Public health contributions of entomological surveillance of WNV in a context of climate change

 Download this article as a PDF (247 KB)
Download this article as a PDF (247 KB)Published by: The Public Health Agency of Canada
Issue: CCDR Volume 50-9, September 2024: Health Risk Assessment
Date published: September 2024
ISSN: 1481-8531
Submit a manuscript
About CCDR
Browse
Volume 50-9, September 2024: Health Risk Assessment
Surveillance
Public health contributions of entomological surveillance of West Nile virus (WNV) and other mosquito-borne arboviruses in a context of climate change
Bouchra Bakhiyi1, Alejandra Irace-Cima1,2, Antoinette Ludwig3,4, Miarisoa Rindra Rakotoarinia1,4, Christian Therrien5, Isabelle Dusfour6, Ariane Adam-Poupart1,2
Affiliations
- 1 Department of Biological Risks, Institut national de santé publique du Québec (INSPQ), Montréal, QC
- 2 School of Public Health of the Université de Montréal (ESPUM), Université de Montréal, Montréal, QC
- 3 Public Health Risk Sciences Division, National Microbiology Laboratory, Public Health Agency of Canada, Saint-Hyacinthe, QC
- 4 Research Group on Epidemiology of Zoonoses and Public Health, Faculty of Veterinary Medicine, Université de Montréal, Saint-Hyacinthe, QC
- 5 Laboratoire de santé publique du Québec, Institut national de santé publique du Québec, Sainte-Anne-de-Bellevue, QC
- 6 Independent medical entomologist, Montpellier, France
Correspondence
Suggested citation
Bakhiyi B, Irace-Cima A, Ludwig A, Rakotoarinia MR, Therrien C, Dusfour I, Adam-Poupart A. Public health contributions of entomological surveillance of West Nile virus (WNV) and other mosquito-borne arboviruses in a context of climate change. Can Commun Dis Rep 2024;50(9):294–304. https://doi.org/10.14745/ccdr.v50i09a02
Keywords: mosquitoes, surveillance, arbovirus, public health, scoping review
Abstract
Background: Climate change is likely to increase the risk of human transmission of arboviruses endemic to Canada, including West Nile virus (WNV), Eastern equine encephalitis virus (EEEV) and California serogroup virus (CSV), calling for enhanced surveillance, including entomological surveillance targeting mosquito vectors. A scoping review was carried out to document the public health contributions of entomological surveillance of arboviruses of importance in Canada.
Methods: The Ovid® and EBSCO platforms and the grey literature were searched to identify documents published between 2009 and 2023, in English or French, dealing with entomological surveillance of arboviruses of interest, conducted annually for human health purposes under the aegis of a government authority, with specified public health objectives and actions.
Results: The 42 selected publications mainly reported two public health objectives of adult mosquito surveillance: early warning of viral circulation and assessment of the level of risk of human transmission. Recommended actions included clinical preparedness, risk communication, promotion of personal protection measures and vector control. The main objectives of immature mosquito surveillance were to identify sites with high larval densities, in order to reduce/eliminate them and target the application of larvicides.
Conclusion: In a context of climate change favouring the spread of arboviruses, this study highlights the potential public health contributions of regular entomological surveillance of endemic arboviruses of importance in Canada. It helps support concrete actions to protect the health of the population from the risks of arboviral transmission.
Introduction
Increased ambient temperatures and variability in precipitation patterns associated with climate change are conducive to an expansion in the geographic range of mosquito vectors of arboviruses endemic to Canada, an increase in their local abundance and a reduction in the extrinsic incubation period, enabling them to become infectious earlierFootnote 1Footnote 2. This greater dispersion would contribute to an increased risk of human transmission, particularly of West Nile virus (WNV), Eastern equine encephalitis virus (EEEV) and California serogroup viruses (CSV)Footnote 1Footnote 2Footnote 3.
These changes call for enhanced surveillance of such arboviruses to better assess the health risks to the Canadian populationFootnote 1 and target interventions more effectively. To the best of our knowledge, no synthesis of the public health objectives of entomological surveillance of mosquito-borne arboviruses has been published in Canada.
The aim of this scoping review was to document, as comprehensively as possible, the public health objectives of the entomological component of surveillance for arboviruses of interest, namely WNV, EEEV, Cache Valley virus (CVV) and the CSVs, including Jamestown Canyon virus (JCV) and Snowshoe Hare virus (SSHV). In other words, the aim was to show how entomological surveillance data can be used to support various actions designed to protect the population from the risk of arbovirus transmission. The purpose of this study is therefore to support thinking on the potential of surveillance of mosquito vectors of these arboviruses of importance in Canada by examining the relevance of such surveillance to concrete action by the authorities concerned, including the implementation of appropriate preventive measures and vector control.
Methods
Search strategy
A scoping review was conducted based on the methodological framework suggested by Arksey and O'MalleyFootnote 4 and improved by Levac et al.Footnote 5. Its specific aims were 1) to synthesize the public health objectives targeted by entomological surveillance of mosquito-borne arboviruses under different arboviral transmission scenarios as detailed below and 2) to describe how the resulting data can help support actions to protect the population. The public health objective implies, in fact, that the entomological surveillances reported in the literature are carried out with the aim of supporting concrete actions.
The research question was: "What are the contributions of the entomological component in the surveillance of WNV, EEEV, CVV and the CSVs, including JCV and SSHV, in a context of climate change?". For this research, the term "surveillance" refers to any process of ongoing data collection, carried out under the aegis of a governmental authority, particularly a public health authority, in order to guide its decisions, policies and responsesFootnote 6. The research question identified three major concepts that were combined as follows: "arboviruses transmitted by mosquitoes," "surveillance" and "mosquito vectors."
For each of these major concepts, a list of synonymous keywords was drawn up for searching the bibliographic databases of the Ovid® (Embase, Global Health and Medline®) and EBSCO (CINAHL® Complete, Environment Complete and GreenFILE) platforms, as well as CAB Abstracts (CABI), Engineering Village, Pascal and Francis, PubMed and Web of Science. No geographical restrictions were applied and the literature search covered the period from 2009 to 2023. A complementary search was carried out in the grey literature for the same time interval. It considered mainly Google and Google Scholar search engines, in addition to grey literature resources, including government websites, notably those of health agencies in Canadian provinces and US states, among which are those bordering Canada.
