Burden of disease of RSV in infants, children and pregnant women and people

 Download this article as a PDF (844 KB)
Download this article as a PDF (844 KB)Published by: The Public Health Agency of Canada
Issue: Volume 50–1/2, January/February 2024: Respiratory Syncytial Virus (RSV)
Date published: January/Fabruary 2024
ISSN: 1481-8531
Submit a manuscript
About CCDR
Browse
Volume 50-1/2, January/February 2024: Respiratory Syncytial Virus (RSV)
Overview
Burden of disease of respiratory syncytial virus in infants, young children and pregnant women and people
Elissa M Abrams1,2,3, Pamela Doyon-Plourde1, Phaedra Davis1,4, Nicholas Brousseau5, Andrea Irwin6, Winnie Siu1,4, April Killikelly1*
Affiliations
1 Centre for Immunization Programs, Public Health Agency of Canada, Ottawa, ON
2 University of Manitoba, Department of Pediatrics, Section of Allergy and Clinical Immunology, Winnipeg, MB
3 University of British Columbia, Department of Pediatrics, Division of Allergy and Immunology, Vancouver, BC
4 University of Ottawa, School of Epidemiology and Public Health, Ottawa, ON
5 Institut national de santé publique du Québec, Québec, QC
6 Yukon Communicable Disease Control, Health and Social Services, Government of Yukon, Whitehorse, YT
Correspondence
Suggested citation
Abrams EM, Doyon-Plourde P, Davis P, Brousseau N, Irwin A, Siu W, Killikelly A. Burden of disease of respiratory syncytial virus in infants, young children and pregnant women and people. Can Commun Dis Rep 2024;50(1/2):1–15. https://doi.org/10.14745/ccdr.v50i12a01
Keywords: respiratory syncytial virus, infants, burden of disease, surveillance, epidemiology
Abstract
Background: Passive immunization products for infants and pregnant women and people have sparked interest in understanding Canada's respiratory syncytial virus (RSV) burden. This rapid review examines RSV burden of disease in infants, young children and pregnant women and people.
Methods: Electronic databases were searched to identify studies and systematic reviews reporting data on outpatient visits, hospitalizations, intensive care unit admissions, deaths and preterm labour associated with RSV. We also contacted Canadian respiratory virus surveillance experts for additional data.
Results: Overall, 17 studies on infants and young children and 10 studies on pregnant women and people were included, in addition to primary surveillance data from one Canadian territory (Yukon). There were higher rates of medical utilization for infants than older children. Hospitalization rates were highest in infants under six months (more than 1% annually), with 5% needing intensive care unit admission, but mortality was low. Severe outcomes often occurred in healthy full-term infants and burden was higher than influenza. Respiratory syncytial virus attack rate was 10%–13% among pregnant women and people. Only one study found a higher hospitalization rate in pregnant women and people compared to non-pregnant women and people. Limited evidence was found on intensive care unit admission, death and preterm birth for pregnant women and people.
Conclusion: While risk of severe outcomes is larger in high-risk infants and children, healthcare burden is greatest in healthy term infants. The RSV severity for pregnant women and people appears to be similar to that for non-pregnant women and people.
Introduction
Respiratory syncytial virus (RSV) is a common respiratory virus, affecting nearly all children younger than two years of age Footnote 1. Globally, RSV contributes to 31% of pneumonia cases, causing 33 million acute respiratory infections (ARI), 3.1 million hospitalizations and 118,200 deaths annually Footnote 2. Respiratory syncytial virus ranks as the third leading cause of lower respiratory deaths in children younger than five years of age, after Streptococcus pneumoniae and Haemophilus influenzae type b Footnote 3.
The RSV vaccine landscape has evolved. Previously, only one passive immunization product (palivizumab; a monoclonal antibody) was available for high-risk infants. Canada anticipates at least two new products; nirsevimab, a long-acting monoclonal antibody, and an RSV stabilized pre-fusion subunit protein vaccine for pregnant women and people (Pfizer RSVpreF vaccine, Abrysvo), offering both active and passive immunity for newborns. As the indication for the new passive immunization product includes healthy infants, and as the vaccine for pregnant individuals would protect both healthy and higher-risk infants, there is a need for an understanding of RSV's burden in infants, young children and pregnant women and people.
Throughout this article we will refer to “pregnant women and people” and intend it to be an inclusive term to include people of all gender identities who are pregnant. We recognize this language is evolving and our aim is to use language that removes barriers to care.
While a recent review focused on high-risk infants (including prematurity, cardiopulmonary disease and immunocompromised), less data exists on RSV's burden in healthy infants and young children in Canada Footnote 4. To inform recommendations for RSV prevention, we conducted literature reviews on RSV's burden focusing on healthy infants (younger than 12 months of age) and young children (12–24 months of age). Since one approach involves vaccinating pregnant women and people, we also explored RSV's burden in this group. This rapid review aims to summarize the available evidence on RSV burden of disease in infants, young children and pregnant women and people in Canada and other high-income countries.
Methods
Search strategies
Three search strategies were developed by a research librarian from Health Canada and the Public Health Agency of Canada. One focused on systematic reviews (SR) of RSV burden in infants and young children (Supplemental material S1). Two targeted RSV burden in pregnant women and people, with one concentrating on primary evidence studies and the other on SRs (Supplemental material S2). Embase, MEDLINE, Global Heath and ProQuest Public Health databases were searched for studies published from January 1, 1995, to April 10, 2023. We also contacted Canadian respiratory virus surveillance experts for additional data. After removal of duplicates, references were uploaded in DistillerSR online software (Evidence Partners, Ottawa, Ontario).
Study selection
Two reviewers (for pregnant women and people and for infants and young children) screened titles and abstracts for study eligibility. The articles pertaining to infants and young children focused on healthy infants younger than 12 months and healthy young children 12–24 months of age but did not exclude articles that captured high-risk infants. Full texts of selected articles were then evaluated. A second independent reviewer assessed citations marked for exclusion, with disagreements resolved through discussion. The reference lists of included studies were also screened for relevant articles on RSV burden in high-income countries including Canada and the United States (US) for infants and young children; due to a paucity of data, we did not restrict articles pertaining to pregnant women and people to high income countries.
