergence from manufacturer's recommendations and did not employ a nucleic acid extraction step. Furthermore, the reported LOD for the Seegene Allplex 2019 nCoV Assay is approximately 4,000 copies/ml, which is higher than the Sample G titer and is likely responsible for the failure to detect SARS-CoV-2 in this sample Footnote 7 (Table 4). Conversely, Sample I was associated with n=1/19 E target failures and n=2/19 RdRp and N target failures; while Sample K had n=2/19 E target failures, n=3/19 RdRp target failures and n=1/19 N target failures. Sample L, H and J were also associated with one discordant result for each target due to the inability to acquire a valid result. These remaining failures to detect SARS-CoV-2 were all associated with off-label use of not employing a nucleic acid extraction procedure, and are likely the cause of the discordant result since sample titers were all above 4,000 copies/ml. The practice of not implementing an extraction protocol was not observed in the subsequent test scheme. Overall, the E, RdRp and N targets produced discordances of 4.37%, 5.56% and 3.52%, respectively (Figure 1, Table 2).
Two different Roche assays were utilized during the CLRN SARS-CoV-2 PT schemes, Roche Cobas SARS-CoV-2 Test, a fully automated, high throughput assay intended for use with the Roche Cobas 5800/6800/8800, and Roche Cobas SARS-CoV-2 & Influenza A/B Assay for Liat, a fully automated qualitative point of care test to be used on the Cobas Liat. The Roche Cobas SARS-CoV-2 Test for Cobas 5800/6800/8800 was employed during all three test schemes, producing accurate and consistent results with the coefficient of variations less than 3% (Figure 1). The Roche Cobas SARS-CoV-2 Test for Liat accurately detected all test samples from nine users (Table 1 and Table 3). Overall, the Roche Cobas SARS-CoV-2 Test for use on the Cobas 5800/6800/8800 performed the best when comparing commercial platforms across the CLRN SARS-CoV-2 PT schemes; it demonstrated 100% accuracy and produced the most reproducible results across users.
The LDT were also employed during the CLRN SARS-CoV-2 PT Scheme from May 2020 to June 2021. Data sets obtained using LDTs that have at least three sets of submitted results in any given test scheme are presented (Figure 2). In all cases, all tests were able to detect SARS-CoV-2 effectively and accurately from the test samples provided (Figure 2). The E and RdRp targets were used in all test schemes (Table 1). The reproducibility of the E target and RdRp target ranged from coefficients of variation between 3.9% and 8.4% and between 3.2% and 10.2%, respectively (Figure 2). The use of the 5' UTR target emerged during the November 2020 test scheme and results were consistently detected with coefficients of variation less than 7% (Figure 2). Laboratories began employing the N target test during the June 2021 test scheme with coefficients of variation ranging between 6.9% and 9.6% (Figure 2). It should be noted that, apart from the targeted gene, we do not have the specific details regarding the primer/probe sequences implemented by each user and it is possible that the sequences utilized are different. In general, Ct values were similar between all the target tests indicating similar detection affinities; however, a more detailed direct comparative analysis was not conducted, since the assays were not identical. Furthermore, shifts between gene targets are expected, as individual gene expression may differ during viral replication; but this finding could also be attributed to technical variations in the threshold/detection settings by different laboratories. Overall, the 5' UTR target on average demonstrated the most consistent results with an average coefficient of variation of 4.3%, followed by the RdRp (4.7%), E (5.2%) and N (7.9%) targets. All targets performed within designated specifications of coefficients of variation of less than 10%.
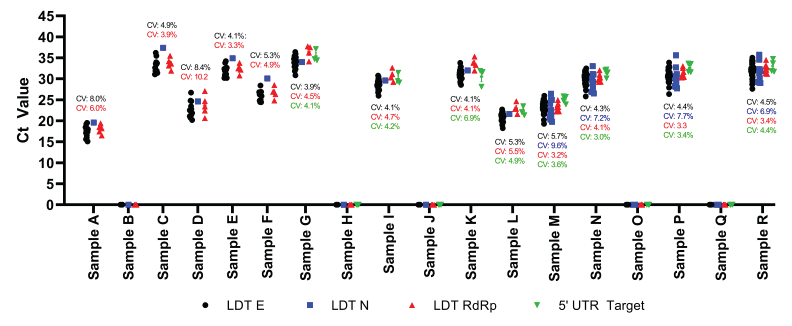
Figure 2 - Text description
This figure presents the data obtained using laboratory develop nucleic acid amplification tests for the detection of SARS-CoV-2. The data presented include the Ct values for each sample based on the target gene that was obtained during the Canadian Laboratory Response Network’s SARS-CoV-2 Proficiency Test Program from May 2020 to June 2021. All laboratory developed tests targeting the E gene (black circle), the N gene (blue square), the RdRp gene (red triangle), and the 5’ untranslated region (green inverted triangle) are presented. Data points located on the x-axis indicate that there was no detectable SARS-CoV-2 RNA present in the sample. The text above the data point is the coefficient of variation (CV) for each respective gene target set using the same representative colour. Within one sample, typically the gene targets cluster together indicating similar detection of the viral RNA. Samples containing low amounts of virus had Ct values between 35 and 40, samples containing moderate amounts of virus had Ct values between 25 and 34, and samples containing high amounts of virus had Ct values less than 25.