Relevant publications were selected initially by evaluating titles and abstracts, and then by reading the full text, where necessary. Inclusion and exclusion criteria required that publications 1) be written in English or French; 2) deal with entomological surveillance for human health purposes of our arboviruses of interest with field collection of mosquitoes; 3) be regularly conducted each year during the mosquito season and initiated, supervised, requested, required or supported by one or more government entity(ies); and 4) have explicit public health objectives and possible subsequent actions implemented or recommended. Publications dealing, for example, with pure research activities, such as the advancement of knowledge on the ecology of mosquito vectors or trapping techniques, without mentioning public health objectives/actions, were excluded.
Descriptive knowledge synthesis
A summary table was developed to report relevant data extracted from the selected publications. These data include the arboviruses and developmental stages targeted, the epidemiological situation during which entomological surveillance was carried out (i.e., no human cases, sporadic, endemic or epidemic human cases), the public health objectives targeted by this surveillance and the subsequent actions that can derive from the entomological data obtained.
The objectives identified were then classified according to four types of arboviral transmission scenarios based on the epidemiological situations described in the literature:
- No arboviral transmission: No human cases reported, no apparent arboviral transmission to the human population and low or unknown levels among reservoir hosts
- Sporadic: Human cases reported anecdotally, arboviral transmission considered sporadic, low level in the human population and among reservoir hosts
- Endemic: Human cases reported on a recurrent basis with no sign of sudden or rapid increase, arboviral transmission considered to be persistent in the human population and among reservoir hosts
- Epidemic: Sudden and rapid increase in human cases, arboviral transmission considered high and persistent in the human population and among reservoir hosts
Results
Figure 1 shows a flow chart illustrating the search and selection of relevant publications. Bibliographic database queries yielded 15,112 results. After removing duplicates, 7,392 scientific publications were evaluated by title and abstract. Only 121 were finally screened for eligibility by full text reading, including 10 literature reviews. These reviews were initially retained in an attempt to find relevant references not detected in the bibliographic databases. They were afterwards excluded. Of these 121 scientific publications, 23 were deemed eligible. Consultation of grey literature sources led to the addition of 18 documents, mainly recent entomological surveillance and intervention plans or reports. A grey literature document was also found in one of the accepted scientific articles. A total of 42 publications were included in the knowledge synthesis. The scientific articles are mainly from European countries, while the grey literature is more from North America. Most of these publications focused on two or even three mosquito-borne arboviruses. However, the vast majority of cases involved WNV (n=38), with the remainder involving EEEV (n=12) and JCV (n=3). No relevant documents on SSHV or CVV were identified (n=0).
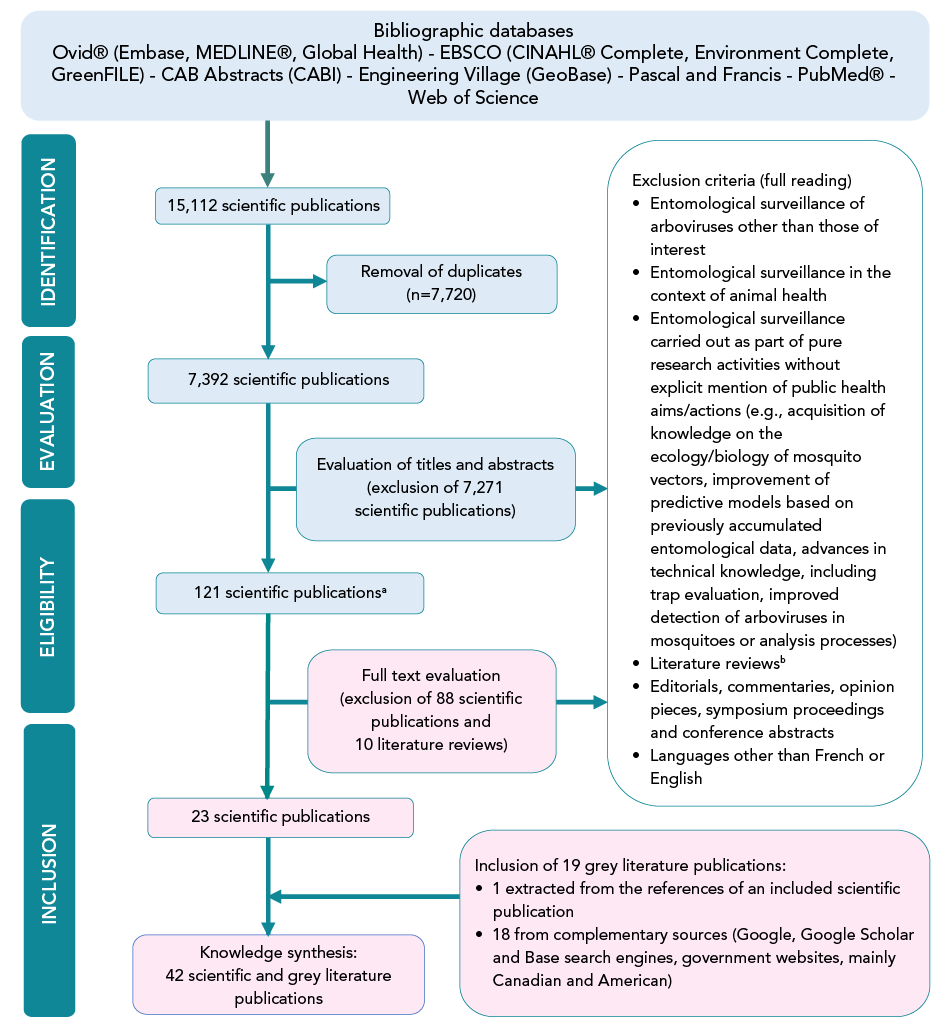
Figure 1 - Text description
Bibliographic database queries yielded 15,112 results. After removing duplicates, 7,392 scientific publications were evaluated by title and abstract. Only 121 were finally screened for eligibility by full text reading, including 10 literature reviews. These reviews were initially retained in an attempt to find relevant references not detected in the bibliographic databases. They were afterwards excluded. Of these 121 scientific publications, 23 were deemed eligible. Consultation of grey literature sources led to the addition of 18 documents, mainly recent entomological surveillance and intervention plans or reports. A grey literature document was also found in one of the accepted scientific articles. A total of 42 publications were included in the knowledge synthesis.