Eligibility criteria
Observational studies, randomized controlled trials (RCTs) and SRs that met the criteria outlined in Table 1 were included. Inclusion was limited to studies conducted after 1995 to capture the most recent evidence. The evaluation of RSV burden focused on clinical outcomes of interest in infants, young children and pregnant women and people and considered emergency department (ED) or outpatient visits, hospitalizations, intensive care unit (ICU) admissions, death and preterm labour associated with RSV.
| PICOS | Inclusion | Exclusion |
|---|---|---|
Population |
Infants and children (focus on 24 months of age and younger) |
Adults only |
Intervention |
N/A |
N/A |
Control |
N/A |
N/A |
Outcome |
Emergency department or other ambulatory visits due to RSV |
Outcome not associated with RSV infection |
Study design |
Systematic reviews and/or meta-analyses |
Narrative reviews |
|
||
Data extraction and data synthesis
One reviewer extracted data from each article, verified by a second reviewer. Disagreements were resolved through discussion. Data included event number, sample size and effect measures. Results were synthesized narratively based on the study population and outcomes. Due to the value of Canadian data on RSV's burden in healthy infants and young children, surveillance data from one territory (Yukon Communicable Disease Control) were included in this literature review.
Results
Infants and young children
Study selection: After deduplication, 389 references underwent screening (Figure 1). Seventeen articles, including five SRs, were incorporated into the narrative synthesis of RSV burden in infants and young children (Table 2).
Figure 1: Study selection PRISMA flow diagram of infants and young children
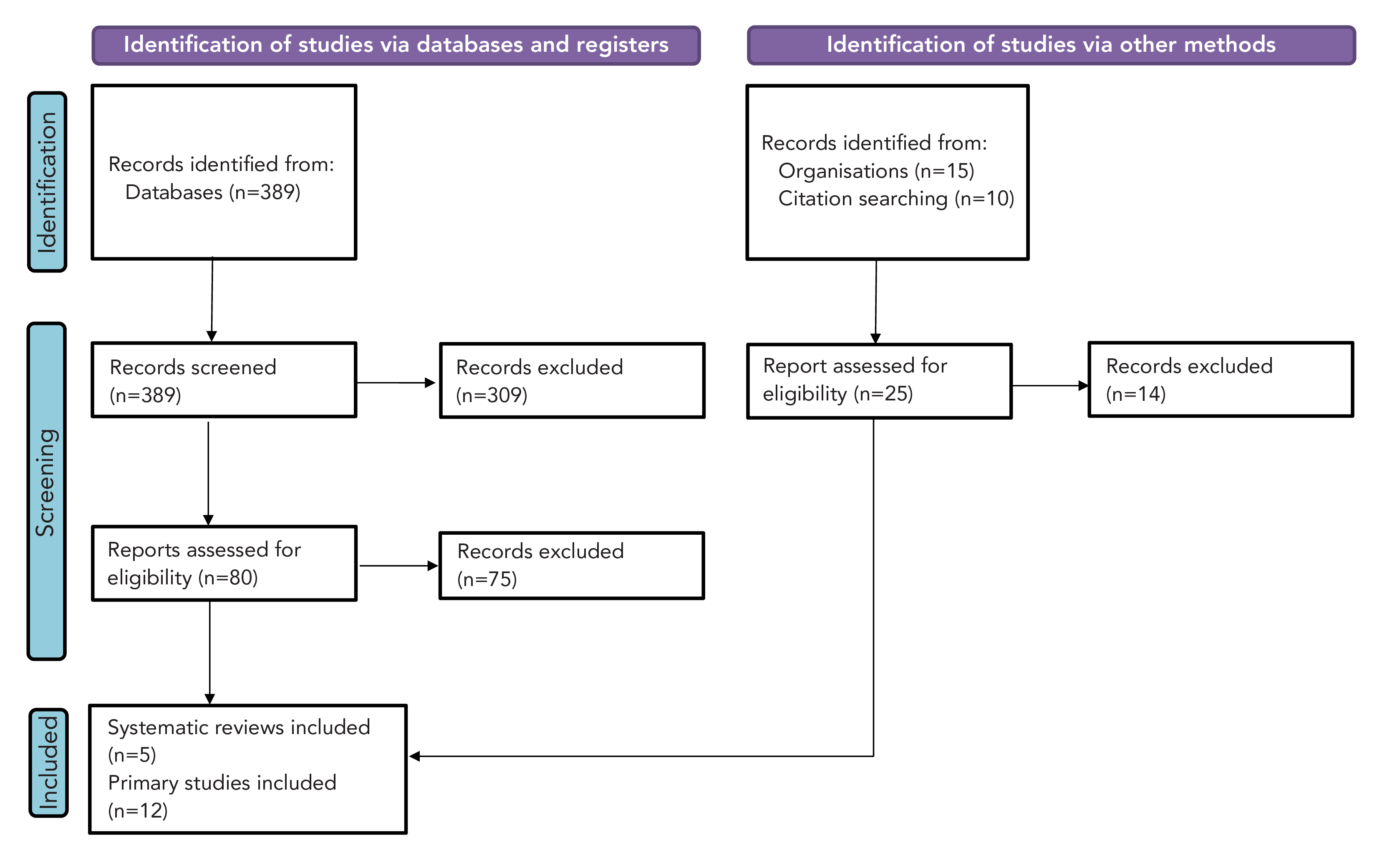
Figure 1 - Text description
This figure shows the process for identification of studies for infants and young children. There were 389 studies identified from databases and 25 from other methods. From the databases, 80 were assessed for eligibility and 75 excluded. From other methods, 25 were assessed for eligibility and 14 excluded. In total, five systematic reviews and 12 primary studies were included.