Overall, these results provide insights into test sensitivity; each test scheme involved testing a sample, which contained low concentrations of virus particles, ranging from 1,100 to 1,600 copies/ml (Sample C, 1,600 copies/ml, Sample G, 1,100 copies/ml or Sample P, 1,600 copies/ml). Effective test sensitivity was observed across all presented commercial and LDT assays employed across the country. A 100% concordance rate for these low concentration samples was observed for all SARS-CoV-2 targets, with a few exceptions. The BioFire Film Array RP2.1 test kit missed detecting Sample G 1/414 times (Table 3); however, this error occurred due to a procedural mishandling of the sample, and upon repetition for remediation purposes, it was detected. Therefore, this error was not included in the general assessment of sensitivity (Table 3).
The Diasorin Simplexa COVID-19 Direct Molecular Assay missed detecting two low concentration samples, both targets were unable to detect Sample G on two occurrences and the S target failed to detect Sample R (3,500 copies/ml) in one instant (Table 2); however, these discordant results did not cause the 95% limit of detection rate to be affected. The Seegene Allplex 2019 nCoV Assay was associated with a number of failures to detect Sample G. The majority of these failures were attributed to off-label use, where a required nucleic acid extraction process was omitted; for this reason, these results were removed from the subsequent analysis of sensitivity. However, there were two instances associated with proper use, where the RdRp target failed to identify SARS-CoV-2 and were included in the analysis. These discordant results elicited a minor effect on test sensitivity; a 95% detection limit was determined to be 1,358 copies/ml (Table 2). With the exception of the Seegene Allplex 2019 nCoV assay, all other assays had 95% detection limits below 1,100 copies/ml. These observed results are in line with the manufacturers reported limits of detection for their respective assays Footnote 6 Footnote 7 Footnote 8 Footnote 9 Footnote 10 Footnote 11 Footnote 12 Footnote 13 Footnote 14 Footnote 15 Footnote 16. While outside of the scope of the intended use of this PT scheme, this study was not able to calculate the limit of detection for all the assays due to lack of samples below detectable levels and therefore further comparison of assay sensitivity was not possible.
In addition to test sensitivity, specificity of the assays was also assessed during the PT schemes. More specifically, the May 2020 PT scheme focused on positive and negative agreement, while the November 2020 test scheme added a component for the detection of other respiratory pathogens of significance, and finally the June 2021 test scheme built upon the last by including relevant SARS-CoV-2 variants of concern (Table 4). Negative agreement for Sample B was 100% across all platforms. The November 2020 test scheme consisted of two samples, neither of which contained SARS-CoV-2: instead, Sample H contained a moderate dose of influenza A virus (Ct 27) and Sample J contained the negative nasal secretion/UTM matrix only. Sample J had 100% negative agreement across all platforms; however, Sample H demonstrated some inconsistencies when the Cepheid Xpert Xpress SARS-CoV-2 platform was employed. In six instances, according to the manufacturer's instructions for reporting, the N target incorrectly identified the presence of SARS-CoV-2 in a sample that only contained influenza A virus (Table 2). In each circumstance, the Ct values were >40 and suggested that there was some degree of cross reactivity with influenza A virus, as this was never observed with any of the negative samples. Since, all discordant results were above the 40 Ct value, recommendations were made to investigate modifying the Ct cut-off to 40 instead of 45, as recommended by the manufacturer to avoid reporting false positives Footnote 17. Over the course of the three PT schemes, the Cepheid Xpert Xpress SARS-CoV-2 platform had a 100% negative agreement for the E target and a 97% negative agreement for the N target. Negative agreement for Sample O and Q were 100% across all platforms.
All commercial and laboratory developed tests were successfully able to detect the variants of concern. Of note, the Thermo Fisher TaqPath COVID-19 Combo Kit had a drop off in one of its three target genes; the S gene was not able to detect the B.1.1.7 variant, while the other two target genes were successfully identified. According to the manufacturer's recommendations for reporting, a positive result requires n=2/3 targets to have Ct values less than 37; therefore, the loss of the S gene did not impair the assays ability to detect the presence of SARS-CoV-2 in Sample N Footnote 14. Failure of the BioFire Film Array RP2.1 to detect SARS-CoV-2 P.1 was attributed to a technical error and not an assay failure; therefore, this test was not included in the analysis. The BioFire Film Array RP2.1 successfully detected the P.1 variant in all other attempts (n=48).