Footnotes
- Footnote a
-
The 121 articles retained following title and abstract evaluation included 111 to be assessed for eligibility by full text review and 10 literature reviews in an attempt to find any relevant publications not detected in the bibliographic databases
- Footnote b
-
Literature reviews were excluded after extracting 13 scientific publications which were not retained after reading of the full text
Restrictions used: from 2009 to 2023; no geographical restrictions were applied for document searches
A summary of the public health objectives of entomological surveillance of arboviruses of interest is presented in Table 1 (adult mosquitoes) and Table 2 (immature forms of mosquitoes). Surveillance of these different developmental stages is usually carried out concomitantly in order to consider the entire vector lifecycleFootnote 7Footnote 8Footnote 9Footnote 10Footnote 11Footnote 12Footnote 13Footnote 14Footnote 15Footnote 16Footnote 17Footnote 18Footnote 19Footnote 20. The two tables also include the number of publications reporting on each of the public health objectives, the arboviral transmission scenarios concerned and the arbovirus(es) of interest targeted per scenario, as well as examples of entomological indicators that help to achieve the targeted objectives.
| Public health objectives | nFootnote a | Arboviral transmission scenarios concerned | Example of entomological indicators used | ||||
|---|---|---|---|---|---|---|---|
| None | Sporadic | Endemic | Epidemic | ||||
| Early warning of viral circulation before the first human cases appear | 20 | WNV | WNV | WNV, EEEV, JCV | - | First positive mosquito pools for one and/or another of the arbovirusesFootnote 7Footnote 9Footnote 10Footnote 11Footnote 14Footnote 18Footnote 19Footnote 21Footnote 22Footnote 23Footnote 24Footnote 25Footnote 26Footnote 27Footnote 28Footnote 29Footnote 30Footnote 31Footnote 32Footnote 33 | |
| Human risk assessment | Assessing the level of risk of human transmissionFootnote b | 22 | WNV | WNV | WNV, EEEV, JCV | - | Spatiotemporal distribution and abundance of mosquitoes by identified species, number of mosquitoes per trap, number of positive mosquito pools, number of traps with positive mosquitoes, species type of positive mosquitoes (more ornithophilic or more that feed on mammals, including mosquitoes that feed on humans), number of weeks with positive mosquito pools, infection rateFootnote c, vector indexFootnote d Footnote 7Footnote 8Footnote 9Footnote 10Footnote 11Footnote 12Footnote 13Footnote 14Footnote 15Footnote 16Footnote 17Footnote 18Footnote 19Footnote 21Footnote 32Footnote 34Footnote 35Footnote 36Footnote 37Footnote 38Footnote 39Footnote 40 |
| Mapping levels of viral circulation intensity | 3 | - | - | WNV | - | Mosquito infection rate (maximum likelihood estimation and minimum infection rate)Footnote c Footnote 22Footnote 23Footnote 24 | |
| Predicting an outbreak of human cases | 2 | - | - | WNV | - | Proportion of positive mosquito pools, minimum infection rate, vector indexFootnote 22Footnote 33 | |
| Assessing resistance to insecticides used in vector control | 7 | - | - | WNV, EEEV, JCV | WNV | Mosquito abundance before and after insecticide treatment, presence and frequency of mutation genesFootnote 9Footnote 11Footnote 16Footnote 17Footnote 20Footnote 41Footnote 42 | |
| Real-time monitoring and support for efforts to reduce human transmission | 3 | - | - | - | WNV, EEEV | Mosquito abundance, number of positive mosquito pools, vector index, minimum infection rateFootnote 43Footnote 44Footnote 45 | |
| Contribution to the declaration of a health emergency linked to arboviruses | 1 | - | - | WNV, EEEV | - | Proportion of positive mosquito pools (health emergency declared as soon as 10% of bridge vectorFootnote e mosquito pools tested positive for WNV or EEEV)Footnote 14 | |
| Controlling the spread of the Culex population from flooded areasFootnote f | 1 | - | - | WNV | - | Abundance of Culex species per trapFootnote 35 | |
| Update of the list of potential vector species | 1 | - | - | WNV | - | Adult mosquito abundance by identified species, minimum infection rateFootnote 46 | |
| Documentation of WNV transmission and overwintering mechanisms in competent vectorsFootnote g | 1 | - | - | WNV | - | Presence of WNV in hibernating mosquitoesFootnote 9 | |
| Documenting the intensity of viral circulation during an epidemic year at international airportsFootnote h | 1 | - | - | - | WNV | Mosquito abundance by identified species, minimum infection rateFootnote 47 | |
| Warning of a potentially increased risk of arboviral transmission for next year's mosquito seasonFootnote i | 1 | - | - | - | WNV | Mosquito abundance by identified species, minimum infection rateFootnote 48 | |
| Abbreviations: EEEV, Eastern equine encephalitis virus; JCV, Jamestown Canyon virus; WNV, West Nile virus; -, not applicable Footnotes
|
|||||||
| Public health objectives | nFootnote a | Arboviral transmission scenarios concerned | Example of entomological indicators used | |||
|---|---|---|---|---|---|---|
| None | Sporadic | Endemic | Epidemic | |||
| Identifying larval breeding sitesFootnote b and determining high larval density areas | 16 | WNV | WNV | WNV, EEEV, JCV | - | Presence of eggs, larvae and pupae; abundance (or density) by identified species and developmental stageFootnote c Footnote 7Footnote 8Footnote 9Footnote 10Footnote 11Footnote 12Footnote 13Footnote 14Footnote 15Footnote 16Footnote 17Footnote 18Footnote 19Footnote 20Footnote 32Footnote 37 |
| Mapping of breeding sitesFootnote d | 6 | - | - | WNV, EEEV, JCV | - | Presence of breeding sites Footnote 12Footnote 15Footnote 17Footnote 18Footnote 19Footnote 20 |
| Abbreviations: EEEV, Eastern equine encephalitis virus; JCV, Jamestown Canyon virus; WNV, West Nile virus; -, not applicable Footnotes
|
||||||
The majority of public health objectives identified in the consulted literature concern the scenario of arboviral transmission at endemic level. Those most documented for adult mosquito surveillance, and common to WNV, EEEV and JCV, are:
- Early warning of viral circulation (n=20) before the first human cases appear and thanks to the first positive mosquito vectors pools for one and/or another of the arboviruses under surveillance.
- Assessment of the level of risk of human transmission (n=22) based on, among others, entomological indicators such as mosquito abundance, infection rate of arbovirus in mosquito population and vector index. These risk levels are generally described as low, moderate, high or very high.
The main objectives reported for surveillance of immature forms of the vectors of one and/or another of these three arboviruses are the identification of artificial and natural breeding sites and the determination of areas with high larval densities (n=16).
Table 3 and Table 4 summarize the main public health actions that can derive from adult and immature mosquito surveillance data, respectively. These actions are presented for each surveillance objective. For adult forms, they include:
- Clinical preparedness to strengthen human surveillance, particularly through greater vigilance in recognizing and diagnosing illnesses linked to these three arboviruses, as well as increased laboratory resources for confirmatory testing of human cases.
- Real-time risk communication by the responsible authorities to local authorities, healthcare providers, the media and the general public.