| Author, year (reference), country | Study design | Study period | Population | Outcome definition | Results |
|---|---|---|---|---|---|
| Medically attended RSV respiratory tract infection | |||||
Hall et al., 2009 Footnote 5 |
Prospective population-based surveillance study (NVSN) |
October 2000 to September 2004, during the winter months (November–April) |
Children under five years of age and had received a diagnosis of acute respiratory infection (n=5,067) |
Specimens were defined as positive if RSV was detected by viral isolation or by duplicate RT-PCR assays |
Of 5,067 participants, 1,014 (20%) were treated in ED and 1,161 (23%) were treated in paediatric offices:
18% of ED visits (184/1,014) and 15% of paediatric office visits (171/1,161) were RSV-associated |
Bourgeois et al., 2009 Footnote 6 |
Prospective cohort study |
2003–2005 |
Children seven years of age and younger and treated in the ED for an acute respiratory infection (n=895) |
Nasopharyngeal specimens were considered RSV-positive if RSV was detected through direct immunofluorescent antibody stain and/or RT-PCR |
ED visit rates:
Children 0–23 months: 64.4 ED visits per 1,000 attributable to RSV |
Wildenbeest et al., 2023 Footnote 7 |
Multicentre, prospective birth cohort study |
2017/07/01–2020/07/31 |
Healthy term infants, defined as children born at 37 weeks or more of gestation with no evidence of significant disordersTable 2 footnote a, were included in the active surveillance cohort (n=993) |
An RSV-positive ARI episode was defined as a positive test result from either in-house RT-qPCR or POCT or both |
Medically attended RSV-positive ARI:
RSV-positive ARI:
|
Li et al., 2022 Footnote 8 |
Systematic review of studies published 2017/01/01–2020/12/31 |
2019 or before (i.e., before the onset of the COVID-19 pandemic) |
Children 0–60 months of age |
RSV-associated acute lower respiratory infection was defined as acute lower respiratory infection with lab-confirmed RSV infection RSV-attributable acute lower respiratory infection was defined as acute lower respiratory infection that could be causally attributable to lab-confirmed RSV-infection |
Incidence rate (UR) of RSV-associated acute lower respiratory infection in high-income regions (number of studies):
|
| RSV respiratory tract infection with hospitalization | |||||
Schanzer et al., 2006 Footnote 9 |
Retrospective population-based study |
September 1994 to August 2000 (six influenza seasons, 1994/1995–1999/2000) |
Hospitalized children younger than 19 years old |
Diagnostic codes (ICD-9) selected based on their association with viral respiratory illness in children. RSV-attributable bronchiolitis admissions provided a better proxy for RSV activity than RSV positive specimens alone |
RSV rates were highest in infants younger than six months of age at approximately 2,000 per 100,000 |
Papenburg et al., 2012 Footnote 10 |
Prospective cohort study |
Four consecutive winter seasons (2006/07–2009/10) |
Children aged 0–35 months presenting as outpatients to paediatric clinic or hospitalized for RTI (n=1,039 episodes; 305 in the clinic and 734 in the hospital) |
PCR/DNA microarray hybridization assay Hospitalization was defined as admission for more than 24 hours to a short-stay unit, paediatric ward or PICU |
RSV was the most frequently identified virus in infants and young children in hospital (n=467/734, 63.6%) with age younger than six months and prematurity associated with severe RSV cases among hospitalized children |
Gilca et al., 2020 Footnote 11 |
Retrospective cohort study |
2012/11/01–2019/06/30 |
Nunavik infants less than one year of age hospitalized for a respiratory illness (ICD-10 codes J00-J22 at any point, n=354) |
RSVH was defined as hospitalization lasting 24 hours or longer with at least one positive RSV specimen collected during hospitalization or within four days prior to admission |
113 (25%) of 458 episodes had RSV; annual average was 2.5 RSV-positive hospitalizations in high-risk infants and 16 RSV-positive hospitalizations in healthy full-term infants The overall RSVH rate per 1,000 live births in children younger than one year of age (adjusted for missed cases):
|
Piesky et al., 2016 Footnote 12 |
Retrospective chart review |
2010/01/01–2011/12/31 |
Children younger than three years of age residing within the Ottawa region potentially hospitalized for RSV (true positive cohort: n=1,119, and annual incidence estimates: n=19,815) |
RSV hospitalization was defined as a positive test for RSV within 72 hours of admission and if the signs and symptoms responsible for hospital admission were consistent with RSV pathophysiology |
Hospital admissions in children attributable to RSV:
Incidence of RSV hospitalization per 1,000 children from 2005 to 2012:
|
Buchan et al., 2023 Footnote 13 |
Population-based birth cohort study |
First hospitalization in children born between 2009/05–2019/06 |
Children born May 2009 to June 2015 (n=826,140) |
RSV hospitalizations were identified using a validated algorithm based on ICD-10 codes and/or laboratory-confirmed outcomes |
12,573 (1.4%) incident cases of RSV hospitalization Rate of RSV-hospitalization per 1,000 patient-year (95% CI):
RSV hospitalization rates varied inversely with gestational age |
McLaughlin et al., 2022 Footnote 14 |
Systematic review and meta-analysis |
Studies identified were published 2000–2020, and reported and collected 1989–2016 |
Children younger than five years of age (n=25 studies gave 31 estimates) |
RSV hospitalization:
|
Pooled rate of RSV-associated hospitalization per 1,000 (95% CI), n=31:
|
Stein et al., 2017 Footnote 15 |
Systematic review and meta-analysis |
Studies published 2000–2015 |
Children younger than five years of age not receiving RSV immunoprophylaxis with palivizumab (n=55 studies, of those 34 reported on hospitalization for severe RSV-ARI) |
Case of severe ARI included hospitalized ARI or hospitalized lower or acute lower respiratory infection, pneumonia, and bronchitis |
RSV-associated ARI hospitalization per 1,000 children-year (95% CI), (number of studies):
|
Suh et al., 2022 Footnote 16 |
Systematic review |
Studies published 2000/01/01–2021/06/11 (data 1979–2020) |
Studies of US infants younger than one year of age with clinical sequelae of RSV, and bronchiolitis (n=141 studies) |
Lab-confirmed or ICD diagnostic codes for RSV hospitalization or bronchiolitis hospitalization |
Five studies provided nationally representative data on annual average RSVH rates per year ranging from 11.6 (95% CI: 6.9–16.3) per 1,000 per year among infants 6–11 months of age to 50.1 (95% CI: 35.6–64.6) per 1,000 per year among infants 0–2 months of age |