Overall, test specificity was comparable across all three PT schemes and platforms; a 99.5% negative agreement was observed.
Conclusion
Over the course of three PT schemes conducted across Canada between May 2020 and June 2021, the average score obtained by participants was 99.3%, demonstrating consistent testing between laboratories and testing platforms. Similarly high levels of agreement have been observed internationally. The American Proficiency Institute conducted a study across the United States and reported an overall score greater than 97% Footnote 3. Similarly, the Royal College of Pathologists of Australasia conducted three PT schemes within Australia and New Zealand between March 2020 and November 2020, with an initial score of 75% concordance early in the pandemic but then dramatically increasing to 95% concordance in the two latter test schemes Footnote 2. Finally, a third program from South Korea demonstrated 93% agreement Footnote 1. While each program varied in its sample composition and intended uses, it is encouraging to see that rapid deployment of SARS-CoV-2 testing resulted in consistently high degrees of agreement across the globe.
The ability to support quality assurance of testing measures through the provision of an external PT Program is essential during a novel or emerging public health threat. CLRN provides a framework to support the quality assurance required for the decentralization and increase in testing capacity within Canada. All Canadian public health laboratories follow a quality management program required by their respective jurisdictions, and on-site verification and validation schemes are essential to achieve these processes. Furthermore, the comparison of PT panel results allows for the assessment of various NAAT platforms at different locations across multiple users providing an overall assessment of platform performance. The cumulative performance of the NAAT employed during the three CLRN SARS-CoV-2 PT schemes was 99.3% concordant. A future consideration would be to collect additional data from participants to gain a greater scope of demographics, population statistics and accreditation status. This study demonstrates the rapid and successful implementation of a Canadian PT Program and provided comparative analysis of the various emergency use authorized and laboratory developed tests employed for the detection of SARS-CoV-2.
Authors' statement
CR — Conceptualization, data analysis, writing–original draft, writing–review
KA — Conceptualization, writing–review
CC — Conceptualization, writing–review
Competing interests
None.
Acknowledgments
We would like to thank the following individuals, organizations, and networks involved with the execution of the Canadian Laboratory Response Network (CLRN) SARS-CoV-2 Proficiency Test Program:
The National Microbiology Laboratory: N Bastien (Influenza, Respiratory Virus and Coronavirus Section), Special Pathogens Program, Specimen Shipping/Receiving team, Office of Intellectual Property Management & Business Development, Safety and Environmental Services, and the Emergency Operations Centre.
Canadian Public Health Laboratory Network's Respiratory Virus Infection Working Group: N Bastien (National Microbiology Laboratory, Winnipeg, Manitoba), P Levett (BC Centre for Disease Control Public Health Laboratory, Vancouver, British Columbia), N Zeylas (Alberta Provincial Laboratory for Public Health, Alberta Precision Laboratories, Edmonton, Alberta), A Lang (Roy Romanow Provincial Laboratory, Regina, Saskatchewan), K Dust (Cadham Provincial Laboratory, Winnipeg, Manitoba), J Gubbay (Public Health Ontario Laboratories, Toronto, Ontario), J Fafard and V Dikimpe (Laboratoire de santé publique du Québec, Ste-Anne-de-Bellevue, Québec), G German and V Arseneau (Queen Elizabeth Hospital-Health Prince Edward Island, Charlottetown, Prince Edward Island), J LeBlanc (Queen Elizabeth II Health Science Centre, Halifax, Nova Scotia), Y Yu (Newfoundland and Labrador Public Health Laboratory, St. John's, Newfoundland and Labrador), G Desnoyers (Centre hospitalier universitaire Dr. Georges L. Dumont, Moncton, New Brunswick), K Dionne (Qikiqtani General Hospital, Iqaluit, Nunavut), L Steven (Stanton Territorial Hospital, Yellowknife, Northwest Territories), and P Rodgers (Whitehorse General Hospital, Whitehorse, Yukon).
Funding
This work was supported by the Public Health Agency of Canada.