- Ongoing education/awareness campaigns, using a variety of communication channels to increase outreach efforts, aimed at the general population and healthcare professionals. These campaigns focus mainly on personal protection measures (e.g., long-sleeved clothing, mosquito nets, use of repellents) and participation in source reduction efforts by eliminating peridomestic stagnant water (e.g., emptying artificial containers, recovering used tires, swimming pool maintenance).
- Vector control, including the ground-based and/or aerial application of larvicidal treatments or even of adulticides, when the level of risk of human transmission is deemed high or critical.
| Surveillance objectives | Public health actions | ||||
|---|---|---|---|---|---|
| Clinical preparednessFootnote a | Risk communicationFootnote b | Ongoing awareness/education campaignsFootnote c | Vector controlFootnote d | ||
| Early warning of viral circulation before the first human cases appear | XFootnote e | XFootnote e | XFootnote e | XFootnote e | |
| Human risk assessment | Assessment of the level of risk of human transmission | XFootnote e | XFootnote e | XFootnote e | XFootnote e |
| Mapping levels of viral circulation intensity | - | - | - | XFootnote e | |
| Predicting an outbreak of human cases | - | - | - | XFootnote e | |
| Evaluation of resistance to insecticides used in vector controlFootnote f | - | - | - | XFootnote e | |
| Real-time monitoring and support for efforts to reduce human epidemic transmissionFootnote g | XFootnote e | XFootnote e | XFootnote e | XFootnote e | |
| Contribution to the declaration of a health emergency linked to arbovirusesFootnote h | - | XFootnote e | XFootnote e | XFootnote e | |
| Controlling the spread of the Culex population from flooded areas | - | XFootnote e | XFootnote e | XFootnote e | |
| Update of the list of potential vector speciesFootnote f | - | - | - | XFootnote e | |
| Documentation of WNV transmission and overwintering mechanisms in competent vectors | - | - | - | XFootnote e | |
| Documenting the intensity of viral circulation during an epidemic year at international airports | - | - | - | XFootnote e | |
| Warning of a potentially increased risk of arboviral transmission for next year's mosquito seasonFootnote i | - | XFootnote e | XFootnote e | - | |
| Abbreviations: WNV, West Nile virus; -, not applicable Footnotes
|
|||||
| Surveillance objectives | Public health actions | |||
|---|---|---|---|---|
| Source reductionFootnote a | Targeted larvicidal treatmentsFootnote b | Evaluation of larvicidal treatmentsFootnote c | Real-time monitoring of larvicide deploymentFootnote d | |
| Identifying larval breeding sites and determining high larval density areas | XFootnote e | XFootnote e | XFootnote e | - |
| Mapping of breeding sitesFootnote f | - | - | - | XFootnote e |
Footnotes
|
||||
Discussion
Entomological surveillance of mosquito-borne arboviruses: a valuable contribution to public health
This literature review has documented the public health objectives that can be achieved through entomological surveillance of arboviruses of interest, as well as the subsequent actions that can derive from the resulting data. These objectives were reported by developmental stage monitored, i.e., adult and immature mosquitoes, and by arboviral transmission scenario. This strategic breakdown of public health objectives/actions according to various scenarios offers some avenues for reflection to carry out mosquito surveillance. Any authority concerned could then, according to its priorities, opt for the appropriate objective(s) in line with the arboviral transmission scenario prevailing in the region targeted for entomological surveillance of mosquito-borne arboviruses.
The major finding of this study is that mosquito surveillance can help support the implementation of actions to protect human health from the risk of arboviral transmission. Real-time exploitation of entomological data from adult mosquito surveillance provides useful and rapid information to the authorities concerned by contributing to early warning of viral circulation and by helping to assess the level of risk of human transmission to support more prompt and informed management. The aim is to decrease arboviral transmission and limit human cases by implementing a range of preventive public health actions, including vector control. The latter intends to reduce the abundance of infected or potentially infected vectors, thereby lowering the environmental viral load.
Early warning of viral circulation is based on the detection of the first positive mosquito pools for the arboviruses under surveillance, which usually occur a few days or even several weeks before human cases appearFootnote 9Footnote 22Footnote 23Footnote 26Footnote 30Footnote 31Footnote 42. In New York City, for example, data collected between 2000 and 2022 showed that WNV was detected in mosquitoes weeks before any risk of human transmission became significantFootnote 9. The main purpose of this alert is to rapidly initiate clinical preparedness activities, as described above, risk communication, coordination and training of local health officials and personnel involved in entomological surveillance, as well as the development of materials for public awareness/education campaigns on preventive measures. Early warning also enables vector control to be initiated, including source reduction, to limit the spread of infected mosquitoes to densely populated areasFootnote 7Footnote 8Footnote 9Footnote 14Footnote 17Footnote 18Footnote 19Footnote 21Footnote 22Footnote 23Footnote 24Footnote 25Footnote 26Footnote 27Footnote 28Footnote 29Footnote 30Footnote 31Footnote 32Footnote 33
The level of risk of human transmission is assessed throughout the mosquito season using real-time entomological data from the current year, often combined with those from previous yearsFootnote 10Footnote 11Footnote 12Footnote 13Footnote 16Footnote 17Footnote 18. These data are most often also combined with other parameters, as no single indicator can provide an accurate measure of riskFootnote 10Footnote 18. These parameters includeFootnote 9Footnote 11Footnote 13Footnote 14Footnote 16Footnote 17Footnote 18Footnote 35:
- Immature mosquito surveillance data, including type and location of breeding sites and their proximity to the human population at risk and larval abundance
- Human and animal surveillance data (wild birds, chickens, horses, etc.)
- The time of year
- Current and projected local weather conditions (degree-day accumulation, precipitation, wind speed, etc.)
- The density of the human population at risk, particularly those close to larval breeding sites
The use of entomological data to estimate the level of risk of human transmission is justified by the statistically positive correlation between mosquito abundance, vector index and/or mosquito infection rate, on the one hand, and the number of human cases, on the other. This correlation has been well documented for WNV in CanadaFootnote 49Footnote 50 and elsewhere in the worldFootnote 33Footnote 44Footnote 51Footnote 52.
This risk level assessment helps guide the rapid implementation and gradual, targeted and proportionate intensification of public health actions, including regular risk communication and updates, education/awareness-raising through public outreach campaign on preventive measures and vector control. The focus is put on a gradual reinforcement of personal protection measures for humans and source reduction measures, or even a possible restriction of outdoor activities to decrease exposure risksFootnote 10Footnote 13Footnote 16Footnote 17Footnote 18Footnote 34Footnote 35Footnote 36Footnote 37Footnote 38. The states of MassachusettsFootnote 10, VermontFootnote 17, New HampshireFootnote 18 and Rhode IslandFootnote 38 have, in fact, developed guidelines revealing the entomological data, combined or not with other parameters, that define levels of risk of human transmission and the subsequent public health responses.