Wingert et al., 2021 Footnote 4 |
Rapid review |
Studies published 2014/01/01–2018/09/06 |
Children 24 months of age and younger, with or without a risk factor of interest, or immunocompromised children 18 years of age and younger without palivizumab prophylaxis with lab-confirmed RSV infection (n=29 cohort studies) |
Lab-confirmed RSV-hospitalization, ICU admission, oxygen support, mechanical ventilation, extracorporeal membrane oxygenation, case fatality and complications from RSV infections (e.g., secondary infection) |
RR (95% CI) RSV-hospitalization, (number of studies):
|
Li et al., 2022 Footnote 8 |
Systematic review of studies published 2017/01/01–2020/12/31 |
2019 or before (i.e., before the onset of the COVID-19 pandemic) |
Children 0–60 months of age |
RSV-associated acute lower respiratory infection was defined as acute lower respiratory infection with lab-confirmed RSV infection RSV-attributable acute lower respiratory infection was defined as acute lower respiratory infection that could be causally attributable to lab-confirmed RSV-infection |
Hospital admission rate per 1,000 children per year due to RSV-associated acute lower respiratory infection in high income countries (number of studies):
|
Bont et al., 2016 Footnote 17 |
Systematic review |
Studies published 1995/01/01–2015/12/31 |
Children 18 years or younger |
Hospitalization for RSV-related ARI or RSV-related bronchiolitis |
RSV was associated with 19%–81% of all viral ARIs causing hospitalization Annual hospitalization rates per 1,000 children per year for RSV-associated ARIs:
More than 70% of children hospitalized with RSV-associated ARIs had no underlying medical conditions Compared to influenza, RSV causes up to 16 times more hospitalizations and ED visits in children younger than five years |
| RSV respiratory tract infection with intensive care unit admissions | |||||
Papenburg et al., 2012 Footnote 10 |
Prospective cohort study |
Four consecutive winter seasons (2006/07–2009/10) |
Children aged 0–35 months presenting as outpatients to paediatric clinics or hospitalized for RTI (n=1,039 episodes; 305 in the clinic and 734 in the hospital) |
PCR/DNA microarray hybridization assay Hospitalization was defined as admission for more than 24 hours to a PICU |
63.6% (n=467) were RSV-positive hospitalization 5.2% (n=24/460) of hospital admissions for RSV had ICU admission (similar for hMPV) |
Piesky et al., 2016 Footnote 12 |
Retrospective chart review |
2010/01/01–2011/12/31 |
Children younger than three years of age residing within the Ottawa region potentially hospitalized for RSV (true positive cohort: n=1,119) |
RSV hospitalization was defined as a positive test for RSV within 72 hours of admission and if the signs and symptoms responsible for hospital admission were consistent with RSV pathophysiology |
Of hospitalized cohort, 5.6% (95% CI: 5.2–5.9) were admitted to PICU and 3.1% (95% CI: 2.9–3.3) were intubated |
Buchan et al., 2019 Footnote 18 |
Retrospective multicentre cohort study |
2009/05/01–2014/05/31 |
Hospitalized children aged 0–59 months tested for respiratory viruses including RSV (n=6,364) |
Monoplex or multiplex PCR, viral culture or direct immunofluorescence |
ICU admission:
|
Buchan et al., 2023 Footnote 13 |
Population-based birth cohort study |
First hospitalization in children born 2009/05–2019/06 |
Children born between May 2009 and June 2015 (n=826,140) |
RSV hospitalizations were identified using a validated algorithm based on ICD-10 codes, and/or laboratory-confirmed outcomes |
8.1% required intensive care during their hospitalizations (from 22% in those fewer than 28 weeks to 7% in those 37 weeks or more gestational age) |
Amini et al., 2019 Footnote 19 |
Prospective surveillance study |
Peak weeks of five influenza seasons (2012/2013, 2014/2015–2017/2018) |
Children younger than 24 months hospitalized with respiratory symptoms (n=546) |
Multiplex PCR |
ICU admissions rates (p=0.07):
|
Wildenbeest et al., 2023 Footnote 7 |
Multicentre, prospective birth cohort study |
2017/07/01–2020/04/01 |
Healthy term infants, defined as children born 37 weeks or more of gestation with no evidence of significant disordersTable 2 footnotea, were included in the active surveillance cohort (n=993) |
Parental questionnaire and hospital chart reviews, active RSV surveillance in nested cohort |
Eight PICU admissions, corresponding to 5.5% of 145 RSV-associated hospitalizations and 0.09% of the total cohort Six of eight infants admitted to the ICU were younger than three months of age (median one month) |
Suh et al., 2022 Footnote 16 |
Systematic review |
Studies published 2000/01/01–2021/06/11 (data 1979–2020) |
Studies of US infants younger than one year of age with RSV, clinical sequelae of RSV and bronchiolitis (n=141 studies) |
RSV and bronchiolitis defined as lab-confirmed and/or ICD codes |
No studies reported nationally representative data. Twenty-two studies reported proportions of ICU admissions among RSV hospitalized infants (range: 6.3%–71.4%) Higher ICU admissions were observed in younger vs. older infants (up to 64.3% in those younger than six months vs. 54.5% in those six months and older; 2013–2016), preterm vs. full-term infants (52.2% vs. 33.3%; 1992–2017) From 2003 to 2007, 21.8% of infants with CHD and 13.3% of infants with CLD hospitalized for RSV had ICU admissions |
| RSV respiratory tract infection with death | |||||
Schanzer et al., 2018 Footnote 20 |
Retrospective population-based study |
September 2003 to August 2014 (nine influenza seasons, excluding the 2008/2009 and 2009/2010 seasons) |
All patients admitted to an acute care hospital for a respiratory condition |
Hospitalization with an ICD-10 code for RSV (J12.1, J20.5, J21.0, B97.4) |
RSV-attributed inpatient death rate: 0.6 (95% CI: −0.1–1.3) per 100,000 population (not limited to paediatric) |
Buchan et al., 2023 Footnote 13 |
Population-based birth cohort study |
First hospitalization in children born 2009/05–2019/06 |
Children born May 2009 to June 2015 (n=826,140) |
RSV hospitalizations were identified using a validated algorithm based on ICD-10 codes and/or laboratory-confirmed outcomes |
12,573 (1.4%) incident cases of RSV hospitalization A small proportion of those (0.2%) died within 30 days of discharge |
Reichert et al., 2022 Footnote 21 |
Population-based birth cohort study |
1999–2018 |
All infants born to residents of the US and those who died at younger than one year of age with RSV, bronchiolitis or influenza as the cause of death (n=80,764,705 live births, 510,502 total infant deaths from all causes) |
RSV was defined by at least one ICD-10 cause of death codes: B97.4 (RSV), J12.1 (RSV, influenza), J20.5 (acute bronchitis due to RSV) and J21.0 (acute bronchiolitis due to RSV) |