References
- Footnote 1
-
Sung H, Han MG, Yoo CK, Lee SW, Chung YS, Park JS, Kim MN, Lee H, Hong KH, Seong MW, Lee K, Chun S, Lee WG, Kwon GC, Min WK. Nationwide External Quality Assessment of SARS-CoV-2 Molecular Testing, South Korea. Emerg Infect Dis 2020;26(10):2353–60. https://doi.org/10.3201/eid2610.202551
- Footnote 2
-
Lau KA, Kaufer A, Gray J, Theis T, Rawlinson WD. Proficiency testing for SARS-CoV-2 in assuring the quality and overall performance in viral RNA detection in clinical and public health laboratories. Pathology 2022;54(4):472–8. https://doi.org/10.1016/j.pathol.2022.01.006
- Footnote 3
-
Edson DC, Casey DL, Harmer SE, Downes FP. Identification of SARS-CoV-2 in a Proficiency Testing Program. Am J Clin Pathol 2020;154(4):475–8. https://doi.org/10.1093/ajcp/aqaa128
- Footnote 4
-
Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DK, Bleicker T, Brünink S, Schneider J, Schmidt ML, Mulders DG, Haagmans BL, van der Veer B, van den Brink S, Wijsman L, Goderski G, Romette JL, Ellis J, Zambon M, Peiris M, Goossens H, Reusken C, Koopmans MP, Drosten C. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill 2020;25(3):2000045. https://doi.org/10.2807/1560-7917.ES.2020.25.3.2000045
- Footnote 5
-
Public Health Ontario. Probit Analysis Tool for LOD data. [Accessed 2023 Jan 23]. https://biostats.shinyapps.io/LOD_probit/
- Footnote 6
-
DiaSorin Molecular LLC. Simplexa® COVID-19 Direct Kit. Cypress, CA; Diasorin; 2021. [Accessed 2022 Feb 25]. https://molecular.diasorin.com/us/kit/simplexa-covid-19-direct-kit/
- Footnote 7
-
Seegene Inc. AllplexTM 2019-nCoV Assay (version 2.3; Dec 13, 2022) Instructions for Use. Seoul (KR): Seegene; 2020. [Accessed 2022 Feb 25]. https://www.fda.gov/media/137178/download
- Footnote 8
-
Ag RD. cobas® SARS-CoV-2. Qualitative assay for use on the cobas® 6800/8800 Systems. Roche; 2022; [Accessed 2023 Jan 27]. https://www.fda.gov/media/136049/download
- Footnote 9
-
Hologic. Aptima SARS-CoV-2 Assay (Panther® system). Hologic; 2022. [Accessed 2023 Jan 27]. https://www.fda.gov/media/138096/download
- Footnote 10
-
GeneXpert. Xpert® Xpress SARS-CoV-2 Instructions for Use. Cepheid Innovation; April 2022. [Accessed 2023 Jan 27]. https://www.fda.gov/media/136314/download#:~:text=Insert%20the%20swab%20into%20either,or%203%20mL%20of%20saline
- Footnote 11
-
Molecular Diagnostics BD. BD SARS-CoV-2 Reagents for BD MAX™ System. BD Max; 2022. [Accessed 2023 Jan 27]. https://www.fda.gov/media/136816/download
- Footnote 12
-
Abbott Molecular Inc. Abbott RealTime SARS-CoV-2 Instructions for Use. Abbott; 2022. [Accessed 2023 Jan 27]. https://www.fda.gov/media/136258/download
- Footnote 13
-
Abbott Molecular Inc. Alinity m SARS-CoV-2 AMP Kit. Abbott; 2022. [Accessed 2023 Jan 27]. https://www.fda.gov/media/137979/download
- Footnote 14
-
Thermo Fisher Scientific. Applied Biosystems. TaqPath™ COVID-19 Combo Kit, Instructions for use. Multiplex real-time RT-PCR test intended for the qualitative detection of nucleic acid from SARS-CoV-2. Thermo Fisher; 2020. [Accessed 2022 Feb 25]. https://assets.thermofisher.com/TFS-Assets/LSG/manuals/MAN0019211_TaqPath_COVID-19_IFU_Canada.pdf
- Footnote 15
-
BioFire Diagnostics L. BioFire Respiratory Panel 2.1 (RP2.1). Instructions for Use. Biofire; 2022. https://www.biofiredx.qarad.eifu.online/ITI/CA/en/all?keycode=ITI0105
- Footnote 16
-
Quidel Corp. Lyra® SARS-CoV-2 Assay. Instructions For Use. Quidel; 2022. [Accessed 2023 Jan 27]. https://www.fda.gov/media/136820/download
- Footnote 17
-
Public Health Ontario. An Overview of Cycle Threshold Values and their Role in SARS-CoV-2 Real-Time PCR Test Interpretation. Toronto, ON: PHO; 2020. [Accessed 2023 Jan 27]. https://www.publichealthontario.ca/-/media/documents/ncov/main/2020/09/cycle-threshold-values-sars-cov2-pcr.pdf?la=en