Outbreak risk assessment is generally based on the vector index, which predicts an increase in the number of human cases over the following two to three weeksFootnote 33Footnote 43Footnote 50. This predictive effort can be used to guide vector control strategies in order to prioritize areas identified as being most at riskFootnote 32Footnote 33.
Other public health objectives were identified in the consulted literature. Although few publications have reported on them, they remain relevant. One example is the assessment of resistance to insecticides used in vector control, which is essential for evidence-based strategy revision. Another example would be the contribution to the declaration of a health emergency linked to arboviruses, prompting the creation of a panel of experts (epidemiologists, veterinarians, vector control experts, biologists, local representatives) and the setting up of an emergency operations centre for coordinated, faster and more effective public health interventionsFootnote 14.
Finally, surveillance of immature mosquitoes is essential, as it enables targeted larvicidal treatments to help reduce the adult mosquito population, particularly when the level of risk of human transmission is deemed highFootnote 11Footnote 17. Detailed documentation of the presence and abundance of immature mosquitoes, the developmental stages treated by larvicides, the size of breeding sites and the effectiveness of vector control is considered of great value in continuously estimating the likely size of future adult mosquito populationsFootnote 10Footnote 14Footnote 18.
Optimal conditions for more effective entomological surveillance
The literature review also identified relevant information on the optimal conditions for strengthening the efficiency of entomological surveillance strategies for adult mosquitoes to achieve the main public health objectives documented.
As an early warning tool for viral circulation
As reported for WNV, early warning of viral circulation depends on certain operational modalities, in particular:
- Intensive trapping to increase the number of mosquitoes to be collected and tested, as this parameter is crucial for the sensitivity of early arbovirus detectionFootnote 21Footnote 28Footnote 30. This condition implies a substantial number of mosquito traps located in "hot spots," selected according to a multifactorial approach (e.g., presence of wetlands and other water bodies, human population density, meteorological parameters)Footnote 21Footnote 30. Thomas-Bachli et al.Footnote 53 demonstrated that increasing the number of traps in Ontario, combined with shifting their locations to areas where WNV had been detected in previous years, improved detection times for arbovirus in mosquitoes, which became similar to or even shorter than those associated with dead corvid surveillanceFootnote 53. A judicious choice of the type of mosquito traps and their wide deployment also enhances the ability of entomological surveillance to provide early warning of viral circulationFootnote 25.
- Rapid acquisition, ideally within a few days, of results of WNV screening in mosquito populationFootnote 9Footnote 26Footnote 27.
- Maintain regular surveillance on an annual basis, preferably from May until the end of the mosquito season (usually late September), in order to improve strategy and refine early detection capabilities and sensitivityFootnote 24.
- Collaboration between veterinary and human health services, as well as between medical entomologists and ornithologists, in addition to coordination and data management at national, regional and local levelsFootnote 24.
- Regular updating of the entomological surveillance program in line with available data (results from the previous year and those obtained from research studies) and funding opportunitiesFootnote 24Footnote 27.
As a tool for assessing the level of risk of human transmission
The development of appropriate models for assessing human transmission risk levels, using entomological data, also requires ongoing surveillance carried out every year during the mosquito season. It also calls for rapid processing and analysis of entomological data, so that the necessary preventive measures can be implemented without delayFootnote 27Footnote 50. It is also strongly recommended that monitoring programs include permanent traps placed at fixed stations, with a long-term perspective, in order to develop a historical baseline for detecting spatiotemporal trends in mosquito abundance and arbovirus prevalence within their populations. In fact, the assessment of the level of risk of human transmission generally incorporates the results of mosquito surveillance from previous years. The constant accumulation of entomological data, year after year, also offers the opportunity to improve the robustness of predictive models for more accurate estimates of human risk, including the occurrence of outbreaksFootnote 10Footnote 14Footnote 18Footnote 19Footnote 24Footnote 27. In New York City, comprehensive vector and human surveillance data collected over the years 2006 to 2022 enabled health authorities to develop a more sensitive protocol for assessing the level of WNV activity and human disease risk across the entire cityFootnote 9. In Massachusetts, routine seasonal data collection over a period of several years also considerably improved the accuracy of assessing the level of risk of human transmission at the municipal levelFootnote 10.
Karki et al.Footnote 54 have demonstrated, for the state of Illinois, that the power of predictive models based on vector index increases in regions with abundant entomological data. The authors also highlighted their usefulness, when collected over the long term, for developing risk assessments at specific times and in specific regions to guide an appropriate public health responseFootnote 54.
On the other hand, Kilpatrick and PapeFootnote 52 warned against the loss of data resulting from discontinuous entomological surveillance. Thus, a pronounced decline in the predictive power of the models used is expected in the absence of arbovirus prevalence and mosquito abundance data for the surveillance year. Moreover, the decline in predictive power is exacerbated by delays in processing and analyzing entomological dataFootnote 52.
Finally, it should be noted that the consulted literature also highlighted the importance of sharing roles and responsibilities for the smooth running of entomological surveillance programs, including the operational aspect (e.g., selection of locations and installation of mosquito traps), the assessment of the level of risk of human transmission and the resulting public health responses, particularly the application of larvicides and adulticides. Mosquito surveillance programs can therefore involve various levels of health authorities, as well as other government bodies, notably from the agricultural and environmental sectors, and local administrations such as municipalitiesFootnote 14Footnote 16Footnote 17Footnote 19Footnote 38Footnote 45.
Strengths and limitations
The main strength of this work lies in the inclusion of publications dealing with entomological surveillance carried out annually, on a regular and uninterrupted basis, while involving government authorities at the national, regional or local level. This approach lent greater weight to the public health objectives of this surveillance and subsequent actions documented in the literature. Furthermore, the surveillance programs described are still underway in the countries/regions concerned, as they are considered to be relevant, which further strengthens this knowledge synthesis. However, this review cannot claim to be exhaustive, as additional results could have been identified with less stringent inclusion criteria. In addition, there have been few publications on EEEV and the CSVs and no relevant literature on SSHV or CVV has been identified. These factors could make it harder to infer about these arboviruses, which, however, are likely to grow in importance in the coming years. Beyond these limitations, we are confident in the relevance and significance of the results obtained and believe that this review remains a first description of the synthesis of the objectives of entomological surveillance of arboviruses of public health interest and endemic to Canada.