The overall infant mortality rates from 1999 to 2018:
Infant RSV mortality rates by birth year from 2008 to 2018 ranged from 8.1 (95% CI: 5.5–11.4) to 3.4 (95% CI: 1.9–5.7) per 1,000,000 live births Infant RSV mortality rates among younger than 29 wGA infants was 103.5 (95% CI: 81.8–129.1) RSV mortality burden was greatest in full-term (53.7%) infants |
Li et al., 2022 Footnote 8 |
Systematic review of studies published 2017/01/01–2020/12/31 |
2019 or before (i.e., before the onset of the COVID-19 pandemic) |
Children 0–60 months of age |
RSV-associated acute lower respiratory infection was defined as acute lower respiratory infection with lab-confirmed RSV infection RSV-attributable acute lower respiratory infection was defined as acute lower respiratory infection that could be causally attributable to lab-confirmed RSV-infection |
Case fatality rate of in-hospital deaths in high-income countries for children 0–12 months with RSV-associated acute lower respiratory infection: 0.1% (95% CI: 0.1–0.3) (n=29 studies) |
|
|||||
Medically attended RSV respiratory tract infection: Three prospective observational studies demonstrated a high incidence of medically attended RSV infections. A US-based surveillance system from 2002 to 2004 found that RSV accounted for 18% of ED visits and 15% of office visits for ARI from November through April with higher rates in infants Footnote 5. More than 70% of the outpatients were previously healthy. Another US study from 2003 to 2005 reported 21.5 RSV-related ED visits per 1,000, higher than influenza (n=10.2 per 1,000), particularly in children younger than 24 months (n=64.4 visits per 1,000) Footnote 6. A European birth cohort in healthy term infants from 2017 to 2020 found a 26.2% (95% confidence interval [CI]: 24.0–28.6) RSV infection incidence and 14.1% (95% CI: 12.3–16.0) medically attended RSV incidence during the first year of life Footnote 7. Global data for children younger than five years aligned with these findings, reporting 38.5 (95% CI: 21.6–68.8) RSV-associated ARI per 1,000 children younger than one year of age in high-income countries Footnote 8.
Over five respiratory seasons, from 2018 to 2023, in Yukon, there were a total of 73 RSV infections in children 24 months and younger, which was higher than the number of influenza infections (n=20). Among infants younger than 12 months of age, the highest number of RSV infections occurred in those younger than three months of age. In summary, medically attended RSV infections are significant during infancy and early childhood, with approximately 10%–20% of infants seeking care for RSV in a season, surpassing medically attended influenza.
Hospitalization associated with RSV respiratory tract infection: Several Canadian studies highlight a substantial incidence of RSV-related hospitalizations in infants and young children. Schanzer et al.'s pan-Canadian study showed RSV as a major cause of hospitalization (n=130 per 100,000), with the highest rates in infants younger than six months Footnote 9. Papenburg et al.'s Québec-based study found RSV was the most common virus (63.6%) in children hospitalized for ARI, with higher severity linked to age under six months and prematurity Footnote 10. In Nunavik, RSV hospitalization rates were higher in high-risk infants (147.6 per 1,000 live births) compared to healthy term infants (n=64.8 per 1,000) Footnote 11. An Ontario study by Pisesky et al. reported RSV hospitalization rates of 10.2 per 1,000 children younger than one year and 4.8 per 1,000 in children one to three years of age Footnote 12. Buchan et al.'s Ontario cohort study revealed varying RSV hospitalization rates across age groups, with the highest in one-month-olds (n=29.55 per 1,000) and declining with age, with rates highest among children born at younger gestational ages Footnote 13. Over five respiratory seasons from 2018 to 2023 in Yukon, there were 27 severe RSV cases (non-ICU hospitalizations, ICU admissions and deaths), of which 18 were non-ICU hospitalizations. During that same time period, in children 24 months and younger, the number of severe RSV (n=27) cases was higher than the number of severe influenza cases (n=7).
Authors of systematic reviews and meta-analyses have also examined RSV hospitalization rates in infants and young children. McLaughin et al. reported US rates of 26.2 (95% CI: 24.2–28.2) and 19.4 (95% CI: 17.9–20.9) per 1,000 infants younger than six months and younger than 12 months, respectively Footnote 14. Stein et al. found rates of 20.01 (95% CI: 9.65–41.31) and 19.19 (95% CI: 15.04–24.48) per 1,000 children-years in the same groups Footnote 15. United States national studies reported annual RSV hospitalization rates ranging from 11.6 to 50.1 per 1,000 among infants Footnote 16. A rapid review showed varying incidence rates, from 1.2% in healthy term infants to 2.8%–5.1% in preterm infants Footnote 4. Global analysis found similar rates in high-income countries, with 28.4 (95% CI: 20.2–40.0) and 22.0 (95% CI: 17.1–28.4) per 1,000 in infants younger than six months and 12 months, respectively Footnote 8. A systematic review identified that RSV was associated with 19%–81% of all viral ARI causing hospitalization Footnote 17. While rates varied by a factor of 2–3 over seasons, they decreased significantly with increasing age. The majority (more than 70%) of children hospitalized had no underlying risk factors. Compared to influenza, RSV caused up to 16 times more hospitalizations in children younger than five years of age Footnote 17. In summary, RSV-related hospitalization rates vary by age and risk factors, with consistent trends of decreasing rates with increasing age. Despite vulnerability in high-risk groups, the majority of hospitalized children have no underlying medical conditions and RSV tends to lead to more hospitalizations compared to influenza.
Intensive care unit admission associated with RSV respiratory tract infection: Canadian studies indicate that approximately 5% of RSV-hospitalized children required ICU admission. Papenburg et al. found that 5.2% needed ICU care Footnote 10, while Pisesky et al. reported 5.6% ICU admission among RSV-hospitalized children younger than three years Footnote 12. Buchan et al. reported 5% ICU admission for healthy children younger than five years, increasing to 10% with comorbidities Footnote 18. In their 2023 study, ICU admission reached 8.1% among RSV-hospitalized children under five, with higher rates for premature births Footnote 13. In one Canadian study, ICU admission was more common with RSV compared to influenza Footnote 19.
International data align with this rate. A European birth cohort study in healthy term infants found 5.5% of RSV-associated hospitalizations led to ICU admissions Footnote 7. An SR of RSV disease in the US identified ICU admission proportions ranging from 6.3% to 71.4% and linked risk factors to younger age, prematurity, congenital heart disease and chronic lung disease Footnote 16. In summary, Canadian research suggests approximately 5% of RSV-hospitalized children required ICU admission, with higher rates among those with risk factors. In comparison to influenza, there is some evidence that RSV leads to more ICU admissions.
Death associated with RSV respiratory tract infection: Existing literature suggests a low risk of RSV-related mortality in both Canada and the US. An overall mortality rate of 0.6 per 100,000 population was reported by Schanzer et al.'s 2018 Canadian model of all patients in Canada admitted to hospital with a respiratory condition (from infancy to older than 65 years) Footnote 20. Buchan et al.'s 2023 Ontario cohort found a 0.2% mortality rate within 30 days of discharge from RSV hospitalization Footnote 13. In the US, an infant cohort followed from 1999 to 2018 showed an RSV mortality rate of 6.9 (95% CI: 6.4–7.5) per one million live births, with preterm infants at the highest risk Footnote 21; however, the majority of deaths occurred in full-term infants (53.7%), primarily those between one and four months of age (63.8%). Globally, a systematic analysis reported a 0.1% (95% CI: 0.1–0.3) case fatality rate for in-hospital RSV deaths in children 0–12 months of age Footnote 8.
Pregnant women and people
Study selection: After removing duplications, 474 primary evidence studies and 28 systematic reviews underwent screening (Figure 2). In total, two SRs and eight studies were included in the narrative synthesis of RSV burden in pregnant women and people (Table 3). No data on RSV-related mortality was identified in pregnant women and people.
Figure 2: Study selection PRISMA flow diagram in pregnant women and people
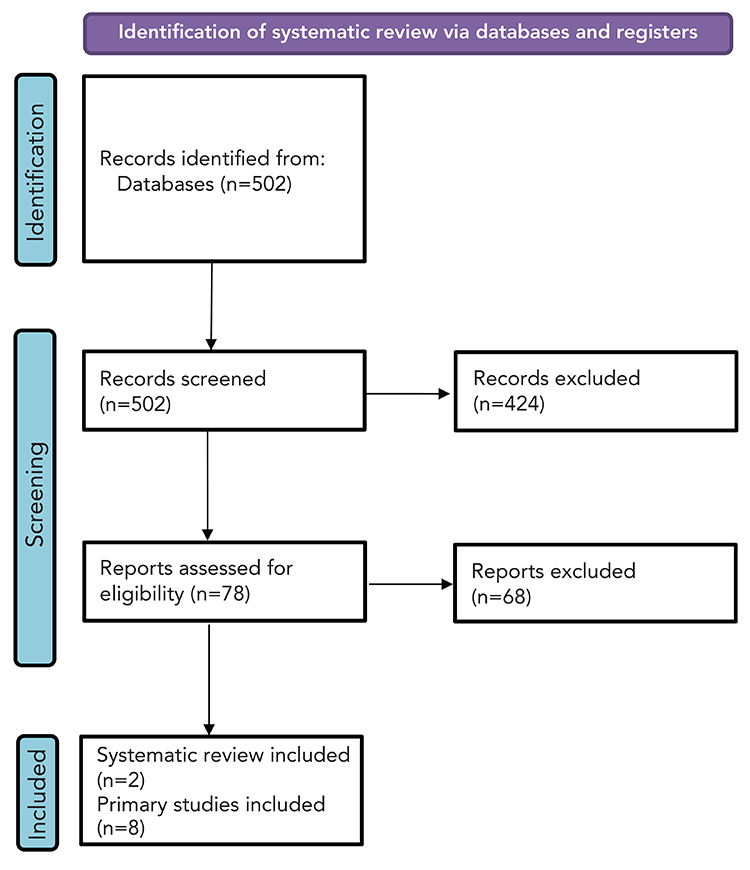
Figure 2 - Text description
This figure shows the process for identification of studies of pregnant women and people. There were 502 records identified, of which 424 were excluded and 78 were assessed for eligibility. Of these, 68 were excluded. In total, two systematic reviews and eight primary studies were included.