Conclusion
In a context of climate change conducive to the spread of arboviruses, this knowledge synthesis supports the usefulness and relevance of entomological surveillance of arboviruses of interest in Canada, namely WNV, EEEV and JCV. Its contribution to public health is nevertheless grounded in a regular annual deployment during the mosquito season, according to the objectives pursued by the authorities concerned, while using a judicious number and locations of mosquito traps. For optimum benefit, it is also vital that entomological data are analyzed and shared rapidly to support effective actions, integrating clinical preparedness, real-time and ongoing risk communication as well as timely implementation of preventive measures. Entomological surveillance of arboviruses of public health importance should be maintained and strengthened, taking into consideration expected changes, due to climate variations, in mosquito populations and the diseases they carry in Canada.
Authors' statement
- BB — Conceptualization, writing–original draft, revision and critical review
- AIC — Conceptualization, revision and critical review
- AL — Revision and critical review
- MRR — Revision and critical review
- CT — Revision and critical review
- ID — Revision and critical review
- AAP — Conceptualization, revision and critical review
The contents and opinions expressed in this article are those of the authors and do not necessarily reflect those of the Government of Canada.
Competing interests
None.
Funding
This publication was carried out through funding from the Green Fund as part of action 6.4.1 of the Government of Québec's Climate Change Action Plan (MI-PACC).
References
- Footnote 1
-
Ludwig A, Zheng H, Vrbova L, Drebot MA, Iranpour M, Lindsay LR. Increased risk of endemic mosquito-borne diseases in Canada due to climate change. Can Commun Dis Rep 2019;45(4):91–7. https://doi.org/10.14745/ccdr.v45i04a03
- Footnote 2
-
Ogden NH, Gachon P. Climate change and infectious diseases: What can we expect? Can Commun Dis Rep 2019;45(4):76–80. https://doi.org/10.14745/ccdr.v45i04a01
- Footnote 3
-
Rosenkrantz L, National Collaborating Centre for Environmental Health. Impacts of Canada's changing climate on West Nile Virus vectors. Vancouver, BC: NCCEH; 2022. https://ncceh.ca/sites/default/files/Mosquito_EvidenceReview_Nov1_EN.pdf
- Footnote 4
-
Arksey H, O'Malley L. Scoping studies: towards a methodological framework. International journal of social research methodology. Int J Soc Res Methodol 2005;8(1):19–32. https://doi.org/10.1080/1364557032000119616
- Footnote 5
-
Levac D, Colquhoun H, O'Brien KK. Scoping studies: advancing the methodology. Implement Sci 2010;5(69):69. https://doi.org/10.1186/1748-5908-5-69
- Footnote 6
-
Lussier MT, Richard C, Bennett TL, Williamson T, Nagpurkar A. Surveillance or research: what's in a name? Can Fam Physician 2012;58(1):117. https://pubmed.ncbi.nlm.nih.gov/22267632
- Footnote 7
-
Osório HC, Zé-Zé L, Amaro F, Alves MJ. Mosquito surveillance for prevention and control of emerging mosquito-borne diseases in Portugal - 2008-2014. Int J Environ Res Public Health 2014;11(11):11583–96. https://doi.org/10.3390/ijerph111111583
- Footnote 8
-
Medlock JM, Guillem R, Johnston C, Gandy S, Findlay-Wilson S, Desoisa K, Schaffner F, Vaux AG. The first 6 years of surveillance of Aedes albopictus (Diptera: Culicidae) in Gibraltar. J Eur Mosq Control Assoc 2022;40:23–35. https://doi.org/10.52004/JEMCA2022.0001
- Footnote 9
-
Bajwa W, Slavinski S, Shah Z, Zhou L, Herbert V. Comprehensive Mosquito Surveillance and Control Plan. New York City Department of Health and Mental Hygiene; 2023. p. 41. https://www.nyc.gov/assets/doh/downloads/pdf/wnv/2023/wnvplan2023.pdf
- Footnote 10
-
Brown CM, DeMaria A Jr, Gallagher GR, Osborne M, Stinson C, Smole S, Werner BG. Massachusetts Arbovirus Surveillance and Response Plan. Bureau of infectious Disease and Laboratory Sciences, Massachusetts Department of Public Health; 2023. https://www.mass.gov/lists/arbovirus-surveillance-plan-and-historical-data
- Footnote 11
-
California Department of Public Health. California Mosquito-borne Virus Surveillance & Response Plan. Mosquito & Vector Control Association of California; 2023. https://westnile.ca.gov/pdfs/CAMosquitoSurveillanceResponsePlan.pdf
- Footnote 12
-
City of Fort Collins. West Nile Virus Program Manual. Fort Collins, CO: City of Fort Collins; 2014. https://www.fcgov.com/westnile/pdf/wnv_program_manual.pdf
- Footnote 13
-
Government of Saskatchewan. West Nile Virus (WNV). Saskatoon, SK: Government of Saskatchewan; 2023. https://www.saskatchewan.ca/residents/health/diseases-and-conditions/west-nile-virus
- Footnote 14
-
Maine Department of Health and Human Services/Maine Center for Disease Control and Prevention. Arboviral (Mosquito-Borne) Illness, Surveillance, Prevention, and Response Guidance for Maine Towns and Communities. Augusta, ME: Maine DHHS; 2021. https://www.maine.gov/dhhs/mecdc/infectious-disease/epi/vector-borne/documents/2022-Arbo-Plan.pdf
- Footnote 15
-
Manitoba Health. West Nile Virus Program 2023: Planning Documents for Municipalities: I. Provincial West Nile Viruses Program Information. Winnipeg, MB: Manitoba Health; 2023. https://www.gov.mb.ca/health/wnv/docs/wnv_program_information_2023.pdf
- Footnote 16
-
Ontario Ministry of Health. West Nile Virus Preparedness and Prevention Plan. Toronto, ON: King's Printer for Ontario; 2023. https://files.ontario.ca/moh-ophs-ref-west-nile-virus_plan-2023-en.pdf
- Footnote 17
-