| Author, year (reference), country | Study design | Study period | Population | Outcome definition | Results |
|---|---|---|---|---|---|
| Medically attended RSV respiratory tract infection | |||||
Hause et al., 2019 Footnote 22 |
Cross-sectional study |
2015/11/03–2016/05/10 |
Pregnant women and people in their 2nd or 3rd trimester enrolled prospectively during their regular prenatal visits (n=155) |
Lab-confirmed acute respiratory illness |
Seven of 65 (11%) pregnant women and people with ARI at their initial enrollment and eight of 77 (10%) pregnant women and people with ARI during the study period (initial or re-enrollment) had PCR-confirmed RSV infection Four (50%) PCR-confirmed RSV ARI cases reported symptoms of a LRTI, one was hospitalized RSV had an attack rate of 10%–13% among ambulatory pregnant women and people receiving routine prenatal care during the respiratory virus season |
Hause et al., 2018 Footnote 23 |
Cross-sectional study |
2015/10/01–2016/05/10 |
Pregnant women and people in their 2nd or 3rd trimester enrolled prospectively during their regular prenatal visits (n=155) |
RSV infection was determined by PCR or serology |
Of the 81 ARI cases, 52 (64%) respiratory pathogens were detected: The most frequently detected viruses were rhinovirus (n=22; 27%), coronavirus (n=14; 17%) and RSV (n=8; 10%) 12 patients had fever; 17 had symptoms of LRTI Of the seven cases with fever in the ALRTI group, three were RSV-positive (one had HRV coinfection) Of those patients with LRTI, two reported decreased fetal heart rate and one RSV-positive case was hospitalized for respiratory illness |
| RSV respiratory tract infection with hospitalization | |||||
Regan et al., 2018 Footnote 24 |
Retrospective database study |
2010–2016 |
Pregnant women and people aged 18–50 years who were admitted to hospital with an ARFI (n=1,604,206 pregnant women and people) |
An RSV-positive ARFI hospitalization was defined as a positive RT-PCR test result within three days of hospital admission |
13,694 hospitalized acute respiratory tract/febrile illness; 846 tested for RSV and influenza 2.5% (n=21) tested positive for RSV 51% (n=430) tested positive for influenza Fewer than 1% tested positive for both influenza and RSV |
Nowalk et al., 2022 Footnote 25 |
Population-based retrospective aggregate cohort study |
2015/09/01–2018/08/31 |
Adults ages 18–64 years, 65 years and older and including pregnant women and people (n=13,174 pregnant women and people) |
Aggregate data used to determine population-based RSV hospital burden |
RSV burden of hospitalization ranged from 0 to 808 per 100,000 pregnant women and people:
Average burden from 2015 to 2018 of 620/100,000 in pregnant women and people which was higher than the burden for non-pregnant adults 18 years and older (n=320/100,000) |
Hause et al., 2021 Footnote 26 |
Retrospective case series |
2010/08/01–2017/04/30 |
Pregnant women and people aged 14–49 years who tested positive for RSV and were hospitalized for RSV infection during pregnancy (n=10) |
Variable |
275,349 pregnant women and people; 1,057 tested for RSV; 25 (2%) tested positive; 10 hospitalized during pregnancy and tested positive within two weeks prior to or during hospitalization Diagnoses: pneumonia/atelectasis (n=5), upper respiratory tract infection (n=2), asthma exacerbation (n=2), respiratory failure (n=2), sepsis (n=2) Six had obstetrical complications (one exacerbation of pre-existing short cervix with preterm labour, three preterm contractions (two of which had co-infections), one induction for preeclampsia); one preterm birth; one ICU admission/mechanical ventilation |
| RSV respiratory tract infection with intensive care unit admissions | |||||
Hause et al., 2021 Footnote 26 |
Retrospective case series |
2010/08/01–2017/04/30 |
Pregnant women and people whose pregnancy ended in live birth (n=10) |
Hospitalization during pregnancy and positive RSV test by culture or PCR |
275,349 pregnant women and people; 1,057 tested for RSV; 25 (2%) tested positive; 10 hospitalized during pregnancy and tested positive within two weeks prior to or during hospitalization One of 10 (10%) required ICU admission and mechanical ventilation |
Wheeler et al., 2015 Footnote 27 |
Case series |
Winter 2014 |
Antepartum RSV infection treated at single tertiary care facility (n=3) |
N/A |
Two of three cases required ICU admission and mechanical ventilation; all three cases complicated by pre-existing lung conditions (asthma, comorbid influenza, group A streptococcus infection) |
Deshmukh et al., 2014 Footnote 28 |
Case report |
Not stated |
40-year-old pregnant person (n=1) |
N/A |
Pregnant person admitted to hospital in UK requiring ICU admission, mechanical ventilation, and emergency C-section at 33 weeks for maternal reasons (RSV pneumonitis and sepsis) |
| Preterm labour/birth with RSV infection | |||||
Regan et al., 2018 Footnote 24 |
Retrospective database study |
2010–2016 |
Pregnant women and people 18–50 years of age who were admitted to hospital with an ARFI (n=1,604,206 pregnant women and people) |
An RSV-positive ARFI hospitalization was defined as a positive RT-PCR test result within three days of hospital admission |
13,694 hospitalized acute respiratory tract/febrile illness; 846 tested for RSV; 2.5% (n=21) tested positive by RT-PCR No difference in preterm, small-for-gestational age and low birth weight births between RSV-positive and RSV-negative participants Association observed between RSV positivity and subsequent preterm birth (p=0.034):
|
Chu et al., 2016 Footnote 29 |
Prospective randomized trial |
April 2011 to May 2014 |
Pregnant women and people in the 2nd trimester of pregnancy and followed until six months postpartum (n=3,693; 14 RSV illness episodes over 3,554 person-year surveillance) |
RSV-positive tests were determined by rt-PCR |
Seven (50%) pregnant participants sought care for RSV illness; none died Of the seven (50%) illness episodes during pregnancy, all had live births with two (29%) preterm births and a median birth weight of 3,060 grams. This compares to 469 (13%) preterm births and a median birth weight of 2,790 grams in persons without RSV during pregnancy |
Hause et al., 2021 Footnote 26 |
Retrospective case series |
2010/08/01–2017/04/30 |
Pregnant women and people whose pregnancy ended in live birth (n=10) |
Hospitalization during pregnancy and positive RSV test by culture or PCR |
275,349 pregnant women and people; 1,057 tested for RSV; 25 (2%) tested positive; 10 hospitalized during pregnancy and tested positive within two weeks prior to or during hospitalization One of 10 (10%) participants had pneumonia and preeclampsia and was induced between 36 and 37 weeks |
|
|||||
Medically attended RSV respiratory tract infection: Two US cross-sectional studies by Hause et al. investigated RSV infection rates in pregnant women and people in their second or third trimester during the 2015–2016 RSV season. In one study, with combined PCR and serological data, the RSV attack rate among ambulatory pregnant women and people receiving routine prenatal care was estimated at 10%–13% Footnote 22. In the second study, approximately 10% of acute lower respiratory tract illness cases in pregnant women and people were confirmed as RSV Footnote 23.
Hospitalization associated with RSV respiratory tract infection: The literature on RSV-associated hospitalizations presents a broad range of rates. A retrospective study within the Pregnancy Influenza Vaccine Effectiveness Network (PREVENT) 2010–2016 found a 2.5% RSV-positive rate, contrasting with a 51% influenza-positive rate Footnote 24. A US population-based study from 2015 to 2018 revealed higher hospitalization rates among pregnant women and people compared to non-pregnant adults (average rate of 620 vs. 320 per 100,000) Footnote 25. Additionally, one retrospective case series documented adverse pregnancy outcomes in ten pregnant individuals hospitalized with RSV, including pneumonia, respiratory failure and sepsis, with six experiencing obstetrical complications during hospitalization, including preterm contractions, coinfections and preeclampsia Footnote 26. In summary, the literature suggests a wide range of possible RSV hospitalization rates among pregnant women and people, with one study indicating a higher burden compared to non-pregnant adults.