Vermont Agency of Agriculture, Food, and Markets/Vermont Department of Public Health. State of Vermont. Arbovirus Surveillance and Response Plan. Vermont, NH: Vermont Department of Public Health; 2022. https://www.healthvermont.gov/sites/default/files/documents/pdf/HS-ID-Arbovirus-Surveillance-Response-Plan.pdf
- Footnote 18
-
State of New Hampshire. Arboviral Illness Surveillance, Prevention and Response Plan. Department of Health and Human Services, Division of Public Health Service; 2023. https://www.dhhs.nh.gov/sites/g/files/ehbemt476/files/documents/2021-11/arboviralresponse.pdf
- Footnote 19
-
New York State Department of Health. Mosquito Borne Illness Surveillance & Response Plan. New York, NY: New York State Department of Health; 2012. https://www.health.ny.gov/diseases/west_nile_virus/docs/2012_mosquito_borne_illness_surveillance_and_response_plan.pdf
- Footnote 20
-
Saginaw County Mosquito Abatement Comission. Surveillance Biology. Saginaw County, MI: Saginaw County Mosquito Abatement Comission; 2024. https://www.saginawmosquito.com/programs/surveillance
- Footnote 21
-
Pautasso A, Radaelli MC, Ballardini M, Francese DR, Verna F, Modesto P, Grattarola C, Desiato R, Bertolini S, Vitale N, Ferrari A, Rossini I, Accorsi A, Mosca A, Monaco F, Savini G, Prearo M, Mignone W, Chiavacci L, Casalone C. Detection of West Nile and Usutu Viruses in Italian Free Areas: Entomological Surveillance in Piemonte and Liguria Regions, 2014. Vector Borne Zoonotic Dis 2016;16(4):292–4. https://doi.org/10.1089/vbz.2015.1851
- Footnote 22
-
Calzolari M, Angelini P, Bolzoni L, Bonilauri P, Cagarelli R, Canziani S, Cereda D, Cerioli MP, Chiari M, Galletti G, Moirano G, Tamba M, Torri D, Trogu T, Albieri A, Bellini R, Lelli D. Enhanced West Nile Virus Circulation in the Emilia-Romagna and Lombardy Regions (Northern Italy) in 2018 Detected by Entomological Surveillance. Front Vet Sci 2020;7:243. https://doi.org/10.3389/fvets.2020.00243
- Footnote 23
-
Petrović T, Šekler M, Petrić D, Lazić S, Debeljak Z, Vidanović D, Ignjatović Ćupina A, Lazić G, Lupulović D, Kolarević M, Plavšić B. Methodology and results of integrated WNV surveillance programmes in Serbia. PLoS One 2018;13(4):e0195439. https://doi.org/10.1371/journal.pone.0195439
- Footnote 24
-
Petrović T, Šekler M, Petrić D, Vidanović D, Debeljak Z, Lazić G, Lupulović D, Kavran M, Samojlović M, Ignjatović Ćupina A, Tešović B, Lazić S, Kolarević M, Labus T, Djurić B. Intensive West Nile Virus Circulation in Serbia in 2018-Results of Integrated Surveillance Program. Pathogens 2021;10(10):1294. https://doi.org/10.3390/pathogens10101294
- Footnote 25
-
Verna F, Modesto P, Radaelli MC, Francese DR, Monaci E, Desiato R, Grattarola C, Peletto S, Mosca A, Savini G, Chianese R, Demicheli V, Prearo M, Chiavacci L, Pautasso A, Casalone C. Control of Mosquito-Borne Diseases in Northwestern Italy: Preparedness from One Season to the Next. Vector Borne Zoonotic Dis 2017;17(5):331–9. https://doi.org/10.1089/vbz.2016.2047
- Footnote 26
-
Papa A, Gewehr S, Tsioka K, Kalaitzopoulou S, Pappa S, Mourelatos S. Detection of flaviviruses and alphaviruses in mosquitoes in Central Macedonia, Greece, 2018. Acta Trop 2020;202:105278. https://doi.org/10.1016/j.actatropica.2019.105278
- Footnote 27
-
Patsoula E, Vakali A, Balatsos G, Pervanidou D, Beleri S, Tegos N, Baka A, Spanakos G, Georgakopoulou T, Tserkezou P, Van Bortel W, Zeller H, Menounos P, Kremastinou J, Hadjichristodoulou C. West Nile Virus Circulation in Mosquitoes in Greece (2010-2013). BioMed Res Int 2016;2016:2450682. https://doi.org/10.1155/2016/2450682
- Footnote 28
-
Tsioka K, Gewehr S, Pappa S, Kalaitzopoulou S, Stoikou K, Mourelatos S, Papa A. West Nile Virus in Culex Mosquitoes in Central Macedonia, Greece, 2022. Viruses 2023;15(1):224. https://doi.org/10.3390/v15010224
- Footnote 29
-
Alba A, Allepuz A, Napp S, Soler M, Selga I, Aranda C, Casal J, Pages N, Hayes EB, Busquets N. Ecological surveillance for West Nile in Catalonia (Spain), learning from a five-year period of follow-up. Zoonoses Public Health 2014;61(3):181–91. https://doi.org/10.1111/zph.12048
- Footnote 30
-
Calzolari M, Bonilauri P, Bellini R, Albieri A, Defilippo F, Maioli G, Galletti G, Gelati A, Barbieri I, Tamba M, Lelli D, Carra E, Cordioli P, Angelini P, Dottori M. Evidence of simultaneous circulation of West Nile and Usutu viruses in mosquitoes sampled in Emilia-Romagna region (Italy) in 2009. PLoS One 2010;5(12):e14324. https://doi.org/10.1371/journal.pone.0014324
- Footnote 31
-
Calzolari M, Monaco F, Montarsi F, Bonilauri P, Ravagnan S, Bellini R, Cattoli G, Cordioli P, Cazzin S, Pinoni C, Marini V, Natalini S, Goffredo M, Angelini P, Russo F, Dottori M, Capell G, Savini G. New incursions of West Nile virus lineage 2 in Italy in 2013: the value of the entomological surveillance as early warning system. Vet Ital 2013;49(3):315–9. https://doi.org/10.12834/VetIt.1308.04
- Footnote 32
-
Saginaw County Mosquito Abatement Commission. Annual Report. Saginaw County, MI: Saginaw County Mosquito Abatement Commission; 2022. https://www.saginawmosquito.com/news/publications/annualreport
- Footnote 33
-
Gobbi F, Capelli G, Angheben A, Giobbia M, Conforto M, Franzetti M, Cattelan AM, Raise E, Rovere P, Mulatti P, Montarsi F, Drago A, Barzon L, Napoletano G, Zanella F, Pozza F, Russo F, Rosi P, Palù G, Bisoffi Z. Summer Fever Study Group. Human and entomological surveillance of West Nile fever, dengue and chikungunya in Veneto Region, Italy, 2010-2012. BMC Infect Dis 2014;14:60. https://doi.org/10.1186/1471-2334-14-60
- Footnote 34
-