Intensive care unit admission associated with RSV respiratory tract infection: Evidence on RSV-related ICU admissions is limited. In a retrospective case series focusing on adverse pregnancy outcomes, one of 10 pregnant women and people required ICU admission and mechanical ventilation Footnote 26. Another case series of three pregnant women and people with RSV found that two required ICU admission and mechanical ventilation, while the third was monitored as an outpatient Footnote 27. A case report describes a pregnant person admitted with RSV pneumonitis and sepsis, requiring ICU admission, mechanical ventilation and emergency C-section Footnote 28. However, data regarding the risk of ICU admission among pregnant women and people remain scarce.
Outcome for both infants and pregnant women and people—preterm labour/birth: Three studies reported data on the risk of preterm labour/birth associated with RSV infection. In the Pregnancy Influenza Vaccine Effectiveness Network study, no difference was observed in preterm, small for gestational age, and low birth weight births between RSV-positive and RSV-negative pregnant women and people Footnote 24. However, among ARI admissions without delivery during the hospital admission, RSV positivity was associated with subsequent preterm birth (29% vs. 15%). A study from Nepal showed a higher rate of preterm birth with RSV illness episodes during pregnancy (29% vs. 13%) Footnote 29. In a case series of ten pregnant women and people hospitalized with RSV, one had preterm birth (10%) Footnote 26. In summary, available evidence is insufficient to assess the risk of preterm labour/birth due to RSV infection during pregnancy.
Discussion
This rapid review offers insight into RSV burden in predominantly high-income countries, with a focus on Canada, the US and Europe. More robust evidence was available for infants and young children, with Canadian studies contributing significantly, while evidence for pregnant women and people primarily stemmed from small observational studies outside Canada. In infants and young children, medically attended RSV was common, and RSV hospitalization rates varied but generally decreased with age. Most hospitalized children had no underlying medical conditions. Approximately 5% of RSV-hospitalized children in Canada required ICU admission, and the risk of death was low. Respiratory syncytial virus caused a higher burden of disease than influenza in this population. Novel and previously unpublished data from the Yukon support the conclusions of this literature review, noting a higher burden of RSV than influenza and the highest burden in younger age groups. For pregnant women and people, RSV severity appeared to be similar to non-pregnant women and people, with an attack rate of 10%–13% during the respiratory virus season. One study reported higher RSV hospitalization rates than those for non-pregnant women and people. Data on ICU admission, death and preterm birth related to RSV in pregnancy were limited, although two studies suggested an association with preterm birth.
This rapid review highlights limitations in characterizing RSV burden in Canada. Studies often focused on RSV-associated hospitalization and ICU admission, which are critical outcomes for assessing severe clinical consequences. However, it is also essential to grasp the significance of other outcomes in the Canadian context, in particular medically attended RSV and death related to RSV infection. Currently, Canada has limited enhanced national RSV surveillance data. Recent research initiatives have leveraged existing healthcare administrative databases to characterize RSV burden; however, those data are expected to underestimate RSV disease especially in the community and outpatient setting due to undertesting in routine clinical care, the lack of generalizability to the Canadian population and healthcare coding systems that do not capture all possible contributors to RSV-related complications Footnote 30.
Limitations
This rapid review has limitations. It primarily focused on short-term outcomes and did not consider potential long-term effects such as asthma, which may be associated with early-life RSV infection Footnote 31Footnote 32. Detection of RSV infection was not limited to laboratory confirmation; some studies relied on clinical diagnostic codes, potentially inflating RSV incidence. Estimates were imprecise. Robust data on severe RSV outcomes in pregnant women and people were lacking; however, historically, pregnant women and people have not been known to specifically be at higher risk of RSV infection. Although the goal of forthcoming RSV immunization products is to reduce complications of RSV in infants, it is essential to also consider the potential benefits of an RSV vaccine for pregnant people, given their increased susceptibility to certain respiratory pathogens such as influenza resulting from pregnancy-related changes in anatomy and the immune and cardiovascular systems. This review did not specifically focus on RSV burden during the coronavirus disease 2019 (COVID-19) pandemic. The public health measures in place during the early phase of the pandemic led to a significant reduction of seasonal respiratory virus circulation Footnote 33. In recent seasons, there has been a substantial increase in RSV cases, with changes in age distribution and atypical seasonality patterns compared to prior to the COVID-19 pandemic, attributed to larger populations of RSV-naive children Footnote 34Footnote 35. For example, a recent publication from 13 paediatric centres in Canada noted a significant burden of RSV hospitalizations, with a significant increase in hospitalizations in 2021–2022 compared to pre-pandemic Footnote 36. Despite these limitations, the data presented here provide a foundation for understanding the typical RSV burden in infants and young children.
Conclusion
A high incidence of medically attended RSV is observed in infants and young children, with hospitalization rates decreasing with age. Approximately 5% of hospitalized infants and young children with RSV required ICU admission. The risk of death appears to have been low. Pregnant and non-pregnant women and people showed similar RSV severity, although data were limited for pregnant individuals. With the introduction of interventions, RSV's disease burden is expected to change; robust surveillance systems at the provincial, territorial and national levels will be crucial for evaluating the public health impact of RSV immunization programs. This review contributes to the literature, aiding in characterizing RSV's burden in Canada and guiding RSV immunization strategies for infant protection.
Authors' statement
- EMA — Conceptualization, data analysis, data interpretation, writing–original draft
- PDP — Conceptualization, data analysis, data interpretation, writing–original draft
- PD — Data analysis, data interpretation, writing–reviewing
- NB —Writing–reviewing
- AI — Writing–reviewing
- WS — Conceptualization, writing–reviewing
- AK — Conceptualization, writing–reviewing
Competing interests
None.
Acknowledgements
The authors wish to acknowledge the National Advisory Committee on Immunization (NACI) Secretariat team, Matthew Tunis, Kelsey Young, Mona Hersi, Adrienne Stevens, Anastassia Howarth, Su Hyun Lim, the Health Canada Library (Shannon Hayes), Liza Lee, Steven Buckrell, Michele Caws and the NACI RSV Working Group.
Funding
None.
Supplemental material
These documents can be accessed on the Supplemental material file.