City of Fort Collins. Program Response Guidelines to Mosquito-Borne Arboviral Activity. Fort Collins, CO: City of Fort Collins; 2018. https://www.fcgov.com/westnile/pdf/WNV_Response_Plan_March_2018.pdf?1568214777
- Footnote 35
-
Kansas Department of Health and Environment. Arboviral Disease Surveillance. Kansas, KS: Kansas Department of Health and Environment; 2019. https://www.kdhe.ks.gov/DocumentCenter/View/23922/Arboviral-Surveillance-Report-2019-PDF
- Footnote 36
-
Kansas Department of Health and Environment. West Nile Virus. Kansas, KS: Kansas Department of Health and Environment; 2023. https://www.kdhe.ks.gov/1519/West-Nile-Virus
- Footnote 37
-
Ginsberg HS, Gettman A, Becker E, Bandyopadhyay AS, Lebrun RA. Environmental management of mosquito-borne viruses in Rhode Island. R I Med J 2013;96(7):37–41. http://www.rimed.org/rimedicaljournal/2013/07/2013-07-37-cont-mosquitos.pdf
- Footnote 38
-
Rhode Island Department of Health. Guidelines for Phased Response to Eastern Equine Encephalitis (EEE) Surveillance Data. Division of Preparedness, Response, Infectious Disease, and EMS Center for Acute Infectious Disease Epidemiology. Rhode Island, RI: Rhode Island Department of Health; 2019. https://health.ri.gov/publications/guidelines/PhasedResponseToEEESurveillanceData.pdf
- Footnote 39
-
Oliver J, Lukacik G, Kokas J, Campbell SR, Kramer LD, Sherwood JA, Howard JJ. Twenty years of surveillance for Eastern equine encephalitis virus in mosquitoes in New York State from 1993 to 2012. Parasit Vectors 2018;11(1):362. https://doi.org/10.1186/s13071-018-2950-1
- Footnote 40
-
Public Health Ontario. Why Is It Important to Monitor Ontario's Mosquitoes? Ottawa, ON: PHO; 2018. https://www.publichealthontario.ca/en/about/news/2017/monitor-mosquitoes
- Footnote 41
-
Fotakis EA, Mavridis K, Kampouraki A, Balaska S, Tanti F, Vlachos G, Gewehr S, Mourelatos S, Papadakis A, Kavalou M, Nikolakakis D, Moisaki M, Kampanis N, Loumpounis M, Vontas J. Mosquito population structure, pathogen surveillance and insecticide resistance monitoring in urban regions of Crete, Greece. PLoS Negl Trop Dis 2022;16(2):e0010186. https://doi.org/10.1371/journal.pntd.0010186
- Footnote 42
-
Mavridis K, Fotakis EA, Kioulos I, Mpellou S, Konstantas S, Varela E, Gewehr S, Diamantopoulos V, Vontas J. Detection of West Nile Virus - Lineage 2 in Culex pipiens mosquitoes, associated with disease outbreak in Greece, 2017. Acta Trop 2018;182:64–8. https://doi.org/10.1016/j.actatropica.2018.02.024
- Footnote 43
-
Kretschmer M, Ruberto I, Townsend J, Zabel K, Will J, Maldonado K, Busser N, Damian D, Dale AP. Unprecedented Outbreak of West Nile Virus - Maricopa County, Arizona, 2021. MMWR Morb Mortal Wkly Rep 2023;72(17):452–7. https://doi.org/10.15585/mmwr.mm7217a1
- Footnote 44
-
Martinez D, Murray KO, Reyna M, Arafat RR, Gorena R, Shah UA, Debboun M. West Nile Virus Outbreak in Houston and Harris County, Texas, USA, 2014. Emerg Infect Dis 2017;23(8):1372–6. https://doi.org/10.3201/eid2308.170384
- Footnote 45
-
Quilliam DN, Gosciminski M, Bandy U. Eastern Equine Encephalitis Surveillance and Response, Rhode Island, 2019. R I Medicine 2020;103(3):68–70.
- Footnote 46
-
Noden BH, Coburn L, Wright R, Bradley K. An Updated Checklist of the Mosquitoes of Oklahoma Including New State Records and West Nile Virus Vectors, 2003-06. J Am Mosq Control Assoc 2015;31(4):336–45. https://doi.org/10.2987/moco-31-04-336-345.1
- Footnote 47
-
Bakran-Lebl K, Camp JV, Kolodziejek J, Weidinger P, Hufnagl P, Cabal Rosel A, Zwickelstorfer A, Allerberger F, Nowotny N. Diversity of West Nile and Usutu virus strains in mosquitoes at an international airport in Austria. Transbound Emerg Dis 2022;69(4):2096–109. https://doi.org/10.1111/tbed.14198
- Footnote 48
-
Kemenesi G, Krtinić B, Milankov V, Kutas A, Dallos B, Oldal M, Somogyi N, Németh V, Bányai K, Jakab F. West Nile virus surveillance in mosquitoes, April to October 2013, Vojvodina province, Serbia: implications for the 2014 season. Euro Surveill 2014;19(16):20779. https://doi.org/10.2807/1560-7917.ES2014.19.16.20779
- Footnote 49
-
Giordano BV, Kaur S, Hunter FF. West Nile virus in Ontario, Canada: A twelve-year analysis of human case prevalence, mosquito surveillance, and climate data. PLoS One 2017;12(8):e0183568. https://doi.org/10.1371/journal.pone.0183568
- Footnote 50
-
Albrecht L, Kaufeld KA. Investigating the impact of environmental factors on West Nile virus human case prediction in Ontario, Canada. Front Public Health 2023;11:1100543. https://doi.org/10.3389/fpubh.2023.1100543
- Footnote 51
-
Bolling BG, Barker CM, Moore CG, Pape WJ, Eisen L. Seasonal patterns for entomological measures of risk for exposure to Culex vectors and West Nile virus in relation to human disease cases in northeastern Colorado. J Med Entomol 2009;46(6):1519–31. https://doi.org/10.1603/033.046.0641
- Footnote 52
-
Kilpatrick AM, Pape WJ. Predicting human West Nile virus infections with mosquito surveillance data. Am J Epidemiol 2013;178(5):829–35. https://doi.org/10.1093/aje/kwt046
- Footnote 53
-
Thomas-Bachli AL, Pearl DL, Berke O, Parmley EJ, Barker IK. A comparison of West Nile virus surveillance using survival analyses of dead corvid and mosquito pool data in Ontario, 2002-2008. Prev Vet Med 2015;122(3):363–70. https://doi.org/10.1016/j.prevetmed.2015.10.007
- Footnote 54
-
Karki S, Westcott NE, Muturi EJ, Brown WM, Ruiz MO. Assessing human risk of illness with West Nile virus mosquito surveillance data to improve public health preparedness. Zoonoses Public Health 2018;65(1):177–84. https://doi.org/10.1111/zph.12386
